Abstract
The recent announcement of the production of new low-cost continuous glucose monitoring (CGM) sensors, the approval of marketed CGM sensors for making treatment decisions, and new reimbursement criteria have the potential to revolutionize CGM use. After briefly summarizing current CGM applications, we discuss how, in our opinion, these changes are expected to extend CGM utilization beyond diabetes patients, for example, to subjects with prediabetes or even healthy individuals. We also elaborate on how the integration of CGM data with other relevant information, for example, health records and other medical device/wearable sensor data, will contribute to creating a digital data ecosystem that will improve our understanding of the etiology and complications of diabetes and will facilitate the development of data analytics for personalized diabetes management and prevention.
Keywords: continuous glucose monitoring, data integration, diabetes management, precision medicine, proactive medicine
Current Status
Glucose monitoring in diabetes therapy has been revolutionized by the introduction of continuous glucose monitoring (CGM) sensors.1-4 Most of the CGM sensors currently on the market are minimally invasive systems that measure glucose concentrations in the subcutis by a needle electrochemical sensor, but other systems based on optical sensing technologies (including both implantable fluorescence sensors and noninvasive optical sensors) have also been proposed (see, eg, Lee et al5 and Chen et al6 for reviews of current and future trends in CGM sensor technologies).
The use of CGM sensors is very beneficial for glycemic control, particularly for reducing HbA1c and the frequency of hypoglycemic events,7-11 and is potentially cost effective in terms of the ratio between costs and quality-adjusted life-years.12-15 Nevertheless, the distribution of CGM sensors has remained limited to a small segment of the population with diabetes, mainly type 1 diabetes (T1D) subjects.16 The most recent data from T1D Exchange17 suggest that only 17%-25% of participants use CGM.18 The use of CGM is even less widespread among type 2 diabetes (T2D) subjects.19-21 A recent study conducted among T1D Exchange participants22 reported that the most common barriers to CGM use were related to high device costs and lack of insurance coverage,23,24 followed by the hassle of wearing devices and the dislike of having devices on the body; the most common reasons for stopping CGM use were cost, alarm fatigue, perceived sensor inaccuracy, and dislike of wearing devices.
However, the CGM usability is rapidly improving because of increases in sensor accuracy,25-32 extension of the sensor wear time, and decreases in calibration requirements.33-37 Moreover, some CGM devices received regulatory approval for nonadjunctive use; that is, the patient can make treatment decisions without using confirmatory self-monitoring of blood glucose (SMBG) measurements obtained by finger stick, except in certain situations (eg, rapidly changing glucose levels or CGM readings that do not match the patient symptoms).38-41 The nonadjunctive indication together with the improved CGM usability and increasing evidence for its clinical safety and efficacy has stimulated new policies for system reimbursement.12 For instance, Medicare recently announced criteria for Dexcom G5 Mobile (Dexcom, San Diego, CA) reimbursement to T1D and T2D patients on intensive insulin therapy.42 These important achievements will likely contribute to expanding CGM use in the next years.
The increasing availability of CGM sensors is also stimulating the development of several interesting applications, for example, programs for retrospective CGM data visualization,43 mobile apps integrating CGM data with other relevant information for diabetes management (eg, medications, food intake and exercise),44-46 decision support system prototypes providing physicians with personalized recommendations for therapy adjustment,47-49 and algorithms for automatic CGM-based regulation of basal insulin delivery.50 Other interesting projects underway include the development of algorithms for automatic optimization of insulin dosing parameters,51-53 and advisory applications for smart insulin pens and artificial pancreas systems.54 Table 1 summarizes the current CGM-based applications.
Table 1.
Summary of the Current CGM-Based Applications and Possible Future Applications, Both for Data Integration and for Utilization of Such Data Integration.
| Application | Benefits | |
|---|---|---|
| Current applications | Programs for CGM data download and retrospective analysis | Retrospective visualization of daily glucose patterns and statistical summaries of glycemic excursions for improved therapeutic decision making |
| Decision support systems for caregivers | Assistance for health care professionals with the periodic adjustment of patient-specific insulin therapy parameters | |
| Mobile apps for diabetes management | Integration of data for daily diabetes management for visualization in portable devices | |
| Online therapy optimization strategies | Automatic adjustment of patient-specific insulin therapy parameters | |
| Automatic insulin delivery algorithms | Automatic regulation of insulin delivery in fully or hybrid closed-loop systems | |
| Future applications for data integration | Platform for the integration of diabetes management data collected by medical devices or mobile apps | Potential new insight into the physiological/behavioral patterns associated with hypo/hyperglycemic events |
| Software for the integration of clinical data | Identification of clinical patterns associated with the development of diabetes, its comorbidities and its complications | |
| Programs for quality of life and health survey data integration | Potential identification of socioeconomic, behavioral and health risk factors for the onset of diabetes and its complications | |
| Applications for wearable sensor data integration | Monitoring of signals/indicators (eg, physical activity and stress) useful for potentially improving therapeutic decision making | |
| Georeferencing programs allowing neighborhood and environmental data integration | Potential identification of environmental and socioeconomic risk factors for the onset of diabetes and its complications | |
| Future applications taking advantage of data integration | Personalized patient decision support systems for daily diabetes management | Personalized recommendations for daily diabetes management, including smart hypo/hyperglycemic alerts and smart bolus/carbohydrate advisors |
| Personalized decision support systems for subjects without diabetes | Personal health risk assessment, subject education about health risk factors and recommendations for healthy behaviors | |
| Gamified health apps | Subject engagement in a collaborative data collection process | |
| Smart decision support systems for caregivers | Assistance for health care professionals in personalized therapy optimization, identification of high-risk patients and optimal visit scheduling | |
| Public health big-data analytics | Identification of high-risk populations, proactive monitoring of subjects, assistance in the design and assessment of intervention plans to reduce health risks |
The purpose of this commentary is not to exhaustively review current and developing CGM technologies and applications but rather to discuss how, in our opinion, the current trends in CGM technological development are expected to further increase CGM utilization in the future, possibly targeting not only subjects with diabetes but also people with prediabetes or even healthy individuals (Section Future Developments in CGM Technology to Target New Population and Markets ). We will also propose a perspective on how the integration of CGM data with other relevant patient/environment data could open the way for the development of applications for personalized diabetes management and prevention within the context of building a learning health care system (Section Building a Digital Ecosystem of Diabetes and Health Data ).
Future Developments in CGM Technology to Target New Populations and Markets
Current research in CGM technology is expected to further expand the adoption of CGM beyond the T1D population. Indeed, some of the leading companies in CGM manufacturing are working on developing new products that were announced to be smaller, less expensive and factory calibrated to target not only people with T1D but also the much wider market of people with T2D. Reduced cost and factory calibration are two of the most innovative features of the Freestyle Libre Flash (Abbott Diabetes Care, Alameda, CA), launched by Abbott in 2014, although this system does not provide continuous glucose and trend data in real time but instead requires the patient to place the receiver close to the sensor for data visualization.55 The Freestyle Libre Flash costs approximately one-half to one-third of the price of other commercial CGM systems23 (this cost difference is market dependent) and is now being used by more than 400,000 people across more than 40 countries.56 In 2015, Dexcom (San Diego, CA) and Verily Life Sciences (Mountain View, CA) announced a collaboration for developing next-generation CGM products designed to be miniaturized, factory calibrated, inexpensive and less obtrusive than current systems.57 Similarly, in 2016, Medtronic and Qualcomm Life (San Diego, CA) announced a collaboration to jointly develop a future generation of CGM sensors for T2D; these sensors are supposed to be single use, as well as smaller, easier to use and more affordable than current sensors.58 These systems could all become accessible not only to people with diabetes but also to subjects affected by prediabetes or obesity, thus transitioning from reactive to proactive health care.
Other emerging companies, such as Glucovation (San Diego, CA)59 and Nemaura Medical (Loughborough, United Kingdom),60 are working on novel low-cost CGM products devised not only for the medical market but also for the consumer market as tools to track health and well-being. Possible users for these applications are people in weight-loss programs or athletes, for whom a CGM system could be beneficial for monitoring metabolism and making informed nutritional decisions, although evidence for the benefits of CGM use in healthy individuals has not yet been reported.
Building a Digital Ecosystem of Diabetes and Health Data
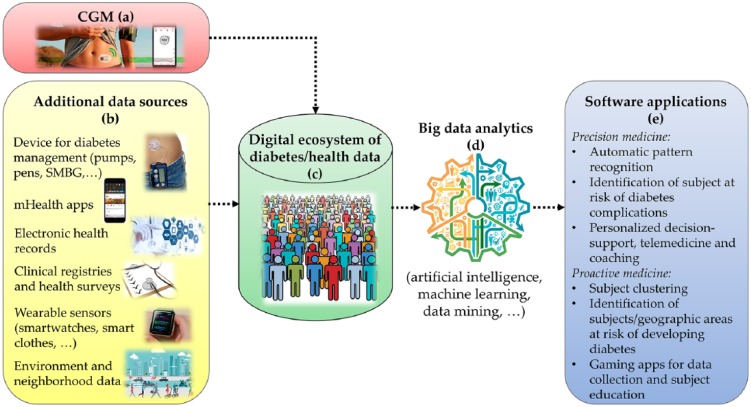
The availability of smartphone- and internet-connected CGM sensors,61,62 together with the extension of their use to a larger population, will allow the creation of large databases in which CGM data (Figure 1a) are integrated with several other data sources (Figure 1b). For example, CGM data can be merged with information provided by other medical devices for diabetes, for example, SMBG devices, insulin pumps and pens, and the variables collected by mobile apps for diabetes management,63-67 including information on medications, diet and exercise. The integration of other clinical data sources, such as clinical registries,17 electronic health records, prescription registries and biomarkers collected in laboratory tests, will provide important clinical contextualization for CGM data. The integration with clinical data would facilitate the design of new strategies for diabetes management, improving the engagement of patients with their clinical care data,68 and the investigation of clinical patterns in the development of diabetes and its comorbidities and complications.69 Information gathered by quality of life and health surveys70-72 will further enrich the database, providing useful insights into psychosocial and economic factors influencing the behavior (eg, diet and exercise habits) and, as a consequence, health (eg, diabetes complications) of subjects.
Figure 1.
Possible data sources (a) that can be integrated with CGM data (b) to create a digital ecosystem of diabetes and health data (c) and possible applications (e) for precision and proactive medicine relying on big data analytics (d).
CGM data can also be combined with measurements collected by several other wearable sensors, such as currently available smartwatches, which can track heart rate, steps/motion, energy consumption, sleep and biometric parameters such as weight and body mass index,73,74 or with measurements collected by new prototypes that can even measure blood oxygenation, skin temperature, skin blood perfusion, respiratory rate, cutaneous sweat, stress and exposure to addictive substances.75,76 A new generation of smart clothes is also entering the market that can provide biometrics and activity data from textile-sensing technologies.77,78 Finally, new wearable and miniaturized sensors currently under development will provide real-time measurements of biomarkers with potential clinical utility based on tear, saliva, sweat, or breath analysis.79
The connection of CGM and other wearable sensors to smartphones equipped with GPS connectivity allows data georeferencing. CGM data can thus be integrated with information about the quality of the living environment (eg, pollution data collected by air quality sensors) and neighborhood (eg, presence of parks and healthy food shops). The geolocalization of CGM data will allow a better understanding of the environmental factors influencing the onset of metabolic disorders and their course.80,81 In one study employing CGM geolocalization,82 CGM, insulin pump and exercise tracker data were geolocalized to characterize patients with diabetes during exercise. Other studies in which health factors were geolocalized and connected to environmental/neighborhood factors are the Seattle Obesity Study83 (obesity in relation to food environment, diet quality and disparities) and MOSAIC84 (correlation between T2D glycemic control and air pollution maps). Interesting scenarios may be revealed by other projects underway, such as the Kavli HUMAN Project,85 which aims to understand how biology, behavior, and environment affect health and well-being in New York City, and PULSE,86 which investigates novel insights into the relationship between environmental/behavioral factors and risk of developing T2D and asthma in urban environments.
The integration of CGM data with this variety of other patient/environment data is certainly not straightforward, and it would require the development of ad hoc software applications (summarized in Table 1) allowing interoperability between the different data collection systems. Such data integration will contribute to the generation of a digital ecosystem of diabetes data87 or more general health and well-being data (Figure 1c); these data can be used to improve our understanding of the biological, behavioral and environmental factors driving the development of diabetes and its complications. This integration will be part of a wider trend that is already observed in other medical areas: to create learning health care systems exploiting data analytics to extract new medical knowledge that cannot be discovered by relying on a single source of information.88-90 In particular, diabetes data integration will offer the opportunity to design data analytics (Figure 1d) for personalized diabetes management and prevention solutions (Figure 1e).
The integration of CGM data with other information relevant to diabetes management, for example, insulin administrations, diet, exercise and stress, will provide new insight into the physiological/behavioral patterns associated with hypo/hyperglycemic events, which are not possible to identify by relying on a single data source. This insight would allow the development of telemedicine services91,92 and personalized decision support systems, which can implement, for example, real-time algorithms to generate smart hypo/hyperglycemia alerts and smart bolus/carbohydrate advisors, with the aim of tailoring the therapy to the personal exercise habits, stress triggers and circadian rhythms of the patient.93 Caregivers could also benefit from the development of decision support systems for health care providers, such as dashboards implementing automatic pattern recognition to identify patients with poor glycemic control and models predicting diabetes complications to identify patients at high risk of developing diabetes-related complications.94 Via patient risk stratification, tools for optimal visit scheduling, for example, giving higher priority to high-risk patients, can be designed. Moreover, software providing personalized therapy adjustment recommendations can be useful to assist the caregiver in revising therapy for the patient. Such applications can exploit not only the information available for a specific patient but also the evidence obtained from patients with similar characteristics.
The extension of CGM technology beyond diabetes will enable the development of software solutions for proactive medicine. For example, mobile decision support systems could be designed not only for people with diabetes but also for healthy individuals as tools to educate subjects about their personal risk factors for diabetes (and other health conditions) and to promote positive behavioral changes. In addition, software applications to identify subjects at high risk of developing diabetes can be designed by implementing models for predicting the onset of diabetes;94 these models currently include mostly demographics, biometrics and blood test biomarkers as risk factors but can be potentially improved by the incorporation of CGM-based glucose variability indices,95,96 as well as behavioral, socioeconomic, and environmental factors. This development will allow health care agencies to devise targeted prevention and screening plans for diabetes, thus promoting the efficient use of resources. The geolocalization of data will allow public health analysts to identify geographic areas where the population is at high risk of diabetes and more efficiently plan initiatives to reduce the relevant risk factors.97
However, several important challenges related to data integration need to be faced.88 The collection of large amounts of individual data will not be trivial, and strategies to engage the subjects in a collaborative data collection process need to be designed, for example, the use of gamified apps.98-100 In addition, data security and veracity cannot be neglected.101 The necessity of handling the copious varied and complex data will require the use of big data analytics tools based on artificial intelligence techniques80,102,103 to overcome the limitations of traditional hypothesis-driven statistical approaches. Real-time applications will also need sophisticated data-storage infrastructures to easily pull and retrieve information. Furthermore, effective integration of signals that have temporal information, such as CGM time series, with static data that do not always provide the true time context, such as health records, can be challenging.89 Last but not least, evidence about the clinical effectiveness and safety of such big data analytics, as well as proof of their clinical utility, must be provided to support employment of these applications in health care.88
Conclusion
The use of CGM sensors will certainly grow significantly in the next years in response to improvements in accuracy, regulatory approval for making treatment decisions and expansions of reimbursement criteria. Moreover, cost decreases will extend the CGM market to new populations, possibly including subjects with prediabetes and even healthy people, as a part of well-being programs, although the effectiveness of CGM use in these populations has not yet been proved.
The availability of a vast amount of CGM data will contribute to new insights into diabetes onset and progression mechanisms, especially when these data are integrated with data from other sources, for example, wearable sensors and health records. The thus-obtained digital ecosystem of diabetes and health data will enable the development of big data analytics tools for precision and proactive medicine, for example, decision support systems, proactive patient monitoring systems and public health applications. Nevertheless, several delicate issues, such as technical challenges (from security to data volume, velocity, veracity, and variety) and the need to provide evidence of their clinical safety and utility, must be carefully addressed.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; T1D, type 1 diabetes; T2D, type 2 diabetes; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Giacomo Cappon  https://orcid.org/0000-0003-4358-9268
https://orcid.org/0000-0003-4358-9268
References
- 1. Gifford R. Continuous glucose monitoring: 40 years, what we’ve learned and what’s next. Chemphyschem. 2013;14:2032-2044. [DOI] [PubMed] [Google Scholar]
- 2. Lodwig V, Kulzer B, Schnell O, Heinemann L. Current trends in continuous glucose monitoring. J Diabetes Sci Technol. 2014;8:390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kropff J, DeVries JH. Continuous glucose monitoring, future products, and update on worldwide artificial pancreas projects. Diabetes Technol Ther. 2016;18(suppl 2):s53-s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappon G, Acciaroli G, Vettoretti M, Facchinetti Sparacino G. Wearable continuous glucose monitoring sensors: a revolution in diabetes treatment. Electronics. 2017;6:65. [Google Scholar]
- 5. Lee H, Hong YJ, Baik S, Hyeon T, Kim DH. Enzyme-based glucose sensor: from invasive to wearable device. Adv Healthc Mater. 2018;7(8):1701150. [DOI] [PubMed] [Google Scholar]
- 6. Chen C, Zhao X-L, Li Z-H, Zhu Z-G, Qian S-H, Flewitt AJ. Current and emerging technology for continuous glucose monitoring. Sensors (Basel). 2017;17:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 8. Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44-50. [DOI] [PubMed] [Google Scholar]
- 9. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379-387. [DOI] [PubMed] [Google Scholar]
- 11. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371-378. [DOI] [PubMed] [Google Scholar]
- 12. Graham C. Continuous glucose monitoring and global reimbursement: an update. Diabetes Technol Ther. 2017;19(suppl 3):s60-s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang ES, O’Grady M, Basu A, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33:1269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roze S, Smith-Palmer J, Valentine WJ, et al. Long-term health economic benefits of sensor-augmented pump therapy versus continuous subcutaneous insulin infusion alone in type 1 diabetes: a UK perspective. J Med Econ. 2016;19:236-242. [DOI] [PubMed] [Google Scholar]
- 15. Fonda SJ, Graham C, Munakata J, Powers JM, Price D, Vigersky RA. The cost-effectiveness of real-time continuous glucose monitoring (RT-CGM) in type 2 diabetes. J Diabetes Sci Technol. 2016;10:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slattery D, Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(suppl 2):s55-s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Type 1 diabetes clinic registry. Unitio; 2017. Available at: https://t1dexchange.org/pages/resources/clinic-registry/. Accessed January 15, 2018.
- 18. Forlenza GP, Argento NB, Laffel LM. Practical considerations on the use of continuous glucose monitoring in pediatrics and older adults and nonadjunctive use. Diabetes Technol Ther. 2017;19(suppl 3):s13-s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(suppl 2):s2-3-s2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications. 2017;31:280-287. [DOI] [PubMed] [Google Scholar]
- 21. Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19(suppl 2):s4-s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heinemann L, DeVries JH. Reimbursement for continuous glucose monitoring. Diabetes Technol Ther. 2016;18(suppl 2):s248-s252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heinemann L, Franc S, Phillip M, et al. Reimbursement for continuous glucose monitoring: a European view. J Diabetes Sci Technol. 2012;6:1498-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bailey TS, Ahmann A, Brazg R, et al. Accuracy and acceptability of the 6-day Enlite continuous subcutaneous glucose sensor. Diabetes Technol Ther. 2014;16:277-283. [DOI] [PubMed] [Google Scholar]
- 26. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGarraugh G, Brazg R, Weinstein R. FreeStyle Navigator continuous glucose monitoring system with TRUstart algorithm, a 1-hour warm-up time. J Diabetes Sci Technol. 2011;5:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey TS. Clinical implications of accuracy measurements of continuous glucose sensors. Diabetes Technol Ther. 2017;19(suppl 2):s51-s54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ólafsdóttir AF, Attvall S, Sandgren U, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor Freestyle Libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faccioli S, Del Favero S, Visentin R, et al. Accuracy of a CGM sensor in pediatric subjects with type 1 diabetes. Comparison of three insertion sites: arm, abdomen, and gluteus. J Diabetes Sci Technol. 2017;11(6):1147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boscari F, Galasso S, Facchinetti A, et al. FreeStyle Libre and Dexcom G4 Platinum sensors: accuracy comparisons during two weeks of home use and use during experimentally induced glucose excursions. Nutr Metab Cardiovasc Dis. 2018;28(2):180-186. [DOI] [PubMed] [Google Scholar]
- 33. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G, Cobelli C. From two to one per day calibration of Dexcom G4 Platinum by a time-varying day-specific Bayesian prior. Diabetes Technol Ther. 2016;18:472-479. [DOI] [PubMed] [Google Scholar]
- 35. Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G, Cobelli C. Reduction of blood glucose measurements to calibrate subcutaneous glucose sensors: a Bayesian multi-day framework. IEEE Trans Biomed Eng. 2018;65(3):587-595. [DOI] [PubMed] [Google Scholar]
- 36. Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G. Toward calibration-free continuous glucose monitoring sensors: Bayesian calibration approach applied to next-generation Dexcom technology. Diabetes Technol Ther. 2018;20:59-67. [DOI] [PubMed] [Google Scholar]
- 37. Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G. Calibration of minimally invasive continuous glucose monitoring sensors: state-of-the-art and current perspectives. Biosensors (Basel). 2018;8:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castle JR, Jacobs PG. Nonadjunctive use of continuous glucose monitoring for diabetes treatment decisions. J Diabetes Sci Technol. 2016;10:1169-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. FDA approves first continuous glucose monitoring system for adults not requiring blood sample calibration. US Food and Drug Administration; 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm577890.htm Accessed January 15, 2018.
- 40. US Food and Drug Administration approval of the Dexcom G5 Mobile premarket approval application. US Food and Drug Administration; 2016. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf12/P120005S041a.pdf. Accessed January 15, 2018.
- 41. Edelman SV. Regulation catches up to reality. J Diabetes Sci Technol. 2017;11:160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. CGM Rulings. Ruling No. [CMS-1682-R]. Centers for Medicare & Medicaid Services; 2017. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Rulings/Downloads/CMS1682R.pdf. Accessed March 14, 2018. [PubMed]
- 43. Scheiner G. CGM retrospective data analysis. Diabetes Technol Ther. 2016;18(suppl 2):s2-14-s2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. mySugr App. mySugr; 2017. Available at: https://mysugr.com/apps/. Accessed January 16, 2018.
- 45. One Drop. Informed Data System; 2017. Available at: http://onedrop.today. Accessed January 16, 2018.
- 46. Glooko mobile app. Glooko; 2017. Available at: https://www.glooko.com/diabetes-coaching/people-with-diabetes/. Accessed March 14, 2018.
- 47. Salzsieder E, Augstein P, Vogt L, et al. Telemedicine-based KADIS® combined with CGMS™ has high potential for improving outpatient diabetes care. J Diabetes Sci Technol. 2007;1:511-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salzsieder E, Augstein P. The Karlsburg Diabetes Management System: translation from research to eHealth application. J Diabetes Sci Technol. 2011;5:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. DreaMed Diabetes receives CE mark for platform for the management of type 1 Diabetes. PRNewswire; 2018. Available at: https://www.prnewswire.com/news-releases/dreamed-diabetes-receives-ce-mark-for-platform-for-the-management-of-type-1-diabetes-674136813.html. Accessed March 14, 2018.
- 50. Stone JY, Haviland N, Bailey TS. Review of a commercially available hybrid closed-loop insulin-delivery system in the treatment of type 1 diabetes. Ther Deliv. 2018;9:77-87. [DOI] [PubMed] [Google Scholar]
- 51. Reddy M, Pesl P, Xenou M, et al. Clinical safety and feasibility of the Advanced Bolus Calculator for type 1 diabetes based on case-based reasoning: a 6-week nonrandomized single-arm pilot study. Diabetes Technol Ther. 2016;18:487-493. [DOI] [PubMed] [Google Scholar]
- 52. Schiavon M, Dalla Man C, Cobelli C. Insulin sensitivity index-based optimization of insulin to carbohydrate ratio: in silico study shows efficacious protection against hypoglycemic events caused by suboptimal therapy. Diabetes Technol Ther. 2018;20:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cappon G, Vettoretti M, Marturano F, Facchinetti A, Sparacino G. A neural-network-based approach to personalize insulin bolus calculation using continuous glucose monitoring. J Diabetes Sci Technol. 2018;12:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. TypeZero. TypeZero Technologies; 2018. Available at: http://typezero.com/#products. Accessed January 16, 2018.
- 55. FreeStyle Libre Flash Glucose Monitoring System. Abbott; 2017. Available at: https://freestylediabetes.co.uk/our-products/freestyle-libre. Accessed January 16, 2018. [PubMed]
- 56. Abbott’s revolutionary continuous glucose monitoring system, FreeStyle® Libre, now available to Medicare patients. PRNewswire; 2018. Available at: https://www.prnewswire.com/news-releases/abbotts-revolutionary-continuous-glucose-monitoring-system-freestyle-libre-now-available-to-medicare-patients-300577201.html. Accessed March 14, 2018.
- 57. DexCom to collaborate with the Life Sciences team at Google on the development of breakthrough technologies to change the future of diabetes management. Dexcom; 2015. Available at: http://www.prnewswire.com/news-releases/dexcom-to-collaborate-with-the-life-sciences-team-at-google-on-the-development-of-breakthrough-technologies-to-change-the-future-of-diabetes-management-300126661.html. Accessed January 16, 2018.
- 58. Medtronic and Qualcomm collaborate to aim to improve care and health outcomes for people with type 2 diabetes. Medtronic; 2016. Available at: http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=2172203. Accessed January 16, 2018.
- 59. Hoskins M. Glucovation: A CGM Sensor for Non-Diabetics? 2014. Available at: https://www.healthline.com/diabetesmine/glucovation-a-cgm-sensor-for-non-diabetics#1. Accessed January 16, 2018.
- 60. SugarBEAT®. SugarBEAT; 2017. Available at: http://sugarbeat.com. Accessed January 16, 2018.
- 61. The Minimed® Connect Accessory and App now available for Android!. Medtronic; 2016. Available at: https://www.medtronicdiabetes.com/loop-blog/minimed-connect-accessory-app-now-available-android/. Accessed January 16, 2018.
- 62. FDA approves Dexcom G4 Platinum continuous glucose monitoring system with share. Dexcom; 2015. Available at: https://www.dexcom.com/news/fda-approves-dexcom-g4-platinum-continuous-glucose-monitoring-system-share. Accessed January 16, 2018.
- 63. Klonoff DC. The current status of mHealth for diabetes: will it be the next big thing? J Diabetes Sci Technol. 2013;7:749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. El-Gayar O, Timsina P, Nawar N, Eid W. Mobile applications for diabetes self-management: status and potential. J Diabetes Sci Technol. 2013;7:247-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arnhold M, Quade M, Kirch W. Mobile applications for diabetics: a systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older. J Med Internet Res. 2014;16:e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shah VN, Garg SK. Managing diabetes in the digital age. Clin Diabetes Endocrinol. 2015; 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brzan PP, Rotman E, Pajnkihar M, Klanjsek P. Mobile applications for control and self management of diabetes: a systematic review. J Med Syst. 2016;40:210. [DOI] [PubMed] [Google Scholar]
- 68. Patel V, Reed ME, Grant RW. Electronic health records and the evolution of diabetes care: a narrative review. J Diabetes Sci Technol.2015;9:676-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pantalone KM, Hobbs TM, Wells BJ, et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res Care. 2015;3:e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schatz BR. National surveys of population health: big data analytics for mobile health monitors. Big Data. 2015;3:219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. National Health and Nutrition Examination Survey. CDC/National Center for Health Statistics; 2017. Available at: https://www.cdc.gov/nchs/nhanes/. Accessed January 16, 2018.
- 72. Behavioral Risk Factor Surveillance System. CDC/National Center for Health Statistics; 2017. Available at: https://www.cdc.gov/brfss/. Accessed January 16, 2018.
- 73. Fitbit. Fitbit; 2018. Available at: https://www.fitbit.com/home. Accessed January 16, 2018.
- 74. Apple Watch. Apple; 2018. Available at: https://www.apple.com/watch/. Accessed January 16, 2018.
- 75. Ertin E, Stohs N, Kumar S, et al. AutoSense: unobtrusively wearable sensor suite for inferring the onset, causality, and consequences of stress in the field. In: Proceedings of the 9th ACM Conference on Embedded Networked Sensor Systems Seattle, WA, 2011: 274-287. [Google Scholar]
- 76. Biovotion. Biovotion; 2018. Available at: http://www.biovotion.com. Accessed January 16, 2018.
- 77. Castano LM, Flatau AB. Smart fabric sensors and e-textile technologies: a review. Smart Mater Struct. 2014;23:053001. [Google Scholar]
- 78. Sawh M. The best smart clothing: from biometric shirts to contactless payment jackets. 2017. Available at: https://www.wareable.com/smart-clothing/best-smart-clothing. Accessed January 16, 2018.
- 79. Tricoli A, Nasiri N, Saya D. Wearable and miniaturized sensor technologies for personalized and preventive medicine. Adv Funct Mater. 2017;27:1605271. [Google Scholar]
- 80. Bellazzi R, Dagliati A, Sacchi L, Segagni D. Big data technologies: new opportunities for diabetes management. J Diabetes Sci Technol. 2015;9:1119-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Drewnowski A, Kawachi I. Diets and health: how food decisions are shaped by biology, economics, geography, and social interactions. Big Data. 2015;3:193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wendel J, Heintzman ND. Open-source framework for integrating and visualising multimodal data from geolocation, diabetes and exercise devices. Diabetes Technol Ther. 2013;15:A117-A118. [Google Scholar]
- 83. Seattle Obesity Study. University of Washington Center for Public Health Nutrition; 2018. Available at: http://depts.washington.edu/uwcphn/work/cor/diet_disparities.shtml. Accessed January 16, 2018.
- 84. Dagliati A, Marinoni A, Cerra C, et al. Integration of administrative, clinical, and environmental data to support the management of type 2 diabetes mellitus: from satellites to clinical care. J Diabetes Sci Technol. 2016;10:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. The HUMAN Project. The Human Project; 2018. Available at: https://thehumanproject.org/about/. Accessed January 16, 2018.
- 86. PULSE. PULSE project; 2018. Available at: http://www.project-pulse.eu/. Accessed March 22, 2018.
- 87. Heintzman ND. A digital ecosystem of diabetes data and technology: services, systems, and tools enabled by wearables, sensors and apps. J Diabetes Sci Technol. 2016;10:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rumsfeld JS, Joynt KE, Maddox TM. Big data analytics to improve cardiovascular care: promise and challenges. Nat Rev Cardiol. 2016;13:350-359. [DOI] [PubMed] [Google Scholar]
- 89. Belle A, Thiagarajan R, Soroushmehr SMR, Navidi F, Beard DA, Najarian K. Big data analytics in healthcare. BioMed Research International. 2015;2015:370194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dagliati A, Sacchi L, Tibollo V, et al. A dashboard-based system for supporting diabetes care. J Am Med Inform Assoc. 2018;25(5):538-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Klonoff DC. Telemedicine for diabetes: current and future trends. J Diabetes Sci Technol. 2016;10:3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database of Sys Rev. 2015;9:CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Klonoff DC. Precision medicine for managing diabetes. J Diabetes Sci Technol. 2015;9:3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cichosz SL, Johansen MD, Hejlesen O. Toward big data analytics: review of predictive models in management of diabetes and its complications. J Diabetes Sci Technol. 2016;10:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Acciaroli G, Sparacino G, Hakaste L, et al. Diabetes and prediabetes classification using glycemic variability indices from continuous glucose monitoring data. J Diabetes Sci Technol. 2018;12:105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Longato E, Acciaroli G, Facchinetti A, et al. Glycaemic variability-based classification of impaired glucose tolerance vs. type 2 diabetes using continuous glucose monitoring data. Comput Biol Med. 2018;96:141-146. [DOI] [PubMed] [Google Scholar]
- 97. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA 2013;309:1351-1352. [DOI] [PubMed] [Google Scholar]
- 98. Ruggiero L. Diabetes prevention and management: what does a serious game have to do with it? Games Health J. 2015;4:333-334. [DOI] [PubMed] [Google Scholar]
- 99. de Ridder M, Kim J, Jing Y, Khadra M, Nanan R. A systematic review on incentive-driven mobile health technology: as used in diabetes management. J Telemed Telecare. 2017;23:26-35. [DOI] [PubMed] [Google Scholar]
- 100. Theng Y, Lee JW, Patinadan PV, Foo SS. The use of videogames, gamification, and virtual environments in the self-management of diabetes: a systematic review of evidence. Games Health J. 2015;4:352-361. [DOI] [PubMed] [Google Scholar]
- 101. Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kavakiotis I, Tsave O, Salifoglou A, Maglaveras N, Vlahavas I, Chouvarda I. Machine learning and data mining methods in diabetes research. Comput Struct Biotechnol J. 2017;15:104-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rigla M, García-Sáez G, Pons B, Hernando ME. Artificial intelligence methodologies and their application to diabetes. J Diabetes Sci Technol. 2018;12:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]