Abstract
Runx2 and Sp7 are essential transcription factors for osteoblast differentiation. However, the molecular mechanisms responsible for the proliferation of osteoblast progenitors remain unclear. The early onset of Runx2 expression caused limb defects through the Fgfr1–3 regulation by Runx2. To investigate the physiological role of Runx2 in the regulation of Fgfr1–3, we compared osteoblast progenitors in Sp7−/− and Runx2−/− mice. Osteoblast progenitors accumulated and actively proliferated in calvariae and mandibles of Sp7−/− but not of Runx2−/− mice, and the number of osteoblast progenitors and their proliferation were dependent on the gene dosage of Runx2 in Sp7−/− background. The expression of Fgfr2 and Fgfr3, which were responsible for the proliferation of osteoblast progenitors, was severely reduced in Runx2−/− but not in Sp7−/− calvariae. Runx2 directly regulated Fgfr2 and Fgfr3, increased the proliferation of osteoblast progenitors, and augmented the FGF2-induced proliferation. The proliferation of Sp7−/− osteoblast progenitors was enhanced and strongly augmented by FGF2, and Runx2 knockdown reduced the FGF2-induced proliferation. Fgfr inhibitor AZD4547 abrogated all of the enhanced proliferation. These results indicate that Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation, at least partly, by regulating Fgfr2 and Fgfr3 expression.
Introduction
Osteoblast differentiation is regulated by Runx2, Sp7, and canonical Wnt signaling1. Since Runx2 is expressed in Sp7-deficient (Sp7−/−) mice and Ctnnb1 conditional knockout mice, Runx2 is the furthest upstream transcription factor in the regulation of osteoblast differentiation, and Runx2, Sp7, and canonical Wnt signaling are mutually regulated and maintain their expression1,2. Both osteoblast differentiation and the expansion of osteoblast progenitors are essential for bone development and bone regeneration. Although the mechanism for osteoblast differentiation has been well studied, the mechanism for the proliferation of osteoblast progenitors remains to be clarified1.
Many in vitro studies of Runx2 in the proliferation of osteoblastic cells has been reported. The proliferation of Runx2−/− calvarial cells was greater than that of wild-type calvarial cells; Runx2 induces G1 cell-cycle arrest through the induction of p27KIP1 in osteosarcoma cells; Runx2 expression is up-regulated in the cessation of cell proliferation and down-regulated to minimal levels during the early S phase and mitosis in MC3T3-E1 preosteoblastic cells; Runx2 suppresses the proliferation of cells with osteogenic potential and osteosarcoma cells, and the introduction of siRNA against Runx2 into human mesenchymal stem cells increases proliferation3–7. Thus, all previous reports show that Runx2 inhibits the proliferation of osteoblastic cells in vitro.
Four fibroblast growth factor receptor (Fgfr) genes have been identified in mammals (Fgfr1 to Fgfr4). The affinity and specificity of Fgfr1–3 are regulated by tissue-specific alternative splicing, which occurs in the region encoding the carboxyl-terminal half of Ig domain III creating the different isoforms, IIIb and IIIc8. Fgf2, Fgf4, Fgf9, Fgf18, Fgfr1, Fgfr2, and Fgfr3 are expressed in mesenchymal cells in the calvaria9–16. The importance of FGF signaling in human skull development has been revealed by the identification of gain-of-function mutations in the FGFR1, FGFR2, and FGFR3 genes in a number of craniosynostosis syndromes, such as Apert, Crouzon, Pfeiffer, and Muenke syndromes and Thanatophoric dysplasia17. Fgf10, Fgfr1c, and Fgfr2c, which are expressed in mesenchyme, and Fgf8, Fgf4, and Fgfr2b, which are expressed in ectoderm, form an epithelial-mesenchymal interaction loop during the proximodistal and anteroposterior patterning of the limb bud18,19, and craniosynostosis is accompanied by limb defects in Apert and Pfeiffer syndromes17.
FGF signals trigger a number of responses in target cells, including stemness, proliferation, anti-apoptosis, drug resistance, angiogenesis, epithelial-to-mesenchymal transition, and invasion, through RAS-MAPK, PI3K-AKT, PLCγ and DAG, and PLCγ and IP3. Furthermore, the FGF signaling pathway has crosstalk with the canonical Wnt signaling cascade. In cell proliferation, FGF signaling plays important roles through RAS-MAPK, PI3K-AKT, and canonical Wnt signaling20,21. Genomic alterations in FGFRs are associated with various cancers, including breast cancer, lung cancer, gastric cancer, multiple myeloma, myeloproliferative syndrome, rhabdomyosarcoma, peripheral T-cell lymphoma, uterine tumors, and bladder tumors20,21.
We previously reported that Runx2 transgenic mice under the control of the Prrx1 promoter, which directs transgene expression to the limb bud mesenchyme and cranial mesenchyme from embryonic day (E) 9.522, exhibit craniosynostosis, ectopic bone formation, and limb defects23. Since FGF signaling plays an important role in limb development, we examined the involvement of Runx2 in the FGF signaling pathway in this study. Runx2 directly regulated the Fgfr1, Fgfr2, and Fgfr3 genes. In order to elucidate the physiological role of Runx2 in the regulation of Fgfr1–3 expression, we further examined the proliferation of osteoblast progenitors. We found that Runx2 was required for the proliferation of osteoblast progenitors, and also that it induced proliferation, at least in part, through the regulation of Fgfr2 and Fgfr3.
Results
Defects in Fgf signaling for limb development in Tg(Prrx1-Runx2) mice
We previously reported that the early onset of Runx2 expression causes craniosynostosis, ectopic bone formation, and limb defects, and also that the severity of limb defects depends on the expression levels of the transgene23. An epithelial-mesenchymal interaction loop formed by Fgfs and Fgfrs is essential for limb development. Fgf10, which first appears in the mesenchyme, has affinity for Fgfr2b in the apical ectodermal ridge (AER), which is a thickening of the ectoderm at the apex of the developing limb bud and is formed along the border of dorsal and ventral ectoderm, and induces Fgf8 and Fgf4 in the AER. Fgf8 and Fgf4 have affinity for Fgfr1c and Fgfr2c expressed in the mesenchyme, and promote mesenchymal proliferation and the outgrowth of limb buds10,24–32. The Fgf8 and Fgf4, which are expressed in AER, and Shh, which is expressed in the zone of polarizing activity (ZPA), mutually support the expression33–35. In order to elucidate the mechanisms responsible for limb defects, we examined the expression of Fgf10 at E10.0 and that of Fgf8, Fgf4, and Shh at E10.5 in Tg(Prrx1-EGFP-Runx2) mice with high expression levels using whole mount in situ hybridization (Fig. 1A–H). Since Tg(Prrx1-EGFP-Runx2) mice were lethal at birth, we analyzed F0 littermates of wild-type and Tg(Prrx1-EGFP-Runx2) mice in each whole mount in situ hybridization. Therefore, the severity of the defect in limb development was different among F0 Tg(Prrx1-EGFP-Runx2) mice depending on the expression level of the transgene as previously described23. Fgf10 mRNA was detected in wild-type and Tg(Prrx1-EGFP-Runx2) mice, while Fgf8, Fgf4, and Shh mRNA was detected in wild-type mice, but not in Tg(Prrx1-EGFP-Runx2) mice. In histological analyses, the AER was observed in the limb buds of wild-type mice, but was not apparent in Tg(Prrx1-EGFP-Runx2) mice at E10.5 (Fig. 1I,J). The endogenous Runx2 protein was undetectable in wild-type mice, while the Runx2 protein was present in mesenchymal cells, but not in the epithelium of the limb buds of Tg(Prrx1-EGFP-Runx2) mice (Fig. 1K,L). Fgf8 mRNA was detected in the AER of the limb buds of wild-type mice, but not in Tg(Prrx1-EGFP-Runx2) mice (Fig. 1M,N), and the number of TUNEL-positive cells was higher in the presumptive AER region of Tg(Prrx1-EGFP-Runx2) mice than in the AER of the limbs of wild-type mice (Fig. 1O,P). Enhanced apoptosis in the AER region was also observed in Fgf4 and Fgf8 double mutant mice32. Therefore, these results suggest that FGF10, which was expressed in mesenchymal cells, failed to induce Fgf8 and Fgf4 mRNA expression in the ectoderm, leading to the apoptosis of cells in the AER region of Tg(Prrx1-EGFP -Runx2) mice with high expression levels. Thus, ectopic expression of Runx2 in limb bud mesenchyme disturbed the induction of Fgf8 and Fgf4 expression in ectoderm by Fgf10 produced in mesenchyme.
Figure 1.
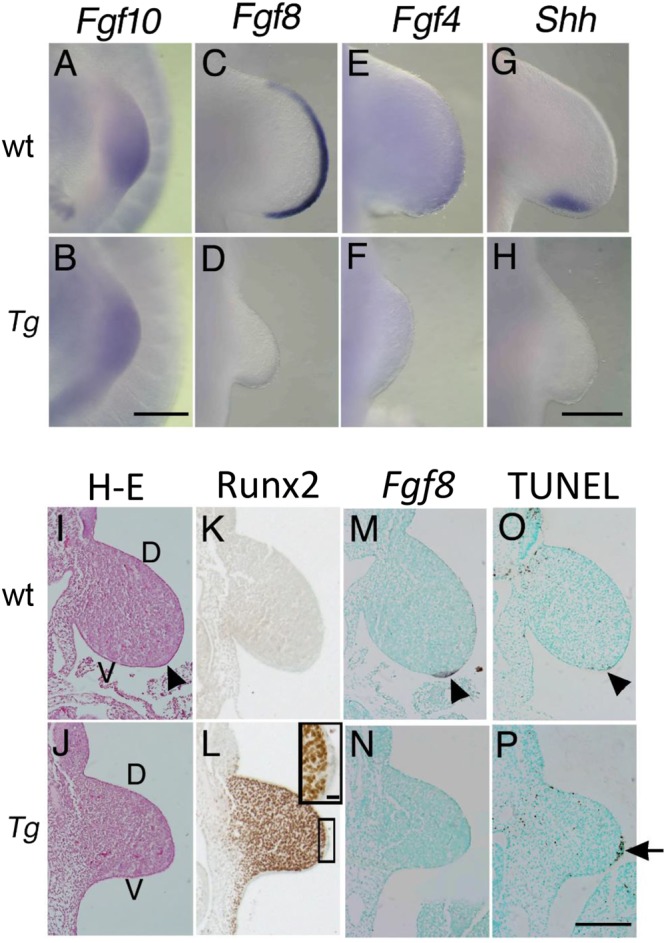
Limb development in Tg(Prrx1-EGFP-Runx2) mice (A–H) Whole mount in situ hybridization. Whole mount in situ hybridization of the forelimb buds of wild-type mice (wt) (A,C,E,G) and Tg(Prrx1-EGFP-Runx2) mice with strong expression (Tg) (B,D,F,H) at E10.0 (A,B) and E10.5 (C–H) using Fgf10 (A,B), Fgf8 (C,D), Fgf4 (E,F), and Shh (G,H) probes. (I–P) Histological analysis. A histological analysis of the forelimb buds of a wild-type mouse (wt) (I,K,M,O) and Tg(Prrx1-EGFP-Runx2) (Tg) mouse with strong expression (Tg) (J,L,N,P) at E10.5. (I,J) H-E staining. The AER is observed in the wild-type mouse (I, arrowhead), but not in the Tg(Prrx1-EGFP-Runx2) mouse (J). D, dorsal; V, ventral. (K,L) Immunohistochemical analysis of Runx2 protein expression. The boxed region in L is amplified in the window. (M,N) In situ hybridization using the Fgf8 probe. (O,P) TUNEL staining. F0 littermates of wild-type and Tg(Prrx1-EGFP-Runx2) mice were compared in each whole mount in situ hybridization and histological analysis. Scale bars: 20 μm (A,B); 200 μm (C–H); 200 μm (I–P); 20 μm (inset in L).
Runx2 regulates the expression of Fgfr1, Fgfr2, and Fgfr3
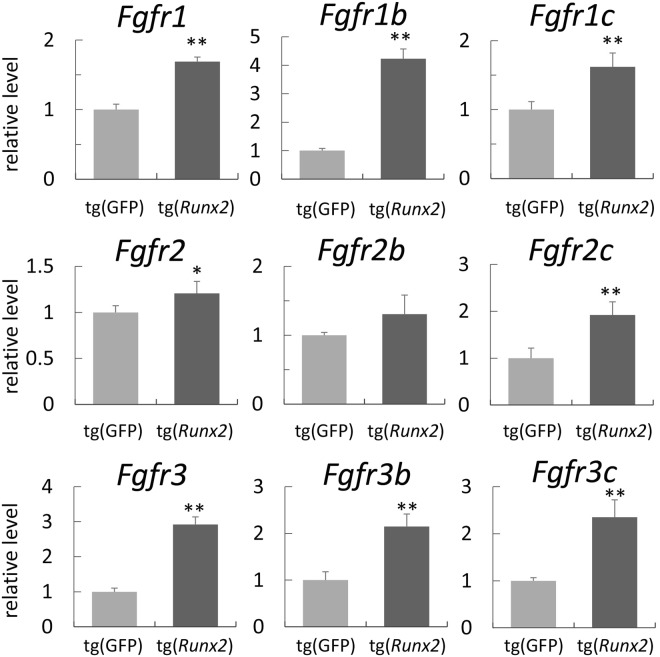
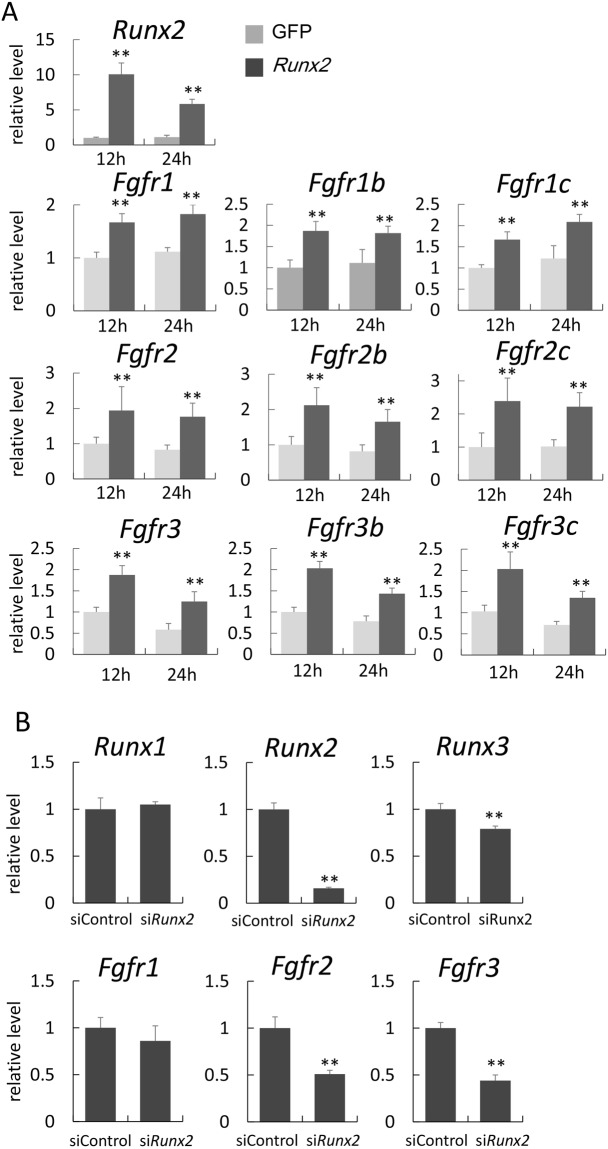
Since Fgf10 mRNA was detected in mesenchymal cells, whereas Fgf8 and Fgf4 mRNA was not observed in the epithelium in Tg(Prrx1-EGFP-Runx2) mice, the expression of Fgfrs or their isoforms might have been disturbed in Tg(Prrx1-EGFP-Runx2) mice. Therefore, we examined the expression of Fgfr1–3 and their isoforms by real-time RT-PCR using RNA from EGFP-positive cells sorted from the cell suspensions of Tg(Prrx1-EGFP) mice and Tg(Prrx1-EGFP-Runx2) mice at E10.5 (Fig. 2). Fgfr1b, Fgfr1c, Fgfr2c, Fgfr3b, and Fgfr3c mRNA levels were significantly higher in Tg(Prrx1-EGFP-Runx2) mice than in Tg(Prrx1-EGFP) mice. The Runx2 expression in Tg(Prrx1-EGFP-Runx2) mice was 16 times higher than that in Tg(Prrx1-EGFP) mice in the microarray analysis using the sorted EGFP-positive cells (data not shown). Since Runx2 expression was not detected in wild-type limb buds (Fig. 1K), the regulation of Fgfrs at this developmental stage is not a physiological function of Runx2. Therefore, we examined whether Runx2 induces the expression of Fgfr1–3 in osteoblast progenitors prepared from wild-type calvarial cells as described in the Materials and methods (Fig. 3A). Infection with type II Runx2-expressing adenovirus induced Fgfr1, Fgfr2, and Fgfr3 mRNA and their IIIb and IIIc isoform mRNA. Since the induction of Fgfr2 by Runx2 in osteoblast progenitors was more apparent than that in limb bud mesenchymal cells at E10.5 (Figs 2 and 3A), it is likely that the transcription factors and/or co-factors collaborating with Runx2 for Fgfr2 expression is more abundant in osteoblast progenitors than limb bud mesenchymal cells at E10.5. Further, the expression of the molecules related to alternative splicing may be also different, because Fgfr2b expression was not significantly upregulated in limb bud mesenchymal cells at E10.5 (Fig. 2). The induction of Fgfr1, Fgfr2, Fgfr3, and Fgfr4 mRNA by Runx2 has also been reported by microarray analysis using RNA from immortalized Runx2−/− calvarial cells infected with Runx2-expressing adenovirus36. The introduction of Runx2 siRNA in osteoblast progenitors reduced the expression of Fgfr2 and Fgfr3 (Fig. 3B). However, Runx2 siRNA reduced Runx3 mRNA as well as Runx2 mRNA, although the siRNA sequence was specific for Runx2. Therefore, we examined whether Runx2 regulates Runx3 expression (Supplemental Fig. 1). Runx3, but not Runx1 expression was markedly weaker in Runx2−/− calvariae than in wild-type and Sp7−/− calvariae (Supplemental Fig. 1A). The overexpression of Runx2 induced the expression of Runx3, but not that of Runx1 in wild-type osteoblast progenitors (Supplemental Fig. 1B). The overexpression of Runx3 induced neither Runx1 nor Runx2. Furthermore, the overexpression of Runx3 failed to induce Fgfr1, Fgfr2, and Fgfr3 (Supplemental Fig. 1B). These results indicated that Runx2, but not Runx3 regulates Fgfr1, Fgfr2, and Fgfr3.
Figure 2.
Real-time RT-PCR analyses of the expression of Fgfr1, Fgfr2, and Fgfr3 The expression of Fgfr1, Fgfr2, and Fgfr3 and their respective IIIb and IIIc isoform mRNA in Tg(Prrx1-EGFP) and Tg(Prrx1-EGFP-Runx2) mice was measured by real-time RT-PCR in triplicate. EGFP-positive cells were collected from limb buds of more than 70 F0 EGFP-positive embryos each in Tg(Prrx1-EGFP-Runx2) mice and Tg(Prrx1-EGFP) mice at E10.5 by sorting EGFP-positive cells using FACS, the EGFP-positive cells obtained in each sorting were pooled, and mRNA was extracted from the pooled cells. We normalized values to that of Gapdh. Values in wild-type mice were defined as 1, and the relative levels are shown. Data are the mean ± SD. *p < 0.05, **p < 0.01.
Figure 3.
(A) Induction of Fgfr1, Fgfr2, and Fgfr3 by Runx2 in vitro. RNA was extracted from wild-type osteoblast progenitors that had been infected with an adenovirus expressing type II Runx2 and EGFP or EGFP alone. Samples were harvested 12 and 24 hrs after infection. Values in cells infected with the EGFP-expressing adenovirus were defined as 1, and the relative levels are shown. Data are the mean ± SD of 4 wells. **p < 0.01. (B) Suppression of Fgfr1, Fgfr2, and Fgfr3 expression by Runx2 siRNA. Osteoblast progenitors were prepared from the calvariae of wild-type newborn mice, and transfected with Runx2 siRNA. RNA was extracted 48 hours after transfection, and real-time RT-PCR was performed. Values in siRNA for the control were defined as 1, and the relative levels are shown. Data are the mean ± SE of 3 wells. **p < 0.01. Similar results were obtained in three independent experiments and representative data are shown.
Direct regulation of promoters of Fgfr1, Fgfr2, and Fgfr3 by Runx2
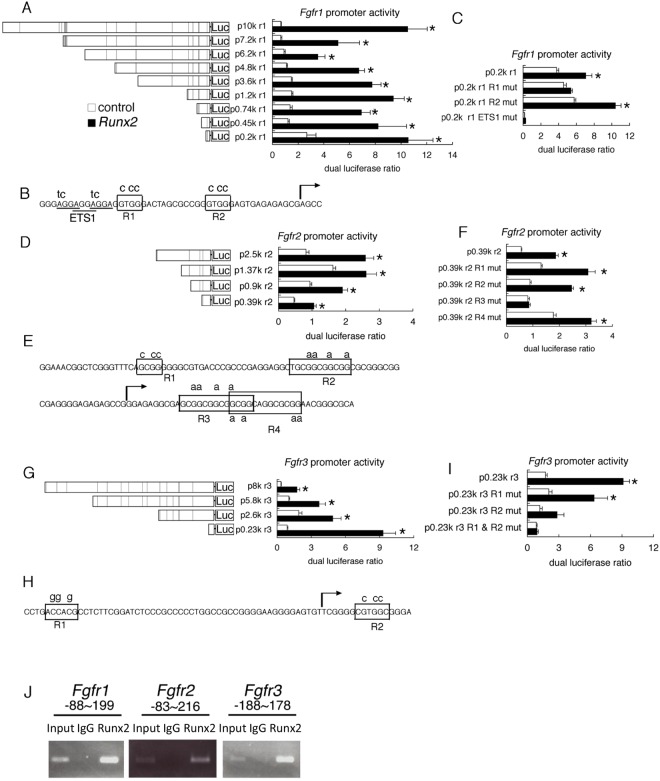
Since Runx2 induced the expression of Fgfr1, Fgfr2, and Fgfr3 in vivo and in vitro, we performed reporter assays using the promoter regions of Fgfr1, Fgfr2, and Fgfr3 (Fig. 4). In the reporter assay using a 10-kb fragment of the Fgfr1 promoter region, which contains eighteen consensus Runx2-binding motifs (TGPyGGPy), Runx2 strongly induced reporter activity (Fig. 4A). Serial deletions of the 10-kb fragment showed that the distal 4 kb is, in part, responsible for Runx2-dependent transcriptional activation; however, further deletions augmented Runx2-dependent transcriptional activation, and Runx2 still activated the reporter activity of the 0.2-kb fragment. In the 0.2-kb fragment, there was one overlapping Ets1-binding site and two putative Runx-binding sites, which contained the core four nucleotides of the consensus Runx2-binding motifs, R1 and R2 (Fig. 4B). The mutation of R1, but not R2 completely abolished Runx2-dependent transcriptional activation (Fig. 4C). Since R1 and the Ets1-binding sites are closely located, Runx2 and Ets1 may co-operatively bind to and activate the Fgfr1 promoter, as previously described for the Spp1 promoter37. It was not possible to confirm this because the mutation of the Ets1-binding site completely abolished the basal activity of the promoter (Fig. 4C).
Figure 4.
Reporter and ChIP assays of Fgfr1, Fgfr2, and Fgfr3 promoters. (A–C) Reporter assays of the Fgfr1 promoter. (A) Schematic diagrams of the reporter vectors of the Fgfr1 promoter and their luciferase (Luc) activities. (B) The nucleotide sequence containing putative Runx2-binding sites in the 0.2-kb fragment. Ets1-binding sites are underlined and putative Runx2-binding sites (R1 and R2) are boxed. The mutated sequences are shown above the boxes and lines. (C) Reporter activities of the 0.2-kb construct (p0.2k r1) and the 0.2-kb constructs carrying a mutated R1, R2, or Ets1 site. (D–F) Reporter assays of the Fgfr2 promoter. (D) Schematic diagrams of the reporter vectors of the Fgfr2 promoter and their luciferase activities. (E) The nucleotide sequence containing putative Runx2-binding sites in the 0.39-kb fragment. The putative Runx2-binding sites (R1–4) are boxed, and the mutated sequences are shown above or below the boxes. (F) Reporter activities of the 0.39-kb construct (p0.39k r2) and the 0.39-kb constructs carrying mutated R1, R2, R3, or R4. (G–I) Reporter assays of the Fgfr3 promoter. (G) Schematic diagrams of the reporter vectors of the Fgfr3 promoter and their luciferase activities. (H) The nucleotide sequence containing putative Runx2-binding sites in the 0.23-kb fragment. The putative Runx2-binding sites (R1, R2) are boxed, and the mutated sequences are shown above the boxes. (I) Reporter activities of the 0.23-kb construct (p0.23k r3) and the 0.23-kb constructs carrying mutated R1, R2, or R1 and R2. Vertical lines in the diagrams represent the positions of consensus Runx2-binding motifs (A,D,G), and arrows indicate reported transcription start sites (B,E,H). In all reporter assays, C3H10T1/2 cells were transfected with an empty (open column) or Runx2-expressing (closed column) vector. Data are the mean ± SD of 4 wells. *p < 0.01 versus the control. Three independent experiments were performed and representative data are shown. (J) ChIP assays. DNA before (input) and after immunoprecipitation with a monoclonal anti-Runx2 antibody (Runx2) or mouse IgG (IgG) was amplified by PCR using primers that amplify the sequences of Fgfr1 (−88 ~+199), Fgfr2 (−83 ~+216), and Fgfr3 (−188 ~+178). Similar results were obtained in three independent experiments and representative data are shown.
In the reporter assay using a 2.5-kb fragment of the Fgfr2 promoter region, which contains four consensus Runx2-binding sites, Runx2 induced reporter activity (Fig. 4D). Serial deletions of the 2.5-kb fragment showed that the distal 1.13 kb was, in part, responsible for Runx2-dependent transcriptional activation; however, Runx2 maintained the ability to enhance the transcription of the reporter vector containing a 0.39-kb deletion fragment (Fig. 4D). The 0.39-kb fragment contained one consensus Runx2-binding motif (R2) and three putative Runx2-binding sites consisting of the four core nucleotides of the consensus Runx2-binding sequences (R1, R3, R4) (Fig. 4E). The mutation of R3 abolished Runx2-dependent transcriptional activation (Fig. 4F).
In the reporter assay using an 8-kb fragment of the Fgfr3 promoter region, which contained fourteen consensus Runx2-binding motifs, Runx2 strongly induced reporter activity (Fig. 4G). Serial deletions of the 8-kb fragment to the 2.6-kb fragment reduced Runx2-dependent transcriptional activation; however, further deletions to the 0.23-kb fragment augmented Runx2-dependent transcriptional activation. Thus, we focused on the 0.23-kb fragment, which contained two putative Runx2-binding sites, R1 and R2 (Fig. 4H). The mutation of either R1 or R2 partly reduced Runx2-dependent transcriptional activation, and the mutations of both R1 and R2 completely abolished Runx2-dependent transcriptional activation (Fig. 4I).
We then examined the binding of endogenous Runx2 in the promoter regions of Fgfr1, Fgfr2, and Fgfr3 by ChIP assays (Fig. 4J). The promoter regions of Fgfr1 (−88 ~+199), Fgfr2 (−83 ~+216), and Fgfr3 (−188 ~+178), which contained the putative Runx2-binding sites responsible for Runx2-dependent transcriptional activation, were amplified by PCR using DNA immunoprecipitated with the anti-Runx2 antibody, but not with IgG.
Accumulation of proliferating osteoblast progenitors, which express Runx2 and Fgfr2, in calvariae and mandibles in Sp7−/− mice, but not in Runx2−/− mice
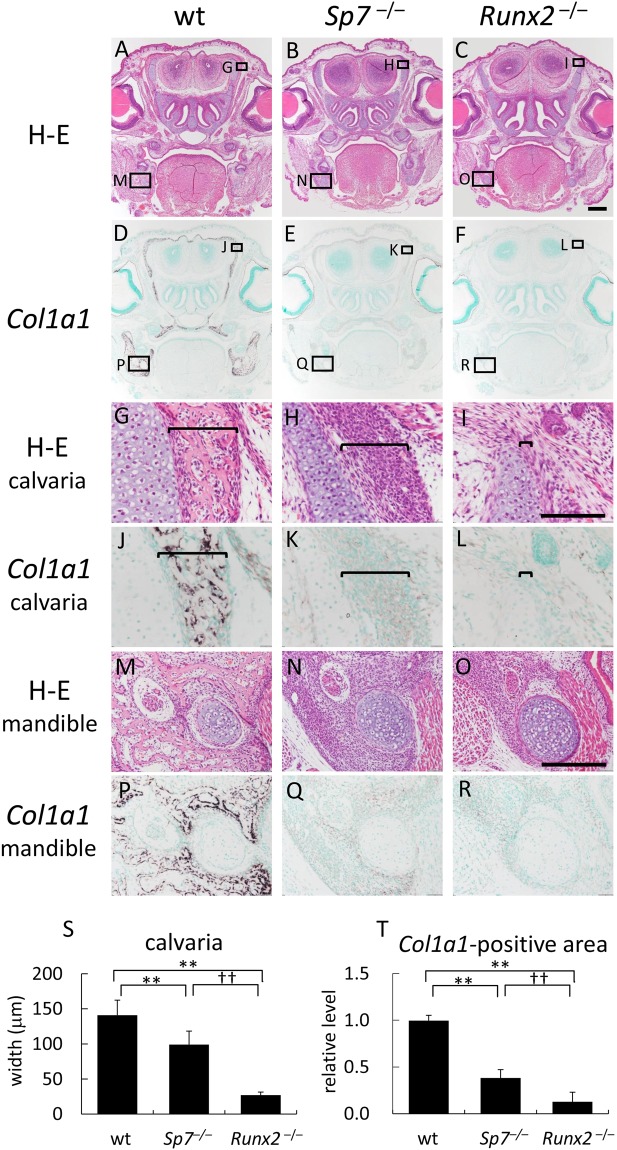
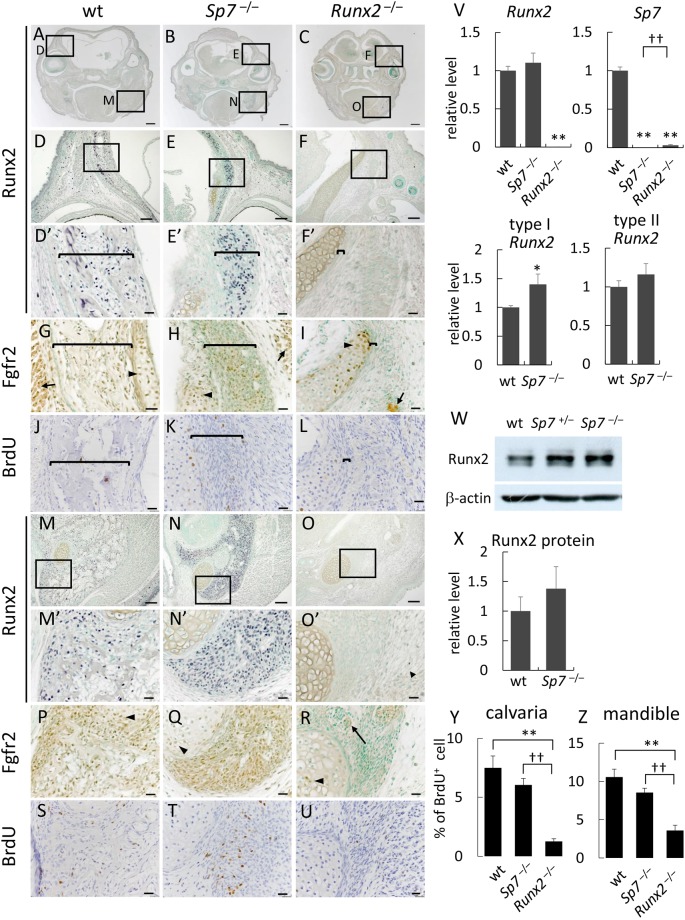
Although Runx2 is expressed in mesenchymal cells in the maxilla, mandible, and perichondrium of the humerus in Sp7−/− mice, their differentiation into osteoblasts is completely blocked38. Since Runx2 regulated Fgfrs in osteoblast progenitors (Figs 3 and 4), we focused on mesenchymal cells in intramembranous bone regions, including calvariae and mandibles, in Sp7−/− mice in order to clarify the physiological roles of Fgfr gene regulation by Runx2. We compared the intramembranous bone area including the calvariae and mandibles in wild-type, Sp7−/−, and Runx2−/− mice at E18.5 (Fig. 5). In wild-type mice, bone structures were established and osteoblasts strongly expressed Col1a1 (Fig. 5G,J,M,P). Although Sp7−/− mice and Runx2−/− mice both showed no bone structure, the layer of mesenchymal cells in the calvarial region in Sp7−/− mice was much thicker than that in Runx2−/− mice (Fig. 5G-I,S). The expression of Col1a1 in mesenchymal cells was weak in Sp7−/− mice and Runx2−/− mice, but stronger in Sp7−/− mice than in Runx2−/− mice, and the Col1a1-positive area in Sp7−/− mandibles was much larger than that in Runx2−/− mandibles (Fig. 5J–L,P–R,T). Therefore, mesenchymal cells, which are considered to be osteoblast progenitors, were accumulated in calvariae and mandibles of Sp7−/− mice but not of Runx2−/− mice.
Figure 5.
Histological analysis of Sp7−/− and Runx2−/− mice Frontal sections of wild-type (wt) (A,D,G,J,M,P), Sp7−/− (B,E,H,K,N,Q), and Runx2−/− (C,F,I,L,O,R) mice at E18.5 were stained with H-E (A–C,G–I,M–O), or subjected to in situ hybridization using the Col1a1 probe (D–F,J–L,P–R). The boxed regions in A, B, and C are magnified in G and M, H and N, and I and O, respectively. The boxed regions in D, E, and F are magnified in J and P, K and Q, and L and R, respectively. Brackets in (G–L) indicate the layers of osteoblastic cells or osteoblast progenitors in the calvarial region. The widths of calvariae (wt: n = 8, Sp7−/−: n = 6, Runx2−/−: n = 5), and Col1a1-positive area in mandibles (wt: n = 4, Sp7−/−: n = 3, Runx2−/−: n = 3) were measured and shown in S and T, respectively. The values in wild-type mice were set as 1, and the relative levels are shown in T. Bars: 500 μm (A–F), 100 μm (G–L), 200 μm (M–R).
In limb bone development in wild-type mice at E18.5, chondrocytes in epiphysis expressed Col2a1, and metaphysis and diaphysis were already replaced with bone and occupied by osteoblasts, which expressed Col1a1 (Supplemental Fig. 2A,D,G,J,M,P). Sp7−/− mice completely lacked bone formation, and the limb skeletons were cartilaginous at E18.5 (Supplemental Fig. 2B,E), as previously described38. Chondrocytes were maturated in the diaphysis, in which Col2a1 expression was absent, in Sp7−/− mice (Supplemental Fig. 2H,K). Osteoblast progenitors, which expressed Col1a1, were accumulated in the perichondrium of Sp7−/− mice and some of them differentiated into morphologically chondrogenic cells, which expressed both Col2a1 and Col1a1 (Supplemental Fig. 2H,K,N,Q). Although the limb skeletons were also cartilaginous in Runx2−/− mice at E18.5, chondrocyte maturation was inhibited and chondrocytes in the entire femurs expressed Col2a1 (Supplemental Fig. 2C,F,I,L), as previously described39,40. The accumulation of mesenchymal cells in the perichondrium was absent, and osteoblast progenitors, which expressed Col1a1, were few (Supplemental Fig. 2O,R). These findings indicate that Runx2 is required for the expansion of osteoblast progenitors in the perichondrium of endochondral bones.
We then performed immunohistochemistry using the anti-Runx2 and anti-Fgfr2 antibodies to examine the expression of Runx2 and Fgfr2 in osteoblast progenitors in Sp7−/− mice. Runx2 and Fgfr2 were strongly detected in osteoblasts in wild-type mice and osteoblast progenitors in Sp7−/− mice, but undetectable in the mesenchymal cells in Runx2−/− mice, although Fgfr2 was detected in chondrocytes of Runx2−/− mice (Fig. 6A–I, M–R). Real-time RT-PCR and Western blot analyses showed that total Runx2 mRNA, type II Runx2 mRNA, and Runx2 protein are expressed at similar levels in wild-type and Sp7−/− mice, that type I Runx2 mRNA is expressed at slightly higher levels in Sp7−/− mice than wild-type mice, and that Sp7 mRNA levels are extremely low in Runx2−/− mice (Fig. 6V–X).
Figure 6.
Runx2 and Fgfr2 expression and BrdU labeling in the calvaria and mandible of wild-type, Sp7−/−, and Runx2−/− mice (A–U) Frontal sections of wild-type (A,D,G,J,M,P,S) and Sp7−/− (B,E,H,K,N,Q,T), and Runx2−/− (C,F,I,L,O,R,U) mice at E18.5 were reacted with the anti-Runx2 antibody (A–F,M–O) and anti-Fgfr2 antibody (G–I,P–R) or subjected to BrdU labeling (J–L,S–U). The boxed regions in A are magnified in D and M, the boxed regions in B are magnified in E and N, and the boxed regions in C are magnified in F and O. The boxed regions in (D–F) and (M–O) are magnified in (D’–F’) and (M’–O’), respectively. The similar regions of (D’–F’) are shown in (G–I) and (J–L), and those of (M’–O’) are shown in (P–R) and (S–U). Brackets in (D’–L) indicate the layers of osteoblastic cells or osteoblast progenitors in the calvarial region, short arrows in (G–I) indicate muscle fibers, arrowheads in (G–I) and (P–R) indicate chondrocytes, and long arrows in R indicate neurons. Bars: 500 μm (A–C), 100 μm (D–F,M–O), 20 μm (D’-L, M’–U). (V) Real-time RT-PCR analysis. RNA was extracted from the calvariae of wild-type, Sp7−/−, and Runx2−/− mice at E18.5. The values in wild-type mice were set as 1, and relative levels are shown. Data are the mean ± SE of 4–5 mice. *vs. wild-type mice. *p < 0.05, **,††p < 0.01. (W) Western blot analysis. Protein was extracted from the calvariae of wild-type, Sp7+/−, and Sp7−/− mice at E18.5. β-actin was used as an internal control. (X) Quantification of Western blot bands. The normalized values of Runx2 protein bands in wild-type mice were set as 1, and the relative levels in Sp7−/− embryos are shown. Data are the mean ± SE of 3 bands. (Y and Z) BrdU-positive osteoblastic cells and osteoblast progenitors in calvariae (Y) and mandibles (Z) were counted and shown as a percentage of the number of osteoblastic cells and osteoblast progenitors. n = 6. **,††p < 0.01.
BrdU-positive osteoblasts or osteoblast progenitors were observed at similar frequencies in wild-type and Sp7−/− mice, whereas BrdU-positive cells were severely reduced in mesenchymal cells in the calvarial region and mandible of Runx2−/− mice (Fig. 6J–L,S–U,Y,Z). These results indicate that Runx2 is required for the proliferation of osteoblast progenitors and the expansion of osteoblast progenitors in Sp7−/− mice.
Runx2 enhances the proliferation of osteoblast progenitors through Fgf signaling pathway
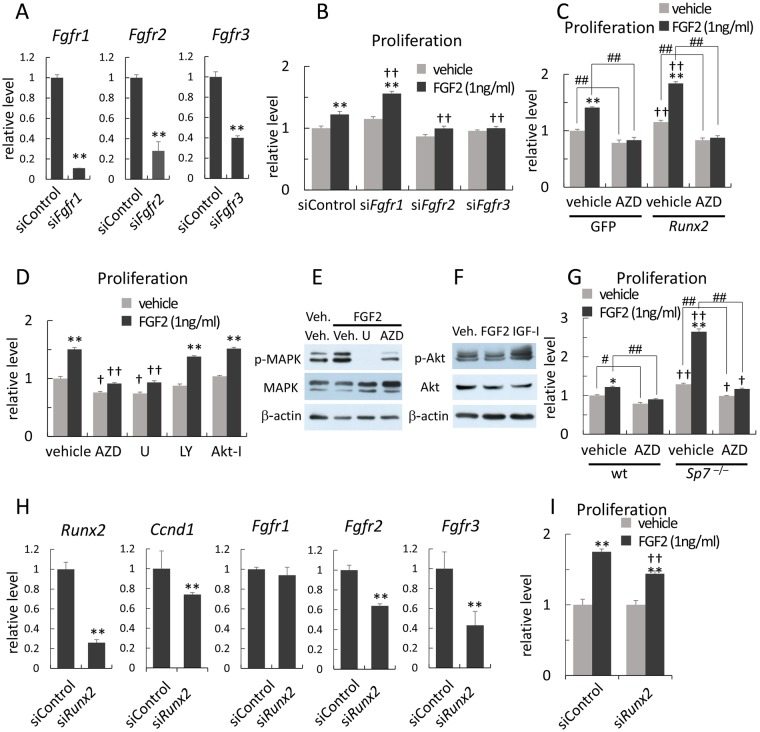
In order to investigate the functions of Fgfr1–3 in the proliferation of osteoblast progenitors, siRNA for Fgfr1, Fgfr2, or Fgfr3 was introduced by electroporation into wild-type osteoblast progenitors and cells were stimulated with FGF2 (Fig. 7A,B). siRNA for Fgfr1 augmented FGF2-induced proliferation, while siRNAs for Fgfr2 and Fgfr3 inhibited FGF2-induced proliferation. The FGF2 treatment or the transfection of Runx2-expression vector by electroporation increased the proliferation of wild-type osteoblast progenitors, and the transfection of Runx2-expression vector enhanced FGF2-induced proliferation. The treatment with AZD4547, which is a specific Fgfr tyrosine kinase inhibitor that inhibits Fgfr1–3, reduced the proliferation of wild-type osteoblast progenitors and abrogated increases by FGF2 and/or Runx2 (Fig. 7C).
Figure 7.
Analyses of the proliferation of osteoblast progenitors in vitro (A) Reductions in Fgfr1, Fgfr2, and Fgfr3 mRNA by the introduction of respective siRNA into wild-type osteoblast progenitors. Each value of Fgfr1–3 in the introduction of control siRNA was set as 1 and the relative levels are shown. n = 3. **p < 0.01. (B) Effects of FGF2 and each siRNA for Fgfr1, Fgfr2, and Fgfr3 on the proliferation of wild-type osteoblast progenitors. The values of the vehicle in the control siRNA were set as 1, and the relative levels are shown. n = 4. * vs. the respective vehicle. †vs. the respective experiment in the control siRNA. **,††p < 0.01. (C) Effects of Runx2 on proliferation and the FGF2-induced proliferation of wild-type osteoblast progenitors and the inhibition by AZD4547 (50 nM). The values of the vehicle in the GFP group were set as 1, and the relative levels are shown. n = 4. * vs. the respective vehicle. †vs. the respective experiment in the GFP group. **,††,##p < 0.01. (D) Effects of inhibitors on the FGF2-induced proliferation of wild-type osteoblast progenitors. AZD: AZD4547 (50 nM), U: U0126 (50 μM), LY: LY294002 (1 μM), Akt-I: Akt inhibitor (2.5 μM). The values in the vehicle were set as 1, and the relative levels are shown. n = 4. * vs. the respective vehicle. †vs. the respective experiment in the vehicle group. †p < 0.05, **,††p < 0.01. (E,F) Western blots of activated p42/44 MAP kinase (E) and Akt (F). IGF-1 was used as a positive control for Akt activation (F). (G) Effects of FGF2 and AZD4547 (50 nM) on the proliferation of Sp7−/− osteoblast progenitors. The values in the vehicle of wild-type osteoblast progenitors were set as 1, and relative levels are shown. n = 4. * vs. the respective vehicle. †vs. the respective experiment in the wild-type group. *,†,#p < 0.05, **,††,##p < 0.01. (H) Real time RT-PCR analysis using RNA from Sp7−/− osteoblast progenitors. The values for control siRNA were set as 1 and the relative levels are shown. n = 3. **p < 0.01. (I) The effects of siRNA for Runx2 on the FGF2-induced proliferation of Sp7−/− osteoblast progenitors. The values for the vehicle were set as 1 and the relative levels are shown. n = 4. * vs. the respective vehicle. †vs. the respective experiment in the control siRNA. **,††p < 0.01. Similar results were obtained in two to four independent experiments and representative data are shown in A–I.
The MAPK inhibitor, U0126, exerted similar effects to AZD4547, whereas the PI3K inhibitor, LY294002, and Akt inhibitor failed to inhibit proliferation and FGF2-induced proliferation of wild-type osteoblast progenitors (Fig. 7D). In accordance with these results, the treatment with FGF2 enhanced the phosphorylation of MAPK, and this phosphorylation was strongly inhibited by U0126 and AZD, whereas the treatment with FGF2 failed to enhance the phosphorylation of Akt (Fig. 7E,F). These results indicate that Fgfr signaling through Fgfr2 and Fgfr3 regulates the proliferation of osteoblast progenitors mainly through the MAPK pathway, and that Runx2 enhances proliferation and FGF2-induced proliferation through the regulation of the Fgfr signaling pathway.
Involvement of Runx2 in the enhancement of the proliferation of Sp7−/− osteoblast progenitors by FGF2
The proliferation of osteoblast progenitors from Sp7−/− calvaria was faster than wild-type osteoblast progenitors, enhanced proliferation by FGF2 was markedly stronger in Sp7−/− osteoblast progenitors than in wild-type osteoblast progenitors, and the treatment with AZD4547 abolished accelerated proliferation and enhanced FGF2-induced proliferation (Fig. 7G). The introduction of siRNA for Runx2 into Sp7−/− osteoblast progenitors reduced the expression of Ccnd1, Fgfr2, and Fgfr3 (Fig. 7H) as well as FGF2-induced proliferation (Fig. 7I).
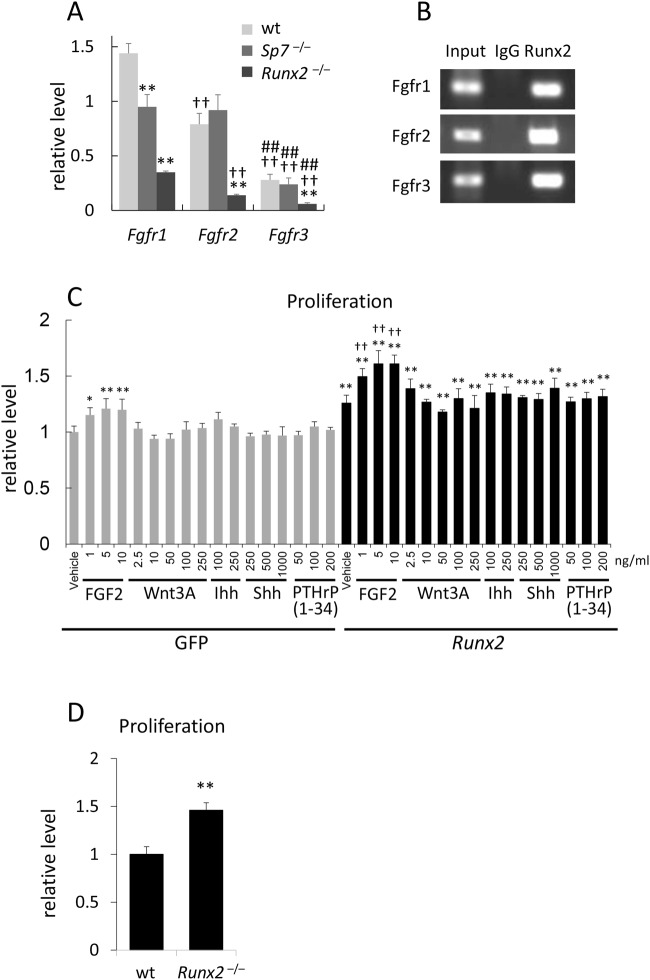
We compared the expression of Fgfr1–3 in the calvariae of wild-type, Sp7−/−, and Runx2−/− mice using a droplet digital RT-PCR analysis, which detects the absolute number of each mRNA. The expression levels of Fgfr1–3 were Fgfr1 > Fgfr2 > Fgfr3 in wild-type and Runx2−/− calvariae, and Fgfr1 = Fgfr2 > Fgfr3 in Sp7−/− calvariae (Fig. 8A). The expression of Fgfr4 was undetectable in wild-type calvariae (data not shown). Fgfr1, Fgfr2, and Fgfr3 mRNA levels were markedly lower in Runx2−/− calvariae than in wild-type and Sp7−/− calvariae. Although the level of Fgfr1 was lower in Sp7−/− calvariae than in wild-type calvariae, those of Fgfr2 and Fgfr3 were similar between wild-type and Sp7−/− calvariae (Fig. 8A). In the ChIP assay using Sp7−/− calvariae, Runx2-binding regions in Fgfr1, Fgfr2, and Fgfr3 were amplified by PCR in DNA precipitated with the anti-Runx2 antibody, but not with IgG (Fig. 8B). These results indicate that Runx2 also directly regulates the expression of Fgfr2 and Fgfr3 in Sp7−/− calvariae and enhances the FGF2-induced proliferation of Sp7−/− osteoblast progenitors.
Figure 8.
Droplet digital RT-PCR, ChIP, and cell proliferation analyses (A) Droplet digital RT-PCR analysis. The expression levels of Fgfr1–3 were compared among wild-type, Sp7−/−, and Runx2−/−calvariae. n = 4. *vs. wild-type mice. †vs. the respective mouse in Fgfr1. #vs. the respective mouse in Fgfr2. **,††,##p < 0.01. (B) ChIP assay. DNA was extracted from Sp7−/− calvariae, and DNA before (input) and after immunoprecipitation with the monoclonal anti-Runx2 antibody (Runx2) or mouse IgG (IgG) was amplified by PCR using the same primers as those in Fig. 4J. (C) The effects of FGF2, Wnt3a, Ihh, Shh, and PTHrP (1–34) in the proliferation of GFP- or Runx2-transfected wild-type osteoblast progenitors. n = 4. * vs. the vehicle in GFP-transfected cells. †vs. the vehicle in Runx2-transfected cells. *p < 0.05, **,††p < 0.01. (D) Proliferation of wild-type and Runx2−/− osteoblast progenitors. n = 4. **p < 0.01. Similar results were obtained in three independent experiments and representative data are shown in B-D.
We also examined whether FGFs enhances the Runx2 capacity for transcriptional activation. Either FGF2 or FGF18 enhanced Runx2-dependent transcription in the reporter assays using the luciferase vector containing six tandem repeats of a Runx2 binding site (6XOSE2) (Supplemental Fig. 3).
Signaling pathways involved in the proliferation of osteoblast progenitors
To investigate other signaling pathways than Fgf involved in the proliferation of osteoblast progenitors, the expression of cell proliferation-related genes in calvarial tissues of Sp7−/− mice and Runx2−/− mice was compared by microarray (Supplemental Tables 1 and 2). The expression of the genes in Wnt (Wnt10b, Lef1), hedgehog (Ihh), and Phtlh (Pth1r, Pthlh) signaling pathways was increased more than two times in Sp7−/− calvarial tissues compared with Runx2−/− calvarial tissues. Therefore, the effects of FGF2, Wnt3a, Ihh, Shh, and PTHrP (1–34) on the proliferation of wild-type osteoblast progenitors, which were transfected with either GFP- or Runx2-expression vector, were compared. Runx2 induced proliferation, and FGF2 but not Wnt3a, Ihh, Shh, and PTHrP (1–34) increased the proliferation in either GFP- or Runx2-transfected cells (Fig. 8C).
Differential expression of the genes related to cell proliferation in Runx2−/− osteoblast progenitors in vitro and in vivo
The proliferation of Runx2−/− osteoblast progenitors was increased in vitro as previously described (Fig. 8D)6. To clarify the reason for the discrepancy of the proliferation capacity of Runx2−/− osteoblast progenitors in vitro and in vivo, the expression of the cell proliferation-related genes in Runx2−/− osteoblast progenitors was compared with that in wild-type osteoblast progenitors in vitro, and that in Runx2−/− calvarial tissues was compared with that in wild-type calvarial tissues by cap analysis of gene expression (CAGE). And the genes, in which the expression ratios in vitro were more than two times or less than half compared with the expression ratios in vivo, were selected (Supplemental Tables 3 and 4). Many genes related to cell proliferation were differentially expressed in Runx2−/− osteoblast progenitors in vitro and in vivo. Further, the expression of Myc, Ccnd1, many growth factor genes, including Ereg, Hbegf, Tgfb1, Vegfa, Fgf7, Csf1, Fgf2, and Pdgfc, and a growth factor receptor gene Pth1r was upregulated in Runx2−/− osteoblast progenitors in vitro than in vivo. The differential expression of many cell proliferation-related genes in vitro and in vivo may explain why Runx2−/− osteoblast progenitors acquired augmented capacity for proliferation in vitro.
The proliferation of osteoblast progenitors in Sp7−/− mice is dependent on the gene dosage of Runx2
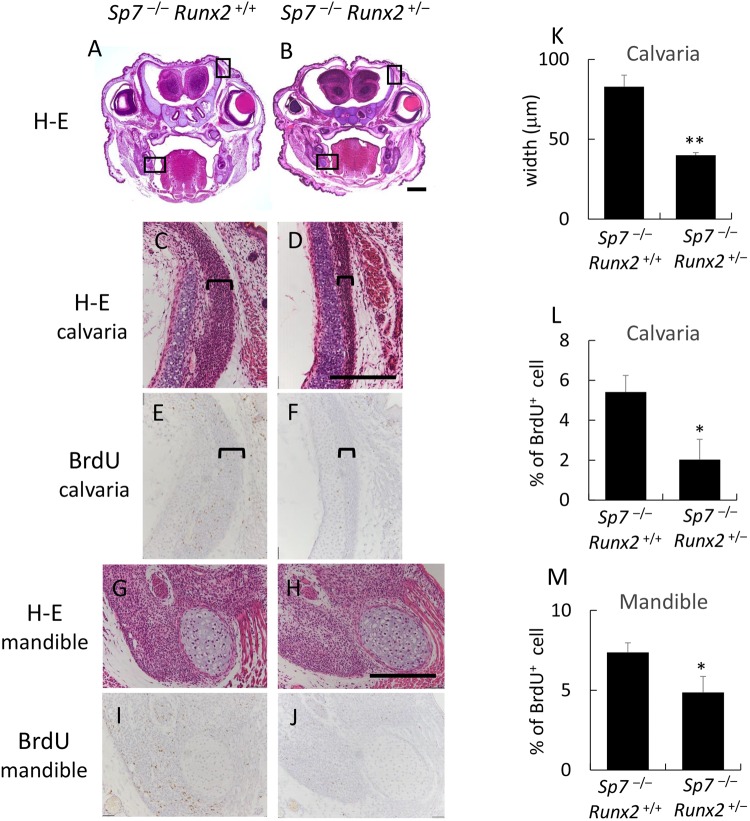
In order to investigate whether the proliferation of osteoblast progenitors in Sp7−/− mice is dependent on Runx2, we generated Sp7−/−Runx2+/− mice and compared them with Sp7−/−Runx2+/+ mice (Fig. 9). The width of the layer of osteoblast progenitors in calvariae in Sp7−/−Runx2+/− mice was about half of that in Sp7−/−Runx2+/+ mice, and the number of BrdU-positive osteoblast progenitors in calvariae was markedly lower in Sp7−/−Runx2+/− mice than in Sp7−/−Runx2+/+ mice (Fig. 9K,L). The number of BrdU-positive osteoblast progenitors in the mandible was also lower in Sp7−/−Runx2+/– mice than in Sp7−/−Runx2+/+ mice, but not by as much as that in calvariae (Fig. 9L,M). Interestingly, the tibiae and fibulae in Sp7−/−Runx2+/+ mice were severely bent due to the accumulation of osteoblast progenitors, whereas those in Sp7−/−Runx2+/– mice were not bent due to the reduction in the amount of osteoblast progenitors compared with Sp7−/−Runx2+/+ mice (Supplemental Fig. 4). These results indicate that the proliferation of osteoblast progenitors in Sp7−/− mice is dependent on the gene dosage of Runx2, and that the proliferation of osteoblast progenitors in calvariae is more dependent on the gene dosage of Runx2 than that in mandibles.
Figure 9.
Comparison of the proliferation of osteoblast progenitors in Sp7−/−Runx2+/+ and Sp7−/−Runx2+/– mice (A–J) Frontal sections of Sp7−/−Runx2+/+ (A,C,E,G,I) and Sp7−/−Runx2+/– (B,D,F,H,J) mice at E18.5 were stained with H-E (A–D,G,H), or subjected to BrdU labeling (E,F,I,J). The upper boxed regions in A and B are magnified in C and D, and the lower boxed regions in A and B are magnified in G and H, respectively. Serial sections were used for BrdU labeling (E,F: calvarial region; I,J: mandibles). The brackets in (C–F) indicate the layers of osteoblast progenitors in the calvarial region. (K–M) The width of calvariae (K) and the percentage of BrdU-positive osteoblast progenitors in calvariae (L) and mandibles (M). The data are the mean ± SE of 3 mice. Bars: 500 μm (A,B), 200 μm (C–J).
Discussion
Although Runx2−/− mice and Sp7−/− mice both completely lack osteoblasts and bone formation, osteoblast progenitors, which abundantly expressed Runx2, accumulated and actively proliferated in the calvaria and mandible of Sp7−/− mice, and the number of osteoblast progenitors and their proliferation were dependent on the gene dosage of Runx2. Runx2 directly regulated Fgfr1–3 expression, enhanced the proliferation of osteoblast progenitors, and augmented the FGF2-induced proliferation through the Fgfr2/3-MAPK signaling pathway. Further, FGF2 but not Wnt3a, Ihh, Shh, and PTHrP (1–34) increased the proliferation and augmented Runx2-induced proliferation. These results indicate that Runx2 is a requisite transcription factor not only for osteoblast differentiation but also for proliferation of osteoblast progenitors, and that Runx2 regulates the proliferation of osteoblast progenitors, at least partly, through the induction of Fgfr2 and Fgfr3 expression.
Since FGF10 binds with high affinity to Fgfr1b and Fgfr2b41, limb defects were likely to have been caused by the up-regulated expression of Fgfr1b in the mesenchyme of the limb buds of Tg(Prrx1-EGFP-Runx2) mice (Figs 1 and 2), which will interrupt the translocation of Fgf10 to the ectoderm leading to the failure of the induction of Fgf8 and Fgf4 expression in the ectoderm. Limb defects in Pfeiffer and Apert syndromes are similar to those in Tg(Prrx1-EGFP-Runx2) mice with low expression levels17,23,42. FGF2 phosphorylates Runx2 through the MAPK pathway and enhances the transcriptional activity of Runx2, ERK-dependent phosphorylation stabilizes the Runx2 protein, and Runx2 is activated through the PI3K-Akt pathway43–46. We also confirmed that Runx2 capacity for the transcriptional activation is enhanced by FGF2 and FGF18 (Supplemental Fig. 3). Since Runx2 directly regulated the expression of Fgfr1–3, a positive feedback loop between FGFR signaling and RUNX2 may play an important role in the pathogenesis of craniosynostosis and limb defects caused by gain-of-function mutations in FGFR1–3.
Runx2−/− calvaria-derived osteoblast progenitors proliferated faster than wild-type osteoblast progenitors in vitro (Fig. 8D), as previously reported6. However, the number of osteoblast progenitors in calvariae and mandibles of Runx2−/− mice was quite low, and the BrdU+ cells were severely reduced (Figs 5 and 6), indicating that there is a discrepancy in the proliferation of Runx2−/− osteoblast progenitors in vitro and in vivo. It was previously reported that the expression of Cdkn1a (p21CIP1) and Cdkn2a (p19ARF) is reduced in Runx2−/− osteoblast progenitors in vitro, and the introduction of Runx2 induces the expression of Cdkn1b (p27KIP1), Cdkn1a, and Cdkn2a, which prevent cell cycle progression through the inhibition of cyclin-dependent kinases (CDKs) or the stabilization of p53 by inhibiting Mdm27,47. The report also showed that severe reduction of Cdkn1a and Cdkn2a expression in Runx2−/− osteoblast progenitors occurs after six passages of the cells47. We examined the proliferation and gene expression of Runx2−/− osteoblast progenitors after one passage of the cells, trying to mimic in vivo situation. Cdkn1b expression in Runx2−/− osteoblast progenitors was greater than that in wild-type osteoblast progenitors, Cdkn1a, Cdkn2a (p19ARF), and Cdkn2a (p16Ink4a) expression in Runx2−/− osteoblast progenitors was about 75% of wild-type osteoblast progenitors, and the introduction of Runx2 failed to induce their expression in vitro (Supplemental Table 5). Further, their expression ratios in Runx2−/− and wild-type osteoblast progenitors in vitro were much higher than those in Runx2−/− and wild-type calvarial tissues in vivo (Supplemental Table 5), indicating that the enhanced proliferation of Runx2−/− osteoblast progenitors in vitro cannot be explained by the expression levels of the CDK inhibitors. Since many genes related to cell proliferation were differentially expressed in Runx2−/− osteoblast progenitors in vitro and in vivo (Supplemental Table 3 and 4), it seemed to be difficult to reveal the function of Runx2 in the proliferation of osteoblast progenitors by investigating Runx2−/− osteoblast progenitors in vitro.
Reductions in the volume of osteoblast progenitors and their frequencies in BrdU uptake in the calvariae were greater than those in the mandibles in Sp7−/−Runx2+/– mice relative to the respective tissues in Sp7−/−Runx2+/+ mice (Fig. 9), indicating that osteoblast progenitor proliferation is more dependent on the gene dosage of Runx2 in calvariae than mandibles. It may partly explain why open fontanelles and sutures are prominent phenotypes in cleidocranial dysplasia, which is caused by heterozygous mutation of RUNX248. High dependency on the amount of Runx2 protein among Runx family transcription factors in calvarial bone development is also shown in the comparison of Runx2+/– mice with conditional Cbfb knockout mice or Cbfb isoform knockout mice49,50. As osteoblast progenitors were scarce and the BrdU-positive cells were few in both regions of calvaria and mandible in Runx2−/− mice (Figs 5 and 6), however, our findings also indicate that Runx2 is required for the proliferation of osteoblast progenitors in both calvaria and mandible. During endochondral bone development, osteoblast differentiation occurs first in the perichondrium, and Sp7-expressing preosteoblasts invade the cartilage with blood vessels and give rise to trabecular osteoblasts51. The accumulation of osteoblast progenitors was observed in the perichondrium of cartilaginous limb skeletons of Sp7−/− mice but not in Runx2−/− mice, and the accumulation of osteoblast progenitors in Sp7−/− limb skeletons was also dependent on the gene dosage of Runx2 (Supplemental Figs 2 and 4), indicating that Runx2 is also required for the proliferation of osteoblast progenitors for trabecular osteoblasts.
Runx2 is required for mammary gland development, and Runx2 deletion increased animal survival in a mouse model of breast cancer with reduced proliferation and cyclin D expression52. The strong expression of Runx2 is associated with estrogen receptor/progesterone receptor/HER2-negative breast cancer and patients with strong Runx2 expression have a poorer survival rate than those with negative or weak expression53. The FGFR2 gene has been identified as a locus associated with an increased risk of developing breast cancer, and a single nucleotide polymorphism in the FGFR2 gene, which enhances RUNX2 binding, increases FGFR2 expression54–57. Furthermore, the knockdown of RUNX2 reduced the expression of FGFR2 in the breast cancer cell line MCT-758. Since Runx2 directly regulated Fgfr2 and the knockdown of Fgfr2 was effective for inhibiting the proliferation of osteoblast progenitors, the regulation of FGFR2 by RUNX2 may also play an important role in the development and progression of some breast cancers by enhancing cell proliferation.
In conclusion, the comparison of Sp7−/− mice, Sp7−/−Runx2+/– mice, and Runx2−/− mice revealed the requirement of Runx2 in the proliferation of osteoblast progenitors. Runx2 regulated it, at least partly, through the regulation of Fgfr2 and Fgfr3. Since Fgf signaling enhances the ability of Runx2 for transcriptional activation, the reciprocal regulation of Runx2 and Fgf signaling will play important roles in skeletal development, the pathogenesis of craniosynostosis, and the progression of some breast cancers.
Materials and Methods
Generation of transgenic and gene-targeting mice
We generated Runx2 transgenic mice under the control of the Prrx1 promoter using enhanced green fluorescent protein (EGFP)-Runx2 fusion DNA {Tg(Prrx1-EGFP-Runx2) mice}, EGFP transgenic mice under the control of the Prrx1 promoter {Tg(Prrx1-EGFP) mice}, and Runx2−/− mice as previously described23,59. In briefly, Prrx1 promoter- EGFP-Runx2 DNA fragment or Prrx1 promoter-EGFP DNA fragment was injected into the pronuclei of fertilized eggs from C57BL/6 x C3H F1. As Tg(Prrx1-EGFP-Runx2) mice died at birth, we analyzed the F0 generation. The expression of transgenic embryos were screened by EGFP observation or real-time RT-PCR. Sp7−/− mice were generated as previously described60. Runx2+/– mice were backcrossed with C57BL/6 more than ten times. Sp7+/– mice were generated and maintained in C56BL/6 background. Prior to the study, all experiments were reviewed and approved by the Animal Care and Use Committee of Nagasaki University Graduate School of Biomedical Sciences (No. 1403111129-21). All animal experiments were performed in accordance with the law of humane treatment and management of animals in Japan, the standards for the breeding, maintenance and reducing pains of experimental animals by Ministry of the Environment in Japan, the basic guidelines of animal experiments by Ministry of Education, Culture, Sports, Science and Technology in Japan, and the rules for animal experiments in Nagasaki University.
Histological and immunohistochemical examinations
Tissues were fixed in 4% paraformaldehyde/0.1 M phosphate buffer and embedded in paraffin, and sections (thickness of 4 μm) were stained with hematoxylin and eosin (H-E). Immunohistochemical analyses were performed using a monoclonal anti-Runx2 antibody (1:200 dilution; Medical & Biological Laboratories, Nagoya, Japan) and polyclonal rabbit anti-Fgfr2 antibody (1:1000 dilution; GeneTex, Inc., Irvine, USA) as previously described61. Sections were counterstained with Methyl green. Immunohistochemistry without the anti-Runx2 antibody or anti-Fgfr2 antibody showed no significant signals (data not shown). TUNEL staining was performed using the ApopTag® system (Intergen, Burlington, MA). For analysis of BrdU incorporation, we subcutaneously injected pregnant mice with 100 μg BrdU/g body weight 1 h before sacrifice. We processed the embryos for histological analysis and detected BrdU incorporation using BrdU staining kit (Invitrogen). The sections were counterstained with hematoxylin.
In situ hybridization
In in situ hybridization, single-stranded RNA probes labeled with digoxigenin-11-UTP were prepared using a DIG RNA labeling kit (Roche, Basel Switzerland) according to the manufacturer’s instructions. Sections were hybridized using mouse Fgf8 and Col1a1 antisense probes as described previously39. Whole mount in situ hybridization was performed using Fgf10, Fgf8, Fgf4, and Shh antisense probes as described previously62. The in situ hybridization of sections and whole embryos using sense probes showed no significant signals (data not shown).
Real-time RT-PCR
Real-time RT-PCR was performed using a THUNDERBIRD SYBR qPCR Mix (Toyobo) and Light Cycler 480 real-time PCR system (Roche Diagnostics). TaqMan PCR for Fgfr1, Fgfr2, Fgfr3, Runx1, Runx2, Runx3, and Sp7 was performed using a THUNDERBIRD Probe qPCR Mix (Toyobo). Primer sequences and information on TaqMan Probes are shown in Supplemental Table 6. We normalized values to that of β-actin.
Cell culture and adenoviral transfer
Wild-type, Sp7−/−, and Runx2−/− osteoblast progenitors were prepared from calvariae at E18.5. The calvariae were cut into small pieces and cultured for 10–14 days in three-dimensional collagen gel (Cell matrix, Nitta Gelatin, Co., Osaka, Japan) with α-modified Minimum (α-MEM) containing 10% FBS. The cells outgrowing from the explants were retrieved by incubation for 30 min with 0.2% collagenase (Wako Pure Chemical Industries, Osaka, Japan) in PBS(−) at 37 °C. In this method, the main cell types isolated were osteoblast progenitors and osteoblasts at an early differentiation stage with low alkaline phosphatase activity and virtually no osteocalcin production59. Cells were plated in 24-well plates at a density of 5 × 104/well in αMEM supplemented with 10% fetal bovine serum (FBS). At confluency, cells were infected with an adenovirus expressing EGFP or type II Runx2-EGFP at a multiplicity of infection of 10 for 2 hrs. The mouse pluripotent mesenchymal cell line, C3H10T1/2, was purchased from the RIKEN Cell Bank (Tsukuba, Japan), and cultured in BME supplemented with 10% FBS.
Reporter Assays
A 10-kb fragment of the Fgfr1 promoter region was subcloned into the firefly luciferase reporter vector pGL4.10Luc2 (Promega, Madison, WI) from the BAC clone. A 2.5-kb fragment of the Fgfr2 promoter region and 8-kb fragment of the Fgfr3 promoter region were amplified by PCR using mouse genomic DNA and cloned into pGL4.10Luc2. All truncated constructs were prepared using the restriction enzyme sites or by PCR amplification. C3H10T1/2 cells were transfected with plasmid DNAs (each luciferase reporter vector 0.1 μg; pRL-Tk Renilla 0.1 μg; pSG5 or pSG5-Runx2 0.05 μg) using FuGENE 6 Transfection Reagent (Roche). Luciferase activities were examined by using Dual-Luciferase Reporter Assay System (Promega), and normalized to Renilla luciferase activity.
ChIP assay
Chromatin immunoprecipitation (ChIP) was performed with a Chromatin Immunoprecipitation Assay Kit (Upstate Biotechnology, Billerica, MA) using the anti-Runx2 monoclonal antibody in Fig. 4J (Medical & Biological Laboratories), anti-Runx2 antibody in Fig. 8B (Santa Cruz Biotechnology, Santa Cruz, CA), or mouse IgG (Cell Signaling, Danvers, MA), using primers in Supplemental Table 6.
Western blot analysis
A Western blot analysis was performed using anti-Runx2 (Cell Signaling), rabbit anti-phospho-p44/42 MAPK (Thr202/Tyr204) (Cell Signaling), rabbit anti-phospho-Akt (ser473) (Cell Signaling), rabbit anti-p44/42 MAPK (Cell Signaling), rabbit anti-Akt (Cell Signaling), and anti-β-actin (Santa Cruz Biotechnology) antibodies.
Cell proliferation assay
A total of 5 × 105 cells were subjected to electroporation with 1.0 μg of either the EGFP or type II Runx2 expression vector or with 10 pmol of siRNA for Fgfr1, Fgfr2, Fgfr3 (Bonac, Kurume, Japan), or Runx2 (Thermo scientific, Waltham, MA) using the Neon Transfection System (Invitrogen) and cultured on 100-mm dishes for 24 h, and then the transfected cells were seeded at 1 × 104 cells/well on 96-well plates. After 6 hrs, FGF2 (PeproTech Inc. Rocky Hill NJ), Wnt3a (R&D Systems, Minneapolis, MN), Ihh (R&D Systems), Shh (R&D Systems), or PTHrP(1–34) (PeproTech Inc.) was added. After 48 hrs, cell numbers were counted using Cell Counting Kit-8 (DOJINDO, Kumamoto, Japan). Inhibitors (AZD4547: Abcom, Cambridge, UK; U0126: Wako Pure Chemical Industries, Osaka, Japan; LY294002: Merck Calbiochem, Darmstadt, Germany; Akt inhibitor: Merck Calbiochem) were added 1 h before the addition of FGF2.
Droplet digital PCR
The absolute quantity of mRNA was measured by the QX200 Droplet Digital PCR System (BIO-RAD) using EvaGreen application reagents (BIO-RAD). The absolute values of mRNA were normalized to those of β-actin mRNA.
Statistical analysis
Data are described as the mean ± SEM, if not specified. Statistical analyses were performed using an analysis of variance followed by the Tukey-Kramer test. P < 0.05 was considered to be significant.
Electronic supplementary material
Acknowledgements
We thank J.F. Martin for the Prrx1 promoter, H. Hamada for the Shh probe, G. Martin for the Fgf4 probe, S. Kato for the Fgf10 probe, N. Kanatani for the generation of transgenic mice, and S. Hamachi for secretarial assistance. This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology to TK (Grant number: 26221310) and to TK (Grant number: 15K11048).
Author Contributions
T.K. and X.Q. contributed to most of the experiments. Q.J., C.S., and Y.D. contributed to the cell proliferation assay. T.M. contributed to the immunohistochemistry. H.K. and R.N. contributed to the generation of mice. C.Y. contributed to the repoter assay. V.M. contributed to ChIP. Y.M. contributed to skeletal preparation and microarray data analysis. K.N. contributed to the cell sorting. T.M. contributed to whole mlount in situ hybridization. T.K. designed and supervised the project and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31853-0.
References
- 1.Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–755. doi: 10.1002/jcb.22994. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends in Genetics. 2003;19:458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 3.Galindo M, et al. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J Biol Chem. 2005;280:20274–20285. doi: 10.1074/jbc.M413665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghali O, Chauveau C, Hardouin P, Broux O, Devedjian JC. TNF-α’s effects on proliferation and apoptosis in human mesenchymal stem cells depend on RUNX2 expression. J Bone Miner Res. 2010;25:1616–1626. doi: 10.1002/jbmr.52. [DOI] [PubMed] [Google Scholar]
- 5.Lucero CM, et al. The cancer‐related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J Cell Physiol. 2013;228:714–723. doi: 10.1002/jcp.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratap J, et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003;63:5357–5362. [PubMed] [Google Scholar]
- 7.Thomas DM, et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol. 2004;167:925–934. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ornitz, D. M. & Itoh, N. Fibroblast growth factors. Genome Biol2, Reviews 3005 (2001). [DOI] [PMC free article] [PubMed]
- 9.Britto, J. A., Evans, R. D., Hayward, R. D. & Jones, B. M. From genotype to phenotype: the differential expression of FGF, FGFR, and TGFbeta genes characterizes human cranioskeletal development and reflects clinical presentation in FGFR syndromes. Plast Reconstr Surg 108, 2026–2039, discussion 2040–2046 (2001). [DOI] [PubMed]
- 10.Iseki S, Wilkie AO, Morriss-Kay GM. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development. 1999;126:5611–5620. doi: 10.1242/dev.126.24.5611. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D, Iseki S, Wilkie AO, Morriss-Kay GM. Expression patterns of Twist and Fgfr1, -2 and -3 in the developing mouse coronal suture suggest a key role for twist in suture initiation and biogenesis. Mech Dev. 2000;91:341–345. doi: 10.1016/S0925-4773(99)00278-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Rice DP, Kettunen PJ, Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohbayashi N, et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quarto N, Behr B, Li S, Longaker MT. Differential FGF ligands and FGF receptors expression pattern in frontal and parietal calvarial bones. Cells Tissues Organs. 2009;190:158–169. doi: 10.1159/000202789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice DP, et al. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127:1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- 17.Cohen M. M. FGFs/FGFRs and associated disorders. In: Epstein, C. J., Erickson, R. P. & Wynshaw-Boris, A. (eds). In inborn errors of development Oxford Univeristy Press: New York (2004).
- 18.Ohuchi H, et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, et al. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- 20.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth F R. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34:280–300. doi: 10.1002/med.21288. [DOI] [PubMed] [Google Scholar]
- 22.Martin JF, Olson EN. Identification of a prx1 limb enhancer. Genesis. 2000;26:225–229. doi: 10.1002/(SICI)1526-968X(200004)26:4<225::AID-GENE10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Maeno T, et al. Early onset of Runx2 expression caused craniosynostosis, ectopic bone formation, and limb defects. Bone. 2011;49:673–682. doi: 10.1016/j.bone.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Ornitz DM, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 25.Yu K, Herr AB, Waksman G, Ornitz DM. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc Natl Acad Sci USA. 2000;97:14536–14541. doi: 10.1073/pnas.97.26.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orr-Urtreger A, et al. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2) Dev Biol. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi M, Finch PW, Aaronson SA. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7) J Biol Chem. 1998;273:13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- 28.Min H, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nature genetics. 2000;26:455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nature genetics. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 32.Boulet AM, Moon AM, Arenkiel BR, Capecchi MR. The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol. 2004;273:361–372. doi: 10.1016/j.ydbio.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Todt WL, Fallon JF. Posterior apical ectodermal ridge removal in the chick wing bud triggers a series of events resulting in defective anterior pattern formation. Development. 1987;101:501–515. doi: 10.1242/dev.101.3.501. [DOI] [PubMed] [Google Scholar]
- 34.Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371:609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- 35.Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 36.Teplyuk NM, et al. The osteogenic transcription factor Runx2 regulates components of the fibroblast growth factor/proteoglycan signaling axis in osteoblasts. J Cell Biochem. 2009;107:144–154. doi: 10.1002/jcb.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato M, et al. Transcriptional regulation of osteopontin gene in vivo by PEBP2alphaA/CBFA1 and ETS1 in the skeletal tissues. Oncogene. 1998;17:1517–1525. doi: 10.1038/sj.onc.1202064. [DOI] [PubMed] [Google Scholar]
- 38.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 39.Inada M, et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 40.Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80:159–170. doi: 10.1016/S0925-4773(98)00210-X. [DOI] [PubMed] [Google Scholar]
- 41.Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274:12827–12834. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- 42.Wilkie AO, Patey SJ, Kan SH, van den Ouweland AM, Hamel BC. FGFs, their receptors, and human limb malformations: clinical and molecular correlations. Am J Med Genet. 2002;112:266–278. doi: 10.1002/ajmg.10775. [DOI] [PubMed] [Google Scholar]
- 43.Fujita T, et al. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge C, et al. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem. 2009;284:32533–32543. doi: 10.1074/jbc.M109.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park OJ, Kim HJ, Woo KM, Baek JH, Ryoo HM. FGF2-activated ERK mitogen-activated protein kinase enhances Runx2 acetylation and stabilization. J Biol Chem. 2010;285:3568–3574. doi: 10.1074/jbc.M109.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002;277:36181–36187. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- 47.Zaidi, S. K. et al. Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proceedings of the National Academy of Sciences, 104, 19861–19866 (2007). [DOI] [PMC free article] [PubMed]
- 48.Mundlos S, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/S0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 49.Qin X, et al. Cbfb regulates bone development by stabilizing Runx family proteins. J Bone Miner Res. 2015;30:706–714. doi: 10.1002/jbmr.2379. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Q, et al. Cbfb2 Isoform Dominates More Potent Cbfb1 and Is Required for Skeletal Development. J Bone Miner Res. 2016;31:1391–1404. doi: 10.1002/jbmr.2814. [DOI] [PubMed] [Google Scholar]
- 51.Maes C, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owens TW, et al. Runx2 Is a Novel Regulator of Mammary Epithelial Cell Fate in Development and Breast Cancer. Cancer Research. 2014;74(18):5277–5286. doi: 10.1158/0008-5472.CAN-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald L, et al. RUNX2 correlates with subtype-specific breast cancer in a human tissue microarray, and ectopic expression of Runx2 perturbs differentiation in the mouse mammary gland. Dis Model Mech. 2014;7:525–534. doi: 10.1242/dmm.015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Easton DF, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunter DJ, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer KB, et al. Allele-specific up-regulation of FGFR2 increases susceptibility to breast cancer. PLoS Biol. 2008;6:e108. doi: 10.1371/journal.pbio.0060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer KB, et al. Fine-scale mapping of the FGFR2 breast cancer risk locus: putative functional variants differentially bind FOXA1 and E2F1. Am J Hum Genet. 2013;93:1046–1060. doi: 10.1016/j.ajhg.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu X, Asa SL, Ezzat S. Histone-acetylated control of fibroblast growth factor receptor 2 intron 2 polymorphisms and isoform splicing in breast cancer. Mol Endocrinol. 2009;23:1397–1405. doi: 10.1210/me.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura R, et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem. 2012;287:33179–33190. doi: 10.1074/jbc.M111.337063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maruyama Z, et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn. 2007;236:1876–1890. doi: 10.1002/dvdy.21187. [DOI] [PubMed] [Google Scholar]
- 62.Ueta C, et al. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J Cell Biol. 2001;153:87–100. doi: 10.1083/jcb.153.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.