Abstract
East Asia has experienced an excessive increase in myopia in the past decades with more than 80% of the younger generation now affected. Environmental and genetic factors are both assumed to contribute in the development of refractive errors, but the etiology is unknown. The environmental factor argued to be of greatest importance in preventing myopia is high levels of daylight exposure. If true, myopia prevalence would be higher in adolescents living in high latitude countries with fewer daylight hours in the autumn-winter. We examined the prevalence of refractive errors in a representative sample of 16–19-year-old Norwegian Caucasians (n = 393, 41.2% males) in a representative region of Norway (60° latitude North). At this latitude, autumn-winter is 50 days longer than summer. Using gold-standard methods of cycloplegic autorefraction and ocular biometry, the overall prevalence of myopia [spherical equivalent refraction (SER) ≤−0.50 D] was 13%, considerably lower than in East Asians. Hyperopia (SER ≥ + 0.50 D), astigmatism (≥1.00 DC) and anisometropia (≥1.00 D) were found in 57%, 9% and 4%. Norwegian adolescents seem to defy the world-wide trend of increasing myopia. This suggests that there is a need to explore why daylight exposure during a relatively short summer outweighs that of the longer autumn-winter.
Introduction
East and Southeast Asia have experienced an excessive increase in myopia in the past few decades, with more than 80% of the younger generation now affected1,2. Myopia is a major health concern3–5, as myopia, and in particular high myopia, may lead to potentially sight-threatening secondary ocular pathology6. The “epidemic” scale of myopia is most commonly observed in highly economically developed countries, where children complete secondary education and many undertake upper- and post-secondary studies, combined with limited time spent outdoors7,8.
Environmental and genetic factors are both assumed to contribute in the development of refractive errors9,10, although there is no general agreement on the etiology of myopia. The environmental factor argued to be of greatest importance in preventing myopia is time spent outdoors prior to myopia onset11–13 (it is debated whether time outdoors has an effect on myopia progression14–19). A dose-response relationship between daylight (outdoor) exposure and ocular axial elongation (associated with developing myopia) has been inferred17. Reported seasonal variation in axial length growth and myopia progression (with decreased eye growth and decreased myopia progression in periods with increased number of daylight hours20,21) is often cited in support of the protective effect of outdoors. Such an explanation warrants further examination and calls for refractive error data from different parts of the world3,22, in particular countries with high performing education systems and differing levels of seasonal variation in daylight.
Norway’s northern latitude stretches from 58° to 71° North, with even those living in Southeast Norway (60° North) experiencing large seasonal variation in daylight exposure, from less than 6 hours in December to around 19 hours in June (Fig. 1)23. Norway is a highly economically developed country, ranked as number 1 in the Human Development report 2016, with high gender equality24. Norwegian children start primary school at age 6 years and complete 10 years of compulsory schooling before reaching upper secondary school, at age 16 years. Most of today’s adolescents will also have attended kindergartens from age 1–5 years (76.2% in 2005)25. The Norwegian education system is high-performing, as classified by the Organisation for Economic Co-operation and Development (OECD) Programme for International Student Assessment (PISA), with both mean performance and the proportion of top performers above the OECD average in science, reading and mathematics26. Near work includes high usage of near electronic devices (NED) at school and at home, with the use of NED reported to be above the OECD average27.
Figure 1.
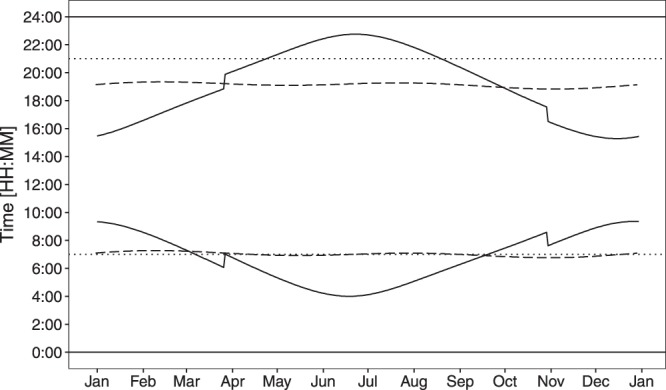
Seasonal variation in sunrise and sunset time. The solid line shows the seasonal variation in sunrise and sunset time in Southeast Norway (60° North, 9° East; range of daylight hours: 5 h 59 min – 18 h 44 min). The sudden change in late March and October is due to daylight saving time. For a comparison, the dashed line shows the sunrise and sunset time in Singapore (1° North, 103° East; range of daylight hours: 12 h 3 min – 12 h 12 min)23. The dotted lines show the amount of daylight available for a child sleeping 10 hours each night.
If high levels of daylight exposure are necessary to protect against myopia, it is reasonable to hypothesize that myopia onset will occur earlier, progression will be faster, and prevalence will be higher in adolescents living in countries with relatively few daylight hours across an extended (5–6 months of autumn-winter) period28, particularly so, if combined with a high level of near work29,30. The current study tested this hypothesis. Its aim, therefore, was to examine the prevalence of refractive errors in adolescents in Southeast Norway and assess the relationship between refractive errors, ocular biometry, sex and environmental factors such as self-reported time spent on activities outdoors and indoors.
Methods
Study Population and Recruitment
A cross-sectional study was carried out on students from the only two upper secondary schools within a catchment area comprising five municipalities in Southeast Norway during 2015–2016. The catchment area is representative of the Norway population in terms of socio-demographic status (details are given in Supplementary Tables S1–S4), with 70.7% living in urban settlements and an average population densities of 4–36 persons/km2 31. The total population of the region was 49,293 in 2016, with 1,737 of these aged 16–19 years32,33. The total student population of the two schools was 1,970 (age 16–24 years), 676 and 1,294 in the first and second schools respectively. The students attend school 5 days a week for 5–8 hours per day, with the school day beginning no earlier than 8 am; in addition, students undertake homework in the evenings and on weekends. By agreement with school administrators, we were given access to 898 students (45.6%) who were all invited to participate; all students in all three years in the first school and those in their first year (typical age 16–17 years) in the second school. The sample was representative of the school’s catchment area with respect to ethnicity and grade point averages (see Supplementary Tables S2 and S5). The study was carried out at the schools during normal school hours.
Verbal and written information about the study was given, and possible consequences of the study were explained to all participants before written informed consent was obtained. The research was approved by the Regional Committee for Medical Research Ethics for the Southern Norway Regional Health Authority and carried out in accordance with the principles embodied in the Declaration of Helsinki. A person aged 16 years or older is considered an adult and fully competent to consent to participate in research according to the Norwegian Health Research Act.
Participants
Of those invited, a sample of 439 (48.9%) students aged 16–19 years [mean age (SD): 16.7 (±0.9) years, 41.9% males] agreed to participate in the study. Self-reported ethnicity was mainly European Caucasians (90.9%); other ethnicities were Asian (5.5%), African (1.4%), South American (0.9%), or mixed (defined as having parents of two different ethnicities, 1.4%).
Analysis beyond calculation of prevalence of hyperopia and myopia was limited to the participants who reported to have both grown up in Norway and who were of Northern European (Caucasian) ethnicity [n = 393, mean age 16.7 (±0.9) years, 41.2% males], hereafter termed Norwegians. This group included participants born in Norway (98.7%) and five participants born in a different Northern European country (1.3%; born in Denmark, Iceland, Germany and Holland), all of whom reported to have moved to Norway during their childhood. Removal of these five participants from the group had no overall effect on the results. The Norwegian participants were grouped according to sex and age for the purpose of analysis (16-years-olds: n = 224, 42.4% males; 17–19-years-olds: n = 169, 39.6% males).
Cycloplegic Autorefraction and Other Measurements
Cycloplegic autorefractions were obtained with a Huvitz HRK-8000A Auto-REF Keratometer (Huvitz Co. Ltd., Gyeonggi-do, Korea), 15–20 minutes after instillation of topical 1% cyclopentolate hydrochloride (Minims single dose; Bausch & Lomb UK Ltd, England). One drop of cyclopentolate was used for blue- and green-eyed participants, and two drops for brown-eyed participants. The mean of five measurements automatically performed by the instrument (Huvitz HRK-8000A) were used for further analyses. One qualified optometrist (author JVBG) performed all autorefraction and biometry measurements.
Ocular axial lengths (AL) and corneal radii (CR) were measured with Zeiss IOLMaster (Carl Zeiss Meditec AG, Jena, Germany). Body height was measured with the Seca 217 stable stadiometer for mobile height measurement (Seca Deutschland, Hamburg, Germany).
Questionnaire
Participants completed an online questionnaire, an adapted version of the one used in the Sydney Myopia study34, to obtain demographic data and to quantify the amount of time spent on various indoor and outdoor activities. Demographic data included place of birth, number of years lived in Southeast Norway, house type and distance to school. Information about access to, and use of, near electronic devices (NED; smart phones, tablets, computers) was also collected.
The reported mean hours per day spent on outdoor- and indoor- activities were calculated for those participants who completed all questions related to time spent on various activities [68.4%, n = 269, 40.1% males, mean age 16.7 (±0.9) years]. Indoor activities included mean time spent on reading and writing on paper (books, newspapers, magazines), use of NED, indoor sport (gymnastics, dance, ball games, etc) and other indoor activities (watching television, playing video games, hobbies, cooking, etc). Outdoor activities included mean time spent on outdoor sport (cycling, skiing, running, etc) and other outdoor activities (walking to school, hiking, fishing, hunting, spending time in the garden etc). The participants were asked to estimate the daily time usually spent on these activities for both weekdays and weekends and about what they do in the school’s recess time. They were given four categorical response options for the estimate of activity hours per day; “Not at all”, “Less than 1 hour”, “1–2 hours”, or “3 hours or more”. The mean numbers of activity hours per day were calculated using “0 hour”, “1 hour”, “2 hours” or “3 hours” for each option, respectively, as follows:
| 1 |
Finally, the participants were asked to estimate the ratio of indoor to outdoor activities during their school holidays. Data were collected during February and March at both schools.
Analysis
Spherical equivalent refractive errors (SER = sphere + ½ cylinder), specified in terms of a 13.5 mm vertex distance, were used to classify refractive errors. Myopia was defined as SER ≤ −0.50 D, emmetropia as −0.50 D < SER < + 0.50 D, and hyperopia as SER ≥ + 0.50 D. The most positive meridian of the autorefractor measurement was defined as the sphere, and the prevalence of refractive astigmatism is reported as negative cylinder refraction ≥1.00 DC. SER, sphere and refractive astigmatism were all well correlated between the right and left eyes (SER: Spearman rho (ρ) = 0.94; sphere: ρ = 0.92; refractive astigmatism: ρ = 0.59; all p < 0.001), and thus only data from the right eye are presented. A SER-difference ≥1.00 D between right and left eye was defined as anisometropia. CR data represent the mean of the corneal radii measured in the flattest and steepest meridians. AL/CR-ratios were also calculated.
The Clopper-Pearson interval method and the method of Sison and Glaz were used for calculation of 95% binomial and multinomial proportion confidence intervals (CI), respectively. QQ-plots, histograms and the Shapiro-Wilk test were used to assess the normality of the variables. Means (±SD) are reported, in addition to the median (50th percentile) for non-normal data. The chi-square test, Fisher’s exact test, and independent sample t-test were used to assess differences in prevalence and mean values between groups. Maximum likelihood estimate was used to fit a suitable distribution to the data for SER35.
Linear regression analyses were performed with SER, AL, AL/CR-ratio and cylinder as the dependent outcome variables. Multivariate logistic regression analyses were performed, with the presence of myopia as the dependent outcome variable. Likelihood ratio tests were performed to compare models. Odds ratios (OR) and 95% CI are presented, with the significance level set at 0.05. All statistical analyses were performed using R statistical software, version 3.4.036 including the packages MASS35 and gmodels37.
Results
Refractive Errors
Table 1 shows an overview of the prevalence of refractive errors by age and sex, independent of ethnicity (a) and for those defined as Norwegians (b). The overall prevalence of hyperopia and myopia was 55.4% and 13.4%, respectively. All results are from here on related to those defined as Norwegians.
Table 1.
Mean spherical equivalent error SER (standard deviation, SD) in diopters [D] and the prevalence of refractive error type (%) for the right eyes categorized by age and sex of (a) all 16–19-year-olds, independent of ethnicity (n = 439), and (b) 16–19-year-old Norwegians (n = 393).
| Age (years) | Group | n | Mean (SD) SER [D] | Myopia % (CI) | Emmetropia % (CI) | Hyperopia % (CI) | |
|---|---|---|---|---|---|---|---|
| (a) ALL ETHNICITIES | 16–19 | All | 439 | +0.51 (1.29) | 13.4 (8.7–18.3) | 31.2 (26.4–36.1) | 55.4 (50.6–60.2) |
| Females | 255 | +0.39 (1.30) | 16.9 (10.6–23.1) | 27.5 (21.2–33.7) | 55.7 (49.4–62.0) | ||
| Males | 184 | +0.67 (1.25) | 8.7 (1.6–16.4) | 36.4 (29.3–44.1) | 54.9 (47.8–62.6) | ||
| 16 | All | 246 | +0.59 (1.23) | 11.0 (4.9–17.5) | 31.3 (25.2–37.8) | 57.7 (51.6–64.3) | |
| Females | 139 | +0.50 (1.10) | 14.4 (6.5–22.9) | 25.9 (18.0–34.4) | 59.7 (51.8–68.2) | ||
| Males | 107 | +0.72 (1.37) | 6.5 (0.0–16.3) | 38.3 (29.0–48.1) | 55.1 (45.8–64.9) | ||
| 17–19 | All | 193 | +0.40 (1.35) | 16.6 (9.3–23.9) | 31.1 (23.8–38.5) | 52.3 (45.1–59.7) | |
| Females | 116 | +0.26 (1.50) | 19.8 (11.2–30.0) | 29.3 (20.7–39.5) | 50.9 (42.2–61.1) | ||
| Males | 77 | +0.60 (1.06) | 11.7 (1.3–23.8) | 33.8 (23.4–45.8) | 54.5 (44.2–66.6) | ||
| (b) NORWEGIANS | 16–19 | All | 393 | +0.55 (1.29) | 12.7 (7.9–18.0) | 30.5 (25.7–35.8) | 56.7 (51.9–62.0) |
| Females | 231 | +0.45 (1.27) | 15.6 (9.1–22.2) | 28.1 (21.6–34.8) | 56.3 (49.8–62.9) | ||
| Males | 162 | +0.70 (1.30) | 8.6 (1.2–16.7) | 34.0 (26.5–42.1) | 57.4 (50.0–65.5) | ||
| 16 | All | 224 | +0.63 (1.23) | 10.3 (4.0–17.1) | 30.8 (24.6–37.7) | 58.9 (52.7–65.8) | |
| Females | 129 | +0.56 (1.05) | 13.2 (5.4–22.2) | 25.6 (17.8–34.6) | 61.2 (53.5–70.3) | ||
| Males | 95 | +0.74 (1.43) | 6.3 (0.0–17.0) | 37.9 (28.4–48.6) | 55.8 (46.3–66.5) | ||
| 17–19 | All | 169 | +0.44 (1.37) | 16.0 (8.3–23.7) | 30.2 (22.5–37.9) | 53.8 (46.2–61.6) | |
| Females | 102 | +0.31 (1.50) | 18.6 (8.8–28.9) | 31.4 (21.6–41.7) | 50.0 (40.2–60.3) | ||
| Males | 67 | +0.65 (1.12) | 11.9 (1.5–24.7) | 28.4 (17.9–41.1) | 59.7 (49.3–72.5) |
Prevalence is given with 95% confidence intervals (CI). Myopia was defined as SER ≤ −0.50 D, emmetropia as −0.50 D < SER < + 0.50 D, and hyperopia as SER ≥ + 0.50 D.
The prevalence of hyperopia and myopia in Norwegians was 56.7% and 12.7%, respectively. Figure 2 shows the leptokurtic distribution of SER [D] for 16–19-year-old Norwegians. The SER mean (±SD) was +0.55 (±1.29) D and median was +0.61 D (range: −6.45–7.71 D). Myopia was more prevalent among females than males [15.6% versus 8.6%, Fisher’s exact test, p = 0.046]. The prevalence of hyperopia decreased with age, with the prevalence of myopia increasing in parallel (Table 1b, column 6 and 8). However, the prevalence of high myopia, defined as SER ≤ −6.00 D, was very low, at 0.5% (CI: 0.1–1.8%). In contrast, the prevalence of moderate to high hyperopia, defined as SER ≥ + 2.00 D, was higher, at 6.4% (CI: 4.2–9.2%). Refractive astigmatism (≥1.00 DC) was found in 8.9% (CI: 6.3–12.2%) and anisometropia (≥1.00 D) in 3.6% (CI: 2.0–5.9%) of participants.
Figure 2.
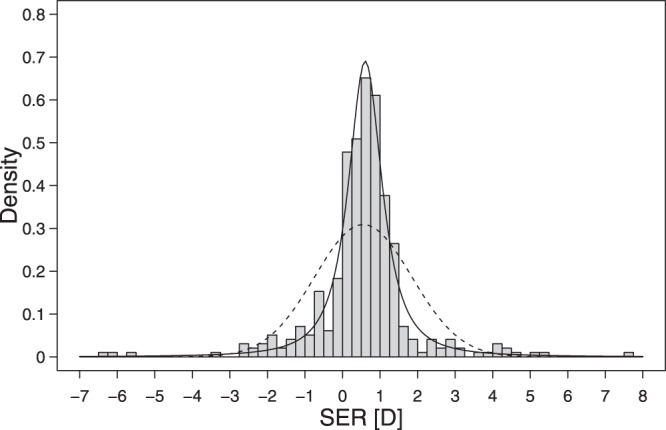
Distribution of SER. The leptokurtic distribution of cycloplegic SER [D] for the right eyes of 16–19-year-old Norwegians (n = 393; skewness = −0.24, kurtosis = 11.3). The dashed curve shows a normal distribution with the same mean and standard deviation as the data, and the solid curve shows a t-distribution fitted to the data by maximum likelihood [degrees of freedom (df) = 1.63, location (m) = 0.61, scale (s) = 0.50]35.
Ocular Biometry and Body Height
Table 2 shows mean AL, CR and AL/CR categorized by age, sex, and refractive error. Mean AL was significantly longer (23.66 vs. 23.28 mm, t(391) = −4.46, p < 0.001) and mean corneal curvature (CR) was significantly flatter (7.87 vs. 7.78 mm, t(305) = −3.00, p = 0.003) in males compared with females. Overall, AL and CR were highly correlated (Pearson; r = 0.53 in females, r = 0.69 in males, p < 0.001), and both AL and AL/CR were significantly negatively correlated with SER in both males and females (AL: r = −0.62, (females), r = −0.47 (males), p < 0.001; AL/CR: r = −0.84 (females), r = −0.77 (males), p < 0.001).
Table 2.
Mean (SD) axial length (AL), corneal radius (CR) and AL/CR-ratio for the right eye of 16–19-year-old Norwegians (n = 393) categorized by age, sex, and refractive error.
| Age | n | SER [D] Mean (SD) | AL [mm] Mean (SD) | CR [mm] Mean (SD) | AL/CR Mean (SD) | |
|---|---|---|---|---|---|---|
| 16–19 | All | 393 | +0.55 (1.29) | 23.44 (0.86) | 7.82 (0.27) | 3.00 (0.09) |
| Females | 231 | +0.45 (1.27) | 23.28 (0.83) | 7.78 (0.25) | 2.99 (0.10) | |
| Males | 162 | +0.70 (1.30) | 23.66 (0.86) | 7.87 (0.30) | 3.01 (0.09) | |
| Myopes | 50 | −1.60 (1.34) | 24.22 (0.79) | 7.74 (0.25) | 3.13 (0.09) | |
| Emmetropes | 120 | +0.18 (0.23) | 23.51 (0.75) | 7.77 (0.27) | 3.03 (0.07) | |
| Hyperopes | 223 | +1.23 (0.97) | 23.22 (0.83) | 7.86 (0.27) | 2.95 (0.07) | |
| 16 | All | 224 | +0.63 (1.23) | 23.38 (0.82) | 7.81 (0.28) | 3.00 (0.09) |
| Females | 129 | +0.56 (1.05) | 23.21 (0.76) | 7.78 (0.26) | 2.99 (0.09) | |
| Males | 95 | +0.74 (1.43) | 23.62 (0.85) | 7.85 (0.31) | 3.01 (0.10) | |
| 17–19 | All | 169 | +0.44 (1.37) | 23.51 (0.91) | 7.83 (0.26) | 3.00 (0.10) |
| Females | 102 | +0.31 (1.50) | 23.36 (0.91) | 7.79 (0.24) | 3.00 (0.11) | |
| Males | 67 | +0.65 (1.12) | 23.73 (0.88) | 7.89 (0.28) | 3.01 (0.08) |
The mean height of participants was 172.2 (±8.7) cm, with males being on average taller than females [179.2 (±7.1) cm vs. 167.3 (±6.0) cm, t(309) = 17.3, p < 0.001]. Height correlated with AL overall (Pearson; r = 0.28, p < 0.001) and in females (Pearson; r = 0.23, p < 0.001), but not in males (Pearson; r = 0.14, p = 0.08). Height did not correlate with SER.
Outdoor and Indoor Activity Time
Times spent doing outdoor and indoor activities were calculated for the subset of Norwegian participants who answered all questions related to time spent on various activities. Although this subgroup represented only 68% of the total group, there were no differences between this smaller sample (n = 269) and the whole sample of Norwegian participants (n = 393) in prevalence of myopia (12.3% vs. 12.7%), emmetropia (30.9% vs. 30.5%) or hyperopia [56.9% vs. 56.9%; χ2(2) = 0.03, p = 0.984]. These participants reported to spend, on average, 3.8 (±1.8) and 10.5 (±2.4) hours per day outdoors and indoors, respectively. Most of the participants (93%) reported staying indoors in their school recess time. Myopes spent, on average, less time doing outdoor sport per day [0.93 (±0.8) h] than non-myopes [emmetropes and hyperopes combined: 1.32 (±1.0) h; t(267) = −2.24, p = 0.03], but total time spent outdoors was not associated with myopia [myopes: 3.65 (±1.5) h; non-myopes: 3.81 (±1.9) h; t(267) = 0.47, p = 0.64], neither was time spent on other activities. The hours spent on various indoor or outdoor activities also showed no significant correlations with either SER, astigmatism, AL or AL/CR-ratio.
Females and males spent, on average, the same amount of time outdoors [females: 3.71 (±1.7) h; males: 3.91 (±2.0) h] and indoors [females: 10.68 (±2.3) h; males: 10.26 (±2.4) h]. More than 97% of the students had both their own smart phone and laptop for use at school and for homework. The time spent using NED each day was the same for females and males [females: 5.01 (±1.5) h; males: 4.97 (±1.5) h].
Table 3 shows the models from the multivariate logistic regression, with myopia as the outcome variable, sex as potential confounder, and mean hours of different indoor and outdoor activities as the predictors (Model A). Likelihood ratio tests were used for manual backward selection (Model B). Model B confirmed a lack of significant association of myopia with indoor activities, but showed myopia to be associated with less time spent on outdoor sport (OR = 0.51, CI: 0.30–0.82, p = 0.007) and more time spent on other outdoor activities (OR = 1.49, CI: 1.04–2.15, p = 0.030), after adjustment for sex.
Table 3.
Multivariate logistic regression models with myopia as the outcome variable. (Model A) mean hour of activity [h/day] as predictors and sex as a potential confounder.
| Model A | Model B | |||||
|---|---|---|---|---|---|---|
| β | OR (95% CI) | p | β | OR (95% CI) | p | |
| Intercept | −2.150 | 0.12 (0.02–0.75) | 0.026 | −2.041 | 0.13 (0.05–0.33) | <0.001 |
| Sex, male | −0.625 | 0.54 (0.21–1.25) | 0.164 | −0.636 | 0.53 (0.21–1.21) | 0.146 |
| Sport outdoors | −0.754 | 0.47 (0.27–0.78) | 0.005 | −0.678 | 0.51 (0.30–0.82) | 0.007 |
| Other outdoors | 0.438 | 1.55 (1.07–2.28) | 0.022 | 0.400 | 1.49 (1.04–2.15) | 0.030 |
| Read paper | 0.260 | 1.30 (0.75–2.23) | 0.344 | |||
| NED | 0.013 | 1.01 (0.78–1.31) | 0.922 | |||
| Other indoors | −0.176 | 0.84 (0.59–1.18) | 0.311 | |||
| Sport indoors | 0.099 | 1.10 (0.72–1.72) | 0.654 | |||
AIC = 201.0. (Model B) mean hours of sport and other outdoor activities as the predictors, adjusted for sex. AIC = 195.1. Odds ratios (OR) and confidence intervals (CI) are presented.
Table 4 shows that 94% and 64% reported to spend half or more of the day outdoors in the summer and Easter holidays, respectively. More myopes (14%) than non-myopes (4%) reported to spend most of their time indoors during the summer holidays (Fisher’s exact test, p = 0.01), with no difference for the other holidays.
Table 4.
Overview of duration, time and mean number of daylight hours for the school holidays in Norwegian upper secondary school, including proportion of students who reported to spend half of the day or more than half of the day outdoors in these periods.
| Duration of holiday (time of the year) | Mean # daylight hours in the period | Proportion (%) | Proportions (%) who spend most time indoors | ||||
|---|---|---|---|---|---|---|---|
| Spend half of the day outdoors | Spend more than half of the day outdoors | Myopes | Non-myopes | p-value | |||
| Summer | 8 weeks (mid June–mid August) | 17 h 35 min | 45 | 49 | 14 | 4 | 0.007* |
| Autumn | 1 week (October) | 11 h 5 min | 38 | 9 | 47 | 53 | 0.447 |
| Winter | 1 week (February) | 10 h 36 min | 35 | 8 | 67 | 56 | 0.164 |
| Spring (Easter) | 1.4 weeks (March–April) | 14 h 21 min | 52 | 12 | 41 | 35 | 0.428 |
Proportions of the students who spend most time indoors are categorized as myopes and non-myopes (p-values were calculated using Fisher’s exact test for count data).
Discussion
This is the first report on refractive errors in a representative sample of adolescents in Southeast Norway, with hyperopia found to be the most common type of refractive error. How does the refractive error profile of this adolescent population compare with other adolescent populations? The prevalence of moderate to high hyperopia (SER ≥ + 2.00 D) in this sample (6.4%) is higher than that reported for adolescents in both Asia (0.5–4.0%)38–40 and Australian European Caucasians (2.0%)5, but lower than among white adolescents in the UK (17.7%)41. Comparative data from other published studies on myopia prevalence are summarized in Table 5, with matched myopia definition. The prevalence of myopia is comparable with, albeit slightly lower than for Australian European Caucasians in Sydney5 and white adolescents in the UK41. It was lower than the 27.4% point estimate for myopia in the 15–19-year age group across Europe, calculated by random-effect meta-analysis and age-standardization by Williams et al.42 (mean SER for the two eyes ≤−0.75 D). The prevalence of myopia was also lower than that reported in a study of Swedish 12–13-year-olds43, though that study’s use of tropicamide 0.5% for accommodation control may have resulted in an artificially high myopia prevalence. The prevalence of myopia observed in the Southeast-Norwegian 16-year-olds is only slightly higher than that reported for 1-year-younger adolescents in rural Nepal, Iran and rural India44–46 (all considerably lower HDI than Norway). Noteworthy, the prevalence of myopia is considerably lower than that generally reported for adolescents in rural and urban parts of Asia12,38–40,47–49 [with comparable or lower human development index (HDI) than Norway]24, and Chile50 (considerably lower HDI than Norway). The ocular biometry data are consistent with the low myopia prevalence, with shorter axial lengths and lower average AL/CR than groups with higher myopia prevalence [cf. Table 2 with Lu et al.51 and Li et al.52].
Table 5.
Summary of myopia prevalence (%) from this study (four leftmost columns) and from other studies (rows, bold), matched on myopia definition and best matched on age.
| Age (years) | n | Myopia definition (SER) | Myopia prevalence (%) matched on myopia definition | Age (years) | n | Country | Ethnicity | HDI 201524 | Mean score in PISA 201582 | Average scale score TIMSS 201583 | Latitude | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study | Other studies | (HDI rank) | Science/Reading/Mathematics | Mathematics 8th grade | ||||||||
| 16 | 224 | <0.00 | 17.4 | 27.5 | 12–14 | 102 | Norway 53 | Not given | 0.949 (1) | 498/513/502 | 512 | 60.4° N |
| <−1.00 | 5.8 | 13.7 | 12–14 | 102 | Norway 53 | Not given | 0.949 (1) | 498/513/502 | 512 | 60.4° N | ||
| ≤−0.50 | 10.3 | 44.9 | 12–13 | 1045 | Sweden 43 | Not given | 0.913 (14) | 493/500/494 | 501 | 57.7° N | ||
| <−0.50 | 10.3 | 52.1 | 13–16 | 2069 | Rural China 47 | Han, Dai, Yi, Bai and other | 0.738 (90) | 518/494/531 | N/A | 24.5° N | ||
| ≤−0.50 | 10.3 | 38.8 | 14–15 | 905 | Rural China 38 | Not given | 0.738 (90) | 518/494/531 | N/A | 40.1° N | ||
| ≤−0.50 | 10.3 | 16.7 | 15 | 395 | Suburban Chile 50 | Not given | 0.847 (38) | 447/459/423 | 427 | 33.5° S | ||
| ≤−0.50 | 10.3 | 0.79 | 15 | 386 | Rural Nepal 44, 84 | Mixed Mongoloid, Aryan, and Aboriginal ancestry | 0.558 (144) | N/A | N/A | 26.6° N | ||
| ≤−0.50 | 10.3 | 6.72 | 15 | 258 | Rural India 46 | Not given | 0.624 (131) | N/A | N/A | 16.4° N | ||
| ≤−0.50 | 10.3 | 10.8 | 15 | 381 | Urban India 40 | Not given | 0.624 (131) | N/A | N/A | 28.6° N | ||
| ≤−0.50 | 10.3 | 9.6 | 15 | 326 | Semi-urban South Africa 85 | African, Indian, mixed | 0.666 (119) | N/A | 372 | 29.9° S | ||
| ≤−0.50 | 10.3 | 78.4 | 15 | 376 | Urban China 48 | Han (Chinese) | 0.738 (90) | 518/494/531 | N/A | 23.1° N | ||
| ≤−0.50 | 10.3 | 32.5 | 15 | 321 | Urban Malaysia 49 | Malay, Chinese, Indian and other | 0.789 (59) | N/A | 465 | 3.3° N | ||
| ≤−0.50 | 10.3 | 4.9 | 15 | 120 | Iran 45 | Not given | 0.774 (69) | N/A | 436 | 32.4° N | ||
| ≤−0.50 | 10.3 | 46.8 | 16 | 452 | Rural China 39 | Not given | 0.738 (90) | 518/494/531 | N/A | 21.8° N | ||
| 16–19 | 393 | ≤−0.50 | 12.7 | 69.5 | 11–20 | 1249 | Singapore 12 | Chinese, Malay, Indian and others | 0.925 (5) | 556/535/564 | 631 | 1.4° N |
| 17 | 80 | ≤−0.50 | 15.0 | 17.7 | 17 | <1202 | Australia 5 | European Caucasian | 0.939 (2) | 510/503/494 | 505 | 33.9° S |
| 18–19 | 89 | ≤−0.50 | 16.9 | 18.6 | 18–20 | 226 | UK 41 | White UK children | 0.909 (16) | 500/497/493 | N/A | 54.8° N |
| [18.2 (±0.4)] | 89 | ≤−0.25 | 18.0 | 33.0 | 21.7 (±0.3) | 112 | Norway 55 | Not given | 0.949 (1) | 498/513/502 | 512 | 63.4° N |
All results are based on cycloplegic autorefraction measurement, except for a few studies that used cycloplegic retinoscopy38,40,46,50,53, retinoscopy with tropicamide43 or cycloplegic subjective refraction55. Human Development Index (HDI) 201524, mean score in Programme for International Student Assessment (PISA) 201582, and average scale score for Trends in International Mathematics and Science Study (TIMSS) 201583 for each country are listed (results for Norway in top row). N/A = Not participated (except from Malaysia which participated in PISA 2015, but did not meet the PISA response-rate standards). PISA results given for China are from the area Beijing-Shanghai-Jiangsu-Guangdong, and PISA results for UK are from Northern Ireland. The PISA 2015 OECD average in science/reading/mathematics = 493/493/49082, and TIMSS 2015 Scale Centerpoint for Mathematics 8th grade = 50083. Highest score is best. Latitude for each study region is given in the rightmost column (latitude for present study is 59.7–60.0° N).
While the prevalence of myopia is reported to have been rising around the world, a similar trend in Southeast Norway appears to be absent. Specifically, a 1971 study of 12–14-year-old Norwegian children in West Norway (latitude 60.4°) reported similar cycloplegic SERs to that found here (at latitude 59.7–60.0°), and similarly low myopia prevalence (SER ≤ −1.0) of 13.7% (Table 5)53. Interestingly, Fledelius reported stability in the myopia prevalence of Danish medical students over the period 1968–199854. Moreover, the low rate of high myopia (0.5%; SER ≤ −6 D) observed here and the reported higher myopia prevalence in 21-year-olds in mid-Norway [myopia prevalence (SER ≤ −0.25) was 33% in the general population, latitude 63.4°]55 suggest that myopia onset is significantly delayed in Norwegians compared with East-Asians and some other Europe based populations12,38,39,41,43,47. The narrow range in refractive errors, higher prevalence of emmetropia with a hyperopic mean SER, coupled with a low prevalence of anisometropia and astigmatism lend support to this suggestion56–58. A further increase in myopia prevalence may be expected when the adolescents enter higher education55.
The education system in Norway is classified as high-performing26. The adolescents in this study spent >10 hours per day indoors doing near work including working on NED for >5 hours per day, which was comparable with the amount of time spent on NED reported in a study of sleep in 16–19-year-olds in West Norway (latitude 60.4°, n = 9,846)59. But, time spent on near work was not associated with myopia, as reported by others60,61, neither was total time spent on outdoor activities in the winter — the multivariate analyses showed that the association for other activities outdoors outweighed that of doing sports outdoors. There was, however, an association between myopia and less time spent outdoors in the summer holiday. Interestingly, the mean time spent outdoors in the winter [3.79 (±1.8) hours per day; data collection was February–March] was similar to that reported for East-Asian adolescents [n = 267; mean 3.79 (±1.9) hours per day]12 in Singapore, where there is no difference in daylight hours (12 hours per day) between seasons (Fig. 1). This parallel raises the question for Norwegian adolescents, as to why the potential negative consequences of limited daylight exposure during the long autumn-winter period, when there are fewer than 12 hours daylight per day (174 days, including 82 days in November–January with only 6–8 hours daylight per day), do not override the potential positive benefits of the long days during the shorter summer period (124 days with 15–19 hours daylight per day). Note that there is a ceiling effect to the benefits of long summer days, since several hours of the daylight are in the late evening or early hours of the morning when children and adolescents sleep62,63. Norwegian children most likely only have access to about 12 hours of the daylight available to them in the spring-summer period (Fig. 1), which is comparable to what the children in Singapore have access to every day of the year. Can the difference in myopia prevalence between Norwegian and for example Singaporean adolescents (12.7% versus 69.5%12) be down to the increased time Norwegian adolescents spend outdoors in the 8-week summer holiday only? Considering the effect on myopia progression reported from the outdoor activity clinical trials in East Asia18, it seems unlikely that this can be the case. This raises the further question in relation to whether exposure to daylight per se is the most important factor in the protective effect of outdoor activity [cf. Guggenheim et al.64]. Could the state of being well adapted to seasonal variations (circannual rhythms) be as important for coordinated eye growth as it is for general health65? Is this to a larger degree preserved in Norwegian adolescents, because of more outdoor time since early childhood?
Being outdoors is a part of the Norwegian culture and a major part of growing up. For example, children in Norwegian kindergartens are reported to spend 2 hours per day outdoors in the winter and at least 4 hours in the summer66. Furthermore, children are required to stay outdoors during school recess (three to five breaks that accumulates to at least 1 hour per day) all the way through primary school (6–12 years of age), and all year long67. Pre-adolescent children spend on average an additional 2 hours outdoors per day after school68. These exposure patterns are quite different from those of children attending East-Asian schools where recess time usually is spent indoors13,17,18. It has been suggested that 2 hours spent outdoors per day is needed to prevent onset of myopia17, with outdoor activities having a stronger protective effect in younger children (age 6 years vs. age 11–12 years)19,69. Our data for Norwegian adolescents represent further supportive evidence from a real-life experiment. Nonetheless, it is also possible that the early onset of myopia as observed in many East Asian populations may be driven by genetic predisposition more than by environmental factors10,30.
Sex differences in myopia prevalence have been reported previously70–72. As in past studies, females were found to have a higher prevalence of myopia than males. There was a significant correlation between AL and height in females, but not males, which may be related to the age of onset of the childhood growth spurt. Specifically, girls usually show an earlier growth spurt, starting approximately two years ahead of boys73–75. There is a parallel here with myopia onset for females, which has been reported to be two years ahead of males54,75. The implication of the earlier onset of myopia in females is that they have a higher risk for developing larger myopic errors and secondary ocular pathology — indeed, as reported for older age groups76–78.
Our study had several limitations. The sample size could have been larger with an even higher response rate, but this is comparable to other studies when considering the narrow age range (Table 5). The population studied may be biased in its representation, although we have shown our sample to be representative for the region of Norway from which it was drawn (see Supplementary Material). It was not representative in terms of sex, with a slightly higher number of females, but considering that more females were myopic this, if anything, might suggest that the true overall prevalence of myopia may be lower. The use of questionnaires for quantifying time outdoors is common in studies of refractive errors11,69,79, even though there are inherent limitations associated with such an instrument compared with objective measures, for example wearable light meters80. This includes analytical problems arising from the use of categorical responses to a continuous event. Nonetheless, the comparisons made above were limited to studies that also made use of questionnaires for quantifying time in the same way.
In summary, this cross-sectional study of adolescents in Southeast Norway revealed hyperopia to be the most common refractive error, with the prevalence of myopia being quite low, despite the few daylight hours in the autumn-winter period and high levels of indoor activity and near work. While the origin of refractive errors is likely multifactorial56, a dose-response relationship between daylight (outdoor exposure) and ocular axial elongation alone cannot explain the low prevalence in myopia, anisometropia and astigmatism in this population. Genetic and environmental risk factors may impact how refractive errors develop differently81, and our results may point to a lower genetic predisposition to myopia in this population. Alternatively, perhaps there is a particular combination of genetic predisposition, circannual adaptation, timing and pattern of exposure to myopia-generating environmental triggers that are effective in protecting the population at this latitude against myopia.
Electronic supplementary material
Supplementary information about representativeness in the data.
Acknowledgements
The authors thank Kenneth Knoblauch for statistical advice and constructive feedback on the manuscript. The study was funded by the University of South-Eastern Norway and Regional Research Funds: The Oslofjord Fund Norway Grant No. 249049 (RCB). LAH holds a PhD position funded by the Norwegian Ministry of Education and Research.
Author Contributions
R.C.B. and L.A.H. conceived and designed the study, analyzed/interpreted the data, wrote the manuscript, and prepared the figures and tables. R.C.B., L.A.H., J.V.B.G., S.A., H.R.P., S.J.G. conducted the experiments, interpreted data and reviewed the manuscript.
Data Availability
Supplementary data on the community profile and demographics, a more detailed summary of refractive errors, time spent on indoor and outdoor activities, and refractive errors of non-Norwegians (n = 46) are available at usn.figshare.com [10.23642/usn.6022790].
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31790-y.
References
- 1.Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom. Vis. Sci. 2015;92:258–266. doi: 10.1097/OPX.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 2.Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann. Acad. Med. Singapore. 2004;33:27–33. [PubMed] [Google Scholar]
- 3.Holden BA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Williams KM, et al. Increasing Prevalence of Myopia in Europe and the Impact of Education. Ophthalmology. 2015;122:1489–1497. doi: 10.1016/j.ophtha.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013;120:1482–1491. doi: 10.1016/j.ophtha.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005;25:381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 7.Mirshahi A, et al. Myopia and level of education: results from the Gutenberg Health Study. Ophthalmology. 2014;121:2047–2052. doi: 10.1016/j.ophtha.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Rose KA, French AN, Morgan IG. Environmental Factors and Myopia: Paradoxes and Prospects for Prevention. Asia Pac J Ophthalmol. 2016;5:403–410. doi: 10.1097/APO.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 9.Pan C-W, Ramamurthy D, Saw S-M. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol. Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 10.Jin ZB, et al. Trio-based exome sequencing arrests de novo mutations in early-onset high myopia. Proc. Natl. Acad. Sci. USA. 2017;114:4219–4224. doi: 10.1073/pnas.1615970114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose KA, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Dirani M, et al. Outdoor activity and myopia in Singapore teenage children. Br. J. Ophthalmol. 2009;93:997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- 13.Jin JX, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC Ophthalmol. 2015;15:73. doi: 10.1186/s12886-015-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones-Jordan LA, et al. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest. Ophthalmol. Vis. Sci. 2012;53:7169–7175. doi: 10.1167/iovs.11-8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li SM, et al. Time Outdoors and Myopia Progression Over 2 Years in Chinese Children: The Anyang Childhood Eye Study. Invest. Ophthalmol. Vis. Sci. 2015;56:4734–4740. doi: 10.1167/iovs.14-15474. [DOI] [PubMed] [Google Scholar]
- 16.Guggenheim JA, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest. Ophthalmol. Vis. Sci. 2012;53:2856–2865. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 18.He M, et al. Effect of Time Spent Outdoors at School on the Development of Myopia Among Children in China: A Randomized Clinical Trial. JAMA. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 19.Xiong S, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;96:551–556. doi: 10.1111/aos.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwiazda J, Deng L, Manny R, Norton TT. Seasonal variations in the progression of myopia in children enrolled in the correction of myopia evaluation trial. Invest. Ophthalmol. Vis. Sci. 2014;55:752–758. doi: 10.1167/iovs.13-13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulk GW, Cyert LA, Parker DA. Seasonal variation in myopia progression and ocular elongation. Optom. Vis. Sci. 2002;79:46–51. doi: 10.1097/00006324-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Morgan IG, Rose KA. Myopia and international educational performance. Ophthalmic Physiol. Opt. 2013;33:329–338. doi: 10.1111/opo.12040. [DOI] [PubMed] [Google Scholar]
- 23.Cornwall, C., Horiuchi, A. & Lehman, C. NOAA Solar Calculator, https://www.esrl.noaa.gov/gmd/grad/solcalc/sunrise.html (2017).
- 24.The United Nations Development Programme (UNDP). Human Development Report 2016, Human Development for Everyone. (New York, USA, 2016).
- 25.Statistics Norway. Kindergartens, 2016, final figures, https://www.ssb.no/en/utdanning/statistikker/barnehager/aar-endelige/2017-03-21-content (2017).
- 26.OECD. PISA 2015, PISA, Results in Focus. (OECD Publisher, 2016).
- 27.OECD. Students, Computers and Learning: Making the Connection. (PISA, OECD Publishing, 2015).
- 28.Ramamurthy D, Lin Chua SY, Saw SM. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin. Exp. Optom. 2015;98:497–506. doi: 10.1111/cxo.12346. [DOI] [PubMed] [Google Scholar]
- 29.Tkatchenko AV, et al. APLP2 Regulates Refractive Error and Myopia Development in Mice and Humans. PLoS Genet. 2015;11:e1005432. doi: 10.1371/journal.pgen.1005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Q, et al. Childhood gene-environment interactions and age-dependent effects of genetic variants associated with refractive error and myopia: The CREAM Consortium. Sci. Rep. 2016;6:25853. doi: 10.1038/srep25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Statistics Norway. Population and land area in urban settlements, 1 January 2016, https://www.ssb.no/en/befolkning/statistikker/beftett (2017).
- 32.Statistics Norway. Population and area, by municipality (SY 57), http://www.ssb.no/303784/population-and-area-by-municipality-sy-57 (2017).
- 33.Statistics Norway. Tabell: 07459: Folkemengde, etter kjønn og ettårig alder. 1. januar (K) [Table: 07459: Population, by sex and age (1-year step). 1 January (M)], https://www.ssb.no/statistikkbanken/selectvarval/Define.asp?subjectcode=&ProductId=&MainTable=NY3026&nvl=&PLanguage=0&nyTmpVar=true&CMSSubjectArea=befolkning&KortNavnWeb=folkemengde&StatVariant=&checked=true (2016).
- 34.Ojaimi E, et al. Methods for a population-based study of myopia and other eye conditions in school children: the Sydney Myopia Study. Ophthalmic Epidemiol. 2005;12:59–69. doi: 10.1080/09286580490921296. [DOI] [PubMed] [Google Scholar]
- 35.Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. Fourth edn, (Springer, 2002).
- 36.R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2016).
- 37.gmodels: Various R Programming Tools for Model Fitting (2015).
- 38.Zhao J, et al. Refractive Error Study in Children: results from Shunyi District, China. Am. J. Ophthalmol. 2000;129:427–435. doi: 10.1016/S0002-9394(99)00452-3. [DOI] [PubMed] [Google Scholar]
- 39.He M, Huang W, Zheng Y, Huang L, Ellwein LB. Refractive error and visual impairment in school children in rural southern China. Ophthalmology. 2007;114:374–382. doi: 10.1016/j.ophtha.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Murthy GV, et al. Refractive error in children in an urban population in New Delhi. Invest. Ophthalmol. Vis. Sci. 2002;43:623–631. [PubMed] [Google Scholar]
- 41.McCullough SJ, O’Donoghue L, Saunders KJ. Six Year Refractive Change among White Children and Young Adults: Evidence for Significant Increase in Myopia among White UK Children. PLoS One. 2016;11:e0146332. doi: 10.1371/journal.pone.0146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams KM, et al. Prevalence of refractive error in Europe: the European Eye Epidemiology (E(3)) Consortium. Eur. J. Epidemiol. 2015;30:305–315. doi: 10.1007/s10654-015-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villarreal MG, Ohlsson J, Abrahamsson M, Sjostrom A, Sjostrand J. Myopisation: the refractive tendency in teenagers. Prevalence of myopia among young teenagers in Sweden. Acta Ophthalmol. Scand. 2000;78:177–181. doi: 10.1034/j.1600-0420.2000.078002177.x. [DOI] [PubMed] [Google Scholar]
- 44.Pokharel GP, Negrel AD, Munoz SR, Ellwein LB. Refractive Error Study in Children: results from Mechi Zone, Nepal. Am. J. Ophthalmol. 2000;129:436–444. doi: 10.1016/S0002-9394(99)00453-5. [DOI] [PubMed] [Google Scholar]
- 45.Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. The prevalence of refractive errors among schoolchildren in Dezful, Iran. Br. J. Ophthalmol. 2007;91:287–292. doi: 10.1136/bjo.2006.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dandona R, et al. Refractive error in children in a rural population in India. Invest. Ophthalmol. Vis. Sci. 2002;43:615–622. [PubMed] [Google Scholar]
- 47.Qian DJ, et al. Myopia among school students in rural China (Yunnan) Ophthalmic Physiol. Opt. 2016;36:381–387. doi: 10.1111/opo.12287. [DOI] [PubMed] [Google Scholar]
- 48.He M, et al. Refractive error and visual impairment in urban children in southern china. Invest. Ophthalmol. Vis. Sci. 2004;45:793–799. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- 49.Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Ophthalmology. 2005;112:678–685. doi: 10.1016/j.ophtha.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 50.Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from La Florida, Chile. Am. J. Ophthalmol. 2000;129:445–454. doi: 10.1016/S0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- 51.Lu TL, et al. Axial Length and Associated Factors in Children: The Shandong Children Eye Study. Ophthalmologica. 2016;235:78–86. doi: 10.1159/000441900. [DOI] [PubMed] [Google Scholar]
- 52.Li SM, et al. Corneal Power, Anterior Segment Length and Lens Power in 14-year-old Chinese Children: the Anyang Childhood Eye Study. Sci. Rep. 2016;6:20243. doi: 10.1038/srep20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsen JS. The sagittal growth of the eye. 1. Ultrasonic measurement of the depth of the anterior chamber from birth to puberty. Acta Ophthalmol. (Copenh) 1971;49:239–262. doi: 10.1111/j.1755-3768.1971.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 54.Fledelius HC. Myopia profile in Copenhagen medical students 1996–98. Refractive stability over a century is suggested. Acta Ophthalmol. Scand. 2000;78:501–505. doi: 10.1034/j.1600-0420.2000.078005501.x. [DOI] [PubMed] [Google Scholar]
- 55.Kinge B, Midelfart A, Jacobsen G. Refractive errors among young adults and university students in Norway. Acta Ophthalmol. Scand. 1998;76:692–695. doi: 10.1034/j.1600-0420.1998.760612.x. [DOI] [PubMed] [Google Scholar]
- 56.Flitcroft DI. Emmetropisation and the aetiology of refractive errors. Eye. 2014;28:169–179. doi: 10.1038/eye.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng L, Gwiazda JE. Anisometropia in children from infancy to 15 years. Invest. Ophthalmol. Vis. Sci. 2012;53:3782–3787. doi: 10.1167/iovs.11-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehrlich DL, et al. Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optom. Vis. Sci. 1997;74:822–843. doi: 10.1097/00006324-199710000-00022. [DOI] [PubMed] [Google Scholar]
- 59.Hysing, M. et al. Sleep and use of electronic devices in adolescence: results from a large population-based study. BMJ Open5, 10.1136/bmjopen-2014-006748 (2015). [DOI] [PMC free article] [PubMed]
- 60.Lu B, et al. Associations between near work, outdoor activity, and myopia among adolescent students in rural China: the Xichang Pediatric Refractive Error Study report. Arch. Ophthalmol. 2009;127:769–775. doi: 10.1001/archophthalmol.2009.105. [DOI] [PubMed] [Google Scholar]
- 61.Tan NW, et al. Temporal variations in myopia progression in Singaporean children within an academic year. Optom. Vis. Sci. 2000;77:465–472. doi: 10.1097/00006324-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Galland BC, Taylor BJ, Elder DE, Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Med. Rev. 2012;16:213–222. doi: 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Hysing M, Pallesen S, Stormark KM, Lundervold AJ, Sivertsen B. Sleep patterns and insomnia among adolescents: a population‐based study. J. Sleep Res. 2013;22:549–556. doi: 10.1111/jsr.12055. [DOI] [PubMed] [Google Scholar]
- 64.Guggenheim JA, et al. Assumption-free estimation of the genetic contribution to refractive error across childhood. Mol. Vis. 2015;21:621–632. [PMC free article] [PubMed] [Google Scholar]
- 65.Evans, J. A. & Davidson, A. J. In Prog. Mol. Biol. Transl. Sci. Vol. 119 (ed Martha U. Gillette) 283–323 (Academic Press, 2013). [DOI] [PubMed]
- 66.Moser T, Martinsen M. The outdoor environment in Norwegian kindergartens as pedagogical space for toddlers’ play, learning and development. European Early Childhood Education Research Journal. 2010;18:457–471. doi: 10.1080/1350293X.2010.525931. [DOI] [Google Scholar]
- 67.Vaage, O. F. Tidene skifter. Tidsbruk 1971–2010 [Time use 1971–2010]. (Statistisk sentralbyrå, 2012).
- 68.Vaage OFT. 2010. Utendørs 2 1/2 time - menn mer enn kvinner [Time use 2010. Outdoors 2 1/2 hour - males more than females] Samfunnsspeilet. 2012;26:37–42. [Google Scholar]
- 69.Shah RL, Huang Y, Guggenheim JA, Williams C. Time Outdoors at Specific Ages During Early Childhood and the Risk of Incident Myopia. Invest. Ophthalmol. Vis. Sci. 2017;58:1158–1166. doi: 10.1167/iovs.16-20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saw SM, et al. Incidence and progression of myopia in Singaporean school children. Invest. Ophthalmol. Vis. Sci. 2005;46:51–57. doi: 10.1167/iovs.04-0565. [DOI] [PubMed] [Google Scholar]
- 71.Rudnicka, A. R. et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br. J. Ophthalmol. 10.1136/bjophthalmol-2015-307724 (2016). [DOI] [PMC free article] [PubMed]
- 72.Congdon N, et al. Visual disability, visual function, and myopia among rural chinese secondary school children: the Xichang Pediatric Refractive Error Study (X-PRES)–report 1. Invest. Ophthalmol. Vis. Sci. 2008;49:2888–2894. doi: 10.1167/iovs.07-1160. [DOI] [PubMed] [Google Scholar]
- 73.Liu YX, Wikland KA, Karlberg J. New reference for the age at childhood onset of growth and secular trend in the timing of puberty in Swedish. Acta Paediatr. 2000;89:637–643. doi: 10.1111/j.1651-2227.2000.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 74.Lyu IJ, et al. The Association Between Menarche and Myopia: Findings From the Korean National Health and Nutrition Examination, 2008–2012. Invest. Ophthalmol. Vis. Sci. 2015;56:4712–4718. doi: 10.1167/iovs.14-16262. [DOI] [PubMed] [Google Scholar]
- 75.Yip VC, et al. The relationship between growth spurts and myopia in Singapore children. Invest. Ophthalmol. Vis. Sci. 2012;53:7961–7966. doi: 10.1167/iovs.12-10402. [DOI] [PubMed] [Google Scholar]
- 76.Willis JR, et al. The Prevalence of Myopic Choroidal Neovascularization in the United States: Analysis of the IRIS((R)) Data Registry and NHANES. Ophthalmology. 2016;123:1771–1782. doi: 10.1016/j.ophtha.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 77.Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am. J. Ophthalmol. 2014;157:9–25.e12. doi: 10.1016/j.ajo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Asakuma T, et al. Prevalence and risk factors for myopic retinopathy in a Japanese population: the Hisayama Study. Ophthalmology. 2012;119:1760–1765. doi: 10.1016/j.ophtha.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 79.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp. Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 80.Alvarez AA, Wildsoet CF. Quantifying light exposure patterns in young adult students. J Mod Opt. 2013;60:1200–1208. doi: 10.1080/09500340.2013.845700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y, Chang BH, Ding X, He M. Patterns in longitudinal growth of refraction in Southern Chinese children: cluster and principal component analysis. Sci. Rep. 2016;6:37636. doi: 10.1038/srep37636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.OECD. PISA 2015 Results (Volume I): Excellence and Equity in Education. (PISA, OECD Publishing, 2016).
- 83.Mullis, I. V. S., Martin, M. O., Foy, P. & Hooper, M. TIMSS2015 InternationalResults in Mathematics. (TIMSS & PIRLS, International Study Center, Lynch School of Education, Boston College, USA, 2016).
- 84.Morgan IG, Rose KA, Ellwein LB. Is emmetropia the natural endpoint for human refractive development? An analysis of population-based data from the refractive error study in children (RESC) Acta Ophthalmol. 2010;88:877–884. doi: 10.1111/j.1755-3768.2009.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naidoo KS, et al. Refractive error and visual impairment in African children in South Africa. Invest. Ophthalmol. Vis. Sci. 2003;44:3764–3770. doi: 10.1167/iovs.03-0283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information about representativeness in the data.
Data Availability Statement
Supplementary data on the community profile and demographics, a more detailed summary of refractive errors, time spent on indoor and outdoor activities, and refractive errors of non-Norwegians (n = 46) are available at usn.figshare.com [10.23642/usn.6022790].


