Abstract
Clostridium difficile is a Gram-positive spore-forming anaerobe and a major cause of antibiotic-associated diarrhoea. Disruption of the commensal microbiota, such as through treatment with broad-spectrum antibiotics, is a critical precursor for colonisation by C. difficile and subsequent disease. Furthermore, failure of the gut microbiota to recover colonisation resistance can result in recurrence of infection. An unusual characteristic of C. difficile among gut bacteria is its ability to produce the bacteriostatic compound para-cresol (p-cresol) through fermentation of tyrosine. Here, we demonstrate that the ability of C. difficile to produce p-cresol in vitro provides a competitive advantage over gut bacteria including Escherichia coli, Klebsiella oxytoca and Bacteroides thetaiotaomicron. Metabolic profiling of competitive co-cultures revealed that acetate, alanine, butyrate, isobutyrate, p-cresol and p-hydroxyphenylacetate were the main metabolites responsible for differentiating the parent strain C. difficile (630Δerm) from a defined mutant deficient in p-cresol production. Moreover, we show that the p-cresol mutant displays a fitness defect in a mouse relapse model of C. difficile infection (CDI). Analysis of the microbiome from this mouse model of CDI demonstrates that colonisation by the p-cresol mutant results in a distinctly altered intestinal microbiota, and metabolic profile, with a greater representation of Gammaproteobacteria, including the Pseudomonales and Enterobacteriales. We demonstrate that Gammaproteobacteria are susceptible to exogenous p-cresol in vitro and that there is a clear divide between bacterial Phyla and their susceptibility to p-cresol. In general, Gram-negative species were relatively sensitive to p-cresol, whereas Gram-positive species were more tolerant. This study demonstrates that production of p-cresol by C. difficile has an effect on the viability of intestinal bacteria as well as the major metabolites produced in vitro. These observations are upheld in a mouse model of CDI, in which p-cresol production affects the biodiversity of gut microbiota and faecal metabolite profiles, suggesting that p-cresol production contributes to C. difficile survival and pathogenesis.
Author summary
Clostridium difficile is a bacterium responsible for causing the majority of antibiotic associated diarrhoea outbreaks world-wide. In the United States of America, C. difficile infects half a million people annually. Antibiotics disrupt the natural protective gut microbiota, rendering people susceptible to C. difficile infection, which leads to potentially life-threatening disease and complications. C. difficile is transmitted by spores, which are able to survive in harsh environments for long periods of time. After initial treatment for C. difficile, up to 35% of patients develop the disease again, thus requiring additional and more successful treatment. Here, we use novel techniques to show that C. difficile produces a compound, p-cresol, which has detrimental effects on the natural protective gut bacteria. We show that p-cresol selectively targets certain bacteria in the gut and disrupts their ability to grow. By removing the ability of C. difficile to produce p-cresol, we show that it makes C. difficile less able to recolonise after an initial infection. This is linked to significant alterations in the natural healthy bacterial composition of the gut. Our study provides new insights into the effects of p-cresol production on the healthy gut microbiota and how it contributes to C. difficile survival and pathogenesis.
Introduction
Clostridium difficile is a Gram-positive spore-forming enteric pathogen and the leading cause of antibiotic-associated diarrhoea worldwide[1]. C. difficile infection (CDI) ranges from self-limiting diarrhoea to severe and life threatening pseudomembranous colitis[2]. C. difficile spores are the aetiological agent of CDI transmission and are resistant to desiccation, environmental stress, disinfectants and heat[3, 4]. These spores, present in both hospitals and the environment are transmitted via the faecal-oral route, contributing to both nosocomial and community acquired CDI [3]. Infection with C. difficile is frequently preceded by treatment with broad-spectrum antibiotics, which eliminate discrete taxa of the commensal intestinal microbiota resulting in dysbiosis and permitting colonisation by C. difficile. Certain bacterial taxa have been highlighted as important in the prevention of C. difficile colonisation[5–7]. Since restoration of microbial diversity can resolve recurrent infections, faecal transplantation is viewed as an effective treatment strategy[8]. However, a greater understanding of how C. difficile is able to influence the gut microbiota and disrupt intestinal homeostasis is a current imperative.
Human intestinal bacteria have been shown to ferment dietary-derived carbohydrates[9] and proteins[10], producing short chain fatty acids (SCFA), as well as an array of metabolites via fermentation of aromatic amino acids[11]. The secondary metabolites of this highly diverse microbial community have the potential to either positively or negatively influence many aspects of human health [12], with some demonstrated to possess toxic and carcinogenic properties [11, 13, 14]. Aromatic amino acids such as phenylalanine, tryptophan and tyrosine are important sources of phenyl metabolites. These metabolites can be absorbed in the small intestine or pass through to the colon[15] to be excreted in faeces. One such fermentation product, phenylacetic acid (PAA), is the most commonly detected secondary metabolite in healthy human faeces, with reported concentrations of 479 μM[15]. C. difficile ferments tyrosine, via p-hydroxyphenylacetate (p-HPA), to produce p-cresol. Para-cresol is a phenolic compound [16] that has been demonstrated to inhibit the growth of a range of bacterial species and other microorganisms[17, 18]. To date, the capacity to produce p-cresol has only been demonstrated in a select number of organisms[19, 20], including eighteen intestinal commensal species[11]. However, the in vitro production of p-cresol by these species was relatively low (ranging from 0.06–1.95 μg/ml)[11]. Furthermore, C. difficile can tolerate relatively high concentrations (1 mg/ml) of p-cresol[21, 22]. As such, the ability to synthesise and tolerate high concentrations of p-cresol has led to the hypothesis that it may provide C. difficile with a competitive advantage over other microorganisms.
The enzyme responsible for the decarboxylation of p-HPA is a member of the glycyl radical family, 4-hydroxyphenylacetate decarboxylase, which is encoded by three genes hpdB (CD630_01530), hpdC (CD630_01540) and hpdA (CD630_01550), which are co-transcribed in an operon. The hpdBCA operon is highly conserved in all the sequenced C. difficile isolates. We have previously shown that disruption of any of the three genes renders C. difficile incapable of synthesising p-cresol[22]. In this study, we demonstrate that production of p-cresol by C. difficile confers a fitness advantage over other intestinal bacteria both in vitro and in vivo, specifically those with a Gram-negative cell envelope. The treatment of human faecal samples with exogenous p-cresol significantly modified the cultivable bacteria therein, in a species-specific manner. Furthermore, a p-cresol deficient mutant showed a modest but significant reduction in viable counts in a relapse mouse model of CDI. Comparisons of the metabolic and 16S rRNA profiles identified variation in the biochemical and bacterial composition between mice infected with the C. difficile strain 630Δerm and the p-cresol deficient mutant (hpdC::CT) following infection and relapse. This is the first study to show that p-cresol production is a mechanism by which C. difficile confers a competitive advantage over other gut bacteria.
Results
Exogenous p-cresol inhibits the growth of Gram-negative intestinal commensal bacteria
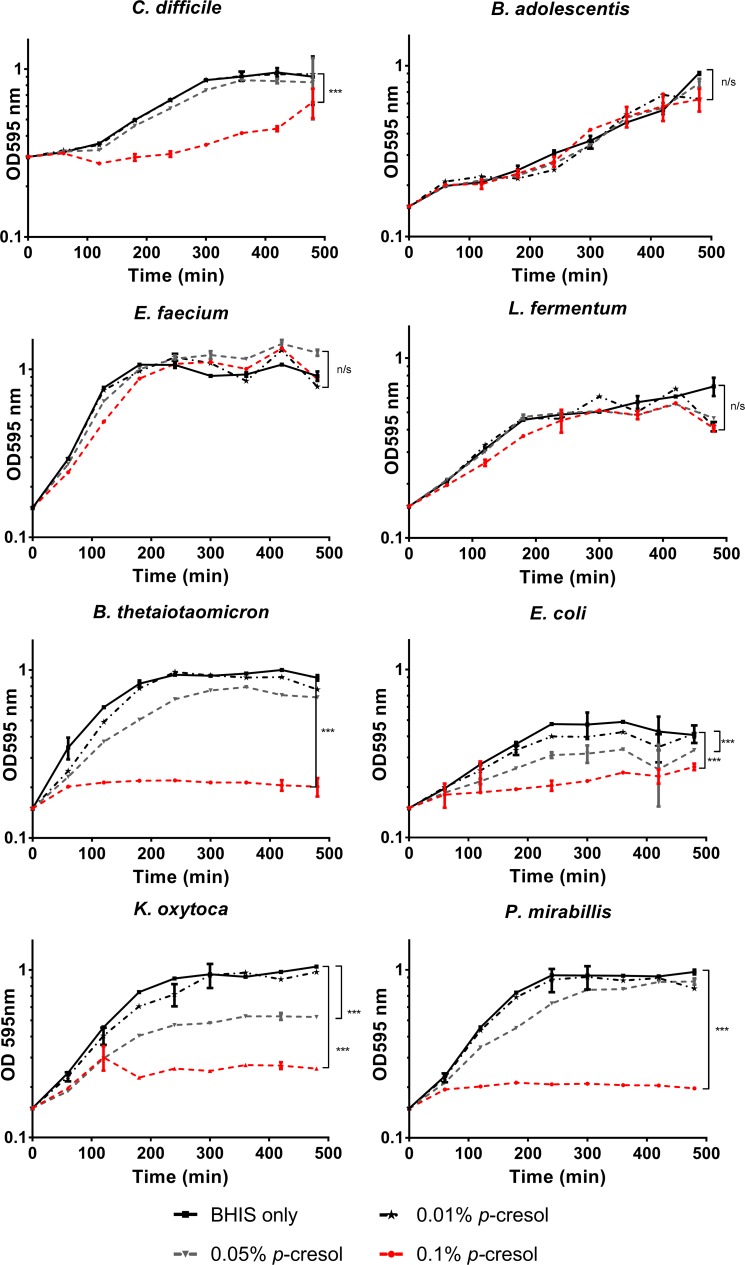
It has been hypothesised that p-cresol production provides C. difficile with a selective advantage over competitors in the human gut. To investigate this, we assessed the effect of exogenous p-cresol on the in vitro growth dynamics of selected intestinal commensal species (S1 Table) compared to C. difficile strain 630Δerm (Fig 1 & S2 Table). The data shows a clear pattern whereby sensitivity to p-cresol correlated with bacterial cell envelope structure. We observed that Gram-positive bacteria were significantly more tolerant to p-cresol than Gram-negative bacteria (Coefficient of variance (COV) = 0.599, p<0.001). Growth of the Gram-negative species, including members of the Bacteroidaceae (Bacteroides thetaiotaomicron) and Enterobacteriaceae (Escherichia coli, Klebsiella oxytoca and Proteus mirabilis) families were inhibited by the addition of exogenous p-cresol in a dose-dependent manner (Fig 1 & S3 Table) and demonstrated a significant decrease in cell growth compared to C. difficile (p<0.005). In contrast, the Gram-positive species including those from the Bifidobacteriaceae (Bifidobacterium adolescentis), Enterococcaceae (Enterococcus faecium) and Lactobacillaceae (Lactococcus fermentum) families displayed no significant reduction in growth rate, even at 0.1% (v/v) p-cresol (Fig 1). Interestingly, E. faecium displayed greater tolerance to p-cresol than C. difficile itself (COV = 0.6 p = 0.002, S3 Table).
Fig 1. Growth of gut commensals in medium supplemented with exogenous p-cresol.
Growth of gut commensals in BHIS compared to media containing 0.01%, 0.5% and 0.1% (v/v) p-cresol. Each curve represents the mean growth rate of three independent replicates. Regression analysis was used to determine significant differences in growth rate compared to the BHIS control over the course of the experiment and marked *** p<0.01. Error bars represent the standard deviation.
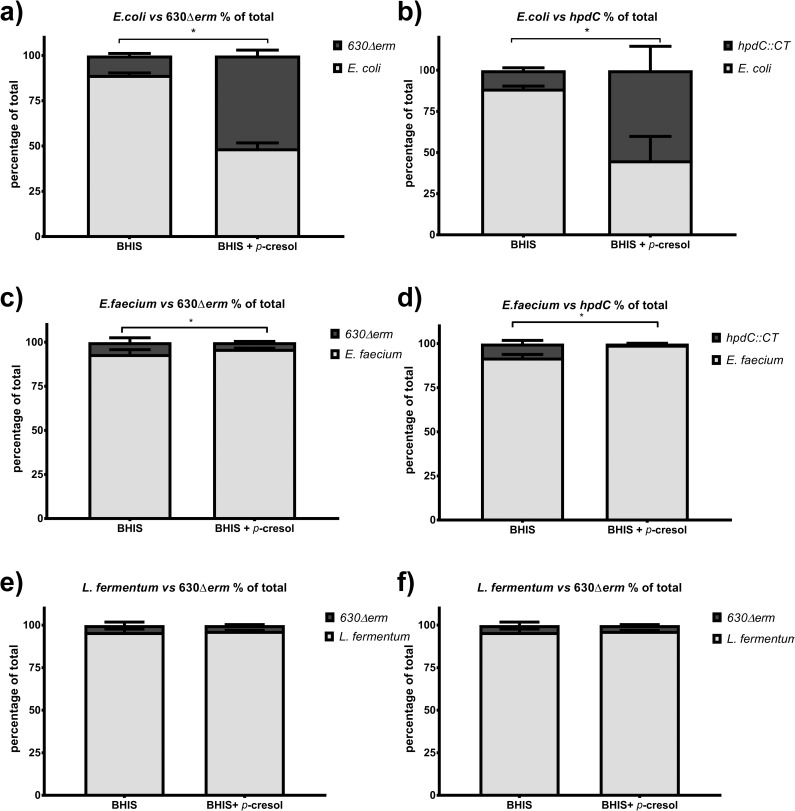
We had previously constructed a ClosTron inactivation mutant in the hpdC decarboxylase gene (strain hpdC::CT), which renders C. difficile unable to produce p-cresol[22]. To investigate whether production of p-cresol contributes to fitness in vitro, we performed co-culture assays with 630Δerm and hpdC::CT cultured with a selection of intestinal commensal species, supplemented with exogenously added p-cresol. Brain heart infusion media with yeast extract (BHIS) was chosen for these co-culture experiments, as we have previously shown that intrinsic production of p-cresol under these conditions is negligible[22], therefore any observed effect could be attributed to the exogenously added p-cresol. We observed no difference in growth rate between 630Δerm and hpdC::CT in these conditions [22]. To establish the comparable growth conditions, each species was normalised to the same starting optical density (OD595 0.5) and starting CFU/ml was determined (S4 Table). The competitors were mixed in a 1:1 ratio at matched OD, and were grown for 24 hours in media supplemented with 0.05% (v/v) p-cresol. Viable counts for each species were determined by plating serial dilutions onto media supplemented with and without D-cycloserine and cefoxitin, facilitating differentiation between C. difficile and the competitor. When C. difficile 630Δerm was grown in co-culture with E. coli in the absence of exogenous p-cresol, E. coli was the dominant organism, represented by a significantly higher CFU/ml than C. difficile (8:1 ratio of E. coli to C. difficile) (COV = 1.02, p = 0.003; Fig 2, S5 Table). However, when the medium was supplemented with exogenous p-cresol, the relative proportion of C. difficile increased to a ratio of 1:1, representing an 8-fold increase in the number of viable C. difficile (Fig 2A) (COV = -1.38, p<0.001). A similar profile was observed when E. coli was co-cultured with the hpdC::CT mutant (Fig 2B) (COV = -0.27, p = 0.882). This suggests that 630Δerm and the hpdC::CT mutant displayed comparable fitness when grown in co-culture with E. coli. When C. difficile was grown in co-culture with a Gram-positive bacterium, E. faecium, C. difficile was significantly less abundant compared with the competitor (COV = 3.41, p<0.001). Here, we observed a ratio of 1:10 of C. difficile to E. faecium (Fig 2C & S5 Table). The growth dynamics of the C. difficile hpdC::CT mutant and E. faecium (Fig 2D) were also indistinguishable from the 630Δerm grown in competition with E. faecium (COV = -0.33, p = 0.283). However, when the medium was supplemented with p-cresol, the relative proportion of E. faecium increased significantly (COV = 1.44, p = 0.010). This suggests that the growth conditions were more permissive for E. faecium. However, this was not the case for all Gram-positive species tested. The relative ratio in co-culture of C. difficile (630Δerm and hpdC::CT mutant) to L. fermentum was not significantly altered by exogenous p-cresol (COV = -0.058, p = 0.818) (Fig 2E & 2F and S5 Table). These data indicate that p-cresol had a range of effects on growth dynamics depending on the Phylum of bacteria and their susceptibility to p-cresol.
Fig 2. Co-culture assays in medium supplemented with exogenous p-cresol.
Relative fitness of C. difficile 630Δerm and hpdC::CT when grown in co-culture with gut commensals, with and without 0.05% (v/v) p-cresol. The relative fitness of 630Δerm and hpdC::CT grown in co-culture with a&b) E. coli, c&d) E. faecium, e&f) L. fermentum (e&f). Viable counts are represented by CFU/ml and displayed as a percentage of the total culture. Error bars are representative of three independent replicates. Regression analysis was used to determine significant differences in growth taking strain and media into consideration ***p<0.001.
Intrinsic production of p-cresol gives C. difficile a competitive growth advantage in vitro
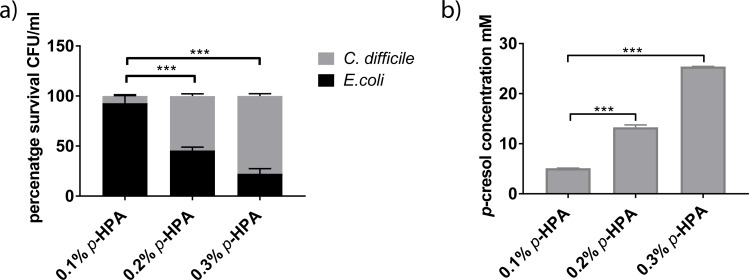
We observed no difference in competitive fitness between wild type and p-cresol mutant when grown in BHIS supplemented with 0.5% (w/v) p-cresol. Therefore, we developed an additional in vitro competition assay to determine whether intrinsic p-cresol production by C. difficile conferred a competitive advantage over other intestinal commensal species. To achieve this we measured the growth rate of E. coli and K. oxytoca in monoculture and compared this to that of C. difficile (S1A Fig). Under these conditions, C. difficile reached exponential growth at a later time point than the other species tested. Furthermore, we have previously shown that p-cresol is detected in C. difficile cultures at around 4 hours (or OD595 0.5) [22]. In order to limit the dominance of competitor species, and ensure optimal p-cresol production, we grew C. difficile to exponential phase (OD595 0.6) before inoculating the medium with the competitor (at OD595 0.05). We also supplemented the growth medium with p-HPA to drive production of p-cresol. To determine a concentration of p-HPA that resulted in inhibitory p-cresol production, competitive co-culture experiments with C. difficile and E.coli were performed in a range of p-HPA concentrations (0.1%, 0.2% or 0.3% (w/v)) (Fig 3). When the medium was supplemented with 0.1% (6.5 mM) p-HPA we observed a ratio of 13:1 (E. coli:C. difficile) (Fig 3A). When the concentration of p-HPA was increased to 0.2% (13.1 mM), we observed a significant difference (p<0.001) in the ratio of E. coli: C. difficile (1:1), compared to the ratio in 0.1% p-HPA (13:1)(Fig 3A). Further increasing the concentration of p-HPA to 0.3% (19.7 mM) resulted in culture conditions that favoured C. difficile, reflected by a ratio of 1:4 (E. coli:C. difficile) (p<0.001)(Fig 3A). Thus, we observed a positive correlation between the proportion of p-HPA supplemented in the growth medium and the survival of C. difficile compared to E. coli (Fig 3A). To determine whether this effect was linked to the level of p-cresol production, we quantified p-cresol in these culture supernatants by High Performance Liquid Chromatography (HPLC) (Fig 3B). Fig 3B demonstrates that increasing p-HPA concentration correlated with a significant increase in p-cresol production (p<0.001). We observed 25 ±0.04 mM p-cresol when the growth medium was supplemented with 0.3% p-HPA (Fig 3B).
Fig 3. The effect of p-HPA supplementation on competitive co-culture of C. difficile with E. coli and detection of p-cresol production.
a) A comparison of the percentage survival of C. difficile strain 630Δerm and E. coli in competitive co-culture for 24 hours, performed in 0.1%, 0.2% and 0.3% p-HPA. Statistical difference in percentage survival was calculated using regression analysis taking strain and media into consideration ***p<0.001. b) HPLC quantification of p-cresol production in competitive co-culture of C. difficile strain 630Δerm with E. coli in media supplemented with 0.1%, 0.2% and 0.3% p-HPA. Statistical difference in p-cresol production was calculated using a two tailed t-test with Welch’s correction, comparing individual p-cresol concentrations to the level produced in 0.1% p-HPA ***p<0.001.
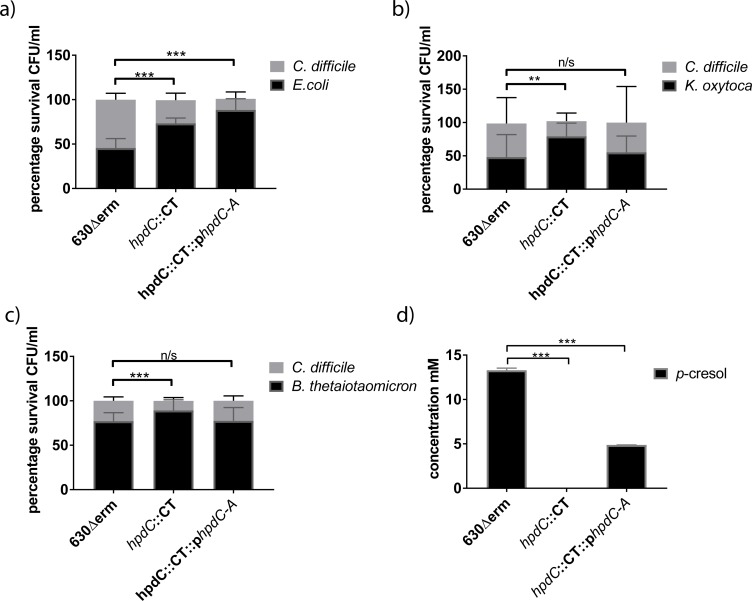
Next, we investigated whether the p-cresol mutant displayed reduced fitness when grown in competitive co-culture with other gut competitor species. Furthermore, we constructed a complement by expressing the hpdC and hpdA genes from a tetracycline-inducible promoter using a plasmid based system (generating strain hpdC::CT::phpdCA). We compared the growth of C. difficile strains 630Δerm, hpdC::CT and the complement (hpdC::CT::phpdCA), in competition with E. coli, K. oxytoca or B. thetaiotaomicron in media supplemented with 0.2% p-HPA (Fig 4). The number of viable counts for each species was determined as outlined above. When 630Δerm was grown in co-culture with E. coli, we observed a 1:1 ratio of C. difficile to E. coli (Figs 4A & 3A). This was consistent with the co-culture assays supplemented with exogenous p-cresol (Fig 2). However, competitive co-culture between hpdC::CT and E. coli resulted in a decrease in the relative proportion of C. difficile to ca. 25% of the total culture (1:4, C. difficile:E. coli). This indicates that the mutant was significantly less viable than the wild type (COV = -1.06, p<0.001). 630Δerm demonstrated comparable relative fitness in competitive co-culture with K. oxytoca (1:1 ratio) (Fig 3B). However, we observed proportionally fewer CFUs of 630Δerm when grown in co-culture with B. thetaiotaomicron (1:4 ratio) (Fig 3C). By contrast, hpdC::CT displayed reduced fitness relative to 630Δerm when grown in competition with both K. oxytoca (COV = -1.40, p<0.001) and B. thetaiotaomicron (COV = -0.79, p = 0.001). This fitness defect was restored when the complement was grown in competition with K. oxytoca and B. thetaiotaomicron (Fig 3). However, complementation of the hpdC mutation, did not restore C. difficile fitness to wild-type levels in competitive co-cultured with E. coli. Therefore, we quantified both p-cresol production and p-HPA utilisation by HPLC (Figs 4D, 3B & S2). Quantification of 630Δerm supernatants grown in both monoculture and competitive co-culture supplemented with 0.2% p-HPA revealed an average p-cresol concentration of 13.3 ±0.1 mM. In contrast, the concentration in supernatants of the complemented mutant (at 0.2% p-HPA) was only 4.8 ±0.2 mM, representing a significant 2.7 fold reduction (p<0.01). Therefore, we conclude that under competitive co-culture conditions, 4.8 ±0.2 mM p-cresol was sufficient to have a deleterious effect on the growth of K. oxytoca and B. thetaiotaomicron, but not on E. coli. Increasing the concentration of the transcriptional inducer (anhydrotetracycline) and p-HPA resulted in increased p-cresol production (from 4.8 ±0.2 mM to 15.6 ±3.9 mM) by the complement and restoration of the phenotype (S2 Fig). Here, the level of p-cresol production directly correlated with the concentration of the transcriptional inducer (S2C and S2D Fig). As expected, this suggests that complementation was more greatly influenced by transcript expression rather than availability of the p-HPA precursor. Furthermore, we observed no difference in growth rate between the three C. difficile strains at any tested concentration of anhydrotetracycline (S1 Fig). Taken together, these data suggest that production of p-cresol by C. difficile confers a competitive growth advantage over susceptible bacterial species (specifically, Gram-negative species) under our in vitro conditions.
Fig 4. Competition co-culture assays in the presence of endogenous p-cresol.
Relative fitness of C. difficile strains 630Δerm, hpdC::CT and the complement hpdC::CT::phpdC-A were grown in competitive co-culture for 24 hours with gut commensal species a) E.coli, b) K. oxytoca and c) B. thetaiotaomicron. The growth medium was supplemented with the intermediate in the p-cresol pathway, pHPA (at 0.2% v/v) and 50 ng/ml anhydrotetracycline to induce expression of hpdCA, encoded in trans. The relative representation of each strain was expressed as a percentage of the total CFU count. Error bars are representative of three independent replicates and show the variation across replicates and co-culture conditions. Regression analysis was used to determine significant differences in growth taking strain into consideration and marked ** p<0.01 and ***p<0.001. d) The concentration of p-cresol produced in the co-cultures of three independent replicates was quantified by HPLC and plotted in GraphPad Prism7. Statistical analysis was performed by Linear regression, significant differences in p-cresol production compared to 630Δerm are indicated *** p<0.001.
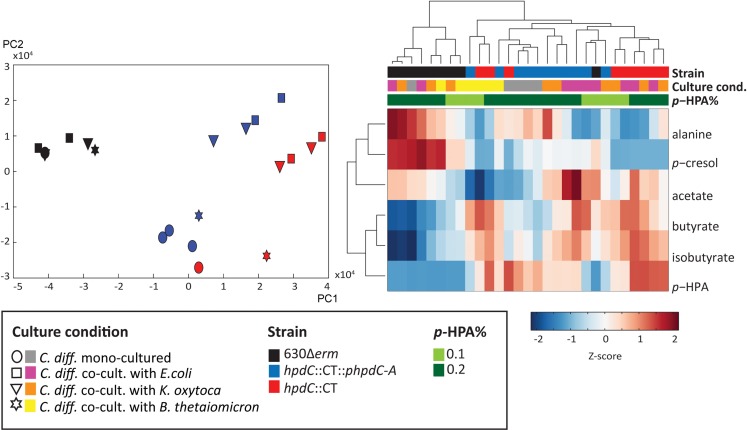
Metabolic profiling of C. difficile and the intestinal commensal species
To further understand the effect of p-cresol production on the interaction between C. difficile and these intestinal species, we characterised the metabolic content of both mono-culture and competitive co-culture supernatants using 1H nuclear magnetic resonance (NMR) spectroscopy. We analysed the culture supernatants described in Fig 4 and performed principal component analysis (PCA) to identify metabolic variation across the profiles. The scores plot from the PCA model comparing all profiles showed that the largest variation in the metabolic data (Principal Component 1 (PC1)) was between the C. difficile strain 630Δerm samples and those from the hpdC::CT strain (Fig 5A). The metabolic profiles from the complement samples clustered between 630Δerm and hpdC mutant samples. The loadings for PC1 describe the metabolites varying between the strains. This indicated that culture supernatants from 630Δerm contained significantly greater amounts of p-cresol and alanine compared to the other strains, but lower amounts of p-HPA, butyrate and isobutyrate (Fig 5B). This was consistent with the notion that p-HPA is being depleted in order to produce p-cresol. The 630Δerm samples clustered together regardless of whether the bacteria were grown in mono-culture or co-cultured with E. coli, K. oxytoca or B. thetaiotaomicron. In contrast, hpdC::CT and complement (hpdC::CT::phpdC-A) strains were separated in the second principal component (PC2) based on the competitive co-culture conditions. The loadings for PC2 indicated that the mono-cultured C. difficile and B. thetaiotaomicron competitive co-culture samples contained lower amounts of acetate compared to the competitive co-cultures from E.coli and K. oxytoca. This metabolic variation between strains and competitive co-culture conditions is summarised in the clustergram shown in Fig 5B, which was constructed from the Z-scores of 1H NMR peak integrals measured for each metabolite across all samples. The dendrogram shows that the 630Δerm metabolic profiles clearly cluster away from those of the other two strains (Fig 5) and the variation in p-cresol production between samples is apparent in the 1H NMR spectrum (S3 Fig). The dendrogram also showed that p-cresol and alanine clustered together as did butyrate and isobutyrate (Fig 5B). We also assessed the effect of altered p-HPA concentration on metabolic profiles. The PCA of metabolites produced in media supplemented with 0.1% and 0.2% p-HPA demonstrated no clear metabolic variation between these samples. However, we did observe clustering within the 0.1% p-HPA samples, driven by increased p-cresol and alanine (S4 Fig). These data suggest that under these growth conditions p-cresol is one of the most abundant metabolites in culture supernatants. This is reflected by both metabolic profiling and HPLC quantification, which correlates to p-cresol susceptibility observed in both competitive co-culture and monoculture of Gram-negative bacteria.
Fig 5. Metabolic profiling of C. difficile in monoculture and competitive co-culture with intestinal commensal species.
a) Principle component analysis (PCA) score plot based on the 1H NMR spectra of the three C. difficile strains (630Δerm (black), hpdC::CT (red) and the complemented mutant (hpdC::CT::phpdC-A) in (blue), grown in monoculture (circle symbol) or in competitive co-culture with intestinal commensal species, E. coli (square), K. oxytoca (triangle) and B. thetaiotaomicron (star), in BHIS media supplemented with 0.2% p-HPA. b) Metabolite clustergram represented as a heatmap of Z-scores of the 1H NMR peak integrals, derived from the quantity of six main metabolites driving the separation between the samples (observed on the loading plots of the model) and a dendrogram showing the hierarchical clustering of the samples. The culture conditions, bacterial strains and p-HPA concentration are colour coded in a key on the heatmap.
Mouse relapse model of C. difficile infection
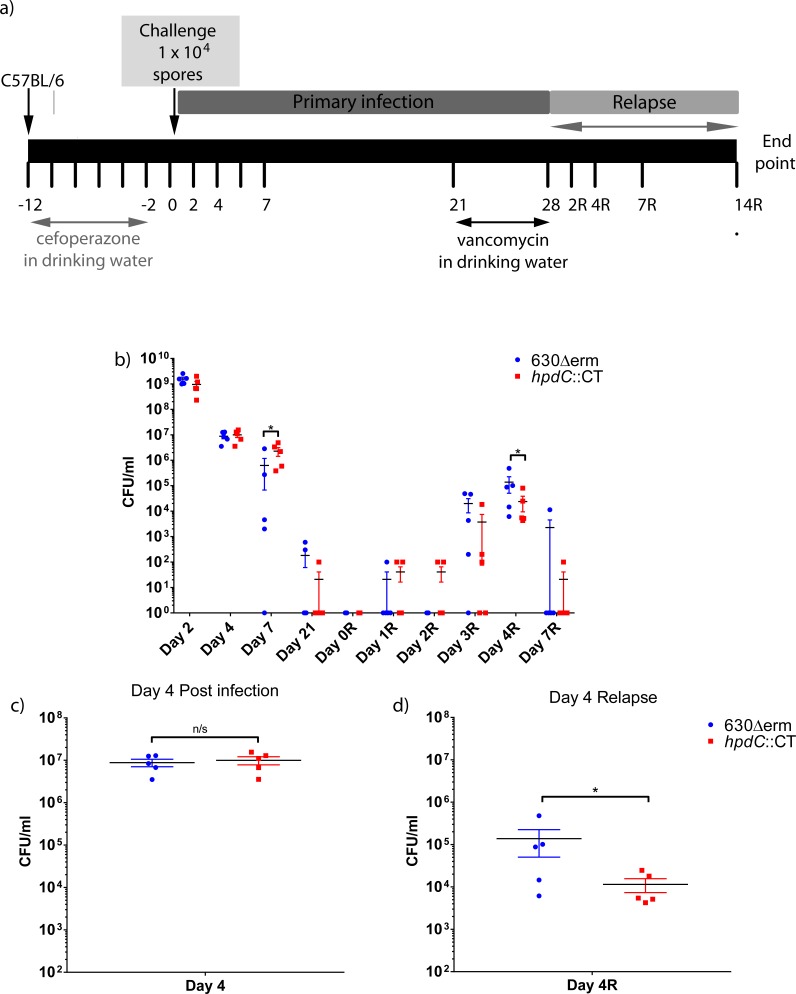
Our results have demonstrated that p-cresol production confers a fitness advantage over discrete bacterial species in vitro. Therefore, we sought to determine whether this was also true in vivo. Individually caged C57BL/6 mice were infected in parallel with 1x104 spores of C. difficile strain 630Δerm (n = 5) or the hpdC::CT mutant (n = 5) and compared to uninfected naïve control mice (n = 5), in a relapse model of infection. Mice were given cefoperazone in their drinking water for 10 days to stimulate gut dysbiosis, before infection by oral gavage with C. difficile spores (Fig 6A)[23, 24]. Stool samples were collected throughout the experiment for analysis. Twenty-eight days post-infection, mice were treated with vancomycin in their drinking water for 7 days to encourage recurrence of infection (Fig 6A). Infection was monitored by enumeration of spores isolated from faeces on C. difficile selective media (Fig 6B). We observed no significant difference in the number of spores recovered from faeces of mice infected with either 630Δerm or hpdC::CT following cefoperazone treatment, indicating that the hpdC::CT mutant and 630Δerm were equally competent at initial colonisation (Fig 6C). This was consistent with the notion that these strains demonstrate similar resistance to cefoperazone and vancomycin (S6 Table) and no differences in sporulation in vitro (S5 Fig). However, at day 7 post-infection we observed a modest difference in colonisation, represented by significantly fewer 630Δerm CFUs compared to the hpdC::CT mutant (p<0.05). Relapse was detected by enumeration of C. difficile spores post-vancomycin treatment. Three days following removal of vancomycin (D3R), spores were detected in all but one 630Δerm infected mouse and three out of five hpdC::CT mutant infected mice. By day 4 post-relapse (D4R) C. difficile spores were recovered from the faeces of all mice and we observed a significant reduction (p<0.05) in the number of spores recovered from the faeces of mice infected with the hpdC::CT mutant relative to 630Δerm infected mice (Fig 6D). Both infections followed a broadly comparable progression, however, we observed modest but significant differences in the number of spores recovered both pre- and post- relapse between 630Δerm infected and hpdC::CT infected mice at discrete time points. Interestingly during relapse, the spore density remained lower in hpdC::CT compared to 630Δerm, despite an initially higher CFU at D7 post-infection, indicating that these differences are a result of in vivo fitness.
Fig 6. Infection of mice with 630Δerm and hpdC::CT.
a) A schematic of the C. difficile relapse infection in C57BL/6 mice. b) CFU counts enumerated from the faeces of individually caged animals (n = 5) infected with either wild-type C. difficile strain (630Δerm) or the corresponding p-cresol null mutant (hpdC::CT) over the duration of the primary infection (days 1–21) and relapse (days 1R-7R). c) CFU enumerated from the faeces day 4 post-infection and d) day 4 relapse. Mann Whitney U test was used to calculate significant differences between infection with wild-type C. difficile and the hpdC mutant marked *p<0.05. Graphs were produced in GraphPad Prism 6.0.
Composition of the intestinal microbiota
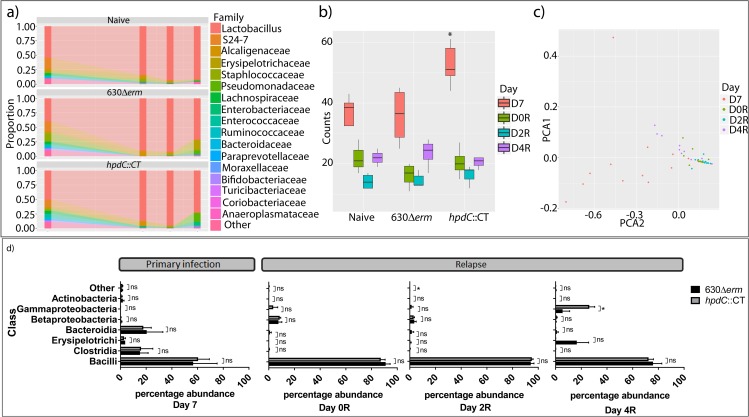
Given that the hpdC::CT mutant displayed an altered colonisation profile during relapse and that p-cresol displays bacteriostatic properties against a number of species, this led us to postulate that production of p-cresol may alter the composition of the intestinal microbiota in such a way that favoured C. difficile re-colonisation. We isolated bacterial DNA from four key time points during the relapse model of CDI; day 7 post-cefoperazone treatment, immediately upon cessation of vancomycin treatment (D0R), day 2 post-relapse (D2R) and day 4 post-relapse (D4R), when all the mice were colonised with C. difficile (1.6 x 106 WT and 2.3 x 105 hpdC::CT mutant spores/g faeces). To assess the community structure of the microbiota, 16S rRNA sequencing was performed by sequencing the V5-V7 region of 16S rRNA gene. The data was grouped with distance-based similarity of 97% into operational taxonomic units (OTUs), using Greengenes and associated summaries and diversity analyses were performed in QIIME. Consistent with previous studies, the microbiota of cefoperazone-treated mice was dominated by Lactobacillaceae (Fig 7A and S7 Table)[24–26], which comprised 39.7% (mean relative abundance) of the total microbiota in 630Δerm infected mice. The microbiota was also populated by Bacteroidetes, including members of the S24-7 (an uncultured commensal of homeothermic animals[27]) (17.6%) and Paraprevotellaceae (1.2%) families. Furthermore, Firmicutes, including Staphylococcaceae (12.75%), other Clostridiales (7.2%), Lachnospiraceae (4.2%), Erysipelotrichaceae (3.7%), Ruminococcaceae (2.9%), Enterococcaceae (2.2%), Turicibacteraceae (1.2%), and Actinobacteria including Bifidobacteriaceae (1.9%) (Fig 7A) also contributed to the microbiota composition. However, animals infected with the hpdC::CT mutant demonstrated a significant increase in microbial diversity at D7 (ANOVA p<0.05), compared to 630Δerm infected and naïve mice (Fig 7B and S7 Table), which is also upheld with an ANOSIM population analysis p<0.05 (S6 Fig). Consistent with the notion that p-cresol prevents outgrowth of Proteobacteria, the majority of the families that were only present in the hpdC mutant infected animals were members of the Proteobacteria Phylum (S7 Table), albeit at low abundance.
Fig 7. 16S relative abundance.
The diversity of the intestinal microbiota was assessed using 16S rRNA sequencing to determine the bacterial operational taxonomic units present during a relapse model of C. difficile infection. a) Composition of the intestinal microbiota, at the Family level, between wild type C. difficile 630Δerm and p-cresol mutant (hpdC::CT) infected mice and the naïve control at day 7 post-infection, day 0, day 2 and day 4 post relapse. b) Box and whisker plots displaying the total number of operational taxonomic units (OTUs) at the Family level. Statistically significant differences between the diversity of OTU’s are labelled * p<0.05 (ANOVA). c) Principle component analysis of the microbial diversity at the Family level. Each dot represents a single mouse at a given time point, colour-coded for day 7 post-infection, and days 0, day 2 and day 4 post-relapse. d) The relative prevalence of bacterial Class in wild type C. difficile and hpdC::CT infected mice at day 7 post-infection, day 0, day 2 and day 4 post relapse. Statistical analysis was performed in Stata15 by regression analysis taking strain and bacterial class into consideration * p<0.05.
Treatment with vancomycin significantly reduced diversity of both uninfected (naïve) and infected mice (630Δerm and hpdC::CT) (Fig 7B & 7C), resulting in a dramatic increase in the relative representation of Lactobacillaceae, specifically the Lactobacillus genus, which constituted ≥85% of the microbiota of all the mice examined (90% 630Δerm, 87% hpdC::CT mutant and 85% naïve at D0R). Principle component analysis demonstrated clustering of D0 and D2 post relapse (Fig 7C). At D2R, the diversity of the microbiota remained low. At D4R, partial recovery of the microbial diversity was observed (Fig 7A and 7B), which coincided with the detection of C. difficile spore in faeces (Fig 6D). At D4R there were distinct differences in population composition in the intestinal bacteria of these animals (Fig 7D). We observed an increase in spread on the PCA plot at D4R (Fig 7C), compared to the clustering observed at D0R and D2R. Although the mean relative proportions of Lactobacillaceae were similar (73.9% in 630Δerm and 72.3% in the hpdC::CT mutant infected animals), there were clear differences in the representation of other taxa, including other Firmicutes, Proteobacteria and Bacteroidetes (Fig 7D). In 630Δerm infected mice there was an increase in representation of Firmicutes from the Erysipelotrichales (16.5% 630Δerm and 0.1% hpdC::CT mutant), Bacillales (1.9% 630Δerm and 0% hpdC::CT mutant) and Clostridiales orders, and the Bacteroidetes (Fig 7D). Conversely, in the p-cresol mutant infected mice, we observed an increase in the representation of Proteobacteria, specifically, the Gammaproteobacteria of the Pseudomonadales (5.5% 630Δerm and 18% hpdC::CT mutant) and Enterobacteriales (0% 630Δerm, 6.75% hpdC::CT mutant) order, and the Betaproteobacteria of the Burkholderiales order (Fig 7D). Consistent with the notion that Gram-negative species were more susceptible to the effects of p-cresol, Gammaproteobacteria formed 26.2% of the total microbiome in hpdC::CT infected animals D4R, compared with 5.5% in 630Δerm infected mice (COV = 9.37, p = 0.023), suggesting that p-cresol may inhibit their outgrowth following treatment with vancomycin.
Metabolic profiling of the mouse model of CDI
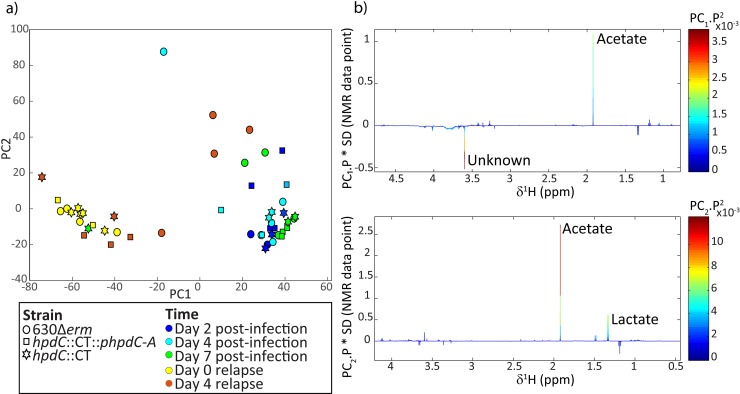
Our data suggest that p-cresol production by C. difficile influenced the composition of the mouse faecal microbiota. Therefore, we investigated whether these differences resulted in an altered biochemical profile. Stool samples collected throughout the duration of the initial mouse infection (at day 2 (D2), 4 (D4) and 7 (D7)) and during relapse (at days 0 (D0R) and 4 (D4R)) were analysed using 1H NMR spectroscopy (Fig 8). The PCA scores plot identified biochemical variation between the faecal profiles collected D2-D7 versus D0R-D4R in the control mice and those infected with the hpdC::CT mutant. The D2-D7 samples contained greater acetate compared to the relapse time points, and lower amounts of an unknown metabolite (δ 3.59, singlet). Vancomycin induced perturbations in the metabolic activity of the intestinal bacteria are likely to underlie these changes[28].
Fig 8. Metabolic profiling of the in vivo mouse relapse model of CDI.
1H NMR spectroscopy was used to investigate metabolic differences between the individually caged mice infected with C. difficile strain 630Δerm, hpdC::CT or uninfected naïve mice. a) Principle component analysis of the metabolite diversity. Each dot represents a single sample at a given time point. A symbol code was used for the strains 630Δerm (circle), hpdC::CT (square) and naïve mice (star), and a colour code was used to represent the different time points, Day 2 post-infection (dark blue), Day 4 post-infection (light blue), Day 7 post-infection (green), Day 0 relapse (yellow) and Day 4 relapse (orange). b) Loading plots of the PCA model, representing the metabolites driving the distribution of the samples along principal component 1 (PC 1) and principal component 2 (PC 2).
The faecal profiles from mice infected with the 630Δerm strain showed similar metabolic alterations to the control mice and those infected with the mutant strain during the initial infection (D2-D7). However, the response was different 4 days after relapse. At D4R, the faecal profiles from mice infected with the 630Δerm strain were more variable than the uninfected mice and those infected with the mutant strain and were similar in composition to the initial infection profiles (Fig 8).
Effect of p-cresol on viability and diversity of the human microbiome
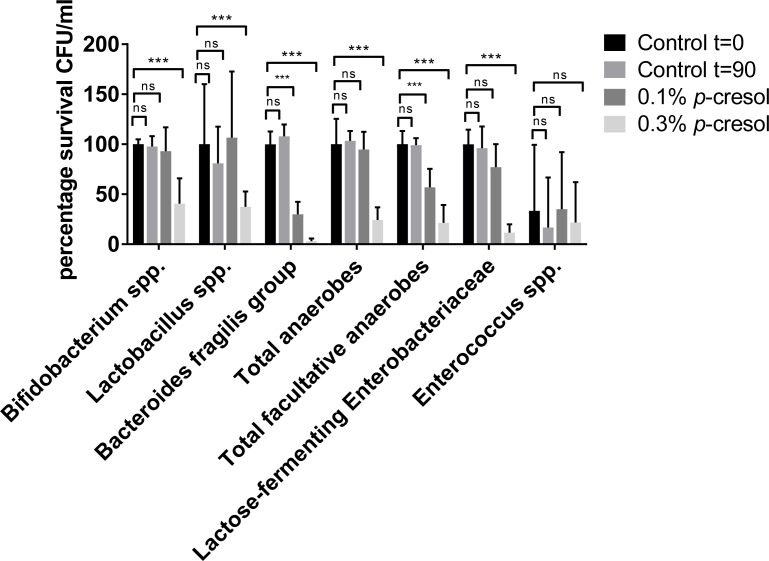
We have shown that p-cresol production has deleterious effects on the outgrowth of Gram-negative bacterial species both in vitro (Figs 1–4), and in an in vivo mouse infection model (Fig 7). Thus, we sought to determine the effect of exogenously added p-cresol on biodiversity of the human microbiome. Healthy human stool samples were taken from donors ranging from 60–65 years old, who had not received antibiotic treatment in the last 3 months, eliminating possible perturbations by antibiotic therapy. We measured the effect that exogenously-added p-cresol (at 0.1% and 0.3%) had on faecal microbiota, compared to a Phosphate Buffered Saline (PBS) control. Differential plating revealed that the facultative anaerobes were particularly sensitive to p-cresol at both 0.1% (COV = -0.61, p = 0.006) and 0.3% (COV = -1.82, p<0.001), represented by a significant reduction in viable counts (Fig 9). The Bacteroides fragilis group was also significantly reduced after exposure to both 0.1% (COV = -1.29, p = 0.009) and 0.3% (COV = -4.39, p<0.001) p-cresol (Fig 9). The total anaerobes and lactose-fermenting Enterobacteriaceae were also significantly reduced after exposure to 0.3% p-cresol (COV = -1.48, p<0.001, COV = -2.36, p<0.001, respectively) (Fig 9). Consistent with the mouse model of CDI, p-cresol at 0.1% had a limited effect on the survival of Lactobacillus (COV = -0.045, p = 0.890) and Bifidobacterium species (COV = -0.100, p = 0.642). However, a significant decrease in survival was observed for both groups when they were incubated in 0.3% p-cresol (p<0.01). In line with our in vitro co-culture data (Fig 2), Enterococcus species present in human faecal samples were not adversely affected by the addition of p-cresol (Fig 8), even at the highest concentrations tested (COV = 0.48, p = 0.873).
Fig 9. Percentage survival of bacterial phyla from healthy human faecal slurry treated with p-cresol.
Healthy human donor stool samples were emulsified and treated with exogenously added p-cresol (0.1% or 0.3% v/v) for 90 minutes and the CFU counts were expressed as percentage survival relative to the CFU of the PBS control at t = 0. Faecal emulsions were plated onto differentially selective agar to determine the survival of endogenous intestinal bacteria to p-cresol stress. Error bars are representative of triplicate experiments. Statistical analysis was performed by linear regression to determine the correlation between survival in 0.1% and 0.3% (v/v) p-cresol relative to the PBS control. Significant differences are indicated with ***p<0.001. Error bars are Standard error of the Mean (SEM).
p-cresol compromises the integrity of Gram-negative cell envelope
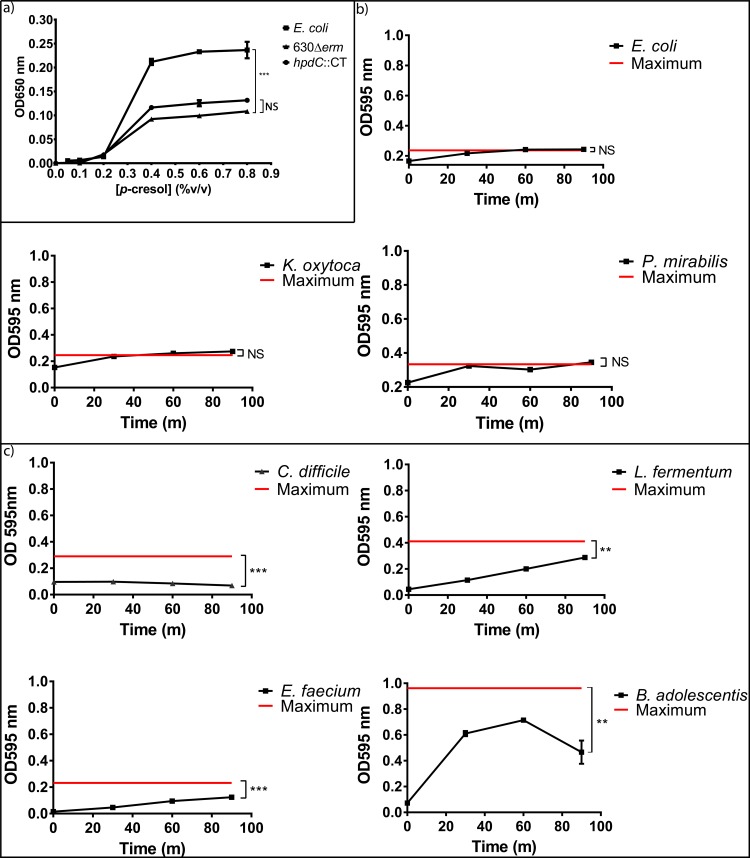
Given that we observed a clear distinction in the nature of species that displayed tolerance to p-cresol, we reasoned that the cell envelope would be an obvious target for its mode of action. Phenolic compounds that target membranes typically induce a rapid loss of low molecular weight compounds from within the cell as a result of increased membrane permeability[29–31]. Thus, we used the release of inorganic phosphate (Pi) as a metric for determining membrane integrity in the presence of p-cresol. Initially, we compared the release of phosphate from E. coli and C. difficile strains (630Δerm and hpdC::CT) in increasing concentrations of p-cresol (Fig 10A). We observed a significant increase in the amount of phosphate released by E. coli compared to C. difficile (COV = 0.868, p = 0.005). Only 16% of the total intracellular phosphate of C. difficile was released upon contact with p-cresol. Furthermore, the hpdC::CT mutant displayed a similar phosphate release profile to 630Δerm C. difficile (COV = 0.201, p = 0.444), which was not significantly different (Fig 10A). This indicates that disruption of p-cresol production had little bearing on p-cresol tolerance, under these conditions. Our data suggests that cells can tolerate p-cresol up to a threshold level (~0.4% v/v), after which the amount of phosphate released becomes saturated. Therefore, we selected a concentration of 0.3% (v/v) to determine phosphate release over time in a selection of Gram-positive and Gram-negative gut bacteria. Membrane integrity was measured in the presence of p-cresol by comparing the amount of p-cresol induced phosphate release, to the total intracellular phosphate, which was determined by boiling cell suspensions for 15 minutes. Fig 10 demonstrates that species with a Gram-positive cell envelope display greater tolerance to p-cresol than Gram-negative species, represented by significantly less phosphate release (COV = -2.478, p<0.001), (Lactobacillales: E. faecium (p = 0.005) and L. fermentum (p = 0.003), the Bifidobacteriales: B. adolescentis (p = 0.01) and the Clostridiales: C. difficile (p<0.01)) corroborating previous observations. Conversely, the Gram-negative Gammaproteobacteria: P. mirabilis, E. coli and K. oxytoca released their total intracellular pool of phosphate over the course of the assay. P. mirabilis and K. oxytoca released 68% and 60% of their total phosphate respectively immediately upon contact with p-cresol (Fig 10B). Both species released >90% of their total phosphate pool following 30 minutes contact with p-cresol. In contrast, no Gram-positive species analysed released their total intracellular phosphate pool over the course of the assay (Fig 10C). However, B. adolescentis released 63% of its total phosphate at 30 minutes compared to 20% for E. faecium, 27% for L. fermentum and 33% for C. difficile, indicating that B. adolescentis is more sensitive to p-cresol than other Gram-positive species. Prolonged exposure to p-cresol resulted in other Gram-positive species releasing a greater portion of their intracellular pool of phosphate, however, the level of Pi released by C. difficile never exceeded its initial level of release (Fig 10). In conclusion, our data demonstrate a clear correlation between bacterial cell envelope structure and susceptibility to p-cresol.
Fig 10. Phosphate release in Gram-positive and Gram-negative common gut commensal species.
The release of intracellular phosphate was determined spectroscopically (OD650) using malachite green and ammonium molybdate from: a) C. difficile 630Δerm, hpdC::CT and E. coli stationary phase cultures in various concentrations of p-cresol. Significant differences in phosphate release compared to 630Δerm were calculated taking concentration into account using linear regression and are marked *** p<0.001. b) phosphate relapse was measured from Gram-negative common gut commensals and c) phosphate relapse was measured from Gram-positive common gut commensals following incubation with 0.3% (v/v) p-cresol over a 90 minute time period. Red line indicates maximum phosphate release determined by boiling a comparable cell suspension. Curves and error bars are representative of three independent replicates. Significant differences in phosphate release from the maximum were calculated using regression analysis taking time points into consideration, significant differences are marked **p<0.01, ***p<0.001. Error bars are SEM.
Discussion
The indigenous microbiota has been shown to form an ecological barrier that prevents the ingress of pathogenic bacteria such as C. difficile [32]. However, the specific components of the intestinal microbiota that facilitate colonisation resistance are only recently becoming clear [5–7, 25, 33–36]. Both the treatment with broad-spectrum antibiotics and the availability of specific metabolites has been shown to play a role in the expansion of particular bacterial species within the human microbiota [37, 38]. Here, we present compelling evidence that C. difficile may directly modify the intestinal microbiota through production of p-cresol. We demonstrate that C. difficile displays a greater degree of tolerance to p-cresol compared to other common intestinal species, including the Gammaproteobacteria: E. coli, K. oxytoca and P. mirabilis, as well as the Bacteroidetes, B. thethaiotaomicron. We show that these bacterial species are susceptible to the effects of both endogenous and exogenous p-cresol, which was reflected in reductions of viable counts when these intestinal microbiota species were grown in competitive co-culture with C. difficile. Using a plasmid based complementation system to restore the expression of the p-HPA decarboxylase, we have shown that p-cresol production by C. difficile must exceed 5 mM to elicit a significant alteration in competitive growth dynamics. We have shown that C. difficile is able to utilise all the available p-HPA supplemented in the growth medium, which results in the production of up to 25 ±0.04 mM p-cresol in vitro (Fig 3), which is 1000-fold more than the amount of p-cresol produced from tyrosine metabolism by other organisms cultured from the intestinal microbiota (range 0.06–1.95 μg/ml)[11]. There is evidence that p-HPA is present in the human colon and detected in healthy human stool samples at 19 μM[15], therefore C. difficile can potentially utilise free tyrosine and p-HPA to produce p-cresol in vivo.
We expanded our investigation of the influence of p-cresol on the growth of other bacterial species, to identify other metabolites influencing growth. In particular, alanine, p-cresol, acetate, butyrate, isobutyrate and p-HPA were the six main metabolites that were differentially modulated in mono-culture and co-culture of C. difficile with intestinal bacteria. The abundance of these metabolites in vitro was altered in the presence of the p-cresol mutant compared to C. difficile strain 630Δerm. Acetate and butyrate are the most common end products of fermentation in the gut[39]. C. difficile can use amino acids as the sole energy source via Stickland fermentation, in which amino acid acceptors (such as glycine, proline and hydroxyproline) are reduced in a paired metabolism with electron donors (such as leucine, isoleucine or alanine). This can result in the conversion of alanine to acetate [40]. However, in C. difficile monoculture, we did not observe an inverse association between acetate and alanine (Fig 5B), suggesting that C. difficile is not utilising alanine in stickland fermentation under nutrient rich conditions (in BHIS media). This suggests that the competitor species, may have been responsible for the increased utilisation of alanine in co-culture with the p-cresol mutant and complement, where the competitor is more abundant. The reduction of the Stickland acceptors glycine and proline in C. sticklandii and C. difficile requires two selenium dependant reductases, glycine reductase and D-proline reductase [40, 41], highlighting the importance of selenium in growth and metabolism in C. difficile, particularly in glycine reduction and selenocysteine production [40, 42]. Nutrient availability has been linked to virulence in C. difficile in a number of different ways, via the global transcriptional regulators CodY, CcpA, PrdR and Rex, which are involved in overlapping cellular processes including toxin production, amino acid biosynthesis, stickland fermentation, nutrient transport, fermentation and cell membrane components[43]. The hypervirulent C. difficile strains (RT027 and RT078) have also developed the ability to metabolise low concentrations of trehalose, via acquisition of a single point mutation in the trehalose repressor (treA), which increases virulence of these ribotypes in vivo[44]. C. difficile is also capable of utilising ethanolamine as a carbon source[45] and the ethanolamine genes are upregulated in vivo in the presence of B. thetaiotaomicron when animals were fed on a standard polysaccharide diet [38].
Cysteine is involved in amino acid and energy metabolism in C. difficile [46], modulating processes such as carbon transfer, electron transport, butyric acid and butanol production. Cysteine results in increased levels of intracellular tyrosine [47]. Cysteine also down-regulated 4p-hydroxyphenylacetate-3-hydroxylase [48], which may reduce p-HPA availability for the p-HPA decarboxylase and thus would decrease flux to p-cresol. Therefore, cysteine-regulated pathways may result in increased p-cresol production. This is consistent with the notion that the production of butyrate and p-cresol are inversely regulated.
C. difficile and other opportunistic gut bacteria have developed metabolic strategies that differ in response to environmental signals, one such strategy is the production of short chain fatty acids [38]. Studies using a simplified gnotobiotic mouse model, have shown that succinate produced by B. thetaiotaomicron is used by C. difficile to produce butyrate, boosting C. difficile titres [38]. However, in our experimental conditions (nutrient rich conditions) succinate was detected at very low levels by 1H NMR spectroscopy. Butyrate has anti-inflammatory properties [49] and is produced by a diverse array of bacterial phyla [50]. Yet, butyrate stimulates C. difficile toxin production in the absence of rapidly metabolised carbohydrates (e.g. glucose) [48]. The production of butyrate from acetyl-CoA or succinate by C. difficile is suppressed by the transcriptional regulators CcpA, CodY and Rex [51], however, if proline is limited then alternative pathways for NAD+ regeneration are used including glycine reductase, alcohol dehydrogenase and butyrate production from acetyl-CoA and succinate are induced [51]. We observed lower butyrate and isobutyrate concentrations in the 630Δerm cultures (mono- and co-cultures), which implies that C. difficile is unable to synthesise butyrate under these conditions. However, we observed high butyrate and isobutyrate concentrations in co-culture with the p-cresol mutant and complement, where the competitor is more abundant, suggesting that the competitors are responsible for the increase in butyrate in co-culture.
Inter-C. difficile strain variation in the metabolic profiles included altered abundance of alanine, isobutyrate, p-cresol and p-HPA. We observed an increase in the production of p-cresol in monocultures and co-cultures containing the C. difficile 630Δerm and an absence of p-cresol in all cultures with the hpdC::CT mutant, consistent with the inability of the mutant to synthesise p-cresol (Fig 5B). The complement produced an intermediate amount of p-cresol (Figs 5B and S3) and therefore clustered between the wild type and mutant in the PCA plot, suggesting that p-cresol and p-HPA were the main metabolites driving separation between C. difficile strains. This observation was corroborated when p-cresol production was increased in co-culture by increasing the supply of p-HPA, which resulted in a decrease in the competitor relative to wild-type C. difficile.
In this study, we present evidence that p-cresol production by C. difficile prevents outgrowth of discrete taxa of bacteria in vitro and that p-cresol production may modulate composition of the mouse microbiota. Our data showed clear differences in the composition of the microbiota of mice infected with the p-cresol mutant compared with the 630Δerm strain pre- and post-relapse in our infection model (Fig 7). Recovery of the microbial community to its pre-dysbiotic state is often a slow process and, consequently, susceptibility to C. difficile colonisation can be increased for weeks and even months following cessation of antibiotics[52, 53]. Both the 630Δerm and the hpdC::CT mutant successfully colonised mice at the initial infection stage. However, we observed increased microbial diversity in hpdC::CT infected mice, at day 7 post-infection. This diversity was largely driven by OTUs that each constituted <0.1% of the total microbiota. Despite relatively low abundance of these OTUs, they could have important consequences for the microbial ecosystem. These Families include Corynebacteriaceae, Propionibacteriaceae (both Actinobacteria), Bradyrhizobiaceae, Burkholderiaceae, Comamonadaceae, Oxalobacteraecea, Rhodocyclaceae, Bdellovibrionaceae and Enterobacteriaceae (all Proteobacteria). Consistent with the notion that p-cresol prevents outgrowth of Proteobacteria, the majority of these Families were members of the Proteobacteria Phylum. Vancomycin treatment reduced microbial diversity, altered the metabolic content of the stool samples, and resulted in a microbiome that was susceptible to relapse with C. difficile. The remaining microbial community was dominated by Lactobacillaceae (consistent with previous publications [24]), which was insufficient to restore colonisation resistance following vancomycin withdrawal. Upon cessation of vancomycin treatment, we observed an expansion of microbial diversity (D2R and D4R). There were clear differences in microbiota composition at D4R between animals infected with the p-cresol mutant and 630Δerm C. difficile. The second most abundant class present in the microbiome of hpdC mutant-infected mice was Gammaproteobacteria (26.2%). In contrast, the second most abundant class in animals infected with 630Δerm C. difficile was the Erysipelotrichia (16.5%). Other studies have shown that without FMT, dysbiosis is maintained in mice with two main OTUs, Lactobacillus and Turicibacter (Erysipelotrichia order)[24, 35], which we observed in the 630Δerm infected mice, but not mice infected with the p-cresol mutant. We have shown in vitro that Gammaproteobacteria, including K. oxytoca, E. coli and P. mirabilis are more sensitive to p-cresol than C. difficile, while Gram-positive bacteria from the Lactobacillales family are more resistant to p-cresol. This is particularly pertinent as the majority of the microbiota post-vancomycin treatment was comprised almost exclusively of Lactobacillus and an increased expansion of the Gammaproteobacteria was only seen in p-cresol mutant-infected mice. The faecal metabolic profiles from all animals post-vancomycin treatment (D0R) were clearly distinct from those collected post-infection (D2, D4 and D7; Fig 7). However, the metabolic profiles of mice infected with the 630Δerm strain 4 days after withdrawal of vancomycin were more variable than those infected with the mutant strain. The 630Δerm infected mice also had a metabolic signature more similar in composition to those samples collected post-infection (D2, D4 and D7). This suggests that the biomolecular perturbations following re-establishment of infection with 630Δerm were more closely related to those observed with the initial infection. In contrast, the hpdC::CT-infected mice had profiles more closely related to the uninfected mice.
To complement the in vitro co-culture assay and mouse model of CDI we assessed the effect of exogenous p-cresol on the human microbiota using ex vivo healthy human faecal samples. We observed a reduction in the number of viable total anaerobes, facultative anaerobes and lactose fermenting enterobacteriacea (LFE). The LFE are comprised of the Gammaproteobacteria E. coli, Klebsiella spp, Enterobacteria spp, Citrobacter spp and Serratia spp. This observation corroborates previous findings that demonstrate a significantly reduced viability of Gram-negative bacteria by in vitro growth kinetic analysis, competitive co-culture and in a mouse model of CDI. In contrast, the Gram-positive bacteria isolated from the ex vivo healthy human faecal samples (Bifidobacteriaceae, Lactobacillales and Enterococcaceae) were consistently less sensitive to p-cresol in the assays we performed.
In this study, we have shown that C. difficile displays a greater degree of tolerance to p-cresol when compared to a selection of other common intestinal bacterial species. Our data suggest a clear distinction between the fundamental properties of the organisms susceptible to the p-cresol, whereby Gram-positive species displayed greater tolerance than Gram-negative species. We demonstrate that p-cresol affects the integrity of surface barriers resulting in a concentration-dependant leakage of small molecules such as phosphate. Similar effects have been observed with m-cresol and chloro-cresol on bacterial cell membranes[29]. p-cresol was recently shown to inhibit proliferation of colonic epithelial cells and induce necrotic leakage of protons through the inner mitochondrial membrane[54]. Pseudomonas putida strain P8, which has the capacity to degrade p-cresol, modifies its fatty acid composition by increasing the abundance of 9-trans hexadeconoic acid and decreasing the abundance of 9-cishexadeconoic acid when grown in the presence of sub-lethal concentrations of phenol[30, 55, 56]. Previous work has demonstrated that sublethal concentrations of phenolics, including p-cresol, resulted in an increase in the degree of saturation of cell membrane lipids, which is thought to counteract the increase in membrane fluidity [30, 57].
In conclusion, we demonstrate that the production of p-cresol by C. difficile alters the composition and recovery of diversity in the intestinal microbiota. A p-cresol deficient mutant has a reduced ability to compete with other intestinal microbiota species in vitro. We have shown that the effect of p-cresol is more detrimental to the growth of Gram-negative bacteria, differentially inhibiting proliferation of various bacterial Phyla. Exposure to p-cresol resulted in release of cellular phosphate, suggesting that it disrupts cell envelope integrity. This study provides evidence that p-cresol production by C. difficile provides it with a competitive survival advantage over other intestinal bacterial species.
Materials and methods
Bacterial strains and culture
C. difficile strains 630Δerm[58] and hpdC::CT[22] have been previously described. The intestinal microbiota species used in the study were obtained from Mark Wilcox and Simon Baines at the University of Leeds isolated from a gut soup model of CDI (S1 Table). All bacteria were cultured in pre-reduced Brain Heart Infusion (BHI) (Oxoid), supplemented with 0.5% (w/v) yeast extract (BHIS) and 0.05% (w/v) L-cysteine (Sigma), at 37°C and under anaerobic conditions. For growth rate analysis, our collection of gut bacteria was grown in 100 ml tissue culture flasks with shaking at 50 rpm, 37°C and under anaerobic conditions. Pre-reduced growth media was supplemented with 0.1%, 0.05% and 0.01% (v/v) p-cresol as indicated. OD595 was determined every hour for 8 hours with a final reading at 24 hours, growth curves were performed in triplicate. A p-cresol complement strain (hpdC::CT::phpdCA) was made, using an inducible plasmid based system derived from pRPF185[59] (S2 Table). The hpdCA genes were PCR amplified and cloned downstream of a tetracycline inducible promoter (ptet) in pRPF185 [59] to produce the plasmid phpdCA. This was then conjugated into the hpdC::CT mutant to create a complement[60]. This was performed alongside an empty plasmid pLDempty, which was derived from pRPF185[59], to contain the ptet promoter, but without a gene (S2 Table). This was transferred into C. difficile using competent E.coli CA434[60] into both 630Δerm and hpdC::CT mutant as controls. Linear regression analysis was performed using Stata15; data was transformed using Log10 to approximate a normal distribution. The data was mined to determine if there was a significant difference in; a) strains, the growth of all bacteria strain compared to the reference strain C. difficile strain 630, b) p-cresol concentration compared to the BHIS untreated control, c) the Gram-negative bacteria compared to the Gram-positive bacteria. The COV indicates whether the growth is higher (positive number) or lower (negative number) than the reference and the p-value indicates the probability, a minimum cut off of p<0.05 was used throughout for significance (S3 Table).
Minimum inhibitory concentrations (MIC) assays
All experiments were performed in pre-reduced media. Primary C. difficile strains were grown in BHIS broth until OD595 0.3 on a shaking platform at 50 rpm, then were diluted 1/100 into 24-well plates containing pre-reduced BHIS media supplemented with vancomycin or cefoperazone at a range of 1–256 μg/ml. These plates were incubated on a shaking platform at 50 rpm for 24 hours under anaerobic conditions at 37°C before OD595 was used to measure growth compared to an un-inoculated blank control. The MIC was taken as the lowest concentration to completely inhibit visible growth. The MICs were calculated as the mean of the three independent biological and technical replicates. There is no significant difference in the MICs between the C. difficile strains (630Δerm, hpdC::CT, hpdC::CT_phpdCA) (S6 Table).
Growth rate analysis
All experiments were performed in pre-reduced media. Growth rates of C. difficile strains (630Δerm, hpdC::CT and hpdC::CT::phpdCA) and intestinal species (E. coli, K. oxytoca, E. faecium and L. fermentum), were grown for 8 hours in BHIS media to compare growth rates, by measuring OD595. CFUs were calculated at OD 0.5 for each of the strains tested (S4 Table). The effect of anhydrotetracycline (ATC) was assessed by growing overnight primary cultures of C. difficile strains (630Δerm, hpdC::CT and hpdC::CT::phpdCA) in 10 ml BHIS supplemented with 0, 100 and 250ng/ml ATC. OD595 was measured and each strain diluted to an OD595 0.5, and used to inoculate secondary cultures at OD595 0.05 into pre-reduced BHIS, supplemented with ATC as outlined above. The OD595 was determined every hour for 8 hours with a final reading at 24 hours, growth curves were performed in triplicate.
Co-culture with extrinsic p-cresol
Under anaerobic conditions, an overnight culture of C. difficile and a gut competitor were normalised to a starting OD595 of 0.5 and were used to inoculate 1/10 into 15 ml of BHI + 0.05% (w/v) L-cysteine supplemented with 0.05% (v/v) p-cresol, which was grown with shaking (50 rpm) for 24 hours. The total number of colony forming units (CFU) was determined by plating serial dilutions on non-selective BHI + 0.05% (w/v) L-cysteine. The total number of C. difficile was determined by plating serial dilutions on BHIS + 0.05% (w/v) L-cysteine and cycloserine/cefoxitin supplement (BHIS CC) (Sigma), which could, in turn, be used to determine the total number of CFU for the competitor. Serial dilutions were plated in triplicate and an average of the three technical replicates was used to determine total CFU. Each experiment was performed in triplicate. Linear regression analysis was performed in Stata15 on the log10 of the CFU as outlined above.
Competitive co-culture with intrinsic p-cresol
Under anaerobic conditions, individual overnight cultures of C. difficile and an intestinal competitor were grown in 10 ml of BHI + 0.05% (w/v) L-cysteine supplemented with 100 ng/ml or 250 ng/ml ATC (to induce expression of hpdCA expressed in trans in the complement strain). Individual monocultures were normalised to a starting optical density (OD600) of 0.5, and were inoculated 1/10 into BHIS broth supplemented with 0.05% L-cysteine (w/v) and either 0.1, 0.2 or 0.3% p-HPA (w/v) (6.5 mM, 13.1 mM and 19.7 mM, respectively), these monocultures were grown until C. difficile reached an OD 0.5–0.6 (~7 hours). The competitor was back diluted to an OD595 0.5 and inoculated 1:10 into the C. difficile culture, to create a competitive co-culture. These co-cultures were grown anaerobically, shaking (50 rpm) for 24 hours and were plated onto both BHIS non-selective plates and BHIS CC plates. CFU counts of both C. difficile and the competitor were determined and processed as outlined above. Each experiment was performed in triplicate and Linear regression analysis was performed in Stata15 on the log10 of the CFU as outlined above, statistically significant differences were observed p<0.05.
Metabolic profiling of supernatants
Culture supernatant from mono-culture and co-culture experiments in media supplemented with 0.1 and 0.2% (v/v) p-HPA were filter sterilised. Samples were diluted into 400 μL of phosphate buffer (pH 7.4, 100% D2O, 3 mM of NaN3, 1 mM of 3-(trimethyl -silyl)-[2,2,3,3-2H4]-propionic acid (TSP) for the chemical shift reference at δ0.0) according a 1:2 ratio. Samples were transferred to 5 -mm tubes for 1H nuclear magnetic resonance (NMR) spectroscopic analysis, which was performed on a Bruker 600 MHz spectrometer (Bruker Biospin, Karlsruhe, Germany) at 300K (26.85°). The parameters of the acquisition were as previously reported for urine[61]. Each spectrum was acquired with 4 dummy scans followed by 32 scans. Spectra were automatically phased, baseline corrected and calibrated to the internal standard (TSP) using Topspin (Bruker Biospin, Karlsruhe, Germany). The processed spectral data was imported into Matlab (version R2014a, The Mathworks Inc.). The region δ4.84–4.76 was removed to eliminate the residual water signal. Principal Components Analysis (PCA) was performed using pareto scaling, due to the significant intensity of the acetate signal (δ 1.92, single. Based on the PCA loadings, spectral peaks contributing to the principal components were integrated using an in-house script. These metabolite peak integrals were used to construct a clustergram in Matlab using the clustergram script.
Sporulation assays
Primary cultures were inoculated from a single colony of C. difficile strains (630Δerm, hpdC::CT, hpdC::CtphpdCA) into pre-reduced BHIS broth and grown to an OD595 0.3 on a shaking platform at 50 rpm. These were inoculated 1/100 into pre-equilibrated BHIS broth which was incubated statically for 72 h under anaerobic conditions at 37°C. Total counts (vegetative cells and spores) and spore counts were then determined using CFU assays in 1X PBS (1/10 dilutions from 0 to -5). All dilutions were plated onto BHIS plates supplemented with 1% taurocholate. The spore counts were performed by heat inactivation of vegetative cells at 65°C for 20 minutes, these were then serially diluted and CFU counts determined on BHIS taurocholate plates. All experiments were performed with duplicate technical replicates and triplicate biological replicates. All data was analyzed in Excel, plotted in GraphPad Prism 7 and statistical analysis was performed in Stata15 using regression analysis p<0.05 were considered significantly different (S5 Fig).
Mouse relapse model of CDI
Female C57BL/6 mice (Charles River; 7–9 weeks old) were kept in independently ventilated cages under sterile conditions. As outlined by Theriot et al.[62], mice were treated with cefoperazone in the drinking water (50 mg/litre) for 10 days to disrupt their normal microflora, rested for two days, before they were infected with 104 C. difficile spores by oral gavage. After 28 days, vancomycin was added to the drinking water for 7 consecutive days (400 mg/litre) to induce relapse of CDI. Fresh faecal samples from individually infected mice were collected throughout the time course to be utilised for determining the C. difficile load, 16S rRNA sequencing of the microflora and metabolite profiling by 1H spectroscopy. Stool samples were plated onto C. difficile selective plates to determine the bacterial load (CFU/g). Statistical analysis was performed using a one tailed Mann Whitney U test, p<0.05 were considered significantly different.
Ethics statement
All animal procedures were performed at Royal Holloway in accordance with the Home Office project license PPL 70/8276, that enables work to be conducted under the UK “Animal (Scientific Procedures) Act 1986”. This work was approved by the Royal Holloway, University of London Ethics Committee.
Healthy human donor faecal samples were collected and processed using different healthy donors who had not received antibiotic treatment in the preceding 3 months in accordance with the University of Hertfordshire Ethics committee guidelines and approval (UH Ethics Approval Number: aLMS/SF/UH/00103). All donors provided informed written consent.
Microbiota analysis
DNA was extracted from faecal samples using a combined method based on phenol:chloroform:isoamyl alcohol extraction, ethanol precipitation and FastDNA SPIN kit for soil (MPBiomedicals). Briefly, an equal mass of faecal material was suspended in 50 mM Tris-HCl pH7.5, 10 mM EDTA, homogenised and bacterial cells were lysed using a FastPrep-24 Classic Instrument (MPBiomedicals). Nucleic acid was extracted using a standard phenol:chloroform:isoamyl alcohol procedure, followed by ethanol precipitation and was suspended in nuclease free dH2O. Faecal DNA was subsequently purified using the DNA binding matrix from the FastDNA SPIN kit for soil with minor modifications of the manufacturer’s instructions. Briefly, DNA samples were added to sodium phosphate buffer, MT buffer and protein precipitation solution supplied in the kit and this was added directly to the binding matrix. DNA was subsequently purified according to the manufacturer’s instructions and eluted in DNase-free water. Library preparations for the MiSeq were performed as outlined in Rosser et al[63]. Briefly, an amplification step was used to add Illumina compatible adaptors, with a unique 12 bp individual barcodes for each sample, with an extra pad and linker sequence. The V5-7 regions of the 16S rRNA genes were then amplified using 785F: 5ʹ-GGATTAGATACCCBRGTAGTC-3ʹ, 1175R: 5 ʹ-ACGTCRTCCCCDCCTTCCTC-3ʹ primers, where the reverse primer (1175R) contained the individual error-corrected barcode: 25 μl reactions were comprised of 1x Molzym PCR buffer, 0.025 μM Moltaq (Molzym), 200 μM dNTPs (Bioline), 0.4 μM forward and reverse primer, 2 μl DNA and nuclease free water (Bioline)[63]. Cycling parameters for each reaction were 94°C x 3 min, then 30 cycles of 94°C x 30 s, 60°C x 40 s, 72°C x 90 s and final extension at 72°C for 10 min. Samples were purified and normalised using a SeqPrep normalisation plate kit (Invitrogen), and quantified using a Qubit2.0 (Life technologies), and further purified using 0.6 X Agencourt AMPure Beads (Beckman Coulter), a selection of samples were run on an Agilent high sensitivity DNA chip (Agilent Technologies), samples were quantified again using a Qubit 2.0 (Life Technologies), and were pooled in equimolar solution, then diluted to a 2 nM library, with 10% PhiX control and loaded into the MiSeq run cartridge in accordance with the manufacturer’s instructions (Illumina). The MiSeq runs produced 250 bp paired end reads, with a 12 bp individual index for each sample. The sequence reads generated were de-multiplexed and quality filtered using QIIME (version 1.9.1 [64]) following the standard pipeline to assign Illumina reads to operational taxonomic units (OTUs) using the Greengenes database [65]). Associated summaries and diversity analyses were also performed in QIIME. Subsequent analyses were performed in R [66] and visualised with ggplot2 [67]. We selected families to include in our 16S plots (Fig 7A) if they had mean proportion of greater than 1% in any of the 12 day/type phenotype combinations. Box plots (Fig 7B) and PCA (Fig 7C) were calculated from the full 16S rRNA sequence dataset at the family level. ANOSIM analysis was used to identify variation in species abundance and composition between strains 630Δerm and hpdC::CT, as well as between time points D7, D0R, D2R and D4R. Significant differences were indicated with a circle p<0.001.
Metabolic profiling of mouse faecal samples
Faecal samples were defrosted and mixed with 400 μL of phosphate buffer (pH 7.4, 100% D2O, 3 mM of NaN3, 1 mM of 3-(trimethyl-silyl)-[2,2,3,3-2H4]-propionic acid (TSP) for the chemical shift reference at δ0.0) and Zirconium beads (0.45 g ±0.1). The samples were vortexed and then homogenised with a FastPrep-24 Classic Instrument (MP BIOMEDICALS) (30 sec per cycle, speed 6.0, 2 cycles). After a centrifugation (13,000 xg, 15 min), 180 μL of the supernatants were collected and transferred in 3-mm tubes for 1H nuclear magnetic resonance (NMR) spectroscopic analysis, which was performed on a Bruker 600 MHz spectrometer (Bruker Biospin, Karlsruhe, Germany) at 300K (26.85°). The parameters of the acquisition were as previously reported for urine[61]. 4 dummy scans followed per 64 scans were acquired for each spectrum which were then imported into Matlab (version R2014a, The Mathworks Inc.). The region δ4.82–4.76 was removed to eliminate residual water signal. All spectra were normalised according probabilistic quotient method and automatically aligned. Principal Components Analysis (PCA) was performed with mean-centring and Pareto scaling.
HPLC
Frozen culture supernatants were defrosted on ice and were mixed in a 1:1 ratio with methanol: water, transferred to HPLC tubes and processed immediately by HPLC. Mouse faecal samples were defrosted, and added to a 2 ml screw cap tube containing 2 mm beads. These were weighed before and after addition of the faecal sample. To these, 400 μl 1:1 methanol:water was added to the pellet, then ribolysed twice using a FastPrep-24 Classic Instrument at speed 6.0 m/s for 30 sec. Tubes were transferred to ice and centrifuged at 14000 xg for 20 minutes. 250 μl of the supernatant was transferred to a clean sterile HPLC tube and were transferred immediately for HPLC. Each experiment was performed in triplicate. All HPLC equipment, software, solvents, columns and vials were from Thermo Fisher Scientific, UK. Separations were performed utilising an Acclaim 120, C18, 5 μm Analytical (4.6 x 150 mm) and the mobile phase consisting of ammonium formate (10 mM, pH 2.7) and menthol (v/v; 40:60) at a flow rate of 2 ml/min. p-HPA and p-cresol were detected by the photo-diode array detector (UV-PDA; DAD 3000) set at 280 nm. Peak identity was confirmed by measuring the retention time, spiking the sample with commercially available p-HPA and p-cresol and determination of absorbance spectra using the UV-PDA. A calibration curve of each compound was generated by Chromeleon (Dionex software) using known amounts of the reference standards (0–100 mg/ml) in methanol/water (v/v; 1:1) injected onto the column to determine the amount in the samples. The lower limit of detection was determined for p-HPA to be 0.03 mg/ml, for p-cresol to be 0.02 mg/ml. The concentration in mM was determined in Excel, using the molecular weight of the compounds and the quantity in mg/ml. The data was analysed in GraphPad Prism7 and statistical analysis was performed in Stata15 using linear regression analysis.
Cultivatable human bacteria following p-cresol stress
Healthy human donor faecal samples were collected and processed using three different healthy donors who had not received antibiotic treatment in the preceding 3 months. Faecal samples (5 g) were emulsified in sterile pre-reduced PBS (50 ml) and faecal material was coarse filtered by passing the 10% emulsion through sterile muslin cloth to remove larger particle matter and leave a bacterial suspension. Faecal emulsions were incubated for 1 hour and 30 minutes in 1X PBS, or PBS containing 0.1% (v/v) p-cresol or 0.3% (v/v) p-cresol. Samples were then sedimented by centrifugation at 14000 x g for 5 minutes and the supernatant were removed. Pellets were resuspended in 1 ml 1X PBS and viable counts (CFU/ml) were performed on differentially selective agar, both anaerobically and aerobically. Each experiment was performed in triplicate. Serial 10-fold dilutions of re-suspended faecal emulsions in sterile pre-reduced peptone water were inoculated onto: fastidious anaerobe agar (total anaerobes), nutrient agar (total facultative anaerobes), kanamycin aesculin azide agar (Enterococci), LAMVAB agar (Lactobacilli), Beeren’s agar (Bifidobacteria), MacConkey agar number 3 (lactose-fermenting Enterobacteriaceae), Bacteroides bile aesculin agar (B. fragilis group), and total viable counts were determined in triplicate, and normalised to the starting CFU.
Phosphate release
Release of cellular phosphate was investigated using a Colorimetric Phosphate Assay Kit (Abcam). The assay involved treating samples with ammonium molybdate and malachite green which forms a chromogenic complex with phosphate ions which can be detected at a wavelength of 650 nm. An overnight culture of each bacterial strain was sedimented by centrifugation and re-suspended in Tris-buffered saline (TBS, 50 mM Tris-HCl pH7.5, 150 mM NaCl) and subsequently washed two further times to remove traces of the growth medium. OD595 was determined and cell suspensions were normalised to an OD595 of 1.0. Five hundred microliter aliquots of cell suspension were sedimented by centrifugation and re-suspended in either TBS alone or TBS + p-cresol. Cell suspensions were incubated for the indicated time, cells were sedimented and 30 μl of supernatant was removed and added to 170 μl H2O and 30 μl ammonium molybdate and malachite green reagent. Absorbance was read at 650 nm in a 96-well microtitre plate reader. Phosphate release was determined by normalising the optical density from cell suspensions incubated with p-cresol against cell suspensions that were incubated with TBS alone. The assay was performed under anaerobic conditions except for spectrophotometry and sedimentation steps, for which tubes and flasks were sealed with parafilm to prevent oxygen infiltration. The maximum intracellular phosphate pool was determined by boiling a 500 μl cell suspension (OD595 1.0) for 15 minutes. All assays were performed in triplicate.
Supporting information
Growth curves were performed in BHIS media under anaerobic conditions to determine the differential growth rate between C. difficile strains 630Δerm, hpdC::CT and the complemented mutant (hpdC::CT::phpdC-A and the intestinal microbiota species a) E. coli and K. oxytoca, and b) E. faecium and L. fermentum. The time, in minutes, that each strain reached OD595 0.5 is indicated by the purple hash line. C) The growth rate of C. difficile strains (630Δerm, hpdC::CT and complement hpdC::CT::phpdC-A) was assessed in BHIS supplemented with a range of anhydrotetracycline concentrations (0, 100 and 250 ng/ml). All experiments were performed in triplicate. Error bars are Standard Error of Mean (SEM).
(TIF)
Relative fitness of C. difficile 630Δerm, hpdC::CT and hpdC::CT complement in competitive co-culture for 24 hours with gut commensal species was performed in media supplemented with p-HPA (0.3% v/v). Expression of the hpdCA in trans from a plasmid-borne tetracycline-inducible promoter in the hpdC::CT background was evaluated by varying the concentration of anhydrotetracycline (100 and 250 ng/ml). The relative fitness of the C. difficile strains (wild-type, p-cresol mutant and complement) was compared in competition with a) E.coli and b) K. oxytoca. The relative proportion of each strain was expressed as a percentage of the total CFU count. Error bars are representative of three independent replicates. Regression analysis was used to determine significant differences in growth taking strain into consideration and marked ** p<0.01 and ***p<0.001. c&d) The concentration of p-cresol produced in the co-cultures quantified by HPLC. e&f) The concentration of p-HPA remaining in the media after the co-cultures was quantified by HPLC. Regression analysis was used to determine significant differences in p-cresol production and p-HPA utilisation compared to 630Δerm *** p<0.001. Error bars are SEM.
(TIF)
a) 1H NMR spectra doublet of pHPA (δ6.867ppm) and p-cresol (δ6.831ppm), with C. difficile strains highlighted in different colours 630Δerm (black), hpdC::CT (red) and the complemented mutant hpdC::CT::phpdC-A (blue). Each line represents an individual 1H NMR spectrum of the culture supernatants. The arrows on the right indicate the differences between the p-cresol levels produced from either 0.1% pHPA or 0.2% pHPA.
(TIF)
1H NMR spectroscopy was used to determine the metabolite profiles of co-culture samples in media containing 0.1% and 0.2% p-HPA. a) PCA demonstrating metabolic variation across the profiles in media containing 0.1% or 0.2% (v/v) p-HPA. b) Loading plots of the PCA model showing the metabolites driving the groupings along principal component 1 (PC1) and principal component 2 (PC2).
(TIF)
The sporulation frequency of strains 630Δerm, hpdC::CT and the complemented mutant hpdC::CT::phpdC-A, were determine in BHIS media grown for 72 hours in BHIS broth. a) total cell counts were enumerated on BHIS plates containing 0.1% taurocholate and spore counts were determined by heat inactivation of vegetative cells at 65°C for 20 minutes before enumerated on BHIS taurocholates plates. b) percentage sporulation was calculated as a proportion of the total cell counts for each strain respectively. Statistical analysis was performed in Stata15 using linear regression and p<0.05 was considered a statistically significant difference. n/s indicates no significant differences.
(TIF)
ANOSIM was performed to identify differences in bacterial population between 630Δerm and hpdC::CT infected animals across the experiment as well as at given time points, D7, D0R, D2R and D4R. Significant differences are indicated with a circle p<0.001.
(TIF)
(XLSX)
(XLSX)
The top table shows the output of a Linear Regression analysis to compare the growth of a particular strain of bacteria, at a given concentration of p-cresol against the reference strain, C. difficile. Grey shading indicates analyses where the p<0.05. The bottom table indicates the relative effect of p-cresol concentration on the growth of a particular strain, relative to the growth rate in BHIS as a reference. This analysis was performed in Stata15 using linear regression, p<0.05 are shaded grey. COV is the co-efficient of variance of the test from the reference.
(XLSX)
(XLSX)
CFU/ml was determined each bacterial species grown in co-culture in the presence or absence of extrinsic p-cresol.
(XLSX)
(XLSX)
A table demonstrating the relative abundance of families comprising the faecal microbiome at day 7 for the wild-type infected, hpdC::CT infected and naïve mice.
(XLSX)
Acknowledgments
The authors thank R. Doyle and N. Klein at the UCL Institute for Child Health for technical assistance setting up the 16S rRNA sequencing protocols, and M. Wilcox (University of Leeds) for providing human intestinal microbiota strains isolated from their gut soup model.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by an ISSF fellowship from the Wellcome Trust: 105609/Z/14/Z (LFD), https://wellcome.ac.uk/funding) and an Athena Swan Career Restart Fellowship (from London School of Hygiene and Tropical Medicine) (LFD), https://www.lshtm.ac.uk/. Additional support was received from the Medical Research Council grants: MR/K000551/1 (BWW) and MR/K015354/1 (SMC), https://www.mrc.ac.uk/ Funding for MPML was provided by the STRATiGRAD PhD training programme, https://www.imperial.ac.uk/stratigrad/ and Servier, https://servier.com/en/home/. Funding for MAH was provided by Medical Research Council/LSHTM studentship MR/N013638/1, https://www.mrc.ac.uk/ and https://www.lshtm.ac.uk/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Leffler DA, Lamont JT. Clostridium difficile Infection. N Engl J Med. 2015;373(3):287–8. [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7(7):526–36. 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- 3.Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, et al. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun. 2012;80(8):2704–11. 10.1128/IAI.00147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson LF, Valiente E, Donahue EH, Birchenough G, Wren BW. Hypervirulent Clostridium difficile PCR-ribotypes exhibit resistance to widely used disinfectants. PLoS One. 2011;6(10):e25754 10.1371/journal.pone.0025754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8(10):e1002995 10.1371/journal.ppat.1002995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–8. 10.1086/525047 [DOI] [PubMed] [Google Scholar]
- 7.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517(7533):205–8. 10.1038/nature13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Nood E, Dijkgraaf MG, Keller JJ. Duodenal infusion of feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(22):2145. [DOI] [PubMed] [Google Scholar]
- 9.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. 10.4161/gmic.19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan MT, Duncan SH, Stams AJ, van Dijl JM, Flint HJ, Harmsen HJ. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 2012;6(8):1578–85. 10.1038/ismej.2012.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57(3):523–35. 10.1002/mnfr.201200594 [DOI] [PubMed] [Google Scholar]
- 12.Russell WR, Hoyles L, Flint HJ, Dumas M-E. Colonic bacterial metabolites and human health. Current Opinion in Microbiology. 2013;16(3):246–54. 10.1016/j.mib.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 13.Gill CI, Rowland IR. Diet and cancer: assessing the risk. Br J Nutr. 2002;88 Suppl 1:S73–87. [DOI] [PubMed] [Google Scholar]
- 14.Hwang MK, Bode AM, Byun S, Song NR, Lee HJ, Lee KW, et al. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010;70(17):6859–69. 10.1158/0008-5472.CAN-09-4393 [DOI] [PubMed] [Google Scholar]
- 15.Jenner AM, Rafter J, Halliwell B. Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic Biol Med. 2005;38(6):763–72. 10.1016/j.freeradbiomed.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 16.Elsden SR, Hilton MG, Waller JM. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976;107(3):283–8. [DOI] [PubMed] [Google Scholar]
- 17.Fung DY, Lin CC, Gailani MB. Effect of phenolic antioxidants on microbial growth. Crit Rev Microbiol. 1985;12(2):153–83. 10.3109/10408418509104428 [DOI] [PubMed] [Google Scholar]
- 18.Erb RW, Eichner CA, Wagner-Dobler I, Timmis KN. Bioprotection of microbial communities from toxic phenol mixtures by a genetically designed pseudomonad. Nat Biotechnol. 1997;15(4):378–82. 10.1038/nbt0497-378 [DOI] [PubMed] [Google Scholar]
- 19.Doerner KC, Mason BP, Kridelbaugh D, Loughrin J. Fe(III) stimulates 3-methylindole and 4-methylphenol production in swine lagoon enrichments and Clostridium scatologenes ATCC 25775. Lett Appl Microbiol. 2009;48(1):118–24. 10.1111/j.1472-765X.2008.02500.x [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama MT, Carlson JR. Production of Skatole and para-Cresol by a Rumen Lactobacillus sp. Applied and Environmental Microbiology. 1981;41(1):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafiz S, Oakley CL. Clostridium difficile: isolation and characteristics. J Med Microbiol. 1976;9(2):129–36. 10.1099/00222615-9-2-129 [DOI] [PubMed] [Google Scholar]
- 22.Dawson LF, Donahue EH, Cartman ST, Barton RH, Bundy J, McNerney R, et al. The analysis of para-cresol production and tolerance in Clostridium difficile 027 and 012 strains. BMC Microbiol. 2011;11:86 10.1186/1471-2180-11-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phetcharaburanin J, Hong HA, Colenutt C, Bianconi I, Sempere L, Permpoonpattana P, et al. The spore-associated protein BclA1 affects the susceptibility of animals to colonization and infection by Clostridium difficile. Mol Microbiol. 2014;92(5):1025–38. 10.1111/mmi.12611 [DOI] [PubMed] [Google Scholar]
- 24.Seekatz AM, Theriot CM, Molloy CT, Wozniak KL, Bergin IL, Young VB. Fecal Microbiota Transplantation Eliminates Clostridium difficile in a Murine Model of Relapsing Disease. Infect Immun. 2015;83(10):3838–46. 10.1128/IAI.00459-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theriot CM, Koenigsknecht MJ, Carlson PE Jr., Hatton GE, Nelson AM, Li B, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114 10.1038/ncomms4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassis CM, Theriot CM, Young VB. Alteration of the murine gastrointestinal microbiota by tigecycline leads to increased susceptibility to Clostridium difficile infection. Antimicrob Agents Chemother. 2014;58(5):2767–74. 10.1128/AAC.02262-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD, et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4(1):36 10.1186/s40168-016-0181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vernocchi P, Del Chierico F, Putignani L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front Microbiol. 2016;7:1144 10.3389/fmicb.2016.01144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston MD, Hanlon GW, Denyer SP, Lambert RJ. Membrane damage to bacteria caused by single and combined biocides. J Appl Microbiol. 2003;94(6):1015–23. [DOI] [PubMed] [Google Scholar]
- 30.Heipieper HJ, Keweloh H, Rehm HJ. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Applied and Environmental Microbiology. 1991;57(4):1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campos FM, Couto JA, Figueiredo AR, Tóth IV, Rangel AOSS, Hogg TA. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. International Journal of Food Microbiology. 2009;135(2):144–51. 10.1016/j.ijfoodmicro.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 32.Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, et al. Antibiotic treatment of clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immun. 2009;77(9):3661–9. 10.1128/IAI.00558-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves AE, Koenigsknecht MJ, Bergin IL, Young VB. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect Immun. 2012;80(11):3786–94. 10.1128/IAI.00647-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis BB, Buffie CG, Carter RA, Leiner I, Toussaint NC, Miller LC, et al. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection With Oral Vancomycin Compared With Metronidazole. J Infect Dis. 2015;212(10):1656–65. 10.1093/infdis/jiv256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koenigsknecht MJ, Theriot CM, Bergin IL, Schumacher CA, Schloss PD, Young VB. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect Immun. 2015;83(3):934–41. 10.1128/IAI.02768-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, et al. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80(1):62–73. 10.1128/IAI.05496-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–9. 10.1038/nature12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell host & microbe. 2014;16(6):770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43. [DOI] [PubMed] [Google Scholar]
- 40.Jackson S, Calos M, Myers A, Self WT. Analysis of proline reduction in the nosocomial pathogen Clostridium difficile. J Bacteriol. 2006;188(24):8487–95. 10.1128/JB.01370-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabisch UC, Gräntzdörffer A, Schierhorn A, Rücknagel KP, Andreesen JR, Pich A. Identification of d-Proline Reductase fromClostridium sticklandii as a Selenoenzyme and Indications for a Catalytically Active Pyruvoyl Group Derived from a Cysteine Residue by Cleavage of a Proprotein. Journal of Biological Chemistry. 1999;274(13):8445–54. [DOI] [PubMed] [Google Scholar]
- 42.Jackson-Rosario S, Cowart D, Myers A, Tarrien R, Levine RL, Scott RA, et al. Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct. Journal of biological inorganic chemistry: JBIC: a publication of the Society of Biological Inorganic Chemistry. 2009;14(4):507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dineen SS, McBride SM, Sonenshein AL. Integration of Metabolism and Virulence by Clostridium difficile CodY. Journal of Bacteriology. 2010;192(20):5350–62. 10.1128/JB.00341-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins J, Robinson C, Danhof H, Knetsch CW, van Leeuwen HC, Lawley TD, et al. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature. 2018;553:291 10.1038/nature25178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitts AC, Tuck LR, Faulds-Pain A, Lewis RJ, Marles-Wright J. Structural Insight into the Clostridium difficile Ethanolamine Utilisation Microcompartment. PLOS ONE. 2012;7(10):e48360 10.1371/journal.pone.0048360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Verstraete I, Peltier J, Dupuy B. The Regulatory Networks That Control Clostridium difficile Toxin Synthesis. Toxins. 2016;8(5):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubois T, Dancer-Thibonnier M, Monot M, Hamiot A, Bouillaut L, Soutourina O, et al. Control of Clostridium difficile Physiopathology in Response to Cysteine Availability. Infection and Immunity. 2016;84(8):2389–405. 10.1128/IAI.00121-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlsson S, Lindberg A, Norin E, Burman LG, Åkerlund T. Toxins, Butyric Acid, and Other Short-Chain Fatty Acids Are Coordinately Expressed and Down-Regulated by Cysteine in Clostridium difficile. Infection and Immunity. 2000;68(10):5881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118(4):724–34. [DOI] [PubMed] [Google Scholar]
- 50.Vital M, Penton CR, Wang Q, Young VB, Antonopoulos DA, Sogin ML, et al. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome. 2013;1(1):8 10.1186/2049-2618-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouillaut L, Dubois T, Sonenshein AL, Dupuy B. Integration of metabolism and virulence in Clostridium difficile. Research in Microbiology. 2015;166(4):375–83. 10.1016/j.resmic.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67(3):742–8. 10.1093/jac/dkr508 [DOI] [PubMed] [Google Scholar]
- 53.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280 10.1371/journal.pbio.0060280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andriamihaja M, Lan A, Beaumont M, Audebert M, Wong X, Yamada K, et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic Biol Med. 2015;85:219–27. 10.1016/j.freeradbiomed.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 55.Hopper DJ. The hydroxylation of P-cresol and its conversion to P-hydroxybenzaldehyde in Pseudomonas putida. Biochem Biophys Res Commun. 1976;69(2):462–8. [DOI] [PubMed] [Google Scholar]
- 56.Cho AR, Lim EJ, Veeranagouda Y, Lee K. Identification of a p-cresol degradation pathway by a GFP-based transposon in Pseudomonas and its dominant expression in colonies. J Microbiol Biotechnol. 2011;21(11):1179–83. [DOI] [PubMed] [Google Scholar]
- 57.Keweloh H, Diefenbach R, Rehm HJ. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch Microbiol. 1991;157(1):49–53. [DOI] [PubMed] [Google Scholar]
- 58.Hussain HA, Roberts AP, Mullany P. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Deltaerm) and demonstration that the conjugative transposon Tn916DeltaE enters the genome of this strain at multiple sites. J Med Microbiol. 2005;54(Pt 2):137–41. 10.1099/jmm.0.45790-0 [DOI] [PubMed] [Google Scholar]
- 59.Fagan RP, Fairweather NF. Clostridium difficile has two parallel and essential Sec secretion systems. J Biol Chem. 2011;286(31):27483–93. 10.1074/jbc.M111.263889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Purdy D, O'Keeffe TA, Elmore M, Herbert M, McLeod A, Bokori-Brown M, et al. Conjugative transfer of clostridial shuttle vectors from Escherichia coli to Clostridium difficile through circumvention of the restriction barrier. Mol Microbiol. 2002;46(2):439–52. [DOI] [PubMed] [Google Scholar]
- 61.Dona AC, Jimenez B, Schafer H, Humpfer E, Spraul M, Lewis MR, et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem. 2014;86(19):9887–94. 10.1021/ac5025039 [DOI] [PubMed] [Google Scholar]
- 62.Theriot CM, Koumpouras CC, Carlson PE, Bergin II, Aronoff DM, Young VB. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes. 2011;2(6):326–34. 10.4161/gmic.19142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat Med. 2014;20(11):1334–9. 10.1038/nm.3680 [DOI] [PubMed] [Google Scholar]
- 64.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME Journal. 2012;6(3):610–8. 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2014. [Google Scholar]
- 67.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verl; N Y: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth curves were performed in BHIS media under anaerobic conditions to determine the differential growth rate between C. difficile strains 630Δerm, hpdC::CT and the complemented mutant (hpdC::CT::phpdC-A and the intestinal microbiota species a) E. coli and K. oxytoca, and b) E. faecium and L. fermentum. The time, in minutes, that each strain reached OD595 0.5 is indicated by the purple hash line. C) The growth rate of C. difficile strains (630Δerm, hpdC::CT and complement hpdC::CT::phpdC-A) was assessed in BHIS supplemented with a range of anhydrotetracycline concentrations (0, 100 and 250 ng/ml). All experiments were performed in triplicate. Error bars are Standard Error of Mean (SEM).
(TIF)
Relative fitness of C. difficile 630Δerm, hpdC::CT and hpdC::CT complement in competitive co-culture for 24 hours with gut commensal species was performed in media supplemented with p-HPA (0.3% v/v). Expression of the hpdCA in trans from a plasmid-borne tetracycline-inducible promoter in the hpdC::CT background was evaluated by varying the concentration of anhydrotetracycline (100 and 250 ng/ml). The relative fitness of the C. difficile strains (wild-type, p-cresol mutant and complement) was compared in competition with a) E.coli and b) K. oxytoca. The relative proportion of each strain was expressed as a percentage of the total CFU count. Error bars are representative of three independent replicates. Regression analysis was used to determine significant differences in growth taking strain into consideration and marked ** p<0.01 and ***p<0.001. c&d) The concentration of p-cresol produced in the co-cultures quantified by HPLC. e&f) The concentration of p-HPA remaining in the media after the co-cultures was quantified by HPLC. Regression analysis was used to determine significant differences in p-cresol production and p-HPA utilisation compared to 630Δerm *** p<0.001. Error bars are SEM.
(TIF)
a) 1H NMR spectra doublet of pHPA (δ6.867ppm) and p-cresol (δ6.831ppm), with C. difficile strains highlighted in different colours 630Δerm (black), hpdC::CT (red) and the complemented mutant hpdC::CT::phpdC-A (blue). Each line represents an individual 1H NMR spectrum of the culture supernatants. The arrows on the right indicate the differences between the p-cresol levels produced from either 0.1% pHPA or 0.2% pHPA.
(TIF)
1H NMR spectroscopy was used to determine the metabolite profiles of co-culture samples in media containing 0.1% and 0.2% p-HPA. a) PCA demonstrating metabolic variation across the profiles in media containing 0.1% or 0.2% (v/v) p-HPA. b) Loading plots of the PCA model showing the metabolites driving the groupings along principal component 1 (PC1) and principal component 2 (PC2).
(TIF)
The sporulation frequency of strains 630Δerm, hpdC::CT and the complemented mutant hpdC::CT::phpdC-A, were determine in BHIS media grown for 72 hours in BHIS broth. a) total cell counts were enumerated on BHIS plates containing 0.1% taurocholate and spore counts were determined by heat inactivation of vegetative cells at 65°C for 20 minutes before enumerated on BHIS taurocholates plates. b) percentage sporulation was calculated as a proportion of the total cell counts for each strain respectively. Statistical analysis was performed in Stata15 using linear regression and p<0.05 was considered a statistically significant difference. n/s indicates no significant differences.
(TIF)
ANOSIM was performed to identify differences in bacterial population between 630Δerm and hpdC::CT infected animals across the experiment as well as at given time points, D7, D0R, D2R and D4R. Significant differences are indicated with a circle p<0.001.
(TIF)
(XLSX)
(XLSX)
The top table shows the output of a Linear Regression analysis to compare the growth of a particular strain of bacteria, at a given concentration of p-cresol against the reference strain, C. difficile. Grey shading indicates analyses where the p<0.05. The bottom table indicates the relative effect of p-cresol concentration on the growth of a particular strain, relative to the growth rate in BHIS as a reference. This analysis was performed in Stata15 using linear regression, p<0.05 are shaded grey. COV is the co-efficient of variance of the test from the reference.
(XLSX)
(XLSX)
CFU/ml was determined each bacterial species grown in co-culture in the presence or absence of extrinsic p-cresol.
(XLSX)
(XLSX)
A table demonstrating the relative abundance of families comprising the faecal microbiome at day 7 for the wild-type infected, hpdC::CT infected and naïve mice.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.