Highlights
-
•
Current practice lacks rapid detection tools to screen for seizures.
-
•
High agreement exists between neurologists’ diagnoses using full and reduced montage EEG.
-
•
Reduced channel EEG can be used to screen for generalized or hemispheric or rhythmic and periodic abnormalities.
Keywords: Electroencephalography, Reduced channel montage, Seizure detection, Rhythmic and periodic patterns, Sensitivity, Specificity
Abstract
Objectives
To compare the diagnostic utility of electroencephalography (EEG) using reduced, 8-channel montage (rm-EEG) to full, 18-channel montage (fm-EEG) for detection of generalized or hemispheric seizures and rhythmic periodic patterns (RPPs) by neurologists with extensive EEG training, neurology residents with minimal EEG exposure, and medical students without EEG experience.
Methods
We presented EEG samples in both fm-EEG (bipolar montage) and rm-EEG (lateral leads of bipolar montage) to 20 neurologists, 20 residents, and 42 medical students. Unanimous agreement of three senior epileptologists defined samples as seizures (n = 7), RPPs (n = 10), and normal or slowing (n = 20). Differences in median accuracy, sensitivity, and specificity were assessed using Wilcoxon signed-rank tests.
Results
Full and reduced EEG demonstrated similar accuracy when read by neurologists (fm-EEG: 95%, rm-EEG: 95%, p = 0.29), residents (fm-EEG: 80%, rm-EEG: 80%, p = 0.05), and students (fm-EEG: 60%, rm-EEG: 51%, p = 0.68). Moreover, neurologists’ sensitivity for detecting seizure activity was comparable between fm-EEG (100%) and rm-EEG (98%) (p = 0.17). Furthermore, the specificity of rm-EEG for seizures and RPP (neurologists: 100%, residents: 90%, students: 86%) was significantly greater than that of fm-EEG (neurologists: 93%, p = 0.03; residents: 80%, p = 0.01; students: 69%, p < 0.001).
Conclusions
The reduction of the number of EEG channels from 18 to 8 does not compromise neurologists’ sensitivity for detecting seizures that are often a core reason for performing urgent EEG. It may also increase their specificity for detecting rhythmic and periodic patterns, and thereby providing important diagnostic information to guide patient’s management.
Significance
Our study is the first to document the utility of a reduced channel EEG above the hairline compared to full montage EEG in aiding medical staff with varying degrees of EEG training to detect generalized or hemispheric seizures.
1. Introduction
Scalp electroencephalography (EEG) is commonly used in the evaluation of altered mental status (AMS) to determine the presence of functional abnormalities such as seizures. Recent consensus opinion from the American Clinical Neurophysiology Society (ACNS) states that “electrode template systems” may be used when rapid placement of EEG electrodes is essential or when EEG technicians are not immediately available (Herman et al., 2015). Such solutions will be especially valuable in the diagnosis of non-convulsive seizures, which can only be detected with EEG and have a significant impact on morbidity, mortality and neuronal health (Kaplan, 1996, DeLorenzo et al., 1998, Towne et al., 2000, Claassen et al., 2004, Meierkord and Holtkamp, 2007, Vespa et al., 2007). In fact, the majority of urgent EEGs are performed to rule out the most serious abnormalities such as non-convulsive status epilepticus (NCSE) (Quigg et al., 2001, Praline et al., 2007). However, current EEG practice with a full set of EEG electrodes is hampered by limited availability of technicians, machines, and trained neurologists, resulting in long delays in setup, recording and interpretation (Quigg et al., 2001, Kämppi et al., 2013, Gururangan et al., 2016). These delays in EEG acquisition limit its utility in emergent situations to detect pathological activity that are more likely to be present close to the onset of AMS and whose early detection may significantly impact both clinical management and patient outcomes (King et al., 1998, Quigg et al., 2001, Kolls and Husain, 2007, Ziai et al., 2012, Betjemann and Lowenstein, 2015, Paliwal et al., 2015, Gururangan et al., 2016, Sofat et al., 2016, Fernández et al., 2017, Jordan, 2017).
Less resource-intensive and point-of-care EEG electrode templates, per the ACNS consensus, may have a reduced number of electrodes that could be deployed more rapidly at the bedside without waiting for trained technicians. Such EEG tools might shorten the delays for acquiring EEG, which may facilitate early detection of seizures or epileptiform activity and guide treatment decisions while awaiting the resources for a full diagnostic study (McMullan et al., 2010, Paliwal et al., 2015). While other rapid bedside diagnostics, such as those for cardiac emergencies, can reduce patients’ length of hospital stay, guide clinical management, and expedite completion of other diagnostic tests, no such point-of-care tool has been widely adopted for epileptic emergencies (McMullan et al., 2010, McMullan et al., 2012, Chan et al., 2013, Singer et al., 2015). This is partly due to controversies in the literature about the value of recording EEG with a non-conventional reduced number of electrodes (Kolls and Husain, 2007, Rubin et al., 2014, Tanner et al., 2014, Jordan, 2017).
Though more EEG sensors would yield better spatial resolution and more sensitive detection of focal abnormalities, it remains controversial whether half of the number of conventional EEG electrodes can be used with equal utility in emergency situations to detect gross abnormalities – such as seizures, hemispheric slowing, or rhythmic and periodic patterns. These findings may have pathognomonic value in guiding the diagnostic workup and management of patients with altered mental status. Prior studies of reduced electrode arrays have been limited by their focus on differentiating patterns that might result in similar management, such as distinguishing between seizures and seizure-like patterns that lie on the ictal-interictal continuum (for example, periodic epileptiform discharges), which is a non-trivial task even with a full set of EEG channels (Chong and Hirsch, 2005, Foldvary-Schaefer et al., 2006, Kolls and Husain, 2007, Young et al., 2009, Tanner et al., 2014). Of these investigations, only one to our knowledge studied the utility of hairline EEG (as opposed to sub-hairline) to screen for NCSE, and while the authors concluded that hairline EEG lacked sensitivity for seizures, especially focal seizures, they did not demonstrate whether hairline EEG preserved sensitivity for generalized or hemispheric patterns (Kolls and Husain, 2007). We hypothesize that reducing the number of EEG sensors (i.e., only the temporal chains of a double banana montage) will still enable trained readers to identify hemispheric or bilateral seizures or seizure-like abnormalities, as well as the majority of seizures that originate in the temporal and frontal lobes (King et al., 1998). After all, the utility of point-of-care EEG is in identifying conditions that need urgent treatment with antiepileptic drugs (AEDs) or triaging patients who will need long term monitoring with a conventional EEG setup rather than in describing minor nuances, such as the presence of a single sharp wave, or subtle abnormalities of EEG background activity.
A related question is whether reducing the number of EEG sensors affects the difficulty of making such diagnostic assessments. This is especially important if untrained individuals (such as junior residents or medical students) are to judge the presence of gross EEG abnormalities, a common request during off-hours when senior specialists may not be immediately available. We hypothesize that reducing the number of sensors will facilitate interpretation of EEG by untrained personnel because it decreases the amount of redundant (and potentially misleading) information that the individual would have to interpret (Ebersole and Bridgers, 1985). In the setting of a stat EEG study, the utility of the study lies in its ability to relay diagnostic information without too much delay (e.g., if EEG-untrained healthcare staff at the bedside can interpret seizures without waiting for the specialist to review the recordings).
This study was designed to test the above hypotheses by comparing the diagnostic utility of reduced 8-channel montage (rm-EEG) and full 18-channel montage (fm-EEG) to detect seizures and rhythmic and periodic patterns (RPP) when read by neurologists with extensive EEG training, neurology residents with minimal EEG training, and medical students without EEG training.
2. Methods
2.1. Study protocol and approval
This study was conducted with the approval of the Stanford University Institutional Review Board. This report describes a retrospective diagnostic study based on a survey and was prepared using the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines (Bossuyt et al., 2015).
2.2. Montage design
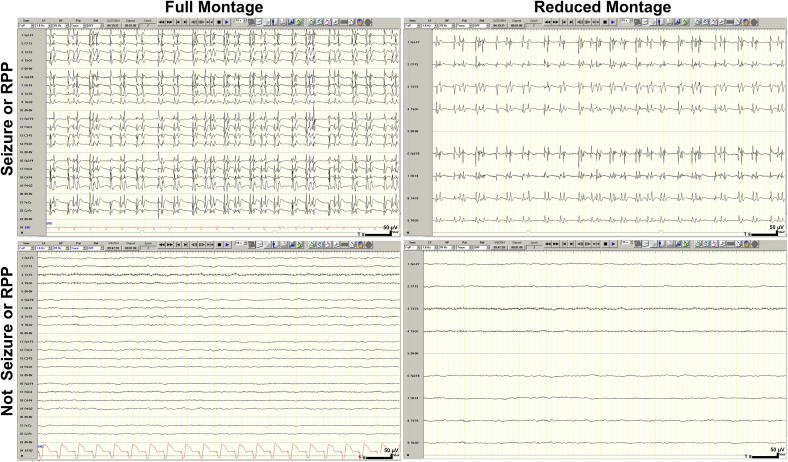
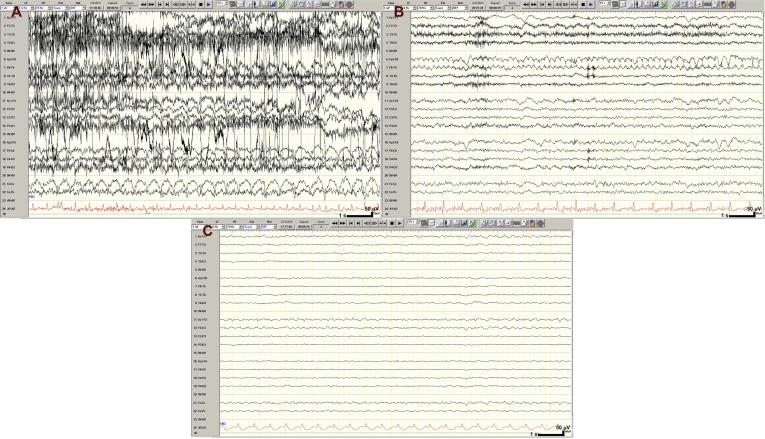
Continuous EEG recordings obtained with electrodes placed according to standard 10–20 system (Acharya et al., 2016a). Samples of these recordings were displayed separately in both the full 18-channel longitudinal bipolar (double banana) montage (fm-EEG) and a reduced 8-channel montage comprising only the lateral chains of the double banana montage (rm-EEG) (Acharya et al., 2016b). Examples of the fm-EEG and rm-EEG images are shown in Fig. 1.
Fig. 1.
Training samples of full and reduced montage EEG. Calibration bar: time scale represents 1 s (s), voltage scale represents 50 microvolts (μV). Abbreviations: RPP, rhythmic and periodic pattern.
2.3. Study participants
Survey responses were collected from 23 neurologists with extensive EEG training (10 epileptologists, 12 epilepsy fellows, 1 neurology resident [who was treated as a fellow for analysis]) from 7 institutions (California Pacific Medical Center, n = 1; Kaiser Permanente Redwood City, n = 1; Rush University, n = 4; Stanford University, n = 3; University of California, Los Angeles, n = 3; University of California, San Francisco, n = 4; Yale University, n = 7), 20 resident physicians in neurology training with minimal EEG training from Stanford University (n = 9) and Yale University (n = 11), and 42 medical students without EEG training from Stanford University. Demographic information was collected, including gender, level of training (i.e., medical student, resident, fellow, attending), years of EEG experience (i.e., none, less than 1, 1 to 2, 2 to 10, more than 10), and pediatric versus adult specialization.
2.4. Survey design
Each survey contained 44 unique EEG samples, each 15-s in duration, from continuous EEG recordings acquired in routine patient care. We felt that these 44 samples facilitated a representative range of electrographic abnormalities while keeping the duration of the survey practical. A single 15-s epoch was selected to mimic the standard presentation of a single page of EEG recording and to limit the ability of each rater to experience the EEG information differently from the others (e.g., by scrolling between pages). Samples were presented in an online survey as static images in a random order, and each sample was presented twice (non-sequentially), once in fm-EEG format and once in rm-EEG format. Participants were unable to adjust the layout of the channels or other settings. For each of the 88 sample presentations, both physicians and students were asked to indicate whether the sample contained seizure activity (question 1). Physicians (both epilepsy-trained neurologists and residents) were additionally asked whether the sample contained any other pathological activity (question 2), and to indicate the presence and laterality of the following specific findings (question 3): seizure, slowing, lateralized periodic discharges (LPDs), generalized periodic discharges (GPDs), triphasic waves (TWs), and burst suppression. Physicians were also able to enter other specific findings or comments. Two fm-EEG training samples with answers, one of generalized seizure and one of diffuse slowing, were shown to acquaint respondents with the survey; these training samples were not repeated in the actual survey. In addition, the time spent on each sample (in seconds) was recorded.
2.5. Classification of survey responses
Physicians’ (both epilepsy-trained neurologists and residents) responses were classified as seizure or RPP (vs. not) based on their responses to questions 1 and 2, selecting the specific findings of seizure, LPD, GPD, or TW in question 3, or any comment indicating that the sample contained seizure or RPP. For medical students, we classified samples as seizure or RPP based on their response to question 1 indicating seizure activity.
2.6. Reference standard for EEG findings
Of the 23 epilepsy-trained neurologists who participated in the study, we identified three senior epileptologists from different institutions with at least 10 years of experience in adult epilepsy. We defined a reference standard based on the unanimous agreement (3/3) of these epileptologists on the classification of each fm-EEG sample as seizure or RPP (vs. not) and as generalized (vs. focal). We grouped seizure-like patterns (i.e., periodic epileptiform discharges), under the ACNS definition of rhythmic and periodic patterns, which encompasses EEG abnormalities with prognostic significance, association with seizures, and potential risk for neuronal injury (Chong and Hirsch, 2005, Hirsch et al., 2013, Pedersen et al., 2013, Koren et al., 2016, Ruiz et al., 2017). The reference standard defined samples as seizure (n = 7; 6 generalized, 1 focal), RPP (n = 10; 8 generalized, 2 focal), and other non-rhythmic, non-periodic activity (e.g., normal or slowing; n = 20; 18 generalized, 2 focal); 7 samples were excluded due to disagreement among the reference standard epileptologists. Substantial agreement was observed between the three epileptologists who formed the reference standard (Fleiss’ kappa 0.79).
2.7. Statistical analysis
We calculated the accuracy, sensitivity, and specificity (reported as mean ± SD and median [IQR]) of fm-EEG and rm-EEG for the detection of generalized or hemispheric seizures and rhythmic periodic patterns by calculating the percentage of respondents who correctly detected the presence or absence of rhythmic and periodic abnormalities (according to the reference standard) and averaging across all samples. The concordance between fm-EEG and rm-EEG was measured by calculating the percentage of neurologists who provided the same classification of an EEG sample in both formats, averaged across samples, irrespective of whether it matched the reference standard. In order to demonstrate the impact of reducing the number of EEG channels on each rater’s determination of seizure or RPP, we calculated a false negative rate (FNR) for each rater by scoring each rater’s rm-EEG responses against their own fm-EEG responses for samples determined by the reference standard to represent seizure or RPP (i.e., if the rater detected a seizure or RPP using the standard EEG setup, what percentage missed this activity in the limited montage). We also calculated overall accuracy (against the reference standard) of fm-EEG and rm-EEG for each participant (except for the three epileptologists who formed the reference standard). The time spent per sample (reported as mean ± SD) was calculated by averaging across raters for each sample and then across samples. We excluded outliers over 1000 s (which might indicate that the rater may have been interrupted during the survey); overall, 23 outliers were removed (<1% of total data).
Differences between fm-EEG and rm-EEG in these diagnostic statistics were assessed using Wilcoxon signed-rank tests with continuity correction for tied comparisons (Shapiro-Wilk tests demonstrated significant deviations from the normal distribution). Differences in overall accuracy between raters associated with demographic characteristics were evaluated using Kruskal-Wallis tests, and Mann-Whitney tests were used to perform post hoc pairwise comparisons if the omnibus Kruskal-Wallis test was significant. Differences in average time spent per sample, as well as differences in time spent per sample between correct and incorrect responses, were assessed using two-tailed paired t-tests. A significance level of α=0.05 was used with Bonferroni correction for multiple comparisons.
3. Results
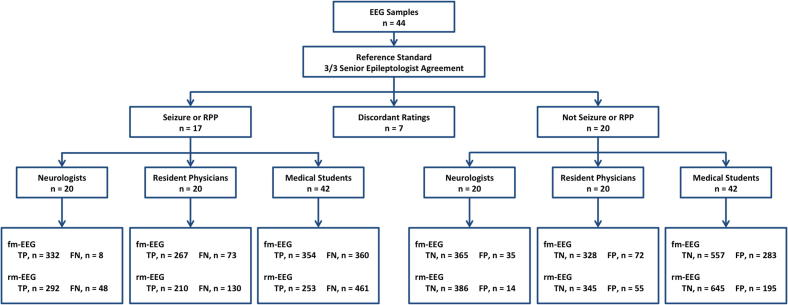
Characteristics of our survey participants are shown in Table 1. All neurologists (i.e., epilepsy attendings and fellows) had some EEG experience, including fellows within the first year of EEG fellowship training (30%) and fellows and attendings with more than one year of experience (70%); 15% of neurologists were pediatric epilepsy specialists and 35% were attending epileptologists. Among resident physicians, 35% had no EEG experience (of whom 71% were post-graduate year 2), and all residents with one year or more of EEG training were in their final year of residency (post-graduate year 4). None of the medical students had experience in reading EEG. The STARD flow diagram is shown in Fig. 2.
Table 1.
Characteristics of survey respondents.
| Neurologists (n = 20) | Neurology residents (n = 20) | Medical students (n = 42) | |
|---|---|---|---|
| Female, n (%) | 13 (65) | 13 (65) | 25 (60) |
| Level of medical training, n (%) | |||
| Attending | 7 (35) | — | — |
| Fellow | 13 (65) | — | — |
| Resident, PGY4 | — | 6 (30) | — |
| Resident, PGY3 | — | 5 (25) | — |
| Resident, PGY2 | — | 9 (45) | — |
| Pediatric specialist, n (%) | 3 (15) | 1 (5) | — |
| Years of EEG experience, n (%) | |||
| None | 0 (0) | 7 (35) | 42 (100) |
| Less than 1 | 6 (30) | 9 (45) | 0 (0) |
| 1–2 | 10 (50) | 2 (10) | 0 (0) |
| 2–10 | 3 (15) | 2 (10) | 0 (0) |
| More than 10 | 1 (5) | 0 (0) | 0 (0) |
Abbreviations: PGY, post-graduate year.
Fig. 2.
STARD flow diagram. Abbreviations: FN, false negative; FP, false positive; RPP, rhythmic and periodic pattern; TN, true negative; TP, true positive.
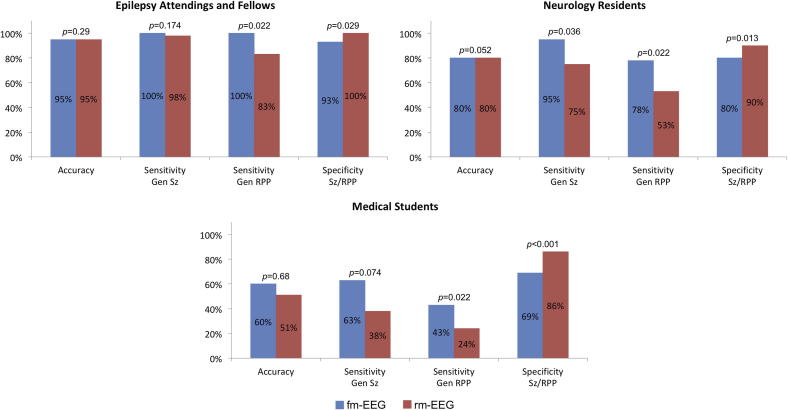
The diagnostic utility (i.e., accuracy, sensitivity, and specificity) of fm-EEG and rm-EEG for detecting seizures and RPP are shown in Fig. 3. Overall, 92 ± 11% of epilepsy-trained neurologists correctly evaluated all brief, 15-s EEG samples using rm-EEG (95% [15%], range: 60–100%), compared to 94 ± 8% (95% [10%], range: 70–100%) using fm-EEG. Resident physicians judged these samples with similar accuracy using rm-EEG (75 ± 21%, 80% [25%], range: 25–100%) and fm-EEG (80 ± 13%, 80% [15%], range: 45–100%), and likewise, medical student accuracy for assessing the 15-s EEG samples was comparable between rm-EEG (58 ± 28%, 57% [52%], range: 14–93%) and fm-EEG (59 ± 22%, 60% [33%], range: 14–90%). We then analyzed the sensitivity for generalized or hemispheric seizures and RPP, separately, and the specificity for seizures and RPP. Neurologists detected seizure activity with comparable sensitivity using both fm-EEG (98 ± 4%, 100% [0%]) and rm-EEG (93 ± 9%, 98% [13%]), however residents identified such activity with greater sensitivity using fm-EEG (90 ± 12%, 95% [15%]) compared to rm-EEG (76 ± 15%, 75% [21%]). Medical student sensitivity for seizures was only slightly above chance using fm-EEG (62 ± 12%, 63% [18%]) and below chance using rm-EEG (46 ± 20%, 38% [11%]). All three groups demonstrated significantly higher sensitivity for diagnosing generalized or hemispheric RPP (not including seizures) using fm-EEG (neurologists: 97 ± 7%, 100% [1%]; residents: 74 ± 17%, 78% [26%]; students: 43 ± 18%, 43% [29%]) compared to rm-EEG (neurologists: 82 ± 15%, 83% [23%]; residents: 56 ± 24%, 53% [40%]; students: 30 ± 17%, 24% [19%]), however we observed significantly greater specificity for RPP (i.e., detection of normal or slow activity) using rm-EEG (neurologists: 97 ± 5%, 100% [5%]; residents: 86 ± 9%, 90% [15%]; students: 77 ± 19%, 86% [13%]) compared to fm-EEG (neurologists: 91 ± 8%, 93% [8%]; residents: 82 ± 10%, 80% [11%]; students: 66 ± 23%, 69% [27%]).
Fig. 3.
Diagnostic utility of full and reduced montage EEG for detection generalized or hemispheric seizures and rhythmic periodic patterns. Data (accuracy, sensitivity, and specificity) are given as median values, p values calculated using Wilcoxon signed-rank tests. Abbreviations: Gen, generalized; RPP, rhythmic or periodic patterns (not including seizure); Sz, seizure.
The percentage of neurologists who provided the same diagnosis using fm-EEG and rm-EEG (concordance) was high overall (88 ± 12%, 90% [20%]), and higher for generalized seizure samples (92 ± 11%, 98% [16%]) and generalized activity (91 ± 10%, 95% [15%]) than for those showing seizure-like RPP (83 ± 14%, 83% [19%]) or focal patterns (70 ± 14%, 70% [10%]). The concordance of resident diagnoses using fm-EEG and rm-EEG was also considerable (78 ± 12%, 80% [20%]). Similarly, samples showing generalized seizures (78 ± 14%, 80% [14%]) displayed greater concordance than those showing RPP (71 ± 15%, 70% [24%]). The concordance for medical students was 70 ± 11% (69% [17%]), and similar concordance was observed for samples showing generalized seizures (67 ± 6%, 68% [2%]) and RPP (67 ± 10%, 68% [10%]).
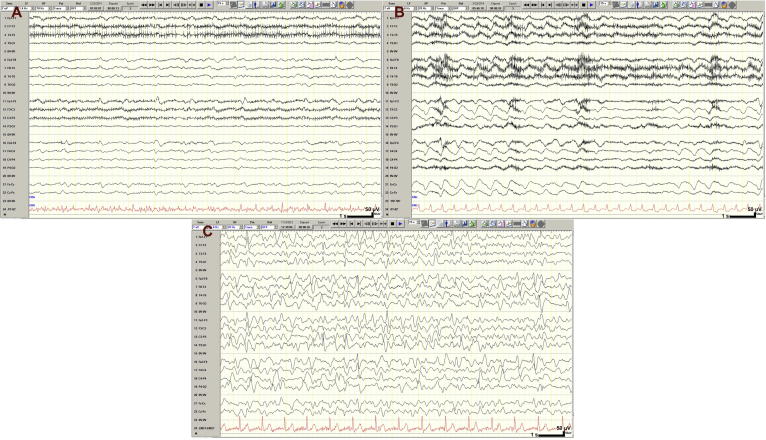
In a more granular analysis, we identified the reason for lower accuracy of all three groups of respondents in evaluation of rm-EEG samples with seizure-like (but not seizure) activity. By scoring each rater’s rm-EEG responses against his/her fm-EEG responses on samples showing seizures or generalized/hemispheric RPP, we found that the FNR associated with a reduction in the number of channels was significantly greater for RPP than for seizure. While using rm-EEG resulted in neurologists missing only 7 ± 10% (7% [17%]) of generalized seizure samples that they correctly identified on fm-EEG, their FNR was 17 ± 14% (17% [16%], p < 0.003) for generalized RPP. Similarly, the FNR for residents was significantly greater for RPP (34 ± 27%, 34% [36%]) than for seizure activity (22 ± 23%, 22% [43%], p = 0.041). Students, however, did not display a significantly different FNR among seizure (43 ± 36%, 43% [67%]) or RPP (56 ± 56%, 56% [67%]) samples (p = 0.099). We identified three samples with RPP that were missed by the majority of neurologists using rm-EEG (Fig. 4). Panels 4A (missed by 65% of neurologists, 80% of residents, and 52% of students using fm-EEG, and by 95% of neurologists, 70% of residents, and 79% of students using rm-EEG) and 4B (missed by 45% of neurologists, 50% of residents, and 21% of students using fm-EEG, and by 75% of neurologists, 90% of residents, and 33% of students using rm-EEG) show focal RPP that is less obvious on lateral channels of the double banana montage (i.e., restricted to the medial channels). Panel 4C was originally interpreted as generalized seizure by the original interpreting epileptologist and was defined as RPP (but not seizure) by our reference standard; however, while 95% of neurologists, 85% of residents, and 69% of students diagnosed this pattern as RPP using fm-EEG, only 50% of neurologists and residents, and 45% of students, did so using rm-EEG.
Fig. 4.
Rhythmic or periodic EEG samples misclassified on reduced montage by the majority of neurologists. (Panel A) Periodic activity missed by 95% on rm-EEG (fm-EEG: 65%). (Panel B) Frontal intermittent rhythmic delta activity (FIRDA) classified by reference standard as rhythmic, periodic activity but labeled as normal/slow by 75% on rm-EEG (fm-EEG: 45%). (Panel C) Rhythmic and periodic activity identified by 95% on fm-EEG but classified as normal/slow by 50% on rm-EEG. Calibration bar: time scale represents 1 s (s), voltage scale represents 50 microvolts (μV).
Additionally, in two other EEG samples (Fig. 5A and B), the ratings of the majority of neurologists differed from the diagnoses provided by the three reference standard epileptologists, suggesting that the referees may have incorrectly classified these samples; in one EEG sample (Fig. 5C), our review of the sample and the attending physician interpretation of the original EEG from which the sample was drawn resulted in a similar conclusion. The clinical EEG report for these samples noted focal seizures during the displayed epochs, however these samples were all classified as non-rhythmic, non-periodic activity (e.g., normal or slowing) by the senior epileptologists who formed our reference standard. Despite the abundance of myogenic artifact, the focal seizure contained in Panel 5A was detected by 75% of neurologists and 85% of residents using fm-EEG and 35% of neurologists and 30% of residents using rm-EEG, but these responses were scored as incorrect based on the reference standard; this sample was associated with the lowest observed concordance between fm-EEG and rm-EEG among both neurologists (50%) and residents (45%). The focal rhythmic abnormality in channel F8-T4 of Panel 5B was detected by only 1 of the reference standard epileptologists, but was detected by the vast majority of neurologists (85% on fm-EEG and 95% on rm-EEG), as well as the majority of residents (70% on fm-EEG and 60% on rm-EEG). Panel 5C displays a left medial frontal seizure that is also visible in the reduced montage (channel Fp1-F7), however this seizure was missed by all three reference standard epileptologists, 80% of neurologists and residents using fm-EEG, and 95% of neurologists and 80% of residents using rm-EEG. With the exception of Panel 5C, none of the samples in Figs. 4 and 5 had 100% agreement among the senior epileptologists who formed the reference standard.
Fig. 5.
Samples with seizure activity misclassified by reference standard epileptologists. (Panel A) Right temporal seizure obscured by abundant myogenic artifact (due to associated automatisms) detected by 75% and 35% of neurologists using fm-EEG and rm-EEG, respectively. (Panel B) Right frontotemporal seizure detected by 95% of neurologists on rm-EEG (fm-EEG: 80%), but classified as non-seizure/seizure-like by reference standard. (Panel C) Patient had continuous multifocal nonconvulsive seizures, seen during this epoch in left frontal and medial channels, that was missed by all three senior epileptologists who formed the reference standard and the majority of neurologists (fm-EEG: 80%, rm-EEG: 95%). Calibration bar: time scale represents 1 s (s), voltage scale represents 50 microvolts (μV).
Overall diagnostic accuracy for seizures and RPP using fm-EEG differed significantly (p < 0.001 for overall Kruskal-Wallis test), as might be expected, between neurologists (94 ± 5%, 95% [3%]), residents (80 ± 12%, 81% [20%], p < 0.001 vs. neurologists), and medical students (59 ± 11%, 59% [16%], p < 0.001 vs. neurologists, p < 0.001 vs. residents). Otherwise, no significant differences were associated with demographic characteristics. Attendings and fellows displayed comparable accuracy using fm-EEG (attendings: 95 ± 5%, 95% [4%]; fellows: 94 ± 5%, 95% [5%]; p = 0.46), as did pediatric (93 ± 6%, 95% [5%]) and adult (97 ± 3%, 97% [5%]) attending epilepsy specialists (p = 0.27). Among residents, differences in post-graduate training year (PGY2: 79 ± 10%, 81% [IQR 14%]; PGY3: 81 ± 15%, 84% [IQR 3%]; PGY4: 82 ± 14%, 85% [IQR 22%]) were not associated with significant differences in accuracy (p = 0.79). Neurologists and residents with different years of EEG experience also evaluated fm-EEG with similar accuracy (neurologists: p = 0.56, residents: p = 0.33), and there were no significant institutional differences in neurologists’ or residents’ judgments (neurologists: p = 0.49, residents: p = 0.079).
Neurologists spent, on average, 42.4 ± 14.7 s on fm-EEG samples (correct: 44.3 ± 17.1 s, incorrect: 46.3 ± 45.7 s, p = 0.79) compared to 45.2 ± 17.4 s on rm-EEG samples (correct: 46.9 ± 20.7 s, incorrect: 48.9 ± 43.1 s, p = 0.79) (difference between overall fm-EEG and rm-EEG: p = 0.33). Residents took 32.3 ± 14.2 s to evaluate fm-EEG samples (correct: 33.5 ± 18.2 s, incorrect: 38.2 ± 72.2 s, p = 0.68) compared to 28.9 ± 12.9 s for rm-EEG samples (correct: 30.7 ± 17.3 s, incorrect: 29.8 ± 18.4 s, p = 0.82) (difference between overall fm-EEG and rm-EEG: p = 0.140). Medical students spent 9.6 ± 3.5 s (correct: 9.4 ± 3.5 s, incorrect: 9.7 ± 5.9 s, p = 0.79) using fm-EEG and 8.9 ± 4.1 s (correct: 8.5 ± 4.3 s, incorrect: 10.2 ± 8.7 s, p = 0.24) using rm-EEG (difference between overall fm-EEG and rm-EEG: p = 0.34).
4. Discussion
4.1. Summary of findings
Our study compared the diagnostic utility of EEG with a reduced number of sensors (rm-EEG) to conventional EEG with a full set of sensors (fm-EEG) in detecting abnormalities such as seizures and seizure-like rhythmic and periodic patterns. We compared responses from EEG-trained neurologists (epileptologists and epilepsy fellows), minimally trained neurology residents, and EEG-untrained medical students to describe the range of the utility of reduced electrode arrays when read by providers of varying levels of expertise. Using rm-EEG, we report comparable accuracy in all three groups for seizures and RPPs, preserved sensitivity for seizures when read by neurologists, and greater specificity (and consequently positive predictive value) for seizures and RPPs across training levels. We also highlight samples that were misclassified using the full EEG montage by the expert epileptologists that formed our reference standard, indicating the impact of inter-rater variability on EEG interpretation and fundamental issues with assuming a “gold standard” for EEG patterns.
4.2. Comparison with prior reports
To date, several studies of reduced electrode arrays in the evaluation of adult patients have been published (Bridgers and Ebersole, 1988, Foldvary et al., 2000, Foldvary-Schaefer et al., 2006, Kolls and Husain, 2007, Young et al., 2009, Karakis et al., 2010, Nitzschke et al., 2012, Grant et al., 2014a, Rubin et al., 2014, Tanner et al., 2014, Brenner et al., 2015, Lepola et al., 2015, Muraja-Murro et al., 2015, Herta et al., 2017). Many of these have found higher specificity and lower sensitivity for reduced montage EEG configurations. To our knowledge, only three studies described devices with sensitivity comparable to their specificity (Karakis et al., 2010, Grant et al., 2014a, Brenner et al., 2015). However, these studies have significant variability in their methodology, such as electrode number and coverage, montage construction, EEG sample duration, presentation of other clinical or video information, reference standard definition, EEG patterns of interest, and threshold for clinical significance (Jordan, 2017). As such, the prior studies did not use the same experimental restrictions to compare reduced montage diagnoses to interpretations obtained using full montage EEG. Thus, it has remained unclear whether the limited performance of raters in these studies was associated with the reduction of channels, or whether it was also a characteristic of raters’ performance in general, even using full montage EEG (Tu et al., 2017).
Some recent studies have compared automated computer detection with reduced channels to physician diagnosis have reflected more on the characteristics of proprietary algorithms than on the value of reduced electrode arrays (Herta et al., 2017). In the recent study by Herta and colleagues, it was shown that certain epileptic activities could in fact be recognized with similar sensitivity using a reduced number of electrodes. However, in that study and other previous studies, the performance of the model was not measured using the EEG montage that we utilized here (i.e., ten electrodes above the hairline corresponding to the lateral leads of the double banana montage).
Our study is different from previous reports in at least five important domains. First, our rm-EEG configuration used the lateral leads of double banana montage (i.e., not sub-hairline). Second, our work focused on the yield of rm-EEG in identifying epileptic abnormalities, such as generalized and hemispheric seizures, that will need urgent medical interventions or might benefit from long-term monitoring with conventional EEG rather than the yield of rm-EEG in detecting all kinds of abnormalities. We are mindful that rm-EEG may not have the same utility as fm-EEG in detecting focal events, especially if they are solely present in areas where our rm-EEG did not provide coverage (e.g., parasagittal regions) (Ebersole and Bridgers, 1985, Kolls and Husain, 2007). It is also important to note that our motivation for the current study was not to test whether rm-EEG has the same utility as fm-EEG in all aspects of clinical practice, for instance, in the detection of single epileptic spikes or in pre-surgical localization of epileptic foci. Third, we examined if the utility of rm-EEG is preserved in situations when EEG-untrained individuals, such as junior residents or medical students, have to decide on the presence or absence of these abnormalities, demonstrating the spectrum of utility. Individuals with minimal EEG exposure are particularly susceptible to information overload (i.e., being unable to see the forest for the trees) when interpreting EEG recordings, and the reduction of channels might serve to reduce the amount of visual information, and therefore the cognitive load, with which untrained individuals must make a diagnostic assessment (Hall and Walton, 2004). Fourth, we presented EEG data as fixed 15-s snapshots to make it difficult for reviewers to make a diagnostic assessment based on a single page of EEG. Since the overarching goal of this study was to compare the utility of rm-EEG and fm-EEG, we made every attempt to provide the reviewers with the same data (except for the number of channels) in both rm-EEG and fm-EEG and to minimize their freedom to alter EEG parameters during their review. Lastly, we aimed to minimize rater bias by recruiting EEG readers across several institutions.
4.3. Limitations of the study
The novelty of our design aside, we are mindful that, in clinical practice, EEG readers have the option of scrolling back and forth between pages to observe evolution of activity, changing the montage or the amplitude of waveforms on visual display, and reviewing video information, clinical context, or hours of preceding EEG data. Thus, our experimental design might have made the reading of EEGs more difficult, particularly with regard to viewing evolution of ictal activity in such a short sample, and given rise to underestimates of diagnostic utility. It is also possible that recruiting EEG readers from several major institutions and across the experience spectrum might have also contributed to significant inter-rater variability that has been reported in the literature to exist even between expert epileptologists in applying EEG terminology or in classifying specific patterns (Mani et al., 2012, Gaspard et al., 2014, Grant et al., 2014b, Halford et al., 2015, Tu et al., 2017). Future studies might explore the impact of these factors (e.g., sample duration, visual display settings, presence of absence of other clinical information, visualization of seizure onset and evolution) on physician confidence in assigning a diagnosis. Finally, the limited number of focal abnormalities (n = 5) in our sample prevented us from assessing the utility of reduced electrode arrays for aiding in the detection of focal seizures or rhythmic and periodic patterns.
5. Conclusion
Reduced channel EEG has reasonable utility for detecting generalized or hemispheric seizures and rhythmic periodic patterns, and it has the potential to provide physicians with quick and pragmatic diagnostic information.
Acknowledgments
Acknowledgements
The authors would like to thank Felice Sun for her assistance with statistical analysis. This work was supported in part by Stanford University’s Medical Scholars Fellowship Program. The funding source had no role in the collection, analysis, or interpretation of the data, in the writing of this report, or in the decision to submit this report for publication.
Conflict of interest
K. Gururangan and B. Razavi have no conflicts of interest to disclose. J. Parvizi is the co-founder of Ceribell, an early-stage startup company that is developing Brain Stethoscope, a new technology to acquire EEG with rm-EEG and sonify it in the evaluation of patients with altered mental status.
References
- Acharya J.N., Hani A., Cheek J., Thirumala P., Tsuchida T.N. American clinical neurophysiology society guideline 2: guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 2016;33:308–311. doi: 10.1097/WNP.0000000000000316. [DOI] [PubMed] [Google Scholar]
- Acharya J.N., Hani A.J., Thirumala P.D., Tsuchida T.N. American clinical neurophysiology society guideline 3: a proposal for standard montages to be used in clinical EEG. J. Clin. Neurophysiol. 2016;33:312–316. doi: 10.1097/WNP.0000000000000317. [DOI] [PubMed] [Google Scholar]
- Betjemann J.P., Lowenstein D.H. Status epilepticus in adults. Lancet Neurol. 2015;14:615–624. doi: 10.1016/S1474-4422(15)00042-3. [DOI] [PubMed] [Google Scholar]
- Bossuyt P.M., Reitsma J.B., Bruns D.E., Gatsonis C.A., Glasziou P.P., Irwig L. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner J.M., Kent P., Wojcik S.M., Grant W. Rapid diagnosis of nonconvulsive status epilepticus using reduced-lead electroencephalography. West. J. Emerg. Med. 2015;16:442–446. doi: 10.5811/westjem.2015.3.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgers S.L., Ebersole J.S. EEG outside the hairline: detection of epileptiform abnormalities. Neurology. 1988;38:146–149. doi: 10.1212/wnl.38.1.146. [DOI] [PubMed] [Google Scholar]
- Chan C.P., Mak W.C., Cheung K.Y., Sin K.K., Yu C.M., Rainer T.H. Evidence-based point-of-care diagnostics: current status and emerging technologies. Annu. Rev. Anal. Chem. 2013;6:191–211. doi: 10.1146/annurev-anchem-062012-092641. [DOI] [PubMed] [Google Scholar]
- Chong D.J., Hirsch L.J. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J. Clin. Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- Claassen J., Mayer S.A., Kowalski R.G., Emerson R.G., Hirsch L.J. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R.J., Waterhouse E.J., Towne A.R., Boggs J.G., Ko D., DeLorenzo G.A. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–840. doi: 10.1111/j.1528-1157.1998.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Ebersole J.S., Bridgers S.L. Direct comparison of 3- and 8-channel ambulatory cassette EEG with intensive inpatient monitoring. Neurology. 1985;35:846–854. doi: 10.1212/wnl.35.6.846. [DOI] [PubMed] [Google Scholar]
- Fernández I.S., Sansevere A.J., Guerriero R.M., Buraniqi E., Pearl P.L., Tasker R.C. Time to electroencephalography is independently associated with outcome in critically ill neonates and children. Epilepsia. 2017;58:420–428. doi: 10.1111/epi.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldvary N., Caruso A.C., Mascha E., Perry M., Klem G., McCarthy V. Identifying montages that best detect electrographic seizure activity during polysomnography. Sleep. 2000;23:221–229. [PubMed] [Google Scholar]
- Foldvary-Schaefer N., Ocampo J.D., Mascha E., Burgess R., Dinner D., Morris H. Accuracy of seizure detection using abbreviated EEG during polysomnography. J. Clin. Neurophysiol. 2006;23:68–71. doi: 10.1097/01.WNP.0000174544.86406.8D. [DOI] [PubMed] [Google Scholar]
- Gaspard N., Hirsch L.J., LaRoche S.M., Hahn C.D., Westover M.B. Critical care EEGMRC. Interrater agreement for critical care EEG terminology. Epilepsia. 2014;55:1366–1373. doi: 10.1111/epi.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A.C., Abdel-Baki S.G., Omurtag A., Sinert R., Chari G., Malhotra S. Diagnostic accuracy of microEEG: a miniature, wireless EEG device. Epilepsy Behav. 2014;34:81–85. doi: 10.1016/j.yebeh.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A.C., Abdel-Baki S.G., Weedon J., Arnedo V., Chari G., Koziorynska E. EEG interpretation reliability and interpreter confidence: a large single-center study. Epilepsy Behav. 2014;32:102–107. doi: 10.1016/j.yebeh.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururangan K., Razavi B., Parvizi J. Utility of electroencephalography: experience from a U.S. tertiary care medical center. Clin. Neurophysiol. 2016;127:3335–3340. doi: 10.1016/j.clinph.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Halford J.J., Shiau D., Desrochers J.A., Kolls B.J., Dean B.C., Waters C.G. Inter-rater agreement on identification of electrographic seizures and periodic discharges in ICU EEG recordings. Clin. Neurophysiol. 2015;126:1661–1669. doi: 10.1016/j.clinph.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Walton G. Information overload within the health care system: a literature review. Health Inf. Libraries J. 2004;21:102–108. doi: 10.1111/j.1471-1842.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- Herman S.T., Abend N.S., Bleck T.P., Chapman K.E., Drislane F.W., Emerson R.G. Consensus statement on continuous EEG in critically ill adults and children, Part II: personnel, technical specifications, and clinical practice. J. Clin. Neurophysiol. 2015;32:96–108. doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herta J., Koren J., Fürbass F., Hartmann M., Gruber A., Baumgartner C. Reduced electrode arrays for the automated detection of rhythmic and periodic patterns in the intensive care unit: frequently tried, frequently failed? Clin. Neurophysiol. 2017;128:1524–1531. doi: 10.1016/j.clinph.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Hirsch L.J., LaRoche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T. American clinical neurophysiology society's standardized critical care EEG terminology: 2012 version. J. Clin. Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- Jordan K.G. Reduced electrode arrays for acute electroencephalography: can less be more? Clin. Neurophysiol. 2017;128:1519–1521. doi: 10.1016/j.clinph.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Kämppi L., Mustonen H., Soinila S. Analysis of the delay components in the treatment of status epilepticus. Neurocrit. Care. 2013;19:10–18. doi: 10.1007/s12028-013-9862-x. [DOI] [PubMed] [Google Scholar]
- Kaplan P.W. Nonconvulsive status epilepticus in the emergency room. Epilepsia. 1996;37:643–650. doi: 10.1111/j.1528-1157.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Karakis I., Montouris G.D., Otis J.A.D., Douglass L.M., Rinat J., Velez-Ruiz N. A quick and reliable EEG Montage for the detection of seizures in the critical care setting. J. Clin. Neurophysiol. 2010;27:100–105. doi: 10.1097/WNP.0b013e3181d649e4. [DOI] [PubMed] [Google Scholar]
- King M.A., Newton M.R., Jackson G.D., Fitt G.J., Mitchell L.A., Silvapulle M.J. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352:1007–1011. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- Kolls B.J., Husain A.M. Assessment of hairline EEG as a screening tool for nonconvulsive status epilepticus. Epilepsia. 2007;48:959–965. doi: 10.1111/j.1528-1167.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- Koren J.P., Herta J., Pirker S., Furbass F., Hartmann M., Kluge T. Rhythmic and periodic EEG patterns of 'ictal-interictal uncertainty' in critically ill neurological patients. Clin. Neurophysiol. 2016;127:1176–1181. doi: 10.1016/j.clinph.2015.09.135. [DOI] [PubMed] [Google Scholar]
- Lepola P., Myllymaa S., Töyräs J., Hukkanen T., Mervaala E., Määttä S. A Handy EEG Electrode Set for patients suffering from altered mental state. J. Clin. Monit. Comput. 2015;29:697–705. doi: 10.1007/s10877-014-9652-9. [DOI] [PubMed] [Google Scholar]
- Mani R., Arif H., Hirsch L.J., Gerard E.E., LaRoche S.M. Interrater reliability of ICU EEG research terminology. J. Clin. Neurophysiol. 2012;29:203–212. doi: 10.1097/WNP.0b013e3182570f83. [DOI] [PubMed] [Google Scholar]
- McMullan J.T., Beyette F.R., Jr., Shutter L.A. Assessing the clinical needs for point of care technologies in neurologic emergencies. Neurocrit. Care. 2012;17:231–235. doi: 10.1007/s12028-012-9705-1. [DOI] [PubMed] [Google Scholar]
- McMullan J.T., Knight W.A., Clark J.F., Beyette F.R., Pancioli A. Time-critical neurological emergencies: the unfulfilled role for point-of-care testing. Int. J. Emerg. Med. 2010;3:127–131. doi: 10.1007/s12245-010-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierkord H., Holtkamp M. Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol. 2007;6:329–339. doi: 10.1016/S1474-4422(07)70074-1. [DOI] [PubMed] [Google Scholar]
- Muraja-Murro A., Mervaala E., Westeren-Punnonen S., Lepola P., Toyras J., Myllymaa S. Forehead EEG electrode set versus full-head scalp EEG in 100 patients with altered mental state. Epilepsy Behav. 2015;49:245–249. doi: 10.1016/j.yebeh.2015.04.041. [DOI] [PubMed] [Google Scholar]
- Nitzschke R., Muller J., Maisch S., Schmidt G.N. Single-channel electroencephalography of epileptic seizures in the out-of-hospital setting: an observational study. Emerg. Med. J. 2012;29:536–543. doi: 10.1136/emj.2010.102855. [DOI] [PubMed] [Google Scholar]
- Paliwal P., Wakerley B.R., Yeo L.L.L., Ali K.M., Ibrahim I., Wilder-Smith E. Early electroencephalography in patients with Emergency Room diagnoses of suspected new-onset seizures: diagnostic yield and impact on clinical decision-making. Seizure. 2015;31:22–26. doi: 10.1016/j.seizure.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Pedersen G.L., Rasmussen S.B., Gyllenborg J., Benedek K., Lauritzen M. Prognostic value of periodic electroencephalographic discharges for neurological patients with profound disturbances of consciousness. Clin. Neurophysiol. 2013;124:44–51. doi: 10.1016/j.clinph.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Praline J., Grujic J., Corcia P., Lucas B., Hommet C., Autret A. Emergent EEG in clinical practice. Clin. Neurophysiol. 2007;118:2149–2155. doi: 10.1016/j.clinph.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Quigg M., Shneker B., Domer P. Current practice in administration and clinical criteria of emergent EEG. J. Clin. Neurophysiol. 2001;18:162–165. doi: 10.1097/00004691-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Rubin M.N., Jeffery O.J., Fugate J.E., Britton J.W., Cascino G.D., Worrell G.A. Efficacy of a reduced electroencephalography electrode array for detection of seizures. Neurohospitalist. 2014;4:6–8. doi: 10.1177/1941874413507930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A.R., Vlachy J., Lee J.W., Gilmore E.J., Ayer T., Haider H.A. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74:181–188. doi: 10.1001/jamaneurol.2016.4990. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Williams J, Taylor M, Le Blanc D, Thode HC, Jr., 2015. Comprehensive bedside point of care testing in critical ED patients: a before and after study. Am. J. Emerg. Med. 33, 776–780. [DOI] [PubMed]
- Sofat P., Teter B., Kavak K.S., Gupta R., Li P. Time interval providing highest yield for initial EEG in patients with new onset seizures. Epilepsy Res. 2016;127:229–232. doi: 10.1016/j.eplepsyres.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Tanner A.E.J., Särkelä M.O.K., Virtanen J., Viertiö-Oja H.E., Sharpe M.D., Norton L. Application of subhairline EEG montage in intensive care unit: comparison with full montage. J. Clin. Neurophysiol. 2014;31:181–186. doi: 10.1097/WNP.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Towne A.R., Waterhouse E.J., Boggs J.G., Garnett L.K., Brown A.J., Smith J.R. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–345. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- Tu B., Young G.B., Kokoszka A., Rodriguez-Ruiz A., Varma J., Eerikäinen L.M. Diagnostic accuracy between readers for identifying electrographic seizures in critically ill adults. Epilepsia Open. 2017;2:67–75. doi: 10.1002/epi4.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P.M., Miller C., McArthur D., Eliseo M., Etchepare M., Hirt D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit. Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- Young G.B., Sharpe M.D., Savard M., Al Thenayan E., Norton L., Davies-Schinkel C. Seizure detection with a commercially available bedside EEG monitor and the subhairline montage. Neurocrit. Care. 2009;11:411–416. doi: 10.1007/s12028-009-9248-2. [DOI] [PubMed] [Google Scholar]
- Ziai W.C., Schlattman D., Llinas R., Venkatesha S., Truesdale M., Schevchenko A. Emergent EEG in the emergency department in patients with altered mental states. Clin. Neurophysiol. 2012;123:910–917. doi: 10.1016/j.clinph.2011.07.053. [DOI] [PubMed] [Google Scholar]