Version Changes
Revised. Amendments from Version 1
We have adapted our manuscript according to the reviewers’ comments. In addition to providing a response to each reviewer’s comment on the web page, we have made to following changes to the paper:
In the data analysis methods paragraph, we explained why a list of 22 studies appeared in tables and figures, while only 21 studies were included in the meta-analysis: the Blanco study provided two study populations with different treatment strategies.
In table 2, we corrected two minor typos.
In the drug resistance results paragraph, we clarified the characteristics of the subjects that developed InSTI mutations.
In the discussion section, we made the following slight changes: inclusion of information on virological failure rates in trials evaluating InSTI-based triple maintenance combinations and on the activity of DTG-based simplified regimens in compartments other than blood; replacement of the XTC acronym by 3TC or FTC; inclusion of two references on the impact of the 184V mutation on viral fitness.
Abstract
Background: Dolutegravir-containing maintenance therapy is a promising simplification strategy for virologically suppressed HIV-infected individuals. However, most of the available data to inform this strategy come from small, uncontrolled studies. We estimated the proportion of HIV-infected patients experiencing virological failure (VF) and developing drug resistance on dolutegravir (DTG)-based maintenance therapy.
Methods: We searched Medline, Embase, Cochrane Central, Web of Science, and conference abstracts for studies assessing VF on DTG-based maintenance therapy. Studies including ≥5 adults with an undetectable viral load on antiretroviral therapy (ART) who switched to a DTG-based mono- or dual therapy were included. Pooled proportions of VF were estimated using random-intercept logistic meta-regression and acquired drug resistance mutations described for each strategy.
Results: Of 1719 studies considered, 21 met our selection criteria, including seven interventional and 14 observational studies. Eight studies including 251 patients assessed VF on DTG monotherapy and fourteen studies including 1670 participants VF on dual therapy. The participant’s median age ranged from 43 to 63 years, their median nadir CD4 count from 90 to 399 cells/µl, and 27.6% were female. The proportion of participants experiencing VF on DTG-monotherapy was 3.6% (95% confidence interval [CI] 1.9-6.7) at 24 weeks and 8.9% (95% CI 4.7-16.2) at 48 weeks. Resistance mutations developed in seven (3.6%) participants on DTG-monotherapy. Among patients on dual therapy, ten (0.7%, 95% CI 0.4-1.3) experienced VF by 48 weeks and none developed resistance to DTG. In adjusted analyses, VF at 24 weeks was less likely on dual therapy than on monotherapy (adjusted odds ratio: 0.10, 95% CI 0.03-0.30).
Conclusions: Whereas VF is relatively common on DTG maintenance monotherapy, DTG-based dual therapy appears to be a promising simplification strategy for individuals with a suppressed HIV viral load on triple-ART.
Keywords: Dolutegravir, simplified therapy, HIV, meta-analysis
Introduction
The concept of combination antiretroviral therapy (ART) for the treatment of HIV infection was established twenty years ago, when the results of the first studies evaluating protease inhibitor-based regimens were published 1. In recent years, several strategies of treatment optimization and simplification gained interest, with the objectives of improving quality of life, minimizing ART-related toxicity and drug-drug interactions (DDI), as well as reducing health-related costs. So far, ART de-escalation from three to one (mono-) or two drugs (dual-) therapies has mainly been evaluated in virologically suppressed patients. The first simplified maintenance strategy studied included a boosted protease inhibitor (bPI), with the hope that the high genetic barrier to resistance would help achieve durable virological suppression. In a meta-analysis including ten studies, bPI monotherapy was found to be inferior to triple ART for the maintenance of viral suppression 2, but non-inferior with regards to loss of future treatment options 3. In contrast, dual therapy with bPI and lamivudine (3TC) was found to be non-inferior to triple ART 4– 6 and is now recognized as a valid switch strategy by current HIV treatment guidelines in selected situations 7. However, bPI-based maintenance strategies are not widely applicable because of cost, toxicity and DDI.
Due to its interesting pharmacokinetic profile, good tolerability and high barrier to resistance, dolutegravir (DTG), a new integrase strand transfer inhibitor (InSTI), has attracted much interest for its use in simplified treatment regimens. While preliminary analyses of a Dutch DTG monotherapy simplification trial seemed encouraging at 24 weeks, rates of virological failures increased significantly by week 48, suggesting a sub-optimal potency of this regimen 8. On the other hand, several studies evaluating DTG-based dual therapy with either 3TC or rilpivirine (RPV), showed a high virological efficacy 9– 12. However, most reports were from small, observational cohort studies, with the exception of one DTG-RPV industry-sponsored randomized controlled trial (RCT) 11.
We performed a systematic review of the literature and a meta-analysis to provide precise estimates of the rate of virological failure (VF) and drug resistance in patients switched to a DTG-based maintenance mono- or dual therapy, and to clarify which drugs or combinations should be evaluated in further studies and implemented in clinical practice.
Methods
The protocol for this systematic review was written and registered with the International Prospective Register of Systematic Reviews (PROSPERO registration number CRD42017070045) 13. The reporting of the review followed the PRISMA guidelines 14 ( Supplementary File 1).
Search strategy and selection criteria
We searched Medline, EMBASE, Cochrane Central and Web of Science, as well as abstracts of major HIV conferences (CROI, AIDS, HIV Glasgow, AFRAVIH, IAS and EACS between 2013 and 2017) on 4. January 2018 for studies assessing the proportion of individuals developing VF on DTG-based maintenance therapy. In Medline we combined free text words and medical subject headings (MESH) describing the study population and the outcome ( Supplementary File 2). This search strategy was adapted for the other databases. We considered RCTs, single-arm clinical trials, cohort studies, and case-series that included at least five HIV-infected adults (≥18 years) on DTG-based simplified therapy. No language restrictions were applied. Studies had to report on virological outcomes of patients who switched to a DTG monotherapy or dual therapy after having an undetectable VL on triple ART. We excluded studies that only reported in vitro data and those selecting participants based on the outcome during DTG-based maintenance therapy. Two investigators (MB and GW) independently selected studies based on titles and abstracts, and, in a second step, based on the full text of potentially eligible articles. Discrepancies in study selection were resolved through discussions with a third investigator (AC).
Data extraction
The following data were extracted independently for each study by two reviewers (GW and MB), using a standardized spreadsheet: bibliographic details, study design, inclusion and exclusion criteria, definitions of outcomes, country, number of participants and their main demographic and clinical characteristics, including duration since HIV diagnosis, ART history, immunological status (CD4 cell count at switch and nadir) and virological parameters (HIV RNA peak and at baseline, HIV-DNA at baseline and changes during the study, VF as defined by the study, and the presence at drug resistance at switch). Again, discrepancies in data extracted were resolved through discussions with a third investigator (AC).
Assessment of risk of bias
A checklist for the assessment of risk of bias was designed to ensure data quality assessment for each study was included. The form for RCTs included information on the sequence generation, allocation concealment, blinding (participants, personnel and outcome assessor), incomplete outcome data, selective outcome reporting and other sources of bias. The methodological components of the randomized trials were assessed by two independent authors and classified as high, low or unclear risk of bias, as recommended by the Cochrane collaboration 15. For observational studies it was not appropriate to use the ROBINS-I tool 16, as we only considered data from the group of patients on simplified, maintenance therapy. Thus, we assessed the population characteristics and missing outcome data for each study.
Data analysis
We described the study design as well as the demographic and clinical characteristics of the population from each study by type of maintenance therapy (DTG-based monotherapy or dual therapy). Pooled proportions of VF and treatment failure (VF or departure from simplified strategy due to toxicity, loss to follow-up, patient’s or physician’s decision), and 95% confidence intervals (CI) were estimated using random intercept logistic meta-regression. These analyses were performed separately at 24 weeks and 48 weeks after the switch from triple ART to maintenance therapy. For all models, statistical evidence for heterogeneity between studies was assessed using the tau-squared statistics 17. Of note, we separated data from Blanco et al. 18 according to the strategy used (DTG-monotherapy and DTG-based dual therapy). We evaluated the association between type of maintenance strategy and VF using random intercept logistic meta-regression (binomial-normal) models. All models were adjusted for potential confounders, including age (median or mean), sex (proportion of female participants) and study type (interventional or observational). Furthermore, the proportion of participants acquiring new drug resistance mutations was assessed for each treatment strategy and the mutations described in detail. Statistical analyses were conducted in STATA version 14.1 (StataCorp, Texas, USA) and R version 3.2.3 (R Core Team, Vienna, Austria).
Role of the funding source
The funder of the study had no role in the design, data collection, data analysis, data interpretation of the results or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study and participant characteristics
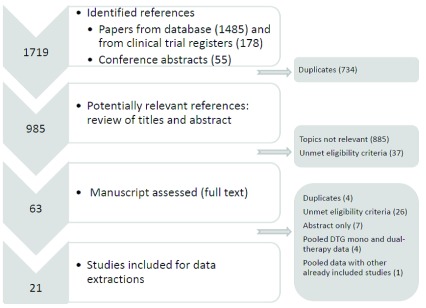
Of 1719 single studies identified, 63 remained potentially eligible after the screening of titles and abstracts. Of these, 21 studies, including four RCTs, three single-arm clinical trials and 14 observational studies met our inclusion criteria 8, 11, 12, 18– 35 ( Figure 1). A description of the main study characteristics by type of maintenance strategy is given in Table 1. Eight studies (two from France, two from The Netherlands, one from Germany, one from Switzerland and two from Spain) including 251 patients assessed the switch to DTG monotherapy and 14 (five from Italy, four from France, three for Spain, one from US, and one multi-country study), including 1670 participants, the switch to DTG-based dual therapy. Dual therapy consisted of DTG + 3TC (seven studies) or RPV (four studies) or atazanavir (ATV, two studies) or darunavir (DRV, one study). Overall, 14 studies allowed the inclusion of patients with previous virological failure, including five monotherapy studies 18– 20, 22– 24. In one study, patients with previous InSTI failure were also included 12. Nineteen studies assessed virological outcomes at six months of maintenance therapy, whereas ten of them additionally showed outcomes at one year 8, 11, 12, 24, 27, 29– 33, 35. Two studies assessed virological outcomes only at 48 weeks 25, 27. Median (or mean) age of participants included in the studies varied from 43 years 11 to 63 years 24 and 27.6% of them were female. 16 studies reported on the median nadir CD4 cell count, which ranged from 90 cells/µl 12 to 399 cells/µl 29.
Figure 1. Flow chart of study selection process.
Table 1. Study characteristics, by treatment group.
| Study | Country | Patients
included (N) |
Median/
mean age (years) |
Female
(%) |
VF definition | Study
type |
Eligibility criteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous
VF allowed |
Previous
resistances allowed |
Months
of stable ART |
Months
with HIV VL<50 |
CD4
nadir (cells/ µl) |
Other | |||||||
| DTG-Mono | ||||||||||||
| Katlama
et al.
JAC 2016 |
F | 28 | 48 | 46.4 | 2× ≥ 50 cp/ml or
1× > 200 cp/ml |
O | Yes | - | - | ≥ 12 | - | - |
| Wijting
et al.
Lancet HIV |
NL | 96 | 45.5 | 8.5 | 2× ≥ 200 cp/ml | I | No | No | - | ≥ 6 | >200 | VL zenith <100.000 |
| Gubavu
et al.
JAC 2016 |
F | 21 | 47 | 38 | 2× ≥ 50 cp/ml | O | Yes | - | - | - | - | - |
| Oldenbüttel
et al.
AVT 2016 |
D | 31 | 44.5 | 32 | 2× ≥ 20 cp/ml | O | Yes | Not to InSTI | - | ≥ 6 | - | no AIDS history |
| Rokx
et al.
JAC 2016 |
NL | 5 | 63 | 0 | 2× ≥ 50 cp/ml | I | Yes | - | - | ≥ 12 | - | - |
| Rojas
et al.
JAC 2016 |
E | 31 | 56 | 55 | 2× ≥ 37 cp/ml | O | Yes | - | - | - | - | - |
| Lecompte
et al.
IAS 2017 |
CH | 8 | 44.5 | 28.5 | 1× ≥ 200cp/ml | I | No | No | ≥ 24 | - | - | - |
| Blanco
et al.
JAC 2018 |
E | 31 | 47 | 10 | 2× ≥ 50 cp/ml or
1× > 1000 cp/ml |
I | Yes | - | - | ≥ 12 | >200 | - |
| DTG-3TC | ||||||||||||
| Borghetti
et al.
JAC 2016 |
I | 36 | 53 | 19.4 | 2× ≥ 50 cp/ml | O | Yes | - | - | - | - | |
| Maggiolo
et al.
BMC ID 2017 |
I | 94 | 52 | 32.3 | 2× ≥ 50 cp/ml | O | Yes | Not to 3TC
or InSTI |
> 6 | ≥ 6 | - | - |
| Joly
et al.
CROI 2017 |
F | 104 | 45 | 14.4 | 2× ≥ 50 cp/ml | I | No | No | - | ≥ 24 | >200 | no HIV encephalitis £ |
| Reynes
et al.
HIV Glasgow 2016 |
F | 27 | 59 | 25.9 | 2× ≥ 50 cp/ml | O | Yes | Not to InSTI | > 12 | - | - | - |
| Blanco
et al.
JAC 2018 |
E | 29 | 44 | 21 | I | Yes | - | - | ≥ 12 | >200 | - | |
| Maggiolo
et al.
EACS 2017 |
I | 203 | 52 | 24.6 | - | O | Yes | No M184V | - | ≥ 6 | - | - |
| Taiwo
et al.
CID 2017 |
US | 44 | 46 | 17 | 2× ≥ 50 cp/ml | I | No | No | ≥ 12 | ≥ 12 | ||
| DTG-RPV | ||||||||||||
| Llibre
et al.
Lancet 2018 |
Multi-
country |
513 | 43 | 23 | 1× ≥ 50 cp/ml | I | No | - | > 6 | ≥ 12 | - | - |
| Gantner
et al.
HIV Med 2017 |
F | 116 | 55 | 44 | 2× ≥ 50 cp/ml or
1× ≥ 1,000 cp/ml |
O | Yes | - | - | - | - | - |
| Bonijoly
et al.
EACS 2017 |
F | 268 | 55 | 44 | 2× ≥ 50 cp/ml | O | Yes | - | - | ≥ 6 | - | On ART for ≥ 12
months |
| Revuelta
et al.
Ann pharmacol 2018 |
E | 32 | 49 | 37 | 2× ≥ 50 cp/ml | O | Yes | Not to InSTI
or RPV |
- | - | - | - |
| DTG-ATV | ||||||||||||
| Riva
et al.
HIV Glasgow 2016 |
I | 61 | 52.1 | 39 | - | O | - | - | - | - | - | - |
| Castagna
et al.
EACS 2017 |
I | 116 | 53 | 13 | 2× ≥ 50 cp/ml | O | - | - | - | ≥ 12 | - | - |
| DTG-DRV | ||||||||||||
| Navarro
et al.
EACS 2017 |
E | 27 | 52 | 30 | 2× ≥ 50 cp/ml | O | Yes | Only to one
ART class |
- | ≥ 6 | - | - |
Abbreviations: VF: virologic failure, ART: antiretroviral therapy, DTG: dolutegravir, 3TC: lamivudine, FTC: emtricitabine, RPV: rilpivirine, ATV: atazanavir, InSTI: integrase stand transfert inhibitor, F: France, NL: The Netherlands, D: Germany, E: Spain, CH: Switzerland, I: Italy, USA: United States of America, O: observational study; I: interventional study
£: no abnormal standard biological parameter
Risk of bias
All RCTs were open-label non-inferiority trials 8, 18, 35, of which one was a single center trial 18 and three were multicenter trials 8, 11, 35. They reported adequate generation of random allocation sequences and allocation concealment. Three single-arm trials were included, of which two included less than 10 patients 21, 24, 29. All interventional studies adequately addressed incomplete outcome data: proportions of drop-outs were low and outcome data were missing for less than 20% of participants in all studies. Five of seven trials reported on virological outcomes at both time-points of interest for this study (24 and 48 weeks) 8, 11, 24, 29, 35. There was no evidence of selective reporting in any of the studies. In each of the 14 observational studies included in this review, the main demographic and clinical characteristics of the study populations were similar and patients were followed for 24 weeks in most studies. Among the observational studies, the majority did not report detailed inclusion and exclusion criteria. Five observational studies reported virological outcomes at both time-points 12, 30– 33. Amplification for drug resistance testing was successful for 19 of the 27 (70%) patients with VF. Finally, patient retention was over 90% in all 14 cohort studies.
Virological and treatment failure
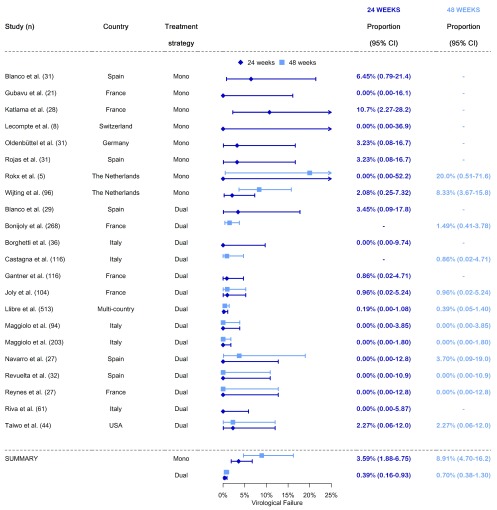
The pooled estimate of the proportion of participants who experienced a VF on DTG-based monotherapy was 3.6% (95% CI 1.9-6.7) at 24 weeks and 8.9% (95% CI 4.7-16.2) at 48 weeks ( Figure 2). The high proportion of treatment failures among patients on monotherapy at 48 weeks was driven by the two studies from the Netherlands, which observed between 8 and 20% of VF 8, 24. Among patients on dual therapy, an estimated 0.4% (95% CI 0.2-0.9) experienced a VF at 24 weeks and 0.7% (95% CI 0.4-1.3) at 48 weeks. Independently of the combination used (DTG/3TC, DTG/RPV, DTG/ATV or DTG/DRV), 11 of 14 studies evaluating the effectiveness of dual therapy had less than 1% of patients developing VF. Compared to patients on monotherapy, those on dual therapy were less likely to experience VF by 24 weeks (odds ratio [OR] 0.10, 95% CI 0.03-0.32, p<0.001) and 48 weeks (OR 0.07, 95% CI 0.03-0.18, p<0.001). In analyses adjusted for study type (interventional or observational), age (median or mean) and sex (proportion of female participants), the OR for VF at 24 weeks and 48 weeks were very similar to the unadjusted estimates (aOR 0.10, 95% CI 0.03-0.30 for 24 weeks and aOR 0.06, 95% CI 0.01-0.30 for 48 weeks, respectively). The only variable that contributed to explaining the between-study heterogeneity in both the 24 and 48-week analyses was treatment strategy. When including this variable, the tau-squared were reduced from 1.17 (95% CI 0.33-2.19) to 0.00 (95% CI 0.00-1.11) in the 24 week analysis and from 1.37 (95% CI 0.54-2.15) to 0.00 (95% CI 0.00-1.00) in the 48 week analysis. The inclusion of other variables did not impact the estimates of tau-squared.
Figure 2. Meta-analysis of virological failure among patients on single or dual DTG-based simplification therapy.
Treatment failure occurred in 5.2% (2.0–12.9) of patients at 24 weeks and 12.3% (4.5–29.4) at 48 weeks on DTG-monotherapy, whereas this outcome was observed in 2.8% (1.4–5.7) of patients at 24 weeks and 6.5% (4.3–9.6) at 48 weeks on DTG-based dual therapy. At 24 weeks, patients on dual therapy tended to be less likely to experience treatment failure compared to those on monotherapy (aOR 0.52, 95% CI 0.15-1.85). Due to multi-collinearity in the model, we were not able to report on multivariable analyses comparing treatment failure between mono and dual therapy at 48 weeks.
Drug resistance
Acquired resistance mutations to InSTI developed in 9/251 (3.6%) participants on DTG-based monotherapy, which corresponded to 56% of the cases of VF ( Table 2). Three individuals developed the Q148R or Q148H mutation in combination with other resistance mutations, conferring high-level resistance to DTG 20, 22. Two of these three patients were previously exposed to InSTI and none had a history of previous VF. They all had a suppressed HIV viral load for several years before switching to DTG-monotherapy. No InSTI resistance mutations developed in patients on dual therapy. Of 962 patients on RPV/DTG, only one developed a major drug resistance mutation to non-nucleoside reverse transcriptase inhibitors (K101E). No resistance was observed in plasma among 237 individuals on DTG/3TC.
Table 2. Virological outcomes and drug resistance, by study.
| Study | Follow-up
(weeks) |
N°
patients |
N° treatment
failures (%) |
N° virological
failures (%) |
N°
amplified |
N° patients
with resistance |
Resistance patterns
(one line per patient) * |
||
|---|---|---|---|---|---|---|---|---|---|
| 24
weeks |
48
weeks |
24
weeks |
48
weeks |
||||||
| DTG-Mono | |||||||||
| Katlama et al. | 24 | 28 | 4 (14.3) | - | 3 (10.7) | - | 3 | 3 |
E138K,G140A, Q148R
E92Q N155H |
| Wijting et al. | 48 | 96 | - | 11 (11.5) | 2 (2.1) | 8 (8.3) | 6 | 3 |
S230R
R263K N155H |
| Gubavu et al. | 24 | 21 | 0 | - | 0 | - | - | - | |
| Oldenbüttel
et al. |
24 | 31 | 2 (6.5) | - | 1 (3.2) | - | 1 | 1 | Q148H, G140S |
| Rokx et al. | 48 | 5 | 0 | 1 (20) | 0 | 1 (20.0) | 1 | 0 | |
| Rojas et al. | 24 | 31 | 1 (3.2) | - | 1 (3.2) | - | 1 | 0 | 118R ** |
| Lecompte et al. | 24 | 8 | 1 (12.5) | - | 0 | - | - | - | |
| Blanco et al. | 24 | 31 | 2 (6.5) | - | 2 (6.4) | - | 2 | 2 |
E138A, S147G, N155H,
Q148R 138K, 155H, 140S |
| DTG-3TC | |||||||||
| Borghetti et al. | 24 | 36 | 3 (8.3) | - | 0 | - | - | - | |
| Maggiolo et al. | 48 | 94 | 0 | 3 (3.2) | 0 | 0 | - | - | |
| Joly et al. | 48 | 104 | 1 (1.0) | 3 (2.9) | 1 (1.0) | 1 (1.0) | 0 | - | |
| Reynes et al. | 48 | 27 | 3 (11.1) | 3 (11.1) | 0 | 0 | - | - | |
| Blanco et al. | 24 | 29 | 1 (3.5) | - | 1 (3.4) | - | 1 | 0 | K70E
***, K219E
***,
G190R $, M230I $ |
| Maggiolo et al. | 48 | 203 | 0 | 12 (6.0) | 0 | 0 | - | - | |
| Taiwo et al. | 48 | 44 | 1 (2.3) | 3 (6.9) | 1 | 1 | 1 | 0 | |
| DTG-RPV | |||||||||
| Llibre et al. | 48 | 513 | - | 27 (5.3) | 1 (0.2) | 2 (0.4) | 2 | 1 | K101K/E |
| Gantner et al. | 24 | 116 | 11 (9.5) | - | 1 (0.9) | - | 0 | - | |
| Bonijoly et al. | 24 | 268 | - | 51 (19.0) | - | 4 (1.5) | - | - | |
| Revuelta et al. | 48 | 32 | - | 2 (6.5) | 0 | 0 | - | - | |
| DTG-ATV | |||||||||
| Riva et al. | 24 | 61 | 3 (4.9) | - | 0 | - | - | - | |
| Castagna et al. | 48 | 116 | 5 (4.3) | 6 (5.2) | - | 1 (0.9) | - | - | |
| DTG-DRV | |||||||||
| Navarro et al. | 48 | 27 | - | 2 (7.4) | 0 | 1 (3.7) | 1 | 0 | |
*bold: InSTI resistance
**in 7% of integrated DNA in PBMC
*** in ≤1.5% of integrated DNA in PBMC
$ in integrated DNA in PBMC
This table shows summary measures, including the number of virological and treatment failures in each study.
Copyright: © 2019 Wandeler G et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Discussion
We performed a comprehensive systematic review of studies that reported on VF among patients switched to DTG-based maintenance therapy. Our meta-analysis shows that DTG-based dual therapy is successful in sustaining virological control in ART-experienced HIV-infected patients: only 12 of 1670 (0.7%) experienced a VF and none of them developed resistance mutations to DTG. On the contrary, 16 of 251 (6.4%) individuals switched to DTG monotherapy had a VF, of which more than one-half developed resistance to DTG. In comparison, recent trials evaluating InSTI-based triple maintenance combinations showed a similar virological failure rates 36, 37. Although the proportion of patients experiencing a confirmed viral rebound on DTG-monotherapy does not seem to be higher than in patients on PI-monotherapy, the risk of losing future treatment options is higher with DTG-monotherapy 3. Overall, our findings suggest that DTG-based monotherapy is not an appropriate simplification strategy and that further studies are urgently needed to confirm the long-term efficacy of DTG-based dual therapy.
DTG-based dual therapy is a promising simplification strategy, especially when combined with 3TC, as the likelihood of developing toxicity events and DDI on such regimens is very low. No drug resistance mutations to DTG developed among more than 1600 patients on dual therapy followed for 24 to 48 weeks and only one had a resistance mutation to another drug class. Although based on very few patients, the results seemed to be independent of previous virological failures. For instance, no virological failures were noted among patients on DTG/3TC despite the presence of a 184V mutation at the time of simplification in several studies. The impact of the latter mutation on viral fitness has been extensively described both in vitro 38 and in vivo 39, and could also potentially explain the improved treatment outcomes in these patients compared to those switched to DTG-monotherapy without any previous failures. Interestingly, similar observations were made for bPI-based regimens, for which efficacy was improved when 3TC was added, despite the presence of the 184V mutation 39.
We also report on estimates of treatment failure, which includes other reasons for treatment interruptions, such as toxicity or loss to follow-up. In our meta-analysis, the proportion of patients experiencing this combined outcome was more than twice as high among patients on monotherapy compared to those on DTG-based dual therapy. Although this outcome is important in evaluating the clinical efficacy of a novel ART strategy, our capacity to analyze this outcome in detail was limited by the missing information on the specific reasons for treatment interruptions in many studies and by the small number of events, especially at 48 weeks of therapy.
Of all simplification strategies evaluated to date, the DTG/3TC combination could be the one most readily accessible for patients in low- and middle-income countries: both DTG and 3TC are available and prequalified by stringent regulatory authorities in generic formulations. In order to be widely implemented, the efficacy of this dual combination should first be evaluated in large studies among different patient populations. The results from the studies included in our meta-analysis are mainly based on selected populations of HIV-infected individuals from European cohorts, and are not generalizable. Furthermore, long-term data are needed, as most treatment failures occurred after the first 24 weeks in several monotherapy studies. Recently, results from the only study which assessed 96-week outcomes with this regimen to date were reported: among 27 ART-experienced individuals with previous VF, DTG/3TC was 100% efficacious virologically 12. However, despite these encouraging results, data from larger studies are needed. In addition, more data on the activity of DTG-based simplified regimens in compartments other than blood are needed. Letendre et al. showed that DTG achieved therapeutic concentrations in the central nervous system (CNS), with a CNS penetration effectiveness score of four 40. In the MONODO study, all patients had an undetectable plasma HIV viral load at week 24 on DTG maintenance monotherapy, whereas only one had a detectable viral load in the cerebrospinal fluid 21. Moreover, levels of HIV-RNA in the genital tract on DTG monotherapy 41 and on DTG-3TC 42 were comparable to those on standard cART. However, these results were based on a very small sample of patients and data from individuals on simplified, DTG-based therapies are lacking.
As a wealth of data on the efficacy of DTG-maintenance strategies from small studies are being disseminated at a fast pace, this systematic review is the first analysis to provide comparative estimates of virological failure between DTG-based monotherapy and dual therapy. More than 1700 studies were screened, including abstracts from all important HIV conferences in the past years. As our meta-analysis included studies with diverse study designs and populations, it could be argued that the comparison of studies with such differences might be problematic. However, the estimates of VF were very similar across studies, especially in the DTG-based dual therapy arm. This finding highlights the potency of this combination, even in the presence of previous drug resistance mutations or multiple co-morbidities. Unfortunately, only studies including low numbers of patients reported outcomes from individuals on DTG-monotherapy, and data on dual therapy was dominated by one large study that assessed the efficacy of the DTG/RPV combination. As a consequence, the comparison of DTG-monotherapy vs. DTG/3TC, which would have been the most interesting one, was not possible. Furthermore, the lack of availability of individual data from the different studies precluded the analysis of risk factors of VF in the different simplification regimens. As most studies were observational, it is possible that the investigators mainly included patients with good adherence, which may have limited the generalizability of their findings. Finally, our results might have slightly under-estimated the proportion of patients with VF as individuals who were lost to follow-up might have experienced this outcome without them being accounted for. However, our treatment failure estimates showed that even when other reasons of treatment failure were considered, DTG-based dual therapy was superior to monotherapy.
In summary, DTG-based dual maintenance therapy seems to be a promising simplification strategy with high virological efficacy and low potential for DDI and toxicity. Such a treatment regimen could be an interesting alternative to classical triple-ART in selected patients. Furthermore, dual therapy might be a cost-effective global ART strategy 43. A number of large prospective studies evaluating the efficacy of DTG-based dual therapy are under way and will inform its potential implementation at a large scale. In addition to the studies using DTG-emtricitabine(FTC 44) and DTG-3TC 45 maintenance therapy, clinical trials are also assessing the efficacy of DTG/3TC in treatment-naïve patients 46. Furthermore, it will be critical to evaluate the efficacy of DTG-3TC or FTC dual therapy in specific sub-groups such as pregnant and breast-feeding women, adolescents, patients with previous failure to standard triple regimens and harboring the M184V resistance mutation, as well as in patients with HIV associated neurocognitive disorder and tuberculosis coinfection.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2019 Wandeler G et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
F1000Research: Dataset 1. Dolutegravir meta-analysis summary data. 10.5256/f1000research.15995.d215724 47
Funding Statement
This study was supported by the Swiss National Science Foundation (Ambizione-PROSPER fellowship PZ00P3_154730 to GW, grant 32FP30-174281 to ME, and grant 33IC30_166819 to AC).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 3 approved]
Supplementary-material
Supplementary File 1: Completed PRISMA checklist.References
- 1. Gulick RM, Mellors JW, Havlir D, et al. : Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–9. 10.1056/NEJM199709113371102 [DOI] [PubMed] [Google Scholar]
- 2. Mathis S, Khanlari B, Pulido F, et al. : Effectiveness of protease inhibitor monotherapy versus combination antiretroviral maintenance therapy: a meta-analysis. PLoS One. 2011;6(7):e22003. 10.1371/journal.pone.0022003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paton NI, Stöhr W, Arenas-Pinto A, et al. : Protease inhibitor monotherapy for long-term management of HIV infection: a randomised, controlled, open-label, non-inferiority trial. Lancet HIV. 2015;2(10):e417–26. 10.1016/S2352-3018(15)00176-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pulido F, Ribera E, Lagarde M, et al. : Non-inferiority of dual-therapy (DT) with darunavir/ritonavir (DRV/r) plus 3TC versus triple-therapy (TT) with DRV/r plus TDF/FTC or ABC/3TC for maintenance of viral suppression: 48-week results of the DUAL-GESIDA 8014 trial - abstract n°: 0331. HIV Glasgow Conference Glasgow, 23–26 October 2016.2016. Reference Source [Google Scholar]
- 5. Arribas JR, Girard PM, Landman R, et al. : Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(7):785–92. 10.1016/S1473-3099(15)00096-1 [DOI] [PubMed] [Google Scholar]
- 6. Perez-Molina JA, Rubio R, Rivero A, et al. : Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(7):775–84. 10.1016/S1473-3099(15)00097-3 [DOI] [PubMed] [Google Scholar]
- 7. EACS Guidelines version 9.0.2017. Reference Source [Google Scholar]
- 8. Wijting I, Rokx C, Boucher C, et al. : Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV. 2017;4(12):e547–e554. 10.1016/S2352-3018(17)30152-2 [DOI] [PubMed] [Google Scholar]
- 9. Cahn P, Rolón MJ, Figueroa MI, et al. : Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc. 2017;20(1):21678. 10.7448/IAS.20.01.21678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charpentier C, Peytavin G, Lê M, et al. : High virological suppression rates regardless to the genotypic susceptibility score after switching to a dolutegravir-based regimen: W48 results in a prospective cohor.Abstract number: MOPEB0316. International AIDS Society (IAS) conference Paris, 23–26 July 2017.2017. Reference Source [Google Scholar]
- 11. Llibre JM, Hung CC, Brinson C, et al. : Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–49. 10.1016/S0140-6736(17)33095-7 [DOI] [PubMed] [Google Scholar]
- 12. Reynes J, Meftah N, Tuaillon E, et al. : Dual regimen with dolutegravir and lamivudine maintains virologic suppression even in heavily treatment experienced HIV-infected patients: 96 weeks results from maintenance DOLULAM study. Abstract n°: MOPEB0322. International AIDS Society (IAS) conference Paris, 23–26 July 2017.2017. Reference Source [Google Scholar]
- 13. Booth A, Clarke M, Dooley G, et al. : The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. 10.1186/2046-4053-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Altman DG, Gøtzsche PC, et al. : The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JA, Hernán MA, Reeves BC, et al. : ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, et al. : Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blanco JL, Rojas J, Paredes R, et al. : Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother. 2018;73(7):1965–1971. 10.1093/jac/dky093 [DOI] [PubMed] [Google Scholar]
- 19. Gubavu C, Prazuck T, Niang M, et al. : Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71(4):1046–50. 10.1093/jac/dkv430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katlama C, Soulié C, Caby F, et al. : Dolutegravir as monotherapy in HIV-1-infected individuals with suppressed HIV viraemia. J Antimicrob Chemother. 2016;71(9):2646–50. 10.1093/jac/dkw186 [DOI] [PubMed] [Google Scholar]
- 21. Sculier D, Doco-Lecompte T, Yerly S, et al. : Stable HIV-1 reservoirs on dolutegravir maintenance monotherapy: the MONODO study. HIV Med. 2018;19(8):572–577. 10.1111/hiv.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oldenbuettel C, Wolf E, Ritter A, et al. : Dolutegravir monotherapy as treatment de-escalation in HIV-infected adults with virological control: DoluMono cohort results. Antivir Ther. 2017;22(2):169–72. 10.3851/IMP3082 [DOI] [PubMed] [Google Scholar]
- 23. Rojas J, Blanco JL, Marcos MA, et al. : Dolutegravir monotherapy in HIV-infected patients with sustained viral suppression. J Antimicrob Chemother. 2016;71(7):1975–81. 10.1093/jac/dkw078 [DOI] [PubMed] [Google Scholar]
- 24. Rokx C, Schurink CA, Boucher CA, et al. : Dolutegravir as maintenance monotherapy: first experiences in HIV-1 patients. J Antimicrob Chemother. 2016;71(6):1632–6. 10.1093/jac/dkw011 [DOI] [PubMed] [Google Scholar]
- 25. Bonijoly T CA, Cheret A, Cotte L, et al. : Week-48 efficacy and safety of dolutegravir + rilpivirine dual therapy as switch strategy in a real-life cohort study.Abstract n°: PE9/15 European AIDS Clinical Society (EACS) conference, Milano, 25–27 October 2017.2017. [Google Scholar]
- 26. Borghetti A, Baldin G, Ciccullo A, et al. : Virological control and metabolic improvement in HIV-infected, virologically suppressed patients switching to lamivudine/dolutegravir dual therapy. J Antimicrob Chemother. 2016;71(8):2359–61. 10.1093/jac/dkw147 [DOI] [PubMed] [Google Scholar]
- 27. Castagna AGR, Rusconi S, Riva A, et al. : Durability and Safety of a Dual Antiretroviral Regimen with Dolutegravir and Unboosted Atazanavir in HIV-1 Infected Patients with Virological Suppression. Abstract n°: PE9/63. European AIDS Clinical Society (EACS) conference, Milano, 25–27 October 2017.2017. [Google Scholar]
- 28. Gantner P, Cuzin L, Allavena C, et al. : Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: a cohort study. HIV Med. 2017;18(9):704–8. 10.1111/hiv.12506 [DOI] [PubMed] [Google Scholar]
- 29. Joly V, Burdet C, Landman R, et al. : Promising Results of Lamivudine + DolutegravirMaintenance Therapy in ANRS 167 Lamidol Trial.Abstract number: 458. Conference on Retrovirus and Opportunistic Infections (CROI), Seattle, 13–16 February 2017. 2017. Reference Source [Google Scholar]
- 30. Maggiolo F, Gulminetti R, Pagnucco L, et al. : Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis. 2017;17(1):215. 10.1186/s12879-017-2311-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maggiolo FGR, Pagnucco L, Digaetano M, et al. : Durability of dolutegravir + lamivudine as simplification cART in patients with suppressed HIV-RNA.Abstract number: PE9/49. European AIDS Clinical Society (EACS) conference, Milano, 25–27 October 20172017. [Google Scholar]
- 32. Navarro JSJ, Silva A, Burgos J, et al. : Efficacy of Once-daily Dolutegravir Plus Boosted-Darunavir as a Switch Strategy in HIV-infected Heavily-treated Patients.Abstract n°: PE9/93. European AIDS Clinical Society (EACS) conference, Milano, 25–27 October 2017.2017. [Google Scholar]
- 33. Revuelta-Herrero JL, Chamorro-de-Vega E, Rodríguez-González CG, et al. : Effectiveness, Safety, and Costs of a Treatment Switch to Dolutegravir Plus Rilpivirine Dual Therapy in Treatment-Experienced HIV Patients. Ann Pharmacother. 2018;52(1):11–8. 10.1177/1060028017728294 [DOI] [PubMed] [Google Scholar]
- 34. Riva AP RS, Bonora S, Cattelan M, et al. : Dolutegravir and unboosted atazanavir: a dual NRTI‐ and booster‐free antiretroviral regimen simplification in HIV-1 infected patients with viral suppression.Abstract number: P090. HIV Glasgow Conference, Glasgow, 23–26 October 2016.2016. [Google Scholar]
- 35. Taiwo BO, Marconi VC, Berzins B, et al. : Dolutegravir Plus Lamivudine Maintains Human Immunodeficiency Virus-1 Suppression Through Week 48 in a Pilot Randomized Trial. Clin Infect Dis. 2018;66(11):1794–1797. 10.1093/cid/cix1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arribas JR, Pialoux G, Gathe J, et al. : Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014;14(7):581–9. 10.1016/S1473-3099(14)70782-0 [DOI] [PubMed] [Google Scholar]
- 37. Pozniak A, Markowitz M, Mills A, et al. : Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis. 2014;14(7):590–9. 10.1016/S1473-3099(14)70796-0 [DOI] [PubMed] [Google Scholar]
- 38. Oliveira M, Ibanescu RI, Pham HT, et al. : The M184I/V and K65R nucleoside resistance mutations in HIV-1 prevent the emergence of resistance mutations against dolutegravir. AIDS. 2016;30(15):2267–73. 10.1097/QAD.0000000000001191 [DOI] [PubMed] [Google Scholar]
- 39. Ciaffi L, Koulla-Shiro S, Sawadogo AB, et al. : Boosted protease inhibitor monotherapy versus boosted protease inhibitor plus lamivudine dual therapy as second-line maintenance treatment for HIV-1-infected patients in sub-Saharan Africa (ANRS12 286/MOBIDIP): a multicentre, randomised, parallel, open-label, superiority trial. Lancet HIV. 2017;4(9):e384–e92. 10.1016/S2352-3018(17)30069-3 [DOI] [PubMed] [Google Scholar]
- 40. Letendre SL, Mills AM, Tashima KT, et al. : ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis. 2014;59(7):1032–7. 10.1093/cid/ciu477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hocqueloux L, Gubavu C, Lefeuvre S, et al. : HIV-1-infected patients under successful less-drug regimens have similar genital shedding and residual viremia than those under triple therapy. International AIDS Society (IAS) conference, Paris, 23–26 July 20172017. Reference Source [Google Scholar]
- 42. Gianella S, Wilkin T, Berzins B, et al. : Genital HIV-1 shedding with Dolutegravir (DTG) plus Lamivudine (3TC) dual therapy. International AIDS Conference, Amsterdam, 23–27 Juy 20182018. Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Girouard MP, Sax PE, Parker RA, et al. : The Cost-effectiveness and Budget Impact of 2-Drug Dolutegravir-Lamivudine Regimens for the Treatment of HIV Infection in the United States. Clin Infect Dis. 2016;62(6):784–91. 10.1093/cid/civ981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Camly A BM, Sculier D, Wandeler G: Evaluation of a simplified strategy for the long-term management of HIV infection: a non-inferiority, randomized, controlled, open-label clinical trial: The Simpl'HIV Trial. NCT03160105. [Google Scholar]
- 45. E. M. Dolutegravir-based Simplification Strategies: DOLAM. EudraCT 2015-000274-35. [Google Scholar]
- 46. Taiwo BO, Zheng L, Stefanescu A, et al. : ACTG A5353: A Pilot Study of Dolutegravir Plus Lamivudine for Initial Treatment of Human Immunodeficiency Virus-1 (HIV-1)-infected Participants With HIV-1 RNA <500000 Copies/mL. Clin Infect Dis. 2018;66(11):1689–1697. 10.1093/cid/cix1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wandeler G, Buzzi M, Anderegg N, et al. : Dataset 1 in: Virologic failure and HIV drug resistance on simplified, dolutegravir-based maintenance therapy: Systematic review and meta-analysis. F1000Research. 2018. 10.5256/f1000research.15995.d215724 [DOI] [PMC free article] [PubMed] [Google Scholar]