BIG contributes to the dynamic adjustment of the circadian period to establish the correct phase of daily rhythms.
Abstract
Circadian clocks drive rhythms with a period near 24 h, but the molecular basis of the regulation of the period of the circadian clockis poorly understood. We previously demonstrated that metabolites affect the free-running period of the circadian oscillator of Arabidopsis (Arabidopsis thaliana), with endogenous sugars acting as an accelerator and exogenous nicotinamide acting as a brake. Changes in circadian oscillator period are thought to adjust the timing of biological activities through the process of entrainment, in which the circadian oscillator becomes synchronized to rhythmic signals such as light and dark cycles as well as changes in internal metabolism. To identify the molecular components associated with the dynamic adjustment of circadian period, we performed a forward genetic screen. We identified Arabidopsis mutants that were either period insensitive to nicotinamide (sin) or period oversensitive to nicotinamide (son). We mapped son1 to BIG, a gene of unknown molecular function that was shown previously to play a role in light signaling. We found that son1 has an early entrained phase, suggesting that the dynamic alteration of circadian period contributes to the correct timing of biological events. Our data provide insight into how the dynamic period adjustment of circadian oscillators contributes to establishing a correct phase relationship with the environment and show that BIG is involved in this process.
The circadian clock is an endogenous oscillator that, in Arabidopsis (Arabidopsis thaliana), consists of nuclear and cytosolic feedback loops. It is often considered that the circadian oscillator runs with a period of 24 h, but the circadian period is plastic, depending on environmental conditions. For example, in diurnal organisms such as Arabidopsis, the circadian clock has a reduced period with increased light intensity (Aschoff, 1960). This is commonly referred to as Aschoff’s rule and was the foundation for the model of parametric entrainment that describes how the circadian oscillator synchronizes with environmental cycles (Aschoff, 1960). We have discovered that exogenous application of two common metabolites also regulates the circadian period in Arabidopsis. Suc reduces the circadian period under dim light conditions (Haydon et al., 2013), whereas nicotinamide makes the circadian clock run more slowly, with a period near 27 h (Dodd et al., 2007).
The way in which circadian clocks regulate and adjust the circadian period is unknown. We refer to this ability of the circadian clock to adapt to environmental conditions as dynamic adjustment of circadian period. To investigate this dynamic adjustment, we have used nicotinamide as a tool that increases the circadian period. Previously, we proposed that nicotinamide affects circadian period through its action as an antagonist of Ca2+ signaling (Dodd et al., 2007). There is circadian regulation of cytosolic free calcium ([Ca2+]cyt) in mesophyll cells (Martí et al., 2013), and this encodes information about light intensity and quality (Love et al., 2004; Xu et al., 2007). In Arabidopsis, circadian regulation of [Ca2+]cyt is driven by the second messenger cADP ribose (cADPR) under the control of the morning oscillator gene CIRCADIAN CLOCK ASSOCIATED1 (CCA1; Dodd et al., 2007; Xu et al., 2007). Nicotinamide, the by-product of cADPR synthesis, inhibits both cADPR accumulation (Dodd et al., 2007) and ADPR cyclase activity (Abdul-Awal et al., 2016). There is no gene in Arabidopsis with homology to any of the known ADPR cyclases (Hunt et al., 2007). However, the existence of a completely novel ADPR cyclase in the green lineage cannot be ruled out, as many cyclases have yet to be characterized at the genetic level in mammals (Masuda et al., 1997).
Nicotinamide increases circadian period in all organisms tested, including Arabidopsis (Dodd et al., 2007), mouse (Asher et al., 2008), and Ostreococcus tauri (O’Neill et al., 2011). In animals, nicotinamide has been hypothesized to affect both circadian period and amplitude through the inhibition of poly-ADP-ribose polymerase (Ramsey et al., 2009) or SIRTUINs (Asher et al., 2008). Similar to ADPR cyclase, SIRTUINS are enzymes belonging to the NADase superfamily that release nicotinamide as a by-product of ADPR production. However, consistent with the effect of nicotinamide on circadian period being due to the inhibition of ADPR cyclase, a knockout mutation of CD38, the main mammalian ADPR cyclase, causes a long circadian period in mice (Sahar et al., 2011).
We have used nicotinamide as a tool to understand the potential mechanisms that regulate the dynamic adjustment of circadian period and to determine how nicotinamide regulates the circadian clock. We performed a forward genetic screen to identify loci that affect the sensitivity of the circadian oscillator to nicotinamide. Previous genetic analyses of the circadian system have focused on the identification of components of the circadian oscillator through screens for a short or long circadian period in constant light (Millar et al., 1995; Somers et al., 2000; Panda et al., 2002; Hazen et al., 2005) or constant darkness (Kevei et al., 2007; Martin-Tryon et al., 2007; Hong et al., 2010; Ashelford et al., 2011). We have taken a different approach by screening for mutations that are affected in their ability to change circadian period in response to altered conditions. We predicted that such a screen might identify genes involved in the response to nicotinamide and, more importantly, genes that participate in the dynamic adjustment of circadian period. We report the mapping by sequencing of an Arabidopsis mutant that is oversensitive to the effect of nicotinamide on circadian period and identification of the causal mutation in the gene BIG. Phenotypic and genotypic analyses of the mutant indicate a wider role for BIG in the dynamic adjustment of circadian period. We tested the hypothesis that mutations in this gene that affect the dynamic adjustment of free-running period also affect the entrained phase. We find that this dynamic adjustment of circadian period is associated with establishing the correct phase relationship with the environment. Therefore, our data identify a genetic component required for the correct regulation of circadian period and suggest that circadian period is not fixed at 24 h, thus permitting entrainment to different photoperiods. Our screen has provided important insight into how circadian clocks entrain to environmental cycles and, therefore, how plants tell the time.
RESULTS
A Forward Genetic Screen Identifies Mutants That Are Compromised in Their Ability to Adjust Circadian Period in Response to Nicotinamide
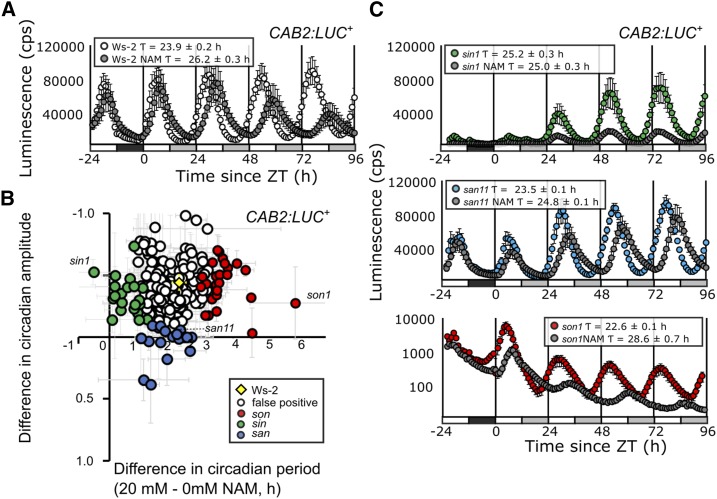
To identify mutants with an altered response of circadian period to nicotinamide, we mutated a Wassilewskija-2 (Ws-2) dual reporter line with ethyl methanesulfonate (EMS), which generates A-G and C-T transitions in base sequence. This line carries both the CHLOROPHYLL A/B BINDING PROTEIN2 promoter:LUCIFERASE+ (CAB2:LUC+; Hall et al., 2003) and the CAULIFLOWER MOSAIC VIRUS 35S promoter:APOAEQUORIN (35S:AEQ; Xu et al., 2007) reporters. The EMS population was initially screened in the M2 generation for period and amplitude of CAB2:LUC+ in the presence of 10 mm nicotinamide. We used the CAB2:LUC+ reporter because this had been used previously to study the effect of nicotinamide on the Arabidopsis circadian clock (Dodd et al., 2007). By measuring the behavior of circadian clock output in CAB2, we could examine the consequence of the entire oscillator dynamics, which is not possible when measuring the behavior of a single oscillator component. This screen of 16,000 M2 plants identified 372 putative mutants. These mutants were categorized as follows: is period insensitive to nicotinamide (sin), is period oversensitive to nicotinamide (son), or is amplitude insensitive to nicotinamide (san), based upon a circadian period that was outside of 2 sd of the Ws-2 circadian period (sin < 24 h, son > 26.1 h) or amplitude (san > 0.4 or < 0.18) of CAB2:LUC+ in the presence of 10 mm nicotinamide. The nature of the M2 screen meant that, in addition to nicotinamide response mutants, it was possible that mutations affecting free-running period also could have been selected.
We performed a rescreen of the M3 generation to confirm initial mutants and exclude those that were just free-running circadian period mutants. In the M3 screen, wild-type Ws-2 plants responded to 20 mm nicotinamide with an increase in circadian period of CAB2:LUC+ from 23.9 ± 0.2 to 26.2 ± 0.3 h and amplitude reduced from 1.1 ± 0.04 to 0.6 ± 0.03 normalized luminescence counts (n.c.; Fig. 1A). Sixty-three mutants were confirmed by the rescreening of the M3 generation, which also allowed the exclusion of false positives from the M2 screen (Fig. 1B; Supplemental Table S1).
Figure 1.
A forward genetic screen separates period and amplitude effects of nicotinamide. A, CAB2:LUC+ rhythms in wild-type Ws-2 in the presence or absence of 20 mm nicotinamide (NAM) in one entraining 12:12 light/dark cycle (black and white bars) and transferred into 4 d in constant light (white and gray bars) at dawn (Zeitgeber time [ZT] 0). Mean fast Fourier transform-nonlinear least squares (FFT-NLLS) period estimates are shown ± se (n = 8). B, Free-running circadian period and amplitude difference of M3 plants in a forward genetic screen for the effect of 20 mm nicotinamide on circadian oscillations of CAB2:LUC+. Period-insensitive mutants (sin) are indicated in green, period-oversensitive mutants (son) in red, and amplitude-sensitive mutants (san) in blue. Plants with no detectable nicotinamide-response phenotype in the screen of the M3 population are shown in white, and the mean wild-type Ws-2 ± se from all experiments (n = 64) is shown overlaid in yellow. Data are pooled from eight separate experiments. C, CAB2:LUC+ rhythms in sin1, son1, and san11 mutants (labeled in B) in the presence or absence of 20 mm nicotinamide in one entraining 12:12 light/dark cycle and 4 d in 70 µmol m−2 s−1 constant light (n = 8). Data are representative of two independent experiments in the M3 generation.
Twenty-five mutants were confirmed for the sin phenotype with either no significant period increase in the presence of 20 mm nicotinamide or with a reproducibly smaller increase in period than Ws-2 (Supplemental Table S1). Sixteen mutants were confirmed for the son phenotype, with significantly greater period in the presence of 20 mm nicotinamide compared with Ws-2 (Supplemental Table S1). Similarly, 25 san mutants were confirmed to have either no significant decrease in amplitude in response to nicotinamide or significantly smaller amplitude than Ws-2 (Supplemental Table S1).
The strongest phenotypes (Fig. 1C) were seen in son1, with a nicotinamide-induced circadian period increase of 6.02 ± 0.75 h (son1 water, 22.6 ± 0.1 h; 20 mm nicotinamide, 28.6 ± 0.7 h; P < 0.01, T = 8.05), sin1 with no period increase (sin1 water, 25.2 ± 0.3 h; 20 mm nicotinamide, 25.0 ± 0.3 h; P = 0.19, T = 0.92), and san11, which had a circadian period increase of 1.3 h but with a rising amplitude of CAB2:LUC+ compared with damping amplitude in the wild type (san11 water, 1.08 ± 0.03 n.c.; 20 mm nicotinamide, 1.05 ± 0.0 n.c.; T = 0.83, P = 0.21). The san lines all had very low amplitude compared with Ws-2 in the absence of nicotinamide, making the phenotypes difficult to measure robustly and map in segregating populations. Therefore, we focused our attention on the sin and son period mutant classes.
Dose-response curves demonstrated that son1 was hypersensitive to nicotinamide, with significant increases in circadian period with the addition of 1 mm nicotinamide (Supplemental Fig. S1A; ANOVA: F statistic = 6.87, degrees of freedom [df] = 15, P = 0.02), while the Ws-2 circadian period of CAB2:LUC+ did not vary significantly until the addition of 10 mm nicotinamide (ANOVA: F = 7.85, df = 19, P < 0.01). sin1 was hyposensitive to nicotinamide, as there was no variation in the circadian period of CAB2:LUC+ between 0.1 mm and 20 mm nicotinamide (Supplemental Fig. S1B; ANOVA: F = 2.15, df = 18, P = 0.11). The mutants were backcrossed twice to the parental Ws-2 line carrying 35S:AEQ and CAB2:LUC+ for mapping. Here, we describe our findings for son1, the first mutant that we mapped from the population, which has the strongest phenotype of all those identified.
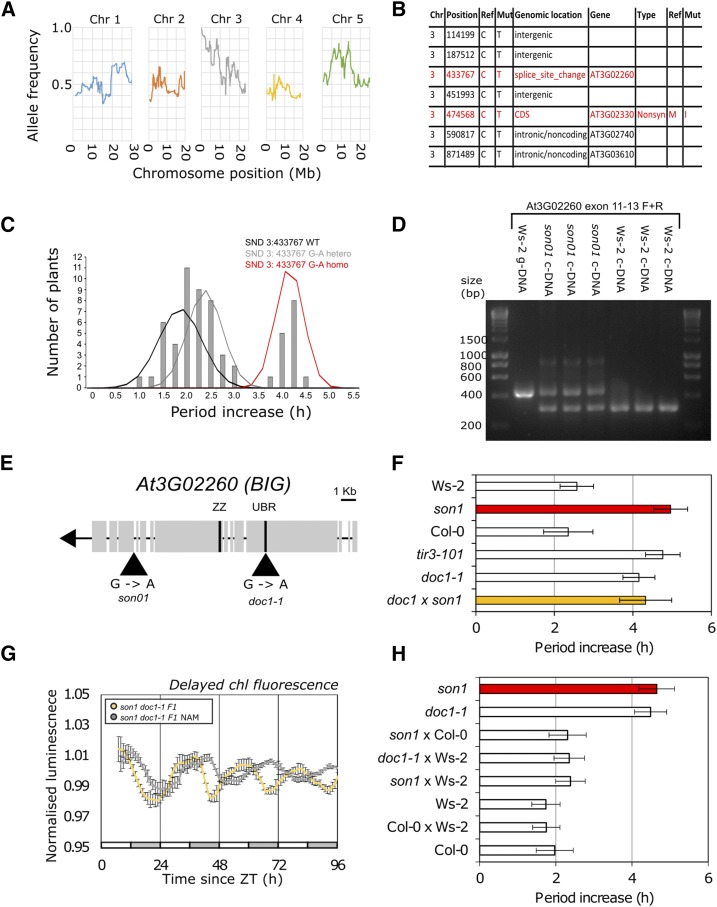
son1 Maps to a Mutation in a Splice Acceptor in BIG
We mapped the causal mutation for son1 using a mapping population of 25 BC1F2 plants clearly displaying the mutant phenotype and sequenced pooled DNA to 50-fold coverage. SHOREmap analysis (Schneeberger et al., 2009) using a sliding window of allele frequency identified a region on the long arm of chromosome 3 where no recombination had occurred (Fig. 2A). Underlying this region was a 750-kb interval containing eight single-nucleotide polymorphisms (SNPs), with three mutations at positions 433,767, 474,568, and 697,938 predicted to cause functional changes to gene products (Fig. 2B). We confirmed the existence of these SNPs using derived cleaved amplified polymorphic sequence (dCAPS) analysis and Sanger sequencing and fine-mapped the mutation by analyzing the segregation pattern in the BC2F3 plants using the M3 screening conditions (Fig 2C; Supplemental Fig. S2). When the segregation pattern of the SNPs was compared with the segregation of the son1 phenotype, the SNP on chromosome 3 at position 433,767 was the only SNP that segregated with the son1 phenotype in the BC2F3 plants (Fig. 2C; Supplemental Fig. S2, E and F). The wild-type and heterozygous 3:433,767 populations were not distinct from one another, with wild-type period difference (plus and minus nicotinamide) of 1.9 ± 0.5 h and heterozygous period difference of 2.5 ± 0.4 h compared with the homozygous 3:433,767 period difference of 4.3 ± 0.3 h.
Figure 2.
A causal mutation in BIG underlies son1. A, SHOREmap backcross mapping by sequencing analysis of son1 generated from 25-fold coverage Illumina sequence data obtained from 25 BC1F2 individual plants with the son1 phenotype. Individual chromosomes are shown separately with an allele frequency sliding window generated with a moving average of 50 kb. A region with an allele frequency of 1 is found on the long arm of chromosome 3. B, SNPs found on chromosome 3 in son1 with an allele frequency of 1. Mutations highlighted in red are predicted to cause a functional change in gene product. C, BC2F3 segregation of the son1 phenotype with mutation at 3:433,767. Period difference in the presence of 20 mm nicotinamide was calculated by FFT-NLLS and plotted rounded to the nearest 0.2 h. Each line consisted of eight biological replicates, and 45 lines were genotyped and phenotyped. Mean and sd for each 3:433,767 G-A genotypic subpopulation (wild type [WT], heterozygous, and homozygous) were used to plot normal distributions overlaid onto a period histogram. D, Reverse transcription (RT)-PCR of BIG exons 11 to 13 showing the effect of son1 on BIG transcript isoforms. Lane 1 has Ws-2 genomic DNA product of predicted size 458 bp; lanes 2 to 4 have son1 cDNA products of 314 and 458 bp; lanes 5 to 7 have Ws-2 cDNA product of 316 bp. A 1-kb ladder annotated with fragment sizes is shown. Independently isolated BC2F3 pedigrees were used. E, Gene structure of At3G02260 (BIG), the potential UBR- and ZZ-type zinc finger domains, and positions of son1 and doc1-1 mutations are labeled. F, Circadian period difference between the presence and absence of 20 mm nicotinamide for delayed chlorophyll fluorescence rhythms in Ws-2, son1, Col-0, doc1-1, and son1 doc1-1 F1. Period estimates were calculated using FFT-NLLS analysis (means ± se; n = 10). Data are representative of two independent experiments. G, Delayed chlorophyll fluorescence rhythm for son1 × doc1-1 F1 in the presence or absence of 20 mm nicotinamide (NAM) across 4 d in constant light. White and gray bars show subjective day and night. Means ± se are shown for n = 10. Data are representative of two independent crosses. H, Circadian period difference between the presence and absence of 20 mm nicotinamide for delayed chlorophyll fluorescence rhythms of Col-0, Ws-2, Col-0 × Ws-2 F1, son1, doc1, son1 × Ws-2 F1, and doc1 × Ws-2 F1. Period estimates were calculated using FFT-NLLS analysis (means ± se; n = 10).
This SNP resulted in a G-A transition causing a mutation in the 3′ splice acceptor site of exon 12 of At3G02260 (Fig. 2D). At3G02260 encodes BIG, a callosin-like protein of 5,098 amino acids and unknown molecular function (Gil et al., 2001). The M3 line carrying son1 is slightly short period (Fig. 1). This short-period phenotype in the M3 generation was reproducible but not significant (period difference = 0.53, P > 0.05; Supplemental Fig. S3). However, the short-period phenotype was not present in the M4 generation or in the BC1F3 (Supplemental Fig. S3) or BC2F3 (Supplemental Fig. S2) plants, indicating that the phenotype was not linked to the son1 phenotype after backcrossing to Ws-2 and that the son1 mutation does not cause a classical circadian period phenotype
To test the effect of the 3:433,767 mutation on transcript splicing in the son1 mutant, PCR products were amplified from cDNA using primers spanning exon 11-12 of BIG in three independent BC2F3 pedigrees. In addition to the 316-bp product amplified from wild-type cDNA (Fig. 2D, lanes 6–8), an additional product was amplified from son1 mutants (Fig. 2D, lanes 3–5) that was of equivalent size to the 458-bp PCR product amplified from wild-type genomic DNA (Fig. 2D, lane 2), indicating that it represented an unspliced transcript. Sequencing of both At3G02260 splice variants in son1 demonstrated that there was a G-A transition corresponding to 3:433,737 in both products (Supplemental Fig. S4). The smaller fragment was 2 bp smaller than the Ws-2 product, with a second AG immediately downstream of the first being used as a splice acceptor instead, while the larger 458-bp product contained the full sequence of intron 11-12, suggesting that it is retained in son1 due to inefficient splicing. Thus, the G-A 3:433,767 causes both the production of an unspliced transcript and the use of a cryptic splice site in son1, both of which result in frame shifts and are predicted to cause premature stop codons.
To confirm that the son1 phenotype was due to the G-A transition in BIG, we assessed the response to nicotinamide in mutants in BIG identified from previous mutant screens, dark overexpresser of cab1-1 (doc1-1; Li et al., 1994) and auxin transport inhibitor response3 (tir3-101; Ruegger et al., 1997), using delayed chlorophyll fluorescence (Gould et al., 2009). doc1-1 has an increase in photosynthesis-related gene expression, including CAB genes, in etiolated seedlings in the dark (Li et al., 1994; Gil et al., 2001) caused by a G-A transition resulting in a Cys-to-Thr amino acid substitution in the first Cys-rich domain (CRD-1, also known as a UBR box). tir3-101 is reported to have impaired polar auxin transport, giving rise to a dwarf phenotype (Ruegger et al., 1997; Prusinkiewicz et al., 2009). Both doc1-1 and tir3-101 were oversensitive to nicotinamide compared with their respective wild types (Fig. 2F; Supplemental Fig. S5; Columbia-0 [Col-0], 2.9 ± 0.5 h; doc1-1, 4.5 ± 0.4 h; tir3-101, 4.4 ± 0.9 h). The increased response of circadian period in the three different son1, doc1-1, and tir3-101 alleles of BIG suggested that the mutations in BIG are causal for the nicotinamide-oversensitive phenotype, and none have a circadian period phenotype in constant high light.
As confirmation, we tested whether son1 is allelic to doc1-1. The doc1-1 son1 F1 plants had significantly greater period increase in the presence of nicotinamide (4.3 ± 0.7 h) than either Ws-2 (1.7 ± 1.2 h; T = 2.22, df = 19, P < 0.05) or Col-0 (2.4 ± 0.6 h; T = 2.16, df = 19, P < 0.05) and were not statistically different from either doc1-1 (4.2 ± 0.4 h; T = 0.22, df = 19, P = 0.42) or son1 (4.9 ± 0.4 h; T = 0.81, df = 19, P = 0.21) in the presence of 20 mm nicotinamide (Fig. 2, F and G). To control for ecotype or dominance effects, we analyzed delayed fluorescence in the presence and absence of nicotinamide for F1 of crosses between son1 and Ws-2, son1 and Col-0, and doc1 and Ws-2 (Fig. 2H; Supplemental Fig. S6). These crosses all behaved like the wild type and had circadian period increases that corresponded to the heterozygous BC2F3 on the segregation analysis (Fig. 2C). This demonstrates that doc1-1 is allelic to son1 and that BIG regulates the sensitivity of the circadian oscillator to nicotinamide.
Having established that son1 and doc1-1 are both nicotinamide oversensitive for circadian period, we tested whether son1 plants exhibit the doc1 phenotype of increased photosynthesis-related gene expression in etiolated seedlings in the dark (Li et al., 1994; Gil et al., 2001). CAB2:LUC+ expression was higher in etiolated seedlings of son1 than the wild type in constant dark (Supplemental Fig. S7, A–C), indicating that son1 also had a dark overexpresser of CAB phenotype consistent with allelism to doc1-1. Similar to doc1-1, higher CAB2 expression in constant dark was not associated with premature deetiolation (Supplemental Fig. S7, D and E).
To test if BIG could be part of the transcriptional feedback loops of the oscillator, we looked at the transcript profile for BIG in the publicly available diurnal transcriptomic data sets under long and short days (Supplemental Fig. S8). BIG did not oscillate in either long or short photoperiods in two separate 48-h microarray experiments (Mockler et al., 2007; Endo et al., 2014). The abundance of BIG transcript also does not appear to be regulated by the circadian oscillator, with no detectable oscillations using JTK_CYCLE (P > 0.05) in a circadian transcriptome taken over 48 h in constant conditions (Dalchau et al., 2010). The lack of circadian or diel changes in BIG transcript abundance suggests that BIG is not part of the transcriptional feedback loops in the circadian oscillator.
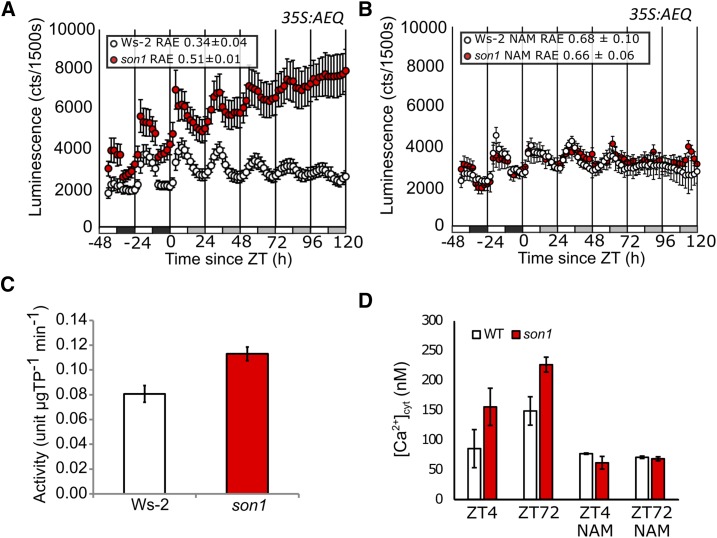
son1 Affects Circadian [Ca2+]cyt Signaling
In addition to increasing circadian period, nicotinamide abolishes the circadian regulation of [Ca2+]cyt, potentially through inhibition of the ADPR cyclase activity that generates the Ca2+ agonist cADPR (Dodd et al., 2007; Abdul-Awal et al., 2016). We investigated the effect of son1 on Ca2+ signaling using the 35S:AEQ reporter. In Ws-2, there was sinusoidal circadian regulation of [Ca2+]cyt, which had an estimated period of 24.2 ± 0.9 h and relative amplitude error (RAE) of 0.3 ± 0 (Fig. 3A). son1 affected the circadian regulation of [Ca2+]cyt, leading to a nonsinusoidal oscillation with an increasing basal level and dampening over time. This resulted in FFT-NLLS analysis estimating the rhythm as only weakly rhythmic, with RAE of 0.5 ± 0.1 (period = 23.1 ± 0.2 h; Fig. 3A). Both son1 and Ws-2 circadian [Ca2+]cyt signals were inhibited by 20 mm nicotinamide (Fig. 3B; Ws-2, RAE = 0.7 ± 0.1; son1, RAE = 0.7 ± 0.1).
Figure 3.
son1 affects circadian [Ca2+]cyt signals. A and B, Bioluminescence (photon counts per 1,500 s) from son1 and Ws-2 expressing 35S:AEQUORIN across two light/dark cycles and 5 d in constant 70 µmol m−2 s−1 white light grown on 20 mm mannitol (A) or 20 mm nicotinamide (NAM; B). Mean luminescence ± se is shown; n = 8. Data are representative of three independent experiments in the BC2F3 generation. C, ADPR cyclase activity measured using nicotinamide guanine dinucleotide assay at ZT4 in 70 µmol m−2 s−1 white light from 3- to 4-week-old Ws-2 (white) and son1 (red) seedlings, given as means of three biological replicates shown ± se. D, [Ca2+]cyt measured at ZT4 and ZT72 in constant 70 µmol m−2 s−1 white light from 11- and 14-d-old Ws-2 (white) and son1 (red) seedlings (n = 12). Data are representative of three independent experiments in the BC2F3 generation. WT, Wild type.
Because we have proposed previously that the circadian regulation of [Ca2+]cyt arises from cADPR-mediated Ca2+ release, we measured the activity of ADPR cyclase in wild-type and mutant plants. son1 had significantly higher ADPR cyclase activity compared with Ws-2 (P = 0.01) in the middle of the photoperiod representing peak [Ca2+]cyt (Fig. 3C). [Ca2+]cyt also was elevated at the same time point in son1 compared with Ws-2 (Fig. 3D; P < 0.05). This effect was more pronounced after 72 h in constant light, where the 35S:AEQ data previously indicated there to be a much higher basal level of [Ca2+]cyt (P < 0.05). [Ca2+]cyt at both time points was reduced by incubation with 20 mm nicotinamide (Fig. 3D). Thus, although nicotinamide reduced [Ca2+]cyt to similar concentrations in the wild type and son1, the change in [Ca2+]cyt in son1 was greater as untreated plants have higher [Ca2+]cyt, indicating that the [Ca2+]cyt increase in son1 might be ADPR cyclase dependent.
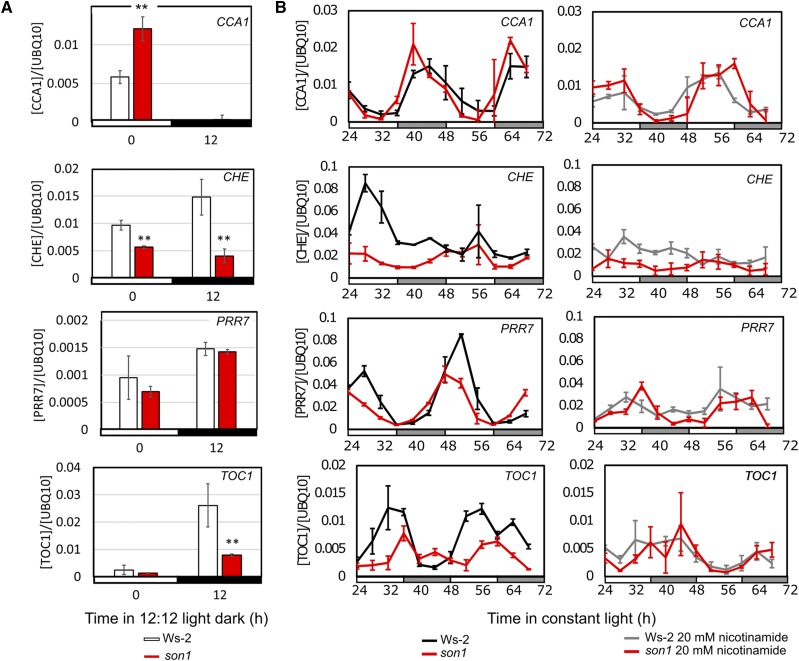
son1 Affects Circadian Oscillator Gene Expression
Circadian clocks evolved to provide competitive advantages in light and dark cycles; therefore, to investigate the role of BIG in the daily timing of Arabidopsis, we examined the effect of son1 on oscillator gene transcript abundance in light and dark cycles and in constant light. As our phenotype was based on the CAB2 gene, we measured the abundance of CCA1, a main circadian regulator of CAB2, and also the direct regulators of CCA1: TOC1, PRR7, and CHE. son1 affected circadian oscillator transcript levels in light/dark cycles. The expression of CCA1 immediately before dawn was higher in son1 compared with Ws-2 (Fig. 4A; P < 0.01), which corresponded to a reduction in TOC1 expression immediately before dusk (Fig. 4A; P = 0.01) and with a significant reduction in CHE expression at both dawn and dusk in son1 (Fig. 4A; P < 0.01). We also measured the expression of CCA1, TOC1, CHE, and PRR7 in constant light across a 48-h time course in son1 and Ws-2 in the presence and absence of 20 mm nicotinamide and estimated circadian period using JTK_CYCLE (Hughes et al., 2010). We performed this to confirm the son1 phenotype at the level of gene expression and to identify if there were any changes in gene expression between the mutant and the wild type in the absence of nicotinamide (Fig. 4B). In Ws-2, nicotinamide treatment significantly reduced the peak expression of all the genes in the first cycle (P < 0.05). Nicotinamide also significantly reduced peak CCA1 and PRR7 transcript levels in son1; however, there was no significant change in TOC1 and CHE at any time point. In son1, CCA1 and PRR7 rhythms had increased circadian period in the presence of nicotinamide compared with Ws-2, with period of 28 h in son1 (P < 0.001) but 24 h in the wild type (P < 0.001). CHE was not rhythmic with JTK_CYCLE in either Ws-2 (P = 1) or son1 (P = 0.08). TOC1 was rhythmic with JTK_CYCLE in Ws-2, with period of 24 h (P < 0.05), but was not rhythmic in son1 (P = 0.16). Thus, the son1 phenotype can be seen in rhythms of CCA1 and PRR7, but in the presence of nicotinamide, the rhythms of CHE and TOC1 were suppressed, with TOC1 also being suppressed in son1 in the absence of nicotinamide.
Figure 4.
son1 affects circadian clock gene expression in light/dark cycles and constant light. A, CCA1, PRR7, TOC1, and CHE expression from son1 (red) and Ws-2 (white) samples harvested immediately preceding dawn and dusk. Asterisks represents significance at P < 0.01 with Student’s t test. Relative expression of genes normalized to UBQ10f expression is given ± sd (n = 3). B, CCA1, PRR7, TOC1, and CHE expression from Ws-2 and son1 in the absence (left) and presence (right) of 20 mm nicotinamide across 48 h in constant 70 µmol m−2 s−1 light from ZT24 to ZT72. Relative expression of genes normalized to UBQ10f expression is given ± sd (n = 3). Plants were grown as clusters of five plants for 11 d in light/dark cycles prior to the experiment.
son1 Affects Dynamic Period Adjustment of the Circadian Oscillator to Regulate the Entrained Phase
As son1 is compromised in the ability to regulate changes in circadian period in response to nicotinamide, we tested whether it also was affected in its ability to adjust period correctly to other stimuli. Response to light is the most well-characterized dynamic adjustment of the circadian period and is described by Aschoff’s rule (Aschoff, 1960). We tested the hypothesis that son1 might be compromised in the ability to regulate circadian period at different light intensities by performing a fluence response curve (Fig. 5A).
Figure 5.
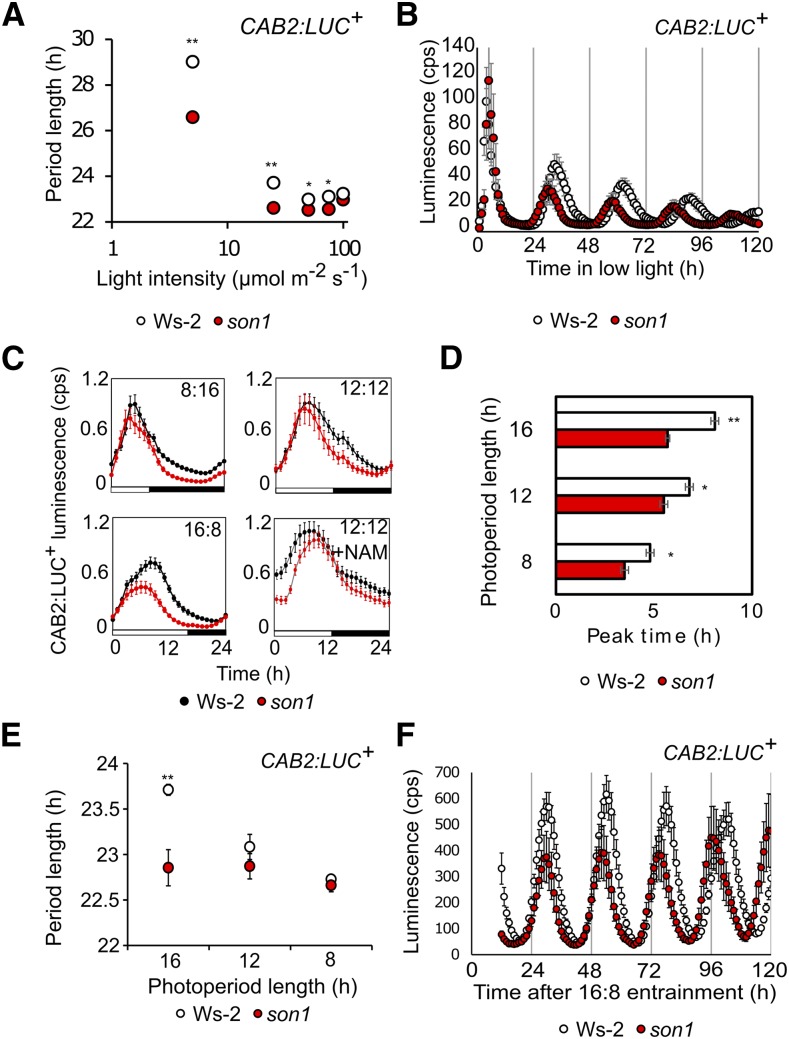
son1 affects dynamic circadian period adjustment by light and photoperiod. A, Fluence response curve for circadian period of CAB2:LUC+ in Ws-2 and son1 estimated in equal mixed red and blue light (means ± se; n = 8–12). Data are pooled from three independent experiments. B, CAB2:LUC+ rhythm of son1 and Ws-2 assayed over 5 d in constant 5 µmol m−2 s−1 equal mixed red and blue light (means ± se; n = 8). Data are representative of two independent experiments in the BC2F3 generation. In A, the small error bars are obscured by symbols. C, CAB2:LUC+ luminescence (counts s−1) from son1 (red) and Ws-2 (black) seedlings grown in 8:16, 12:12, and 16:8 photoperiods. Plants grown in 12:12 were treated with medium supplemented with or without 20 mm nicotinamide (NAM) 2 d prior to entrainment in a camera chamber (means ± se; n = 8). Plants were grown in entrainment conditions from germination and transferred to the camera chamber 1 d before imaging, maintaining the same entrainment regime. Data are representative of three independent experiments. D, Peak time of CAB2:LUC+ from the light/dark cycles in C. The mean peak time of CAB2:LUC+ ± se is plotted (n = 8). E, Photoperiod response curve for the circadian period of CAB2:LUC+ in Ws-2 and son1 estimated in equal mixed 80 µmol m−2 s−1 red and blue light (means ± se; n = 8). Data are pooled from two independent experiments. Plants were entrained in photoperiods of 16, 12, or 8 h prior to transfer to constant light. F, CAB2:LUC+ rhythm of son1 and Ws-2 entrained in a 16:8 light/dark cycle and released into constant light for 5 d (means ± se; n = 8). Asterisks indicate P < 0.05 with Student’s t test.
There was no difference between the period length of CAB2:LUC+ rhythms in Ws-2 and son1 at 100 µmol m−2 s−1 light (Ws-2, 23.2 ± 0.1 h; son1, 23 ± 0.1 h), which was the intensity of light used for entrainment, indicating again that son1 is not a circadian period mutant. However, son1 had a significantly shorter circadian period compared with the wild type under low fluence rates (Fig. 5A; Supplemental Fig. S9): under 5 µmol m−2 s−1 light, son1 had a period of 26.6 ± 0.8 h and Ws-2 had a period of 29 ± 0.3 h (P < 0.01; Fig. 5B). This indicates that son1 cannot properly regulate circadian period in response to changes in light intensity. A similar phenotype was detected in doc1-1: under 5 µmol m−2 s−1 light, doc1-1 had a circadian period of CAB2:LUC+ of 25.7 ± 0.1 h and Col-0 had a period of 29.2 ± 0.2 h (P < 0.01; Supplemental Fig. S9).
Having previously established that son1 affects the expression of circadian clock genes in a light/dark cycle, we next investigated the effect of son1 on the entrained phase to investigate the potential roles of BIG in the daily timing of Arabidopsis. Wild-type Ws-2 had a typical phase shift of later phase with increasing photoperiod (Fig. 5C, peak at 4.8 ± 0.2 h [8:16], peak at 6.8 ± 0.2 h [12:12], and peak at 8.1 ± 0.2 h [16:8]). By contrast, son1 was an early phase mutant (Fig. 5, C and D; peak at 3.5 ± 0.2 h [8:16], peak at 5.5 ± 0.2 h [12:12], and peak at 5.7 ± 0.1 h [16:8]). These data demonstrate that BIG is required for correct circadian entrainment. Lastly, we measured the effect of nicotinamide on entrained phase under 12:12 (Fig. 5C) and found that it caused a phase delay of CAB2:LUC+ peak expression of 1 h in Ws-2 and 3 h in son1 (Ws-2, ZT 7.8 ± 0.2; son1, ZT 8.8 ± 0.2), consistent with the effect of nicotinamide on free-running period in both backgrounds.
Finally, having identified that BIG regulates the dynamic adjustment of circadian period and that it is required for correct circadian entrainment, we wanted to investigate whether oscillator period is associated with entrainment and whether the effect of BIG on phase could be involved in this regulation. To do this, we studied whether entrainment photoperiod affects free-running period in Ws-2 and son1 (Fig. 5E). The results showed that there is a relationship between the length of the entrainment photoperiod and the length of the circadian period in Ws-2 (Fig. 5E). However, this relationship was lost in son1, whose circadian period was not affected by the duration of the photoperiod during entrainment. As a result of this, son1 did not have a significantly shorter free-running period of CAB2:LUC+ compared with Ws-2 when released from entrainment cycles of 8:16 and 12:12 (son1, 22.6 ± 0.07 h [8:16] and 22.8 ± 0.14 h [12:12]; Ws-2, 22.7 ± 0.04 h [8:16] and 23.1 ± 0.14 h [12:12]). However, when plants were entrained in 16:8, son1 had a free-running period of 22.7 ± 0.2 h, 1 h shorter than Ws-2 (23.7 ± 0.06 h; P < 0.05; Fig. 5F). This shows that the photoperiod-determined entrained phase of the circadian clock affects the free-running period in constant light, and son1 does not adjust circadian period correctly in 16:8. We performed the same series of experiments but with circadian free run in constant darkness in the presence of Suc to sustain the oscillation of CAB2:LUC+ (Dalchau et al., 2011). Similar to the result in constant light, we saw that, in wild-type plants, free-running period length increased with longer entraining photoperiod (Supplemental Fig. S10), and for plants entrained in 16:8, son1 had a significantly shorter free-running period than the wild type (Ws-2, 27 ± 0.4 h; son1, 25.7 ± 0.5 h; P < 0.05).
Thus, son1 cannot correctly adjust period and has impaired phase in response to photoperiod. Collectively, these data demonstrate that nicotinamide targets a pathway involved in establishing the phase relationship between the circadian oscillator and the external environment and that BIG contributes to the correct timing of physiology in light/dark cycles, through regulating the pace of the oscillator.
DISCUSSION
Using a forward genetic screen, we found that BIG is a regulator of the dynamic adjustment of circadian period and phase. The period of the circadian oscillator is not fixed to 24 h but, instead, is a dynamically plastic phenotype and dependent on environmental conditions. Typically, experimentalists measure circadian period in constant conditions that allow the circadian oscillator to free run. In these constant conditions, the period of the Arabidopsis circadian oscillator decreases with increasing light intensity (Somers et al., 1998b), temperature (Salomé et al., 2010), and Suc (Haydon et al., 2013) and increases with nicotinamide (Dodd et al., 2007). We have identified a nicotinamide-oversensitive phenotype resulting from a mutation in BIG. son1 is allelic to doc1-1, a previously characterized mutation in BIG, confirming that BIG is a regulator of the sensitivity of the circadian oscillator to nicotinamide.
While NAD is an abundant metabolite, we do not suggest that cellular nicotinamide derived from NAD breakdown directly regulates the pace of the circadian oscillator as part of the normal functioning of the plant. Instead, we consider nicotinamide as a probe that can be used to understand the potential mechanisms by which the circadian oscillator dynamically adjusts circadian period. Previously, we proposed that nicotinamide affects circadian period through the inhibition of ADPR cyclase activity and, therefore, the production of cADPR, which is a Ca2+ agonist (Dodd et al., 2007; Abdul-Awal et al., 2016). Our demonstration that mutations in BIG affecting the sensitivity of the circadian oscillator to nicotinamide also affect the regulation of [Ca2+]cyt are supportive of the hypothesis that nicotinamide regulates circadian period through a Ca2+-sensitive mechanism. son1 has higher [Ca2+]cyt and ADPR cyclase activity than the wild type, and that increased [Ca2+]cyt is nicotinamide sensitive. This might indicate that the increased effect of nicotinamide on circadian period is related to the altered [Ca2+]cyt in the mutant. However, we do not exclude the possibility of additional Ca2+-insensitive modes of action of nicotinamide on the circadian system (Malapeira et al., 2012).
Animal homologs of BIG, UBR4/p600 in mammals and Calossin/Pushover in Drosophila melanogaster, are confirmed calmodulin-binding proteins (Xu et al., 1998; Nakatani et al., 2005; Belzil et al., 2013) and have been proposed to act as part of a Ca2+ sensing/signaling mechanism. In mammalian neurons, UBR4, calmodulin, and calmodulin-dependent protein kinase IIα form a complex upon Glu-induced Ca2+ entry through NMDA receptors or inositol trisphosphate receptor-mediated Ca2+ release from the endoplasmic reticulum (Belzil et al., 2013). Since BIG has a putative calmodulin-binding domain (Yap et al., 2000), it is tempting to speculate that this also could play a role in Ca2+ signaling, although the interacting molecular players will be different in plants.
BIG was identified originally as a light signaling regulator (Li et al., 1994) and later was shown to also control multiple hormone signaling pathways (Kanyuka et al., 2003), including auxin transport (Guo and Tan, 2013), and recently was implicated in CO2-induced stomatal closure (He et al., 2018). The precise biochemical functions of BIG are unknown, but mutations in Pushover and knockout or down-regulation of UBR4 also produce pleiotropic phenotypes (Richards et al., 1996; Sekelsky et al., 1999; Yager et al., 2001; Nakatani et al., 2005; Belzil et al., 2014). BIG, UBR4, and Pushover contain a zinc finger-like domain, the UBR box, found in ubiquitin E3 ligases specific to the N-end rule for targeted protein degradation (Gil et al., 2001; Tasaki et al., 2005, 2009). The N-end rule is a conserved pathway in which proteins are targeted for destruction dependent on their N-terminal residue and has diverse roles in different organisms (Bachmair et al., 1986; Gibbs et al., 2014). While UBR4 is required for the degradation of model and physiological N-end rule substrates, it contains no HECT or RING domains and, hence, is considered unlikely to act as an E3 ligase in isolation; rather, it may act as a substrate (N-degron) recognition subunit of a complex (Tasaki et al., 2005, 2009). It is not known whether BIG belongs to an E3 ligase complex or whether it has intrinsic E3 ligase activity. Direct evidence for the ability of the recombinant UBR box of mammalian UBR4 to bind N-degrons is lacking (Tasaki et al., 2009), but a previous bioinformatics analysis identified a ZZ domain in BIG (Gil et al., 2001). The ZZ domain is structurally and evolutionarily related to the UBR box (Kaur and Subramanian, 2015) and recently was shown to bind N-degrons in the autophagic adaptor protein p62 (Cha-Molstad et al., 2017). There is a precedent for the regulation of circadian period through the control of protein turnover, since a double mutant lacking two ubiquitin-specific proteases, UBP12 and UBP13, exhibits a short-period circadian clock phenotype (Cui et al., 2013). Thus, one potential mode of action of BIG on the circadian oscillator is through a role in protein degradation, but further study will be required to confirm or reject this hypothesis.
The effect of the son1 mutation on levels of [Ca2+]cyt was greater during the night or subjective night than during the day or subjective day. This is indicative of a time-dependent effect of BIG in the circadian system. Similarly, the doc1-1 allele of BIG specifically affects the expression of CAB and other photosynthetic genes at night rather than in the day. These data suggest that BIG acts at night in the circadian system. Previous studies have demonstrated that BIG plays a role in conveying light information and partially suppresses the phenotype of phytochromeA and phytochromeB mutations on hypocotyl length (Kanyuka et al., 2003). Thus, BIG may be involved in conveying light signaling for circadian entrainment. However, it is likely that BIG regulates period or entrainment more widely, due to the effect of son1 on both nicotinamide period lengthening and photoperiod regulation of period, indicating that BIG has a further role outside of light signaling.
Time-dependent effects on the circadian oscillator also are sometimes associated with entrainment, which is the matching of the phase and period of the oscillator with that of the external photoperiod. Synchronization of the circadian oscillator through entrainment ensures that cellular events occur at the right time of day and ensures that the circadian oscillator can track dawn and dusk as they change through the year. This is essential to coordinate whole-organism responses, as circadian period is different between organs (Takahashi et al., 2015) and is age dependent (Kim et al., 2016). We found that son1 has an early-entrained phase in long-day cycles, suggesting an impact on entrainment. The early phase of son1 and the reduced ability to dynamically alter circadian period to light and nicotinamide might be related through parametric entrainment. The inability of son1 to adjust period depending on entrainment photoperiod strongly suggests this. A previous study demonstrated that tissue-specific changes in circadian period are accompanied by corresponding changes in entrained phase (Takahashi et al., 2015). The effect of photoperiod on the entrained phase of the oscillator has been widely reported (Millar and Kay, 1996; Millar et al., 2015; Yeang, 2015). Importantly, Millar et al. (2015) report that the circadian mutant cca1 lhy has the same phase under 8:16, 12:12, and 16:8 photoperiods and, thus, is unable to adjust phase to entrainment photoperiod, unlike the wild type, which had a 2.6-h difference. This is similar to the result we find here for son1, which has the same phase under 12:12 and 16:8 photoperiods. Unlike CCA1, the transcript of BIG does not oscillate either in light/dark cycles or in constant light and shows no modulation by photoperiod. This indicates that BIG is not part of the transcription-based oscillator loops.
When previously identified Arabidopsis circadian mutants are viewed in the context of phenotypic plasticity to light, they can be assigned to one of four categories (Supplemental Table S2). Mutants can have a constitutive effect on circadian period at all intensities of light; therefore, the mutation has no effect on the dynamic plasticity of the circadian oscillator. Alternatively, mutants might have no plastic response to light, appearing insensitive, with period unchanging at all light intensities. Finally, using the conventions in the literature (Martin-Tryon and Harmer, 2008), we have defined mutations as hyposensitive, with a shallow response curve to light, or hypersensitive, in which the response curve is steep. Ten mutations do not affect dynamic adjustment to either red or blue light, including four mutations that do not affect the response to both wavelengths: the toc1-1 allele (Somers et al., 1998b), cry2-1 (Somers et al., 1998a), fio1-1 (Kim et al., 2008), and tej (Panda et al., 2002). There are eight mutations reported to cause insensitivity to either red or blue light, including prr7-11 to red light (Farré et al., 2005) and gi-200 (Martin-Tryon et al., 2007) to both red and blue light. Seven mutations cause hypersensitivity to either red or blue light, including toc1-2 (Martin-Tryon and Harmer, 2008), lwd1 lwd2 (Wang et al., 2011), and the light-signaling mutants phyA-201 and cry1-1 (Somers et al., 1998a). The hypersensitivity in terms of the effect of light on circadian period for phyA-201 and cry1-1 is caused by a very steep fluence response curve due to the inability to sense low light intensities. However, only three reported mutations cause hyposensitivity to light. rve4 rve6 rve8 (Gray et al., 2017) and phyB-1 (Somers et al., 1998a) confer hyposensitivity to red light, and prr7-3 confers hyposensitivity to blue light (Farré et al., 2005). The phenotype of son1 for the white light fluence response curve also is hyposensitive. However, as shown in Supplemental Table S2, rve4 rve6 rve8 (Gray et al., 2017) and phyB-1 (Somers et al., 1998a) both have long-period phenotypes in addition to hyposensitivity phenotypes, whereas son1 has no period phenotype under the light intensity used for the initial entrainment in 12:12 (Fig. 5; Supplemental Fig. S3). Thus, the phenotype of son1 indicates a function in adjusting period to stimuli, rather than being a core oscillator component, as under normal conditions there is no evidence for it being an oscillator component, since period defects are conditional and the transcript abundance does not oscillate. The short period of son1 after entrainment only to long days, or through maintenance in constant low light (Fig. 5) demonstrates that the effect of son1 is conditional on environmental input, suggesting that BIG is associated with the regulation of the plastic period of the oscillator by environmental signals, rather than acting as a core oscillator component. There is variability in the reported phenotypes of prr7 mutants, with them being described as long period (Farré et al., 2005) or wild type (Nakamichi et al., 2005; Seki et al., 2017). This and the hyposensitivity to light suggest that prr7 mutants also might have a defect in plasticity similar to son1 in terms of responses to light. The mechanisms might be different because PRR7 is an oscillator component, while there is no evidence for BIG being so.
Alterations in circadian period are thought to be required for entrainment, although there is not yet a consensus on how this is achieved. It is envisaged that changes in circadian period are a result of phase adjustment of the oscillator. For example, a phase advance will reduce the period of the cycle in which the advance occurred by an amount equal to the phase advance (Johnson, 1992). Additionally, changes in the velocity of the oscillator can affect period. While changes in period are associated with entrainment, it is not known if this is due to changes in velocity, phase, or both and whether these occur continuously or discontinuously (Daan, 2000). Our discovery of a mutant that is specifically compromised in the ability to dynamically alter circadian period and has altered entrained phase provides a tool with which to study the mechanism of entrainment and the pathways of this essential feature of the circadian oscillator. The study of how the circadian clock establishes a correct phase relationship with the environment is essential to understand the role of the circadian oscillator in the plant, because the timing of events within the diel cycle constitutes the likely evolutionary pressure that resulted in the emergence and optimization of circadian clocks.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Ws-2 carrying CAB2:LUC+ (Hall et al., 2003) and transformed with 35S:AEQ was described previously (Xu et al., 2007). doc1-1 (Gil et al., 2001) was obtained from the Nottingham Arabidopsis Seed Stock Centre (Arabidopsis.org). tir3-101 was a gift from Ottoline Leyser (Sainsbury Laboratory at Cambridge University). Plant growth on agar or soil was as described previously (Xu et al., 2007).
Mutagenesis
Ws-2 seeds homozygous for CAB2:LUC+ and 35S:AEQ were mutagenized using EMS (Sigma). Seeds were suspended in 150 mm EMS and 0.1% (v/v) KCl for 4 h in an AtmosBag (Sigma). Seeds were washed three times in 100 mm sodium thiosulfate (Fisher) before overnight stratification at 4°C and sowing on soil at a density of 10 seeds per 4 cm2 of soil. Ten percent of the M1 seedlings had regions of chlorosis, indicative of EMS-induced alterations to the genomic sequence. Seeds were harvested in 10 plant M2 pools. One hundred seeds were screened from 160 pools, with a total of 16,000 M2 seeds screened.
Circadian Phenotyping
Luciferase Imaging
CAB2:LUC+ luminescence was imaged from either clusters of 10 seedlings or individual seedlings (Haydon et al., 2017). Nicotinamide treatment was applied by transferring membranes (1 µm; Sefar) with 7-d-old seedlings to 10 mm nicotinamide-containing medium. Clusters of plants were transferred to nicotinamide-containing plates at 7 d old using a sterile toothpick, lifting plants under the hypocotyls. Treatment with luciferin and imaging with a Nightshade CCD camera and imaging chamber (Berthold) mounted with an 18-mm lens were as described by Haydon et al. (2017). Where the effect of light intensity was investigated, the assay plates were covered with combinations of the following neutral density filters: Lee Technical Filter #211 (Lee Filters) and Roscolux #397, #97, and #98 (Rosco). Light intensity was measured using a Skye Quantum Sensor (Skye Instruments).
Delayed Chlorophyll Fluorescence Imaging
Delayed chlorophyll fluorescence was measured from excised leaves of 28-d-old plants. Leaves were excised at the petiole and transplanted to fresh medium on 25-well plates at dawn. The camera chamber was supplied with constant RB LED light at 70 μmol m−2 s−1 and was cooled to 20°C. Measurements were automated and data extracted using IndiGO software (Berthold). Delayed chlorophyll fluorescence measurements were taken by acquiring luminescence for 60 s immediately following illumination.
Aequorin Bioluminescence Imaging
Aequorin bioluminescence was imaged from clusters of 15 seedlings as described by Hearn and Webb (2014).
Genetic Mapping
Segregation Analysis
Crosses were made with paternal Ws-2 and maternal mutant. BC1F2 seedlings were screened as individual seedlings for circadian period of CAB2:LUC+ on 10 mm nicotinamide. BC2F3 seedlings were screened as clusters of seedlings for circadian period of CAB2:LUC+ in the presence or absence of 20 mm nicotinamide.
Mapping by Sequencing
Genomic DNA was extracted from 20-d-old plants using the Qiagen Plant Maxi Kit and quantified using a Nanodrop. Sequencing libraries were prepared using Ilumina Tru-seq. DNA was sequenced by VIB Nucleomics using an Illumina HiSeq 2000. Paired-end reads supplied in fastq format were trimmed using Fast X0.0.13 to remove reads with Q < 20 or read length greater than 35 bp. Adapters were removed using cutadapt 1.2.1. Reads were filtered further to remove those with greater than 90% A content (poly-A reads), all ambiguous reads containing an N in any position, reads with Q < 25, and artifact reads using FastX 0.0.13 and ShortRead 1.20.0. Contaminant reads were removed by discarding reads that aligned to phix_illumina using Bowtie 2.1.0. Sequencing data in fastq format can be obtained from NCBI SRA (ncbi.nlm.nih.gov/sra) under accession SRP119118. Paired-end reads were aligned to the TAIR10 reference genome (Arabidopsis.org) using Bowtie2 version 2.0.2 (Langmead, 2010). SNP calling was performed using SAMtools 0.1.18 mpileup and bcftools (Li, 2011). Vcf files were converted to SHORE format using SHOREmap 2.1 convert. Allele frequency estimation and plots were generated using SHOREmap backcross. The Ws-2 parental strain and Ws-2 1001 Genomes project (http://1001genomes.org/data/MPI/MPIcollab2011/releases/current/strains/Ws-2/) were used for background correction for BC1F2 in SHOREmap backcross. SNPs with background frequency less than 16 were discarded. The workflow was automated in a pipeline using bpipe 0.9.8.5. (Supplemental Table S3). Sliding allele frequencies were generated for SNPs based on the R statistic in SHOREmap.
SNP Verification with dCAPS and Sanger Sequencing
Genomic DNA was extracted from 300 µg of plant material using the Plant Mini Kit (Qiagen). DNA was eluted into 150 µL of deionized water (Sigma). dCAPS was used to verify and genotype SNPs in the wild type, BC1F2 pools, and BC2F3 pedigrees. Primers and restriction enzymes used for dCAPS were as follows, with product sizes once amplicons had been digested given in parentheses: AT3G02260 F, 5′-TTAACATGTAATGTATTCCTCTGCA-3′ and R, 5′-TCCAGTTTCCTCGTTACTGAC-3′, HindIII 300 bp (276 and 24 bp); AT3G02330 F, 5′-GAGATTTCGTGACCTGGAACG-3′ and R, 5′-GCATCTCTCGAATAAGCTCTAATG-3′, TasI 300 bp (276 and 24 bp); and AT3G03070 F, 5′-CTAGTCGGCAATCACACCG-3′ and R, 5′-TTTCAGAAATGAACAATTCCCTGT-3′, BsmI 300 (275 and 25 bp). PCR reagents were purchased as part of the Biotaq Kit (Bioline) or as part of the Phusion Polymerase Kit (New England Biolabs). PCR products were purified using the QIAquick PCR Purification Kit (Qiagen) and quantified using a Nanodrop 2000 (Thermo Scientific). HindIII (Fisher Scientific), TasI (New England Biolabs), and BsmI (New England Biolabs) reactions were prepared for 100 µg of DNA in their optimal buffers as specified in their instructions. Restriction digestions were run in a Darwin thermocycler for 4 h at 37°C (HindIII) or 72°C (TasI and BsmI). Restriction enzyme reactions were deactivated by the addition of 4 m Tris, pH 8.4, and purple loading dye (Bioline). Digested and undigested products were run on 2.5% and 4% (w/v) fine molecular biology grade agarose (Bioline) 1× Tris-acetate EDTA buffer for resolution of small fragments. Hyperladder 100 bp (Bioline) was used for size comparison. Gels were imaged using a transilluminator controlled by GeneSnap software with 80-s exposure. Alternatively, purified PCR products were Sanger sequenced using reverse primers as the sequencing primers. Sequencing was performed by Source Bioscience. Sequencing of SNPs was accepted if the chromatograph had a quality score greater than 20.
Isolation of RNA, and Determination of Size and Abundance
RNA Extraction and Reverse Transcription
RNA was extracted using the RNeasy Plant Mini Kit (Qiagen) and the RNase-free DNase set (Qiagen). RNA was double eluted into 30 µL of RNase-free water. cDNA was generated from RNA using the RevertAid First Strand cDNA Synthesis Kit (K1622; Fermentas) using 0.5 µg of RNA in a 10-µL reaction volume.
RT-PCR and RT-Quantitative PCR
Primers were generated using NCBI-primer BLAST as follows: AT3G02260 F, 5′-GATGGTGAAGCTACTGAGCCT-3′ and R, 5′-CTTCAGCTGGCTCCATAGCA-3′ (predicted product size for gDNA of 458 bp and for cDNA of 316 bp); UBQ10 F, 5′-GGCCTTGTATAATCCCTGATGAATAAG-3′ and R, 5′-AAAGAGATAACAGGAACGGAAACATAGT; CCA1 F, 5′-GATGATGTTGAGGCGGATG-3′ and R, 5′-TGGTGTTAACTGAGCTGTGAAG-3′, TOC1 F, 5′-TCTTCGCAGAATCCCTGTGAT-3′ and R, 5′-GCTGCACCTAGCTTCAAGCA-3′; PRR7 F, 5′-GGAAACTTGGCGGATGAAAA-3′ and R, 5′-CGAGGGCGTTGTTCTGCT-3′; and CHE F, 5′-TCCACCGGAAATGGTTTTTG-3′ and R, 5′-GGCGGAAGCTTGCTGTTG-3′. RT-PCR was performed using the PCR settings and electrophoresis described above. RT-quantitative PCR was performed as described previously (Haydon et al., 2013).
Cytosol-Free Calcium Measurements
Plants grown on agar plates for 11 d were transferred to cuvettes and dosed with coelenterazine to determine the free Ca2+ as described by Martí et al. (2013).
Nicotinamide Guanine Dinucleotide Assay of ADPR Cyclase Activity
ADPR cyclase activity was measured using the nicotinamide guanine dinucleotide assay as described by Abdul-Awal et al. (2016) from 3- to 4-week-old plants grown on agar plates. Rosette tissue (5–10 g) pooled from at least 25 rosettes was harvested as a single biological replicate. Data were collected from three biological replicates.
Estimation of Circadian Parameters
Data were analyzed using the BRASS plug-in for MS Excel (http://www.amillar.org) to carry out FFT-NLLS analysis and manual phase estimation (Plautz et al., 1997). Rhythms were analyzed for at least three cycles in constant light after the first 24 h. FFT-NLLS was performed with period limits between 18 and 35 h at a 95% confidence level. Phase was calculated using the BRASS peak time analysis function. Rhythms in RT-quantitative PCR and microarray time courses were analyzed using JTK_CYCLE (Hughes et al., 2010) with period limits between 20 and 32 h.
Microarray Analysis
Microarray data sets were downloaded from array express (E-GEOD-19271 and E-GEOD-50438) and the DIURNAL long-day and short-day expression sets.
Statistical Tests
Two-sample Student’s t tests, single-factor ANOVA, and χ2 statistical tests were performed using MS Excel. The probability of rejecting the null hypothesis (P), calculated T, F, or χ2 statistic, and df are given in the text for each analysis in the form T = n, df = n, P = n.
Accession Numbers
Sequence data for the genes used in this study can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers/locus identifiers: BIG (AT3G02260), CAB2 (AT1G29920), CCA1 (AT2G46830), TOC1 (AT5G61380), CHE (AT5G08330), PRR7 (AT5G02810), and ZTL (AT5G57360). Sequencing data in fastq format can be obtained from NCBI SRA (ncbi.nlm.nih.gov/sra) under accession number SRP119118.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Dose response of circadian period to nicotinamide in Ws-2, sin1, and son1.
Supplemental Figure S2. son1 segregates with 3:433,767 in BC2F3.
Supplemental Figure S3. son1 plants do not have a circadian period phenotype in the absence of nicotinamide.
Supplemental Figure S4. Sequencing of cDNA for son1 fragments.
Supplemental Figure S5. Effects of nicotinamide on delayed chlorophyll fluorescence rhythms in son1, doc1-1, and tir3-101.
Supplemental Figure S6. Allelism of son1 and doc1 is not due to ecotype differences.
Supplemental Figure S7. doc1-1 phenotype in son1.
Supplemental Figure S8. BIG expression does not oscillate in long- or short-day photoperiods or in constant light.
Supplemental Figure S9. Circadian rhythms of CAB2:LUC+ in BIG mutants under different light intensities.
Supplemental Figure S10. son1 does not adjust period due to photoperiod entrainment.
Supplemental Table S1. Results of an M3 forward genetic screen for the effect of nicotinamide on the circadian clock.
Supplemental Table S2. Arabidopsis circadian clock genes with circadian and entrainment phenotypes.
Supplemental Table S3. bpipe script for mapping by sequencing.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Ian Henderson for advice on genetic mapping.
Footnotes
T.J.H. was supported by Biotechnology and Biological Sciences Research Council (BBSRC) CASE studentship 1090203 supported by Bayer Cropscience and BBSRC Grant BB/M006212/1. M.C.M. was supported by a University of Cambridge Broodbank Fellowship.
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Abdul-Awal SM, Hotta CT, Davey MP, Dodd AN, Smith AG, Webb AAR (2016) NO-mediated [Ca2+]cyt increases depend on ADP-ribosyl cyclase activity in Arabidopsis. Plant Physiol 171: 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. (1960) Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 25: 11–28 [DOI] [PubMed] [Google Scholar]
- Ashelford K, Eriksson ME, Allen CM, D’Amore R, Johansson M, Gould P, Kay S, Millar AJ, Hall N, Hall A (2011) Full genome re-sequencing reveals a novel circadian clock mutation in Arabidopsis. Genome Biol 12: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328 [DOI] [PubMed] [Google Scholar]
- Bachmair A, Finley D, Varshavsky A (1986) In vivo half-life of a protein is a function of its amino-terminal residue. Science 234: 179–186 [DOI] [PubMed] [Google Scholar]
- Belzil C, Neumayer G, Vassilev AP, Yap KL, Konishi H, Rivest S, Sanada K, Ikura M, Nakatani Y, Nguyen MD (2013) A Ca2+-dependent mechanism of neuronal survival mediated by the microtubule-associated protein p600. J Biol Chem 288: 24452–24464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzil C, Asada N, Ishiguro K, Nakaya T, Parsons K, Pendolino V, Neumayer G, Mapelli M, Nakatani Y, Sanada K, et al. (2014) p600 regulates spindle orientation in apical neural progenitors and contributes to neurogenesis in the developing neocortex. Biol Open 3: 475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha-Molstad H, Yu JE, Feng Z, Lee SH, Kim JG, Yang P, Han B, Sung KW, Yoo YD, Hwang J, et al. (2017) p62/SQSTM1/Sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nat Commun 8: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Lu F, Li Y, Xue Y, Kang Y, Zhang S, Qiu Q, Cui X, Zheng S, Liu B, et al. (2013) Ubiquitin-specific proteases UBP12 and UBP13 act in circadian clock and photoperiodic flowering regulation in Arabidopsis. Plant Physiol 162: 897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S. (2000) The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jürgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms 15: 195–207 [DOI] [PubMed] [Google Scholar]
- Dalchau N, Hubbard KE, Robertson FC, Hotta CT, Briggs HM, Stan GB, Gonçalves JM, Webb AA (2010) Correct biological timing in Arabidopsis requires multiple light-signaling pathways. Proc Natl Acad Sci USA 107: 13171–13176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau N, Baek SJ, Briggs HM, Robertson FC, Dodd AN, Gardner MJ, Stancombe MA, Haydon MJ, Stan GB, Gonçalves JM, et al. (2011) The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc Natl Acad Sci USA 108: 5104–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Gardner MJ, Hotta CT, Hubbard KE, Dalchau N, Love J, Assie JM, Robertson FC, Jakobsen MK, Gonçalves J, et al. (2007) The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 318: 1789–1792 [DOI] [PubMed] [Google Scholar]
- Endo M, Shimizu H, Nohales MA, Araki T, Kay SA (2014) Tissue-specific clocks in Arabidopsis show asymmetric coupling. Nature 515: 419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA (2005) Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol 15: 47–54 [DOI] [PubMed] [Google Scholar]
- Gibbs DJ, Bacardit J, Bachmair A, Holdsworth MJ (2014) The eukaryotic N-end rule pathway: conserved mechanisms and diverse functions. Trends Cell Biol 24: 603–611 [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould PD, Diaz P, Hogben C, Kusakina J, Salem R, Hartwell J, Hall A (2009) Delayed fluorescence as a universal tool for the measurement of circadian rhythms in higher plants. Plant J 58: 893–901 [DOI] [PubMed] [Google Scholar]
- Gray JA, Shalit-Kaneh A, Chu DN, Hsu PY, Harmer SL (2017) The REVEILLE clock genes inhibit growth of juvenile and adult plants by control of cell size. Plant Physiol 173: 2308–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Tan J (2013) Applications of delayed fluorescence from photosystem II. Sensors (Basel) 13: 17332–17345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Bastow RM, Davis SJ, Hanano S, McWatters HG, Hibberd V, Doyle MR, Sung S, Halliday KJ, Amasino RM, et al. (2003) The TIME FOR COFFEE gene maintains the amplitude and timing of Arabidopsis circadian clocks. Plant Cell 15: 2719–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AAR (2013) Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502: 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon MJ, Mielczarek O, Frank A, Román Á, Webb AAR (2017) Sucrose and ethylene signaling interact to modulate the circadian clock. Plant Physiol 175: 947–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA (2005) LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc Natl Acad Sci USA 102: 10387–10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhang RX, Peng K, Tagliavia C, Li S, Xue S, Liu A, Hu H, Zhang J, Hubbard KE, et al. (2018) The BIG protein distinguishes the process of CO2-induced stomatal closure from the inhibition of stomatal opening by CO2. New Phytol 218: 232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn TJ, Webb AA (2014) Measuring circadian oscillations of cytosolic-free calcium in Arabidopsis thaliana. Methods Mol Biol 1158: 215–226 [DOI] [PubMed] [Google Scholar]
- Hong S, Song HR, Lutz K, Kerstetter RA, Michael TP, McClung CR (2010) Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana. Proc Natl Acad Sci USA 107: 21211–21216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Hogenesch JB, Kornacker K (2010) JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms 25: 372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L, Holdsworth MJ, Gray JE (2007) Nicotinamidase activity is important for germination. Plant J 51: 341–351 [DOI] [PubMed] [Google Scholar]
- Johnson CH. (1992) Phase response curves: what can they tell us about circadian clocks? In Hiroshige T, Honma K, eds, Circadian Clocks from Cell to Human. Hokkaido University Press, Sapporo, Japan, pp 209–249 [Google Scholar]
- Kanyuka K, Praekelt U, Franklin KA, Billingham OE, Hooley R, Whitelam GC, Halliday KJ (2003) Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J 35: 57–70 [DOI] [PubMed] [Google Scholar]
- Kaur G, Subramanian S (2015) The UBR-box and its relationship to binuclear RING-like treble clef zinc fingers. Biol Direct 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevei E, Gyula P, Fehér B, Tóth R, Viczián A, Kircher S, Rea D, Dorjgotov D, Schäfer E, Millar AJ, et al. (2007) Arabidopsis thaliana circadian clock is regulated by the small GTPase LIP1. Curr Biol 17: 1456–1464 [DOI] [PubMed] [Google Scholar]
- Kim H, Kim Y, Yeom M, Lim J, Nam HG (2016) Age-associated circadian period changes in Arabidopsis leaves. J Exp Bot 67: 2665–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim Y, Yeom M, Kim JH, Nam HG (2008) FIONA1 is essential for regulating period length in the Arabidopsis circadian clock. Plant Cell 20: 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B. (2010) Aligning short sequencing reads with Bowtie. Curr Protoc Bioinformatics 11: 11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. (2011) A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27: 2987–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Altschmied L, Chory J (1994) Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Dev 8: 339–349 [DOI] [PubMed] [Google Scholar]
- Love J, Dodd AN, Webb AAR (2004) Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 16: 956–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapeira J, Khaitova LC, Mas P (2012) Ordered changes in histone modifications at the core of the Arabidopsis circadian clock. Proc Natl Acad Sci USA 109: 21540–21545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí MC, Stancombe MA, Webb AAR (2013) Cell- and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Plant Physiol 163: 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Tryon EL, Harmer SL (2008) XAP5 CIRCADIAN TIMEKEEPER coordinates light signals for proper timing of photomorphogenesis and the circadian clock in Arabidopsis. Plant Cell 20: 1244–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Tryon EL, Kreps JA, Harmer SL (2007) GIGANTEA acts in blue light signaling and has biochemically separable roles in circadian clock and flowering time regulation. Plant Physiol 143: 473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda W, Takenaka S, Inageda K, Nishina H, Takahashi K, Katada T, Tsuyama S, Inui H, Miyatake K, Nakano Y (1997) Oscillation of ADP-ribosyl cyclase activity during the cell cycle and function of cyclic ADP-ribose in a unicellular organism, Euglena gracilis. FEBS Lett 405: 104–106 [DOI] [PubMed] [Google Scholar]
- Millar AJ, Kay SA (1996) Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA 93: 15491–15496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AJ, Carré IA, Strayer CA, Chua NH, Kay SA (1995) Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267: 1161–1163 [DOI] [PubMed] [Google Scholar]
- Millar AJ, Carrington JT, Tee WV, Hodge SK (2015) Changing planetary rotation rescues the biological clock mutant lhy cca1 of Arabidopsis thaliana. bioRxiv [Google Scholar]
- Mockler TC, Michael TP, Priest HD, Shen R, Sullivan CM, Givan SA, McEntee C, Kay SA, Chory J (2007) The DIURNAL project: DIURNAL and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb Symp Quant Biol 72: 353–363 [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Kita M, Ito S, Yamashino T, Mizuno T (2005) PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol 46: 686–698 [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Konishi H, Vassilev A, Kurooka H, Ishiguro K, Sawada J, Ikura T, Korsmeyer SJ, Qin J, Herlitz AM (2005) p600, a unique protein required for membrane morphogenesis and cell survival. Proc Natl Acad Sci USA 102: 15093–15098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ (2011) Circadian rhythms persist without transcription in a eukaryote. Nature 469: 554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Poirier GG, Kay SA (2002) tej defines a role for poly(ADP-ribosyl)ation in establishing period length of the Arabidopsis circadian oscillator. Dev Cell 3: 51–61 [DOI] [PubMed] [Google Scholar]
- Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA (1997) Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms 12: 204–217 [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324: 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Hillman T, Stern M (1996) Mutations in the Drosophila pushover gene confer increased neuronal excitability and spontaneous synaptic vesicle fusion. Genetics 142: 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Nin V, Barbosa MT, Chini EN, Sassone-Corsi P (2011) Altered behavioral and metabolic circadian rhythms in mice with disrupted NAD+ oscillation. Aging (Albany NY) 3: 794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé PA, Weigel D, McClung CR (2010) The role of the Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. Plant Cell 22: 3650–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Ossowski S, Lanz C, Juul T, Petersen AH, Nielsen KL, Jørgensen JE, Weigel D, Andersen SU (2009) SHOREmap: simultaneous mapping and mutation identification by deep sequencing. Nat Methods 6: 550–551 [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, McKim KS, Messina L, French RL, Hurley WD, Arbel T, Chin GM, Deneen B, Force SJ, Hari KL, et al. (1999) Identification of novel Drosophila meiotic genes recovered in a P-element screen. Genetics 152: 529–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Ohara T, Hearn TJ, Frank A, da Silva VCH, Caldana C, Webb AAR, Satake A (2017) Adjustment of the Arabidopsis circadian oscillator by sugar signalling dictates the regulation of starch metabolism. Sci Rep 7: 8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Devlin PF, Kay SA (1998a) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282: 1488–1490 [DOI] [PubMed] [Google Scholar]
- Somers DE, Webb AA, Pearson M, Kay SA (1998b) The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development 125: 485–494 [DOI] [PubMed] [Google Scholar]
- Somers DE, Schultz TF, Milnamow M, Kay SA (2000) ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101: 319–329 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Hirata Y, Aihara K, Mas P (2015) A hierarchical multi-oscillator network orchestrates the Arabidopsis circadian system. Cell 163: 148–159 [DOI] [PubMed] [Google Scholar]
- Tasaki T, Mulder LCF, Iwamatsu A, Lee MJ, Davydov IV, Varshavsky A, Muesing M, Kwon YT (2005) A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons. Mol Cell Biol 25: 7120–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki T, Zakrzewska A, Dudgeon DD, Jiang Y, Lazo JS, Kwon YT (2009) The substrate recognition domains of the N-end rule pathway. J Biol Chem 284: 1884–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu JF, Nakamichi N, Sakakibara H, Nam HG, Wu SH (2011) LIGHT-REGULATED WD1 and PSEUDO-RESPONSE REGULATOR9 form a positive feedback regulatory loop in the Arabidopsis circadian clock. Plant Cell 23: 486–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Wes PD, Chen H, Li HS, Yu M, Morgan S, Liu Y, Montell C (1998) Retinal targets for calmodulin include proteins implicated in synaptic transmission. J Biol Chem 273: 31297–31307 [DOI] [PubMed] [Google Scholar]
- Xu X, Hotta CT, Dodd AN, Love J, Sharrock R, Lee YW, Xie Q, Johnson CH, Webb AAR (2007) Distinct light and clock modulation of cytosolic free Ca2+ oscillations and rhythmic CHLOROPHYLL A/B BINDING PROTEIN2 promoter activity in Arabidopsis. Plant Cell 19: 3474–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager J, Richards S, Hekmat-Scafe DS, Hurd DD, Sundaresan V, Caprette DR, Saxton WM, Carlson JR, Stern M (2001) Control of Drosophila perineurial glial growth by interacting neurotransmitter-mediated signaling pathways. Proc Natl Acad Sci USA 98: 10445–10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M (2000) Calmodulin target database. J Struct Funct Genomics 1: 8–14 [DOI] [PubMed] [Google Scholar]
- Yeang HY. (2015) Cycling of clock genes entrained to the solar rhythm enables plants to tell time: data from Arabidopsis. Ann Bot 116: 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]