Abstract
Background and aims
While environmental factors are presumed to be primary drivers of food timing, preliminary evidence suggests that genetics may be an additional determinant. The aim was to explore the relative contribution of genetics and environmental factors to variation in the timing of food timing in a Spanish twin population. Because chronotype, bedtime and wake time are related to food timing, covariance with food timing was further assessed.
Methods
In this observational study, 53 pairs of adult (mean(SD)=52(6.03) years) female twins (28 monozygotic; 25 dizygotic) were recruited from the Murcia Twin Register. Zygosity was determined by DNA-testing. Timing of the three main meals of the day was assessed via 7-day dietary records, and the midpoint of food intake was computed by calculating the midpoint between breakfast and dinner times. Chronotype, bedtime and wake time were self-reported. Heritability of food timing and related traits were estimated by comparing monozygotic and dizygotic twin correlations and fitting genetic structural equation models to measured variables.
Results
We observed genetic influences for food timing, with highest heritability for the midpoint of food intake (64%) in an overweight/obese population (BMI=26.01±3.77). Genetic factors contributed to a higher degree to the timing of breakfast (56%) than the timing of lunch (38%) or dinner (n.s.). Similarly, heritability estimates were larger in behavioral traits earlier on in the day (i.e. wake time, (55%)), than those later on in the day (i.e. bedtime, (38%)). Bivariate analyses revealed a significant genetic overlap between food timing and bedtime and chronotype (rg between .78 and .91).
Conclusions
Genetic influences appear to account for a significant proportion of the variaibility in food timing, particularly breakfast. Thus, interventions related to food timing may be more effective when targeting afternoon/evening traits, such as lunch or dinner times. Furthermore, our data suggest shared genetic architecture underlying food timing and phenotypically related traits.
Clinical trial
NCT03059576. https://clinicaltrials.gov/ct2/show/NCT03059576
Keywords: Food timing, Dietary intake, Heritability, Twins
Introduction
Secular trends from national surveys indicate shifts in the timing of food intake towards later timing [1]. This late eating habit has been associated with adverse health outcomes such as estimated higher odds of being overweight/obese [2, 3] and impaired glucose tolerance and insulin secretion [4, 5]. Moreover, later consumption of the main meals of the day, as determined by self-reported food timing, has also been shown to hinder weight loss during a dietary intervention [6, 7] and following bariatric surgery [8]. Adverse effects of later meals have also been suggested by experimental studies. In randomized, crossover studies, it was shown that a later lunch decreases resting-energy expenditure, fasting carbohydrate oxidation and glucose tolerance [5, 9, 10], later dinner times worsens postprandial glucose profiles for the following morning’s breakfast [11], and later consumption of the main meal of the day inverts the salivary microbiota 24-rhythm [12]. Moreover, to include a high-energy breakfast plus a low-energy dinner reduced metabolic risk compared with a meal pattern with a low-energy breakfast plus a high-energy dinner [13].
These recent findings emphasize the importance of food timing as a novel dimension in nutrition science [6, 14, 15]. Indeed, the timing of food intake is newly proposed as a modifiable risk factor for weight management and chronic disease prevention [16]. As food timing is likely a complex trait, like food composition [17], elucidating the genetic and environmental components that contribute to the variability in food timing for individual eating episodes is necessary. Unraveling those components is relevant in designing more effective and individually tailored therapeutic strategies related to food timing and developing public health initiatives tackling later food intake and understanding biological pathways regulating decisions related to food timing [18, 19]. Whereas environmental determinants of food timing such as chronotype, caloric density [20], and sleep [21–24], have been explored in epidemiological studies, genetics remains under-investigated [9].
Thus far, only a single study has investigated the heritability of food timing [25]. In that twin study from the United States, the highest heritability was observed for the timing of breakfast (24%), while lunch and dinner timing showed lower heritability estimates (ranging from 18–22%) [25]. Other related studies provide additional support for the putative genetic component of food timing. For instance, genetic influences have also been suspected for night eating syndrome (NES) and sleep-related eating disorder (SRED), two eating disorders with evening eating preference [26]. We have previously reported an association between a genetic variant in CLOCK (rs4580704) and lunch time [6]. Moreover, we reported that food timing modifies the association between a genetic variant in the PLIN locus and the efficacy of a weight loss intervention [27]. In addition, no study to our knowledge has investigated the genetics of food timing along with closely related heritable traits that may explain the metabolic implications of later food intake and unravel shared genetic architecture among those traits.
Findings from twin studies have indicated that genetics plays a major role in several diet-related phenotypes including energy and macronutrient intakes, dietary patterns, and the intake of specific foods [28]. Twins provide a naturally unique case-control experiment whereas the classical twin design compares the similarity of identical/monozygotic (MZ) and dizygotic (DZ) twins. Genetics are implicated in the investigated trait when MZ twins are observed to be considerably more similar than DZ twins. The aim of our current investigation was to explore the relative contribution of genetics and environmental factors to variation in the timing of the three main meals of the day (i.e., breakfast, lunch and dinner) in a twin population. Because chronotype, bedtimes and wake times are related to food timing, co-variation with these traits was further assessed.
Methods
Subjects
In this observational study, a sample of female twins selected from the Murcia Twin Register (MTR) participated in this study. The MTR is a population-based registry of people born between 1940 and 1966 in the region of Murcia, southeast Spain. The twin pairs that form the MTR are assumed to be representative of the general Spanish population [29]. The registry has collected information from >2200 individual twins. More detailed description regarding characteristics and procedures of the MTR can be found elsewhere [30, 31]. Written informed consent was obtained from all participants. The Committee of Research Ethics of the University of Murcia has approved MTR data collection procedures and management; the protocol follows national regulations regarding personal data protection.
Using a regional health system database, female pairs living within the same geographical area, and within a 30-km radius from the recruitment center, and free from severe health condition that may impede or hinder participation such as cognitive disorders, diabetes mellitus, chronic renal failure, hepatic diseases or cancer were selected for inclusion in this study. A total of 118 twin pairs were recontacted (between 2012 and 2014), and a total of 53 pairs of adult female twins (N=106) volunteered for this study (28 MZ; 25 DZ). This sample size has been shown to be enough to assess the heritability of cronotype and other related features [32, 33]. Zygosity was confirmed by DNA testing.
Timing of food intake
The primary outcome of the present study was the timing of food intake. The timing of food intake was self-reported via a 7-day food record. Specifically, participants recorded the start time, finish time, and duration of individual food intake episodes during 5 weekdays and 2 weekend days. Midpoint of intake was ascertained by calculating the midpoint between breakfast and dinner times (first and last eating episode). Participants were instructed and trained on how to accurately complete the food records at the start of the study, and collected data were later reviewed with a technician.
Sleep and Chronotype
Participants also recorded information related to sleep including bedtime and wake time during the same 7-day period. Chronotype was assessed using the Morningness-Eveningness (ME) questionnaire, a 19-item scale developed by Horne and Östberg, and an ME score was computed [34]. A higher ME score reflects more morning (earlier) chronotype.
General characteristics of the sample/subjects and procedures
Body weight was estimated in barefooted participants wearing light clothes using a digital scale accurate to the nearest 0.1 kg. Height was determined using a portable stadiometer (rank, 0.14–2.10) and subjects were positioned upright, relaxed, and with the head in the Frankfort plane. Body Mass Index (BMI) was calculated by weight (kg) divided by height (m2). Total body fat was determined by bioelectrical impedance, using TANITA TBF-300 (Tanita Corporation of America, Arlington Heights, IL, USA) equipment. In addition, waist to hip ratio was calculated using waist circumference (cm), at level of the umbilicus, and hip circumference (cm) [35].
Statistical Analyses
First, differences between MZ and DZ general characteristics were assessed by t-test. Heritability analysis was based on the basic logic of twin studies and can be summarized as follows: MZ twins (identical) share 100% of their genetic makeup, while DZ twins (non-identical) share on average 50% of their segregating genes [36]. Comparing the resemblance (correlation) of MZ twins for a trait with the resemblance of DZ twins for that trait the total variance of a trait can be partitioned into genetic and environmental factors, following a variance components approach. Observed MZ and DZ correlations generally reflect a combination of additive (A; i.e., summed allelic effects across multiple genes) and non-additive (D; i.e., genetic dominance, possibly including epistasis) genetic factors, as well as shared (C; i.e., common/family environment) and individual (E; i.e., idiosyncratic experiences, including measurement error) environmental factors. A greater phenotypic resemblance in MZ twin pairs compared with DZ twin pairs must be due to genetic influences (A or D components), considering the assumption that both MZ and DZ twins are exposed to equal shared environments during childhood [37]. It is not possible to estimate C and D simultaneously in a classical twin model and the choice of modelling C or D depends on the pattern of MZ and DZ correlations; usually C is estimated if the DZ twin correlation is more than half of the MZ twin correlation (ACE model), and D is estimated if the DZ twin correlation is less than half of the MZ correlation (ADE model) [38].
Structural equation models (SEM) offer a precise way to estimate the variance explained by each of the latent components (A, C, D and E) and determines the combination that best matches the observed data. For each variable, the full models (ACE/ADE) were estimated and tested against nested sub-models, where A component, C/D component or both (AC/AD) were fixed to zero. The log-likelihood ratio test (LRT) was used to compare the fit of the different models and sub-models. The difference in minus two times the log-likelihood (−2LL) between two models has a χ2 distribution with the degrees of freedom (df) equaling the difference in df between the two models. Additionally, model fit was evaluated using Akaike’s information criterion (AIC) which is a parsimony-adjusted statistic used to select among competing models.
In the present study, all SEM were fitted to the raw data employing the full information maximum likelihood (FIML) method within the Open-Mx package v2.7.9 [39] for R v3.3.3 [40]. The accuracy of the obtained parameters was assessed using likelihood-based 95% confidence intervals. Effect of age was regressed out from the raw scores using also the FIML procedure in Open-Mx. Subsequently, SEM were fitted to the residual scores. Data preparation and descriptive analyses were performed in SPSS v19 [41].
Results
The MTR population included in the present study comprised of 53 adult female twin pairs (n=106) with overweight/obesity (BMI= 26.01±3.77) and their general characteristics are presented in Table 1. Mean age of the selected participants was 52 years (SD: 6.0; Range: 46–69). Mean timing of food intake was 8:43±00:53 for breakfast, 14:53±00:31 for lunch, and 21:29±00:41 for dinner. The mean midpoint of intake was estimated at 15:20±00:36. Significant weekday and weekend differences were observed for breakfast timing only. The timing of breakfast was significantly earlier on weekdays (8:33±1:03) compared to weekends (9:12±1:06) (P=0.001). No significant differences were observed between MZ and DZ twins for food timing. Furthermore, no differences were observed between the two groups for anthropometric measures, sleep timing, and chronotype.
Table 1.
General characteristics of 53 twin pairs.
| Monozygotic (n =56) |
Dizygotic (n =50) |
p values |
|
|---|---|---|---|
| Age (years) | 51±6 | 53±6 | 0.066 |
| Weight (kg) | 64.12±8.56 | 63.44±7.91 | 0.370 |
| Height (cm) | 156.43±6.84 | 157.52±5.61 | 0.369 |
| BMI (kg/m) | 26.30±3.89 | 25.66±3.65 | 0.404 |
| Body fat (%) | 32.99±5.89 | 32.96±6.72 | 0.979 |
| Waist (cm) | 90.56±8.76 | 90.08±10.66 | 0.805 |
| Hip (cm) | 103.68±7.15 | 102.39±8.10 | 0.379 |
| WHR | 1.15±0.06 | 1.14±0.09 | 0.742 |
| Timing of food intake | |||
| Breakfast | 08:49±00:54 | 08:36±00:52 | 0.209 |
| Lunch | 14:31±00:33 | 14:32±00:30 | 0.904 |
| Dinner | 21:36±00:40 | 21:22±00:41 | 0.072 |
| Midpoint of intake | 15:16±00:32 | 15:23±00:40 | 0.335 |
| Sleep | |||
| Wake-time (hh:mm) | 07:33±01:09 | 07:38±01:00 | 0.684 |
| Bed-time (hh:mm) | 24:18±00:56 | 24:28±00:59 | 0.288 |
| Chronotype score | 55.21±8.67 | 56.44±7.56 | 0.442 |
Data are represented as means ± SD.
Abbreviations: BMI, body mass index, WHR, waist-to-hip ratio
MZ twins showed higher intra-pair correlations than DZ twins for breakfast and lunch timing, but not for dinner timing. In addition, MZ twins showed higher intra-pair correlations than DZ twins for wake and bed times and chronotype (Table 2). AE models, where phenotypic variance is explained by additive genetic and non-shared environmental factors, showed the best fit in every case. The only exception was for dinner timing, where a CE model showed a better fit accordingly to the higher DZ correlation compared to MZ (Table 3).
Table 2.
Twin intra-pair correlations with 95% CI for timing of food intake and related traits
| Intra-pair correlation coefficients | ||
|---|---|---|
|
| ||
| r MZ (CI 95%) |
r DZ (CI 95%) |
|
| Food intake timing | ||
| Breakfast timing | 0.56 (0.26, 0.74) | 0.29 (−0.12, 0.59) |
| Lunch timing | 0.40 (0.06, 0.63) | 0.15 (−0.26, 0.50) |
| Dinner timing | 0.36 (−0.03, 0.63) | 0.42 (0.08, 0.66) |
| Midpoint of food intake | 0.64 (0.39, 0.79) | 0.438 (−0.16, 0.61) |
|
| ||
| Sleep and wake timing | ||
| Wake timing | 0.54 (0.26, 0.73) | 0.37 (−0.06, 0.65) |
| Bed timing | 0.42 (0.10, 0.65) | 0.02 (−0.36, 0.40) |
|
| ||
| Chronotype (MEQ) | 0.42 (0.11, 0.64) | 0.23 (−0.22, 0.57) |
r MZ: monozygotic intra-pair correlation coefficient, r DZ: dizygotic intra-pair correlation coefficient, CI (95%): confidence interval, MEQ; Morning-Evening Questionnaire.
Table 3.
Model-fitting results for univariate models, and proportions of variance (parameter estimates) explained by additive genetic influences (A), shared-environmental (C) and residual variation (E) with 95% confidence intervals (CI).
| Goodness-of-fit index | Parameter estimates (CI = 95%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Model | −2LL | df | AIC | ΔX2 | Δdf | p | A | C/D | E | |
| Breakfast timing | ACE | 258.11 | 101 | 56.11 | - | 0.53 (0, 0.74) | 0.02 (0, 0.59) | 0.45 (0.26, 0.74) | ||
| AE | 258.12 | 102 | 54.12 | 0.003 | 1 | .954 | 0.56 (0.28, 0.74) | -- | 0.44 (0.26, 0.72) | |
| CE | 259.78 | 102 | 55.78 | 1.67 | 1 | .196 | 1 | |||
| E | 271.01 | 103 | 65.01 | 12.89 | 1 | < .001 | 1 | |||
|
| ||||||||||
| Lunch timing | ADE | 155.69 | 101 | −46.31 | 0.21 (0, 0.62) | 0.19 (0, 0.63) | 0.60 (0.37, 0.92) | |||
| AE | 155.74 | 102 | −48.26 | 0.05 | 1 | .830 | 0.38 (0.07, 0.62) | -- | 0.62 (0.38, 0.93) | |
| E | 161.46 | 103 | −44.54 | 5.72 | 1 | .017 | 1 | |||
|
| ||||||||||
| Dinner timing | ACE | 205.29 | 101 | 3.29 | - | 0 (0, 0.60) | 0.39 (0, 0.60) | 0.61 (0.37, 0.86) | ||
| AE | 206.97 | 102 | 2.97 | 1.68 | 1 | .195 | 1 | |||
| CE | 205.29 | 102 | 1.29 | < 0.01 | 1 | 1 | -- | 0.39 (0.14, 0.59) | 0.61 (0.40, 0.86) | |
| E | 214.17 | 103 | 8.17 | 8.88 | 1 | .003 | 1 | |||
|
| ||||||||||
| Midpoint of food intake | ADE | 168.56 | 101 | −33.44 | 0.57 (0, 0.79) | 0.07 (0, 0.85) | 0.36 (0.21, 0.60) | |||
| AE | 168.57 | 102 | −35.43 | 0.008 | 1 | .930 | 0.64 (0.40, 0.79) | -- | 0.36 (0.21, 0.60) | |
| E | 187.79 | 103 | −18.21 | 19.225 | 1 | <.0001 | - | - | 1 | |
|
| ||||||||||
| Wake timing | ACE | 299.92 | 101 | 97.92 | - | 0.34 (0, 0.72) | 0.20 (0, 0.64) | 0.46 (0.27, 0.73) | ||
| AE | 300.17 | 102 | 96.17 | 0.25 | 1 | .618 | 0.55 (0.29, 0.73) | -- | 0.45 (0.27, 0.70) | |
| CE | 300.64 | 102 | 96.64 | 0.72 | 1 | .395 | 1 | |||
| E | 314.24 | 103 | 108.24 | 14.08 | 1 | < .001 | 1 | |||
|
| ||||||||||
| Bed timing | ADE | 287.83 | 101 | 85.83 | 0 (0, 0.61) | 0.42 (0, 0.65) | 0.58 (0.35, 0.91) | |||
| AE | 288.44 | 102 | 84.44 | 0.61 | 1 | .436 | 0.38 (0.06, 0.63) | -- | 0.62 (0.37, 0.94) | |
| E | 293.80 | 103 | 87.80 | 5.36 | 1 | .021 | 1 | |||
|
| ||||||||||
| Chronotype | ACE | 729.27 | 101 | 527.27 | - | 0.38 (0, 0.64) | 0.04 (0, 0.55) | 0.58 (0.35, 0.88) | ||
| AE | 729.28 | 102 | 525.28 | 0.01 | 1 | .922 | 0.43 (0.13, 0.64) | -- | 0.57 (0.35, 0.86) | |
| CE | 729.90 | 102 | 525.90 | 0.62 | 1 | .430 | 1 | |||
| E | 736.99 | 103 | 530.99 | 7.09 | 1 | .008 | 1 | |||
−2LL: twice negative log-likelihood; df: degrees of freedom; AIC: Akaike Information Criterion; ΔX2: difference in X2 to full model; Δdf: difference in degrees of freedom to full model. Bold values indicate best fitting model.
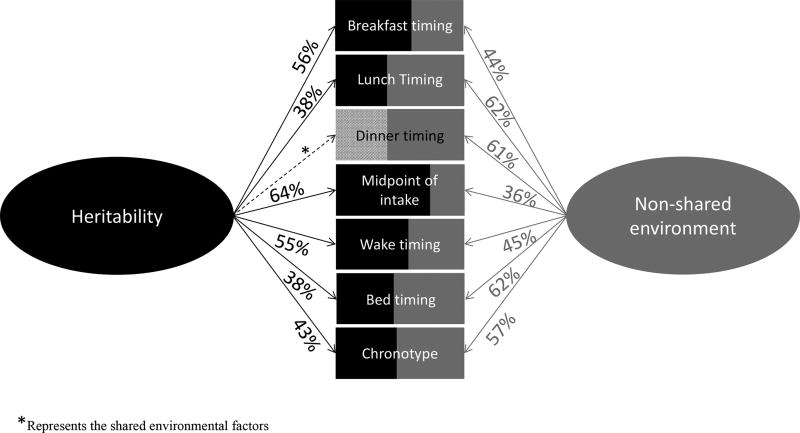
Higher heritability was observed for investigated traits made earlier on in the day (Figure 1). Indeed, heritability was higher for the timing of breakfast (56%) compared to lunch (38%), and the timing of dinner was not determined to be heritable. Similarly, the heritability of wake time was higher (55%) compared to bedtime (38%). Furthermore, we observed the highest overall heritability for the midpoint of food intake (64%).
Figure 1.
Broad heritability and environmental effect estimates for food timing and related variables analyzed. The rectangles represent the contribution (percentage) of heritability (A: additive genetic factor + D: non-additive genetic factors) in black and non-shared environmental factors (E) in grey of the different variables. The asterisk represents the share environmental factors (C) in diagonals lines.
Further bivariate analyses for midpoint of food intake and the other timing-related factors – sleep timing and chronotype – rendered high genetic correlation estimates in the range of 0.78 and 0.91. Environmental correlations, however, were smaller and non-significant (Table 4). Hence 85% of the covariance between midpoint of intake and chronotype could be attributed to common genetic variation. Genetic contribution to covariance between midpoint of intake and wake and bed time was 90% and 75%, respectively.
Table 4.
Phenotypic (rP), genetic (rG), and unique environmental (rE) correlations from bivariate AE models for midpoint food intake and circadian-timing related traits.
| Midpoint of food intake | |||
|---|---|---|---|
|
|
|||
| rP (CI 95%) |
rG (CI 95%) |
rE (CI 95%) |
|
| Sleep and wake timing | |||
| Wake timing | 0.56 (0.40, 0.69) | 0.79 (0.53, 1.00) | 0.15 (−0.17, 0.45) |
| Bed timing | 0.53 (0.37, 0.66) | 0.78 (0.41, 1.00) | 0.28 (−0.55, 0.56) |
|
| |||
| Chronotype (MEQ) | −0.45 (−0.60, −0.27) | −0.91 (−1.00, −0.60) | 0.23 (−0.10, 0.49) |
MEQ: Morning-Evening Questionnaire
Discussion
The present study provides supporting evidence that the timing of food intake is indeed heritable, and thus has an underlying genetic component. We observe that the estimated heritability of food timing varies by meal, and ranges from 56% for breakfast to non-significant heritability for dinner. Heritability estimates are higher for meals earlier on in the day (breakfast), than later on in the day (lunch and dinner). Similarly, heritability plays a larger role in other behaviors specific to the morning, such as wake times. Conversely, the environmental component is larger for the timing of dinner and other evening behaviors, such as bedtime. This variation in heritability suggests that interventions geared towards modifying behaviors later on in the day, and those less predetermined by genetics, may be more successful. Lastly, bivariate analyses for midpoint of food intake and sleep timing and chronotype suggest shared genetic architecture and likely common biological pathways underlying those phenotypically related traits.
Our data support the simultaneous interplay between genetic and environmental factors in contrast to earlier presumptions that the timing of food intake is determined by cultural factors alone. In twin studies, any learned habit should have an equal effect on MZ and DZ pairs and as such should have produced a significant effect of common (familial) environment in the analysis. Because the adult twins participants in the present study live separately and away from the familial environment, the higher intra-pair correlations found in MZ siblings suggests that food timing, like food composition [17], is a heritable trait.
Our results show that the timing of intake for breakfast, lunch, and dinner are differentially influenced by genetics with higher heritability for meals earlier on in the day, confirming previous results [25]. Consistent with the timing of meals, we also observe that other traits related to later on in the day tend to be more driven by environmental factors. Secular trends from US national surveys indicate shifts in food timing. For example, data from the National Health and Nutrition Examination Survey analyzing over a 40-year span from 1971–1974 to 2009–2010 observed later intakes of breakfast, snacks between breakfast and lunch, lunch, and snacks between lunch and dinner (among men), in addition to earlier intakes of snacks after dinner in 2009–2010 compared to 1971–1974 [42]. Our heritability results suggest that intervening for the purpose of advancing late lunch and dinner may be more achievable than changing breakfast time. Moreover, it is not clearly demonstrated that breakfast timing may impact health, but rather the prolongation of an overnight fast, which depends on the timing of both the first and the last meal, may be beneficial [43]. Our previous study on weight loss showed that a delayed breakfast time was not significantly associated with lower weight loss effectiveness [6]. Nevertheless, other breakfast habits such as skipping breakfast [44–46] or a lower energy intake at breakfast relative to at dinner [13, 47] may yield adverse metabolic consequences. Thus, targeting the timing of breakfast intake might be less effective than targeting the timing of lunch and dinner for the purpose of achieving overall health: first because of its genetic influence and, second due to its unclear health benefits. By contrast, targeting the timing of lunch and dinner may be crucial as the timing of lunch has been observed to associate with weight loss success [6], and late or night-time eating was found to be linked to night-time hunger, body image distortions, and mood disorders [48], as well as elevated fasting blood levels of insulin and glucose that characterize metabolic syndrome [49].
In the current work, we aimed to study the relationship between the heritability of the timing of food intake and other phenotypically related traits, particularly sleep timing and chronotype. These traits have been associated with food timing in epidemiological studies [6, 24, 42, 50]. There is also evidence for the heritability of sleep rhythms [51] and chronotype [52, 53]. Our results confirm the importance of genetic factors for sleep timing phenotypes and chronotype. We detect moderate heritability for wake and bed times (55% and 38%, respectively), and for chronotype (43%), corroborating previous studies [52–56]. Furthermore, when analyzing the genetic and environmental contribution to covariation between those variables, we find high and significant genetic correlations (.78 to .91) of the timing of food intake (midpoint) with sleep timing and chronotype. The genetic contribution to phenotypic correlation was 3–5 fold larger than that of the environment. Such outcome indicates that it is likely that a common set of genes underlies timing decisions regarding food intake, sleep timing and chronotype. Thus, future analyses in population-based studies equipped with genome-wide genetic data are warranted to confirm these genetic correlations.
Our results on the high heritability in food timing may be surprising, considering anecdotal evidence that food timing is driven primarily by cultural factors. However, studies performed under laboratory conditions with a protocol that controlled for several behaviors, including meal content and sleep periods, showed that the internal circadian clock controls the temporality of hunger and appetite independent of other behaviors [57]. Moreover, a recent study has demonstrated that adipose tissue specific deletion of BMAL1, a core molecular clock component, is able to impact the timing of food intake in mice [58]. Both studies indicate that the temporality of food intake is influenced by the internal circadian clock.
The present findings on the relative contribution of genetics and environmental factors to the timing of different meals may have relevance to the prevention and treatment of metabolic disorders considering the emerging evidence implicating food timing with metabolic diseases. Later timing of food intake has been associated with: (a) a substantial increase in the odds of being overweight/obese [2]; whereas, a later endogenous circadian timing of food intake, relative to melatonin onset, has been associated with increased body fat [3] (b) weight loss impairment during a dietary intervention [6, 47] and following bariatric surgery [8]; (c) and decreased insulin sensitivity [6, 47]. In addition, a large epidemiological study performed in 61,364 participants showed that late-night dinner consumption is associated with hyperglycemia, independent of relevant confounders, including BMI [59].
Some limitations need to be considered when interpreting the results of our study. Food and sleep timing were self-reported and are prone to measurement error, however these self-reported measures were previously found to be associated with metabolic diseases and weight-loss difficulty [6, 8, 9]. Furthermore, timing is a single dimension of diet. Finally, our study was limited to adult female twin pairs in Spain, and thus findings may not be generalizable to individuals of different gender, age, and BMI groups.
In conclusion, our data support that genetics may account for a large proportion of the variation in food timing, particularly for breakfast, whereas the environment appears to be a more important determinant of lunch and dinner timing. These results suggest that intervention studies targeting food timing may be most effective if focused on modifiable factors later on in the day, such as lunch or dinner, rather than breakfast. In addition, future efforts should attempt to unravel specific genetic variants associated with food timing and disclose the shared genetic architecture underlying food timing and phenotypically related traits.
Acknowledgments
We are grateful to the people from the Murcia Twin Register who participated in this experiment.
Founding Sources
This work has been supported in part by The Spanish Government of Science and Innovation (Project No. SAF2014-52480-R) including FEDER co-funding, and NIDDK R01DK105072 granted to M. Garaulet. The Ministry of Economy and Competitiveness and the Instituto de Salud Carlos III – RETICEF (The Ageing and Frailty Cooperative Research Network, RD12/0043/0011), the Ministry of Education and Science and the Ministry of Economy and Competitiveness (BFU2010-21945-C02-01, IPT-2011-0833-900000), including FEDER co-funding granted to J. A. Madrid. The Murcia Twin Register is supported by the Seneca Foundation, the Regional Agency for Science and Technology, Murcia, Spain (19479/PI/2014) and the Ministry of Economy and Competitiveness, Spain (PSI2014-56680-R) including FEDER co-funding granted to J. R. Ordoñana. FAJLS was supported in part by NHLBI R01 HL094806, NHLBI R01 HL118601, NIDDK R01 DK099512, and RS and FAJLS were supported in part by NIDDK R01 DK102696 and NIDDK R01DK105072.
Abbreviations
- CLOCK
Circadian Locomotor Output Cycles Kaput
- MZ
Monozygotic
- DZ
Dizygotic
- MTR
Murcia Twin Register
- BMI
Body Mass Index
- ME
Morningness-Eveningness
- SEM
Structural Equation Models
- FIML
Full Information Maximum Likelihood
Footnotes
Conflict of interest: The authors declare no conflict of interest
Statement of authorship
Jesus Lopez-Minguez conducted research, analyzed data and wrote the paper; Hassan S Dashti analyzed data and wrote the paper, Juan J Madrid-Valero performed statistical analyses, Juan A Madrid analyzed data, Richa Saxena wrote the paper, Frank A Scheer wrote the paper, Juan R Ordoñana designed research, performed statistical analyses and wrote the paper, Marta Garaulet designed research, analyzed data and wrote the paper.
References
- 1.Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet. 2015;115:50–63. doi: 10.1016/j.jand.2014.06.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2014;27(Suppl 2):255–62. doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 3.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, et al. Later circadian timing of food intake is associated with increased body fat. The American journal of clinical nutrition. 2017 doi: 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan LM, Shi JW, Hampton SM, Frost G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. The British journal of nutrition. 2012;108:1286–91. doi: 10.1017/S0007114511006507. [DOI] [PubMed] [Google Scholar]
- 5.Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A, Fedele D, et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int J Obes (Lond) 2015;39:1689–95. doi: 10.1038/ijo.2015.138. [DOI] [PubMed] [Google Scholar]
- 6.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–11. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versteeg RI, Ackermans MT, Nederveen AJ, Fliers E, Serlie MJ, la Fleur SE. Meal timing effects on insulin sensitivity and intrahepatic triglycerides during weight loss. Int J Obes (Lond) 2018;42:156–62. doi: 10.1038/ijo.2017.199. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Lozano T, Vidal J, de Hollanda A, Scheer FA, Garaulet M, Izquierdo-Pulido M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin Nutr. 2016;35:1308–14. doi: 10.1016/j.clnu.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Bandin C, Scheer FA, Luque AJ, Avila-Gandia V, Zamora S, Madrid JA, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int J Obes (Lond) 2015;39:828–33. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 10.Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life sciences. 2003;73:2467–75. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida Y, Hata S, Sone Y. Effects of a late supper on digestion and the absorption of dietary carbohydrates in the following morning. Journal of physiological anthropology. 2013;32:9. doi: 10.1186/1880-6805-32-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collado MC, Engen PA, Bandin C, Cabrera-Rubio R, Voigt RM, Green SJ, et al. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2018 doi: 10.1096/fj.201700697RR. fj201700697RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakubowicz D, Wainstein J, Ahren B, Bar-Dayan Y, Landau Z, Rabinovitz HR, et al. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia. 2015;58:912–9. doi: 10.1007/s00125-015-3524-9. [DOI] [PubMed] [Google Scholar]
- 14.Grant CL, Coates AM, Dorrian J, Kennaway DJ, Wittert GA, Heilbronn LK, et al. Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiology international. 2017:1–11. doi: 10.1080/07420528.2017.1335318. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Yasuda J, et al. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. European journal of applied physiology. 2013;113:2603–11. doi: 10.1007/s00421-013-2702-z. [DOI] [PubMed] [Google Scholar]
- 16.St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2017;135:e96–e121. doi: 10.1161/CIR.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. The American journal of clinical nutrition. 2013;97:1395–402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danilenko KV, Plisov IL, Hebert M, Krauchi K, Wirz-Justice A. Influence of timed nutrient diet on depression and light sensitivity in seasonal affective disorder. Chronobiology international. 2008;25:51–64. doi: 10.1080/07420520801903976. [DOI] [PubMed] [Google Scholar]
- 19.Nohara K, Yoo SH, Chen ZJ. Manipulating the circadian and sleep cycles to protect against metabolic disease. Front Endocrinol (Lausanne) 2015;6:35. doi: 10.3389/fendo.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutrition research (New York, NY) 2014;34:930–5. doi: 10.1016/j.nutres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossavar-Rahmani Y, Weng J, Wang R, Shaw PA, Jung M, Sotres-Alvarez D, et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueno ancillary study. Journal of sleep research. 2017 doi: 10.1111/jsr.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil J, Forest G, Hintze LJ, Brunet JF, Finlayson G, Blundell JE, et al. The effects of partial sleep restriction and altered sleep timing on appetite and food reward. Appetite. 2017;109:48–56. doi: 10.1016/j.appet.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Komada Y, Narisawa H, Ueda F, Saito H, Sakaguchi H, Mitarai M, et al. Relationship between Self-Reported Dietary Nutrient Intake and Self-Reported Sleep Duration among Japanese Adults. Nutrients. 2017;9 doi: 10.3390/nu9020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broussard JL, Van Cauter E. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23:353–9. doi: 10.1097/MED.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Castro JM. Heritability of diurnal changes in food intake in free-living humans. Nutrition. 2001;17:713–20. doi: 10.1016/s0899-9007(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 26.Lundgren JD, Allison KC, Stunkard AJ. Familial aggregation in the night eating syndrome. The International journal of eating disorders. 2006;39:516–8. doi: 10.1002/eat.20269. [DOI] [PubMed] [Google Scholar]
- 27.Garaulet M, Vera B, Bonnet-Rubio G, Gomez-Abellan P, Lee YC, Ordovas JM. Lunch eating predicts weight-loss effectiveness in carriers of the common allele at PERILIPIN1: the ONTIME (Obesity, Nutrigenetics, Timing, Mediterranean) study. The American journal of clinical nutrition. 2016;104:1160–6. doi: 10.3945/ajcn.116.134528. [DOI] [PubMed] [Google Scholar]
- 28.Pallister T, Spector TD, Menni C. Twin studies advance the understanding of gene-environment interplay in human nutrigenomics. Nutr Res Rev. 2014;27:242–51. doi: 10.1017/S095442241400016X. [DOI] [PubMed] [Google Scholar]
- 29.Ordonana JR, Sanchez Romera JF, Colodro-Conde L, Carrillo E, Gonzalez-Javier F, Madrid-Valero JJ, et al. The Murcia Twin Registry. A resource for research on health-related behaviour. Gac Sanit. 2017 doi: 10.1016/j.gaceta.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Ordonana JR, Perez-Riquelme F, Gonzalez-Javier F, Carrillo E, Gomez-Amor J, Martinez-Selva JM. An initiative in Spain for the study of women's health: the Murcia Twin Registry. Twin Res Hum Genet. 2006;9:865–7. doi: 10.1375/183242706779462534. [DOI] [PubMed] [Google Scholar]
- 31.Ordonana JR, Rebollo-Mesa I, Carrillo E, Colodro-Conde L, Garcia-Palomo FJ, Gonzalez-Javier F, et al. The Murcia Twin Registry: a population-based registry of adult multiples in Spain. Twin Res Hum Genet. 2013;16:302–6. doi: 10.1017/thg.2012.66. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Minguez J, Ordonana JR, Sanchez-Romera JF, Madrid JA, Garaulet M. Circadian system heritability as assessed by wrist temperature: A twin study. Chronobiology international. 2014:1–10. doi: 10.3109/07420528.2014.955186. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Minguez J, Morosoli JJ, Madrid JA, Garaulet M, Ordonana JR. Heritability of siesta and night-time sleep as continuously assessed by a circadian-related integrated measure. Scientific reports. 2017;7:12340. doi: 10.1038/s41598-017-12460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International journal of chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- 35.Ferrario VF, Sforza C, Schmitz JH, Miani A, Jr, Taroni G. Fourier analysis of human soft tissue facial shape: sex differences in normal adults. J Anat. 1995;187(Pt 3):593–602. [PMC free article] [PubMed] [Google Scholar]
- 36.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–82. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 37.Verweij KJ, Mosing MA, Zietsch BP, Medland SE. Estimating heritability from twin studies. Methods Mol Biol. 2012;850:151–70. doi: 10.1007/978-1-61779-555-8_9. [DOI] [PubMed] [Google Scholar]
- 38.M N, L C. Methodology for genetic studies of twins and families. 1992 [Google Scholar]
- 39.Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, et al. OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika. 2016;81:535–49. doi: 10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2016. [Google Scholar]
- 41.Arbuckle JL. IBM SPSS® Amos™ 19 User’s Guide. 1995 [Google Scholar]
- 42.Holmback U, Forslund A, Lowden A, Forslund J, Akerstedt T, Lennernas M, et al. Endocrine responses to nocturnal eating--possible implications for night work. Eur J Nutr. 2003;42:75–83. doi: 10.1007/s00394-003-0386-6. [DOI] [PubMed] [Google Scholar]
- 43.Zilberter T, Zilberter EY. Breakfast: to skip or not to skip? Front Public Health. 2014;2:59. doi: 10.3389/fpubh.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Lippevelde W, Te Velde SJ, Verloigne M, Van Stralen MM, De Bourdeaudhuij I, Manios Y, et al. Associations between family-related factors, breakfast consumption and BMI among 10- to 12-year-old European children: the cross-sectional ENERGY-study. PloS one. 2013;8:e79550. doi: 10.1371/journal.pone.0079550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.So HK, Nelson EA, Li AM, Guldan GS, Yin J, Ng PC, et al. Breakfast frequency inversely associated with BMI and body fatness in Hong Kong Chinese children aged 9–18 years. The British journal of nutrition. 2011;106:742–51. doi: 10.1017/S0007114511000754. [DOI] [PubMed] [Google Scholar]
- 46.Laska MN, Murray DM, Lytle LA, Harnack LJ. Longitudinal associations between key dietary behaviors and weight gain over time: transitions through the adolescent years. Obesity (Silver Spring) 2012;20:118–25. doi: 10.1038/oby.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21:2504–12. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 48.Boseck JJ, Engel SG, Allison KC, Crosby RD, Mitchell JE, de Zwaan M. The application of ecological momentary assessment to the study of night eating. The International journal of eating disorders. 2007;40:271–6. doi: 10.1002/eat.20359. [DOI] [PubMed] [Google Scholar]
- 49.Allison KC, Ahima RS, O'Reardon JP, Dinges DF, Sharma V, Cummings DE, et al. Neuroendocrine profiles associated with energy intake, sleep, and stress in the night eating syndrome. The Journal of clinical endocrinology and metabolism. 2005;90:6214–7. doi: 10.1210/jc.2005-1018. [DOI] [PubMed] [Google Scholar]
- 50.Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, et al. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PloS one. 2013;8:e56519. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 52.Vink JM, Groot AS, Kerkhof GA, Boomsma DI. Genetic analysis of morningness and eveningness. Chronobiology international. 2001;18:809–22. doi: 10.1081/cbi-100107516. [DOI] [PubMed] [Google Scholar]
- 53.Lane JM, Vlasac I, Anderson SG, Kyle SD, Dixon WG, Bechtold DA, et al. Genome wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Commun. 2016;7:10889. doi: 10.1038/ncomms10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hur Y-M. Stability of genetic influence on morningness–eveningness: a cross-sectional examination of South Korean twins from preadolescence to young adulthood. Journal Sleep Research. 2007 doi: 10.1111/j.1365-2869.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 55.Sletten TL, Rajaratnam SM, Wright MJ, Zhu G, Naismith S, Martin NG, et al. Genetic and environmental contributions to sleep-wake behavior in 12-year-old twins. Sleep. 2013;36:1715–22. doi: 10.5665/sleep.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 57.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013;21:421–3. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nature medicine. 2012;18:1768–77. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakajima K, Suwa K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J Diabetes Metab Disord. 2015;14:16. doi: 10.1186/s40200-015-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]