Abstract
Background
Vitamin D is a hormone with immunomodulatory properties. Vitamin D deficiency has been reported for patients with inflammatory bowel disease (IBD). In this cross-sectional study, 25-hydroxyvitamin D3 (25-OH-D3) levels in patients with IBD were compared to those in patients with irritable bowel syndrome (IBS).
Methods
A total of 181 patients, 156 with IBD and 25 with IBS, were included. The influence of disease activity, inflammatory markers, physical activity, and season were assessed.
Results
A total of 58.6$ (n = 58) of the patients with Crohn's disease (CD) and 44.6$ (n = 25) of the patients with ulcerative colitis (UC) had a 25-OH-D3 level < 50 nmol/L. CD patients showed significantly decreased 25-OH-D3 levels compared to the IBS patients (p = 0.018), but no significant difference was found for UC patients. In a linear regression model adjusted for age, gender, and BMI, a significant inverse association of C-reactive protein (CRP) (p = 0.031) and faecal calprotectin (FC) (p = 0.025) with 25-OH-D3 levels was observed for CD patients. Seasonal variation in 25-OH-D3 levels was found in CD patients, with significantly lower values in spring than in summer (p = 0.04).
Conclusion
Vitamin D deficiency was common in all IBD patients, but more pronounced in CD patients, in whom it also showed a significant inverse association with inflammatory markers such as CRP and FC.
Keywords: Inflammatory bowel disease, Vitamin D, Crohn's disease, Ulcerative colitis
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gut related to a combination of genetic and environmental factors that have an impact on host-microbe interactions [1]. IBD includes two different diseases: Crohn's disease (CD) and ulcerative colitis (UC). Epidemiologic studies have demonstrated that IBD is more frequently diagnosed in northern countries and at higher altitudes, and frequently diagnosed during winter [2, 3, 4, 5]. It has been observed that vitamin D deficiency occurs more frequently among IBD patients than in the general population, and has therefore recently received more attention as a potential environmental factor in the pathogenesis of IBD [6, 7, 8]. As vitamin D is mainly produced in the skin upon exposure to sunlight (UVB) or is absorbed by food intake in the small intestine, several factors such as malabsorption, reduced vitamin D in the diet, insufficient physical activity, and insufficient exposure to sunlight or intestinal inflammation may cause the deficiency [9]. Vitamin D is involved in a wide range of physiological processes and especially well characterized in the regulation of calcium homoeostasis. It is recommended to IBD patients with active disease and those who are treated with steroids to monitor for serum calcium as well as vitamin D and to supplement it if required to help prevent low bone mineral density [10]. However, low serum vitamin D levels have also been reported in various immune-related diseases, pointing to an immunomodulatory function. Vitamin D receptors are located in most tissues and cells of the body and in the intestine, with high expression in the small intestine and colon. Vitamin D and its receptor are known to interact with different players of the immune system involved in innate immune functions and adaptive T-cell responses, as well as in maintenance of the integrity of the intestinal barrier [11, 12, 13].
Different clinical studies confirmed an association between vitamin D status and markers of disease activity and inflammation [14, 15, 16, 17]. IBD patients with low serum vitamin D required more drugs such as steroids and biological treatment, and they showed an increased risk of relapse with an increased rate of emergency department visits and hospital admissions, suggestive of a more severe disease course [18, 19]. Normalization of serum vitamin D levels was associated with a lower risk of CD-related surgery when compared to patients with a low serum vitamin D level [20]. These data suggest that lack of vitamin D relevantly contributes to inflammatory processes in the gut and therefore may be a detrimental environmental factor for the course of the disease in patients with IBD.
The aim of this single-centre, cross-sectional study was to assess the vitamin D status of patients with IBD compared to patients with irritable bowel syndrome (IBS). Additional relevant confounders were examined in the IBD group (e.g., disease activity, inflammatory markers, BMI, age, gender, physical activity, and season) to identify possible determinants of vitamin D deficiency in IBD patients.
Methods
Study Design and Subjects
This single-centre, prospective, cross-sectional study was performed at the Department of Gastroenterology and Hepatology, University Hospital Basel, Basel, Switzerland. Between April 2016 and January 2017, 277 patients with CD, UC, or IBS who were treated at the University Hospital Basel were asked to participate. Of these, 181 agreed to participate, signed the informed consent form, and were included in the study.
The inclusion criteria were the following: IBD patients in clinical remission, IBD patients with a chronically active but medically controlled disease status, IBD patients hospitalized due to active exacerbated disease (evaluation within the first 3 days of hospitalization), and patients with IBS. Excluded were patients unwilling to participate and those aged < 18 years. The diagnosis of IBD was confirmed by endoscopic and histological analysis. For a diagnosis of IBS, the Rome III criteria were applied, and IBS patients had to undergo at least an endoscopic and histological analysis and had to have levels of faecal calprotectin (FC) < 20 μg/g stool and tissue transglutaminase IgA < 5 IU/mL to exclude the presence of inflammation in the gastrointestinal tract or coeliac disease. All procedures, laboratory evaluations, and epidemiological data were assessed on the day of examination.
Assessments
Analytical Assays
Blood samples were analysed for laboratory indices including albumin and markers of systemic inflammation (C-reactive protein [CRP], leucocytes, and platelet count) through routine laboratory techniques.
Serum 25-hydroxyvitamin D3 (25-OH-D3) was measured with a competitive electrochemiluminescence binding assay using the cobas® modular analyser platform (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Results are expressed in nanomole per litre.
For evaluation of intestinal inflammation, the patients received a small tube to store a stool sample at home which was collected the day before consultation. After extraction from stool, FC was assessed using a competitive chemiluminescent immunoassay (DiaSorin LIAISON® Calprotectin; DiaSorin Inc., Stillwater, MN, USA). Values are expressed as micrograms per gram stool. Values were quantified within the range of 20–1,500 µg/g stool, with values below this range expressed as < 20 µg/g stool.
Definition of Disease Activity and 25-OH-D3 Deficiency
The Harvey Bradshaw Index (HBI) for CD and the Modified Truelove and Witts Severity Index (MTWSI) for UC were used for determining disease activity [21, 22, 23]. The HBI is divided into four categories: remission (< 5), mild disease (5–7), moderate disease (8–16), and severe disease (> 16). The MTWSI is defined as remission (≤3), mild disease (4–6), moderate disease (7–11), and severe disease (≥12). Each score was assessed on the day of examination. CRP levels > 5 mg/L were defined as active inflammation [21]. FC levels > 100 μg/g stool were considered a relevant elevation [24]. Vitamin D deficiency was considered with 25-OH-D3 values < 50 nmol/L [25, 26].
Vitamin D Supplementation and Physical Activity
On the day of examination, the patients were asked about vitamin D supplementation and registered as “yes” or “no” in the database. The average physical activity per week in minutes was assessed, and was then divided into three categories: low, < 30 min 5× per week; moderate, 30–60 min 5× per week; and high, ≥60 min 5× per week. Physical activity included sport (e.g., running, gym, and cycling) and general movement during the day (e.g., walking or riding to work by bike).
Statistical Methods
Descriptive statistics are presented as means (SD) for metric variables and medians (IQR) for ordinal variables and clinical parameters. The prediction variable 25-OH-D3 and the cofactors CRP and calprotectin were log transformed, as verified by preliminary analysis including quantile comparison plots. Hierarchical variable clustering analysis using Spearman's rank correlation revealed no significant collinearity between the covariates.
For comparing vitamin D levels between the three study groups (reference group: IBS), linear models predicting log-transformed 25-OH-D3 levels were performed. To separately study the influence of selected covariates on log-transformed 25-OH-D3 levels in patients with CD and UC, multiple linear models were performed using a nested model design. All models were adjusted for age, gender, and BMI. Back-transformed estimates represent ratios for categorical parameters and slopes for continuous variables, with corresponding 95$ CI and p values.
Seasonal effects were studied by pooling individual study times into three periods: spring (April to June), summer (July to September), and autumn/winter (October to January). Physical activity values were pooled in two groups (≥60 and < 60 min).
A p value < 0.05 was considered significant. Adjustment of the significance level for multiple comparisons was omitted because of the descriptive nature of the study. All analyses were performed with the statistical program R version 3.1.2 (R Core Team 2014; R Foundation for Statistical Computing, Vienna, Austria).
Results
Subject Characteristics
A total of 277 consecutive patients were asked to participate in our study; 181 (66.3$) gave written informed consent and were included in the study. Ninety-nine of the 181 participants were diagnosed with CD, 57 with UC, and 25 with IBS. The baseline characteristics of all patients are shown in Table 1; the specific baseline characteristics of the patients with CD and those with UC are shown in Table 2.
Table 1.
Baseline characteristics of the IBD and IBS patients
| CD (n = 99) | UC (n = 57) | IBS (n = 25) | |
|---|---|---|---|
| Age, years | 41.2 (14.5) | 41.5 (13.6) | 48.2 (15.2) |
| Gender | |||
| Female | 48 (48.5) | 31 (54.4) | 18 (72) |
| Male | 51 (51.5) | 26 (45.6) | 7 (28) |
| Disease duration, years | 13.8 (10.8) | 10.2 (9.4) | 4.4 (8.8) |
| BMI | 25.4 (6) | 24.2 (4.3) | 23.7 (4.7) |
| Smoking status | |||
| Current smoker | 28 (28.3) | 11 (19.3) | 8 (32) |
| Former smoker | 30 (30.3) | 16 (28.1) | 9 (36) |
| Never smoked | 41 (41.4) | 30 (52.6) | 8 (32) |
| Serum 25-OH-D3, nmol/L | 46 (34–63.5) | 52.5 (35.5–69.2) | 67 (47–78) |
| Serum 25-OH-D3 level | |||
| <50 nmol/L | 58 (58.6) | 25 (44.6) | 7 (28) |
| ≥50 nmol/L | 41 (41.4) | 31 (55.4) | 18 (72) |
| Serum 25-OH-D3 level <50 nmol/L | |||
| 25-OH-D3 supplementation | |||
| No | 46 (79.3) | 21 (84) | 6 (85.7) |
| Yes | 12 (20.7) | 4 (16) | 1 (14.3) |
| Serum 25-OH-D3 level >50 nmol/L | |||
| 25-OH-D3 supplementation | |||
| No | 22 (53.7) | 17 (54.8) | 11 (61.1) |
| Yes | 19 (46.3) | 14 (45.2) | 7 (38.9) |
Values are presented as mean (SD), median (IQR), or n (%). Age, gender, BMI, and smoking status were not significantly different between the study groups (p > 0.1). Disease duration was significantly different (CD vs. IBS, UC vs. IBS) at a p value <0.001. CD, Crohn's disease; UC, ulcerative colitis; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; 25-OH-D3, 25-hydroxyvitamin D3.
Table 2.
Baseline characteristics of the patients with CD and UC
| CD (n = 99) | UC (n = 57) | |
|---|---|---|
| Montreal classification | ||
| Age at diagnosis | ||
| A1 (<16 years) | 18 (18.2) | |
| A2 (16–40 years) | 69 (69.7) | |
| A3 (>40 years) | 12 (12.1) | |
| Disease location (UC) | ||
| E1 (proctitis) | 7 (12.3) | |
| E2 (left colitis) | 30 (52.6) | |
| E3 (pancolitis) | 20 (35.1) | |
| Disease location (CD) | ||
| L1 (terminal ileum) | 19 (19.4) | |
| L2 (colon) | 25 (25.5) | |
| L3 (ileum-colon) | 39 (39.8) | |
| L4 (upper gastrointestinal tract) | 1 (1) | |
| Disease behaviour (CD) | ||
| B1 (non-stenotic, non-penetrating) | 52 (53.1) | |
| B2 (stenotic) | 24 (24.5) | |
| B3 (penetrating) | 11 (11.2) | |
| B3p (perianal penetrating) | 11 (11.2) | |
| Number of drugs per patient | ||
| 0 | 9 (9.1) | 5 (8.8) |
| 1 | 77 (77.8) | 37 (64.9) |
| 2 | 13 (13.1) | 10 (17.5) |
| ≥3 | 0 | 5 (8.8) |
| Current use of medication for IBD | ||
| 5-Aminosalicylic acid | 2 (2) | 21 (28.8) |
| Immunomodulators | 11 (10.7) | 8 (10.9) |
| Integrin antagonists | 6 (5.8) | 11 (15.1) |
| TNF-α blockers | 71 (68.9) | 25 (34.2) |
| Glucocorticoids | 13 (12.6) | 8 (11) |
| Disease activity1 | ||
| Remission | 70 (70.7) | 31 (54.4) |
| Mild | 13 (13.1) | 16 (28.1) |
| Moderate | 14 (14.1) | 9 (15.8) |
| Severe | 2 (2) | 1 (1.8) |
| Calprotectin, µg/g stool | 187.5 (53–394.8) | 137 (60–702) |
| ≤100 µg/g stool | 38 (38.8) | 24 (42.1) |
| »100 µg/g stool | 60 (61.2) | 33 (57.9) |
| CRP, mg/L | 1.7 (0.8–5) | 1.5 (0.6–3.8) |
| ≤5 mg/L | 75 (75.8) | 46 (80.7) |
| »5 mg/L | 24 (24.2) | 11 (19.3) |
| Leucocytes, ×109/L | 6.3 (5–8.2) | 5.7 (4.7–7.5) |
| ≤10 × 109/L | 89 (89.9) | 53 (93) |
| »10 × 109/L | 10 (10.1) | 4 (7) |
Values are presented as median (IQR) or n (%). CD, Crohn's disease; UC, ulcerative colitis; IBD, inflammatory bowel disease; HBI, Harvey Bradshaw Index for CD (remission <5, mild 5–7, moderate 8–16, and severe >16); MTWSI, Modified Truelove and Witts Severity Index for UC (remission <3, mild 4–6, moderate 7–11, and severe ≥12); CRP, C-reactive protein.
According to the HBI for CD and the MTWSI for UC.
Most patients with CD (70.7$; n = 70) and UC (54.4$; n = 31) were in clinical remission according to the clinical scores on the HBI and MTWSI, respectively.
Vitamin D Deficiency in IBD Compared to IBS Patients
The first analysis showed that vitamin D levels were decreased in the patients with CD (median: 46 nmol/L; IQR: 34–63.5) and UC (median: 52.5 nmol/L; IQR: 35.5–69.2) when compared to the IBS patients (median: 67 nmol/L; IQR: 47–78). A distinction between insufficient and adequate vitamin D levels (< 50 vs. ≥50 nmol/L) was made, and it was observed that 58.6$ (58/99) of the patients with CD, 44.6$ (25/57) of the patients with UC, and 28$ (7/25) of the IBS patients had an insufficient 25-OH-D3 level (< 50 nmol/L) (Table 1). Of these patients, 31.3$ (n = 31) of those with CD and 31.6$ (n = 18) of those with CU had a prescription for vitamin D supplementation at the time of analysis. A higher percentage of sufficient vitamin D levels (> 50 nmol/L) - i.e., 46.3$ of the CD and 45.2$ of UC patients - was observed among the patients with vitamin D supplementation.
In the linear regression analysis (adjusted for age, gender, and BMI), 25-OH-D3 levels were significantly different between CD patients and IBS controls (ratio: 0.761; 95$ CI: 0.608–0.954; p = 0.018) (Table 3; Fig. 1), but not between UC patients and IBS controls. As some patients had vitamin D substitution at the time of evaluation, the same analysis was also performed only for patients without vitamin D supplementation, which still demonstrated a significant difference between CD and IBS patients (ratio: 0.723; 95$ CI: 0.549–0.952; p = 0.021). No significant difference was observed between UC and IBS patients (Table 3).
Table 3.
Linear regression model predicting log-transformed 25-OH-D3 by comparing the three study groups (reference group: IBS) adjusted for age, gender, and BMI
| Comparison | Ratio | 95% CI | p value | |
|---|---|---|---|---|
| With vitamin D supplementation | CD vs. IBS UC vs. IBS |
0.761 0.829 |
0.608–0.954 0.653–1.052 |
0.018 0.121 |
| Without vitamin D supplementation | CD vs. IBS UC vs. IBS |
0.723 0.816 |
0.549–0.952 0.608–1.094 |
0.021 0.172 |
Bold type denotes significance. CD, Crohn's disease; UC, ulcerative colitis; IBS, irritable bowel syndrome; 25-OH-D3, 25-hydroxyvitamin D3.
Fig. 1.

Boxplot of 25-OH-D3 levels in the three study groups (IBS, CD, and UC). 25-OH-D3, 25-hydroxyvitamin D3; CD, Crohn's disease; UC, ulcerative colitis; IBS, irritable bowel syndrome.
Association between Inflammatory Markers and 25-OH-D3 Levels
Most of the patients with CD and UC were in clinical remission. For CD patients, a median FC level of 187.5 μg/g stool (IQR: 53–394.8) was observed; for UC patients, the median FC level was 137 μg/g stool (IQR: 60–702) (Table 2). Approximately 60$ of CD and UC patients had an FC level > 100 μg/g stool at the time of analysis. The median CRP levels were 1.7 mg/L (IQR: 0.8–5) for CD patients and 1.5 mg/L (IQR: 0.6–3.8) for UC patients. Only 24.2$ of CD patients and 19.3$ of UC patients had a CRP level > 5 mg/L.
Linear regression analysis demonstrated that serum 25-OH-D3 levels were significantly inversely correlated with FC levels (slope: 0.918; 95$ CI: 0.852–0.989; p = 0.025) in CD patients (Fig. 2a; Table 4). However, no correlation was found between FC and 25-OH-D3 levels in UC patients. Further analysis showed that the correlation for CD patients appeared only in those patients with acute inflammation, and therefore we differentiated inflammation from no inflammation by an FC cut-off at 100 µg/g stool, which showed that an FC level > 100 µg/g stool had a significant influence on 25-OH-D3 levels in CD patients (ratio: 0.802; 95$ CI: 0.647–0.995; p = 0.045).
Fig. 2.
a Inverse correlation of calprotectin with 25-OH-D3 levels in the patients with CD. The ellipse represents 95$ of the given data. b Inverse correlation of 25-OH-D3 levels with log-transformed CRP for CD (dotted line), but no correlation for UC (full line). 25-OH-D3, 25-hydroxyvitamin D3; CD, Crohn's disease; UC, ulcerative colitis; CRP, C-reactive protein.
Table 4.
Multiple linear model of log-transformed 25-OH-D3 and selected inflammatory markers in CD and UC adjusted for age, gender, and BMI including subgroup analysis
| Parameter | Group | Comparison | Slope/ratio | 95% CI | p value |
|---|---|---|---|---|---|
| log calprotectin | CD | 0.918 | 0.852–0.989 | 0.025 | |
| UC | 0.987 | 0.903–1.079 | 0.767 | ||
| Calprotectin | CD1 | >100 vs. ≤100 µg/g stool | 0.802 | 0.647–0.995 | 0.045 |
| log CRP | CD | 0.91 | 0.835–0.992 | 0.031 | |
| UC | 1.101 | 0.979–1.239 | 0.107 | ||
| CRP | CD1 | >5 vs. ≤5 mg/L | 0.822 | 0.642–1.054 | 0.121 |
| Leucocytes | CD | 1.016 | 0.975–1.059 | 0.434 | |
| UC | 1.057 | 0.993–1.124 | 0.08 | ||
| CD1 | >10 vs. ≤10 × 109/L | 1.148 | 0.820–1.609 | 0.418 | |
| UC1 | >10 vs. ≤10 × 109/L | 1.487 | 0.875–2.530 | 0.142 | |
Bold type denotes significance. CD, Crohn's disease; UC, ulcerative colitis; 25-OH-D3, 25-hydroxyvitamin D3; CRP, C-reactive protein.
Subgroup analysis.
Similar to FC levels, CRP levels were significantly inversely correlated with serum 25-OH-D3 levels in CD patients (slope: 0.91; 95$ CI: 0.835–0.992; p = 0.031) (Fig. 2b; Table 4). Again, no correlation was found between CRP and 25-OH-D3 levels in UC. The differentiation between inflammation (> 5 mg/L) and no inflammation (≤5 mg/L) was not associated with significant differences (slope: 0.822; 95$ CI: 0.642–1.054; p = 0.121) for CD patients.
No significant correlation was observed between leucocyte count (> 10 × 109/L) and 25-OH-D3 levels in both UC and CD patients. In addition, in a subgroup analysis the correlation between high leucocyte levels and intake of steroids was examined. Systemic steroids were taken by 8/57 (14$) of UC patients and by 13/99 (13.1$) of CD patients, and these patients expectedly showed a significant correlation (p < 0.001) when compared to the patients who did not take steroids.
Association between Disease Activity Scores and 25-OH-D3 Levels
Disease activity was determined by clinical scores on the HBI for CD patients and the MTWSI for UC patients. The analysis of 25-OH-D3 deficiency in correlation to the HBI showed an inverse trend (slope: 0.976; 95$ CI: 0.951–1.002; p = 0.074) (Fig. 3; Table 5). A subgroup analysis was performed for the HBI to see if there were differences between subgroups concerning disease activity (remission, mild disease, moderate, and severe disease) and 25-OH-D3 levels, but no differences were observed. There was no correlation between MTWSI and 25-OH-D3 levels in UC patients.
Fig. 3.

Inverse correlation of the disease activity score on the HBI and 25-OH-D3 levels in the patients with CD. The ellipse represents 95$ of the given data. HBI, Harvey Bradshaw Index; 25-OH-D3, 25-hydroxyvitamin D3; CD, Crohn's disease.
Table 5.
Multiple linear model of log-transformed 25-OH-D3 and selected variables in CD and UC adjusted for age, gender, and BMI
| Parameter | Group | Comparison | Ratio/slope | 95% CI | p value |
|---|---|---|---|---|---|
| Clinical | CD | HBI | 0.976 | 0.951–1.002 | 0.074 |
| UC | MTWSI | 0.965 | 0.918–1.014 | 0.16 | |
| Season | CD | Apr–Jun vs. Jul–Sep | 0.801 | 0.648–0.99 | 0.04 |
| Oct–Jan vs. Jul–Sep | 0.623 | 0.364–1.067 | 0.084 | ||
| UC | Apr–Jun vs. Jul–Sep | 0.933 | 0.689–1.263 | 0.65 | |
| Oct–Jan vs. Jul–Sep | 0.913 | 0.563–1.48 | 0.71 | ||
| Physical activity | CD | Physical activity | 1.101 | 0.893–1.358 | 0.365 |
| UC | Physical activity | 1.218 | 0.891–1.666 | 0.214 | |
| Disease duration | CD | Disease duration | 0.993 | 0.984–1.003 | 0.181 |
| UC | Disease duration | 1.005 | 0.99–1.02 | 0.484 | |
Bold type denotes significance. Physical activity values were pooled in two groups (≥60 vs. <60 min). Seasonal effects were studied by pooling individual study times per month in three periods: spring (Apr–Jun), summer (Jul–Sept), and autumn/winter (Oct–Jan). Summer was set as the reference period. CD, Crohn's disease; UC, ulcerative colitis; HBI, Harvey Bradshaw Index for CD; MTWSI, Modified Truelove and Witts Severity Index for UC; 25-OH-D3, 25-hydroxyvitamin D3.
Association between Season and 25-OH-D3 Levels
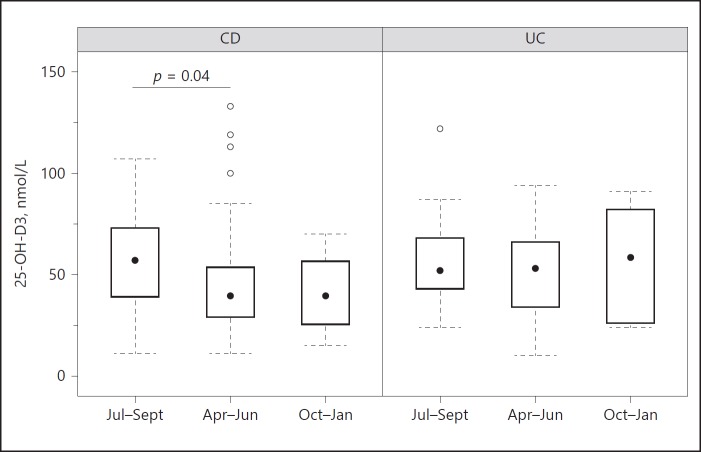
The patients in this study were enrolled between April 2016 and January 2017. Therefore, we investigated whether seasonal differences could be responsible for any variation in 25-OH-D3 serum levels. As the patients were enrolled within a time frame of 10 months, the following seasons were set for analysis: spring (April to June), summer (July to September), and autumn/winter (October to January); summer was used as the reference. The assessment showed that 25-OH-D3 levels were significantly lower in spring (ratio: 0.801; 95$ CI: 0.648–0.99; p = 0.04) and that there was a trend for low 25-OH-D3 levels in autumn/winter (ratio: 0.623; 95$ CI: 0.364–1.067; p = 0.084) in CD patients. No associations between the different seasons and 25-OH-D3 levels were found for UC patients (Fig. 4; Table 5).
Fig. 4.
Boxplots showing 25-OH-D3 levels in spring (Apr–Jun), summer (Jul–Sept), and autumn/winter (Oct–Jan) in the patients with CD and UC. In the patients with CD, 25-OH-D3 levels were significantly enhanced in summer compared to spring, as revealed by linear regression adjusted for age, gender, and BMI. CD, Crohn's disease; UC, ulcerative colitis; 25-OH-D3, 25-hydroxyvitamin D3.
Associations of Physical Activity (< 60 vs. ≥60 min) and Disease Duration with 25-OH-D3 Levels
There was no significant association between physical activity (< 60 vs. ≥60 min) and 25-OH-D3 levels for both UC and CD patients (Table 5). Disease duration showed a significant inverse association with serum 25-OH-D3 levels in CD patients (slope: 0.983; 95$ CI: 0.972–0.994; p = 0.003) in the model that was adjusted for age, gender, and BMI. Because disease duration and age may be highly correlated, a single-model analysis adjusted for gender and BMI was performed. In this model, there was no significant effect for CD (slope: 0.993; 95$ CI: 0.984–1.003; p = 0.181). No correlation was seen between disease duration and 25-OH-D3 levels in UC patients (Table 5).
Discussion
Vitamin D is a fat-soluble secosteroid hormone that has been attributed to immunomodulatory functions in patients with IBD [27]. High prevalence rates of vitamin D deficiency among IBD patients ranging between 22 and 63$ - depending on which threshold value for serum vitamin D was used to signify deficiency - indicate its significance as an environmental factor in IBD [7, 28, 29, 30, 31]. The data from our cross-sectional analysis confirm the findings regarding vitamin D deficiency from previous reports, since up to 60$ of the patients with CD and approximately 45$ of the patients with UC had serum 25-OH-D3 levels < 50 nmol/L as compared to only 25$ of the patients in the IBS group. The 25$ prevalence rate of vitamin D deficiency among our IBS patients is comparable to the prevalence rate reported for the general population [32]. Independently of vitamin D supplementation, we found a significant difference in serum 25-OH-D3 levels between the CD and IBS patients. Similar to our findings, results from other reports more frequently showed insufficient 25-OH-D3 serum levels in CD patients than in UC patients [33].
The result that CD patients have a lower vitamin D level suggests that the resorption of vitamin D in the small intestine in these patients may be compromised by inflammatory activity or for other reasons, and it may indicate the need for additional vitamin D substitution in order to reach a sufficient level > 50 nmol/L. A recently published review of two small open-label trials and one randomized controlled trial found a positive effect of vitamin D supplementation on disease activity at least in patients with CD [34]. Oral supplementation for CD patients in remission with 1,200 IU vitamin D3 was shown to significantly increase serum vitamin D levels and to reduce the risk of relapse in these patients [35]. In our study, only approximately 30$ of the IBD patients were supplemented with 1,000–1,500 IU vitamin D3 per day at the time of the analysis, and in a substantial proportion of these IBD patients we did not reach a sufficient vitamin D level > 50 nmol/L. Although adherence issues and the exact dosing of vitamin D supplementation was not fully addressed in our evaluation, the above suggests that the recommended level of supplementation may not prevent vitamin D deficiency in all patients and that higher supplementation and/or better monitoring may therefore be needed especially in IBD patients at risk [36]. A small study on patients with active disease supplemented with vitamin D with a targeted vitamin D serum level of 100–125 nmol/L over a 12-week treatment period showed an improvement in symptom-based activity scores but no alteration in inflammatory markers [37].
Our analysis revealed that in CD patients, disease activity was inversely correlated with serum 25-OH-D3 levels, as shown for the inflammation markers FC and CRP. By differentiating between inflammation (FC > 100 µg/g stool) and no inflammation (FC < 100 µg/g stool) in our study, higher FC values were associated with lower 25-OH-D3 serum levels, indicating that a low vitamin D status is associated with active disease in patients with CD. Other authors reported that FC levels were significantly inversely associated with 25-OH-D3 levels in CD patients [15, 17], and this confirms an association of low vitamin D level with increased disease activity [7, 16]. A correlation of cytokine levels with vitamin D status has been shown in patients with CD, with the finding that a low vitamin D status is associated with reduced levels of the anti-inflammatory cytokine interleukin-10 but with no association with serum TNF-α levels [38]. However, also in UC patients an inverse association of mucosal inflammation with serum 25-OH-D3 levels was reported [15, 39]. Other studies showed that a low quality of life correlates with a low serum vitamin D level [14, 31, 33].
Besides its absorption in the small intestine, vitamin D is produced in the skin upon exposure to sunlight, and a lack of it is associated with an increased risk of osteoporosis and fractures, as well as of autoimmune and infectious diseases [40, 41]. Our study has shown that patients with CD had fluctuating 25-OH-D3 levels during different seasons. When comparing the different periods of spring, summer, and autumn/winter, patients with CD had lower 25-OH-D3 levels in spring and autumn than in the summer months, suggesting that exposure to sunlight influences vitamin D levels in patients with IBD. A study from Germany showed that adults have low vitamin D levels especially at higher altitudes, during winter and spring, and when having a low physical activity level [42]. Another study showed that patients with CD had low vitamin D levels in winter, but there was no statistically significant correlation between seasonal vitamin D level and disease activity [43]. In our study, we included questions about physical activity, which was measured by asking patients how often they did sports or generally moved in everyday life in an average week. Nonetheless, in our cohort physical activity did not seem to have influenced 25-OH-D3 levels in the patients with CD and UC. However, as a limitation we should state that we did not distinguish between indoor and outdoor sports activity in order to exactly quantify any outdoor activity as a possible measure of exposure to sunlight; our evaluation permitted only to assess for physical activity in two categories (≥60 vs. < 60 min). Therefore, it cannot be concluded whether physical activity is associated with vitamin D levels.
Disease duration has been reported to influence 25-OH-D3 levels in CD patients. As different studies showed that older people more often have a low vitamin D status [44], we performed a single-model analysis which was adjusted for gender and BMI to exclude any influence of age. We did not observe any correlation between disease duration and 25-OH-D3 level. As IBD is mostly diagnosed in young people (< 40 years), it could be logically concluded that patients with a longer disease duration are older and that consequently their risk of vitamin D deficiency increases [41].
An important limitation of this study is its cross-sectional design with voluntary participation and consequently an uneven distribution of patient groups. However, we believe that our evaluation is representative of the population of IBD patients treated at tertiary referral centres. Since many of the IBD patients were in remission, our statistical analysis of inflammatory parameters, especially among the patients with UC, may limit conclusions about an association of inflammation with vitamin D levels in UC patients. For organizational reasons, patient inclusion was set between April 2016 and January 2017, which limited the evaluation of seasonal effects especially during the winter months of February and March, when vitamin D deficiency may be even more pronounced in our region with a low average sunshine duration of 90–120 h per month.
In conclusion, our cross-sectional analysis of IBD patients revealed that 25-OH-D3 deficiency is found more frequently among CD patients than among UC patients. An inverse association of the inflammatory parameters calprotectin and CRP with vitamin D levels was found for patients with CD. Seasonal variation in 25-OH-D3 levels was observed among CD patients, which suggests consideration of a general substitution of IBD patients with vitamin D at least during the winter months with less exposure to sunlight.
Statement of Ethics
The study was approved by the Ethics Commission Northwest and Central Switzerland (EKNZ BASEC 2016-00071).
Disclosure Statement
All authors declare that they have no financial and non-financial competing interests or benefits from this project.
Funding Sources
The Swiss National Science Foundation (SIBDCS 2015–2020) and Takeda Pharmaceutical Company provided a research grant to support this study, but they did not have any active role in the design, collection, analysis, and interpretation of data, or in the writing of the manuscript or the decision to submit the manuscript for publication.
Author Contributions
Petr Hruz is acting as the submission's guarantor. All authors have made substantial contributions to (1) the conception and design of the study, or acquisition of data or analysis and interpretation of data; (2) drafting the article and revising it critically; and (3) final approval of the version to be submitted. All authors had full access to the data of the study and have approved the final version of the article, including the authorship list.
Acknowledgements
We acknowledge Urs Simmen for performing the statistical analysis.
References
- 1.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–36. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–36. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Economou M, Pappas G. New global map of Crohn's disease: genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis. 2008;14:709–36. doi: 10.1002/ibd.20352. [DOI] [PubMed] [Google Scholar]
- 4.Moum B, Aadland E, Ekbom A, Vatn MH. Seasonal variations in the onset of ulcerative colitis. Gut. 1996;38:376–378. doi: 10.1136/gut.38.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–697. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25 (OH) vitamin D level in Crohn's disease: association with sun exposure and disease activity. Indian J Med Res. 2009;130:133–36. [PubMed] [Google Scholar]
- 7.Sadeghian M, Saneei P, Siassi F, Esmaillzadeh A. Vitamin D status in relation to Crohn's disease: meta-analysis of observational studies. Nutrition. 2016;32:505–36. doi: 10.1016/j.nut.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Silvennoinen J. Relationships between vitamin D, parathyroid hormone and bone mineral density in inflammatory bowel disease. J Intern Med. 1996;239:131–137. doi: 10.1046/j.1365-2796.1996.420765000.x. [DOI] [PubMed] [Google Scholar]
- 9.Narula N, Marshall JK. Management of inflammatory bowel disease with vitamin D: beyond bone health. J Crohns Colitis. 2012;6:397–36. doi: 10.1016/j.crohns.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Forbes A, Escher J, Hébuterne X, Klek S, Krznaric Z, Schneider S, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321–36. doi: 10.1016/j.clnu.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Ardesia M, Ferlazzo G, Fries W. Vitamin D and inflammatory bowel disease. Biomed Res Int. 2015;2015 doi: 10.1155/2015/470805. 470805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbáchano A, Fernández-Barral A, Ferrer-Mayorga G, Costales-Carrera A, Larriba MJ, Muñoz A. The endocrine vitamin D system in the gut. Mol Cell Endocrinol. 2017;453:79–36. doi: 10.1016/j.mce.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 14.Blanck S, Aberra F. Vitamin D deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013;58:1698–36. doi: 10.1007/s10620-012-2531-7. [DOI] [PubMed] [Google Scholar]
- 15.Garg M, Rosella O, Lubel JS, Gibson PR. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2634–36. doi: 10.1097/01.MIB.0000436957.77533.b2. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF. Active Crohn's disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7:e407–e413. doi: 10.1016/j.crohns.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Raftery T, Merrick M, Healy M, Mahmud N, O'Morain C, Smith S, et al. Vitamin D status is associated with intestinal inflammation as measured by fecal calprotectin in Crohn's disease in clinical remission. Dig Dis Sci. 2015;60:2427–36. doi: 10.1007/s10620-015-3620-1. [DOI] [PubMed] [Google Scholar]
- 18.Gubatan J, Mitsuhashi S, Zenlea T, Rosenberg L, Robson S, Moss AC. Low serum vitamin D during remission increases risk of clinical relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2017;15:240–246. doi: 10.1016/j.cgh.2016.05.035. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabbani TA, Koutroubakis IE, Schoen RE, Ramos-Rivers C, Shah N, Swoger J, et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. Am J Gastroenterol. 2016;111:712–36. doi: 10.1038/ajg.2016.53. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflamm Bowel Dis. 2013;19:1921–36. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–36. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 23.Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol. 2010;8:357–36. doi: 10.1016/j.cgh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen V, Klepp P, Cvancarova M, Røseth A, Skar V, Moum B. Prediction of endoscopic disease activity in ulcerative colitis by two different assays for fecal calprotectin. J Crohns Colitis. 2015;9:164–36. doi: 10.1093/ecco-jcc/jju015. [DOI] [PubMed] [Google Scholar]
- 25.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–36. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 26.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–36. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limketkai BN, Mullin GE, Limsui D, Parian AM. Role of vitamin D in inflammatory bowel disease. Nutr Clin Pract. 2017;32:337–36. doi: 10.1177/0884533616674492. [DOI] [PubMed] [Google Scholar]
- 28.Frigstad SO, Høivik M, Jahnsen J, Dahl , SR, Cvancarova M, Grimstad T, et al. Vitamin D deficiency in inflammatory bowel disease: prevalence and predictors in a Norwegian outpatient population. Scand J Gastroenterol. 2017;52:100–36. doi: 10.1080/00365521.2016.1233577. [DOI] [PubMed] [Google Scholar]
- 29.Siffledeen JS, Siminoski K, Steinhart H, Greenberg G, Fedorak RN. The frequency of vitamin D deficiency in adults with Crohn's disease. Can J Gastroenterol. 2003;17:473–36. doi: 10.1155/2003/391308. [DOI] [PubMed] [Google Scholar]
- 30.Suibhne TN, Cox G, Healy M, O'Morain C, O'sullivan M. Vitamin D deficiency in Crohn's disease: prevalence, risk factors and supplement use in an outpatient setting. J Crohns Colitis. 2012;6:182–36. doi: 10.1016/j.crohns.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–36. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 32.Greene-Finestone LS, Berger C, de Groh M, Hanley DA, Hidiroglou N, Sarafin K, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22:1389–36. doi: 10.1007/s00198-010-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro FD, Magalhães J, Carvalho PB, Moreira MJ, Mota P, Cotter J. Lower levels of vitamin D correlate with clinical disease activity and quality of life in inflammatory bowel disease. Arq Gastroenterol. 2015;52:260–36. doi: 10.1590/S0004-28032015000400003. [DOI] [PubMed] [Google Scholar]
- 34.Hlavaty T, Krajcovicova A, Payer J. Vitamin D therapy in inflammatory bowel diseases: who, in what form, and how much? J Crohns Colitis. 2015;9:198–209. doi: 10.1093/ecco-jcc/jju004. [DOI] [PubMed] [Google Scholar]
- 35.Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, et al. Clinical trial: vitamin D3 treatment in Crohn's disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–36. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 36.Reboul E. Intestinal absorption of vitamin D: from the meal to the enterocyte. Food Funct. 2015;6:356–36. doi: 10.1039/c4fo00579a. [DOI] [PubMed] [Google Scholar]
- 37.Garg M, Rosella O, Rosella G, Wu Y, Lubel JS, Gibson PR. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr. 2017 doi: 10.1016/j.clnu.2017.06.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Kelly P, Suibhne TN, O'Morain C, O'sullivan M. Vitamin D status and cytokine levels in patients with Crohn's disease. Int J Vitam Nutr Res. 2011;81:205–36. doi: 10.1024/0300-9831/a000066. [DOI] [PubMed] [Google Scholar]
- 39.Meckel K, Li YC, Lim J, Kocherginsky M, Weber C, Almoghrabi A, et al. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am J Clin Nutr. 2016;104:113–36. doi: 10.3945/ajcn.115.123786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80((suppl)):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 41.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 42.Rabenberg M, Scheidt-Nave C, Busch MA, Rieckmann N, Hintzpeter B, Mensink GB. Vitamin D status among adults in Germany - results from the German Health Interview and Examination Survey for Adults (DEGS1) BMC Public Health. 2015;15:641. doi: 10.1186/s12889-015-2016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kini GP, Young B, Herbison P, Schultz M. Does seasonal level of serum 25-OH vitamin D correlate with the activity of Crohn's disease? NZ Med J. 2014;127:51–59. [PubMed] [Google Scholar]
- 44.Meehan M, Penckofer S. The role of vitamin D in the aging adult. J Aging Gerontol. 2014;2:60–36. doi: 10.12974/2309-6128.2014.02.02.1. [DOI] [PMC free article] [PubMed] [Google Scholar]