Abstract
Background
The effectiveness of smoking cessation treatment is limited in real-world use, perhaps because we have not selected the components of such treatments optimally nor have treatments typically been developed for and evaluated in real-world clinical settings.
Purpose
To validate an optimized smoking cessation treatment package that comprises intervention components identified as effective in factorial screening experiments conducted as per the Multiphase Optimization Strategy (MOST).
Methods
Adult smokers motivated to quit were recruited from primary care clinics (N = 623). Participants were randomized to receive either recommended usual care (R-UC; 10 min of in-person counseling, 8 weeks of nicotine patch, and referral to quitline services) or abstinence-optimized treatment (A-OT; 3 weeks of prequit mini-lozenges, 26 weeks of nicotine patch + mini-lozenges, three in-person and eight phone counseling sessions, and 7–11 automated calls to prompt medication use). The key outcomes were self-reported and biochemically confirmed (carbon monoxide, CO <6 ppm) 7-day point-prevalence abstinence.
Results
A-OT participants had significantly higher self-reported abstinence rates than R-UC participants at 4, 8, 16, and 26 weeks (ORs: 1.91–3.05; p <. 001). The biochemically confirmed 26-week abstinence rates were lower than the self-reported 26-week rates, but revealed a similar treatment effect size (OR = 2.94, p < .001). There was no moderation of treatment effects on 26-week abstinence by demographic, psychiatric, or nicotine dependence variables. A-OT had an incremental cost-effectiveness ratio for 26-week CO-confirmed abstinence of $7,800.
Conclusions
A smoking cessation treatment that is optimized via MOST development meaningfully enhances cessation rates beyond R-UC smoking treatment in smokers seen in primary care.
Clinical Trial Registration
Keywords: Smoking cessation, Multiphase optimization strategy, Combination nicotine replacement, Primary care, Randomized controlled trial
A smoking cessation intervention engineered using a new methodology helped significantly more smokers in primary care successfully quit smoking than did typical clinical smoking cessation treatment.
Introduction
Smoking prevalence has declined steadily in the USA over the past 50 years [1]. However, ~36.5 million adults in the USA continue to smoke, making smoking the leading preventable cause of morbidity and mortality [2]. Moreover, an estimated 70% of smokers visit a primary care physician each year [3, 4], underscoring the need for especially effective treatments for smoking that are feasible for use in healthcare settings.
Various pharmacotherapy and counseling approaches are efficacious for smoking cessation [3, 5–7] with estimates of long-term abstinence rates ranging from 12.7% for quitline counseling to 21.7% for medication alone to 27.6% for counseling plus medication [3]. However, there are myriad potential combinations of such interventions and a host of strategies to deliver them. For instance, some evidence supports prequit delivery of smoking cessation medication ([8, 9] cf. [10, 11]), different combinations of medications [3, 12], extended medication [13, 14], in-person counseling [6, 15], and phone counseling [6]. Although the extant literature does suggest that more intensive interventions are more efficacious [3], little research has directly evaluated the effects of individual intervention components or their joint and interactive effects, making the development of a treatment package a “best guess” exercise rather than a process directly informed by experimental evidence.
Furthermore, there are limited data regarding the effectiveness of these components when they are provided in primary care. This lack of evidence demonstrating real-world effectiveness may contribute to the fact that medical professionals provide advice to quit to fewer than two-thirds of smokers and even fewer smokers receive evidence-based treatment. A national survey found only 4.7% of smokers who tried to quit in the past year used the recommended combination of cessation medication plus counseling [4]. Another study found that 77% of primary care patients received advice to quit, but only one-third of them used medication and only 16% used counseling, resulting in less than 9% quitting for more than 30 days [16]. Without counseling and medication, long-term quit rates among patients who receive only advice to quit smoking or “usual care” range between 2% and 10% [3, 17, 18].
The Multiphase Optimization Strategy (MOST; [19]) is an innovative framework that can be used to engineer treatment packages that improve upon current treatment. In the first step in MOST, Preparation, the investigator uses theory and extant data to identify potential intervention components to include in an optimized treatment package. In the next step, Optimization, these components are evaluated empirically, and based on the results, components are selected to form the optimized treatment package. Different strategies can be used for empirical evaluation of the components depending on the type of package to be optimized and the research questions at hand. In the current research, factorial screening experiments were used [20–25]. A single factorial experiment can efficiently yield data on both the main and interactive effects of multiple intervention components [20, 26–28]. In the final step in MOST, evaluation, the optimized treatment package is evaluated in a randomized controlled trial (RCT). The current research constitutes the first RCT of a smoking cessation treatment engineered based on MOST.
In our prior research using MOST [29, 30], we experimentally evaluated 11 smoking cessation intervention components in two factorial screening experiments [23, 24]. Because primary care settings offer an excellent opportunity to deliver smoking treatment [3, 31], the components were developed for and evaluated in such settings. Thus, the components involved few in-person meetings, and some elements were delivered via phone or automated calls; we did not include varenicline or bupropion due to the need for heightened medical screening and monitoring, and participants were patients making primary care visits. Components were evaluated based on their main and interactive effects on 6-month self-reported smoking abstinence [23, 24]. With the objective of maximizing abstinence at 6 months postquit, we identified five especially effective components that worked well together across the Prequit, Cessation, and Maintenance phases of smoking treatment [29]. These components were combined into an abstinence-optimized treatment (A-OT): (a) 3 weeks of preparation-phase mini-lozenges; (b) 26 weeks of postquit combination nicotine replacement (NRT; nicotine patch + nicotine mini-lozenges); (c) three cessation-phase in-person counseling sessions; (d) eight maintenance-phase counseling calls; and (e) 7–11 automated medication adherence calls.
This RCT represents the final step in one cycle of MOST—determining whether the optimized treatment improves upon current practice. We compared the A-OT with a smoking treatment that reflects a recommended standard of care for the primary care setting. Traditional deductive treatment development strategies (e.g., review of extant literature [20]) were used to develop the comparison treatment. It should be noted that the recommended usual care treatment (R-UC; 8 weeks of nicotine patch, brief in-person counseling, fax referral to a tobacco quitline for phone counseling, and assistance with accessing the quitline’s digital intervention resources for additional follow-up support) was more intense than what is typically provided in primary care (e.g., advice to quit, self-help material, possibly pharmacotherapy [32, 33]), but consistent with recommended practice [3]. This relatively intensive usual care treatment serves as a more rigorous comparator than simple advice to quit or advice plus referral to a quitline. Nevertheless, the A-OT was itself more intense than the R-UC treatment. Thus, differences between the two treatments may reflect both the way in which the treatments were developed (via MOST vs. deductively) and intensity per se. Despite the greater intensity of the A-OT, we hypothesized that primary care patients would engage in it satisfactorily based upon the engagement rates we obtained in prior research [23, 24] and that it would produce higher abstinence rates than R-UC.
Methods
Participants
Participants were patients from seven primary care clinics within two Wisconsin healthcare systems. Smokers who expressed interest in quitting smoking during a clinic visit were referred via the electronic health record (EHR) to the research study. Other smokers from these clinics were recruited via mailings and EHR messaging. Research personnel called all interested patients and assessed initial eligibility. Inclusion criteria were as follows: >17 years old; smoke >4 cigarettes/day for the previous 6 months; able to read, write, and speak English; plan to remain in the area for at least 12 months; have reliable phone access and agree to respond to interactive voice response phone prompts; be a patient at a participating clinic; not currently taking bupropion; agree to use only study medication for the duration of the study; have no history of stroke, heart attack, transient ischemic attack nor an abnormal electrocardiogram in the past 4 weeks; have no hospitalizations for diabetes or congestive heart failure in the past 4 weeks; have no diagnosis of or treatment for schizophrenia, a psychotic disorder or bipolar disorder in the last 10 years; and, for women of childbearing potential, use of an approved method of birth control during treatment.
Primary care patients who passed the phone screen were invited to attend a study visit at their referring clinic (where all treatment visits occurred) to learn more about the study, have eligibility confirmed, and provide written informed consent. Following consent, participants were randomized to one of two treatment conditions: R-UC or A-OT. Computer-based randomization used a 1:1 randomization within blocks of six participants, stratified by gender. This research was approved by the University of Wisconsin, Madison’s Institutional Review Board.
Treatments
Participants assigned to R-UC received the following treatment: (a) 8 weeks of nicotine patch (participants who smoked >10 cigarettes/day: 4 weeks of 21 mg, 2 weeks of 14 mg, and 2 weeks of 7 mg nicotine patches; participants who smoked ≤10 cigarettes/day: 6 weeks of 14 mg, and 2 weeks of 7 mg nicotine patches); (b) a single, 10-min in-person counseling session with a bachelor’s level trained smoking cessation counselor that included setting a quit date, discussing reasons for quitting, and preparing for the quit date; (c) a faxed referral to the Wisconsin Tobacco Quit Line (WTQL) with a time for the WTQL to call the participant for additional counseling and to provide access to Web Coach; and (d) the instructions for downloading the QUITNOW app, a free smoking cessation app, from the WTQL, that provides access to evidence-based tools to help smokers quit and active support for the first 2 weeks postquit.
Participants randomized to A-OT received five intervention components: (i) Preparation-phase nicotine mini-lozenges; (ii) 26-weeks of combination NRT (nicotine patch + nicotine mini-lozenges); (iii) intensive cessation-phase in-person counseling; (iv) intensive maintenance-phase phone counseling; and (v) automated medication adherence calls. Participants received 3 weeks of prequit, preparation-phase, nicotine mini-lozenges dosed per mini-lozenge labeling (smoke within 30 min of waking, 4 mg; smoke more than 30 min after waking, 2 mg). Participants were instructed to use one mini-lozenge every 1–2 h and up to 12 daily, with a goal of using at least 5 daily. Participants were advised to try to reduce their smoking, reduce the range of contexts in which they smoked, and substitute mini-lozenges for cigarettes.
Starting on the target quit day (TQD), A-OT participants were instructed to use both the nicotine patch and the nicotine mini-lozenges for 26 weeks, regardless of whether they returned to regular smoking. Dosing was consistent with medication labeling (patch dosing: smoke >10 cigarettes/day = 22 weeks of 21-mg, 2 weeks of 14-mg, and 2 weeks of 7-mg nicotine patches; smoke ≤10 cigarettes/day = 24 weeks of 14-mg and 2 weeks of 7-mg nicotine patches; mini-lozenge dosing: see the previous paragraph). Health counselors urged participants to use an average of 9 mini-lozenges/day, unless they experienced negative health effects, and instructed participants to taper their mini-lozenge use to zero over Weeks 24–26. All participants received instructions on proper NRT use, including an NRT information sheet with study staff contact information.
A-OT participants received three 20-min in-person cessation counseling sessions during the cessation phase (prequit Week –1, TQD, and postquit Week 1) from a bachelor’s level trained smoking cessation counselor. The goals of the counseling were to prepare for the TQD (e.g., reinforce motivation, remove cigarettes and smoking paraphernalia from the environment, plan for the quit day), develop techniques to cope with withdrawal symptoms and smoking triggers, including negative affect and withdrawal, and provide support. Participants then received eight maintenance-phase smoking cessation phone counseling sessions (Weeks 3, 4, 6, 8, 10, 14, 18, and 22). These 15-min calls emphasized content similar to that provided in the in-person counseling, namely support and problem solving. Finally, participants received 11 brief, automated calls reminding them to use their medications properly (Days 1, 3, 10, 17, 24, 31, 45, 73, 101, 129, and 157). Participants who reported no smoking during the Week 8 assessment call did not receive further automated adherence messages (i.e., they only received 7 automated calls during the first 6 weeks of their quit attempt). This decision was based on unpublished data from our prior study [24] that suggested that continued automated adherence calls undermined long-term self-reported cessation among smokers who achieved abstinence early in the quit attempt.
Assessments and Outcome Measures
Participants completed baseline assessments of demographics, tobacco use history, tobacco dependence (Fagerstrom Test of Nicotine Dependence, FTND, [34]; Brief Wisconsin Inventory of Smoking Dependence Motives, WISDM, [35]), medication beliefs, motivation, self-efficacy, confidence, withdrawal, affect, pleasure, and self-reported lifetime diagnosis of, or treatment for, the following psychiatric disorders: depression, bipolar, schizophrenia, anxiety, panic, post-traumatic stress, and attention deficit. Exhaled carbon monoxide (CO) was assessed using the Bedfont Micro+ Smokerlyzer (Bedfont Scientific, Rochester, England). Follow-up calls at Weeks 4, 8, 16, 26, 39, and 52 assessed recent tobacco use, motivation, self-efficacy, confidence, withdrawal, affect, pleasure, and medication use and adverse events. During each follow-up call, participants reported cigarettes per day in the last 7 days and completed a time-line follow-back calendar to capture smoking status (yes or no) cumulatively for each day of study participation. At the Week 26 follow-up call, participants who reported no smoking in the last 7 days were invited to their clinic to provide a breath sample for CO-verification of abstinence. Biochemically confirmed (CO <6 ppm) 7-day point-prevalence abstinence at Week 26 was the primary outcome. The study sample size (minimum of ~300 per group) was based on powering the study at 0.80 (α = .05, two-tailed test) to detect a 10% increase in long-term abstinence over and above the 20% abstinence rate that we projected conservatively for the usual care control [36].
Analytic Plan
Descriptive statistics were used to examine participant characteristics, treatment engagement (i.e., completion of study visits and calls), medication adherence, and adverse events. Group differences in patch use at 4 and 8 weeks were examined using chi-square tests. Self-reported point-prevalence abstinence rates at Weeks 4, 8, 16, 26, 39, and 52, and CO-confirmed point-prevalence abstinence rates at Week 26, were examined using logistic regression with an intent-to-treat approach where participants missing data were assumed to be smoking. Moderation of treatment effects at each follow-up time point was examined using logistic regression with treatment, the potential moderator, and the interaction term included in each moderator model. Finally, cost per quit and the incremental cost-effectiveness ratio (ICER) were calculated for each intervention from the payer’s perspective. Costs for the counseling interventions were derived from the Medicaid portal based on a bachelor’s level trained provider. Phone counseling costs were based on 50% of the billed Wisconsin Tobacco Quit Line rate, a formula approved by Medicaid. Finally, costs for the medications were estimated using the maximum allowed drug costs from the Medicaid portal.
Role of the Funding Source
This research was funded by grants from the National Institutes of Health. The funder had no role in the development, implementation, analysis, or reporting of this research.
Results
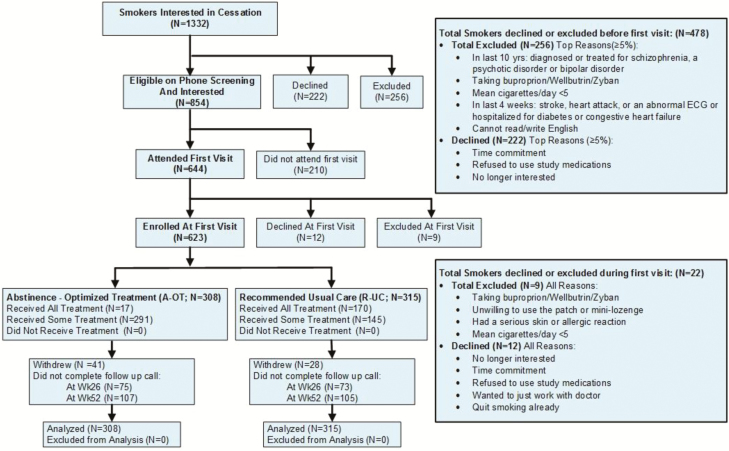
A total of 623 eligible participants were randomized to receive either R-UC (n = 315) or A-OT (n = 308; see Table 1). Figure 1 presents the CONSORT diagram. Data were collected from January 2015 through December 2016.
Table 1.
Participant characteristics
| Total sample (n = 623) | Recommended usual care (n = 315) | Abstinence-optimized treatment (n = 308) | |
|---|---|---|---|
| Women (%) | 357 (57.3) | 184 (58.4) | 173 (56.2) |
| Hispanic (%) | 21 (3.4) | 12 (4.0) | 9 (3.0) |
| White (%) | 431 (69.2) | 211 (67.0) | 220 (71.4) |
| African-American (%) | 161 (25.8) | 92 (29.2) | 69 (22.4) |
| Less than high school education (%) | 98 (15.7) | 53 (16.8) | 45 (14.6) |
| High school education (%) | 248 (39.8) | 135 (42.9) | 113 (36.7) |
| More than high school education (%) | 276 (44.3) | 126 (40.0) | 150 (48.7) |
| Married/living with partner (%) | 298 (47.8) | 149 (47.3) | 149 (48.4) |
| Age (M [SD]) | 49.7 (12.7) | 49.4 (12.9) | 50.0 (12.5) |
| Cigarettes per day (M [SD]) | 16.8 (9.4) | 17.1 (9.8) | 16.5 (9.0) |
| Smoke menthol cigarettes (%) | 304 (48.8) | 161 (51.1) | 143 (46.4) |
| Motivation to quit (M [SD]) | 6.4 (1.0) | 6.4 (1.0) | 6.5 (0.9) |
| Baseline CO (M [SD]) | 17.9 (11.0) | 17.9 (11.0) | 17.9 (11.0) |
| FTND Score (M [SD]) | 4.8 (2.2) | 4.9 (2.2) | 4.8 (2.2) |
| Live with a smoker (%) | 295 (47.5) | 143 (45.4) | 152 (49.4) |
| History of depression (%) | 245 (39.3) | 121 (38.4) | 124 (40.3) |
| History of anxiety or panic (%) | 161 (25.8) | 80 (25.4) | 81 (26.3) |
| Reported no psychiatric history (%) | 308 (49.4) | 159 (50.5) | 149 (48.4) |
There were no statistically significant differences between the abstinence-optimized treatment and recommended usual care groups on any variable.
CO, carbon monoxide; FTND, Fagerstrom Test of Nicotine Dependence.
Fig. 1.
CONSORT diagram.
Treatment Engagement, Medication Adherence, and Adverse Events
All R-UC participants received the 10-min in-person counseling session, 8 weeks of nicotine patches, and referral to the WTQL at the initial study visit. WTQL data showed that 170 participants (54.0%) completed a WTQL call; 49 (15.6%) declined services when the WTQL called them, and 96 (30.5%) were unreachable. Fifty-one of the 241 participants who completed the Week 4 follow-up call (21.2%) reported that they had downloaded the QuitNow WTQL app. Of these 51, self-reported use rates in the previous 4 weeks ranged from less than once a week (35%) to at least once a day (29.4%).
Among A-OT participants, engagement rates for the in-person Cessation-phase counseling sessions were as follows: 58.1% attended all three sessions, 20.5% attended two, 11.4% attended one, and 10.1% attended none. They also completed a mean of 4.4 maintenance-phase counseling calls (SD = 3.2); 60.7% completed ≥4 calls, 21.8% completed no calls, and 29.5% completed all eight calls. Finally, A-OT participants completed a mean of 4.2 (SD = 3.4) medication automated adherence calls (7 or 11 possible); 24.0% of participants completed none.
More A-OT participants than R-UC participants reported using patches every day for the last 7 days at Week 4 (71.0% vs. 49.8%), and fewer reported using patches on none of the days (9.5% vs. 25.7%; χ2 = 30.02, p < .001). Similar differences in patch use occurred at Week 8 (χ2 = 30.82, p < .001). Among A-OT participants, daily patch use in the last 7 days decreased to 52.6% by Week 16 and 37.6% by Week 26.
Two-thirds of A-OT participants used mini-lozenges in the Preparation phase. By Week 4, only 53.9% reported using lozenges in the last 7 days; that declined to 35.7% by Week 26. A-OT participants reported using a mean 5.1 (SD = 3.8) lozenges per day in the Preparation phase, and 5.7 (SD = 4.2) lozenges per day at Week 4, which decreased to 2.6 (SD = 3.2) lozenges per day by Week 26.
The most common adverse events reported by R-UC participants over the course of 8 weeks of nicotine patch were: itching/hives (7%), skin rash (6%), headache (5%), and vivid dreams (4%). The most common adverse events reported by A-OT participants over the course of 26 weeks of nicotine patch + mini-lozenges were: nausea (12%), indigestion (11%), skin rash (11%), itching/hives (7%), hiccups (7%), insomnia (6%), and vivid dreams (5%).
Cessation Outcome, Misreporting, and Moderation
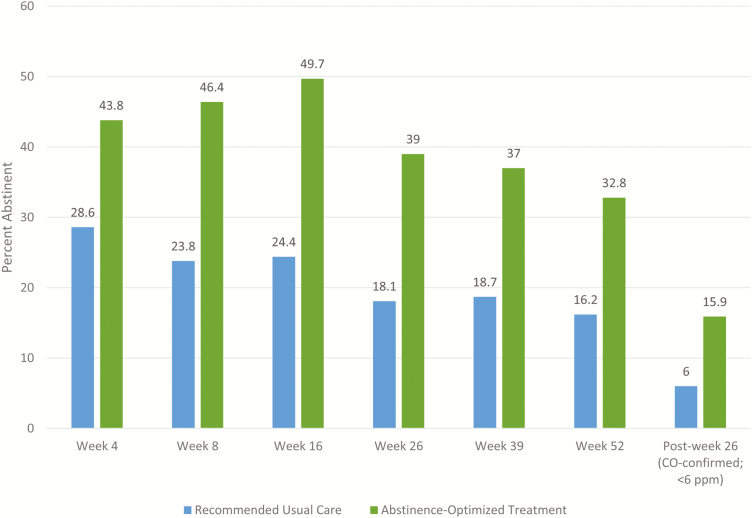
Abstinence rates were significantly higher among A-OT participants than R-UC participants at all follow-up time points, for both self-reported and 26-week biochemically confirmed outcomes (see Fig. 2 and Table 2). In fact, abstinence rates for the A-OT group are twice those of the R-UC group at most time points. Odds ratios ranged from 1.95 at Week 4 to 3.09 at Week 16.
Fig. 2.
Mean self-reported 7-day point-prevalence abstinence rates at Weeks 4, 8, 16, 26, 39, and 52, and carbon monoxide (CO)-confirmed point-prevalence abstinence at Week 26 for the recommended usual care and abstinence-optimized treatments.
Table 2.
Seven-day point-prevalence abstinence rates over time for both treatment groups
| Abstinence type | Time | Recommended usual care | Abstinence-optimized treatment | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Self-reported | Week 4 | 28.6 | 43.8 | 1.95 | 1.40, 2.72 | <.001 |
| Week 8 | 23.8 | 46.4 | 2.77 | 1.97, 3.91 | <.001 | |
| Week 16 | 24.4 | 50.0 | 3.09 | 2.20, 4.35 | <.001 | |
| Week 26 | 18.4 | 39.3 | 2.87 | 1.99, 4.13 | <.001 | |
| Week 39 | 18.7 | 37.0 | 2.55 | 1.77, 3.68 | <.001 | |
| Week 52 | 16.2 | 32.8 | 2.53 | 1.72, 3.70 | <.001 | |
| CO-confirmed (<6 ppm) | Post-week 26 | 6.0 | 15.9 | 2.95 | 1.69, 5.14 | <.001 |
CO, carbon monoxide.
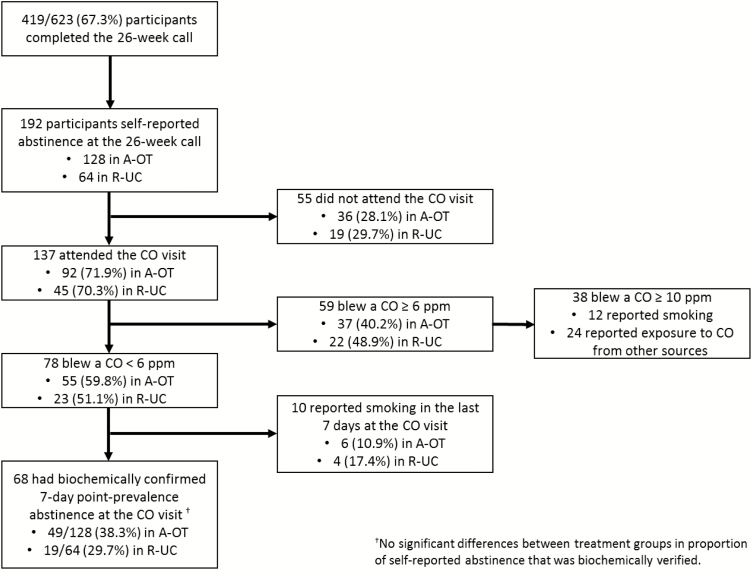
Table 2 also shows that the Week 26 biochemically confirmed abstinence rates (the primary outcome) for the two treatment conditions were substantially lower than the self-reported rates. One hundred ninety-two participants reported 7-day point-prevalence abstinence at the Week 26 follow-up call (Fig. 3); all these participants were invited to attend an in-person visit for CO confirmation. Of the 192, 137 attended the CO visit, with both treatment conditions participating at an equivalent rate (71.4% of those claiming abstinence in each group). The following variables were tested as predictors of not confirming self-reported Week 26 abstinence (due to nonattendance or a positive CO test): treatment condition, gender, race, education, age, cigarettes per day, menthol use, motivation, Week 16 self-reported point-prevalence abstinence, living with a smoker, anxiety, depression, and income. Income was the only significant predictor in a multivariable model (OR = 1.30, p = .001); participants in households earning less than $20,000 were less likely to biochemically verify self-reported abstinence compared to those living in households with incomes of $20,000 or more.
Fig. 3.
Participant flow for establishing biochemically confirmed abstinence at Week 26.
Significant moderation of treatment effects was found for only race and education and only at Week 16 (race OR = 0.41, 95% CI = 0.20, 0.84, p = .02; education OR = 0.49, 95% CI 0.25 0.98, p = .045). Specifically, self- reported abstinence rates among Whites and non-Whites were similar if they received A-OT (50.5% vs. 49.4%); however, non-Whites had higher abstinence rates than Whites if they received R-UC (35.6% vs. 19.0%). A-OT participants had higher self-reported abstinence rates than R-UC participants across education levels, but those with more than a high school education were especially likely to benefit from A-OT versus those with a high school education or less (57.3% vs. 43.0% abstinent at Week 16, χ2 = 6.29, p = .01). There was no significant moderation of the primary outcome, CO-confirmed 26-week abstinence.
Cost per Quit
Mean estimated payer costs were $138.48/person for R-UC and $900.31/person for A-0T (see Table 3). The mean cost per biochemically verified quit was $2,295.82 for R-UC and $5,659.12 for A-OT. The ICER (the cost/quit for additional quitters beyond the comparison group) was $3,709.15 for self-reported 26-week abstinence and $7,789.22 for CO-confirmed 26-week abstinence. Therefore, using biochemically confirmed abstinence, it cost ~$2,300/quit to get a quit rate of 6% with R-UC, but to raise the quit rate from 6% to 16% with A-OT, it cost an additional $7,800/quit.
Table 3.
Cost estimates for the two treatments as delivered
| Costs as delivered | Recommended usual care | Abstinence-optimized treatment |
|---|---|---|
| Patches | $94.76 (SD = 7.19) | $195.05 (SD = 112.82) |
| In-person counseling | $21.96 (SD = 0.00) | $68.67 (SD = 30.75) |
| Fax-to-quit | $21.76 (SD = 12.73) | – |
| Mini-lozenges | – | $536.81 (SD = 278.13) |
| Phone counseling | – | $68.79 (SD = 49.21) |
| Automated adherence calls | – | $30.00 (SD = 0.00) |
| Mean cost per participant | $138.48 (SD = 14.01) | $900.31 (SD = 428.55) |
| Total cost | $43,620.27 | $2,77,296.79 |
| Cost per quit (self-report at 26 weeks) |
$752.08 | $2,310.81 |
| Cost per quit (biochemically verified at 26 weeks) |
$2,295.82 | $5,659.12 |
| Incremental cost per quit (self-report at 26 weeks) |
$3,709.15 | |
| Incremental cost per quit (biochemically verified at 26 weeks) | $7,789.22 |
Conclusions
This research represents the first application of MOST [37, 38] to evaluate an engineered, optimized smoking cessation treatment. Intervention components that produced promising main and/or interaction effects on long-term abstinence in prior factorial experiments [23, 24] were combined to form an A-OT. The A-OT was compared in an RCT with R-UC, which was developed via a traditional, informal deductive treatment development strategy ([20]; i.e., via an appraisal of extant research and consideration of current practice and compatibility with use in primary care). Key outcomes included whether primary care patients would engage in an optimized treatment and whether it would produce higher abstinence rates than the R-UC. Thus, this research addresses the relative effectiveness of two treatments that differed in both intensity and development strategy but were both deemed appropriate for primary care.
A-OT produced self-reported and biochemically confirmed abstinence rates that were approximately double those produced by the R-UC. While the absolute levels of biochemically confirmed abstinence at Week 26 were not high for A-OT smokers, it is important to observe that this trial was conducted not with “treatment seekers,” but with primary care patients who expressed interest in quitting smoking when asked about it during clinic visits. More than half of participants lived with smokers, reported low socio-economic status, and/or had positive psychiatric histories (although those who reported a diagnosis of or treatment for psychosis, schizophrenia, or bipolar disorder in the last 10 years were excluded at screening). Furthermore, this intervention worked equally well for all patients; no significant moderators of treatment effects (self-reported or biochemically confirmed) were found at Week 26.
A-OT was engineered to maximize abstinence rates and therefore was more intensive than the R-UC treatment. This raises the question of whether smokers are likely to engage in such an intensive treatment. Our results suggest that participants were more likely to engage in A-OT than in R-UC. Only about half of participants assigned to the R-UC treatment completed a single counseling call with the WTQL. This is consistent with research reporting that only 30–50% of quitline referrals convert to enrollment [32, 39]. On the other hand, 90% of A-OT participants completed at least one in-person counseling session; >75% attended two to three in-person counseling sessions, and more than half completed four or more counseling calls. While these data reflect treatment engagement among those who volunteer for treatment, this study does not reflect how willing smokers would be to volunteer for the two different types of treatment if each were offered under real-world conditions.
While A-OT is, no doubt, cost-effective in absolute terms [40, 41], its cost and cost-effectiveness from a payer perspective, relative to the R-UC, might limit its adoption. However, cheaper but less effective treatments can be costly in the long run because healthcare costs are ~40% higher for smokers than for non-smokers [42]. Studies have shown that smoking cessation treatment in primary care clinics lowers healthcare costs within 18 months of quitting [43], by at least 10% [44]. As the ICER shows, it cost ~$2,300 per quit for the first 6% of successful biochemically verified quitters with R-UC, but $7,800 per quit for the next 10% of biochemically verified quitters with A-OT. These costs must be weighed against the costs of continued smoking by more patients.
While cost-effectiveness is clearly relevant from the treatment payer’s perspective, many other factors influence the selection of a healthcare system-wide cessation treatment, including the staffing needed to implement an intensive intervention, downstream health effects, and costs of continued smoking. One response to the lower cost-effectiveness of the A-OT would be to generate an additional optimized treatment, one based on the earlier factorial experiments [23, 24] and that selects intervention components based on their cost-effectiveness versus on long-term abstinence per se. It is possible that similar abstinence rates could be produced by a treatment with fewer and less costly elements. A similar approach could be taken to develop a less staff-intensive optimized treatment. This illustrates a virtue of MOST: viz. multiple treatments may be developed from the same set of factorial experiments using different optimization criteria (e.g., long-term abstinence, cost-effectiveness, minimal staff burden). Of course, any new treatment package would need to be evaluated in a new RCT.
Another concern regarding this research is the discrepancy between the self-reported and biochemically confirmed 26-week abstinence rates. Studies of low-contact treatments, such as quitline and primary care smoking treatments, typically do not use biochemical verification [45–47]. This may lead to over-estimation of abstinence, especially when financial incentives are contingent upon reporting abstinence. However, biochemical confirmation may be difficult to implement in real-world studies. Ferguson and colleagues [48] tried to verify abstinence in their quitline study, but only 52% of the self-reported quitters provided samples for confirmation. Our results are consistent with those of Scheuermann et al. [49] who found that among hospitalized smokers who claimed abstinence, only 70% returned a saliva sample for biochemical confirmation; only 58%–61% of study participants were biochemically confirmed abstinent, and bioverification was related to education (i.e., a marker of socio-economic status) but not treatment. It should be noted that our results might actually underestimate the occurrence of recent smoking since CO has a relatively short half-life [50]. While cotinine has a longer half-life, it would detect the use of NRT which would have occurred in close proximity to the 26-week follow-up. Taken together, these results suggest that biochemical confirmation may be vital to an accurate assessment of abstinence rates in real-world effectiveness studies. It may be especially important for studies that occur in clinical settings where patients may be concerned about providers’ reactions to their relapse.
Two additional limitations of this research are that, first, treatment was provided by research staff embedded within the primary care clinics rather than by existing clinic personnel. It is unknown whether this intervention would be similarly effective when delivered by healthcare system employees outside of a research context. Future research is needed to address the effects of translating this optimized treatment into standard clinical practice where treatment is provided by clinic staff and where its effectiveness is gauged in a broader sample of primary care patients (i.e., those with serious mental illness, those not interested in participating in a research study). Second, we conducted basic cost-effectiveness analyses using only the payer’s costs for providing treatment. Further cost-effectiveness and cost–benefit analyses are needed to understand the full economic costs and benefits of the two treatments.
This study demonstrates that a treatment engineered using the methodologically principled MOST produced significantly higher long-term smoking abstinence rates than did a treatment intended to represent somewhat intensive evidence-based clinical care. This is the first program of research to investigate the effectiveness of individual treatment components using efficient factorial experiments, assemble an optimized integrated treatment package based on the results, and then compare the performance of the optimized treatment package to a high level of standard care. While the MOST-engineered treatment produced significantly higher abstinence rates than did the traditionally designed comparison treatment, and produced higher rates of treatment engagement, it was clearly more expensive. A future step in our research program is to develop a new smoking treatment designed to optimize effectiveness while controlling costs.
Although A-OT self-reported and 26-week biochemically confirmed abstinence rates were double those of R-UC, they were still fairly low, especially the biochemically confirmed rate. This finding shines light on the need to increase treatment reach and initial engagement in primary care [51, 52]. That is, the modest effectiveness of even an optimized, relatively intense treatment suggests that the most likely route to significant reductions in primary care smoking prevalence is engaging more smokers in treatment. In the current study, smokers were recruited during their primary care visits when they were not seeking cessation treatment, thereby demonstrating one way to expand reach. Further, these abstinence rates were obtained among smokers who had high levels of risk factors for treatment failure (e.g., low socio-economic status, psychiatric history). Therefore, the A-OT is a viable option for use by healthcare systems that are interested in reducing smoking prevalence in their patient populations.
Acknowledgments
The study was supported by National Cancer Institute [grant P01CA180945], National Institute on Alcohol Abuse and Alcoholism [grant R01AA022931], National Institute on Drug Abuse [grants R01DA040480 and P50DA039838], and National Institute of Diabetes and Digestive and Kidney Disease [grant R01DK097364].
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Study procedures were approved by the University of Wisconsin IRB.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1. U.S. Department of Health and Human Services. In: National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Source, ed. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA:Centers for Disease Control and Prevention (US)2014. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults in the United States, 2016http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/.Accessibility verified September 15, 2017.
- 3. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 4. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults – United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. [DOI] [PubMed] [Google Scholar]
- 5. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2015 Oct 12;(10): CD009670. [DOI] [PubMed] [Google Scholar]
- 7. Siu AL, U.S. Preventive Services Task Force Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(8):622–634. [DOI] [PubMed] [Google Scholar]
- 8. Shiffman S, Ferguson SG. Nicotine patch therapy prior to quitting smoking: a meta-analysis. Addiction. 2008;103(4):557–563. [DOI] [PubMed] [Google Scholar]
- 9. Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD000146. [DOI] [PubMed] [Google Scholar]
- 10. Lindson N, Aveyard P. An updated meta-analysis of nicotine preloading for smoking cessation: investigating mediators of the effect. Psychopharmacology (Berl). 2011;214(3):579–592. [DOI] [PubMed] [Google Scholar]
- 11. Hawk LW Jr, Ashare RL, Rhodes JD, Oliver JA, Cummings KM, Mahoney MC. Does extended pre quit bupropion aid in extinguishing smoking behavior?Nicotine Tob Res. 2015;17(11):1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175(4):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. 2016;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2017;3:CD001292. [DOI] [PubMed] [Google Scholar]
- 16. Quinn VP, Hollis JF, Smith KS, et al. Effectiveness of the 5-As tobacco cessation treatments in nine HMOs. j Gen Intern Med. 2009;24(2):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Law M, Tang JL. An analysis of the effectiveness of interventions intended to help people stop smoking. Arch Intern Med. 1995;155(18):1933–1941. [PubMed] [Google Scholar]
- 18. Naughton F, Jamison J, Boase S, et al. Randomized controlled trial to assess the short-term effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care (iQuit in practice). Addiction. 2014;109(7):1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins LM, Kugler KC, Gwadz MV. Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS Behav. 2016;20(Suppl 1):S197–S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baker TB, Smith SS, Bolt DM, et al. Implementing clinical research using factorial designs: a primer. Behav Ther. 2017;48(4):567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cook JW, Collins LM, Fiore MC, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction. 2016;111(1):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClure JB, Peterson D, Derry H, et al. Exploring the “active ingredients” of an online smoking intervention: a randomized factorial trial. Nicotine Tob Res. 2014;16(8):1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piper ME, Fiore MC, Smith SS, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction. 2016;111(1):129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schlam TR, Fiore MC, Smith SS, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. 2016;111(1):142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strecher VJ, McClure JB, Alexander GL, et al. Web-based smoking-cessation programs: results of a randomized trial. Am j Prev Med. 2008;34(5):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. 2014;47(4):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(Suppl 5):S112–S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins LM, Trail JB, Kugler KC, Baker TB, Piper ME, Mermelstein RJ. Evaluating individual intervention components: making decisions based on the results of a factorial screening experiment. Transl Behav Med. 2014;4(3):238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41(2):192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baker TB, Collins LM, Mermelstein R, et al. Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction. 2016;111(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jamal A, Dube SR, Malarcher AM, Shaw L, Engstrom MC. Tobacco use screening and counseling during physician office visits among adults—National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005–2009. MMWR. 2012;61(Suppl):38–45. [PubMed] [Google Scholar]
- 32. Zwar NA, Richmond RL, Halcomb EJ, et al. Quit in general practice: a cluster randomized trial of enhanced in-practice support for smoking cessation. Fam Pract. 2015;32(2):173–180. [DOI] [PubMed] [Google Scholar]
- 33. Martín Cantera C, Puigdomènech E, Ballvé JL, et al. Effectiveness of multicomponent interventions in primary healthcare settings to promote continuous smoking cessation in adults: a systematic review. BMJ Open. 2015;5(10):e008807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 35. Smith SS, Piper ME, Bolt DM, et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob Res. 2010;12(5):489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169(22):2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14(3):202–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins LM, Nahum-Shani I, Almirall D. Optimization of behavioral dynamic treatment regimens based on the sequential, multiple assignment, randomized trial (SMART). Clin Trials. 2014;11(4):426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Transl Behav Med. 2014;4(3):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for health care policy and research. JAMA. 1997;278(21):1759–1766. [PubMed] [Google Scholar]
- 41. Maciosek MV, LaFrance AB, Dehmer SP, et al. Health benefits and cost-effectiveness of brief clinician tobacco counseling for youth and adults. Ann Fam Med. 2017;15(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barendregt JJ, Bonneux L, van der Maas PJ. The health care costs of smoking. N Engl J Med. 1997;337(15):1052–1057. [DOI] [PubMed] [Google Scholar]
- 43. Hockenberry JM, Curry SJ, Fishman PA, et al. Healthcare costs around the time of smoking cessation. Am j Prev Med. 2012; 42(6): 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leif Assiciates. The Business Case for Coverage of Tobacco Cessation, 2012 Update http://www.ctri.wisc.edu/documents/Actuarial.Analysis.pdf. Accessibility verified September 15, 2017.
- 45. Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013 Aug 12;(8):CD002850. [DOI] [PubMed] [Google Scholar]
- 46. Park ER, Gareen IF, Japuntich S, et al. Primary care provider-delivered smoking cessation interventions and smoking cessation among participants in the national lung screening trial. JAMA Intern Med. 2015;175(9):1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith SS, Keller PA, Kobinsky KH, et al. Enhancing tobacco quitline effectiveness: identifying a superior pharmacotherapy adjuvant. Nicotine Tob Res. 2013;15(3):718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferguson J, Docherty G, Bauld L, et al. Effect of offering different levels of support and free nicotine replacement therapy via an English national telephone quitline: randomised controlled trial. BMJ. 2012;344:e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheuermann TS, Richter KP, Rigotti NA, et al. ; Consortium of Hospitals Advancing Research on Tobacco (CHART). Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandberg A, Sköld CM, Grunewald J, Eklund A, Wheelock ÅM. Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS One. 2011;6(12):e28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Institute of Medicine. In: Bonnie RJ, Stratton K, Wallace RB, eds. Ending the Tobacco Problem: A Blueprint for the Nation. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 52. Abrams DB, Graham AL, Levy DT, Mabry PL, Orleans CT. Boosting population quits through evidence-based cessation treatment and policy. Am J Prev Med. 2010;38(Suppl 3):S351–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]