Diatom aquaporin-like channels mediate the permeability of cell membranes to CO2 and NH3 and help in mitigating high light stress.
Abstract
Aquaporins (AQPs) are ubiquitous water channels that facilitate the transport of many small molecules and may play multiple vital roles in aquatic environments. In particular, mechanisms to maintain transmembrane fluxes of important small molecules have yet to be studied in marine photoautotrophic organisms. Here, we report the occurrence of multiple AQPs with differential cellular localizations in marine diatoms, an important group of oceanic primary producers. The AQPs play a role in mediating the permeability of membranes to CO2 and NH3. In silico surveys revealed the presence of five AQP orthologs in the pennate diatom Phaeodactylum tricornutum and two in the centric diatom Thalassiosira pseudonana. GFP fusions of putative AQPs displayed clear localization to the plasma membrane (PtAGP1 and PtAQP2), the chloroplast endoplasmic reticulum (CER; PtAGP1 and PtAQP3), and the tonoplast (PtAQP5) in P. tricornutum. In T. pseudonana, GFP-AQP fusion proteins were found on the vacuole membrane (TpAQP1) and CER (TpAQP2). Transcript levels of both PtAQP1 and PtAQP2 were highly induced by ammonia, while only PtAQP2 was induced by high (1%[v/v]) CO2. Constitutive overexpression of GFP-tagged PtAQP1 and PtAQP2 significantly increased CO2 and NH3 permeability in P. tricornutum, strongly indicating that these AQPs function in regulating CO2/NH3 permeability in the plasma membrane and/or CER. Cells carrying GFP-tagged PtAQP1 and PtAQP2 had higher nonphotochemical quenching under high light relative to that of wild-type cells, suggesting that these AQPs are involved in photoprotection. These AQPs may facilitate the efflux of NH3, preventing the uncoupling effect of high intracellular ammonia concentrations.
In marine environments, diatoms are one of the most important primary producers, and their photosynthetic activity plays a vital function in the global carbon cycle, which concomitantly drives global cycles of elements such as silica and nitrogen (Falkowski et al., 2000; Mock et al., 2017). The efficiency of photosynthesis and metabolism depends upon numerous environmental conditions, such as CO2 concentration, nitrogen availability, light intensity, and many other factors. The constraints of the aquatic environment on CO2 supply typically require photoautotrophs to employ a regulated acquisition system for dissolved inorganic carbon (DIC) to maintain rapid and efficient photosynthesis. The high salinity and alkalinity of seawater greatly slow down the replenishment of CO2 from the bulk medium (Zeebe and Wolf-Gladrow, 2001). To compensate, marine diatoms take up both CO2 and HCO3− from the medium and efficiently accumulate DIC in the chloroplast for photosynthesis (Colman and Rotatore, 1995; Hopkinson et al., 2011; Nakajima et al., 2013). In the model pennate diatom Phaeodactylum tricornutum, a Solute-Carrier4 (SLC4) family protein, PtSLC4-2, was shown to be an Na+-dependent plasma membrane HCO3− transporter, which enables the direct uptake of abundant HCO3− in seawater to overcome the limited availability of dissolved CO2 (Nakajima et al., 2013). Some diatoms also possess extracellular carbonic anhydrase (CA), which allows the rapid formation of CO2 from abundant HCO3− in the periplasmic space and may provide cells a nearly unlimited supply of CO2 (Martin and Tortell, 2008; Hopkinson et al., 2011; Samukawa et al., 2014). Tsuji et al. (2017) demonstrated that some evolutionarily distant marine diatoms mainly take up CO2 formed from HCO3− by external CA. However, the mechanisms that support the efficient movement of CO2 across the plasma membrane and four-layered membranes of secondary plastids have yet to be clarified.
Ammonium is one of the most important nitrogen sources for autotrophic organisms. Most phytoplankton prefer to take up NH3 relative to NO3−, which needs to be reduced prior to incorporation into proteins and other molecules (Cresswell and Syrett, 1979). On the other hand, excess internal NH3 is toxic for photoautotrophs, notably as a dissipating reagent of the proton gradient across the thylakoid lumen or as a binding factor for the Mn cluster of the oxygen-evolving complex in PSII (Pérez-Navarro et al., 2013; Oyala et al., 2015). The thylakoidal proton gradient is essential for photoprotection in the diatoms, as it activates the xanthophyll cycle (Caron et al., 1987) by triggering the deepoxidation of the xanthophyll diadinoxanthin to synthesize diatoxanthin, which induces the nonphotochemical quenching (NPQ) of PSII (Lavaud et al., 2002). To protect the xanthophyll cycle and PSII from NH3 toxicity, a futile cycling of excess NH3 might have evolved in numerous different organisms (Britto et al., 2001; Coskun et al., 2013). Somewhat paradoxically, NH3 release often has been observed in phytoplankton; for example, in the green alga Chlamydomonas reinhardtii, NH3 was released from the cell under nitrogen starvation and in the presence of a glutamine synthase (GS) inhibitor (Florencio and Vega, 1983); in the marine diatoms Chaetoceros spp., Skeletonema costatum, and Thalassiosira weissflogii, NO2− and NH3 release was reported in cultures after a rapid transition to high irradiance (Lomas et al., 2000). Release of these inorganic nitrogen compounds was hypothesized to be facilitated by an NH3 channel, such as an aquaporin (AQP), to maintain NO3−, NO2−, or NH3 levels in relation to the levels of reductants such as ferredoxin and NADPH under fluctuating irradiance (Lomas et al., 2000).
Major intrinsic protein (MIP; also denoted as AQP) is known to be a ubiquitous water channel, which was found initially in bovine lens fiber cells (Gorin et al., 1984). The basic structure of AQP contains two Asn-Pro-Ala (NPA) domains, which are highly conserved among the MIP family (Jung et al., 1994). AQPs form homotetramers or heterotetramers with one water/glycerol pore per subunit and one intersubunit pore at the center of the tetramer structure, which is believed to facilitate the passage of several small, nonionic gaseous molecules such as CO2 and NH3 (Nakhoul et al., 2001; Wang et al., 2007). It also was reported, however, that some monomeric AQPs facilitate the movement of gaseous NH3, which has a similar electron configuration to water (Musa-Aziz et al., 2009). The two NPA motifs, together with conserved aromatic amino acids and Arg (ar/R) residues, are known to control the size and substrate permeability of the intersubunit pore (Sui et al., 2001; Beitz et al., 2006). In land plants, there are seven subclasses of AQPs, which are classified and named by their subcellular locations and sequence similarity. The plasma membrane intrinsic proteins (PIPs), the tonoplast intrinsic proteins (TIPs), and the nodulin26-like intrinsic proteins have been well described (Johanson and Gustavsson, 2002). The small basic intrinsic proteins (SIPs) have more basic amino acids and are smaller than other AQPs (Johanson and Gustavsson, 2002). GlpF-like intrinsic proteins (GIPs) are similar to the bacterial glycerol channel GlpF (Fu et al., 2000). Hybrid intrinsic proteins were found only in bryophytes and were named for their similarities to both TIPs and PIPs (Danielson and Johanson, 2008). The uncategorized X intrinsic proteins were identified from many dicots, including tobacco (Nicotiana tabacum), potato (Solanum tuberosum), tomato (Solanum lycopersicum; Bienert et al., 2011), and poplar (Populus trichocarpa; Lopez et al., 2012).
In tobacco, the PIP-type NtAQP1 was reported to be a CO2 channel (Uehlein et al., 2003), and knockdown of this protein decreased mesophyll CO2 conductance (Flexas et al., 2006). The Arabidopsis (Arabidopsis thaliana) TIP-type AQPs, AtTIP2;1 and AtTIP2;3, are known to be NH3 channels located in the tonoplast in roots (Daniels et al., 1996; Loqué et al., 2005). In silico analysis has revealed many putative algal AQPs, including the alga-specific subfamilies MIPA through MIPE present in chlorophytes (Anderberg et al., 2011). Likely substrates transported by these AQPs were determined based on comparison of the NPA motif, ar/R filter, and C-terminal region of these algal AQPs with those of other biochemically characterized MIPs: MIPAs were inferred to transport water, urea, and ammonia; MIPBs, glycerol and water; MIPCs, unknown; MIPDs, water and glycerol; and MIPEs, water (Anderberg et al., 2011). In several Heterokontophyta, a new subfamily of putative AQPs named large intrinsic proteins (LIPs) was found (Khabudaev et al., 2014), although little is known about the functions of these alga-type AQPs.
In this study, the localization of diatom AQP candidates was determined in two marine diatoms, P. tricornutum and Thalassiosira pseudonana, and the functions of two plasma membrane-associated AQPs, which showed transcriptional responses to nitrogen and/or CO2, were determined in P. tricornutum. The results revealed that these two AQPs function as channels for CO2 and/or NH3.
RESULTS
AQP Candidates in Diatoms
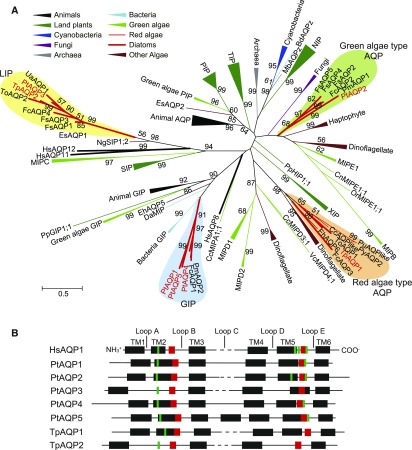
Using human HsAQP1, tobacco NtAQP1, and Arabidopsis AtPIP1;2 as reference sequences, putative AQPs were identified in the genomes of P. tricornutum and T. pseudonana. P. tricornutum and T. pseudonana respectively possessed five and two candidate AQP genes, and phylogenetic analysis showed that PtAQP1, PtAQP4, and PtAQP5 were grouped in the GIP clade, while PtAQP3 and TpAQP2 were grouped with other diatom AQP candidates in the LIP clade (Fig. 1A; Supplemental Table S1). On the other hand, TpAQP1 and PtAQP2 were more similar to the red algae and green algae MIPE-type AQPs, respectively (Fig. 1A). These associations were identical regardless of whether the phylogeny was built using full-length sequences or sequences from which the highly variable N and C termini had been deleted (Supplemental Fig. S1). In silico translation revealed that all AQP candidates contained four to seven transmembrane domains, ar/R selectivity filter residues, and two well-conserved NPA motifs, which are highly conserved characteristics of MIP family proteins (Fig. 1B; Supplemental Fig. S2).
Figure 1.
Comparison of AQP sequences from diatoms with those of different AQP families. A, Phylogenetic tree of AQP family proteins derived from animals (black), land plants (deep green), cyanobacteria (deep blue), fungi (purple), bacteria (light blue), archaea (gray), green algae (light green), red algae (thin red line), diatoms (thick red line), and other eukaryotic algae (brown; Supplemental Table S1). PtAQP1 to PtAQP5, TpAQP1, and TpAQP2 are indicated with red text. Diatom clades are colored as follows: green algae-type AQP, light green; GIP, light blue; red algae-type AQP, orange; LIP, yellow. The phylogenetic tree was constructed using the maximum-likelihood method based on the Whelan and Goldman evolutionary model (Whelan and Goldman, 2001). Clades with greater than 50% bootstrap support (using 1,000 replicates) are indicated at the nodes of the tree. The branch lengths represent the distance between sequences in the number of substitutions per site as indicated by the scale bar. B, Schematic of the structure of specific regions of AQPs in human AQP1 (HsAQP1) and diatom AQPs (PtAQP1–PtAQP5, TpAQP1, and TpAQP2). Black boxes indicate transmembrane (TM) domains, red boxes indicate NPA motifs, and green boxes indicate ar/R residues.
The ar/R residues control substrate specificity and generally are well conserved among phylogenetically related plant MIP families (Hove and Bhave, 2011). Hence, we compared the ar/R residues of diatom AQPs with those of characterized AQPs to help identify their substrate specificity. The ar/R residues in human AQP1, which is known to be a CO2 channel (Nakhoul et al., 1998), are composed of a Phe in the second transmembrane domain (TM2), a His in TM5, a Cys before the second NPA in loop E, and an Arg after the second NPA in loop E (abbreviated F-H-C-R; Supplemental Fig. S2; Supplemental Table S2). Plant AQPs that facilitate CO2 flux, including NtAQP1 from tobacco (Uehlein et al., 2003; Flexas et al., 2006), Arabidopsis AtPIP1;2 (Aharon et al., 2003), and barley (Hordeum vulgare) HvPIP2;1 (Hanba et al., 2004), instead possess Phe, His, Thr, and Arg (F-H-T-R) as their ar/R residues (Hove and Bhave, 2011). In PtAQP2, the ar/R residues were F-H-C-R, as in human HsAQP1 (Supplemental Table S2; Supplemental Fig. S1). Arabidopsis AtTIP2;1 and AtTIP2;3 and wheat (Triticum aestivum) TaTIP2;1 are known to be NH3 channels and possess His, Ile, Gly, and Arg (H-I-G-R) as their ar/R residues, but no diatom AQPs containing these residues were found. However, a soybean (Glycine max) nodulin, GmNOD26, which also is an NH3 channel (Niemietz and Tyerman, 2000), possesses another ar/R variant: Trp, Val, Ala, and Arg (W-V-A-R; Hove and Bhave, 2011), which matched that of PtAQP1 and PtAQP4 (Supplemental Table S2). The ar/R residues of PtAQP3, PtAQP5, TpAQP1, and TpAQP2 had no similarity to any plant ar/R residues (Supplemental Fig. S2).
Localization of Exogenously Introduced Diatom AQPs
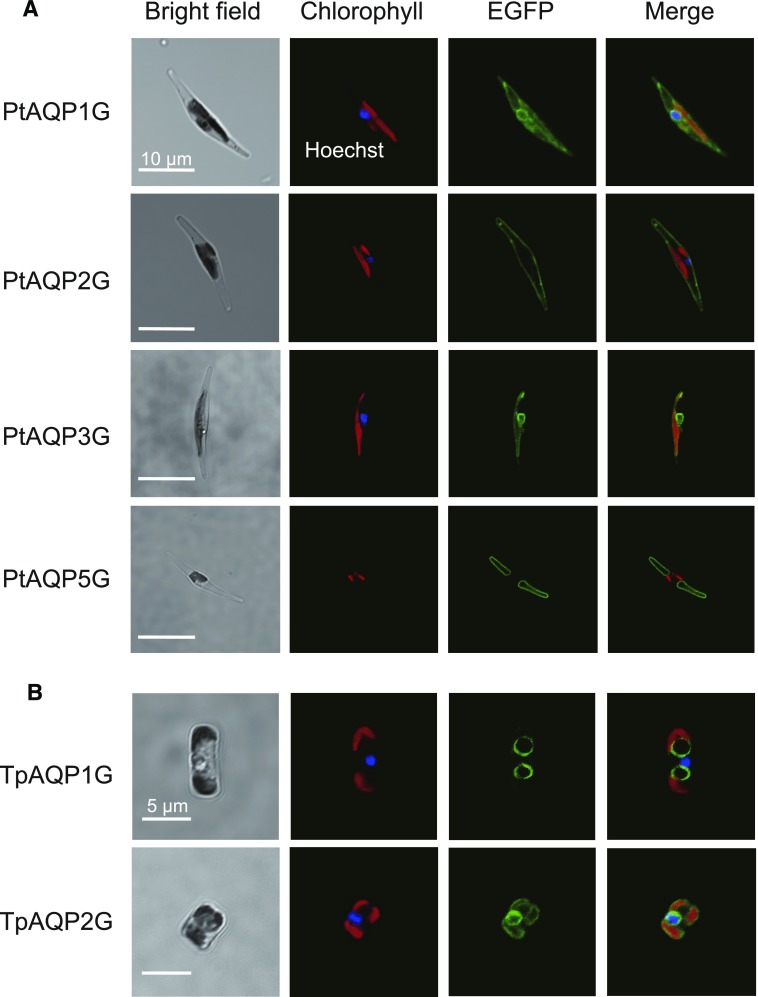
Translational fusions of the enhanced GFP gene (eGFP) and the full-length PtAQP or TpAQP cDNAs were transformed into P. tricornutum or T. pseudonana cells under the control of the promoters of the fucoxanthin-chlorophyll a/c-binding-protein gene (PfcpA) for P. tricornutum and Pfcp8 for T. pseudonana (Zaslavskaia et al., 2000; Poulsen et al., 2006). The resulting mutants expressing the PtAQPs:EGFP or TpAQPs:EGFP fusions are denoted, respectively, as PtAQPnG and TpAQPnG cells. In PtAQP1G cells, the EGFP signal was present at both the plasma membrane and the chloroplast endoplasmic reticulum (CER), a two-layered membrane system surrounding the chloroplast envelope of secondary endosymbiotic chloroplasts, indicating a dual localization of PtAQP1 (Fig. 2A). In contrast, PtAQP2G cells displayed specific localization of the EGFP signal at the plasma membrane (Fig. 2A). EGFP signals in both PtAQP3G and TpAQP2G cells were found at the CER (Fig. 2). In PtAQP5G and TpAQP1G cells, the EGFP signal was localized in a ring shape, distinct from either the chloroplast fluorescence or Hoechst signal (Fig. 2), strongly suggesting that these proteins are localized on the tonoplast or vacuole membrane. Despite several attempts, PtAQP4G cells expressing EGFP could not be obtained in this study; thus, PtAQP4 could not be localized. Overexpression of PtAQP4 apparently is detrimental to the cells, and alternative approaches will need to be used to localize this protein. The possibility of dual localization of PtAQP1 was confirmed further by confocal microscopy with a scanning depth of focus (Supplemental Fig. S3A). In several PtAQP1G#3 cells, EGFP signal was observed clearly at both the CER and the plasma membrane (Supplemental Fig. S3A).
Figure 2.
Subcellular localization of PtAQPs and TpAQPs. From left, bright field, Hoechst-stained nucleus (blue) and autofluorescence of chlorophyll (red), EGFP signal (green), and merged image. A, Images of P. tricornutum heterologously expressing EGFP-tagged PtAQP1, PtAQP2, PtAQP3, or PtAQP5. B, Images of T. pseudonana heterologously expressing EGFP-tagged TpAQP1 and TpAQP2. Bars = 10 µm (A) and 5 µm (B).
Transcriptional Responses of AQP Genes to Changing CO2 or Nitrogen Conditions
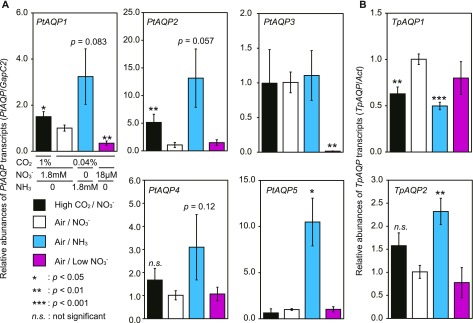
Reverse transcription quantitative PCR (RT-qPCR) analysis of the seven diatom AQPs revealed clear transcriptional responses of most of the AQPs to CO2 and/or nitrogen. Glyceraldehyde-3-phosphate dehydrogenase (Gap) and Actin (Act) were used as reference genes, and we verified that these genes were generally unresponsive to carbon and nitrogen variations by comparing their expression levels with those of two other constitutive genes (PtGapC2 versus PtHistone H4 [His] and PtAct1; TpAct versus TpHis and TpGapC3; Supplemental Fig. S4). In fact, the expression profiles were comparable between the 2−ΔΔCt method (Livak and Schmittgen, 2001; Fig. 3) and the geometric mean method using three reference genes (Supplemental Fig. S5).
Figure 3.
Gene expression levels of PtAQPs and TpAQPs. The responses of PtAQP and TpAQP transcript levels to CO2 and nitrogen were quantified by RT-qPCR. Transcript levels are shown for PtAQP1, PtAQP2, PtAQP3, PtAQP4, or PtAQP5 in P. tricornutum and TpAQP1 or TpAQP2 in T. pseudonana grown in 1% CO2 (high CO2/NO3−; black bars), 0.04% CO2 (air/NO3−; white bars), with the addition of 1.8 mm NH4Cl (air/NH3; cyan bars) or 18 µm NaNO3 (air/low NO3−; magenta bars). The transcripts of GapC2 in P. tricornutum and Act in T. pseudonana were used as constitutive internal controls. A, PtAQPs in P. tricornutum. B, TpAQPs in T. pseudonana. All data are means ± sd (n = 3). Welch's t tests were performed between the air/NO3− condition as the reference and all treatment conditions (*, P < 0.05; **, P < 0.01; ***, P < 0.001; and n.s., not significant).
The PtAQP1 gene was affected by CO2 availability, showing a slightly higher transcript level under 1% [v/v] (high) CO2 relative to atmospheric CO2 (air; 0.04% [v/v]) when the diatoms were grown at high nitrate concentrations (1.8 mm; Fig. 3A, high CO2/NO3− and air/NO3−, respectively). PtAQP1 also was responsive to nitrogen, with transcript levels increasing roughly 3-fold when NO3− was replaced with 1.8 mm NH4Cl (NH3) in air-grown cells (Fig. 3A, white bars versus cyan bars) and decreasing to about 10% in 18 µm (low) NO3− cells relative to NH3 cells grown in air (Fig. 3, cyan bars versus magenta bars). Similar expression profiles were observed for PtAQP2, PtAQP4, and PtAQP5 (Fig. 3A). Notably, PtAQP2 showed the most evident up-regulation under high CO2 relative to air-grown cells, while PtAQP5 was up-regulated most specifically under NH3 (Fig. 3A). All PtAQPs, except PtAQP3, were up-regulated by NH3 relative to growth on 1.8 mm NO3− (Fig. 3A). PtAQP1 and PtAQP3 were repressed under air/low NO3− compared with the air/NO3− condition (Fig. 3A). The expression profile of TpAQP1 was quite unusual, displaying down-regulation under high CO2/NO3− and under air/NH3 compared with that under air/NO3− and under air/low NO3− conditions (Fig. 3B). In contrast, TpAQP2 displayed a similar expression profile to that of PtAQP1, PtAQP2, and PtAQP4, although its extent of induction by NH3 was muted compared with those of these three PtAQPs (Fig. 3).
Assessment of the CO2 Permeability of PtAQP1G and PtAQP2G Cells
The RT-qPCR results in Figure 3 clearly indicated that all AQPs analyzed in this study exhibited some transcriptional response to CO2 and nitrogen. CO2 and NH3 are critical molecules for primary production; thus, their efficient mobility across the plasma membrane and subcellular compartments is critical for the survival of aquatic autotrophs. Of particular interest is the permeability of the plasma membrane, which is known in P. tricornutum to be extremely permeable to CO2 (Hopkinson et al., 2011), although the molecular bases of this high permeability are not yet known.
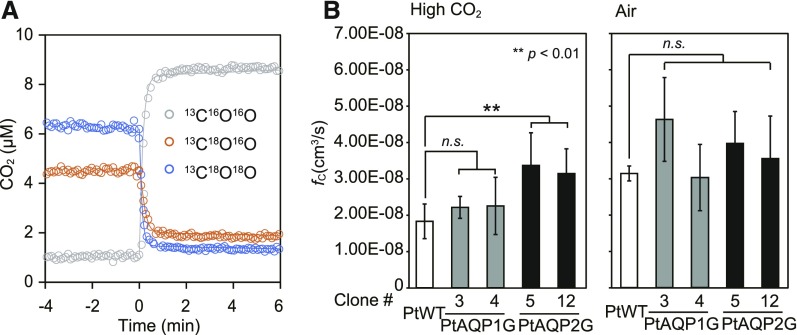
In order to analyze the CO2 channel activity of the plasma membrane-type PtAQP1 and PtAQP2, the cellular CO2 permeability of P. tricornutum AQP mutants was assessed via a C18O2/C16O2 equilibration assay using membrane-inlet mass spectrometry (MIMS; Fig. 4A). The assay uses the fact that intracellular CA accelerates the exchange of 18O from labeled CO2 for 16O from water to infer rates of CO2 flux into the cell and intracellular CA activity (Tu et al., 1978; Hopkinson et al., 2011). PtAQP1 and PtAQP2 were fused to EGFP and expressed in P. tricornutum under the control of the CO2-independent PfcpA promoter. Heterologous expression of PtAQP1:EGFP and PtAQP2:EGFP in these mutants was confirmed by western-blot analysis (Supplemental Fig. S3B). Overexpression of PtAQP2G increased the CO2 cellular transfer coefficient in two separate mutant lines (#5 and #12) compared with the wild type under high CO2 concentrations (Fig. 4B). The cellular transfer coefficient includes the barrier effects of both the diffusive boundary layer and the cell membrane on CO2 fluxes, but as there should be no change in the diffusive boundary layer in the mutants, changes in CO2 cellular transfer coefficient are interpreted as changes in membrane permeability. When grown at air CO2, wild-type CO2 permeability increased, which likely masked any effect of the overexpression. Overexpression of PtAQP1G did not affect CO2 permeability significantly.
Figure 4.
MIMS 18O exchange analysis with P. tricornutum wild-type (WT) cells and transformant cells heterologously expressing PtAQP1 or PtAQP2 under dark conditions. A, Time-dependent changes in the concentrations of extracellular 13C18O18O (blue circles), 13C18O16O (orange circles), and 13C16O16O (gray circles) were monitored with MIMS. The addition of P. tricornutum cells (0 min) induced immediate 18O exchange. B, The CO2 influx rate constants (fc; cm3 s−1) of PtAQP1G#3 and PtAQP1G#4 (gray bars) and PtAQP2G#5 and PtAQP2G#12 (black bars), grown under high (1%) CO2 or air (0.04% CO2), were compared with those of PtWT (white bars) grown under the same conditions. Values are means ± sd (n = 4–9). Statistical significance was determined using a Student t test (**, P < 0.01 and n.s., not significant).
NH3 Permeability in PtAQP1G and PtAQP2G Cells
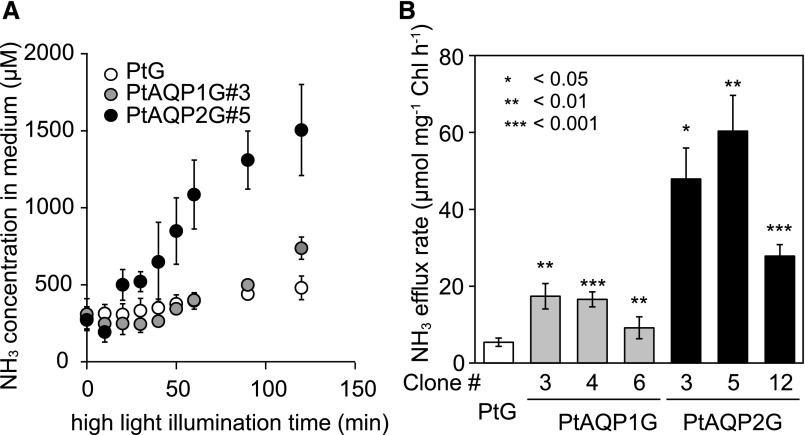
Previous reports have demonstrated that NH3 is released from diatoms under nitrogen-rich and high-light irradiance conditions (Lomas et al., 2000). To test the involvement of AQP in controlling NH3 permeability, an NH3 efflux assay was carried out with three clones of PtAQP1G and PtAQP2G cells under high-light and air CO2 conditions in the presence of 1.8 mm NO3−. Ammonium efflux was detected with a lag of 30 to 50 min after the application of high light (1,500 μmol photons m−2 s−1; Fig. 5A). Following the lag, significantly higher rates of NH3 efflux were observed in both PtAQP1G#3 and PtAQP2G#5 cells relative to that of the control cells, in which only GFP was overexpressed (PtG); the NH3 efflux started leveling off in PtAQP2G#5 cells after 60 min of high-light illumination, while PtAQP1G#3 cells showed increased efflux until 120 min of high-light treatment (Fig. 5A). The maximum rates of NH3 efflux calculated with PtAQP1G#3, PtAQP1G#4, and PtAQP1G#6 were 2 to 3 times faster than that of PtG cells (about 6 µmol mg−1 chlorophyll h−1), while those in PtAQP2G#3, PtAQP2G#5, and PtAQP2G#12 cells ranged from 5 to 11 times faster than that in PtG cells (Fig. 5B).
Figure 5.
NH3 efflux in diatom cells expressing PtAQP1 or PtAQP2. A, Time course of changes in NH3 concentration in the external medium of cell cultures of PtG (white circles), PtAQP1G#3 (gray circles), and PtAQP2G#5 (black circles) grown in 0.04% CO2. NH3 concentration was monitored from the start of high-light illumination (1,500 μmol m−2 s−1) for 120 min. B, Maximum rates of NH3 efflux in cell cultures of PtG (white bar), PtAQP1G#3, PtAQP1G#4, and PtAQP1G#6 (gray bars), and PtAQP2G#3, PtAQP2G#5, and PtAQP2G#12 (black bars). Student's t tests were performed between PtG and PtAQPnGs (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
Effect of Overexpressed AQP on NPQ
Photosynthetic organisms, in general, possess defense systems against excess light energy, and these systems typically are enhanced when the energy flow to carbon and nitrogen assimilation is limited (Khamis et al., 1990). To protect PSII from light stress, photoautotrophs dissipate excess light energy as heat, which can be measured as a major component of NPQ of chlorophyll fluorescence (Külheim et al., 2002).
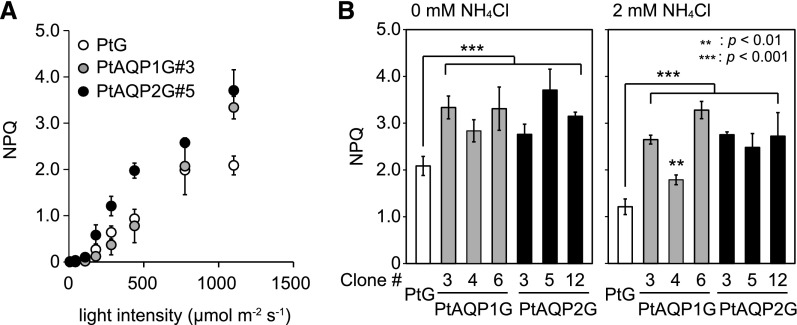
Ammonium is known to be a strong uncoupler of thylakoidal ΔpH, which suppresses NPQ (Young and Beardall, 2003). To determine if AQP-mediated NH3 fluxes contribute to the maintenance of NPQ in diatoms, chlorophyll fluorescence was measured in three clones of PtAQP1G and PtAQP2G mutants. In all diatom lines, NPQ increased roughly proportionally to the intensity of actinic light (Fig. 6). Illumination with 1,100 µmol m−2 s−1 actinic light induced 1.4 to 2 times higher NPQ in all PtAQPnG mutants compared with that of PtG cells (Fig. 6B). External addition of 1 to 5 mm NH4Cl resulted in decreased NPQ in an NH3 concentration-dependent manner in all cells (Supplemental Fig. S6). Nonetheless, cells heterologously expressing AQPs unequivocally showed a significantly higher NPQ relative to that of PtG cells even in the presence of NH4Cl (Fig. 6B; Supplemental Fig. S6), clearly displaying a high basal level and tolerance of NPQ against NH3. This stimulated tolerance was not observed in cells treated with another uncoupler, carbonylcyanide m-chlorophenylhydrazone (Supplemental Fig. S6).
Figure 6.
Pulse amplitude modulator (PAM) assays in diatom cells expressing PtAQP1 or PtAQP2. A, NPQ of cell cultures of PtG (white circles), PtAQP1G#3 (gray circles), and PtAQP2G#5 (black circles) with a spectrum of actinic light from 7 to 1,100 μmol m−2 s−1. B, NPQ of PtG (white bars), PtAQP1G (#3, #4, and #6; gray bars), and PtAQP2G cells (#3, #5, and #12; black bars) exposed to an actinic light intensity of 1,100 μmol m−2 s−1 in the absence (left) or presence (right) of 2 mm NH4Cl. All values are means ± sd (n = 3). Student's t tests were performed between PtG and PtAQPnGs (**, P < 0.01 and ***, P < 0.001).
DISCUSSION
In this study, we have identified several AQP proteins in the marine diatoms P. tricornutum and T. pseudonana, which are distributed among multiple subcellular compartments. In P. tricornutum, plasma membrane-type AQPs seem to regulate membrane permeability to both CO2 and NH3 in order to facilitate the desired fluxes of these substrates. AQPs help support rapid CO2 influx to sustain photosynthesis, whereas the NH3 permeability may help reduce toxic buildup under high-light conditions. There are five putative AQP proteins, which were classified into three clades in P. tricornutum and two homologs in two independent clades in T. pseudonana (Fig. 1A). Of these seven putative AQPs, only one, TpAQP1, was related to a red algal AQP-like protein, strongly suggesting that AQPs in the ancestral red alga might largely have been lost during the endosymbiotic events that led to the evolution of diatoms.
Current diatom AQPs are likely derived from the ancestral heterotrophic host cell or acquired by horizontal gene transfer. PtAQP1, PtAQP4, and PtAQP5 are included in a tightly clustered diatom-specific group (Fig. 1A). However, ancestral to this diatom-specific cluster are a wide variety of AQPs, mostly GIPs, from diverse organisms such as bacteria, green algae, haptophytes, and humans (Fig. 1A). Given the phylogenetic ambiguity of this GIP branch, its ultimate origin is still an open question. A previous study suggested that the bryophytic AQP (annotated as PpGIP1;1) and green algal GIPs in this branch were obtained by a horizontal gene transfer event from bacteria, based upon close alignment analysis (Gustavsson et al., 2005; Anderberg et al., 2011). The ar/R motifs of these GIPs, including PtAQP1, PtAQP4, and PtAQP5, are W-V-G/A-R, suggesting their potential role as a glycerol channel (Danielson and Johanson, 2010; Khabudaev et al., 2014). GmNOD26, a known glycerol and NH3 channel (Supplemental Table S2; Weaver et al., 1991; Niemietz and Tyerman, 2000; Wallace et al., 2002), has the same ar/R filter. PtAQP3 and TpAQP2 were included in a diatom-specific clade of LIPs, which is related to the green algal MIPCs and land plant SIP families (Fig. 1A). Plant SIPs have been localized at the ER, where they serve to regulate water permeability (Noronha et al., 2014), in agreement with our finding that PtAQP3 and TpAQP2 were localized to the CER in P. tricornutum and T. pseudonana, respectively (Fig. 2). The ar/R filters of PtAQP3, TpAQP1, and TpAQP2 have no similarity to those of the other AQPs; however, the ar/R filter of PtAQP2 is identical to that of HsAQP1 (Supplemental Table S2), suggesting that PtAQP2 has water channel activity.
Although PtAQP1 and PtAQP5 are phylogenetically similar, these AQP candidates were targeted to different subcellular loci: PtAQP5 was localized at the tonoplast, while, interestingly, PtAQP1 was targeted dually to the plasma membrane and CER (Fig. 2; Supplemental Fig. S3A). Such dual localization of AQP also has been reported in plants. Tobacco NtAQP1 is targeted to both the plasma membrane and the inner chloroplast membranes (Biela et al., 1999; Uehlein et al., 2008). In Arabidopsis seeds, AtTIP3;1 and AtTIP3;2 are targeted to both the plasma membrane and the tonoplast (Gattolin et al., 2011). The mechanisms for plasma membrane targeting and this type of dual targeting of proteins are not understood in diatoms, but some examples have been investigated in land plants. An N-terminal diacidic motif (D/EXD/E) was determined to be a recognition motif for coat protein II, directing proteins with this motif to the plasma membrane via exocytosis through the ER and Golgi apparatus (Zelazny et al., 2009; Sorieul et al., 2011). AtPIP2;1 has a phosphorylated Ser in the C-terminal region that is necessary for exocytosis to the plasma membrane (Prak et al., 2008). In maize (Zea mays) ZmPIP2;5, an LXXXA motif in TM3 was hypothesized to interact with an export-competent domain of the ER membrane (Chevalier et al., 2014). Furthermore, it has been reported that a Q-SNARE of the syntaxin family can mediate the exocytosis process of plant plasma membrane-type AQPs (Besserer et al., 2012). Plant AQPs exported by this process also were partially sorted to the chloroplastic envelope or tonoplast (Tyrrell et al., 2007; Hachez et al., 2013). The diatom AQP candidates possess no diacidic motif in their N-terminal regions, but there are some potentially phosphorylated Ser residues and an LXXXA motif in the predicted TM3 region of PtAQP1 (Supplemental Fig. S7). Also, diatom genomes possess SNARE family proteins (Tanaka et al., 2015a), suggesting that a mechanism for dual targeting to the plasma membrane and other organelle membranes via an exocytosis process similar to that in plants may be utilized by diatom PtAQP1.
AQPs have been shown to facilitate CO2 flux through membranes (Nakhoul et al., 1998; Uehlein et al., 2003; Endeward et al., 2008). Although this finding has been somewhat controversial because lipid bilayers are inherently permeable to CO2 (Verkman, 2002), several lines of evidence support a role for AQPs in CO2 passage through membranes. We also found that overexpression of PtAQP2 resulted in increased membrane permeability to CO2 (Fig. 4B), which suggests that AQPs contribute to the high permeability of diatom membranes to CO2. However, at this point, we cannot rule out the possibility that overexpression of PtAQP2 indirectly altered membrane permeability, for example by altering the lipid composition of the membrane due to changes in water permeability. Diatoms are believed to take up CO2 through an inward diffusive gradient, which is ultimately generated by the export of HCO3− from the cytoplasm into the chloroplast (Hopkinson et al., 2011). A membrane highly permeable to CO2 minimizes the diffusive gradient needed to achieve a given rate of CO2 influx, and PtAQP2 appears to contribute to this high permeability. Consistent with this finding, the ar/R residues of PtAQP2, which regulate substrate specificity, match those in the human AQP1 that facilitates CO2 flux (Supplemental Table S2).
PtAQP2 transcripts increased under high-CO2 conditions (Fig. 3A), strongly suggesting that PtAQP2 works to increase the CO2 permeability of the plasma membrane in CO2-rich environments. However, the CO2 permeability of wild-type diatoms increased in low-CO2 cells relative to high-CO2 cells (Fig. 4B). It is possible that posttranslational regulation alters PtAQP2 function in response to the extracellular environment. In fact, there are posttranslational gating systems to control substrate movement by plant and human AQPs (Törnroth-Horsefield et al., 2006). However, there are no apparent regulatory residues in the diatom AQPs examined here.
As some AQPs act as NH3 pores, we explored the potential involvement of diatom AQPs in nitrogen metabolism. RT-qPCR data strongly suggested an association of PtAQP1, PtAQP2, PtAQP5, and TpAQP2 with nitrogen acquisition/assimilation (Fig. 3). Indeed, the overexpression of PtAQP1 and PtAQP2 increased the rate of NH3 efflux when cells were exposed to high irradiance (Fig. 5). Together with their sequence characteristics, these data strongly suggested that PtAQP4 and PtAQP5 act as NH3 channels (Fig. 1A; Supplemental Table S2). Ammonia efflux has been reported as a general phenomenon in unicellular algae (e.g. the freshwater green alga C. reinhardtii released NH3 in nitrogen-starved conditions under GS inhibitor treatment [Florencio and Vega, 1983] or under low-CO2 conditions [Azuara and Aparicio, 1983]). Several reports have documented NH3 efflux in diatoms. The marine diatoms S. costatum, T. weissflogii, and Chaetoceros spp. exhibited NH3 efflux when nitrogen-replete cells were exposed to an abrupt increase in irradiance, as may occur in the oceanic mixed layer (Lomas et al., 2000). In P. tricornutum, NH3 efflux also was observed in the dark under sodium-free conditions (Alwyn and Rees, 1995), which probably induced CO2 limitation by inhibiting Na+-dependent HCO3− transport (Nakajima et al., 2013). NH3 release under a spectrum of stress conditions is considered to be a futile cycling, induced by an imbalance of nitrogen, carbon, and light energy, with the goal of rebalancing these energetics by consuming excess ATP and reductants for inorganic substrate acquisition (Coskun et al., 2013).
The rate of DIC influx or NH3 uptake (Supplemental Fig. S8) during photosynthesis was not altered by the overexpression of PtAQP1 or PtAQP2. It is probable that the steady-state photoautotrophic influx of such inorganic substrates is supported primarily by active transporters for bicarbonate and nitrate in P. tricornutum.
NH3 futile cycling is hypothesized to operate when NH3-consuming metabolism is damaged or repressed by factors such as a rapid increase in irradiance, low CO2, low sodium concentration, or inhibition of the GS enzyme (Florencio and Vega, 1983). Ammonia is assimilated by the GS-GOGAT system into Glu within the chloroplast, but any inhibition of metabolic steps after GS-GOGAT results in an accumulation of stromal NH3, which could uncouple the thylakoidal proton gradient (Crofts, 1966). In good agreement with these studies, high light and limited DIC induced NH3 efflux from diatom cells in this study (Fig. 5; Supplemental Fig. S8), strongly suggestive of the occurrence of an NH3 futile cycle under conditions where light energy inputs exceed the supply of inorganic carbon sinks. It is probable that the suppression of carbon fixation under high-light and low-DIC conditions would limit the supply of carbon skeletons to amino acid metabolism, thus leaving unassimilated NH3 accumulated in the stroma. This accumulated NH3 needs to be removed from the cells, presumably with the aid of AQPs. Supporting this hypothesis, higher initial [DIC] delayed NH3 efflux concomitantly with the decrease in levels of external [DIC] (Supplemental Fig. S8).
A futile NH3 cycle is thought to develop as part of an acclimation process to high-light stress. Such cycles, mediated by AQP, have been speculated to occur in diatoms and cyanobacteria (Alwyn and Rees, 1995; Lomas et al., 2000; Ritchie, 2013), although, to date, there has been little direct molecular evidence. Our data demonstrated a close relationship between NH3 efflux capacity and NPQ (Fig. 6B), strongly suggesting that the overexpression of PtAQPs efficiently mitigated NH3 toxicity and increased the photoacclimation capacity in the marine diatom P. tricornutum by promoting NH3 efflux. These results provide strong molecular evidence for the function of plasma membrane- and CER-type AQPs as critical components of photoprotection by driving NH3 futile cycling in marine diatoms.
MATERIALS AND METHODS
Phylogenetic Analysis of AQP Family Proteins
For phylogenetic analysis of the AQP family proteins, full-length AQP protein sequences from various organisms (Supplemental Table S1) were aligned using Clustal Omega (Sievers et al., 2011). Maximum-likelihood phylogenetic analyses were performed using MEGA7 (Kumar et al., 2016). The phylogenetic analysis was initiated with a neighbor-joining method (BioNJ; Saitou and Nei, 1987) using the Jones-Taylor-Thornton substitution model (Jones et al., 1992). The fast algorithm performing nearest-neighbor interchange was used to provide a reasonable starting tree topology. Subsequently, a maximum-likelihood phylogenetic analysis was performed using the Whelan and Goldman model for protein evolution (Whelan and Goldman, 2001). The gaps or missing regions were deleted with the setting Site Coverage Cutoff, 80%. The phylogeny was bootstrapped with 1,000 replicates, and branches with greater than 50% bootstrap support are indicated in the phylogenetic trees.
Cells and Culture Conditions
The marine diatom Phaeodactylum tricornutum (UTEX 642) was obtained from the University of Texas Culture Collection, and Thalassiosira pseudonana (CCMP1335) was obtained from the Provasoli-Guillard National Center for Culture of Marine Algae and Microbiota. These diatoms were cultured in artificial seawater supplemented with one-half-strength Guillard’s f solution (F/2ASW; Guillard and Ryther, 1962; Harrison et al., 1980) under continuous illumination (30–75 µmol m−2 s−1) at 20°C and constant aeration with 1% (v/v) CO2 (high CO2-grown cells) or ambient air containing 0.04% (v/v) CO2 (air-grown cells). For expression analysis, 1.8 mm NH4Cl was added as a nitrogen source, and 18 µm NaNO3 was added for the low-nitrogen condition.
RT-qPCR
Thirty-milliliter cultures of P. tricornutum cells or 60-mL cultures of T. pseudonana were harvested at the midlogarithmic phase (OD730 = 0.2–0.3 for P. tricornutum and 0.1–0.15 for T. pseudonana) by centrifugation at 1,700g at 20°C for 10 min. Harvested cells were frozen with liquid nitrogen. Total RNA was isolated from frozen cells using the RNeasy Plant Mini Kit (Qiagen). To synthesize the single-stranded cDNA, 1 μg of total RNA was reverse transcribed using oligo(dT)20 primers and ReverTra Ace (Toyobo). The entire process of RT-qPCR was carried out with a Thermal Cycler Dice (TaKaRa Bio) and GeneAce SYBR qPCR Mix α No ROX (Nippon Gene) under the following PCR conditions: after an initial heating at 95°C for 10 min, up to 45 cycles of denaturing (95°C for 30 s) and annealing/elongation (60°C for 1 min) were performed. RT-qPCR results were calculated with the 2−ΔΔCt method (Livak and Schmittgen, 2001). PtGapC2 and TpAct were used as reference genes, which were confirmed to have little response to changes in carbon and/or nitrogen conditions by the geometric mean method according to Vandesompele et al. (2002); Supplemental Figs. S4 and S5). The specificity of products amplified by the primers was confirmed by melting curve profiling, and all PCR products revealed a single product melting in the range of 80°C to 87°C (Supplemental Table S3). The trials were replicated more than three times.
Construction of Transformation Vectors Containing the PtAQPs::egfp and TpAQPs::egfp Fusions
For the transformation of P. tricornutum, inverse PCR was carried out using the pFcpApGFP vector (Kitao et al., 2008) as a template with high-fidelity PrimeSTAR HS DNA polymerase (TaKaRa) and the following primer pair: eGFP forward (5′-ATGGTGAGCAAGGGCGAGGAGCTGTTC-3′) and fcpAp reverse (5′-TCGAAACGGCAGACAAATTTGTG-3′). For T. pseudonana transformation, the PCR-amplified eGFP gene with adjacent EcoRV and XhoI sites was inserted between EcoRV and NotI sites in the transformation vector pTha-K1, which is equipped with the Nourseothricin acetyltransferase gene cassette as a selection maker (Tachibana et al., 2011). AQP fragments from either P. tricornutum or T. pseudonana were amplified from cDNA synthesized using the SMARTer RACE cDNA Amplification Kit (TaKaRa). The PCR products were phosphorylated and ligated to the upstream region of eGFP in the inverse PCR product from the pFcpApeGFP vector or the EcoRV and XhoI sites of the pTha-K1 vector in frame with eGFP, respectively.
Transformation of Wild-Type Cells and Screening of Transformants
Air-grown cells of P. tricornutum or T. pseudonana were harvested at the midlogarithmic phase (OD730 = 0.2–0.3 for P. tricornutum and 0.1–0.15 for T. pseudonana). Approximately 5 × 107 cells were spotted as a plaque of 3.1-cm diameter on the surface of the F/2ASW agar plate. A total of 500 µg of tungsten microcarriers (particle size, 0.79 µm; Japan New Metals) was coated with 1 µg of plasmid DNA in an aqueous solution of 1 m CaCl2 and 16 mm spermidine (Sigma-Aldrich). The Biolistic PDS-1000/He Particle Delivery System (Bio-Rad Laboratories) was used for microprojectile bombardment of the microcarrier. The bombardment was performed at a pressure of 10.7 MPa under a negative pressure of 92 kPa at a target distance of 6 cm. After bombardment, P. tricornutum cells were cultured for 1 d in the dark and suspended in 5 mL of F/2ASW. Bombarded T. pseudonana cells were suspended in 50 mL of F/2ASW immediately and then were allowed to recover for 1 d in the dark before being resuspended in 0.3 mL of F/2ASW. The transformants were screened on agar plates containing 100 µg mL−1 zeocin (Invitrogen) for P. tricornutum and 150 µg mL−1 nourseothricin (Werner BioAgents) for T. pseudonana. GFP-positive clones were further screened from the zeocin- or nourseothricin-resistant clones after culturing for 3 to 4 weeks under continuous illumination. The numbers of transformants were counted and are reported in Supplemental Table S4.
Confocal Laser Microscopy
Cells at the midlogarithmic growth phase were harvested and resuspended in a small volume of F/2ASW. The nucleus in PtAQPnG and TpAQPnG cells was stained with 5 μm Hoechst 33342 (Sigma-Aldrich) at room temperature for 30 min. The stained cells were washed three times with F/2ASW. Specimens were observed using a Nikon A-1R laser confocal microscope with Apo 60 × Oil λS (Nikon). Chlorophyll autofluorescence was detected in the 662.5- to 735.5-nm emission range following excitation with a 640-nm laser; for Hoechst 33342 fluorescence, the emission was detected at 425 to 475 nm with excitation by a 405-nm laser; for eGFP, fluorescence emission was monitored at 500 to 550 nm following excitation at 488 nm. All settings of the microscope, objective, pinhole, and image sampling were identical to those used for the bright field.
MIMS
18O-labeled DIC (2 mm) was added to assay buffer (DIC-free artificial seawater, 20 mm Bicine, pH 8, and 100 μm dextran-bound acetazolamide [Ramidus]) in the MIMS chamber (Reinfelder et al., 2004). The temperature was maintained at 20°C in the chamber. 18O was monitored by MIMS for ∼10 min to detect the CO2/HCO3− interconversion before the addition of concentrated cells pretreated with 50 μm dextran-bound acetazolamide. After the addition of the cells in the chamber, 18O removal catalyzed by intercellular CA was monitored for 10 to 20 min in the dark. The rate of 18O removal was analyzed using the model described by Hopkinson et al. (2011) to determine the membrane permeability to CO2.
NH3 Efflux Measurement
Cell cultures at the midlogarithmic growth phase were washed with NH3-free F/2ASW three times and concentrated to 20 μg mL−1 chlorophyll in 2 mL of F/2ASW. In a chamber equipped with a Clark-type oxygen electrode (Hanzatech), 1 mL of the cells was incubated while illuminated with 1,500 μmol m−2 s−1 for 120 min. Every 10 or 30 min, samples were taken and filtered by centrifugation at 6,000g for 5 s, and 20 µL of the filtered external medium was mixed with 80 µL of 77 mm Na2B4O7, 0.78 μm Na2SO3, and 3.7 μm o-phthalaldehyde (Kérouel and Aminot, 1997) on a 96-well plate. After more than 2 h in the dark, the fluorescence was measured at 460 nm and excited by 355 nm with a plate reader (2104 EnVision; PerkinElmer Japan) to determine the dissolved NH3 concentration. The ammonia efflux rate was calculated at maximum slope from the concentration of the NH3 increasing curve.
PAM Assay of Chlorophyll Fluorescence
Cell cultures at the midlogarithmic growth phase were concentrated to 5 μg mL−1 chlorophyll in 2 mL of F/2ASW. The characteristics of PSII were measured with the PAM approach using a PAM-2100 (Walz). In a chamber with a Clark-type oxygen electrode, the cells were incubated without light for longer than 10 min at 20°C. After the dark adaptation, a saturation pulse (3,000 μmol m−2 s−1) was applied for 1 s to determine Fv/Fm. After a stable baseline was obtained, cells were exposed to increasing intensities of actinic light (7, 44, 110, 180, 280, 440, 775, and 1,100 μmol m−2 s−1). Under each light intensity, the saturation pulses were applied in 1-min intervals for 5 to 10 min. NPQ was calculated with the formula NPQ = Fm/Fm′ − 1 according to Bilger and Björkman (1990). The addition of uncouplers (1–5 mm NH4Cl or 0.1 mm carbonylcyanide m-chlorophenylhydrazone) was performed before turning on the actinic light.
Statistical Analysis
All statistical analyses were performed with F tests and t tests using Microsoft Excel 2013. Homoscedastic data comprising two groups and having greater than three replicates were compared with Student’s t test. The heteroscedastic data were compared with Welch’s t test.
Accession Numbers
The sequences reported in this article have been deposited in the GenBank database as follows: LC341922 (PtAQP1), LC341923 (PtAQP2), LC341924 (PtAQP3), LC341925 (PtAQP4), LC341926 (PtAQP5), LC341927 (TpAQP1), and LC341928 (TpAQP2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phylogenetic tree of AQP family proteins without N- and C-terminal regions.
Supplemental Figure S2. Comparison of amino acid sequences of AQP candidates in diatoms and CO2 or NH3 AQPs in human or plants.
Supplemental Figure S3. Localization of heterologously expressed PtAQP1:EGFP and the accumulation of EGFP-tagged PtAQPs.
Supplemental Figure S4. Relative expression levels of reference genes.
Supplemental Figure S5. Gene expression levels of PtAQPs and TpAQPs normalized by geometric mean.
Supplemental Figure S6. Effects of inhibitors of chlorophyll fluorescence on NPQ in PtAQP1G and PtAQP2G cells.
Supplemental Figure S7. Comparison of potential targeting regions of AQP candidates in diatoms and human HsAQP1 or plant AQPs, Arabidopsis AtPIP2;1, and maize ZmPIP2;5.
Supplemental Figure S8. Influence of PtAQP1 or PtAQP2 expression on DIC or NH3 uptake.
Supplemental Table S1. Accession numbers of AQPs.
Supplemental Table S2. Characteristics of PtAQPs, TpAQPs, CO2, or NH3 channels.
Supplemental Table S3. Primers used for RT-qPCR.
Supplemental Table S4. Number of obtained clones of transformants.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Nobuko Higashiuchi for technical assistance and Miyabi Inoue for skillful secretarial aid.
Footnotes
This work was supported by the Japan Society for the Promotion of Science (KAKENHI Grants JP24310015 and JP16H06557 to Y.M.), by the Promotion and Mutual Aid Corporation for Private Schools of Japan supported Science Research Promotion Fund (Y.M.), and by Hyogo Science and Technology Association (STA) Grant 29105 (to K.N.). B.M.H. acknowledges support from the National Science Foundation (EF 1041023 and MCB 1129326).
Articles can be viewed without a subscription.
References
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwyn T, Rees V (1995) On ammonia futile cycling in a marine unicellular alga. Biochim Biophys Acta 1228: 254–260 [Google Scholar]
- Anderberg HI, Danielson JÅ, Johanson U (2011) Algal MIPs, high diversity and conserved motifs. BMC Evol Biol 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuara MP, Aparicio PJ (1983) In vivo blue-light activation of Chlamydomonas reinhardtii nitrate reductase. Plant Physiol 71: 286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T (2006) Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 103: 269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer A, Burnotte E, Bienert GP, Chevalier AS, Errachid A, Grefen C, Blatt MR, Chaumont F (2012) Selective regulation of maize plasma membrane aquaporin trafficking and activity by the SNARE SYP121. Plant Cell 24: 3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18: 565–570 [DOI] [PubMed] [Google Scholar]
- Bienert GP, Bienert MD, Jahn TP, Boutry M, Chaumont F (2011) Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J 66: 306–317 [DOI] [PubMed] [Google Scholar]
- Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25: 173–185 [DOI] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass AD, Kronzucker HJ (2001) Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA 98: 4255–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron L, Berkaloff C, Duval JC, Jupin H (1987) Chlorophyll fluorescence transients from the diatom Phaeodactylum tricornutum: relative rates of cyclic phosphorylation and chlororespiration. Photosynth Res 11: 131–139 [DOI] [PubMed] [Google Scholar]
- Chevalier AS, Bienert GP, Chaumont F (2014) A new LxxxA motif in the transmembrane Helix3 of maize aquaporins belonging to the plasma membrane intrinsic protein PIP2 group is required for their trafficking to the plasma membrane. Plant Physiol 166: 125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman B, Rotatore C (1995) Photosynthetic inorganic carbon uptake and accumulation in two marine diatoms. Plant Cell Environ 18: 919–924 [Google Scholar]
- Coskun D, Britto DT, Li M, Becker A, Kronzucker HJ (2013) Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiol 163: 1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell R, Syrett P (1979) Ammonium inhibition of nitrate uptake by the diatom, Phaeodactylum tricornutum. Plant Sci Lett 14: 321–325 [Google Scholar]
- Crofts AR. (1966) Uptake of ammonium ion by chloroplasts, and the mechanism of amine uncoupling. Biochem Biophys Res Commun 24: 127–134 [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Chaumont F, Mirkov TE, Chrispeels MJ (1996) Characterization of a new vacuolar membrane aquaporin sensitive to mercury at a unique site. Plant Cell 8: 587–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson JÅ, Johanson U (2008) Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson JÅ, Johanson U (2010) Phylogeny of major intrinsic proteins. Adv Exp Med Biol 679: 19–27 [DOI] [PubMed] [Google Scholar]
- Endeward V, Cartron JP, Ripoche P, Gros G (2008) RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22: 64–73 [DOI] [PubMed] [Google Scholar]
- Falkowski P, Scholes RJ, Boyle E, Canadell J, Canfield D, Elser J, Gruber N, Hibbard K, Högberg P, Linder S, et al. (2000) The global carbon cycle: a test of our knowledge of earth as a system. Science 290: 291–296 [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48: 427–439 [DOI] [PubMed] [Google Scholar]
- Florencio FJ, Vega JM (1983) Utilization of nitrate, nitrite and ammonium by Chlamydomonas reinhardtii: photoproduction of ammonium. Planta 158: 288–293 [DOI] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM (2000) Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290: 481–486 [DOI] [PubMed] [Google Scholar]
- Gattolin S, Sorieul M, Frigerio L (2011) Mapping of tonoplast intrinsic proteins in maturing and germinating Arabidopsis seeds reveals dual localization of embryonic TIPs to the tonoplast and plasma membrane. Mol Plant 4: 180–189 [DOI] [PubMed] [Google Scholar]
- Gorin MB, Yancey SB, Cline J, Revel JP, Horwitz J (1984) The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell 39: 49–59 [DOI] [PubMed] [Google Scholar]
- Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol 8: 229–239 [DOI] [PubMed] [Google Scholar]
- Gustavsson S, Lebrun AS, Nordén K, Chaumont F, Johanson U (2005) A novel plant major intrinsic protein in Physcomitrella patens most similar to bacterial glycerol channels. Plant Physiol 139: 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Besserer A, Chevalier AS, Chaumont F (2013) Insights into plant plasma membrane aquaporin trafficking. Trends Plant Sci 18: 344–352 [DOI] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45: 521–529 [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Waters RE, Taylor F (1980) A broad spectrum artificial sea water medium for coastal and open ocean phytoplankton. J Phycol 16: 28–35 [Google Scholar]
- Hopkinson BM, Dupont CL, Allen AE, Morel FM (2011) Efficiency of the CO2-concentrating mechanism of diatoms. Proc Natl Acad Sci USA 108: 3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove RM, Bhave M (2011) Plant aquaporins with non-aqua functions: deciphering the signature sequences. Plant Mol Biol 75: 413–430 [DOI] [PubMed] [Google Scholar]
- Johanson U, Gustavsson S (2002) A new subfamily of major intrinsic proteins in plants. Mol Biol Evol 19: 456–461 [DOI] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8: 275–282 10.1093/bioinformatics/8.3.275 [DOI] [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P (1994) Molecular structure of the water channel through aquaporin CHIP: the hourglass model. J Biol Chem 269: 14648–14654 [PubMed] [Google Scholar]
- Kérouel R, Aminot A (1997) Fluorometric determination of ammonia in sea and estuarine waters by direct segmented flow analysis. Mar Chem 57: 265–275 [Google Scholar]
- Khabudaev KV, Petrova DP, Grachev MA, Likhoshway YV (2014) A new subfamily LIP of the major intrinsic proteins. BMC Genomics 15: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamis S, Lamaze T, Lemoine Y, Foyer C (1990) Adaptation of the photosynthetic apparatus in maize leaves as a result of nitrogen limitation: relationships between electron transport and carbon assimilation. Plant Physiol 94: 1436–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao Y, Harada H, Matsuda Y (2008) Localization and targeting mechanisms of two chloroplastic β-carbonic anhydrases in the marine diatom Phaeodactylum tricornutum. Physiol Plant 133: 68–77 [DOI] [PubMed] [Google Scholar]
- Külheim C, Ågren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaud J, Rousseau B, Etienne AL (2002) In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Lett 523: 163–166 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-Δ Δ CT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lomas MW, Rumbley CJ, Glibert PM (2000) Ammonium release by nitrogen sufficient diatoms in response to rapid increases in irradiance. J Plankton Res 22: 2351–2366 [Google Scholar]
- Lopez D, Bronner G, Brunel N, Auguin D, Bourgerie S, Brignolas F, Carpin S, Tournaire-Roux C, Maurel C, Fumanal B, et al. (2012) Insights into Populus XIP aquaporins: evolutionary expansion, protein functionality, and environmental regulation. J Exp Bot 63: 2217–2230 [DOI] [PubMed] [Google Scholar]
- Loqué D, Ludewig U, Yuan L, von Wirén N (2005) Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol 137: 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CL, Tortell PD (2008) Bicarbonate transport and extracellular carbonic anhydrase in marine diatoms. Physiol Plant 133: 106–116 [DOI] [PubMed] [Google Scholar]
- Mock T, Otillar RP, Strauss J, McMullan M, Paajanen P, Schmutz J, Salamov A, Sanges R, Toseland A, Ward BJ, et al. (2017) Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 541: 536–540 [DOI] [PubMed] [Google Scholar]
- Musa-Aziz R, Chen LM, Pelletier MF, Boron WF (2009) Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tanaka A, Matsuda Y (2013) SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proc Natl Acad Sci USA 110: 1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF, Boron WF (1998) Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol 274: C543–C548 [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL (2001) Transport of NH3/NH in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281: F255–F263 [DOI] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD (2000) Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett 465: 110–114 [DOI] [PubMed] [Google Scholar]
- Noronha H, Agasse A, Martins AP, Berny MC, Gomes D, Zarrouk O, Thiebaud P, Delrot S, Soveral G, Chaumont F, et al. (2014) The grape aquaporin VvSIP1 transports water across the ER membrane. J Exp Bot 65: 981–993 [DOI] [PubMed] [Google Scholar]
- Oyala PH, Stich TA, Debus RJ, Britt RD (2015) Ammonia binds to the dangler manganese of the photosystem II oxygen-evolving complex. J Am Chem Soc 137: 8829–8837 [DOI] [PubMed] [Google Scholar]
- Pérez-Navarro M, Ames WM, Nilsson H, Lohmiller T, Pantazis DA, Rapatskiy L, Nowaczyk MM, Neese F, Boussac A, Messinger J, et al. (2013) Ammonia binding to the oxygen-evolving complex of photosystem II identifies the solvent-exchangeable oxygen bridge (μ-oxo) of the manganese tetramer. Proc Natl Acad Sci USA 110: 15561–15566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen N, Chesley PM, Kröger N (2006) Molecular genetic manipulation of the diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 42: 1059–1065 [Google Scholar]
- Prak S, Hem S, Boudet J, Viennois G, Sommerer N, Rossignol M, Maurel C, Santoni V (2008) Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol Cell Proteomics 7: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Reinfelder JR, Milligan AJ, Morel FM (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol 135: 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RJ. (2013) The ammonia transport, retention and futile cycling problem in cyanobacteria. Microb Ecol 65: 180–196 [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425 [DOI] [PubMed] [Google Scholar]
- Samukawa M, Shen C, Hopkinson BM, Matsuda Y (2014) Localization of putative carbonic anhydrases in the marine diatom, Thalassiosira pseudonana. Photosynth Res 121: 235–249 [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorieul M, Santoni V, Maurel C, Luu DT (2011) Mechanisms and effects of retention of over-expressed aquaporin AtPIP2;1 in the endoplasmic reticulum. Traffic 12: 473–482 [DOI] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414: 872–878 [DOI] [PubMed] [Google Scholar]
- Tachibana M, Allen AE, Kikutani S, Endo Y, Bowler C, Matsuda Y (2011) Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynth Res 109: 205–221 [DOI] [PubMed] [Google Scholar]
- Tanaka A, De Martino A, Amato A, Montsant A, Mathieu B, Rostaing P, Tirichine L, Bowler C (2015a) Ultrastructure and membrane traffic during cell division in the marine pennate diatom Phaeodactylum tricornutum. Protist 166: 506–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Maeda Y, Veluchamy A, Tanaka M, Abida H, Maréchal E, Bowler C, Muto M, Sunaga Y, Tanaka M, et al. (2015b) Oil accumulation by the oleaginous diatom Fistulifera solaris as revealed by the genome and transcriptome. Plant Cell 27: 162–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P (2006) Structural mechanism of plant aquaporin gating. Nature 439: 688–694 [DOI] [PubMed] [Google Scholar]
- Tsuji Y, Mahardika A, Matsuda Y (2017) Evolutionarily distinct strategies for the acquisition of inorganic carbon from seawater in marine diatoms. J Exp Bot 68: 3949–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C, Wynns GC, McMurray RE, Silverman DN (1978) CO2 kinetics in red cell suspensions measured by 18O exchange. J Biol Chem 253: 8178–8184 [PubMed] [Google Scholar]
- Tyrrell M, Campanoni P, Sutter JU, Pratelli R, Paneque M, Sokolovski S, Blatt MR (2007) Selective targeting of plasma membrane and tonoplast traffic by inhibitory (dominant-negative) SNARE fragments. Plant J 51: 1099–1115 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425: 734–737 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R (2008) Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20: 648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. (2002) Does aquaporin-1 pass gas? An opposing view. J Physiol 542: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IS, Wills DM, Guenther JF, Roberts DM (2002) Functional selectivity for glycerol of the nodulin 26 subfamily of plant membrane intrinsic proteins. FEBS Lett 523: 109–112 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cohen J, Boron WF, Schulten K, Tajkhorshid E (2007) Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J Struct Biol 157: 534–544 [DOI] [PubMed] [Google Scholar]
- Weaver CD, Crombie B, Stacey G, Roberts DM (1991) Calcium-dependent phosphorylation of symbiosome membrane proteins from nitrogen-fixing soybean nodules: evidence for phosphorylation of nodulin-26. Plant Physiol 95: 222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S, Goldman N (2001) A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18: 691–699 [DOI] [PubMed] [Google Scholar]
- Young EB, Beardall J (2003) Rapid ammonium-and nitrate-induced perturbations to Chl a fluorescence in nitrogen-stressed Dunaliella tertiolecta (Chlorophyta). J Phycol 39: 332–342 [Google Scholar]
- Zaslavskaia LA, Lippmeier JC, Kroth PG, Grossman AR, Apt KE (2000) Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J Phycol 36: 379–386 [Google Scholar]
- Zeebe RE, Wolf-Gladrow DA (2001) CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Gulf Professional Publishing, Houston, TX [Google Scholar]
- Zelazny E, Miecielica U, Borst JW, Hemminga MA, Chaumont F (2009) An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J 57: 346–355 [DOI] [PubMed] [Google Scholar]