Abstract
Regenerative medicine is currently the focus of global attention. Countries all around the world are actively working to create new regenerative treatment modalities through pioneering research and novel technologies. This is wonderful news for patients who could not be treated with existing medical options. New venture businesses and companies are being established in regenerative medicine and their rapid industrialization is anticipated. However, to ensure high-quality products, human resources qualified in research and development and the manufacturing of these products are essential. The Forum for Innovative Regenerative Medicine (FIRM) conducted a questionnaire of its industry members to examine the training and hiring of people in research and development, product creation, manufacturing, and more. Regenerative medicine is a brand new field; thus, many different businesses will need to cooperate together. People with a broad range of technical skills, abilities, and knowledge will be in demand, with various levels of expertise, from basic to advanced.
Keywords: Regenerative medicine, Human resource, Education, Training
Abbreviations: FIRM, Forum for Innovative Regenerative Medicine; iPS, induced pluripotent stem; GMP, Good Manufacturing Practice; GCTP, Good Gene, Cellular, and Tissue-based Products Manufacturing Practice
Highlights
-
•
A questionnaire was used to clarify human resource needs in regenerative medicine.
-
•
Participants were asked about the skills and experiences needed in human resources.
-
•
Participants were asked about the state of education and training for personnel.
-
•
Participants were asked about systems for developing human resources.
1. Introduction
Ever since Yamanaka et al. discovered induced pluripotent stem (iPS) cells [1], regenerative medicine has advanced in leaps and bounds, with extensive progress in clinical research and studies in Japan and globally [2]. In Japan, two laws were established in November 2014 [3], [4], [5], [6]. The Act on the Safety of Regenerative Medicine and The Act on Pharmaceuticals and Medical Devices (PMD Act) were implemented to ensure an infrastructure existed that was conducive to the development of innovative regenerative medicine products. The research and development, clinical research, clinical studies, and marketing of Japan's regenerative medicine products are currently drawing attention from around the globe [7]. In Japan, two products were newly available in 2015, HeartSheet® and Temcell® [5], [8], after JACE® and JACC®, showing amazing progress in this field.
Regenerative medicine has revived hope in many patients who, until now, lacked viable therapy options other than highly-advanced treatments such as organ transplantation. These new technologies have the potential to offer alternative treatment modalities to these patients. Those who, until now, expected to require life-long treatment may now have alternative options, which will also offer medical economic benefits [5], [9], [10]. However, regenerative medicine itself still carries a high price-tag, which must be addressed [11]. In addition, the shortage of human resources is a barrier for the development of regenerative medicine.
Regenerative medicine is a rapidly developing field and its progress has come primarily from within academia and venture businesses where the necessary human talent had been nurtured on an individual basis. As industrialization progresses, however, many venture businesses and corporations will engage in product development and marketing, focusing attention on the need for human resource development. Industrial associations must identify the type and number of people that will be required in key positions. For future progress in the regenerative therapy industry, not only will we need to identify what sort of personnel will become necessary, but also how to train people and how to undertake initiatives to meet these needs.
The Forum for Innovative Regenerative Medicine (FIRM) is a federation of approximately 200 venture businesses and companies that work towards the industrial promotion of regenerative medicine [12]. The FIRM comprises not only highly-specialized venture businesses and companies, but also includes those that deal in pharmaceutical products and medical devices and want to enter this field; businesses in related fields such as those creating machines, devices, reagents, and media; those that distribute these products; health insurance companies that provide medical insurance; human resource companies; and other businesses in a diverse range of fields. All these institutions have joined our association because they are invested in the future development of regenerative medicine.
The FIRM will need to acknowledge the challenges facing these businesses and to clarify their ideals and the type of employees that they seek. Thus, we conducted a survey of all the corporate members to gather this information. As technology grows ever more sophisticated, employees in these fields are required to maintain cutting-edge technology and expertise in these specialties. The highly diverse industries that are part of FIRM revealed that regenerative medicine calls for cooperation among various companies with distinct specialties and each will require highly-specialized personnel. This survey was carefully constructed to gain a better understanding of the talent cultivation needed, the type of opportunities required to train these personnel, the needed certification programs, and the way to establish the training systems.
2. Methods
This questionnaire study was conducted, by the education board at FIRM, to survey the special need for human resources among the fields of regenerative medicine. This survey was conducted from November 2–16, 2015. A questionnaire was sent to all full members and supporting members of FIRM, a total of 175 companies. The questionnaire consisted of 100 items relevant to (1) the background of the responder company, (2) plans for training personnel and hiring, (3) plans for a training program and method, (4) required human resources, and (5) the need for a certification and training system. All questions had some alternatives, and responders could select one or more (when indicated) options. Responders could also add comments with sentences for some questions. Questionnaire sheets were sent to the FIRM office by mail, and the answers were aggregated. The results were monitored and we received a quality certification by STATcom Co. LTD.
3. Results
3.1. Background
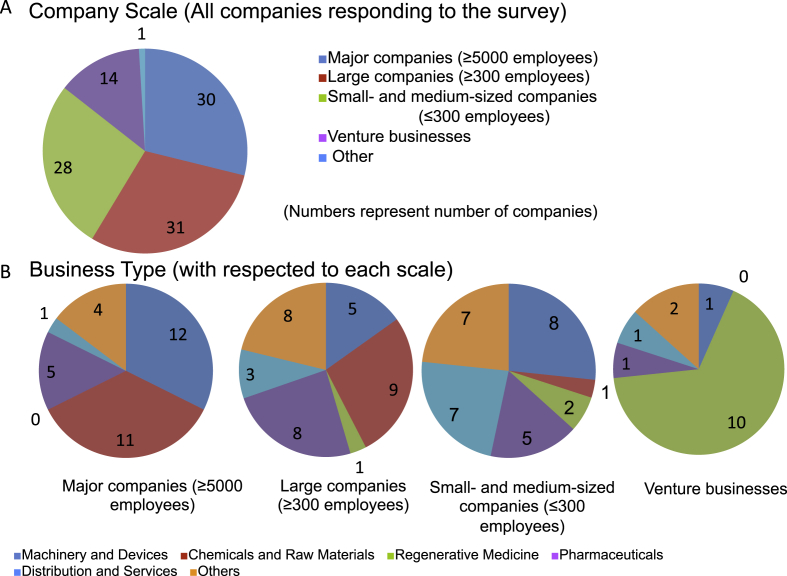
We received replies from 110 of the 175 companies, for a very high recovery rate of 63% that reflected the high level of interest among FIRM member companies in training personnel in this field. Companies that responded to the questionnaire were classified by scale. The largest companies with ≥5000 employees accounted for 31%, those with ≥300 accounted for 30%, small- to medium-sized businesses accounted for 28%, and ventures and miscellaneous businesses made up the remaining 15%; therefore, company sizes were evenly distributed. Industry types were analyzed based on company size and we found major corporations tended to be involved in peripheral industries such as machinery, devices, chemicals, and raw materials. As the company scale grew smaller, the more likely they were to be dealing directly in regenerative medicine, and companies focused on regenerative medicine were primarily venture businesses (Fig. 1).
Fig. 1.
Background of responders: Company scale and business. A. Company scales of responders. Indicated numbers show numbers of responder companies fitting into respective options. Company size is distributed almost uniformly. B. Type of business in each scale. Most of the major companies were in the machinery, devices, chemicals, raw materials and related industries, while the smaller-sized companies tended to be in the regenerative medicine industry.
3.2. Plans for personnel training and hiring
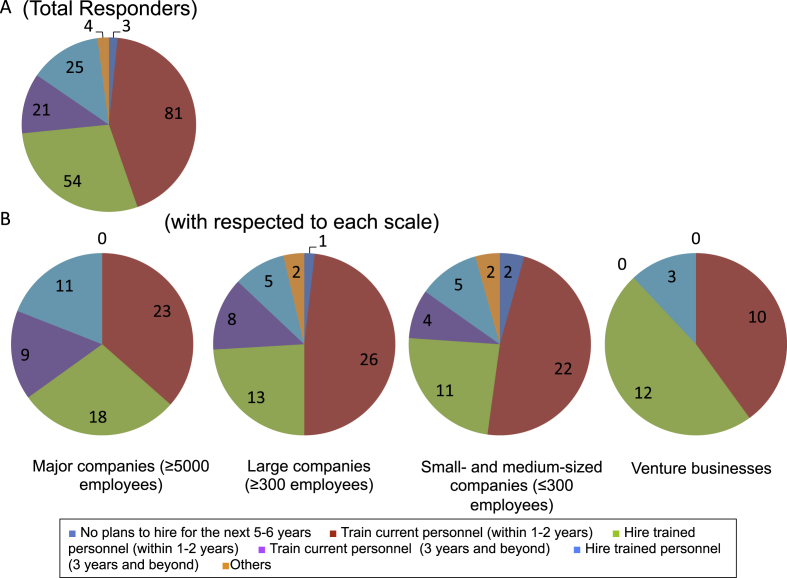
When participants were allowed multiple options regarding future personnel training and hiring plans for regenerative fields and business development, we found that 70% or more of all venture businesses and companies were thinking of training the personnel that they had already hired as well as hiring people that had been trained in relevant fields within the next 1–2 years. When these data were analyzed based on company size, many venture businesses and companies regardless of size were seeking to train and hire people within the next 1–2 years, but this tendency was more prominent in smaller companies (Fig. 2).
Fig. 2.
Plans for personnel training and hiring. Responder companies were asked if they were planning to train current employees or to hire already trained personnel. A. Total responders. At least 70% plan to train and hire personnel within the next 1–2 years. B. Analysis based on company size. Regardless of company size, most companies plan to hire or train personnel, but this tendency was more prominent in the smaller-sized companies.
3.3. Plans for training programs and methods
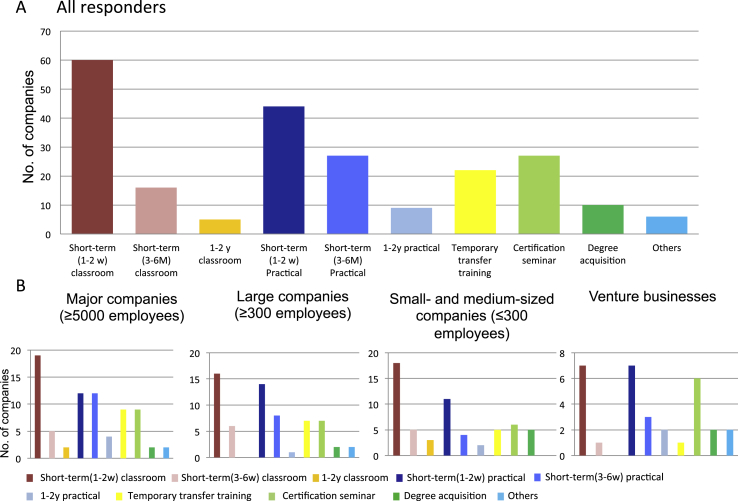
In training human resources, companies were asked what types of employee training programs or measures were in place or ideal with the option for multiple replies. We found that most felt the best option would be short-term classroom-based learning or practice-based human resource training (1–2 weeks); while, there was little interest in long-term personnel training programs (1–2 years). Although the demand is smaller, there were some who felt a mid- to long-term temporary assignment for training purposes would prove useful, and this tendency was seen regardless of the company size (Fig. 3). Based on these results, the availability of human resources to meet the need for rapid developments in regenerative medicine is an issue that must be addressed immediately. People with highly-specialized training are in great demand in regenerative medicine. Human resource training programs and other means of meeting these needs are also in demand.
Fig. 3.
Training program and methods (Choose up to 3 answers). All responders were asked what kind of training they were planning to perform to their employees. Upper panel shows total answers from all responders, and lower panels show those in each business scale. Short-term (1–2 weeks) of classroom or practical training was most popular. Only a few respondents wished for long-term personnel training options. On the other hand, there were some who wished for training through temporary transfers. This tendency was seen regardless of company size.
3.4. Human resources required
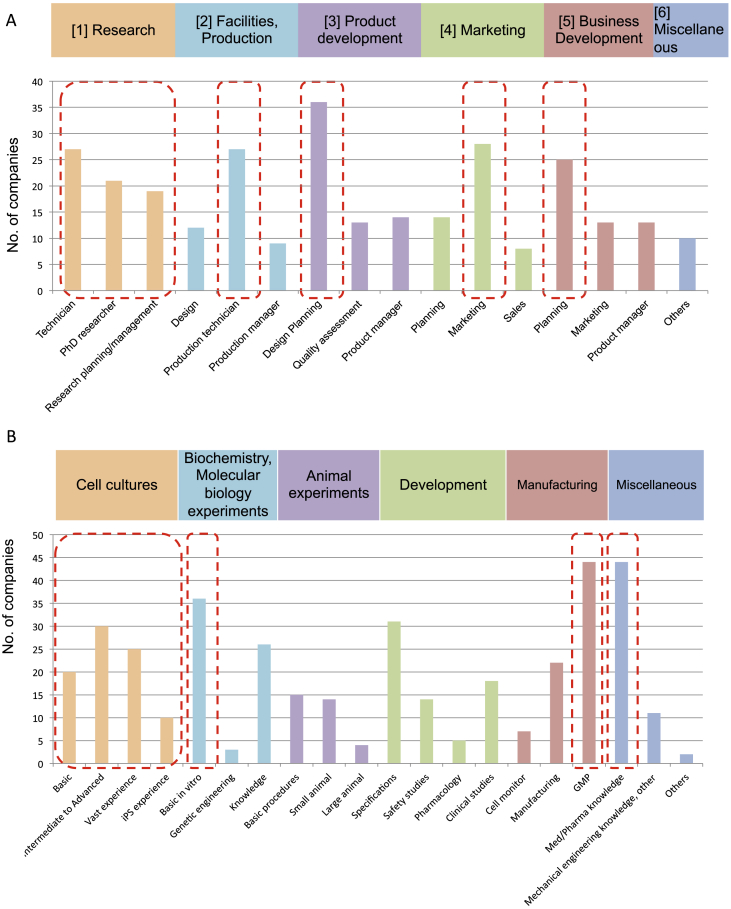
Next, in starting up businesses in the regenerative medicine field, we surveyed what specific types of personnel these companies were looking for, allowing multiple replies. Needs in research, facilities and manufacturing, product development, sales, and business expansion were surveyed and it appears people are in demand in all these fields. When the fields are broken down further, there is a need for researchers with a broad range of skill levels, ranging from basic technicians to PhD-level personnel who are capable of research planning and management. In the field of manufacturing facilities and production, when given a choice of personnel with skills in design, production technologists, and production management personnel, production technologists were in highest demand. It was assumed that they meant cell processing technicians. In product development, the choices were design and planning, quality evaluation, and product management. Design and planning skills were in highest demand. For sales, when planning, marketing, and sales personnel were given as choices, marketing was in greatest demand. The business development choices included planning, marketing, and product management, and planning was in the highest demand. These results demonstrated that all types of personnel training are needed in the regenerative medicine field (Fig. 4A).
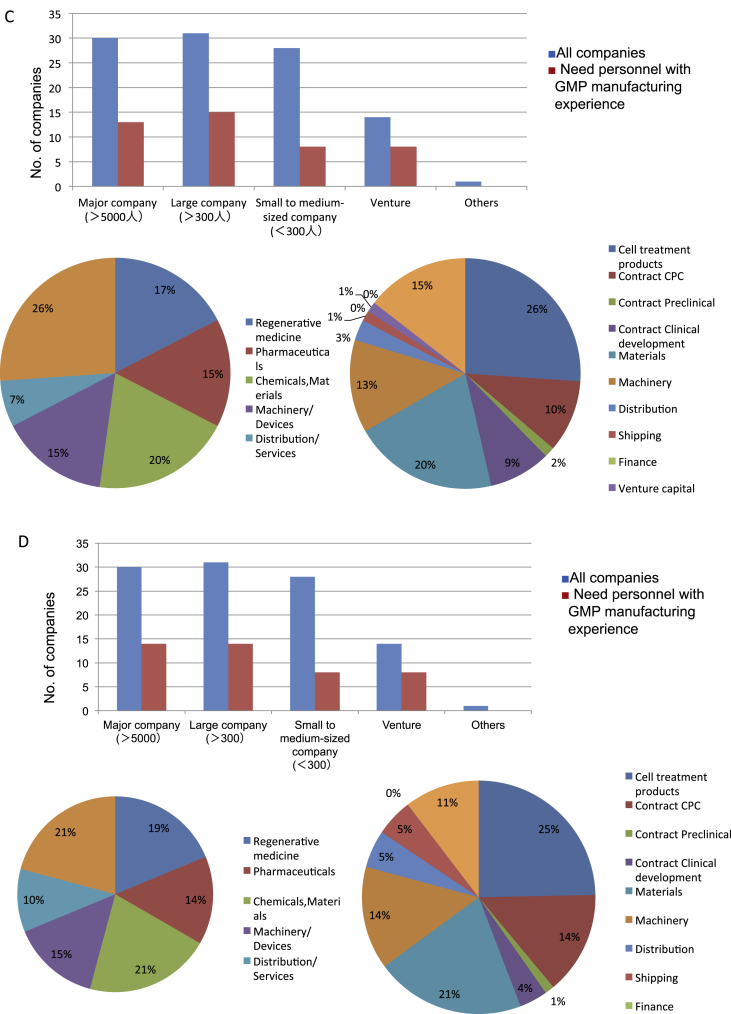
Fig. 4.
What kind of personnel is needed in companies related in regenerative medicine? A. Job type (Choose up to 3 answers). In research field, people are needed at all levels from novices to highly trained personnel to management class. Those who can perform cellular manipulation are the production technicians in need. In product development, design and planning abilities are in demand. In sales, marketing, and business development, people who are capable of planning are in demand. B. Persons with the necessary knowledge, experience, and technology (1 answer). Scientists experienced in various forms of cell culturing are in high demand. Basic in vitro tests are believed to be basic experiments using cells. There is not much need for animal experimentation experience. Establishment of planning in development and GMP manufacturing experience are believed to be in need to handle product manufacturing. People with Med/Pharma knowledge are in need. C. Needs for personnel with GMP manufacturing experiences (Includes negotiations with authorities, notification, etc.). Upper panel: Blue bars show all companies, and red bars show companies that require personnel with GMP manufacturing experiences. As many as 1/3 to 1/2 of all respondents said they needed employees with GMP manufacturing experiences. Lower left panel: Business fields of companies that required GMP experience and knowledge in their employees. Lower right panel: Business contents of companies that required GMP experience and knowledge in their employees. They were expected in broad range of business. In particular, people with GMP experience and knowledge are needed in companies seeking to enter industries that are directly involved in the creation of regenerative products. D. Needs for personnel with Medical/Pharmaceutical knowledge. Upper panel: Blue bars show all companies, and red bars show companies that require personnel with medical and pharmaceutical knowledge. Just as observed in demand for GMP experiences, as many as 1/3 to 1/2 of all respondents said they needed employees with med/pharm knowledge. Lower left panel: Business fields of companies that required med/pharm knowledge in their employees. Lower right panel: Business contents of companies that required med/pharm knowledge in their employees. They were expected in broad range of business. In particular, people with med/pharm knowledge are needed in companies seeking to enter industries that are directly involved in the creation of regenerative products.
Next, we looked at the knowledge, experience, and technology needs after detailed stratification. First, we surveyed what type of cell culture technology was required in research and production and found that a very broad range of expertise was in demand. The specific requirements ranged from basic cell-culturing experience to intermediate and advanced skills, including the ability to isolate cells from tissue or create primary cultures and experience in culturing iPS cells. For biochemical and molecular biology experimentation, the ability to perform basic in vitro assays and FACS analysis were in demand. We believe this referred to personnel who could conduct basic experiments and procedures involving cells. The demand for very advanced genetic manipulation was not necessarily high. Although we asked about experience in animal experimentation at various levels from those with experience using small animals to those with experience handling large animals, results showed that the need for people with animal experimentation experience was limited.
In development, we inquired how companies viewed experience in the design and planning of cellular medicine, in safety studies and pharmacology studies, and in clinical studies (including investigator-led studies). Although there was a demand for all these specialties, it was only seen in a limited percentage of the overall FIRM companies. However, we believe this demand will grow as the research and development of regenerative medicine move forward. For manufacturing, a survey investigated the need for people with three abilities: those developing technology to monitor administered cells for tissue distribution and imaging of graft survival, those capable of mass scale culturing and changes in culture morphology, and those with experience in Good Manufacturing Practice (GMP). The highest demand was for individuals with GMP experience. Moreover, an overwhelming demand for personnel with medical or pharmaceutical knowledge came to light from a question on the demand for personnel with expertise in various areas such as medical/pharmaceutical, mechanical or engineering, or those with other specialties (Fig. 4B).
We decided to look a little further into the skills that were in highest demand, namely GMP (or Good Gene, Cellular, and Tissue-based Products Manufacturing Practice [GCTP]) manufacturing experience and those with medical and pharmaceutical knowledge. Cell product manufacturing is new to this industry, and so all companies must prepare their facilities to meet GMP (or GCTP) standards for these products. We confirmed that the companies realize they will need to hire people with experience and knowledge in GMP (or GCTP) standards and plan to prepare their facilities to handle the manufacture of cell products. These people are needed by one-third to one-half of all the companies who answered our survey. Although the businesses that require these human resources may be diverse, it can be assumed that these personnel will be required by all businesses that are directly involved in the creation of regenerative medicine products or are seeking to enter this field (Fig. 4C).
Regenerative therapy differs from pharmaceuticals or medical devices in that the machinery, raw materials, and distribution are intricately interrelated. Many venture businesses and companies that have never dealt in pharmaceuticals and medical device before are now joining the field; thus, there is an extremely high level of interest in employing people with medical and pharmaceutical knowledge. These needs are reflected in the fact that one-third to one-half of all companies who participated in the survey reported a demand for people with these backgrounds (Fig. 4D). The current workforce lacks many of the requirements to enter the novel field known as regenerative medicine, revealing the demand for people with broader technical expertise, skills, knowledge, and experience.
3.5. Need for certification and current training methods
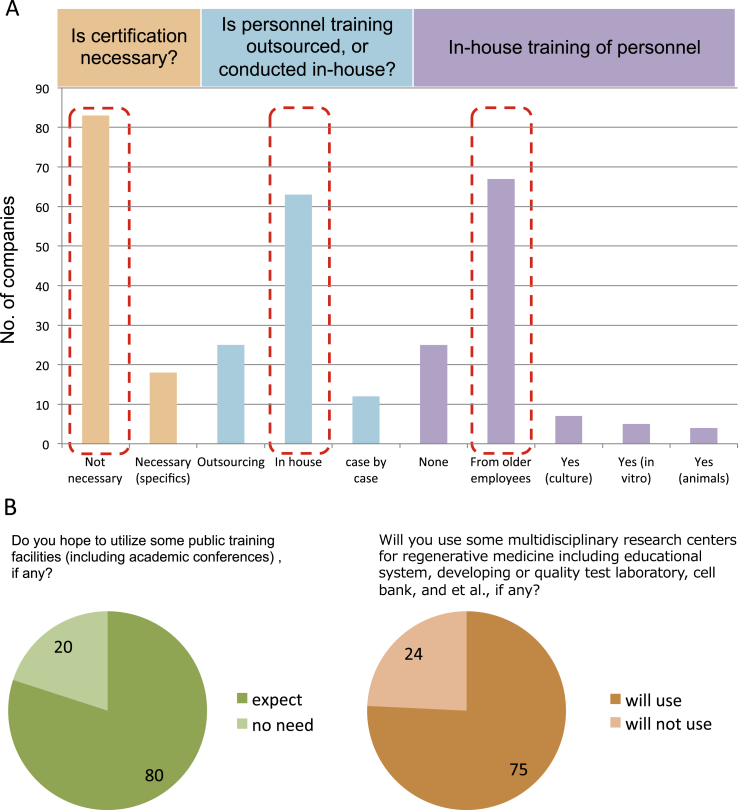
Next, in order to find and train the necessary manpower for regenerative medicine, we asked what kind of system or certification would be required. However, an extremely large number of respondents replied that such certification was unnecessary. This means that even if a person lacks specific qualifications for regenerative therapy, this would not be an issue in terms of employment if they have the necessary potential. This may be because there is no commonly accepted certification for regenerative medicine. Current programs rely on a leader or more-experienced employees to conduct in-house training. Few, if any, have developed a systematized training program within their companies (Fig. 5A). These tendencies are common regardless of the company's size and areas of specialization (Supplemental Fig. 5). As industrialization of regenerative medicine progresses, we expect this is an area in which a solution is necessary. Importantly, the majority of responders answered that they are expecting some public support in education/training and other systems, regardless of the company's scale (Fig. 5B, Supplemental Figs. 5 and 6).
Fig. 5.
Need for Certification and public support. A. Need for certification and current personnel training methods. Orange bars show the need for certification to their employees. Most portion answered that certification is not needed. Blue and violet bars show current training systems they adopt. Currently, personnel is trained by in-house mentors as part of the corporate education program, but many companies do not have any training structure in place. These are common tendencies regardless of company scale or industry. B. Expectation of public support for education/training and others in regenerative medicine. For last questions, needs for public supports in regenerative medicine were asked. Indicated numbers show numbers of total responders.
4. Discussion
As described above, great strides are rapidly being made in regenerative medicine. However, for true industrialization of this technology, the lack of highly-specialized personnel must be addressed. Thus, there is an immediate call for the training and education of such workers. Academia and industry must collaborate to create the necessary means and structures to train and educate the type of professionals who can ensure progress and disseminate regenerative therapies. It is interesting that the large majority of responders regarded some kinds of certification or license as not necessary. However, they expect some public training system to be established. It means that really substantial but not merely formal training systems are desired to meet the urgent demands for substantive and practical human resources.
5. Conclusion
We conducted a questionnaire survey aimed at discerning the need for training and education among companies related to regenerative medicine. The results stress that a firm foundation for human resource development has never been established in the field of regenerative medicine. We clearly must build the necessary infrastructure to train people in various fields to meet the needs of regenerative medicine and the industry. Hence, we must move forward rapidly to address this problem and ensure that highly effective, top quality regenerative medicine products can be delivered as soon as possible to waiting patients.
Acknowledgments
The authors wish to thank all the participating companies.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.reth.2017.06.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Takahashi K., Tanaka K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y., Inoue H., Wu J.C., Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. Dec. 2016;16(2):115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda D., Yamaguchi T., Ishizuka T., Hirata M., Takekita K., Sato D. Regulatory frameworks for gene and cell therapies in Japan. Adv Exp Med Biol. 2015;871:147–162. doi: 10.1007/978-3-319-18618-4_8. [DOI] [PubMed] [Google Scholar]
- 4.Okada K., Miyata T., Sawa Y. Insurance systems and reimbursement concerning research and development of regenerative medicine in Japan. Regen Med. Mar. 2017;12(2):179–186. doi: 10.2217/rme-2016-0124. [DOI] [PubMed] [Google Scholar]
- 5.Tsukamoto A., Abbot S.E., Kadyk L.C., DeWitt N.D., Schaffer D.V., Wertheim J.A. Challenging regeneration to transform medicine. Stem Cells Transl Med. Jan. 2016;5(1):1–7. doi: 10.5966/sctm.2015-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobita M., Konomi K., Torashima Y., Kimura K., Taoka M., Kaminota M. Japan's challenges of translational regenerative medicine: act on the safety of regenerative medicine. Regen Ther. 2016;4:78–81. doi: 10.1016/j.reth.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inokuma Y. Pharmacovigilance of regenerative medicine under the amended pharmaceutical affairs act in Japan. Drug Saf. Mar. 2017;40(6):475–482. doi: 10.1007/s40264-017-0517-2. [DOI] [PubMed] [Google Scholar]
- 8.Coghlan A. First fully approved ‘off the shelf’ stem cells launch in Japan. New Sci. 2016:8–9. [Google Scholar]
- 9.Faulkner A. Opening the gateways to market and adoption of regenerative medicine? The UK case in context. Regen Med. Apr. 2016;11(3):321–330. doi: 10.2217/rme-2015-0046. [DOI] [PubMed] [Google Scholar]
- 10.Okano T., Sawa Y., Barber E., Umezawa A. Regenerative therapy by fusion of medicine and engineering: first-in-human clinical trials with induced pluripotent stem cells and cell sheet technology: a report of the Symposium of Regenerative Medicine for Patients. Regen Ther. 2015;2:2–5. doi: 10.1016/j.reth.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culme-Seymour E.J., Mason K., Vallejo-Torres L., Carvalho C., Partington I., Crowley C. Cost of stem cell-based tissue-engineered airway transplants in the United Kingdom: case series. Tissue Eng Part A. Feb. 2016;22(3–4):208–213. doi: 10.1089/ten.tea.2015.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa T., Harris I., Joung J., Kanai N., Kawamata S., Kellathur S. Report of the International regulatory forum on human cell therapy and gene therapy products. Biologicals. Sep. 2016;44(5):467–479. doi: 10.1016/j.biologicals.2016.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.