Key Points
Question
Is airway bioengineering using stented aortic matrices for complex tracheal and bronchial reconstruction feasible in humans?
Findings
In this uncontrolled cohort study that included 20 patients, 13 were able to undergo tracheal, bronchial, or carinal transplantation. The overall 90-day mortality rate was 5%.
Meaning
This preliminary study suggests feasibility of human airway bioengineering using stented aortic matrices for tracheal and bronchial reconstruction.
Abstract
Importance
Airway transplantation could be an option for patients with proximal lung tumor or with end-stage tracheobronchial disease. New methods for airway transplantation remain highly controversial.
Objective
To establish the feasibility of airway bioengineering using a technique based on the implantation of stented aortic matrices.
Design, Setting, and Participants
Uncontrolled single-center cohort study including 20 patients with end-stage tracheal lesions or with proximal lung tumors requiring a pneumonectomy. The study was conducted in Paris, France, from October 2009 through February 2017; final follow-up for all patients occurred on November 2, 2017.
Exposures
Radical resection of the lesions was performed using standard surgical techniques. After resection, airway reconstruction was performed using a human cryopreserved (−80°C) aortic allograft, which was not matched by the ABO and leukocyte antigen systems. To prevent airway collapse, a custom-made stent was inserted into the allograft. In patients with proximal lung tumors, the lung-sparing intervention of bronchial transplantation was used.
Main Outcomes and Measures
The primary outcome was 90-day mortality. The secondary outcome was 90-day morbidity.
Results
Twenty patients were included in the study (mean age, 54.9 years; age range, 24-79 years; 13 men [65%]). Thirteen patients underwent tracheal (n = 5), bronchial (n = 7), or carinal (n = 1) transplantation. Airway transplantation was not performed in 7 patients for the following reasons: medical contraindication (n = 1), unavoidable pneumonectomy (n = 1), exploratory thoracotomy only (n = 2), and a lobectomy or bilobectomy was possible (n = 3). Among the 20 patients initially included, the overall 90-day mortality rate was 5% (1 patient underwent a carinal transplantation and died). No mortality at 90 days was observed among patients who underwent tracheal or bronchial reconstruction. Among the 13 patients who underwent airway transplantation, major 90-day morbidity events occurred in 4 (30.8%) and included laryngeal edema, acute lung edema, acute respiratory distress syndrome, and atrial fibrillation. There was no adverse event directly related to the surgical technique. Stent removal was performed at a postoperative mean of 18.2 months. At a median follow-up of 3 years 11 months, 10 of the 13 patients (76.9%) were alive. Of these 10 patients, 8 (80%) breathed normally through newly formed airways after stent removal. Regeneration of epithelium and de novo generation of cartilage were observed within aortic matrices from recipient cells.
Conclusions and Relevance
In this uncontrolled study, airway bioengineering using stented aortic matrices demonstrated feasibility for complex tracheal and bronchial reconstruction. Further research is needed to assess efficacy and safety.
Trial Registration
clinicaltrials.gov Identifier: NCT01331863
This case series describes 90-day mortality of patients who required tracheal, bronchial, or carinal reconstruction using stented aortic allografts supported by muscle flaps for treatment of inoperable airway disease including lung cancer, tracheal stenosis, or tracheomalacia.
Introduction
Over the last decade, new procedures in the field of airway transplantation have gained attention primarily through case reports or small studies.1,2,3,4,5,6 Due to the lack of prospective data, these interventions have failed to achieve standard of care status. An efficient airway replacement could potentially benefit many patients with lung cancer and eliminate the need for high-risk thoracic surgical procedures including pneumonectomies. Airway replacement also could be an option for patients with end-stage tracheobronchial disease for whom palliative treatment is often proposed.
In 1997, a research program on airway transplantation using stented aortic matrices was initiated in the laboratory of the Alain Carpentier Foundation. A series of 7 preclinical animal studies using a sheep model showed that autologous and fresh or cryopreserved allogeneic aortic grafts could be valuable airway substitutes.7 The regeneration of epithelium and de novo generation of cartilage were observed within the aortic matrices, thus allowing stent removal after only 6 months. Although controversial, the hypothesis that bone marrow mesenchymal stem cells play a role in this process of in vivo tissue engineering has been proposed.8 These favorable results led to the first human applications in patients with extensive tracheal diseases or complex tumors that would otherwise necessitate pneumonectomies.9,10,11,12
De novo generation of cartilage within cryopreserved aortic allografts has been observed recently.12 This process originated from the recipient cells, which was observed in animal models. Moreover, remaining viable matrix cells have been identified to play a critical role in the regenerative process as a way to release proangiogenic, chemoattractant, proinflammatory or proimmunomodulatory cytokines, and growth factors. This prospective study was designed to evaluate the feasibility of airway bioengineering using stented cryopreserved aortic allografts as biological matrices.
Methods
Study Design
The study was sponsored by Direction de la Recherche Clinique at Assistance Publique–Hôpitaux de Paris, which is a consortium of university hospitals in Paris, France. The protocol was written by 2 of the principal investigators (E.M. and E.V.), it was approved by the French national institutional ethical review board on March 14, 2011, and it appears in Supplement 1. The initial protocol focused on bronchial transplantation to avoid a pneumonectomy in patients with extensive lung cancer.
To extend the study to all types of major (malignant or benign) lesions of the trachea and bronchi requiring airway transplantation, 3 amendments to the protocol were approved by the institutional ethical review board on March 27, 2012, October 25, 2012, and June 5, 2013. Before approval of the study and its amendments, 4 procedures had been accepted by the ethical review board. An independent data and safety monitoring board periodically reviewed the study outcomes. Written informed consent was obtained from all patients.
Patients
Eligible patients underwent a standard preoperative evaluation and cardiopulmonary tests. A multidisciplinary team approved the inclusion and exclusion criteria. Patients were included in the study if they (1) had proximal lung tumors requiring a surgical resection (pneumonectomy, carinal resection, or sleeve lobectomy) that may or may not have been treated with neoadjuvant chemotherapy and had adequate or compromised preoperative lung function tests; or (2) had significant major malignant or benign lesions of the trachea and bronchi untreated with conventional therapeutic approaches.
Patients were excluded from the study if they (1) were younger than 18 years; (2) were unable to give consent or not affiliated with the French Social Security System; (3) had a lung tumor requiring a standard lobectomy; (4) had nonresectable major locally invasive tumors; (5) had contralateral lymph node invasion; (6) had metastatic disease with the exception of a unique resectable brain metastasis; (7) had tracheal lesions requiring standard resection with direct anastomosis; (8) had an iodine allergy; or (9) received a preoperative evaluation indicating an inability to undergo a standard lobectomy.
Treatment
After enrollment in the study, patients underwent an intensive preoperative respiratory conditioning program. The human cryopreserved (−80°C) aortic allograft, which was not matched by the ABO and leukocyte antigen systems, was ordered from a certified tissue bank (Saint-Antoine, Etablissement Français du Sang, Assistance Publique–Hôpitaux de Paris). To prevent airway collapse, a custom-made, fully covered conical nitinol stent (Silmet, Novatech) or a silicone stent (Tracheobronxane Dumon, Novatech) were manufactured according to preoperative measurements obtained via computed tomography.
The first step was to evaluate whether a complete surgical resection with adequate margins was needed. The second step was to determine whether a conventional approach should be taken using direct end-to-end anastomosis for tracheal lesions or a lobectomy or bilobectomy for lung tumors. The third step was to determine whether an airway transplantation was needed.
Radical resection of the lesions was performed using standard surgical techniques. A conventional solution for airway reconstruction was preferred when feasible. If used, the cryopreserved aortic allografts were removed from the dry ice, then thawed in container bags for 10 minutes at room temperature, followed by 10 minutes in a water bath at 37°C. Next, the allografts were washed with a sterile saline solution for 5 minutes before implantation and were used for airway reconstruction, pulmonary artery reconstruction, or both.
At the end of the operations, the allografts were covered circumferentially with a local muscle flap to promote neovascularization and prevent fistulization. Sterno-omo-thyrohyoid muscle flaps were used for tracheal reconstructions; pectoralis major muscle flaps were used for carinal reconstructions; and latissimus dorsi or intercostal muscle flaps were used for bronchial reconstructions. The techniques used followed guidelines established during human and other experimental studies.7,8,9,10,11,12,13,14,15,16,17,18,19,20
In Figure 1, the final aspect of the airways after surgical resection and reconstruction is shown schematically. None of the patients received immunosuppressive therapy per the usual recommendations when cryopreserved aortic allografts are used in vascular surgery. Low-molecular-weight heparin was administered for postoperative venous thromboembolism prophylaxis. Until stent removal, an aerosolized saline solution was administrated 3 times daily to prevent airway obstruction.
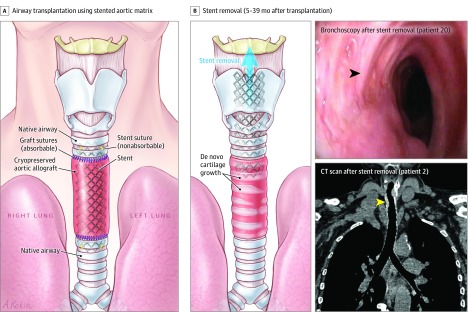
Figure 1. De Novo Generation of Cartilage Within Stented Cryopreserved Aortic Matrices After Surgical Resection of End-Stage Tracheal Lesions or Proximal Lung Tumors.
A, Schematic view of airway transplantation using a stented aortic matrix. After resection with adequate surgical margins of end-stage tracheal lesions or proximal lung tumors, the native airway was reconstructed using a cryopreserved aortic allograft. This biological matrix was supported by a stent to prevent airway collapse. The native airway and the aortic graft were anastomosed with continuous absorbable sutures. Both ends of the stent were fixed to the native airway using 4 nonabsorbable sutures (yellow stitches).
B, Schematic view of stent removal after airway reconstruction and clinical follow-up. De novo generation of cartilage within the aortic matrix allowed removal of stents 5 to 39 months after transplantation. Top right, patient 20 had complete resection of recurrent thyroid carcinoma with tracheal invasion followed by tracheal transplantation using a cryopreserved aortic allograft. Bronchoscopy at 8 months after tracheal transplantation showed de novo generation of tracheal cartilage (black arrowhead) (Video). The patient was able to breathe and speak normally through newly formed airways at last follow-up after both stent removal at 5 months and tracheostomy tube decannulation at 7 months. Bottom right, patient 2 had end-stage postintubation stenosis that was treated with tracheal transplantation. Chest computed tomographic (CT) scan at last follow-up (7 years 1 month) after stent removal showed a newly formed trachea (yellow arrowhead).
Follow-up and Assessment of Outcomes
Patients were followed up for 90 days. The outcomes of mortality and morbidity were assessed, including complications directly related to the allograft, the stent, or both. Patients were systematically examined at 30, 60, and 90 days. There was a 7-day window around each time point during which patients could have been examined (eg, on day 23 or on day 37 for the 30-day time point). If required, complementary examinations were performed such as chest radiography, computed tomography, and bronchoscopy. The primary outcome was 90-day mortality. The secondary outcome was 90-day morbidity. After 90 days, all patients were followed up for long-term mortality and morbidity outcomes.
Biopsy specimens were obtained from cryopreserved aortic allografts after stent removal in a woman (patient 2) who received an allograft from a male donor at 15 months and in a man (patient 4) who received an allograft from a female donor at 39 months. Tissues identified as neoepithelium, neocartilage, and granuloma were isolated and used for histological studies. Biopsy specimens from patient 2 were used for engraftment (chimerism) studies. The biological study protocols are detailed in the eMethods in Supplement 2.
For all patients, the last follow-up visit occurred on November 2, 2017. The following data were collected for each patient: sex, age, medical history, type of airway disease, tumor localization, preoperative treatment, indication for inclusion in the protocol, date of the procedure, type of operation, duration of hospitalization, 90-day mortality and morbidity, histopathological specimen examination, postoperative treatment, delayed complications (after 90 days), stent removal timing, and status at last follow-up visit.
Statistical Analysis
The predefined sample size was 20 patients. This sample size enables estimation of a 90-day survival rate with a maximal half-width of its 2-sided 95% CI equal to ± 17.5%. The reported results include assessments from all 20 patients. All analyses were made using SAS version 9.4 (SAS Institute Inc).
Results
Patients
From October 2009 through February 2017, 20 patients were included in the study. Eight patients (40%) were referred from other medical centers. There were 13 male and 7 female patients ranging in age from 24 to 79 years with a mean age of 54.9 years. The patient characteristics, types of preoperative treatments, and indications for inclusion appear in Table 1.
Table 1. Patient Characteristics, Preoperative Treatment, and Indication for Inclusion in the Study.
| Patient No. |
Sex | Age, y | Medical History | Type of Airway Disease | Description of Localization | Type of Preoperative Treatment | Indication for Study Inclusion |
|---|---|---|---|---|---|---|---|
| 1 | M | 78 | Prostatic adenoma, past smoker (50 pack-years), and COPD | Non–small cell lung cancer, large cell subtype | Right lung and extending to main bronchus and left atrium | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 2 | F | 58 | Thyroidectomy for hyperthyroidism and idiopathic laryngeal edema | Postintubation benign tracheal stenosis and upper airway malacia | Laryngotracheal | Conventional endoscopic and surgical treatment | Extensive lesion and failure of previous treatment |
| 3 | F | 24 | Toll-like receptor deficiency and fulminant hepatitis (HSV type2) | Postintubation benign tracheal stenosis | Laryngotracheal | Conventional endoscopic and surgical treatment | Extensive lesion and failure of previous treatment |
| 4 | M | 33 | Severe epilepsy and tracheal intubation in ICU | Postintubation benign tracheal stenosis | Laryngotracheal | Conventional endoscopic and surgical treatment | Extensive lesion and failure of previous treatment |
| 5 | M | 59 | Alcoholic cirrhosis, obesity, past smoker (80 pack-years), and COPD | Non–small cell lung cancer, squamous cell subtype | Right lung and extending to proximal bronchi and pulmonary artery | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 6 | F | 41 | Obesity (BMI of 37.5a) and gestational diabetes | Extended carcinoid tumor for >3 y | Lower trachea, carina, right lung, and mediastinal nodes | Palliative endoscopic treatment | Not accessible to conventional surgery |
| 7 | M | 62 | Coronary stenting, past smoker (40 pack-years), and COPD | Non–small cell lung cancer, squamous cell subtype | Left lung, upper lobe extending to main bronchus | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 8 | M | 57 | Carotid surgery, past smoker (70 pack-years), and COPD | Non–small cell lung cancer, squamous cell subtype | Left lung, lower lobe extending to the origin of upper bronchus | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 9 | M | 50 | Past smoker (45 pack-years) and COPD | Non–small cell lung cancer, squamous cell subtype | Right lung and extending to proximal bronchi and pulmonary artery | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 10 | F | 79 | Middle lobectomy for carcinoid tumor and thyroidectomy | Recurrence of carcinoid tumor | Right lung and extending to proximal bronchi and pulmonary artery | None | To avoid pneumonectomy |
| 11 | M | 54 | Alcohol use disorder, past smoker (60 pack-years), and COPD | Non–small cell lung cancer, squamous cell subtype | Right lung and extending to proximal bronchi and pulmonary artery | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 12 | F | 41 | Thyroidectomy and ablation of atrial tachycardia | Carcinoid tumor | Left lung, lower lobe extending to superior pulmonary vein | None | To avoid pneumonectomy |
| 13 | M | 60 | Diabetes, coronary stenting, past smoker (80 pack-years), and COPD | Non–small cell lung cancer, squamous cell subtype | Right lung, lower and middle lobe extending to proximal bronchi | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 14 | M | 59 | Cardiac arrhythmia, past smoker (60 pack-years), and COPD | Non–small cell lung cancer, squamous cell subtype | Right lung, lower and middle lobe extending to proximal bronchi | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 15 | M | 68 | Endovascular treatment of aortic aneurysm | Non–small cell lung cancer, squamous cell subtype | Left lung, lower lobe extending to the origin of upper bronchus | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 16 | M | 68 | Hypertension, past smoker (50 pack-years), and COPD | Non–small cell lung cancer, adenocarcinoma subtype | Left lung, upper lobe extending to main bronchus | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 17 | M | 45 | Hypertension and thyroid carcinoma | Anaplastic thyroid carcinoma | Tracheal invasion left in place at initial surgical resection (R2)b | None | Extensive lesion and failure of previous treatment |
| 18 | F | 49 | Amygdalectomy | Non–small cell lung cancer, large cell subtype | Left lung, lower lobe extending to the origin of upper bronchus | Neoadjuvant chemotherapy (3 cycles) | To avoid pneumonectomy |
| 19 | M | 50 | Amygdalectomy and past smoker (34 pack-years) | Rhabdomyosarcoma | Right lung and extending to proximal bronchi and thoracic wall | Neoadjuvant chemotherapy (5 cycles) | To avoid pneumonectomy |
| 20 | F | 64 | Recurrent thyroid carcinoma and right vocal cord paralysis | Papillary thyroid carcinoma | Invasion on right side of trachea left in place at iterative surgical resection (R2)b | Refractory to iterative iodine therapy | Extensive lesion and failure of previous treatment |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; HSV, herpes simplex virus; ICU, intensive care unit; R2, macroscopic residual tumor.
Calculated as weight in kilograms divided by height in meters squared.
Found during pathological examination of the resected specimens.
Fourteen patients were included to avoid a pneumonectomy for non–small cell lung cancer (n = 11), carcinoid tumor (n = 2), or rhabdomyosarcoma (n = 1). Five patients had extensive benign (n = 3) or malignant (n = 2) tracheal lesions for which previous treatment had failed. One patient presented with a carcinoid tumor extending to the distal trachea, carina, and main right bronchus.
Procedures
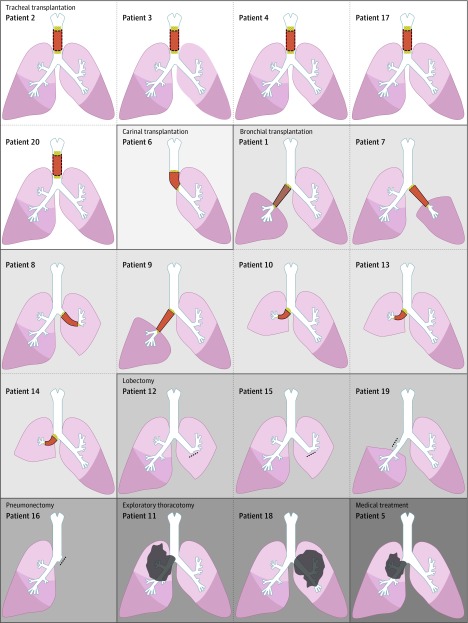
The interventions performed for each patient are detailed in Table 2 and Figure 2. Thirteen patients underwent a tracheal (n = 5), carinal (n = 1), or bronchial (n = 7) transplantation using a stented cryopreserved aortic allograft. Two of the patients who underwent bronchial reconstruction also underwent a partial replacement of the pulmonary artery using the same allograft to avoid a pneumonectomy. Seven patients did not receive airway transplantation for the following reasons: medical contraindication appeared after enrollment (n = 1), unavoidable pneumonectomy (n = 1), exploratory thoracotomy only (n = 2), and a lobectomy or bilobectomy was possible (n = 3).
Table 2. Primary and Secondary Outcomes by Type of Intervention.
| Type of Intervention | Date of Operation |
Patient No. |
Hospital Length of Stay, d |
Primary Oucome: 90-d Mortality |
Secondary Outcome: 90-d Morbidity |
Cancer Pathology | Type of Postoperative Cancer Treatment |
|---|---|---|---|---|---|---|---|
| Tracheal transplantation | Oct 2010 | 2 | 14 | No | No | NA | NA |
| Tracheal transplantation | Jan 2011 | 3 | 11 | No | No | NA | NA |
| Tracheal transplantation | Oct 2011 | 4 | 10 | No | No | NA | NA |
| Tracheal transplantation | Jun 2016 | 17 | 6 | No | Subcutaneous emphysema | R0 resectiona | None |
| Tracheal transplantation | Feb 2017 | 20 | 10 | No | Tracheal granuloma, laryngeal edema at months 2 and 3, bronchoscopy, and tracheostomy | R0 resectiona | None |
| Venovenous ECMO, right pneumonectomy extended to trachea, and carinal transplantation | May 2012 | 6 | NA | Yes on day 1 | Cerebrovascular accident and intracranial carotid artery occlusion | Pathological T4N2M0 | NA |
| Intrapericardial upper bilobectomy and bronchial transplantation | Oct 2009 | 1 | 16 | No | Atrial fibrillation, acute lung edema, atelectasis, and urinary retention | Pathological T4N0M0 | None |
| Left upper lobectomy and bronchial transplantation | Oct 2012 | 7 | 12 | No | Atelectasis | Clinical T2N2M0 and pathological T3N0M0 | Radiotherapy |
| Left lower lobectomy and bronchial and pulmonary artery transplantation | Jan 2013 | 8 | 8 | No | No | Clinical T2N2M0 and pathological T2N1M0 | Radiotherapy |
| Intrapericardial upper bilobectomy and bronchial transplantation | Mar 2013 | 9 | 9 | No | No | Clinical T3N1M0 and pathological T3N1M0 | None |
| Right lower lobectomy and bronchial and pulmonary artery transplantation | Dec 2013 | 10 | 22 | No | Atrial fibrillation and acute respiratory distress syndrome | 2 Atypical carcinoid tumors: pathological T3N0M0 | None |
| Lower bilobectomy and bronchial transplantation | Nov 2014 | 13 | 12 | No | Atelectasis | Clinical T3N0M0 and pathological T3N1M0 | Radiotherapy |
| Lower bilobectomy and bronchial transplantation | Jan 2015 | 14 | 9 | No | Anemia necessitating blood transfusion | Clinical T3N0M0 and pathological T0N0M0 | Radiotherapy |
| Standard lobectomy but transplantation not performed | Apr 2014 | 12 | 10 | No | No | Pathological T2N0M0 | None |
| Standard lobectomy but transplantation not performed | Mar 2016 | 15 | 6 | No | No | Pathological T2N2M0 | Radiochemotherapy |
| Standard lobectomy extended to thoracic wall but transplantation not performed | Jul 2016 | 19 | 9 | No | No | Clinical T3N0M0 and pathological T3N0M0 | Radiotherapy |
| Pneumonectomy but transplantation not performed | Apr 2016 | 16 | 11 | No | Atrial fibrillation | Pathological T2N1M0 | None |
| Exploratory thoracotomy but transplantation not performed | Jun 2014 | 11 | 8 | No | No | Clinical T4N2M0 | Radiochemotherapy |
| Exploratory thoracotomy but transplantation not performed | Jun 2016 | 18 | 6 | No | No | Clinical T4N2M0 | Radiochemotherapy |
| Myocardial ischemia but operation not performed | Jul 2011 | 5 | NA | No | No | Clinical T3N2M0 | NA |
Abbreviations: ECMO, extracorporeal membrane oxygenation; NA, not applicable.
Indicates no residual tumor was found during pathological examination of the resected specimens.
Figure 2. Schematic Illustrations of the Intervention Performed for Each of the 20 Patients With End-Stage Tracheal Lesions or Proximal Lung Tumors .
In patients 12, 15, 16, and 19, dashed lines indicate bronchial sutures. In patients 5, 11, and 18, gray irregular shapes represent unresectable proximal lung tumors.
Primary Outcome
The details regarding the primary outcome for each patient appear in Table 2. Among all 20 patients, the 90-day mortality rate was 5% (1 patient underwent a carinal transplantation and died). Among the 13 patients who underwent airway transplantation, the 90-day mortality rate was 7.7% (n = 1). The 90-day mortality rate was 0% for the patients who had a tracheal (n = 5) or bronchial transplantation (n = 7).
Secondary Outcomes
The details regarding the secondary outcomes for each patient appear in Table 2. The mean length of hospitalization was 10.5 days and ranged from 6 to 22 days. Among the 13 patients who underwent airway transplantation, major 90-day morbidity events occurred in 4 (30.8%) and included laryngeal edema, acute lung edema, acute respiratory distress syndrome, and atrial fibrillation. There was no adverse event directly related to the surgical technique. All general complications resolved except for a massive cerebrovascular accident, resulting in the death of the patient.
Long-term Follow-up
Long-term follow-up did not identify any major complications specifically related to the allograft or the stent (Table 3). Minor stent-related complications (tracheal or bronchial granulomas) were found in 7 patients and required treatment management with bronchoscopy. A temporary tracheostomy, via the stented allograft, was required due to severe laryngeal edema in patient 2. Extensive granulomas required early stent removal in patient 20 at 5 months and a temporary tracheostomy from months 5 to 7 until the allograft was structurally strong enough.
Table 3. Long-term Follow-up of Patients Who Had a Tracheal or Bronchial Transplantation.
| Patient No. | Types of Complications During Long-Term Follow-up | Stent Removed? | Last Follow-upa | Status | Clinical Status |
|---|---|---|---|---|---|
| Tracheal Transplantation | |||||
| 2 | Laryngeal edema and stent bacterial infection | Yes at 15 mo | 7 y and 1 mo | Alive | Breathes and speaks normally through newly formed airways |
| 3 | Tracheal granuloma and graft malacia requiring repeated bronchoscopies | No | 6 y and 10 mo | Alive | Breathes and speaks through a translaryngeal stent |
| 4 | Tracheal granuloma and graft malacia requiring repeated bronchoscopies | Yes at 39 mo | 6 y and 1 mo | Alive | Breathes and speaks normally through newly formed airways |
| 17 | Tracheal granuloma related to the stent requiring bronchoscopy | No | 1 y and 6 mo | Alive | No cancer recurrence; breathes and speaks through the stented aortic graft |
| 20 | Tracheal granuloma related to the stent requiring bronchoscopy and tracheostomy during months 5-7 | Yes at 5 mo | 9 mo | Alive | No cancer recurrence; breathes and speaks normally through newly formed airways |
| Bronchial Transplantation | |||||
| 1 | Bronchial granuloma related to the stent requiring bronchoscopy | No | 2 y and 6 mo | Dead | Diffuse metastases |
| 7 | Bronchial granuloma related to the stent requiring bronchoscopy | Yes at 15 mo | 5 y and 1 mo | Alive | No cancer recurrence; breathes normally through newly formed airways |
| 8 | None | Yes at 22 mo | 3 y and 2 mo | Dead | No cancer recurrence but had a myocardial infarction |
| 9 | Bronchial granuloma related to the stent requiring bronchoscopy | Yes at 22 mo | 4 y and 8 mo | Alive | No cancer recurrence; breathes normally through newly formed airways |
| 10 | None | Yes at 17 mo | 3 y and 11 mo | Alive | No tumor recurrence; breathes normally through newly formed airways |
| 13 | None | Yes at 15 mo | 3 y | Alive | No cancer recurrence; breathes normally through newly formed airways |
| 14 | None | Yes at 14 mo | 2 y and 10 mo | Alive | No cancer recurrence; breathes normally through newly formed airways |
From the date of the operation.
Stent removal was possible in the majority of patients (n = 9; 69.2%). It was performed postoperatively between months 5 and 39 at a mean of 18.2 months. At a median follow-up of 3 years 11 months (maximal follow-up of 7 years 1 month), 10 patients (76.9%) were alive. Of these 10 patients, 8 (80%) breathed normally through newly formed airways after stent removal (Video). As of November 2, 2017, the stent was still in place in patients 3 and 17.
Video. Videobronchoscopy Visualization of De Novo Cartilage Formation After Stent Removal Following Airway Reconstruction Using a Stented Aortic Matrix.
Videobronchoscopy imaging from 2 representative patients (patients 20 and 8) who underwent airway reconstruction with a stented aortic matrix. At the time of imaging, the patients were breathing normally through newly formed airways. Generated cartilage was strong enough to avoid airway collapse.
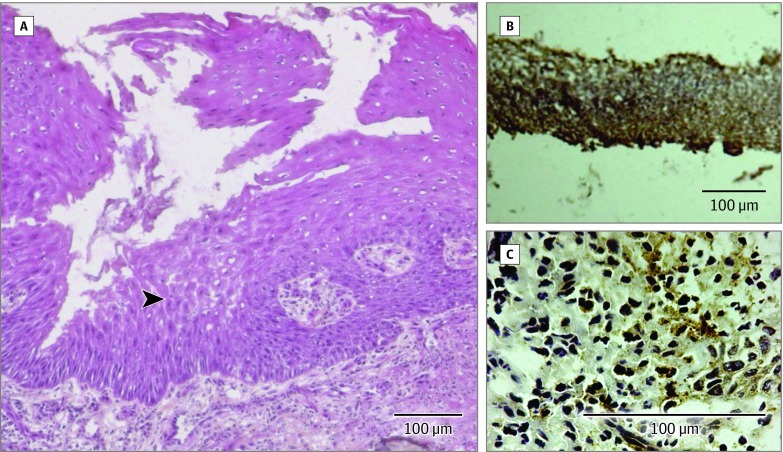
In biological studies, the explant cell culture demonstrated that the cells contained within the cryopreserved aortic allografts were viable and able to proliferate after thawing. The absence of SRY donor DNA 15 months after surgery in biopsy specimens from patient 2 suggested a progressive disappearance of donor cells from the cryopreserved aortic allograft in parallel to its colonization by the host’s cells. Pathological examination of superficial allograft biopsies showed the regeneration of mixed respiratory epithelium (Figure 3A).
Figure 3. Histology and Immunohistochemistry of Biopsy Specimens of Aortic Allografts.
A, Superficial graft biopsy specimen from patient 2 obtained 15 months after tracheal transplantation showed regeneration of a mixed respiratory neoepithelium-like structure (black arrowhead) (hematoxylin-eosin-safran stain). B and C, Cryopreserved aortic allograft biopsy specimens from patient 4 obtained 39 months after tracheal transplantation showed type 2 collagen (B) and Sox9 (C) immunoreactivity (brown). The positive labeling of the cryopreserved aortic allograft for these 2 specific collagen markers suggested the presence of cartilage-like cells. Biopsy specimens from nonimplanted cryopreserved aortic allografts were negative for these markers. Immunohistochemistry for collagen type 2 used goat polyclonal anticollagen type 2 with horseradish peroxidase (HRP)-labeled polymer-antigoat antibody detection system (Envision+ Kit, Dako K4011), 3,3′-diaminobenzidine (DAB+) as the chromogen (brown-colored precipitate at the antigen site), and Mayer hematoxylin counterstain. Immunohistochemistry for Sox9 used a rabbit polyclonal anti-Sox9 with HRP-labeled polymer-antirabbit antibody detection system (Envision+ Kit, Dako K4011), DAB+ as the chromogen (brown-colored precipitate at the antigen site), and Mayer hematoxylin counterstain.
The positive labeling of a biopsy sample from the cryopreserved aortic allograft at 39-month implantation for specific cartilage markers (type 2 collagen and Sox9) suggested the presence of cartilage-like cells (Figure 3B, C). Nonimplanted cryopreserved aortic allografts were negative for these markers.
Discussion
In this uncontrolled study, airway bioengineering using stented aortic matrices demonstrated feasibility for complex tracheal and bronchial reconstruction. Prospective protocols for surgical innovations before their introduction into clinical practice remain the exception rather than the rule despite international recommendations.21
In the field of airway transplantation, recent advances have been associated with important scientific and ethical controversies.22,23 In 2014, the International Society of Cell Therapy in association with other regulators and ethics experts proposed specific recommendations for human airway bioengineering.24 Since the establishment of this research initiative in 1997, all investigators have strictly adhered to the international scientific and ethical principles of surgical innovation.21
In this study, the 90-day mortality rate was low among patients undergoing the innovative surgical approach. The 90-day mortality rate was used as the primary outcome rather than the 30-day mortality rate because it is more suitable for the evaluation of surgical interventions. The only death reported during the study was observed after a complex carinal reconstruction with extracorporeal membrane oxygenation. However, the mechanism of the cerebrovascular event remains unclear. Despite the fact that the death was not directly associated with the surgical technique, carinal reconstruction should be approached with extreme caution. In a retrospective study25 of 12 patients who had autologous tracheal substitution, the 90-day mortality rate was 16.6%; 2 patients who underwent carinal resection died.
In the present study, the 90-day mortality rate was 0% in the subgroup of patients who underwent bronchial transplantation to avoid a high-risk pneumonectomy. The mortality rate was lower than the rate observed in a retrospective series of patients who required pneumonectomies (varying from 10.5% to 26% at 90 days).11,26,27 This is important to note because pneumonectomy remains a frequent operation in the United States and in Europe.28 Tan et al29 developed a closed technique of lung-sparing pulmonary resection of malignant tumors using a different type of tissue-engineered bronchus.
In the subgroup of patients who had tracheal transplantation (n = 5) in the present study, the 90-day mortality rate was 0%. Regarding this mortality rate, it is challenging to compare the results from the present study with those obtained by retrospective case reports or series. Since the review of airway transplantation by Grillo,30 recent advances have been observed and include the development of modern tissue engineering techniques.7,24,31,32 The results obtained by Macchiarini et al1 have been critically debated following the revelation that the majority of patients died after the implantation of tissue-engineered airways, which is in contrast to the initial reports.3,5,22,23,24
Other case studies have been reported by multiple groups using various conduit types including a Marlex mesh patch with spiral rings covered by a collagen sponge (Omori et al33,34; n = 7), tracheal allograft after withdrawal of immunosuppressive therapy (Delaere et al2,6,35; n = 6), and a decellularized cadaveric trachea (Elliott et al,4 Hamilton et al,36 and Steinke et al37; n = 2). These reports have led some groups to start prospective observational studies.24 The 90-day morbidity rate in the present series was not directly linked to the surgical technique but rather to common complications of thoracic surgery. Importantly, there were no major complications related to the allograft or the stent.
The present study demonstrated positive long-term results. No death was related to airway transplantation using stented cryopreserved aortic allografts. Furthermore, stent removal was feasible in the majority of patients in this study as well as in preclinical studies7,11 and in a recent report.12 The majority of patients were breathing and speaking normally without a tracheostomy or stent at long-term follow-up. Moreover, postoperative examinations did not detect a definitive malacia of the cryopreserved aortic allografts after stent removal.
The present study confirmed prior biological observations.12 The regeneration of a mixed respiratory epithelium was observed on superficial allograft biopsies. Immunodetection of type 2 collagen, Sox9-specific markers, and engraftment (chimerism) studies from samples of neotissues demonstrated de novo generation of cartilage within the aortic allografts from recipient cells. Aortic matrices played a significant role in this observation as illustrated by the release of proangiogenic, chemoattractant, proinflammatory and immunomodulatory cytokines, and growth factors.12 The main hypothesis was that this phenomenon promoted progenitor or stem cell homing followed by de novo generation of cartilage. In this scenario, the human body was used as a natural bioreactor and allowed in vivo airway tissue engineering.
The role of stem cells in airway tissue regeneration has been widely debated in recent years. Some have argued that airway regeneration should be regarded as hypothetical and scientifically unfounded.35 To date, there has been no further explanation of the process observed since the beginning of the experiments. The mechanism of epithelium regeneration is less controversial. Epithelial cells have gradually repopulated the allograft lumen by direct migration, by expansion from adjacent native airways (as observed after epithelium destruction), or by both.38
The technique of in vivo bioengineering using an aortic matrix has been applied to the replacement of a small bowel segment with encouraging results in a porcine model.39 Compared with other techniques, the present solution did not require the use of decellularized cadaveric tracheal allografts, recipient cells, artificial bioreactors, or immunosuppressive treatment.7,24,30,31,32
Areas for additional research include the possibility to accelerate de novo generation of cartilage for early stent removal; study of mechanisms of airway regeneration within cryopreserved aortic matrices; assessment of long-term quality of life among patients after receiving a transplantation; and development of clinical applications using multicenter studies, in particular for patients with end-stage tracheal lesions or proximal lung tumors requiring a pneumonectomy.
Limitations
This study has several limitations. First, this is a feasibility study with a limited number of patients. Larger studies are needed for a validated estimate of this technique. Second, along with many surgical innovations, this a monocentric study, which implies that the results cannot be generalized without further evaluation involving a larger number of centers. Third, this is a noncomparative study. Considering these limitations, further studies and multicenter randomized clinical trials are necessary to evaluate the benefit-risk balance of this approach for specific indications such as end-stage tracheal diseases, locally advanced thyroid cancer, and proximal lung cancer.
Conclusions
In this uncontrolled study, airway bioengineering using stented aortic matrices demonstrated feasibility for complex tracheal and bronchial reconstruction. Further research is needed to assess efficacy and safety.
Trial protocol
eMethods. Follow-up and outcome assessment
References
- 1.Macchiarini P, Jungebluth P, Go T, et al. . Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023-2030. [DOI] [PubMed] [Google Scholar]
- 2.Delaere P, Vranckx J, Verleden G, et al. . Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362(2):138-145. [DOI] [PubMed] [Google Scholar]
- 3.Jungebluth P, Alici E, Baiguera S, et al. . Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite [published corrections appear in Lancet. 2016;387(10022):944 and 2016;387(10025):1276]. Lancet. 2011;378(9808):1997-2004. [DOI] [PubMed] [Google Scholar]
- 4.Elliott MJ, De Coppi P, Speggiorin S, et al. . Stem-cell-based, tissue engineered tracheal replacement in a child. Lancet. 2012;380(9846):994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonfiotti A, Jaus MO, Barale D, et al. . The first tissue-engineered airway transplantation. Lancet. 2014;383(9913):238-244. [DOI] [PubMed] [Google Scholar]
- 6.Delaere PR, Vranckx JJ, Den Hondt M, et al. . Tracheal allograft after withdrawal of immunosuppressive therapy. N Engl J Med. 2014;370(16):1568-1570. [DOI] [PubMed] [Google Scholar]
- 7.Martinod E, Seguin A, Radu DM, et al. . Airway transplantation. Eur J Med Res. 2013;18:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seguin A, Baccari S, Holder-Espinasse M, et al. . Tracheal regeneration. J Thorac Cardiovasc Surg. 2013;145(5):1297-1304.e2. [DOI] [PubMed] [Google Scholar]
- 9.Wurtz A, Porte H, Conti M, et al. . Tracheal replacement with aortic allografts. N Engl J Med. 2006;355(18):1938-1940. [DOI] [PubMed] [Google Scholar]
- 10.Wurtz A, Porte H, Conti M, et al. . Surgical technique and results of tracheal and carinal replacement with aortic allografts for salivary gland-type carcinoma. J Thorac Cardiovasc Surg. 2010;140(2):387-393.e2. [DOI] [PubMed] [Google Scholar]
- 11.Martinod E, Radu DM, Chouahnia K, et al. . Human transplantation of a biologic airway substitute in conservative lung cancer surgery. Ann Thorac Surg. 2011;91(3):837-842. [DOI] [PubMed] [Google Scholar]
- 12.Martinod E, Paquet J, Dutau H, et al. . In vivo tissue engineering of human airways. Ann Thorac Surg. 2017;103(5):1631-1640. [DOI] [PubMed] [Google Scholar]
- 13.Martinod E, Aupècle B, Zegdi R, et al. . Segmentary replacement of the trachea with an aortic autograft [in French]. Presse Med. 1999;28(30):1638. [PubMed] [Google Scholar]
- 14.Martinod E, Zakine G, Fornes P, et al. . Metaplastic transformation of an aortic autograft into a tracheal tissue [in French]. C R Acad Sci III. 2000;323(5):455-460. [DOI] [PubMed] [Google Scholar]
- 15.Martinod E, Zegdi R, Zakine G, et al. . A novel approach to tracheal replacement. J Thorac Cardiovasc Surg. 2001;122(1):197-198. [DOI] [PubMed] [Google Scholar]
- 16.Martinod E, Seguin A, Pfeuty K, et al. . Long-term evaluation of the replacement of the trachea with an autologous aortic graft. Ann Thorac Surg. 2003;75(5):1572-1578. [DOI] [PubMed] [Google Scholar]
- 17.Martinod E, Seguin A, Holder-Espinasse M, et al. . Tracheal regeneration following tracheal replacement with an allogenic aorta. Ann Thorac Surg. 2005;79(3):942-948. [DOI] [PubMed] [Google Scholar]
- 18.Seguin A, Martinod E, Kambouchner M, et al. . Carinal replacement with an aortic allograft. Ann Thorac Surg. 2006;81(3):1068-1074. [DOI] [PubMed] [Google Scholar]
- 19.Seguin A, Radu D, Holder-Espinasse M, et al. . Tracheal replacement with cryopreserved, decellularized, or glutaraldehyde-treated aortic allografts. Ann Thorac Surg. 2009;87(3):861-867. [DOI] [PubMed] [Google Scholar]
- 20.Radu DM, Seguin A, Bruneval P, et al. . Bronchial replacement with arterial allografts. Ann Thorac Surg. 2010;90(1):252-258. [DOI] [PubMed] [Google Scholar]
- 21.McCulloch P, Altman DG, Campbell WB, et al. . No surgical innovation without evaluation. Lancet. 2009;374(9695):1105-1112. [DOI] [PubMed] [Google Scholar]
- 22.Claesson-Welsh L, Hansson GK; Royal Swedish Academy of Sciences . Tracheobronchial transplantation. Lancet. 2016;387(10022):942. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira da Silva JA. Ethical perspectives and ramifications of the Paolo Macchiarini case. Indian J Med Ethics. 2017;2(4):270-275. [DOI] [PubMed] [Google Scholar]
- 24.Weiss DJ, Elliott M, Jang Q, et al. . Tracheal bioengineering. Cytotherapy. 2014;16(12):1601-1613. [DOI] [PubMed] [Google Scholar]
- 25.Fabre D, Kolb F, Fadel E, et al. . Successful tracheal replacement in humans using autologous tissues. Ann Thorac Surg. 2013;96(4):1146-1155. [DOI] [PubMed] [Google Scholar]
- 26.Albain KS, Swann RS, Rusch VW, et al. . Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer. Lancet. 2009;374(9687):379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansour Z, Kochetkova EA, Santelmo N, et al. . Risk factors for early mortality and morbidity after pneumonectomy. Ann Thorac Surg. 2009;88(6):1737-1743. [DOI] [PubMed] [Google Scholar]
- 28.Seder CW, Wright CD, Chang AC, et al. . The society of thoracic surgeons general thoracic surgery database update on outcomes and quality. Ann Thorac Surg. 2016;101(5):1646-1654. [DOI] [PubMed] [Google Scholar]
- 29.Tan Q, Liu R, Chen X, et al. . Clinic application of tissue engineered bronchus for lung cancer treatment. J Thorac Dis. 2017;9(1):22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grillo HC. Tracheal replacement In: Grillo HC, ed. Surgery of the Trachea and Bronchi. Hamilton, ON: BC Decker Inc; 2004:839-854. [Google Scholar]
- 31.Chiang T, Pepper V, Best C, Onwuka E, Breuer CK. Clinical translation of tissue engineered trachea grafts. Ann Otol Rhinol Laryngol. 2016;125(11):873-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abouarab AA, Elsayed HH, Elkhayat H, et al. . Current solutions for long-segment tracheal reconstruction. Ann Thorac Cardiovasc Surg. 2017;23(2):66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omori K, Nakamura T, Kanemaru S, et al. . Regenerative medicine of the trachea. Ann Otol Rhinol Laryngol. 2005;114(6):429-433. [DOI] [PubMed] [Google Scholar]
- 34.Kanemaru S, Hirano S, Umeda H, et al. . A tissue-engineering approach for stenosis of the trachea and/or cricoid. Acta Otolaryngol Suppl. 2010;563(563)(suppl):79-83. [DOI] [PubMed] [Google Scholar]
- 35.Delaere P, Van Raemdonck D. Tracheal replacement. J Thorac Dis. 2016;8(suppl 2):S186-S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton NJ, Kanani M, Roebuck DJ, et al. . Tissue-engineered tracheal replacement in a child. Am J Transplant. 2015;15(10):2750-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinke M, Dally I, Friedel G, et al. . Host-integration of a tissue-engineered airway patch. Tissue Eng Part A. 2015;21(3-4):573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coraux C, Roux J, Jolly T, Birembaut P. Epithelial cell-extracellular matrix interactions and stem cells in airway epithelial regeneration. Proc Am Thorac Soc. 2008;5(6):689-694. [DOI] [PubMed] [Google Scholar]
- 39.Chouillard E, Chahine E, Allaire E, et al. . Small bowel in vivo bioengineering using an aortic matrix in a porcine model. Surg Endosc. 2016;30(11):4742-4749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods. Follow-up and outcome assessment