Short abstract
Objective
This study was performed to investigate the role of probiotics (Clostridium butyricum combined with Bifidobacterium infantis) in the treatment of minimal hepatic encephalopathy (MHE) in patients with hepatitis B virus (HBV)-induced liver cirrhosis.
Methods
Sixty-seven consecutive patients with HBV-induced cirrhosis without overt hepatic encephalopathy were screened using the number connection test and digit symbol test. The patients were randomized to receive probiotics (n = 30) or no probiotics (n = 37) for 3 months. At the end of the trial, changes in cognition, intestinal microbiota, venous ammonia, and intestinal mucosal barriers were analyzed using recommended systems biology techniques.
Results
The patients’ cognition was significantly improved after probiotic treatment. The predominant bacteria (Clostridium cluster I and Bifidobacterium) were significantly enriched in the probiotics-treated group, while Enterococcus and Enterobacteriaceae were significantly decreased. Probiotic treatment was also associated with an obvious reduction in venous ammonia. Additionally, the parameters of the intestinal mucosal barrier were obviously improved after probiotic treatment, which might have contributed to the improved cognition and the decreased ammonia levels.
Conclusion
Treatment with probiotics containing C. butyricum and B. infantis represents a new adjuvant therapy for the management of MHE in patients with HBV-induced cirrhosis.
Keywords: Minimal hepatic encephalopathy, hepatitis B virus, probiotics, Clostridium, Bifidobacterium, liver cirrhosis
Introduction
Long-term chronic hepatitis B virus (HBV) infection, which can evolve into liver cirrhosis and carcinoma, affects an estimated 350 million people worldwide and results in more than 0.5 to 1.0 million deaths per year.1 Growing evidence has shown that an altered gut microbiota profile is associated with cirrhosis and its complications, such as hepatic encephalopathy (HE), spontaneous bacterial peritonitis, and other infections, which might be correlated with disruption of the intestinal barrier.1 HE is a serious neuropsychiatric syndrome that causes central nervous system dysfunction due to severe liver dysfunction, especially decompensated liver cirrhosis.1 According to the West-Haven criteria, minimal HE (MHE) is the mildest form of HE, affecting about one-third of patients with cirrhosis.1 Patients with MHE have no recognizable clinical symptoms of HE; instead, they have mild cognitive and psychomotor deficits that negatively affect their daily functioning and quality of life.1 The prevalence of MHE varies from 30% to 84% in patients with liver cirrhosis.2 No standardized criteria for a diagnosis of MHE have yet been established; the diagnosis depends on the patient’s history and physical examination findings, mental status, and alteration of cognition and/or neurophysiological function as well as the exclusion of concomitant neurological disorders.3 Previous studies have shown that ammonia is a crucial factor in the pathogenesis of MHE: hyperammonemia induces abnormalities such as brain edema and intracranial hypertension in patients with liver cirrhosis, and the blood ammonia levels are correlated with the severity of MHE in patients with cirrhosis.4
The human intestinal microbiota plays vital roles in maintaining intestinal homeostasis, which is central to human general health and wellbeing. The gut–brain axis is characterized by complex bidirectional communication between the gut and brain to maintain intestinal homeostasis and influence cognitive functions. Dysbiosis of the intestinal microbiota alters the microenvironment of the colonic lumen, especially the pH, which contributes to increased production of ammonia from the gut microbiota and increased ammonia absorption from the colonic lumen to the blood. Several studies have confirmed that probiotics are effective and safe, are minimally toxic, and have few adverse effects in the treatment of MHE.5–9 Compared with lactulose, probiotics are associated with no major adverse events and are better tolerated. The present study was performed to evaluate the effects of a probiotic mixture containing Clostridium butyricum combined with Bifidobacterium infantis in patients with MHE with the goal of developing a new adjuvant therapy for MHE in clinical practice.
Methods
Patient recruitment
In the present study, patients with HBV-induced liver cirrhosis without overt encephalopathy were screened for MHE, and those patients with MHE were enrolled. HBV-induced liver cirrhosis was diagnosed on a clinical basis involving laboratory tests, ultrasound imaging, and liver histology, if available. Patients with MHE were diagnosed according to the consensus on the diagnosis and treatment of HE in China,10 which involved the performance of two psychometric tests: the number connection test A (NCT-A) and digit symbol test (DST). Patients with abnormal NCT-A and DST results were diagnosed with MHE. All patients were treated with the same conventional therapy, while a portion was randomized to probiotic treatment and another portion was randomized to no probiotic treatment. The following exclusion criteria were applied: a history of overt HE in the past 2 months; the use of probiotics, prebiotics, synbiotics, or antibiotics during the previous 4 weeks; the presence of any other neurological or psychiatric diseases; a history of spontaneous bacterial peritonitis or gastrointestinal bleeding in the past 2 months; known active microbial infections; and a high-protein diet. The protocols utilized in the present study were approved by the Ethics Committee of the Changxing People’s Hospital (Huzhou, China) and were carried out according to the approved guidelines. Written informed consent was obtained from each patient prior to enrollment.
Probiotics
The probiotic mixture containing Clostridium butyricum (CGMCC0313-1) combined with B. infantis (CGMCC0313-2) used in the present study was produced by Shandong Kexing Bioproducts Co., Ltd. (Shandong, China). The freeze-dried probiotic mixture had more than 1.0 × 107 CFU/g viable C. butyricum and more than 1 × 106 CFU/g viable B. infantis per capsule. The patients treated with the probiotics received a dose of 1500 mg three times daily for 3 months.
Quantitative assessment of predominant fecal bacteria
At the end of the treatment, a fresh fecal sample (approximately 2 g) was collected from each patient for bacterial analysis. Bacterial genomic DNA was extracted using a QIAamp® DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to our previous study.11 The amount of bacterial genomic DNA was analyzed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and the integrity and size of bacterial genomic DNA were checked by electrophoresis. All bacterial genomic DNA was stored at −20°C for further use.
For analysis of the predominant gut bacteria, quantitative polymerase chain reaction (PCR) was performed on an ABI Prism 7900HT real-time PCR system (Applied Biosystems, Carlsbad, CA, USA) with Power SYBR Green PCR Master Mix (Vazyme Biotech, Nanjing, China). The bacterial primer sets and reaction conditions were in accordance with our previous studies.11,12 SDS 2.4 software (Applied Biosystems) was used for data analysis. Triplicate repeats were carried out for all reactions in every analysis with inclusion of a nontemplate. The quantity of these bacteria was presented as log10 bacteria per gram of feces (wet weight).
Measurement of venous ammonia levels
Blood was sampled for blood ammonia analysis at the end of the therapy using a commercial ammonia test kit (ARKRAY, Tokyo, Japan) within 30 minutes after sample collection with a detection range of 10 to 400 µmol/L.
Intestinal mucosal barrier function analysis
At the end of the treatment, serum from each patient was collected for intestinal mucosal barrier function analysis. Three parameters of the intestinal mucosal barrier function [D-lactate, endotoxin (LPS), and diamine oxidase (DAO)] were detected by a dry chemical method using the Intestinal Mucosal Barrier Biochemical Index Analysis System (JY-DLT; Beijing Zhongsheng Jinyu Diagnostic Technology Co., Ltd., Beijing, China).
Statistical analysis
The quantitative data of gut bacteria and other parameters in our study are presented as mean ± standard deviation, and differences between the two groups were evaluated by Student’s t test using IBM SPSS Statistics, version 20.0 (IBM Corp., Armonk, NY, USA). A p value of <0.05 was considered statistically significant for all analyses.
Results
Patients’ characteristics
Sixty-seven patients were enrolled in this study (probiotic treatment, n = 30; no probiotic treatment, n = 37). The characteristics of all patients after 3 months of probiotic treatment are shown in Table 1. All participants were diagnosed with HBV-induced liver cirrhosis. No obvious differences in age or sex were found between the two groups, while there were significant improvements in the serum alanine aminotransferase, aspartate aminotransferase, total bilirubin, and albumin levels after probiotic treatment at the end of the trial (p < 0.05). In addition, the probiotics improved the results of the psychometric tests for MHE (NCT-A and DST). These data suggest that probiotics containing C. butyricum and B. infantis can improve the MHE status and be used as an effective adjuvant therapy for MHE.
Table 1.
Patients’ characteristics
| Control (n = 37) | Treatment (n = 30) | |
|---|---|---|
| Age, y | 43.8 ± 10.3 | 41.5 ± 12.9 |
| Sex, male/female | 20/17 | 18/12 |
| TBIL, µmol/L* | 48.3 ± 6.7 | 17.6 ± 13.5 |
| ALT, U/L* | 89.1 ± 26.4 | 35.9 ± 22.5 |
| AST, U/L* | 95.6 ± 101.7 | 28.5 ± 18.7 |
| Albumin, g/L* | 55.3 ± 25.9 | 62.3 ± 22.5 |
| Child–Pugh score | ||
| B | 15 | 18 |
| C | 22 | 12 |
| Ascites | 15 (40.54) | 8 (26.67) |
| INR | 1.4 ± 0.6 | 1.3 ± 0.4 |
| NCT-A, s* | 72.4 ± 11.5 | 41.2 ± 8.9 |
| DST, points/90 s* | 12.5 ± 8.3 | 29.8 ± 10.7 |
Data are presented as mean ± standard deviation, n, or n (%).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DST, digit symbol test; INR, international normalized ratio of prothrombin time; NCT-A, number connection test A; TBIL, total bilirubin. *p < 0.05
Quantification and comparison of the predominant fecal microbiota between the two groups
The differences in the predominant fecal bacteria in patients with MHE were detected by quantitative PCR (Figure 1). After treatment, the total bacteria of the gut microbiota were similar between the probiotics-treated group and control group. Nine other fecal bacteria were also analyzed to reveal the specific changes in the fecal microbiota. Our data indicated that Clostridium cluster I and Bifidobacterium were significantly increased after probiotic treatment (p < 0.05). Because C. butyricum belongs to Clostridium cluster I and B. infantis belongs to the genus Bifidobacterium, the colonization of the probiotic strains might have contributed to the increase in these two abundant bacteria. In addition, Enterococcus and Enterobacteriaceae were significantly decreased after probiotic treatment (p < 0.05); these microorganisms might be the sources of the opportunistic infections that translocate from the gut to other tissues. There were no significant differences in the other bacteria between the two groups.
Figure 1.
Quantification of the predominant intestinal bacteria in patients with minimal hepatic encephalopathy treated by probiotics (log10 copies per gram of fresh feces). *p < 0.05; #p < 0.01.
Improvement in venous ammonia level
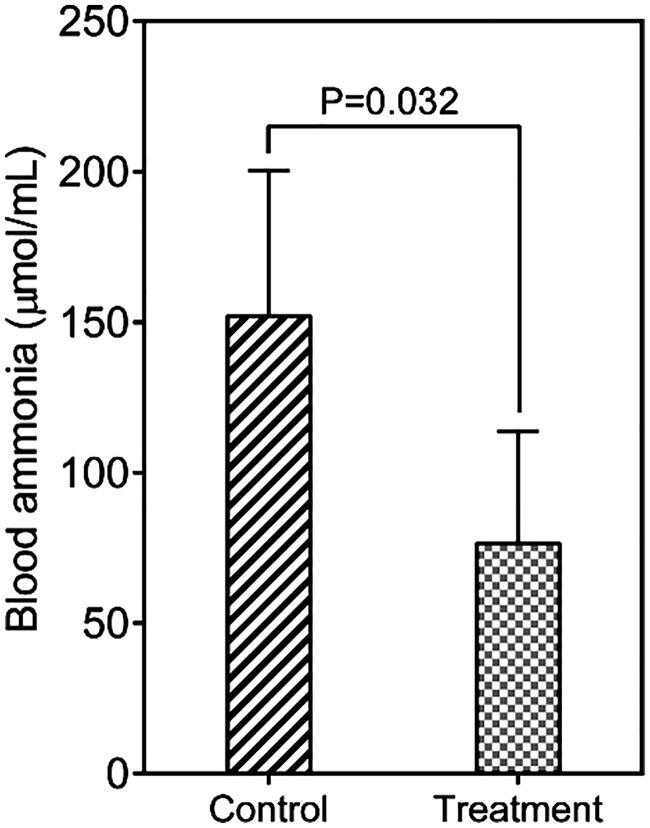
Venous ammonia is thought to play vital roles in the pathogenesis of HE. At the end of the trial, the mean venous ammonia level was significantly lower in the probiotics-treated group than control group (76.4 ± 37.3 vs. 152.0 ± 48.3 μmol/mL, respectively; p = 0.032) (Figure 2). This decrease in venous ammonia might have been associated with the improvement in the NCT-A and DST results for these patients with MHE after probiotic treatment.
Figure 2.
Changes in the blood ammonia level after treatment with probiotics.
Restoration of intestinal mucosal barrier function
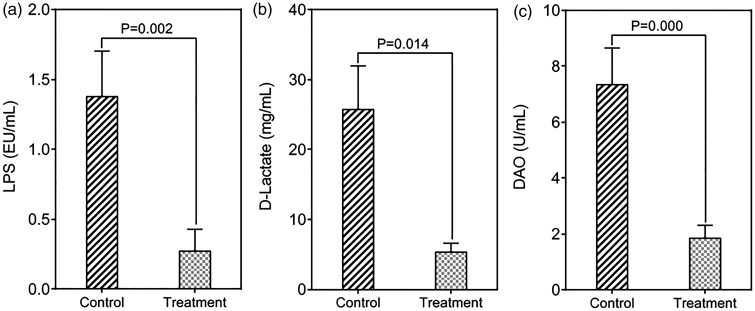
Dysfunction of the intestinal mucosal barrier is associated with the development of liver disease and is more relevant to clinical complications such as HE. Increased intestinal permeability is reportedly associated with bacterial translocation from the lumen to extraintestinal sites, resulting in the development of various complications.13 At the end of the trial, the parameters of the intestinal mucosal barrier were significantly improved. Specifically, after 3 months of probiotic treatment, the LPS level was significantly decreased (0.27 ± 0.16 EU/mL in treatment group vs. 1.38 ± 0.32 EU/mL in control group, p = 0.002) (Figure 3(a)), as was the concentration of D-lactate (5.38 ± 1.23 mg/mL in treatment group vs. 25.76 ± 6.14 mg/mL in control group, p = 0.014) (Figure 3(b)). These findings indicate that the probiotics improved the intestinal permeability. In addition, the level of DAO, an indicator of mucosal damage, was significantly decreased after probiotic treatment (1.84 ± 0.46 U/mL in treatment group vs. 7.35 ± 1.29 U/mL in control group, p = 0.000) (Figure 3(c)). Our data indicate that probiotics can restore the intestinal mucosal barrier in patients with MHE.
Figure 3.
Improvement in the indicators of the intestinal mucosal barrier after treatment with probiotics.
Discussion
MHE is related to poorer quality of life, and the liver must work harder in affected patients. MHE is present in one-third of patients with cirrhosis depending on liver function. The pathogenesis of MHE is thought to be similar to that of overt HE, and higher blood ammonia plays a key role. Dysbiosis of the gut microbiota has recently been observed in patients with liver cirrhosis,14 and such dysbiosis plays a critical role in the generation of ammonia. In patients with HBV-induced cirrhosis, the alterations in the intestinal microbiota are characterized by an obvious increase in potentially pathogenic bacteria and a decrease in commensal bacteria, which may contribute to the elevation of fecal ammonia.15 A previous study demonstrated that the gut microbiota plays a prominent role in gut–brain interactions,16 and perturbations of the intestinal microbiota can impact the gut–brain axis to alter behavioral responses. The bidirectional crosstalk between the gut and brain is critical for maintaining host homeostasis and is regulated at the neural, hormonal, and immunological levels. Alterations in the gut microbiota are associated with disruption of the intestinal barrier, which is related to the development of HE. Increased intestinal permeability can increase the translocation of bacterial products and/or bacteria into the blood. By targeting modulation of the intestinal microbiota, previous randomized controlled trials (RCTs) have estimated the efficacy of probiotics, prebiotics, and synbiotics in patients with MHE and revealed that probiotic treatment is effective in decreasing endotoxin and serum ammonia levels, improving MHE, and preventing overt HE development in patients with liver cirrhosis.17–19 Several RCTs have compared probiotic treatment with placebo or no treatment, and others have compared the therapeutic effect of probiotics and lactulose.5,7,20–23 These RCTs used different bacterial strains as probiotics for MHE treatment, including Lactobacillus, Bifidobacterium, Pediococcus, Streptococcus, Enterococcus, and others. Sharma et al.5 used C. butyricum in combination with other bacteria such as Streptococcus faecalis, Bacillus mesentericus, and lactic acid bacillus as probiotics for MHE treatment and reported improvement in abnormal psychometric parameters and a significant reduction of venous ammonia and the P300 auditory event-related potential. Although the beneficial effects of probiotics on MHE have been verified, the efficacy of the combination of C. butyricum and B. infantis on MHE in patients with HBV-induced liver cirrhosis remains unclear.
The probiotic mixture of C. butyricum combined with B. infantis has been used to modulate the intestinal microbiota for many years. Clostridium butyricum (belonging to Clostridium cluster I) is a typical butyric acid-producing, endospore-producing, gram-positive anaerobe that has been used to modulate the intestinal microbiota and treat intestinal disorders.24–26 As a commensal bacteria of the gut microbiota, B. infantis can produce lactic acid, which is frequently associated with health-promoting effects. A previous study showed that B. infantis can specifically relieve various symptoms of irritable bowel syndrome.27 However, this probiotic has not been used for MHE treatment. In the present study, probiotics were used to treat patients with MHE for a relatively long period of time. Interestingly, the NCT-A and DST psychometric test results were significantly improved in the probiotic-treated group after 3 months of treatment. Compared with the control group, these patients’ clinical symptoms were obviously controlled and their quality of life was significantly improved. In addition, beneficial bacteria such as Clostridium cluster I and Bifidobacterium were significantly increased, while opportunistic pathogens such as Enterococcus and Enterobacteriaceae were significantly decreased after probiotic treatment. The two beneficial bacteria produce wide range of metabolites, mainly short-chain fatty acids such as butyrate and acetate. The production of short-chain fatty acids can alter bacterial adhesion and improve tight-junction integrity, indicating that these metabolites play an important role in the maintenance of the intestinal mucosal barrier function.28,29 After probiotic treatment, the parameters of intestinal permeability (LPS and D-lactate) and the indicator of mucosal damage (DAO) were significantly improved. In addition, the venous ammonia level was obviously decreased after probiotic treatment, which might have been due to restoration of the intestinal mucosal barrier. The decrease in the venous ammonia level effectively relieved the clinical symptoms of MHE.
The present study has several limitations. First, the sample size was relatively small; the treatment of more patients with MHE using this probiotic mixture will validate our conclusions. Second, this was a case-control study involving only one probiotic mixture. Future RCTs with more probiotics, prebiotics, and synbiotics will help to select the best therapy for patients with MHE. Third, the long-term effects of the probiotic treatment should be observed to establish a suitable intervention course.
In summary, the present data have important implications for MHE treatment in clinical practice. Probiotics that contain C. butyricum and B. infantis can modulate the intestinal microbiota, which plays a vital role in re-establishing the intestinal mucosal barrier function. Restoration of the intestinal barrier contributes to a decrease in the blood ammonia level. The present study indicates that probiotics containing C. butyricum and B. infantis can be used as an effective adjuvant therapy for MHE in clinical practice.
Acknowledgements
The authors thank all of the participants in this study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014; 60: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quero JC, Schalm SW. Subclinical hepatic encephalopathy. Semin Liver Dis 1996; 16: 321–328. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz M, Jacas C, Cordoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol 2005; 42(Suppl 1): S45–S53. [DOI] [PubMed] [Google Scholar]
- 4.Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 2003; 114: 188–193. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Sharma BC, Puri V, et al. An open-label randomized controlled trial of lactulose and probiotics in the treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol 2008; 20: 506–511. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A, Sharma BC, Sharma P, et al. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol 2012; 107: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004; 39: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 8.Malaguarnera M, Greco F, Barone G, et al. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci 2007; 52: 3259–3265. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS, Saeian K, Christensen KM, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol 2008; 103: 1707–1715. [DOI] [PubMed] [Google Scholar]
- 10.Chinese Society Of Gastroenterology, Chinese Society of Hepatology. The consensus on the diagnosis and treatment of hepatic encephalopathy in China. Chinese Journal of Digestion 2013; 33: 581–592. [Google Scholar]
- 11.Ling Z, Liu X, Cheng Y, et al. Clostridium butyricum combined with Bifidobacterium infantis probiotic mixture restores fecal microbiota and attenuates systemic inflammation in mice with antibiotic-associated diarrhea. Biomed Res Int 2015; 2015: 582048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011; 54: 562–572. [DOI] [PubMed] [Google Scholar]
- 13.MacFie J, O'Boyle C, Mitchell CJ, et al. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 1999; 45: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014; 513: 59–64. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Christophersen CT, Sorich MJ, et al. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci 2012; 57: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 16.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015; 125: 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla S, Shukla A, Mehboob S, et al. Meta-analysis: the effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Aliment Pharmacol Ther 2011; 33: 662–671. [DOI] [PubMed] [Google Scholar]
- 18.Moratalla A, Ampuero J, Bellot P, et al. Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int 2017; 37: 212–223. [DOI] [PubMed] [Google Scholar]
- 19.Wang JY, Wang JB, Shang J, et al. Lactulose treatment improves cognition, quality of life and intestinal microbiota in minimal hepatic encephalopathy patients: results from a multi-center, randomized, controlled trial. Hepatology 2015; 62: 925A. [Google Scholar]
- 20.Bajaj JS, Heuman DM, Hylemon PB, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther 2014; 39: 1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malaguarnera M, Gargante MP, Malaguarnera G, et al. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol 2010; 22: 199–206. [DOI] [PubMed] [Google Scholar]
- 22.Mittal VV, Sharma BC, Sharma P, et al. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol 2011; 23: 725–732. [DOI] [PubMed] [Google Scholar]
- 23.Pereg D, Kotliroff A, Gadoth N, et al. Probiotics for patients with compensated liver cirrhosis: a double-blind placebo-controlled study. Nutrition 2011; 27: 177–181. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, Taguchi H, Yamaguchi H, et al. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol 2004; 41: 219–226. [DOI] [PubMed] [Google Scholar]
- 25.Kong Q, He GQ, Jia JL, et al. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr Microbiol 2011; 62: 512–517. [DOI] [PubMed] [Google Scholar]
- 26.Yang CM, Cao GT, Ferket PR, et al. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult Sci 2012; 91: 2121–2129. [DOI] [PubMed] [Google Scholar]
- 27.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006; 101: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 28.Jung TH, Park JH, Jeon WM, et al. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract 2015; 9: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng L, Li ZR, Green RS, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009; 139: 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]