Significance
Citrobacter rodentium infection, a murine colitis model to study human intestinal diseases, causes exacerbated apoptosis of intestinal epithelial cells and triggers Th17 immune responses. Here, we identified a heretofore unknown role for the bioactive lipid mediator prostaglandin E2 (PGE2) in the inhibition of Th17 cell differentiation during intestinal C. rodentium infection. When the PGE2 receptor EP4 was antagonized, we detected enhanced colonic Th17 cells, increased expression of antimicrobial peptides, and decreased bacterial numbers in the colon. These results suggest that pharmacological intervention of the PGE2 signaling may be an important target to enhance Th17 actions and improve intestinal host defense.
Keywords: efferocytosis, infected apoptotic cells, prostaglandin E2, Th17 cells, EP4
Abstract
Inflammatory responses are terminated by the clearance of dead cells, a process termed efferocytosis. A consequence of efferocytosis is the synthesis of the antiinflammatory mediators TGF-β, PGE2, and IL-10; however, the efferocytosis of infected cells favors Th17 responses by eliciting the synthesis of TGF-β, IL-6, and IL-23. Recently, we showed that the efferocytosis of apoptotic Escherichia coli-infected macrophages by dendritic cells triggers PGE2 production in addition to pro-Th17 cytokine expression. We therefore examined the role of PGE2 during Th17 differentiation and intestinal pathology. The efferocytosis of apoptotic E. coli-infected cells by dendritic cells promoted high levels of PGE2, which impaired IL-1R expression via the EP4-PKA pathway in T cells and consequently inhibited Th17 differentiation. The outcome of murine intestinal Citrobacter rodentium infection was dependent on the EP4 receptor. Infected mice treated with EP4 antagonist showed enhanced intestinal defense against C. rodentium compared with infected mice treated with vehicle control. Those results suggest that EP4 signaling during infectious colitis could be targeted as a way to enhance Th17 immunity and host defense.
Microorganisms are able to trigger different types of cellular death, such as apoptosis (1, 2). The clearance of dead cells, a process termed efferocytosis, is critical for homeostasis and the prevention of autoimmune disorders (3). Efficient efferocytosis of uninfected apoptotic cells by macrophages or dendritic cells (DCs) is essential to inhibit inflammatory responses (4). Efferocytosis induces the production of TGF-β, IL-10, prostaglandin E2 (PGE2), and platelet-activating factor (PAF) and inhibits the secretion of inflammatory mediators, such as TNF-α, IL-1, GM-CSF, IL-8, and leukotriene C4 (5, 6). However, during some infections, the recognition of infected apoptotic cells by DCs induces a proinflammatory program that leads to T cell immunity (7) and antimicrobial responses (8, 9). For example, the efferocytosis of apoptotic Mycobacterium tuberculosis-infected macrophages helps to kill the bacteria (9) and promotes adaptive immunity by cross-priming CD8+ T cells (10). Moreover, the phagocytosis of herpes simplex virus 1-infected cells leads to viral antigen-specific CD8+ T cell activation in vitro and in vivo (11). When DCs take up apoptotic Escherichia coli-infected cells, they release IL-23, TGF-β, and IL-6, and induce Th17 cell differentiation (7). We recently reported that the efferocytosis of infected cells leads to PGE2 production by DCs (12, 13). PGE2, one of the most abundant lipid mediators produced by both immune and structural cells, acts through four different G protein-coupled receptors (14). The EP1 receptor is coupled to Gαq proteins and promotes the increase of intracellular Ca2+. The EP3 receptor is coupled to Gαi proteins and inhibits cyclic adenosine monophosphate (cAMP) formation (14). The EP2 and EP4 receptors are coupled to Gαs proteins and promote increased cAMP concentrations (15). Changes in cAMP levels induce pleiotropic cellular responses via protein kinase A (PKA) activation and protein exchange via cAMP (EPAC)-dependent and cAMP (EPAC)-independent pathways (16).
Although all four EP receptors are detected in naïve CD4+ T cells (17), EP2 and EP4 are the most abundant and more potent EP receptors expressed on effector Th17 cells (18). The role of PGE2 in the activation, differentiation, and expansion of Th17 cells is both important and controversial (19, 20). Despite the well-known effects of PGE2 in Th17 development, it remains undetermined how the PGE2 produced during the efferocytosis of infected, dead cells is relevant to Th17 cells involved in the host gut defense.
IL-6 and TGF-β are critical for the activation of the transcription factors RORγt and STAT3 to promote Th17 commitment. The up-regulation of IL-1R, IL-23R, and IL-21R expression and IL-21 production by early Th17 cells favors the expansion of those cells and their subsequent expression of IL-17 (21). PGE2 acts synergistically with IL-23 and favors the expression of IL-17A (18, 22). Furthermore, PGE2 directly increases IL-17 expression in human Th17 cells in vitro (23). Moreover, exogenous PGE2 increases IL-23 production in DCs and promotes IL-1 and IL-23 receptor expression, which further drives the expansion of Th17 cells (24, 25). However, Valdez et al. (26) demonstrated that PGE2 via EP2/4 inhibits IRF4 activation and impairs Th17 cell differentiation and immunity against Cryptococcus neoformans infection in mice.
The pathogen Citrobacter rodentium causes intestinal infection in mice and has virulence factors similar to those of the human pathogen, enteropathogenic and enterohemorrhagic E. coli (27). C. rodentium infection is therefore used as a model to study chronic human intestinal diseases, such as ulcerative colitis and Crohn’s disease (28), and intestinal host defense (27). IL-17 and IL-22 play an important role during the resolution of C. rodentium infection (29). The infection causes the apoptosis of intestinal epithelial cells, which is critical for Th17 cell differentiation in vivo, as the inhibition of apoptosis during C. rodentium infection drastically impairs typical colonic Th17 responses (7). It is unknown what role PGE2 produced during the efferocytosis of infected cells plays in Th17 cell differentiation and intestinal host defense. Therefore, we set up experiments to determine whether PGE2 affects Th17 cell commitment to drive host defenses against intestinal C. rodentium infection in mice. Our results reveal a regulatory mechanism by which PGE2 suppresses Th17 cell differentiation during the efferocytosis of infected cells and thus compromises adaptive immunity, suggesting that therapeutically targeting PGE2 actions during infectious colitis may induce microbial clearance and restore intestine homeostasis.
Results
Efferocytosis of Apoptotic E. coli-Infected Cells Induces PGE2 and IL-1β Beyond the Classic Th17-Inducing Cytokines.
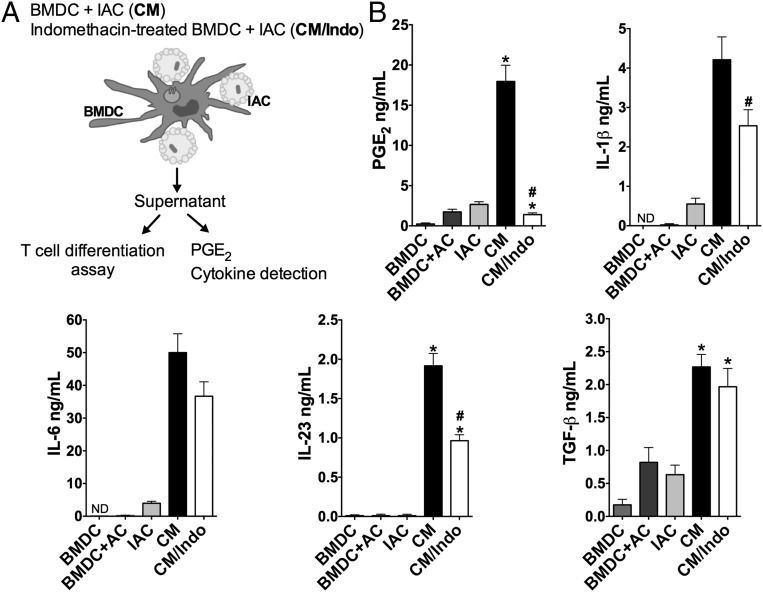
Innate recognition of apoptotic E. coli-infected cells leads to TGF-β, IL-6, and IL-23 production and favors Th17 cell differentiation (7). We previously showed that PGE2 and IL-1β are also produced during the engulfment of infected apoptotic cells by bone marrow-derived DCs (BMDCs) (12). Here, we sought to determine the relevance of PGE2 synthesis in Th17 cell differentiation in the context of infected-cell efferocytosis. We performed efferocytosis assays using either mouse BMDCs or human monocyte-derived DCs (MoDCs) incubated with apoptotic E. coli-infected cells (IACs). As schematized in Fig. 1A, we refer to the supernatants of cocultures of DCs and IACs as conditioned medium (CM) or, when the DCs were previously treated with the COX inhibitor indomethacin, as CM/Indo. Both types of DCs engulfed IACs and produced PGE2 and IL-1β in addition to IL-6, IL-23, and TGF-β (Fig. 1B and SI Appendix, Figs. S1 A–C and S2).
Fig. 1.
Efferocytosis of apoptotic E. coli-infected cells triggers PGE2 production. BMDCs were previously treated or not with indomethacin (10 µM) and then cocultured in the presence of IACs, at the ratio 1:3 for 18 h. (A) Schematic figure illustrating experimental procedure. (B) Concentration of PGE2 and cytokines were measured by ELISA in the supernatants derived from resting BMDC, BMDC cocultured with noninfected apoptotic cells (BMDC+AC), IACs only (IAC), BMDC cocultured with IAC (CM), or indomethacin-treated BMDC cocultured with IAC (CM/Indo). Data represent mean ± SEM of at least three independent experiments performed in triplicate. ND, not detected. *P < 0.05 compared with BMDC; #P < 0.05 compared with CM.
To confirm that efferocytosis was critical for the synthesis of PGE2 and Th17-inducing cytokines, we blocked apoptotic cell recognition by DCs using annexin V microbeads, which bound and thus “hid” the phosphatidylserine on the surface of the apoptotic cells. That strategy impaired the engulfment of the apoptotic cells by BMDCs or MoDCs (SI Appendix, Figs. S1B and S2A) and reduced the levels of PGE2 and Th17-inducing cytokines in the conditioned media (SI Appendix, Figs. S1C and S2B), confirming the importance of efferocytosis in the production of those mediators. We also treated BMDCs or MoDCs with COX inhibitors to decrease the PGE2 levels in the conditioned media. We observed that COX inhibition decreased IL-1β and IL-23 levels as well as PGE2 levels in the media during the uptake of IACs (Fig. 1B). Next, we determined the relevance of the PGE2 produced during the efferocytosis of infected cells to Th17 cell differentiation.
Th17 Cell Differentiation Is Controlled by PGE2 Produced During the Efferocytosis of IACs.
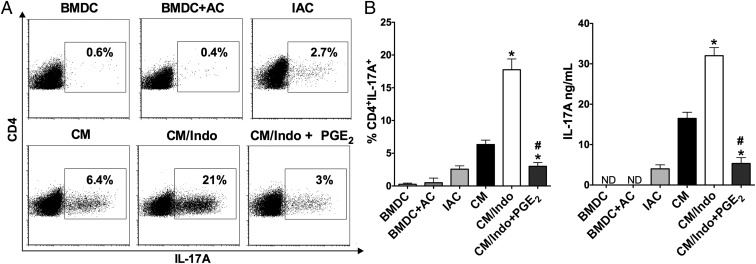
The differentiation of naïve CD4+ T cells in CM resulted in predominantly Th17 cell differentiation (Fig. 2) as well as low levels of IFN-γ and Foxp3 (SI Appendix, Fig. S3 A and B). We confirmed that the products released by BMDCs during the efferocytosis of IACs were crucial to generate a microenvironment suitable for Th17 cell differentiation. Only CM from cocultured IACs and BMDCs was capable of strongly inducing Th17 cell differentiation; that from isolated IACs or BMDCs alone did not strongly induce IL-17–producing CD4+ T cells (Fig. 2 A and B). Moreover, naïve CD4+ T cells exposed to CM in which efferocytosis was impaired produced lower levels of IL-17A than those exposed to CM from cultures with unimpaired efferocytosis (SI Appendix, Fig. S4), confirming the relevance of efferocytosis in the triggering of Th17 cell differentiation.
Fig. 2.
PGE2 inhibits Th17 cell differentiation in the context of the efferocytosis of apoptotic E. coli-infected cells. Naïve CD4+ T cells were differentiated with anti-CD3 and anti-CD28 in the presence of supernatant from resting BMDC, and in the presence of the CM from efferocytosis of noninfected apoptotic cells (BMDC+AC), IACs only (IAC), BMDC efferocytosis of IACs (CM), indomethacin-treated BMDC efferocytosis of IACs (CM/Indo), or CM/Indo with addition of exogenous PGE2 (10 nM) for 72 h. (A) The percentage of CD4+IL-17A+ T cells was determined by flow cytometry and showed by representative dot plots and (B) bar graph as well as the levels of IL-17A released by lymphocytes was measured in the supernatant of cultures by ELISA. Data represent mean ± SEM of at least five independent experiments performed in triplicate. ND, not detected. *P < 0.05 compared with CM; #P < 0.05 compared with CM/Indo.
Surprisingly, naïve CD4+ T cells differentiated in CM/Indo or CM/Ibup (ibuprofen-treated BMDCs cocultured with IACs) showed more Th17 cell differentiation than CD4+ T cells differentiated in CM (Fig. 2 and SI Appendix, Fig. S5). The specific role of PGE2 in the reduction of Th17 cell differentiation was further evidenced when we performed “add-back” experiments. The addition of PGE2 into CM/Indo reduced Th17 cell differentiation compared with that in CM/Indo alone (Fig. 2). Moreover, when we specifically depleted PGE2 from CM using a PGE2 affinity column, Th17 cell differentiation was higher compared with that in nondepleted CM (SI Appendix, Fig. S5). Human T cells produced similar results by autologous mixed lymphocyte reaction in the presence of CM or CM/Indo derived from MoDCs cocultured with IACs (SI Appendix, Fig. S6). Those results further confirm that PGE2 is the main soluble mediator secreted during the efferocytosis of IACs that impairs Th17 cell differentiation.
Although PGE2 may inhibit the proliferation of T cells (30), we did not observe any difference in Th17 cell proliferation between CM and CM/Indo (SI Appendix, Fig. S7), indicating that the reduced Th17 cell differentiation observed in CM was not a result of impaired proliferation of naïve CD4+ T cells.
PGE2-EP4 Signaling Impairs Th17 Cell Differentiation.
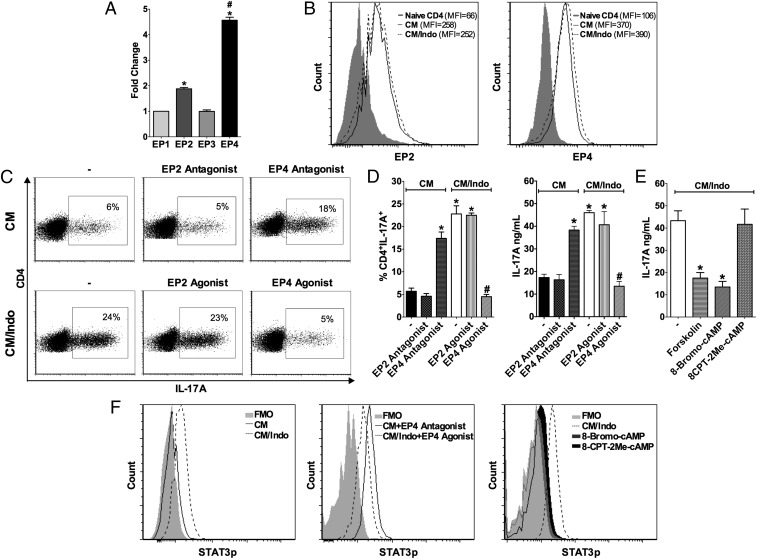
To study which EP receptor could be mediating the inhibitory effect of PGE2, we initially determined the expression profile of EP receptors in T cells during differentiation into Th17 cells in CM. The results confirmed previous reports that EP4 was more abundantly expressed than EP1, EP2, and EP3 in differentiating Th17 cells (Fig. 3 A and B). In addition, we determined which receptor was involved in the suppression of Th17 cell differentiation by treating naïve CD4+ T cells with different EP agonists and antagonists. EP4 antagonist prevented CM-inhibited Th17 cell differentiation, while EP1 and EP2 antagonists did not show any effect on Th17 cell differentiation (Fig. 3 C and D and SI Appendix, Fig. S8).
Fig. 3.
PGE2 impairs STAT3 phosphorylation and Th17 cell differentiation through EP4 activation. Naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence of CM from BMDCs cocultured with apoptotic E. coli-infected cells (IACs), or CM/Indo from indomethacin pretreated BMDC incubated with IACs. (A) The expression of EP1, EP2, EP3, and EP4 receptors on T cell differentiated in the presence of CM was measured by qPCR after 48 h of culture and represented as fold-change compared with EP1 receptor. Data represent mean ± SEM of three independent experiments performed in triplicate. *P < 0.05 compared with EP1; #P < 0.05 compared with EP2. (B) The expression of EP2 (Left) and EP4 (Right) receptor was assessed by flow cytometry on naïve CD4+ T cells or T cells after 72 h of differentiation in the presence of CM or CM/Indo. Data are shown in representative histograms of two independent experiments. (C) Naïve CD4+ T cells were treated with PF04418948 (EP2 antagonist) or L-161,982 (EP4 antagonist) and differentiated in the presence of CM or treated with Butaprost (EP2 agonist) or Cay10598 (EP4 agonist) and differentiated in the presence of CM/Indo, (−) represents only CM or CM/Indo without treatment. After 72 h, the expression of IL-17A was analyzed by flow cytometry and data are shown by representative dot plots from at least five independent experiments as well as D by bar graph showing the percentage of CD4+IL17A+ T cells (Left) and the levels of IL-17A detected by ELISA in the cultures supernatants (Right). Data represent mean ± SEM of three independent experiments performed in triplicate. *P < 0.05 compared with CM (−) or CM (EP2 antagonist); #P < 0.05 compared with CM/Indo (−) or CM/Indo (EP2 agonist). (E) Naïve CD4+ T cells were treated with Forskolin (adenylyl cyclase activator), 8-Bromo-cAMP (PKA activator), or 8-CPT-2Me-cAMP (EPAC activator) and differentiated in the presence of CM/Indo. After 72 h, IL-17A released in the supernatant of the cultures was measured by ELISA. Data represent mean ± SEM of three independent experiments performed in triplicate. *P < 0.05 compared with CM/Indo (−). (F) Naïve CD4+ T cells were cultured with CM in the presence or absence of L-161,982 (EP4 antagonist); or with CM/Indo in the presence or absence of Cay10598 (EP4 agonist), 8-Bromo-cAMP (PKA activator), or 8-CPT-2Me-cAMP (EPAC activator). After 15 min, cells were stained for STAT3 phosphorylation detection by phosflow assay. Data are shown in representative histograms of three independent experiments.
To further address the intracellular mechanism by which EP4 engagement compromises Th17 cell differentiation, we cultured naïve CD4+ T cells in CM/Indo with different concentrations of forskolin (adenylyl cyclase activator), 8-Bromo-cAMP (PKA activator), and 8-CPT-2Me-cAMP (EPAC activator). The forskolin and 8-Bromo-cAMP treatments impaired IL-17A production, while the 8-CPT-2Me-cAMP did not affect CD4+ T cell differentiation into Th17 cells (Fig. 3E). In addition, naïve CD4+ T cells treated with a PKA peptide inhibitor (PKI 14-22 amide-myristoylated) in CM showed enhanced IL-17A production compared with untreated cells (SI Appendix, Fig. S9).
Given that STAT3 dictates Th17 cell differentiation, we determined whether the PGE2-EP4-PKA axis could affect STAT3 phosphorylation and Th17 cell differentiation. Naïve CD4+ T cells cultured in CM showed reduced STAT3 phosphorylation compared with cells cultured in CM/Indo (Fig. 3F). Moreover, the EP4 antagonist restored STAT3 phosphorylation in cells cultured in CM, whereas the EP4 agonist and PKA activator decreased STAT3 phosphorylation in cells cultured in CM/Indo (Fig. 3F). Those results suggest that BMDCs produce PGE2 upon recognition of IACs, which inhibits Th17 cell differentiation via STAT3.
PGE2 Generated During Efferocytosis of IACs Down-Regulates IL-1R Expression in Naïve CD4+ T Cells.
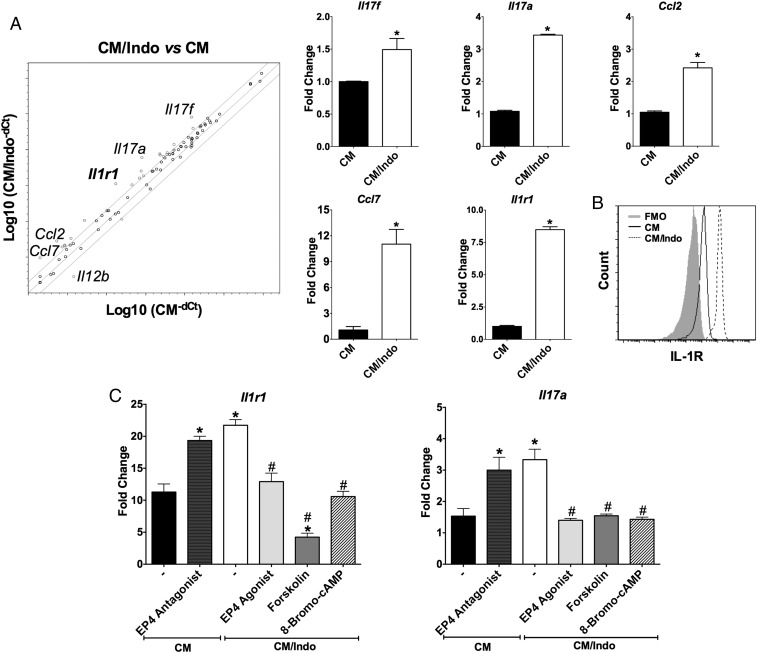
Next, we employed a Th17-focused qPCR gene array to investigate the gene-expression profile by which PGE2 influences Th17 cell commitment. Naïve CD4+ T cells cultured in CM/Indo displayed the up-regulation of 21 genes during Th17 cell differentiation compared with naïve CD4+ T cells cultured in CM alone (SI Appendix, Table S1). The mRNA expression of Il17f, Il17a, Il1r1, Ccl2, and Ccl7 was at least fourfold higher in CD4+ T cells differentiated in CM/Indo or CM/Ibup than in CD4+ T cells differentiated in CM (Fig. 4A and SI Appendix, Fig. S10). We confirmed the expression of Il1r1 by individual qPCR and FACS analysis (Fig. 4 A and B). In naïve CD4+ T cells cultured in CM, pretreatment with EP4 antagonist further enhanced Il1r1 and Il17a expression (Fig. 4C). Additionally, in naïve CD4+ T cells cultured in CM/Indo, pretreatment with EP4 agonist, forskolin (adenylyl cyclase activator), or 8-Bromo-cAMP (PKA activator) reduced Il1r1 and Il17a expression (Fig. 4C).
Fig. 4.
PGE2 compromises the expression of Th17-related genes during the efferocytosis of apoptotic E. coli-infected cells. Naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence of supernatant from efferocytosis of IACs by untreated BMDC (CM) or indomethacin-treated BMDC (CM/Indo). (A) After 48 h, RNA was extracted and the expression of Th17 related genes was measured by qPCR array. Representative scatterplot of expressed genes in T lymphocytes cultured in CM/Indo (y axis) or CM (x axis). Genes fourfold over or down-regulated are highlighted in the scatterplot. Bar graphs are presenting the most expressed genes in the array (Il17f, Il17a, Ccl2, Ccl7, and Il1r1) confirmed by conventional qPCR. Data represent mean ± SEM of three independent experiments performed in triplicate. *P < 0.05 compared with CM. (B) After 72 h of differentiation, the expression of IL-1R was analyzed by flow cytometry on CD4+ T cells cultured in CM or CM/Indo condition. Data are shown in a representative histogram of two independent experiments. (C) Naïve CD4+ T cells were differentiated in CM condition in the presence or not of L-161,982 (EP4 antagonist) or cultured in CM/Indo condition in the presence or not of Cay10598 (EP4 agonist), Forskolin (adenylyl cyclase activator), or 8-Bromo-cAMP (PKA activator). After 48 h of culture, RNA was extracted and the expression of Il1r1 and Il17a genes was measured by qPCR, and represented as fold-change compared with naïve T cells. Data represent mean ± SEM of three independent experiments. *P < 0.05 compared with CM (−); #P < 0.05 compared with CM/Indo (–).
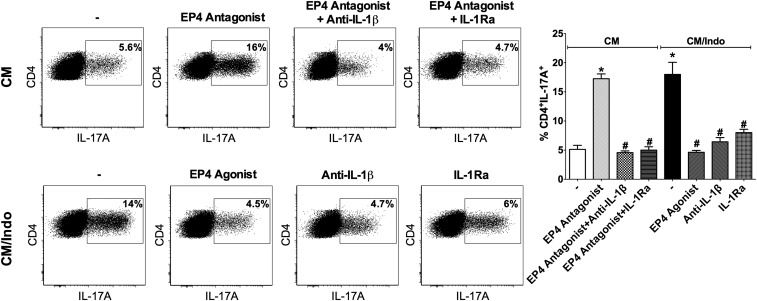
IL-1R signaling is critical during the differentiation, commitment, and maintenance of Th17 cells (31). To determine the cross-talk within the PGE2-EP4-IL-1R axis that may determine the Th17 fate, we treated naïve CD4+ T cells cultured in CM or CM/Indo with the IL-1R antagonist (IL-1Ra, IL-1 receptor antagonist) and with IL-1β neutralizing antibodies (anti–IL-1β) to block IL-1β actions. CD4+ T cells cultured in CM in the presence of EP4 antagonist plus anti–IL-1β or IL-1Ra showed a marked reduction in the percentage of IL-17A–producing lymphocytes compared with those cultured in CM in the presence of EP4 antagonist alone (Fig. 5). Similarly, in CD4+ T cells cultured in CM/Indo, the blocking of IL-1β actions reduced the frequency of Th17 cells, suggesting that IL-1R signaling is required for Th17 cell differentiation in that context. Those results suggest that during the efferocytosis of IACs, PGE2 in the microenvironment might control Th17 cell differentiation by downregulating IL-1R expression via the EP4-PKA axis.
Fig. 5.
IL-1R signaling is critical for Th17 cell differentiation in the context of the efferocytosis of apoptotic E. coli-infected cells. Naïve CD4+ T cells activated with anti-CD3 and anti-CD28 were differentiated in CM condition only (−), in the presence of L-161,982 (EP4 antagonist), L-161,982 plus anti–IL-1β, or L-161,982 plus IL-1Ra; or in CM/Indo condition only (−), in the presence or not of Cay10598 (EP4 agonist), anti–IL-1β or IL-1Ra. After 72 h, cells were stimulated and stained to determine the percentage of CD4+IL17A+ T cells by flow cytometry. Data are shown on representative dot plots (Left) and bar graph (Right) by mean ± SEM of at least three independent experiments. *P < 0.05 compared with CM (-); #P < 0.05 compared with CM/Indo (−).
C. rodentium Intestinal Infection Impairs Th17 Cell Differentiation Through the PGE2-EP4 Signaling Pathway.
The pharmacological inhibition of apoptosis during C. rodentium infection drastically impairs Th17 responses (7). Although Th17 responses are crucial to control C. rodentium infection, the role of PGE2 in the pathogenesis and intestinal host defense against C. rodentium remains unknown. Therefore, to investigate whether PGE2 controls Th17 cell differentiation in vivo, we used C. rodentium to induce infectious colitis in mice.
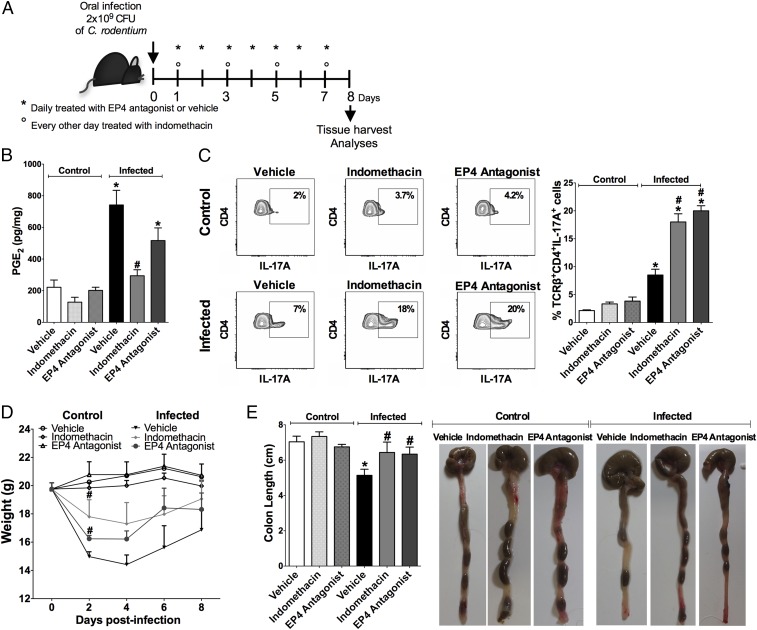
We infected mice with C. rodentium and treated them with indomethacin, EP4 antagonist, or vehicle (Fig. 6A). C. rodentium infection markedly increased PGE2 production, while indomethacin treatment significantly reduced the colonic levels of PGE2 in infected mice compared with those in infected mice treated with vehicle control (Fig. 6B). Moreover, to confirm whether efferocytosis as being the key driver of this response, infected mice were treated with recombinant Annexin V via the intravenous route and lower levels of PGE2, as well as COX-2 (Ptgs2) expression, were observed in the colon compared with that in infected mice that received the vehicle control (SI Appendix, Fig. S11). As expected, the EP4 antagonist did not affect the PGE2 levels in infected mice differently than the vehicle control (Fig. 6B). The frequency of TCRβ+CD4+IL-17+ cells in the colonic lamina propria was at least twofold higher in infected mice that were treated with indomethacin or EP4 antagonist compared with that in infected mice that received the vehicle control (Fig. 6C). Moreover, mPGES-1 knockout mice infected with C. rodentium displayed a similar pattern of TCRβ+CD4+IL-17+ cell frequency (SI Appendix, Fig. S12 A and B). In addition, infected mice treated with EP4 antagonist showed decreased weight loss and colon length reduction compared with vehicle-treated infected mice (Fig. 6 D and E). The treatment of uninfected control mice with indomethacin or EP4 antagonist had no significant effect on weight loss, colon length, or the colonic Th17 cell population (Fig. 6 C–E).
Fig. 6.
In vivo inhibition of PGE2 synthesis or EP4 signaling improves Th17 cell population in the colonic tissue of C. rodentium-infected mice. Mice were orally infected or not with C. rodentium and treated every other day with indomethacin (5 mg/kg), daily with L-161,982 (EP4 antagonist) (10 mg/kg) or vehicle for 7 d. On the eighth day of infection, colons were harvested. (A) Scheme of treatment. (B) PGE2 levels quantified by ELISA in the colonic tissue. (C) The percentage of TCRβ+CD4+IL17A+ T cells in the colon were assessed by flow cytometry and demonstrated by representative dot plots and bar graph. (D) Measurement of body weight throughout the experiment. (E) The colon length of different groups of animals was measured after killing. Data represent mean ± SEM of two independent experiments. n = 7–10. *P < 0.05 compared with control (vehicle); #P < 0.05 compared with infected (vehicle).
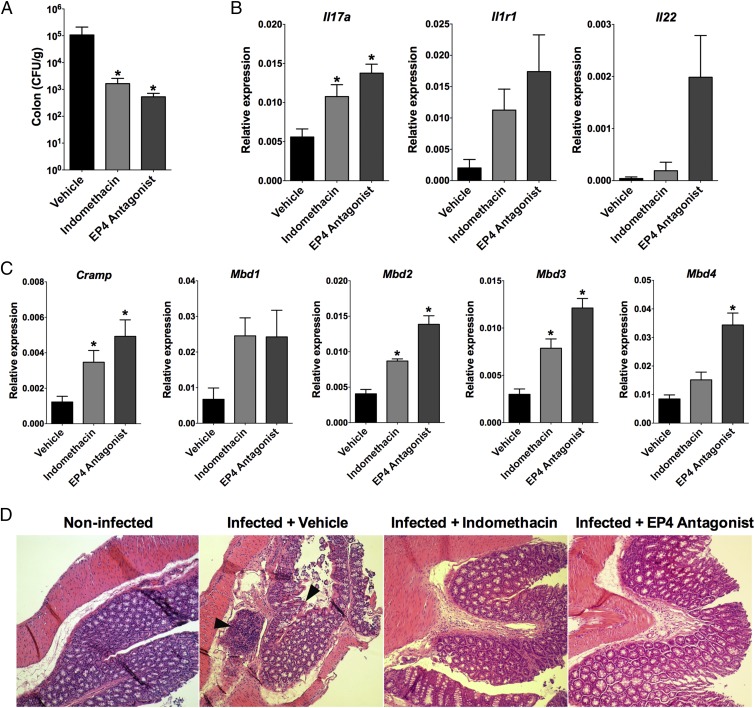
Infected mice that received indomethacin or EP4 antagonist showed decreased numbers of C. rodentium bacteria in the colon compared with infected mice that received the vehicle control (Fig. 7A). Infected mice that were treated with EP4 antagonist had higher levels of Il17a, and a slight increase in Il1r1 and Il22 expression in the colonic tissue (Fig. 7B) and also showed enhanced IL-1R expression on TCRβ+CD4+ T cells (SI Appendix, Fig. S13 A and B). Moreover, infected mice that received indomethacin or EP4 antagonist showed enhanced antimicrobial peptide expression in the colon (Fig. 7C), while the levels of IL-1β and IL-23 had a slightly inhibition compared with infected mice that received the vehicle control (SI Appendix, Fig. S13 C and D).
Fig. 7.
Indomethacin or EP4 antagonist treatment improves host defense against C. rodentium infection in mice. Mice were orally infected with C. rodentium and treated intraperitoneally every other day with indomethacin (5 mg/kg) or daily with L-161,982 (EP4 antagonist) (10 mg/kg) or vehicle for 7 d. On the eighth day of infection, colons were harvested. (A) The colonic tissue homogenates were plated for CFU counting per gram of tissue. (B) The expression of Il1r1, Il17a, Il22, and (C) antimicrobial peptides genes were analyzed by qPCR in the colonic tissue and represented as relative expression to Gapdh. (D) Representative H&E-staining sections of distal colonic tissues from untreated noninfected and vehicle, indomethacin, and EP4 antagonist-treated infected mice are shown. Areas of mononuclear cell infiltration and epithelial tissue injury are indicated by arrowheads. (Magnification: 100×.) Data represent mean ± SEM of two independent experiments. n = 7–10. *P < 0.05 compared with vehicle-treated infected mice group.
We also investigated morphological changes in the colonic tissue of C. rodentium-infected mice. In a representative image of the colon from infected, vehicle-treated mice, there was localized infiltration of mononuclear cells and areas of epithelial tissue injury, whereas the integrity of the muscularis mucosae was preserved (Fig. 7D). Treatment with indomethacin or EP4 antagonist attenuated the mononuclear cell infiltration and damage to the parenchyma (Fig. 7D) and also caused an intense influx of Ly6C+Ly6G+ cells into the colon (SI Appendix, Fig. S14) compared with the vehicle control. Those findings indicate that PGE2 may impair Th17-mediated intestinal host defense by acting on EP4 on CD4+ T cells.
Discussion
Microbial infections that cause host cell apoptosis have been shown to trigger Th17 immune responses (32). The clearance of IACs by BMDCs induces the synthesis of Th17-inducing cytokines, such as TGF-β and IL-6 (7). C. rodentium is an intestinal pathogen that causes apoptosis of epithelial cells, which is a critical process to trigger Th17 cell differentiation (7). We demonstrated that high levels of PGE2 are present during enteric C. rodentium infection in vivo. Targeting PGE2 in vitro and in vivo markedly improved Th17 cell differentiation and intestinal host defense against C. rodentium. The EP4-cAMP-PKA pathway mediated the suppressive effect of PGE2, which impaired IL-1R expression in T cells and compromised the Th17 phenotype. Moreover, selective impairment of EP4 signaling increased the colonic Th17 cell population and antimicrobial peptide expression, resulting in a reduction of the C. rodentium load in the colon.
The uptake of apoptotic cells by phagocytes is capable of modulating different cells of the immune system, resulting in either the suppression or the activation of immune cells (33). The efferocytosis of noninfected cells by DCs or macrophages favors Treg generation and leads to the synthesis of antiinflammatory mediators, such as TGF-β, PAF, and PGE2 (5, 34). However, the capture of apoptotic E. coli-infected cells by BMDCs promotes TGF-β, IL-6, and IL-23 release and triggers inflammation and Th17 cell differentiation (7). We demonstrated that PGE2 produced during the uptake of IACs impairs Th17 cell differentiation and intestinal host defense. A similar effect has been described in Leishmania donovani infection; PGE2 inhibited IL-17 synthesis and compromised the host response against infection, while treatment with COX inhibitors reversed that effect (35). Additionally, during C. neoformans infection in mice, treatment with indomethacin enhanced Th17 responses and promoted the survival of infected mice (26).
The treatment of BMDCs with indomethacin during the uptake of apoptotic E. coli-infected cells decreased the production of IL-23 and IL-1β, which are essential for Th17 cell differentiation. That result is consistent with previous findings that PGE2 acts in an autocrine manner, favoring the synthesis of those mediators (36, 37). Reduced levels of IL-23 and IL-1β in CM/Indo did not affect the differentiation of naïve CD4+ T cells into Th17 cells, while the exogenous addition of PGE2 to CM/Indo decreased the frequency of Th17 cells relative to that in CM/Indo without PGE2.
EP1, EP2, and EP4 are expressed in human naïve CD4+ T cells, whereas EP2 and EP4 are mainly expressed in Th17 cells (18, 38). Furthermore, EP1 signaling increases intracellular Ca2+, whereas the EP2 and EP4 pathways enhance intracellular cAMP levels, resulting in the activation of PKA and EPAC proteins (39). RORγt expression during Th17 cell differentiation impairs EP2 expression but does not affect EP4 expression (40). Consistent with those findings, we observed that EP4 was the most prevalent PGE2 receptor expressed in Th17 cells. EP1 and EP2 antagonists did not influence the inhibitory effect of PGE2 on Th17 cell differentiation in vitro in the context of the efferocytosis of infected cells. Although those results indicate that PGE2 mainly acts via EP4-cAMP-PKA to suppress Th17 cell differentiation and intestinal host defense, the relevance of EP1 or EP2 actions in vivo remains elusive.
IL-6, IL-21, and IL-23 receptor signaling induces IL-17 expression via STAT3 activation, which binds the RORγt promoter and activates RORα and IRF4 expression (41, 42). Indeed, IL-6R signaling promotes IL-1R expression (43). IL-1β signaling plays a critical role during Th17 cell differentiation and is also related to the maintenance and expansion of Th17 cells (31, 44). Our results demonstrated that PGE2 produced by the efferocytosis of infected cells impaired STAT3 phosphorylation and the expression of IL-1R in T cells via the EP4-PKA pathway. Suppressor of cytokine signaling (SOCS) binds to Janus kinase and suppresses STAT activation (45). PGE2 and misoprostol, a PGE analog, induced SOCS1 expression in BM cells during peritonitis (46). Indeed, SOCS3 and SOCS1 can inhibit STAT-1 and STAT-3 phosphorylation (45, 47, 48). Therefore, the early inhibition of STAT3 phosphorylation may have been a critical point in the impairment of IL-1R expression, although the specific mechanism by which EP4 signaling affects STAT3 phosphorylation (via SOCS or other factors) and thereby impairs IL-1R expression remains to be elucidated.
IL-1β receptor-deficient lymphocytes have reduced IL-23R expression and less ability to produce IL-17A (31). That finding is consistent with our qPCR array data showing that, in addition to that of Il1r1, the expression of Il23r was decreased, which may have contributed to the reduced Th17 cell differentiation in CM. Similar data have been reported for experimental autoimmune encephalomyelitis, as IL-1R knockout animals had a lower capacity to induce Th17 responses compared with WT animals and showed resistance to development of experimental autoimmune encephalomyelitis (49).
Th17 cells are important to host defenses against pathogens, such as fungi and extracellular bacteria (41). Th17 cytokines increase granulopoiesis and the expression of chemokines, which coordinate cellular recruitment and neutrophil chemotaxis to the inflammatory site, and IL-17A and IL-22 induce the expression of defensins and cathelicidins by epithelial cells (29). Furthermore, IL-26—also produced by Th17 cells—acts as an antimicrobial peptide capable of destroying bacteria such as Pseudomonas aeruginosa, E. coli, Klebsiella pneumoniae, and Staphylococcus aureus (50). Several studies have shown that protection against C. rodentium infection correlates with IL-17A and IL-22 production (28, 51) and that Th17 cells, as well as other cells, such as innate lymphoid cells and γδ T, NK, and NK-T cells, can produce IL-17A (52). However, CD3+ cell depletion during C. rodentium infection drastically reduces host defense and aggravates infection (53), which demonstrates the relevance of CD3+ lymphocytes in that context. Our results demonstrate that C. rodentium infection enhances colonic Th17 responses compared with those in noninfected control mice. Indeed, the treatment of infected mice with indomethacin or EP4 antagonist markedly increased levels of IL-17A and antimicrobial peptides, while drastically reducing the bacterial load in colonic tissue compared with those in mice treated with a vehicle control.
The recognition of different microorganisms induces PGE2 synthesis by phagocytes and may modulate the function of immune cells (19). In addition, the efferocytosis of cells that undergo pathogen-induced apoptosis greatly increases the levels of PGE2 in the microenvironment. Therefore, the ability of some pathogens to induce apoptosis and consequently favor PGE2 synthesis raises the intriguing possibility that those pathogens use PGE2 as a pathway to manipulate or suppress host defense. For example, PGE2 impairs the phagocytosis and killing of some bacteria, viruses, and fungi by macrophages (54–56). Moreover, PGE2, the levels of which are increased by efferocytosis, compromises the clearance of S. pneumoniae infection by alveolar macrophages (6). The effects of PGE2 or EP4 signaling on macrophage effector functions in colonic tissue and during the efferocytosis of infected cells remain unclear, however.
The roles of PGE2 in Th17 cell differentiation and expansion remain controversial. PGE2 facilitates DC migration to lymph nodes (57, 58) and IL-23 production (59) and directly increases the expansion of Th17 cells (23); however, a suppressive effect of PGE2 on Th17 cells has also been reported (26, 60, 61). For example, using a model of C. neoformans infection in mice, Valdez et al. (26) demonstrated that PGE2 in the early stages of infection negatively influenced the fate of Th17 cells, although there were high levels of IL-17 production from memory Th17 cells. The majority of studies describing the effect of exogenous PGE2 on Th17 cell differentiation or expansion used relatively high PGE2 concentrations (1–10 μM) and mainly preactivated CD4+ T cells (18, 23). In our study, the amount of endogenous PGE2 produced by BMDCs during efferocytosis of apoptotic E. coli-infected cells was between 40 nM and 50 nM. Therefore, the mechanisms that dictate the opposite effects of PGE2 under different conditions may be complex and depend on a variety of factors. For example, the discrepancies among studies may be related to distinct cellular microenvironments (homeostasis or inflammation), maturation and activation states of CD4+ T cells, or types of EP receptors activated, as well as to the different concentrations of exogenous or endogenous PGE2. In our study, in the context of efferocytosis of infected cells, endogenous levels of PGE2 associated with the inflammatory microenvironment, along with the type of receptor engaged (EP4) on naïve CD4+ T cells, may have contributed to the inhibitory actions of PGE2 on Th17 cell differentiation. Interestingly, noninfected mPGES-1−/− mice showed higher Th17 abundance in the colon than naïve WT mice. We also observed a slight, albeit nonsignificant, increase in Th17 cell frequency when noninfected mice were treated with either indomethacin or EP4 antagonist compared with noninfected mice treated with vehicle control. This unexpected finding may open new avenues of research to determine whether mPGES-1–derived PGE2 is critical to control homeostatic colonic inflammation.
Immune and nonimmune cells express EP4 (62). Hence, the modulation of EP4 signaling might affect a variety of cellular functions. The enhancement of Th17 cell differentiation in vivo by EP4 antagonist suggests an interesting strategy to target specific PGE2 actions and immune responses to promote host defense. However, exacerbated Th17 cell responses are also related to chronic inflammation and autoimmune disorders, such as rheumatoid arthritis, psoriasis, and multiple sclerosis (63). Indeed, a recent study demonstrated that the engulfment of IACs by BMDCs may promote the exposure of self- and nonself-antigens to naïve CD4+ T cells in the inflammatory microenvironment, allowing the activation of autoreactive clones (64). Therefore, additional investigations are needed to understand the late effects of enhancement of Th17 cell responses and its link to increased autoimmunity risk.
Our study provides evidence of a regulatory mechanism by which PGE2 produced by the efferocytosis of infected cells suppresses Th17 cell differentiation and compromises adaptive immunity. We demonstrated that EP4 antagonist improved Th17 cell differentiation and intestinal host defense without affecting colonic PGE2 levels. Considering the diversity of prostaglandin actions and receptors in different organs and cell types, the use of an EP4 receptor antagonist may be useful to improve selectivity and avoid unwanted reactions. Targeted inhibition of EP4 signaling during infections that trigger host apoptosis and PGE2 synthesis may be a way to enhance Th17 immunity and host defense.
Methods
Mice.
Female WT C57BL/6 mice (8-wk-old) were obtained from Multidisciplinary Center for Biological Research, University of Campinas. Mice were kept in the animal facility at the School of Pharmaceutical Sciences, São Paulo State University, under pathogen-free conditions in mini-isolators with controlled temperature, dark/light cycle, humidity, and airflow, and with free access to sterilized water and food. Experimental procedures were approved by the Institutional Animal Care and Committee of the School of Pharmaceutical Sciences, São Paulo State University.
Generation of CM from Cocultures of BMDCs and IACs.
BMDCs were differentiated with GM-CSF (PeproTech) for 7 d (12, 13). RAW 264.7 cells were cultured with E. coli (ATCC 25922) (ratio 1:10) for 2 h to allow infection. Then, the cells were washed with PBS to remove bacteria and cellular debris. RAW 264.7 infected cells were exposed to UVC radiation and maintained in a humidified 37 °C 5% CO2 incubator for 4 h, as previously described (12, 13). BMDCs were treated with 10 μM of indomethacin or left untreated. Then, BMDCs were cocultured for 18 h with apoptotic E. coli-infected cells (IACs) at a ratio of 1:3, and the supernatants from each condition were collected for cytokine/PGE2 quantification and used in differentiation assays of naïve CD4+ T cells. For experimental controls, BMDCs were left in resting conditions in the absence of IACs or cocultured with noninfected ACs. More details are described in SI Appendix, Supplemental Methods.
Differentiation of Naïve CD4+ T Cells.
Naïve CD4+ T cells were purified from the spleens of C57BL/6 mice using CD4+CD62L+ T Cell Isolation Kit II (Miltenyi Biotech) according to the manufacturer’s protocol. Approximately 5 × 105 naïve CD4+ T cells were cultured in the presence of 250 μL supernatant from BMDCs, CM, or CM/Indo plus 250 μL fresh Iscove’s Modified Dulbecco’s Medium (IMDM) medium supplemented with 10% FBS, 1 nM nonessential amino acids, 1 mM l-glutamine, 1 nM sodium pyruvate, 55 μM 2-β mercaptoethanol, 4 μg/mL anti-CD3, 2 μg/mL anti-CD28, and 5 μg/mL anti–IL-2, anti–IL-4, and anti–IFN-γ (BD). After 72 h, the supernatant was collected for cytokine quantification by ELISA and the cells were stimulated for flow cytometry analysis. More details are described in SI Appendix, Supplemental Methods.
C. rodentium-Induced Infectious Colitis Model.
Female C57BL/6 mice were infected by gavage with 2 × 109 CFU of C. rodentium (ICC168) in 200 μL of PBS. The control groups were vehicle-treated noninfected mice and noninfected mice treated with indomethacin or EP4 antagonist. The infected groups were vehicle-treated infected mice and infected mice treated with indomethacin or EP4 antagonist. Animals were treated intraperitoneally with vehicle (PBS+2%DMSO) or indomethacin (5 mg/kg) on days 1, 3, 5, and 7 after infection or with EP4 antagonist (L-161,982; 10 mg/kg) daily for 7 d. On the eighth day after infection, colons were collected for different analysis. More details are described in SI Appendix, Supplemental Methods.
Detailed information on cell culture, reagents, flow cytometry analysis, phosflow assay, ELISA, quantitative real-time PCR, qPCR array, lamina propria lymphocyte isolation, histopathological evaluation of colitis, and statistical analysis is available in SI Appendix, Supplemental Methods.
Supplementary Material
Acknowledgments
We thank Prof. Cristiano Gallina Moreira for kindly providing the wild-type strain of Citrobacter rodentium; Prof. Vânia Luiza Deperon Bonato, Prof. Ana Paula Lepique, and José Alexandre Barbuto for supplying the reagents; Prof. João Gustavo P. Amarante-Mendes and Maziar Divangahi for providing microsomal prostaglandin E synthase-1 knockout mice; Valéria Malavolta for helping during mouse experiments; Denise Brufato Ferraz for excellent technical help with flow cytometry; and José A. S. Zuanon for processing the colonic tissues for histology. This work was financially supported through the São Paulo Research Foundation (Grants FAPESP 11/17611-7, 12/23580-0, 14/03967-2, and 16/10964-5) and Brazilian Federal Agency (CAPES).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.L.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722016115/-/DCSupplemental.
References
- 1.Genestier A-L, et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005;115:3117–3127. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: Implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grabiec AM, Hussell T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin Immunopathol. 2016;38:409–423. doi: 10.1007/s00281-016-0555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 8.Divangahi M, Behar SM, Remold H. Dying to live: How the death modality of the infected macrophage affects immunity to tuberculosis. Adv Exp Med Biol. 2013;783:103–120. doi: 10.1007/978-1-4614-6111-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin CJ, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzelepis F, et al. Annexin1 regulates DC efferocytosis and cross-presentation during Mycobacterium tuberculosis infection. J Clin Invest. 2015;125:752–768. doi: 10.1172/JCI77014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian M, et al. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J Clin Invest. 2014;124:1296–1308. doi: 10.1172/JCI72051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penteado LA, et al. Distinctive role of efferocytosis in dendritic cell maturation and migration in sterile or infectious conditions. Immunology. 2017;151:304–313. doi: 10.1111/imm.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejani NN, et al. Topical prostaglandin E analog restores defective dendritic cell-mediated Th17 host defense against methicillin-resistant Staphylococcus aureus in the skin of diabetic mice. Diabetes. 2016;65:3718–3729. doi: 10.2337/db16-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 15.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: A tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagamachi M, et al. Facilitation of Th1-mediated immune response by prostaglandin E receptor EP1. J Exp Med. 2007;204:2865–2874. doi: 10.1084/jem.20070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boniface K, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: Friends or foes? Immunol Cell Biol. 2012;90:579–586. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhaumik S, Basu R. Cellular and molecular dynamics of Th17 differentiation and its developmental plasticity in the intestinal immune response. Front Immunol. 2017;8:254. doi: 10.3389/fimmu.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheibanie AF, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23→IL-17 axis. J Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 23.Yao C, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 24.Myer RG, El Mezayen R, High KP. Prostaglandin E2-dependent IL-23 production in aged murine dendritic cells. Exp Gerontol. 2010;45:834–841. doi: 10.1016/j.exger.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schirmer C, Klein C, von Bergen M, Simon JC, Saalbach A. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood. 2010;116:1715–1725. doi: 10.1182/blood-2010-01-263509. [DOI] [PubMed] [Google Scholar]
- 26.Valdez PA, et al. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity. 2012;36:668–679. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silberger DJ, Zindl CL, Weaver CT. Citrobacter rodentium: A model enteropathogen for understanding the interplay of innate and adaptive components of type 3 immunity. Mucosal Immunol. 2017;10:1108–1117. doi: 10.1038/mi.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins JW, et al. Citrobacter rodentium: Infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12:612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- 29.Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vercammen C, Ceuppens JL. Prostaglandin E2 inhibits human T-cell proliferation after crosslinking of the CD3-Ti complex by directly affecting T cells at an early step of the activation process. Cell Immunol. 1987;104:24–36. doi: 10.1016/0008-8749(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 31.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brereton CF, Blander JM. The unexpected link between infection-induced apoptosis and a TH17 immune response. J Leukoc Biol. 2011;89:565–576. doi: 10.1189/jlb.0710421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devitt A, Marshall LJ. The innate immune system and the clearance of apoptotic cells. J Leukoc Biol. 2011;90:447–457. doi: 10.1189/jlb.0211095. [DOI] [PubMed] [Google Scholar]
- 34.Pujol-Autonell I, et al. Efferocytosis promotes suppressive effects on dendritic cells through prostaglandin E2 production in the context of autoimmunity. PLoS One. 2013;8:e63296. doi: 10.1371/journal.pone.0063296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha A, et al. Prostaglandin E2 negatively regulates the production of inflammatory cytokines/chemokines and IL-17 in visceral leishmaniasis. J Immunol. 2014;193:2330–2339. doi: 10.4049/jimmunol.1400399. [DOI] [PubMed] [Google Scholar]
- 36.Shi Q, et al. PGE2 elevates IL-23 production in human dendritic cells via a cAMP dependent pathway. Mediators Inflamm. 2015;2015:984690. doi: 10.1155/2015/984690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoccal KF, et al. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat Commun. 2016;7:10760. doi: 10.1038/ncomms10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- 39.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: Macrophage inhibition by cyclic AMP (cAMP): Differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174:595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 40.Kofler DM, et al. Decreased RORC-dependent silencing of prostaglandin receptor EP2 induces autoimmune Th17 cells. J Clin Invest. 2014;124:2513–2522. doi: 10.1172/JCI72973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 42.Hirahara K, et al. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21:425–434. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulen MF, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T helper immune responses. Front Immunol. 2013;4:182. doi: 10.3389/fimmu.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: Regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol. 2011;31:980–985. doi: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- 46.Zaslona Z, Serezani CH, Okunishi K, Aronoff DM, Peters-Golden M. Prostaglandin E2 restrains macrophage maturation via E prostanoid receptor 2/protein kinase A signaling. Blood. 2012;119:2358–2367. doi: 10.1182/blood-2011-08-374207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi R, et al. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-gamma and IL-17A production. J Exp Med. 2011;208:2055–2067. doi: 10.1084/jem.20110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piñeros Alvarez AR, et al. SOCS1 is a negative regulator of metabolic reprogramming during sepsis. JCI Insight. 2017;2:92530. doi: 10.1172/jci.insight.92530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutton C, Brereton C, Keogh B, Mills KHG, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meller S, et al. T(H)17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol. 2015;16:970–979. doi: 10.1038/ni.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubino SJ, Geddes K, Girardin SE. Innate IL-17 and IL-22 responses to enteric bacterial pathogens. Trends Immunol. 2012;33:112–118. doi: 10.1016/j.it.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmons CP, et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71:5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serezani CH, et al. PTEN directly activates the actin depolymerization factor cofilin-1 during PGE2-mediated inhibition of phagocytosis of fungi. Sci Signal. 2012;5:ra12. doi: 10.1126/scisignal.2002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coulombe F, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Serezani CH, et al. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37:562–570. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J Immunol. 2006;176:966–973. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- 58.Scandella E, Men Y, Gillessen S, Förster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 59.Khayrullina T, Yen J-H, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E(2) alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen H, et al. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot Essent Fatty Acids. 2009;80:195–200. doi: 10.1016/j.plefa.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Duffy MM, et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41:2840–2851. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- 62.Yokoyama U, Iwatsubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev. 2013;65:1010–1052. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- 63.Waite JC, Skokos D. Th17 response and inflammatory autoimmune diseases. Int J Inflamm. 2012;2012:819467. doi: 10.1155/2012/819467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campisi L, et al. Apoptosis in response to microbial infection induces autoreactive TH17 cells. Nat Immunol. 2016;17:1084–1092. doi: 10.1038/ni.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.