Abstract
Heart failure (HF) affects 23 million people worldwide and results in 300000 annual deaths. It is associated with many comorbidities, such as obstructive sleep apnea (OSA), and risk factors for both conditions overlap. Eleven percent of HF patients have OSA and 7.7% of OSA patients have left ventricular ejection fraction <50% with arrhythmias being a significant comorbidity in HF and OSA patients. Forty percent of HF patients develop atrial fibrillation (AF) and 30%–50% of deaths from cardiac causes in HF patients are from sudden cardiac death. OSA is prevalent in 32%–49% of patients with AF and there is a dose-dependent relationship between OSA severity and resistance to anti-arrhythmic therapies. HF and OSA lead to various downstream arrhythmogenic mechanisms, including metabolic derangement, remodeling, inflammation, and autonomic imbalance. (1) Metabolic derangement and production of reactive oxidative species increase late Na+ currents, decrease outward K+ currents and downregulate connexin-43 and cell-cell coupling. (2) remodeling also features downregulated K+ currents in addition to decreased Na+/K+ ATPase currents, altered Ca2+ homeostasis, and increased density of If current. (3) Chronic inflammation leads to downregulation of both Nav1.5 channels and K+ channels, altered Ca2+ homeostasis and reduced cellular coupling from alterations of connexin expression. (4) Autonomic imbalance causes arrhythmias by evoking triggered activity through increased Ca2+ transients and reduction of excitation wavefront wavelength. Thus, consideration of these multiple pathophysiological pathways (1–4) will enable the development of novel therapeutic strategies that can be targeted against arrhythmias in the context of complex disease, such as the comorbidities of HF and OSA.
Keywords: Arrhythmias, heart failure, obstructive sleep apnea, autonomic nervous system, inflammation, therapeutics, ion channel, cardiac remodeling, oxidative stress
Statement of Significance
There is increasing evidence that patients often present with several comorbidities, impacting therapeutic strategies. Heart failure (HF) patients are more likely to have obstructive sleep apnea (OSA), both of which are strongly associated with cardiac arrhythmias. Whilst research on arrhythmias tends to focus on perturbations at the ion channel level, this review explores several arrhythmogenic pathways at both a molecular and systems level in HF and OSA patients. These mechanisms give insight into how HF and OSA compound management of arrhythmia and could represent useful therapeutic avenues. In a wider clinical setting, it is likely that the multiple mechanisms discussed in this review can be extrapolated to various other pathologies, maximizing potential benefits of future clinical study into these mechanisms.
Obstructive Sleep Apnea in Heart Failure
Epidemiology of heart failure
Approximately 23 million people worldwide suffer from heart failure (HF), with 300000 annual deaths directly attributable to HF. This contributes to an annual cost of $39 billion on the US healthcare system [1]. HF is characterized by the inability of the heart to adequately perfuse the systemic circulation, and can be broadly classified into two major subsets, depending on the left ventricular ejection fraction (LVEF): heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), known as systolic and diastolic HF, respectively. Systolic heart failure (SHF) occurs due to a defect in contractile function during systole and features an LVEF <50%. Diastolic heart failure (DHF) occurs due to increased resistance to ventricular filling during diastole [2]. Approximately half of HF patients have SHF, with the remainder having DHF. However, the number of patients with DHF is increasing, either due to increasing incidence, or increased diagnosis [3]. Furthermore, SHF and DHF, although share pathophysiological processes, have different underlying physiology and can be considered distinct phenotypes within the spectrum of HF [3]. However, most of the published literature relating to HF, OSA, and arrhythmia focus on HFrEF thus most findings discussed in this review will pertain to HFrEF. Importantly, HF rarely occurs in isolation and often presents with other comorbidities sharing similar risk factors, which exacerbate the HF and contribute to further pathophysiological changes [4].
Epidemiology of obstructive sleep apnea
Sleep-disordered breathing (SDB) describes intermittent episodes of apnea, hypopnoea or arousal from sleep as a result of strained breathing. Examples of SDB syndromes are obstructive sleep apnea (OSA) and central sleep apnea (CSA). OSA occurs as a result of physical upper airway obstruction and is characterized by periods of interrupted airflow and strained breathing against an obstructed upper airway [5], whereas CSA occurs due to a reduction in central respiratory drive [6]. Another type of central SDB syndrome is Cheyne-Stokes respiration (CSR), which is characterized by oscillations in breathing punctuated by intermittent periods of apnea and hypopnoea [7].
OSA is the most common SDB syndrome in the general population, occurring in 3%–7% of adult men and 2%–5% of adult women [8–10]. These figures are likely an under-representation as a significant proportion of patients (up to 82% of men and 93% of women) with OSA are undiagnosed [11]. It is estimated that approximately 13% of men and 6% of women have moderate to severe SDB, defined as having an apnea-hypopnea index (AHI) ≥ 15 [12]. The Multi-Ethnic Study of Atherosclerosis (MESA) study demonstrate increased risk of SDB in ethnic minorities. Black populations had higher odds ratios of developing sleep apnea syndrome, and Hispanic and Chinese populations had higher odds ratios of developing SDB or short sleep relative to white populations, highlighting disparities in health [13].
Epidemiology of the HF-OSA overlap
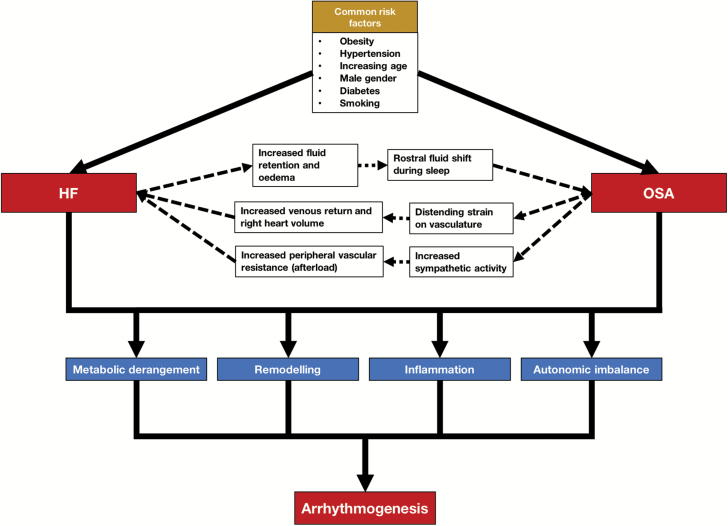
HF has considerable overlap with OSA in terms of common risk factors, including obesity, hypertension, increasing age, male gender, diabetes and smoking (Figure 1). Whereas OSA in itself is an independent risk factor for developing HFrEF, CSA and CSR are thought to be representative prognostic markers for cardiac dysfunction [14, 15]. Thus, the focus of the present review will be on the mechanisms of arrhythmogenesis in a context of HF and OSA.
Figure 1.
There are many risk factors common to HF and OSA. In addition, HF and OSA are interrelated conditions because HF-associated pathophysiology can lead to OSA and vice versa, as indicated by the broken arrows. HF and OSA lead to metabolic derangement, myocardial remodeling, inflammation and autonomic imbalance. These downstream conditions in turn lead to arrhythmogenesis. Abbreviation: LV – left ventricle.
Aside from HF [16], OSA is an independent risk factor for other cardiovascular diseases [17], such as hypertension [18, 19]. Thus, HF and OSA tend to exist together as comorbidities and influence each other in severity. Indeed, 49%–81% of patients with HFrEF suffer from SDB, with 12%–57% of HFrEF patients suffering specifically from OSA [20–22], while 7.7% of patients with OSA have an LVEF <50%, indicative of systolic dysfunction [23]. In a study of patients with SDB (sub-type not specified), those in the upper quartile of the AHI, a measure of the degree of disordered breathing, had a 2.38 multivariable-adjusted relative odds of HF compared to patients in the lower quartile [24]. In patients suffering from HFrEF, several single-center studies [21] and one multi-center study [20] report greater prevalence of OSA than CSA. However, few studies have reported greater prevalence of CSA in similar patient populations [14, 25, 26].
Mechanistic link between HF and OSA
Mechanistically, HF can contribute to OSA through rostral fluid shift during sleep [27]. Patients with HFrEF commonly present with fluid collection in the lower extremities which is distributed in a rostral manner when in a supine position. This results in neck edema during sleep, which may predispose to OSA [15]. This mechanism may explain the reason why OSA can be attenuated in HFrEF patients by administration of diuretics [28].
The mechanisms by which OSA leads to cardiovascular morbidity act locally and systemically. A characteristic feature of OSA is repeated attempts to breathe against an obstructed airway, which causes significant thoracic pressure changes and distending strain on the vasculature. This leads to increased venous return and right heart volume, as well as pressure overload [15]. Further stress on the cardiac wall occurs as a result of acute changes in left ventricular transmural pressure during obstructive apneas. Combined with increased sympathetic outflow during OSA [29], which increases peripheral resistance and hence afterload on the heart, shear stress acting on the cardiovascular endothelium may lead to endothelial dysfunction [15]. These mechanisms contribute to remodeling and impaired function [23, 30, 31], and represent a mechanistic pathway which may explain the bidirectional nature between HF and OSA (Figure 1).
Given the apparent interrelationship between pathophysiological processes of HF and OSA, it is not surprising that patients with OSA are likely to suffer from HF and vice versa. This overlap between HF and OSA poses therapeutic challenges and it is essential to consider pathophysiological mechanisms underlying both conditions. Of particular mechanistic and therapeutic interest is the role of HF and OSA as comorbidities predisposing individuals to cardiac arrhythmia. Cardiac arrhythmias are a significant cause of morbidity and mortality worldwide [32, 33]. From 2004 to 2009, 5750440 people suffered from cardiac arrhythmias in the United States with an annual financial burden of $67.4 billion [34]. In the form of ventricular fibrillation (VF), arrhythmias can lead to sudden cardiac death (SCD) [35] and account for ~30% of deaths resulting from cardiac causes [36].
The consideration of arrhythmia directly relates to the clinical management of HF which typically employs the use of ion channel drugs. However, it is biologically plausible that in HF and OSA, significant alterations in the electrophysiological phenotype may render these drugs therapeutically inefficient or even proarrhythmic. It thus may be of relevance to look at proarrhythmic changes at the crossroads of HF and OSA and explore their molecular and electrophysiological basis.
Cardiac Arrhythmogenesis in OSA and HF
A significant proportion (~40%) of patients with HF will develop atrial fibrillation (AF), and vice versa [37] and there is a strong association between pre-existent AF and heightened risk for HF readmission [38]. Clinical studies such as the Framingham Study show a sixfold increase in risk of AF in HF patients, and there is a relationship between the severity of HF and prevalence of AF (5%, 26% and 50% among patients with mild, moderate, and severe HF, respectively) [39]. Twenty to eighty percent of HF patients display ventricular tachycardia (VT), giving a fivefold increased risk of SCD in patients with HF [40]. Indeed 30%–50% of deaths from cardiac causes are due to SCD in HF patients [41, 42].
The link between arrhythmias and SDB, specifically OSA, is supported by evidence from large multicentre studies such as the Sleep Heart Health Study (SHHS) [43, 44]. Analysis of ECG data from participants of the SHHS revealed that participants with overall SDB (classified as respiratory distress index ≥ 30) had higher odds of having arrhythmic conditions than controls [45]. In the analysis of records from a US Veteran’s Affairs (VA) multicenter trial, the DREAM study [46], an independent association was observed linking overall SDB and nocturnal arrhythmias (supraventricular, ventricular, and conduction delay), with OSA found to be a stronger predictor of nocturnal arrhythmias than CSA [47]. This association included a dose-response relationship influenced by the severity of overall SDB, and hypoxia was found to be a stronger predictor of arrhythmic risk compared to arousal from sleep [47]. A large cross-sectional study looking at arrhythmias in ~3000 older men suffering from SDB showed that increasing severity of overall SDB resulted in increased risk of atrial and ventricular arrhythmia. However, when stratified into type of SDB, increasing severity of OSA resulted in increased odds ratio of complex ventricular ectopy (CVE) alone when adjusted to take into account confounding factors [48]. In contrast, the relationship between SDB severity and AF is largely due to CSA. In both cases, the pathogenesis of arrhythmia is associated with overnight hypoxia [48]. A longitudinal study spanning 12 years and involving predominantly middle-aged male patients referred after OSA diagnosis revealed that an apnea/hypopnoea ratio >5 and time with oxygen saturation < 90% were important predictors of AF [49]. In prospective studies looking at incident AF in middle-aged or older male patient populations, the proportion of patients developing incident AF was correlated with increasing severity of OSA, with OSA being a significant predictor of AF in minimally-adjusted models. However, after adjusting for confounders, there was no significant association between OSA and incident of AF. Instead, significant predictors of AF were CSA and CSR-CSA which were associated with a twofold to threefold increase in odds of AF incidence [50, 51]. Despite these findings, OSA is associated with concomitant CSA and vice-versa [51]. Other studies have suggested that cardiac arrhythmias are a significant cause of death in OSA patients [52, 53]. A study in patients with congenital long QT syndrome (LQTS) demonstrated prolonged durations of electrocardiographic markers of ventricular depolarization and repolarization (QT and QTc intervals) in patients with concomitant OSA (AHI > 5) compared to congenital LQTS sufferers without OSA. Furthermore, prolongation of QTc was positively correlated with two measures of apnea severity, AHI as well as apnea index (AI) [54]. Acute changes in QT interval have also been observed in otherwise healthy individuals with OSA, with QT intervals prolonging during periods of apnea relative to periods of normal sleep [55]. Furthermore, treatment of sleep apnea by continuous positive airway pressure (CPAP) has added cardiac benefit, as CPAP therapy has been shown to decrease QT intervals in patients [56]. The prevalence of OSA lies in the range of 32%–49% in patients with AF, and OSA patients have a 2.57 relative risk of SCD from midnight to 06:00 am compared to healthy controls [57]. Additionally, untreated male OSA patients have higher incidences of fatal and nonfatal cardiovascular events compared to untreated patients with milder disease, treated OSA patients, and healthy controls [58]. Of particular clinical importance, patients with severe OSA respond less well to anti-arrhythmic drugs (AADs) compared to patients with less severe OSA [59, 60], and concurrent cardiac pathology such as HF elevates the severity of arrhythmia in OSA patients [61]. This discussion of therapeutic implications in the intersection of HF, OSA, and arrhythmia will be explored later.
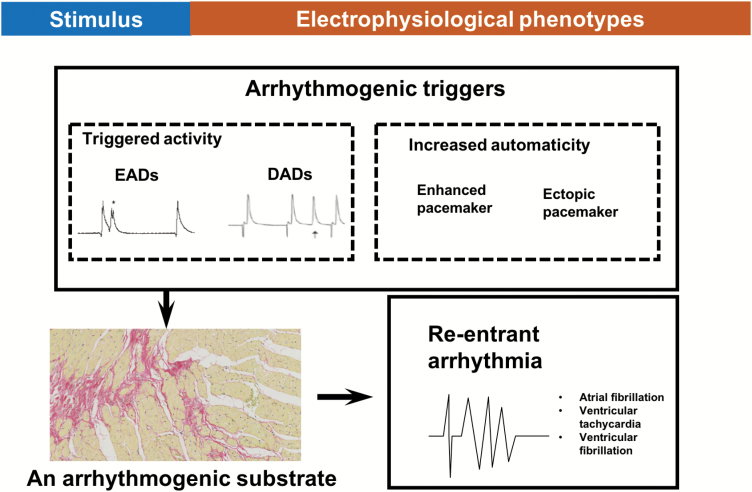
Understanding arrhythmogenic mechanisms in situations of comorbidities is complex. Physiologically, an orderly process of electrical excitation followed by mechanical contraction underlies the ordinary functioning of the heart. Abnormalities in this complex process result in arrhythmias. Arrhythmias can be broadly classified into non-re-entrant extrasystoles and self-sustaining re-entrant arrhythmias, the latter which tend to arise upon the application of an arrhythmic “trigger” to a vulnerable area of myocardium, the arrhythmic “substrate” [62] (Figure 2). Triggers may be due to extrasystoles, which may arise due to reflection and parasystole, or due to early and delayed after-depolarizations (EADs and DADs). EADs can arise due to prolonged action potential duration (APD) (and delayed repolarization) of the myocardium which causes opening of L-type Ca2+ channels. In contrast, DADs arise due to perturbations in Ca2+ homeostasis and aberrant Ca2+ release from the sarcoplasmic reticulum. DADs are often seen in patients suffering from HF [63] as L-type Ca2+ channels have a higher open probability in HF due to chronic catecholaminergic stimulation leading to protein kinase-A (PKA) phosphorylation of these channels [64]. Arrhythmic substrate facilitates re-entry and the formation of circus rhythms by allowing triggered activity to re-excite areas that have recovered from refractoriness. Examples of arrhythmic substrate include slowed AP conduction velocity, prolonged APD, impaired cell-cell coupling, reduced membrane excitability, and increased heterogeneity in repolarization [62, 65].
Figure 2.
A simplified schematic to illustrate how the application of an arrhythmogenic trigger, such as an EADs, DADs, or extrasystolic beats from enhanced or ectopic pacemaker activity to a vulnerable area of myocardium, the arrhythmogenic substrate, can lead to the generation of self-sustaining re-entrant arrhythmias. In this example, the arrhythmogenic substrate is arising from increased myocardial fibrosis.
While the above represents a basic understanding of mechanisms leading to arrhythmogenesis, it is crucial to consider arrhythmogenic mechanisms in more complex disease states such as HF and OSA. These molecular and electrophysiological mechanisms arise as a consequence of several common pathophysiological pathways in HF and OSA. These include (1) metabolic derangement and oxidative stress, (2) tissue remodeling, (3) inflammation, and (4) autonomic imbalance.
Metabolic derangement is a feature of the pathophysiology of chronic HF that worsens with deteriorating cardiac function [66]. Oxidative stress and increased production of reactive oxygen species (ROS) play a significant role in HF-related pathophysiology [67]. Tissue remodeling [68], inflammation [69], and autonomic imbalance [70] associated with HF may additionally result in increased arrhythmogenesis. We will examine the cardiac electrophysiological changes associated with these four pathophysiological features.
Metabolic derangement and oxidative stress
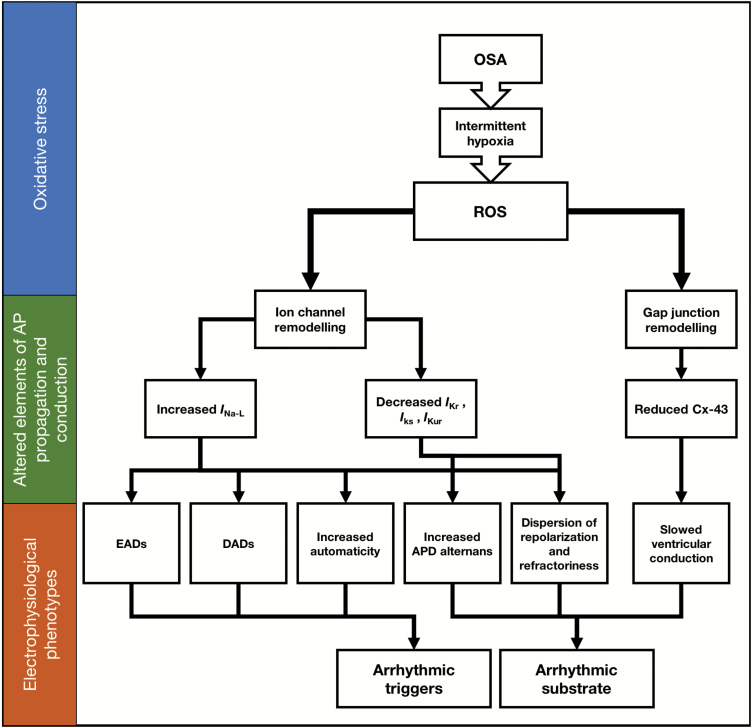
Cellular metabolic dysfunction is a feature seen in OSA patients due to the periods of intermittent hypoxia, placing oxidative stress on cardiac tissue [71]. Analysis of postmortem atrial tissue in a rat model of OSA revealed dysregulation of enzymes involved in glycolysis, the Krebs cycle, and anaerobic glycolysis. This resulted in an overall reduction in ATP generation and impairment of energy metabolism ultimately leading to increased production of ROS [72]. Increased production of ROS is also seen in HF [67], including in rat models with pressure overload-induced HFrEF [73].
There is abundant evidence to suggest that ROS and dysfunction of mitochondria and metabolism contribute to arrhythmogenesis [74–76]. Changes in properties of cardiomyocyte ion handling in the presence of high levels of ROS include increased diastolic release of Ca2+ from sarcoplasmic reticulum via actions on ryanodine (RyR2) receptors, and an increase in the late Na+ current (INa-L), perhaps through actions on modulators of INa-L such as Ca2+-calmodulin-dependent protein kinase II (CaMKII), PKA, and protein kinase-C (PKC) [75]. Evidence exists implicating PKC in upregulating this INa-L; the prolongation of APD during oxidative challenge with H2O2 is attenuated upon inhibition of PKC [77]. In rat ventricular myocytes, INa-L was increased during hypoxia. This is hypothesized to occur as a result of increased PKC activation [78] because cells from rat cerebral cortex show an increase in phosphatidylserine (an activator of PKC) secondary to increased intracellular Ca2+ upon hypoxia [79]. PKC, in turn, phosphorylates the α-subunit of voltage-gated Na+ channels in cells from rat brain, consequently altering kinetics to delay inactivation [80]. Since the α-subunit mediates the voltage-dependent inactivation properties of the channel [81], this phenomenon could be assumed to be similar in heart Na+ channels but this warrants further investigation. Additional support for the role of PKC and CaMKII comes from studies of isolated rabbit ventricular myocytes. Bisindolylmaleimide VI (BIM) and KN-93, inhibitors of PKC and CaMKII respectively, attenuated the increase in INa-L during hypoxia and not during normoxia [82, 83], implicating these proteins as responsible for the increased INa-L during hypoxia.
This INa-L contributes around 50% of Na+ loading during the plateau phase of the AP and when increased, it can lead to both triggers and substrates of arrhythmia, as previously described by Chadda et al. [84]. Thus, increased INa-L leads to arrhythmic triggers through (1) increased automaticity due to depolarization phenomena during diastole, which triggers inappropriate APs in the conducting system, (2) prolonged AP plateaus lead to EADs resulting from regenerative reactivation of L-type Ca2+ channels and (3) increased intracellular Na+ causing Ca2+ overload via altering the gradients acting on the NCX exchanger leading to DADs. Similarly, increased INa-L leads to arrhythmic substrate in the form of (4) increased APD alternans and (5) dispersion of repolarization and refractoriness. Increased INa-L is associated with arrhythmic conditions such as LQTS and sudden infant death syndrome [84–87], and it may be a factor underlying the increased arrhythmogenicity seen in OSA.
In addition to altering INa-L, oxidative stress may play a role in changing cardiac potassium currents. Repolarizing outward currents such as IKr, IKs, and IKur are decreased during exposure to increased ROS in ventricular cardiomyocytes, due to reduced mRNA expression and modulation of the phosphorylation status of these channels through PKA and PKC. These can delay repolarization and increase APD, contributing to arrhythmogenesis [76].
Metabolic dysfunction may also lead to the formation of arrhythmic substrate through the decrease in levels of cell-cell coupling, from reduction of connexin-43 (Cx-43), a gap junction protein. The evidence for this comes from studies of transgenic mouse models possessing high renin-angiotensin system activity [88, 89], which is known to increase levels of ROS. These mice expressed reduced levels of Cx-43, due to increased activity of a redox-sensitive tyrosine kinase (cSrc) [76]. Deficient Cx-43 expression leads to slowing down of conduction in ventricles [90], which is an arrhythmic substrate [91]. Thus, through actions on Na+ channels, K+ channels, and gap junction proteins, increased ROS due to oxidative stress results in creation of both triggers and substrate of arrhythmia and may explain how periods of hypoxia related to OSA contributes to arrhythmogenesis [92] as summarized in Figure 3.
Figure 3.
Generation of arrhythmic triggers and arrhythmic substrate due to ROS generation because of oxidative stress subsequent to metabolic derangement. Changes in ion channel properties and cellular coupling are the basis for this change.
Tissue remodeling
At a systems level, cardiac remodeling mainly occurs due to pressure or volume overload. This leads to downstream structural and electrophysiological changes that are associated with HF, OSA, and arrhythmogenesis. In particular, hearts in patients suffering from HF and OSA are placed under significant oxidative and mechanical stress. OSA causes mechanical stress on the heart due to negative intrathoracic pressure acting on the atrial and ventricular walls, which may predispose to arrhythmias via mechano-electrical feedback mechanisms [93]. OSA-associated intermittent hypoxia and oxidative stress can also cause cardiac hypertrophy. This hypertrophy is associated with ROS-upregulated signaling kinases (mitogen-activated protein kinase [MAPK], c-Jun N-terminal kinases [JNK], p38, Akt) and transcription factors (NF-κB, AP-1, tumor necrosis factor α [TNFα], insulin-like growth factor II [IGF-II], interleukin-6 [IL-6]), in addition to upregulated apoptosis also contributing to structural remodeling [67, 94]. There is atrioventricular remodeling seen in a rabbit model of HFrEF [68] and atrial electrophysiological and molecular remodeling in a rat model of OSA [72].
In failing hearts, the AP profile is altered, APD is lengthened and QT interval prolonged regardless of the cause of HF [95], and experimental models of hypertrophied hearts report spatial and temporal inhomogeneities in APD [95]. Changes observed due to atrioventricular remodeling in rabbit models include a prolonged PR interval and slowing of conduction through the atrioventricular node, hypothesized to be due to a decrease in two ion channels, HCN1 and Cav1.3 [68]. In rat models of OSA, increased P-wave duration is seen [72], which is associated with long-term risk of AF in patients [96]. These are all examples of arrhythmic substrate.
Mechanisms underlying some ventricular electrophysiological phenotypes in hypertrophied and failed hearts are summarized in a review by Tomaselli and Marban [95]. They summarize four central mechanisms, involving (1) downregulated K+ currents (predominantly IK-TO), (2) altered Ca2+ homeostasis via a decrease in L-type current density, reduced rate of decay of whole-cell L-type current and defects in Ca2+ sequestration, (3) increased If current, and (4) decreased Na+/K+ ATPase currents due to reduced expression and function.
These four mechanisms (1–4) culminate in the creation of an arrhythmic substrate through increased APD and subsequent prolonged repolarization, and triggered activity due to increased automaticity, predisposing toward ventricular arrhythmia. Other electrophysiological phenotypes in hypertrophied hearts are prolonged repolarization and increased transmural dispersion of repolarization, which provides an arrhythmic substrate in ventricular conditions such as torsades de pointes [97].
Inflammatory response
The sustained state of metabolic derangement and oxidative stress may lead to chronic inflammation, and evidence suggests a link between inflammation and arrhythmias such as AF [98, 99]. In OSA patients, there are higher levels of inflammatory markers such as TNFα during periods of hypoxia [100, 101] and a critical inflammatory signal pathway regulator, NF-κB, is activated during oxidative stress [102]. Furthermore, in in-vitro models, there is increased activation of NFκB during exposure to intermittent hypoxia [103]. Another study found increased intracellular expression of TNFα and IL-8 in patients with OSA, along with increased production of ROS by monocytes [101]; this provides strong evidence for upregulation of inflammatory pathways during periods of intermittent hypoxia. When HF is present, it is plausible that there may be additional upregulation of this inflammatory state, because in human studies involving HFrEF patients, there are increased levels of inflammatory markers such as TNF-α and IL-6 in the systemic circulation as well as in the myocardium [104, 105].
The electrophysiological effects of inflammatory mediators such as TNFα and IL-1β are well characterized. Binding of NF-κB to the SCN5A gene appears to be increased during oxidative stress, resulting in a reduction in NaV1.5 expression [106]. Nav1.5 channel downregulation is associated with slowed conduction velocity (an arrhythmic substrate) as is seen in genetic arrhythmic conditions such as Brugada syndrome [107, 108]. NF-κB has effects on other elements of AP propagation, and this can be seen during the administration of angiotensin II (AII) because NF-κB is overexpressed following AII administration. Reductions similar to that of Nav1.5 are seen in the outward K+ channel, gap junction proteins such as Cx-40 and Cx-43, and Ca2+ currents during AII administration. These may all contribute to increased risk of arrhythmia [74].
TNFα-upregulated mice have prolonged action potentials (APs) due to a reduction in the Ito and IK-slow1 currents brought on by reduced expression of Kv4.2, Kv4.3, and Kv1.5 proteins [109]. This phenomenon is also seen in HF, where APD is prolonged due to a reduction of Ito, among other K+ channels (IKs, IKr, IK1), reinforcing the idea that there is considerable overlap between pathophysiological processes of the two conditions OSA and HF [110]. In addition to Kv protein downregulation, there is shifting of the inactivation curve in a manner that reduces Ito, as evidenced by studies in rat ventricular myocytes [111]. The upstream pathway for this is thought to be due to iNOS up-regulation, NO production, and superoxide generation—due to the observation that administration of iNOS inhibitors reduces the effect of TNFα on Ito [111]. Another study in canine cardiomyocytes supports this claim, having found that TNFα reduces activity of the hERG channel (responsible for the IKR current) by stimulating the production of ROS [112].
Proinflammatory cytokines affect intracellular Ca2+ handling as well. Transgenic mice overexpressing TNFα show increased diastolic [Ca2+] [113]. Studies in isolated rat ventricular myocytes indicate that when TNFα and IL-1β are administered, there are spontaneous waves of, and increased asynchronous release of Ca2+ during electrical pacing [114]. Inflammatory cytokines such as TNFα and IL-6 also downregulate sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2) transcription, resulting in increased Ca2+ remaining in the cytosol during diastole [115], and could lead to increased incidence of DADs.
The electrical coupling between cardiomyocytes is affected by proinflammatory cytokines. Transgenic mice overexpressing TNFα in cardiac tissue were shown to have significant downregulation of Cx-40, and dispersion of Cx-43 away from the intercalated discs [116]. Canine models of myocardial infarction show reduced cell-cell coupling in cardiomyocytes, through the actions of IL-1β; this may be due to heterogeneous Cx-43 reduction, leading to slow conduction [117].
Autonomic imbalance
Autonomic imbalance refers to a state where one branch of the autonomic nervous system is dominant over the other, with increased morbidity associated with sympathetic hyperactivity and parasympathetic hypoactivity [118]. Frequent episodes of sleep apnea are associated with autonomic imbalance [119], particularly higher levels of sympathetic outflow during day and night. The increase in sympathetic activity could underlie the cardiovascular morbidity and mortality common in OSA patients [29, 120]. These changes may be due to baroreceptor reflexes, chemoreceptor reflexes, and arousal from deep sleep, which arise due to negative thoracic pressure (NTP) and intermittent hypoxia during periods of hypopnoea [121]. A common confounder of the relationship between OSA and increased sympathetic activity is obesity [122]. However, a study by Narkiewicz et al. shows that sympathetic outflow is increased in obese patients who have OSA, compared to obese individuals without OSA and healthy controls [123]. Thus, there is perhaps a causative relationship between OSA and increased sympathetic activity [122]. Intermittent periods of parasympathetic activation may also occur due to NTP [124]. Importantly, HF is worsened due to increased sympathetic activation, via the increased peripheral vascular resistance and afterload on the heart [125]. In addition to the autonomic imbalance seen in OSA, pathologic activation of the sympathetic arm of the autonomic nervous system has been observed in both HFrEF and HFpEF, accompanied with increased activation of the renin-angiotensin-aldosterone system and increased release of various brain natriuretic peptides [3]. However, it is unclear whether this increased sympathetic activation is a cause of or result of HF.
In AF, concurrent activation of both limbs of the autonomic nervous system is thought to precede the development of AF [126, 127]. While the activity of the sympathetic nervous system remains high due to the processes described above, there is also an increase in vagal activation and parasympathetic (i.e. cholinergic) input to the heart [124]. Tan et al. found that there was an increase in both stellate ganglion and vagus nerve activity preceding AF developing from atrial tachycardia. This phenomenon was abolished by bilateral cryo-ablation of the autonomic innervation of the heart, demonstrating a causal relationship [128]. Furthermore, in a study involving a dog model of AF it was established that cholinergic stimulation plays a significant role in AF initiation [129]. Thus, the increased vagal activation associated with OSA could potentially lead AF.
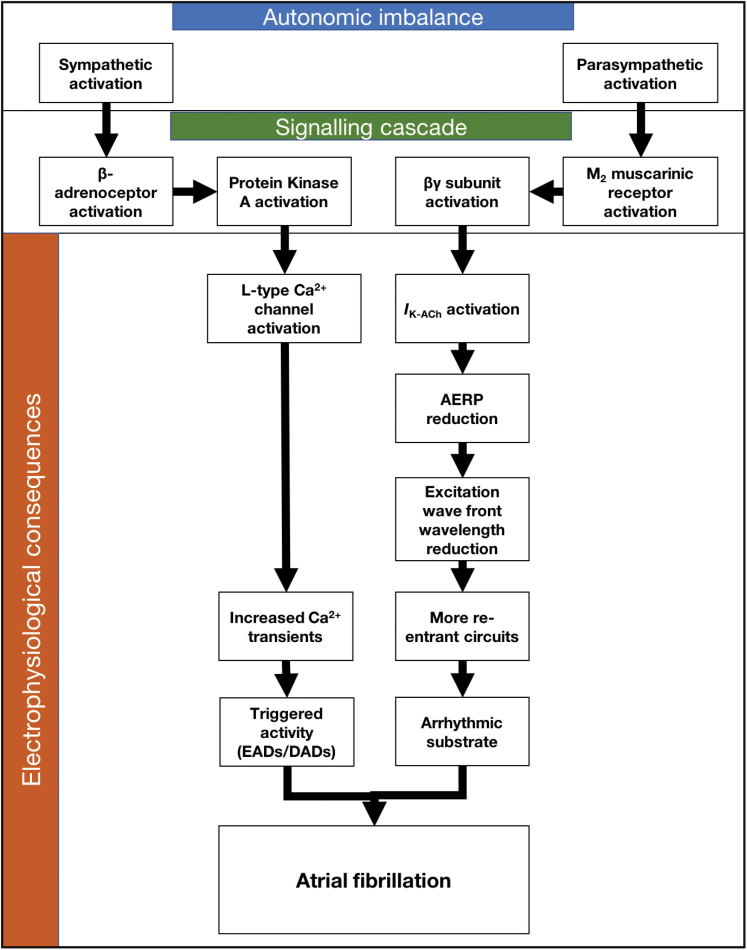
Sympathetic activation causes increased Ca2+ transients, due to the action of β-adrenergic receptors leading to a signaling cascade which ends with PKA phosphorylating several vital proteins including the L-type Ca2+ channel (Figure 4) [130]. The proarrhythmic effects of vagal activation are due to a reduction in the atrial effective refractory period (AERP), which reduces the wavelength of excitation wavefronts in the atria [131]. This reduction in wavelength allows more circuits of re-entrant activity to exist within the myocardium, thereby stabilizing the AF [132]. Activation of the IKACh channel by the βγ subunit of the M2 muscarinic receptor [133] is the cause of AERP reduction following vagal stimulation [134–137]. The loss of coupling between APD and Ca2+ transients allows triggered activity in the form of DADs [64] and the arrhythmic substrate to interact, producing AF [138, 139].
Figure 4.
Summary of mechanisms leading to AF brought on by autonomic imbalance. Alterations in sympathetic and parasympathetic supply will trigger signaling cascades which alter selected ion channel function. Alterations in ion channel functional properties will allow for the development of the trigger-substrate framework for the initiation of AF.
Increased sympathetic nerve activity can lead to VF. Heart rate variability analysis and direct nerve recordings show an increase in sympathetic activity preceding VF [140, 141]. An increased adrenergic tone is associated with arrhythmic conditions such as LQTS (increased QT prolongation) and catecholaminergic polymorphic ventricular tachycardia (CPVT), whereas conditions such as Brugada syndrome and J-wave syndrome are associated with an increased vagal tone [138]. Therefore, due to the sustained periods of autonomic imbalance that is present in OSA patients, there may be a risk of SCD owing to ventricular arrhythmias.
Therapeutic Implications
Angiotensin-converting enzyme (ACE) inhibitors remain the first-line therapy for HF. Other pharmacologic interventions include β-blockers, mineralocorticoid/aldosterone receptor antagonists (MRAs) and diuretics [142]. First-line treatment for OSA utilizes CPAP therapy, and this has cardiovascular and neurological benefits. For example, CPAP management of OSA in HFrEF patients reduces both ventricular arrhythmias and sympathetic nervous system activity compared to untreated controls [143]. However, CPAP is not tolerated by all patients, so medical therapy involves weight loss therapy (diet/exercise/bariatric surgery), pharmacologic agents, supplemental oxygen, treatments to improve nasal patency and positional therapies [144]. Current therapy for AF can be classified as rate control, rhythm control, and hybrid rhythm control. Pharmacologic treatment for acute rate control uses β-blockers, non-dihydropyridine Ca2+ channel blockers (diltiazem, verapamil), and digoxin (in patients with HFrEF who have LVEF <40%) [145]. Many non-AADs may have anti-arrhythmic effects, especially those used in HF; ACE inhibitors, angiotensin receptor blockers (ARBs), and β-blockers are effective at preventing AF onset [145]. Pharmacological treatment of ventricular arrhythmia and prevention of SCD revolves around β-blockers as first-line therapy [146]. Other therapies include class IA AADs (contraindicated in patients suffering from LQTS or taking QT interval-prolonging drugs) and class IB AADs, amiodarone, and sotalol [146]. However, class I AADs are contraindicated in patients post-MI due to a higher incidence of mortality observed in the Cardiac Arrhythmia Suppression Trial (CAST) [146]. Device therapies for ventricular arrhythmias include implantable cardioverter defibrillators (ICDs), subcutaneous implantable cardioverter defibrillators, wearable cardioverter defibrillators and public access defibrillators [146]. Interventional therapies such as catheter ablation and anti-arrhythmic surgery are effective in treating ventricular arrhythmia in patients with incessant VT or who are refractory to pharmacologic treatment [146].
It is crucial to consider therapeutics in a context of HF, OSA, and arrhythmias occurring concurrently. For example, evidence is scarce in the form of studies looking at rate and rhythm control drugs in patients with hypertrophic cardiomyopathy [145]. The European Society of Cardiology guidelines on HF [142] recommends ICDs for patients suffering from HF and AF. It has been found that rates of SCD in patients with HFrEF are reduced when there is an ICD; however, this therapy is not recommended in patients with severe symptomatic HF because these patients have a limited life expectancy and a high likelihood of dying from pump failure [142]. The incidence of AF was also found to be reduced by drugs used in HF treatment, such as ACE inhibitors, ARBs, β-blockers, and MRAs. Ivabradine, in contrast, was found to increase risk of AF. The AAD drug dronedarone is contraindicated in sufferers of concomitant HF and AF [142]. A meta-analysis looking at sleep apnea sufferers treated with a non-pharmacologic treatment for HF, cardiac resynchronization therapy, showed reduced AHI in patients suffering from CSA although no benefit was found in OSA patients [147]. Rhythm control strategies have not been shown to be better in reducing morbidity and mortality in patients with chronic HF, compared to a rate control strategy [142]. Furthermore, class IA antiarrhythmic medications have been found to increase morbidity and mortality in HF and AF patients due to increased risk of ventricular arrhythmias, and data from the CAST showed that class IC AADs were associated with increased mortality in post-MI patients [148]. Although amiodarone has no impact in increasing mortality in patients with HF [149], it has been shown to be no better than placebo in patients with HFrEF and LVEF <35% in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) [150]. Some other studies implicate amiodarone in negatively impacting outcomes in patients with more severe HF [40, 142]. Sotalol is another AAD that although is used to treat VA is not recommended in patients with left ventricular dysfunction unless an ICD is also present [146].
Drugs targeted to reduce inflammation may be beneficial in treating arrhythmias in OSA/HF patients. RAAS modulators (such as ACE inhibitors) are known to have anti-inflammatory properties [99], which may explain why they are beneficial in the treatment of AF. Steroids, which have obvious anti-inflammatory effects, and statins, fish oil and vitamin C, which evidence suggests have anti-inflammatory properties, have also been shown in both human studies and animal models to reduce the onset and severity of AF [99, 151]. Targeting interleukins directly may also represent an attractive future target. The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) reported a reduced risk of cardiovascular death amongst a cohort of post-MI patients when given an inhibitor of IL-1β, canakinumab [152]. Hence, there may be merit in investigating whether IL-1β inhibitors may have an effect on arrhythmogenesis in future trials. Certain anti-inflammatory therapies should be approached with caution, however. H1 antihistamines, for example, have been shown to increase risk of cardiac death due to their effects on HERG1 K+ channels, which lead to potentially fatal cardiac arrhythmias [153].
It should be noted that OSA and its clinical management have significant implications for the treatment of arrhythmias. Pharmacologic rhythm treatment of symptomatic AF by AADs is less effective in patients suffering from more severe OSA compared to those suffering from milder OSA [59]. In addition, the effect of genotype on AF patients’ response to AAD therapy depends partially on the severity of OSA [60]. It is conjectured that AADs which act on IKACh channels, such as amiodarone, are an attractive target for future research in patients with concurrent OSA and HF [154]. This is because, autonomic tone instability and IKACh channels are key aspects of the pathogenesis of AF in OSA patients. However, it has been shown that strong NTP seen in a pig model of OSA evokes shortening of the AERP and increases susceptibility to AF; this AERP shortening is modulated by atropine or atenolol, but notably was not affected by class III AADs (amiodarone and sotalol) [155]. Instead, blockade of K+ channels carrying IKur and IKr have been shown to induce shortening of AERP in a pig model of OSA [156]. Non-pharmacologic treatments of OSA in addition to CPAP such as neurostimulation and oral appliances have shown promising results and may be of use in patients where there is low compliance with CPAP. A study in human patients involving targeted hypoglossal nerve stimulation showed benefit in ameliorating oxygen desaturation and reducing the AHI [157]. Upper airway stimulation in a study of 126 participants yielded similar beneficial results in terms of reducing the severity of OSA [158]. Oral appliances show benefit in some but not all OSA patients and although not as efficacious as CPAP, tend to have better compliance amongst patients [159]. Thus, by reducing the severity of OSA, neurostimulation and oral appliances may potentially improve outcomes relating to HF and arrhythmia in OSA patients, although more research is required in this area.
Non-pharmacologic treatments of arrhythmias are similarly impacted by OSA. Post-electrical cardioversion, patients suffering from untreated AF and OSA have a higher rate of recurrence (82%) compared with AF and CPAP-treated OSA (42%) [160]. OSA, after accounting for several covariates (age, gender, BMI, hypertension, left atrial size and ventricular ejection fraction), is the strongest predictor of AF recurring after catheter ablation, resulting a threefold increase compared to patients without OSA [161]. Thus, OSA confers a 25% greater risk of recurring AF after catheter ablation [162]. Treatment with CPAP therapy increases rate of AF-free survival in post-catheter ablation OSA patients compared to non-CPAP patients (65.6% vs. 33.3%), and in fact increases AF-free survival to a rate similar to OSA-free patients [163]. Furthermore, CPAP appears to be highly successful in reducing the incidence of AF in patients who have not had catheter ablation [164].
The impact of OSA on the activity AADs may perhaps partially explain the results of the SERVE-HF trial, which showed that adaptive servo-ventilation (ASV) therapy in patients with HF suffering from CSA (~80% of cohort) or OSA (~20% of cohort) increased both all-cause and cardiovascular mortality [165]. Given that there was significantly higher rate of AAD usage in the ASV group (19.2%) compared to the control group (13.5%), it is plausible that the AADs themselves may be either inactive or even pro-arrhythmic. This is evidenced by the fact that subgroup analysis showed increased mortality rates in patients with high usage of AADs [166]. Caution should be taken when generalizing the results of this study to other patient populations, however. The study population was composed exclusively of patients with HFrEF, most of whom had CSA. A randomized trial, ADVENT-HF, is underway in patients with HFrEF and CSA or OSA (ClinicalTrials.gov identifier: NCT01128816) [167]. Furthermore, CSR may indeed be a cardioprotective, compensatory process occurring downstream of HF, which is a fundamentally different pathophysiology compared to OSA [14, 168, 169]. Another explanation may be that because CSR can also present in patients with HF when awake, a rebound of CSR during the day might be possible [14]. Thus, ASV treatment may not be addressing this daytime CSR. Finally, CPAP is used in patients who mainly suffer from OSA. In patients with predominantly CSA, CPAP is not sufficient and ASV is used [167]. Thus, CPAP studies would be of more interest than ASV when concerning OSA patients.
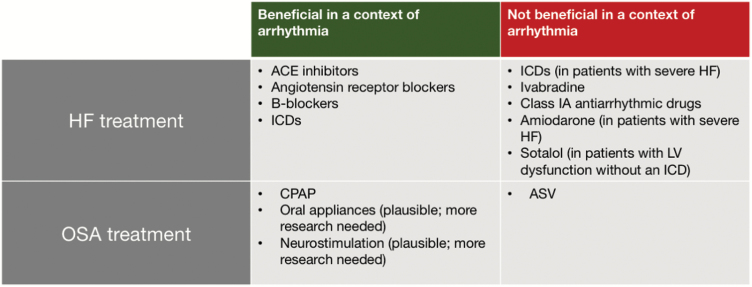
The different therapeutic strategies in HF, OSA and arrhythmias are summarized in Figure 5. Thus, for a therapeutic intervention to be successful in patients with several comorbidities, it should manage multiple risk factors and future therapeutics should be targeted against common pathophysiological pathways, such as the four discussed in this review, to maximize efficacy.
Figure 5.
A summary of HF and OSA treatment and their interactions with arrhythmias, with beneficial or negative effects indicated. Abbreviation: LV – left ventricular.
Conclusion
HF and OSA are conditions that have a significant impact on human health. A substantial body of evidence exists that support a link between these two conditions and cardiac arrhythmias. There are many mechanisms leading to arrhythmogenesis in HF and OSA, such as metabolic derangement, remodeling, inflammation, and autonomic imbalance. These mechanisms lead to the generation of arrhythmic triggers and substrates, resulting in atrial and ventricular arrhythmias that cause significant morbidity and mortality. Changes in therapeutic strategies are essential to consider when treating patients with these concurrent comorbidities. Some pharmacologic interventions used to treat HF, OSA, and arrhythmias in isolation may not be suitable when these conditions exist together, while other interventions such as CPAP for OSA have beneficial effects on atrial arrhythmias. The molecular and electrophysiological basis of the arrhythmogenesis explored in this review highlights novel pathways to consider in future research and therapeutic investigations.
Funding
K.R.C. was funded by the Physiological Society for a summer studentship at the University of Surrey. I.T.F. was funded by the Wellcome Trust Biomedical Vacation Scholarship at the University of Surrey. S.A. was funded by a Medical Research Council Research Fellowship (MR/M001288/1). H.V. was funded by the Wellcome Trust Research Training Fellowship (105727/Z/14/Z) and Sudden Arrhythmic Death Syndrome (SADS), UK. C.L-H.H. is funded by the Medical Research Council (MR/M001288/1), Wellcome Trust (105727/Z/14/Z), British Heart Foundation (PG/14/79/31102), the McVeigh Benefaction and SADS UK. K.J. is funded by the Research Support Fund, Faculty of Health and Medical Science, University of Surrey.
Conflict of interest statement. None declared.
References
- 1. Bui AL, et al. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chatterjee K, et al. Systolic and diastolic heart failure: differences and similarities. J Card Fail. 2007;13(7):569–576. [DOI] [PubMed] [Google Scholar]
- 3. Borlaug BA, et al. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123(18):2006–2013; discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mentz RJ, et al. Noncardiac comorbidities and acute heart failure patients. Heart Fail Clin. 2013;9(3):359–67, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strollo PJ Jr, et al. Obstructive sleep apnea. N Engl J Med. 1996;334(2):99–104. [DOI] [PubMed] [Google Scholar]
- 6. Panossian L, et al. Sleep-disordered breathing. Continuum (Minneap Minn). 2013;19(1 Sleep Disorders):86–103. [DOI] [PubMed] [Google Scholar]
- 7. Brack T, et al. Cheyne-stokes respiration in patients with heart failure: prevalence, causes, consequences and treatments. Respiration. 2012;83(2):165–176. [DOI] [PubMed] [Google Scholar]
- 8. Banno K, et al. Sleep apnea: clinical investigations in humans. Sleep Med. 2007;8(4):400–426. [DOI] [PubMed] [Google Scholar]
- 9. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paiva T, et al. Obstructive sleep apnea and other sleep-related syndromes. Handb Clin Neurol. 2014;119:251–271. [DOI] [PubMed] [Google Scholar]
- 11. Young T, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. [DOI] [PubMed] [Google Scholar]
- 12. Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oldenburg O, et al. Heart failure: central sleep apnoea in HF–what can we learn from SERVE-HF?Nat Rev Cardiol. 2015;12(12):686–687. [DOI] [PubMed] [Google Scholar]
- 15. Selim BJ, et al. Management of sleep apnea syndromes in heart failure. Sleep Med Clin. 2017;12(1):107–121. [DOI] [PubMed] [Google Scholar]
- 16. Gottlieb DJ, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jean-Louis G, et al. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4(3):261–272. [PMC free article] [PubMed] [Google Scholar]
- 18. Lavie P, et al. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brooks D, et al. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99(1):106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulz R, et al. ; working group Kreislauf und Schlaf of the German Sleep Society (DGSM). Sleep apnoea in heart failure. Eur Respir J. 2007;29(6):1201–1205. [DOI] [PubMed] [Google Scholar]
- 21. Paulino A, et al. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009;102(3):169–175. [DOI] [PubMed] [Google Scholar]
- 22. Khayat RN, et al. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15(9):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laaban JP, et al. Left ventricular systolic dysfunction in patients with obstructive sleep apnea syndrome. Chest. 2002;122(4):1133–1138. [DOI] [PubMed] [Google Scholar]
- 24. Shahar E, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. [DOI] [PubMed] [Google Scholar]
- 25. Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106(1):21–28. [DOI] [PubMed] [Google Scholar]
- 26. MacDonald M, et al. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4(1):38–42. [PMC free article] [PubMed] [Google Scholar]
- 27. Yumino D, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121(14):1598–1605. [DOI] [PubMed] [Google Scholar]
- 28. Bucca CB, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132(2):440–446. [DOI] [PubMed] [Google Scholar]
- 29. Somers VK, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fung JW, et al. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest. 2002;121(2):422–429. [DOI] [PubMed] [Google Scholar]
- 31. Oliveira W, et al. Left atrial volume and function in patients with obstructive sleep apnea assessed by real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21(12):1355–1361. [DOI] [PubMed] [Google Scholar]
- 32. Kannel WB, et al. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113(6):1489–1494. [DOI] [PubMed] [Google Scholar]
- 33. Colquitt JL, et al. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess. 2014;18(56):1–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang DH, et al. Economic burden and disparities in healthcare resource use among adult patients with cardiac arrhythmia. Appl Health Econ Health Policy. 2014;12(1):59–71. [DOI] [PubMed] [Google Scholar]
- 35. Rubart M, et al. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115(9):2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Behr E, et al. ; Sudden Arrhythmic Death Syndrome Steering Group. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet. 2003;362(9394):1457–1459. [DOI] [PubMed] [Google Scholar]
- 37. Abraham JM, et al. Atrial fibrillation in heart failure: stroke risk stratification and anticoagulation. Heart Fail Rev. 2014;19(3):305–313. [DOI] [PubMed] [Google Scholar]
- 38. Thihalolipavan S, et al. Atrial fibrillation and heart failure: update 2015. Prog Cardiovasc Dis. 2015;58(2):126–135. [DOI] [PubMed] [Google Scholar]
- 39. Maisel WH, et al. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6A):2D–8D. [DOI] [PubMed] [Google Scholar]
- 40. Saltzman HE. Arrhythmias and heart failure. Cardiol Clin. 2014;32(1):125–33, ix. [DOI] [PubMed] [Google Scholar]
- 41. Mosterd A, et al. The prognosis of heart failure in the general population: the Rotterdam Study. Eur Heart J. 2001;22(15):1318–1327. [DOI] [PubMed] [Google Scholar]
- 42. Ho KK, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. [DOI] [PubMed] [Google Scholar]
- 43. Quan SF, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 44. Redline S, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 45. Mehra R, et al. ; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koo BB, et al. The Determining Risk of Vascular Events by Apnea Monitoring (DREAM) study: design, rationale, and methods. Sleep Breath. 2016;20(2):893–900. [DOI] [PubMed] [Google Scholar]
- 47. Selim BJ, et al. The association between nocturnal cardiac arrhythmias and sleep-disordered breathing: the DREAM Study. J Clin Sleep Med. 2016;12(6):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mehra R, et al. Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169(12):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cadby G, et al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest. 2015;148(4):945–952. [DOI] [PubMed] [Google Scholar]
- 50. May AM, et al. ; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med. 2016;193(7):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tung P, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc. 2017;6(7):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. He J, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94(1):9–14. [PubMed] [Google Scholar]
- 53. Partinen M, et al. Long-term outcome for obstructive sleep apnea syndrome patients. Mortality. Chest. 1988;94(6):1200–1204. [DOI] [PubMed] [Google Scholar]
- 54. Shamsuzzaman AS, et al. Obstructive sleep apnea in patients with congenital long QT syndrome: implications for increased risk of sudden cardiac death. Sleep. 2015;38(7):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gillis AM, et al. Changes in the QT interval during obstructive sleep apnea. Sleep. 1991;14(4):346–350. [DOI] [PubMed] [Google Scholar]
- 56. Dursunoglu D, et al. Effect of CPAP on QT interval dispersion in obstructive sleep apnea patients without hypertension. Sleep Med. 2007;8(5):478–483. [DOI] [PubMed] [Google Scholar]
- 57. Gami AS, et al. Therapy insight: interactions between atrial fibrillation and obstructive sleep apnea. Nat Clin Pract Cardiovasc Med. 2005;2(3):145–149. [DOI] [PubMed] [Google Scholar]
- 58. Marin JM, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. [DOI] [PubMed] [Google Scholar]
- 59. Monahan K, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012;110(3):369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goyal SK, et al. Severity of obstructive sleep apnea influences the effect of genotype on response to anti-arrhythmic drug therapy for atrial fibrillation. J Clin Sleep Med. 2014;10(5):503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vizzardi E, et al. Obstructive sleep apnoea-hypopnoea and arrhythmias: new updates. J Cardiovasc Med (Hagerstown). 2017;18(7):490–500. [DOI] [PubMed] [Google Scholar]
- 62. Kalin A, et al. Cardiac arrhythmia: a simple conceptual framework. Trends Cardiovasc Med. 2010;20(3):103–107. [DOI] [PubMed] [Google Scholar]
- 63. Yano M, et al. Role of ryanodine receptor as a Ca²(+) regulatory center in normal and failing hearts. J Cardiol. 2009;53(1):1–7. [DOI] [PubMed] [Google Scholar]
- 64. Marks AR, et al. Progression of heart failure: is protein kinase a hyperphosphorylation of the ryanodine receptor a contributing factor?Circulation. 2002;105(3):272–275. [PubMed] [Google Scholar]
- 65. Antzelevitch C. Basic mechanisms of reentrant arrhythmias. Curr Opin Cardiol. 2001;16(1):1–7. [DOI] [PubMed] [Google Scholar]
- 66. Wang ZV, et al. Heart failure and loss of metabolic control. J Cardiovasc Pharmacol. 2014;63(4):302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsutsui H, et al. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–H2190. [DOI] [PubMed] [Google Scholar]
- 68. Nikolaidou T, et al. Congestive heart failure leads to prolongation of the PR interval and atrioventricular junction enlargement and ion channel remodelling in the rabbit. PLoS One. 2015;10(10):e0141452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Askevold ET, et al. Interleukin-6 signaling, soluble glycoprotein 130, and inflammation in heart failure. Curr Heart Fail Rep. 2014;11(2):146–155. [DOI] [PubMed] [Google Scholar]
- 70. Florea VG, et al. The autonomic nervous system and heart failure. Circ Res. 2014;114(11):1815–1826. [DOI] [PubMed] [Google Scholar]
- 71. May AM, et al. Obstructive sleep apnea: role of intermittent hypoxia and inflammation. Semin Respir Crit Care Med. 2014;35(5):531–544. [DOI] [PubMed] [Google Scholar]
- 72. Channaveerappa D, et al. Atrial electrophysiological and molecular remodelling induced by obstructive sleep apnoea. J Cell Mol Med. 2017;21(9):2223–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schwarzer M, et al. Mitochondrial reactive oxygen species production and respiratory complex activity in rats with pressure overload-induced heart failure. J Physiol. 2014;592(17):3767–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jeong EM, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52(2):454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Köhler AC, et al. Reactive oxygen species and excitation-contraction coupling in the context of cardiac pathology. J Mol Cell Cardiol. 2014;73:92–102. [DOI] [PubMed] [Google Scholar]
- 76. Yang KC, et al. Mitochondria and arrhythmias. Free Radic Biol Med. 2014;71:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ward CA, et al. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500 (Pt 3):631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ju YK, et al. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497 (Pt 2):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mozzi R, et al. Phosphatidylserine synthesis in rat cerebral cortex: effects of hypoxia, hypocapnia and development. Mol Cell Biochem. 1993;126(2):101–107. [DOI] [PubMed] [Google Scholar]
- 80. West JW, et al. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991;254(5033):866–868. [DOI] [PubMed] [Google Scholar]
- 81. Payandeh J, et al. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475(7356):353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ma J, et al. Calmodulin kinase II and protein kinase C mediate the effect of increased intracellular calcium to augment late sodium current in rabbit ventricular myocytes. Am J Physiol Cell Physiol. 2012;302(8):C1141–C1151. [DOI] [PubMed] [Google Scholar]
- 83. Fu C, et al. Modulation of late sodium current by Ca2+ -calmodulin-dependent protein kinase II, protein kinase C and Ca2+ during hypoxia in rabbit ventricular myocytes. Exp Physiol. 2017;102(7):818–834. [DOI] [PubMed] [Google Scholar]
- 84. Chadda KR, et al. Sodium channel biophysics, late sodium current and genetic arrhythmic syndromes. Pflugers Arch. 2017;469(5–6):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cronk LB, et al. Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm. 2007;4(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vatta M, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114(20):2104–2112. [DOI] [PubMed] [Google Scholar]
- 87. Bennett PB, et al. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376(6542):683–685. [DOI] [PubMed] [Google Scholar]
- 88. Xiao HD, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165(3):1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Donoghue M, et al. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35(9):1043–1053. [DOI] [PubMed] [Google Scholar]
- 90. Thomas SA, et al. Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: evidence for chamber-specific molecular determinants of conduction. Circulation. 1998;97(7):686–691. [DOI] [PubMed] [Google Scholar]
- 91. Vaidya D, et al. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res. 2001;88(11):1196–1202. [DOI] [PubMed] [Google Scholar]
- 92. Rutledge C, et al. Mitochondria and arrhythmias. Expert Rev Cardiovasc Ther. 2013;11(7):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Franz MR. Mechano-electrical feedback in ventricular myocardium. Cardiovasc Res. 1996;32(1):15–24. [PubMed] [Google Scholar]
- 94. Chen LM, et al. Eccentric cardiac hypertrophy was induced by long-term intermittent hypoxia in rats. Exp Physiol. 2007;92(2):409–416. [DOI] [PubMed] [Google Scholar]
- 95. Tomaselli GF, et al. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42(2):270–283. [DOI] [PubMed] [Google Scholar]
- 96. Magnani JW, et al. P wave duration and risk of longitudinal atrial fibrillation in persons ≥ 60 years old (from the Framingham Heart Study). Am J Cardiol. 2011;107(6):917–921.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kozhevnikov DO, et al. Electrophysiological mechanism of enhanced susceptibility of hypertrophied heart to acquired torsade de pointes arrhythmias: tridimensional mapping of activation and recovery patterns. Circulation. 2002;105(9):1128–1134. [DOI] [PubMed] [Google Scholar]
- 98. Boos CJ, et al. The role of inflammation in atrial fibrillation. Int J Clin Pract. 2005;59(8):870–872. [DOI] [PubMed] [Google Scholar]
- 99. Boos CJ, et al. Is atrial fibrillation an inflammatory disorder?Eur Heart J. 2006;27(2):136–149. [DOI] [PubMed] [Google Scholar]
- 100. Vgontzas AN, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82(5):1313–1316. [DOI] [PubMed] [Google Scholar]
- 101. Dyugovskaya L, et al. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. Am J Respir Crit Care Med. 2003;168(2):242–249. [DOI] [PubMed] [Google Scholar]
- 102. Bowie A, et al. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59(1):13–23. [DOI] [PubMed] [Google Scholar]
- 103. Ryan S, et al. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. [DOI] [PubMed] [Google Scholar]
- 104. Torre-Amione G, et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 1996;27(5):1201–1206. [DOI] [PubMed] [Google Scholar]
- 105. Aukrust P, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83(3):376–382. [DOI] [PubMed] [Google Scholar]
- 106. Shang LL, et al. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008;294(1):C372–C379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Capulzini L, et al. Arrhythmia and right heart disease: from genetic basis to clinical practice. Rev Esp Cardiol. 2010;63(8):963–983. [DOI] [PubMed] [Google Scholar]
- 108. Brugada R, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101(5):510–515. [DOI] [PubMed] [Google Scholar]
- 109. Petkova-Kirova PS, et al. Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2006;290(5):H2098–H2107. [DOI] [PubMed] [Google Scholar]
- 110. Jeevaratnam K, et al. Cardiac potassium channels: physiological insights for targeted therapy. J Cardiovasc Pharmacol Ther. 2018;23(2):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fernández-Velasco M, et al. TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am J Physiol Heart Circ Physiol. 2007;293(1):H238–H245. [DOI] [PubMed] [Google Scholar]
- 112. Wang J, et al. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem. 2004;279(14):13289–13292. [DOI] [PubMed] [Google Scholar]
- 113. London B, et al. Calcium-dependent arrhythmias in transgenic mice with heart failure. Am J Physiol Heart Circ Physiol. 2003;284(2):H431–H441. [DOI] [PubMed] [Google Scholar]
- 114. Duncan DJ, et al. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium. 2010;47(4):378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wu CK, et al. Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med. 2011;39(5):984–992. [DOI] [PubMed] [Google Scholar]
- 116. Sawaya SE, et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am J Physiol Heart Circ Physiol. 2007;292(3):H1561–H1567. [DOI] [PubMed] [Google Scholar]
- 117. Baum JR, et al. Myofibroblasts cause heterogeneous Cx43 reduction and are unlikely to be coupled to myocytes in the healing canine infarct. Am J Physiol Heart Circ Physiol. 2012;302(3):H790–H800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thayer JF, et al. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131. [DOI] [PubMed] [Google Scholar]
- 119. Roche F, et al. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol. 2003;26(3):669–677. [DOI] [PubMed] [Google Scholar]
- 120. Narkiewicz K, et al. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177(3):385–390. [DOI] [PubMed] [Google Scholar]
- 121. Smith RP, et al. Obstructive sleep apnoea and the autonomic nervous system. Sleep Med Rev. 1998;2(2):69–92. [DOI] [PubMed] [Google Scholar]
- 122. Schwartz AR, et al. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Narkiewicz K, et al. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–776. [DOI] [PubMed] [Google Scholar]
- 124. Linz D, et al. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8(9):1436–1443. [DOI] [PubMed] [Google Scholar]
- 125. Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59(2):117–122. [DOI] [PubMed] [Google Scholar]
- 126. Amar D, et al. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42(7):1262–1268. [DOI] [PubMed] [Google Scholar]
- 127. Tomita T, et al. Role of autonomic tone in the initiation and termination of paroxysmal atrial fibrillation in patients without structural heart disease. J Cardiovasc Electrophysiol. 2003;14(6):559–564. [DOI] [PubMed] [Google Scholar]
- 128. Tan AY, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118(9):916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sharifov OF, et al. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43(3):483–490. [DOI] [PubMed] [Google Scholar]
- 130. Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. [DOI] [PubMed] [Google Scholar]
- 131. Smeets JL, et al. The wavelength of the cardiac impulse and reentrant arrhythmias in isolated rabbit atrium. The role of heart rate, autonomic transmitters, temperature, and potassium. Circ Res. 1986;58(1):96–108. [DOI] [PubMed] [Google Scholar]
- 132. Allessie MA. Atrial electrophysiologic remodeling: another vicious circle?J Cardiovasc Electrophysiol. 1998;9(12):1378–1393. [DOI] [PubMed] [Google Scholar]
- 133. Krapivinsky G, et al. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995;374(6518):135–141. [DOI] [PubMed] [Google Scholar]
- 134. Wijffels MC, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–1968. [DOI] [PubMed] [Google Scholar]
- 135. Rensma PL, et al. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62(2):395–410. [DOI] [PubMed] [Google Scholar]
- 136. Hashimoto N, et al. Tertiapin, a selective IKACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacol Res. 2006;54(2):136–141. [DOI] [PubMed] [Google Scholar]
- 137. Kovoor P, et al. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol. 2001;37(8):2136–2143. [DOI] [PubMed] [Google Scholar]
- 138. Shen MJ, et al. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114(6):1004–1021. [DOI] [PubMed] [Google Scholar]
- 139. Patterson E, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47(6):1196–1206. [DOI] [PubMed] [Google Scholar]
- 140. Shusterman V, et al. Autonomic nervous system activity and the spontaneous initiation of ventricular tachycardia. ESVEM Investigators. Electrophysiologic study versus electrocardiographic monitoring trial. J Am Coll Cardiol. 1998;32(7):1891–1899. [DOI] [PubMed] [Google Scholar]
- 141. Zhou S, et al. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm. 2008;5(1):131–139. [DOI] [PubMed] [Google Scholar]
- 142. Ponikowski P, et al. ; ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. [DOI] [PubMed] [Google Scholar]
- 143. Ryan CM, et al. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60(9):781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Veasey SC, et al. Medical therapy for obstructive sleep apnea: a review by the medical therapy for obstructive sleep apnea task force of the standards of practice committee of the American Academy of Sleep Medicine. Sleep. 2006;29(8):1036–1044. [DOI] [PubMed] [Google Scholar]
- 145. Kirchhof P, et al. ; ESC Scientific Document Group. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 146. Priori SG, et al. ; ESC Scientific Document Group. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–2867. [DOI] [PubMed] [Google Scholar]
- 147. Lamba J, et al. Cardiac resynchronization therapy for the treatment of sleep apnoea: a meta-analysis. Europace. 2011;13(8):1174–1179. [DOI] [PubMed] [Google Scholar]
- 148. Echt DS, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324(12):781–788. [DOI] [PubMed] [Google Scholar]
- 149. Singh SN, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival trial of antiarrhythmic therapy in congestive heart failure. N Engl J Med. 1995;333(2):77–82. [DOI] [PubMed] [Google Scholar]
- 150. Bardy GH, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. [DOI] [PubMed] [Google Scholar]
- 151. Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52(4):306–313. [DOI] [PubMed] [Google Scholar]
- 152. Ridker PM, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 153. Leurs R, et al. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002;32(4):489–498. [DOI] [PubMed] [Google Scholar]
- 154. Goyal SK, et al. Atrial fibrillation in obstructive sleep apnea. World J Cardiol. 2013;5(6):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Linz D. Atrial fibrillation in obstructive sleep apnea: atrial arrhythmogenic substrate of a different sort. Am J Cardiol. 2012;110(7):1071. [DOI] [PubMed] [Google Scholar]
- 156. Linz D, et al. Combined blockade of early and late activated atrial potassium currents suppresses atrial fibrillation in a pig model of obstructive apnea. Heart Rhythm. 2011;8(12):1933–1939. [DOI] [PubMed] [Google Scholar]
- 157. Mwenge GB, et al. Targeted hypoglossal neurostimulation for obstructive sleep apnoea: a 1-year pilot study. Eur Respir J. 2013;41(2):360–367. [DOI] [PubMed] [Google Scholar]
- 158. Strollo PJ Jr, et al. ; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. [DOI] [PubMed] [Google Scholar]
- 159. Ferguson KA, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–262. [DOI] [PubMed] [Google Scholar]
- 160. Kanagala R, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. [DOI] [PubMed] [Google Scholar]
- 161. Jongnarangsin K, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19(7):668–672. [DOI] [PubMed] [Google Scholar]
- 162. Ng CY, et al. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108(1):47–51. [DOI] [PubMed] [Google Scholar]
- 163. Fein AS, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62(4):300–305. [DOI] [PubMed] [Google Scholar]
- 164. Fox H, et al. Sleep-disordered breathing and arrhythmia in heart failure patients. Sleep Med Clin. 2017;12(2):229–241. [DOI] [PubMed] [Google Scholar]
- 165. Cowie MR, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Randerath W, et al. Sleep-disordered breathing in patients with heart failure. Curr Sleep Med Rep. 2016;2(2):99–106. [Google Scholar]
- 167. Carmo J, et al. Sleep-disordered breathing in heart failure: the state of the art after the SERVE-HF trial. Rev Port Cardiol. 2017;36(11):859–867. [DOI] [PubMed] [Google Scholar]
- 168. Naughton MT. Cheyne-Stokes respiration: friend or foe?Thorax. 2012;67(4):357–360. [DOI] [PubMed] [Google Scholar]
- 169. Emdin M, et al. After the SERVE-HF trial, is there still a need for treatment of central apnea?J Card Fail. 2015;21(11):903–905. [DOI] [PubMed] [Google Scholar]