Abstract
Lipid microdomains (“rafts”) are dynamic, nanoscale regions of the plasma membrane enriched in cholesterol and glycosphingolipids, that possess distinctive physicochemical properties including higher order than the surrounding membrane. Lipid microdomain integrity is thought to affect neurotransmitter signaling by regulating membrane-bound protein signaling. Among the proteins potentially affected are monoaminergic receptors and transporters. As dysfunction of monoaminergic neurotransmission is implicated in major depressive disorder and other neuropsychiatric conditions, interactions with lipid microdomains may be of clinical importance. This systematic review evaluates what is known about the molecular relationships of monoamine transporter and receptor regulation to lipid microdomains. The PubMed/MeSH database was searched for original studies published in English through August 2017 concerning relationships between lipid microdomains and serotonin, dopamine and norepinephrine transporters and receptors. Fifty-seven publications were identified and assessed. Strong evidence implicates lipid microdomains in the regulation of serotonin and norepinephrine transporters; serotonin 1A, 2A, 3A, and 7A receptors; and dopamine D1 and β2 adrenergic receptors. Results were conflicting or more complex regarding lipid microdomain associations with the dopamine transporter, D2, D3, and D5 receptors; and negative with respect to β1 adrenergic receptors. Indirect evidence suggests that antidepressants, lipid-lowering drugs, and polyunsaturated fatty acids may exert effects on depression and suicide by altering the lipid milieu, thereby affecting monoaminergic transporter and receptor signaling. The lipid composition of membrane subdomains is involved in localization and trafficking of specific monoaminergic receptors and transporters. Elucidating precise mechanisms whereby lipid microdomains modulate monoamine neurotransmission in clinical contexts can have critical implications for pharmacotherapeutic targeting.
Introduction
Plasma membrane structure–function relationships
A growing area of research over the last two decades concerns structural and functional characteristics conferred by the lipid composition of plasma membranes. The distinct physicochemical properties of different lipid species are accommodated in ways that impose organization on the membrane, with consequences for the distribution and regulation of proteins within the membrane bilayer [1].
Studies in model membrane systems show that lipid domains rich in sphingolipids and cholesterol are highly ordered, existing in a tightly-packed, gel-like state with an aversion to the unsaturated acyl chains of glycerophospholipids, which occur in a loosely-packed, liquid disordered state (reviewed in refs. [2, 3]). These observations support the paradigm that membrane lipid microdomains (lipid rafts) exist as physically and temporally dynamic, nanoscale membrane assemblies enriched in cholesterol, sphingolipids, and glycosylphosphatidylinositol (GPI)-anchored proteins [4].
Lipid microdomains also have been studied in cell membranes [5–9], leading to a proposed categorization into caveolar and non-caveolar, or planar, rafts [10]. Both raft types exhibit high cholesterol and sphingomyelin content, enrichment in GPI-anchored proteins, cytoskeletal association, and resistance to detergent extraction. Most rafts are reported to be of the caveolar type. Caveolae are small, flask-shaped invaginations of the membrane that function in cell signaling, lipid trafficking, and endocytosis, an important mechanism for downregulating cell-surface levels of proteins such as transporters. Key relevant characteristics are the absence of clathrin in caveolar endocytosis and the presence of caveolin and cavin proteins [11, 12], which are present in brain [11]. Another type of raft-related, non-clathrin-mediated endocytotic mechanism involves flotillin proteins (also known as reggies) that are also present in the central nervous system [13, 14] and analogous functionally, but not homologous structurally, to caveolin [15]. Caveolins and flotillins serve a scaffolding/organizing function, anchoring signaling components to lipid microdomains [10]. In addition to stimulating movement into the cell by endocytosis across the membrane, neurotransmitter signaling also can cause movement of signaling proteins into or out of lipid microdomains by translocation or lateral movement within the membrane bilayer [16].
However, countervailing structural models also have been proposed. One alternative is a continuous bilayer with an outer membrane leaflet rich in cholesterol and sphingolipids, with a large ordered fraction of the plasma membrane [17, 18]. In another suggested model, sphingolipids form distinct domains not dependent on cholesterol [19], and a strong role for the actin cytoskeleton is implicated [19–23]. Some investigators propose that lipid domains are larger than nanoscale assemblies and are not organized on the basis of phase-differentiation [24]. The most extreme view suggests that microdomains do not exist but are an artifact of the methods used to study them [21, 24]. Finally, others have attempted to reconcile disparate observations into an integrative model [4].
This controversy is fueled by the uncertain validity of extrapolating information from artificial model membranes, combined with technical difficulties inherent in the study of submicroscopic, temporally dynamic nanostructures [4]. Common methods used to isolate lipid microdomain-containing cellular fractions have drawbacks; notably, the use of nonionic detergent-resistant membranes (DRM) [25, 26] produce results that are inconsistent [27] and are not be adequately representative of in vivo lipid microdomains [28]. Evidence that the detergent itself induces artifacts was presented over a decade ago [29]. Possibly less perturbative methods for isolating raft-like domains include sucrose gradient ultracentrifugation [30], which ostensibly avoids the pitfalls of DRM, but since this technique includes treating the membranes with alkaline buffer, it may cause saponification, essentially retaining a detergent-like effect. Cholesterol depletion is commonly used under the assumption that subsequent functional loss is evidence for lipid microdomain disruption. Disruptive techniques include: (1) sequestration of cholesterol using filipin or nystatin [31]; (2) depletion and removal of cholesterol using methyl-β-cyclodextrin [32]; (3) inhibition of cholesterol synthesis using HMG-CoA reductase inhibitors (statins) [33]; and (4) genetic approaches using caveolin-1 knockout mice [11] or caveolin RNA interference (siRNA) [34]. However, because the use of cholesterol-disrupting, and thus lipid raft-disrupting, drugs can produce pleiotropic effects, including effects on non-raft domains [32] and on the actin cytoskeleton [23], data from cholesterol depletion experiments ideally require validation. The restoration of cholesterol or replacement with its analog, desmosterol (a non-raft promoting sterol) is sometimes used as an experimental control. Depletion experiments can likewise clarify the role of sphingolipids, using Fumonisin B1 [35, 36], and actin, using the actin filament disrupter cytochalasin D [16].
The resolution of these controversies will require continuing development of technologies to more directly study the lipid–protein milieu, such as super-resolution microscopy coupled with fluorescence and bioluminescence techniques [37] harnessed to protein-conjugated quantum dots, which enables nanoscale and microscale quantitation and visualization of receptor clustering in living cells [38]. Sophisticated techniques also include stimulated emission depletion (STED) super-resolution nanoscopy [39], Förster resonance energy transfer (FRET) [40, 41], fluorescence correlation spectroscopy (FCS) [39], k-space image correlation spectroscopy (kICS) [42], and magic-angle spinning nuclear magnetic resonance (HR-MAS NMR) [43, 44], each of which can provide valuable information on the nanometer scale.
Structural characteristics of lipid microdomains are understood to influence plasma membrane proteins in several ways, including (1) providing a scaffolding for spatially co-localizing the membrane proteins with other elements important in cell-cell signaling [45, 46]; (2) affecting lateral movement of proteins [47]; (3) altering molecular trafficking, e.g., endocytosis of proteins from the cell membrane surface to the cell interior [48–50] to achieve receptor recycling, sequestration, and/or downregulation; and (4) directly regulating activity, e.g., modulation of the transport rate or affinity of transporter proteins for substrates by the relative amounts of cholesterol [51, 52].
Lipid microdomains as regulators of monoaminergic signaling
Among membrane proteins affected by lipid microdomains are a variety of monoamine transporters and receptors, important for emotion regulation. Monoamine neurotransmitters comprise a key family of neural signaling proteins, including serotonin (5-hydroxytryptamine, 5-HT), dopamine (DA), and norepinephrine (NE). Serotonin modulates mood, aggression, motivation, appetite, sleep, cognition, and sexual activity; and altered 5-HT signaling has been implicated in neuropsychiatric diseases [53–57]. DA systems control motor function, mood, reward, and cognition [58, 59], dysregulation of which is linked to attention deficit hyperactivity disorder (ADHD), schizophrenia, substance use disorders, and Parkinson’s disease [60]. NE regulates arousal, mood, attention, and stress-responsiveness [61–64].
Monoaminergic transporters and receptors
Plasma membrane monoamine transporters govern the effects of their respective neurotransmitters within the synaptic cleft by recycling them back into the synaptic boutons, with direct effects on intra-synaptic neurotransmitter concentrations and downstream indirect effects on monoaminergic receptors [65, 66]. Thus, altering the quantity of functional transporters at the neuronal or glial plasma membrane represents one mechanism for regulating synaptic levels of neurotransmitters [67]. These transporters are integral membrane phosphoproteins affected by kinase and phosphatase activities (reviewed in ref. [67]) and are also pharmacological targets of many psychoactive drugs [68–70]. Studies in knockout mice have demonstrated that the mechanisms regulating monoamine neurotransmitter biosynthesis and storage, receptor sensitivity, and transporter expression are interdependent [71–78]. A deeper understanding of lipid microdomain regulation of monoamine transporter function is likely to foster new treatment targets for neuropsychiatric disease.
Monoaminergic receptors exist in multiple subtypes and may be pre-synaptic or post-synaptic. With the exception of 5-HT3, monoaminergic receptors belong to the G-protein-coupled receptor superfamily, with distinct receptor subtype specificity for G-proteins. Depending on receptor subtype and the concomitant G-protein, agonist binding to monoaminergic receptors activates or inhibits second messengers adenylyl cyclase (AC) and cyclic AMP (cAMP), or inositol triphosphate and diacylglycerol.
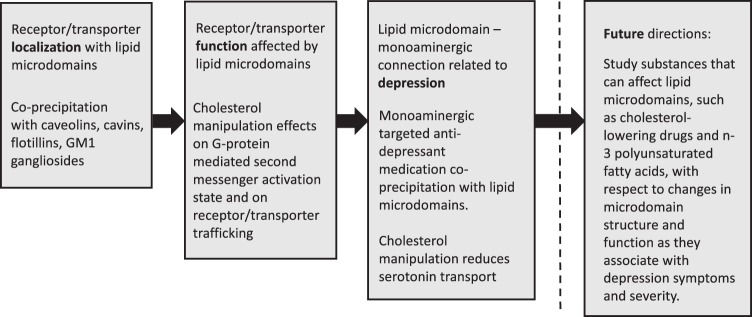
We summarize the literature concerning lipid raft involvement in monoaminergic transporter and receptor regulation and depression (Fig. 1), including (1) co-localization of transporters/receptors with lipid microdomains, ganglioside GM1, and raft-associated proteins in detergent-resistant membranes or low-buoyancy sucrose fractions; (2) lateral transporter movement; (3) association of transporters/receptors with caveolar proteins; (4) lipid influences on receptor transport rate; (5) effects on agonist and antagonist receptor binding; (6) downstream effects on second messengers; and (7) sensitivity to effects of cholesterol depletion (Fig. 1).
Fig. 1.
Trajectory of research on lipid microdomain relationships with monoaminergic receptors/transporters and depression
Methods
Literature search strategy
Animal and human experimental studies on monoamine neurotransmitters, receptors and transporter proteins, and lipid microdomains published in English through August 2017 were identified by searching with MEDLINE Medical Subject Heading (MeSH) terms. In addition, general PubMed searches were conducted. Duplicate papers were removed, and articles were excluded if not clearly germane. The reference lists of prior reviews and research articles were also manually searched to find other potentially relevant studies (see detailed strategy, Table 1.)
Table 1.
Lipid microdomain localization, trafficking, and regulation of monoamine neurotransmitter transporters and receptors
| Study biosystem | Lipid raft co-localization | Effects on receptor/transporter function | Effects of cholesterol manipulation | Lipid raft-mediated mechanism of downregulation | ||
|---|---|---|---|---|---|---|
| Cholesterol depletion (methyl-β-cyclodextrin) | Cholesterol sequestration (filipin, nystatin) | Cholesterol repletion (cholesterol, desmosterol, ergosterol, epicholesterol) | ||||
| Transporters | ||||||
| SERT | ||||||
| •Rat midbrain synaptosomes [90] •RN46A cell line [16] •T-REx [51] •HEK-293 [51, 52] and •RN46A-B14 [51] cells |
•Cav-1 and ganglioside GM1 [90] •Raft-associated SERT are confined in terms of lateral mobility [16] |
•Localization in lipid rafts is essential for serotonin uptake [51] | •Increased lateral mobility [16] •Decreased 5-HT transport rate (Vmax) and altered affinity (Km) [51, 52] |
•Treatment with filipin resulted in decreased affinity for agonist and antagonist binding [52] | •Adding cholesterol, but not other sterols, to depleted membranes restored SERT activity [52] | •PKC-mediated movement from lipid microdomains to non-raft membrane domains [90] |
| DAT | ||||||
| •Lewis Lung Carcinoma-Porcine kidney (LLC-PK1) cells [99] •HEK-293 cells [100, 105, 107, 109, 110] •Pig kidney epithelial cells (LLC-PK) [100] •Rat striatal synaptosomes and immortalized mouse midbrain neuron MN9D cells [105] •Primary mesencephalic •Dopaminergic neurons [107] •HeLa cells [107] •Neuroblastoma (N2a) cells [101] •Porcine aortic endothelial (PAE) cells [102] •Madin–Darby canine kidney (MDCK) cells [104] •PC12 cells [106] •Human neuroblastoma (SK-N-MC) cells and murine striatal slices [108] |
•Equally distributed across raft and non-raft domains [99] | •Outward-facing structural conformation increases DA uptake [100, 105] •Some conflict: •Flotillin depletion does not affect DAT endocytosis but does increase lateral diffusion rates [110] •Flot1 is required for DAT internalization [107] |
•Decreased transport rate (Vmax) and affinity (Km) for DA uptake [101, 107] and efflux [100] •No change in surface expression [99, 101] |
•No effect of nystatin on DA uptake and efflux rates [100, 102] | •Desmosterol restored transport rates [100] | •DAT substrates including DA promote internalization and thus diminishing transport capacity [109] Some conflict: •Clathrin/dynamin-dependent (i.e., non-raft) internalization process [99, 102, 104] •Linked to GTPase (Rin or Rit2) [106] and Flot1 [107] (both localized to lipid rafts) •Different pathways may exist for the “raft” and “non-raft” populations [99] •Basal internalization from raft and non-raft domains. PKC-stimulated internalization only from lipid raft domains [108] |
| NET | ||||||
| •Rat [113] and human [46] placental trophoblast cells •Rat brain synaptosomes [46] |
•Cholesterol-rich lipid rafts [113] | — | — | •Filipin and nystatin block internalization [113] | — | •Dynamin-, clathrin-, and caveolar independent, cholesterol-rich lipid rafts mediate PKC-initiated internalization process [113] •NET complexes with NK1R resulting in redistribution to lipid rafts and internalization [46] |
| Receptors | ||||||
| Serotonin | ||||||
| 5-HT1A | ||||||
| •NIH-3T3 and neuroblastoma N1E-115 cells [93] •Amino acid sequences of GPCRs from NCBI database [120]; •Model membrane systems [121] •Bovine hippocampal membranes [118] |
•Detergent-resistant fraction [93, 117] along with Cav-1[93] •Exhibits a cholesterol consensus motif indicating a cholesterol binding site [120] •Lipid raft localization dependent on palmitoylation [93] |
•Greater membrane order results in increased receptor activity [121] | •Decreased agonist [118] and antagonist [119] binding and signaling •Altered G-protein coupling [118] |
— | •Cholesterol replenishment counteracts methyl-β-cyclodextrin effects on ligand binding and membrane order [118] | — |
| 5-HT2A | ||||||
| •C6 glioma cells, transfected HEK-293 cells, and rat brain synaptic membrane preparations [34] •Rat tail artery devoid of endothelium [122] •H9C2 rat embryonic myoblast-derived cell line [123] •Bovine tracheal smooth muscle [124] |
Some conflict: •Co-localizes with Cav-1 [34, 122], and Cav-3 [123], •Non-caveolar [124] |
•Inhibits expression of the ANF gene [123] •Inhibits activation of nuclear factor of activated T cells in cardiac myocytes [123] •Stimulates calcium flux in synaptic membranes [34] |
•Disrupted physiologic response to stimulation by 5-HT [122, 124] | — | •Cholesterol-loaded methyl-β-cyclodextrin partially restored [124] and exogenous cholesterol restored [122] physiologic response to stimulation |
Some conflict: •Caveolar-dependent upon 5-HT stimulation [123] •Co-localized with Cav-1 but 5-HT stimulation did not induce internalization [34] |
| 5-HT3 | ||||||
| •N1E-115 and HEK-5-HT3A cells [126–128] and •Hippocampal neurons [128] | •Localized in low buoyant sucrose gradient fraction along with cholesterol, Cav-2, Flot1, and multiple psychotropic medications [127] | •Psychotropic drug concentrations in microdomain fractions correlate with magnitude of non-competitive inhibition of 5-HT-induced cation currents [127, 128] •Antidepressant antagonism of 5-HT3 is independent of lipid subdomains [126–128] |
•Decreased functioning by reducing peak amplitude and kinetics of 5-HT stimulated cation currents [126] | — | — | •Non-competitive 5-HT3 functional antagonism does not induce internalization [127] |
| 5-HT7 | ||||||
| •HeLa cells stably transfected with the human 5-HT7 receptor [33, 36] | •Localized by detergent extraction with Cav-1 [36, 129] | •Decreased 5-HT7 agonist and antagonist binding; decreased Bmax without change in 5-HT binding affinity; and decreased 5-HT-induced CREB phosphorylation [33] | •Cholesterol [36, 129] and sphingomyelin [36, 129] depletion affect binding of 5-HT to 5-HT7 | — | — | •Clathrin-independent internalization [36, 129] |
| Dopamine | ||||||
| D1-class | ||||||
| D1 | ||||||
| •Rat frontal cortex [80]; •HEK-293 [35, 134], •hRPT [136], N2a, and COS-7 [135] cells |
•Predominantly localized to lipid microdomains [80] along with Gαs and Gαi3 as well as Cav-2 and Flot1 [81] •Localization depends on the receptor conformation [35] |
•Receptor binding of D1-class agonist increases adenylyl cyclase exclusively in lipid raft domains [136] | •In HEK cells molecular size of adenylyl cyclase was reduced; in hRPT cells, all adenylyl cyclase was shifted to non-lipid rafts [136] | — | — | Some conflict: •Clathrin-dependent [134] and caveolar-dependent [81, 135] endocytosis have been reported. Possible cell line-specific differences |
| D5 | ||||||
| •Rat [80] and primate [138] prefrontal cortex; •HEK-293 cells [136] |
•Predominantly in cytoplasmic fractions and not present in density gradient buoyant fraction lipid raft-like subdomains [80, 138] | •Enhancement of anti-oxidizing enzyme paraoxonase [139] and adenylyl activity [136] does not take place in lipid raft domains | •Reduced adenylyl cyclase activity in cells heterologously expressing D5 [136] | — | — | — |
| D2-class | ||||||
| D2 | ||||||
| •Rat prefrontal cortex [80] •Mouse striatum [141]; •HEK-293 [79, 140, 141], •CHO [142, 144], and •COS-7 [143, 144] cells |
•Diffusely distributed across membrane domains [80] Some conflict: •Not found in buoyant fractions [80] •Majority found in detergent-insoluble fraction [140, 141] |
•Presence of dopamine decreased levels of detergent-insoluble plasma membrane-expressed receptor by >50% [140] •Activation of D2 causes G-protein-coupled receptor kinase-dependent receptor phosphorylation and robust receptor internalization [144] |
•Did not increase the detergent solubility of D2 [140] | — | — | •Both caveolar-dependency [142] based on glycosylation state [79], and dynamin-dependency [143, 144] have been reported |
| D3 | ||||||
| •CHO [144], •HEK-293 [79, 146] •hRPT [145], and •COS-7 [146] cells |
•Co-localizes with G-protein-coupled receptor kinase 4 predominately in non-raft fractions but also (10%) in raft fractions with Cav-1 [145] | — | •Redistribution of raft-associated receptor, Cav-1, and G-protein-coupled receptor kinase 4 (GRK4) to non-raft fractions [145] | — | — | •Clathrin-dependent (glycosylation of receptor is essential mediator) [79], consistent with dynamin-dependency [144] •Low susceptibility to downregulation through internalization [144, 146] |
| Norepinephrine | ||||||
| β1-AR | ||||||
| •Neonatal myocytes isolated from β1-AR and β2-AR knockout mice [147] | •β1-AR is primarily located in non-raft membrane regions [147] | — | — | •Filipin has no effect on β1-AR [147] | — | •Agonist stimulation does not affect abundance of β1-AR [150] |
| β2-AR | ||||||
| •Neonatal mouse [147] and rat [149, 150] myocytes •Adult rat ventricular myocytes [156] •HEK-293 [148, 154, 155] cells •Sf9 insect cell membranes [153] •Rat H9c2(2-1) myoblast cells [152] |
•Co-localized into caveolae shown by confocal imaging [147] and co-immunoprecipitation with Cav-3 [147], flotillin and AC [150] and other signaling components [148, 149] •β2-AR exhibits a cholesterol consensus motif indicating a cholesterol binding site [120, 153] Conflicting results: •Confinement of β2-AR does not depend on caveolae but rather on scaffold proteins [152] |
•Redistribution of β-AR out of caveolae inhibits cAMP signaling [150] •This is consistent with findings that overexpression of Cav-3 results in increased caveolae, lower β-AR and decreased downstream cAMP signaling; and blocking Cav-3 expression upregulates the cAMP signal [156] |
•AC activity and cAMP production are variously reported as inhibited [149], unaffected [148], or enhanced [150, 154, 155] •Decreased β2-AR-induced ERK phosphorylation but this may be non-specific to caveolae [148] •Lateral diffusion of β2-AR was decreased [148] |
Filipin reduces co-immunoprecipitation of β-AR with Cav-3 [147] | — | •Agonist stimulation causes β2-AR to redistribute out of caveolae [150] |
Articles were identified by searching with the following MEDLINE Medical Subject Heading (MeSH) terms: (“membrane microdomains” OR “caveolae”) AND (“biogenic monoamines” OR “catecholamines” OR “tryptamines” OR “serotonin” OR “receptors, serotonin” OR “serotonin plasma membrane transport proteins” OR “dopamine” OR “receptors, dopamine” OR “dopamine plasma membrane transport proteins” OR “norepinephrine” OR “receptors, adrenergic” OR “norepinephrine plasma membrane transport proteins”). In addition, general PubMed searches were conducted using the following search terms as key words in titles/abstracts: (“membrane microdomain” OR “lipid rafts” OR “caveolae” OR “signalosomes” OR “cholesterol-enriched domains” OR “ordered domains”) AND (“biogenic monoamines” OR “catecholamines” OR “tryptamines” OR “serotonin receptor” OR “serotonin transporter” OR “dopamine receptor” OR “norepinephrine transporter” OR “norepinephrine receptor” OR “adrenergic receptor”)
— Effects have not yet been studied or determined, β-AR beta-adrenergic receptors, 5-HT serotonin, 5-HT1A serotonin receptor subtype 1A, 5-HT2A serotonin receptor subtype 2A, 5-HT3gA serotonin receptor subtype 3A, 5-HT7A serotonin receptor subtype 7A, AC adenylyl cyclase, ANF atrial natriuretic factor, cAMP cyclic adenosine monophosphate, C6 rat glioma cell line, Cav caveolin, COS-7 an acronym for fibroblast-like cells derived from simian kidney tissue, Cv-1 in Origin carrying monkey virus Sv40 genetic material, CHO Chinese hamster ovary cells, D1 dopamine receptor subtype 1, D2 dopamine receptor subtype 2, D3 dopamine receptor subtype 3, D5 dopamine receptor subtype 5, DA dopamine, DAT dopamine transporter, Flot1 flotillin-1, HEK-293 human embryonic kidney cells, hRPT human renal proximal tubule, MCF-7 human breast adenocarcinoma cell line, NE norepinephrine, NET norepinephrine transporter, NK1R neurokinin-1 receptor, RN46A immortalized serotonergic neural cell line, SERT serotonin transporter, Sf9 Spodoptera frugiperda insect cells
Results
Literature search
Combined PubMed/MeSH searches identified 250 potentially eligible reports, of which 27 review articles were eliminated; 183 were excluded as not directly concerning monoaminergic systems, were focused narrowly on non-brain systems, or were primarily concerned with other elements of the signaling pathways, yielding 37 articles. Twenty additional studies were identified through manual bibliography searching, yielding 57 reports considered in the Results section of this systematic review, which we view as appropriately summarizing the current understanding of lipid microdomain associations with monoaminergic transporters and receptors. Of these investigations into the interactions with lipid microdomains, 21 studies reported on serotonergic systems [16, 33, 34, 36, 51, 52, 90, 93, 117–124, 126–129]; 25 reported on dopaminergic systems [35, 79–81, 99–102, 105–108, 110, 134–136, 138–146]; and 12 reported on noradrenergic systems [46, 113, 147–156].
Monoamine transporters and lipid microdomains
Transporter relationship to plasma membrane is modulated by phosphatidylinositol 4,5-bisphosphate (PIP2)
The monoaminergic transporters for serotonin (SERT), dopamine (DAT), and norepinephrine (NET) are all examples of neurotransmitter:sodium symporters with a 12-transmembrane domain structure that form oligomers in intact cells [82, 83]. One important mechanism whereby these transporters are anchored in lipid microdomains is through binding to PIP2 [84]. Found in low concentration in lipid microdomains [85, 86], in the cytoplasmic leaflet of the cell membrane [87], PIP2 nevertheless has an important role in monoaminergic signaling by stabilizing transporter oligomerization [84, 88, 89] and regulating neurotransmitter efflux [88, 89].
SERT
Co-localization of SERT with caveolin and the ganglioside known as GM1 has been demonstrated in rat brain synaptosomes [90]. SERT proteins exist in two states with respect to their degree of lateral mobility: more mobile transporters that can diffuse relatively freely, and less mobile transporters localized to cholesterol and GM1 ganglioside-enriched microdomains [16]. In RN46A serotonergic neuronal cells labeled with quantum dots conjugated to the SERT ligand IDT318, confocal imaging reveals SERT clusters in cholesterol-rich membrane microdomains. Studied before and after cholesterol depletion with methyl-β-cyclodextrin, SERT molecules evince greater lateral movement after reduction of cholesterol, consistent with lipid raft disaggregation. In addition, actin filaments have a role in anchoring SERT molecules via their C terminus, since the actin filament disrupter cytochalasin D also results in increased SERT diffusion rates [16].
Increased SERT mobility after cholesterol depletion or actin disruption is accompanied by decreased transport activity [16]. Functional changes induced by cholesterol depletion result in a mean decrease of 50% in the transport rate (Vmax) of SERT [51, 52], and a concurrent reduction in SERT affinity (Km) for 5-HT, suggesting that lipid microdomains may regulate SERT by promoting a high-affinity state [51, 52]. Consistent with this hypothesis, signaling pathways that upregulate SERT activity, particularly p38 MAPK, act on SERT localized within membrane microdomains [16]. Conversely, in synaptosomes, activation of PKC downregulates SERT, causing redistribution from lipid raft to non-raft fractions [90].
Trafficking of SERT appears to be homeostatically regulated, as platelet studies have found that application of 5-HT variously induces SERT upregulation [91, 92] or downregulation [93, 94], which may depend on complex factors including 5-HT concentration and acute vs. chronic exposure [94]. SERT downregulation can be mediated by 5-HT binding to 5-HT2A autoreceptors via activation of Gαq proteins and downstream PKC activation [95, 96]. PKC exerts its effects on SERT by phosphorylating the transporter [97]; under some conditions, the SERT internalization can be blocked by 5-HT [98]. An analogous process has been reported for DAT (see below).
DAT
DAT are equally distributed across raft and non-raft domains [99], and their function is regulated by cholesterol [100]. Cholesterol depletion with methyl-β-cyclodextrin decreases Vmax and Km values for DA uptake [101] and efflux [100, 101] via DAT. However, in contrast to the SERT findings, cholesterol effects on DAT may not be mediated by caveolar endocytosis, as DAT downregulation induced by methyl-β-cyclodextrin does not occur by reduction of surface DAT expression [99, 101]. Moreover, although methyl-β-cyclodextrin depletes cholesterol across both raft and non-raft membrane domains [32], cholesterol chelation with nystatin or filipin primarily disrupts raft-localized cholesterol [31] and has no effect on DA uptake and efflux rates [100, 102]. Additionally, supranormal repletion with desmosterol—a sterol that does not form highly ordered domains and thus does not affect lipid rafts [103]—was as effective as cholesterol in restoring DAT transport rates [100]. Finally, DAT is internalized in a clathrin/dynamin-dependent process [104]. As an alternative explanation to lipid raft- mediated effects, studies with DAT mutations suggest that cholesterol may impact DA uptake by promoting an outward-facing conformation [100, 105].
Contradictory evidence exists concerning a lipid raft role in DAT endocytosis. Lipid raft-mediated DAT endocytosis is linked to the GTPase known as Rin or Rit2 [106], and to flotillin-1 [107], both reportedly localized in raft domains. Different regulatory pathways have been suggested for the “raft” and “non-raft” DAT populations [99], since basal DAT internalization occurs similarly from raft and non-raft domains, while PKC-stimulated DAT internalization occurs only from lipid raft domains [108]. The latter finding does not agree with reports that (1) the clathrin-mediated (i.e., non-raft) endocytosis inhibitor concanvalin A blocks PKC-stimulated loss of surface DAT [99]; (2) coexpression with a dominant-negative mutant of dynamin I inhibits internalization of DAT [109]; and (3) interference with clathrin and dynamin significantly reduces PKC-dependent DAT endocytosis, but flotillin depletion does not [110]. However, depletion of flotillins does decrease lateral mobility of DAT [110], suggesting a limited role for lipid microdomains.
Similar to effects of 5-HT on SERT, DAT substrates, DA and amphetamines, induce internalization and thus downregulation of DA transport [109, 111]. This DA-stimulated trafficking of DAT is accomplished by DA binding to D2 autoreceptors, triggering the downstream activation of Gαi and protein kinase C [112].
NET
Like SERT, NET is found in lipid microdomains, and NET downregulation is triggered by PKC via a cholesterol-dependent mechanism blocked by filipin and nystatin and accomplished through a lipid raft-mediated internalization that is surprisingly independent not only of dynamin and clathrin but also of caveolae [113]. However, this NET internalization depends on complexing with the neurokinin-1 receptor (NK1R), which occurs in non-raft domains; after agonist binding to NK1R, the NET-NK1R complex translocates to lipid raft membrane domains [46].
Mechanisms of NET inhibition through internalization appear to differ from those described above for SERT and DAT, in that NE does not demonstrate homeostatic regulation of NET [114]. Treatment of PC12 cells with even high concentrations of NE does not alter NET binding properties [115]. Additionally, in 293-hNET cells without the ability to produce NE, NET is downregulated by desipramine or nisoxetine equally as well as are NE-synthesizing PC12 cells [115].
Monoamine receptors and lipid microdomains
Serotonin (5-HT) receptors
5-HT1A receptor
The 5-HT1A receptor, an ion channel ligated autoreceptor, is coupled to Gαi, which mediates inhibitory effects of 5-HT binding on AC and cAMP [116]. Evidence for involvement of lipid microdomains in 5-HT1A regulation includes findings in cell cultures that 5-HT1A receptors distribute to detergent-resistant lipid microdomain fractions together with caveolin-1 [93, 117]. Cholesterol depletion with methyl-β-cyclodextrin inhibits both agonist [118] and antagonist [119] binding and G-protein coupling [118] to 5-HT1A receptors, effects that are reversible with exogenous cholesterol [118]. More direct evidence is the identification of a cholesterol recognition amino acid consensus motif in the 5-HT1A receptor [120]. In a model membrane system, increasing membrane order by manipulating cholesterol, ergosterol, epicholesterol, or sphingomyelin results in increased 5-HT1A receptor activity [121].
5-HT2A receptor
The 5-HT2 receptor couples to Gαq, increasing inositol triphosphate and diacylglycerol levels [116]. 5-HT2A receptors are abundantly expressed in brain and targeted by virtually all atypical antipsychotic agents. Co-localization of 5-HT2A receptors with caveolins occurs in sucrose gradient fractions in a rat tail artery preparation [122]; as co-immunoprecipitated in C6 glioma cells, human embryonic kidney (HEK-293) cells, or rat brain synaptic membrane preparations [34]; and as co-immunoprecipitated in cardiac myocytes [123]. A single study in bovine tracheal muscle, however, found that 5-HT2A co-immunoprecipitated in the non-caveolar fraction [124], although the immunoprecipitation was not studied after 5-HT stimulation. This may be relevant since in cardiac myocytes, 5-HT2A receptors only co-immunoprecipitate with caveolin-3 (a muscle-specific caveolin) upon serotonin stimulation, and are then redistributed into caveolae microdomains, trafficking that is abolished by caveolin-3 knockdown [123]. However, in C6 glioma cells, 5-HT2A and caveolin-1 co-localize, on the cell surface and in intracellular vesicles, exclusively in the unstimulated condition, as 5-HT agonists administration does not induce 5-HT2A receptor internalization [34]. These dissimilarities may be tissue-specific or may reflect methodological differences.
The association of 5-HT2A receptors with caveolae microdomains has functional consequences, inhibiting 5-HT2A receptor-mediated expression of the atrial natriuretic factor (ANF) gene and activation of nuclear factor of activated T cells (NFAT) in cardiac myocytes [123]; and stimulating calcium flux in rat brain synaptic membranes [34]. Physiologic responses to 5-HT stimulation are disrupted by the cholesterol depletion with methyl-β-cyclodextrin and restored by cholesterol repletion in the bovine airway smooth muscle [124] and rat tail artery [122] preparations, and are abolished by caveolin-1 [34] or caveolin-3 [123] knockdown.
5-HT3 receptor
The only 5-HT receptor that does not bind G-proteins, 5-HT3 is a member of the Cys-loop pentameric receptor family consisting primarily of five 5-HT3A monomers (although some also contain 5-HT3B). Serotonin binding to 5-HT3 triggers fast signal transduction across synapses by opening Ca2+ channels presynaptically and Ca2+, Na+, or K+ channels postsynaptically [125]. Using fluorescence resonance energy transfer technology (FRET), lipid microdomains can be measured on a nanometer scale [40], and ligand binding to 5-HT3A receptors can be quantitated [41]. Cholesterol depletion with methyl-β-cyclodextrin reduces 5-HT3 function, affecting peak amplitude and kinetics of serotonin-stimulated cation currents [126]. In HEK-293 and neuroblastoma (N1E-115) cells transfected with 5-HT3A receptors, 5-HT3 receptor proteins localize exclusively in the low buoyant, lipid raft-containing fractions of the sucrose gradient, along with a high content of cholesterol, caveolin-2, and flotillin-1, and a number of antidepressant and antipsychotic medications [127]. In addition to co-localization of these drugs with 5-HT3 receptors, drug concentrations within lipid microdomain fractions correlated highly with their inhibition of serotonin-induced cation currents. This non-competitive antagonism at the 5-HT3 receptor was not caused by increased receptor internalization, as demonstrated by immunofluorescence, receptor density in clathrin-coated vesicles, and electrophysiology [127]; the antidepressant antagonism of 5-HT3 also was shown to be independent of the association of 5-HT3 with lipid microdomains [127, 128].
5-HT7 receptor
The 5-HT7 receptor is coupled to Gαs, stimulating cAMP levels [116]. Caveolin-1, cholesterol, sphingomyelin, and gangliosides all regulate 5-HT binding to the 5-HT7 receptor as well as agonist-induced internalization and signaling. Caveolin-1 and 5-HT7 receptors co-localize in lipid microdomains [129]. Reduction of cholesterol, by methyl-β-cyclodextrin treatment or cholesterol synthesis inhibition, reduces agonist and antagonist binding to the 5-HT7 receptor in cell culture, with downstream decreases of 5-HT-induced CREB phosphorylation [33]. Independent of cholesterol-mediated effects, inhibition of sphingolipids and gangliosides attenuates agonist binding at 5-HT7 receptors [36].
Dopamine (DA) receptors
Dopamine receptors are categorized as D1-class (D1 and D5) or D2-class (D2, D3, D4) based on G-protein receptor coupling and associated respective stimulation or inhibition of AC and production of cAMP: stimulating via Gαs/Gαolf with D1-class, and inhibiting via Gαi/Gαo with D2-class [130], although D3 may also couple to Gαs [131, 132]. In the central nervous system, D1-class receptors are primarily post-synaptic, while D2-class have both pre- and post-synaptic distributions [133].
D1-class (D1 and D5) receptors
D1 receptor: Using both detergent solubilization of rat brain [80] and detergent-free HEK-293 preparations [35, 81], D1 receptors have been predominantly localized to lipid microdomains. Within the membrane, D1 localization depends on receptor conformation [35]. As expected, D1 co-fractionates with Gαs, caveolin-2, flotillins, and other signal transduction proteins [81]. However, D1 receptor endocytosis can occur via both clathrin-dependent (i.e., non-lipid raft) [134, 135] and caveolar (raft) [81, 135] mechanisms. In heterologously D1-expressing human renal proximal tubule (hRPT) and HEK-293 cells, a D1-class receptor agonist fenoldopam increases AC protein in lipid rafts, but not in non-lipid raft domains [136]. Consistent with lipid raft localization, disruption of lipid microdomains with methyl-β-cyclodextrin reduces basal AC activity [136].
D5 receptor: The D5 subtype is a high-affinity receptor [137] occurring predominantly in cytoplasmic fractions and not in lipid raft-like subdomains in two studies of rat frontal cortex [80, 138]. Like D1 receptors, D5 receptors co-localize with and enhance the activity of anti-oxidizing enzyme paraoxonase in the plasma membrane of hRPT cells and HEK-293 cells heterologously expressing D5. However, in contrast to D1 receptors, D5 agonist binding in HEK-293 cells increases paraoxonase activity [139] and AC protein [136] only in non-rafts although cholesterol depletion with methyl-β-cyclodextrin does reduce AC activity [136].
D2-class (D2, D3, D4) receptors
D2 receptor: Studies of the D2 receptor subtype have produced conflicting results. Immunoreactivity assays find a diffuse distribution of the D2 receptor subtype spanning cytoplasmic, detergent-soluble and detergent-resistant membrane fractions in rat frontal cortex [80]. D2 receptors are expressed either endogenously in brain or exogenously in HEK-293T cells and localize to lipid microdomains [140, 141], but are unaffected by cholesterol depletion using methyl-β-cyclodextrin [140]. Internalization of D2 receptors through caveolar endocytosis [142] depends on glycosylation state [79], but dynamin-dependent internalization is also reported [143, 144].
D3 receptor: Although less abundant than other DA receptors, the D3 receptor has a much higher affinity for agonists than D2 receptors. In human proximal tubule cells, D3 receptors co-localize with G-protein-coupled receptor kinase 4 (GRK4) both in non-raft fractions (90%) and, together with caveolin-1, in lipid raft fractions (10%) isolated using detergent-free sucrose fractionation and co-immunoprecipitation methods [145]. Cholesterol depletion using methyl-β-cyclodextrin causes redistribution of raft-associated D3 to non-raft fractions [145]. In contrast to D2 receptors, glycosylation of the D3 receptor is essential for clathrin-dependent internalization [79]. In brain, D3 receptors are expressed mainly in limbic regions; they have a low susceptibility to downregulation through internalization, consistent with a role as autoreceptors to monitor DA concentrations [144, 146].
Norepinephrine (NE, adrenergic) receptors
Agonist binding to β1 and β2 adrenergic receptors (β-AR), linked to Gαs proteins and thence to AC, catalyzes the formation of cAMP, with downstream effects such as heart muscle contraction and smooth muscle relaxation. In cardiac myocytes, β1-AR is primarily located in non-raft membrane regions, whereas β2-AR is exclusively localized to caveolae [147] along with signaling components such as AC and Gαs proteins [148, 149]. However, in cardiomyocytes, agonist binding to β2-AR triggers redistribution of the receptor out of caveolae [150], causing sequestration of β2-AR receptors away from the effectors and reducing downstream signaling events [151]. A dissenting view is that localization and signaling of β2-AR do not depend on caveolae, but rather are controlled by protein signaling complexes comprised of scaffold proteins interacting with the receptor carboxy-terminus [152].
Crystallographic studies identify a consensus motif in β2-AR [153] indicating a cholesterol binding site. The effects of β2-AR on cAMP are altered by cholesterol depletion, with disagreement as to whether AC activity and cAMP production are inhibited [148, 149] or enhanced [150, 154, 155]. A disinhibitory mechanism is proposed for enhanced cAMP production, in which β2-AR-induced caveolar endocytosis and concomitant reduction in receptor number is interrupted by the loss of cholesterol [150].
Studies with caveolin-3 dominant-negative mutants in rat cardiomyocytes find that caveolin-3 directly regulates β2-AR functioning. Caveolin-3 loss leads to β2-AR-cAMP signaling that mimics heart failure, which is rescued by caveolin-3 overexpression [156].
Discussion
Summary of findings
Monoaminergic transporters
The evidence supports an association of lipid microdomains with SERT and NET, but is less clear with respect to DAT. SERT co-localizes with caveolin and GM1 and is visualized in cholesterol-rich membrane microdomains [16, 90], and its functioning is adversely affected by cholesterol depletion [51, 52]. NET demonstrates cholesterol-dependent trafficking [46, 113] and internalization that is mediated neither by clathrin nor caveolin [113]. In contrast, DAT occurs across raft and non-raft domains [99], and, although DAT are affected by changes in cholesterol [100], these effects are not clearly lipid raft-mediated.
Monoaminergic receptors
There is strong evidence for lipid microdomain regulation of serotonin function for 5-HT1A, 5-HT2A, and 5-HT7 receptors, although leading to very different downstream effects: 5-HT1A couples to Gαi, with inhibitory effects on AC and cAMP; 5-HT7 couples to Gαs, stimulating cAMP levels; and 5-HT2 couples to Gαq, increasing inositol triphosphate and diacylglycerol levels [116]. The 5-HT1A receptors co-localize with caveolin-1 in a palmitoylation-dependent manner [93], and cholesterol depletion affects binding to 5-HT1A receptors and signaling [118, 119], effects that are reversed by replenishing cholesterol [118]. Most studies of 5-HT2A [34, 122, 123] but not all [124] find co-localization with caveolins. Findings also are conflicting with regard to caveolin association in agonist-stimulated [123] vs. unstimulated [34] conditions; however, in all studies responses to agonist binding to 5-HT2A receptors were affected by cholesterol depletion or caveolin knockdown [34, 122–124]. Lipid rafts regulate 5-HT7 binding, the ensuing functional sequelae, and receptor internalization, although it should be noted that all studies have been performed by one group [33, 36, 129]. Lipid microdomain associations with 5-HT3 have been studied primarily in the context of psychotropic medications, many of which co-localize with the 5-HT3 receptor in lipid rafts (see section “Lipid microdomains and antidepressant treatment” below).
The role of lipid microdomains in the regulation of both D1- and D2-class dopaminergic receptors is complex. D1 receptors localize in raft domains [80, 81], and agonist binding to AC protein increases in lipid rafts, but raft localization has only been observed in certain receptor conformations [35]. Both caveolar [81, 135] and clathrin-dependent [134, 135] endocytosis have been reported. The D5 receptor, on the other hand, does not localize in lipid microdomains [80, 138]; its effects on paraoxonase activity are seen only in non-rafts [139], yet it does mediate effects on AC activity that are attenuated with cholesterol depletion [136]. Also poorly understood are lipid raft effects on D2 receptors, which are diffusely distributed across membrane domains [80, 140, 141], but are unaffected by cholesterol depletion [140]. Endocytosis of D2 receptors can be both caveolar [142] and dynamin-dependent [143, 144].
The evidence strongly supports a key role of lipid microdomains with regard to β2-AR but not β1-AR adrenergic (norepinephrine) receptors: β2-AR are localized to caveolar lipid microdomains [147] along with relevant signaling components [148, 149], and are dependent on cholesterol [150, 153] and caveolin-3 [156], with downstream effects on AC and cAMP [148–150, 154, 155].
One caveat in interpreting the possible importance of these findings for depression is that studies of lipid microdomains with respect to 5-HT7 and D3 receptors were carried out only in cells derived from kidney, and likewise β-AR were studied exclusively in cardiomyocytes. Therefore, assuming that lipid microdomain effects on 5-HT7, D3, and β-AR have ramifications for brain function and depression, is premature.
Mechanisms of lipid microdomain effects in monoaminergic receptor signaling
Early studies focused on establishing co-localization of receptor and transporter molecules in lipid microdomains. More recent research has expanded the field of study to include related elements of signaling complexes and to begin exploration of structure–function relationships related to lipid microdomains. For instance, a salient characteristic of most monoaminergic receptors is coupling to G-proteins at the cytoplasmic membrane leaflet, and it turns out that lipid microdomains have regulatory effects on G-proteins, impacting localization, trafficking, and signaling. Among the G-protein isoforms associated with monoaminergic receptors, studies in rat lung homogenate find that Gαs (with which 5-HT7 and β2-AR associate) and Gαi (5-HT1A) tend to localize in lipid microdomains, while Gαq (5-HT2A) more than Gαs or Gαi, are enriched in caveolae [157]. As an example of lipid microdomain-mediated trafficking of G-proteins related to monoaminergic signaling, in C6 glioma and MCF-7 adenoma cells that express only endogenous β2-AR, Gαs undergoes internalization within vesicles in response to agonist binding [158], a process blocked by caveolin knockdown or cholesterol depletion [159]. Furthermore, lipid microdomains can have effects downstream from agonist-receptor binding, e.g., in C6 cells, caveolin knockout or knockdown interferes directly with Gαs stimulation of adenylyl cyclase [159].
A growing body of research shows that monoaminergic receptors in the membrane not only interact with G-proteins, but also form complexes with a variety of other molecules in association with lipid microdomains, including pre-synaptic receptors [46], membrane proteins involved in exocytosis [45, 160, 161], and hundreds of adaptor proteins that modulate maturation, internalization, targeting for degradation, and recycling of monoamine receptors [162].
Indirect effects on monoaminergic function via lipid microdomains also have been observed. For example, the raft-associated cellular prion protein (PrPC) inhibits GSK3β kinase in serotonergic cells in the raphe region, with the downstream effects of suppressing 5-HT1B receptor activity [163]. As 5-HT1B receptors are inhibitory, the net effect is an increase in serotonergic activity.
Lipid microdomains and antidepressant treatment
Although selective serotonin re-uptake inhibitors (SSRI) act acutely on SERT in pre-synaptic neurons, the therapeutic effects of these antidepressants occur after chronic exposure and are believed to occur via downstream effects on post-synaptic 5-HT receptors [164]. Consistent with this hypothesis, accumulation of the active S- but not the inactive R- enantiomer of citalopram triggers Gαs translocation from raft to non-raft regions in C6 glioma cells that do not express SERT, and is not enhanced in cells that contain transfected SERT [165].
Studies of postmortem brain tissue from suicide decedents with confirmed unipolar major depression find increased accumulation of Gαs in lipid microdomains [166]. Treatment with several chemically distinct antidepressants facilitates translocation of Gαs, but not other G-proteins, from lipid microdomains to non-raft fractions and into closer association with AC, potentiating its activity [165, 167–170]. This translocation might contribute to the increased cAMP tone and synaptic changes observed subsequent to chronic antidepressant treatment [171]. These studies are also in agreement with observations that rolipram, a cAMP-phosphodiesterase inhibitor, has antidepressant effects [172]. On the other hand, mood stabilizers lithium and valproate have the opposite effect, causing increased Gαs in lipid microdomains [173], thus suggesting a biphasic effect at the molecular level for treatment of the two “poles” of bipolar illness. Among monoaminergic receptors, D1 and D3, 5-HT7, and β1-AR and β2-AR are known to couple with Gαs and cause increased cAMP, although only D1, 5-HT7, and β2-AR co-localize predominantly in lipid raft fractions. Possible connections between these elements and antidepressant mechanisms could be a promising area of future investigation.
In cell culture, the 5-HT3 receptor protein localizes exclusively to fractions containing lipid microdomains, which are also the same fractions robustly enriched in antidepressants (desipramine, fluoxetine, and reboxetine) and antipsychotics (fluphenazine, haloperidol, and clozapine) [127]. Concentrations of these psychotropic medications correlate with the magnitude of non-competitive inhibition of 5-HT3 serotonin-induced cation currents [127]. However, the ability of lipid raft co-localizers desipramine and fluoxetine to counter 5-HT3 effects in N1E-115 cells was not affected by cholesterol depletion [126]. Thus, although lipid rafts have an impact on 5-HT3 function, antidepressant antagonism of 5-HT3 appears to be independent of lipid microdomain status [126–128].
In addition to studies in postmortem brain and in vitro cell cultures, ex vivo studies of human tissue such as platelets [44] and B-cells [174] are now feasible approaches to the study of lipid microdomains in humans. Outcome measures of these approaches are physicochemical microdomain properties such as membrane order [44] and microdomain clustering [174] that could be tested alone or in combination with monoaminergic parameters as potential biomarkers for depression or as predictors of antidepressant response.
Lipid microdomains as a target for polyunsaturated fatty acid (PUFA) effects in depression
Lipid raft molecular organization and raft-associated protein distribution are highly susceptible to modulation by long chain n-3 PUFAs, dietarily essential fatty acids that are generally low in Western diets [175], and very low in major depressive disorder [176] and in patients at high risk for suicide [177–179]. There is strong evidence that n-3 PUFAs, upon incorporation into the plasma membrane as acyl chains of glycerophospholipids, can either prevent or promote lipid raft formation, depending on several factors: cell type, the relative concentrations of eicosapentaenoic acid (EPA, 20:5,n-3), docosahexaenoic acid (DHA, 22:6,n-3), and docosapentaenoic acid (DPA, 22:5,n-3), and the phosphatidyl moiety to which the n-3 PUFA is bound [180–183].
It has been hypothesized that n-3 PUFA effects on depression [184–186] are mediated by their ability to modify lipid rafts, with downstream effects on G-protein signaling [187]. If correct, monoaminergic transporters and receptors are possible candidates as mechanistic intermediaries. Membrane n-3 PUFAs affect oligomerization kinetics of adenosine A2A and D2 receptors via effects on biophysical membrane properties [188], and in rodent models in a cardiovascular context, α-linolenic acid (ALA, 18:3,n-3) enhances caveolin-3 expression [189] and prevents cardiac damage due to β-adrenergic overstimulation, an effect blocked by preadministration with methyl-β-cyclodextrin [190]. Other possible lipid microdomain-associated targets for PUFA effects in depression relate to the anti-inflammatory and pro-resolving properties of n-3 PUFA, as inflammation likely contributes to depression etiology in a subset of patients [191, 192]. For example, in B-cell cultures, DHA inhibits Toll-like receptor (TLR) dimerization and recruitment into lipid microdomains [193].
Antihyperlipidemic drugs and suicide risk
Lower cholesterol is linked to suicide risk [194–196], and in nonhuman primates, low cholesterol is associated with lower CSF levels of the 5-HT metabolite 5-HIAA, blunted prolactin response to fenfluramine-stimulated 5-HT release, and increases in aggressive behaviors [197].
The aggregate evidence presented herein supports a hypothesis that effects of cholesterol-lowering therapies on suicide risk are mediated by influencing lipid raft regulation of plasma membrane serotonergic proteins, since cholesterol depletion decreases the transport rate (Vmax) and affinity (Km) of 5-HT for SERT [51, 52], agonist and antagonist binding to 5-HT1A and 5-HT7 [33, 118, 119], and physiologic responses of 5-HT2A to 5-HT stimulation [122, 124].
Conclusions
Our literature review suggests that lipid microdomains are a significant modulator of monoaminergic neurotransmission, influencing co-localization of transporters/receptors with other signaling proteins and adaptor molecules; trafficking by endocytosis; agonist and antagonist binding; and downstream second messenger-mediated events. Limitations on knowledge reflect the limitations of current experimental methodologies for nanoscale discrimination of spatial and temporal aspects of neuronal signaling. In addition, there is a paucity of clinical studies, which are needed to advance understanding of lipid microdomain effects on monoaminergic signaling with respect to etiology, diagnosis, and treatment of major depression. Thus, the next wave of research should seek to move toward the study of monoaminergic–lipid microdomain interactions in humans with and without depression. One approach would be to measure physicochemical effects on microdomain structure, using ex vivo techniques, of substances already known to be safe for human use that affect lipid microdomains, such as cholesterol-lowering drugs and n-3 PUFAs.
Acknowledgments
Funding
This study was supported in part by the following grants: K08 MH079033 (PI, MES), NIH R01AT008375 (PI, SRS), NIH 8G12 MD007603 (Area Leader, RES). Dr. JJM receives royalties for commercial use of the Columbia-Suicide Severity Rating Scale (C-SSRS) from the Research Foundation for Mental Hygiene.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lorent JH, Levental I. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem Phys Lipids. 2015;192:23–32. doi: 10.1016/j.chemphyslip.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Levental I, Veatch SL. The continuing mystery of lipid rafts. J Mol Biol. 2016;428:4749–64. doi: 10.1016/j.jmb.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–40. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 4.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–62. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 7.Wilson BS, Pfeiffer JR, Oliver JM. Observing FcepsilonRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–42. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–5. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 9.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–42. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838:532–45. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trushina E, Du Charme J, Parisi J, McMurray CT. Neurological abnormalities in caveolin-1 knock out mice. Behav Brain Res. 2006;172:24–32. doi: 10.1016/j.bbr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM. Cavin family proteins and the assembly of caveolae. J Cell Sci. 2015;128:1269–78. doi: 10.1242/jcs.167866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokubo H, Helms JB, Ohno-Iwashita Y, Shimada Y, Horikoshi Y, Yamaguchi H. Ultrastructural localization of flotillin-1 to cholesterol-rich membrane microdomains, rafts, in rat brain tissue. Brain Res. 2003;965:83–90. doi: 10.1016/s0006-8993(02)04140-9. [DOI] [PubMed] [Google Scholar]
- 14.Lang DM, Lommel S, Jung M, Ankerhold R, Petrausch B, Laessing U, et al. Identification of reggie-1 and reggie-2 as plasma membrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol. 1998;37:502–23. doi: 10.1002/(sici)1097-4695(199812)37:4<502::aid-neu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 16.Chang JC, Tomlinson ID, Warnement MR, Ustione A, Carneiro AM, Piston DW, et al. Single molecule analysis of serotonin transporter regulation using antagonist-conjugated quantum dots reveals restricted, p38 MAPK-dependent mobilization underlying uptake activation. J Neurosci. 2012;32:8919–29. doi: 10.1523/JNEUROSCI.0048-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–88. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 18.Owen DM, Williamson DJ, Magenau A, Gaus K. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat Commun. 2012;3:1256. doi: 10.1038/ncomms2273. [DOI] [PubMed] [Google Scholar]
- 19.Frisz JF, Klitzing HA, Lou K, Hutcheon ID, Weber PK, Zimmerberg J, et al. Sphingolipid domains in the plasma membranes of fibroblasts are not enriched with cholesterol. J Biol Chem. 2013;288:16855–61. doi: 10.1074/jbc.M113.473207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisz JF, Lou K, Klitzing HA, Hanafin WP, Lizunov V, Wilson RL, et al. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc Natl Acad Sci USA. 2013;110:E613–22. doi: 10.1073/pnas.1216585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraft ML. Plasma membrane organization and function: moving past lipid rafts. Mol Biol Cell. 2013;24:2765–68. doi: 10.1091/mbc.E13-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, et al. Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson's fluid-mosaic model. Annu Rev Cell Dev Biol. 2012;28:215–50. doi: 10.1146/annurev-cellbio-100809-151736. [DOI] [PubMed] [Google Scholar]
- 23.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci USA. 2003;100:13964–9. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevcsik E, Brameshuber M, Folser M, Weghuber J, Honigmann A, Schutz GJ. GPI-anchored proteins do not reside in ordered domains in the live cell plasma membrane. Nat Commun. 2015;6:6969. doi: 10.1038/ncomms7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, et al. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206–15. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–44. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 27.Babiychuk EB, Draeger A. Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem J. 2006;397:407–16. doi: 10.1042/BJ20060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–6. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83:2693–701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–7. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 31.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–24. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjogren B, Hamblin MW, Svenningsson P. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur J Pharmacol. 2006;552:1–10. doi: 10.1016/j.ejphar.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 34.Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem. 2004;279:34614–23. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- 35.Mystek P, Dutka P, Tworzydlo M, Dziedzicka-Wasylewska M, Polit A. The role of cholesterol and sphingolipids in the dopamine D1 receptor and G protein distribution in the plasma membrane. Biochim Biophys Acta. 2016;1861:1775–86. doi: 10.1016/j.bbalip.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Sjogren B, Svenningsson P. Depletion of the lipid raft constituents, sphingomyelin and ganglioside, decreases serotonin binding at human 5-HT7(a) receptors in HeLa cells. Acta Physiol. 2007;190:47–53. doi: 10.1111/j.1365-201X.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- 37.Gaus K, Inoue T. New biological frontiers illuminated by molecular sensors and actuators. Biophys J. 2016;111:E01–02. doi: 10.1016/j.bpj.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaikh SR, Boyle S, Edidin M. A high fat diet containing saturated but not unsaturated fatty acids enhances T cell receptor clustering on the nanoscale. PLEFA. 2015;100:1–4. doi: 10.1016/j.plefa.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller V, Honigmann A, Ringemann C, Medda R, Schwarzmann G, Eggeling C. FCS in STED microscopy: studying the nanoscale of lipid membrane dynamics. Methods Enzymol. 2013;519:1–38. doi: 10.1016/B978-0-12-405539-1.00001-4. [DOI] [PubMed] [Google Scholar]
- 40.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–6. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 41.Ilegems E, Pick H, Deluz C, Kellenberger S, Vogel H. Ligand binding transmits conformational changes across the membrane-spanning region to the intracellular side of the 5-HT3 serotonin receptor. Chembiochem. 2005;6:2180–5. doi: 10.1002/cbic.200500191. [DOI] [PubMed] [Google Scholar]
- 42.Abu-Arish A, Pandzic E, Goepp J, Matthes E, Hanrahan JW, Wiseman PW. Cholesterol modulates CFTR confinement in the plasma membrane of primary epithelial cells. Biophys J. 2015;109:85–94. doi: 10.1016/j.bpj.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polozov IV, Gawrisch K. NMR detection of lipid domains. In: McIntosh TJ, editor. Lipid rafts. Totowa: Himana Press; 2007. p. 107–26. [DOI] [PubMed]
- 44.Cenido JF, Itin B, Stark RE, Huang YY, Oquendo MA, John Mann J, et al. Characterization of lipid rafts in human platelets using nuclear magnetic resonance: a pilot study. Biochem Biophys Rep. 2017;10:132–6. doi: 10.1016/j.bbrep.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler B, Saha K, Rana T, Becker JP, Sambo D, Davari P, et al. Dopamine transporter activity is modulated by alpha-synuclein. J Biol Chem. 2015;290:29542–54. doi: 10.1074/jbc.M115.691592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arapulisamy O, Mannangatti P, Jayanthi LD. Regulated norepinephrine transporter interaction with the neurokinin-1 receptor establishes transporter subcellular localization. J Biol Chem. 2013;288:28599–610. doi: 10.1074/jbc.M113.472878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triantafilou M, Morath S, Mackie A, Hartung T, Triantafilou K. Lateral diffusion of Toll-like receptors reveals that they are transiently confined within lipid rafts on the plasma membrane. J Cell Sci. 2004;117:4007–14. doi: 10.1242/jcs.01270. [DOI] [PubMed] [Google Scholar]
- 48.Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–14. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 49.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118:1099–102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- 50.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–26. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 51.Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–8. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- 52.Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40:10507–13. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- 53.Mann JJ. The serotonergic system in mood disorders and suicidal behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120537. doi: 10.1098/rstb.2012.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–95. [PubMed] [Google Scholar]
- 55.Sellers EM, Higgins GA, Tompkins DM, Romach MK. Serotonin and alcohol drinking. NIDA Res Monogr. 1992;119:141–5. [PubMed] [Google Scholar]
- 56.Coccaro EF. Central serotonin and impulsive aggression. Br J Psychiatry. 1989;155:52–62. [PubMed]
- 57.Compagnon P, Ernouf D, Narcisse G, Daoust M. Serotonin in animal models of alcoholism. Alcohol Alcohol Suppl. 1993;2:215–9. [PubMed] [Google Scholar]
- 58.Carlsson A. Perspectives on the discovery of central monoaminergic neurotransmission. Annu Rev Neurosci. 1987;10:19–40. doi: 10.1146/annurev.ne.10.030187.000315. [DOI] [PubMed] [Google Scholar]
- 59.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–76. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 60.Bannon M, Granneman J. The dopamine transport. Potential involvement in neuropsychiatric disorders. In: Bloom F, Kupfer D, editors. Psychopharmacoogy: the fourth generation of progress. New York, NY: Raven Press; 1995. p. 179–87.
- 61.Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46:1219–33. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- 62.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–22. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 63.Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonard BE. The role of noradrenaline in depression: a review. J Psychopharmacol. 1997;11:S39–47. [PubMed] [Google Scholar]
- 65.Blier P, de Montigny C, Chaput Y. A role for the serotonin system in the mechanism of action of antidepressant treatments: preclinical evidence. J Clin Psychiatry. 1990;51:14–20. [PubMed] [Google Scholar]
- 66.Gray NA, Milak MS, DeLorenzo C, Ogden RT, Huang YY, Mann JJ, et al. Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol Psychiatry. 2013;74:26–31. doi: 10.1016/j.biopsych.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–38. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barker EL, Blakely RD. Norephinephrine and serotonin transporters: molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York, NY: Raven Press; 1995. p. 321–33.
- 69.Richelson E. Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry. 2003;64:5–12. [PubMed] [Google Scholar]
- 70.Jayanthi LD, Vargas G, DeFelice LJ. Characterization of cocaine and antidepressant-sensitive norepinephrine transporters in rat placental trophoblasts. Br J Pharmacol. 2002;135:1927–34. doi: 10.1038/sj.bjp.0704658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Q, Ma L, Innis RB, Seneca N, Ichise M, Huang H, et al. Pharmacological and genetic characterization of two selective serotonin transporter ligands: 2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylphenylamine (AFM) and 3-amino-4-[2-(dimethylaminomethyl-phenylthio)]benzonitrile (DASB) J Pharmacol Exp Ther. 2004;308:481–6. doi: 10.1124/jpet.103.058636. [DOI] [PubMed] [Google Scholar]
- 72.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine ("Ecstasy") in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 73.Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, et al. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–16. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- 74.Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, et al. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5300–05. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adriani W, Boyer F, Gioiosa L, Macri S, Dreyer JL, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats' nucleus accumbens. Neuroscience. 2009;159:47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 76.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–84. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 77.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 78.Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, et al. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–61. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- 79.Min C, Zheng M, Zhang X, Guo S, Kwon KJ, Shin CY, et al. N-linked glycosylation on the N-terminus of the dopamine D2 and D3 receptors determines receptor association with specific microdomains in the plasma membrane. Biochim Biophys Acta. 2015;1853:41–51. doi: 10.1016/j.bbamcr.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 80.Voulalas PJ, Schetz J, Undieh AS. Differential subcellular distribution of rat brain dopamine receptors and subtype-specific redistribution induced by cocaine. Mol Cell Neurosci. 2011;46:645–54. doi: 10.1016/j.mcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, et al. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–80. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- 82.Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278:2731–9. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 83.Kocabas AM, Rudnick G, Kilic F. Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters. J Neurochem. 2003;85:1513–20. doi: 10.1046/j.1471-4159.2003.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderluh A, Hofmaier T, Klotzsch E, Kudlacek O, Stockner T, Sitte HH, et al. Direct PIP2 binding mediates stable oligomer formation of the serotonin transporter. Nat Commun. 2017;8:14089. doi: 10.1038/ncomms14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, Casey L, Pike LJ. Compartmentalization of phosphatidylinositol 4,5-bisphosphate in low-density membrane domains in the absence of caveolin. Biochem Biophys Res Commun. 1998;245:684–90. doi: 10.1006/bbrc.1998.8329. [DOI] [PubMed] [Google Scholar]
- 86.Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271:26453–6. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- 87.Khelashvili G, Weinstein H. Functional mechanisms of neurotransmitter transporters regulated by lipid–protein interactions of their terminal loops. Biochim Biophys Acta. 2015;1848:1765–74. doi: 10.1016/j.bbamem.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buchmayer F, Schicker K, Steinkellner T, Geier P, Stubiger G, Hamilton PJ, et al. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proc Natl Acad Sci USA. 2013;110:11642–7. doi: 10.1073/pnas.1220552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamilton PJ, Belovich AN, Khelashvili G, Saunders C, Erreger K, Javitch JA, et al. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat Chem Biol. 2014;10:582–9. doi: 10.1038/nchembio.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitworth TL, Herndon LC, Quick MW. Psychostimulants differentially regulate serotonin transporter expression in thalamocortical neurons. J Neurosci. 2002;22:RC192. doi: 10.1523/JNEUROSCI.22-01-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carneiro AM, Blakely RD. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. J Biol Chem. 2006;281:24769–80. doi: 10.1074/jbc.M603877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Renner U, Glebov K, Lang T, Papusheva E, Balakrishnan S, Keller B, et al. Localization of the mouse 5-hydroxytryptamine(1A) receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol Pharmacol. 2007;72:502–13. doi: 10.1124/mol.107.037085. [DOI] [PubMed] [Google Scholar]
- 94.Jorgensen TN, Christensen PM, Gether U. Serotonin-induced down-regulation of cell surface serotonin transporter. Neurochem Int. 2014;73:107–12. doi: 10.1016/j.neuint.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Myers CL, Lazo JS, Pitt BR. Translocation of protein kinase C is associated with inhibition of 5-HT uptake by cultured endothelial cells. Am J Physiol. 1989;257:L253–8. doi: 10.1152/ajplung.1989.257.4.L253. [DOI] [PubMed] [Google Scholar]
- 96.Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sorensen L, Stromgaard K, Kristensen AS. Characterization of intracellular regions in the human serotonin transporter for phosphorylation sites. ACS Chem Biol. 2014;9:935–44. doi: 10.1021/cb4007198. [DOI] [PubMed] [Google Scholar]
- 98.Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–6. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 99.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–99. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones KT, Zhen J, Reith ME. Importance of cholesterol in dopamine transporter function. J Neurochem. 2012;123:700–15. doi: 10.1111/jnc.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, et al. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–97. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- 102.Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–70. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 103.Vainio S, Jansen M, Koivusalo M, Rog T, Karttunen M, Vattulainen I, et al. Significance of sterol structural specificity. Desmosterol cannot replace cholesterol in lipid rafts. J Biol Chem. 2006;281:348–55. doi: 10.1074/jbc.M509530200. [DOI] [PubMed] [Google Scholar]
- 104.Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 105.Hong WC, Amara SG. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J Biol Chem. 2010;285:32616–26. doi: 10.1074/jbc.M110.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navaroli DM, Stevens ZH, Uzelac Z, Gabriel L, King MJ, Lifshitz LM, et al. The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. J Neurosci. 2011;31:13758–70. doi: 10.1523/JNEUROSCI.2649-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–77. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gabriel LR, Wu S, Kearney P, Bellve KD, Standley C, Fogarty KE, et al. Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J Neurosci. 2013;33:17836–46. doi: 10.1523/JNEUROSCI.3284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, et al. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci USA. 2000;97:6850–5. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sorkina T, Caltagarone J, Sorkin A. Flotillins regulate membrane mobility of the dopamine transporter but are not required for its protein kinase C dependent endocytosis. Traffic. 2013;14:709–24. doi: 10.1111/tra.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–36. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- 112.Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, et al. Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem. 2013;125:663–72. doi: 10.1111/jnc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279:19315–26. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- 114.Mandela P, Ordway GA. The norepinephrine transporter and its regulation. J Neurochem. 2006;97:310–33. doi: 10.1111/j.1471-4159.2006.03717.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhu MY, Ordway GA. Down-regulation of norepinephrine transporters on PC12 cells by transporter inhibitors. J Neurochem. 1997;68:134–41. doi: 10.1046/j.1471-4159.1997.68010134.x. [DOI] [PubMed] [Google Scholar]
- 116.Fantini J, Barrantes FJ. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim Biophys Acta. 2009;1788:2345–61. doi: 10.1016/j.bbamem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 117.Kalipatnapu S, Chattopadhyay A. Membrane organization of the human serotonin(1A) receptor monitored by detergent insolubility using GFP fluorescence. Mol Membr Biol. 2005;22:539–47. doi: 10.1080/09687860500421738. [DOI] [PubMed] [Google Scholar]
- 118.Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim Biophys Acta. 2004;1663:188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 119.Sjogren B, Csoregh L, Svenningsson P. Cholesterol reduction attenuates 5-HT1A receptor-mediated signaling in human primary neuronal cultures. Naunyn Schmiede Arch Pharmacol. 2008;378:441–6. doi: 10.1007/s00210-008-0323-6. [DOI] [PubMed] [Google Scholar]
- 120.Jafurulla M, Tiwari S, Chattopadhyay A. Identification of cholesterol recognition amino acid consensus (CRAC) motif in G-protein coupled receptors. Biochem Biophys Res Commun. 2011;404:569–73. doi: 10.1016/j.bbrc.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 121.Gutierrez MG, Mansfield KS, Malmstadt N. The functional activity of the human serotonin 5-HT1A receptor is controlled by lipid bilayer composition. Biophys J. 2016;110:2486–95. doi: 10.1016/j.bpj.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol. 2002;22:1267–72. doi: 10.1161/01.atv.0000023438.32585.a1. [DOI] [PubMed] [Google Scholar]
- 123.Mialet-Perez J, D'Angelo R, Villeneuve C, Ordener C, Negre-Salvayre A, Parini A, et al. Serotonin 5-HT2A receptor-mediated hypertrophy is negatively regulated by caveolin-3 in cardiomyoblasts and neonatal cardiomyocytes. J Mol Cell Cardiol. 2012;52:502–10. doi: 10.1016/j.yjmcc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 124.Sommer B, Montano LM, Carbajal V, Flores-Soto E, Ortega A, Ramirez-Oseguera R, et al. Extraction of membrane cholesterol disrupts caveolae and impairs serotonergic (5-HT2A) and histaminergic (H1) responses in bovine airway smooth muscle: role of Rho-kinase. Can J Physiol Pharmacol. 2009;87:180–95. doi: 10.1139/y08-114. [DOI] [PubMed] [Google Scholar]
- 125.Wu ZS, Cheng H, Jiang Y, Melcher K, Xu HE. Ion channels gated by acetylcholine and serotonin: structures, biology, and drug discovery. Acta Pharmacol Sin. 2015;36:895–907. doi: 10.1038/aps.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nothdurfter C, Tanasic S, Di Benedetto B, Rammes G, Wagner EM, Kirmeier T, et al. Impact of lipid raft integrity on 5-HT3 receptor function and its modulation by antidepressants. Neuropsychopharmacology. 2010;35:1510–9. doi: 10.1038/npp.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eisensamer B, Uhr M, Meyr S, Gimpl G, Deiml T, Rammes G, et al. Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains. J Neurosci. 2005;25:10198–206. doi: 10.1523/JNEUROSCI.2460-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nothdurfter C, Tanasic S, Rammes G, Rupprecht R. Modulation of ligand-gated ion channels as a novel pharmacological principle. Pharmacopsychiatry. 2011;44:S27–34. doi: 10.1055/s-0031-1271704. [DOI] [PubMed] [Google Scholar]
- 129.Sjogren B, Svenningsson P. Caveolin-1 affects serotonin binding and cell surface levels of human 5-HT7(a) receptors. FEBS Lett. 2007;581:5115–21. doi: 10.1016/j.febslet.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 130.Beaulieu JM, Espinoza S, Gainetdinov RR. Dopamine receptors—IUPHAR review 13. Br J Pharmacol. 2015;172:1–23. doi: 10.1111/bph.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Obadiah J, Avidor-Reiss T, Fishburn CS, Carmon S, Bayewitch M, Vogel Z, et al. Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell Mol Neurobiol. 1999;19:653–64. doi: 10.1023/a:1006988603199. [DOI] [PubMed] [Google Scholar]
- 132.Ilani T, Fishburn CS, Levavi-Sivan B, Carmon S, Raveh L, Fuchs S. Coupling of dopamine receptors to G proteins: studies with chimeric D2/D3 dopamine receptors. Cell Mol Neurobiol. 2002;22:47–56. doi: 10.1023/a:1015341712166. [DOI] [PubMed] [Google Scholar]
- 133.Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–19. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 134.Vickery RG, von Zastrow M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–3. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kong MM, Hasbi A, Mattocks M, Fan T, O'Dowd BF, George SR. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Mol Pharmacol. 2007;72:1157–70. doi: 10.1124/mol.107.034769. [DOI] [PubMed] [Google Scholar]
- 136.Yu P, Sun M, Villar VA, Zhang Y, Weinman EJ, Felder RA, et al. Differential dopamine receptor subtype regulation of adenylyl cyclases in lipid rafts in human embryonic kidney and renal proximal tubule cells. Cell Signal. 2014;26:2521–9. doi: 10.1016/j.cellsig.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–9. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 138.Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci. 2004;24:5292–300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang S, Yang Y, Yu P, Yang J, Jiang X, Villar VA, et al. Dopamine D1 and D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free Radic Res. 2015;49:397–410. doi: 10.3109/10715762.2015.1006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sharma M, Celver J, Octeau JC, Kovoor A. Plasma membrane compartmentalization of D2 dopamine receptors. J Biol Chem. 2013;288:12554–68. doi: 10.1074/jbc.M112.443945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Celver J, Sharma M, Kovoor A. D(2)-dopamine receptors target regulator of G protein signaling 9-2 to detergent-resistant membrane fractions. J Neurochem. 2012;120:56–69. doi: 10.1111/j.1471-4159.2011.07559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Genedani S, Guidolin D, Leo G, Filaferro M, Torvinen M, Woods AS, et al. Computer-assisted image analysis of caveolin-1 involvement in the internalization process of adenosine A2A-dopamine D2 receptor heterodimers. J Mol Neurosci. 2005;26:177–84. doi: 10.1385/JMN:26:2-3:177. [DOI] [PubMed] [Google Scholar]
- 143.Iwata K, Ito K, Fukuzaki A, Inaki K, Haga T. Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. Eur J Biochem. 1999;263:596–602. doi: 10.1046/j.1432-1327.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 144.Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–14. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- 145.Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, et al. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem. 2009;284:21425–34. doi: 10.1074/jbc.M109.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim KM, Gainetdinov RR, Laporte SA, Caron MG, Barak LS. G protein-coupled receptor kinase regulates dopamine D3 receptor signaling by modulating the stability of a receptor-filamin-beta-arrestin complex. A case of autoreceptor regulation. J Biol Chem. 2005;280:12774–80. doi: 10.1074/jbc.M408901200. [DOI] [PubMed] [Google Scholar]
- 147.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277:34280–6. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 148.Oner SS, Kaya AI, Onaran HO, Ozcan G, Ugur O. beta2-Adrenoceptor, Gs and adenylate cyclase coupling in purified detergent-resistant, low density membrane fractions. Eur J Pharmacol. 2010;630:42–52. doi: 10.1016/j.ejphar.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 149.Ostrom RS, Bundey RA, Insel PA. Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J Biol Chem. 2004;279:19846–53. doi: 10.1074/jbc.M313440200. [DOI] [PubMed] [Google Scholar]
- 150.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–57. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 151.Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem. 2001;276:42063–9. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- 152.Valentine CD, Haggie PM. Confinement of beta(1)- and beta(2)-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol Biol Cell. 2011;22:2970–82. doi: 10.1091/mbc.E11-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, et al. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pontier SM, Percherancier Y, Galandrin S, Breit A, Gales C, Bouvier M. Cholesterol-dependent separation of the beta2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J Biol Chem. 2008;283:24659–72. doi: 10.1074/jbc.M800778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.DiPilato LM, Zhang J. The role of membrane microdomains in shaping beta2-adrenergic receptor-mediated cAMP dynamics. Mol Biosyst. 2009;5:832–7. doi: 10.1039/b823243a. [DOI] [PubMed] [Google Scholar]
- 156.Wright PT, Nikolaev VO, O'Hara T, Diakonov I, Bhargava A, Tokar S, et al. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol. 2014;67:38–48. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–98. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Allen JA, Yu JZ, Donati RJ, Rasenick MM. Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67:1493–504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- 159.Allen JA, Yu JZ, Dave RH, Bhatnagar A, Roth BL, Rasenick MM. Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling. Mol Pharmacol. 2009;76:1082–93. doi: 10.1124/mol.109.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Muller HK, Wiborg O, Haase J. Subcellular redistribution of the serotonin transporter by secretory carrier membrane protein 2. J Biol Chem. 2006;281:28901–9. doi: 10.1074/jbc.M602848200. [DOI] [PubMed] [Google Scholar]
- 161.Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci USA. 2001;98:5619–24. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bjork K, Svenningsson P. Modulation of monoamine receptors by adaptor proteins and lipid rafts: role in some effects of centrally acting drugs and therapeutic agents. Annu Rev Pharmacol Toxicol. 2011;51:211–42. doi: 10.1146/annurev-pharmtox-010510-100520. [DOI] [PubMed] [Google Scholar]
- 163.Hernandez-Rapp J, Martin-Lanneree S, Hirsch TZ, Pradines E, Alleaume-Butaux A, Schneider B, et al. A PrP(C)-caveolin-Lyn complex negatively controls neuronal GSK3beta and serotonin 1B receptor. Sci Rep. 2014;4:4881. doi: 10.1038/srep04881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fakhoury M. Revisiting the serotonin hypothesis: implications for major depressive disorders. Mol Neurobiol. 2016;53:2778–86. doi: 10.1007/s12035-015-9152-z. [DOI] [PubMed] [Google Scholar]
- 165.Erb SJ, Schappi JM, Rasenick MM. Antidepressants accumulate in lipid rafts independent of monoamine transporters to modulate redistribution of the G protein, Galphas. J Biol Chem. 2016;291:19725–33. doi: 10.1074/jbc.M116.727263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Donati RJ, Dwivedi Y, Roberts RC, Conley RR, Pandey GN, Rasenick MM. Postmortem brain tissue of depressed suicides reveals increased Gs alpha localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J Neurosci. 2008;28:3042–50. doi: 10.1523/JNEUROSCI.5713-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Menkes DB, Rasenick MM, Wheeler MA, Bitensky MW. Guanosine triphosphate activation of brain adenylate cyclase: enhancement by long-term antidepressant treatment. Science. 1983;219:65–7. doi: 10.1126/science.6849117. [DOI] [PubMed] [Google Scholar]
- 168.Toki S, Donati RJ, Rasenick MM. Treatment of C6 glioma cells and rats with antidepressant drugs increases the detergent extraction of G(s alpha) from plasma membrane. J Neurochem. 1999;73:1114–20. doi: 10.1046/j.1471-4159.1999.0731114.x. [DOI] [PubMed] [Google Scholar]
- 169.Zhang L, Rasenick MM. Chronic treatment with escitalopram but not R-citalopram translocates Galpha(s) from lipid raft domains and potentiates adenylyl cyclase: a 5-hydroxytryptamine transporter-independent action of this antidepressant compound. J Pharmacol Exp Ther. 2010;332:977–84. doi: 10.1124/jpet.109.162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Donati RJ, Rasenick MM. Chronic antidepressant treatment prevents accumulation of gsalpha in cholesterol-rich, cytoskeletal-associated, plasma membrane domains (lipid rafts) Neuropsychopharmacology. 2005;30:1238–45. doi: 10.1038/sj.npp.1300697. [DOI] [PubMed] [Google Scholar]
- 171.Donati RJ, Rasenick MM. G protein signaling and the molecular basis of antidepressant action. Life Sci. 2003;73:1–17. doi: 10.1016/s0024-3205(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 172.Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, et al. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64. doi: 10.1159/000118897. [DOI] [PubMed] [Google Scholar]
- 173.Donati RJ, Schappi J, Czysz AH, Jackson A, Rasenick MM. Differential effects of antidepressants escitalopram versus lithium on Gs alpha membrane relocalization. BMC Neurosci. 2015;16:40. doi: 10.1186/s12868-015-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guesdon W, Kosaraju R, Brophy P, Clark A, Dillingham S, Aziz S, et al. Effects of fish oils on ex vivo B-cell responses of obese subjects upon BCR/TLR stimulation: a pilot study. J Nutr Biochem. 2018;53:72–80. doi: 10.1016/j.jnutbio.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;44:203–15. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 176.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–7. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 177.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–2. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 178.Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, et al. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–6. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 179.Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J Clin Psychiatry 2011;72:1585–90. [DOI] [PMC free article] [PubMed]
- 180.Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim Biophys Acta. 2014;1848:211–9. [DOI] [PubMed]
- 181.Shaikh SR, Wassall SR, Brown DA, Kosaraju R. N-3 polyunsaturated fatty acids, lipid microclusters, and vitamin E. Curr Top Membr. 2015;75:209–31. doi: 10.1016/bs.ctm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 182.Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, et al. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012;103:228–37. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harris M, Kinnun JJ, Kosaraju R, Leng X, Wassall SR, Shaikh SR. Membrane disordering by eicosapentaenoic acid in B lymphomas is reduced by elongation to docosapentaenoic acid as revealed with solid-state nuclear magnetic resonance spectroscopy of model membranes. J Nutr. 2016;146:1283–9. doi: 10.3945/jn.116.231639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28:525–42. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 185.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144–9. doi: 10.1038/mp.2012.25. [DOI] [PubMed] [Google Scholar]
- 186.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–84. [DOI] [PMC free article] [PubMed]
- 187.Czysz AH, Rasenick MM. G-protein signaling, lipid rafts and the possible sites of action for the antidepressant effects of n-3 polyunsaturated fatty acids. CNS Neurol Disord Drug Targets. 2013;12:466–73. doi: 10.2174/1871527311312040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guixa-Gonzalez R, Javanainen M, Gomez-Soler M, Cordobilla B, Domingo JC, Sanz F, et al. Membrane omega-3 fatty acids modulate the oligomerisation kinetics of adenosine A2A and dopamine D2 receptors. Sci Rep. 2016;6:19839. doi: 10.1038/srep19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Carotenuto F, Minieri M, Monego G, Fiaccavento R, Bertoni A, Sinigaglia F, et al. A diet supplemented with ALA-rich flaxseed prevents cardiomyocyte apoptosis by regulating caveolin-3 expression. Cardiovasc Res. 2013;100:422–31. doi: 10.1093/cvr/cvt211. [DOI] [PubMed] [Google Scholar]
- 190.Folino A, Sprio AE, Di Scipio F, Berta GN, Rastaldo R. Alpha-linolenic acid protects against cardiac injury and remodelling induced by beta-adrenergic overstimulation. Food Funct. 2015;6:2231–9. doi: 10.1039/c5fo00034c. [DOI] [PubMed] [Google Scholar]
- 191.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Raison CL, Miller AH. Do cytokines really sing the blues? Cerebrum. 2013;2013:10. [PMC free article] [PubMed] [Google Scholar]
- 193.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–92. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301:309–14. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Muldoon MF, Manuck SB, Mendelsohn AB, Kaplan JR, Belle SH. Cholesterol reduction and non-illness mortality: meta-analysis of randomised clinical trials. BMJ. 2001;322:11–15. doi: 10.1136/bmj.322.7277.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu S, Ding Y, Wu F, Xie G, Hou J, Mao P. Serum lipid levels and suicidality: a meta-analysis of 65 epidemiological studies. J Psychiatry Neurosci. 2016;41:56–69. doi: 10.1503/jpn.150079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaplan JR, Shively CA, Fontenot MB, Morgan TM, Howell SM, Manuck SB, et al. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med. 1994;56:479–84. doi: 10.1097/00006842-199411000-00001. [DOI] [PubMed] [Google Scholar]