Summary
Age-related macular degeneration is caused by dysfunction and loss of retinal pigment epithelium (RPE) cells, and their transplantation may rescue visual functions and delay disease progression. Human embryonic stem cells (hESCs) may be an unlimited source of RPE cells for allotransplantation. We analyzed the immunomodulatory properties of hESC-derived RPE (hESC-RPE) cells, and showed that they inhibited T cell responses. Co-culture experiments showed that RPE cells inhibited interfon-γ secretion and proliferation of activated T cells. Furthermore, hESC-RPE cells enhanced T cell apoptosis and secretion of the anti-inflammatory cytokine interleukin-10 (IL-10). In addition, RPE cells altered the expression of T cell activation markers, CD69 and CD25. RPE cells transplanted into RCS rats without immunosuppression survived, provided retinal rescue, and enhanced IL-10 blood levels. Our data suggest that hESC-RPE cells have immunosuppressive properties. Further studies will determine if these properties are sufficient to alleviate the need for immunosuppression therapy after their clinical allotransplantation.
Keywords: retinal pigment epithelium, human embryonic stem cells, immunomodulation, immune-privilege
Highlights
-
•
T cells proliferation and IFN-γ secretion are inhibited by hESC-RPE cells
-
•
T cells apoptosis and secretion of IL-10 are enhanced by hESC-RPE cells
-
•
RPE cells survive, provide retinal rescue, and enhance IL-10 blood levels in vivo
-
•
These findings are relevant to immunosuppressive regimens for RPE cell therapies
In this article, Reubinoff and colleagues describe the immunomodulatory properties of hESC-RPE cells. They show that the RPE cells inhibit IFN-γ secretion and proliferation of T cells and enhance T cells apoptosis and secretion of IL-10. In the absence of immunosuppression therapy, RPE cells survive, provide retinal rescue, and enhance IL-10 blood levels in a rat model of retinal degeneration. These findings are relevant to designing immunosuppressive regimens for RPE cell allotransplantation therapies.
Introduction
Retinal pigment epithelium (RPE) cells comprise the layer lying adjacent to retinal photoreceptors and playing important role in the precise functioning of the retina. The functions of these cells include formation of blood-retina barrier, absorption of stray light, supply of nutrients to the neural retina, recycling of the components of visual cycle, and phagocytosys of shed photoreceptor outer segments. Dysfunction and loss of RPE cells are prominent features of age-related macular degeneration, Best disease, and subtypes of retinitis pigmentosa as well as Leber congenital amaurosis. Transplantation of RPE cells is considered as a potential therapeutic approach. Human embryonic stem cell (hESCs) may serve as an unlimited donor source of RPE cells for transplantation. We previously developed specific culture conditions, which allow efficient, directed differentiation of hESCs into functional RPE cells. Following transplantation of these cells into the subretinal space of RCS rats, a model of retinal degeneration caused by RPE dysfunction, functional and structural rescue was observed (Idelson et al., 2009). Other groups have obtained similar results (Lu et al., 2009, Lund et al., 2006, Vugler et al., 2008), and recent clinical trials involving the use of hESC-RPE cells have been initiated (Schwartz et al., 2012, Song et al., 2015).
A key issue in safe and efficient application of hESC-based treatments is the interaction of transplanted cells with the host immune system and potential graft rejection. The eye is considered an immune privileged site. RPE cells are suggested to participate in the blood-retina barrier, to possess immunomodulatory properties and to protect the eye against T cells using various mechanisms (Jorgensen et al., 1998, Kaestel et al., 2002). Whether hESC-RPE cells possess similar immunomodulatory properties is of paramount importance for their use in ocular transplantation. Previous studies characterized some of the properties of hESC or induced pluripotent stem cell (iPSC)-derived RPE cells including expression of human leukocyte antigen (HLA) molecules and inhibition of T cells proliferation (Kamao et al., 2014, Sugita et al., 2015). In the present study we confirm these observations and further explore the immunomodulatory properties of hESC-RPE cells. We show their capability to induce T cell apoptosis, interleukin-10 (IL-10) secretion in vitro and in vivo and to alter the expression of T cell activation markers, CD69 and CD25.
Our results demonstrate the immunosuppressive capabilities of hESC-RPE cells. They support the exploration of the need and extent of immunosuppression regimens in clinical allotransplantation trials of hESC-RPE.
Results
Expression of HLA Class I and Class II Molecules by hESC-RPE Cells
Differentiation of hESCs toward RPE fate was induced as described previously (Idelson et al., 2009). Clumps of pigmented cells were mechanically isolated from differentiating floating clusters of hESCs, plated and propagated into homogeneous cultures of pigmented cells with typical polygonal shape and phenotype of RPE cells (Figure S1).
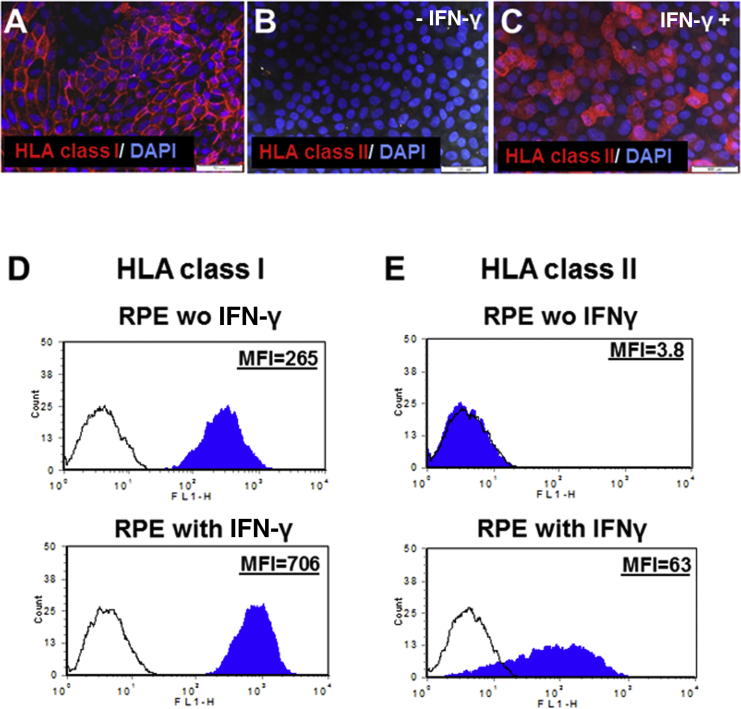
We initiated the characterization of the immunogenicity of hESC-RPE cells by fluorescence-activated cell sorting (FACS) analysis of the expression of HLA class I and class II molecules. The expression of HLA molecules on the surface of RPE cells was also analyzed using immunofluorescence stainings (Figure 1).
Figure 1.
Expression of HLA Class I and Class II Proteins by hESC-RPE Cells
(A–C) Immunostaining showing that the hESC-RPE cells express HLA class I (HLA-ABC) (A). Immunostaining showing that the hESC-RPE cells express HLA class II (HLA- DR, DP, DQ) molecules after stimulation with IFN-γ (25 nM) (C), but not without IFN-γ stimulation (B).
(D and E) Representative FACS analysis of the expression of HLA class I (D) and class II molecules (E). The hESC-RPE cells were incubated without or with IFN-γ (25 nM) for 2 days. Histogram of the mean fluorescence intensity (MFI) in cells stained with anti-HLA (solid blue) and with isotype control antibodies (black line).
Scale bars, 50 μm in (A) and 100 μm in (B and C).
The expression of HLA class I antigens, as determined by FACS and immunostaining with anti-HLA-ABC antibody was demonstrated in 100% of RPE cells (Figures 1A and 1D). We further analyzed expression of HLA molecules in the presence of interfon-γ (IFN-γ), which is known to increase immunogenicity of cells and was used in our system to model inflammatory state. We showed that following stimulation with IFN-γ, the hESC-RPE cells enhanced the expression of HLA class I antigens by about 2-fold (mean fluorescence intensity [MFI] = 731.3 ± 29.9 versus 324.0 ± 34.5, p = 0.00082; Figure 1D).
We also analyzed the expression of HLA class II (HLA-DR, DP, DQ) antigens that are usually present on the surface of antigen-presenting cells. We showed that hESC-RPE cells do not express HLA class II molecules (Figures 1B and 1E). However, in the presence of IFN-γ, hESC-RPE cells expressed HLA class II molecules (Figures 1C, 1E, and S2; HLA-DR).
Our results demonstrated that the immunogenicity of the hESC-RPE cells, exemplified by HLA class I and II expression, was enhanced by treatment with IFN-γ.
Expression of Immunomodulatory Molecules by hESC-RPE Cells
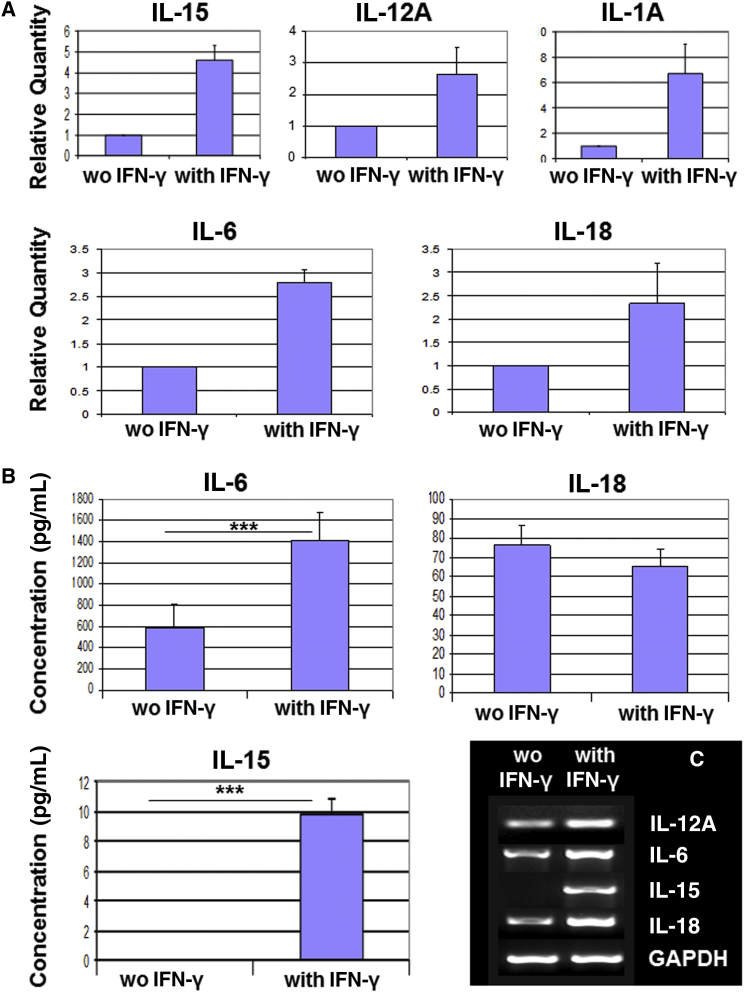
We sought to elucidate the potential mechanisms underlying immunomodulation by RPE cells. We profiled the cytokine expression of hESC-RPE cells by real-time PCR using the human cytokine network TaqMan array plate. We demonstrated the expression of IL-15, IL-18, IL-12A, IL-6, and IL-1A, which was further confirmed by RT-PCR. We showed that the expression of these cytokines was enhanced following treatment of the RPE cells with IFN-γ for 3 days (Figures 2A and 2C). We further showed by ELISA analysis the secretion of IL-6, IL-18, and IL-15 to the medium (Figure 2B). The secretion of IL-6 was high and was significantly enhanced by IFN-γ treatment (1,408.20 ± 120.15 pg/mL compared with 580.40 ± 105.63 pg/mL in treated versus untreated, respectively; p ≤ 0.001). Low secretion of IL-15 was demonstrated only after IFN-γ stimulation (9.79 ± 0.50 pg/mL). The secretion of IL-18 was similar in the absence and presence of IFN-γ (76.55 ± 9.85 pg/mL and 65.36 ± 8.45 pg/mL, respectively).
Figure 2.
Expression of Cytokines by hESC-RPE Cells
(A) Real-time PCR analysis of the expression of cytokines by RPE cells cultured for 3 days in the presence or absence of IFN-γ. Relative quantity is the relative expression level of each gene in comparison with its expression level in unstimulated RPE cells that was set at 1.
(B) ELISA analysis of the secretion of cytokines, IL-6, IL-18, and IL-15 by RPE cells cultured for 3 days in the presence or absence of IFN-γ.
(C) RT-PCR analysis of the expression of IL-12A, IL-6, IL-15, and IL-18 by RPE cells cultured for 3 days with or without IFN-γ.
Data are presented as means ± SEM of at least 3 independent experiments. ∗∗∗p ≤ 0.001.
We further analyzed the expression of molecules known to participate in suppression of immune response: Fas ligand, transforming growth factor β (TGF-β), Arginase I, TRAIL, PDL1, IDO, vascular endothelial growth factor (VEGF), and MIF, in IFN-γ-stimulated or non-stimulated hESC-RPE cells (Figure S2). We showed the expression of Fas ligand, TGF-β, VEGF, and MIF by the unstimulated and IFN-γ-stimulated hESC-RPE cells. The expression of TRAIL, PDL1, and IDO was induced by treatment with IFN-γ. The expression of the immunomodulatory enzyme, Arginase I, which was previously shown by us to have a role in the immunosuppressive properties of hESCs (Yachimovich-Cohen et al., 2009), was not detected in unstimulated as well as in IFN-γ-stimulated RPE cells.
Our experiments demonstrated that RPE cells expressed various immunomodulatory molecules and cytokines that could potentially have a role in their “immune privileged” properties.
Attenuation of T Cell Responses by hESC-RPE Cells
To further characterize the immunosuppressive properties of hESC-RPE cells, we analyzed their potential to attenuate T cell responses in co-culture experiments with human peripheral blood mononuclear cells (PBMCs).
To elucidate whether the immunomodulatory activity of RPE cells is mediated by membrane-associated factor or soluble secreted factor, we either co-cultured PBMCs with hESC-RPE cells or separated them by transwells. The RPE cells were either unstimulated or pre-stimulated for 3 days with IFN-γ prior to co-culture with PBMCs. During co-culture with RPE cells, PBMCs were non-activated or activated with anti-CD3/anti-CD28 antibodies known to promote extensive T cell expansion. We analyzed the influence of hESC-RPE cells on CD3+ T cell proliferation, apoptosis, activation, and secretion of cytokines.
RPE Cells Reduce T Cell Proliferation
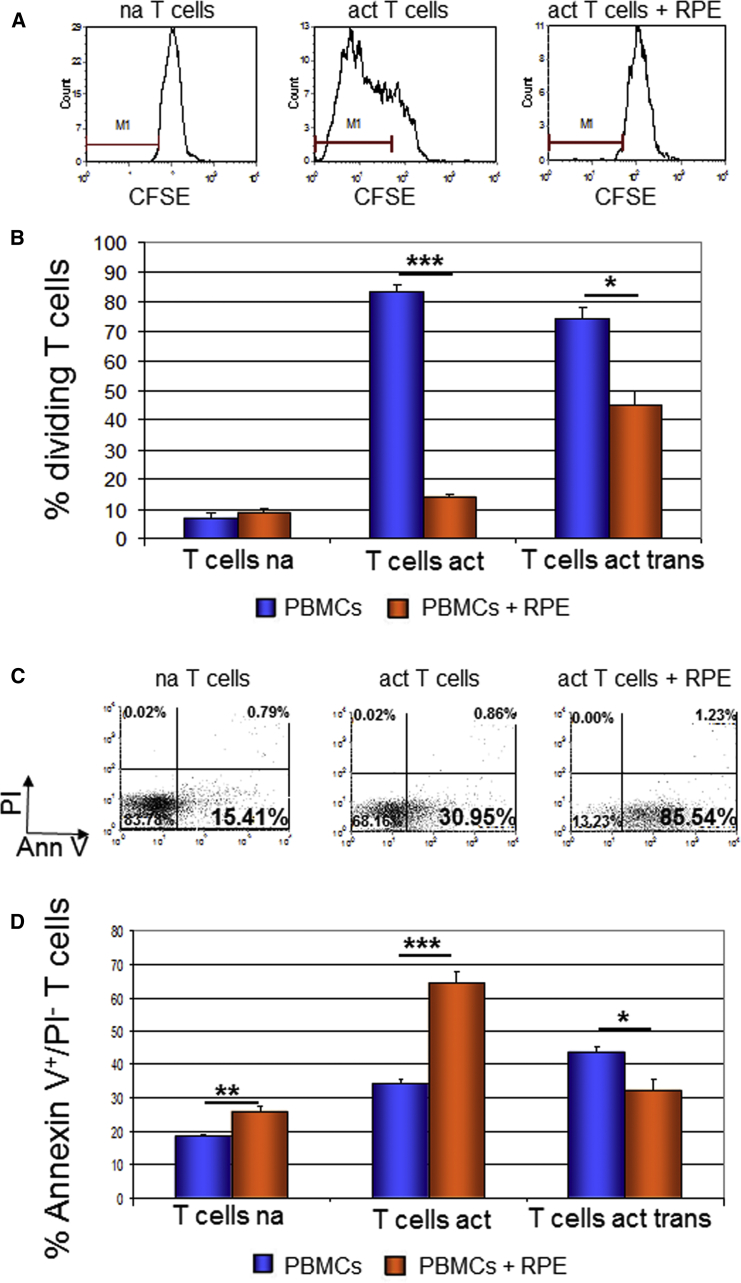
T cell proliferation was analyzed by FACS analysis of carboxyfluorescein succinimidyl ester (CFSE) dilution. We cultured PBMCs for 4 days either without or with RPE cells, as above, and analyzed the effect of co-culture on T cell proliferation.
We showed that RPE cells inhibited the proliferation of activated CD3+ T cells (Figures 3A, 3B, and S3). In the case of direct contact between the RPE cells and PBMCs (co-culture without transwell), the percentage of dividing T cells was reduced dramatically (from 83.17% ± 2.88% in control PBMCs to 13.94% ± 1.20% and 15.38% ± 0.46% in co-culture with RPE cells from HES1 and HADC102, respectively). In the transwell system where direct contact is prevented, the inhibition of proliferation was less pronounced (74.16% ± 4.01% without RPE compared with 44.84% ± 5.04% with RPE; p ≤ 0.05; Figures 3A and 3B). When the RPE cells were pre-stimulated with IFN-γ prior to co-culture, the inhibition of proliferation was profound also in the transwell system (Figure S3). These results suggest the involvement of both contact-dependent and contact-independent mechanisms in the inhibition of proliferation.
Figure 3.
T Cell Proliferation Was Highly Attenuated and T Cell Apoptosis Was Enhanced by Co-culture with hESC-RPE Cells
(A and B) Histogram plots presentation of carboxyfluorescein succinimidyl ester (CFSE) fluorescence (A) and histogram presentation of the percentage of dividing CD3+ T cells (B). PBMCs were labeled with CFSE and cultured for 4 days with or without RPE cells. Co-culture with RPE cells was either direct or using transwells (trans).
(C) Dot plots presentation of CD3+ T cells detected by Annexin V-FITC and PI staining after culture for 1 day with or without RPE cells.
(D) Quantification of the percentage of apoptotic annexin V+/PI− T cells following co-culture with RPE. PBMCs were co-cultured with RPE cells directly or using transwells (trans). na, non-activated T cells; act, cells activated with anti-CD3/anti-CD28 antibodies; AnnV, AnnexinV.
Data are presented as means ± SEM of at least 3 independent experiments. ∗p ≤ 0.05; ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
RPE Cells Enhance T Cell Apoptosis
The potential of hESC-RPE cells to induce T cell apoptosis was analyzed in co-culture experiments with human PBMCs.
We analyzed the apoptosis of CD3+ T cells that were cultured for 1–3 days either without or with RPE cells. We focused on the early apoptotic CD3+ T cells that were annexin V+/PI−.
We demonstrated that hESC-RPE cells induced T cell apoptosis at day 1 of co-culture (Figures 3C and 3D). At this time, in the presence of RPE cells from HES1 and HADC102, 64.33% ± 3.30% and 47.97% ± 3.20%, respectively, of activated CD3+ T cells were early apoptotic cells compared with 34.31% ± 1.52% in the absence of RPE. During the following 2 days the apoptosis level was gradually reduced (Figure S4). When PBMCs were co-cultured with RPE cells using the transwell system, the level of apoptosis was decreased (32.21% ± 3.53% of co-cultured CD3+ T cells versus 43.72% ± 1.52% in control cells; p ≤ 0.05). In light of these findings, it seems that RPE cells induced apoptosis of activated T cells in a contact-dependent mechanism.
RPE Cells Modulate the Secretion of Pro- and Anti-inflammatory Cytokines
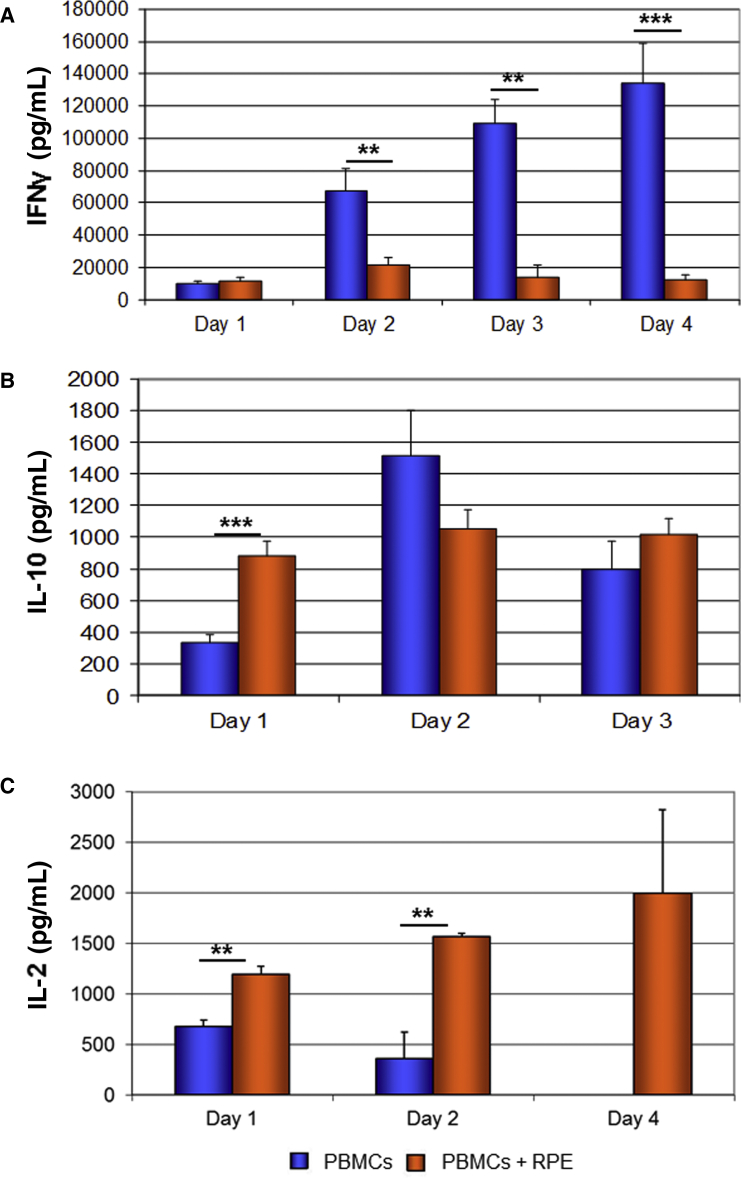
We analyzed the secretion of pro- and anti-inflammatory cytokines by activated PBMCs that were cultured for up to 4 days either with or without RPE cells.
We demonstrated that hESC-RPE cells inhibited the secretion of the pro-inflammatory cytokine IFN-γ, beginning from day 2 of co-culture and reaching the most dramatic inhibition at day 4 (133.88 ± 24.96 ng/mL secreted from control PBMCs compared with 11.88 ± 3.17 ng/mL from PBMCs in co-culture with RPE; p ≤ 0.001; Figure 4A).
Figure 4.
Decrease in Secretion of IFN-γ and Increase in Secretion of IL-10 and IL-2 by PBMCs following Co-culture with hESC-RPE Cells
ELISA analysis of the secretion of IFN-γ (A), IL-10 (B), and IL-2 (C) by activated PBMCs that were co-cultured for 1–4 days with RPE cells. (C) At day 4 no secretion of IL-2 by control PBMCs was detected (n = 2). Data are presented as means ± SEM of at least 3 independent experiments. ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
We further analyzed the secretion of the anti-inflammatory immunoregulatory cytokine IL-10 by PBMCs at days 1–3 of co-culture. We demonstrated that hESC-RPE cells enhanced the secretion of IL-10 by PBMCs at day 1 of co-culture (879.59 ± 92.69 pg/mL compared with 337.14 ± 44.52 pg/mL secretion from control PBMCs; p ≤ 0.001; Figure 4B). At the following 2 days the concentrations of IL-10 were not significantly different in dishes of co-cultured and control PBMCs.
Finally, we analyzed the secretion of IL-2 by activated PBMCs that were cultured for 1–4 days either with or without RPE cells. HESC-RPE cells significantly enhanced the secretion of IL-2 by PBMCs. The level of IL-2 in activated PBMCs cultures peaked at day 1 and decreased in the next days to non-detectable levels at day 4. In the presence of hESC-RPE cells the level of IL-2 was significantly increased already at day 1 of co-culture (1.19 ± 0.08 ng/mL in the presence of RPE versus 0.68 ± 0.06 ng/mL in the absence of RPE; p ≤ 0.05; Figure 4C), and in contrast to PBMC cultured alone the level of IL-2 increased in the next 3 days.
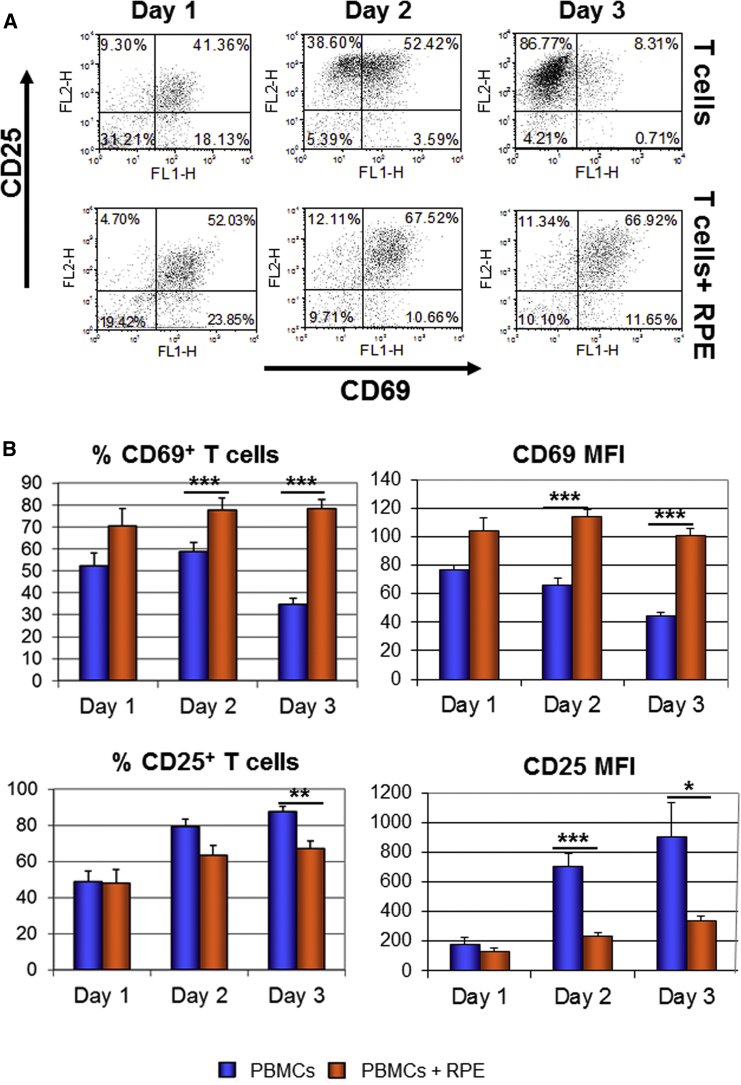
RPE Cells Modify T Cell Activation Process
We analyzed whether co-culture with RPE cells modifies the expression of T cell activation markers. We quantified the expression of CD69, an early activation marker, which is expressed shortly after activation and its expression decreases after 2 days of activation (Werfel et al., 1997). It was suggested that sustained expression of CD69 defines T cells with immunoregulatory properties (Saldanha-Araujo et al., 2012, Sancho et al., 2005). RPE cells increased the percentage of co-cultured T cells that expressed CD69 at every time point analyzed (Figure 5). At day 3, the percentage of T cells expressing CD69 was 78.23% ± 1.87% and 59.21% ± 2.10% in the presence of RPE cells from HES1 and HADC102, respectively, in comparison with 34.59% ± 7.13% of control T cells. Furthermore, co-culture with RPE cells from HES1 and HADC102 enhanced the expression level of CD69 (MFI = 101.38 ± 4.32 and 91.23 ± 6.79, respectively, versus 44.85 ± 2.41; p ≤ 0.001; Figure 5B). We also analyzed the expression of CD25, an intermediate activation marker (Sancho et al., 1999). Co-culture with RPE cells from HES1 and HADC102 reduced the percentage of T cells expressing CD25 (from 87.69% ± 2.78% to 67.19% ± 4.23% and 62.19% ± 1.57%, respectively) and significantly reduced the expression level of the marker on T cells (905.08 ± 275.42 MFI without RPE versus 276.21 ± 30.72 and 255.98 ± 13.54 with RPE from HES1 and HADC102, respectively) at day 3 (Figure 5B). CD25 is the high-affinity receptor for IL-2 and its downregulation was suggested to decrease IL-2 uptake. In line with this suggestion, we demonstrated accumulation of free IL-2 in the medium following co-culture with RPE (Figure 4).
Figure 5.
Expression of T Cell Activation Markers Was Changed following Co-culture with hESC-RPE Cells
Activated PBMCs were co-cultured for 1–3 days with or without RPE cells. The expression of activation markers, CD69 and CD25 was analyzed within the population of CD3+ T cells.
(A) Representative dot plots of CD3+ T cells stained with anti-CD69 and anti-CD25 antibodies.
(B) Histogram presentation of the percentage of CD25 and CD69+ cells as well as the level of the markers expression referred to as MFI.
Data are presented as means ± SEM of at least 3 independent experiments. ∗p ≤ 0.05; ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Elucidating the Mechanisms of RPE Immunomodulation
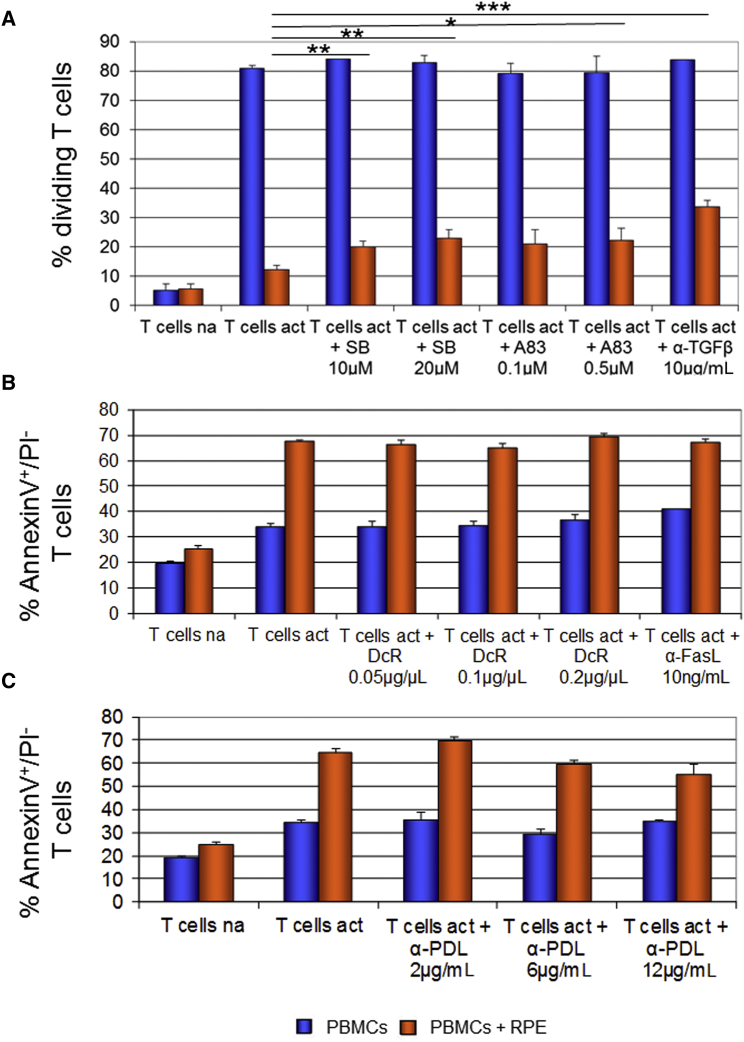
To identify the mechanisms underlying potential immunomodulation by hESC-RPE cells, we inhibited signal activation pathways of the immunomodulatory molecules, FasL, PDL1, and TGF-β in co-cultured T cells and monitored their proliferation and apoptosis.
We first inhibited TGF-β signaling using either anti-TGF-β antibody or, alternatively, with two small inhibitory molecules, SB-431542 and A-83-01. All three inhibitors induced partial but significant increase in proliferation of T cells that were co-cultured with RPE cells in the presence compared with absence of the inhibitory molecules (Figure 6A). Our results suggested a role for TGF-β signaling in the inhibition of T cells proliferation by hESC-RPE cells. Since blocking of TGF-β signaling could not completely restore the percentage of proliferating T cells, there are probably other factors that contribute to the inhibition of proliferation induced by RPE cells. We also found that blocking of TGF-β signaling had no influence on RPE-dependent T cell apoptosis (data not shown).
Figure 6.
The Role of TGF-β, FasL, and PDL1 Signaling in RPE Immunomodulation
(A) The percentage of dividing CD3+ T cells was analyzed following staining of PBMCs with CFSE, activation and co-cultured for 4 days with RPE cells in the presence or absence of inhibitors of TGF-β signaling.
(B and C) The percentage of apoptotic annexin V+/PI− cells was analyzed after activation and co-cultured for 1 day with RPE cells in the presence or absence of inhibitors of FasL signaling (B) or anti-PDL1 blocking antibody (α-PDL) (C); na, non-activated T cells; act, cells activated with anti-CD3/anti-CD28 antibodies; SB, SB-431542; A83, A-83-01; α-TGF-β, anti-TGF-β antibody; DcR, recombinant human DcR3; α-FasL, anti-FasL antibody.
Data are presented as means ± SEM of at least 3 independent experiments. ∗p ≤ 0.05; ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
We then analyzed the role of FasL signaling in the induction of T cell apoptosis by RPE cells. We inhibited FasL signaling using a blocking anti-FasL antibody or inhibitory molecule DcR (Figure 6B). We did not observe any differences in the RPE-induced apoptosis of T cells following inhibition of FasL signaling suggesting that FasL signaling probably does not mediate the induction of apoptosis in our system.
We also analyzed the role of PDL1 signaling in the induction of T cell apoptosis. We inhibited PDL1 signaling using neutralizing anti-PDL1 antibody. We did not observe a significant alteration in the RPE-dependent apoptosis of T cells following inhibition of PDL1 signaling at various concentrations of the antibody (Figure 6C).
In Vivo Analysis of the Immunomodulatory Properties of Subretinally Transplanted hESC-RPE Cells
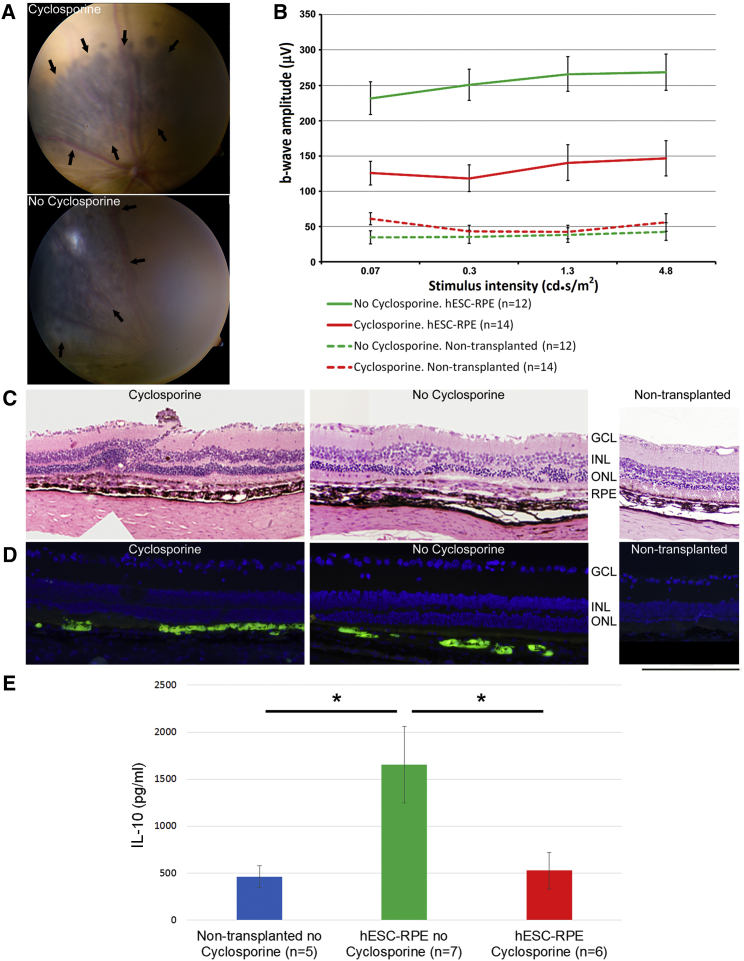
We further analyzed the immunomodulatory properties of the hESC-RPE cells in vivo after subretinal transplantation in RCS rats, an animal model of retinal degeneration.
It was previously shown that the immune privileged properties of the rodent eye are not sufficient to prevent the rejection of subretinally transplanted human cells (Zhu et al., 2017). In line with these results, we previously showed in RCS rats that subretinal transplantation of human fibroblasts was associated with marked inflammatory response despite cyclosporine treatment (Idelson et al., 2009). In contrast, we showed prolonged survival of transplanted hESC-RPE cells that was not associated with inflammatory response in the presence of cyclosporine (Idelson et al., 2009, McGill et al., 2017). Here we sought to test whether subretinally transplanted RPE cells will survive and function without cyclosporine. We transplanted GFP+ RPE cells into RCS rats in the presence or absence of cyclosporine treatment. Retinal function and structure were studied 4–6 weeks post-transplantation (Figure 7). The transplanted pigmented cells could be readily identified using standard color fundus imaging both in cyclosporine-treated and untreated animals (Figure 7A). Immunostaining analysis further showed subretinally transplanted GFP+ cells both with and without cyclosporine treatment (Figure 7D). Histological analysis showed preservation of the ONL in proximity to subretinal grafts in the presence and absence of immunosuppression in comparison with non-transplanted eyes (Figures 7C and 7D). Electroretinographic (ERG) analysis showed functional rescue in transplanted eyes both in the presence and absence of cyclosporine treatment. Interestingly, functional rescue after hESC-RPE transplantation was more prominent in the absence of cyclosporine treatment (Figure 7B). To assess whether an anti-inflammatory state is induced by hESC-RPE cells after subretinal transplantation, we examined the peripheral blood IL-10 levels. In the absence of cyclosporine treatment, RPE-transplanted animals showed significantly higher IL-10 serum levels compared with non-transplanted controls, suggesting an RPE-induced anti-inflammatory state (Figure 7E). These in vivo results were in line with the RPE-PBMCs co-culture data that showed the potential of RPE cells to augment IL-10 secretion in vitro (Figure 4). In the presence of cyclosporine, transplantation of RPE cells had no effect on the IL-10 levels.
Figure 7.
Transplanted hESC-RPE Cells Provide Functional Retinal Rescue in the Absence of Systemic Immunosuppression
(A) Color fundus photographs show subretinal grafts of pigmented cells (marked by arrows) in RCS rats in the presence (upper image) or absence (lower image) of systemic cyclosporine immunosuppression 5–6 weeks post-transplantation.
(B) Mean b-wave amplitudes in response to white flashes of increasing intensity. The mean b-wave amplitudes were significantly higher both in cyclosporine-treated (red line) and non-treated (green line) animals compared with non-transplanted fellow eyes (red and green dashed lines, respectively).
(C) Representative H&E-stained retinal sections show relative preservation of photoreceptors layer (ONL) in proximity to subretinal RPE grafts in cyclosporine-treated and non-treated animals, in comparison with a thinner ONL in a non-transplanted eye.
(D) Anti-GFP immunostaining show presence of transplanted cells in the subretinal space of RCS rats either treated or untreated with systemic immunosuppression.
(E) ELISA analysis of the peripheral blood IL-10 levels in RCS rats 4–5 weeks post-transplantation. Levels of anti-inflammatory interleukin IL-10 were statistically higher in non-cyclosporine group compared with the cyclosporine-treated group and non-transplanted control. ∗p ≤ 0.05.
Data are presented as means ± SEM of at least 3 independent experiments.
Given these results in vivo, we further explored the effect of cyclosporine on RPE immunosuppressive properties in vitro in co-culture experiments of hESC-RPE cells with PBMCs. We demonstrated that cyclosporine significantly reduced the RPE-induced T cells apoptosis (Figure S5A). Moreover, cyclosporine decreased the secretion of IL-10 by PBMCs both in the presence or absence of RPE cells (Figure S5B).
Taken together, our data demonstrated the survival and function of subretinally transplanted hESC-RPE cells in the absence of cyclosporine treatment, supporting their immunosuppressive properties in vivo. Furthermore, the RPE-mediated induction of IL-10 secretion that was shown in vitro was also observed in vivo.
Discussion
In the present study, we analyzed the immunomodulatory properties of hESC-RPE cells. We demonstrated the expression of HLA molecules by hESC-RPE cells as well as the expression and secretion of various immunomodulatory molecules. We demonstrated that in a co-culture system, hESC-RPE cells inhibited IFN-γ secretion and proliferation of activated T cells while enhancing T cell apoptosis. Moreover, our studies provide insights regarding potential mechanisms that mediate the immunosuppressive properties of hESC-RPE cells. We showed that hESC-RPE cells augmented the secretion of the anti-inflammatory cytokine IL-10 by activated PBMCs. Furthermore, hESC-RPE cells altered the expression of markers of T cell activation, CD69 and CD25. We demonstrated the involvement of TGF-β, but not FasL and PDL1 signaling in the immunosuppressive activity of RPE cells. RPE cells transplanted into RCS rats without immunosuppression survived, provided retinal rescue and enhanced IL-10 blood levels.
We demonstrated that similarly to human native RPE cells and iPSC-derived RPE (iPSC-RPE) cells, hESC-RPE cells express HLA class I and following stimulation with IFN-γ they express HLA class II molecules (Osusky et al., 1997, Sugita et al., 2016). The expression of HLA class II antigens as well as additional molecules, such as CD40 and CD54, can be associated with antigen-presenting properties of RPE cells (Elner et al., 1992, Osusky et al., 1997, Willermain et al., 2000). This suggestion is in line with previously published data showing that major histocompatibility complex class II-expressing RPE cells could contribute to immune and inflammatory activity in the eye by presenting superantigens to T lymphocytes (Osusky et al., 1997). Our data suggest a potential enhancement of antigen presentation properties of RPE cells in the course of inflammation associated with elevated levels of IFN-γ.
RPE transplantation has a risk of intraocular complications, such as ocular trauma from cell injection, hemorrhage, retinal detachment, and proliferative vitreoretinopathy, which are associated with inflammation and elevated levels of IFN-γ (Morescalchi et al., 2013). As a consequence, the expression of HLA class II antigens may be induced on RPE cells, increasing the risk of their rejection.
The main focus of this manuscript was to study the effect of hESC-RPE cells on co-cultured activated human T cells. We showed that RPE cells inhibit the proliferation of the T cells. Our data suggest that TGF-β signaling has a role in the suppressive effect of RPE cells on proliferation of T cells. Blocking TGF-β signaling by various approaches attenuated the suppressive effect of RPE cells on T cell proliferation. These data are in line with the observations of a previous study that demonstrated the involvement of TGF-β signaling in the inhibition of T cell proliferation by iPSC-RPE cells (Sugita et al., 2015).
We demonstrated that RPE cells could efficiently inhibit the proliferation of activated T cells in a co-culture system where the inhibitory effect could be mediated by both secreted molecules and cell-cell contact. To differentiate between these two mechanisms, we evaluated the role of secreted molecules by using a transwell system. Under these conditions, we showed that secreted molecules inhibited the proliferation of activated T cells, although to a less prominent level compared with direct contact co-culture system. However, when RPE cells were pre-incubated with IFN-γ prior to introduction to the transwell system, the inhibition of proliferation was augmented to a level similar to the direct contact co-culture system. Our data suggest a role of both contact-dependent and contact-independent mechanisms in the inhibition of proliferation. These two mechanisms could be potentially mediated by the same molecule in the soluble or membrane-bound form.
We analyzed the involvement of FasL signaling in the immunosuppressive functions of hESC-RPE cells. There are controversial reports in the literature regarding the expression of FasL in RPE cells. In a paper published by Jorgensen et al. (1998), the expression of FasL was demonstrated in cultured fetal RPE cells, and blocking of FasL signaling reduced RPE-mediated T cell apoptosis. On the other hand, Kaestel et al. (2001) demonstrated a discrepancy between RPE cells in vitro and in vivo with regard to the expression of FasL molecule. They showed that whereas in vivo the RPE cells expressed FasL molecule, cultured fetal and adult RPE cells did not express FasL, however, they still possessed their immunosuppressive properties. We demonstrated the expression of FasL transcripts by hESC-RPE cells, however, we could not detect the expression of FasL protein on cell surface of the RPE cells (data not shown) and inhibition of FasL signaling did not reverse the ability of RPE cells to induce T cell apoptosis. Our results suggest that FasL is not involved in the induction of RPE-mediated T cell apoptosis in our system.
We further analyzed the involvement of PDL1 in the immunomodulatory properties of RPE cells. PDL1 was shown to be involved in the suppression of T cells. Its expression on the surface of tumor cells allows them to escape immune response (Iwai et al., 2002). Our results did not show significant alteration in the RPE-dependent apoptosis of T cells after inhibiting PDL1 signaling. We concluded that PDL1 has no role in the induction of RPE-dependent T cell apoptosis.
Our results clearly demonstrated that RPE cells dramatically modulated T cell activation. We focused on the analysis of the expression of two T cell activation markers, CD69 and CD25. We demonstrated an increased and sustained expression of CD69 marker following co-culture with RPE cells. It was previously suggested that stable CD69 expression defines cells with immunoregulatory properties (Sancho et al., 2005). Furthermore, a novel subset of CD69+ regulatory T cells was reported and was shown to have the capability to maintain immune tolerance and protect against inflammation (Cortes et al., 2014). In addition, it was shown that mesenchymal stem cells promote sustained CD69 expression in T cells as part of their immunomodulatory activity (Saldanha-Araujo et al., 2012).
In contrast to the enhancement in the expression of CD69, the expression of CD25 by T cells was significantly decreased following co-culture of PBMCs with RPE cells. In contrast to our results, Kaestel et al. (2002) as well as Sugita et al. (2015), demonstrated that the T cells' expression of CD25, which is an IL-2R-alpha, was upregulated or unchanged after co-culture with human RPE cells. Chao et al. (2002) demonstrated that the expression of CD25 is reduced and the expression of CD69 is markedly increased during normal pregnancy. These changes could be suggested to result in maternal tolerance of the fetal allograft.
In our study, the decreased expression of CD25 on T cell membrane was correlated with enhanced levels of IL-2 in the medium. Possible explanations to the excess of IL-2 could be increased secretion or reduced uptake by T cells that could be related to the lower expression of IL-2R (CD25).
IL-2 signaling is essential for T cell survival, proliferation, and amplifies T cell responses (de Oliveira et al., 2011, Lin et al., 2000). Hence, it may be speculated that its reduced uptake and signaling contributed to the attenuated proliferation of T cells co-cultured with RPE cells.
IL-2 is also found to promote differentiation and maintenance of regulatory T cells, which induce tolerance by suppressing T cell responses (de la Rosa et al., 2004, Furtado et al., 2002).
We showed that hESC-RPE cells also enhanced the secretion of the cytokine IL-10, which was suggested as a factor that promotes regulatory T cells. Taken together, these data support a potential role of RPE cells in the induction of regulatory phenotype of T cells as suggested previously (Imai et al., 2012, Kawazoe et al., 2012, Sugita et al., 2015).
In this study, we mainly concentrated on the effects of hESC-RPE cells on T cell responses in vitro. It is possible that interactions between transplanted hESC-RPE cells and T cells may be more complex in vivo, given potential involvement of other cell types in the transplantation niche. RPE cells were reported to interact with other cells of the immune system. Primary cultured mouse RPE cells were shown to suppress B cell activation (Sugita et al., 2010). It was also demonstrated that mouse RPE cells could activate macrophages to function similar to myeloid suppressor cells. This effect was mediated by alpha-melanocyte-stimulating hormone as an immunosuppressive regulator (Taylor, 2013). Similarly, the mechanism of immunomodulaton by hESC-RPE cells may be complex and may involve regulation of various immune cells. Further studies are needed to elucidate potential interactions between hESC-RPE cells and other immune cells.
We further explored the immunomodulatory properties of hESC-RPE cells in vivo after subretinal transplantation in RCS rats. Ours and others previous data showed prolonged (19–25 weeks) survival of transplanted functional hESC-RPE cells without evidence of immune cell infiltrates in the area of transplantation in the presence of cyclosporine immunosuppression (Idelson et al., 2009, Lu et al., 2009, McGill et al., 2017). In support of the immunomodulatory properties of hESC-RPE cells in vivo, here we further showed their survival and functional rescue after subretinal transplantation in the absence of cyclosporine immunosuppression.
Following RPE transplantation, we demonstrated increased blood levels of the anti-inflammatory cytokine IL-10 in the absence of cyclosporine treatment. A previous study showed that the vitreous level of IL-10 was not enhanced after allogeneic subretinal transplantation of iPSC-RPE cells in pigs (Sohn et al., 2015). This discrepancy may be related to the different sites of IL-10 measurement in the two studies: local vitreal fluid versus systemic blood. It could also result from the different animal models: healthy pigs versus a rat model of retinal degeneration. Our in vivo results were in line with our co-culture experiments that showed increased secretion of IL-10 from PBMCs in the presence of RPE cells. In the presence of cyclosporine, an increase in IL-10 levels was not observed in transplanted animals. These results were supported by in vitro data showing significant reduction of IL-10 secretion by PBMCs in the presence of cyclosporine. In addition, RPE-induced T cell apoptosis was also significantly reduced by cyclosporine. Cyclosporine, similarly to other immunosuppressive agents, tacrolimus and Mycophenolate mofetil, suppresses T cell activation and proliferation. It is possible that the immunomodulatory properties of RPE cells that are mediated by regulatory T cells are abrogated at least in part in the presence of cyclosporine.
Zhao et al. (2015) evaluated the immunogenicity of differentiated cells derived from autologous iPSCs. The authors showed that autologous RPE cells were not rejected after transplantation into skeletal muscle of humanized mice in contrast to smooth muscle cells. It was suggested that the differential immunogenicity was related in part to abnormal increased expression of the antigens Zg16 and Hormad1 in the smooth muscle cells (Zhao et al., 2015). In this study, allogeneic hESC-RPE cells were not rejected after subretinal transplantation in humanized mice in the absence of cyclosporine. This is in line with our results, showing the survival of hESC-RPE cells and functional rescue in the absence of cyclosporine after subretinal transplantation in RCS rats.
Sugita et al. (2016) reported that iPSC-RPE cells can induce T cell immune responses in mixed lymphocyte reaction assay with allogeneic HLA mismatched PBMCs. T cell responses did not occur when iPSC-RPE cells from HLA-matched donors were used (Sugita et al., 2016). These results are in contrast to our co-culture studies where hESC-RPE cells did not induce the proliferation of non-activated CD3+ T cells. Further studies are needed to compare the immune properties of hESC- and iPSC-RPE cells. It is unclear whether hESC-, iPSC-RPE cells or both will be routinely used in the future for the treatment of retinal degenerations, and whether HLA-matched iPSC-RPE cells will be available for all patients.
Understanding the immune properties of hESC-RPE cells is relevant and valuable for clinical transplantation of hESC-RPE cells in retinal degenerations caused by RPE dysfunction. The immunosuppressive properties of hESC-RPE cells, demonstrated in this manuscript, and in particular, their survival and therapeutic effect in RCS rats in the absence of cyclosporine highlight the importance of exploring the need and mode of immunosuppressive treatment in clinical transplantation of RPE cells.
Experimental Procedures
Cell Culture
HES1 and HADC102 hESC lines were derived and characterized previously (Reubinoff et al., 2000, Tannenbaum et al., 2012). Human ESCs were maintained as described (Tannenbaum et al., 2012). For differentiation into RPE cells, hESCs colonies were picked up and cultured as described (Idelson et al., 2009) using differentiation protocol including treatment with nicotinamide and activin A. The RPE cell lines were cultured on gelatin (Sigma), passaged every 2 weeks and frozen after 2 passages. Thawed cells were studied between passages 4 and 7. For some experiments, RPE cells were pre-treated with 25 nM IFN-γ (PeproTech, Rocky Hill, NJ) for 2–4 days. All the experiments were done with multiple batches of RPE cells independently derived from HES1 or HADC102 cell lines (seven and two RPE batches, respectively).
Immunofluorescent Stainings of RPE Cells
To characterize HLA and RPE markers by immunofluorescence, the RPE cells were plated on poly-D-lysine (30–70 kDa, 10 mg/mL) and laminin (4 mg/mL; both from Sigma, St. Louis, MO) and cultured for 2 weeks in KO medium. The cells were stained with mouse monoclonal anti-human HLA-ABC Ab (1:50; no. 555551) or anti-human HLA-DR, DQ, DP Ab (1:50, no. 555557, both from BD Biosciences, San Jose, CA). Cy3-conjugated goat anti-mouse Ab (1:100; no. 115-165-146, Jackson ImmunoResearch Laboratories, PA) was used for detection. Nuclei were counterstained with DAPI (Vector Laboratories, Burlingame, CA). Immunofluorescent staining for RPE markers was as described (Idelson et al., 2009). The specimens were visualized with Olympus BX61 fluorescent microscope (Olympus, Hamburg, Germany).
Co-culture of PBMCs with RPE Cells
Blood was collected under approval of the Hadassah Medical Center Helsinki Ethics Committee and was supplied from Hadassah or Sheba Medical Centers. Seventeen different PBMCs donors were used in the study. The same donors were used for the experiments with RPE cells derived from HES1 and HADC102 cell lines. Most of the experiments were done with RPE cells derived from HES1 cell line and most of the data summarize the results obtained from at least three different RPE batches and at least three different PBMC donors. The most important results of the study were confirmed in HADC102 line as indicated in the Results section. PBMCs were purified using Histopaque-1077 (Sigma), according to manufacturer's instructions. PBMCs were cultured alone or, alternatively, the cells were added to the wells with pre-seeded RPE cells. In each co-culture experiments, fresh RPE medium was added to PBMCs to control for non-specific effects of the medium on T cell proliferation, apoptosis, secretion of cytokines and activation. PBMCs were co-cultured with RPE cells either directly or using a 0.4 μm transwell tissue culture inserts (24-well plates, Corning). PBMCs were incubated for 1–4 days with or without (w/or w/o) 10 ng/mL anti-CD3 Ab (OKT3, no. 16–0037) and 10 ng/mL anti-CD28 Ab (no. 16–0289, both from eBiosciences, San Diego, CA).
We inhibited TGF-β, FasL, and PDL1 signaling pathways with specific inhibitors and analyzed apoptosis and proliferation of T cells. The following inhibitors were used: neutralizing anti-PDL1 Ab (2–12 μg/mL, no. AF156), neutralizing anti-FasL Ab (10 ng/mL, no. MAB126), recombinant human DcR3/TNFRSF6B chimeric protein acting as an inhibitor of FasL signaling (0.05–0.2 μg/mL, no. 142-DC-100), neutralizing anti-TGF-β1,2,3 Ab (10 μg/mL, no. MAB1835; all the inhibitors from R&D Systems, Minneapolis, MN), and two small molecules inhibiting TGF-β signaling, A-83-01 (0.1–0.5 μM) and SB-431542 (10 or 20 μM; both from Tocris Bioscience, Bristol, UK).
Flow Cytometry
RPE cells w/or w/o IFN-γ pre-treatment were immunostained using either mouse anti-human HLA-ABC (1:2,000, no. 555551) or mouse anti-human HLA-DR, DQ, DP (1:1,200, no. 555557) primary Abs (both from BD Biosciences) or the appropriate isotype control Abs (all from Chemicon International, Temecula, CA), and fluorescein isothiocyanate (FITC)-conjugated polyclonal goat anti-mouse immunoglobulins (1:100, no. F0479; DakoCytomation, Glostrup, Denmark) for detection; 5 mg/mL propidium iodide (PI) (Sigma) served for dead cell exclusion.
PBMCs, non-activated or activated with OKT3 and anti-CD28, that were cultured alone or in the presence of RPE cells, were stained with APC-labeled mouse anti-human CD3 (1:50, no. IQP-519A, IQ Products, Houston, TX, FITC-labeled mouse anti-human CD69 or PE-labeled mouse anti-human CD25 Abs; all 1:50, eBiosciences).
For each sample, at least 104 cells were analyzed on a FACS Calibur flow cytometer (BD Biosciences) using CELLQuest (BD Biosciences) or FCS Express V3 (De Novo Software, Thornhill, ON, Canada) software.
Cytokine Quantification
The amount of cytokines in the samples collected from RPE cells and from PBMCs cultured w/or w/o RPE cells was quantified using commercial human ELISA systems (human IL-15, R&D Systems; human IL-6, IL-10, IL-2, and IFN-γ, Ready-Set-Go, eBioscience; IL-18, Medical and Biological Laboratories, Nagoya, Aichi, Japan).
Determination of T Cell Proliferation
To track PBMCs proliferation, CFSE-labeled (Molecular Probes, Leiden, The Netherlands) PBMCs were cultured w/or w/o RPE cells and activated or not with OKT3 and anti-CD28. After 96 hr, the cells were collected and fluorescence intensity of CD3+ T cells as well as the percentage of dividing cells were determined by flow cytometry.
Determination of T Cell Apoptosis
Flow cytometry analysis was used for the quantification of apoptotic CD3+ T cells after Annexin V-FITC and PI labeling as described in the manufacturer's protocol (MEBCYTO Apoptosis Annexin V-FITC Kit, Medical and Biological Laboratories). As a control, we used PBMCs cultured in the fresh RPE medium. As an additional control, we co-cultured PBMCs with foreskin cells (Figure S4).
PCR Analysis
Total RNA was extracted from RPE cells cultured for 3 days in the presence or absence of IFN-γ using TRIzol (Gibco-BRL). cDNA was synthesized and PCR was carried out as described (Idelson et al., 2009). The list of primer sequences is provided in the Supplemental Experimental Procedures. For qPCR TaqMan Human Cytokine Gene Expression Plate, TaqMan Universal PCR Master Mix and ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) were used.
Transplantation of hESC-RPE Cells into RCS Rats and Their Analysis In Vivo
All animal experiments were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Institutional Committee for Animal Research of the Hebrew University-Hadassah Medical School.
GFP+ cryopreserved hESC-RPE cells were thawed and transplanted into RCS rats as described in Idelson et al. (2009). One day prior to the surgery, animals allocated for cyclosporine therapy were started on a maintenance dose of oral cyclosporine A (210 mg/L; Sandimmune, Novartis Pharma AG, Basel, Switzerland) in the drinking water until sacrificing. In vivo imaging, ERG recordings, and histological and immunohistochemical examinations were done as described previously (Idelson et al., 2009). Mouse anti-GFP FITC-labeled antibody (1:100, no. sc-9996 FITC, Santa Cruz Biotechnology, Santa Cruz, CA) was used for immunohistochemical analysis. Serum samples were analyzed for IL-10 concentration by using ELISA kit (DRG Instruments, Marburg, Germany).
Statistical Methods
All data are presented as means ± SEM of at least three independent experiments. The statistical analysis was performed using the Student's t test.
Author Contributions
M.I. and B.R. designed and analyzed the experiments and wrote the manuscript. M.I. performed the in vitro experiments. N.Y.C., J.R., and E. Beider contributed to the design and analysis of the in vitro experiments. E. Banin designed and analyzed the in vivo experiments. R.A., A.O., and A.E. performed and analyzed the in vivo experiments.
Acknowledgments
We gratefully acknowledge Oliver Stevens (Oxford University), Ariel Berl (Bar Ilan University), Dr. Michal Gropp, Dr. Etti Ben-Shushan, Dr. Hanita Khaner, Prof. Eithan Galun, Evelyne Zeira, Yael Berman-Zaken, Hagit Yotvat, Anastasija Birger, Itai Waldhorn, Dr. Elina Zorde, Dr. Zohar Bromberg, Dr. Doron Kabiri, and Dr. Matan Elami-Suzin (Hadassah Medical Center) for technical support. We thank Shelly Tannenbaum (Hadassah Medical Center) for editing the manuscript. We thank Prof. Amnon Peled (Hadassah Medical Center) and Dr. Osnat Bohana-Kashtan (CellCure Neurosciences) for discussion. This research was supported by a kind donation of Judy and Sidney Swartz, The Foundation Fighting Blindness Grant (TA-RA-0321-0568-HUJ), a GT Foundation Grant, a Moxie Foundation Grant, and a Yedidut Research Grant.
Benjamin Reubinoff is a founder and chief scientific officer of and holds shares in CellCure Neurosciences. The focus of CellCure Neurosciences is the development of hESC-derived RPE cells for cell therapy in retinal degeneration. The study was not funded by CellCure Neurosciences, and scientists from the company did not participate in the study.
Published: August 16, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.07.009.
Supplemental Information
References
- Chao K.H., Wu M.Y., Yang J.H., Chen S.U., Yang Y.S., Ho H.N. Expression of the interleukin-2 receptor alpha (CD25) is selectively decreased on decidual CD4+ and CD8+ T lymphocytes in normal pregnancies. Mol. Hum. Reprod. 2002;8:667–673. doi: 10.1093/molehr/8.7.667. [DOI] [PubMed] [Google Scholar]
- Cortes J.R., Sanchez-Diaz R., Bovolenta E.R., Barreiro O., Lasarte S., Matesanz-Marin A., Toribio M.L., Sanchez-Madrid F., Martin P. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 2014;55:51–62. doi: 10.1016/j.jaut.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa M., Rutz S., Dorninger H., Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- de Oliveira C.M., Sakata R.K., Issy A.M., Gerola L.R., Salomao R. Cytokines and pain. Rev. Bras. Anestesiol. 2011;61:255–259. doi: 10.1016/S0034-7094(11)70029-0. 260-255, 137-242. [DOI] [PubMed] [Google Scholar]
- Elner S.G., Elner V.M., Pavilack M.A., Todd R.F., 3rd, Mayo-Bond L., Franklin W.A., Strieter R.M., Kunkel S.L., Huber A.R. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab. Invest. 1992;66:200–211. [PubMed] [Google Scholar]
- Furtado G.C., Curotto de Lafaille M.A., Kutchukhidze N., Lafaille J.J. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J. Exp. Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelson M., Alper R., Obolensky A., Ben-Shushan E., Hemo I., Yachimovich-Cohen N., Khaner H., Smith Y., Wiser O., Gropp M. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Imai A., Sugita S., Kawazoe Y., Horie S., Yamada Y., Keino H., Maruyama K., Mochizuki M. Immunosuppressive properties of regulatory T cells generated by incubation of peripheral blood mononuclear cells with supernatants of human RPE cells. Invest. Ophthalmol. Vis. Sci. 2012;53:7299–7309. doi: 10.1167/iovs.12-10182. [DOI] [PubMed] [Google Scholar]
- Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A., Wiencke A.K., la Cour M., Kaestel C.G., Madsen H.O., Hamann S., Lui G.M., Scherfig E., Prause J.U., Svejgaard A. Human retinal pigment epithelial cell-induced apoptosis in activated T cells. Invest. Ophthalmol. Vis. Sci. 1998;39:1590–1599. [PubMed] [Google Scholar]
- Kaestel C.G., Jorgensen A., Nielsen M., Eriksen K.W., Odum N., Holst Nissen M., Ropke C. Human retinal pigment epithelial cells inhibit proliferation and IL2R expression of activated T cells. Exp. Eye Res. 2002;74:627–637. doi: 10.1006/exer.2002.1183. [DOI] [PubMed] [Google Scholar]
- Kaestel C.G., Madsen H.O., Prause J.U., Jorgensen A., Liang Y., la Cour M., Lui G.M., Odum N., Nissen M.H., Ropke C. Lack of FasL expression in cultured human retinal pigment epithelial cells. Exp. Clin. Immunogenet. 2001;18:34–41. doi: 10.1159/000049085. [DOI] [PubMed] [Google Scholar]
- Kamao H., Mandai M., Okamoto S., Sakai N., Suga A., Sugita S., Kiryu J., Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazoe Y., Sugita S., Keino H., Yamada Y., Imai A., Horie S., Mochizuki M. Retinoic acid from retinal pigment epithelium induces T regulatory cells. Exp. Eye Res. 2012;94:32–40. doi: 10.1016/j.exer.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lin E., Calvano S.E., Lowry S.F. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117–126. doi: 10.1067/msy.2000.101584. [DOI] [PubMed] [Google Scholar]
- Lu B., Malcuit C., Wang S., Girman S., Francis P., Lemieux L., Lanza R., Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- Lund R.D., Wang S., Klimanskaya I., Holmes T., Ramos-Kelsey R., Lu B., Girman S., Bischoff N., Sauve Y., Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- McGill T.J., Bohana-Kashtan O., Stoddard J.W., Andrews M.D., Pandit N., Rosenberg-Belmaker L.R., Wiser O., Matzrafi L., Banin E., Reubinoff B. Long-term efficacy of GMP grade xeno-free hESC-derived RPE cells following transplantation. Transl. Vis. Sci. Technol. 2017;6:17. doi: 10.1167/tvst.6.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morescalchi F., Duse S., Gambicorti E., Romano M.R., Costagliola C., Semeraro F. Proliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloid. Mediators Inflamm. 2013;2013:269787. doi: 10.1155/2013/269787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osusky R., Dorio R.J., Arora Y.K., Ryan S.J., Walker S.M. MHC class II positive retinal pigment epithelial (RPE) cells can function as antigen-presenting cells for microbial superantigen. Ocul. Immunol. Inflamm. 1997;5:43–50. doi: 10.3109/09273949709085049. [DOI] [PubMed] [Google Scholar]
- Reubinoff B.E., Pera M.F., Fong C.Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Saldanha-Araujo F., Haddad R., Farias K.C., Souza Ade P., Palma P.V., Araujo A.G., Orellana M.D., Voltarelli J.C., Covas D.T., Zago M.A. Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: roles of canonical and non-canonical NF-kappaB signalling. J. Cell. Mol. Med. 2012;16:1232–1244. doi: 10.1111/j.1582-4934.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D., Gomez M., Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Sancho D., Yanez-Mo M., Tejedor R., Sanchez-Madrid F. Activation of peripheral blood T cells by interaction and migration through endothelium: role of lymphocyte function antigen-1/intercellular adhesion molecule-1 and interleukin-15. Blood. 1999;93:886–896. [PubMed] [Google Scholar]
- Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Sohn E.H., Jiao C., Kaalberg E., Cranston C., Mullins R.F., Stone E.M., Tucker B.A. Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: a pilot study. Sci. Rep. 2015;5:11791. doi: 10.1038/srep11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.K., Park K.M., Kim H.J., Lee J.H., Choi J., Chong S.Y., Shim S.H., Del Priore L.V., Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Horie S., Yamada Y., Mochizuki M. Inhibition of B-cell activation by retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2010;51:5783–5788. doi: 10.1167/iovs.09-5098. [DOI] [PubMed] [Google Scholar]
- Sugita S., Iwasaki Y., Makabe K., Kimura T., Futagami T., Suegami S., Takahashi M. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Reports. 2016;7:619–634. doi: 10.1016/j.stemcr.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Kamao H., Iwasaki Y., Okamoto S., Hashiguchi T., Iseki K., Hayashi N., Mandai M., Takahashi M. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Invest. Ophthalmol. Vis. Sci. 2015;56:1051–1062. doi: 10.1167/iovs.14-15619. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S.E., Turetsky T.T., Singer O., Aizenman E., Kirshberg S., Ilouz N., Gil Y., Berman-Zaken Y., Perlman T.S., Geva N. Derivation of xeno-free and GMP-grade human embryonic stem cells – platforms for future clinical applications. PLoS One. 2012;7:e35325. doi: 10.1371/journal.pone.0035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.W. Alpha-melanocyte stimulating hormone (alpha-MSH) is a post-caspase suppressor of apoptosis in RAW 264.7 macrophages. PLoS One. 2013;8:e74488. doi: 10.1371/journal.pone.0074488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugler A., Carr A.J., Lawrence J., Chen L.L., Burrell K., Wright A., Lundh P., Semo M., Ahmado A., Gias C. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp. Neurol. 2008;214:347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Werfel T., Boeker M., Kapp A. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy. 1997;52:465–469. doi: 10.1111/j.1398-9995.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Willermain F., Caspers-Velu L., Baudson N., Dubois C., Hamdane M., Willems F., Velu T., Bruyns C. Role and expression of CD40 on human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2000;41:3485–3491. [PubMed] [Google Scholar]
- Yachimovich-Cohen N., Even-Ram S., Shufaro Y., Rachmilewitz J., Reubinoff B. Human embryonic stem cells suppress T cell responses via arginase I-dependent mechanism. J. Immunol. 2009;184:1300–1308. doi: 10.4049/jimmunol.0804261. [DOI] [PubMed] [Google Scholar]
- Zhao T., Zhang Z.N., Westenskow P.D., Todorova D., Hu Z., Lin T., Rong Z., Kim J., He J., Wang M. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 2015;17:353–359. doi: 10.1016/j.stem.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Cifuentes H., Reynolds J., Lamba D.A. Immunosuppression via loss of IL2rgamma enhances long-term functional integration of hESC-derived photoreceptors in the mouse retina. Cell Stem Cell. 2017;20:374–384.e5. doi: 10.1016/j.stem.2016.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.