Abstract
Hypoxia-inducible factor (HIF) is a main heterodimeric transcription factor that regulates the cellular adaptive response to hypoxia by stimulating the transcription of a series of hypoxia-inducible genes. HIF is frequently upregulated in solid tumors, and the overexpression of HIF can promote tumor progression or aggressiveness by blood vessel architecture and altering cellular metabolism. In this review, we focused on the pivotal role of HIF in tumor angiogenesis and energy metabolism. Furthermore, we also emphasized the possibility of HIF pathway as a potential therapeutic target in cancer.
Keywords: Angiogenesis, HIF, Hypoxia, Metabolism, VHL
Introduction
Solid tumors are known for a long time to contain poorly vascularized regions, including low pH, severe hypoxia and nutrient starvation.1 The unlimited proliferation of tumor cell results in increased oxygen consumption, thus, most part of solid tumors develop hypoxia as compared to surrounding normal tissue.2 Tumor hypoxia is typically correlated with poor prognosis, partly because of refractory to therapy, in particular to radiotherapy that kills tumor cells by generating reactive oxygen species (ROS). In laryngeal cancer, hypoxia has been shown to promote cell invasion and metastasis via epithelial–mesenchymal transition (EMT).3 Hypoxia-inducible factor (HIF) plays an important role in the adaptive cellular response to hypoxia in tumor microenvironment.4
Hypoxia-inducible factors (HIFs) are a heterodimer consisting of an oxygen-dependent α-subunit (HIF-α) and an oxygen-independent β-subunit (HIF-β). The HIF-α has three isoforms, HIF-1α, HIF-2α, and HIF-3α.5 There are two isoforms of HIF-β that also called as the aryl hydrocarbon receptor nuclear translocator (ARNT), namely, HIF-1β and HIF-2β.6 Among those HIFs, the most important is HIF-1α, responsible for activating transcriptional responses under hypoxia. Similar to HIF-1α, HIF-2α is involved in the regulation of hypoxia. Nevertheless, their ability to transcriptionally regulate specific hypoxia-responsive genes, HIF-2α and HIF-1α have distinct functions and only partially overlap. For example, HIF-2 is the predominant regulator of fatty acid storage, whereas glycolytic genes appear to be primarily regulated by HIF-1.5 HIF-3α acts as a down-regulator, reducing the anoxia response by a HIF-1α inhibitor mediator.7 As a consequence of the hypoxia-inducible transcription factors stabilization, the cell constitutively upregulates the hypoxic response pathway resulting in gene expression programs, which are responsible for global shift in glucose uptake, cell proliferation, differentiation, apoptosis, energy metabolism, erythropoiesis, and angiogenesis.8 These physiological adaptive responses are also commonly observed in human cancer. Thus, Warburg effect (altering glucose metabolism), resistance to apoptosis and angiogenic switch are all hallmarks of cancer. Recently, HIFs have been shown to control cancer stem cells (CSCs) proliferation, differentiation and pluripotency through activating specific signaling pathways such as Oct4, Wnt and Notch.1, 9, 10 Furthermore, these features are important during tumor progression. Accordingly, hypoxia-inducible factor (HIF) contributes to tumor progression in a positive feedback loop.11, 12
HIF expression in cancer
A recent survey found that HIF-1α and HIF-2α were commonly upregulated in a variety of human tumors, including breast, bladder, brain, glial, hepatocellular, colon, ovarian, pancreatic, prostate, and renal tumors.13 Clinically, high levels of HIF-1α expression positively correlate with tumor progression and poor patient outcome in non-small cell lung cancer,14 head and neck cancer,15 colon cancer,16 gastric cancer,17 breast cancer,18, 19 prostate cancer,20 pancreatic cancer,21 esophageal cancer,22 osteosarcoma,23 endometrial carcinoma,24 ovarian carcinoma,25 bladder carcinoma,26 and nasopharyngeal carcinoma,27 while elevated HIF-2α expression correlate with tumor progression and poor patient outcome in non-small cell lung cancer,14 bladder cancer,28, 29 breast cancer,30 colorectal cancer,31 clear cell renal cell carcinoma (ccRCC),32 and hepatocellular carcinoma,33 HIF-3α expression is commonly found in various human tissues and cancer cell lines, and while the dominant-negative HIF-3α inhibits the transcriptional activity of HIF-1α.34, 35 Moreover, HIF-3α was discovered to be down-regulated in renal cell carcinomas.34 Generally, HIFs activation is a common incident in human cancer and the overexpression of HIFs may play a significant role in tumorigenesis. However, there are a couple of examples to the contrary. A study by Acker et al reported that overexpression of HIF-2α reduced the growth of rat glioma tumors, in part by increasing tumor cell apoptosis, and Knock-down of HIF-2α in human malignant glioblastoma reduce apoptosis.36 Nevertheless, Raval et al showed that overexpression of HIF-2α promoted tumor growth, while overexpression of HIF-1α inhibited tumor progression.37
HIF activation in cancer
Hypoxia, is the most common mechanism of HIF activation in neoplasms. Vaupel, et al estimated that hypoxic and/or anoxic tissues, developing as a consequence of an imbalance between tumor cell oxygen consumption and supply, was present in 50–60% of solid tumors.38 Hypoxia is defined as having an internal partial pressure of oxygen of less than 10–15 mm Hg in solid tumors.39 Under hypoxic conditions or in VHL−/− cells, stabilized HIF-1α dimerizes with HIF-1β and interacts with the transcriptional coactivators p300/Creb-binding protein (p300/CBP) before binding to DNA on hypoxia-response elements (HREs), finally activating target gene transcription and mRNA, and protein synthesis.40, 41
The activation of HIF also can be influenced in tumor with normoxia conditions by genetic alterations in its oxygen-signaling pathway. As mentioned before, VHL (von Hippel–Lindau) plays a central role in the oxygen-sensing pathway promoting HIFα proteosome-mediated degradation.42 Therefore, inactivation or loss of VHL results in activation of the HIF pathway in normoxia,43 which in turn results in transactivation of HIF target genes. Germline mutations in the VHL tumor-suppressor gene lead to VHL disease, a familial cancer syndrome that associates with the development of multiple vascular and benign tumors. The main tumor manifestations include central nervous system and retina haemangioblastomas, phaeochromocytomas, clear cell renal cell carcinoma (CCRCC), pancreatic islet tumors, endolymphatic sac tumors, epididymal cystadenomas and pancreatic and renal cystadenomas.44
There are also some evidence that HIF activity can be induced by oxygen-independent manner, such as the activation of the mitogen-activated protein kinase (MAPK) and phosphoinositol 3-kinase (PI3K) pathways. It was found that HIF activity was increased via the activation of PI3K/Akt pathway through enhancing positive regulators such as Ras and receptor tyrosine kinases or inactivation of negative regulators include tensin homolog (PTEN) and tuberous sclerosis (TSC) 1 or 2.45 The PI3K signaling pathway can regulates HIF activity in mammalian target of rapamycin (mTOR)-independent or mTOR-dependent mechanisms. All together, the activation of HIF is a complex process and may involve different tumor-suppressor genes and relevant oncogenes during tumor progression.
HIF and angiogenesis
Angiogenesis is critical for the process of solid tumor formation and progression since the supply of oxygen and nutrients is inadequate in tumor cell. Ultimately, beyond a certain size, the stimulation of vasculogenesis and/or neoangiogenesis is required for a tumor to grow. This ability of tumor cell involves a multistep process, termed the ‘angiogenic switch’, a phenomenon occurs when the balance of pro-angiogenic factors outweighs anti-angiogenic factors.46 HIF can induce the expression of a large number of pro-angiogenic factors, including vascular endothelial growth factor (VEGF), VEGF receptors FLT-1 and FLK-1, platelet-derived growth factor B (PDGF-B), plasminogen activator inhibitor-1 (PAI-1), the TIE-2 receptor, matrix metalloproteinases (MMP-2 and MMP-9) and angiopoietins (ANG-1 and ANG -2).47 Among all of these pro-angiogenic factors activated by HIF, VEGF-A, a potent endothelial mitogen, is the most notable protein since it is highly expressed in many human tumors.45, 48
In both physiological and pathophysiological angiogenesis, HIF-1α pathway has been shown to be a master regulator of vasculature formation by upregulating other proangiogenic factors such as VEGF.49 HIF-1α plays an important role of in endothelial cells (EC) biology and angiogenesis. Tang et al found that loss of HIF-1α prevented EC angiogenesis behavior, including proliferation, migration, chemotaxis and wound healing.50 Additionally, HIF-2α has also been shown to relate to tumor angiogenesis. Skuli et al found that HIF-2α-deficient in murine EC could decrease vascular function and tumor angiogenesis.51 Raval et al noted that the expression of VEGF was positively regulated by HIF-2α in VHL-deficient RCC cells.37 In addition, Geis et al showed that HIF-2α promoted angiogenesis in HepG2 cells via increasing PAI-1 to inhibit concentrations of active plasmin.52 Collectively, these findings suggest that both HIF-1α and HIF-2α contribute to tumor angiogenesis.
HIF and tumor metabolism
In 1924, Otto Warburg observed that tumor cells converted much more glucose to produce lactic acid even under normal oxygen tension (aerobic glycolysis). This finding, known as the Warburg effect, is an almost universal feature of solid tumors and first explained the shift of glucose metabolism in tumor cells. However, in normal tissue, cells prefer to oxidize glucose to carbon dioxide by coupling glycolysis to oxidative phosphorylation and citric acid cycle. Warburg believed that this metabolic transition was important for tumorigenesis.53 It has been shown that HIF, particularly HIF-1α, induces the expression of glucose transporters (Glut1 and Glut3) and glycolytic enzymes including lactate dehydrogenase A (LDHA), phosphoglycerate kinase 1 (PGK-1) and hexokinases (HK1 and HK2) and plays an important role in the switch from oxidative phosphorylation to anaerobic glycolysis.54, 55 Moreover, besides blocking tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) in mitochondria, HIF-1α is shown to suppress mitochondrial biogenesis itself. Zhang et al observed that HIF-1α in VHL-deficient renal carcinoma cells negatively regulated mitochondrial mass and oxygen consumption by inhibition of PGC-1b through c-Myc.56 In addition, accumulated evidence indicated that many cancer genes, including cMyc, p53, and Ras, are all associated with the regulation of aerobic glycolysis.57 As a capital regulator of tumor hypoxic response, HIF also is a significant mediator of metabolism in human cancer.
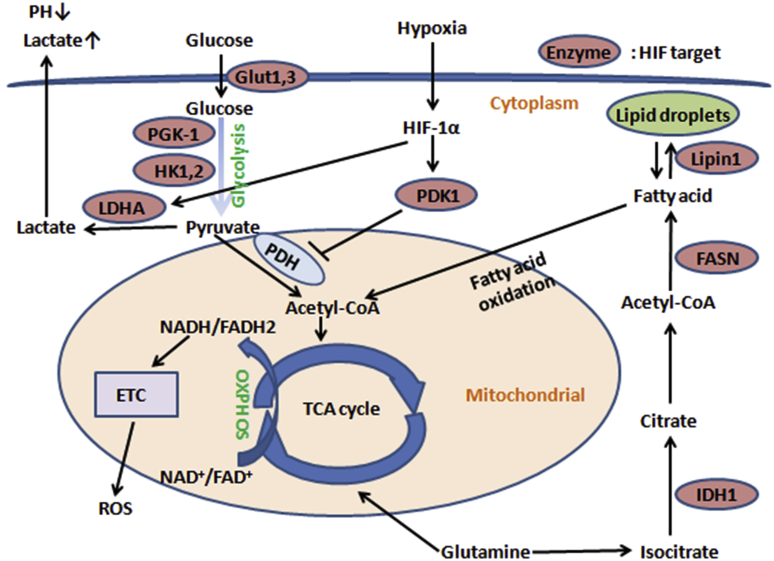
A well-recognized key process for cellular energy metabolism is that, under normoxic conditions, glucose is transformed into pyruvate in the cytoplasm and then pyruvate is catabolized to acetyl-CoA by pyruvate dehydrogenase (PDH) complex and enter the TCA cycle and OXPHOS in the mitochondria. However, in solid tumors, HIF-1α activates pyruvate dehydrogenase kinase 1 (PDK1), which inhibits PDH by phosphorylation.58 Therefore, pyruvate can not be converted to acetyl-CoA, as a consequence, preventing the production of ATP through TCA cycle and OXPHOS (Fig. 1). HIF-1α protects cells from reactive oxygen species (ROS) damage by activating PDK-1, which can attenuate ROC production. HIF-1α also fine-tunes hypoxic cell respiration by regulating the switch of cytochrome oxidase (COX-4) subunit.59 Futhermore, Chen et al shown that HIF-1α regulated the expression of microRNA-210, which decreased COX10 (cytochrome c oxidase assembly protein) and ISCU (iron-sulfur cluster scaffold homolog) expression, two important composition of the mitochondria electron transport chain (ETC) and the TCA cycle.60 MiR-210 is highly expressed in hypoxia, which can inhibit cell proliferation by promoting cell cycle arrest and apoptosis through the targeting of fibroblast growth factor receptor-like 1 (FGFRL1) in human Laryngocarcinoma cancer.61 All together, miR-210 as a robust target of HIF plays an important role in mitochondrial metabolism, DNA damage response, angiogenesis, apoptosis, and cell survival.62
Fig. 1.
Effects of hypoxia on tumor cells metabolism. Under hypoxic conditions, glucose is converted to lactate rather than to metabolized acetyl-CoA to enter TCA cycle and OXPHOS in the mitochondria. A number of the effects of hypoxia on cancer metabolism are explained by the HIF-1α-mediated activation of glycolytic enzymes and LDHA and inhibition of PDH, and this phenomenon that tumor cells shift from oxidative phosphorylation to glycolysis, even under normoxia, was termed Warburg effect. As a consequence, tumor cells are protected from ROS damage generated from ETC in hypoxic condition.
In addition, hypoxic tumor cells have its unique characteristics of lipid metabolism via HIF pathway. Recently, Furuta et al found that hypoxia promoted fatty acid synthesis through activation of sterol regulatory-element binding protein (SREBP)-1 and up-regulation of fatty acid synthase (FASN) in HIF signaling pathway.63 It has been reported that tumor cells hypoxia results in accumulation of triglycerides and lipid droplets. In this process, Mylonis et al have shown that HIF-1α directly contributed to the stimulation of phosphatidate phosphatase isoform 1 (Lipin1), a phosphatidate phosphatase isoform that catalyzes triglyceride biosynthesis during the penultimate step.64 Overall, all of these studies indicate that hypoxia can stimulate fatty acid synthesis and triglyceride storage via HIF-1- mediated pathway in tumor cells. Otherwise, one recent research revealed that HIF-2α in liver-specific VHL-knockout mouse model was identified as a central regulator of hepatic lipid metabolism, including synthesis, oxidation, and storage. In this model, acyl-CoA synthase long-chain family member 1 (Acsl1) and carnitine palmitoyltransferase I (Cpt-1) were both decreased by activation of HIF-2α. Notably, HIF-2α activation caused severe hepatic steatosis by increasing hepatic lipid storage and suppressing lipid synthesis and fatty acid–oxidation.65
HIF as targets for cancer therapy
Hypoxia is a common feature in all solid tumors, and it is correlated with tumor progression and metastasis and poor patient survival, as well as the resistance of cancer to chemotherapy and radiotherapy.66, 67 As we have mentioned previously, HIF is a central mediator in regulating tumor survival and growth under low oxygen tension condition. Therefore, HIF is considered as an attractive target for anticancer agents. In recent years, there are several small molecules identified as HIF-1α inhibitors, which can inhibit the HIF-1α pathway. Among these molecules are inhibitors of HIF-1α mRNA expression (EZN-2698 and Aminoflavone), inhibitors of HIF-1α translation (Camptothecins, LY294002, Temsirolimus, 2-Methoxyestradiol and Cardiac glycosides), inhibitors of HIF-1α stabilization (Geldanamycins, Radicicols, SCH66336), inhibitors of HIF-1 dimerization (Acriflavin), inhibitors of HIF-1/DNA binding (Anthracyclines and Echinomycin), inhibitors of HIF-1 transcriptional activity (Chetomin and Bortezomib), and inhibitors of HIF-1α at multiple levels (YC-1 and PX-478).68 All of these inhibitors of HIF-1α can be divided into two classes: indirect and direct inhibitors. Nevertheless, these indirect inhibitors represent pleiotropic effect and affect other signaling pathways, which indicates that development of selective HIF inhibitors is crucially important.69
Future perspectives
Hypoxia has been known as a basic hallmark of human cancer. HIF is a key mediator of cellular response to hypoxia. The activation of HIF contributes to cancer progression by promoting angiogenesis, induction of glycolysis, alteration of EMT and inhibition of apoptosis. Futhermore, HIF can also modulate tumor progression through interactions with many oncogenic pathways, such as c-Myc and p53. Thus, the mechanisms of HIF in tumor initiation/progression are multiple and complex, which need more profound research to find the details of HIF pathway. Interestingly, many of agents that have been shown to inhibit HIF pathway have presented significant anti-cancer activity in clinic trials. HIF would be a novel target for tumor drug discovery and development, although further studies are needed.
Conflicts of interest
None.
Acknowledgement
This work was supported by National Nature Science Foundation of China (81272960), Key Research Program from Science and Technology Department of Hunan Province, China (2013WK2010 and 2014SK2015). Key Research Program from Ministry of human Resources and Social Security of the People's Republic of China (2016)176.
Footnotes
Grant sponsor: National Nature Science Foundation of China; Grant number: 81272960; Key Research Program from Science and Technology Department of Hunan Province China; Grant number: 2013WK2010 and 2014SK2015. The fund of Tianqing liver disease research.
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Meiling Wen, Email: 1115910801@qq.com.
Jianhong Zuo, Email: 632138414@qq.com.
References
- 1.Mathieu J., Zhang Z., Zhou W. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71(13):4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh Mei Yee, Spivak-Kroizman Taly R., Powis G. HIF-1alpha and cancer therapy. Recent Results Cancer Res. 2010;180:15–34. doi: 10.1007/978-3-540-78281-0_3. [DOI] [PubMed] [Google Scholar]
- 3.Zuo J., Wen J., Lei M. Hypoxia promotes the invasion and metastasis of laryngeal cancer cells via EMT. Med Oncol. 2016;33(2):15. doi: 10.1007/s12032-015-0716-6. [DOI] [PubMed] [Google Scholar]
- 4.Kumar V., Gabrilovich D.I. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143(4):512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito S., Lin Y.C., Tsai M.H. Emerging roles of hypoxia-inducible factors and reactive oxygen species in cancer and pluripotent stem cells. Kaohsiung J Med Sci. 2015;31(6):279–286. doi: 10.1016/j.kjms.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng W., Wan R., Zheng Y. Hypoxia, stem cells and bone tumor. Cancer Lett. 2011;313(2):129–136. doi: 10.1016/j.canlet.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Sayans M., Suarez-Penaranda J.M., Pilar G.D. Hypoxia-inducible factors in OSCC. Cancer Lett. 2011;313(1):1–8. doi: 10.1016/j.canlet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risbud M.V., Schipani E., Shapiro I.M. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176(4):1577–1583. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majmundar A.J., Lee D.S., Skuli N. HIF modulation of Wnt signaling regulates skeletal myogenesis in vivo. Development. 2015;142(14):2405–2412. doi: 10.1242/dev.123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto Y., Ibusuki M., Okumura Y. Hypoxia-inducible factor 1alpha is closely linked to an aggressive phenotype in breast cancer. Breast Cancer Res Treat. 2008;110(3):465–475. doi: 10.1007/s10549-007-9742-1. [DOI] [PubMed] [Google Scholar]
- 12.Rankin E.B., Giaccia A.J. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15(4):678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talks K.L., Turley H., Gatter K.C. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157(2):411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X.H., Qian C., Yuan K. Correlations of hypoxia-inducible factor-1alpha/hypoxia-inducible factor-2alpha expression with angiogenesis factors expression and prognosis in non-small cell lung cancer. Chin Med J (Engl) 2011;124(1):11–18. [PubMed] [Google Scholar]
- 15.Bache M., Reddemann R., Said H.M. Immunohistochemical detection of osteopontin in advanced head-and-neck cancer: prognostic role and correlation with oxygen electrode measurements, hypoxia-inducible-factor-1alpha-related markers, and hemoglobin levels. Int J Radiat Oncol Biol Phys. 2006;66(5):1481–1487. doi: 10.1016/j.ijrobp.2006.07.1376. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y., Jin M., Xu H. Clinicopathologic significance of HIF-1alpha, CXCR4, and VEGF expression in colon cancer. Clin Dev Immunol. 2010:2010. doi: 10.1155/2010/537531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Li Z., Zhang H. HIF-1alpha and HIF-2alpha correlate with migration and invasion in gastric cancer. Cancer Biol Ther. 2010;10(4):376–382. doi: 10.4161/cbt.10.4.12441. [DOI] [PubMed] [Google Scholar]
- 18.Marana H.R., Tiezze D.G., de Andrade J.M., da Silva J.S. HIF-1alpha and locally advanced breast cancer. Breast J. 2010;16(5):569–570. doi: 10.1111/j.1524-4741.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 19.Dales J.P., Beaufils N., Silvy M. Hypoxia inducible factor 1alpha gene (HIF-1alpha) splice variants: potential prognostic biomarkers in breast cancer. BMC Med. 2010;8:44. doi: 10.1186/1741-7015-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitani T., Harada N., Tanimori S. Resveratrol inhibits hypoxia-inducible factor-1alpha-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer. J Nutr Sci Vitaminol (Tokyo) 2014;60(4):276–282. [PubMed] [Google Scholar]
- 21.Hao J. HIF-1 is a critical target of pancreatic cancer. Oncoimmunology. 2015;4(9):e1026535. doi: 10.1080/2162402X.2015.1026535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa K., Chiba I., Morioka T. Clinical significance of HIF-1alpha expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Anticancer Res. 2011;31(6):2351–2359. [PubMed] [Google Scholar]
- 23.El Naggar A., Clarkson P., Zhang F. Expression and stability of hypoxia inducible factor 1alpha in osteosarcoma. Pediatr Blood Cancer. 2012;59(7):1215–1222. doi: 10.1002/pbc.24191. [DOI] [PubMed] [Google Scholar]
- 24.Abouhashem N.S., Ibrahim D.A., Mohamed A.M. Prognostic implications of epithelial to mesenchymal transition related proteins (E-cadherin, Snail) and hypoxia inducible factor 1alpha in endometrioid endometrial carcinoma. Ann Diagn Pathol. 2016;22:1–11. doi: 10.1016/j.anndiagpath.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Osada R., Horiuchi A., Kikuchi N. Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: nuclear expression of hypoxia-inducible factor 1alpha is an independent prognostic factor in ovarian carcinoma. Hum Pathol. 2007;38(9):1310–1320. doi: 10.1016/j.humpath.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Theodoropoulos V.E., Lazaris A.C., Kastriotis I. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005;95(3):425–431. doi: 10.1111/j.1464-410X.2005.05314.x. [DOI] [PubMed] [Google Scholar]
- 27.Wan X.B., Fan X.J., Huang P.Y. Aurora-A activation, correlated with hypoxia-inducible factor-1alpha, promotes radiochemoresistance and predicts poor outcome for nasopharyngeal carcinoma. Cancer Sci. 2012;103(8):1586–1594. doi: 10.1111/j.1349-7006.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onita T., Ji P.G., Xuan J.W. Hypoxia-induced, perinecrotic expression of endothelial Per-ARNT-Sim domain protein-1/hypoxia-inducible factor-2alpha correlates with tumor progression, vascularization, and focal macrophage infiltration in bladder cancer. Clin Cancer Res. 2002;8(2):471–480. [PubMed] [Google Scholar]
- 29.Koga F., Kageyama Y., Kawakami S. Prognostic significance of endothelial Per-Arnt-sim domain protein 1/hypoxia-inducible factor-2alpha expression in a subset of tumor associated macrophages in invasive bladder cancer. J Urol. 2004;171(3):1080–1084. doi: 10.1097/01.ju.0000110541.62972.08. [DOI] [PubMed] [Google Scholar]
- 30.Koga F., Kageyama Y., Kawakami S. Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res. 2008;68(22):9212–9220. doi: 10.1158/0008-5472.CAN-08-1135. [DOI] [PubMed] [Google Scholar]
- 31.Xue X., Taylor M., Anderson E. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72(9):2285–2293. doi: 10.1158/0008-5472.CAN-11-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroeger N., Seligson D.B., Signoretti S. Poor prognosis and advanced clinicopathological features of clear cell renal cell carcinoma (ccRCC) are associated with cytoplasmic subcellular localisation of hypoxia inducible factor-2alpha. Eur J Cancer. 2014;50(8):1531–1540. doi: 10.1016/j.ejca.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Bangoura G., Liu Z.S., Qian Q. Prognostic significance of HIF-2alpha/EPAS1 expression in hepatocellular carcinoma. World J Gastroenterol. 2007;13(23):3176–3182. doi: 10.3748/wjg.v13.i23.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maynard M.A., Evans A.J., Hosomi T. Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma. FASEB J. 2005;19(11):1396–1406. doi: 10.1096/fj.05-3788com. [DOI] [PubMed] [Google Scholar]
- 35.Pasanen A., Heikkilä M., Rautavuoma K. Hypoxia-inducible factor (HIF)-3alpha is subject to extensive alternative splicing in human tissues and cancer cells and is regulated by HIF-1 but not HIF-2. Int J Biochem Cell Biol. 2010;42(7):1189–1200. doi: 10.1016/j.biocel.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Acker T., Diez-Juan A., Aragones J. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell. 2005;8(2):131–141. doi: 10.1016/j.ccr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Raval R.R., Lau K.W., Tran M.G. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25(13):5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaupel P., Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 39.Khan N., Mupparaju S., Hou H. Repeated assessment of orthotopic glioma pO(2) by multi-site EPR oximetry: a technique with the potential to guide therapeutic optimization by repeated measurements of oxygen. J Neurosci Methods. 2012;204(1):111–117. doi: 10.1016/j.jneumeth.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greer S.N., Metcalf J.L., Wang Y., Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin D., Wu J. Hypoxia inducible factor in hepatocellular carcinoma: a therapeutic target. World J Gastroenterol. 2015;21(42):12171–12178. doi: 10.3748/wjg.v21.i42.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim W.Y., Kaelin W.G. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22(24):4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell P.H., Wiesener M.S., Chang G.W. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Skowronek I., Kozaczuk S. Von Hippel-Lindau syndrome. Horm Res Paediatr. 2015;84(3):145–152. doi: 10.1159/000431323. [DOI] [PubMed] [Google Scholar]
- 45.Maynard M.A., Ohh M. The role of hypoxia-inducible factors in cancer. Cell Mol Life Sci. 2007;64(16):2170–2180. doi: 10.1007/s00018-007-7082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeriswyl V., Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Hickey M.M., Simon M.C. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217–257. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- 48.Prager G.W., Poettler M., Unseld M., Zielinski C.C. Angiogenesis in cancer: anti-VEGF escape mechanisms. Transl Lung Cancer Res. 2012;1(1):14–25. doi: 10.3978/j.issn.2218-6751.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimna A., Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015:549412. doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang N., Wang L., Esko J. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Skuli N., Liu L., Runge A. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114(2):469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geis T., Döring C., Popp R. HIF-2alpha-dependent PAI-1 induction contributes to angiogenesis in hepatocellular carcinoma. Exp Cell Res. 2015;331(1):46–57. doi: 10.1016/j.yexcr.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 54.Gordan J.D., Thompson C.B., Simon M.C. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12(2):108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semenza G.L. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H., Gao P., Fukuda R. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11(5):407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Huang D., Li C., Zhang H. Hypoxia and cancer cell metabolism. Acta Biochim Biophys Sin (Shanghai) 2014;46(3):214–219. doi: 10.1093/abbs/gmt148. [DOI] [PubMed] [Google Scholar]
- 58.Kirito K., Hu Y., Komatsu N. HIF-1 prevents the overproduction of mitochondrial ROS after cytokine stimulation through induction of PDK-1. Cell Cycle. 2009;8(17):2844–2849. doi: 10.4161/cc.8.17.9544. [DOI] [PubMed] [Google Scholar]
- 59.Fukuda R., Zhang H., Kim J.W. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 60.Chen Z., Li Y., Zhang H. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29(30):4362–4368. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 61.Zuo J., Wen M., Lei M. MiR-210 links hypoxia with cell proliferation regulation in human laryngocarcinoma cancer. J Cell Biochem. 2015;116(6):1039–1049. doi: 10.1002/jcb.25059. [DOI] [PubMed] [Google Scholar]
- 62.Huang X., Zuo J. Emerging roles of miR-210 and other non-coding RNAs in the hypoxic response. Acta Biochim Biophys Sin (Shanghai) 2014;46(3):220–232. doi: 10.1093/abbs/gmt141. [DOI] [PubMed] [Google Scholar]
- 63.Furuta E., Pai S.K., Zhan R. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68(4):1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 64.Mylonis I., Sembongi H., Befani C. Hypoxia causes triglyceride accumulation by HIF-1-mediated stimulation of lipin 1 expression. J Cell Sci. 2012;125(Pt 14):3485–3493. doi: 10.1242/jcs.106682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rankin E.B., Rha J., Selak M.A. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29(16):4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rankin E.B., Giaccia A.J. Hypoxic control of metastasis. Science. 2016;352(6282):175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 68.Masoud G.N., Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang R., Zhou S., Li S. Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr Med Chem. 2011;18(21):3168–3189. doi: 10.2174/092986711796391606. [DOI] [PubMed] [Google Scholar]