Abstract
Purpose
The unfavorable safety profiles of commonly prescribed knee osteoarthritis (OA) treatments have led clinicians and patients to seek safer alternatives. Research has suggested that curcuminoid and boswellia formulations could moderate key inflammatory pathways that are associated with worsening symptoms and disease progression. We conducted a systematic review and meta-analysis to assess the efficacy and safety of these treatments vs. placebo or NSAIDs for knee OA.
Methods
We searched Medline, EMBASE, Google Scholar, Web of Science and the Cochrane database from inception to February 21, 2018. We also hand searched reference lists and reviewed conference proceedings. We included randomized clinical trials (RCTs) comparing curcuminoid or boswellia formulations with placebo or NSAIDs for knee OA. We calculated standardized mean differences (SMD) or risk ratios (RR) for all relevant outcomes. Meta-analyses were conducted using random effects models. Heterogeneity was assessed using the I2 statistic.
Results
Eleven RCTs (N= 1,009) were eligible for analysis. Study quality was low overall, and most included RCTs were conducted on fewer than 100 participants. Both curcuminoid and boswellia formulations were statistically significantly more effective than placebo for pain relief and functional improvement. There were no significant differences between curcuminoids or boswellia and placebo in safety outcomes. Curcuminoids showed no statistically significant differences in efficacy outcomes compared to NSAIDs; patients receiving curcuminoids were significantly less likely to experience gastrointestinal adverse events. No RCTs compared boswellia against approved NSAIDs.
Conclusions
The results of our study suggest that curcuminoid and boswellia formulations could be a valuable addition to the knee OA treatment regimens by relieving symptoms while reducing safety risks. The current body of evidence is not adequate in size or quality to make any meaningful clinical practice recommendations. Further research through large, high quality RCTs probably investigating the synergistic effect of these products with other OA treatments is warranted.
Keywords: Curcumin, Boswellia, Knee Osteoarthritis, Meta-Analysis
Introduction
Knee osteoarthritis (OA) affects nearly 40% of adults over the age of 60 in the United States1. In the absence of effective disease modifying treatments, current standards of care for knee OA are primarily aimed at pain relief and functional improvement. Non-steroidal anti-inflammatory drugs (NSAIDs) are the therapeutic agents of choice for many clinicians due to their widely reported efficacy, despite the well-documented safety risks of these treatments, particularly with respect to gastrointestinal (GI) adverse events2–6. In response to the safety risks associated with these and other commonly prescribed treatments for knee OA, clinicians and patients have begun to shift focus to complementary therapies which may have more favorable safety profiles7.
Curcuminoids and the gum resin of boswellia have been used for thousands of years in the practice of Ayurveda, an ancient system of medicine with origins in the Indian subcontinent, to bring relief to those suffering from inflammatory and degenerative disorders8,9. These phytochemicals are being explored with renewed interest by OA researchers as safer alternatives for effective symptom management.
Though the primary driver of knee OA symptoms and radiographic progression has long been considered the “wear and tear” of cartilage over time, advances in molecular biology have produced evidence indicating that the pathogenesis of OA may be considerably impacted by inflammatory processes. In many cases, decreasing levels of proteoglycan within the articular cartilage are the earliest detectable change signaling the onset of knee OA, which is succeeded by degradation of type II collagen, bone remodeling, and synovial inflammation, among other joint abnormalities10. Some researchers have posited that continuing degradation of cartilage is driven by the release inflammatory cytokines and exacerbated by the subsequent activation of other inflammatory mediators, such as matrix metalloproteinases (MMPs)11.
In vivo and in vitro studies have suggested that the use of curcuminoid and boswellia formulations could halt or slow the catabolic actions of key inflammatory mediators in the early stages of disease, and could continue to block inflammatory pathways that have been associated with the progression of knee OA12–15. Both curcuminoid and boswellia formulations have been shown to counteract decreases in glycosaminoglycan levels and impede the secretion and activity of MMPs, which could potentially forestall further degradation of cartilaginous tissue9,16,17. Certain NSAIDs, in contrast, can disrupt the synthesis of glycosaminoglycan which could act to hasten or intensify cartilage damage18,19. Additionally, curcuminoids act as inhibitors to the enzyme cyclooxygenase-2 (COX-2), which is associated with inflammatory processes and pain, by blocking the action of tumor necrosis factor15. Boswellic acid has been shown to inhibit the 5-lipooxygenase (LOX) pathway, which is a primary source of pro-inflammatory leukotrienes20,21. Curcuminoids and acetyl-keto-beta-boswellic acid (AKBA), an active ingredient of Boswellia serrata gum resin, also have inhibitory effects on inflammatory nuclear factor κB (NF-κB) and its gene products, some of which are directly involved in the processes of osteoclastogenesis and the resorption of bone12,13,22. In summary, many studies suggest that curcuminoids and boswellia could provide a therapeutic benefit that extends beyond symptom relief to disease modification. Such effects would demonstrate a clear superiority of these formulations over conventional NSAID treatment, particularly in light of the evidence that some NSAIDs may have deleterious effects on cartilage metabolism over time and the well-known toxicity of conventional NSAIDs.
The extensive history of curcuminoid and boswellia use for pain relief coupled with the recent findings showing that these phytochemicals may directly act upon several inflammatory processes offers compelling evidence that these products could reduce pain and may even slow cartilage degradation in patients with knee OA. There are, however, very few randomized controlled trials (RCTs) which have tested the efficacy of curcuminoid and boswellia formulations in humans with knee OA, and further few have reported the comparative efficacy and safety of these treatments against conventional treatments like NSAIDs. Three recent meta-analyses have attempted to assess the efficacy and safety of boswellia and/or curcuminoids. However, these meta-analyses have methodological flaws including a lack of up to date RCT data, analysis techniques that do not allow for pooling of effects, or presentation of results that could not be replicated23–25. Therefore we conducted an updated systematic review and meta-analysis to assess the efficacy and safety of these treatments, alone or in combination, in comparison to placebo and NSAIDs for knee OA. We conducted a joint review of curcuminoid and boswellia formulations primarily based on their similar biochemical targets, and in light of their respective prevalence in the literature as mono-therapies (compared to other herbal formulations) and due to the presence of RCTs examining these specific formulations used in combination with each other. The protocol for this study was registered in PROSPERO (CRD42017073911) before commencing data extraction.
Methods
Data Sources/Searches
We searched Medline, EMBASE, Google Scholar, Web of Science, and the Cochrane Database from inception to February 21, 2018. We hand-searched reference lists of relevant systematic reviews and meta-analyses, and whenever necessary, we searched within supplements of conference proceedings that had been published up until February 21, 2018. Wherever additional information was required, we contacted the authors electronically. The systematic search was limited to randomized controlled trials (RCTs) in human subjects with knee OA which involved treatment with orally administered curcuminoid or boswellia formulations. We did not restrict our search by publication date, status, or language.
Study Selection
RCTs that compared curcuminoid or boswellia formulations administered alone or in combination against either a matching placebo or NSAIDs were included. Exclusion criteria were non-randomized study design, treatment protocol involving concomitant medications such as NSAIDs or other analgesics (unless administered as rescue medication), and use of an intervention which included additional nutraceuticals or herbal supplements. Two independent reviewers (FA, MO) screened each abstract recovered by the search in accordance with the inclusion and exclusion criteria. Abstracts included at this stage underwent a second round of screening, during which full manuscripts were gathered and thoroughly reviewed for eligibility by the same two reviewers (FA, MO). Inclusion or exclusion conflicts which occurred during either screening stage were resolved by a third reviewer (RB).
Data Extraction and Quality Assessment
From each RCT, two reviewers (FA, MO) independently extracted data on study and population characteristics, specific curcumin and/or boswellia formulation, dosage and frequency, rescue medication use, improvement of symptoms, and relevant safety outcomes. To establish an a priori extraction hierarchy for pain and functional outcome scales, we referenced the Cochrane Musculoskeletal research group’s List of Proposed Outcomes26. For our main analyses, we prioritized data which were presented in tabular format or which were written within manuscript text over graphical data to ensure accuracy and objectivity. Wherever necessary, we recovered data which were presented graphically using Engauge Digitizer27. Data were extracted into RevMan software28, and study quality was assessed in RevMan using the Cochrane Risk of Bias Tool29. A third reviewer (RB) evaluated all data extraction and quality ratings for consistency and resolved discordant responses.
Outcome Definitions
Outcomes of interest included pain, function, use of protocol-assigned rescue medication (including NSAIDs, opioids, or acetaminophen), rate of discontinuation due to adverse events, incidence of serious adverse events and gastrointestinal adverse events. We collected pain and functional outcomes assessed by any scale and reported the mean change from baseline to the study endpoint. Rates of discontinuation were reported as the number of participants who discontinued treatment or withdrew from the study due to any adverse event, irrespective of an association to the given intervention. Incidence of serious adverse events was defined as the number of patients reporting at least one adverse event which was explicitly designated by the outcome assessor(s) as a “Serious Adverse Event” within the study period. We collected gastrointestinal (GI) adverse events on the basis of the inherent potential for gastrointestinal risk associated with use of substances which act as COX- and LOX-inhibitors, and in order to assess the comparative GI safety of curcumin and/or boswellia formulations against NSAIDs, since GI adverse events are a well-established risk of NSAID use4.
Statistical analysis
For all continuous outcomes, we calculated standardized mean differences (SMDs) and 95% Confidence Intervals (CIs) based on the mean change from baseline to the study end point. Meta-analyses were conducted using random effects models, in the anticipation of methodological and clinical heterogeneity. Standardized mean differences were used in all analyses of continuous outcome measures, regardless of variation in their scales, in order to facilitate straightforward comparability of effect sizes across different outcomes. We planned a priori sensitivity analyses based on pain outcome measure used, study sample size (≤30 vs. >30), study quality (High vs. Low), rescue analgesia accommodations (allowed vs. not allowed), and bioavailability of curcuminoid preparations (enhanced vs. not enhanced) all of which were contingent upon the availability of data. Dichotomous outcomes were analyzed using the Mantel-Haenszel method and were reported as risk ratios (RR) and 95% CI30. We evaluated heterogeneity using the I2 statistic31. All analyses were conducted using RevMan software29.
Evidence Grading
In an attempt to objectively measure the overall quality of evidence constituting our analyses, two independent reviewers (MO, RB) evaluated the evidence quality for each outcome using GRADE quality assessment criteria, and discrepancies were resolved by consensus32. RevMan files were exported onto online GRADEpro software to formulate a GRADE evidence profile for each overarching comparison (i.e. Treatment vs. Placebo or Treatment vs. NSAID)33. GRADE methodology assesses quality over four principal domains: risk of bias, inconsistency, indirectness, and imprecision. The overall grade of the evidence is assessed as either “High”, “Moderate”, “Low”, or “Very Low”. “High” grade evidence is designated a numerical equivalent of 4, with subsequent downgrades carrying a weight of −1 for “serious” risk or −2 for “very serious” risk; once the quality rating has reached “Very Low” (numerical equivalent of 1), the evidence can be downgraded no further.
By establishing and adhering to strict inclusion criteria, we eliminated the need to reassess indirectness. In assessing risk of bias, we tailored our downgrades by outcome to the dimension of bias for which there was a direct concern; for example, if a study was noted as showing potential risk of reporting bias in its efficacy analyses, a High risk of bias downgrade would apply solely to the affected efficacy outcomes. We established the following a priori I2 cutoff values for inconsistency ratings: ≤50%= low/acceptable heterogeneity, no quality downgrade; >50% and ≤75%= moderate heterogeneity, “serious” quality downgrade; >75%= high heterogeneity, “very serious” quality downgrade. We applied the effect size magnitude cutoffs proposed by Cohen in grading the imprecision of continuous outcomes34: 95% Confidence Interval of an SMD extending between >0.2-≤0.5 points in either direction, “serious” quality downgrade; 95% Confidence Interval of an SMD extending >0.5 points in either direction, “very serious” quality downgrade. We adhered strictly to GRADE imprecision guidelines when evaluating risk ratios and applied additional downgrades for outcomes assessed by exceptionally small numbers of patients: sample size in one study arm <50, “serious” quality downgrade; total sample size ≤30, “very serious” quality downgrade. Wherever applicable, we made note of industry funding or conflicts of interest. Due to the small number of published studies for each comparison, we lacked sufficient power to objectively assess publication bias using Egger’s test35.
Results
Our initial search yielded 92 references; 14 RCTs (N= 1,215) were included in the systematic review, and 11 (N= 1,009) adequately reported outcomes of interest and were eligible for meta-analysis (Figure 1). Five RCTs compared curcuminoid formulations against placebo (N= 331)36–40, and four trials compared boswellia formulations against placebo (N= 216)41–44. Two trials compared Curcuma domestica extract against Ibuprofen (N= 438)45,46. We found one trial which compared Boswellia serrata extract against Valdecoxib (N=66)47, but since Valdecoxib has been withdrawn from several markets, this RCT was included in our systematic review, but not included in our analysis. We included three RCTs (N= 208) involving curcumin and boswellia combination therapies- two compared against placebo and one compared against Celecoxib40,48–49. Only one of these studies reported pain and functional outcomes in a manner that was appropriate for analysis. Since the other two studies did not report pain as a continuous outcome and did not report an appropriate functional outcome, these two studies were not eligible for analysis. Given the fact that no pooled analyses were possible for these comparisons, the three trials were qualitatively summarized.
Figure 1.
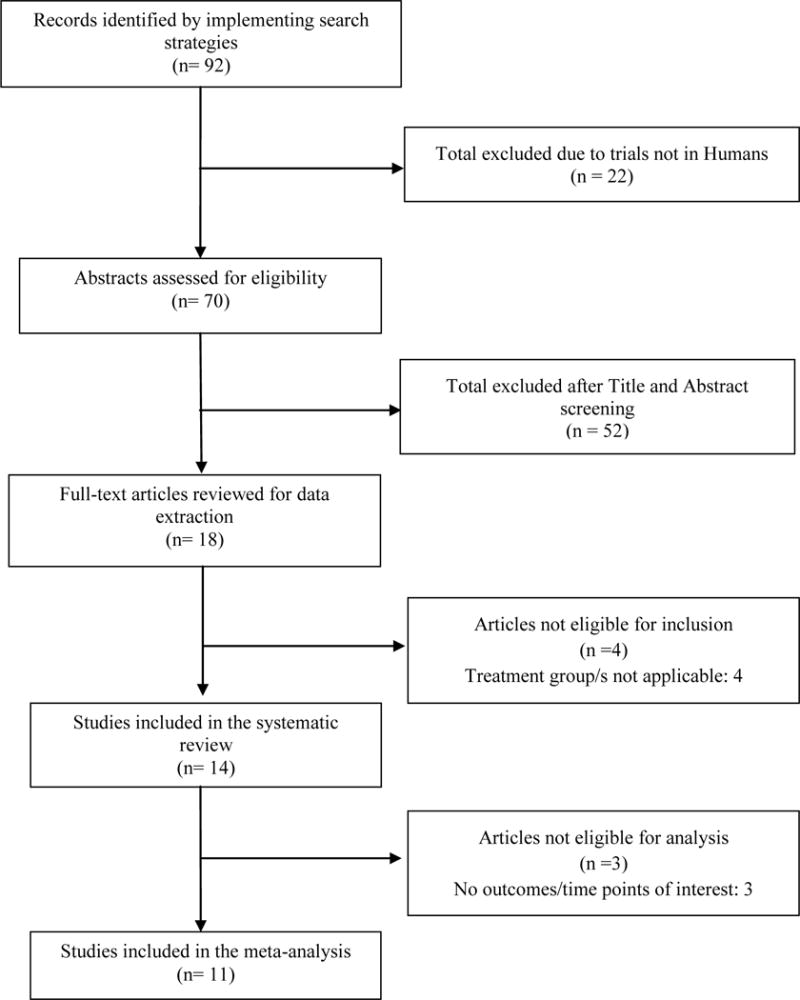
Study Selection Flow Chart
Table 1 describes the study and patient characteristics of the included studies. They were published between 2003 and 2018. The total sample size of included RCTs ranged from 30 to 331 (median: 60). Included trials for all comparisons were generally of short duration, with a range of 4 to 12 weeks (median: 9 weeks). The mean age ranged from 52 to 69 years (median: 57 years), and the percentage of females ranged from 57% to 89% (median: 71%). Mean body mass index (BMI) of patients ranged from 24.9 kg/m2 to 29.2 kg/m2 (median: 26.5 kg/m2).
Table 1.
Characteristics of included studies
| Author, year, location |
Included Analysis | N Patients |
Age (yr) |
% Female |
Intervention | Bioavailability Information, Curcumin |
Comparator | Rescue Analgesia |
Baseline Radiographic Status |
Treatment duration (wk) |
Industry Sponsored? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boswellia vs. Placebo | |||||||||||
| Kimmatkar, et al., 200341 India |
Yes | 30 | 59.0 | 60.0 | Boswellia serrata extract, 333 mg capsule TID | NA | Placebo capsule TID | Current use of NSAIDs and physiotherapy were inclusion criteria | “clinico-radiological osteoarthritis” | 8 | Yes |
| Sengupta, et al., 200842 India |
Yes | 70 | 52.7 | 71.0 |
Boswellia serrata extract
(5-Loxin), 50 mg capsule BID Boswellia serrata extract (5-Loxin), 125 mg capsule BID |
NA | Placebo capsule BID | Eligibility required patients to meet
specific flare criteria upon medication washout. Ibuprofen rescue medication (maximum 1,200 mg/day) was allowed ≥ 3 days prior to examination. |
ND | 12 | Yes |
| Sengupta, et al., 201043 India |
Yes | 57 | 52.4 | 66.7 |
Boswellia serrata extract
(5-Loxin), 50 mg capsule BID Boswellia serrata extract (Aflapin), 50 mg capsule BID |
NA | Placebo capsule BID | Eligibility required patients to meet
specific flare criteria upon medication washout. Ibuprofen rescue medication (maximum 1,200 mg/day) was allowed ≥ 3 days prior to examination. |
ND | 12 | Yes |
| Vishal, et al., 201144 India |
Yes | 59 | 54.2 | 62.7 | Boswellia serrata extract (Aflapin), 50 mg capsule BID | NA | Placebo capsule BID | Ibuprofen rescue medication (maximum 1,200 mg/day) was allowed ≥ 3 days prior to examination. | ND | 4 | Yes |
| Curcumin vs. Placebo | |||||||||||
| Haroyan, et al., 201840 (Arm 1 and Placebo) Armenia |
Yes | 134 | 55.4 | 93.3 | CuraMed capsule (contains 552–578 mg of BCM-95 as a dry extract, corresponding to 500 mg curcuminoids, and 49–52 mg volatile oil from Curcuma longa L. rhizome, 22–23.4 mg aromatic turmerone) TID | “… studies… have indicated that the relative bioavailability of curcumin from BCM-95 complex is approximately 6.93-fold greater than that of normal curcumin…” | Placebo capsule TID | Washout of NSAIDs and/or turmeric required
1 week prior to randomization Rescue medication use not described/indicated |
Participants with “degenerative hypertrophic knee OA”… Kellgren - Lawrence grades I–III | 12 | Yes |
| Madhu, et al., 201336 India |
Yes | 60 | 56.7 | 56.7 | Curcuma longa extract (NR-INF-02), 500 mg capsule BID | Capsules contained 12.6 % w/w polysaccharides “These polysaccharides have been demonstrated to have good bioavailability after oral administration” | Placebo capsule BID | Acetaminophen rescue medication (2,000–4,000 mg/day) was allowed ≥24 hours before clinical exam | “…tibiofemoral osteophytes of at least 1 mm and Kellgren and Lawrence grade 2 or 3.” | 6 | Not reported |
| Moharam zad, et al., 201139 Iran |
Yes | 67 | ND | ND | Curcumin capsule, 600 mg/day | Not reported | Placebo capsule | ND | “…documented knee osteoarthritis (using knee x-ray).” | 10 | Not reported |
| Nakagawa, et al., 201438 Japan |
Yes | 41 | 68.6 | 78.9 | Highly-bioavailble curcumin (Theracurmin), 180 mg capsule six times/day | Surface-controlled water-dispersible curcumin (Theracurmin) | Placebo capsule six times/day | Oral Celecoxib rescue medication (maximum 200 mg/day) or pain relief patches were permitted | Kellgren Lawrence grade II (80.5%) or III (19.5%) | 8 | Yes |
| Panahi, et al., 201437 Iran |
Yes | 53 | 57.4 | 77.0 | C3 curcuminoid complex, 500 mg capsule TID | Each curcuminoid capsule contained 5 mg Bioperine to enhance oral bioavailability | Placebo capsule TID | Naproxen rescue medication was permitted | “…radio logical criteria defined by the American College of Rheumatology. | 6 | No |
| Curcumin+Boswellia vs. Placebo | |||||||||||
| Badria, et al., 201348 Egypt |
No | 45 | ND | ND | Turmeric+Boswellia carteri extract, 500 mg capsule TID | Turmeric powder; no additional processing | Placebo capsule TID | ND | ND | 12 | Not reported |
| Haroyan, et al., 201840 (Arm 2 and Placebo) Armenia |
Yes | 135 | 57.0 | 94.1 | Curamin capsule (350 mg BCM-95 and 150 mg Boswellia serrata gum resin extract consisting of 75% boswellic acids and 10% 3-O-AKBA) TID | “…studies…have indicated that the relative bioavailability of curcumin from BCM-95 complex is approximately 6.93-fold greater than that of normal curcumin…” | Placebo capsule TID | Washout of NSAIDs and/or turmeric required
1 week prior to randomization Rescue medication use not described/indicated |
Participants with “degene rative hypertrophic knee OA”…Kellgren Lawrence grades I-III | 12 | Yes |
| Boswellia vs. NSAID | |||||||||||
| Sontakke, et al., 200747 India |
No | 66 | ND | ND | Boswellia serrata extract, 333 mg capsule TID | NA | Valdecoxib, 10 mg capsule QD | Ibuprofen 400 mg TID was provided as rescue medication and given until symptomatic improvement | “…diag nosis was based on clinical presentation and X-ray findings and confirmed by the orthopedician.” | 24 | Not reported |
| Curcumin vs. NSAID | |||||||||||
| Kuptniratsaikul, et al., 200945 Thailand |
Yes | 107 | 60.7 | 80.4 | Curcuma domestica extract, 500 mg capsule QID | Not reported | Ibuprofen, 400 mg capsule BID | 1 week washout of NSAIDs and other OA
medication Use of rescue medication was not permitted. |
Evidence of radiographic osteophytes at baseline required | 6 | No |
| Kuptniratsaikul, et al., 201446 Thailand |
Yes | 331 | 60.6 | 89.4 | Curcuma domestica extract, 250 mg capsule six times/day | Not reported | Ibuprofen, 200 mg capsule six times/day | Tramadol rescue medication was allowed, but other medications were not permitted | Kellgren Lawrence grade I (13%), II (40%), III (31%), IV (16%) | 4 | No |
| Kizhakkedath, 201349 India |
No | 28 | 48.5 | 57.0 | Curcuma longa extract + Boswellia serrata extract, 500 mg capsule BID | Curcumin formulation is described as having “enhanced bioavailability.” | Celecoxib, 100 mg capsule BID | ND | “…moderate form of OA, evidenced by the narrowing of the medial joint space. | 12 | No |
N= “number of…”; yr= years; wk= weeks; ND= “No data”; NA= “Not applicable”; TID= three times per day; NSAID= non-steroidal anti-inflammatory drug; BID= twice per day; AKBA= acetyl-11-keto-boswellic acid; QID= four times per day
Table 2 describes the study quality and Figure 2 describes the overall risk of bias distribution with in this evidence base. Overall quality was low due to selection bias, selective reporting bias and sponsorship bias concerns. Of 11 trials included in our analyses, six (55%) reported industry sponsorship or direct industry involvement of one or more investigator(s), three (27%) were not industry funded, and two trials (18%) did not adequately report their funding sources. Due to a limited number of RCTs and an overall low quality of evidence, sensitivity analyses based on study quality were not justified. Similarly, we were unable to conduct sensitivity analyses limiting by rescue analgesia protocols due to insufficient variation between studies.
Table 2.
Risk of Bias Summary
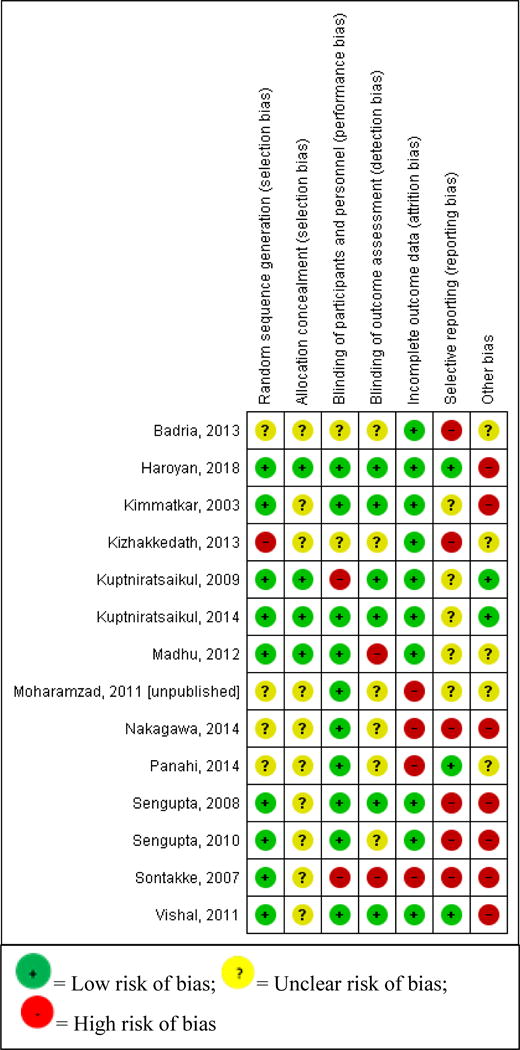
|
Figure 2.
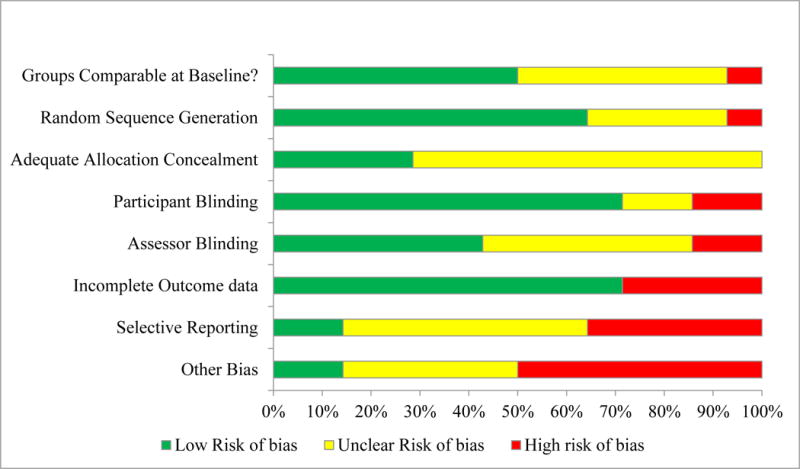
Risk of Bias Distribution Graph
Curcuminoid vs. Placebo
Five trials (N=331) compared curcuminoid formulations against placebo36–40. Two studies reported funding from the drug manufacturer38,40, and another study did not report its funding source but received study medication from the manufacturer36. One study, an unpublished abstract, did not provide sufficient information to ascertain source of funding39. The other study was not funded by a drug manufacturer37.
The analysis revealed a substantial and statistically significant beneficial effect on pain in favor of curcuminoid (Standardized Mean Difference: −0.81 [95% Confidence Interval: −1.25, −0.37]) (Table 3). Since all five RCTs included at least 30 patients in total, sensitivity analyses limited by sample size were not conducted. Four trials reported pain using VAS scales (Table 4)36–39. The effect size for pain as measured by VAS scales alone was much larger (SMD: −1.16 [95% CI: −1.71, −0.62]), favoring curcuminoids. Pooled analysis of two studies that reported pain using the WOMAC scale (N=165)37,40 demonstrated a statistically significant, but noticeably smaller, effect on pain (SMD: −0.47 [95% CI: −0.78, −0.16]). The overall quality of evidence for pain was assessed to be “Very Low” due to high risk of bias, moderate heterogeneity (I2= 71%), and imprecision of the estimate (Table 5a).
Table 3.
Results
| Outcome | N RCTs | N Patients | Effect estimate (95% CI)* |
|---|---|---|---|
| Curcuminoid vs. Placebo | |||
| Pain36–40 | 5 | 331 | SMD −0.81 (−1.25, −0.37), I2= 71% |
| Function37,39,40 | 3 | 232 | SMD −0.48 (−0.74, −0.22), I2= 0% |
| Use of Rescue Medication36–38 | 3 | 141 | RR 0.65 (0.48, 1.05), I2= 74% |
| Serious Adverse Events37,38,40 | 3 | 237 | Due to zero events, an effect estimate was not estimable. |
| Withdrawals due to Adverse Events36–38,40 | 4 | 288 | RR 0.90 (0.21, 3.79), I2= 14% |
| Gastrointestinal Adverse Events36,37,40 | 3 | 247 | RR 2.22 (0.94, 5.26), I2= 0% |
| Boswellia vs. Placebo | |||
| Pain41–44 | 4 | 216 | SMD −2.04 (−2.81, −1.27), I2= 79% |
| Function41–44 | 4 | 216 | SMD −1.52 (−2.24, −0.79), I2= 80% |
| Use of Rescue Medication | ND | ND | ND |
| Serious Adverse Events43,44 | 2 | 120 | Due to zero events, an effect estimate was not estimable. |
| Withdrawals due to Adverse Events41–44 | 4 | 255 | RR 0.75 (0.13, 4.20), I2= 0% |
| Gastrointestinal Adverse Events41,43,44 | 3 | 180 | RR 0.93 (0.17, 5.10), I2= 0% |
| Curcuminoid vs. NSAID | |||
| Pain45,46 | 2 | 422 | SMD −0.05 (−0.41, 0.31), I2= 60% |
| Function46 | 1 | 331 | SMD −0.02 (−0.24, 0.19), I2= NA |
| Time to complete 100 meter walk (seconds)45 | 1 | 91 | SMD −0.30 (−0.71, 0.11), I2= NA |
| Use of Rescue Medication46 | 1 | 367 | RR 2.46 (0.48, 12.52), I2= NA |
| Serious Adverse Events45 | 1 | 100 | Due to zero events, an effect estimate was not estimable. |
| Withdrawals due to Adverse Events45,46 | 2 | 474 | RR 0.22 (0.05, 0.99), I2= 0% |
| Gastrointestinal Adverse Events45,46 | 2 | 467 | RR 0.74 (0.60, 0.91), I2= 0% |
Statistically significant effects are written in bold font. Negative standardized mean differences favor Treatment, and positive standardized mean differences favor Placebo. Risk ratios less than one favor Treatment, and risk ratios greater than one favor Placebo.
RCT= Randomized controlled trial; N= “number of…”; CI= Confidence Interval; SMD= Standardized Mean Difference; I2= measure of heterogeneity, with 100% being the maximum possible heterogeneity; NA= Not applicable; RR= Risk Ratio; ND= No data
Table 4.
Sensitivity Analyses
| Outcome | N RCTs | N patients | Effect estimate (95% CI)* |
|---|---|---|---|
| Curcuminoid vs. Placebo | |||
| WOMAC Pain only37,40 | 2 | 165 | SMD −0.47 (−0.78, −0.16), I2= 0% |
| VAS Pain only36–39 | 4 | 206 | SMD −1.16 (−1.71, −0.62), I2= 69% |
| Pain-Bioavailable curcumin only36–38,40 | 4 | 264 | SMD −0.77 (−1.32, −0.22), I2= 76% |
| Function-Bioavailable curcumin only37,40 | 2 | 165 | SMD −0.50 (−0.93, −0.06), I2= 37% |
| Boswellia vs. Placebo | |||
| WOMAC Pain only42–44 | 3 | 186 | SMD −1.37 (−1.70, −1.04), I2= 0% |
| VAS Pain studies with Total N >3042–44 | 3 | 186 | SMD −1.67 (−2.16, −1.17), I2= 50% |
| Function by any scale in studies with Total N >3042–44 | 3 | 186 | SMD −1.10 (−1.42, −0.78), I2= 0% |
| Curcuminoid vs. NSAID | |||
| WOMAC Pain only46 | 1 | 331 | SMD 0.09 (−0.12, 0.31), I2= NA |
| VAS Pain only45 | 1 | 91 | SMD −0.28 (−0.70, 0.13), I2= NA |
Statistically significant effects are written in bold font. Negative standardized mean differences favor Treatment, and positive standardized mean differences favor NSAID. Risk ratios less than one favor Treatment, and risk ratios greater than one favor NSAID.
RCT= Randomized controlled trial; N= “number of…”; CI= Confidence Interval; SMD= Standardized Mean Difference; I2= measure of heterogeneity, with 100% being the maximum possible heterogeneity; NA= Not applicable; RR= Risk Ratio
Table 5.
GRADE Quality Assessment
| A. Treatment vs. Placebo | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessment | №
of events/№ of patients |
Effect | Quality | Importance | ||||||||
| № of studies |
Study design | Risk of bias |
Inconsistency | Indirectness | Imprecision | Other considerations |
Treatment | Placebo | Relative (95 %CI) |
Absolute (95% CI) |
||
| Pain - Curcuminoid vs. Placebo (Lower scores indicate better Pain outcome) (follow up: range 6 weeks to 12 weeks) | ||||||||||||
| 5 | randomized trials | very seriousa | seriousb | not serious | seriousc | none | N=161 | N=170 | SMD 0.81 lower (1.25 lower to 0.37 lower) | ⊕○○○ VERY LOW | CRITICAL | |
| Function - Curcuminoid vs. Placebo (Lower scores indicate better Functional outcome) (follow up: range 6 weeks to 12 weeks) | ||||||||||||
| 3 | randomized trials | very seriousa | not serious | not serious | seriousc | none | N=114 | N=118 | SMD 0.48 lower (0.74 lower to 0.22 lower) | ⊕○○○ VERY LOW | CRITICAL | |
| Use of Rescue Medication - Curcuminoid vs. Placebo (Risk ratios less than one favor Curcuminoid) (follow up: range 6 weeks to 8 weeks) | ||||||||||||
| 3 | randomized trials | very seriousa | seriousd | not serious | seriouse | none | 35/67 (52.2%) | 60/74 (81.1%) | RR 0.65 (0.40 to 1.05) | 284 fewer per 1,000 (from 41 more to 486 fewer) | ⊕○○○ VERY LOW | IMPORTANT |
| Withdrawals due to Adverse Events - Curcuminoid vs. Placebo (Risk ratios less than one favor Curcuminoid) (follow up: range 6 weeks to 12 weeks) | ||||||||||||
| 4 | randomized trials | very seriousa | not serious | not serious | seriouse | none | 4/141 (2.8%) | 6/147 (4.1%) | RR 0.90 (0.21 to 3.79) | 4 fewer per 1,000 (from 32 fewer to 114 more) | ⊕○○○ VERY LOW | CRITICAL |
| Serious Adverse Events - Curcuminoid vs. Placebo (Risk ratios less than one favor Curcuminoid) (follow up: range 6 weeks to 12 weeks) | ||||||||||||
| 3 | randomized trials | not serious | not serious | not serious | not serious | none | 0/118 (0.0%) | 0/119 (0.0%) | Due to zero events, an absolute risk reduction was not estimable. | ⊕⊕⊕⊕ HIGH | CRITICAL | |
| Gastrointestinal Adverse Events - Curcuminoid vs. Placebo (Risk ratios less than one favor Curcuminoid) (follow up: range 6 weeks to 12 weeks) | ||||||||||||
| 3 | randomized trials | very serious a | not serious | not serious | seriouse | none | 15/123 (12.2%) | 6/124 (4.8%) | RR 2.22 (0.94 to 5.26) | 59 more per 1,000 (from 3 fewer to 206 more) | ⊕○○○ VERY LOW | CRITICAL |
| Pain - Boswellia vs. Placebo (Lower scores indicate better Pain outcome) (follow up: range 4 weeks to 12 weeks) | ||||||||||||
| 4 | randomized trials | very seriousf | very seriousg | not serious | very serioush | none | N=130 | N=86 | SMD 2.04 lower (2.81 lower to 1.27 lower) | ⊕○○○ VERY LOW | CRITICAL | |
| Function - Boswellia vs. Placebo (Lower scores indicate better Functional outcome) (follow up: range 4 weeks to 12 weeks) | ||||||||||||
| 4 | randomized trials | very serious f | very serious i | not serious | very serioush | none | N=130 | N=86 | SMD 1.52 lower (2.24 lower to 0.79 lower) | ⊕○○○ VERY LOW | CRITICAL | |
| Withdrawals due to Adverse Events - Boswellia vs. Placebo (Risk ratios less than one favor Boswellia) (follow up: range 4 weeks to 12 weeks) | ||||||||||||
| 4 | randomized trials | not serious | not serious | not serious | seriouse | none | 3/150 (2.0%) | 2/105 (1.9 %) | RR 0.75 (0.13 to 4.20) | 5 fewer per 1,000 (from 17 fewer to 61 more) | ⊕⊕⊕○ MODE RATE | CRITICAL |
| Serious Adverse Events - Boswellia vs. Placebo (Risk ratios less than one favor Boswellia) (follow up: range 4 weeks to 12 weeks) | ||||||||||||
| 2 | randomized trials | not serious | not serious | not serious | not applicable | none | 0/70 (0.0%) | 0/50 (0.0%) | Due to zero events, an absolute risk reduction was not estimable. | ⊕⊕⊕⊕ HIGH | CRITICAL | |
| Gastrointestinal Adverse Events - Boswellia vs. Placebo (Risk ratios less than one favor Boswellia) (follow up: range 4 weeks to 12 weeks) | ||||||||||||
| 3 | randomized trials | not serious | not serious | not serious | seriouse | none | 3/100 (3.0%) | 2/80 (2.5%) | RR 0.93 (0.17 to 5.10) | 2 fewer per 1,000 (from 21 fewer to 103 more) | ⊕⊕⊕○ MODE RATE | CRITICAL |
| B. Treatment vs. NSAID | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessment | № of events/№ of patients | Effect | Quality | Importance | ||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Treatment | NSAID | Relative (95% CI) | Absolute (95% CI) | ||
| Pain - Curcuminoid vs. NSAID (Lower scores indicate better Pain outcome) (follow up: range 4 weeks to 6 weeks) | ||||||||||||
| 2 | randomized trials | seriousa | seriousb | not serious | seriousc | none | N=216 | N=206 | SMD 0.05 lower (0.41 lower to 0.31 higher) | ⊕○○○ VERY LOW | CRITICAL | |
| Thai WOMAC Function [0–10] - Curcuminoid vs. NSAID (Lower scores indicate better Functional outcome) (follow up: 4 weeks) | ||||||||||||
| 1 | randomized trials | not serious | not assessable | not serious | seriousc | none | N=171 | N=160 | SMD 0.02 lower (0.24 lower to 0.19 higher) | ⊕⊕⊕○ MODE RATE | CRITICAL | |
| Time to complete 100 m walk [sec] - Curcuminoid vs. NSAID (Lower scores indicate better outcome) (follow up: 6 weeks) | ||||||||||||
| 1 | randomized trials | seriousa | not assessable | not serious | very seriousd,e | none | N=45 | N=46 | SMD 0.3 lower (0.71 lower to 0.11 higher) | ⊕○○○ VERY LOW | IMPORTANT | |
| Use of Rescue Medication - Curcuminoid vs. NSAID (Risk ratios less than one favor Curcuminoid) (follow up: 4 weeks) | ||||||||||||
| 1 | randomized trials | seriousa | not assessable | not serious | seriousf | none | 5/185 (2.7%) | 2/182 (1.1%) | RR 2.46 (0.48 to 12.5 2) | 16 more per 1,000 (from 6 fewer to 127 more) | ⊕⊕○○ LOW | IMPORTANT |
| Withdrawals due to Adverse Events - Curcuminoid vs. NSAID (Risk ratios less than one favor Curcuminoid) (follow up: range 4 weeks to 6 weeks) | ||||||||||||
| 2 | randomized trials | seriousa | not serious | not serious | not serious | none | 2/237 (0.8%) | 10/2 37 (4.2 %) | RR 0.22 (0.05 to 0.99) | 33 fewer per 1,000 (from 0 fewer to 40 fewer) | ⊕⊕⊕○ MODE RATE | CRITICAL |
| Serious Adverse Events - Curcuminoid vs. NSAID (Risk ratios less than one favor Curcuminoid) (follow up: 6 weeks) | ||||||||||||
| 1 | randomized trials | not serious | not assessable | not serious | not applicablee | none | 0/48 (0.0%) | 0/52 (0.0 %) | Due to zero events, an absolute risk reduction was not estimable. | ⊕⊕⊕○ MODE RATE | CRITICAL | |
| Gastrointestinal Adverse Events - Curcuminoid vs. NSAID (Risk ratios less than one favor Curcuminoid) (follow up: range 4 weeks to 6 weeks) | ||||||||||||
| 2 | randomized trials | seriousa | not serious | not serious | not serious | none | 87/233 (37.3%) | 118/234 (50.4%) | RR 0.74 (0.60 to 0.91) | 131 fewer per 1,000 (from 45 fewer to 202 fewer) | ⊕⊕⊕○ MODE RATE | CRITICAL |
CI: Confidence interval; SMD: Standardized mean difference; RR: Risk ratio
All trials received at least one High Risk of Bias rating, and >50% of trials received ≥3 Unclear Risk of Bias ratings outside of the “Other” dimension. With particular reference to Use of Rescue Medication and Withdrawal due to Adverse Events outcomes, two studies were marked down for potential attrition bias, and one study provided no data on study discontinuation.
I2= 71%; moderate heterogeneity.
95% Confidence Interval of an SMD extends between >0.2-≤0.5 points in either direction (Cohen 1988*)
I2= 74%; moderate heterogeneity
95% Confidence Interval of a Risk Ratio crosses null.
Three of four studies received at least one High risk of bias rating due to very small sample size and/or potential reporting bias.
I2= 79%
95% Confidence Interval of an SMD extends between >0.5 points in either direction (Cohen 1988*)
I2= 80%
One study received a High risk of bias rating due to potentially inadequate blinding.
I2= 60%; moderate heterogeneity.
95% Confidence Interval of an SMD extends between >0.2-≤0.5 points in either direction (Cohen 1988*)
95% Confidence Interval of an SMD extends between >0.5 points in either direction (Cohen 1988*)
Sample size in each study arm <50.
95% Confidence Interval of a Risk Ratio crosses null.
Sample size in one study arm <50.
The effect of curcuminoids on function (3 RCTs, N= 232) was statistically significantly better than placebo (SMD: −0.48 [95% CI: −0.74, −0.22]; I2= 0%) (Table 3). The overall quality of evidence for function was assessed to be “Very Low” due to high risk of bias and imprecision of the estimate (Table 5a).
Subgroup analyses analyzing only RCTs that explicitly involved curcuminoid formulations with some manner of enhanced bioavailability produced similar results to analyses of all included studies with regard to pain and functional outcomes (Table 4).
Three RCTs (N= 141) reported on the use of rescue medication (Table 3). Patients receiving curcuminoids were less likely to use rescue medication than patients who received placebo, but the difference was not significant (Risk Ratio: 0.65 [95% CI: 0.48, 1.05]). Three RCTs (N= 237) reported on the incidence of serious adverse events; no serious adverse event was reported in any trial. There was no difference between patients receiving curcuminoids and those receiving placebo with regard to incidence of treatment withdrawal due to adverse events (RR: 0.90 [95% CI: 0.21, 3.79]). Patients receiving curcuminoids showed a higher, but non-significant risk of experiencing gastrointestinal (GI) adverse events than patients receiving placebo (RR: 2.22 [95% CI: 0.94, 5.26]).
Boswellia vs. Placebo
Four RCTs (N=216) compared boswellia formulations against placebo41–44. Three studies were funded by the drug manufacturers42–44, and the other included a co-author who was employed by the drug manufacturer41.
Patients receiving boswellia formulations had a large reduction in pain compared to those receiving placebo which was statistically significant (SMD: −2.04 [95% CI: −2.81, −1.27]) (Table 3). Sensitivity analyses restricted by sample size larger than 30 (3 RCTs, N= 186) showed similar results, with a slightly smaller effect size (SMD: −1.67 [95% CI: −2.16, −1.17]) (Table 4). Since all four trials reported pain using VAS in tabular format, sensitivity analyses restricting by VAS scale were not conducted. Three trials also reported the WOMAC pain subscale, and once again, boswellia performed statistically significantly better than placebo (SMD: −1.37 [95% CI: −1.70, −1.04]). The overall quality of evidence for pain was assessed to be “Low” due to high risk of bias and high heterogeneity (I2= 79%) (Table 5a).
Four RCTs (N= 216) compared the effects of boswellia formulations versus placebo on functional outcomes and reported statistically significant beneficial effects of boswellia (SMD: −1.52 [95% CI: −2.24, −0.79]) (Table 3). Sensitivity analyses restricting by sample size (3 RCTs, N=186) also showed a statistically significant benefit on functional outcomes (SMD: −1.10 [95% CI: −1.42, −0.78]; I2= 0%) (Table 4). The overall quality of evidence for function was also assessed to be “Low” due to high risk of bias and high heterogeneity (I2= 80%) (Table 5a).
Use of rescue medication was not adequately reported by any of the four trials. Two trials reported incidence of serious adverse events (N= 120); none of the patients in either group reported any serious adverse event in either trial (Table 3). Participants receiving boswellia formulations were slightly less likely to withdraw due to adverse events (4 RCTs, N= 255), but the difference was not statistically significant (RR: 0.75 [95% CI: 0.13, 4.20]). The risk of gastrointestinal adverse events (3 RCTs, N=180) was the same for patients receiving boswellia formulations or placebo (RR: 0.93 [95% CI: 0.17, 5.10]).
Curcuminoid vs. NSAID
Two RCTs compared Curcuma domestica extract against Ibuprofen (N= 422)45,46. Neither study was industry funded. Reported benefits on pain were no different between patients receiving Curcuma longa extract or Ibuprofen (SMD: −0.05 [95% CI: −0.41, 0.31]) (Table 3). Since both trials included over thirty participants, sensitivity analyses limiting by sample size were not conducted. Sensitivity analyses produced slightly different results based on pain outcome, though only one trial reported each respective scale (Table 4). The effects of curcuminoid on pain were not significantly better than NSAIDs using either scale. The overall quality of evidence contributing to the analysis of pain outcomes was rated “Very Low” due to high risk of bias, moderate heterogeneity (I2= 60%), and imprecision (Table 5b).
Only one RCT contributed to the analysis of function (N= 331); there was no significant difference between Curcuma longa extract and Ibuprofen with regard to functional benefit (SMD: −0.02 [95% CI: −0.24, 0.19]) (Table 3). The overall quality assessment for this outcome was “Moderate”, as it received one downgrade for imprecision (Table 5b).
Patients receiving curcuminoid were more likely to use rescue medication within the course of the study period (1 RCT, N=367), but the relative risk was not statistically significant (RR: 2.46 [95% CI: 0.48, 12.52]) (Table 3). One trial reported on serious adverse events (N= 100), and none of the patients in either group reported any serious adverse event. Withdrawals due to adverse events (2 RCTs, N= 474) were statistically significantly less likely in the Curcuma longa extract group versus the Ibuprofen group (RR: 0.22 [95% CI: 0.05, 0.99]). Patients receiving curcuminoid were also statistically significantly less likely to experience any GI adverse event (2 RCTs, N= 467) during the course of treatment (RR: 0.74 [95% CI: 0.60, 0.91]).
Curcuminoid + Boswellia vs. Placebo or NSAID
Two RCTs (N= 180) compared the clinical effects of treatment with curcuminoid and boswellia formulations in combination versus placebo40,48. One RCT involving “Turmeric” (specific curcuminoid formulation not indicated) in combination with Boswellia carteri extract reported highly significant decreases in pain on active movement (p<0.001) and on passive movement (p<0.001) in the treatment group after 3 months, with no significant changes occurring in the placebo group48. This study did not collect safety data. The other study (N= 135) was a three-armed trial that compared CuraMed curcumin capsules and Curamin curcumin and boswellia combination capsules against a matching placebo. The CuraMed results are included in the pooled efficacy analyses for curcuminoids versus placebo, where safety data are also provided (Tables 3 & 4). A significant improvement in pain was noted in Curamin (boswellia and curcuminoid combination) group versus placebo over 12 weeks (p<0.05), but Curamin did not demonstrate statistically significant benefits on functional outcomes compared to placebo. Only two patients in the Curamin group (3%) experienced adverse events, both of which were gastrointestinal in nature; 10.6% of CuraMed patients and 5.9% of Placebo patients experienced at least one adverse event. Only one RCT (N= 28) compared a combination of Curcuma longa extract and Boswellia serrata extract against Celecoxib for 12 weeks49. The authors reported significant improvement in pain scores in both groups over the course of 12 weeks of treatment, but there was not a statistically significant difference between the two groups. No adverse events were reported by either study group.
Discussion
The results of our meta-analysis indicate that both curcuminoid and boswellia formulations administered as mono-therapy are significantly more effective than placebo in relieving the symptoms of knee OA, and that they do not pose significant safety risks. Our results also suggest that curcuminoid formulations have comparable efficacy profiles to NSAID treatments, with significantly fewer adverse events.
Our results are concordant with a recent meta-analysis assessing the efficacy of curcuminoid products conducted by Onakpoya, et al., in the sense that we found significant benefits of curcuminoids on pain and function23. However, our risk of bias assessments, included studies, and, most notably, the magnitudes of our effect sizes were different. The majority of these differences arose from our use of strict inclusion criteria, standardized and validated data extraction and risk of bias assessment methods, and analysis techniques. For example, they included a study which compared curcuminoid and NSAID combination therapy against NSAIDs as part of their Curcumin vs. placebo analysis, whereas we excluded it due to the standardized use of NSAIDs in both treatment groups. Though the strictness of inclusion criteria are subjective, the trial’s exclusion from our study undoubtedly led to differences in our results50. The key difference between our two meta-analyses, and the most obvious contributing factor to the large differences in effect size magnitudes, is the difference in standard deviations (SDs) extracted from the included RCTs. In the majority of occasions, we were unable to replicate the SDs they have extracted or confirm from where the values were obtained36,37,39. In our analyses comparing Curcumin to Ibuprofen, we were able to collect and combine the data on pain and functional outcomes from the 2009 and 2014 studies by Kuptniratsaikul, whereas Onakpoya did not collect pain from either study. Despite the fact that our study focused on patients with knee OA only, our results were also concordant with two other meta-analyses assessing the efficacy of curcuminoid formulations against placebo in mixed groups of patients, in that curcuminoids demonstrated significant benefits on pain and no significant safety concerns51,52.
We also compared our study against a Cochrane review24. Our analyses of boswellia consisted of the same references, with the exception of a study by Sontakke, et al., which compared Boswellia serrata extract against Valdecoxib. We excluded it because Valdecoxib has been removed from the market and is not FDA approved. This Cochrane review did not reference any of the trials we included which compared curcuminoid against placebo; for the majority of these trials, the likely reason for this is due to publication status39 or because the publication date extended beyond their search date37,38,40. The main methodological difference between the Cochrane review and our own is that their team chose to separately analyze different doses, formulations, and time points, as well as analyzing only like scales as mean differences. As a result, many of the effect sizes reported for these formulations represent the results of single studies.
The most recently published meta-analysis of curcuminoid and boswellia products reported “large and clinically important effects for pain reduction” at short-term follow-up times25. Though our results were similar, the effect sizes reported in our study differed from those reported by Liu, et al. because we included a larger number of studies in each analysis. In addition, our assessments of study quality for trials evaluating the effectiveness of boswellia formulations versus placebo were stricter than theirs, and our GRADE quality assessments were similarly harsher, in general.
The generalizability of our results may be limited by the quality, sample size, and duration of the available RCT evidence. The RCTs which comprised our analyses were moderately to highly heterogeneous. Heterogeneity between studies can arise from a number of sources, including differences in treatment protocol, population, and/or the analytic methods used in each study. One source of heterogeneity in our analyses comparing curcuminoid or boswellia formulations to placebo could be differences in treatment formulations used. In the comparison of Curcuma domestica extract against Ibuprofen, for which treatment formulation differences were not applicable, differences in rescue medication protocols could have introduced heterogeneity, with one trial requiring NSAID washout prior to study onset45 and another trial allowing for use of tramadol as rescue medication46. Unfortunately, we are unable to definitively identify or confirm causative factors of heterogeneity.
We could not rule out potential small study effect bias, as the majority (79%) of included RCTs randomized less than 100 participants36–39,41–44,47–49. A meta-epidemiological study found that smaller trials, particularly those involving complementary medical interventions presented effect sizes that were 50% larger than that of a typical effect found for OA interventions53. Since all studies which compared either curcuminoid or boswellia against a placebo were relatively small, we were only able to conduct a sensitivity analysis which excluded exceptionally small studies (N≤ 30), and found an 18% reduction in the effect size magnitude in estimates of pain and functional improvement in studies comparing boswellia against placebo when these studies were removed (Table 4). We could not assess the effects of exceptionally small studies for placebo-controlled trials of curcuminoid formulations, because all of the included trials randomized over 30 participants. We did not assess small study effects for Curcumin vs. NSAIDs comparison because both studies involving this comparison randomized over 100 participants. In addition to small sample size, only 43% of included studies were of duration of 12 weeks or longer (Table 1). Since knee osteoarthritis is a chronic disease, longer term studies are desirable to assess the efficacy of curcuminoid and boswellia formulations on lasting symptom relief.
We observed a lack of treatment response in the placebo groups of many of the included placebo-controlled trials, which was interesting in light of the fact that most participants in all trials were experienced NSAID users, and that the majority of studies allowed for use of rescue medication (Figure 3). Even in the absence of rescue medication use, modest treatment benefits are expected to occur for patients receiving placebo treatments as a result of regression to the mean, and also in accordance with the natural disease progression of knee OA54. With longer term use of curcuminoid or boswellia formulations, it is possible that the disparities in treatment effects we observed may dissipate. Trials of such short duration may also be unable to adequately measure the impact of treatments on patient safety. The gastrointestinal safety profile of NSAIDs, for example, has been established by a number of observational studies and randomized trials over the course of years of research in thousands of patients. Unlike the established safety profile of NSAIDs, the data we have presented on the comparative safety of curcuminoids versus NSAIDs may not be generalizable to the knee OA population, because it is based on data from two RCTs of 4 and 6 weeks in length, respectively, which were conducted by the same study team and assessed a total of only 467 patients. Likewise, longer term research in a larger number of patients is needed to assess the relative safety of curcuminoid and boswellia formulations against placebo.
Figure 3.
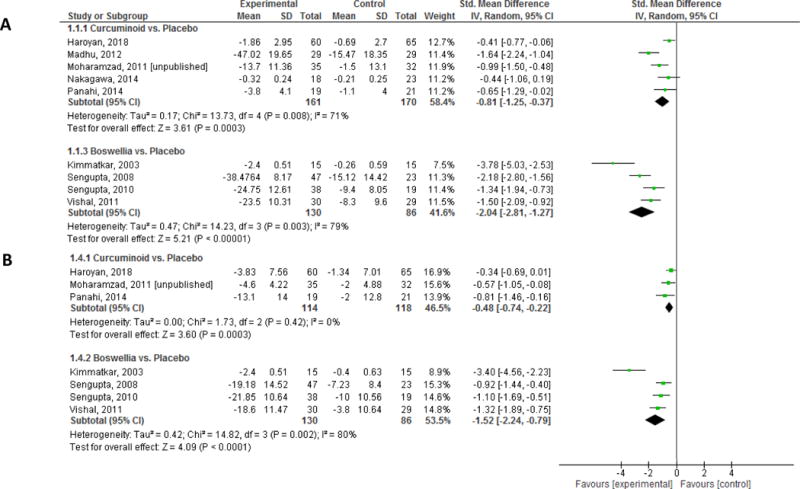
Forest Plot of the effects of Treatment vs. Placebo on: A) Pain and B) Function
Our study was also limited by the small number of randomized trials assessing the efficacy and safety of curcuminoid and boswellia formulations. Potential publication bias could have had a considerable impact on the number of overwhelmingly positive studies we encountered in the small body of evidence we accrued, but we lacked sufficient power to objectively assess publication bias.
Our results also suggest that curcuminoids may be as effective as NSAIDs, while posing significantly less safety risk. These results are promising, in the sense that herbal formulations using phytochemicals may provide respite for OA patients from long-term NSAID use and its well-established safety risks. It is worth noting that a follow-up study of one of the RCTs included in our analysis was conducted to assess whether 6 weeks of treatment with an oral curcuminoid formulation would have a significant effect on serum levels of inflammatory biomarkers in knee OA patients compared to placebo, given the fact that these patients experienced significant improvements in clinical symptoms during the trial period37,55. The authors found no significant differences between the treatment groups with regard to changes in serum levels of IL-4, IL-6, tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), high-sensitivity C-reactive protein (hs-CRP), or erythrocyte sedimentation rate (ESR), and concluded that the clinical improvements observed in participants of their study could not be ascribed to the anti-inflammatory effects of the curcuminoid treatment55. Larger, higher quality randomized trials with a longer duration of follow-up are needed to fully evaluate safety and confirm effectiveness of these treatments as stand-alone therapeutic options, and to further explore the mechanisms of action of such phytochemicals with these properties.
In contrast to our study, some studies have focused on exploring the value of curcuminoids or boswellia as adjuvant therapies. A study conducted in mice compared the anti-nociceptive activity of NSAIDs (nimesulide, naproxen, or rofecoxib) against acetyl 11-keto-beta boswellic acid (AKBA), and compared each of these drugs in combination with AKBA against the others56. The authors reported evidence of an enhanced anti-nociceptive effect when AKBA was combined with NSAIDs, and concluded that boswellic acid could help in reducing therapeutic doses of NSAIDs, leading to a reduction in side effects. Two RCTs conducted in humans assessed the effectiveness of Curcuma longa extract as an adjuvant therapy with NSAIDs50,57. One trial reported that combination treatment with Curcuma longa extract and Diclofenac showed similar benefits in pain and functional outcomes to Diclofenac, but no statistically significant differences57. A higher quality, more recent study, conducted in a larger number of patients, found statistically significant benefits in patients who used combination treatment with Curcuma longa extract and Diclofenac compared to patients using Diclofenac and Placebo50. With more robust research, it may be possible to determine a synergistic effect between curcuminoid or boswellia formulations with other OA treatments, to maximize pain relief and functional benefits while minimizing safety risks.
Conclusions
The results of this meta-analysis suggest that curcuminoid and boswellia formulations could be a valuable addition to pharmacological treatment regimens for knee OA by reducing pain and improving function, while reducing the risk of adverse events. The current body of evidence is not adequate in size or quality to make any meaningful clinical practice recommendations. Future research should consist of larger, higher quality RCTs that specifically examine the role of curcuminoid and boswellia formulations as adjuvant treatments for knee OA patients who are dependent upon NSAID treatment and should focus on their potential to reduce these patients’ risk of serious gastrointestinal adverse events.
Acknowledgments
Anbuselvan Dharmarajan assisted in initial screening and collection of data.
Funding source
Supported by: National Center for Complementary and Integrative Health (K23AT009374 and K24AT007323). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
Raveendhara Bannuru made substantial contributions to: conception and design; acquisition of data; analysis and interpretation of the data; statistical expertise; drafting of the article; critical revision of the article for important intellectual content; and final approval of the article.
Fatimah Al-Eid and Mikala Osani made substantial contributions to all of the following: conception and design; acquisition of data; analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; and final approval of the article.
Chenchen Wang made substantial contributions to: conception and design; analysis and interpretation of the data; critical revision of the article for important intellectual content; and final approval of the article.
Competing interests
None.
References
- 1.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33(11):2271–2279. [PubMed] [Google Scholar]
- 2.Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. The Lancet. 2013;382(9894):769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52(18):1502–1517. doi: 10.1016/j.jacc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S3. doi: 10.1186/ar4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis–an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:55. doi: 10.1186/s12916-015-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mobasheri A, Henrotin Y, Biesalski HK, Shakibaei M. Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int J Mol Sci. 2012;13(4):4202–4232. doi: 10.3390/ijms13044202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 9.Aggarwal BB, Prasad S, Reuter S, et al. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr Drug Targets. 2011;12(11):1595–1653. doi: 10.2174/138945011798109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makela JT, Han SK, Herzog W, Korhonen RK. Very early osteoarthritis changes sensitively fluid flow properties of articular cartilage. J Biomech. 2015;48(12):3369–3376. doi: 10.1016/j.jbiomech.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004;172(10):5940–5947. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 13.Takada Y, Ichikawa H, Badmaev V, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J Immunol. 2006;176(5):3127–3140. doi: 10.4049/jimmunol.176.5.3127. [DOI] [PubMed] [Google Scholar]
- 14.Clutterbuck AL, Allaway D, Harris P, Mobasheri A. Curcumin reduces prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in the secretome of interleukin 1beta-treated articular cartilage. F1000Res. 2013;2:147. doi: 10.12688/f1000research.2-147.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2(1):56. doi: 10.1186/2193-1801-2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy GK, Chandrakasan G, Dhar SC. Studies on the metabolism of glycosaminoglycans under the influence of new herbal anti-inflammatory agents. Biochem Pharmacol. 1989;38(20):3527–3534. doi: 10.1016/0006-2952(89)90124-x. [DOI] [PubMed] [Google Scholar]
- 17.Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58(12):899–908. doi: 10.1007/s00011-009-0063-1. [DOI] [PubMed] [Google Scholar]
- 18.Dingle JT. The effect of nonsteroidal antiinflammatory drugs on human articular cartilage glycosaminoglycan synthesis. Osteoarthritis Cartilage. 1999;7(3):313–314. doi: 10.1053/joca.1998.0176. [DOI] [PubMed] [Google Scholar]
- 19.Hauser RA. The acceleration of articular cartilage degeneration in osteoarthritis by nonsteroidal anti-inflammatory drugs. Journal of Prolotherapy. 2010;2(1):305–322. [Google Scholar]
- 20.Hedi H, Norbert G. 5-Lipoxygenase Pathway, Dendritic Cells, and Adaptive Immunity. J Biomed Biotechnol. 2004;2004(2):99–105. doi: 10.1155/S1110724304310041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schweizer S, von Brocke AF, Boden SE, Bayer E, Ammon HP, Safayhi H. Workup-dependent formation of 5-lipoxygenase inhibitory boswellic acid analogues. J Nat Prod. 2000;63(8):1058–1061. doi: 10.1021/np000069k. [DOI] [PubMed] [Google Scholar]
- 22.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11(6):R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onakpoya IJ, Spencer EA, Perera R, Heneghan CJ. Effectiveness of curcuminoids in the treatment of knee osteoarthritis: a systematic review and meta-analysis of randomized clinical trials. Int J Rheum Dis. 2017;20(4):420–433. doi: 10.1111/1756-185X.13069. [DOI] [PubMed] [Google Scholar]
- 24.Cameron M, Chrubasik S. Oral herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2014;(5):Cd002947. doi: 10.1002/14651858.CD002947.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2017 doi: 10.1136/bjsports-2016-097333. [DOI] [PubMed] [Google Scholar]
- 26.The Cochrane Musculoskeletal Group. List of Proposed Outcomes. http://musculoskeletal.cochrane.org/proposed-outcomes.
- 27.Markum Mitchell. Engauge Digitizer. https://github.com/markummitchell/engauge-digitizer.
- 28.The Cochrane Collaboration. RevMan 5.3. http://tech.cochrane.org/revman.
- 29.The Cochrane Collaboration. The Cochrane Risk of Bias Tool. http://handbook.cochrane.org.
- 30.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool [Software] McMaster University; 2015. https://gradepro.org. [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences 2nd edn. Erlbaum Associates; Hillsdale: 1988. [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madhu K, Chanda K, Saji MJ. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology. 2013;21(2):129–136. doi: 10.1007/s10787-012-0163-3. [DOI] [PubMed] [Google Scholar]
- 37.Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res. 2014;28(11):1625–1631. doi: 10.1002/ptr.5174. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa Y, Mukai S, Yamada S, et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19(6):933–939. doi: 10.1007/s00776-014-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moharamzad Y, Panahi Y, Rahimnia A, Beiraghdar F. Clinical Efficacy of Curcumin in Knee Osteoarthritis: A Double-Blind Randomized Clinical Trial. Baqiyatallah Medical Sciences University; 2011. [Google Scholar]
- 40.Haroyan A, Mukuchyan V, Mkrtchyan N, et al. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study. BMC Complement Altern Med. 2018;18(1):7. doi: 10.1186/s12906-017-2062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee–a randomized double blind placebo controlled trial. Phytomedicine. 2003;10(1):3–7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- 42.Sengupta K, Alluri KV, Satish AR, et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4):R85. doi: 10.1186/ar2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengupta K, Krishnaraju AV, Vishal AA, et al. Comparative efficacy and tolerability of 5-Loxin and AflapinAgainst osteoarthritis of the knee: a double blind, randomized, placebo controlled clinical study. Int J Med Sci. 2010;7(6):366–377. doi: 10.7150/ijms.7.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vishal AA, Mishra A, Raychaudhuri SP. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee. Int J Med Sci. 2011;8(7):615–622. doi: 10.7150/ijms.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuptniratsaikul V, Thanakhumtorn S, Chinswangwatanakul P, Wattanamongkonsil L, Thamlikitkul V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med. 2009;15(8):891–897. doi: 10.1089/acm.2008.0186. [DOI] [PubMed] [Google Scholar]
- 46.Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451–458. doi: 10.2147/CIA.S58535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sontakke S, Thawani V, Pimpalkhute S, Kabra P, Babhulkar S, Hingorani L. Open, randomized, controlled clinical trial of Boswellia serrata extract as compared to valdecoxib in osteoarthritis of knee. Indian Journal of Pharmacology. 2007;39(1):27. [Google Scholar]
- 48.Badria FA, El-Farahaty T, Shabana AA, Hawas SA, El-Batoty MF. Boswellia–curcumin preparation for treating knee osteoarthritis: a clinical evaluation. Alternative & Complementary Therapies. 2002;8(6):341–348. [Google Scholar]
- 49.Kizhakkedath R. Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol Med Rep. 2013;8(5):1542–1548. doi: 10.3892/mmr.2013.1661. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology. 2016;24(6):377–388. doi: 10.1007/s10787-016-0289-9. [DOI] [PubMed] [Google Scholar]
- 51.Sahebkar A, Henrotin Y. Analgesic Efficacy and Safety of Curcuminoids in Clinical Practice: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Med. 2016;17(6):1192–1202. doi: 10.1093/pm/pnv024. [DOI] [PubMed] [Google Scholar]
- 52.Daily JW, Yang M, Park S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Med Food. 2016;19(8):717–729. doi: 10.1089/jmf.2016.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nuesch E, Trelle S, Reichenbach S, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. Bmj. 2010;341:c3515. doi: 10.1136/bmj.c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bannuru RR, McAlindon TE, Sullivan MC, Wong JB, Kent DM, Schmid CH. Effectiveness and Implications of Alternative Placebo Treatments: A Systematic Review and Network Meta-analysis of Osteoarthritis Trials. Ann Intern Med. 2015;163(5):365–372. doi: 10.7326/M15-0623. [DOI] [PubMed] [Google Scholar]
- 55.Rahimnia AR, Panahi Y, Alishiri G, Sharafi M, Sahebkar A. Impact of Supplementation with Curcuminoids on Systemic Inflammation in Patients with Knee Osteoarthritis: Findings from a Randomized Double-Blind Placebo-Controlled Trial. Drug Res (Stuttg) 2015;65(10):521–525. doi: 10.1055/s-0034-1384536. [DOI] [PubMed] [Google Scholar]
- 56.Bishnoi M, Patil CS, Kumar A, Kulkarni SK. Potentiation of antinociceptive effect of NSAIDs by a specific lipooxygenase inhibitor, acetyl 11-keto-beta boswellic acid. Indian J Exp Biol. 2006;44(2):128–132. [PubMed] [Google Scholar]
- 57.Pinsornsak P, Niempoog S. The efficacy of Curcuma Longa L. extract as an adjuvant therapy in primary knee osteoarthritis: a randomized control trial. J Med Assoc Thai. 2012;95(Suppl 1):S51–58. [PubMed] [Google Scholar]


