Abstract
The present review summarizes recent experimental evidences about the existence of the non-cell-autonomous death entosis in physiological and pathophysiological contexts, discusses some aspects of this form of cell death, including morphological, biochemical and signaling pathways that distinguish non-cell-autonomous demises from other death modalities and propose to define this new modality of death as type IV programmed cell death.
Keywords: Entosis, Cellular cannibalism, Non-cell-autonomous death
Over past decades, there have been continuing researches focusing on the appraisal of cell death under physiological and pathological conditions and the underlying molecular pathways. This scientific interest is justified by the emerging therapeutic potential of cell death modulation. In particular, the elucidation of how tumor cells escape from cell death and how cell death processes can be immunogenic or not have become the major challenges in oncology and underscore the requirement to better understand molecular mechanisms and cell biology involved in these lethal processes.
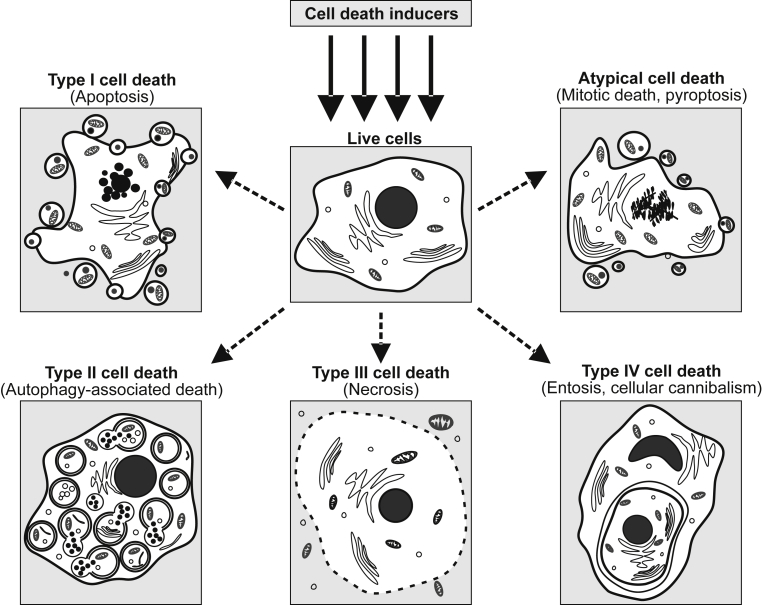
The knowledge of cell death mechanisms has evolved over the past decade and distinct cell death mechanisms have been described. Classically, cell death in mammalian cells was classified into three types of cell death: Apoptosis (Type I cell death), Autophagy-associated cell death (Type II cell death) and Necrosis (Type III cell death) [Fig. 1] [1]. However, this morphological-based classification of cell death is often questioned by the scientific community. It is now obvious that there are sophisticated interconnections and overlaps between different types of cell death [2], [3], [4], [5]. The respective prevalence of each death mechanism may depend on various factors, such as cell-dependent characteristics (including cell cycle progression and cell type), specificity and intensity of the death signals, and also micro-environmental conditions.
Fig. 1.
New classification proposed for cell death modalities.
Apoptosis is a genetically controlled lethal process that contributes to the elimination of undesired (infected, damaged or mutated) cells from multicellular organisms [6], [7]. Historically defined as the first programmed cell death, this cell-autonomous process requires catabolic activities of several enzymes (including caspases and nucleases) and is characterized by specific morphological and biochemical features (such as membrane blebbing, cell shrinkage, nuclear dismantling, chromatin condensation (also known as pyknosis), DNA fragmentation, phosphatidylserine exposure on the outer leaflet of the plasma membrane and changes in mitochondrial membrane permeability). Although the complete landscape of the underlying mechanisms has not been fully understood, the execution of this type of cell death is largely modulated by the members of BCL-2 protein family including pro-apoptotic members (e.g. PUMA, BAX, BAK) and anti-apoptotic members (e.g. BCL-2 and BCL-XL) [8], [9], [10], [11], [12], [13]. Apoptosis is generally considered as a major process that contributes to tissue and cellular homeostasis. Therefore, its deregulation is also associated with numerous human diseases (such as AIDS, neurodegenerative disorders and cancer) [6], [7], [14].
Autophagy-associated cell death (ACD) is another form of programmed cell death process that is triggered by self-digestion of cellular contents through autophagy. Autophagy was initially described as a homeostatic strategy to remove protein aggregates and dysfunctional organelles (such as mitochondria or endoplasmic reticulum) and, thus, to provide an alternative source of energy when nutrients are scarce. Autophagic processes are involved in a wide range of normal physiological situations (such as mammalian development, metabolism, immunity and aging) [15], but can also be stimulated in response to cellular stresses (such as starvation), enhanced or impaired to favor the establishment of infectious diseases caused by Mycobacterium tuberculosis [16], Toxoplasma gondii [17], Listeria monocytogenes [18] or Brucella abortus [19]. Autophagy can be elicited through distinct subtypes such as macroautophagy, chaperone-mediated autophagy and microautophagy [20]. Among these processes, the macroautophagy, which is believed to be more important, is initiated by the formation of autophagosome, a double-membrane vesicle containing cytoplasmic materials. The outer membrane of the autophagosome subsequently fuses with the membrane of lysosomes to form the acid single membrane enclosed vacuoles, termed “autolysosomes” (also called autophagolysosomes). The sequestered material is then hydrolyzed by the lysosomal enzymes (in particular by hydrolases), and released in the cytoplasm following degradation of the autolysosome membrane [21]. Autophagy activity is mainly controlled by the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway, which regulates cell growth and protein synthesis in response to nutrient and growth factor availability [22]. Autophagy is strongly regulated by a family of “autophagy-related” proteins (also named ATG proteins) [23] and relies on a complex machinery which includes the ULK1–ATG13–FIP200–ATG101 protein kinase complex, the class III PI3K complex (containing the core proteins VPS34, VPS15 and BECLIN-1), the PI3P-binding WIPI/ATG18–ATG2 complex, the trans-membrane protein ATG9A and two ubiquitin-like (UBL) protein conjugation systems (the ubiquitin-like ATG5/ATG12 system and the ubiquitin-like ATG8/LC3 conjugation system) [23]. Macroautophagy-associated proteins are also involved in autophagy-associated cell death (ACD) [24]. Overactivated autophagic flux may promote ACD, which is morphologically defined by the appearance of autophagosomes in the cytoplasm without chromatin condensation [24]. The term ACD described exclusively the cell death, induced by autophagy, which is completely suppressed by inhibition of the autophagy pathway. ACD has been described in mammalian cells treated with chemotherapeutic agents or other toxic molecules [24]. In physiological conditions, this process is involved in animal metamorphosis and development (which requires massive cell elimination) and in homeostatic processes during adulthood [25]. Recently, autosis, a new form of non-apoptotic cell death, was defined as a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia–ischemia [25], [26]. Autotic death is characterized by focal plasma membrane rupture, nuclear membrane convolution and shrinkage, focal swelling of the perinuclear space and the disappearance of endoplasmic reticulum [25], [26].
Necrosis (Type III cell death) has been characterized as accidental, uncontrolled, passive and energy-independent cell death. This necrotic process has long been described as a consequence of extreme physicochemical stress (like osmotic shock, heat and high concentration of hydrogen peroxide). The observed morphological changes comprise rapid cytoplasmic swelling with the organelle breakdown and the rupture of the plasma membrane releasing damage-associated molecular pattern molecules (DAMPs) that alert the innate immune system within the stroma [27]. This process can also be observed at late stages of autophagic or apoptotic cell death, termed secondary necrosis, when dead cells fail to be cleared by the immune system. Necrosis has been commonly viewed as an unregulated event. However, this is now challenged by a recently discovered programmed necrosis, which occurs under caspase deficient conditions. This programmed necrosis was called necroptosis [28]. The necroptotic process involves activation of the multiprotein complex necrosome, comprising the receptor-interacting protein (RIP)1 and RIP3, and mixed-lineage kinase domain-like protein (MLKL), and it can be specifically inhibited by RIP1 inhibitor necrostatin-1 [28]. Necroptosis involves the loss of membrane integrity and the release of DAMPs, and is therefore associated with the development of inflammatory or anticancer immune responses [29], [30], [31], [32].
Lethal mechanisms that do not or partially reveal morphological/biochemical characteristics of apoptosis, autophagy or necrosis, define a growing subgroup of atypical cell death forms [33], such as anoikis [34], cornification [35], entosis [36], excitotoxicity [37], mitotic catastrophe [38], netosis [39], paraptosis [40], parthanatos [41], pyronecrosis [42], pyroptosis [43] and wallerian degeneration [44]. Further morphological, molecular and functional characterizations of these processes are required to identify precisely specific signaling pathways and define their physiological and pathological functions. Several atypical cell death processes are initiated by the engulfment of live cells by neighboring live cells, underling the existence of non-cell-autonomous deaths (such as emperipolesis, cannibalism, emperitosis, phagoptosis and entosis) among these processes [Table 1]. These processes that can occur after the interaction between epithelial cells or between epithelial cells and immune cells may be triggered by cellular invasion or cannibalistic activities [45]. Here we review major highlights on entosis, which can be described as a non-autonomous cell death elicited by cellular invasion.
Table 1.
Characteristics of non-cell-autonomous deaths.
| Name | Cell types | Death effectors of target cell | Molecules participating in the process | References |
|---|---|---|---|---|
| Emperipolesis | Live T cell | Lysosome-mediated Bim independent cell death | Rho-ROCK independent, Caveolin-1 independent | [53], [54], [55], [68] |
| Entosis | Live cells | Lysosome-mediated caspase-3 independent cell death | LC3, Atg5, Atg7, Rho, ROCK, Vps34, Cadherin, MCAK, TIP150, Aurora-A | [36], [47], [52], [62] |
| Cannibalism | Dead or live cells | Lytic enzymes mediated degradation | Caveolin-1, Actin, Ezrin, Cathepsin B, TM9SF4, Vimentin | [56], [57], [69], [70] |
| Emperitosis | Live cytotoxic cells (NK or T) | Granzyme-B induces apoptosis | LFA-1, ICAM-1, CD62, ICAM-2,E-cadherin, Rho, ROCK, Actin, Myosin, Ezrin, Granzyme-B | [49], [71], [72] |
| Phagoptosis | Live cells | Lysosome-mediated degradation | Phosphatidylserine, CD14, CD68, Vitronectin receptor (VNR) | [73], [74], [75] |
Morphological and biochemical features of entosis
It is well known that apoptotic cells are commonly engulfed by professional phagocytes (such as macrophages) or by non-professional neighboring cells. However, living cells may be also engulfed by other cells, which induce an atypical cell death process [36], [46]. This lethal mechanism is termed entosis (from the Greek word entos, which means inside, into, or within). Entosis provides an interesting case of xenophagy (intracellular destruction of foreign organisms) in cell biology and was first discovered in human tumors [36]. Interactions between cancer cells initiate the formation of adherent junctions containing E-cadherins and β-catenins allowing a cellular invasion which is characterized by “cell-in-cell” cytological features. There are growing preclinical evidences that adherent junctions play a fundamental role in the formation of these cell-in-cell cytostructures [47]. The main morphological change that defines entosis is the cell-in-cell structure in which viable cells invaded other cells. These structures have been also described as “bird's eye cells” or signet ring cells. Once internalized, engulfed cells are eliminated through lysosomal degradation by host cells. Sometimes, after escaping from death or dividing inside “host” cells, target cells can be released and restart to proliferate [48]. However, most frequently, entotic tumor cells are destroyed, which suggests a tumor suppressive role for entosis [36].
The characterization of entotic death is still in its infancy. It was shown from data obtained in MCF10A cancer cells that homotypic invasion of a cell within another cell was not dependent on apoptotic pathway. Indeed, unlike the phagocytic ingestion of apoptotic cells, the death of target cells is not inhibited by the anti-apoptotic protein BCL-2 or by the pan-caspase inhibitor zVAD-fmk [36]. After loss of attachment to the extracellular matrix, tumor cells that invaded neighboring cells are completely internalized into the phagosome, whose membrane is generated from invagination of the engulfing cell plasma membrane. Then, the fusion of lysosomes to vacuolar membranes depending on autophagy-related proteins contributes to the death of internalized cells. Engulfed cells are eliminated through lysosomal enzymes mediated degradation, such as cathepsins [36]. However, the contribution of apoptotic pathway is not exclusive, and its role has not been definitively resolved. Recent researches have shown that apoptosis might be also involved in the destruction of internalized cells under certain conditions, such as heterotypic cell-in-cell invasion. Engulfed cells could inversely induce host cell death. Natural killer (NK) cells could be internalized within tumor cells, leading to either tumor cell death or self-destruction within target cells. In this case, apoptotic pathway might be involved in the cell death mechanisms since NK cell death after internalization could be partially attenuated by caspase inhibition [49].
Although no specific biochemical markers for entosis have yet been described, this process is a genetically controlled form of cell death because it is repressed by the chromatin factor Nuclear Protein 1 (NUPR1) [50]. In addition, the induction of entosis is also controlled by multiple signaling proteins including actin/myosin, cadherins and ezrin [36]. Actin polymerization and myosin II play critical roles during cell engulfment. Ezrin, which is an actin binding protein of the ezrin-radixin-moesin (ERM) family that can positively regulate the Rho signaling pathways, is also required for the internalization of NK cells into tumors [49]. In addition, the cell permeable inhibitor Tat-C3 that is known to repress the adhesion and the phagocytosis of zymosan particles, and the binding of complement to macrophages reduces cell-in-cell internalization, thus demonstrating that Rho GTPases favor entosis [36]. In addition, ROCK1 and ROCK2 that are the downstream effectors of Rho GTPase signaling pathways, also contribute to entosis as revealed by inhibitory effects of pharmacological inhibitors (Y27632 and H-1152) or the knockdown of ROCK1 and ROCK2 expressions [36]. Adherent junctions containing E-cadherin and β-catenin that are formed between epithelial cells have been also involved in the induction of cell invasion because blocking antibodies against cadherins reduced cell-in-cell internalization [36]. Apart from above mentioned mechanisms, entosis also shares some features with classical phagocytosis including phagocytic related genes such as CDC42, CXCL1 and CXCL6 were also found upregulated during entosis of pancreatic adenocarcinoma. In contrast to phagocytosis, target cell death is not primodial. Indeed, Overholtzer and colleagues did not detect signs of cell death during interactions between cells (as revealed by the absence of the detection of caspase-3 (CASP3) cleavage, nuclear condensation and fragmentation on interacting cells) [36]. Moreover, internalized cells maintained their mitochondrial membrane potential during 6 h after engulfment, suggesting that engulfed cells are not dying when they are internalized. Overexpression of anti-apoptotic BCL-2 factor, pan-caspase inhibitor (zVAD-fmk) and recombinant Annexin-V did not reduce target cell internalization and entosis, demonstrating that cell engulfment is independent of phagocytic uptake of apoptotic bodies. In the case of breast cancer cell entosis, cell death of internalized cells did not required apoptotic signaling pathways. Majority (85%) of target cells displayed nuclear disintegration without DNA fragmentation and the activating cleavage of CASP3, the downstream effector of apoptosis [36]. However, Wang et al. demonstrated that most of the NK cells that are internalized into tumor cells died through programmed cell death that required CASP3. These studies suggest that the internalization steps are independent of the death of target cells and the cell death mechanism is context-dependent, saying once internalized, different cell death modalities could be engaged [49].
Entosis involves the invasion of a living cell into another living cell. After cell-in-cell internalization, lysosomal cell death of internalized cells was studied. The recruitment of lysosomes to internalized cells was evaluated by using antibodies against the lysosomal-associated membrane protein 1 (LAMP1) in fixed cells and a red-fluorescent LysoTracker dye for labeling and tracking acidic organelles in live entotic cells, demonstrating that internalized cell shows a clear circled staining of lysosome markers inside the host cells. These results were further confirmed by electronic microscopy analysis. In addition, the activation of the lysosomal enzyme cathepsin B was also detected on entotic cells and the depletion of cathepsin B activity by pharmacological inhibition reduced internalized cell degradation, indicating that cathepsin B contributes to target cell degradation after entosis [36]. Overholtzer et al. have identified the lipid kinase PIKfyve as a critical regulator of vacuole maturation and nutrient recovery during engulfment in macropinocytosis, entosis, and phagocytosis processes [51]. Recently, experimental evidences have shown that autophagy machinery also contributes to the cell death of internalized cells. Entotic vacuole membranes surrounding internalized cells recruit LC3 through a mechanism that depends on the autophagy machinery involving ATG5, ATG7 and VPS34. LC3-targeted entotic vacuoles thus recruit lysosomes and lead to the degradation of internalized cells. Internalized cells require macroautophagy to survive within entotic vacuoles. Indeed, internalized cells do exhibit some features of autophagic cell death, as an accumulation of autophagosomes and a high rate of autophagy flux. Destruction of internalized cells by macroautophagy is observed during starvation. The inhibition of autophagy machinery in internalized cells leads to the death of target cells by apoptosis. The combined inhibition of apoptosis and autophagy machinery of internalized cells significantly increased the percentage of internalized cells that are released from their host cells [52]. It has been also suggested that LC3 proteins are recruited to ingested apoptotic bodies or single-membrane vacuoles containing apoptotic cells to facilitate corpse degradation [52]. Autophagy protein inhibitors significantly increase the growth of transformed cells undergoing high rates of entosis, suggesting that entosis suppresses transformed uncontrollable growth by inducing cell death that takes advantage of the machinery of the autophagy pathway. Under some circumstances, some internalized cells divide and are further released from the host cells. Related signaling pathways involved in this escaping process remain elusive [36].
Physiological and pathological relevances of entosis
Cell-in-cell structures have been reported in human malignancies for many years (see Ref. [45] for review). Different processes have been reported to give rise to cell-in-cells, including emperipolesis [53], [54], [55], heterotypic cell cannibalism [49], [56], [57], homotypic cell-in-cells and/or entosis [36], [45], [50], [58], [59], [60], [61] [Table 1]. Depending on the type of internalized cells, entosis could be involved in both tumor suppressive and pro-tumorigenesis mechanisms [36], [50], [62], [63].
Physiological role of entosis during embryo implantation
During implantation of the mouse embryo, uterine luminal epithelial cells that are in direct contact with the blastocyst undergo entosis into trophoblast cells. This non-apoptotic process occurs without CASP3 activation. The disappearance of these cells allows trophoblast cells to contact the underneath stroma for successful implantation [64]. Li et al. showed that implantation does not take place in mice where ROCK activity is inhibited. Entosis was thus identified as an essential mechanism for embryo implantation, conferring for the first time an important physiological role to entosis [64].
Entosis triggers the killing of target cells
Cell-in-cell structures were observed in different types of cancers. Recently, it has reported that this phenotype arised from breast cancer cells that invade each other in vitro leading to entotic cell death [36]. Despite the fact that pathologists have observed cases of pancreatic adenocarcinoma cells with atypical morphology characterized by enlarged size that contained other cells in the past decades [65], cell-in-cell structures do not only arise from the invasion of one cell by another cell. Interestingly, Cano et al. detected cell-in-cell forms after homotypic cell cannibalism. In addition, pancreatic adenocarcinoma patients whose tumor displays cell-in-cell features develop less metastasis than those without the similar phenotype, suggesting a protective role for non-cell-autonomous death in pancreatic adenocarcinoma [50]. In vitro, homotypic cell cannibalism was promoted by the genetic inactivation of the NUPR1, leading to the death of pancreatic adenocarcinoma. Additionally, Sun et al. recently showed that the tumor suppressor epithelial cadherin (E-cadherin) could increase entosis [47]. The role of entosis in human cancers is highly context-dependent. Several studies have suggested a tumor-promoting role for non-cell-autonomous death (such as entosis). Among them, it was convincingly reported that melanoma cells are able to cannibalize immune cells and to feed on themselves, thereby ensuring their survival upon starvation [57]. Similarly, it was shown that the activated form of the Kras oncogene can induce entosis [47] in a consequence to increase the mechanic deformability of the cell. Tanjoni et al. have used a syngenic mouse model system and reported that FAK inhibition could inhibit spontaneous breast to lung metastasis. Of interest, they found that loss of integrin activation associated with FAK-p130Cas-Rap1 inhibition triggered entosis within spheroids. As spheroids are usually associated with relative chemoresistance, this highlights potential therapeutic perspectives that FAK signaling could be involved in modulation of the non-canonical survival pathway [66].
Entosis may contribute to the aneuploidy of host cell
Recently, Krajcovic et al. demonstrated that cell-in-cell internalization induces genomic instability of host cells through the alteration of cytokinesis of the host cell and could therefore contribute to the formation of aneuploid cells. It has been reported that an increase in the number of centrosomes causes multipolar divisions and generates aneuploid cells, which are characterized by an abnormal chromosome numbers. In addition, cytokinesis failure, chromosome missegregation and rearrangements also contribute to genomic instability. During in vitro assays of breast cancer cell fate, detected entotic cells are frequently multi-nucleated [62]. Time-lapse microscopy analysis of the entotic host cells revealed that host cells frequently failed to undergo cell division through incomplete formation of the contractile ring [62], [63]. Thus, internalized cells induce the disruption of furrow formation. This concept has been further enforced by the existence of strong correlation between the multi-nucleation of host cell by target cell stress (in vitro) and the existence of multinucleated host cells in different human tumors suggested that non-cell-autonomous death (such as entosis) might be also induced in numerous human tumors [62]. Wang et al. have demonstrated that NK cells are internalized into the tumor cells without alterations of host entotic cells but it may lead to host cell aneuploidy [49]. In conclusion, entosis is one example of non-cell-autonomous mechanisms that could contribute to generation of aneuploid cells, which is frequently considered as a driver of human oncogenesis through the promotion of tumor progression [63]. Gene dysregulation, endoreplication and cell fusion were previously involved in cytokinesis failure. The contribution of these biological processes to non-cell-autonomous genomic instability remains to be determined. To date, there are very scare data regarding the role of non-cell-autonomous death and entosis in pathology or in cancer treatment.
The entotic process contributes to cancer cell competition
Human carcinomas showed a strong heterogeneity in both morphological and physiological features. Therefore, heterogeneous cells could compete with each other during the tumor evolution [67]. Sun et al. showed that several culture cell lines compete by entosis. They showed that mechanical deformability controlled by RhoA and actomyosin dictate the identity of engulfing (“winner”) and engulfed (“loser”) cells. Thus, tumor cells with high deformability preferentially engulf neighboring cells with low deformability in heterogeneous populations. The consequence of this competition is that entosis leads to the cell death of the “loser” cells and therefore its elimination. Interestingly, it was observed that malignant cells engulf systematically the non-transformed associated cells, suggesting an association between oncogenic transformation and the “winner” identity [67].
Conclusion
The Nomenclature Committee on Cell Death proposed a set of recommendations for the definition of distinct cell death morphologies without taking into account the non-autonomous cell death. Regarding the seminal works on entosis, we encourage researchers working on cell death mechanisms to consider the complexity of cell death modalities by analyzing simultaneously the cell-autonomous death subroutines and non-cell-autonomous deaths (NCADs). This anti-dogmatic strategy will with no doubt help to better decipher the molecular basis and the biological consequences of NCADs in numerous physiological and physiopathological situations and ultimately lead to define NCADs as new type IV cell death [Fig. 1]. The study of cell death processes should take into account all processes both autonomous and non-autonomous cell death. Unfortunately, the current methods used do not permit to analyze all these processes simultaneously and entosis is not systematically studied. Although the cell-in-cell structures resulting from entosis are frequently observed in human cancers, their function and clinical relevance remain largely unknown [52]. To date, no pharmacological agent has been shown to induce entosis and it is still uncertain whether this phenomenon could be used for therapeutics applications. However, a better understanding of underlying molecular mechanisms will bring novel perspectives for researchers, leading ultimately benefit for clinical therapeutics.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
This work was supported by funds from Agence Nationale de la Recherche (ANR), Cancéropôle Ile de France, Fondation Gustave Roussy, French National Agency for Research on AIDS and viral Hepatitis (ANRSH), Institut National du Cancer (INCA), Laboratory of Excellence (LabEx) LERMIT, NATIXIS and the SIDACTION (to J-L.P.). S.Q.R is supported by Higher Education Commission (Pakistan) and by the LabEx LERMIT with a grant from ANR (ANR-10-LABX-33) under the program “Investissements d'Avenir” ANR-11-IDEX-0003-01. I.M. and L.V. are funded by INCA (INCA-DGOS-INSERM 6043 and 2015-1- PL BIO-07-IGR-1). H.D and D.D. are respectively recipients of PhD fellowships from LabEx LERMIT and Fondation Philantropia.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H. Classification of cell death: recommendations of the nomenclature committee on cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheller C., Knoferle J., Ullrich A., Prottengeier J., Racek T., Sopper S. Caspase inhibition in apoptotic T cells triggers necrotic cell death depending on the cell type and the proapoptotic stimulus. J Cell Biochem. 2006;97:1350–1361. doi: 10.1002/jcb.20670. [DOI] [PubMed] [Google Scholar]
- 3.Vercammen D., Beyaert R., Denecker G., Goossens V., Van Loo G., Declercq W. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vercammen D., Brouckaert G., Denecker G., Van de Craen M., Declercq W., Fiers W. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vercammen D., Vandenabeele P., Declercq W., Van de Craen M., Grooten J., Fiers W. Cytotoxicity in L929 murine fibrosarcoma cells after triggering of transfected human p75 tumour necrosis factor (TNF) receptor is mediated by endogenous murine TNF. Cytokine. 1995;7:463–470. doi: 10.1006/cyto.1995.0063. [DOI] [PubMed] [Google Scholar]
- 6.Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 7.Golstein P. Cell death in us and others. Science. 1998;281:1283. doi: 10.1126/science.281.5381.1283. [DOI] [PubMed] [Google Scholar]
- 8.Perfettini J.L., Kroemer R.T., Kroemer G. Fatal liaisons of p53 with Bax and Bak. Nat Cell Biol. 2004;6:386–388. doi: 10.1038/ncb0504-386. [DOI] [PubMed] [Google Scholar]
- 9.Perfettini J.L., Roumier T., Castedo M., Larochette N., Boya P., Raynal B. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med. 2004;199:629–640. doi: 10.1084/jem.20031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villunger A., Michalak E.M., Coultas L., Mullauer F., Bock G., Ausserlechner M.J. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 11.Wei M.C., Zong W.X., Cheng E.H., Lindsten T., Panoutsakopoulou V., Ross A.J. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaux D.L., Cory S., Adams J.M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama N., Wang F., Roth K.A., Sawa H., Nakayama K., Nakayama K. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 14.Perfettini J.L., Castedo M., Roumier T., Andreau K., Nardacci R., Piacentini M. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005;12:916–923. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- 15.Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 16.Deretic V., Delgado M., Vergne I., Master S., De Haro S., Ponpuak M. Autophagy in immunity against mycobacterium tuberculosis: a model system to dissect immunological roles of autophagy. Curr Top Microbiol Immunol. 2009;335:169–188. doi: 10.1007/978-3-642-00302-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Weiss L.M., Orlofsky A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J Biol Chem. 2009;284:1694–1701. doi: 10.1074/jbc.M807890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa M., Yoshikawa Y., Mimuro H., Hain T., Chakraborty T., Sasakawa C. Autophagy targeting of Listeria monocytogenes and the bacterial countermeasure. Autophagy. 2011;7:310–314. doi: 10.4161/auto.7.3.14581. [DOI] [PubMed] [Google Scholar]
- 19.Starr T., Child R., Wehrly T.D., Hansen B., Hwang S., Lopez-Otin C. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe. 2012;11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z., Klionsky D.J. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 22.Sarbassov D.D., Ali S.M., Sabatini D.M. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 24.Levine B., Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Levine B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015;22:367–376. doi: 10.1038/cdd.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Shoji-Kawata S., Sumpter R.M., Jr., Wei Y., Ginet V., Zhang L. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA. 2013;110:20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss R.S., Strasser A., McDunn J.E., Swanson P.E. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chemical Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 29.Cho Y.S., Challa S., Moquin D., Genga R., Ray T.D., Guildford M. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He S., Wang L., Miao L., Wang T., Du F., Zhao L. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D.W., Shao J., Lin J., Zhang N., Lu B.J., Lin S.C. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 32.Aaes T.L., Kaczmarek A., Delvaeye T., De Craene B., De Koker S., Heyndrickx L. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15:274–287. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reginato M.J., Mills K.R., Paulus J.K., Lynch D.K., Sgroi D.C., Debnath J. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 35.Candi E., Schmidt R., Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 36.Overholtzer M., Mailleux A.A., Mouneimne G., Normand G., Schnitt S.J., King R.W. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 37.Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 38.Vakifahmetoglu H., Olsson M., Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell death and Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg B.E., Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007:pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 40.Sperandio S., de Belle I., Bredesen D.E. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.David K.K., Andrabi S.A., Dawson T.M., Dawson V.L. Parthanatos, a messenger of death. Front Biosci. 2009;14:1116–1128. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willingham S.B., Bergstralh D.T., O'Connor W., Morrison A.C., Taxman D.J., Duncan J.A. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan M.A., Cookson B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 44.Raff M.C., Whitmore A.V., Finn J.T. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 45.Overholtzer M., Brugge J.S. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008;9:796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- 46.Janssen W.J., Barthel L., Muldrow A., Oberley-Deegan R.E., Kearns M.T., Jakubzick C. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Q., Cibas E.S., Huang H., Hodgson L., Overholtzer M. Induction of entosis by epithelial cadherin expression. Cell Res. 2014;24:1288–1298. doi: 10.1038/cr.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishna S., Overholtzer M. Mechanisms and consequences of entosis. Cell Mol Life Sci. 2016;73:2379–2386. doi: 10.1007/s00018-016-2207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S., Guo Z., Xia P., Liu T., Wang J., Li S. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 2009;19:1350–1362. doi: 10.1038/cr.2009.114. [DOI] [PubMed] [Google Scholar]
- 50.Cano C.E., Sandi M.J., Hamidi T., Calvo E.L., Turrini O., Bartholin L. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol Med. 2012;4:964–979. doi: 10.1002/emmm.201201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishna S., Palm W., Lee Y., Yang W., Bandyopadhyay U., Xu H. PIKfyve regulates vacuole maturation and nutrient recovery following engulfment. Dev Cell. 2016;38:536–547. doi: 10.1016/j.devcel.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Florey O., Kim S.E., Sandoval C.P., Haynes C.M., Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13:1335–1343. doi: 10.1038/ncb2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns E.R. Tumor cell-tumor cell emperipolesis. Exp Cell Res. 1967;48:229–231. doi: 10.1016/0014-4827(67)90310-2. [DOI] [PubMed] [Google Scholar]
- 54.Humble J.G., Jayne W.H., Pulvertaft R.J. Biological interaction between lymphocytes and other cells. Br J Haematol. 1956;2:283–294. doi: 10.1111/j.1365-2141.1956.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 55.Shamoto M. Emperipolesis of hematopoietic cells in myelocytic leukemia. Electron microscopic and phase contrast microscopic studies. Virchows Archiv B, Cell Pathol Incl Mol Pathol. 1981;35:283–290. doi: 10.1007/BF02889168. [DOI] [PubMed] [Google Scholar]
- 56.Lozupone F., Perdicchio M., Brambilla D., Borghi M., Meschini S., Barca S. The human homologue of Dictyostelium discoideum phg1A is expressed by human metastatic melanoma cells. EMBO Rep. 2009;10:1348–1354. doi: 10.1038/embor.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lugini L., Matarrese P., Tinari A., Lozupone F., Federici C., Iessi E. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629–3638. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- 58.Abodief W.T., Dey P., Al-Hattab O. Cell cannibalism in ductal carcinoma of breast. Cytopathology. 2006;17:304–305. doi: 10.1111/j.1365-2303.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 59.Gupta K., Dey P. Cell cannibalism: diagnostic marker of malignancy. Diagn Cytopathol. 2003;28:86–87. doi: 10.1002/dc.10234. [DOI] [PubMed] [Google Scholar]
- 60.Kojima S., Sekine H., Fukui I., Ohshima H. Clinical significance of “cannibalism” in urinary cytology of bladder cancer. Acta Cytol. 1998;42:1365–1369. doi: 10.1159/000332169. [DOI] [PubMed] [Google Scholar]
- 61.Kumar P.V., Hosseinzadeh M., Bedayat G.R. Cytologic findings of medulloblastoma in crush smears. Acta Cytol. 2001;45:542–546. doi: 10.1159/000327862. [DOI] [PubMed] [Google Scholar]
- 62.Krajcovic M., Johnson N.B., Sun Q., Normand G., Hoover N., Yao E. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13:324–330. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krajcovic M., Overholtzer M. Mechanisms of ploidy increase in human cancers: a new role for cell cannibalism. Cancer Res. 2012;72:1596–1601. doi: 10.1158/0008-5472.CAN-11-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y., Sun X., Dey S.K. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 2015;11:358–365. doi: 10.1016/j.celrep.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silverman J.F., Dabbs D.J., Finley J.L., Geisinger K.R. Fine-needle aspiration biopsy of pleomorphic (giant cell) carcinoma of the pancreas. Cytologic, immunocytochemical, and ultrastructural findings. Am J Clin Pathology. 1988;89:714–720. doi: 10.1093/ajcp/89.6.714. [DOI] [PubMed] [Google Scholar]
- 66.Tanjoni I., Walsh C., Uryu S., Tomar A., Nam J.O., Mielgo A. PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biol Ther. 2010;9:764–777. doi: 10.4161/cbt.9.10.11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Q., Huang H., Overholtzer M. Cell-in-cell structures are involved in the competition between cells in human tumors. Mol Cell Oncol. 2015;2:e1002707. doi: 10.1080/23723556.2014.1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sierro F., Tay S.S., Warren A., Le Couteur D.G., McCaughan G.W., Bowen D.G. Suicidal emperipolesis: a process leading to cell-in-cell structures, T cell clearance and immune homeostasis. Curr Mol Med. 2015;15:819–827. doi: 10.2174/1566524015666151026102143. [DOI] [PubMed] [Google Scholar]
- 69.Caruso R.A., Muda A.O., Bersiga A., Rigoli L., Inferrera C. Morphological evidence of neutrophil-tumor cell phagocytosis (cannibalism) in human gastric adenocarcinomas. Ultrastruct Pathol. 2002;26:315–321. doi: 10.1080/01913120290104593. [DOI] [PubMed] [Google Scholar]
- 70.Fais S. Cannibalism: a way to feed on metastatic tumors. Cancer Lett. 2007;258:155–164. doi: 10.1016/j.canlet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 71.Wang S., He M.F., Chen Y.H., Wang M.Y., Yu X.M., Bai J. Rapid reuptake of granzyme B leads to emperitosis: an apoptotic cell-in-cell death of immune killer cells inside tumor cells. Cell Death Dis. 2013;4:e856. doi: 10.1038/cddis.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeuchi M., Inoue T., Otani T., Yamasaki F., Nakamura S., Kibata M. Cell-in-cell structures formed between human cancer cell lines and the cytotoxic regulatory T-cell line HOZOT. J Mol Cell Biol. 2010;2:139–151. doi: 10.1093/jmcb/mjq002. [DOI] [PubMed] [Google Scholar]
- 73.Brown G.C., Neher J.J. Eaten alive! Cell death by primary phagocytosis: 'phagoptosis'. Trends Biochem Sci. 2012;37:325–332. doi: 10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Brown G.C., Neher J.J. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- 75.Brown G.C., Vilalta A., Fricker M. Phagoptosis – cell Death by phagocytosis – plays central roles in physiology, host defense and pathology. Curr Mol Med. 2015;15:842–851. doi: 10.2174/156652401509151105130628. [DOI] [PubMed] [Google Scholar]