Abstract
The purpose of the Society of Anesthesia and Sleep Medicine Guideline on Intraoperative Management of Adult Patients With Obstructive Sleep Apnea (OSA) is to present recommendations based on current scientific evidence. This guideline seeks to address questions regarding the intraoperative care of patients with OSA, including airway management, anesthetic drug and agent effects, and choice of anesthesia type. Given the paucity of high-quality studies with regard to study design and execution in this perioperative field, recommendations were to a large part developed by subject-matter experts through consensus processes, taking into account the current scientific knowledge base and quality of evidence. This guideline may not be suitable for all clinical settings and patients and is not intended to define standards of care or absolute requirements for patient care; thus, assessment of appropriateness should be made on an individualized basis. Adherence to this guideline cannot guarantee successful outcomes, but recommendations should rather aid health care professionals and institutions to formulate plans and develop protocols for the improvement of the perioperative care of patients with OSA, considering patient-related factors, interventions, and resource availability. Given the groundwork of a comprehensive systematic literature review, these recommendations reflect the current state of knowledge and its interpretation by a group of experts at the time of publication. While periodic reevaluations of literature are needed, novel scientific evidence between updates should be taken into account. Deviations in practice from the guideline may be justifiable and should not be interpreted as a basis for claims of negligence.
The purpose of the Society of Anesthesia and Sleep Medicine (SASM) Guideline on Intraoperative Management of Adult Patients With Obstructive Sleep Apnea (OSA) is to present recommendations based on the available scientific evidence. In light of a paucity of well-designed, high-quality studies in this perioperative field, a large part of the present recommendations was developed by experts in the field taking into account published evidence in the literature and utilizing consensus processes, including the grading of the level of evidence. At times, when specific information on patients with OSA was not available in the literature, evidence in highly correlated patient populations, specifically those with obesity, was considered if appropriate. When this was the case, it is explicitly stated in various parts of this document.
The guideline presented may not be suitable for all clinical settings and patients. Thus, its consideration requires an assessment of appropriateness by clinicians on an individualized basis. Among many factors, the existence of institutional protocols, individual patient-related conditions, the invasiveness of an intervention, and the availability of resources need to be considered. The present practice guideline is not intended to define standards or represent absolute requirements for patient care. Adherence to this guideline cannot guarantee successful outcomes but rather should aid health care professionals and institutions to formulate plans for improved management of patients with OSA. The present recommendations reflect the current state of knowledge and its interpretation by a group of experts in the field at the time of publication. Periodic reevaluations of the literature will be needed, and novel scientific evidence should be considered between updates. Deviations from this guideline in the practical setting may be justifiable, and such deviations should not be interpreted as a basis for negligence claims.
OSA is a common and frequently undiagnosed disorder defined by the repeated collapse of the upper airway with resultant blood oxygen desaturation events during sleep.1,2 OSA has been associated with adverse long-term health outcomes and has been linked to increased perioperative complication risk.3–5 Indeed, a comprehensive review of the literature performed by a task force appointed by SASM revealed substantial risk for adverse events, especially pulmonary complications, to be associated with OSA in the perioperative period.6 Based on the elevated risk for perioperative complications, the recently published SASM Guideline on Preoperative Screening and Assessment of Adults With Obstructive Sleep Apnea recommends that attempts should be made to appropriately identify patients with OSA, with the goal to raise awareness among providers, mitigate risk, and improve outcomes.7 While recommendations for preoperative screening and assessment of patients with OSA and their optimal preparation for surgery are now available, there is a paucity of evidence-based guidance for the intraoperative management of this patient population. Thus, there remains a lack of evidence-based practice recommendations regarding techniques for airway management, selection of anesthetic agents, and drugs, as well as choice of anesthetic technique.
This document is derived from results of an extensive consensus process based on a systematic literature search, review, and analysis performed by experts in the field. It is a follow-up to the previously published SASM Guideline on Preoperative Screening and Assessment of Adult Patients With Obstructive Sleep Apnea.7 Given the large amount of related literature in this arena, this study focuses only on intraoperative patient care. Postoperative care issues are not considered and may be the subject of future projects.
What Other Guidelines and Reviews Are Available?
Previous OSA-related practice guidelines8–12 have been published by the American Society of Anesthesiologists,8,9 the Society for Ambulatory Anesthesia,10 the American Academy of Sleep Medicine,11 the SASM,7 the International Bariatric Consensus Guideline Group,13 and the task force on best practice recommendations for the anesthetic perioperative care and pain management in weight loss surgery.14
Why Was This Guideline Developed and How Does It Differ From Existing Guidelines?
This guideline was developed to provide evidence-based recommendations for the intraoperative management of patients with OSA. Therefore, a careful examination of the current literature using a systematic review approach with a focus on airway management, commonly used anesthesia-related drugs and agents, and anesthetic techniques in this patient population was conducted. The task force recognizes that there has been recent progress in attempts to subcategorize patients with OSA according to anatomic predisposition, arousal thresholds, muscle responsiveness, and ventilatory control characteristics.15 However, given the lack of evidence in this context, statements were made referring to patients with OSA as a general group. Nevertheless, phenotypic subcategorization may allow the development of individual risk profiling in the future.
Aims
The aim of this guideline was to present recommendations based on the best current evidence. Clinical research as it relates to best perioperative practices in OSA is burdened by numerous difficulties. The intraoperative setting involves a multitude of concurrent interventions and use of anesthetic medications, making it difficult to single out specific factors that potentially drive the adverse outcome. Lack of preoperative polysomnography data within publications represents a further challenge, making it difficult to include information of the impact of disease severity. Ethical considerations in study designs regarding the randomization of patients with known OSA were additional obstacles in this context. Furthermore, the task force recognizes that there is a tendency to underreport medical complications, rendering it difficult to establish the true perioperative risk.16 Presenting the current available evidence and its limitations should raise awareness regarding the need for high-quality studies in the future.
Specific aims were to: (1) evaluate considerations of difficult airway management in patients with OSA, (2) assess the impact of individual anesthesia-related drugs and agents in the care of patients with OSA, and (3) evaluate best anesthetic techniques in this patient population. To achieve these aims, a question-driven approach was sought.
In areas lacking sufficient published evidence, the task force sought to establish expert consensus while considering related literature. Patients affected by sleep-disordered breathing unrelated to OSA, including hypoventilation syndromes, periodic breathing, and central apnea unrelated to OSA, were not considered in this project. This decision was made a priori to reduce the influence of heterogeneity in our assessment given the lack of evidence on which to base recommendations for these specific populations.
GUIDELINE TASK FORCE
The task force was comprised of 14 members of SASM, an international society devoted to advancing the care for clinical problems shared by anesthesiology and sleep medicine clinicians. Given that this project included only intraoperative aspects, the task force included 12 anesthesiologists and 2 anesthesiology research fellows. Members of the task force share expertise on the topic of sleep-disordered breathing in the perioperative setting and included practitioners from both academic and nonacademic settings from various parts of the United States, Canada, and Europe.
METHODS
Research Questions
A systematic review of the literature addressing the intraoperative management of patients with OSA was conducted after search terms were developed by the task force. Three groups were established, each focusing on one of the focus areas (Table 1). Group 1 investigated whether patients with OSA are at increased risk for difficult airway management. Group 2 investigated the impact of various anesthesia-related drugs and agents used in the intraoperative care of patients with OSA. Group 3 evaluated the effect of anesthesia technique in patients with OSA. Leaders and group members are listed in the acknowledgments section of the article.
Table 1.
Selection Criteria and Study Questions

Literature Search Strategy
With the help of a research librarian, a literature search was performed for each group, including publications from 1946 to September 2016. Databases searched included (1) Medline, (2) ePub Ahead of Print/Medline In-process, (3) Embase, (4) Cochrane Central Register of Controlled Trials, (5) Cochrane Database of Systematic Reviews, (6) PubMed-NOT-Medline, and (7) ClinicalTrials.Gov. The search focused on studies of adult individuals (≥18 years of age) and published in English. Continued literature surveillance was done through January 2018.
Excerpt of the Controlled Vocabulary Terms and Key Words Included in the Systematic Search.
Group 1: “sleep apnea, obstructive,” “obstructive sleep apnea,” “obstructive sleep apnea syndrome,” “sleep disordered breathing,” “obesity hypoventilation syndrome,” “apnoea or apnea,” “hypopnoea or hypopnea,” “airway,” “intubation,” “extubation,” “airway management,” “airway obstruction,” “airway extubation,” “intubation, intratracheal,” “intubation.mp,” “laryngeal masks,” “respiration, artificial,” “positive pressure respiration,” “respiratory mechanics,” “continuous positive airway pressure,” “supine position,” “apap.mp,” “bipap.mp,” “cpap.mp,” “facemask,” “ventilat.mp,” “patient positioning,” “difficult mask ventilation,” “supraglottic airway devices,” and “surgical airway.”
Group 2: “sleep apnea, obstructive,” “obstructive sleep apnea,” “obstructive sleep apnea syndrome,” “sleep disordered breathing,” “obesity hypoventilation syndrome,” “apnoea or apnea,” “hypopnoea or hypopnea,” “postoperative period,” “complications or outcome,” “perioperative care,” “perioperative complications,” “intraoperative complications,” “postoperative complications,” “outcome,” “risk,” “morbidity,” “mortality and death,” “anesthesia,” “anesthetics,” “anesthetics, intravenous,” “inhalational anesthesia,” “volatile anesthesia,” “anesthetics local,” “analgesia, opioid,” “hypnotics and sedatives,” “adverse effects,” “intravenous regional anesthesia,” “sedation,” “sedatives,” “short acting,” “nonsteroid of nonsteroid or nasaids,” “opioid,” “complication,” “muscle relaxant,” “rocuronium, atracurium,” “cis-atracurium,” “vecuronium,” “mivacurium,” “suxamethonium or succinylcholine,” “rapacuronium,” “pancuronium,” “skeletal muscle relaxant,” “neuromuscular reversal agents,” “sugammadex,” “residual neuromuscular block,” “drug effects,” “adverse effects,” “adverse drug reactions,” “abnormalities drug induced,” “adverse drug events,” “adverse drug reactions reporting systems,” “morbidity,” and “mortality.”
Group 3: “sleep apnea, obstructive,” “obstructive sleep apnea,” “obstructive sleep apnea syndrome,” “sleep disordered breathing,” “obesity hypoventilation syndrome,” “apnoea or apnea,” “hypopnoea or hypopnea,” “postoperative period,” “complications or outcome,” “perioperative care,” “perioperative complications,” “intraoperative complications,” “postoperative complications,” “outcome,” “risk,” “morbidity,” “mortality and death,” “anesthesia, epidural,” “anesthesia, spinal,” “anesthesia, general,” “major conduction anesthesia,” “treatment outcome,” “treatment failure,” “mortality,” “outcome,” “peripheral nerve blocks,” “nerve blocks,” “anesthesia regional,” “anesthesia technique,” “sedation,” “sedative medication,” “deep sedation,” “secure airway,” “airway,” “multimodal analgesia,” “balanced anesthesia,” “opioid sparing,” and “opioids.”
Full search strategies in Medline for all groups are reported in the Supplemental Digital Content 1, SASM Guideline Intraoperative OSA Appendix, http://links.lww.com/AA/C373; Supplemental Digital Content 2, Search Anesthesia Technique, http://links.lww.com/AA/C374; Supplemental Digital Content 3, Search Difficult Airway and OSA, http://links.lww.com/AA/C375; Supplemental Digital Content 4, Search Intraoperative Medication Use in Patients With OSA, http://links.lww.com/AA/C376; Supplemental Digital Content 5, Search Strategy NMBA, http://links.lww.com/AA/C377.
Furthermore, detailed reviews addressing difficult airway, anesthesia-related drugs and agents, specifically those involving neuromuscular blocking agents (NMBAs) and opioids, were conducted and summarized in separate systematic reviews by the respective SASM focus groups (members listed in the acknowledgments) to share the evidence gathered and expand the scope of the present guideline.
Study Selection
In the respective groups, ≥2 reviewers assessed titles and abstracts for eligibility by using the standardized format of the Covidence platform.17 This step was followed by a full-text review and data extraction. Furthermore, a citation search by a manual review of references from primary or review articles was performed to compile additional relevant results. Any disagreements were resolved by consensus among reviewers or by consulting with the respective SASM groups via face-to-face meetings, teleconferences, or email communications. Study designs considered included randomized controlled trials (RCTs), prospective and retrospective observational studies, case series, systematic reviews, and meta-analyses. Within this literature, the presence or risk for OSA was based on polysomnography, screening questionnaires, clinical assessment, chart diagnosis, medical history, or International Classification of Diseases (ICD)-9 codes from administrative or billing records, while studies reported on at least 1 outcome of interest. Existing guidelines were cross-checked for completeness of references.
Data extracted from these studies included type of study, demographic data, comorbidities, procedure type, anesthesia-related interventions and medications, adverse events, as well as other clinically important outcomes and effects.
Exclusion Criteria.
Exclusion criteria were: nonhuman studies, non-English language, review articles, single case reports, studies reporting on the chronic use of medications commonly used intraoperatively such as chronic opioid medication, and studies without outcome reporting. For group 3, studies not directly comparing anesthesia modalities were also excluded.
Level of Evidence and Recommendations
The Oxford Level of Evidence (Oxford LOE) tool was utilized to evaluate the quality of evidence of individual studies.18 Grading the strength of recommendations and quality of the underlying evidence enhances the usefulness of clinical practice guidelines.19 Therefore, the approach according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system20,21 was utilized with regard to the body of evidence and the development of guideline recommendations.22 As specified by GRADE, the quality of evidence is classified into high, moderate, low, and very low levels, according to factors that include study methodology, consistency and precision of results, and directness of evidence.19 These levels were assigned to the body of evidence of each respective recommendation within their focus area and reflect the confidence in estimates of the true effect.21 When moving from evidence to recommendations, the GRADE approach focuses on 4 factors: balance between benefit and harm, certainty of evidence, values and preferences, and resource considerations.22 The strength of recommendation is separated into strong and weak and defines the extent to which one can be confident that the desirable consequences outweigh its undesirable consequences (Table 2).23
Table 2.
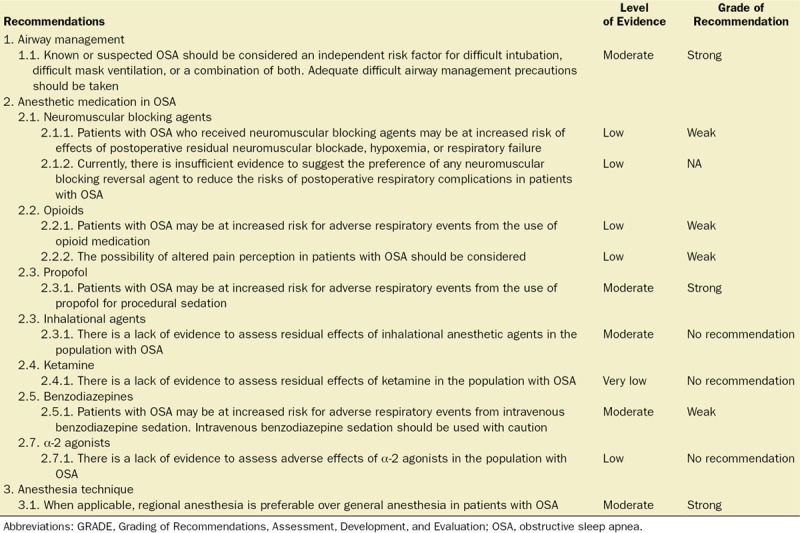
Recommendations for the Intraoperative Management of Patients With Known or Suspected OSA
In-person SASM Intraoperative Guideline Task Force meetings took place at special sessions during the SASM annual meetings in Chicago, IL (2016), and Boston, MA (2017), as well as the International Anesthesia Research Society annual meeting in Washington, DC (2017). Furthermore, multiple teleconferences and electronic communications took place throughout this time period. Preliminary results and implications of findings were presented and discussed at the 2017 SASM annual meeting in Boston, MA.
1. DIFFICULT AIRWAY AND OSA
1.1. Question: Are patients with OSA at increased risk for difficult airway management and do special precautions need to be taken?
1.1. Recommendation: Known or suspected OSA should be considered an independent risk factor for difficult intubation, difficult mask ventilation, or a combination of both. Adequate difficult airway management precautions should be taken.
Level of evidence: Moderate; Grade of recommendation: Strong
Rationale
The perception of OSA as an important risk factor for difficult airway management is widely held among anesthesiologists and intensive care physicians. In the absence of RCTs, several prospective and retrospective controlled studies have supported this assumption.24–39
Association Between OSA and Difficult Airway Management
After applying the designated search strategy and removing duplicates, 4806 references were screened for title and/or abstract. After reviewing 25 full-text articles, 16 studies were identified as reporting on the association between difficult airway management and OSA, while 9 studies were excluded.40–47 A detailed summary of associations between OSA and various difficult airway management components is provided in Supplemental Digital Content, Table A1, http://links.lww.com/AA/C373.
Among the included studies, 5 were retrospective24,25,27,35,36 and 11 were prospective controlled studies.26,28–34,37–39 Ten studies confirmed OSA by overnight polysomnography24,25,27,29,35 or electronic database entries,28,30,31,34,37 3 used the STOP-Bang screening questionnaire,33,38,39 2 identified patients by clinical diagnosis,26,36 and 1 used both polysomnography and the STOP-Bang questionnaire.32
In total, 266,603 patients were included in 16 studies. Of those patients, 32,052 had OSA (identified by polysomnography, electronic database, chart or clinical diagnoses, and STOP-Bang questionnaires) and 234,551 did not. In summary, 12 studies reported on difficult intubation,24–29,31–33,35,38,39 6 on difficult mask ventilation,28,30,31,36,38,39 2 on both difficult intubation and mask ventilation,28,37 and 2 on failed supraglottic airway.27,34 Several studies reported >1 difficult airway outcome. No study was available on the need for a surgical airway (Supplemental Digital Content, Table A1, http://links.lww.com/AA/C373).
Concerning difficult intubation and OSA, 7 of 12 studies showed positive associations.24,25,28,33,35,38,39 Of 6 studies, 5 demonstrated a significant impact of OSA on difficult mask ventilation.28,30,36,38,39 In the 2 studies that reported on combined difficult intubation and mask ventilation, both demonstrated a significant impact of OSA.28,37 Although 5 studies assessing difficult intubation26,27,29,31,32 and 1 study evaluating difficult mask ventilation31 did not find a significant association with OSA, the overall estimates showed a positive association between OSA and difficult airway. This finding suggests that patients with OSA are at increased risk of difficult airway management compared to patients without OSA. Detailed data, analysis, and results on the association between OSA and difficult airway will be reported in a separate systematic review with meta-analysis by the SASM airway focus group (members listed in acknowledgments).
One prospective controlled study34 reported on the use of the LMA Unique® (Teleflex Incorporated, Morrisville, NC), and an additional retrospective investigation27 reported on a separate unspecified supraglottic airway device. No significant association was found between OSA and failed supraglottic devices.
Prevalence of OSA in Patients With Difficult Intubation
Two studies elucidated the association between OSA and difficult intubation in a reverse manner by investigating the rate of OSA among patients with difficult intubation. In a retrospective study, Hiremath et al,24 using an apnea-hypopnea index (AHI) ≥10 as a cutoff, found that 53% of patients with difficult airway had OSA. This finding was confirmed by a prospective controlled study by Chung et al.29 using an AHI ≥5 as a cutoff for OSA diagnosis. Patients who were determined to have a difficult airway were referred for polysomnography after surgery, and 66% were shown to have OSA.
Kim and Lee35 showed that patients with an AHI ≥40 had a significantly higher prevalence of difficult intubation. For patients with OSA with AHIs ≤40, 40–70, and ≥70, the incidence of difficult intubation was 3.3%, 19.3%, and 27.6%, respectively.35 Anatomical skeletal and soft tissue changes may contribute to a difficult airway in OSA. However, these observations are “hypothesis-generating” rather than “hypothesis-proving” findings. The shared anatomical abnormalities explain the positive association between difficult airway and OSA.
A number of studies evaluated the association of difficult airway management with OSA using the STOP-Bang questionnaire to identify patients at high risk of OSA.32,33,38,39 The sensitivity and specificity of the STOP-Bang questionnaire can vary according to the prevalence and severity of OSA.48 This variation can create false-positive and false-negative cases in both OSA and non-OSA groups, leading to potential misclassification bias.
One of the contributing factors for adverse respiratory events in patients with OSA is the increased risk of difficult airway management, such as difficult intubation, difficult mask ventilation, or both. In a recent report, there were 7 litigation cases where OSA was associated with either death or anoxic brain injury due to difficult airway management in the form of failure to reintubate in the postoperative period.49 Knowledge about the association between OSA and difficult airway may improve perioperative airway management and decrease airway-related complications.
In view of ethical considerations, it is difficult to perform RCTs in patients with OSA to determine its associations with difficult airway management. As a result, only observational prospective and retrospective studies are available in the literature. The end estimates of these studies indicate that there is an increased risk of difficult airway management in patients with OSA. Due to the large number of trials and large patient numbers, the overall quality of the body of evidence was considered to be moderate using the GRADE approach20,21 and the Oxford LOE.18
2. INTRAOPERATIVE MEDICATION USE IN PATIENTS WITH OSA
A large body of literature supports the notion that the effects of surgery and anesthesia pose unique hazards to patients with OSA.5,50,51 Anesthetic agents and analgesic drugs interact with consciousness, sleep, and ventilatory drive,52,53 and thus they deserve consideration when caring for patients with OSA. In addition, upper airway and pulmonary physiology, including upper airway dilator muscle activity, are impacted by pharmacological and mechanical elements (airway manipulation) of anesthesia with possible increased detriment in OSA.54–56 The following section discusses questions related to the effects of various agents and drugs commonly utilized intraoperatively in patients with OSA.
2.1 Neuromuscular Blocking Agents
2.1.1 Question: Are patients with OSA at increased risk for postoperative respiratory complications from the use of NMBAs?
2.1.1 Recommendation: Patients with OSA who received NMBAs may be at increased risk of effects of postoperative residual neuromuscular blockade, hypoxemia, or respiratory failure.
Level of evidence: Low; Grade of recommendation: Weak
2.1.2 Question: Does the choice of neuromuscular blocking reversal agent impact the risk of postoperative respiratory complications in patients with OSA?
2.1.2 Recommendation: Currently, there is insufficient evidence to suggest the preference of any neuromuscular blocking reversal agent to reduce the risks of postoperative respiratory complications in patients with OSA.
Level of evidence: Low; Grade of recommendation: No recommendation
Rationale.
NMBAs are commonly used to optimize intubation conditions and provide surgical relaxation for various procedures. However, residual neuromuscular blockade has been reported to occur in ≤64% of patients in postanesthesia care units.57 The use of NMBAs and residual neuromuscular blockade has been associated with significant postoperative respiratory complications such as hypoxemia,58 upper airway obstruction,58 and pneumonia.59 High doses of NMBA given during abdominal surgery were associated with an increased risk of 30-day readmission, increased length of hospital stay, and increased hospital cost.60 A retrospective review of a single-center database showed that patients who required tracheal intubation within the first 3 days after surgery had a significantly higher frequency of NMBA administration and reversal with neostigmine.61 Residual neuromuscular blockade may persist despite the administration of neostigmine reversal, especially when neuromuscular monitoring is not utilized.62
It is unclear whether patients with OSA may be at higher risk for postoperative respiratory complications due to the adverse effects of postoperative residual neuromuscular blockade compared to patients without OSA. Moreover, it is uncertain whether the type of reversal agent impacts the risk of postoperative complications in patients with OSA. Patients with suspected61 or confirmed50,63,64 OSA have been shown to be at increased risk for early postoperative respiratory complications, including emergent intubation,63,64 mechanical ventilation,63,64 noninvasive ventilation,63,64 respiratory failure,50 desaturation,6,50 and pneumonia.64 The use of NMBA was not described in these studies.6,50,63,64 Many patients with OSA are obese and have anatomical risk factors that may increase vulnerability to the effects of residual neuromuscular blockade on the upper airway and pharyngeal function.
Our literature search yielded 5 studies that were heterogeneous in terms of study design, types of surgery, and types of respiratory complications.65–69 Many studies were excluded because OSA diagnosis or use of NMBA was not described.
One RCT11 and 2 observational studies66,67 were included to address the question of whether patients with OSA are at a higher risk for postoperative respiratory complications from the use of NMBA compared to patients without OSA. Although the level of evidence was limited (Oxford LOE 2–3), the studies suggest that patients with OSA who received NMBA may be at increased risk of effects of residual neuromuscular blockade, postoperative respiratory failure, and hypoxemia.65–67 The results of our review are consistent with previous studies showing that patients with OSA are at higher risk of postoperative respiratory failure and hypoxemia than patients without OSA.6,61,70,71 Even partial residual neuromuscular blockade that does not evoke respiratory symptoms can impair upper airway dilator muscle function.72 Minimizing the use and dose of NMBA, monitoring the level of neuromuscular blockade, and complete reversal of NMBA before extubation may be particularly important for patients with OSA.9
While not considering OSA status, reversal of NMBA with sugammadex, a cyclodextrin used to reverse rocuronium,73 has been shown to decrease the incidence of residual paralysis compared to the anticholinesterase inhibitor, neostigmine.74 A recent Cochrane review of 41 studies comparing sugammadex with neostigmine concluded that patients receiving sugammadex versus neostigmine had 40% fewer composite adverse events (bradycardia, postoperative nausea and vomiting, and residual neuromuscular blockade).75 Patients receiving sugammadex had less desaturation and need for transitory oxygen supplementation; however, the OSA status was not reported in these reviews, limiting its value to assess its differential effect in this subpopulation.74,75
There are limited studies comparing the impact of different neuromuscular blocking reversal agents on postoperative respiratory complications in patients with OSA. We identified 1 RCT68 and 1 observational study69 that compared sugammadex to neostigmine. In the 2 studies, 209 patients with OSA and 185 patients without OSA were included.68,69 The RCT (n = 74) found that patients receiving sugammadex versus neostigmine had less postoperative respiratory complications (desaturation, hypoxemia, apnea, airway manipulation, airway usage, reintubation, continuous positive airway pressure [CPAP] therapy, and invasive mechanical ventilation).68 There was no difference in airway obstruction. The observational study (n = 320) compared sugammadex to a historical cohort of patients who received neostigmine reversal for laparoscopic bariatric surgeries. Patients with OSA who received sugammadex versus neostigmine had less postoperative chest radiographic changes (atelectasis, pleural effusions), 6.9% vs 16.3% (odds ratio [OR], 0.36; 95% CI, 0.18–0.8),69 but there were no differences in postoperative mechanical ventilation or hospital length of stay. Although both studies showed a reduction in some postoperative respiratory complications, the evidence is limited because the number of patients included in the RCT (Oxford LOE: 2) was small,68 and the observational study (Oxford LOE: 3) reported no difference in clinical outcomes.69
Currently, there is insufficient evidence to recommend the use of sugammadex over neostigmine to reduce the risk of postoperative respiratory complications in patients with OSA. More trials with larger sample sizes are needed in this patient population.
2.2 Opioids
2.2.1 Question: Are patients with OSA at increased risk for opioid-related respiratory events?
2.2.1 Recommendation: Patients with OSA may be at increased risk for adverse respiratory events from the use of opioid medications.
Level of evidence: Low; Grade of recommendation: Weak
2.2.2 Question: Is pain perception and opioid potency altered in patients with OSA?
2.2.2 Recommendation: The possibility of altered pain perception in patients with OSA should be considered.
Level of evidence: Low; Grade of recommendation: Weak
Rationale.
While opioids are highly effective in treating moderate to severe pain, their intrinsic capacity to suppress ventilatory drive demands caution in OSA. Despite consensus among perioperative physicians to restrict or avoid opioids in OSA,9 the presence of robust, high-quality scientific evidence to demonstrate the merit of heightened concern and guide safe opioid practice in this population is limited.76
Nevertheless, despite limitations with respect to the quality of evidence suggesting an adverse impact of acute opioid administration in OSA, current literature indicates that a heightened concern regarding opioid use in this population may be justified. A summary of evidence is provided in Supplemental Digital Content, Table A2, http://links.lww.com/AA/C373.
Specifically, 17 observational studies exploring the impact of systemic opioid use in OSA were identified. While the majority demonstrated an association between opioid use and adverse perioperative outcomes in OSA,61,77–89 this was not confirmed by all.66,90,91 It should be noted that, particularly among observational analyses, there is notable heterogeneity with regard to the modality of OSA assessment, ranging from the gold standard of polysomnography to identification by screening questionnaires or patient history. Furthermore, potential selection bias should be considered in these studies. In recent publications, a comparison of postoperative complications among patients with and without OSA within the same study cohort revealed that the incidence of postoperative pulmonary (2.49% vs 1.83%), cardiac (2.81% vs 0.23%), gastrointestinal (0.45% vs 0.33%), renal (3.47% vs 1.83%), and thromboembolic (0.41% vs 0.33%) complications was higher in patients with OSA at similar opioid dose levels.88,92 Additional analysis of the impact of opioid dose increase within patients with OSA demonstrated an associated increase in the odds for gastrointestinal complications, prolonged length of stay, and increased hospital cost, while no further increase in risk for pulmonary complications was observed, possibly due to increased levels of monitoring afforded to this population.88 A higher incidence of postoperative complications in OSA versus non-OSA in this context was also found by Blake et al77 and Esclamado et al,80 while the latter conducted their study in upper airway surgery, a procedure with a potentially inherent influence on respiratory outcome.80
Chung et al79 demonstrated an opioid dose-dependent postoperative worsening of sleep-disordered breathing associated with the severity of OSA (expressed by AHI), although this effect may have been fairly small. Male patients with OSA had a significantly higher central apnea index on postoperative night 1 versus female patients with OSA. In this context, numerous other observational studies took a different approach by investigating the occurrence of critical, life-threatening respiratory events, such as respiratory failure and naloxone requirement and identifying drivers for these complications.61,81–84,86,87 Moreover, a recent systematic review reported that the majority of surgical patients with OSA experiencing perioperative death or near-death events received a morphine equivalent dose of <10 mg/d.89 Subramani et al89 suggested that a dose-response pattern with increased odds for complications at increasing opioid dose levels (ORs of 1.0, 1.5, and 3.0 at opioid doses of <10, 10–25, and >25 mg; P for trend <.005) exists.
In contrast, others66,85 who restricted their focus to patients with obesity, a population of high OSA prevalence,2 demonstrated that, although postoperative respiratory complications in the context of opioid analgesia were common, surprisingly, OSA could not be established as an independent risk factor.66,85 However, a factor potentially causing an underestimation of a possible deleterious effect of OSA was the postoperative use of positive airway pressure therapy among patients with OSA.85 Moreover, a proof of concept analysis by Wang et al91 suggested that the experimental oral administration of 30 mg controlled-release morphine in 10 volunteers outside the surgical setting paradoxically improved oxygenation through modulating chemoreflexes.91 In summary, evidence from observational analyses suggests that opioid use in the presence of OSA presents a risk factor for postoperative critical respiratory events (Oxford LOE 3–4).61,79,81–84,86,87,89
With regard to evidence from RCTs, 6 such studies were identified (Oxford LOE 2).93–98 In a volunteer study, Bernards et al94 directly demonstrated that opioid administration during sleep increased the number of central apneas, leading to decreased saturation levels in patients with OSA versus those without OSA.94 Abdelmageed et al93 demonstrated that opioid dose reduction significantly reduced the incidence of central apneas and respiratory events in patients with OSA.93 While interesting, it must be noted that opioid reduction may decrease respiratory depression and related complications in the general population as well.92 Using a nonvalidated OSA prediction instrument, Blake et al95 showed that central apneas and respiratory events were related to the dose of morphine administered postoperatively. However, differences in the occurrence of respiratory complications between patients with standard morphine patient-controlled analgesia and an opioid-sparing regimen could not be established.95
Other studies explored the safety of neuraxial opioid administration in patients with OSA.99–102 In a systematic review, Orlov et al99 found that the incidence of major cardiorespiratory complications after neuraxial opioid administrations was 4.1% among patients with OSA. However, the authors also emphasized that significant limitations in the quality of evidence and persistent underreporting of adverse events prevented an accurate and robust assessment of true perioperative risk.16,99 A prospective study in patients having a cesarean delivery with intrathecal morphine administration demonstrated that OSA and obesity were associated with approximately a 2-fold increase in risk for desaturation.100 However, another observational analysis of 990 patients undergoing orthopedic surgery with intrathecal morphine could not find an association between OSA and adverse pulmonary events.101
In summary, limited literature suggests that patients with OSA may be at increased risk for opioid-related respiratory adverse events. However, high-quality evidence to support and prove this notion is largely lacking (Oxford LOE 2–4).
Pain and Opioid Analgesia in OSA.
A systematic evaluation of opioid-related respiratory effects in OSA requires focused attention on closely related issues such as pain perception and pharmacology of opioid analgesia. A summary of evidence is provided in Supplemental Digital Content, Table A3, http://links.lww.com/AA/C373 (Oxford LOE 3). Characterizing these relationships is important because the dose of opioids that is required to treat pain, as well as the sensitivity to these medications, directly influence the likelihood of opioid-induced respiratory depression.
Disturbed sleep continuity and intermittent hypoxia are 2 important features of OSA. Studies in humans have repeatedly demonstrated that fragmented103,104 or chronically curtailed sleep87,105 and insomnia,106 a condition highly comorbid with OSA,107 are associated with heightened sensitivity to pain.108
Among 3 identified studies examining the response to experimental pain in subjects suffering from OSA, 1 study found that patients with OSA and comorbid temporomandibular joint disorder experienced hypoalgesia to pressure-related pain,109 while another reported a significant increase in pain threshold after restoring sleep continuity with the application of CPAP therapy.110 In contrast, the third investigation found no association between wake-after-sleep-onset or nocturnal nadir blood oxygen saturation (SpO2) polysomnographic parameters and threshold/tolerance to thermal pain.111
In the context of chronic pain, a retrospective analysis of prospectively collected data from the Cleveland Family Study showed that chronic intermittent hypoxia was associated with more frequent chronic pain complaints, even after adjusting for the potentially hyperalgesic effect of sleep fragmentation and systemic inflammation.112
Despite the primary goal to focus on the adult patient population in this guideline, a significant amount of evidence originates from the pediatric population and deserves mention here particularly because they show contradictory findings to those found among adults. In children undergoing adenotonsillectomy for treatment of OSA, 2 case–control studies, 1 retrospective113 and 1 prospective,114 showed that patients with a preoperative nocturnal nadir SpO2 <85% required half the dose of morphine to treat postoperative pain, versus those with a nadir SpO2 ≥85%. Two prospective case–control studies in the same population did not confirm these findings.115,116 In the first study, African American children versus Caucasian children with OSA presented with more pain requiring a higher dose of morphine for postoperative analgesia.115 The second study showed that children with OSA (respiratory disturbance index >5) required more morphine for postoperative analgesia, but they also demonstrated a higher incidence of opioid-related respiratory complications.116
In adults, 1 retrospective analysis found that bariatric patients with nocturnal hypoxemia (expressed as percentage of total sleep time spent at oxygen saturation [SaO2] <90%) required less opioids for postoperative analgesia,117 whereas another prospective study did not detect any association between preoperative nocturnal hypoxemia and postoperative opioid use in general surgical patients with OSA.118 A more detailed and comprehensive summary of evidence on the potential impact of acute opioid analgesia in OSA is provided in a separate systematic review by the SASM opioids focus group (members listed in the acknowledgments).
2.3 Propofol
2.3.1 Question: Are patients with OSA at increased risk for adverse events from the use of propofol for procedural sedation?
2.3.1 Recommendation: Patients with OSA may be at increased risk for adverse respiratory events from the use of propofol for procedural sedation.
Level of evidence: Moderate; Grade of recommendation: Strong
Rationale.
The literature discussed for the purpose of the recommendation reflects evidence of importance for patients receiving propofol for sedation in a procedural setting, that is, drug-induced sleep endoscopy (DISE), gastroenterological endoscopy, or dentistry. The use of propofol to induce general anesthesia purposefully suppresses respiratory activity and was thus deferred in this section.
Propofol is the most commonly used agent for DISE.119,120 A summary of findings from 5 studies120–124 is shown in Supplemental Digital Content, Table A4, http://links.lww.com/AA/C373 (Oxford LOE: 2–4). Both body mass index (BMI) and severity of OSA correlated with a greater likelihood of a patient having multiple sites of airway collapse and a higher possibility of circumferential and total airway obstruction during DISE.119,125 The goal of propofol administration for DISE is to produce a sleep-like loss of consciousness and muscle relaxation to precipitate pharyngeal narrowing and collapse in vulnerable individuals. To avoid the problem of profound relaxation or central apnea, it has been suggested that initial dosing for DISE be judiciously titrated.120,126
Attempts have been made to formulate a mathematical equation to model the pharmacokinetics for propofol in patients with obesity (Supplemental Digital Content, Table A5, http://links.lww.com/AA/C373).127–130 Uncertainty regarding dosing scalar adjustments that may be required in patients with obesity, as well as the concomitant use of depressant drugs with synergistic effects (midazolam,131 ketamine,132,133 dexmedetomidine,134 opioids135), further add to the need for heightened vigilance when using propofol for patients with OSA. Propofol has a relatively steep dose-response curve compared to other sedatives/hypnotics, thus underscoring the importance of careful titration.131,136,137 Adverse effects are not uncommon in patients with OSA undergoing procedures with propofol sedation. A summary of findings from 5 studies138–143 is shown in Supplemental Digital Content, Table A6, http://links.lww.com/AA/C373. OSA, increased BMI, male gender, American Society of Anesthesiologists physical status ≥III, initial dose of propofol, and increased age were found to be independent risk factors for hypoxemic incidents. Airway interventions were common in patients receiving propofol, although indications for airway intervention were left to the discretion of the anesthesia provider. Whether precautionary or subsequent to an obstructed airway, apneic, or desaturation episode, such airway interventions were undoubtedly done to prevent or mitigate a sedation-related adverse event. The use of capnography was associated with a decreased incidence of hypoxic events compared to standard monitoring alone during sedation with propofol144 in patients with OSA.140
2.4 Inhalational Agents
2.4.1 Question: Are patients with OSA at increased risk for residual effects of inhalational anesthetic agents?
2.4.1 Recommendation: There is a lack of evidence to assess residual effects of inhalational anesthetic agents in the population with OSA.
Level of evidence: Moderate; Grade of recommendation: No recommendation
Rationale.
There is a lack of scientific literature to guide best intraoperative practices in OSA regarding the preferred technique among various inhalational agents and intravenous propofol for the maintenance of anesthesia. Nevertheless, a significant amount of evidence has been published on the general population and patients with obesity.145 Evidence from the population with obesity may merit consideration in this context, given the close association to OSA,146 reflected in the substantial OSA prevalence of ≤90% in male bariatric patients.147,148 Notably, there is significant overlap between obesity and OSA with regard to challenges in general anesthesia because of altered cardiorespiratory physiology, including decreased functional residual capacity, upper airway obstruction, and the propensity to hypoxemia in perioperative settings.149,150
This renders the period of emergence and recovery from anesthesia of high concern regarding the risk for detrimental outcomes.56,146
In this context, 25 studies were identified that compared the efficacy and recovery profile among the most common inhalational agents and intravenous propofol.65,151–174 A summary of evidence is provided in Supplemental Digital Content, Tables A7 and A8, http://links.lww.com/AA/C373. Comparing propofol and isoflurane, propofol was suggested to be associated with a faster recovery from anesthesia and improved postoperative respiratory control in 2 RCTs.154,155 However, sevoflurane was found to be superior to propofol in 2 RCTs due to faster anesthesia recovery and improved hemodynamic stability.152,153 In addition, recently Fassbender et al151 reported no difference with regard to postoperative obstructive and hypoxemic events between the 2 anesthetic agents when combined with remifentanil. Furthermore, comparing propofol and desflurane, 1 study demonstrated that the use of propofol impaired pulmonary function and SpO2 to a greater degree than desflurane,157 while another could not confirm these differences.156 Thus, current evidence indicates that sevoflurane and desflurane might be superior to intravenous propofol in terms of anesthesia recovery in patients with obesity (Oxford LOE: 2).
Similarly, 4 RCTs conducted in the population with obesity supported the notion that sevoflurane was associated with favorable features compared to isoflurane.65,158–160 In particular, Sudré et al65 demonstrated that sevoflurane embedded in a short-acting anesthetic regimen comprised of remifentanil, rocuronium, and ropivacaine improved emergence from anesthesia and reduced respiratory complications, postoperative anesthesia care unit stay, and hospital length of stay when compared to isoflurane within a long-acting regimen. This analysis emphasized the plausible benefit of generally utilizing short-acting medications with regard to all anesthetic drug classes, including opioids and NMBA, among patients at higher perioperative risk.65 The majority of studies, however, focused on the comparative effectiveness between sevoflurane and desflurane,161,163,165,167 demonstrating improved anesthesia recovery with desflurane (Oxford LOE: 2).162,164,166,168,169,174 Notably, limitations inherent to the nature of these comparisons can prevent the detection of differences. For instance, Eger and Shafer175 showed that differences in postoperative wake-up times among anesthetics were minimal at lower anesthetic concentrations,175 while the duration of anesthesia176 and BMI present important covariates.174
Summarizing the evidence, a well-designed systematic review by Liu et al171 provided a comprehensive comparison with quantitative analysis of immediate postoperative recovery after desflurane, isoflurane, sevoflurane, and intravenous propofol anesthesia in patients with obesity. In addition, a rather small clinical trial by Juvin et al170 also compared desflurane, isoflurane, and propofol together in 1 analysis. Both Liu et al171 and Juvin et al170 established desflurane as the most favorable anesthetic agent because of its superior postoperative recovery profile. Specifically, it was observed that patients who received desflurane anesthesia required less time to respond to commands, eye opening, hand squeezing, tracheal extubation, and name stating. Moreover, desflurane reduced sedation levels171 and conferred higher postoperative SpO2.170,171
It appears, therefore, that postoperative recovery might occur faster and with improved hemodynamic stability after anesthesia with desflurane followed by sevoflurane (Supplemental Digital Content, Table A7, http://links.lww.com/AA/C373), and these findings have also been observed in the general population.177–180
Consistently, desflurane and sevoflurane feature low blood-gas partition coefficients,171 conferring greater intraoperative control of anesthesia depth, as well as rapid and consistent postoperative emergence and recovery.161,181,182
These properties, in turn, imply earlier achievement of baseline respiratory function with potentially better protection against aspiration and improved oxygenation.183 This has also been supported by the observation of decreases in hypoxemia in clinical trials.170,171 Both obesity and OSA predispose patients to higher risk of postoperative upper airway obstruction and serious hypoxemia,184 thus suggesting a benefit associated with early and rapid recovery of active airway control and alertness.171
Another intervention, possibly promoting increased safety in OSA, is the intraoperative monitoring of anesthesia depth. This has been suggested by Ibraheim et al172 and Freo et al,173 who demonstrated that monitoring for titration of levels of inhalational agents reduced the required anesthetic dosage and improved the postanesthetic recovery in patients with obesity.
Furthermore, Katznelson et al185 suggested that recovery time after general anesthesia in patients with and without obesity can be accelerated using either isocapnic or hypercapnic hyperpnea.185
In summary, the available evidence supports the use of desflurane and sevoflurane in patients with obesity (Oxford LOE: 2). Given the strong association between obesity and OSA, and the benefits of accelerating and improving postoperative anesthesia recovery, these outcomes are desirable and may apply to patients with OSA as well. However, except for 2 RCTs,151,154 no studies specifically in OSA are available, and thus no specific recommendations can be made.
2.5 Ketamine
2.5.1 Question: Are patients with OSA at increased risk for adverse events from the use of ketamine?
2.5.1 Recommendation: There is a lack of evidence to assess residual effects of ketamine in the population with OSA.
Level of evidence: Very low; Grade of recommendation: No recommendation
Rationale.
The literature is scarce with regard to complications associated with ketamine in patients with OSA.
Ketamine has mostly been studied with respect to its potent analgesic effects as a sedative and hypnotic and, more recently, to reduce opioid use.186–188 There are only a few studies involving ketamine use in patients with OSA, but data are insufficient to draw any firm conclusions.189,190
Adverse effects of ketamine, such as neuropsychiatric effects, signs of increased sympathetic system activation (hypertension and tachycardia), and hypersalivation, are well documented in patients without OSA.191,192 Although patients with OSA are not specifically studied, these adverse events most likely translate to increased risk in this patient population as well. Adverse events are mostly seen in patients who received high doses, meaning >0.5 mg/kg boluses and 100 µg/kg/h infusions.193
Ketamine has been shown to have some beneficial effects. Studies demonstrated that ketamine, when combined with other sedative medications, mostly propofol, may decrease respiratory-related adverse effects.194,195 One such prospective observational study looking at sedation-related risk factors (airway obstruction, hypoventilation, and desaturation) for procedural sedation found ketamine to be a protective factor.195 De Oliveira et al194 reported that ketamine decreased duration and severity of hypercapnia in patients undergoing breast surgery under deep sedation.
Furthermore, Drummond196 studied the effect of ketamine versus midazolam on upper airway function. Interestingly, they found decreased upper airway muscle activity in the midazolam group, which resulted in airway obstruction, whereas no change in muscle activity was observed in the ketamine group. In another study, genioglossus muscle activity, tidal volume, and respiratory rate have been shown to be increased after administration of high and low doses of ketamine in rats.197 Upper airway dilator muscle activity plays an important role in patients who are at risk of upper airway obstruction. Despite the lack of data on ketamine in the patient population with OSA, available information suggests that these patients could benefit from potentially favorable respiratory effects over other sedatives. Firm conclusions, however, cannot be drawn at this time.
2.6 Benzodiazepines
2.6.1 Question: Are patients with OSA at increased risk for adverse events from intravenous benzodiazepine sedation?
2.6.1 Recommendation: Patients with OSA may be at increased risk for adverse respiratory events from intravenous benzodiazepine sedation. Intravenous benzodiazepine sedation should be used with caution.
Level of evidence: Moderate; Grade of recommendation: Weak
Rationale.
Although the literature is immature on the topic of differential effects of intravenous benzodiazepine sedation in patients with OSA compared to those without OSA, studies suggest that the use of intravenous benzodiazepines is associated with airway compromise in patients with OSA. Intravenous benzodiazepine sedation is routinely used to induce airway collapse for diagnostic purposes in OSA.
Much of the literature revolves around the use of intravenous benzodiazepines for DISE in a diagnostic context to examine locations and patterns of obstruction in patients with OSA.119,198–210 Midazolam is the most commonly used intravenous benzodiazepine for DISE. In 7 studies,199,200,202,205,207,208,210 the majority of patients had multilevel obstruction, especially those with higher AHI. Two studies evaluated sleep staging during midazolam-induced sleep. The first showed that patients spent the most time in nonrapid eye movement sleep stage N1 and N2 but not in stage N3 and rapid eye movement (REM) sleep.198 The second reported that patients reached N2 sleep without further deepening of sleep stage.201 Because most obstructive events occur in N1 and N2 sleep, DISE with intravenous midazolam is considered a good option to study obstructive events in patients with OSA.102,105
Interestingly, Sadaoka et al209 found that patients with OSA had oxygen desaturation and apneas during DISE with intravenous diazepam more frequently than simple snorers.
Another category of studies described the use of intravenous benzodiazepines for sleep imaging.211–214 Thus, a retrospective analysis by Lee et al213 compared 53 patients with OSA to 10 simple snorers. All patients with OSA had desaturation events after 2 mg of midazolam, but none in the simple snorers group had such events.213
We identified 5 studies evaluating intravenous benzodiazepines in the context of other endoscopic or surgical procedures.215–219 Midazolam was used either alone or in combination with fentanyl. One study did not specify which benzodiazepines were used.218 Three studies215,216,219 compared outcomes between patients with and without OSA. In a retrospective cohort study by Adler et al,215 215 patients undergoing routine endoscopy were randomized to 4 groups: patients with OSA undergoing endoscopy with propofol or midazolam + fentanyl and patients without OSA undergoing endoscopy with propofol or midazolam + fentanyl. A comparison of patients with and without OSA receiving midazolam and fentanyl showed that desaturation events and other complications were not significantly different.215 Notably, doses of midazolam and fentanyl needed for colonoscopy were slightly lower in patients with OSA, although the procedure time was moderately longer.
Cha et al216 published a prospective study that compared cardiopulmonary complications during routine esophagogastroduodenoscopy under sedation with midazolam between 31 patients with OSA and 65 healthy controls. Patients with OSA received a higher dose of midazolam than patients without OSA, but cardiopulmonary complications were not increased in patients with OSA.
Mador et al219 conducted a prospective study in 904 patients undergoing endoscopy to investigate whether OSA, assessed by the Berlin questionnaire, increases the risk of complications during sedation with midazolam and fentanyl. Major complications were observed in 3.25% of patients with low risk for OSA and in 1.9% of patients with high risk for OSA (OR, 0.6; 95% CI, 0.26–1.46; P = .21). Minor complications were observed in 10.56% of patients with low OSA risk and 10.63% of patients with high OSA risk (OR, 1.01; 95% CI, 0.65–1.56; P = 1.0), suggesting that OSA was not associated with increased risk for cardiopulmonary complications during endoscopy under sedation with midazolam and fentanyl in this analysis.
In conclusion, 5 studies directly compared outcomes between patients with and without OSA after intravenous benzodiazepine sedation in the context of anesthesia.209,213,215,216,219 However, only 2 studies209,213 were able to establish a higher risk for respiratory complications in patients with OSA (Oxford LOE: 3). A summary of evidence is provided in Supplemental Digital Content, Table A9, http://links.lww.com/AA/C373.
2.7 α-2 Agonists
2.7.1 Question: Are patients with OSA at increased risk for adverse events from the use of α-2 agonists?
2.7.1 Recommendation: There is a lack of evidence to assess adverse effects of α-2 agonists in the OSA population.
Level of evidence: Low; Grade of recommendation: No recommendation
Rationale.
Dexmedetomidine and clonidine are centrally acting α-2 agonists with sedative, analgesic, and sympatholytic properties. Dexmedetomidine, in particular, has been suggested to cause minimal respiratory depression. Because OSA is associated with an increased risk of adverse postoperative pulmonary events,6 the potentially favorable respiratory profile and analgesic-sparing effects theoretically make α-2 agonists appealing for this population. When assessing the risk of adverse events with the use of α-2 agonists, no eligible studies compared patients with OSA to patients without OSA. The majority of studies focused on OSA or bariatric populations, comparing the use of α-2 agonists to either placebo or other medications. The body of literature is limited by a small total number of subjects, inconsistent results, lack of uniformity in outcomes, and low adverse event rates. Although many studies demonstrate statistical differences in hemodynamic parameters with α-2 agonists, the translation into clinically meaningful outcome differences is not supported at this time.
Four studies123,124,220,221 compared the use of dexmedetomidine to propofol in DISE as summarized in Supplemental Digital Content, Table A10, http://links.lww.com/AA/C373 (propofol in DISE has also been discussed in Section 2.3). In a series by Capasso et al,123 patients receiving propofol had a significantly increased likelihood of complete tongue base obstruction versus partial or no obstruction compared to those receiving dexmedetomidine. The 2 other studies that examined aspects of airway obstruction did not demonstrate significant differences between the dexmedetomidine and comparison groups.220,221
Three DISE studies measured intraprocedural respiratory and hemodynamic parameters. Two studies demonstrated a decrease in respiratory rate and lower SpO2 with propofol compared to dexmedetomidine.124,221 In the study by Cho et al,220 mean SpO2 of the dexmedetomidine-remifentanil and propofol groups did not differ; however, it was significantly lower in the propofol-remifentanil group.220 This study showed no hemodynamic differences, a finding shared by Kuyrukluyildiz et al.124 Conversely, Yoon et al221 observed similar mean arterial pressure (MAP) but lower mean heart rate (HR) with dexmedetomidine and no episodes of clinically significant bradycardia. Kuyrukluyildiz et al124 measured postprocedure outcomes, finding significantly lower MAP and HR with dexmedetomidine. Mean SpO2 and respiratory rate were higher with dexmedetomidine, although only 1 patient receiving propofol required additional oxygen supplementation.124
These 4 studies were examined in a systematic review, which concluded that dexmedetomidine appeared to yield a more stable cardiopulmonary profile, while propofol offered a faster onset, a shorter half-life, and potentially a greater degree of airway obstruction.222 The authors emphasized that neither propofol nor dexmedetomidine has been validated in replicating the obstruction that occurs during sleep. The obstructive patterns could be due to drug effect rather than reflective of the natural sleep state. Consequently, additional investigation is necessary to ascertain the optimal sedative in DISE.
Other Procedures.
For studies involving procedures other than DISE, adverse events were characterized according to respiratory effects, hemodynamic effects, and recovery profile (Supplemental Digital Content, Table A11, http://links.lww.com/AA/C373).
Two studies reported respiratory outcomes during sedation procedures. In a descriptive series of 20 patients at high risk of OSA, 13 required interventions for airway obstruction and 2 for desaturation during endoscopy with combined dexmedetomidine–propofol sedation.134 An RCT in upper respiratory procedures demonstrated that, compared to propofol target-controlled infusion, dexmedetomidine use resulted in lower desaturation incidence, higher SpO2 at most time points, and lower rates of airway obstruction.223
Data are limited regarding respiratory effects of dexmedetomidine in the postoperative recovery period. A descriptive series of bariatric patients reported adequate saturations with supplemental oxygen without the need for CPAP.224 Studies with quantitative data suggest that intraoperative use of dexmedetomidine may not affect the respiratory rate in bariatric patients225 and when compared to placebo may have a better recovery profile in individuals undergoing uvuloplasty.93 In another group of patients receiving postoperative sedation after uvulopalatopharyngoplasty, the dexmedetomidine group experienced less severe and less frequent cough during extubation and less respiratory depression compared to the propofol group.226 Finally, in a retrospective review comparing patients undergoing airway reconstruction surgery who received dexmedetomidine versus those who did not, neither group required interventions for airway compromise.227
Two studies examined the effect of clonidine on respiratory parameters and sleep in patients with OSA.228,229 In an RCT of 8 patients, clonidine compared to placebo suppressed the amount of time in REM sleep and decreased apnea duration during REM while not affecting overall AHI.228 Minimum SpO2 levels were higher in the clonidine group (86% ± 1.5% vs 84% ± 1.0%), reaching statistical but arguably not clinical significance. Pawlik et al229 performed an RCT in patients with OSA undergoing ear, nose, and throat surgery, with patients receiving either oral clonidine or placebo the night before and 2 hours before surgery. AHI in the night of surgery did not differ from baseline or between the 2 treatment groups. In both groups, the desaturation index decreased on the preoperative night, the day of the operation, and the postoperative night compared to their respective baseline measurements but did not differ between groups.
The hemodynamic effects of α-2 agonists were assessed according to varied outcome measures, including vital sign measurements, categorical descriptors, and need for rescue medications. Intraoperatively, 3 studies demonstrated significantly lower MAPs with α-2 agonists,229–231 while 1 study showed no difference.232 Heart rate was significantly lower with dexmedetomidine in 3 studies,223,229,230 while no difference to controls was observed in 2 other studies.231,232 Chawla et al227 reported transient loading dose hypertension followed by “titratable, controlled hypotension, and bradycardia.” Three studies223,227,231 demonstrated less frequent use of rescue antihypertensives or β blockers among α-2 agonist groups intraoperatively; 1 study showed this postoperatively.229 Furthermore, 1 study demonstrated a greater incidence of need for phenylephrine support in patients receiving dexmedetomidine.231 In studies reporting the need for atropine and/or ephedrine, the overall incidence was low, and no differences were reported between treatment groups.223,229 Among studies that measured postoperative hemodynamics, there was inconsistency as to whether MAP was decreased with α-2 agonists229–231 or similar to that of the control patients.93,225 Xu et al226 also characterized outcomes according to categorical variables and found a decreased incidence of hypertension and tachycardia, as well as an increased incidence of bradycardia in the dexmedetomidine-treated group; the frequency of hypotension did not differ.
The potential role of α-2 agonists in modulating the sympathetic response is of clinical interest. Four studies226,229,231,233 examined the effects of α-2 agonists on hemodynamics at points of stimulation, such as intubation, incision, and extubation. Only 1 study compared the measurements of each group to their respective baseline values,226 while all compared the measurements between treatment groups. Blood pressure and HR in the α-2 agonist groups were either lower than or similar to their control groups. Another group observed less frequent spikes in MAP and HR in clonidine-treated patients, but this was not statistically significant.234
The effects of α-2 agonists on recovery profile varied. Three studies demonstrated shorter time to extubation with α-2 agonists,226,230,234 1 showed no difference compared to control patients,231 and another showed increased time to extubation.93 One series described prolonged drowsiness with dexmedetomidine,134 while another study showed no difference in sedation score compared to control patients.93 End points related to postoperative nausea/vomiting were examined in 1 observational study225 and 3 RCTs.93,226,231
In summary, the literature on the differential effect of α-2-agonists in patients with and without OSA is limited and results are nonuniform (Oxford LOE: 2–4). While a trend in statistical outcomes for some cardiorespiratory parameters may be observed, the clinical impact of these findings remains unknown.
3. ANESTHESIA TECHNIQUE
3.1 Question: Should regional anesthesia be preferred over general anesthesia in patients with OSA?
3.1 Recommendation: When applicable, regional anesthesia is preferable over general anesthesia in patients with OSA.
Level of evidence: Moderate; Grade of recommendation: Strong
Rationale
A wide range of literature and earlier guidelines have favored the use of regional anesthesia techniques and multimodal analgesic approaches among patients with OSA despite little scientific evidence to support this practice.8,9 To address this matter, a systematic literature search was performed to summarize evidence on preferable anesthesia techniques in patients with OSA.
Anesthesia Technique as a Modifier of Postoperative Outcome.
With regard to comparative effectiveness between general and regional anesthesia specifically in patients with OSA, 6 observational studies were identified.61,235–239 A summary of evidence is provided in Supplemental Digital Content, Table A12, http://links.lww.com/AA/C373. Overall, studies indicated that the utilization of regional as opposed to general anesthesia would improve postoperative outcome.79,235–239 The largest population-based analysis included >30,000 patients with OSA from >400 US hospitals undergoing joint arthroplasty procedures.235 Adjusted risk of numerous major complications was significantly lower in patients with OSA who received neuraxial anesthesia versus general anesthesia. Furthermore, the addition of neuraxial to general anesthesia versus the use of general anesthesia alone was associated with improved outcome profiles. Additionally, the utilization of peripheral nerve blocks was associated with decreased odds for mechanical ventilation, critical care admissions, and prolonged hospital length of stay.235
Subsequent studies236,239 confirmed the previous findings, while 1 suggested benefits with regard to mortality.239 Notably, in a prospective analysis investigating drivers of postoperative worsening of sleep-disordered breathing, Chung et al79 demonstrated that the utilization of general anesthesia was associated with an increased central apnea index postoperatively, while 72-hour total opioid dose was a driver of increased AHI. This finding suggests that the residual effects of general anesthesia may affect postoperative sleep architecture and sleep-disordered breathing in OSA.
Given the necessity of airway manipulation under general anesthesia, other challenges inherent to OSA should be considered as well. The higher risk for a difficult airway in OSA has been discussed in Section 1. However, challenges with regard to airway complications in patients with OSA appear to also extend to the time for emergence from anesthesia and the immediate postoperative period, potentially leading to the requirement of emergent airway interventions.240,241 Thus, consistent with the underlying pathogenesis of OSA, perioperative complications in these patients may be driven by upper airway obstruction.240,241 Recently, Ramachandran et al61 showed that OSA was an independent predictor of respiratory complications and unplanned intubation after general anesthesia.
Another potential hazard associated with the use of general anesthesia is the frequent need for neuromuscular blockade. As described in Section 2.1, studies suggest that patients with OSA who received NMDA may be at increased risk for effects of residual neuromuscular blockade and respiratory failure compared to the general population.67,242 Therefore, the use of regional anesthesia may offer advantages by virtue of avoiding upper airway effects, although the potential for the need to convert to general anesthesia should always be considered.
Neural stimulation appears to be essential in initiating the surgical catabolic stress response,243,244 and regional anesthesia utilizing local anesthetics seems to reliably block this effect.245 Given the evidence suggesting potential OSA-related alterations in pain perception and opioid potency due to intermittent hypoxia and sleep fragmentation, as discussed in Section 2.2, regional anesthesia confers benefits by providing effective pain relief while reducing opioid requirement,246,247 a key factor to consider in patients with OSA.112,248
In summary, despite the lack of high-quality RCTs, some evidence suggests a higher risk of complications with general compared to regional anesthesia in patients with OSA (Oxford LOE 2–4). Thus, regional anesthesia should be considered by anesthesiologists whenever feasible.
RECOMMENDATIONS: EXECUTIVE SUMMARY
Patients with OSA should be considered at increased risk for difficult airway challenges compared to patients without OSA. This particularly applies to difficult intubation, difficult mask ventilation, or both. Data on the placement of supraglottic airway devices are scarce, but available evidence does not suggest a difference between patients with and without OSA. Adequate difficult airway management precautions should be taken in patients with OSA.
Anesthetic and analgesic drugs can interact with or impact consciousness, sleep, upper airway anatomy and physiology, arousal responses, muscle activation, and ventilatory drive, potentially increasing perioperative risk in patients with OSA.
In patients with OSA, the utilization of NMBA may confer an increased risk for the effects of residual neuromuscular blockade, postoperative respiratory failure, or hypoxemia. Residual neuromuscular blockade could be a driver of the higher incidence of respiratory complications in OSA. While neuromuscular blocking reversal agents can decrease postoperative residual paralysis and respiratory complications, current evidence does not favor any specific neuromuscular reversal agent with regard to outcome.
Given the respiratory depressant effects of opioids, patients with OSA may be at increased risk for respiratory complications from the use of these analgesic drugs. Furthermore, chronic intermittent hypoxia and habitual sleep fragmentation may increase pain perception and augment opioid potency in OSA. These factors should be considered when administering opioids to patients with OSA.
Patients with OSA receiving propofol for procedural sedation may be at increased risk for respiratory compromise and hypoxemic events. In the absence of certainty regarding dosing and scalar adjustments to concomitant use of other drugs and potential concurrent obesity, the utilization of propofol sedation in OSA requires a heightened level of vigilance as well as careful monitoring and titration to achieve desired effects.
There is a lack of evidence on residual effects and anesthesia recovery profiles of inhalational agents and intravenous propofol specifically for the population with OSA. However, evidence in patients with obesity, a population with a high prevalence of OSA, indicates a potential superiority of sevoflurane and desflurane compared to intravenous propofol with regard to emergence and recovery from anesthesia. Comparing sevoflurane and desflurane, the latter has been associated with improved anesthesia recovery in patients with obesity.
Evidence on the impact of ketamine specifically in OSA is largely lacking; however, adverse events such as psychiatric effects, sympathetic system activation, and hypersalivation, as usually observed in the general population during utilization of high doses, likely translate to OSA as well. Notably, however, emerging evidence indicates a potentially favorable impact of ketamine over other sedatives with regard to preservation of upper airway and ventilatory function.
Despite the scarcity of data on the comparative effectiveness of intravenous benzodiazepine sedation among patients with and without OSA, intravenous benzodiazepines are known to and are purposefully utilized to induce upper airway collapse for diagnostic purposes of OSA. Thus, the procedure of intravenous benzodiazepine sedation may be associated with airway compromise in OSA.
The potentially favorable respiratory profile and analgesic-sparing effects of α-2 agonists may render these drugs beneficial to the population with OSA. However, current literature on the effect of α-2 agonists in patients with OSA is limited and provides heterogeneous results. Thus, despite the detection of trends in statistical outcomes for some cardiorespiratory parameters, the clinical relevance of these findings remains unclear.
Evidence on the comparative effectiveness of general versus regional anesthesia in the context of OSA is sparse. Nevertheless, the limited evidence in patients with OSA indicates a higher risk of complications with general compared to regional anesthesia. When feasible, regional anesthesia may confer advantages such as avoidance of upper airway effects and neuromuscular blockade, effective pain management, reduced opioid consumption, and efficient suppression of the systemic stress response. These features may be of benefit to patients with OSA. Given these findings and in the absence of evidence suggesting a disadvantage of regional anesthesia, the utilization of these techniques should be considered preferable over general anesthesia whenever feasible. A summary of evidence is provided in Supplemental Digital Content, Table A9, http://links.lww.com/AA/C373.
ACKNOWLEDGMENTS
The SASM task force is divided into 9 groups addressing the questions surrounding (1) airway, (2) neuromuscular blocking agents, (3) opioids, (4) propofol, (5) inhalational agents, (6) benzodiazepines, (7) ketamine, (8) α-2 agonists, and (9) anesthesia technique. The leaders of the respective groups and its individual members were: (1) Difficult airway in OSA: Mahesh Nagappa (Leader), David T. Wong, Frances Chung, Satya Krishna Ramachandran; (2) NMBAs: Jean Wong (Leader), Frances Chung, Mandeep Singh; (3) Opioids: Crispiana Cozowicz (Leader), Anthony G. Doufas, Frances Chung, Stavros G. Memtsoudis; (4) Propofol: Mark H. Stein (Leader), Frances Chung; (5) Inhalational agents: Girish P. Joshi (Leader), Crispiana Cozowicz, Stavros G. Memtsoudis; (6) Ketamine: Meltem Yilmaz (Leader); (7) Benzodiazepines: Stavros G. Memtsoudis (Leader), Lukas Pichler, Crispiana Cozowicz; (8) α 2-agonists: Megan L. Krajewski (Leader), Satya Krishna Ramachandran, Crispiana Cozowicz; and (9) Anesthesia technique: Stavros G. Memtsoudis (Leader), Crispiana Cozowicz. We would like to express special thanks to the following participants in alphabetical order for their significant contribution in the systematic literature search and data analysis process: Marina Englesakis, Library and Information Services, University Health Network, University of Toronto, Toronto, Ontario, Canada; Rie Goto, Kim Barrett Memorial Library, Hospital for Special Surgery, New York, NY; Bridget Jivanelli, Kim Barrett Memorial Library, Hospital for Special Surgery, New York, NY; Eva E. Mörwald, MD, Department of Anesthesiology, Perioperative Medicine and Intensive Care Medicine, Paracelsus Medical University, Salzburg, Austria; Khawaja Rashid Hafeez, MBBS, FCPS, Toronto Western Hospital, University Health Network, University of Toronto, Toronto, Ontario, Canada; Arvind Tuteja, MBBS, Toronto Western Hospital, University Health Network, University of Toronto, Toronto, Ontario, Canada; Vwaire Urhuru, MD, Department of Anesthesia, Critical Care, and Pain Management, Beth Israel Deaconess Medical Center, Boston, MA; Sarah M. Weinstein, BA, Department of Anesthesiology, Hospital for Special Surgery, New York, NY.
DISCLOSURES
Name: Stavros G. Memtsoudis, MD, PhD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: S. G. Memtsoudis is a director on the boards of the American Society of Regional Anesthesia and Pain Medicine (ASRA) and the Society of Anesthesia and Sleep Medicine (SASM). He is a 1-time consultant for Sandoz Inc and the holder of US Patent Multicatheter Infusion System US-2017-0361063. He is the owner of SGM Consulting, LLC, and co-owner of FC Monmouth, LLC. None of these relations influenced the conduct of the present study.
Name: Crispiana Cozowicz, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Mahesh Nagappa, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Jean Wong, MD, FRCPC.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: J. Wong has received research grants from Acacia Pharma.
Name: Girish P. Joshi, MBBS, MD, FFARCSI.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: G. P. Joshi received an honorarium from Baxter Pharmaceuticals, Mallinckrodt Pharmaceuticals, Merck Pharmaceuticals, and Pacira Pharmaceuticals.
Name: David T. Wong, MD, FRCPC.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Anthony G. Doufas, MD, PhD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Meltem Yilmaz, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: M. Yilmaz serves on the advisory board of VitaHEAT Medical.
Name: Mark H. Stein, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Megan L. Krajewski, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Mandeep Singh, MBBS, MD, MSc, FRCPC.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: None.
Name: Lukas Pichler, MD.
Contribution: This author helped analyze the data and write the manuscript.
Conflicts of Interest: None.
Name: Satya Krishna Ramachandran, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: S. K. Ramachandran funded research from Merck, Sharp & Dohme, New Jersey.
Name: Frances Chung, MBBS, FRCPC.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Conflicts of Interest: F. Chung received research grants from Ontario Ministry of Health and Long-Term Care Innovation Fund, University Health Network Foundation, ResMed Foundation, Acacia Pharma, and Medtronics Inc STOP-Bang tool: proprietary to University Health Network, royalties from Up-To-Date.
This manuscript was handled by: David Hillman, MD.
Supplementary Material
Footnotes
Published ahead of print June 25, 2018.
Funding: None.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
LMA Unique is a registered trademark of Teleflex Incorporated or its affiliates.
Reprints will not be available from the authors.
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053.. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041.. [DOI] [PubMed] [Google Scholar]
- 5.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–121.. [DOI] [PubMed] [Google Scholar]
- 6.Opperer M, Cozowicz C, Bugada D, et al. does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the Society of Anesthesia and Sleep Medicine Task Force on Preoperative Preparation of Patients With Sleep-Disordered Breathing. Anesth Analg. 2016;122:1321–1334.. [DOI] [PubMed] [Google Scholar]
- 7.Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of anesthesia and sleep medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg. 2016;123:452–473.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross JB, Bachenberg KL, Benumof JL, et al. ; American Society of Anesthesiologists Task Force on Perioperative Management. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093.. [DOI] [PubMed] [Google Scholar]
- 9.Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients With Obstructive Sleep Apnea. Anesthesiology. 2014;120:268–286.. [DOI] [PubMed] [Google Scholar]
- 10.Joshi GP, Ankichetty SP, Gan TJ, Chung F. Society for Ambulatory Anesthesia consensus statement on preoperative selection of adult patients with obstructive sleep apnea scheduled for ambulatory surgery. Anesth Analg. 2012;115:1060–1068.. [DOI] [PubMed] [Google Scholar]
- 11.Meoli AL, Rosen CL, Kristo D, et al. ; Clinical Practice Review Committee; American Academy of Sleep Medicine. Upper airway management of the adult patient with obstructive sleep apnea in the perioperative period–avoiding complications. Sleep. 2003;26:1060–1065.. [DOI] [PubMed] [Google Scholar]
- 12.Seet E, Chung F. Management of sleep apnea in adults: functional algorithms for the perioperative period: continuing professional development. Can J Anaesth. 2010;57:849–864.. [DOI] [PubMed] [Google Scholar]
- 13.de Raaff CAL, Gorter-Stam MAW, de Vries N, et al. Perioperative management of obstructive sleep apnea in bariatric surgery: a consensus guideline. Surg Obes Relat Dis. 2017;13:1095–1109.. [DOI] [PubMed] [Google Scholar]
- 14.Schumann R, Jones SB, Cooper B, et al. Update on best practice recommendations for anesthetic perioperative care and pain management in weight loss surgery, 2004-2007. Obesity (Silver Spring). 2009;17:889–894.. [DOI] [PubMed] [Google Scholar]
- 15.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leape LL. Reporting of adverse events. N Engl J Med. 2002;347:1633–1638.. [DOI] [PubMed] [Google Scholar]
- 17.Innovation VH. Covidence systematic review software. Melbourne, Australia. [Google Scholar]
- 18.Group OLoEW. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. Available at: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/. Accessed May 26, 2018. [Google Scholar]
- 19.Schünemann HJ, Jaeschke R, Cook DJ, et al. ; ATS Documents Development and Implementation Committee. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med. 2006;174:605–614.. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406.. [DOI] [PubMed] [Google Scholar]
- 22.Neumann I, Santesso N, Akl EA, et al. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016;72:45–55.. [DOI] [PubMed] [Google Scholar]
- 23.Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719–725.. [DOI] [PubMed] [Google Scholar]
- 24.Hiremath AS, Hillman DR, James AL, Noffsinger WJ, Platt PR, Singer SL. Relationship between difficult tracheal intubation and obstructive sleep apnoea. Br J Anaesth. 1998;80:606–611.. [DOI] [PubMed] [Google Scholar]
- 25.Siyam MA, Benhamou D. Difficult endotracheal intubation in patients with sleep apnea syndrome. Anesth Analg. 2002;95:1098–1102.. [DOI] [PubMed] [Google Scholar]
- 26.Brodsky JB, Lemmens HJ, Brock-Utne JG, Vierra M, Saidman LJ. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94:732–736.. [DOI] [PubMed] [Google Scholar]
- 27.Sabers C, Plevak DJ, Schroeder DR, Warner DO. The diagnosis of obstructive sleep apnea as a risk factor for unanticipated admissions in outpatient surgery. Anesth Analg. 2003;96:1328–1335.. [DOI] [PubMed] [Google Scholar]
- 28.Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–891.. [DOI] [PubMed] [Google Scholar]
- 29.Chung F, Yegneswaran B, Herrera F, Shenderey A, Shapiro CM. Patients with difficult intubation may need referral to sleep clinics. Anesth Analg. 2008;107:915–920.. [DOI] [PubMed] [Google Scholar]
- 30.Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation: a review of 50,000 anesthetics. Anesthesiology. 2009;110:891–897.. [DOI] [PubMed] [Google Scholar]
- 31.Shah PN, Sundaram V. Incidence and predictors of difficult mask ventilation and intubation. J Anaesthesiol Clin Pharmacol. 2012;28:451–455.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toshniwal G, McKelvey GM, Wang H. STOP-Bang and prediction of difficult airway in obese patients. J Clin Anesth. 2014;26:360–367.. [DOI] [PubMed] [Google Scholar]
- 33.Acar HV, Yarkan Uysal H, Kaya A, Ceyhan A, Dikmen B. Does the STOP-Bang, an obstructive sleep apnea screening tool, predict difficult intubation? Eur Rev Med Pharmacol Sci. 2014;18:1869–1874.. [PubMed] [Google Scholar]
- 34.Ramachandran SK, Mathis MR, Tremper KK, Shanks AM, Kheterpal S. Predictors and clinical outcomes from failed Laryngeal Mask Airway Unique™: a study of 15,795 patients. Anesthesiology. 2012;116:1217–1226.. [DOI] [PubMed] [Google Scholar]
- 35.Kim JA, Lee JJ. Preoperative predictors of difficult intubation in patients with obstructive sleep apnea syndrome. Can J Anaesth. 2006;53:393–397.. [DOI] [PubMed] [Google Scholar]
- 36.Cattano D, Killoran P, Cai C, Katsiampoura AD, Corso RM, Hagberg CA. Difficult mask ventilation in general surgical population: observation of risk factors and predictors. F1000Res. 2014;3:1–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kheterpal S, Healy D, Aziz MF, et al. ; Multicenter Perioperative Outcomes Group (MPOG) Perioperative Clinical Research Committee. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology. 2013;119:1360–1369.. [DOI] [PubMed] [Google Scholar]
- 38.Gokay P, Tastan S, Orhan ME. Is there a difference between the STOP-BANG and the Berlin Obstructive Sleep Apnoea Syndrome questionnaires for determining respiratory complications during the perioperative period? J Clin Nurs. 2016;25:1238–1252.. [DOI] [PubMed] [Google Scholar]
- 39.Corso RM, Petrini F, Buccioli M, et al. Clinical utility of preoperative screening with STOP-Bang questionnaire in elective surgery. Minerva Anestesiol. 2014;80:877–884.. [PubMed] [Google Scholar]
- 40.Aceto P, Perilli V, Modesti C, Ciocchetti P, Vitale F, Sollazzi L. Airway management in obese patients. Surg Obes Relat Dis. 2013;9:809–815.. [DOI] [PubMed] [Google Scholar]
- 41.Benumof JL. Obstructive sleep apnea in the adult obese patient: implications for airway management. Anesthesiol Clin North America. 2002;20:789–811.. [DOI] [PubMed] [Google Scholar]
- 42.Biro P, Bloch KE. Case reports patient with obstructive sleep and difficult airway access. 1995;180:417–421.. [DOI] [PubMed] [Google Scholar]
- 43.Corso RM, Cattano D, Buccioli M, Carretta E, Maitan S. Post analysis simulated correlation of the El-Ganzouri airway difficulty score with difficult airway. Braz J Anesthesiol. 2016;66:298–303.. [DOI] [PubMed] [Google Scholar]
- 44.Hukins C. Mallampati class is not useful in the clinical assessment of sleep clinic patients. J Clin Sleep Med. 2010;6:545–549.. [PMC free article] [PubMed] [Google Scholar]
- 45.Kim MK, Park SW, Lee JW. Randomized comparison of the Pentax AirWay Scope and Macintosh laryngoscope for tracheal intubation in patients with obstructive sleep apnoea. Br J Anaesth. 2013;111:662–666.. [DOI] [PubMed] [Google Scholar]
- 46.Neligan PJ, Porter S, Max B, Malhotra G, Greenblatt EP, Ochroch EA. Obstructive sleep apnea is not a risk factor for difficult intubation in morbidly obese patients. Anesth Analg. 2009;109:1182–1186.. [DOI] [PubMed] [Google Scholar]
- 47.Cattano D, Katsiampoura A, Corso RM, Killoran P, Cai C, Hagberg CA. Predictive factors for difficult mask ventilation in the obese surgical population. F1000Research. 2014:1–11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang Questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10:e0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fouladpour N, Jesudoss R, Bolden N, Shaman Z, Auckley D. Perioperative complications in obstructive sleep apnea patients undergoing surgery: a review of the legal literature. Anesth Analg. 2016;122:145–151.. [DOI] [PubMed] [Google Scholar]
- 50.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109:897–906.. [DOI] [PubMed] [Google Scholar]
- 51.Hai F, Porhomayon J, Vermont L, Frydrych L, Jaoude P, El-Solh AA. Postoperative complications in patients with obstructive sleep apnea: a meta-analysis. J Clin Anesth. 2014;26:591–600.. [DOI] [PubMed] [Google Scholar]
- 52.Ankichetty S, Wong J, Chung F. A systematic review of the effects of sedatives and anesthetics in patients with obstructive sleep apnea. J Anaesthesiol Clin Pharmacol. 2011;27:447–458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hillman DR, Chung F. Anaesthetic management of sleep-disordered breathing in adults. Respirology. 2017;22:230–239.. [DOI] [PubMed] [Google Scholar]
- 54.McNicholas WT, Ryan S. Obstructive sleep apnoea syndrome: translating science to clinical practice. Respirology. 2006;11:136–144.. [DOI] [PubMed] [Google Scholar]
- 55.Ciscar MA, Juan G, Martínez V, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86.. [DOI] [PubMed] [Google Scholar]
- 56.Ehsan Z, Mahmoud M, Shott SR, Amin RS, Ishman SL. The effects of anesthesia and opioids on the upper airway: a systematic review. Laryngoscope. 2016;126:270–284.. [DOI] [PubMed] [Google Scholar]
- 57.Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111:120–128.. [DOI] [PubMed] [Google Scholar]
- 58.Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg. 2008;107:130–137.. [DOI] [PubMed] [Google Scholar]
- 59.Bulka CM, Terekhov MA, Martin BJ, Dmochowski RR, Hayes RM, Ehrenfeld JM. Nondepolarizing neuromuscular blocking agents, reversal, and risk of postoperative pneumonia. Anesthesiology. 2016;125:647–655.. [DOI] [PubMed] [Google Scholar]
- 60.Thevathasan T, Shih SL, Safavi KC, et al. Association between intraoperative non-depolarising neuromuscular blocking agent dose and 30-day readmission after abdominal surgery. Br J Anaesth. 2017;119:595–605.. [DOI] [PubMed] [Google Scholar]
- 61.Ramachandran SK, Pandit J, Devine S, Thompson A, Shanks A. Postoperative respiratory complications in patients at risk for obstructive sleep apnea: a single-institution cohort study. Anesth Analg. 2017;125:272–279.. [DOI] [PubMed] [Google Scholar]
- 62.Fuchs-Buder T, Nemes R, Schmartz D. Residual neuromuscular blockade: management and impact on postoperative pulmonary outcome. Curr Opin Anaesthesiol. 2016;29:662–667.. [DOI] [PubMed] [Google Scholar]
- 63.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg. 2013;23:1842–1851.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Memtsoudis SG, Stundner O, Rasul R, et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118:407–418.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sudré EC, de Batista PR, Castiglia YM. Longer immediate recovery time after anesthesia increases risk of respiratory complications after laparotomy for bariatric surgery: a randomized clinical trial and a cohort study. Obes Surg. 2015;25:2205–2212.. [DOI] [PubMed] [Google Scholar]
- 66.Ahmad S, Nagle A, McCarthy RJ, Fitzgerald PC, Sullivan JT, Prystowsky J. Postoperative hypoxemia in morbidly obese patients with and without obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesth Analg. 2008;107:138–143.. [DOI] [PubMed] [Google Scholar]
- 67.Pereira H, Xará D, Mendonça J, Santos A, Abelha FJ. Patients with a high risk for obstructive sleep apnea syndrome: postoperative respiratory complications. Rev Port Pneumol. 2013;19:144–151.. [DOI] [PubMed] [Google Scholar]
- 68.Ünal DY, Baran İ, Mutlu M, Ural G, Akkaya T, Özlü O. Comparison of sugammadex versus neostigmine costs and respiratory complications in patients with obstructive sleep apnoea. Turk J Anaesthesiol Reanim. 2015;43:387–395.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Llauradó S, Sabaté A, Ferreres E, Camprubí I, Cabrera A. Postoperative respiratory outcomes in laparoscopic bariatric surgery: comparison of a prospective group of patients whose neuromuscular blockade was reverted with sugammadex and a historical one reverted with neostigmine. Rev Esp Anestesiol Reanim. 2014;61:565–570.. [DOI] [PubMed] [Google Scholar]
- 70.Dowidar AE-RM, Basuni AS, EL-kalla RS, Eid GM. Influence of severity of obstructive sleep apnea on postoperative pulmonary complications in patients undergoing gastroplasty. Tanta Med J. 2016;44:58. [Google Scholar]
- 71.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56:819–828.. [DOI] [PubMed] [Google Scholar]
- 72.Eikermann M, Vogt FM, Herbstreit F, et al. The predisposition to inspiratory upper airway collapse during partial neuromuscular blockade. Am J Respir Crit Care Med. 2007;175:9–15.. [DOI] [PubMed] [Google Scholar]
- 73.Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009:Cd007362. [DOI] [PubMed] [Google Scholar]
- 74.Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R, et al. ; Evidence Anaesthesia Review Group. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia. 2015;70:1441–1452.. [DOI] [PubMed] [Google Scholar]
- 75.Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017;8:CD012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doufas A. Obstructive sleep apnea, pain, and opioid analgesia in the postoperative patient. Curr Anesthesiol Rep. 2014;4:1–9.. [Google Scholar]
- 77.Blake DW, Chia PH, Donnan G, Williams DL. Preoperative assessment for obstructive sleep apnoea and the prediction of postoperative respiratory obstruction and hypoxaemia. Anaesth Intensive Care. 2008;36:379–384.. [DOI] [PubMed] [Google Scholar]
- 78.Bolden N, Smith CE, Auckley D, Makarski J, Avula R. Perioperative complications during use of an obstructive sleep apnea protocol following surgery and anesthesia. Anesth Analg. 2007;105:1869–1870.. [DOI] [PubMed] [Google Scholar]
- 79.Chung F, Liao P, Elsaid H, Shapiro CM, Kang W. Factors associated with postoperative exacerbation of sleep-disordered breathing. Anesthesiology. 2014;120:299–311.. [DOI] [PubMed] [Google Scholar]
- 80.Esclamado RM, Glenn MG, McCulloch TM, Cummings CW. Perioperative complications and risk factors in the surgical treatment of obstructive sleep apnea syndrome. Laryngoscope. 1989;99:1125–1129.. [DOI] [PubMed] [Google Scholar]
- 81.Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125–132.. [DOI] [PubMed] [Google Scholar]
- 82.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122:659–665.. [DOI] [PubMed] [Google Scholar]
- 83.Melamed R, Boland LL, Normington JP, et al. Postoperative respiratory failure necessitating transfer to the intensive care unit in orthopedic surgery patients: risk factors, costs, and outcomes. Perioper Med (Lond). 2016;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramachandran SK, Haider N, Saran KA, et al. Life-threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth. 2011;23:207–213.. [DOI] [PubMed] [Google Scholar]
- 85.Weingarten TN, Hawkins NM, Beam WB, et al. Factors associated with prolonged anesthesia recovery following laparoscopic bariatric surgery: a retrospective analysis. Obes Surg. 2015;25:1024–1030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weingarten TN, Herasevich V, McGlinch MC, et al. Predictors of delayed postoperative respiratory depression assessed from naloxone administration. Anesth Analg. 2015;121:422–429.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weingarten TN, Chong EY, Schroeder DR, Sprung J. Predictors and outcomes following naloxone administration during phase I anesthesia recovery. J Anesth. 2016;30:116–122.. [DOI] [PubMed] [Google Scholar]
- 88.Mörwald EE, Olson A, Cozowicz C, Poeran J, Mazumdar M, Memtsoudis SG. Association of opioid prescription and perioperative complications in obstructive sleep apnea patients undergoing total joint arthroplasties. Sleep Breath. 2018;22:115–121.. [DOI] [PubMed] [Google Scholar]
- 89.Subramani Y, Nagappa M, Wong J, Patra J, Chung F. Death or near-death in patients with obstructive sleep apnoea: a compendium of case reports of critical complications. Br J Anaesth. 2017;119:885–899.. [DOI] [PubMed] [Google Scholar]
- 90.Madani M. Effectiveness of Stadol NS (butorphanol tartrate) with ibuprofen in the treatment of pain after laser-assisted uvulopalatopharyngoplasty. J Oral Maxillofac Surg. 2000;58:27–31.. [DOI] [PubMed] [Google Scholar]
- 91.Wang D, Somogyi AA, Yee BJ, et al. The effects of a single mild dose of morphine on chemoreflexes and breathing in obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185:526–532.. [DOI] [PubMed] [Google Scholar]
- 92.Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic surgery. Pain. 2017;158:2422–2430.. [DOI] [PubMed] [Google Scholar]
- 93.Abdelmageed WM, Elquesny KM, Shabana RI, Abushama HM, Nassar AM. Analgesic properties of a dexmedetomidine infusion after uvulopalatopharyngoplasty in patients with obstructive sleep apnea. Saudi J Anaesth. 2011;5:150–156.. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Bernards CM, Knowlton SL, Schmidt DF, et al. Respiratory and sleep effects of remifentanil in volunteers with moderate obstructive sleep apnea. Anesthesiology. 2009;110:41–49.. [DOI] [PubMed] [Google Scholar]
- 95.Blake DW, Yew CY, Donnan GB, Williams DL. Postoperative analgesia and respiratory events in patients with symptoms of obstructive sleep apnoea. Anaesth Intensive Care. 2009;37:720–725.. [DOI] [PubMed] [Google Scholar]
- 96.Huang HC, Lee LA, Fang TJ, Li HY, Lo CC, Wu JH. Transnasal butorphanol for pain relief after uvulopalatopharyngoplasty: a hospital-based, randomized study. Chang Gung Med J. 2009;32:390–399.. [PubMed] [Google Scholar]
- 97.Lee LA, Wang PC, Chen NH, et al. Alleviation of wound pain after surgeries for obstructive sleep apnea. Laryngoscope. 2007;117:1689–1694.. [DOI] [PubMed] [Google Scholar]
- 98.Yang L, Sun DF, Wu Y, Han J, Liu RC, Wang LJ. Intranasal administration of butorphanol benefits old patients undergoing H-uvulopalatopharyngoplasty: a randomized trial. BMC Anesthesiol. 2015;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orlov D, Ankichetty S, Chung F, Brull R. Cardiorespiratory complications of neuraxial opioids in patients with obstructive sleep apnea: a systematic review. J Clin Anesth. 2013;25:591–599.. [DOI] [PubMed] [Google Scholar]
- 100.Ladha KS, Kato R, Tsen LC, Bateman BT, Okutomi T. A prospective study of post-cesarean delivery hypoxia after spinal anesthesia with intrathecal morphine 150μg. Int J Obstet Anesth. 2017;32:48–53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson MJ, Clinger BN, Simonds RM, Hochheimer CJ, Lahaye LA, Golladay GJ. Probability of undiagnosed obstructive sleep apnea does not correlate with adverse pulmonary events nor length of stay in hip and knee arthroplasty using intrathecal opioid. J Arthroplasty. 2017;32:2676–2679.. [DOI] [PubMed] [Google Scholar]
- 102.Zotou A, Siampalioti A, Tagari P, Paridis L, Kalfarentzos F, Filos KS. Does epidural morphine loading in addition to thoracic epidural analgesia benefit the postoperative management of morbidly obese patients undergoing open bariatric surgery? A pilot study. Obes Surg. 2014;24:2099–2108.. [DOI] [PubMed] [Google Scholar]
- 103.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151.. [DOI] [PubMed] [Google Scholar]
- 104.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505.. [DOI] [PubMed] [Google Scholar]
- 105.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1667–1672.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16:522–533.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luyster FS, Buysse DJ, Strollo PJ., Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6:196–204.. [PMC free article] [PubMed] [Google Scholar]
- 108.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith MT, Wickwire EM, Grace EG, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779–790.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khalid I, Roehrs TA, Hudgel DW, Roth T. Continuous positive airway pressure in severe obstructive sleep apnea reduces pain sensitivity. Sleep. 2011;34:1687–1691.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doufas AG, Tian L, Padrez KA, et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PLoS One. 2013;8:e54807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Doufas AG, Tian L, Davies MF, Warby SC. Nocturnal intermittent hypoxia is independently associated with pain in subjects suffering from sleep-disordered breathing. Anesthesiology. 2013;119:1149–1162.. [DOI] [PubMed] [Google Scholar]
- 113.Brown KA, Laferrière A, Moss IR. Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia. Anesthesiology. 2004;100:806–810.. [DOI] [PubMed] [Google Scholar]
- 114.Brown KA, Laferrière A, Lakheeram I, Moss IR. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;105:665–669.. [DOI] [PubMed] [Google Scholar]
- 115.Sadhasivam S, Chidambaran V, Ngamprasertwong P, et al. Race and unequal burden of perioperative pain and opioid related adverse effects in children. Pediatrics. 2012;129:832–838.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanders JC, King MA, Mitchell RB, Kelly JP. Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea syndrome. Anesth Analg. 2006;103:1115–1121.. [DOI] [PubMed] [Google Scholar]
- 117.Turan A, You J, Egan C, et al. Chronic intermittent hypoxia is independently associated with reduced postoperative opioid consumption in bariatric patients suffering from sleep-disordered breathing. PLoS One. 2015;10:e0127809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chung F, Liao P, Yegneswaran B, Shapiro CM, Kang W. Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology. 2014;120:287–298.. [DOI] [PubMed] [Google Scholar]
- 119.Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;9:433–438.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Vito A, Agnoletti V, Berrettini S, et al. Drug-induced sleep endoscopy: conventional versus target controlled infusion techniques: a randomized controlled study. Eur Arch Otorhinolaryngol. 2011;268:457–462.. [DOI] [PubMed] [Google Scholar]
- 121.Rabelo FA, Küpper DS, Sander HH, Fernandes RM, Valera FC. Polysomnographic evaluation of propofol-induced sleep in patients with respiratory sleep disorders and controls. Laryngoscope. 2013;123:2300–2305.. [DOI] [PubMed] [Google Scholar]
- 122.Dotan Y, Pillar G, Tov N, et al. Dissociation of electromyogram and mechanical response in sleep apnoea during propofol anaesthesia. Eur Respir J. 2013;41:74–84.. [DOI] [PubMed] [Google Scholar]
- 123.Capasso R, Rosa T, Tsou DY, et al. Variable findings for drug-induced sleep endoscopy in obstructive sleep apnea with propofol versus dexmedetomidine. Otolaryngol Head Neck Surg. 2016;154:765–770.. [DOI] [PubMed] [Google Scholar]
- 124.Kuyrukluyildiz U, Binici O, Onk D, et al. Comparison of dexmedetomidine and propofol used for drug-induced sleep endoscopy in patients with obstructive sleep apnea syndrome. Int J Clin Exp Med. 2015;8:5691–5698.. [PMC free article] [PubMed] [Google Scholar]
- 125.Blumen M, Bequignon E, Chabolle F. Drug-induced sleep endoscopy: a new gold standard for evaluating OSAS? Part II: results. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:109–115.. [DOI] [PubMed] [Google Scholar]
- 126.De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath. 2014;18:453–465.. [DOI] [PubMed] [Google Scholar]
- 127.Cortínez LI, Anderson BJ, Penna A, et al. Influence of obesity on propofol pharmacokinetics: derivation of a pharmacokinetic model. Br J Anaesth. 2010;105:448–456.. [DOI] [PubMed] [Google Scholar]
- 128.Servin F, Farinotti R, Haberer JP, Desmonts JM. Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide: a clinical and pharmacokinetic study. Anesthesiology. 1993;78:657–665.. [DOI] [PubMed] [Google Scholar]
- 129.La Colla L, Albertin A, La Colla G, et al. No adjustment vs adjustment formula as input weight for propofol target-controlled infusion in morbidly obese patients. Eur J Anaesthesiol. 2009;26:362–369.. [DOI] [PubMed] [Google Scholar]
- 130.Dong D, Peng X, Liu J, Qian H, Li J, Wu B. Morbid obesity alters both pharmacokinetics and pharmacodynamics of propofol: dosing recommendation for anesthesia induction. Drug Metab Dispos. 2016;44:1579–1583.. [DOI] [PubMed] [Google Scholar]
- 131.Short TG, Chui PT. Propofol and midazolam act synergistically in combination. Br J Anaesth. 1991;67:539–545.. [DOI] [PubMed] [Google Scholar]
- 132.Thomas MC, Jennett-Reznek AM, Patanwala AE. Combination of ketamine and propofol versus either agent alone for procedural sedation in the emergency department. Am J Health Syst Pharm. 2011;68:2248–2256.. [DOI] [PubMed] [Google Scholar]
- 133.Bojak I, Day HC, Liley DT. Ketamine, propofol, and the EEG: a neural field analysis of HCN1-mediated interactions. Front Comput Neurosci. 2013;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hannallah M, Rasmussen M, Carroll J, Charabaty A, Palese C, Haddad N. Evaluation of dexmedetomidine/propofol anesthesia during upper gastrointestinal endoscopy in patients with high probability of having obstructive sleep apnea. Anaesth Pain Intensive Care. 2013;173. [Google Scholar]
- 135.Vuyk J. Pharmacokinetic and pharmacodynamic interactions between opioids and propofol. J Clin Anesth. 1997;9:23S–26S.. [DOI] [PubMed] [Google Scholar]
- 136.Sneyd JR, Rigby-Jones AE. New drugs and technologies, intravenous anaesthesia is on the move (again). Br J Anaesth. 2010;105:246–254.. [DOI] [PubMed] [Google Scholar]
- 137.Colao J, Rodriguez-Correa D. Rapidly metabolized anesthetics: novel alternative agents for procedural sedation. J Anesth Clin Res. 2016;7:11. [Google Scholar]
- 138.Mehta PP, Kochhar G, Kalra S, et al. Can a validated sleep apnea scoring system predict cardiopulmonary events using propofol sedation for routine EGD or colonoscopy? A prospective cohort study. Gastrointest Endosc. 2014;79:436–444.. [DOI] [PubMed] [Google Scholar]
- 139.Coté GA, Hovis RM, Ansstas MA, et al. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8:137–142.. [DOI] [PubMed] [Google Scholar]
- 140.Friedrich-Rust M, Welte M, Welte C, et al. Capnographic monitoring of propofol-based sedation during colonoscopy. Endoscopy. 2014;46:236–244.. [DOI] [PubMed] [Google Scholar]
- 141.Nagels AJ, Bridgman JB, Bell SE, Chrisp DJ. Propofol-remifentanil TCI sedation for oral surgery. N Z Dent J. 2014;110:85–89.. [PubMed] [Google Scholar]
- 142.Mador MJ, Abo Khamis M, Nag N, Mreyoud A, Jallu S, Mehboob S. Does sleep apnea increase the risk of cardiorespiratory complications during endoscopy procedures? Sleep Breath. 2011;15:393–401.. [DOI] [PubMed] [Google Scholar]
- 143.McVay T, Fang JC, Taylor L, et al. Safety analysis of bariatric patients undergoing outpatient upper endoscopy with non-anesthesia administered propofol sedation. Obes Surg. 2017;27:1501–1507.. [DOI] [PubMed] [Google Scholar]
- 144.Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55:258–264.. [DOI] [PubMed] [Google Scholar]
- 145.Gupta A, Stierer T, Zuckerman R, Sakima N, Parker SD, Fleisher LA. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: a systematic review. Anesth Analg. 2004;98:632–641.. [DOI] [PubMed] [Google Scholar]
- 146.Benumof JL. Obesity, sleep apnea, the airway and anesthesia. Curr Opin Anaesthesiol. 2004;17:21–30.. [DOI] [PubMed] [Google Scholar]
- 147.Peromaa-Haavisto P, Tuomilehto H, Kössi J, et al. Prevalence of obstructive sleep apnoea among patients admitted for bariatric surgery: a prospective multicentre trial. Obes Surg. 2016;26:1384–1390.. [DOI] [PubMed] [Google Scholar]
- 148.Duarte RL, Magalhães-da-Silveira FJ. Factors predictive of obstructive sleep apnea in patients undergoing pre-operative evaluation for bariatric surgery and referred to a sleep laboratory for polysomnography. J Bras Pneumol. 2015;41:440–448.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000;85:91–108.. [DOI] [PubMed] [Google Scholar]
- 150.Rose DK, Cohen MM, Wigglesworth DF, DeBoer DP. Critical respiratory events in the postanesthesia care unit: patient, surgical, and anesthetic factors. Anesthesiology. 1994;81:410–418.. [DOI] [PubMed] [Google Scholar]
- 151.Fassbender P, Bürgener S, Haddad A, Silvanus MT, Peters J. Perioperative incidence of airway obstructive and hypoxemic events in patients with confirmed or suspected sleep apnea: a prospective, randomized pilot study comparing propofol/remifentanil and sevoflurane/remifentanil anesthesia. BMC Anesthesiol. 2018;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Salihoglu Z, Karaca S, Kose Y, Zengin K, Taskin M. Total intravenous anesthesia versus single breath technique and anesthesia maintenance with sevoflurane for bariatric operations. Obes Surg. 2001;11:496–501.. [DOI] [PubMed] [Google Scholar]
- 153.Siampalioti A, Karavias D, Zotou A, Kalfarentzos F, Filos K. Anesthesia management for the super obese: is sevoflurane superior to propofol as a sole anesthetic agent? A double-blind randomized controlled trial. Eur Rev Med Pharmacol Sci. 2015;19:2493–2500.. [PubMed] [Google Scholar]
- 154.Hendolin H, Kansanen M, Koski E, Nuutinen J. Propofol-nitrous oxide versus thiopentone-isoflurane-nitrous oxide anaesthesia for uvulopalatopharyngoplasty in patients with sleep apnea. Acta Anaesthesiol Scand. 1994;38:694–698.. [DOI] [PubMed] [Google Scholar]
- 155.Pizzirani E, Pigato P, Favretti F, et al. The post-anaesthetic recovery in obesity surgery: comparison between two anaesthetic techniques. Obes Surg. 1992;2:91–94.. [DOI] [PubMed] [Google Scholar]
- 156.Tanaka P, Goodman S, Sommer BR, Maloney W, Huddleston J, Lemmens HJ. The effect of desflurane versus propofol anesthesia on postoperative delirium in elderly obese patients undergoing total knee replacement: a randomized, controlled, double-blinded clinical trial. J Clin Anesth. 2017;39:17–22.. [DOI] [PubMed] [Google Scholar]
- 157.Zoremba M, Dette F, Hunecke T, Eberhart L, Braunecker S, Wulf H. A comparison of desflurane versus propofol: the effects on early postoperative lung function in overweight patients. Anesth Analg. 2011;113:63–69.. [DOI] [PubMed] [Google Scholar]
- 158.Sollazzi L, Perilli V, Modesti C, et al. Volatile anesthesia in bariatric surgery. Obes Surg. 2001;11:623–626.. [DOI] [PubMed] [Google Scholar]
- 159.Torri G, Casati A, Albertin A, et al. Randomized comparison of isoflurane and sevoflurane for laparoscopic gastric banding in morbidly obese patients. J Clin Anesth. 2001;13:565–570.. [DOI] [PubMed] [Google Scholar]
- 160.Torri G, Casati A, Comotti L, Bignami E, Santorsola R, Scarioni M. Wash-in and wash-out curves of sevoflurane and isoflurane in morbidly obese patients. Minerva Anestesiol. 2002;68:523–527.. [PubMed] [Google Scholar]
- 161.Arain SR, Barth CD, Shankar H, Ebert TJ. Choice of volatile anesthetic for the morbidly obese patient: sevoflurane or desflurane. J Clin Anesth. 2005;17:413–419.. [DOI] [PubMed] [Google Scholar]
- 162.De Baerdemaeker LE, Struys MM, Jacobs S, et al. Optimization of desflurane administration in morbidly obese patients: a comparison with sevoflurane using an “inhalation bolus” technique. Br J Anaesth. 2003;91:638–650.. [DOI] [PubMed] [Google Scholar]
- 163.De Baerdemaeker LE, Jacobs S, Den Blauwen NM, et al. Postoperative results after desflurane or sevoflurane combined with remifentanil in morbidly obese patients. Obes Surg. 2006;16:728–733.. [DOI] [PubMed] [Google Scholar]
- 164.Bilotta F, Doronzio A, Cuzzone V, Caramia R, Rosa G; PINOCCHIO Study Group. Early postoperative cognitive recovery and gas exchange patterns after balanced anesthesia with sevoflurane or desflurane in overweight and obese patients undergoing craniotomy: a prospective randomized trial. J Neurosurg Anesthesiol. 2009;21:207–213.. [DOI] [PubMed] [Google Scholar]
- 165.Ozdogan HK, Cetinkunar S, Karateke F, Cetinalp S, Celik M, Ozyazici S. The effects of sevoflurane and desflurane on the hemodynamics and respiratory functions in laparoscopic sleeve gastrectomy. J Clin Anesth. 2016;35:441–445.. [DOI] [PubMed] [Google Scholar]
- 166.Strum EM, Szenohradszki J, Kaufman WA, Anthone GJ, Manz IL, Lumb PD. Emergence and recovery characteristics of desflurane versus sevoflurane in morbidly obese adult surgical patients: a prospective, randomized study. Anesth Analg. 2004;99:1848–1853.. [DOI] [PubMed] [Google Scholar]
- 167.Vallejo MC, Sah N, Phelps AL, O’Donnell J, Romeo RC. Desflurane versus sevoflurane for laparoscopic gastroplasty in morbidly obese patients. J Clin Anesth. 2007;19:3–8.. [DOI] [PubMed] [Google Scholar]
- 168.La Colla L, Albertin A, La Colla G, Mangano A. Faster wash-out and recovery for desflurane vs sevoflurane in morbidly obese patients when no premedication is used. Br J Anaesth. 2007;99:353–358.. [DOI] [PubMed] [Google Scholar]
- 169.Kaur A, Jain AK, Sehgal R, Sood J. Hemodynamics and early recovery characteristics of desflurane versus sevoflurane in bariatric surgery. J Anaesthesiol Clin Pharmacol. 2013;29:36–40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Juvin P, Vadam C, Malek L, Dupont H, Marmuse JP, Desmonts JM. Postoperative recovery after desflurane, propofol, or isoflurane anesthesia among morbidly obese patients: a prospective, randomized study. Anesth Analg. 2000;91:714–719.. [DOI] [PubMed] [Google Scholar]
- 171.Liu FL, Cherng YG, Chen SY, et al. Postoperative recovery after anesthesia in morbidly obese patients: a systematic review and meta-analysis of randomized controlled trials. Can J Anaesth. 2015;62:907–917.. [DOI] [PubMed] [Google Scholar]
- 172.Ibraheim O, Alshaer A, Mazen K, et al. Effect of bispectral index (BIS) monitoring on postoperative recovery and sevoflurane consumption among morbidly obese patients undergoing laparoscopic gastric banding. Middle East J Anaesthesiol. 2008;19:819–830.. [PubMed] [Google Scholar]
- 173.Freo U, Carron M, Innocente F, Foletto M, Nitti D, Ori C. Effects of A-line Autoregression Index (AAI) monitoring on recovery after sevoflurane anesthesia for bariatric surgery. Obes Surg. 2011;21:850–857.. [DOI] [PubMed] [Google Scholar]
- 174.McKay RE, Malhotra A, Cakmakkaya OS, Hall KT, McKay WR, Apfel CC. Effect of increased body mass index and anaesthetic duration on recovery of protective airway reflexes after sevoflurane vs desflurane. Br J Anaesth. 2010;104:175–182.. [DOI] [PubMed] [Google Scholar]
- 175.Eger EI, 2nd, Shafer S. The complexity of recovery from anesthesia. J Clin Anesth. 2005;17:411–412.. [DOI] [PubMed] [Google Scholar]
- 176.Ebert TJ, Robinson BJ, Uhrich TD, Mackenthun A, Pichotta PJ. Recovery from sevoflurane anesthesia: a comparison to isoflurane and propofol anesthesia. Anesthesiology. 1998;89:1524–1531.. [DOI] [PubMed] [Google Scholar]
- 177.Dexter F, Tinker JH. Comparisons between desflurane and isoflurane or propofol on time to following commands and time to discharge: a meta-analysis. Anesthesiology. 1995;83:77–82.. [DOI] [PubMed] [Google Scholar]
- 178.Macario A, Dexter F, Lubarsky D. Meta-analysis of trials comparing postoperative recovery after anesthesia with sevoflurane or desflurane. Am J Health Syst Pharm. 2005;62:63–68.. [DOI] [PubMed] [Google Scholar]
- 179.Dexter F, Bayman EO, Epstein RH. Statistical modeling of average and variability of time to extubation for meta-analysis comparing desflurane to sevoflurane. Anesth Analg. 2010;110:570–580.. [DOI] [PubMed] [Google Scholar]
- 180.Agoliati A, Dexter F, Lok J, et al. Meta-analysis of average and variability of time to extubation comparing isoflurane with desflurane or isoflurane with sevoflurane. Anesth Analg. 2010;110:1433–1439.. [DOI] [PubMed] [Google Scholar]
- 181.Yasuda N, Lockhart SH, Eger EI, 2nd, et al. Comparison of kinetics of sevoflurane and isoflurane in humans. Anesth Analg. 1991;72:316–324.. [DOI] [PubMed] [Google Scholar]
- 182.Ogunnaike BO, Jones SB, Jones DB, Provost D, Whitten CW. Anesthetic considerations for bariatric surgery. Anesth Analg. 2002;95:1793–1805.. [DOI] [PubMed] [Google Scholar]
- 183.Eger EI., 2nd. Age, minimum alveolar anesthetic concentration, and minimum alveolar anesthetic concentration-awake. Anesth Analg. 2001;93:947–953.. [DOI] [PubMed] [Google Scholar]
- 184.Juvin P, Marmuse JP, Delerme S, et al. Post-operative course after conventional or laparoscopic gastroplasty in morbidly obese patients. Eur J Anaesthesiol. 1999;16:400–403.. [DOI] [PubMed] [Google Scholar]
- 185.Katznelson R, Naughton F, Friedman Z, et al. Increased lung clearance of isoflurane shortens emergence in obesity: a prospective randomized-controlled trial. Acta Anaesthesiol Scand. 2011;55:995–1001.. [DOI] [PubMed] [Google Scholar]
- 186.De Kock M, Lavand’homme P, Waterloos H. Balanced analgesia” in the perioperative period: is there a place for ketamine? Pain. 2001;92:373–380.. [DOI] [PubMed] [Google Scholar]
- 187.Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006:CD004603. [DOI] [PubMed] [Google Scholar]
- 188.Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–923.. [DOI] [PubMed] [Google Scholar]
- 189.Luscri N, Tobias JD. Monitored anesthesia care with a combination of ketamine and dexmedetomidine during magnetic resonance imaging in three children with trisomy 21 and obstructive sleep apnea. Paediatr Anaesth. 2006;16:782–786.. [DOI] [PubMed] [Google Scholar]
- 190.Cheng X, Huang Y, Zhao Q, Gu E. Comparison of the effects of dexmedetomidine-ketamine and sevoflurane-sufentanil anesthesia in children with obstructive sleep apnea after uvulopalatopharyngoplasty: an observational study. J Anaesthesiol Clin Pharmacol. 2014;30:31–35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26:985–1028.. [DOI] [PubMed] [Google Scholar]
- 192.Melendez E, Bachur R. Serious adverse events during procedural sedation with ketamine. Pediatr Emerg Care. 2009;25:325–328.. [DOI] [PubMed] [Google Scholar]
- 193.Gorlin AW, Rosenfeld DM, Ramakrishna H. Intravenous sub-anesthetic ketamine for perioperative analgesia. J Anaesthesiol Clin Pharmacol. 2016;32:160–167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.De Oliveira GS, Jr, Fitzgerald PC, Hansen N, Ahmad S, McCarthy RJ. The effect of ketamine on hypoventilation during deep sedation with midazolam and propofol: a randomised, double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2014;31:654–662.. [DOI] [PubMed] [Google Scholar]
- 195.Taylor DM, Bell A, Holdgate A, et al. Risk factors for sedation-related events during procedural sedation in the emergency department. Emerg Med Australas. 2011;23:466–473.. [DOI] [PubMed] [Google Scholar]
- 196.Drummond GB. Comparison of sedation with midazolam and ketamine: effects on airway muscle activity. Br J Anaesth. 1996;76:663–667.. [DOI] [PubMed] [Google Scholar]
- 197.Eikermann M, Grosse-Sundrup M, Zaremba S, et al. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116:35–46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Abdullah VJ, Lee DL, Ha SC, van Hasselt CA. Sleep endoscopy with midazolam: sedation level evaluation with bispectral analysis. Otolaryngol Head Neck Surg. 2013;148:331–337.. [DOI] [PubMed] [Google Scholar]
- 199.Bachar G, Feinmesser R, Shpitzer T, Yaniv E, Nageris B, Eidelman L. Laryngeal and hypopharyngeal obstruction in sleep disordered breathing patients, evaluated by sleep endoscopy. Eur Arch Otorhinolaryngol. 2008;265:1397–1402.. [DOI] [PubMed] [Google Scholar]
- 200.Bachar G, Nageris B, Feinmesser R, et al. Novel grading system for quantifying upper-airway obstruction on sleep endoscopy. Lung. 2012;190:313–318.. [DOI] [PubMed] [Google Scholar]
- 201.Carrasco Llatas M, Agostini Porras G, Cuesta González MT, et al. Drug-induced sleep endoscopy: a two drug comparison and simultaneous polysomnography. Eur Arch Otorhinolaryngol. 2014;271:181–187.. [DOI] [PubMed] [Google Scholar]
- 202.De Corsa E, Fiorita A, Rizzotto G, et al. The role of drug-induced sleep endoscopy in the diagnosis and management of obstructive sleep apnoea syndrome: our personal experience. Acta Otorhinolaryngol Ital. 2013;33:405–413.. [PMC free article] [PubMed] [Google Scholar]
- 203.Gregório MG, Jacomelli M, Figueiredo AC, Cahali MB, Pedreira WL, Jr, Lorenzi Filho G. Evaluation of airway obstruction by nasopharyngoscopy: comparison of the Müller maneuver versus induced sleep. Braz J Otorhinolaryngol. 2007;73:618–622.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hamans E, Meeus O, Boudewyns A, Saldien V, Verbraecken J, Van de Heyning P. Outcome of sleep endoscopy in obstructive sleep apnoea: the Antwerp experience. B-ENT. 2010;6:97–103.. [PubMed] [Google Scholar]
- 205.Hessel NS, de Vries N. Diagnostic work-up of socially unacceptable snoring: II. Sleep endoscopy. Eur Arch Otorhinolaryngol. 2002;259:158–161.. [DOI] [PubMed] [Google Scholar]
- 206.Iwanaga K, Hasegawa K, Shibata N, et al. Endoscopic examination of obstructive sleep apnea syndrome patients during drug-induced sleep. Acta Oto laryngol Suppl. 2003;550:36–40.. [DOI] [PubMed] [Google Scholar]
- 207.Koo SK, Choi JW, Myung NS, Lee HJ, Kim YJ, Kim YJ. Analysis of obstruction site in obstructive sleep apnea syndrome patients by drug induced sleep endoscopy. Am J Otolaryngol. 2013;34:626–630.. [DOI] [PubMed] [Google Scholar]
- 208.Ravesloot MJ, de Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope. 2011;121:2710–2716.. [DOI] [PubMed] [Google Scholar]
- 209.Sadaoka T, Kakitsuba N, Fujiwara Y, Kanai R, Takahashi H. The value of sleep nasendoscopy in the evaluation of patients with suspected sleep-related breathing disorders. Clin Otolaryngol Allied Sci. 1996;21:485–489.. [DOI] [PubMed] [Google Scholar]
- 210.Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope. 2014;124:797–802.. [DOI] [PubMed] [Google Scholar]
- 211.Choi JK, Hur YK, Lee JM, Clark GT. Effects of mandibular advancement on upper airway dimension and collapsibility in patients with obstructive sleep apnea using dynamic upper airway imaging during sleep. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:712–719.. [DOI] [PubMed] [Google Scholar]
- 212.Hillarp B, Nylander G, Rosén I, Wickström O. Videoradiography of patients with habitual snoring and/or sleep apnea: technical description and presentation of videoradiographic results during sleep concerning occurrence of apnea, type of apnea, and site of obstruction. Acta Radiol. 1996;37:307–314.. [DOI] [PubMed] [Google Scholar]
- 213.Lee CH, Mo JH, Kim BJ, et al. Evaluation of soft palate changes using sleep videofluoroscopy in patients with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2009;135:168–172.. [DOI] [PubMed] [Google Scholar]
- 214.Lee CH, Hong SL, Rhee CS, Kim SW, Kim JW. Analysis of upper airway obstruction by sleep videofluoroscopy in obstructive sleep apnea: a large population-based study. Laryngoscope. 2012;122:237–241.. [DOI] [PubMed] [Google Scholar]
- 215.Adler DG, Kawa C, Hilden K, Fang J. Nurse-administered propofol sedation is safe for patients with obstructive sleep apnea undergoing routine endoscopy: a pilot study. Dig Dis Sci. 2011;56:2666–2671.. [DOI] [PubMed] [Google Scholar]
- 216.Cha JM, Jeun JW, Pack KM, et al. Risk of sedation for diagnostic esophagogastroduodenoscopy in obstructive sleep apnea patients. World J Gastroenterol. 2013;19:4745–4751.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cillo JE, Jr, Finn R. Hemodynamics and oxygen saturation during intravenous sedation for office-based laser-assisted uvuloplasty. J Oral Maxillofac Surg. 2005;63:752–755.. [DOI] [PubMed] [Google Scholar]
- 218.Madan AK, Tichansky DS, Isom J, Minard G, Bee TK. Monitored anesthesia care with propofol versus surgeon-monitored sedation with benzodiazepines and narcotics for preoperative endoscopy in the morbidly obese. Obes Surg. 2008;18:545–548.. [DOI] [PubMed] [Google Scholar]
- 219.Mador MJ, Nadler J, Mreyoud A, et al. Do patients at risk of sleep apnea have an increased risk of cardio-respiratory complications during endoscopy procedures? Sleep Breath. 2012;16:609–615.. [DOI] [PubMed] [Google Scholar]
- 220.Cho JS, Soh S, Kim EJ, et al. Comparison of three sedation regimens for drug-induced sleep endoscopy. Sleep Breath. 2015;19:711–717.. [DOI] [PubMed] [Google Scholar]
- 221.Yoon BW, Hong JM, Hong SL, Koo SK, Roh HJ, Cho KS. A comparison of dexmedetomidine versus propofol during drug-induced sleep endoscopy in sleep apnea patients. Laryngoscope. 2016;126:763–767.. [DOI] [PubMed] [Google Scholar]
- 222.Chang ET, Certal V, Song SA, et al. Dexmedetomidine versus propofol during drug-induced sleep endoscopy and sedation: a systematic review. Sleep Breath. 2017;21:727–735.. [DOI] [PubMed] [Google Scholar]
- 223.Ma XX, Fang XM, Hou TN. Comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during coblation-assisted upper airway procedure. Chin Med J (Engl). 2012;125:869–873.. [PubMed] [Google Scholar]
- 224.Bamgbade OA, Alfa JA. Dexmedetomidine anaesthesia for patients with obstructive sleep apnoea undergoing bariatric surgery. Eur J Anaesthesiol. 2009;26:176–177.. [DOI] [PubMed] [Google Scholar]
- 225.Dholakia C, Beverstein G, Garren M, Nemergut C, Boncyk J, Gould JC. The impact of perioperative dexmedetomidine infusion on postoperative narcotic use and duration of stay after laparoscopic bariatric surgery. J Gastrointest Surg. 2007;11:1556–1559.. [DOI] [PubMed] [Google Scholar]
- 226.Xu J, Jin C, Cui X, Jin Z. Comparison of dexmedetomidine versus propofol for sedation after uvulopalatopharyngoplasty. Med Sci Monit. 2015;21:2125–2133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chawla S, Robinson S, Norton A, Esterman A, Taneerananon T. Peri-operative use of dexmedetomidine in airway reconstruction surgery for obstructive sleep apnoea. J Laryngol Otol. 2010;124:67–72.. [DOI] [PubMed] [Google Scholar]
- 228.Issa FG. Effect of clonidine in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:435–439.. [DOI] [PubMed] [Google Scholar]
- 229.Pawlik MT, Hansen E, Waldhauser D, Selig C, Kuehnel TS. Clonidine premedication in patients with sleep apnea syndrome: a randomized, double-blind, placebo-controlled study. Anesth Analg. 2005;101:1374–1380.. [DOI] [PubMed] [Google Scholar]
- 230.Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18:24–28.. [DOI] [PubMed] [Google Scholar]
- 231.Tufanogullari B, White PF, Peixoto MP, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008;106:1741–1748.. [DOI] [PubMed] [Google Scholar]
- 232.Feld J, Hoffman WE, Paisansathan C, Park H, Ananda RC. Autonomic activity during dexmedetomidine or fentanyl infusion with desflurane anesthesia. J Clin Anesth. 2007;19:30–36.. [DOI] [PubMed] [Google Scholar]
- 233.Jayaraman L, Sinha A, Punhani D. A comparative study to evaluate the effect of intranasal dexmedetomidine versus oral alprazolam as a premedication agent in morbidly obese patients undergoing bariatric surgery. J Anaesthesiol Clin Pharmacol. 2013;29:179–182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sollazzi L, Modesti C, Vitale F, et al. Preinductive use of clonidine and ketamine improves recovery and reduces postoperative pain after bariatric surgery. Surg Obes Relat Dis. 2009;5:67–71.. [DOI] [PubMed] [Google Scholar]
- 235.Memtsoudis SG, Stundner O, Rasul R, et al. Sleep apnea and total joint arthroplasty under various types of anesthesia: a population-based study of perioperative outcomes. Reg Anesth Pain Med. 2013;38:274–281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ambrosii T, Şandru S, Belîi A. The prevalence of perioperative complications in patients with and without obstructive sleep apnoea: a prospective cohort study. Rom J Anaesth Intensive Care. 2016;23:103–110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu DY, Cai XL, Liu HY. [Obstructive sleep apnea hypopnea syndrome: surgical complications and strategy for avoidance]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:555–560.. [PubMed] [Google Scholar]
- 238.Liu SS, Chisholm MF, Ngeow J, et al. Postoperative hypoxemia in orthopedic patients with obstructive sleep apnea. HSS J. 2011;7:2–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Naqvi SY, Rabiei AH, Maltenfort MG, et al. Perioperative complications in patients with sleep apnea undergoing total joint arthroplasty. J Arthroplasty. 2017;32:2680–2683.. [DOI] [PubMed] [Google Scholar]
- 240.Biddle C. Comparative aspects of the airway during general anesthesia in obese sufferers of sleep apnea and matched normals. Adv Pract Nurs Q. 1996;2:14–19.. [PubMed] [Google Scholar]
- 241.Loube DI, Erman MK, Reed W. Perioperative complications in obstructive sleep apnea patients. Sleep Breath. 1997;2:3–10.. [DOI] [PubMed] [Google Scholar]
- 242.Xará D, Mendonça J, Pereira H, Santos A, Abelha FJ. Adverse respiratory events after general anesthesia in patients at high risk of obstructive sleep apnea syndrome. Braz J Anesthesiol. 2015;65:359–366.. [DOI] [PubMed] [Google Scholar]
- 243.Kehlet H. The stress response to surgery: release mechanisms and the modifying effect of pain relief. Acta Chir Scand Suppl. 1989;550:22–28.. [PubMed] [Google Scholar]
- 244.Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg. 2000;24:690–695.. [DOI] [PubMed] [Google Scholar]
- 245.O’Neill J, Helwig E. Postoperative management of the physiological effects of spinal anesthesia. J Perianesth Nurs. 2016;31:330–339.. [DOI] [PubMed] [Google Scholar]
- 246.Meng T, Zhong Z, Meng L. Impact of spinal anaesthesia vs general anaesthesia on peri-operative outcome in lumbar spine surgery: a systematic review and meta-analysis of randomised, controlled trials. Anaesthesia. 2017;72:391–401.. [DOI] [PubMed] [Google Scholar]
- 247.Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty? Clin Orthop Relat Res. 2009;467:2379–2402.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lam KK, Kunder S, Wong J, Doufas AG, Chung F. Obstructive sleep apnea, pain, and opioids: is the riddle solved? Curr Opin Anaesthesiol. 2016;29:134–140.. [DOI] [PMC free article] [PubMed] [Google Scholar]


