Salicylic acid signaling is integrated with abscisic acid signaling in Arabidopsis guard cells via the Ca2+/CPKdependent signaling branch but not the OST1-dependent signaling branch.
Abstract
The phenolic hormone salicylic acid (SA) induces stomatal closure. It has been suggested that SA signaling is integrated with abscisic acid (ABA) signaling in guard cells, but the integration mechanism remains unclear. The Ca2+-independent protein kinase Open Stomata1 (OST1) and Ca2+-dependent protein kinases (CPKs) are key for ABA-induced activation of the slow-type anion channel SLAC1 and stomatal closure. Here, we show that SA-induced stomatal closure and SA activation of slow-type anion channel are impaired in the CPK disruption mutant cpk3-2 cpk6-1 but not in the OST1 disruption mutant ost1-3. We also found that the key phosphorylation sites of SLAC1 in ABA signaling, serine-59 and serine-120, also are important for SA signaling. Chemiluminescence-based detection of superoxide anion revealed that SA did not require CPK3 and CPK6 for the induction of reactive oxygen species production. Taken together, our results suggest that SA activates peroxidase-mediated reactive oxygen species signal that is integrated into Ca2+/CPK-dependent ABA signaling branch but not the OST1-dependent signaling branch in Arabidopsis (Arabidopsis thaliana) guard cells.
The stomatal aperture in the epidermis of plant leaves is formed by a pair of guard cells. Ion transport across the plasma and vacuolar membranes of guard cells regulates opening and closing the aperture (Jezek and Blatt, 2017). Guard cells can respond to a variety of stimuli such as light, drought, external Ca2+, abscisic acid (ABA), salicylic acid (SA), and methyl jasmonate (MeJA) and, consequently, control gas exchange, transpirational water loss, and innate immunity (Murata et al., 2015; Melotto et al., 2017). To optimize growth under ever-changing environments in nature, plants have developed robust mechanisms that integrate the stress inputs and then output the optimal stomatal aperture in guard cells.
The phytohormone SA is a phenolic compound that triggers systemic acquired resistance (White, 1979; Ward et al., 1991; Uknes et al., 1992) and confers drought tolerance to plants (Miura et al., 2013; Okuma et al., 2014). It also has been reported that SA induces stomatal closure (Lee, 1998; Mori et al., 2001; Zeng and He, 2010; Khokon et al., 2011; Hua et al., 2012). The guard cell SA signaling and its cross talk with other signaling play key roles in stomatal immunity together with other signaling (Melotto et al., 2017), but the molecular mechanism remains to be clarified in detail.
Ca2+-dependent protein kinases (CPKs) such as CPK3 and CPK6 (Mori et al., 2006) and the Ca2+-independent protein kinase Open Stomata1 (OST1; Mustilli et al., 2002) function in ABA signal cascades in guard cells. It has been reported that CPK6 and OST1 also participate in MeJA signaling (Munemasa et al., 2011; Yin et al., 2016) and yeast elicitor signaling (Ye et al., 2013, 2015) in Arabidopsis (Arabidopsis thaliana) guard cells.
Slow anion channel-associated1 (SLAC1) is a plasma membrane anion transporter that is essential for guard cell slow-type (S-type) anion channel function and is involved in ABA-induced stomatal closure (Negi et al., 2008; Vahisalu et al., 2008). Activation of the S-type anion channel triggers plasma membrane depolarization, which is the primary driving force for K+ efflux from guard cells (Schroeder et al., 1987; Schroeder and Keller, 1992; Schmidt et al., 1995; Pei et al., 1997). When SLAC1 was coexpressed with CPKs or OST1 in Xenopus laevis oocytes, large anion currents similar to S-type anion currents in guard cells were observed (Geiger et al., 2009, 2010; Lee et al., 2009; Brandt et al., 2012). In the X. laevis oocyte system, CPKs and OST1 phosphorylate Ser-59 (S59) and Ser-120 (S120) of SLAC1 for the activation, respectively (Geiger et al., 2009, 2010; Brandt et al., 2012). A recent in planta analysis confirmed that the phosphorylation-dependent regulation of SLAC1 at both the two Ser residues is involved in ABA-induced S-type anion channel activation and stomatal closure (Brandt et al., 2015). Similar to ABA, SA activates the S-type anion channel in Arabidopsis guard cells (Khokon et al., 2017).
Reactive oxygen species (ROS) act as a second messenger in both ABA and SA signaling in guard cells. Plasma membrane NAD(P)H oxidases are responsible for ROS production in guard cell ABA signaling (Kwak et al., 2003), while salicylhydroxamic acid (SHAM)-sensitive peroxidases are major ROS sources in guard cell SA signaling (Mori et al., 2001). Guard cell plasma membrane Ca2+-permeable cation (ICa) channels are activated by hyperpolarization and ABA (Grabov and Blatt, 1998, 1999; Hamilton et al., 2000, 2001; Pei et al., 2000). The ICa channel-mediated Ca2+ entry triggers Ca2+ release from intracellular Ca2+ stores, resulting in guard cell cytosolic Ca2+ elevation (Grabov and Blatt, 1998, 1999; Minguet-Parramona et al., 2016). ROS stimulate the ICa channels that mediate guard cell ABA signaling (Pei et al., 2000; Murata et al., 2001). In the NADPH oxidase double disruption mutant rbohD/F, ICa channels are activated by hydrogen peroxide (H2O2) but not by ABA, indicating that NADPH oxidase-derived ROS mediate the activation of ICa channels in guard cell ABA signaling (Kwak et al., 2003).
It has been shown that the second messengers ROS and Ca2+ are crucial for signal integration between ABA signaling and other signaling in guard cells (Mori et al., 2009; Song et al., 2014; Murata et al., 2015; Singh et al., 2017). The integration of SA and ABA signaling in guard cells also was proposed (Zeng and He, 2010), but the mechanism remains unclear.
In order to elucidate the integration mechanism of SA signaling with ABA signaling in guard cells, here we analyzed SA-induced stomatal closure using CPK3- and CPK6-disruption mutants and the OST1-disruption mutant. We found that, different from ABA, SA requires the CPK-dependent pathway but not the OST1-dependent pathway for the induction of stomatal closure. Whole-cell patch-clamp analysis revealed that SA activation of the S-type anion channel is impaired in cpk3-2 cpk6-1 guard cell protoplasts (GCPs) but not in ost1-3 GCPs. Both S59 and S120 of SLAC1 functioned in guard cell SA signaling as shown in ABA signaling. We also found that SA-mediated ROS production is not disrupted in the cpk3-2 cpk6-1 mutant. Based on these results, we propose a new model for SA and ABA signaling integration in guard cells.
RESULTS
SA-Induced Stomatal Closure Is Impaired in cpk3 cpk6 Double Mutants But Not in the ost1 Mutant
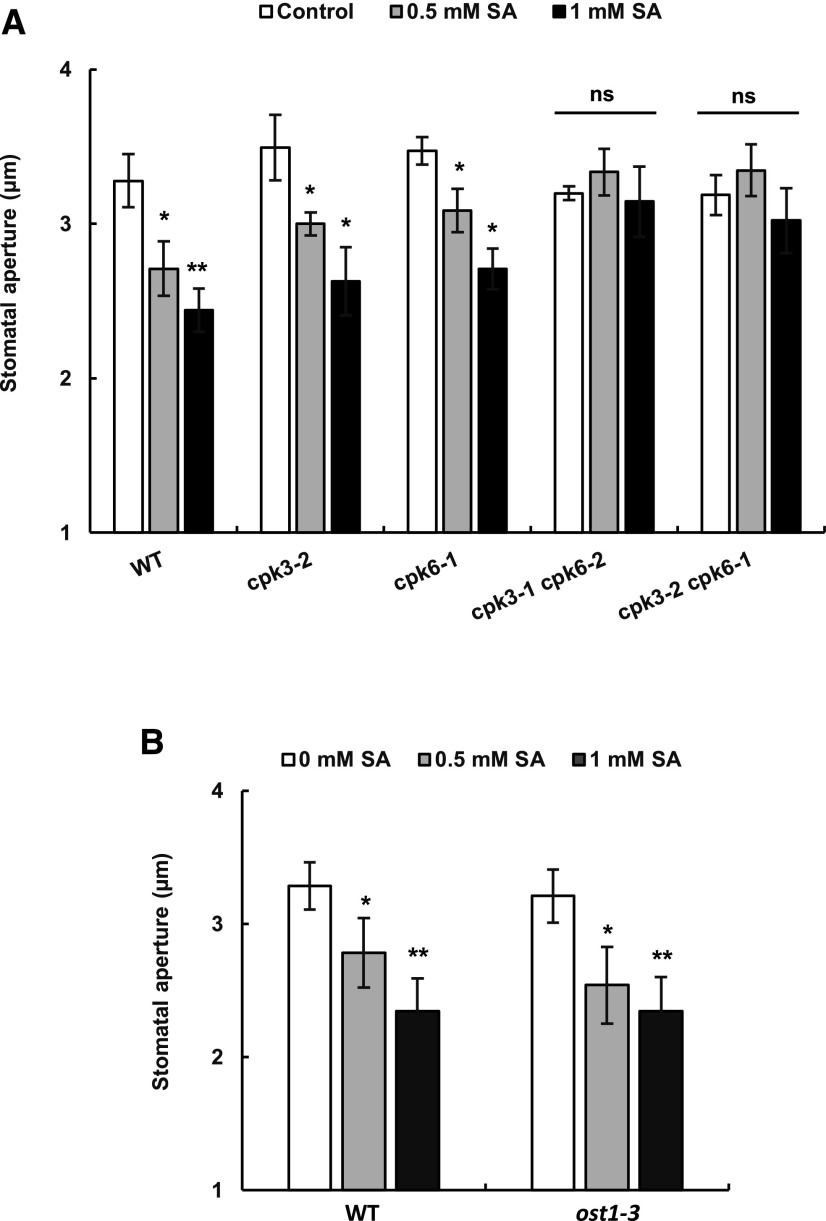
As reported previously (Khokon et al., 2011; Hua et al., 2012; Issak et al., 2013), under our experimental conditions, SA significantly induced stomatal closure in a concentration-dependent manner in Arabidopsis (Supplemental Fig. S1). The impairment of ABA-induced stomatal closure in the cpk3 cpk6 double mutant has been reported (Mori et al., 2006). We found that exogenous application of 0.5 and 1 mm SA significantly induced stomatal closure in the cpk3-2 and cpk6-1 single mutants but not in the cpk3-2 cpk6-1 and cpk3-1 cpk6-2 double mutants (Fig. 1A). These results suggest that CPK3 and CPK6 are involved in SA-induced stomatal closure as well as ABA-induced stomatal closure. Relative transcriptional levels of CPK3 and CPK6 were not changed by 0.5 mm SA treatment in guard cell-enriched epidermal tissues (Supplemental Fig. S2).
Figure 1.
SA-induced stomatal closure responses in Arabidopsis CPK disruption mutants (A) and the OST1 disruption mutant ost1-3 (B). Averages of stomatal apertures from at least three independent experiments (total stomata per bar ≥ 60) are shown. Error bars represent se. **, *, and ns indicate P < 0.01, P < 0.05, and P > 0.05, respectively. WT, Wild type.
Next, we investigated the involvement of OST1 kinase in guard cell SA signaling. It has been reported that stomata of the OST1 disruption mutant do not close in response to ABA (Mustilli et al., 2002), MeJA (Yin et al., 2016), yeast elicitor (Ye et al., 2015), and flg22 (Guzel Deger et al., 2015). However, we found that exogenous application of 0.5 and 1 mm SA significantly induced stomatal closure in wild-type plants as well as in the ost1-3 mutant (Fig. 1B).
Activation of S-Type Anion Channels by SA Is Impaired in the CPK3 CPK6 Disruption Mutant But Not in the OST1 Disruption Mutant
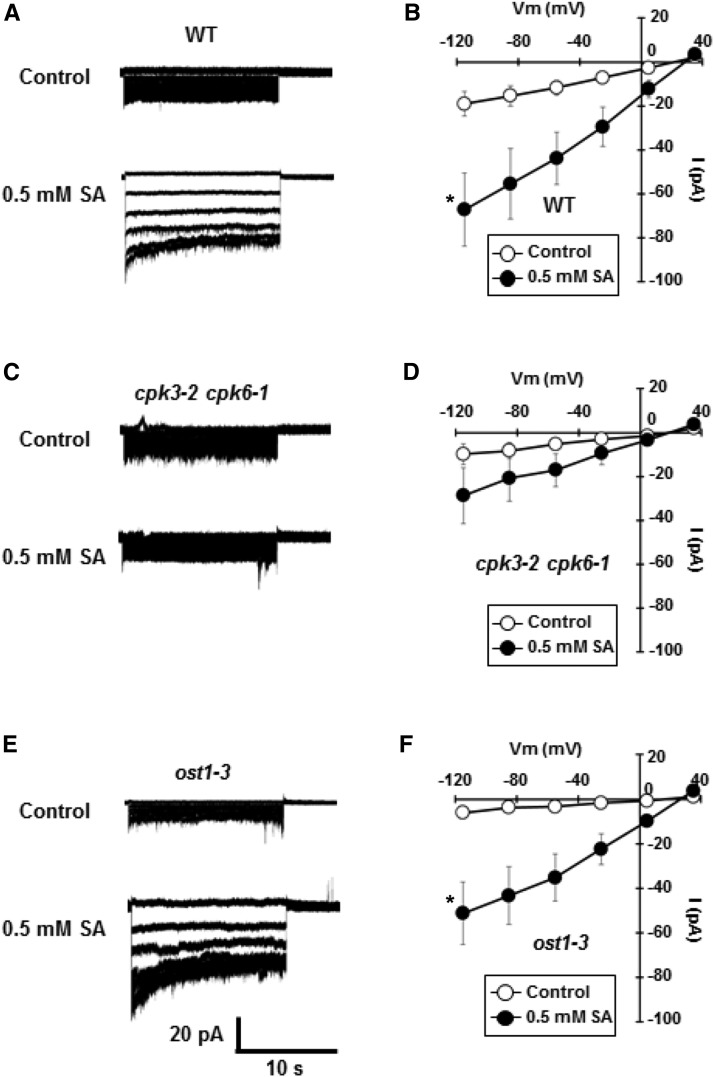
Similar to ABA, SA activates S-type anion channels in guard cells (Khokon et al., 2017), and stomata of the SLAC1-disruption mutants slac1-1 and slac1-3 failed to close in response to SA (Supplemental Fig. S3). These results indicate that SLAC1 S-type anion channels are essential for SA-induced stomatal closure. Next, we tested the SA activation of S-type anion channels in the cpk3-2 cpk6-1 GCPs and ost1-3 GCPs. As reported by Khokon et al. (2017), SA activates S-type anion channels in wild-type GCPs (Fig. 2A). We found that the SA activation of S-type anion channels was disrupted in the cpk3-2 cpk6-1 GCPs (Fig. 2C) but not in ost1-3 GCPs (Fig. 2E), indicating that CPK3 and CPK6, but not OST1, mediate SA signals for the activation of S-type anion channels.
Figure 2.
SA activation of S-type anion currents in wild-type (WT), cpk3-2 cpk-6-1, and ost1-3 GCPs. A, C, and E, Representative whole-cell S-type anion currents of wild-type GCPs (A), cpk3-2 cpk6-1 GCPs (C), and ost1-3 GCPs (E) in the absence (top trace) or presence of 0.5 mm SA (bottom trace). B, D, and F, Average current-voltage curves of wild-type GCPs (B; n = 6 [control] and n = 6 [SA]), cpk3-2 cpk6-1 GCPs (D; n = 7 [control] and n = 6 [SA]), and ost1-3 GCPs (F; n = 5 [control] and n = 7 [SA]). White and black circles indicate the absence and presence of 0.5 mm SA, respectively. GCPs were pretreated with 0.5 mm SA for 10 min before starting patch-clamp analysis. Error bars represent se. *, P < 0.05 (SA versus control).
SA-Induced Stomatal Closure Is Impaired in S59/S120 Double Disruption SLAC1 Mutants But Not in S59 and S120 Single Disruption SLAC1 Mutants
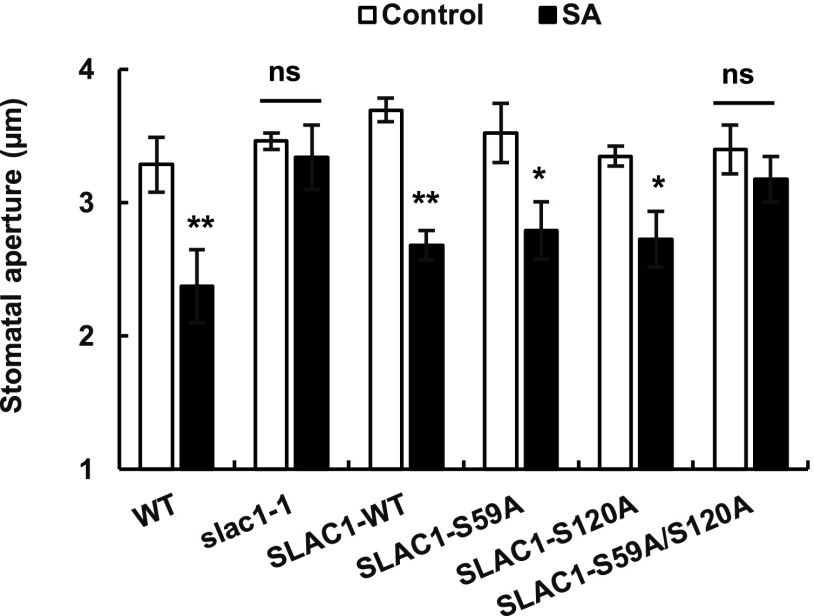
It has been reported that the amino acid residues S59 and S120 are the key phosphorylation sites of SLAC1 and are required for the activation of SLAC1 by CPKs and OST1 protein kinases, respectively (Geiger et al., 2009, 2010; Brandt et al., 2012). Brandt et al. (2015) reported that ABA-induced stomatal closure was impaired in the S59 and S120 double phosphosite disruption mutant SLAC1-S59A/S120A (Ser is mutated to Ala) but not in the S59A or S120A single mutant, proving the in planta function of both phosphorylation sites. Next, to investigate the functions of the two phosphorylation sites S59 and S120 of SLAC1 in guard cell SA signaling, we performed stomatal bioassay analysis using slac1-1 complementation lines with phosphosite-mutated SLAC1, SLAC1-S59A, SLAC1-S120A, and SLAC1-S59A/S120A (Brandt et al., 2015). SLAC1-WT complementation lines showed an SA-sensitive stomatal phenotype similar to that of wild-type plants (Fig. 3). We found that SA induced stomatal closure in the SLAC1-S59A and SLAC1-S120A single mutants but not in the SLAC1-S59A/S120A double mutant (Fig. 3).
Figure 3.
SA-induced responses of stomatal apertures in the slac1-1 complementation lines SLAC1-WT, SLAC1-S59A, SLAC1-S120A, and SLAC1-S59A/S120A. Averages of stomatal apertures from at least three independent experiments (total stomata per bar ≥ 60) are shown. Error bars represent se. **, *, and ns indicate P < 0.01, P < 0.05, and P > 0.05, respectively. SLAC1 complement lines are slac1-1 mutants that express SLAC1 or SLAC1-phosphosite mutants fused with monomeric Venus (C terminally) under the control of the SLAC1 promoter.
SA Elicited ROS Production in the cpk3 cpk6 Mutant as Well as in the Wild-Type Plants
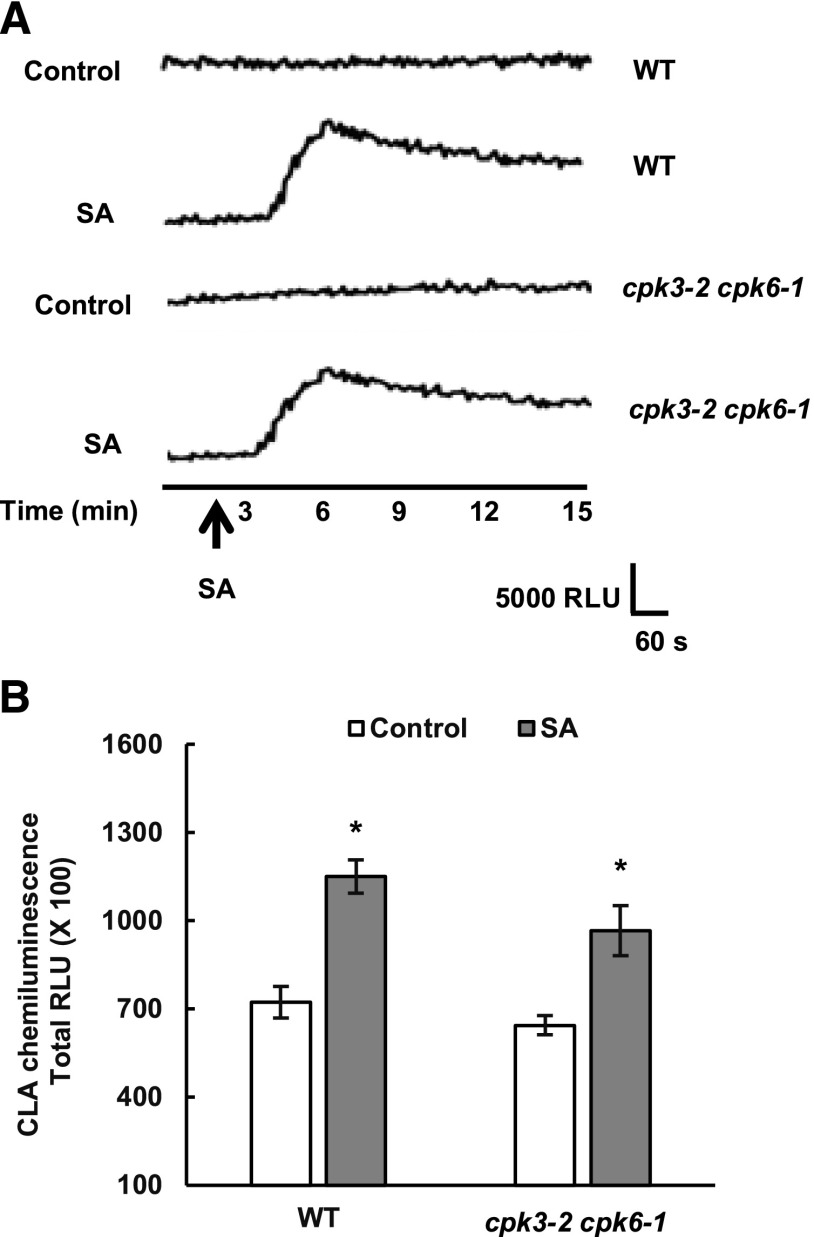
It has been shown that SA triggers the accumulation of ROS such as superoxide anion (O2−) and H2O2 and that the ROS function as second messengers in the promotion of stomatal closure (Lee et al., 1999; Mori et al., 2001; Khokon et al., 2011, 2017). It is reported that SA elicits a SHAM-sensitive peroxidase-mediated ROS burst that triggers the activation of downstream SA signaling components in guard cells (Mori et al., 2001; Khokon et al., 2011). We examined the effects of SA on ROS production in the cpk3-2 cpk6-1 mutants to investigate the positions of ROS production and the two CPKs in the SA signal pathway in guard cells.
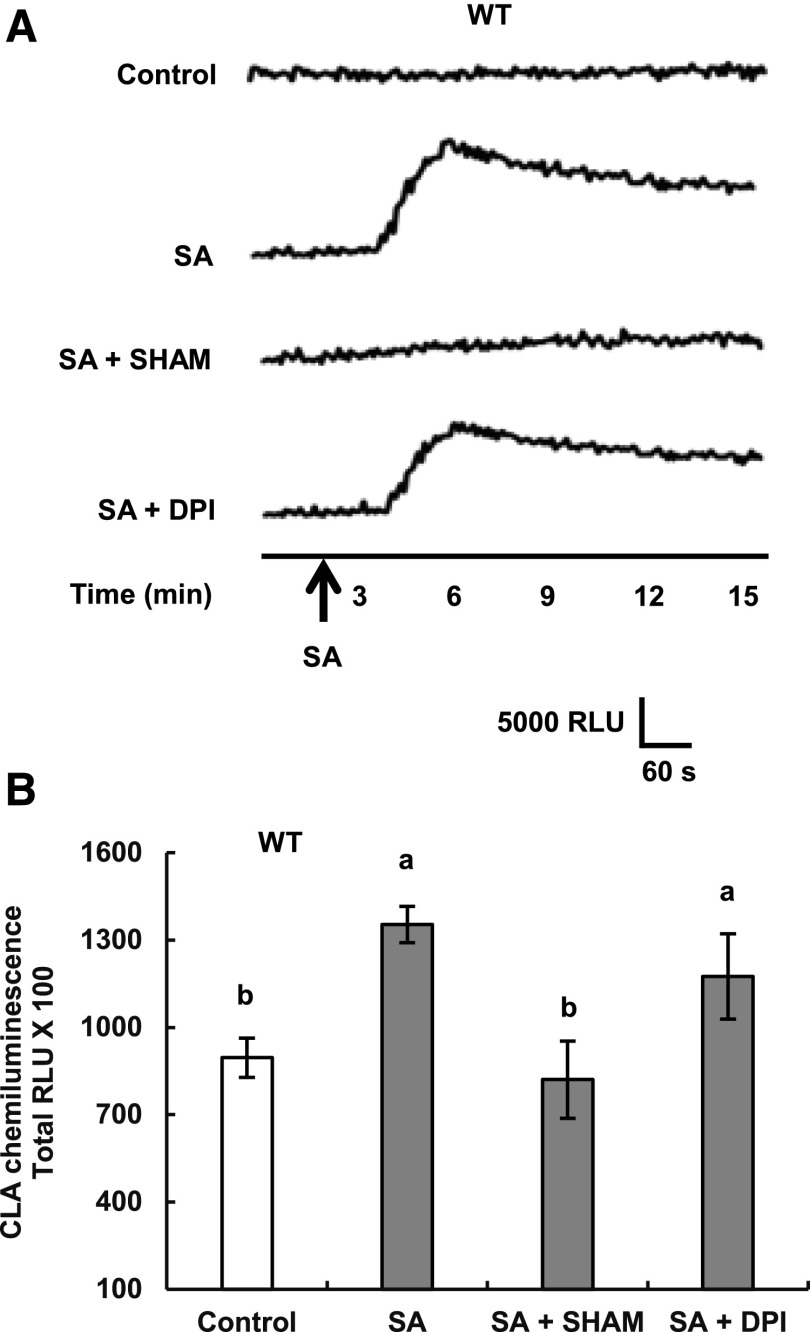
We measured O2− production in guard cell enriched-epidermal tissues using CLA (Cripridina lucigenin-derived chemiluminescent agent: 2-methyl-6-phenyl-3,7- dihydroimidazo[1,2-a]pyrazin-3-one; Kawano et al., 1998; Mori et al., 2001). The application of 0.5 mm SA evoked O2− production in wild-type guard cell-enriched epidermal tissues (Fig. 4), and the O2− production was inhibited by SHAM but not by the NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI), confirming that the SA-triggered O2− production is mediated mainly by SHAM-sensitive peroxidases (Mori et al., 2001; Khokon et al., 2011) but not by DPI-sensitive NADPH oxidases such as RBOHD/F (Kwak et al., 2003). Application of 0.5 mm SA also evoked O2− production in cpk3-2 cpk6-1 guard cell enriched-epidermal tissues (Fig. 5). In previous studies, 3,3′-diaminobenzidine (DAB)-based apoplastic H2O2 measurement in whole leaves also was employed to dissect guard cell SA signaling (Khokon et al., 2011; Prodhan et al., 2017). The DAB staining analysis revealed that disruption of CPK3 and CPK6 did not affect SA-induced H2O2 production in whole leaves (Supplemental Fig. S4). Together, these results indicate that CPK3 and CPK6 protein kinases function downstream of ROS production in guard cell SA signaling.
Figure 4.
Effects of SHAM and DPI on SA-elicited O2− production in guard cell-enriched epidermal peels of Arabidopsis wild-type (WT) plants. A, Typical traces of the CLA chemiluminescence reflecting the production of O2− in the presence and absence of 2 mm SHAM (a peroxidase inhibitor) or 20 μm DPI (an NADPH oxidase inhibitor). SA at 0.5 mm was added at the time shown by the arrow. B, Total CLA chemiluminescence reflecting the production of O2− as recorded in A. Total RLU (relative luminescence units) is the sum of luminescence between 1 and 15 min. Each data point reflects the mean (n = 3). Error bars represent se. Values indicated by the same letter do not differ significantly at the 5% level, as determined by Tukey’s test.
Figure 5.
SA-elicited O2− production in wild-type (WT) and cpk3-2 cpk6-1 mutant plants. A, Typical kinetics of CLA chemiluminescence reflecting O2− production in the presence or absence of SA in the wild type and the cpk3-2 cpk6-1 mutant. Same WT traces are shown as in Fig. 4A. B, Total CLA chemiluminescence reflecting the production of O2− as recorded in A. Total RLU (relative luminescence units) is the sum of luminescence between 1 and 15 min.WT datasets include the same experimental replicates as Figure 4B and two additional experimental replicates. Each data point reflects the mean (n = 5). *, Significant difference (P < 0.05) from controls as assessed by Student’s t test. Error bars represent se.
DISCUSSION
SA and ABA induce stomatal closure in plants and, thus, play a crucial role in adaptation to stress conditions. Significant efforts have been devoted to identifying signaling components in guard cell responses to ABA. In comparison, very few studies have focused on SA signaling in guard cells. Although the roles of CPKs, OST1, and SLAC1 in ABA signaling in guard cells are elucidated, those in SA signaling remain to be examined. In this study, we present the involvement of CPKs and SLAC1 in guard cell SA signaling and elucidate the integration of SA signaling and ABA signaling in guard cells.
SA Requires CPK3 and CPK6 Protein Kinases But Not OST1 for the Induction of Stomatal Closure
It has been reported that SA regulates gene expression involved in the innate response via a Ca2+-dependent pathway (Du et al., 2009; Wang et al., 2009; Coca and San Segundo, 2010). As well, Khokon et al. (2011) reported that the Ca2+ chelator EGTA and the Ca2+ channel blocker LaCl3 significantly suppressed the SA-induced stomatal closure, suggesting the involvement of extracellular free Ca2+ in the modulation of SA-induced stomatal closure. However, the molecular identities of the Ca2+ sensors that are responsible for guard cell SA signaling were unknown. CPKs play a role as cytosolic Ca2+ concentration sensors in many aspects of plant physiological processes (Klimecka and Muszyńska, 2007). Several CPKs are important mediators of Ca2+-dependent stomatal closure and S-type anion channel activation and play roles in ABA signaling in guard cells (Mori et al., 2006; Zhu et al., 2007; Geiger et al., 2010; Brandt et al., 2015). Mori et al. (2006) have reported that CPK3 and CPK6 positively regulate ABA-induced stomatal closure in Arabidopsis. In this study, we found that the disruption of CPK3 and CPK6 also impaired the SA-induced stomatal closure (Fig. 1A). Our findings along with the previous reports suggested that the CPK-dependent Ca2+ recognition could be key for an integration mechanism between SA signaling and ABA signaling in guard cells. It should be noted that, although pharmacological analysis (Khokon et al., 2011) and genetic analysis (this study) provide strong evidence that SA requires cytosolic Ca2+ signals to induce stomatal closure, so far, our Ca2+ imaging analyses using genetically encoded Ca2+ sensors could not detect any Ca2+ increase response in SA-treated Arabidopsis guard cells (yellow cameleon 3.6 [Khokon et al., 2011, 2017] and yellow cameleon 2.6 [data not shown]). Recently, de Jonge et al. (2017) reported that SA interferes with the fluorescence of several fluorescent proteins in planta; therefore, a Ca2+ imaging experiment using different types of Ca2+ biosensors or Ca2+-sensitive fluorescent dyes is needed to validate the true effect of SA on cytosolic Ca2+ in the future. The other possible explanation is that SA does not significantly increase cytosolic Ca2+ but primes the Ca2+ sensitivity of the downstream targets, as proposed in the case of ABA signaling (for review, see Hubbard et al., 2012; Laanemets et al., 2013; Munemasa et al., 2015). Cytosolic Ca2+ regulation of S-type anion channels is primed by ABA in Arabidopsis guard cells (Brandt et al., 2015). It also was reported that phosphorylation enhances the sensitivity of the S-type anion current to cytosolic Ca2+ in Vicia faba guard cells (Chen et al., 2010). Future studies are needed to confirm whether or not SA has the Ca2+ sensitivity priming mechanism similar to that of ABA.
The OST1 protein kinase plays a key role in the ABA responses of Arabidopsis guard cells (Mustilli et al., 2002; Yoshida et al., 2002; Acharya et al., 2013; Imes et al., 2013). It has been reported that OST1 also can be activated independently of ABA (Yoshida et al., 2006), and the ABA-independent OST1 function is involved in stomatal closure induced by MeJA (Yin et al., 2016), CO2 (Xue et al., 2011), and leaf-to-air vapor pressure deficit (Merilo et al., 2018; Pantin and Blatt, 2018). Different from these reports, here we found that SA-induced stomatal closure does not require OST1 (Fig. 1B).
Microbe-associated molecular patterns (MAMPs) such as yeast elicitor (Ye et al., 2015) and flg22 (Guzel Deger et al., 2015) also require OST1 to induce stomatal closure. It has been suggested that SA acts downstream of MAMP perception in MAMP-induced stomatal closure (Montillet et al., 2013; Montillet and Hirt, 2013). Different from the MAMPs, SA-induced stomatal closure does not require OST1 (Fig. 1B), suggesting that MAMPs activate the OST1-dependent pathway via an SA-independent manner in guard cells. The detailed cross talk mechanism for the SA-dependent pathway and the OST1-dependent pathway in guard cell MAMP signaling needs to be analyzed further. Recently, Khokon et al. (2017) showed that two mitogen-activated protein kinases (MAPKs), MPK9 and MPK12, are involved in guard cell SA signaling. The MPK9 and MPK12 double disruption mutant mpk9 mpk12 is defective in SA-induced S-type anion channel activation and stomatal closure but not in ROS production (Khokon et al., 2017). Thus, similar to CPKs, the two MAPKs regulate guard cell SA signaling downstream of ROS production. Several studies report that CPKs directly phosphorylate and activate the SLAC1 S-type anion channel (Geiger et al., 2010; Brandt et al., 2012, 2015; Scherzer et al., 2012; Maierhofer et al., 2014). However, although two studies recently reported that MPK12 indirectly regulates the SLAC1 channel via phosphorylation of the protein kinase High Leaf Temperature1, which functions as a negative regulator of CO2-induced stomatal movements (Hõrak et al., 2016; Jakobson et al., 2016), our current knowledge of the molecular mechanism of how MPK9 and MPK12 regulate SLAC1 activity in guard cell ABA and SA signaling is still limited. A detailed analysis of the underlying mechanisms that integrate MPK9 and MPK12 into the CPK-dependent SLAC1 regulation is an important topic for future research.
SLAC1 and Its Activation by the Phosphorylation of S59 and S120 Are Required for SA Signaling in Guard Cells
SLAC1 mediates S-type anion channel activity and stomatal closure (Negi et al., 2008; Vahisalu et al., 2008). In this study, we found that the SLAC1 S-type anion channel is required for SA-induced stomatal closure (Supplemental Fig. S3), and the SA activation of S-type anion channels was impaired in the cpk3-2 cpk6-1 GCPs (Fig. 2C) but not in ost1-3 (Fig. 2E). Therefore, different from ABA, SA requires CPK3 and CPK6, but not OST1, for the activation of SLAC1 S-type anion channels.
Two-electrode voltage clamp analysis using X. laevis oocytes revealed that CPKs phosphorylate S59 and OST1 phosphorylates S120 of SLAC1, resulting in activation of the channel (Geiger et al., 2009, 2010; Brandt et al., 2012; Maierhofer et al., 2014). Moreover, in planta analysis concludes that both phosphorylation sites, S59 and S120, of SLAC1 are required for ABA-induced stomatal closure in Arabidopsis (Brandt et al., 2015). Similar to ABA (Brandt et al., 2015), SA-induced stomatal closure was impaired in the S59A/S120A double disruption SLAC1 mutant but not in the S59A and S120A single disruption SLAC1 mutants (Fig. 3). These results demonstrated that, in guard cell SA signaling, although OST1 kinase is absent (Fig. 1B), CPKs and other protein kinases (e.g. CIPKs; Maierhofer et al., 2014) can phosphorylate S59 and S120 of SLAC1 to activate the S-type anion channel, resulting in stomatal closure.
CPKs Function Downstream of ROS Production in the Guard Cell SA Signal Cascade
It has been shown that ROS function as important second messengers in guard cells during stomatal closure (Pei et al., 2000; Zhang et al., 2001; Suhita et al., 2004; Khokon et al., 2011). In guard cell ABA signaling, ROS production is mediated by plasma membrane NAD(P)H oxidases (Kwak et al., 2003), while ROS production in guard cell SA signaling is mediated by SHAM-sensitive peroxidases (Mori et al., 2001; Khokon et al., 2011; Fig. 4).
It was suggested that CPK3 and CPK6 function downstream of ROS production in guard cell ABA signaling (Mori et al., 2006) and that CPK6 also functions downstream of ROS production in guard cell MeJA signaling (Munemasa et al., 2011). In this study, we found that SA elicited ROS production in cpk3 cpk6 guard cell-enriched epidermis as well as in wild-type guard cell-enriched epidermis (Fig. 5). This result along with the previous reports indicate that the CPKs function downstream of SHAM-sensitive peroxidase-mediated ROS production as well as NAD(P)H oxidase-mediated ROS production in guard cell signaling. Therefore, the results presented as well as previous reports (Mori et al., 2006; Munemasa et al., 2011) provide evidence that the hormone-triggered ROS signals activate CPKs that function as signal integrators in guard cells. The mechanism by which SA-elicited ROS signal is transduced into the activation of guard cell CPKs remains unclear and needs to be investigated in the future.
Integration of SA and ABA Signaling Pathways in Arabidopsis Guard Cells
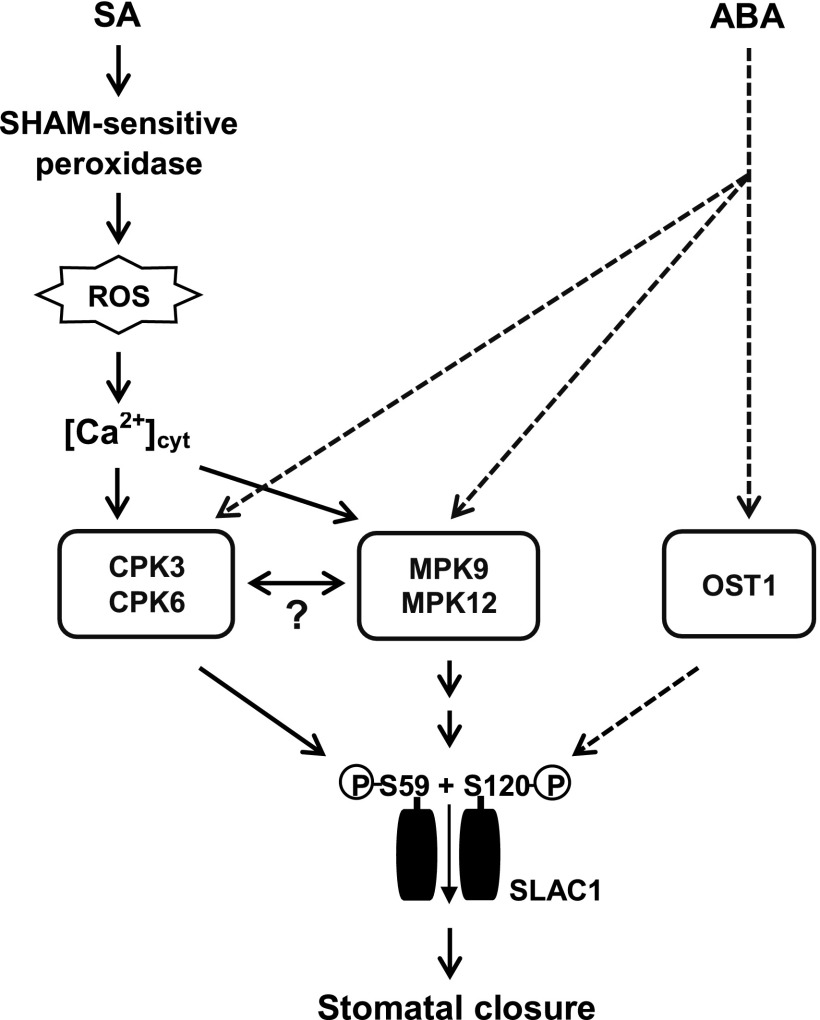
Based on the results discussed here, we propose a model (Fig. 6) showing the overlapping between SA signaling and ABA signaling in guard cells. Briefly, SA triggers SHAM-sensitive peroxidase-mediated ROS signal that activates not OST1 but CPKs and other protein kinases that phosphorylate S59 and S120 of SLAC1 and activate it, whereas ABA needs both OST1 and CPKs for the full activation of SLAC1 (Geiger et al., 2010; Brandt et al., 2015). Thus, the Ca2+/CPK-dependent signaling branch is key for the integration of SA signaling with ABA signaling in guard cells. The involvement of the MAPKs MPK9 and MPK12 in guard cell SA signaling also is reported (Khokon et al., 2017). The two MAPKs function downstream of ROS and Ca2+ and mediate SLAC1 activation indirectly, but the molecular mechanism is unclear. The strong impairment of SA-induced S-type anion channel activation and stomatal closure is caused by the disruption of either the CPKs (this study) or MAPKs (Khokon et al., 2017), suggesting the unknown interdependent mechanism of CPK and MAPK signaling pathways. It has been reported that SA as well as ABA function as key mediators for biotic stress-triggered stomatal closure (Melotto et al., 2006; Zeng et al., 2010; Montillet et al., 2013). In addition, previous research reported an SA-independent biotic signaling that antagonizes Ca2+-dependent but OST1-independent ABA signaling in guard cells (Kim et al., 2011). Therefore, the Ca2+/CPK-dependent integration mechanism of SA signaling with ABA signaling described here might be one of innate immune responses for overcoming the pathogen-induced inhibition of Ca2+-dependent ABA-induced stomatal closure.
Figure 6.
Proposed model of integration between SA signaling and ABA signaling in Arabidopsis guard cells. SA triggers a SHAM-sensitive peroxidase-mediated ROS signal that activates CPKs but not OST1 protein kinase. Then, the CPKs together with other unknown protein kinases (e.g. CIPKs) phosphorylate S59 and S120 of SLAC1 and activate it, whereas ABA requires OST1 as well as CPKs for the activation of SLAC1. These suggest that SA signaling integrates with ABA signaling via the Ca2+/CPK-dependent signaling pathway in guard cells. The two MAPKs, MPK9 and MPK12, also function downstream of ROS and Ca2+ in guard cell SA signaling and regulate SLAC1 activity indirectly. The molecular mechanism underlying the interdependence of the CPK-dependent and MPK-dependent pathways for SLAC1 activation is unknown.
MATERIALS AND METHODS
Plants and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 was used as wild-type plants. cpk3-2 cpk6-1 (Mori et al., 2006), ost1-3 (Yoshida et al., 2002), slac1-1 and slac1-3 (Vahisalu et al., 2008), SLAC1-WT, SLAC1-S59A, SLAC1-S120A, and SLAC1-S59A/S120A (Brandt et al., 2015) mutants were grown in controlled growth conditions in a 1:1 soil:vermiculite (v/v) mixture under a 16-h-light/8-h-dark photoperiod with photon flux density of 80 µmol m−2 s−1. The temperature and relative humidity in the growth chamber were 21°C and 70%. The nutrient solution (0.1%; Hyponex) was provided to the plants twice per week. Four-week-old plants were used for the experiments. SLAC1 complement lines (Fig. 3) are slac1-1 mutants that express SLAC1 or SLAC1-phosphosite mutants fused with monomeric Venus (C terminally) under the control of the SLAC1 promoter. More detailed information regarding the SLAC1 complement lines can be found in Brandt et al. (2015).
Analysis of Stomatal Apertures
Stomatal apertures were measured as reported by Khokon et al. (2011). Excised rosette leaves were blended in tap water for 30 s, and epidermal tissues were collected using a 100-µm nylon mesh. The collected epidermal tissues were dipped into stomatal assay buffer containing 50 mm KCl, 50 µm CaCl2, and 10 mm MES-Tris (pH 5.5) for 2 h in the light to induce stomatal opening. Then, SA was added to the stomatal assay buffer and again incubated 2 h in the light. The epidermal tissues were collected, and 20 stomatal apertures were measured for each individual experiment. Acidification control experiments using HCl confirmed that the pH drop caused by SA in the buffer has no effect on stomatal apertures (data not shown).
Detection of ROS Production
The generation of O2− was measured by monitoring CLA chemiluminescence as described previously (Kawano et al., 1998; Mori et al., 2001). Guard cell-enriched epidermal tissues were prepared by blending the rosette leaves of Arabidopsis. SA at 0.5 mm was applied to epidermal tissues suspended in a medium consisting of 20 µm CLA, 50 mm KCl, 50 µm CaCl2, and 10 mm MES (pH 5.5 with Tris). After the application of SA, the luminescence was measured between 1 and 15 min by a luminometer (AB2200; Atto). The chemiluminescence is presented as relative luminescence units.
The detection of apoplastic H2O2 production was measured using DAB by the method of Thordal-Christensen et al. (1997). The excised leaves were floated on buffer solution containing 50 mm KCl, 50 µm CaCl2, and 10 mm MES (pH 5.5 with Tris) and incubated in the light for 3 h. Then, DAB (1 mg mL−1) was added to the buffer solution and infiltrated gently in a vacuum for 2 h. After that, SA was applied to the buffer solution and again incubated for 2 h. The leaves were then decolorized by boiling in ethanol. ROS was visualized as a reddish-brown color and quantified using ImageJ software (National Institutes of Health).
Electrophysiology
Arabidopsis GCPs for whole-cell patch-clamp analysis were prepared enzymatically from rosette leaves as described previously (Pei et al., 1997). Whole-cell S-type anion currents were recorded as described previously (Munemasa et al., 2007). The patch-clamp solutions contained 150 mm CsCl2, 2 mm MgCl2, 6.7 mm EGTA, 5.58 mm CaCl2 (free Ca2+ concentration, 2 μm), 5 mm ATP, and 10 mm HEPES-Tris (pH 7.1) in the pipette and 30 mm CsCl2, 2 mm MgCl2, 1 mm CaCl2, and 10 mm MES-Tris (pH 5.6) in the bath (Pei et al., 1997). Osmolarity was adjusted to 500 mmol kg−1 (pipette solution) and 485 mmol kg−1 (bath solution) using d-sorbitol. GCPs were pretreated with 0.5 mm SA in the bath solution for 10 min before starting patch-clamp experiments. The application of 0.5 mm SA changed the pH in the bath solution from 5.6 to 5.4, but the pH drop has no effect on S-type anion channel activity, which was confirmed by experiments using HCl (data not shown).
RNA Extraction and Real-Time PCR
Total RNA was isolated from guard cell-enriched tissues using Trizol reagent (Invitrogen), and cDNA was synthesized using PrimeScript RT Master Mix (Perfect Real Time; Takara Bio) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using KOD SYBR qPCR Mix (Toyobo) and the Mx3005P Real-Time QPCR System (Stratagene). The levels of CPK3 and CPK6 transcript were normalized to that of ACTIN2. Relative quantification was performed using the comparative cycle threshold method. Primers used in gene-specific PCR amplification are as follows: for CPK3 (At4g23650), 5′-AGCTGATATGGATGGAGATGG-3′ and 5′-ACGATCGGTGTCTACTTCAGC-3′; for CPK6 (At2g17290), 5′-GTTCATTCTCCTACTACAGACC-3′ and 5′-ACCTGTGGCAATATCAGTACAC-3′; and for ACTIN2 (At3g18780), 5′-AAAGGCCAACAGAGAGAAGATG-3′ and 5′-TGATGTCTCTTACAATTTCCCGC-3′.
Statistical Analysis
Unless stated otherwise, the significance of differences between mean values was assessed by Student’s t test. Differences at the level of P < 0.05 were considered significant.
Accession Numbers
Sequence data from this article can be found in The Arabidopsis Information Resource (https://www.arabidopsis.org/) under accession numbers: CPK3 (At4g23650), CPK6 (At2g17290), OST1 (At4g33950), and SLAC1 (At1g12480).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Dose-dependent SA-induced stomatal closure in Arabidopsis wild-type plants.
Supplemental Figure S2. Effects of SA on CPK3 and CPK6 gene expression in guard cell-enriched tissues.
Supplemental Figure S3. SA-induced stomatal closure in the SLAC1 disruption mutants slac1-1 and slac1-3.
Supplemental Figure S4. SA-elicited apoplastic H2O2 production in wild-type and cpk3-2 cpk6-1 plants.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Footnotes
This work was in part supported by Japan Society for the Promotion of Science KAKENHI Grants 26850233 and 18K05557 (to S.M.).
Articles can be viewed without a subscription.
References
- Acharya BR, Jeon BW, Zhang W, Assmann SM (2013) Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol 200: 1049–1063 [DOI] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B, Munemasa S, Wang C, Nguyen D, Yong T, Yang PG, Poretsky E, Belknap TF, Waadt R, Aleman F, et al. (2015) Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 4: e03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Lim CK, Blatt MR (2010) Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J 61: 816–825 [DOI] [PubMed] [Google Scholar]
- Coca M, San Segundo B (2010) AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J 63: 526–540 [DOI] [PubMed] [Google Scholar]
- de Jonge J, Hofius D, Hennig L (2017) Salicylic acid interferes with GFP fluorescence in vivo. J Exp Bot 68: 1689–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Ali GS, Simons KA, Hou J, Yang T, Reddy AS, Poovaiah BW (2009) Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 457: 1154–1158 [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KAS, Grill E, et al. (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95: 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1999) A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol 119: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzel Deger A, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosché M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MR (2015) Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol 208: 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Köhler B, Blatt MR (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Blatt MR (2001) Extracellular Ba2+ and voltage interact to gate Ca2+ channels at the plasma membrane of stomatal guard cells. FEBS Lett 491: 99–103 [DOI] [PubMed] [Google Scholar]
- Hõrak H, Sierla M, Tõldsepp K, Wang C, Wang YS, Nuhkat M, Valk E, Pechter P, Merilo E, Salojärvi J, et al. (2016) A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 28: 2493–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI (2012) Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann Bot 109: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes D, Mumm P, Böhm J, Al-Rasheid KA, Marten I, Geiger D, Hedrich R (2013) Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J 74: 372–382 [DOI] [PubMed] [Google Scholar]
- Issak M, Okuma E, Munemasa S, Nakamura Y, Mori IC, Murata Y (2013) Neither endogenous abscisic acid nor endogenous jasmonate is involved in salicylic acid-, yeast elicitor-, or chitosan-induced stomatal closure in Arabidopsis thaliana. Biosci Biotechnol Biochem 77: 1111–1113 [DOI] [PubMed] [Google Scholar]
- Jakobson L, Vaahtera L, Tõldsepp K, Nuhkat M, Wang C, Wang YS, Hõrak H, Valk E, Pechter P, Sindarovska Y, et al. (2016) Natural variation in Arabidopsis Cvi-0 accession reveals an important role of MPK12 in guard cell CO2 signaling. PLoS Biol 14: e2000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S (1998) Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: the earliest events in salicylic acid signal transduction. Plant Cell Physiol 39: 721–730 [Google Scholar]
- Khokon AR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2011) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34: 434–443 [DOI] [PubMed] [Google Scholar]
- Khokon MAR, Salam MA, Jammes F, Ye W, Hossain MA, Okuma E, Nakamura Y, Mori IC, Kwak JM, Murata Y (2017) MPK9 and MPK12 function in SA-induced stomatal closure in Arabidopsis thaliana. Biosci Biotechnol Biochem 81: 1394–1400 [DOI] [PubMed] [Google Scholar]
- Kim TH, Hauser F, Ha T, Xue S, Böhmer M, Nishimura N, Munemasa S, Hubbard K, Peine N, Lee BH, et al. (2011) Chemical genetics reveals negative regulation of abscisic acid signaling by a plant immune response pathway. Curr Biol 21: 990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecka M, Muszyńska G (2007) Structure and functions of plant calcium-dependent protein kinases. Acta Biochim Pol 54: 219–233 [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K, Brandt B, Li J, Merilo E, Wang YF, Keshwani MM, Taylor SS, Kollist H, Schroeder JI (2013) Calcium-dependent and -independent stomatal signaling network and compensatory feedback control of stomatal opening via Ca2+ sensitivity priming. Plant Physiol 163: 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS. (1998) The mechanism of stomatal closing by salicylic acid in Commelina communis L. J Plant Biol 41: 97–102 [Google Scholar]
- Lee S, Choi H, Suh S, Doo IS, Oh KY, Choi EJ, Schroeder Taylor AT, Low PS, Lee Y (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maierhofer T, Diekmann M, Offenborn JN, Lind C, Bauer H, Hashimoto K, Al-Rasheid KAS, Luan S, Kudla J, Geiger D, et al. (2014) Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci Signal 7: ra86. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Melotto M, Zhang L, Oblessuc PR, He SY (2017) Stomatal defense a decade later. Plant Physiol 174: 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Yarmolinsky D, Jalakas P, Parik H, Tulva I, Rasulov B, Kilk K, Kollist H (2018) Stomatal VPD response: there is more to the story than ABA. Plant Physiol 176: 851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet-Parramona C, Wang Y, Hills A, Vialet-Chabrand S, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR (2016) An optimal frequency in Ca2+ oscillations for stomatal closure is an emergent property of ion transport in guard cells. Plant Physiol 170: 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM, Murata Y (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J 73: 91–104 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Hirt H (2013) New checkpoints in stomatal defense. Trends Plant Sci 18: 295–297 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C, et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Pinontoan R, Kawano T, Muto S (2001) Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol 42: 1383–1388 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al. (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Uraji M (2009) Integration of ROS and hormone signaling. In del Río LA, Puppo A, eds, Reactive Oxygen Species in Plant Signaling. Springer-Verlag, Berlin, pp 25–42 [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells: specific impairment of ion channel activation and second messenger production. Plant Physiol 143: 1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y (2011) The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol 155: 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI (2015) Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol 28: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S (2015) Diverse stomatal signaling and the signal integration mechanism. Annu Rev Plant Biol 66: 369–392 [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Okuma E, Nozawa R, Murata Y, Miura K (2014) Accumulation of endogenous salicylic acid confers drought tolerance to Arabidopsis. Plant Signal Behav 9: e28085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin F, Blatt MR (2018) Stomatal response to humidity: blurring the boundary between active and passive movement. Plant Physiol 176: 485–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Prodhan MY, Issak M, Nakamura T, Munemasa S, Nakamura Y, Murata Y (2017) Chitosan signaling in guard cells requires endogenous salicylic acid. Biosci Biotechnol Biochem 81: 1536–1541 [DOI] [PubMed] [Google Scholar]
- Scherzer S, Maierhofer T, Al-Rasheid KAS, Geiger D, Hedrich R (2012) Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol Plant 5: 1409–1412 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao YJ, Schroeder JI (1995) Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA 92: 9535–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Keller BU (1992) Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA 89: 5025–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E (1987) Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Parihar P, Singh S, Mishra RK, Singh VP, Prasad SM (2017) Reactive oxygen species signaling and stomatal movement: current updates and future perspectives. Redox Biology 11: 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Miao Y, Song CPP (2014) Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol 201: 1121–1140 [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powder mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J (1992) Acquired resistance in Arabidopsis. Plant Cell 4: 645–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tsuda K, Sato M, Cohen JD, Katagiri F, Glazebrook J (2009) Arabidopsis CaM binding protein CBP60g contributes to MAMP-induced SA accumulation and is involved in disease resistance against Pseudomonas syringae. PLoS Pathog 5: e1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Metraux JP, Ryals JA (1991) Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3: 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF. (1979) Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology 99: 410–412 [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J 30: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Muroyama D, Munemasa S, Nakamura Y, Mori IC, Murata Y (2013) Calcium-dependent protein kinase CPK6 positively functions in induction by yeast elicitor of stomatal closure and inhibition by yeast elicitor of light-induced stomatal opening in Arabidopsis. Plant Physiol 163: 591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Adachi Y, Munemasa S, Nakamura Y, Mori IC, Murata Y (2015) Open stomata 1 kinase is essential for yeast elicitor-induced stomatal closure in Arabidopsis. Plant Cell Physiol 56: 1239–1248 [DOI] [PubMed] [Google Scholar]
- Yin Y, Adachi Y, Nakamura Y, Munemasa S, Mori IC, Murata Y (2016) Involvement of OST1 protein kinase and PYR/PYL/RCAR receptors in methyl jasmonate-induced stomatal closure in Arabidopsis guard cells. Plant Cell Physiol 57: 1779–1790 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Zeng W, He SY (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Melotto M, He SY (2010) Plant stomata: a checkpoint of host immunity and pathogen virulence. Curr Opin Biotechnol 21: 599–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, Shang Y, Du SY, Wang XF, Wu FQ, et al. (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19: 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]