Abstract
The organization of the human insular cortex has traditionally been considered as an anterior–posterior dichotomy, where anterior and posterior subdivisions have unique structural and functional connections. However, recent functional neuroimaging research proposes a tripartite organization where insular subdivisions have both unique and overlapping functional profiles. Studies examining unique profiles show that the dorsal anterior insula (dAI) has connections with frontal areas supporting higher-level cognitive processes, the ventral anterior insula (vAI) has connections with limbic areas supporting affective processes, and the posterior insula (PI) has connections with sensorimotor areas supporting interoceptive processes. Studies examining overlapping profiles demonstrate that all 3 subdivisions can also have similar functional profiles. The structural organization supporting a functional tripartite insula organization presenting with overlapping and unique connections is currently unknown. We used a large HARDI diffusion magnetic resonance imaging (MRI) dataset (n = 199) to demonstrate novel visualizations of insula white matter tracts supporting a tripartite structure–function insula organization. Overlapping connections of all 3 insula subdivisions consisted of association pathways (inferior fronto-occipital fasciculus, uncinate fasciculus, arcuate fasciculus) while unique connections included the corona radiata, subcortical-cortical tracts, and horizontal and u-shaped tracts. These results generally support a tripartite structure–function organization of the insular cortex, with subdivisions that exhibit both overlapping and unique connectivity profiles.
Keywords: diffusion MRI, insula, structure–function
Introduction
The structural and functional subdivisions of the insular cortex have been delineated from 2 different perspectives. In vivo approaches for structural connectivity mapping using diffusion weighted imaging in humans typically converge on an anterior–posterior division, where the anterior insula has structural connections with temporal, frontal, and occipital areas while the posterior insula (PI) has connections with parietal, frontal, and sensorimotor areas (Cerliani et al. 2012). This finding has support from resting-state functional connectivity research (Cauda et al. 2011b, 2012). However, other functional connectivity research has identified unique functional subdivisions according to a tripartite (Deen et al. 2011; Chang et al. 2012), and 4 subdivision (Kurth et al. 2010) parcellation while investigations focusing purely on parcellating the insula have identified up to 13 different insula subdivisions (Kelly et al. 2012; Glasser et al. 2016). It is currently unclear how structural connections underlie such functional parcellations consisting of more than 2 insula subdivisions. The current study aimed to examine the structural connections that could support a tripartite functional parcellation scheme of the human insular cortex.
Only a few in vivo studies in humans have focused on mapping the whole-brain structural connections of the insula (Cerliani et al. 2012; Cloutman et al. 2012; Dennis et al. 2014; Jakab et al. 2012; Ghaziri et al. 2017). Three studies used traditional diffusion tensor imaging (DTI) approaches. Jakab et al. (2012) (n = 40; 12 gradient directions) used a probabilistic DTI approach to demonstrate an anterior–posterior trajectory of connections where the anterior insula had connections with superior temporal, inferior frontal, orbitofrontal, opercular, and occipital areas while the PI had connections with inferior parietal, middle/superior temporal, inferior frontal, and sensorimotor areas. Additionally, the anterior and posterior subdivisions had common occipital connections. Cerliani et al. (2012) (n = 10; 15 gradient directions) used a probabilistic diffusion tensor approach to demonstrate that the anterior insula was connected with inferior frontal, orbitofrontal, and the superior temporal pole, while the PI was connected with posterior parietal and superior/middle temporal areas. Mid-insula areas showed mixed connectivity patterns consisting of both anterior and PI connections. Cloutman et al. (2012) (n = 24; 61 non-collinear directions) used a probabilistic tracking method to demonstrate that the anterior insula had connections with inferior frontal, orbitofrontal, and anterior polar temporal regions, while the PI had connections with posterior and anterior temporal regions. Ghaziri et al. (2017) (n = 46 subjects; 60 gradient directions) used a deterministic algorithm applied to high angular resolution diffusion-weighted imaging (HARDI) data to demonstrate an anterior–posterior organization in accord with previous structural work, and also found connections between the insula and anterior cingulate that were previously identified in only a few studies (van den Heuvel et al. 2009; Uddin 2011; Sotiropoulos et al. 2013; Wiech et al. 2014). Taken together, these structural investigations have identified a general anterior–posterior structural organization of the human insula.
Functional magnetic resonance imaging (fMRI) investigations of insular cortex connectivity have demonstrated the aforementioned anterior–posterior parcellation (Cauda et al. 2011b, 2012), a tripartite parcellation with dorsal anterior, ventral anterior, and posterior subdivisions (Deen et al. 2011; Chang et al. 2012), and a 4 subdivision parcellation consisting of the 3 tripartite subdivisions and a fourth mid-posterior subdivision (Kurth et al. 2010). One well-replicated tripartite cognition-emotion-interoception functional division proposes that the dorsal anterior insula (dAI) is connected to the anterior cingulate and frontal areas involved in higher-level cognition, the ventral anterior insula (vAI) is connected to limbic areas involved in affective processing, and the PI is connected to sensorimotor areas involved in interoceptive processes (Deen et al. 2011; Chang et al. 2012; Uddin et al. 2014; Uddin 2015). Additional recent functional neuroimaging investigations show that these tripartite subdivisions can also behave in a homogeneous manner. For example, Uddin et al. (2014) demonstrated that although each of 3 individual functional subdivisions had unique task-related co-activation patterns consistent with a cognition-emotion-interoception tripartite parcellation, there was also substantial functional profile overlap of all 3 subdivisions. Nomi et al. (2016) demonstrated that while a static resting-state functional connectivity analysis supported a cognition-emotion-interoception parcellation, time-varying functional connectivity analyses demonstrated that tripartite insula subdivisions sometimes present with overlapping functional connectivity profiles. Thus, although there is evidence for a tripartite insula parcellation where each subdivision presents with unique functional profiles, these same subdivisions can also act in concert, presenting at times with similar, overlapping functional profiles.
Although both structural and functional investigations of the insula have developed in parallel, few whole-brain investigations have attempted to directly converge across imaging modalities (for targeted ROI-based structure–function insula investigations, see (Uddin 2011; Wiech et al. 2014). It is important to identify structure–function relationships because such relationships are used to create more sophisticated functional connectivity maps (Calamante et al. 2013) and brain parcellations (Glasser et al. 2016) that better identify developmental trajectories (Supekar et al. 2010; Uddin 2011), and better classify clinical disorders such as schizophrenia (Skudlarski et al. 2010; van den Heuvel et al. 2013), epilepsy (Zhang et al. 2011), and depression (de Kwaasteniet et al. 2013). Thus, developing structure–function models of the insular cortex may offer better predictive power in differentiating developmental stages and identifying clinical biomarkers than either structural or functional measures alone.
The current study builds on previous work by using the largest (n = 199) multi-shell HARDI diffusion dataset (Sotiropoulos et al. 2013) to date to identify whole-brain structural connections of the insula. We employ a standard-space normalization scheme to facilitate visualization of combined individual-level white matter tracts. Visualization of pathway trajectories is important to compare identified fiber bundles with known structural anatomy from the human and non-human primate literature. Additionally, we approach the interpretation of insula structure–function relationships in a novel framework compared with previous structural studies that have proposed an anterior–posterior dichotomy. We build on previous functional work (Deen et al. 2011; Chang et al. 2012; Uddin et al. 2014; Nomi et al. 2016) to determine if structural connections are able to support a tripartite conception of insula organization, where 3 insula subdivisions demonstrate both overlapping and unique connections. To investigate how structural connections arise from functional insular cortex subdivisions in the human brain, the current study used 6 bilateral insula subdivisions derived from a voxel-wise k-means clustering approach applied to resting-state fMRI data (Deen et al. 2011). We used these 6 insula subdivisions in the current study as regions-of-interest (ROIs) to identify structural connections with brain areas delineated by an anatomical parcellation (AAL atlas) (Tzourio-Mazoyer et al. 2002).
We hypothesized a large degree of overlap of structural connections across all 3 tripartite insula functional subdivisions. In addition, we hypothesized that the dAI would have unique structural connections related to network switching and higher-order cognitive processes, the vAI would exhibit unique structural connections related to affective processing, and the PI would have unique connections related to sensorimotor processing (Uddin 2015). Such findings would support a tripartite structure–function organization of the insula with both overlapping and unique structural and functional connections of insula subdivisions, in contrast to an anterior–posterior structure–function organization where anterior and posterior subdivisions demonstrate unique structural and functional connections.
Methods
Participants
Diffusion MRI data for 199 participants (all right-handed; 22–35 years of age: M = 28.60 years old, SD = 3.87; 104 females) were acquired from the Human Connectome Project (https://www.humanconnectome.org) 500 subject data release. The inclusion criteria required that subjects were not twins, right-handed, and had a diffusion MRI dataset.
Data Acquisition and Preprocessing
Diffusion MRI data was collected on a specialized Siemens 3 T Skyra system using a single-shot 2D spin-echo multiband (factor of 3) EPI acquisition (1.25 mm isotropic; FOV PE × Readout = 210 × 180; matrix size PE × Readout = 144 × 168; 111 interleaved slices without gap; left–right and right–left phase encoding; flip angles = 78° and 160°). Sampling in q-space consisted of 3 shells at b = 1000, 2000, and 3000 s/mm2 with TE (89 ms) and TR (5.5 s) matched across all shells. Multiband dMRI acquisition parameters have shown to increase q-space coverage while multi-shell schemes resolve crossing fibers better than single-shell acquisitions (Sotiropoulos et al. 2013). Each shell contains 192 data points representing 90 diffusion gradient directions and 6 b = 0 shells acquired twice resulting in 270 non-conlinear directions for each PE. Total acquisition time was approximately 54 min (6 segments of 9 min each). Preprocessing of dMRI data was conducted by the HCP team, and mainly focused on distortion correction (susceptibility, eddy-current induced, and head-motion). For more details, see (Sotiropoulos et al. 2013).
Regions-of-Interest
Six masks representing a tripartite functional parcellation of the insula into bilateral dAI, vAI, and PI (Deen et al. 2011; Uddin et al. 2014) were used as insula ROIs (Supplementary Fig. 1). The 6 insula ROIs were acquired in 2 mm Montreal Neurological Institute (MNI) space directly from authors of a previous manuscript that applied a k-means clustering algorithm to voxel-wise functional connectivity estimates between the insula and the rest of the brain (for more info see Deen et al. 2011). This clustering approach produced bilateral dAI, vAI, and PI subdivisions that have been replicated in other work (Chang et al. 2012; Nomi et al. 2016) and utilized in subsequent work (Uddin et al. 2014).
The AAL atlas consisted of 116 cortical and subcortical brain areas, of which 114 areas (the 2 insula areas were discarded and replaced with the functional tripartite parcellation) were used in the current study (67 areas per hemisphere) (Supplementary Fig. 1).
Data Analysis
Diffusion data were reconstructed using DSI studio software (http://dsi-studio.labsolver.org) by implementing a q-space diffeomorphic reconstruction algorithm (QSDR) (Yeh and Tseng 2011; Yeh et al. 2013b). The QSDR algorithm transforms diffusion data from subject space into normalized space by producing spin distribution function (SDF) maps in 2 mm MNI space. A separate analysis using diffusion deconvolution (Yeh et al. 2011) and the same analysis parameters can be found in Supplementary Table 1.
White matter tracts were identified using a generalized deterministic fiber-tracking algorithm in DSI studio (Yeh et al. 2013a) using sub-voxel randomized seeding (10^5 seeds) and trilinear interpolation. A large number of seeds were used as increasing the number of seeds has been shown to reduce tract variability and to facilitate the identification of long-distance tracts (Cheng et al. 2012). Tract progression continued with a step size of 1 mm, minimum fiber length of 20 mm, and a turning angle of 60°. Quantitative anisotropy (QA) values were calculated by subtracting reconstructed SDFs from their minimum values in order to estimate the number of spins diffusing preferentially along the fiber orientation. DSI studio automatically calculated the minimum QA values used to terminate tracts based on individual signal-to-noise ratios (Mean subject QA = 0.33, SD = 0.06, subject average range = 0.19–0.55) (Verstynen et al. 2011). Using an individual subject threshold is important to account for differences in signal-to-noise ratios across subjects.
Because disentangling efferent or afferent structural connections using tractography algorithms is not feasible, tracts were calculated twice for each ROI pair; the insula ROI was a seed and the AAL atlas region was an end, and vice versa (Ghaziri et al. 2017). Following previous investigations looking at binary structural connections (connection/no connection), a subject level threshold of at least 1 streamline was used as a criterion to determine if a connection between 2 ROIs existed (Hagmann et al. 2008; Bassett et al. 2011; van Den Heuvel and Sporns 2011; Hermundstad et al. 2013; Betzel et al. 2014). A connection between 2 ROIs at the subject level was considered to exist as long as 1 tract was identified from either analysis (seed-end or end-seed). When calculating the average number of tracts for each connection for descriptive purposes, the number of tracts from both connections (seed-end and end-seed) was used in the average.
Following previous tractography work investigating whole-brain structural connections of the insular cortex, both strict and liberal group thresholds were used to determine if a connection existed at the group level (Cloutman et al. 2012): a stringent criterion where 75% (≥150 subjects) of subjects had at least 1 tract present between ROIs and a more liberal criterion where 50% of subjects (≥100 subjects) had at least 1 tract connecting both ROIs. This helped to enforce sparsity of identified structural connections, as typically reported in diffusion investigations (Bassett et al. 2011).
Results
Streamlines and QA
The average number of streamlines identified for each subject for each possible connection across all ROIs (including connections that did and did not survive the group thresholds) was 270.88 (SD = 91.22, range = 83.16–507.91). Tracts were not normalized to ROI size because the purpose of the current study was to identify connections between ROIs in a binary matter (connection/no connection) rather than a quantitative manner (determining if 1 connection was stronger than another) (Van Den Heuvel and Sporns 2011).
There were 50 connections surpassing the 50% threshold and 63 connections surpassing the 75% threshold (Table 1). The total number of connections that survived both group thresholds was 113 out of 684 (16.52% of possible connections: 6 insula ROIs × 114 AAL ROIs). Thus, structural connections between insula ROIs and AAL ROIs in the current study showed a similar proportion of sparse connections as previous research using diffusion tensor imaging (DTI: 15%) and diffusion spectrum imaging (DSI: 16%) reconstruction techniques across 3 different a priori parcellations; this also included the AAL atlas (Bassett et al. 2011).
Table 1.
Structural connections of dAI, vAI, and PI to AAL atlas ROIs. Each “x” marks a connection at the more strict 75% criterion while each “o” marks a connection at the more liberal 50% criterion. The top section shows connections common to all 3 insula subdivisions while the next 3 sections shows unique connections for the dAI, vAI, and PI, respectively. The last section shows connections that were not overlapping across all 3 insula subdivisions and were not unique to any single insula subdivision
| Left | Right | |||||||
|---|---|---|---|---|---|---|---|---|
| dAI | vAI | PI | dAI | vAI | PI | |||
| Occipital middle | x | x | x | o | x | o | Bilateral | Overlapping |
| Putamen | x | o | x | x | x | x | Bilateral | |
| Temporal middle | o | x | x | o | o | o | Bilateral | |
| Angular | o | o | o | o | o | Right | ||
| Temporal superior | x | x | o | o | o | Right | ||
| Parietal inferior | o | x | o | o | o | Right | ||
| Parietal superior | x | x | o | o | x | Right | ||
| Temporal inferior | o | o | x | x | x | Left | ||
| Temporal pole superior | x | o | x | x | Left | |||
| Frontal inferior orbital | x | x | o | x | x | Left | ||
| Occiptial superior | o | x | o | o | x | Left | ||
| Frontal superior medial | x | o | Bilateral | Unique dAI | ||||
| Frontal inferior operculum | x | x | Bilateral | |||||
| Pallidum | o | o | Bilateral | |||||
| Supramarginal | o | Right | ||||||
| Thalamus | x | left | ||||||
| Amygdala | x | x | Bilateral | Unique vAI | ||||
| Rectus | o | o | Bilateral | |||||
| Frontal superior orbital | x | x | Bilateral | |||||
| Olfactory | o | x | Bilateral | |||||
| Calcarine | o | o | Bilateral | |||||
| Hippocampus | x | Right | ||||||
| Cuneus | o | Right | ||||||
| Parahippocampal | o | Left | ||||||
| Precuneus | o | Left | Unique PI | |||||
| Frontal inferior tri | x | o | ||||||
| Frontal middle | x | o | x | o | ||||
| Frontal superior | x | x | x | o | ||||
| Heschl | x | x | ||||||
| Occipital inferior | o | o | ||||||
| Precentral | x | x | ||||||
| Postcentral | x | x | o | x | ||||
| Rolandic operculum | x | x | x | x | ||||
| Frontal mid orbital | x | x | o | x | ||||
| Temporal pole middle | x | x | o | |||||
| Supplementary motor area | x | x | o | |||||
Although the aim of the current study was not to identify differences in strength between various connections, something that would necessitate quatifying the average number of tracts or anisotropy values for each connection, tables representing the average number of tracts (Supplementary Table 2), and the minimum number of tracts needed for a particular connection to exist at the group level, are provided for the interested reader in Supplementary Materials (Supplementary Tables 3 and 4).
Overlapping Connections Across Insular Subdivisions
All overlapping connections for insula subdivisions are shown together in Figure 1 while overlapping connections in isolation are shown in Supplementary Figures 2–4. Tracts for both overlapping and unique structural connections were visualized by overlaying all individual subject level tracts (acquired in 2 mm MNI space) into a common 2 mm MNI template and then removing any extraneous projections. Note that the figures thus represent the sum of all tracts observed across all individual subjects for each connection. Common structural connections for all 3 insula subdivisions were mainly found when using the more liberal criterion (50% of subjects demonstrated a connection). Overlapping bilateral connections were found for the mid-occipital, mid-temporal AAL ROIs and the putamen. Overlapping connections for all right insula subdivisions were found for the angular, superior temporal, inferior parietal, and superior parietal AAL ROIs. Overlapping connections for all left insula subdivisions were found for the inferior temporal, superior temporal pole, frontal inferior orbital, and superior occipital ROIs. Overall, these findings demonstrate overlapping structural connections for all 3 insular subdivisions to inferior orbital frontal, inferior/middle/superior temporal, inferior/superior parietal, middle/superior occipital, and subcortical brain areas. These analyses identify a common set of fiber pathways that could facilitate coordinated activity across insular subdivisions, allowing them to act in concert in certain circumstances.
Figure 1.
Top: common structural connections of all 3 insula subdivisions consisting mainly of association tracts such as the inferior fronto-occipital fasciculus, arcuate fasciculus, and uncinate fasciculus. Middle: Unique structural connections of the dAI (red) and vAI (green). Numbers refer to figures representing these tracts in isolation. 1: Figure 2A; 2: Figure 2B; 3: Figure 2D; 4: Figure 2C; 5: Figure 2E; 6: Figure 3A; 7: Figure 3B; 8: Figure 3C; 9: Figure 3E; 10: Figure 3F; 11: Figure 3G. Individual tracts were acquired in MNI space and figures were produced by overlaying individual tracts onto a common MNI template, and then removing any extraneous tracts. Bottom: AAL ROIs representing unique and overlapping connections.
Unique Connections for Insular Subdivisions
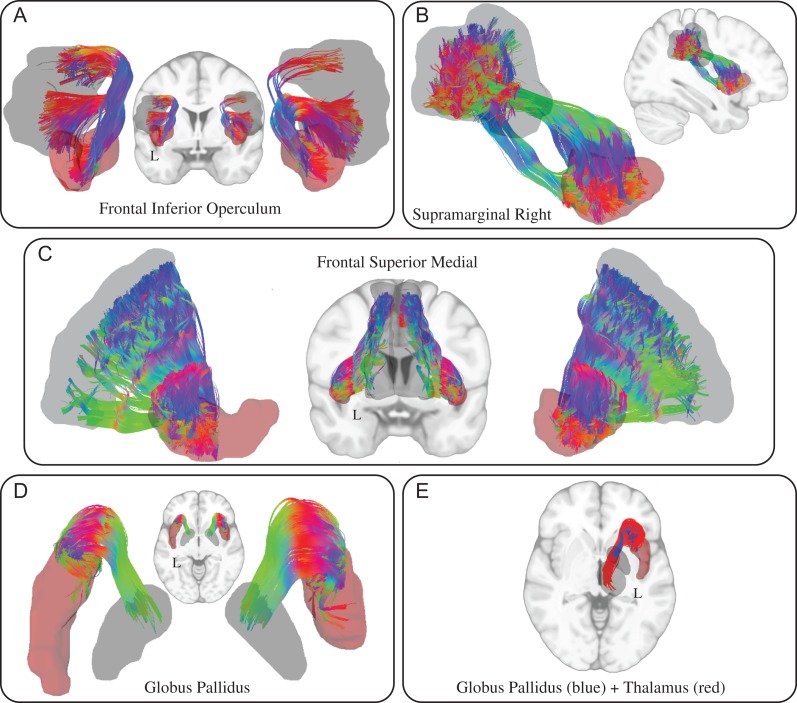
A unique connection was considered to exist only if no other insula subdivision demonstrated that same connection in either hemisphere. For example, the supplementary motor area (SMA) was not considered a unique connection for the right dAI (Table 1) because the left dAI and left PI in the contralateral hemisphere also showed connections to the SMA. This was because it was considered likely that if a connection existed for a subdivision in either hemisphere, that connection was also likely to exist in the other hemisphere, but did not surpass the group threshold cutoff. Unique connections for the dAI were found for bilateral medial pre-frontal ROIs, bilateral frontal inferior operculum (FIO) ROIs, and bilateral thalamus ROIs (Fig. 2). Isolated individual-level tracts demonstrating the heterogeneity of identified pathways are presented in Supplementary Figures 5 and 6. The unique connections of the dAI to bilateral medial pre-frontal cortices and bilateral FIO cortices are in accord with a functional framework demonstrating interactions between the dAI and frontal areas involved in higher-level cognitive processes.
Figure 2.
Combined individual-level tracts representing unique connections for the dAI. Tracts were combined across all subjects in MNI space with any extraneous tracts removed.
Unique connections for the vAI were found bilaterally for the amygdala, superior orbitofrontal cortex, olfactory cortex, calcarine cortex, and the gyrus rectus (Fig. 3). Isolated individual-level tracts demonstrating the heterogeneity of identified pathways are presented in Supplementary Figures 7–9. Unique ipsilateral connections were found for the right vAI to the hippocampus and cuneus, and for the left VAI to the parahippocampal gyrus. The unique connections of the vAI to the amygdala and olfactory cortex are in accord with a functional framework demonstrating interactions between the vAI and limbic areas involved in affective processing.
Figure 3.
Combined individual-level tracts representing selected unique connections for the vAI. Tracts were combined across all subjects in MNI space with any extraneous tracts removed. L = left, R = right.
Finally, there was only 1 unique ipsilateral connection for the left PI that was to the precuneus. Although the PI did have a unique connection not found for the other insula subdivisions, it was not a sensorimotor connection, as might be predicted based on functional connectivity of the PI.
Discussion
The current study sought to determine how structural connections of the human insula are related to a purported tripartite functional parcellation (dAI, vAI, and PI) of this brain region (Deen et al. 2011; Chang et al. 2012; Uddin et al. 2014; Nomi et al. 2016). The findings demonstrated that all 3 insular subdivisions in both hemispheres shared common structural connections to inferior orbitofrontal, middle/superior occipital, inferior/middle/superior temporal, inferior/superior parietal, and subcortical brain areas, in accord with reports of partially overlapping functional profiles of these subdivisions. Additionally, the dAI had unique connections with the medial frontal cortex and FIO, consistent with its proposed role in higher-order cognitive processes and network switching, while the vAI had unique connections with the superior orbitofrontal cortex, amygdala, and olfactory cortex, in accord with its proposed role in affective processing (Uddin 2015). These structural connections in part recapitulate a tripartite cognition-emotion-interoception functional parcellation scheme of the insula. However, there were no unique sensorimotor connections associated with the PI, in contrast to previous work showing unique static functional relationships related to interoceptive processes for the PI (Deen et al. 2011; Uddin et al. 2014).
Fiber Bundles Contributing to Common Structural Connections
Major association pathways such as the inferior fronto-occipital fasciculus (IFOF), the arcuate fasciculus (AF), and the uncinate fasciculus (UF), were largely responsible for a number of overlapping connections to the mid and superior occipital cortices, the inferior and superior parietal cortices, and to the inferior, middle, and superior temporal cortices. In addition, a number of smaller u-shaped fibers that characterized connections of adjacent cortical areas (Catani et al. 2012) formed connections between the insula and the inferior orbitofrontal cortex and the superior temporal pole. The IFOF is a horizontal fiber bundle that connects frontal and occipital/parietal areas of the brain, the UF is a short curved fiber bundle connecting inferior frontal and anterior temporal areas and insula, and the AF is a large curved fiber fundle that connects inferior frontal, parietal, and temporal areas of the brain (Catani et al. 2002).
The IFOF mainly connected all 3 insula subdivisions to the bilateral mid-occipital cortices, the left superior occipital cortex, and the inferior and superior parietal cortices. The AF mainly connected all 3 insula subdivisions with bilateral middle temporal cortices, the left inferior temporal cortex, and the right superior temporal cortex. In addition, the AF also connected all 3 subdivisions to the right angular gyrus and the inferior parietal cortex, and the dAI and PI to the middle occipital cortex in both hemispheres. Finally, the UF supported connections between all 3 insula subdivisions and the left inferior temporal cortex, and between 5 subdivisions (excluding the right PI) with the middle temporal cortex.
Fiber Bundles Contributing to Unique Structural Connections
While common structural connections of all 3 insula subdivisions were mainly supported by major association pathways such as the IFOF, UF, and AF, unique structural connections were largely supported by the corona radiata, cortical-striatal projections, and a number of smaller u-shaped and horizontal localized fiber bundles. The most amount of unique connections originated in the anterior portion of the insula; the dAI and vAI subdivisions had ipsilateral unique structural connections in both hemispheres while the PI only had a single unique ipsilateral structural connection to the precuneus in the left hemisphere.
Unique Structural Connections: dAI
The unique connections of the dAI were found for bilateral FIO cortices, bilateral fontal superior medial cortices, bilateral pallidum, the right supramarginal gyrus, and the left thalamus (Fig. 2). The unique structural connections to frontal brain areas such as the FIO (located in the ventral frontal cortex) and medial pre-frontal cortex may support language processing, attention, and executive function. These unique structural connections in combination with its overlapping structural connections may help to explain the high levels of functional diversity observed for the dAI (Kurth et al. 2010; Uddin et al. 2014; Nomi et al. 2016), and also the dAI’s role in higher-level cognitive processing (Deen et al. 2011; Chang et al. 2012; Yeo et al. 2015; Uddin 2015).
The bilateral FIO cortices were connected to the dAI through 2 u-shaped tracts: 1 connected to the ventral- (Catani et al. 2012) and 1 connected to the dorsal-FIO (Fig. 2A). The left hemisphere dAI-FIO structural connection has been implicated in language processing (Cerliani et al. 2012; Cloutman et al. 2012) while functional fMRI meta-analyses (Ardila et al. 2014), fMRI clinical investigations (Duffau et al. 2001), and clinical lesion studies (Ardila et al. 1997) implicate the left dAI in language and speech processes. The right hemisphere dAI-FIO connection is in accord with previous structural (Cloutman et al. 2012; Wiech et al. 2014) and functional (Farrant and Uddin 2015) investigations. The right dAI and right FIO are key nodes in the salience (Menon and Uddin 2010) and the ventral attention network (Corbetta et al. 2008), respectively. This connection may facilitate the dAI and FIO acting in concert during cognitive control tasks (Levy and Wagner 2011), however, they may also act independently, with the dAI involved in the detection of salient inhibitory cues and the frontal inferior operculum involved in anticipation of inhibitory cues (Cai et al. 2014)
Ipsilateral connections in both hemispheres between the dAI and bilateral frontal medial cortices were supported by frontal-striatal projections consisting of fibers intersecting with the corona radiata, frontal aslant tract (Broce et al. 2015), and corpus collosum (Fig. 2C). The right dAI also had a connection to the ipsilateral supramarginal gyrus through a ventral pathway that was part of the IFOF and a dorsal pathway apparently consisting of the superior longitudinal fasciculus III (Thiebaut de Schotten et al. 2011) (Fig. 2B). The superior medial frontal cortex (medial pre-frontal cortex; MPFC) and supramarginal gyri are major nodes of the default mode network (DMN) and central executive (CE) networks, respectively. Although brain regions such as the dAI comprising the salience network (SN) are typically anti-correlated from the DMN (MPFC) (Fox et al. 2005) in static network connectivity approaches, research suggests 2 ways that communication can occur between the SN and these other 2 networks. First, the right dAI has effective connections with default network nodes, where right dAI activity causally influences activity in DMN nodes (Sridharan et al. 2008). Second, time-varying functional connectivity analysis has shown that the bilateral dAI can have transient functional connections with the MPFC (Chang and Glover 2010; Nomi et al. 2016). The current study demonstrated unique bilateral structural connections between the dAI and MPFC that may help to facilitate effective and time-varying communication between there regions.
Connections between the dAI and subcortical areas such as the globus pallidus in both hemispheres and the thalamus in the left hemisphere were supported by anterior–thalamic (Catani et al. 2012) projections (Fig. 2D and E).
The thalamic connection replicates a similar structural connection in the macaque monkey (Mufson and Mesulam 1984) and humans (Wiech et al. 2014), in addition to a functional relationship related to reward processing in humans (Cauda et al. 2011a; Cho et al. 2013). The thalamus, insula, and nucleus accumbens form the key nodes of a reward processing network identified through meta-analytic techniques (Knutson and Greer 2008; Liu et al. 2011).
Unique Structural Connections: vAI
Unique connections for the vAI were found bilaterally for the amygdala, rectus, frontal superior orbital cortices, olfactory, and calcarine cortices (Fig. 3). Additional unique ipsilateral connections were found for the left vAI with the parahippocampal area and for the right vAI with the cuneus and hippocampus. The vAI-amygdala connection replicates previous structural (Cerliani et al. 2012), functional (Deen et al. 2011; Chang et al. 2012; Uddin et al. 2014; Nomi et al. 2016), and meta-analytic research (Mutschler et al. 2009). The vAI-amgydala connection in the current study was represented through 2 pathways in each hemisphere: a small horizontal fiber bundle and a small u-shaped fiber bundle that was directly adjacent to the UF, but curving inward rather than outward as the UF does. The same u-shaped fiber bundle connecting the vAI and the amygdala also connected the vAI to the parahippocampal cortex in addition to another small horizontal fiber bundle (Fig. 3D).
The vAI-frontal superior orbital and vAI-gyrus rectus connections (Fig. 3C) were both supported by the fronto-orbitopolar tract (Catani et al. 2012) while the vAI-olfactory cortex connection was supported by a horizontal twisted fiber bundle in both hemispheres. The vAI-olfactory connection builds on macaque monkey tracer work where general anterior, but not posterior, insula connections to the olfactory cortex were reported (Mufson and Mesulam 1982). The vAI was also connected to the hippocampus in the right hemisphere by a medial tract that was part of the IFOF, and a more lateral curved horizontal fiber tract and was connected to the calcarine cortex in both hemispheres and the cuneus in the right hemisphere through the IFOF.
The amygdala and the olfactory connections are also consistent with previous research demonstrating a functional relationship between the olfactory cortex, amygdala, and insula when making affective odor decisions (Zald and Pardo 1997; Rolls et al. 2010; Soudry et al. 2011). These connections also support a functional network consisting of the anterior insula, amygdala, MPFC, and anterior cingulate (Seeley et al. 2007) involved in cognitive emotional state appraisal that may also influence cognition (Craig 2009). Thus, the current study identifies structural connections between the anterior insula and olfactory, frontal, thalamic, and limbic structures likely involved in cognitive emotion appraisal, emotional influence on cognition, and reward processing.
Multiple Pathways of a Single Connection and Multiple Connections of a Single Fiber Bundle
In the current study, multiple pathways were found to support overlapping connections for insula subdivisions. The IFOF, UF, and AF association pathways involved in overlapping structural connections demonstrated a number of unique and multiple pathways to various brain areas. The dAI demonstrated 2 pathways (dorsal and ventral) to the FIO in both hemispheres (Fig. 2A) and to the supramarginal gyrus in the right hemisphere (Fig. 2B). The vAI demonstrated unique connections to the amygdala in both hemispheres through horizontal and u-shaped tract pathways (Fig. 3A) and also had medial and lateral pathways to the parhippocampal cortex in the right hemisphere (Fig. 3H).
As multiple pathways may underlie a single connection between 2 ROIs, a single fiber bundle may underlie multiple connections. For example, the same fiber bundle connecting the dAI to the globus pallidus was also responsible for the connection between the left dAI and the ipsilateral thalamus (Fig. 2E). The same u-shaped tract involved in the vAI-amygdala connection also connected the left vAI with the parahippocampal gyrus along with another short horizontal fiber (Fig. 3B and D). Finally, the fronto-orbitopolar tract was responsible for 2 unique connections: the vAI-gyrus rectus and the vAI-frontal superior orbital connections (Fig. 3C). Thus, in addition to multiple pathways forming a connection between 2 ROIs, individual fiber bundles give rise to multiple connections.
The existence of multiple fiber pathways underlying a single connection, and the existence of individual fiber tracts underlying multiple connections, both have implications for structure–function relationships. The fact that multiple pathways underlying a single connection between 2 ROIs should facilitate focus on uncovering more nuanced structure–function relationships involving ROI subdivisions. The fact that a single fiber bundle produces connections between different ROIs means that the integrity of such fiber bundles may have implications for a number of ROIs. Thus, more detailed investigations accounting for multiple pathways and multiple connections are needed to further clarify structure–function relationships of the insula on increasingly finer scales.
The dAI and PI Connections
The dAI and PI demonstrated the most shared structural connections to other brain areas out of any subdivision pair not included in the overlapping or unique connections (bottom of Table 1), with most connections occurring in the left hemisphere. This result is not in line with an anterior–posterior organizational framework, where anterior and posterior insular subdivisions have unique structural and functional connections. An anterior–posterior organization would predict that the dAI and vAI would have more overlapping connections than the dAI and PI. This is likely due to the fact that previous studies have parsed out a “soft” mid-insula “transition area” that most-likely overlaps with the “hard” functional dAI-PI boundary in the current study. Thus, a hard tripartite functional parcellation with clear functional boundaries dictating the groupings of structural connections supports a tripartite structural organization rather than an anterior–posterior structural organization.
Structure–Function Relationships of the Insula
In contrast to an anterior–posterior structure–function framework, where anterior and PI subdivisions have unique functional and structural connections, the current study presents another possible structure–function framework based on a tripartite insula parcellation. In the context of the current study, overlapping structural connections of insula subdivisions may be supported by major association pathways such as the IFOF, UF, and AF while unique structural connections of insula subdivisions were supported by subcortical-cortical connections and unique small fiber bundles. Importantly, the current study demonstrates that the anterior portion of the insula has distinct dorsal and ventral structural connections that may support the functional independence of the dAI and vAI.
The structure–function relationship of insula subdivisions specifically related to white matter projections and functional connectivity has similarities and differences from other structure–function relationships such as the gyri-function relationship and the architectonic-function relationship. Post mortem human brain assessments show that the insula is separated into 2 main structures by a central sulcus; an anterior portion consisting of 4 short gyri and a posterior portion consisting of 2 long gyri (Morel et al. 2013; Uddin et al. 2017). The architectonic organization of the human insula consists of concentric layers of cell structures that radiate outward from the ventral anterior portion of the insula to the dorsal posterior portion of the insula. The ventral anterior portion of the insula consists of initial layers with no granular cells that then transition to layers with increasing granularity until the most dorsal posterior “hypergranular” area (Morel et al. 2013). These concentric layers of granularity cut across the insular gyri as their arrangement orients them in different directions.
The gyral-sulcul organization would predict an anterior–posterior organization of insular structure–function that is in accord with previous studies showing an anterior–posterior organization of white matter connections (Cerliani et al. 2012; Cloutman et al. 2012; Jakab et al. 2012) and functional connections (Cauda et al. 2012). However, the anterior–posterior organization does not account for the dorsal–ventral separation of the anterior portion of the insula that has been observed in other functional investigations (Deen et al. 2011; Chang et al. 2012; Uddin et al. 2014; Nomi et al. 2016) and the dorsal–ventral separation of unique white matter connections found in the current study.
The architectonic organization of cellular structure would predict a concentric organization of structure and function with patterns of connections spreading out concentrically in a ventral anterior to dorsal posterior organization. This arrangement does not fit with the anterior–posterior conception of insula structure–function organization, as the anterior portion of the insula is composed of more agranular layers in the vAI and more granular layers in the dAI. This arrangement fits more closely with the tripartite structure–function organization proposed in the current study. In this organizational framework, the vAI is composed mostly of granular cell structure while the dAI and PI consist of both granular and agranular cells. Thus, the architectonic organization of the insula is in part consistent with the proposed tripartite insula structure–function organization proposed in the current study where the vAI has unique functional connections that may be related to granular cells while the dAI and PI have a number of overlapping connections that may be related to their underlying granular and agranular cell structure.
Although the anterior–posterior and tripartite frameworks both fit white matter structure and functional organization of the insula, the tripartite model offers more specificity due to its division of the anterior insula into dorsal and ventral subdivisions (Deen et al. 2011; Chang et al. 2012; Uddin et al. 2014; Nomi et al. 2016). The current study further demonstrates structural independence of the dAI and vAI. Future work should investigate if parcellations of the insula based on myelin-function relationships (Glasser et al. 2016), or other parcellations that produce more than 4 insula subdivisions (Power et al. 2011; Kelly et al. 2012; Gordon et al. 2016), are able to better describe structure–function relationships of the insula when focusing on white matter projections.
Insula and the Dorsal Anterior Cingulate Cortex
The dorsal ACC and dAI form the 2 major nodes of the SN; numerous studies demonstrate a strong functional relationship between these 2 brain areas (Seeley et al. 2007; Menon and Uddin 2010; Deen et al. 2011; Cauda et al. 2011b; Chang et al. 2012). Despite tracer studies in non-human primates identifying structural connections between these 2 areas (Mesulam and Mufson 1982; Mufson and Mesulam 1982), studies exploring structural connections between them in humans in vivo have been inconsistent. Only a few previous studies have identified structural connections between these 2 areas (van den Heuvel et al. 2009; Uddin 2011; Sotiropoulos et al. 2013; Wiech et al. 2014; Ghaziri et al. 2017). Despite the large sample size and high-quality diffusion data used in the current analysis, there were only a very small number of subjects that presented with ipsilateral connections between the dAI and dorsal ACC (2 in the left hemisphere and 1 in the right hemisphere), but not enough to surpass the 50% liberal threshold. Thus, these connections were not detectable in the majority of subjects in the current dataset.
Although it is possible that the diffusion dataset used in the current study did not have the proper acquisition protocols to identify the dAI-ACC connection, previous exploratory work on this dataset did identify the dAI-ACC tract with a seed-to-ROI probabilistic tractography analysis using FSL software (Sotiropoulos et al. 2013). This suggests that the lack of identification of the dAI-ACC tract was because of reconstruction and analysis approaches rather than acquisition protocols. Future studies should aim to determine the exact data analysis procedure required to identify the dAI-ACC structural connection, and how such analytic approaches influence the identification of other documented tracts of the insula.
Limitations
Despite the high-quality HARDI data provided by the Human Connectome Project, there are inherent limitations in the tractography algorithms used to reconstruct fiber pathways in the human brain in vivo. Limitations exist regarding the algorithm implemented and the parameters used for normalizing data to subject or standard space, reconstruction of SDFs, turning angle thresholds, step-size thresholds, and seeding parameters among many others. Additionally, individual thresholds for QA values, subject level thresholds, and group level thresholds may also interact with tractography parameters to influence the identification (or non-identification) of tracts across subjects.
Another limitation is the type of ROIs used in tractography studies. As different types of atlases (AAL, Broadman, Harvard-Oxford, etc.) and parcellation techniques exist, there can be substantial heterogeneity of the size of regions used in tractography studies. As a result, connections identified in one study may not be identified in another simply because of the size or placement of the ROIs.
These considerations may also help to explain why the current study found no tracts from the insula to the anterior cingulate cortex in the majority of subjects, even though previous studies have identified such a tract at the group level (Ghaziri et al. 2017) and individual level (Uddin et al. 2011). Differences in tractography algorithms and parameters may also explain why contralateral structural connections in the current study were not found as in previous studies (Cerliani et al. 2012; Wiech et al. 2014). It may be of interest to directly compare how probabilistic (Cloutman et al. 2012; Jakab et al. 2012; Wiech et al. 2014) and deterministic (Ghaziri et al. 2017) tractography algorithms differ with respect to insula tractography.
Additional future considerations should be given to individual differences related to psychiatric and medical conditions, as well as any influences of medication and substance abuse. It is currently unknown how such factors would influence the identification of white matter tracts within the framework of the current study.
Conclusions
The current study used high-quality HARDI data acquired from a large sample of subjects to identify structural connections related to functional subdivisions of the insular cortex. The general findings provide an integrated tripartite structure–function framework for demonstrating how functionally distinct subdivisions of the insula can also demonstrate overlapping functional profiles through common structural connections supported by association fiber pathways such as the IFOF, AF, and UF. The unique structural connections found in the current study also align with the tripartite functional framework of unique functional connections, where the right dAI demonstrates unique connections related to its role in higher-level cognitive processing, salience detection, and network switching and the vAI has unique connections related to its role in affective processing. Additionally, the left dAI demonstrated unique connections related to its role in language processing. These findings contribute to building more nuanced models of insula structure–function relationships in the human brain.
Supplementary Material
Footnotes
The authors thank Ben Deen for providing the insula regions-of-interest from his earlier work. Conflict of Interest: None declared.
Funding
This work was supported by the National Institute of Mental Health (R01MH107549) and a NARSAD Young Investigator Award to LQU.
References
- Ardila A, Benson DF, Flynn FG. 1997. Participation of the insula in language. Aphasiology. 11:1159–1169. [Google Scholar]
- Ardila A, Bernal B, Rosselli M. 2014. Participation of the insula in language revisited: a meta-analytic connectivity study. J Neurolinguistics. 29:31–41. [Google Scholar]
- Bassett DS, Brown JA, Deshpande V, Carlson JM, Grafton ST. 2011. Conserved and variable architecture of human white matter connectivity. Neuroimage. 54:1262–1279. [DOI] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, Sporns O. 2014. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 102:345–357. [DOI] [PubMed] [Google Scholar]
- Broce I, Bernal B, Altman N, Tremblay P, Dick AS. 2015. Fiber tracking of the frontal aslant tract and subcomponents of the arcuate fasciculus in 5–8-year-olds: relation to speech and language function. Brain Lang. 149:66–76. [DOI] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li C-SR, Menon V. 2014. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci. 34:14652–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamante F, Masterton RA, Tournier J-D, Smith RE, Willats L, Raffelt D, Connelly A. 2013. Track-weighted functional connectivity (TW-FC): a tool for characterizing the structural–functional connections in the brain. Neuroimage. 70:199–210. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, de Schotten MT. 2012. Short frontal lobe connections of the human brain. Cortex. 48:273–291. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. 2002. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 17:77–94. [DOI] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC. 2011. a. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 23:2864–2877. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D’Agata F, Duca S, Geminiani G, Fox PT, Vercelli A. 2012. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 62:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. 2011. b. Functional connectivity of the insula in the resting brain. Neuroimage. 55:8–23. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, Gazzola V, D’Arceuil H, Keysers C. 2012. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum Brain Mapp. 33:2005–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. 2010. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 50:81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. 2012. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 23:739–749. bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Wang Y, Sheng J, Sporns O, Kronenberger WG, Mathews VP, Hummer TA, Saykin AJ. 2012. Optimization of seed density in DTI tractography for structural networks. J Neurosci Methods. 203:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M. 2013. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 66:508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, Lambon Ralph MA. 2012. The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. Neuroimage. 59:3514–3521. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron. 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AB. 2009. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 59–70. [DOI] [PubMed] [Google Scholar]
- de Kwaasteniet B, Ruhe E, Caan M, Rive M, Olabarriaga S, Groefsema M, Heesink L, van Wingen G, Denys D. 2013. Relation between structural and functional connectivity in major depressive disorder. Biol Psychiatry. 74:40–47. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. 2011. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, McMahon KL, Zubicaray GI, Martin NG, Hickie IB, Toga AW, Wright MJ, Thompson PM. 2014. Development of insula connectivity between ages 12 and 30 revealed by high angular resolution diffusion imaging. Hum Brain Mapp. 35:1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Bauchet L, Lehéricy S, Capelle L. 2001. Functional compensation of the left dominant insula for language. Neuroreport. 12:2159–2163. [DOI] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ. 2015. Asymmetric development of dorsal and ventral attention networks in the human brain. Dev Cogn Neurosci. 12:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Houde J-C, Boucher O, Gilbert G, Descoteaux M, Lippé S, Rainville P, Nguyen DK. 2017. The corticocortical structural connectivity of the human insula. Cereb Cortex. 27(2):1216–1228. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, et al. 2016. A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. 2016. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermundstad AM, Bassett DS, Brown KS, Aminoff EM, Clewett D, Freeman S, Frithsen A, Johnson A, Tipper CM, Miller MB, et al. 2013. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc Natl Acad Sci USA. 110:6169–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab A, Molnár PP, Bogner P, Béres M, Berényi EL. 2012. Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topogr. 25:264–271. [DOI] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. 2012. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 61:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Greer SM. 2008. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 363:3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. 2010. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. 2011. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 1224:40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. 2011. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. 1982. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 212:38–52. [DOI] [PubMed] [Google Scholar]
- Morel A, Gallay M, Baechler A, Wyss M, Gallay D. 2013. The human insula: architectonic organization and postmortem MRI registration. Neuroscience. 236:117–135. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam M. 1982. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 212:23–37. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam M. 1984. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 227:109–120. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. 2009. Functional organization of the human anterior insular cortex. Neurosci Lett. 457:66–70. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Farrant K, Damaraju E, Rachakonda S, Calhoun VD, Uddin LQ. 2016. Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Hum Brain Mapp. 37:1770–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL. 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. 2010. Neural systems underlying decisions about affective odors. J Cogn Neurosci. 22:1069–1082. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. 2010. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 68:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, et al. 2013. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. 80:125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudry Y, Lemogne C, Malinvaud D, Consoli S-M, Bonfils P. 2011. Olfactory system and emotion: common substrates. Eur Ann Otorhinolaryngol Head Neck Dis. 128:18–23. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. 2010. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 52:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M. 2011. A lateralized brain network for visuospatial attention. Nat Neurosci. 14:1245–1246. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. 2011. Brain connectivity and the self: the case of cerebral disconnection. Conscious Cogn. 20:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. 2015. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 16:55–61. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kinnison J, Pessoa L, Anderson ML. 2014. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J Cogn Neurosci. 26:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. 2017. Structure and function of the human insula. J Clin Neurophysiol. 34:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. 2009. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 30:3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Sporns O. 2011. Rich-club organization of the human connectome. J Neurosci. 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goñi J, Pol HEH, Kahn RS. 2013. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 70:783–792. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Jarbo K, Pathak S, Schneider W. 2011. In vivo mapping of microstructural somatotopies in the human corticospinal pathways. J Neurophysiol. 105:336–346. [DOI] [PubMed] [Google Scholar]
- Wiech K, Jbabdi S, Lin C, Andersson J, Tracey I. 2014. Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. Pain. 155:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI. 2013. a. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 8:e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Wedeen VJ, Tseng W-YI. 2011. Estimation of fiber orientation and spin density distribution by diffusion deconvolution. Neuroimage. 55:1054–1062. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Tang PF, Tseng WY. 2013. b. Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. Neuroimage Clin. 2:912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Tseng WY. 2011. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage. 58:91–99. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, Chee MW. 2015. Functional specialization and flexibility in human association cortex. Cereb Cortex. 10:3654–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. 1997. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA. 94:4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liao W, Chen H, Mantini D, Ding J-R, Xu Q, Wang Z, Yuan C, Chen G, Jiao Q. 2011. Altered functional–structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 134:2912–2928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.