Abstract
Background
This study's objective was to evaluate the association between proton-pump inhibitor (PPI) use and bone fracture incidence and bone mineral density (BMD) by meta-analyzing the estimates reported by epidemiological and cohort studies.
Methods
Data were acquired from studies identified after a literature search in electronic databases. Odds ratios (ORs), hazard ratios (HRs), and risk ratios (RRs) between PPI use and bone fracture incidence were pooled under the random effects model, and meta-analysis of standardized mean differences between PPI users and controls in cross-sectional values and BMD changes was conducted.
Results
Thirty-three studies fulfilled the eligibility criteria. These studies provided data from 2,714,502 individuals with a mean age of 66.91 years (95% confidence interval [CI], 63.37–70.46); 33.21% (95% CI, 30.44–35.99) were males and 64.61% (95% CI, 60.73–68.49) were females. Overall, fracture incidence was 22.04% (95% CI, 16.10–27.97) in PPI users and 15.57% (95% CI, 12.28–18.86) in controls. The overall effect size of the point estimate was 1.28 (95% CI, 1.22–1.35) between PPI use and bone fracture incidence. There was a trend towards increased fracture incidence from short duration use: OR 1.29 (95% CI, 1.19–1.40), medium duration use: OR 1.33 (95% CI, 1.12–1.55) and long duration use: OR 1.62 (95% CI, 1.33–1.90). There was no significant difference in the standardized mean differences between PPI users and controls, either in cross-sectional BMD values or in the BMD change observed in longitudinal studies.
Conclusions
Pooling of ORs, HRs, and RRs suggested that PPI use might increase fracture risk. However, there was no effect of PPI use on BMD.
Keywords: Fracture, Meta-analysis, Proton-pump inhibitors
INTRODUCTION
Proton-pump inhibitors (PPIs) are widely prescribed medications used to treat acid-related gastrointestinal diseases and are considered the superior option for anti-secretory therapy against several conditions including: non-erosive gastrointestinal reflux disease, erosive esophagitis, dyspepsia and peptic ulcer in terms of improved symptomatic outcomes [1] and as co-therapy with non-steroidal anti-inflammatory drugs for the prevention of peptic ulcers.[2] PPIs irreversibly block the proton pump (H+-K+-ATPase ion exchanger) in the stomach's acid-secreting parietal cells, leading to a profound inhibition of gastric acid secretion.[3]
In general, PPIs are well tolerated with minimal short-term side effects; therefore, these drugs are considered safe therapeutic regimens.[4] However, many epidemiological and cohort studies have observed an association between PPI use and an increased fracture risk among long-term PPI users,[5] which has raised concerns about their long-term use, especially in individuals with fracture risk. This risk is concerning for patients who prescribe PPIs and wish to balance their efficacy and the possibilities of future metabolic bone disease and fracture.[6]
Whereas many studies have found significant associations between PPI use and fracture risk, others could not endorse these findings. This discrepancy has necessitated a comprehensive review of the literature to synthesize the evidence. Recently, a meta-analysis of relative risk obtained from 18 studies found a modest risk of bone fractures with PPI use.[7] We conducted a systematic review and performed a meta-analysis by including all possible sources of prospective and retrospective data to evaluate the relationship between PPI use and fracture incidence.
METHODS
The present study was performed following the Cochrane Collaboration guidelines for conducting systematic reviews and meta-analysis, and the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement was used as guideline for preparing the present report.
1. Eligibility criteria
Inclusion criteria: (1) the study was general/patient population-based prospective or retrospective examining the association between PPI use and fracture incidence; (2) the study reported fracture incidence (hip, femur, forearm, hindarm, humerus, spine, etc.) in PPI users vs PPI non-users or the odds or hazards of using PPI for fracture incidence; (3) the study reported PPI use in individual with and without fracture incidence; or (4) the study reported either the epidemiological value of bone mineral density (BMD) or the BMD change in PPI users and their non-user controls. Exclusion criteria: (1) the study examined the association between fracture incidence and PPI use in combination with other drugs such as histamine2-receptor antagonists; or (2) the study involved other related measures such as falls or fracture-related mortality but not fractures per se.
2. Literature search
The literature search was conducted in electronic databases including: PubMed, Embase, and Google Scholar using the following relevant keywords and subject headings: PPIs, lansoprazole, dexlansoprazole, rabeprazole, pantoprazole, omeprazole, bone density, fractures, incidence, humans, medical records, BMD, incidence, hazard, odds, cohort, case-control, prospective, retrospective, database, general population, patient population, registry, medical records, trial, and registries. The search encompassed articles published in peer-reviewed journals in the English language before February 2018. Each database was searched for the aforementioned search terms. The search encompassed articles published in peer-reviewed journals in the English language before February 2018. Additional searches included the considerations of software-suggested corroborations and cross references of important research papers and review articles relevant to the present study.
3. Meta-analysis endpoints
For the present study, the meta-analysis endpoint was the attainment of a point estimate by pooling the odds ratio (OR), hazard ratio (HR), and risk ratio (RR) between PPI use and fracture incidence reported in individual studies. Subgroup meta-analysis were performed regarding low/medium/high PPI use, short/medium/long duration PPI use, outcomes of prospective vs. retrospective studies, and the fracture site. An additional endpoint was differences in cross-sectional values of BMD and BMD changes between PPI users and non-users observed in longitudinal studies.
4. Data and analyses
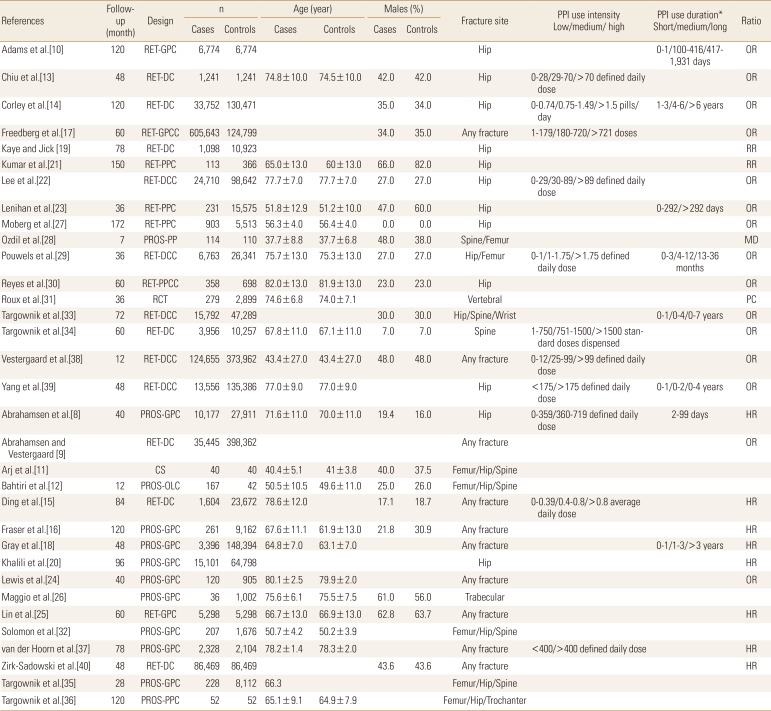
Demographic and clinical characteristics of subjects, study characteristics, and outcomes were extracted from respective research articles using a standardized procedure and were organized in specialized datasheets. Meta-analysis were performed using a random-effects model with STATA software (version 12; Stata Corp., College Station, TX, USA) by pooling the OR, HR, and RR reported by individual studies to achieve the overall effect size (OR approximated RR). The classifications used either for low, medium, and high intensity or for short, medium, and long duration were those of individual studies' authors which are reported in Table 1.
Table 1. Characteristics of the included studies which recruited cases with fractures and non-fracture controls.
RET, retrospective; GPC, general population cohort; DC, database cohort; GPCC, general population case-controlled; PPC, patient population cohort; DCC, database case-control; PROS, prospective; PPCC, patient-population case-controlled; RCT, randomized controlled trial; CS, cross-sectional; OLC, open-label comparative; PPI, proton-pump inhibitor; OR, odds ratio; RR, risk ratio; MD, mean difference; PC, precision; HR, hazard ratio.
For the assessment of the relationship between PPI use and BMD, meta-analyses of standardized mean differences (SMD) were performed by using RevMan software (version 5.3.1; The Cochrane Collaboration, Oxford, UK) to evaluate the significance of differences in BMD between PPI users and non-users or change in BMD after PPI use reported by longitudinal studies.
The overall effect size/s in the meta-analysis were a weighted average of the inverse variance adjusted individual effect sizes. Between-study inconsistency was tested using the I2 index. For the assessment of publication bias, Begg's funnel plot asymmetry tests was performed, and trim-and-fill method was used to estimate the number of missing studies. All data are presented as weighted effect sizes with 95% confidence interval (CI) and P<0.05 were considered statistically significant.
RESULTS
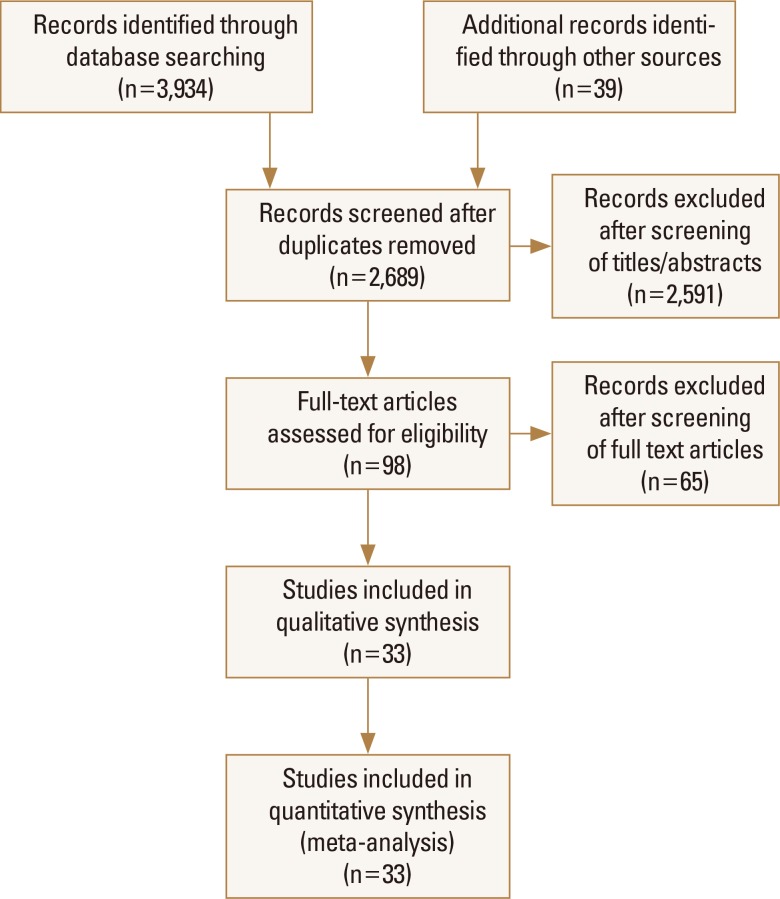
Thirty-three studies [8-40] fulfilled the eligibility criteria (Fig. 1). No significant publication bias was detected with the Begg's test of funnel plot asymmetry (adjusted Kendall's score=58±40.32; P=0.15), but the trim-and-fill method indicated the possibility of up to 4 missing studies (Supplementary Fig. 1). Important characteristics of these studies are presented in Table 1. Study subjects had a mean age of 66.91 years (95% CI, 63.37–70.46). Thirty-three point twenty one percent (95% CI, 30.44–35.99) were males while 64.61% (95% CI, 60.73–68.49) were females. Overall, fracture incidence was 22.04% (95% CI, 16.10–27.97) in 302,522 PPI users and 15.57% (95% CI, 12.28–18.86) in 833,254 controls (data from 14 studies).
Fig. 1. A flowchart of study screening and selection process after the literature search.
1. Relationship between PPI use and fractures
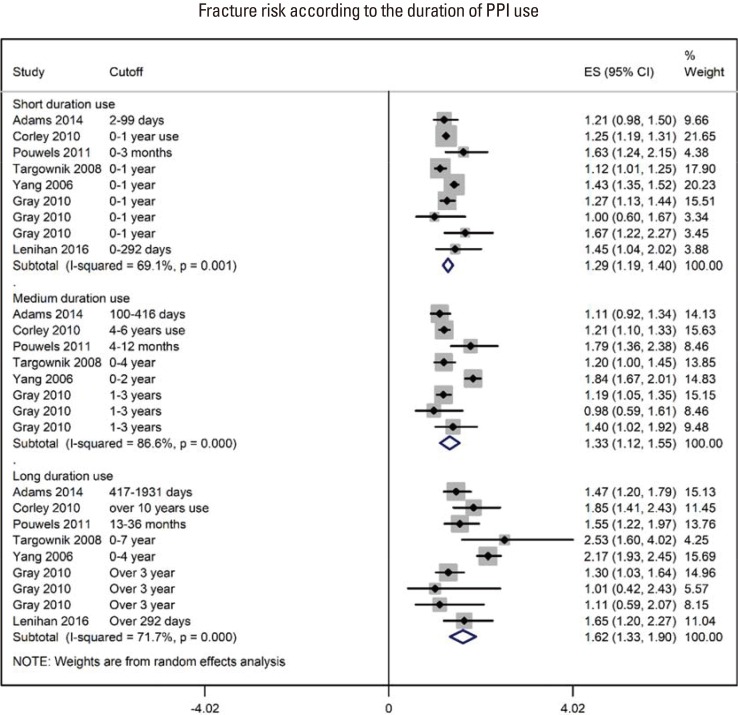
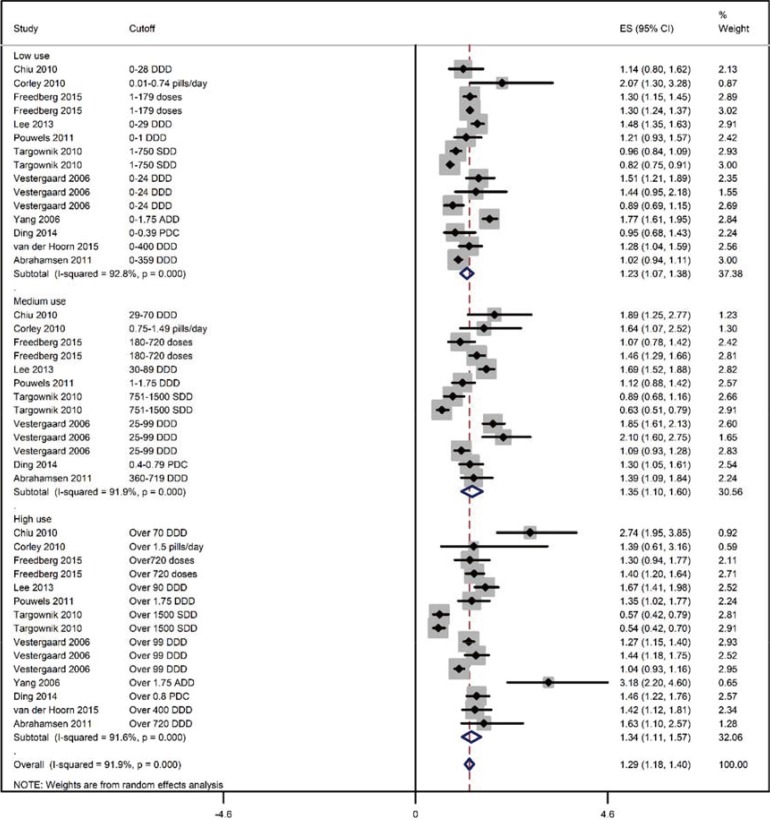
For the point estimation of the relationship between PPI use and fracture risk, pooling of the OR, HR, and RR revealed an effect size of 1.28 (95% CI, 1.22–1.35) (85 ratios from 2,714,502 individuals; I2=89.7%; P<0.00001; Supplementary Fig. 2). The follow-up duration was 73.12 (95% CI, 60.70–85.54) months (range, 12–150 months; data from 23 studies). There was a trend toward increased fracture incidence from short duration use, pooled OR 1.29 (95% CI, 1.19–1.40); I2=69.1%; P=0.001) to medium OR 1.33 (95% CI, 1.12–1.55); I2=86.6%; P<0.00001) and long duration use OR 1.62 (95% CI, 1.33–1.90); I2=71.7% ; P<0.00001) (Fig. 2). There was no difference in fracture incidence with low OR 1.22 (95% CI, 1.078–1.36); I2=91.9%; P<0.00001), medium OR 1.32 (95% CI, 1.08–1.56); I2=91.3%; P<0.00001) and high PPI use OR 1.26 (95% CI, 1.045–1.47); I2=91.1%; P<0.00001; Fig. 3).
Fig. 2. A forest graph showing the outcomes of a subgroup meta-analysis conducted to evaluate the effect of proton-pump inhibitor (PPI) use on fracture incidence with respect to the duration of PPI use. ES, effect sizes; CI, confidence interval.
Fig. 3. A forest graph showing the outcomes of a subgroup meta-analysis conducted to evaluate the effect of proton-pump inhibitor (PPI) use on fracture incidence with respect to the intensity of PPI use. ADD, average daily dose; DDD, defined daily dose; SDD, standard daily dose; PDC, proportion of days covered; ES, effect sizes; CI, confidence interval.
In other subgroup analyses, the effect sizes of the OR, HR and RR between PPI use and fracture incidence were OR 1.33 (95% CI, 1.25–1.41); (I2=92.4%; P<0.00001; 57 OR from 14 studies), HR 1.26 (95% CI, 1.19–1.33); (I2=49%; P=0.005; 23 HR from 8 studies), and RR 0.74 (95% CI, 0.48–0.99); (I2=43.1%; P=0.134; 5 RR from 2 studies), respectively (Supplementary Fig. 2). Outcomes regarding the study design were similar for retrospective studies OR 1.29 (95% CI, 1.21–1.36); (I2=91.5%; P<0.00001; 18 studies) and for prospective studies OR 1.27 (95% CI, 1.16–1.38); (I2=49.7%; P=0.009; 7 studies). Effect sizes regarding the fracture site were hip with OR 1.34 (95% CI, 1.24–1.46); (I2=89.6%; P<0.00001; 15 studies), spine OR 1.18 (95% CI, 0.93–1.42); (I2=91.5%; P<0.00001; 10 studies), and any fracture OR 1.24 (95% CI, 1.18–1.31); (I2=78.6%; P<0.00001; 22 studies).
2. Relationship between PPI use and BMD
In the literature search, 11 studies were identified that reported the association between PPI use and either the cross-sectional BMD values or the BMD changes evaluated in longitudinal designs. Overall, data for 1,863 PPI users and 34,392 controls were used in this meta-analysis.
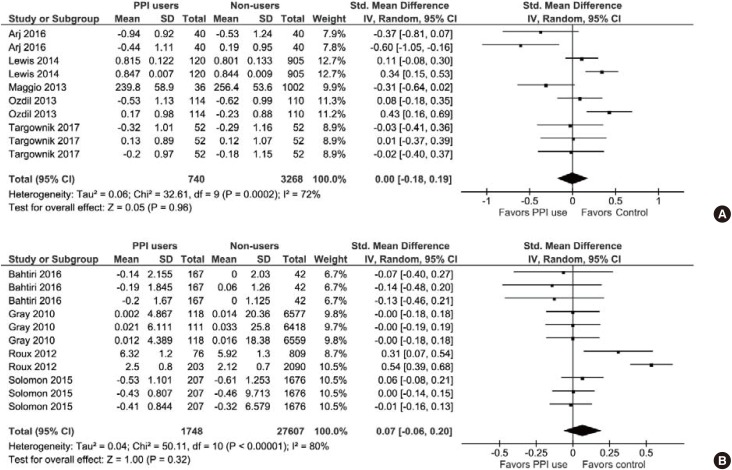
With respect to the cross-sectional BMD values, there was no significant difference between the PPI users and their non-user counterparts (SMD, 0.00; 95% CI, −0.18 to 0.19; P=0.96; I2=72%; P=0.0002; Fig. 4A). Also, there was no significant difference between PPI users and PPI non users in the BMD changes observed in the longitudinal studies (SMD, 0.07; 95% CI, −0.06 to 0.20; P=0.32; I2=80%; P<0.00001; Fig. 4B). In these studies, treatment durations were between 1 and 8 years.
Fig. 4. (A) A forest graph showing the meta-analysis of standardized mean differences between proton-pump inhibitor (PPI) users and non-users in cross-sectional bone mineral density (BMD) values observed in individual studies. (B) A forest graph showing the meta-analysis of standardized mean differences between PPI users and non-users in the BMD changes observed in longitudinal studies. SD, standard deviation; CI, confidence interval; df, degrees of freedom.
DISCUSSION
In this meta-analysis, we found that PPI use might increase fracture risk. A subgroup analysis also showed that the risk of fracture incidence with PPI use increased from short duration use to medium through high duration use. However, there was no significant difference between the PPI users and their non-user counterparts, either in cross-sectional values of BMD or in the change in BMD observed in longitudinal studies. At least 12 of the included studies failed to observe a significant association between PPI use and fracture incidence or BMD.
So far, a mechanistic relationship between PPI use and fracture incidence is lacking. However, many factors are identified that can affect this relationship. PPI therapy may be associated with side effects such as vitamin B12 deficiency, hypomagnesaemia, Clostridium difficile infection, pneumonia, gastrointestinal and cardiovascular risks [41,42] and may also interfere with bioavailability and/or metabolism of minerals such as calcium, iron and magnesium.[43] A review has also found that PPI use is associated with increased risk of chronic kidney disease.[44] Whereas in vitro studies have shown deleterious effects of PPIs on bone cells possibly by affecting bone turnover,[45] in vivo studies have shown that PPIs inhibit osteoclast mediated resorption when delivered to a bony defect in self setting calcium phosphate cements.[46] Bone fragility depends not only on areal BMD but also on other factors including bone quality, which may be affected by other factors such as vitamin B12 levels and modulated skeletal fragility due to collagen cross-linking independent of areal BMD.[47]
Although these results suggest that PPI therapy increases fracture risk, confounding factors may play a role in the overall outcomes. Participant age in individual studies ranged from 38±9 to 82±13 years, for example. Effects of aging, general health conditions and comorbid conditions may affect the actual prevalence of fractures. This presumption is further supported by the fact that PPI use had no effect on BMD. Many drugs, such as antipsychotics, anti-Parkinson's and anti-seizure medications can affect bone strength and are associated with increased fracture risk.[39] Thyroxine replacement therapy and warfarin may also affect the incidence rate of fractures.[48,49] Thus, possible spurious effects of confounders can't be ruled out when interpreting the results of this meta-analysis. Delineation of such effectors may be possible in the future as confounder variable-specific analyzable data from future trials become available or further retrospective analyses are performed.
In the present meta-analysis, the majority of fracture cases were related to the hip. Hip fractures can have several determinants. Falls, muscle weakness, low physical activity levels, suboptimal nutrition, drugs increasing fall risk and comorbid conditions of the neuromuscular system may contribute to hip fracture disability.[50] Neurological and neurodegenerative diseases such as Alzheimer's disease also pose significant fracture risk.[51] These factors may contribute to the presence of high statistical heterogeneity in the meta-analysis.
A significant increase in cardiovascular disease (CVD) incidence has been found with PPI use.[52] Some studies have also reported an increased incidence of major adverse cardiac events in patients who received PPIs along with an antiplatelet drug, clopidogrel.[53,54] A recent meta-analysis has also found that co-prescription of PPI and thienopyridines increases the risk of ischemic and composite stroke.[55] In the present study, four of the included studies reported incidence of CVD events, which was almost double OR 1.90 (1.52–2.37); P<0.00001) in PPI users (n=20,268) compared with non-users (n=21,298). However, the possible influence of confounding factors in the association between PPI use and increased cardiovascular risk cannot be ruled out [56] because fracture incidence has been found to be usually higher in patients with comorbidities,[57,58,59] which could be partially related to physical inactivity following fracture.[60]
There were several limitations to this study. There were a large number of studies included in this meta-analysis and were primarily observational rather than randomized controlled trials. This is the evidence currently available on this topic. Also, in subgroup analysis of the present study, outcomes were mostly associated with moderate to high I2. Sources of statistical heterogeneity could be several but usually originate from clinical and methodological heterogeneity. Clinical heterogeneity may arise from patients' differences, interventions or co-interventions and outcome measures, whereas the methodological heterogeneity may arise from the use of different study designs, cut-offs, and control over bias.
Regardless of the possible impacts of unidentified factors, the outcomes of the present meta-analysis demand a judicious and cautious use of PPIs. Studies have found that inappropriate use of PPIs in the inpatient setting is prevalent and should be discouraged.[61] With some caveats, authors of previous meta-analysis have also suggested that there could be a potential association between PPI use and fracture incidence especially hip and vertebral fractures.[7,62] Moreover, a strong association has been reported between PPI use and the subsequent prescribing of anti-osteoporotic drugs. Such an association has been also found to increase in a dose and time dependent manner.[63] Patients requiring continuous PPI therapy should ensure the recommended daily intake of calcium and vitamin D. However, the pharmacologic osteoprotection or BMD monitoring may not be advisable for chronic PPI users unless other indications necessitate it.[64]
CONCLUSIONS
Data generated from prospective and retrospective studies may be used for better statistical modeling to study potential confounding factors [5] and by arranging more homogeneous sub-datasets. Risk stratification of elderly, frail, malnourished, dialyzed and chronically hospitalized patients will also help in narrowing the conclusive evidence.[43] Prospective studies should establish cohorts of long-term PPI users and their non-user controls to follow BMD changes.[64] Even more useful, although potentially more demanding, would be to conduct randomized controlled trials.[5]
Footnotes
No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Forest graph showing the outcomes of an overall pooling of all odds ratios, hazard ratios, and relative risks for point estimation depicting relationship between the proton-pump inhibitor (PPI) use and the incidence of fracture. DDD, defined daily dose; SDD, standard daily dose; PDC, proportion of days covered; ES, effect sizes; CI, confidence interval.
A funnel plot showing the outcomes of trim and fill method of publication bias assessment. Theta represents the effect sizes of point estimate ratios.
References
- 1.Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354–364. [PMC free article] [PubMed] [Google Scholar]
- 2.Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319:1707–1715. doi: 10.1056/NEJM198812293192605. [DOI] [PubMed] [Google Scholar]
- 4.Eusebi LH, Rabitti S, Artesiani ML, et al. Proton pump inhibitors: risks of long-term use. J Gastroenterol Hepatol. 2017;32:1295–1302. doi: 10.1111/jgh.13737. [DOI] [PubMed] [Google Scholar]
- 5.Richards JB, Goltzman D. Proton pump inhibitors: balancing the benefits and potential fracture risks. CMAJ. 2008;179:306–307. doi: 10.1503/cmaj.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104:S21–S26. doi: 10.1038/ajg.2009.48. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med. 2011;171:998–1004. doi: 10.1001/archinternmed.2011.20. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsen B, Vestergaard P. Proton pump inhibitor use and fracture risk - effect modification by histamine H1 receptor blockade. Observational case-control study using National Prescription Data. Bone. 2013;57:269–271. doi: 10.1016/j.bone.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Adams AL, Black MH, Zhang JL, et al. Proton-pump inhibitor use and hip fractures in men: a population-based case-control study. Ann Epidemiol. 2014;24:286–290. doi: 10.1016/j.annepidem.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Arj A, Razavi Zade M, Yavari M, et al. Proton pump inhibitors use and change in bone mineral density. Int J Rheum Dis. 2016;19:864–868. doi: 10.1111/1756-185X.12866. [DOI] [PubMed] [Google Scholar]
- 12.Bahtiri E, Islami H, Hoxha R, et al. Esomeprazole use is independently associated with significant reduction of BMD: 1-year prospective comparative safety study of four proton pump inhibitors. J Bone Miner Metab. 2016;34:571–579. doi: 10.1007/s00774-015-0699-6. [DOI] [PubMed] [Google Scholar]
- 13.Chiu HF, Huang YW, Chang CC, et al. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:1131–1136. doi: 10.1002/pds.2026. [DOI] [PubMed] [Google Scholar]
- 14.Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Heller DA, Ahern FM, et al. The relationship between proton pump inhibitor adherence and fracture risk in the elderly. Calcif Tissue Int. 2014;94:597–607. doi: 10.1007/s00223-014-9855-6. [DOI] [PubMed] [Google Scholar]
- 16.Fraser LA, Leslie WD, Targownik LE, et al. The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporos Int. 2013;24:1161–1168. doi: 10.1007/s00198-012-2112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedberg DE, Haynes K, Denburg MR, et al. Use of proton pump inhibitors is associated with fractures in young adults: a population-based study. Osteoporos Int. 2015;26:2501–2507. doi: 10.1007/s00198-015-3168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women's Health Initiative. Arch Intern Med. 2010;170:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951–959. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- 20.Khalili H, Huang ES, Jacobson BC, et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372. doi: 10.1136/bmj.e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Drake MT, Schleck CD, et al. Incidence and predictors of osteoporotic fractures in patients with Barrett's oesophagus: a population-based nested case-control study. Aliment Pharmacol Ther. 2017;46:1094–1102. doi: 10.1111/apt.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Youn K, Choi NK, et al. A population-based case-control study: proton pump inhibition and risk of hip fracture by use of bisphosphonate. J Gastroenterol. 2013;48:1016–1022. doi: 10.1007/s00535-012-0722-9. [DOI] [PubMed] [Google Scholar]
- 23.Lenihan CR, Sukumaran Nair S, Vangala C, et al. Proton pump inhibitor use and risk of hip fracture in kidney transplant recipients. Am J Kidney Dis. 2017;69:595–601. doi: 10.1053/j.ajkd.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JR, Barre D, Zhu K, et al. Long-term proton pump inhibitor therapy and falls and fractures in elderly women: a prospective cohort study. J Bone Miner Res. 2014;29:2489–2497. doi: 10.1002/jbmr.2279. [DOI] [PubMed] [Google Scholar]
- 25.Lin SM, Yang SH, Liang CC, et al. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: a population-based cohort study. Osteoporos Int. 2018;29:153–162. doi: 10.1007/s00198-017-4262-2. [DOI] [PubMed] [Google Scholar]
- 26.Maggio M, Lauretani F, Ceda GP, et al. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone. 2013;57:437–442. doi: 10.1016/j.bone.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moberg LM, Nilsson PM, Samsioe G, et al. Use of proton pump inhibitors (PPI) and history of earlier fracture are independent risk factors for fracture in postmenopausal women. The WHILA study. Maturitas. 2014;78:310–315. doi: 10.1016/j.maturitas.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Ozdil K, Kahraman R, Sahin A, et al. Bone density in proton pump inhibitors users: a prospective study. Rheumatol Int. 2013;33:2255–2260. doi: 10.1007/s00296-013-2709-0. [DOI] [PubMed] [Google Scholar]
- 29.Pouwels S, Lalmohamed A, Souverein P, et al. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int. 2011;22:903–910. doi: 10.1007/s00198-010-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes C, Formiga F, Coderch M, et al. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone. 2013;52:557–561. doi: 10.1016/j.bone.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Roux C, Goldstein JL, Zhou X, et al. Vertebral fracture efficacy during risedronate therapy in patients using proton pump inhibitors. Osteoporos Int. 2012;23:277–284. doi: 10.1007/s00198-011-1574-5. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH, Diem SJ, Ruppert K, et al. Bone mineral density changes among women initiating proton pump inhibitors or H2 receptor antagonists: a SWAN cohort study. J Bone Miner Res. 2015;30:232–239. doi: 10.1002/jbmr.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Targownik LE, Lix LM, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012;107:1361–1369. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Targownik LE, Goertzen AL, Luo Y, et al. Long-term proton pump inhibitor use is not associated with changes in bone strength and structure. Am J Gastroenterol. 2017;112:95–101. doi: 10.1038/ajg.2016.481. [DOI] [PubMed] [Google Scholar]
- 37.van der Hoorn MMC, Tett SE, de Vries OJ, et al. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: a prospective cohort study. Bone. 2015;81:675–682. doi: 10.1016/j.bone.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 38.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 39.Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 40.Zirk-Sadowski J, Masoli JA, Strain WD, et al. Proton-pump inhibitors and fragility fractures in vulnerable older patients. Am J Gastroenterol. 2017;112:520–523. doi: 10.1038/ajg.2016.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham NS. Proton pump inhibitors: potential adverse effects. Curr Opin Gastroenterol. 2012;28:615–620. doi: 10.1097/MOG.0b013e328358d5b9. [DOI] [PubMed] [Google Scholar]
- 42.de la Coba Ortiz C, Argüelles Arias F, Martin de Argila de Prados C, et al. Proton-pump inhibitors adverse effects: a review of the evidence and position statement by the Sociedad Espanola de Patologia Digestiva. Rev Esp Enferm Dig. 2016;108:207–224. doi: 10.17235/reed.2016.4232/2016. [DOI] [PubMed] [Google Scholar]
- 43.Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4:125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li T, Xie Y, Al-Aly Z. The association of proton pump inhibitors and chronic kidney disease: cause or confounding? Curr Opin Nephrol Hypertens. 2018;27:182–187. doi: 10.1097/MNH.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 45.Costa-Rodrigues J, Reis S, Teixeira S, et al. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. Febs j. 2013;280:5052–5064. doi: 10.1111/febs.12478. [DOI] [PubMed] [Google Scholar]
- 46.Sheraly AR, Lickorish D, Sarraf F, et al. Use of gastrointestinal proton pump inhibitors to regulate osteoclast-mediated resorption of calcium phosphate cements in vivo. Curr Drug Deliv. 2009;6:192–198. doi: 10.2174/156720109787846225. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama T, Watarai K, Oda T, et al. Proton pump inhibitors and fracture: they impair bone quality and increase fall risk? Osteoporos Int. 2016;27:1675–1676. doi: 10.1007/s00198-016-3509-7. [DOI] [PubMed] [Google Scholar]
- 48.Gage BF, Birman-Deych E, Radford MJ, et al. Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2. Arch Intern Med. 2006;166:241–246. doi: 10.1001/archinte.166.2.241. [DOI] [PubMed] [Google Scholar]
- 49.Turner MR, Camacho X, Fischer HD, et al. Levothyroxine dose and risk of fractures in older adults: nested case-control study. BMJ. 2011;342:d2238. doi: 10.1136/bmj.d2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks R, Allegrante JP, Ronald MacKenzie C, et al. Hip fractures among the elderly: causes, consequences and control. Ageing Res Rev. 2003;2:57–93. doi: 10.1016/s1568-1637(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 51.Tolppanen AM, Taipale H, Tanskanen A, et al. Comparison of predictors of hip fracture and mortality after hip fracture in community-dwellers with and without Alzheimer's disease - exposure-matched cohort study. BMC Geriatr. 2016;16:204. doi: 10.1186/s12877-016-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiraev TP, Bullen A. Proton pump inhibitors and cardiovascular events: a systematic review. Heart Lung Circ. 2018;27:443–450. doi: 10.1016/j.hlc.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 53.Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 54.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 55.Malhotra K, Katsanos AH, Bilal M, et al. Cerebrovascular outcomes with proton pump inhibitors and thienopyridines: a systematic review and meta-analysis. Stroke. 2018;49:312–318. doi: 10.1161/STROKEAHA.117.019166. [DOI] [PubMed] [Google Scholar]
- 56.Sukhovershin RA, Cooke JP. How may proton pump inhibitors impair cardiovascular health? Am J Cardiovasc Drugs. 2016;16:153–161. doi: 10.1007/s40256-016-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menzies IB, Mendelson DA, Kates SL, et al. The impact of comorbidity on perioperative outcomes of hip fractures in a geriatric fracture model. Geriatr Orthop Surg Rehabil. 2012;3:129–134. doi: 10.1177/2151458512463392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roche JJ, Wenn RT, Sahota O, et al. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331:1374. doi: 10.1136/bmj.38643.663843.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang CH, Liu CJ, Chen PJ, et al. Hip fracture and risk of acute myocardial infarction: a nationwide study. J Bone Miner Res. 2013;28:404–411. doi: 10.1002/jbmr.1714. [DOI] [PubMed] [Google Scholar]
- 60.Dharmarajan TS, Banik P. Hip fracture. Risk factors, preoperative assessment, and postoperative management. Postgrad Med. 2006;119:31–38. doi: 10.3810/pgm.2006.06.1638. [DOI] [PubMed] [Google Scholar]
- 61.Durand C, Willett KC, Desilets AR. Proton pump inhibitor use in hospitalized patients: is overutilization becoming a problem? Clin Med Insights Gastroenterol. 2012;5:65–76. doi: 10.4137/CGast.S9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209–1218. doi: 10.1038/ajg.2011.113. quiz 19. [DOI] [PubMed] [Google Scholar]
- 63.McGowan B, Bennett K, Barry M. Prescribing of anti-osteoporotic therapies following the use of proton pump inhibitors in general practice. Pharmacoepidemiol Drug Saf. 2010;19:763–769. doi: 10.1002/pds.1972. [DOI] [PubMed] [Google Scholar]
- 64.Fournier MR, Targownik LE, Leslie WD. Proton pump inhibitors, osteoporosis, and osteoporosis-related fractures. Maturitas. 2009;64:9–13. doi: 10.1016/j.maturitas.2009.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest graph showing the outcomes of an overall pooling of all odds ratios, hazard ratios, and relative risks for point estimation depicting relationship between the proton-pump inhibitor (PPI) use and the incidence of fracture. DDD, defined daily dose; SDD, standard daily dose; PDC, proportion of days covered; ES, effect sizes; CI, confidence interval.
A funnel plot showing the outcomes of trim and fill method of publication bias assessment. Theta represents the effect sizes of point estimate ratios.