Abstract
The eosinophilic gastrointestinal diseases (EGID) represent disorders of the GI tract that result from the local infiltration and aberrant activity of eosinophils and other immune cells. Eosinophilic esophagitis is the most well-characterized EGID and is defined by the presence of intraepithelial eosinophils in the esophagus (≥15 eosinophils per high powered field) and clinical symptoms associated with esophageal dysfunction. The other EGID are rare and lack strong data regarding pathogenesis and management. The incidence and prevalence of EoE are increasing, and EoE is now a major cause of upper GI morbidity. Management is multidisciplinary, with collaboration between gastroenterologists, allergists, pathologists, and dieticians, and is aimed at amelioration of symptoms and prevention of long-term complications such as esophageal stricture. Treatment options for EoE include proton pump inhibitors, swallowed topical corticosteroids, and elimination diets. Esophageal dilation is used when esophageal strictures or fibrostenotic changes are present. Additional therapies targeting eosinophils and other mediators of Th2 inflammation are under development and are promising. Treatment options for other EGIDs typically involve corticosteroids or dietary elimination.
Keywords: eosinophilic esophagitis, eosinophilic gastrointestinal disease
Introduction
Eosinophilic gastrointestinal diseases (EGID), including eosinophilic esophagitis (EoE), gastritis (EG), gastroenteritis (EGE), and colitis (EC), are characterized by GI tract eosinophilia and symptoms that cause significant morbidity in children and adults. EGID definitions and management are evolving as more is learned about the etiology and natural history of these disorders. EGIDs are chronic disorders that are thought to develop in response to an immunogenic trigger. The diagnosis of EGID is contingent upon exclusion of other disorders associated with eosinophilia. This review focuses on the current clinical guidelines, controversial topics, and emerging therapies for the best characterized and most common EGID, EoE. In addition, a brief discussion of what is known, and unknown, about EG, EGE, and EC is presented.
What is known about EGID: EoE
EoE Diagnosis
EoE was recognized as a distinct clinical entity in the early 1990s (1, 2), and since then incidence and prevalence have markedly increased (3). Initial diagnosis and management guidelines were released in 2007 (4) and updated in 2011 (5), 2013 (6), and 2017 (7). Early definitions of EoE required symptoms of esophageal dysfunction and esophageal eosinophilia on biopsy (at least 15 eosinophils per high-power field, eos/hpf), not otherwise explained by a potential competing cause of eosinophilia, be present after a high-dose proton pump inhibitor (PPI) trial. Significant research advances over the past five years, especially those related to the understanding of the role of PPIs (8), led a European task force to eliminate the requirement for a PPI trial. Therefore, those with esophageal eosinophilia who respond to PPI therapy (PPI-responsive esophageal eosinophilia, PPI-REE) exist on the continuum of EoE. This decision was re-emphasized during a recent international consensus conference (9) based on substantial evidence of overlap between PPI-REE and EoE in terms of clinical symptoms, endoscopic findings, pathophysiology, and molecular profiles; updated and operationalized diagnostic guidelines will be released this year.
EoE Pathophysiology
EoE is a non-IgE-mediated allergic immune response. It occurs more often in male patients (3:1 male:female), and its highest prevalence is currently in the 3rd – 5th decades of life (3). Clinical symptoms differ in young children compared to older children and adults, partially due to the progression of EoE from an inflammatory to fibrostenotic phenotype over time (10–12). Young children have feeding difficulties, reflux-like symptoms, vomiting, abdominal pain, food refusal, and failure to thrive (13, 14); older children and adults experience dysphagia, heartburn, chest discomfort, exercise-induced chest pain, and food impaction (15–20).
The natural history of EoE appears to progress from an inflammatory to fibrostenotic phenotype (11, 12, 21–27), but subepithelial fibrosis is detected even in children, suggesting that esophageal remodeling occurs early in the disease process (11, 28). Esophageal remodeling may also contribute to esophageal dysmotility in EoE (27, 29–36). Patients with EoE demonstrate impaired esophageal epithelial barrier integrity (27, 37–41) and increased esophageal sensitivity to acid (42) and local allergen exposure (43). Treatment of esophageal inflammation, either with topical corticosteroids or elimination diets, likely prevents long-term fibrostenotic changes and improves impaired barrier integrity in patients with EoE (39, 41, 44–53). Evidence does not currently support EoE as a premalignant lesion (54), although there are small case studies suggesting an association between esophageal eosinophilia and granular cell tumors (55–59).
The prevalence of atopic disease such as allergic rhinitis, bronchial asthma, IgE-mediated food allergies, and eczema, is far higher in patients with EoE than the general population (60), suggesting a prominent role for the Allergist in treatment of these patients’ allergic comorbidities (61). However, the definition of “food allergy” varies widely across studies (60, 62), which highlights a fundamental misunderstanding of the nature and role of skin prick testing (SPT) and serum food-specific IgE testing in diagnosing IgE-mediated food allergies. Allergists are uniquely qualified to determine “probable”, “possible”, and “unlikely” culprit food allergens from the clinical history and epidemiology of food allergies, which then informs subsequent testing. The 2011 guidelines recommended patients with EoE who previously demonstrated sensitization to a particular food based on allergy testing undergo office-based oral challenge prior to reintroduction of that food into their diet (5), as there are case reports of patients with EoE who develop an IgE-mediated food allergy after avoiding their EoE trigger food (63–67). On the other hand, there are case reports of patients with IgE-mediated food allergy on oral food immunotherapy who develop EoE, although the presence of EoE prior to starting this immunotherapy is not known (68, 69). A recent literature search estimated the prevalence of EoE in patients during oral immunotherapy for IgE-mediated food allergy to be approximately 5% (70). EoE itself is understood as a non-IgE-mediated food allergy; therefore, allergy testing to guide treatment is controversial and discussed below under the Elimination Diet section.
There is evidence suggesting that exposure to aeroallergens may contribute to the pathogenesis of EoE in some individuals, although it is unclear whether aeroallergens alone can cause EoE or if exposure can modify disease in certain patients with food-triggered EoE (71). In a retrospective chart review Gita Ram, et al., identified a subset of patients with EoE and aeroallergen sensitization whose EoE symptoms and histopathologic findings worsened during the season corresponding to their specific aeroallergen sensitization in the absence of dietary or treatment changes (72, 73). Interestingly, there are case reports of patients on oral or sublingual aeroallergen immunotherapy who develop EoE that subsequently resolves with the cessation of the immunotherapy (74–76). It is not known whether EoE disease activity improves in these patients with more aggressive treatment of allergic rhinitis and with counseling on allergen avoidance, but one intriguing case report showed resolution of EoE in one patient after two years of dust mite oral immunotherapy (77). Therefore, the Allergist’s role in management of EoE is multifaceted.
EoE Monitoring
Several validated scoring systems measure symptoms and disease activity in EoE, though most are currently being used primarily for research purposes (78–81). Scoring systems focused on tracking changes in endoscopy/histology findings are being increasingly used in clinical practice, including the EoE Endoscopic Reference Score (EREFS; Figure 1) (82, 83). This classification is increasing being used in endoscopic reports and represents an important way to monitor endoscopic severity over time.
Figure 1.
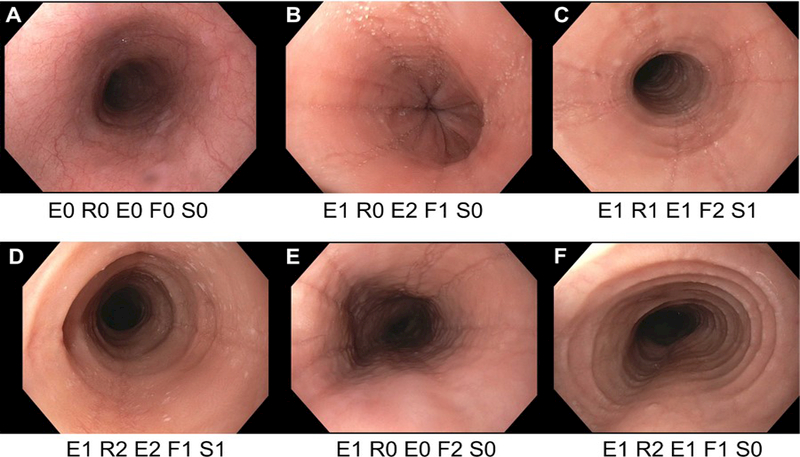
A range of endsocopic findings in EoE with application of the EREFS classification, which measures the 5 main endoscopic features of EoE: Edema, Rings, Exudates, Furrows, and Strictures. Edema is graded as absent (0) or present (1); Rings are graded as absent (0), mild (1), moderate (2), or severe (3); Exudates are graded as absent (0), mild (1), or severe (2); Furrows are graded as absent (0), mild (1), or severe (2); Stricture are graded as absent (0) or present (1), and if present the inner diameter can also be reported. (A) A patient with suspected EoE, but with a normal endoscopy. (B) A patient with edema, exudates, and furrows. (C) A patient with edema, rings, exudates, furrows, and a stricture. (D) A patient with edema, with edema, rings, exudates, furrows, and a stricture. (E) A patient with edema and furrows. (F) A patient with edema, rings, exudates, and furrows.
There can be a disconnect between EoE symptoms and endoscopic or histologic measures of disease activity. For example, patients may be able to minimize symptoms with dietary avoidance or modification (careful chewing, slow eating, avoiding hard or fibrous foods) despite ongoing inflammation, or conversely, if there is an esophageal stricture, symptoms may persist despite resolved inflammation. Therefore, close clinical follow-up of patients is required, and histopathology remains necessary for monitoring EoE disease activity. However, studies have used varying thresholds of eos/hpf to determine treatment response (84). Recent work supports histologic response as an eosinophil count of <15 eos/hpf, as it is modestly predictive of gross endoscopic or symptomatic improvement, but a more stringent level of <5 eos/hpf correlates with combined improvement in both of these parameters (85, 86). While EoE is a chronic disease, the optimal intervals for endoscopic surveillance of patients who have achieved remission is not known and should be an area of future study. However, endoscopic assessments with esophageal biopsy should be made approximately 6–8 weeks after a treatment is initiated or changed in order to evaluate treatment efficacy as measured by endoscopy and histologic response. Once the disease is under control, less frequent intervals are acceptable. There are no guidelines as to the recommended frequency, so surveillance endoscopy may be performed at intervals that is dictated by the clinical picture. Some clinicians opt to do this on a yearly basis, but others perform endoscopy less frequently (87).
EoE Treatment
Management of EoE requires coordination between providers across disciplines and can be time- and resource-intensive. Ultimately, a patient-centric discussion will determine the best approach for individualized care, and gastroenterologists, allergists, dieticians, and nurses are integral to this process. While there are no FDA-approved treatments for EoE, current options include PPI therapy, swallowed topical corticosteroids (tCS), and elimination diets (88, 89). Esophageal dilation is an additional option for treatment of esophageal strictures and fibrostenotic changes.
Proton Pump Inhibitor Therapy
PPIs are inexpensive, effective, and despite recent reports of adverse effects, are still considered generally safe for long-term use (90–92). A novel anti-inflammatory/anti-eosinophil mechanism of PPIs is independent of acid suppression; indeed, histologic remission occurs in up to half of patients with symptomatic esophageal eosinophilia on PPI therapy (93–99), only a proportion of whom have gastroesophageal reflux. Interestingly, the presence of acid reflux does not predict who with EoE will respond to PPI treatment. Future studies may find ways to identify which patients with EoE will respond to PPI therapy as a first-line treatment. Omeprazole 20–40 mg twice daily or its equivalent in adults, or 1 mg/kg/day (max 40 mg once daily) is considered high dose PPI therapy in the setting of esophageal eosinophilia (6), and in a meta-analysis induced histologic remission in up to 50% and symptomatic improvement in up to 60% of adults and children (93, 100, 101). Twice daily dosing is more effective at inducing histologic response than once daily dosing (100). Sustained remission is achieved in more than three-quarters of patients, and a majority of those who relapse on maintenance dosing can achieve remission at a higher treatment dose (101–103). Thus, PPI therapy can be considered as an initial treatment. An important caveat is that most of the known treatment responses of steroids and dietary elimination are in patient populations who were non-responsive to PPI use, and the comparative efficacy of PPIs, steroids, and diet is not known (104). In Table 1, we provide dosing recommendations for PPI therapy in children and adults based on our experience, though we acknowledge there are currently no FDA-approved dosage guidelines for PPI therapy in EoE.
Table 1. UNC Experience: Recommendations for tCS and PPI therapy.
| Topical Corticosteroids (tCS) Dosing typically divided twice daily | |||||
|---|---|---|---|---|---|
| Initial Dosing 6–12 week period |
Maintenance Dosing* |
Notes | |||
| Drug | Children | Adolescents & Adults** |
Children | Adolescents & Adults** |
|
| Fluticasone Proprionate 220 mcg Inhaler |
880–1760 mcg/day |
1760 mcg/day | 440–880 mcg/day |
880–1760 mcg/day |
No eating/drinking/brushing teeth for 30 minutes after each dose Do not use a spacer: Puff directly into mouth & swallow |
| Budesonide Slurry 0.25 mg/2 mL respules OR 0.5 mg/2 mL respules; PLUS thickening agent*** |
1 mg/day | 2 mg/day | 0.5 mg/day |
1 mg/day | No eating/drinking/brushing teeth for 30 minutes after each dose Use 0.25 mg respules instead of 0.5 mg respules if a larger volume desired |
| Proton Pump Inhibitors (PPI) | |||||
| Drug | Children <10 years old | Adolescents & Adults | Notes | ||
| Lansoprazole | 2 mg/kg/day max divided bid | 30 mg twice daily | Initial treatment 8–12 weeks to assess response If response, can attempt to wean dosing and evaluate for minimal effective clinical dose Ideally give PPI 30 minutes before meals |
||
| Omeprazole | 2 mg/kg/day divided bid | 20–40 mg/day divided bid | |||
| Pantoprazole | 2 mg/kg/day max divided bid | 20–40 mg twice daily | |||
| Esomeprazole | 2 mg/kg/day max divided bid | 20–40 mg once daily | |||
| Rabeprazole | -- | 20 mg twice daily | |||
| Dexlansoprazole | -- | 60 mg daily | |||
Goal is to use the lowest effective dose, but few data to support this approach or any maintenance approach; recent literature suggests that some patients relapse with tCS dose reduction (118)
Some have used cut-off from children to adults as >5 feet tall (109)
Example regimens include mixing contents of each respule with 5 grams of sucralose (1 packet of sucralose = 1 gram) or 2.5 mL Neocate Nutra per mg of budesonide to make total volume of 8–12 mL (96)
Swallowed Topical Corticosteroids (tCS)
Multiple systematic reviews and meta-analyses show that tCS induce remission in EoE and are safe and well-tolerated in the short term, although studies vary in medication type, dosing, method of administration, and duration of treatment (105–109), and discontinuation of therapy results in recurrence of active disease (87). Currently available tCS adapt the use of existing asthma preparations for administration to the GI tract. Instead of inhaling fluticasone propionate (FP) for asthma, use for EoE requires spraying the MDI directly to the mouth followed by swallowing. Major placebo-controlled prospective studies examining FP in children used daily doses between 880–1760 mcg per day, divided twice daily (110, 111). Low doses of FP in children may also be supported; partial or complete histologic remission was achieved in 63–83% of children using low swallowed FP dosing, based on age-specific treatment doses for asthma (2–4 years old: 176 mcg/day, 5–11 years old: 440 mcg/day, and ≥12 years old: 880 mcg/day) (112). In adults, high dose FP therapy (1760 mcg/day) when compared to placebo therapy achieved a 90% decrease in mean baseline esophageal eosinophilia in 62% of patients (113). Low dose FP therapy (880 mcg/day) was less effective at inducing histologic remission (114, 115) compared to PPI, though this was not directly compared to high-dose FP therapy.
To minimize pulmonary deposition and maximize esophageal deposition of tCS, budesonide respules can be mixed with either sucralose (5 grams per 2 mL respule), elemental formula, or other thickening agents (116), to prepare a slurry for patients to swallow. The first prospective study examining budesonide slurry (children shorter than 5 feet received 0.5 mg/day and children taller than 5 feet received 1 mg/day) showed reductions in esophageal eosinophilia in 87% of patients (117). Among adult populations, nebulized budesonide 1 mg twice daily for 15 days resulted in histologic remission in 72% of patients with EoE (118). However, oral viscous budesonide 1 mg twice daily was more effective than nebulization of the same dose, corresponding with increased esophageal mucosal contact time as measured by scintigraphy (119). This finding led to the development of novel methods of delivering tCS directly to the esophageal mucosa specifically for EoE. Budesonide effervescent tablets (BET) were effective in inducing histologic remission in 100% and 94% of patients receiving 1 mg BET twice daily or 2 mg BET twice daily, respectively (120), and has recently been approved for EoE treatment in Europe. Pre-mixed viscous budesonide, currently in phase 3 trials, induced histologic remission of EoE and reduced clinical symptoms in both children and adults (121, 122).
After initial treatment and response to tCS, there are relatively few data to guide long-term treatment (87). One strategy is to decrease the dose to the lowest effective level to minimize medication side effects. In one study, high dose FP (1760 mcg/day: 4 puffs of the 220 mcg MDI twice daily) for 12 weeks induced histologic remission in 65%–77% of patients with EoE. Among those who responded to FP therapy, a 50% dose reduction (4 puffs of the 220 mcg MDI once daily) was effective at maintaining histologic remission in 73%–93% of patients (110). This study also demonstrated that patients who failed 12-week high dose FP treatment were unlikely to respond to a prolonged course of this same treatment. Intermittent dosing is not supported by a small study of budesonide slurry every Monday-Wednesday-Friday, which found the majority of patients relapsed (123). A randomized, double-blind, 50-week trial of 0.5 mg daily budesonide maintenance therapy versus placebo showed 36% of patients in the active arm compared to 0% of patients on placebo who maintained remission (124). A retrospective cohort study (125) was performed on patients who were able to achieve “deep remission”, defined as combined clinical, endoscopic, and histologic remission, for six months on tCS. Of the 33 patients (9.4%) who achieved deep remission, only six (18.2%) maintained deep remission off tCS. Other studies have raised the question of whether there might be a loss of response or steroid resistance in some patients during an extended treatment course (52, 126, 127).
In general, tCS appear to be safe and well tolerated, but there are potential side effects. A recent meta-analysis found a 4–5% rate of esophageal candidiasis among children and 5–15% in adults (106). To date, no study has described decreased growth in pediatric patients with EoE receiving tCS (112, 121, 128). While there are some reports of adrenal suppression due to corticosteroid use in EoE (129), a systematic review found that clinically important adrenal insufficiency is rare (130). Given the association of EoE with other atopic diseases that predispose to glucocorticoid use, cortisol monitoring to detect adrenal insufficiency may be considered for children with EoE if they are receiving long-term high-dose tCS therapy or are receiving different steroid formulations for other atopic conditions, but there are no specific recommendations in guidelines to date. In Table 1, we provide dosing recommendations for tCS therapy in children and adults based on our experience.
Elimination Diets
The first clue that EoE was a food antigen-driven allergic disease came from a landmark study where 10 children with esophageal eosinophilia who did not respond to PPI treatment were treated with an elemental diet; 8 had complete resolution of symptoms, and 10 had significant reduction in eosinophil counts (131). Reintroduction of foods in a controlled setting identified trigger foods, and recurrence of GI symptoms occurred. An elemental diet is amino acid-based and devoid of intact food proteins. While the elemental diet is effective (a meta-analysis found 91% histologic response (132)), there remain significant barriers to widespread implementation of this diet, including poor palatability, high financial cost, inconsistent health insurance coverage, and increased psychosocial isolation of the patient (81, 133–136). Thus, alternative elimination diets have been developed that balance time to remission and to identification of the food trigger, minimize number of repeat endoscopy with biopsies, and improve patient adherence.
Serum food-specific IgE, skin prick testing (SPT), atopy patch testing (APT), or combinations thereof, have poor positive predictive value of non-IgE-mediated food triggers in adults with EoE (137, 138). Allergy test-directed elimination diets perform poorly in EoE, inducing histologic remission in approximately 43% of patients with EoE with high variability between studies suggesting low reproducibility (132, 138). However, in the context of a detailed history of possible food triggers along with epidemiologically known culprits, history-directed allergy testing, especially when APT is done by rigorously trained individuals, may help identify food triggers in some patients with EoE (139–141). Practically speaking, APT is technically challenging, and empiric elimination diets have been shown to be effective for the treatment of EoE. In 2006, a retrospective study (142) compared an elemental diet with a diet that eliminated the six most common allergenic foods (the so-called six food elimination diet (SFED)): cow/animal-milk protein, soy, egg, wheat, peanuts/tree nuts, and seafood (143). After six weeks, 74% and 88% of children on the SFED and elemental diet, respectively, achieved histologic remission (<10 peak eos/hpf). This approach was used in other studies, and a meta-analysis showed the SFED is successful at inducing histologic remission in 72% of patients with EoE (132). However, this diet is quite restrictive, requires numerous endoscopies to monitor disease activity and to reintroduce individual foods, and there remains controversy about the extent of elimination with certain food groups (for example, soy or all legumes, or wheat or all cereal grains) (144).
The most common EoE triggers have been reported to be animal-milk protein (adults: 39–64%, children: 65–85%), wheat/gluten-containing cereals (adults: 22–60%, children: 26–37%), egg (adults: 5–44%, children: 17–40%), and soy/legumes (adults: 7–24%, children: 10–38%) (145); 65–85% of patients had a single food trigger (146). The four-food elimination diet (FFED, animal milk, wheat/gluten, egg, soy/legumes) and two-food elimination diet (TFED, animal milk, wheat/gluten) achieved histologic remission in 54% and 43% of adult patients, respectively, and in 64% and 43% of pediatric patients, respectively (132). In a multicenter prospective study (147) utilizing a step-up approach, clinicohistologic remission was achieved in 43%, 60%, and 79% of patients on the TFED, FFED, and SFED, respectively. This approach also had the potential to decrease the number of endoscopies by 20% and time to identification of food trigger(s) by 30% when compared to a SFED. Importantly, a detailed, user-friendly guide was developed for this study (147) that can serve as an important clinical tool as empiric elimination diets become more widely used. Table 2 provides a summary of the TFED, FFED, and SFED.
Table 2. A Practical Guide to the Empiric Elimination Diet in the USa.
| Diet | Two-food group | Four-food group | Six-food group | |
|---|---|---|---|---|
|
Eliminated food groups |
Animal milk, wheatb | Animal milk, wheatb, egg, soyb |
Animal milk, wheatb, egg, soyb, nuts, fish/seafood |
|
|
Allowed food groups (favor raw, fresh, or uncooked) |
Vegetables, tubers, non- heat grainsc, legumesd, meate, egg, fish/seafoodf, fruit, nuts, [dairy made from soy, rice, spelt, quinoa, walnut, nuts] |
Vegetables, tubers, non- wheat grainsc, non-soy legumesd, meate, fish/seafoodf, fruit, nuts, [dairy made from rice, spelt quinoa, walnut, nuts] |
Vegetables, tubers, non- wheat grainsc, non-soy legumesd, meate, fruit, [dairy made from rice, spelt, quinoa] |
|
| Foods include: | Food labels may contain: | |||
|
Animal milk |
Cow’s, goat’s, and sheep’s milk and milk products (cheese, yogurt, butter, margarine, ice creams, milkshakes, custard, crème caramel, rice pudding) |
Milk, cream, caseinates, hydrolysates, lactalbumin, casein, whey, E-4511, E-4512, E-4513, flavor, animal fat, cream, proteins, dehydrated powder or sauce |
||
| Wheat | Wheat, wheat-containing: bread, toast, biscuits, cookies, donuts, muffins, pretzel, pancakes, waffles, crackers, cream desserts, sweets, candies, pasta, cream, soups, sauces, malted food, breaded or floured vegetables, [beer, whiskey] |
Flour or floured, farina, wheat-enriched, malted or malt added, breaded, E-1404, E-1412, E-1413, E- 1414, E-1420, E-1422, E-1440, E-1442, E-1450, starch, fiber, protein, vegetable protein, hydrolyzed protein, malt, malt extract, cous cous, yeast, species, flavour |
||
| Egg | Egg, baked goods, pasta, cakes, biscuits, cookies, donuts, muffins, pretzel, pancakes, waffles, crackers, cream desserts, sweets, candies, processed meat, goose liver, mayonnaise, coat and wrapped in bread food, breaded or creamed vegetables, sauces |
Albumin, apovitellin, binder, coagulant, cholesterol free egg substitute, dried egg, egg white, egg yolk, egg lecithin, egg lysosome, eggnog, egg wash, globulin, lecithin, livetin, lysozyme, meringue, meringue powder, simplesse, surimi, ovoalbumin, ovamucin, ovamucoid, ovotransferrin, ovovitelin, powdered egg, trailblazer, vitellin, whole egg |
||
| Soyg | Soy sauce, tofu, edamame, tempeh, vegetarian meat analogs |
Oil made with soy, hydrolyzed plant, vegetable protein, plant protein, vegetable gum, vegetable starch |
||
| Nutsg | Almond, artificial nuts, Brazil nut, beechnut, butternut, cashew, chestnut, chinquapin nut, coconut, filbert/hazelnut, macadamia nut, marzipan/almond paste, Nangai nut, natural nut extract, nut butters, nut meal, nut meat, nut milk, nut paste, nut pieces, pecan, pesto, pili nut, pine nut (e.g., Indian, pignoli, pinolia, pignon, pinon, and pinyon nut), pistachio, praline, shea nut, walnut |
Oil made with any of the named nuts to the left, nut flavoring |
||
| Fish/ Seafoodg | Fish (anchovies, bass, catfish, cod, flounder, grouper, haddock, hake, halibut, herring, mahi mahi, perch, pike, pollock, salmon, scrod, swordfish, sole, snapper, tilapia, trout, tuna), shellfish (crab, lobster, prawns, shrimps), and mollusks (cockles, mussels, octopus, oyster, snails, squid) |
Oil or gelatin made with named seafood to the left | ||
Adapted from Molina-Infante, J, et al., 2017{Molina-Infante, 2017 #3}, and Kliewer, KL, et al., 2017 {Kliewer, 2017 #159}, for the US population.
Expanded elimination diets, which exclude wheat/gluten/cereal grains and all legumes in place of wheat and soy, respectively, have been proposed despite no studies exploring whether these increasingly restrictive elimination diets are more effective or clinically relevant for EoE treatment {Kliewer, 2016 #184}. In the case of grains, there is a risk of: 1) cross-reactivity between similar grain proteins, and 2) cross-contamination due to processing of different grains within the same facility. However, the clinical relevance of these concerns is not known. Diverse legumes are consumed by Spanish populations and are frequently associated with EoE in these patients {Molina-Infante, 2018 #218}. The provider may take this into consideration when advising a patient to avoid soy or all legumes.
Non-wheat grains include barley, rye, gluten-free oats (due to high risk of cross-contamination during the processing of conventional oats {Kliewer, 2017 #159}), spelt, triticale, semola, semolina, and kamut.
Legumes include soy, lentils, pea, chickpeas, beans, peanuts, lupin, guar gum, carob bean, and alfalfa.
Excepting processed or pre-cooked meats, such as sausages or hamburgers.
Excepting processed or pre-cooked fish.
African and Asian ethnic foods often contain legumes (soy), nuts, and fish/seafood, and may be high-risk foods.
The reintroduction of foods after achieving histologic remission has not been studied in detail and varies among providers. Sequential addition of individual foods into the diet for a period of about six weeks should be followed by endoscopy with biopsy to monitor disease activity. A common method is to introduce two or more foods sequentially at two-week intervals, depending on how many foods were initially eliminated, before undergoing endoscopy at regular intervals. For example, a patient on a SFED may first introduce seafood for two weeks; if asymptomatic, the patient can introduce peanuts/tree nuts for two weeks, followed by an endoscopy to assess the histologic response to these two food groups. For patients who had more extensive elimination diets, introduction of less allergenic foods (lower protein content such as fruits and vegetables) are typically tried before more allergenic foods. An example of a food reintroduction schedule is shown in Figure 2 (104). Foods from each category can be reintroduced every five to seven days (148). The patient should ingest an age-appropriate form and serving size amount of the food on most of the days of the week during the trial period (145). If a patient develops recurrence of symptoms with food reintroduction, they may undergo a wash-out period of six weeks prior to reintroduction of the next food group (149). Of note, if two foods are added back and recurrent esophageal eosinophilia is noted, then it is not possible to determine which of the foods is a trigger. Because of that, the more highly allergenic foods (i.e. Column D of Figure 2) are typically added back individually followed by repeat endoscopy in 6 weeks to confirm histologic remission prior to further food introductions.
Figure 2.
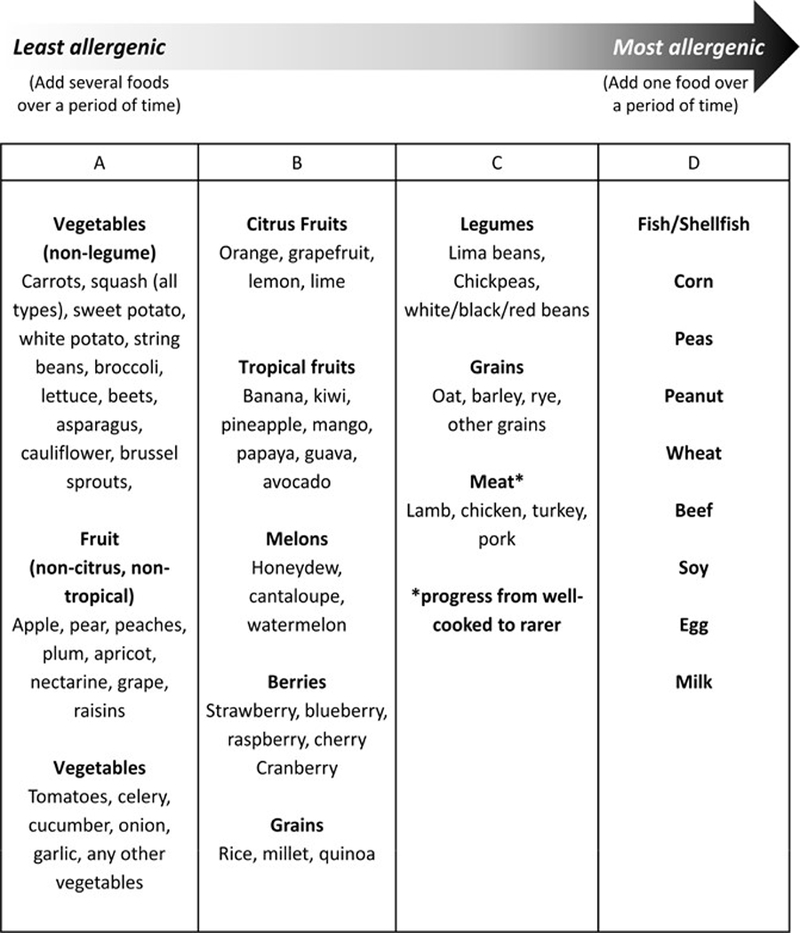
Dietary reintroduction of food allergen groups for patients with EoE. Modified with permission from Gastrointest Endosc Clin N Am. 2008;18(1):179–94; xi (148) and Gastroenterology. 2014;147(6):1238–54 (96).
Complete avoidance of the culprit food should maintain long-term symptomatic and histologic remission without the need for pharmacologic therapy (6, 7). There remains, however, a dearth of studies addressing the efficacy and feasibility of long-term food allergen avoidance in EoE. Adult patients who avoided their food trigger(s) were able to achieve sustained symptomatic and histologic remission for up to three years without the need for pharmacologic therapy (47, 150). However, long-term compliance can be difficult, and lower long-term “real-world” response rates have also been noted (151). In addition, patients with two or more food triggers more often preferred pharmacologic therapy than a continued restricted diet (88).
There are several factors that should be considered before embarking on an elimination diet, particularly in pediatric patients. The choice to pursue dietary therapy may be influenced by the severity of disease, nutritional status of the patient, learned maladaptive feeding behaviors, family dynamics, financial resources, and family preferences (145). Learned maladaptive feeding behaviors are highly prevalent in pediatric patients with EoE and can persist despite histologic remission (152). Many children also exhibited delayed oral motor skills required for feeding, as EoE during early formative years hinders the natural development of these skills (153). Furthermore, compensatory behaviors such as thoroughly chewing food prolongs meal times and may limit caloric intake (154). Individual or group therapy with a pediatric feeding specialist provides re-learning of appropriate feeding behaviors, development of oral motor and sensory skills, allows interaction with peers, and provides communal support for caregivers. Dieticians are key team members for EoE treatment who provide practical education, guidance to ensure adequate nutritional intake, knowledge of community resources, and a unique perspective of the patient’s barriers to care. Current recommendations encourage the involvement of a dietician and other support services if warranted (6, 7).
Esophageal Dilation
It is estimated that esophageal strictures are present in 30–80% of adults with EoE, and prevalence increases proportionally with age and delay in time to diagnosis (25). Multiple meta-analyses and systematic reviews support the safety and efficacy of endoscopic dilation in adult and pediatric patients with EoE (155–157). Some patients, especially those with smaller initial esophageal diameters, will require multiple procedures over several months to achieve improvement in esophageal caliber; others will need repeat dilation due to stricture recurrence (158). Importantly, endoscopic dilatation does not alter the underlying inflammatory process in EoE, and treatment with concomitant pharmacologic therapy or elimination diet reduces the risk of needing subsequent dilations (26, 59). There are no definitive guidelines for when to offer endoscopic dilation, although it is generally recommended for patients with EoE who have food impaction, strictures, or whose dysphagia does not respond to therapy, or when critical strictures are present, even at the time of diagnosis.
Challenges that remain in EGID
Refractory EoE
Refractory disease in EoE is conceptually defined as ongoing clinical symptoms, endoscopic findings, and esophageal eosinophilia despite treatment with either a tCS or elimination diet (159–163). A significant proportion of patients in both observational studies and randomized controlled trials of EoE meet these criteria (159). There is no clear consensus on specific endpoints or response thresholds that should define treatment failure. A recent review on this topic proposed that refractory disease constitutes incompletely resolved esophageal eosinophilia (≥15 eos/hpf) in the setting of persistent endoscopic findings and clinical symptoms (159). However, patients may have discordant findings among these domains, and it is not clear how these patients should be categorized. For instance, a patient may have complete resolution of histologic and endoscopic findings but continue to experience dysphagia. Importantly, the patient’s individual pattern may yield clues as to the underlying cause for their refractory disease; in the previous example of a patient with continued clinical symptoms, one should consider the presence of esophageal strictures.
The first step in managing patients with refractory EoE is to consider patient nonadherence, suboptimal medication dose, ineffective delivery or inappropriate administration of the medication, continued exposure to allergen, concomitant infection, esophageal stricture, or an inappropriate diagnosis of EoE. Patient nonadherence is an especially important consideration in elimination diets, as this therapy is restrictive, expensive, and requires significant nutritional literacy.
Once a patient has confirmed refractory EoE, a careful consideration of the patient’s initial failed treatment (tCS versus elimination diet), disease severity, clinical course, and individual biopsychosocial circumstances should guide treatment. Unfortunately, there are few evidence-based data to guide the management of refractory EoE. Therefore, recommendations are based on logical and systematic progression of therapies. For patients who failed tCS, the dose, mode of delivery, and specific corticosteroid may need adjustment. The addition of an elimination diet or second-line therapy in combination with tCS may be a reasonable approach. Second-line treatments could include systemic corticosteroids, leukotriene antagonists, mast cell stabilizers, immunomodulators, or biologic agents. Patients refractory to an elimination diet should consider the elemental diet, despite significant downsides to this therapy, as it is highly effective (132). Many patients with treatment non-response may be good candidates for clinical trials. A diagnostic and management algorithm for patients with EoE is provided in Figure 3.
Figure 3.
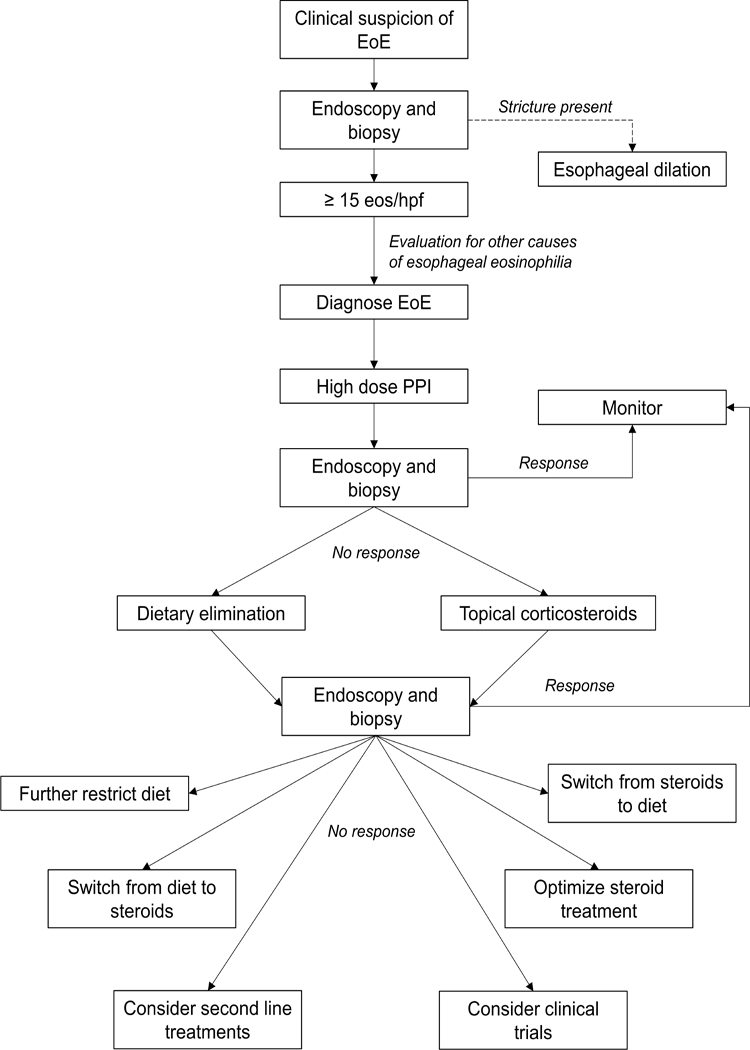
Algorithm for diagnosis and management of EoE. “Response” refers to <15 eos/hpf with resolution of symptoms. “No Response” refers to >15 eos/hpf and persistence of symptoms. For patients with a histologic response but with ongoing symptoms, assessment should be made for a persistent esopahgeal stricture or alternative cause of symptoms (dysmotility, viserceral hypersensitivity, etc). Patients with no histologic reponse but with resolution of symptoms may have undergone prior esophageal dilation and should be assessed for dietary avoidance or modification behaviors.
EGIDs: EG, EGE, and EC
In comparison to EoE, data related to the non-EoE EGIDs are immature, with most information derived from single center case series; there are no RCTs or published guidelines.EG, EGE, and EC are rare, with an estimated prevalence of ~5–10 per 100,000, accounting for ~50,000 cases in the U.S (164, 165). While there is an association with atopic diseases as is seen in EoE, patients with EGID have a unique gene expression signature that does not fully overlap with EoE (166). In addition, there does not seem to be the same male predominance as in EoE.
In general, the presentation of EG, EGE, and EC is dependent on the location of the eosinophilic infiltration (167–172). Symptoms of EG include abdominal pain, nausea, and vomiting. Symptoms are similar for EGE, though diarrhea can also be seen. For EC, symptoms include abdominal pain, diarrhea, and hematochezia. Depending on the extent of eosinophil infiltration though the GI tract wall, there can be malabsorption, protein-losing enteropathy, or ascites. The diagnosis is established after endoscopic biopsies show increased levels of eosinophils in the GI tract and excluding other potential causes of eosinophilia. There is debate as to the eosinophil threshold for diagnosis, and this level depends on the area of the GI tract examined (173). Eosinophils are normally present in the stomach, small bowel, and colon, which is in contrast to the esophagus where they are not normally seen (174). Therefore, a careful histopathologic examination is required to demonstrate pathogenic infiltration. Experts suggest at least 30 eos/5 hpf in the stomach designates EG, at least 50 eos/hpf in the duodenum establishes EGE, and even higher levels in the colon, particularly on the right side, suggest EC (173).
Treatment of EG, EGE, and EC is challenging, particularly as data are limited to case series (170–172, 175). Systemic steroids are a mainstay of therapy, but side effects limit long-term use and disease typically recurs after steroid discontinuation. “Topical steroids” have also been used. For example, enteral release budesonide can be useful for eosinophilic enteritis and proximal colitis, and granules of budesonide can be crushed and swallowed for delivery to the stomach. However, the effect is variable. Dietary elimination has also been reported, with similar strategies as with EoE, including SFED and elemental formula (176). Finally, there are scattered case reports of treatment with immunomodulators and mast cell agents (cromolyn; ketotifen).
Emerging Therapies for EGIDs
There has been keen interest in developing new pharmacologic treatments for EoE and the EGIDs in recent years (177). In addition to the novel topical steroid formulations as noted above, the increasing knowledge of EoE pathogenesis has allowed a number of therapeutic targets to be identified and tested. The anti-IL-5 agents mepolizumab and reslizumab are currently approved for treatment of eosinophilic asthma and have been tested in phase 2 randomized controlled trials in EoE with a moderate impact on tissue eosinophilia (178–180). The anti-IL-5 receptor antagonist benralizumab is also approved for eosinophilic asthma and may be a future treatment for EGIDs. There have been two randomized controlled trials with anti-IL-13 agents (181, 182), the most recent of which was a phase 2 study with RPC4046 showing promising results. The anti-IL-4 receptor antagonist dupilumab, approved for the treatment of eczema, is also in a phase 2 trial of EoE with promising results (183). An anti-TSLP agent has recently shown efficacy for eosinophilic asthma and may be a future treatment for EGIDs. A small molecule antagonist of the chemoattractant receptor-homologous molecule on Th2 cells shows some beneficial effect in a small study (184). Notably, omalizumab and infliximab have not shown efficacy in EoE (185, 186).
Conclusions and Future Directions
Non-IgE mediated eosinophilic infiltration of the GI tract can cause significant morbidity in patients of all ages. This aberrant immune response may induce a progression from inflammatory to fibrostenotic disease in EoE. PPI therapy, tCS, and dietary elimination are effective at inducing remission in many patients with EoE, and adjunctive endoscopic dilation is helpful in cases with esophageal strictures or fibrostenotic changes. Treatment plans should be individualized based on patient values, financial resources, lifestyle, and social support. Future research should attempt to identify patients who will respond best to specific therapies. Novel therapies targeting eosinophil and Th2-mediated inflammation in EoE are currently in development and show therapeutic promise. The other EGIDs are rare and less well studied in terms of both pathogenesis and management. Better understanding of EGID pathogenesis will ultimately lead to the identification of clinically-meaningful outcomes that will inform the guidelines for diagnosis and management of these conditions.
Acknowledgments
Funding: This work is supported, in part, by CEGIR (U54 AI117804) which is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED CURED and EFC; as well as by NIH R01 DK 101856 (ESD).
Abbreviations:
- APT
atopy patch test
- BET
budesonide effervescent tablet
- EC
eosinophilic colitis
- EG
eosinophilic gastritis
- EGE
eosinophilic gastroenteritis
- EGID
eosinophilic gastrointestinal disease
- EoE
eosinophilic esophagitis
- EoEHSS
EOE-specific histologic scoring system
- Eos
eosinophils
- Eos/hpf
eosinophils per high-powered field
- EREFS
EoE Endoscopic Reference Score
- FFED
four food elimination diet
- FP
fluticasone propionate
- GERD
gastroesophageal reflux disease
- GI
gastrointestinal
- PPI-REE
proton pump inhibitor-responsive esophageal eosinophilia
- SFED
six food elimination diet
- SPT
skin prick test
- tCS
topical corticosteroids
- TFED
two food elimination diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Dellon has received research funding from Adare, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; has consulted for Adare, Alivio, Allakos, AstraZeneca, Banner, Enumeral, Celgene/Receptos, GSK, Regeneron, and Shire; and has received educational grants from Banner and Holoclara. The other authors report no potential related competing interests.
References
- 1.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993;38(1):109–16. [DOI] [PubMed] [Google Scholar]
- 2.Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vogtlin J. [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr 1994;124(33):1419–29. [PubMed] [Google Scholar]
- 3.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007;133(4):1342–63. [DOI] [PubMed] [Google Scholar]
- 5.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128(1):3–20 e6; quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108(5):679–92; quiz 93. [DOI] [PubMed] [Google Scholar]
- 7.Lucendo AJ, Molina-Infante J, Arias A, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017;5(3):335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 2016;65(3):524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AGREE Conference. Digestive Diseases Week; 2017 May 5, 2017; Chicago, IL. [Google Scholar]
- 10.Binkovitz LA, Lorenz EA, Di Lorenzo C, Kahwash S. Pediatric eosinophilic esophagitis: radiologic findings with pathologic correlation. Pediatr Radiol 2010;40(5):714–9. [DOI] [PubMed] [Google Scholar]
- 11.Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2007;45(3):319–28. [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79(4):577–85 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aceves SS, Newbury RO, Dohil MA, Bastian JF, Dohil R. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Ann Allergy Asthma Immunol 2009;103(5):401–6. [DOI] [PubMed] [Google Scholar]
- 14.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 2009;48(1):30–6. [DOI] [PubMed] [Google Scholar]
- 15.Rassbach W, Rubenstein JH, Elkins M, DeMatos V, Greenson JK, Greenhawt M. Age-based differences in the diagnosis and management of esophageal eosinophilia. J Allergy Clin Immunol Pract 2015;3(1):81–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohm M, Malik Z, Sebastiano C, Thomas R, Gaughan J, Kelsen S, et al. Mucosal eosinophilia: prevalence and racial/ethnic differences in symptoms and endoscopic findings in adults over 10 years in an urban hospital. J Clin Gastroenterol 2012;46(7):567–74. [DOI] [PubMed] [Google Scholar]
- 17.Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2009;7(12):1305–13; quiz 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croese J, Fairley SK, Masson JW, Chong AK, Whitaker DA, Kanowski PA, et al. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc 2003;58(4):516–22. [DOI] [PubMed] [Google Scholar]
- 19.Kahn J, Bussmann C, Beglinger C, Straumann A, Hruz P. Exercise-induced chest pain: an atypical manifestation of eosinophilic esophagitis. Am J Med 2015;128(2):196–9. [DOI] [PubMed] [Google Scholar]
- 20.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc 2006;63(1):3–12. [DOI] [PubMed] [Google Scholar]
- 21.Koutlas NT, Dellon ES. Progression from an Inflammatory to a Fibrostenotic Phenotype in Eosinophilic Esophagitis. Case Rep Gastroenterol 2017;11(2):382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li-Kim-Moy JP, Tobias V, Day AS, Leach S, Lemberg DA. Esophageal subepithelial fibrosis and hyalinization are features of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2011;52(2):147–53. [DOI] [PubMed] [Google Scholar]
- 23.Lipka S, Kumar A, Richter JE. Impact of Diagnostic Delay and Other Risk Factors on Eosinophilic Esophagitis Phenotype and Esophageal Diameter. J Clin Gastroenterol 2016;50(2):134–40. [DOI] [PubMed] [Google Scholar]
- 24.Menard-Katcher P, Marks KL, Liacouras CA, Spergel JM, Yang YX, Falk GW. The natural history of eosinophilic oesophagitis in the transition from childhood to adulthood. Aliment Pharmacol Ther 2013;37(1):114–21. [DOI] [PubMed] [Google Scholar]
- 25.Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145(6):1230–6 e1–2. [DOI] [PubMed] [Google Scholar]
- 26.Singla MB, Chehade M, Brizuela D, Maydonovitch CL, Chen YJ, Riffle ME, et al. Early Comparison of Inflammatory vs. Fibrostenotic Phenotype in Eosinophilic Esophagitis in a Multicenter Longitudinal Study. Clin Transl Gastroenterol 2015;6:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rhijn BD, Oors JM, Smout AJ, Bredenoord AJ. Prevalence of esophageal motility abnormalities increases with longer disease duration in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil 2014;26(9):1349–55. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Park JY, Huang R, Souza RF, Spechler SJ, Cheng E. Obtaining Adequate Lamina Propria for Subepithelial Fibrosis Evaluation in Pediatric Eosinophilic Esophagitis. Gastrointest Endosc 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassett J, Maydonovitch C, Perry J, Sobin L, Osgard E, Wong R. Prevalence of esophageal dysmotility in a cohort of patients with esophageal biopsies consistent with eosinophilic esophagitis. Dis Esophagus 2009;22(6):543–8. [DOI] [PubMed] [Google Scholar]
- 30.Chen JW, Pandolfino JE, Lin Z, Ciolino JD, Gonsalves N, Kahrilas PJ, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 2016;48(9):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colizzo JM, Clayton SB, Richter JE. Intrabolus pressure on high-resolution manometry distinguishes fibrostenotic and inflammatory phenotypes of eosinophilic esophagitis. Dis Esophagus 2016;29(6):551–7. [DOI] [PubMed] [Google Scholar]
- 32.Korsapati H, Babaei A, Bhargava V, Dohil R, Quin A, Mittal RK. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut 2009;58(8):1056–62. [DOI] [PubMed] [Google Scholar]
- 33.Menard-Katcher C, Benitez AJ, Pan Z, Ahmed FN, Wilkins BJ, Capocelli KE, et al. Influence of Age and Eosinophilic Esophagitis on Esophageal Distensibility in a Pediatric Cohort. Am J Gastroenterol 2017;112(9):1466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moawad FJ, Maydonovitch CL, Veerappan GR, Bassett JT, Lake JM, Wong RK. Esophageal motor disorders in adults with eosinophilic esophagitis. Dig Dis Sci 2011;56(5):1427–31. [DOI] [PubMed] [Google Scholar]
- 35.Nurko S, Rosen R, Furuta GT. Esophageal dysmotility in children with eosinophilic esophagitis: a study using prolonged esophageal manometry. Am J Gastroenterol 2009;104(12):3050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieder F, Nonevski I, Ma J, Ouyang Z, West G, Protheroe C, et al. T-helper 2 cytokines, transforming growth factor beta1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology 2014;146(5):1266–77 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choksi Y, Lal P, Slaughter JC, Sharda R, Parnell J, Higginbotham T, et al. Esophageal Mucosal Impedance Patterns Discriminate Patients With Eosinophilic Esophagitis From Patients With GERD. Clin Gastroenterol Hepatol 2017. [DOI] [PubMed] [Google Scholar]
- 38.Marietta EV, Geno DM, Smyrk TC, Becker A, Alexander JA, Camilleri M, et al. Presence of intraepithelial food antigen in patients with active eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;45(3):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, de Jonge WJ, et al. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol 2015;110(9):1289–97. [DOI] [PubMed] [Google Scholar]
- 40.Warners MJ, van Rhijn BD, Verheij J, Smout A, Bredenoord AJ. Disease activity in eosinophilic esophagitis is associated with impaired esophageal barrier integrity. Am J Physiol Gastrointest Liver Physiol 2017;313(3):G230–G8. [DOI] [PubMed] [Google Scholar]
- 41.Warners MJ, Vlieg-Boerstra BJ, Verheij J, van Hamersveld PHP, van Rhijn BD, Van Ampting MTJ, et al. Esophageal and Small Intestinal Mucosal Integrity in Eosinophilic Esophagitis and Response to an Elemental Diet. Am J Gastroenterol 2017;112(7):1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krarup AL, Villadsen GE, Mejlgaard E, Olesen SS, Drewes AM, Funch-Jensen P. Acid hypersensitivity in patients with eosinophilic oesophagitis. Scand J Gastroenterol 2010;45(3):273–81. [DOI] [PubMed] [Google Scholar]
- 43.Warners MJ, Terreehorst I, van den Wijngaard RM, Akkerdaas J, van Esch B, van Ree R, et al. Abnormal Responses to Local Esophageal Food Allergen Injections in Adult Patients With Eosinophilic Esophagitis. Gastroenterology 2018;154(1):57–60 e2. [DOI] [PubMed] [Google Scholar]
- 44.Abu-Sultaneh SM, Durst P, Maynard V, Elitsur Y. Fluticasone and food allergen elimination reverse sub-epithelial fibrosis in children with eosinophilic esophagitis. Dig Dis Sci 2011;56(1):97–102. [DOI] [PubMed] [Google Scholar]
- 45.Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy 2010;65(1):109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlson DA, Hirano I, Zalewski A, Gonsalves N, Lin Z, Pandolfino JE. Improvement in Esophageal Distensibility in Response to Medical and Diet Therapy in Eosinophilic Esophagitis. Clin Transl Gastroenterol 2017;8(10):e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012;142(7):1451–9 e1; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 48.Helou EF, Simonson J, Arora AS. 3-yr-follow-up of topical corticosteroid treatment for eosinophilic esophagitis in adults. Am J Gastroenterol 2008;103(9):2194–9. [DOI] [PubMed] [Google Scholar]
- 49.Kagalwalla AF, Akhtar N, Woodruff SA, Rea BA, Masterson JC, Mukkada V, et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol 2012;129(5):1387–96 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lieberman JA, Morotti RA, Konstantinou GN, Yershov O, Chehade M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy 2012;67(10):1299–307. [DOI] [PubMed] [Google Scholar]
- 51.Lucendo AJ, Arias A, De Rezende LC, Yague-Compadre JL, Mota-Huertas T, Gonzalez-Castillo S, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol 2011;128(5):1037–46. [DOI] [PubMed] [Google Scholar]
- 52.Rajan J, Newbury RO, Anilkumar A, Dohil R, Broide DH, Aceves SS. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol 2016;137(1):147–56 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf WA, Jerath MR, Sperry SL, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014;12(8):1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipka S, Keshishian J, Boyce HW, Estores D, Richter JE. The natural history of steroid-naive eosinophilic esophagitis in adults treated with endoscopic dilation and proton pump inhibitor therapy over a mean duration of nearly 14 years. Gastrointest Endosc 2014;80(4):592–8. [DOI] [PubMed] [Google Scholar]
- 55.Jingsheng Z, Yuncheng L, Yingye M, Hao L, Congyang L. The mural form of eosinophilic esophagitis is accompanied by superficial esophageal squamous cell carcinoma. Case Rep Pathol 2012;2012:315428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucendo AJ, De Rezende L, Martin-Plaza J, Larrauri J. Esophageal granular cell tumor and eosinophilic esophagitis: two interesting entities identified in the same patient. Case Rep Gastroenterol 2008;2(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nojkov B, Amin M, Ghaith G, Cappell MS. A Statistically Significant Association Between Esophageal Granular Cell Tumors and Eosinophilic Esophagitis: A 16-year Analysis at Two Large Hospitals of 167,434 EGDs. Dig Dis Sci 2017;62(12):3517–24. [DOI] [PubMed] [Google Scholar]
- 58.Pasman EA, Heifert TA, Nylund CM. Esophageal squamous papillomas with focal dermal hypoplasia and eosinophilic esophagitis. World J Gastroenterol 2017;23(12):2246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Runge TM, Eluri S, Woosley JT, Shaheen NJ, Dellon ES. Control of inflammation decreases the need for subsequent esophageal dilation in patients with eosinophilic esophagitis. Dis Esophagus 2017;30(7):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez-Cervera J, Arias A, Redondo-Gonzalez O, Cano-Mollinedo MM, Terreehorst I, Lucendo AJ. Association between atopic manifestations and eosinophilic esophagitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol 2017;118(5):582–90 e2. [DOI] [PubMed] [Google Scholar]
- 61.Aceves SS Food allergy testing in eosinophilic esophagitis: what the gastroenterologist needs to know. Clin Gastroenterol Hepatol 2014;12(8):1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract 2017;5(2):369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill DA, Shuker M, Cianferoni A, Wong T, Ruchelli E, Spergel JM, et al. The development of IgE-mediated immediate hypersensitivity after the diagnosis of eosinophilic esophagitis to the same food. J Allergy Clin Immunol Pract 2015;3(1):123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soller L, Mill C, Avinashi V, Teoh T, Chan ES. Development of anaphylactic cow’s milk allergy following cow’s milk elimination for eosinophilic esophagitis in a teenager. J Allergy Clin Immunol Pract 2017;5(5):1413–4. [DOI] [PubMed] [Google Scholar]
- 65.Alsalamah M, Makhija M, Somers G, Marcon M, Hummel D, Upton J. Anaphylaxis to Milk After Elimination Diet for Eosinophilic Gastrointestinal Disease. Am J Gastroenterol 2016;111(5):752–3. [DOI] [PubMed] [Google Scholar]
- 66.Panel NI- SE, Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010;126(6 Suppl): S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol 2010;126(1):77–82 e1. [DOI] [PubMed] [Google Scholar]
- 68.Gomez Torrijos E, Mendez Diaz Y, Moreno Lozano L, Extremera Ortega AM, Borja Segade J, Feo Brito JF, et al. Frequency and Course of Eosinophilic Esophagitis During Oral Immunotherapy for Cow’s Milk Allergy in a Series of 57 Children. J Investig Allergol Clin Immunol 2017;27(2):132–3. [DOI] [PubMed] [Google Scholar]
- 69.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2014;113(6):624–9. [DOI] [PubMed] [Google Scholar]
- 70.Petroni D, Spergel JM. Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann Allergy Asthma Immunol 2018;120(3):237–40 e4. [DOI] [PubMed] [Google Scholar]
- 71.Guajardo JR, Zegarra-Bustamante MA, Brooks EG. Does Aeroallergen Sensitization Cause or Contribute to Eosinophilic Esophagitis? Clin Rev Allergy Immunol 2018. [DOI] [PubMed] [Google Scholar]
- 72.Ram G, Lee J, Ott M, Brown-Whitehorn TF, Cianferoni A, Shuker M, et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy Asthma Immunol 2015;115(3):224–8 e1. [DOI] [PubMed] [Google Scholar]
- 73.Jensen ET, Shah ND, Hoffman K, Sonnenberg A, Genta RM, Dellon ES. Seasonal variation in detection of oesophageal eosinophilia and eosinophilic oesophagitis. Aliment Pharmacol Ther 2015;42(4):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antico A, Fante R. Esophageal hypereosinophilia induced by grass sublingual immunotherapy. J Allergy Clin Immunol 2014;133(5):1482–4. [DOI] [PubMed] [Google Scholar]
- 75.Miehlke S, Alpan O, Schroder S, Straumann A. Induction of eosinophilic esophagitis by sublingual pollen immunotherapy. Case Rep Gastroenterol 2013;7(3):363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rokosz M, Bauer C, Schroeder S. Eosinophilic esophagitis induced by aeroallergen sublingual immunotherapy in an enteral feeding tube-dependent pediatric patient. Ann Allergy Asthma Immunol 2017;119(1):88–9. [DOI] [PubMed] [Google Scholar]
- 77.Ramirez RM, Jacobs RL. Eosinophilic esophagitis treated with immunotherapy to dust mites. J Allergy Clin Immunol 2013;132(2):503–4. [DOI] [PubMed] [Google Scholar]
- 78.Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017;30(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.ES. RCaD. Patient reported outcomes in esophageal diseases. Clin Gastroenterol Hepatol 2017;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Franciosi JP, Hommel KA, DeBrosse CW, Greenberg AB, Greenler AJ, Abonia JP, et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol 2011;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taft TH, Kern E, Kwiatek MA, Hirano I, Gonsalves N, Keefer L. The adult eosinophilic oesophagitis quality of life questionnaire: a new measure of health-related quality of life. Aliment Pharmacol Ther 2011;34(7):790–8. [DOI] [PubMed] [Google Scholar]
- 82.Dellon ES, Cotton CC, Gebhart JH, Higgins LL, Beitia R, Woosley JT, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin Gastroenterol Hepatol 2016;14(1):31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirano K, Fukae J, Hieda S, Fujimaki M, Ishii H, Tsuboi Y, et al. Eosinophilic meningitis caused by primary angiitis of the central nervous system. Intern Med 2013;52(12):1393–6. [DOI] [PubMed] [Google Scholar]
- 84.Warners MJ, Hindryckx P, Levesque BG, Parker CE, Shackelton LM, Khanna R, et al. Systematic Review: Disease Activity Indices in Eosinophilic Esophagitis. Am J Gastroenterol 2017;112(11):1658–69. [DOI] [PubMed] [Google Scholar]
- 85.Reed CC, Wolf WA, Cotton CC, Rusin S, Perjar I, Hollyfield J, et al. Optimal Histologic Cutpoints for Treatment Response in Patients With Eosinophilic Esophagitis: Analysis of Data From a Prospective Cohort Study. Clin Gastroenterol Hepatol 2018;16(2):226–33 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolf WA, Cotton CC, Green DJ, Hughes JT, Woosley JT, Shaheen NJ, et al. Evaluation of Histologic Cutpoints for Treatment Response in Eosinophilic Esophagitis. J Gastroenterol Hepatol Res 2015;4(10):1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Philpott H, Dellon ES. The role of maintenance therapy in eosinophilic esophagitis: who, why, and how? J Gastroenterol 2018;53(2):165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lucendo AJ, Arias A, Gonzalez-Cervera J, Olalla JM, Molina-Infante J. Dual response to dietary/topical steroid and proton pump inhibitor therapy in adult patients with eosinophilic esophagitis. J Allergy Clin Immunol 2016;137(3):931–4 e2. [DOI] [PubMed] [Google Scholar]
- 89.Sodikoff J, Hirano I. Proton pump inhibitor-responsive esophageal eosinophilia does not preclude food-responsive eosinophilic esophagitis. J Allergy Clin Immunol 2016;137(2):631–3. [DOI] [PubMed] [Google Scholar]
- 90.Reimer C Safety of long-term PPI therapy. Best Pract Res Clin Gastroenterol 2013;27(3):443–54. [DOI] [PubMed] [Google Scholar]
- 91.Scarpignato C, Gatta L, Zullo A, Blandizzi C, Group S-A-F, Italian Society of Pharmacology tIAoHG, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med 2016;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017;152(4):706–15. [DOI] [PubMed] [Google Scholar]
- 93.Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011;9(2):110–7. [DOI] [PubMed] [Google Scholar]
- 94.Francis DL, Foxx-Orenstein A, Arora AS, Smyrk TC, Jensen K, Nord SL, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012;35(2):300–7. [DOI] [PubMed] [Google Scholar]
- 95.Vazquez-Elizondo G, Ngamruengphong S, Khrisna M, Devault KR, Talley NJ, Achem SR. The outcome of patients with oesophageal eosinophilic infiltration after an eight-week trial of a proton pump inhibitor. Aliment Pharmacol Ther 2013;38(10):1312–9. [DOI] [PubMed] [Google Scholar]
- 96.Molina-Infante J, Rivas MD, Hernandez-Alonso M, Vinagre-Rodriguez G, Mateos-Rodriguez JM, Duenas-Sadornil C, et al. Proton pump inhibitor-responsive oesophageal eosinophilia correlates with downregulation of eotaxin-3 and Th2 cytokines overexpression. Aliment Pharmacol Ther 2014;40(8):955–65. [DOI] [PubMed] [Google Scholar]
- 97.Dellon ES, Speck O, Woodward K, Covey S, Rusin S, Gebhart JH, et al. Markers of eosinophilic inflammation for diagnosis of eosinophilic esophagitis and proton pump inhibitor-responsive esophageal eosinophilia: a prospective study. Clin Gastroenterol Hepatol 2014;12(12):2015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol 2015;135(1):187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut 2013;62(6):824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lucendo AJ, Arias A, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2016;14(1):13–22 e1. [DOI] [PubMed] [Google Scholar]
- 101.Gutierrez-Junquera C, Fernandez-Fernandez S, Cilleruelo ML, Rayo A, Echeverria L, Quevedo S, et al. High Prevalence of Response to Proton-pump Inhibitor Treatment in Children With Esophageal Eosinophilia. J Pediatr Gastroenterol Nutr 2016;62(5):704–10. [DOI] [PubMed] [Google Scholar]
- 102.Gomez-Torrijos E, Garcia-Rodriguez R, Castro-Jimenez A, Rodriguez-Sanchez J, Mendez Diaz Y, Molina-Infante J. The efficacy of step-down therapy in adult patients with proton pump inhibitor-responsive oesophageal eosinophilia. Aliment Pharmacol Ther 2016;43(4):534–40. [DOI] [PubMed] [Google Scholar]
- 103.Molina-Infante J, Rodriguez-Sanchez J, Martinek J, van Rhijn BD, Krajciova J, Rivas MD, et al. Long-Term Loss of Response in Proton Pump Inhibitor-Responsive Esophageal Eosinophilia Is Uncommon and Influenced by CYP2C19 Genotype and Rhinoconjunctivitis. Am J Gastroenterol 2015;110(11):1567–75. [DOI] [PubMed] [Google Scholar]
- 104.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology 2014;147(6):1238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chuang MY, Chinnaratha MA, Hancock DG, Woodman R, Wong GR, Cock C, et al. Topical Steroid Therapy for the Treatment of Eosinophilic Esophagitis (EoE): A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol 2015;6:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murali AR, Gupta A, Attar BM, Ravi V, Koduru P. Topical steroids in eosinophilic esophagitis: Systematic review and meta-analysis of placebo-controlled randomized clinical trials. J Gastroenterol Hepatol 2016;31(6):1111–9. [DOI] [PubMed] [Google Scholar]
- 107.Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther 2015;41(9):797–806. [DOI] [PubMed] [Google Scholar]
- 108.Tan ND, Xiao YL, Chen MH. Steroids therapy for eosinophilic esophagitis: Systematic review and meta-analysis. J Dig Dis 2015;16(8):431–42. [DOI] [PubMed] [Google Scholar]
- 109.Cotton CC, Eluri S, Wolf WA, Dellon ES. Six-Food Elimination Diet and Topical Steroids are Effective for Eosinophilic Esophagitis: A Meta-Regression. Dig Dis Sci 2017;62(9):2408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology 2014;147(2):324–33 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006;131(5):1381–91. [DOI] [PubMed] [Google Scholar]
- 112.Andreae DA, Hanna MG, Magid MS, Malerba S, Andreae MH, Bagiella E, et al. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children With Eosinophilic Esophagitis. Am J Gastroenterol 2016;111(8):1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alexander JA, Jung KW, Arora AS, Enders F, Katzka DA, Kephardt GM, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2012;10(7):742–9 e1. [DOI] [PubMed] [Google Scholar]
- 114.Moawad FJ, Veerappan GR, Dias JA, Baker TP, Maydonovitch CL, Wong RK. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol 2013;108(3):366–72. [DOI] [PubMed] [Google Scholar]
- 115.Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci 2010;55(5):1313–9. [DOI] [PubMed] [Google Scholar]
- 116.Lee J, Shuker M, Brown-Whitehorn T, Cianferoni A, Gober L, Muir A, et al. Oral viscous budesonide can be successfully delivered through a variety of vehicles to treat eosinophilic esophagitis in children. J Allergy Clin Immunol Pract 2016;4(4):767–8. [DOI] [PubMed] [Google Scholar]
- 117.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 2010;139(2):418–29. [DOI] [PubMed] [Google Scholar]
- 118.Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010;139(5):1526–37, 37 e1. [DOI] [PubMed] [Google Scholar]
- 119.Dellon ES, Sheikh A, Speck O, Woodward K, Whitlow AB, Hores JM, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012;143(2):321–4 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miehlke S, Hruz P, Vieth M, Bussmann C, von Arnim U, Bajbouj M, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 2016;65(3):390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I, et al. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology 2017;152(4):776–86 e5. [DOI] [PubMed] [Google Scholar]
- 122.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2015;13(1):66–76 e3. [DOI] [PubMed] [Google Scholar]
- 123.Rubinstein E, Hait EE, Mitchell PD, Lee JJ. Every Other Day Dosing of Oral Viscous Budesonide is not Effective in the Management of Eosinophlic Esophagitis. J Pediatr Gastroenterol Nutr 2017. [DOI] [PubMed] [Google Scholar]
- 124.Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2011;9(5):400–9 e1. [DOI] [PubMed] [Google Scholar]
- 125.Greuter T, Bussmann C, Safroneeva E, Schoepfer AM, Biedermann L, Vavricka SR, et al. Long-Term Treatment of Eosinophilic Esophagitis With Swallowed Topical Corticosteroids: Development and Evaluation of a Therapeutic Concept. Am J Gastroenterol 2017;112(10):1527–35. [DOI] [PubMed] [Google Scholar]
- 126.Eluri S, Runge TM, Hansen J, Kochar B, Reed CC, Robey BS, et al. Diminishing Effectiveness of Long-Term Maintenance Topical Steroid Therapy in PPI Non-Responsive Eosinophilic Esophagitis. Clin Transl Gastroenterol 2017;8(6):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dellon ES KD, Collins MH, Hamdi M, Gupta SK, Hirano I. Safety and efficacy of oral budesonide suspension for maintenance therapy in eosinophilic esophagitis: Results from a prospective open-label study of adolescents and adults. . Gastroenterology 2016;150(Suppl 1):S188. [Google Scholar]
- 128.Jensen ET, Kuang KZ, Chen HX, Landes LE, McConnel KA, Almond MA, et al. Longitudinal growth outcomes following first line treatment for pediatric eosinophilic esophagitis patients. J Pediatr Gastroenterol Nutr 2018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, Putnam PE, Backeljauw PF. Adrenal Insufficiency after Chronic Swallowed Glucocorticoid Therapy for Eosinophilic Esophagitis. J Pediatr 2016;170:240–5. [DOI] [PubMed] [Google Scholar]
- 130.Philpott H DM, Reed CC, Caldwell M, Kirk D, Torpy D, Dellon ES. . Adrenal insufficiency secondary to swallowed topical corticosteroids in eosinophilic esophagitis: a systematic review. Aliment Pharmacol Ther 2018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995;109(5):1503–12. [DOI] [PubMed] [Google Scholar]
- 132.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014;146(7):1639–48. [DOI] [PubMed] [Google Scholar]
- 133.Warners MJ, Vlieg-Boerstra BJ, Verheij J, van Rhijn BD, Van Ampting MT, Harthoorn LF, et al. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment Pharmacol Ther 2017;45(6):777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peterson KA, Byrne KR, Vinson LA, Ying J, Boynton KK, Fang JC, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013;108(5):759–66. [DOI] [PubMed] [Google Scholar]
- 135.Franciosi JP, Hommel KA, DeBrosse CW, Greenberg AB, Greenler AJ, Abonia JP, et al. Quality of life in paediatric eosinophilic oesophagitis: what is important to patients? Child Care Health Dev 2012;38(4):477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lucendo AJ, Sanchez-Cazalilla M, Molina-Infante J, Perez-Martinez I, Tenias JM, Barrio J, et al. Transcultural adaptation and validation of the “Adult Eosinophilic Esophagitis Quality of Life Questionnaire” into Spanish. Rev Esp Enferm Dig 2014;106(6):386–94. [PubMed] [Google Scholar]
- 137.Arias A, Perez-Martinez I, Tenias JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2016;43(1):3–15. [DOI] [PubMed] [Google Scholar]
- 138.Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. Allergy tests do not predict food triggers in adult patients with eosinophilic oesophagitis. A comprehensive prospective study using five modalities. Aliment Pharmacol Ther 2016;44(3):223–33. [DOI] [PubMed] [Google Scholar]
- 139.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol 2002;109(2):363–8. [DOI] [PubMed] [Google Scholar]
- 140.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol 2007;119(2):509–11. [DOI] [PubMed] [Google Scholar]
- 141.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol 2012;130(2):461–7 e5. [DOI] [PubMed] [Google Scholar]
- 142.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006;4(9):1097–102. [DOI] [PubMed] [Google Scholar]
- 143.Sampson HA Update on food allergy. J Allergy Clin Immunol 2004;113(5):805–19; quiz 20. [DOI] [PubMed] [Google Scholar]
- 144.Kliewer KL, Venter C, Cassin AM, Abonia JP, Aceves SS, Bonis PA, et al. Should wheat, barley, rye, and/or gluten be avoided in a 6-food elimination diet? J Allergy Clin Immunol 2016;137(4):1011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kliewer KL, Cassin AM, Venter C. Dietary Therapy for Eosinophilic Esophagitis: Elimination and Reintroduction. Clin Rev Allergy Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 146.Molina-Infante J, Arias A, Barrio J, Rodriguez-Sanchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: A prospective multicenter study. J Allergy Clin Immunol 2014;134(5):1093–9 e1. [DOI] [PubMed] [Google Scholar]
- 147.Molina-Infante J, Arias A, Alcedo J, Garcia-Romero R, Casabona-Frances S, Prieto-Garcia A, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2–4-6 study. J Allergy Clin Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 148.Spergel JM, Shuker M. Nutritional management of eosinophilic esophagitis. Gastrointest Endosc Clin N Am 2008;18(1):179–94; xi. [DOI] [PubMed] [Google Scholar]
- 149.Doerfler B, Bryce P, Hirano I, Gonsalves N. Practical approach to implementing dietary therapy in adults with eosinophilic esophagitis: the Chicago experience. Dis Esophagus 2015;28(1):42–58. [DOI] [PubMed] [Google Scholar]
- 150.Lucendo AJ, Arias A, Gonzalez-Cervera J, Yague-Compadre JL, Guagnozzi D, Angueira T, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013;131(3):797–804. [DOI] [PubMed] [Google Scholar]
- 151.Reed CC, Fan C, Koutlas NT, Shaheen NJ, Dellon ES. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;46(9):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mukkada VA, Haas A, Maune NC, Capocelli KE, Henry M, Gilman N, et al. Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics 2010;126(3):e672–7. [DOI] [PubMed] [Google Scholar]
- 153.Haas AM, Maune NC. Clinical presentation of feeding dysfunction in children with eosinophilic gastrointestinal disease. Immunol Allergy Clin North Am 2009;29(1):65–75, ix. [DOI] [PubMed] [Google Scholar]
- 154.Menard-Katcher C, Henry M, Furuta GT, Atkins D, Maune NC, Haas AM. Significance of feeding dysfunction in eosinophilic esophagitis. World J Gastroenterol 2014;20(31):11019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moawad FJ, Cheatham JG, DeZee KJ. Meta-analysis: the safety and efficacy of dilation in eosinophilic oesophagitis. Aliment Pharmacol Ther 2013;38(7):713–20. [DOI] [PubMed] [Google Scholar]
- 156.Moawad FJ, Molina-Infante J, Lucendo AJ, Cantrell SE, Tmanova L, Douglas KM. Systematic review with meta-analysis: endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;46(2):96–105. [DOI] [PubMed] [Google Scholar]
- 157.Moole H, Jacob K, Duvvuri A, Moole V, Dharmapuri S, Boddireddy R, et al. Role of endoscopic esophageal dilation in managing eosinophilic esophagitis: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(14):e5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dellon ES, Gibbs WB, Rubinas TC, Fritchie KJ, Madanick RD, Woosley JT, et al. Esophageal dilation in eosinophilic esophagitis: safety and predictors of clinical response and complications. Gastrointest Endosc 2010;71(4):706–12. [DOI] [PubMed] [Google Scholar]
- 159.Dellon ES Management of refractory eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol 2017;14(8):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leung J, Mehrzad R, Hundal NV, Alejos A, Hesterberg PE, Katz AJ, et al. Longitudinal Perspective on Managing Refractory Eosinophilic Esophagitis. J Allergy Clin Immunol Pract 2015;3(6):951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mukkada VA, Furuta GT. Management of refractory eosinophilic esophagitis. Dig Dis 2014;32(1–2):134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sodikoff J, Hirano I. Therapeutic strategies in eosinophilic esophagitis: Induction, maintenance and refractory disease. Best Pract Res Clin Gastroenterol 2015;29(5):829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wolf WA, Cotton CC, Green DJ, Hughes JT, Woosley JT, Shaheen NJ, et al. Predictors of response to steroid therapy for eosinophilic esophagitis and treatment of steroid-refractory patients. Clin Gastroenterol Hepatol 2015;13(3):452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of Eosinophilic Gastritis, Gastroenteritis, and Colitis: Estimates From a National Administrative Database. J Pediatr Gastroenterol Nutr 2016;62(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mansoor E, Saleh MA, Cooper GS. Prevalence of Eosinophilic Gastroenteritis and Colitis in a Population-Based Study, From 2012 to 2017. Clin Gastroenterol Hepatol 2017;15(11):1733–41. [DOI] [PubMed] [Google Scholar]
- 166.Caldwell JM, Collins MH, Stucke EM, Putnam PE, Franciosi JP, Kushner JP, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol 2014;134(5):1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rothenberg ME Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 2004;113(1):11–28; quiz 9. [DOI] [PubMed] [Google Scholar]
- 168.Prussin C Eosinophilic gastroenteritis and related eosinophilic disorders. Gastroenterol Clin North Am 2014;43(2):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 1990;31(1):54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chang JY, Choung RS, Lee RM, Locke GR 3rd, Schleck CD, Zinsmeister AR, et al. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin Gastroenterol Hepatol 2010;8(8):669–75; quiz e88. [DOI] [PubMed] [Google Scholar]
- 171.Ko HM, Morotti RA, Yershov O, Chehade M. Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol 2014;109(8):1277–85. [DOI] [PubMed] [Google Scholar]
- 172.Reed C, Woosley JT, Dellon ES. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis 2015;47(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Collins MH Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am 2014;43(2):257–68. [DOI] [PubMed] [Google Scholar]
- 174.Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol 2011;18(5):335–48. [DOI] [PubMed] [Google Scholar]
- 175.Lee CM, Changchien CS, Chen PC, Lin DY, Sheen IS, Wang CS, et al. Eosinophilic gastroenteritis: 10 years experience. Am J Gastroenterol 1993;88(1):70–4. [PubMed] [Google Scholar]
- 176.Gonsalves N, Doerfler B, Yang GY, Hirano I. S1861 A Prospective Clinical Trial of Six Food Elimination Diet or Elemental Diet in the Treatment of Adults with Eosinophilic Gastroenteritis. Gastroenterol 2009;136(5 Suppl 1):A–280. [Google Scholar]
- 177.Straumann A Eosinophilic esophagitis: emerging therapies and future perspectives. Gastroenterol Clin North Am 2014;43(2):385–94. [DOI] [PubMed] [Google Scholar]
- 178.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut 2010;59(1):21–30. [DOI] [PubMed] [Google Scholar]
- 179.Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011;141(5):1593–604. [DOI] [PubMed] [Google Scholar]
- 180.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2012;129(2):456–63, 63 e1–3. [DOI] [PubMed] [Google Scholar]
- 181.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2015;135(2):500–7. [DOI] [PubMed] [Google Scholar]
- 182.Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer A, Straumann A, Grimm M, Smith H, Tompkins C, Woo A, Peach R, Frohna P, Gujrathi S, Aranda R, Dellon E. A randomized, double-blind, placebo-controlled trial of a novel recombinant, humanized, anti-interleukin-13 monoclonal antibody (RPC4046) in patients with active eosinophilic esophagitis: Results of the HEROES study. United European Gastroenterol J 2016;4(5 Suppl):A1–156. [Google Scholar]
- 183.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, Schoepfer AM, Safroneeva E, Rothenberg ME, Falk GW, Assouline-Dayan Y, Qing Z, Swanson BN, Pirozzi G, Mannent L, Graham NMH, Akinlade B, Radin A. Dupilumab Efficacy and Safety in Adult Patients with Active Eosinophilic Esophagitis: A Randomized Double-Blind Placebo-Controlled Phase 2 Trial. Am J Gastroenterol 2017;112(S1). [DOI] [PubMed] [Google Scholar]
- 184.Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Antieosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy 2013;68(3):375–85. [DOI] [PubMed] [Google Scholar]
- 185.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014;147(3):602–9. [DOI] [PubMed] [Google Scholar]
- 186.Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol 2008;122(2):425–7. [DOI] [PubMed] [Google Scholar]


