ABSTRACT
Background. Sipuleucel T, an autologous cell-based vaccine targeting prostatic acid phosphatase (PAP), has demonstrated efficacy for the treatment of advanced prostate cancer. DNA vaccines encoding PAP and live attenuated Listeria vaccines have entered clinical trials for patients with prostate cancer, and have advantages in terms of eliciting predominantly Th1-biased immunity. In this study, we investigated whether the immunogenicity and anti-tumor efficacy of a DNA and Listeria vaccine, each encoding PAP, could be enhanced by using them in a heterologous prime/boost approach.
Methods. Transgenic mice expressing HLA-A2.01 and HLA-DRB1*0101 were immunized alone or with a heterologous prime/boost strategy. Splenocytes were evaluated for MHC class I and II-restricted, PAP-specific immune responses by IFNγ ELISPOTs. Anti-tumor activity to a syngeneic, PAP-expressing tumor line was evaluated.
Results. PAP-specific cellular immunity and anti-tumor activity were elicited in mice after immunization with DNA- or listeria-based vaccines. Greater CD4+ and CD8+ responses, and anti-tumor responses, were elicited when mice were immunized first with DNA and boosted with Listeria, but not when administered in the opposite order. This was found to be dependent on CD4+ T cells elicited with DNA priming, and was not due to inflammatory signals by Listeria itself or due to B cells serving as antigen-presenting cells for DNA during priming.
Conclusions. Heterologous prime/boost vaccination using DNA priming with Listeria boosting may provide better anti-tumor immunity, similar to many reports evaluating DNA priming with vaccines targeting foreign microbial antigens. These findings have implications for the design of future clinical trials.
KEYWORDS: DNA vaccine, heterologous prime-boost, Listeria monocytogenes, prostatic acid phosphatase
Introduction
Immunotherapies have demonstrated clinical benefit for many types of cancer. Sipuleucel T, an autologous cell-based vaccine targeting the prostate tumor antigen prostatic acid phosphatase (PAP), received FDA approval in 2010 for the treatment metastatic, castration-resistant prostate cancer, and was the first anti-tumor vaccine to be approved in the U.S.1 While the approval of sipuleucel-T demonstrated that vaccines can have efficacy in the treatment of advanced cancer, and that PAP in particular may be a rational target for anti-tumor vaccines, the cost and logistics of this autologous cell product make off-the-shelf vaccines with the ability to elicit greater anti-tumor efficacy highly desirable.
Our group and others have investigated genetic vaccines as simpler vaccine approaches that favor the generation of antigen-specific CD8+ T cells. In particular, we have focused on plasmid DNA vaccines, with efforts to understand their mechanism of action and improve their immunogenicity.2 We have previously reported that a DNA vaccine encoding PAP (pTVG-HP), the same tumor target as the sipuleucel-T vaccine, could elicit PAP-specific CD8+ T cells in rodent preclinical models and in patients with early recurrent prostate cancer in two phase I clinical trials.3-5 In both trials, the development of persistent PAP-specific Th1-biased immunity was associated with favorable changes in PSA doubling time.5,6 On the basis of these findings, that vaccine is currently being evaluated in a randomized, placebo-controlled phase II clinical trial (NCT01341652).
The use of live bacterial cells as vehicles to deliver tumor-associated antigens, cytokines, DNA, and RNA has emerged over the last two decades.7 In particular, bacterial-based vaccines using Listeria monocytogenes (Lm) as an antigen delivery vector have been investigated in preclinical models and multiple clinical trials.8,9 Upon infection, Lm naturally activates both the innate immune system and permits antigen presentation through the endogenous pathway to elicit both CD4+ and CD8+ T cell antigen-specific immunity. An advantage of Lm over other bacterial and viral vectors is that it directly infects CD8α dendritic cells and is not lytic.10 Consequently, repeated administration of Lm is possible as a vaccine approach without generation of neutralizing antibodies.11 Multiple mutant, attenuated Lm strains have been genetically engineered to maintain immunopotency but decrease potential toxicity associated with infection.12 In particular, a live attenuated double deleted Lm strain (LADD) developed by Aduro Biotech Inc. has deletions in the actA virulence factor and inlB to reduce hepatocyte uptake and toxicity.13 A LADD strain encoding mesothelin (CRS-207) has been shown to stimulate robust innate and adaptive mesothelin-specific T cell immunity in cancer patients and has been evaluated as an anti-cancer therapy in patients with mesothelioma, pancreatic, gastric, and ovarian cancer in phase 1 and 2 clinical trials.9,14 Additionally, a LADD strain expressing four prostate cancer associated antigens has entered clinical development for patients with metastatic castration-resistant prostate cancer (NCT02625857).
Heterologous prime-boost strategies, by administering the same antigen through two different delivery methods, have been reported to induce greater numbers of antigen-specific T cells and increase the quality of the immune response by involving multiple T-cell subsets and stimulating diverse cytokine profiles compared with homologous boosting alone.7 We have previously evaluated prime-boost strategies targeting PAP using a vaccinia viral vector with DNA or protein booster immunizations.3 We found that the generation of vector-specific immunity after multiple vaccinations with vaccinia could be avoided by using a prime-boost approach. Prime-boost strategies using a DNA priming step followed by a boost with a different type of vaccine (ie. viral, bacteria, and protein) targeting the same antigen have been extensively studied in preclinical studies and clinical trials of vaccines for HIV and other pathogens.15 In most cases, these prime-boost approaches generated greater antigen-specific immunity (both humoral and cellular) than elicited by immunization with either delivery vector method alone. Moreover, Listeria vaccines have specifically demonstrated efficacy when used as a boosting agent in prime-boost regimens using dendritic cells (DC), poly-lactic-co-glycolic acid (PLGA) microspheres, and viral vaccines as priming agents, suggesting they might specifically be used in a prime-boost sequence with DNA priming.16-18
While DNA and Lm immunotherapies have each shown promise as monotherapies, we questioned whether the combination of these therapies targeting the same antigen could increase the magnitude or diversity of a Th1-biased cellular immune response and increase anti-tumor responses. In this study, we investigated whether the immunogenicity and anti-tumor efficacy of DNA and Lm vaccines targeting PAP could be enhanced using a heterologous prime/boost vaccination strategy, and if there was a preferred sequence of vaccination. As a tumor model, we used HLA-A2/HLA-DR1 transgenic mice tumors engineered to express human PAP. Mice immunized with either DNA or Lm encoding PAP developed T-cell immunity and anti-tumor responses. However, these responses were augmented when DNA was used as a priming immunization. This was found to be due to the generation of Th1-biased CD4+ T cell immunity during priming.
Results
Prime/boost immunization using Lm- and DNA-based vaccines elicits significant anti-tumor response and broad immune response to PAP-specific MHC class I- and class II-restricted epitopes
We have previously reported that a DNA vaccine encoding PAP (pTVG-HP) can elicit persistent, PAP-specific, Th1 immunity in preclinical models and in patients with prostate cancer.4,5,19 A Lm vaccine that encodes PAP has entered clinical development for patients with advanced prostate cancer (NCT02625857). Consequently, we wished to evaluate whether the anti-tumor efficacy and immunogenicity of either approach could be augmented using a prime/boost vaccination strategy. Specifically, A2/DR1 mice were implanted with a syngeneic tumor cell line engineered to express human PAP, using the same HLA-A2-expressing sarcoma tumor cell line we have previously described engineered to express other tumor antigens.20,21 Mice then received weekly immunization with the LADD-PAP or pTVG-HP alone or in prime/boost schedules using both vaccines. While there was no significant difference between animals receiving pTVG-HP alone or in a prime-boost sequence, the greatest anti-tumor activity against the tumor cell line occurred in mice that were vaccinated first with pTVG-HP followed by LADD-PAP (Fig. 1A). A2/DR1 mice immunized with the alternative prime/boost schedule (LADD-PAP/pTVG-HP) had a significantly inferior anti-tumor response to those immunized with LADD-PAP or pTVG-HP alone (Fig. 1B), suggesting that a prime/boost schedule might provide greater anti-tumor immunity, and the order of vaccination is important, with DNA priming preferred. Tumors were collected at the time of study termination and evaluated for tumor-infiltrating lymphocytes. As shown in Fig. 1C, animals receiving LADD-PAP alone had a lower CD4:CD8 ratio, and this was increased using pTVG-HP alone or in combination with LADD-PAP. No differences were seen in markers of activation (4-1BB or PD-1) on tumor-infiltrating CD4+ or CD8+ T cell subsets among the treatment groups (Supplemental Fig. 1).
Figure 1.
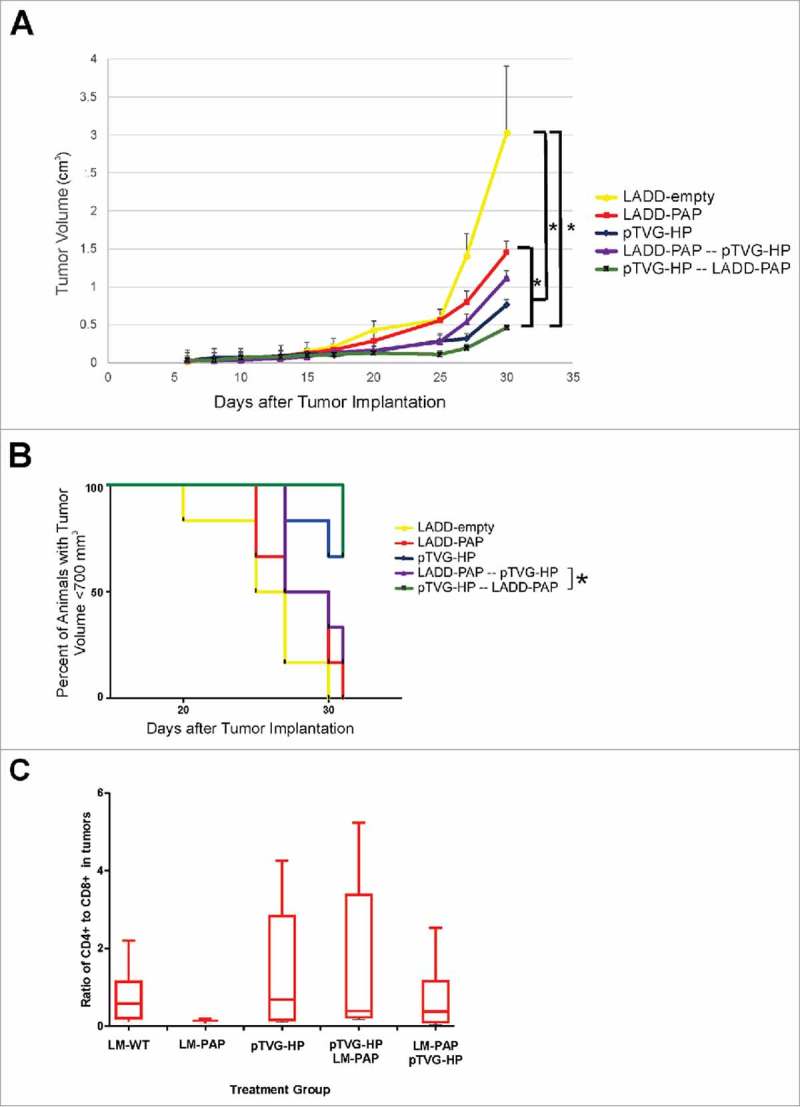
Prime/boost immunization using Lm- and DNA-based vaccines elicits significant antigen-specific anti-tumor response. Six- to ten-week old A2/DR1 mice were implanted with sarcoma tumors expressing PAP. One day after tumor implantation, mice were immunized weekly with 1× 106 cfu LADD-empty (yellow), 1× 106 cfu LADD-PAP (red), 100 µg pTVG-HP (blue), 1× 106 cfu LADD-PAP once followed by weekly immunization with 100 µg pTVG-HP (purple), or 100 µg pTVG-HP once followed by weekly immunization with 1× 106 cfu LADD-PAP (green). Tumor volumes were measured three times a week. Panel A: Shown is the average tumor volume (cm3) +/- SE for each group at each time point. * = p < 0.05 at day 30. Panel B: The same data are shown as time to progression, defined as tumor size ≥ 700 mm3. * = p < 0.05 (log-rank test). Panel C: Tumors collected from these mice were evaluated for CD4+ and CD8+ T cell subsets by flow cytometry. Shown are the CD4:CD8 T cell ratios for each group. Results shown are from one study with 6 animals per group, and are representative of 2 experiments performed with 12 total animals per group.
We next investigated whether the anti-tumor activity was associated with differences in the magnitude or quality of PAP-specific immunity elicited with vaccination. A2/DR1 mice received two immunizations three weeks apart with a Lm-based vaccine alone (LADD-empty) or expressing PAP (LADD-PAP) or in an alternative prime/boost schedule combining pTVG-HP and LADD-PAP. An additional group received four weekly immunizations with pTVG-HP. One week after the last immunization, splenocytes were collected and assessed for peptide-specific T cells by IFNγ ELISPOT using HLA-A2- and HLA-DR1-restricted peptide epitopes previously identified.22,23 Of note, the LADD-PAP construct contained a deletion of the PAP secretory signal, which allowed efficient expression and secretion from the bacterial cell into the cytoplasm of the host antigen-presenting cell (APC). This deletion effectively removed one of the MHC class I restricted epitopes (pp18–26) from the LADD-PAP strain, enabling any response to this epitope to be attributed to DNA immunization alone. Mice immunized with LADD-PAP or pTVG-HP developed IFNγ-secreting PAP-specific T cells to both the whole PAP protein and several epitopes. Mice immunized with the DNA vaccine developed a predominant immune response to the MHC class II-restricted epitopes (HP161–175, HP351–365, and HP191–205) while immunization with LADD-PAP elicited a dominant immune response to the MHC class I-restricted epitope (pp112–120) and a low level response to one of the MHC class II-restricted epitopes (HP351–365) (Fig. 2A). Mice that received an priming immunization with pTVG-HP followed three weeks later with LADD-PAP developed a robust immune response to the whole PAP protein and to both PAP-specific, MHC class I (pp112–120) and class II restricted epitopes (HP161–175, HP351–365, HP191–205) (Fig. 2A). These responses were significantly higher than immune responses elicited by priming and boosting with the individual therapies. Interestingly, the vaccine order in the prime/boost schedule appeared to be important since A2/DR1 mice immunized first with LADD-PAP followed by pTVG-HP developed a lower immune response to the whole PAP protein and to the PAP-specific, MHC class II restricted epitopes (e.g. HP351–365) than if delivered using the opposite schedule (Fig. 2A). Immune responses to the PAP-specific, MHC class I epitope pp112–120 were not augmented using this prime/boost schedule (Fig. 2A). Splenocytes from animals immunized with pTVG-HP alone, or with pTVG-HP followed by LADD-HP, were stimulated with PAP protein or individual peptides and further assessed for release of other cytokines (IL-2, IL-4, IL-6, IL-10, IL-17a, TNFα) in addition to IFNγ. PAP-specific responses observed were mediated by both MHC class I and class II-restricted responses (partially blocked by anti-HLA-A2 or by HLA-DR), and were Th1-biased (secreting IFNγ, IL-2, and TNFα, Fig. 2B). Taken together, these results indicate both Lm-based and DNA vaccines generated antigen-specific Th1-biased T-cell immunity that was systemically detectable directly ex vivo, but the magnitude and breadth of CD4 and CD8 response was greatest following prime/boost using a DNA priming immunization followed by LADD immunization regimen.
Figure 2.
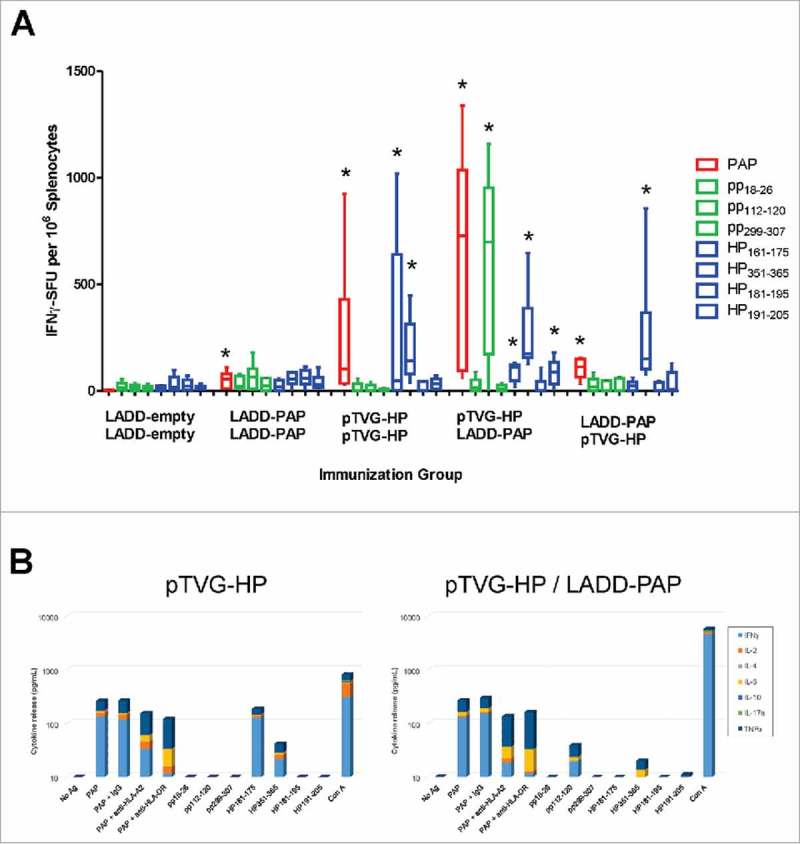
Prime/boost immunization using Lm- and DNA-based vaccines elicits PAP-specific MHC class I- and class II-restricted epitopes. Six- to ten-week old A2/DR1 mice were immunized twice three weeks apart with 1× 106 cfu LADD-empty, 1× 106 cfu LADD-PAP, 100 µg pTVG-HP followed by 1× 106 cfu LADD-PAP, or 1× 106 cfu LADD-PAP followed by 100 µg pTVG-HP (n = 6 per group). An additional group was immunized weekly with 100 µg pTVG-HP. Panel A: One week after the last immunization, splenocytes were harvested and assessed for cellular immunity by INFγ ELISPOT to PAP protein (red), PAP-specific HLA A2-restricted peptides (green, pp18–26, pp112–120, pp299–307), or PAP-specific, HLA-DR1-restricted peptides (blue, HP161–175, HP351–365, HP181–195, and HP191–205). Shown are box and whisker plots with the number of IFNγ spot-forming units (SFU) per million splenocytes for each stimulating antigen for all animals per treatment group, with group median shown by the horizontal bar. Statistical comparisons to mice immunized with LADD-empty control were made using Mann-Whitney U test and comparisons with p < 0.05 are represented with an asterisk. Results shown are from one study with 6 animals per group, and are representative of 3 experiments performed with 18 total animals per group. Panel B: Splenocytes from animals immunized twice with pTVG-HP (left) or pTVG-HP followed by LADD-PAP (right) were assessed for cytokine release in response to PAP protein or peptide stimulation by cytokine bead array. Shown are the individual cytokines (IFNγ, IL-2, IL-4, IL-6, IL-10, IL-17a, and TNFα) detected in culture supernatants following stimulation. Additional groups stimulated with PAP protein were co-cultured with anti-HLA-A2 or anti-HLA-DR.
Increased immunogenicity of prime-boost immunization with LADD boost not due to increased antigen load or inflammatory signals from Listeria alone
We next sought to elucidate the mechanism by which heterologous prime-boost immunization might lead to a greater immune response than immunization with either vector alone, and specifically whether this was due to effects from LADD boosting. CpG-rich bacterial DNA is known to be an agonist for TLR9,24 and Lm is known to be an agonist for TLR2 and TLR5.25 Hence it was conceivable that TLR activation, or other inflammatory signals from Lm treatment, might augment the immune response following DNA immunization. Alternatively, increased expression of the target antigen by LADD might specifically augment immune response following DNA priming. To test these possibilities, A2/DR1 mice received a pTVG-HP immunization followed three weeks later with an immunization with pTVG-HP, LADD-PAP, or pTVG-HP with LADD-PAP (or LADD-empty). Mice immunized twice with pTVG-HP developed an immune response to PAP protein and the PAP-specific MHC class II restricted epitopes (HP161–175 and HP351–365), and mice primed with pTVG-HP followed by LADD-PAP boost developed a greater magnitude and broader immune response, as expected (Fig. 3). When mice were boosted with pTVG-HP along with LADD-empty, there was no increase in response to PAP or MHC class I or class II epitopes compared with immunization with DNA alone, suggesting that TLR activation or other inflammatory signals from Lm were not contributing to the antigen-specific immune response. Similarly, when animals were boosted with pTVG-HP and LADD-PAP, there was no increase in immunity compared with boosting with LADD-PAP alone, suggesting that increased antigen load did not contribute to greater immune response with heterologous prime-boost immunization (Fig. 3).
Figure 3.
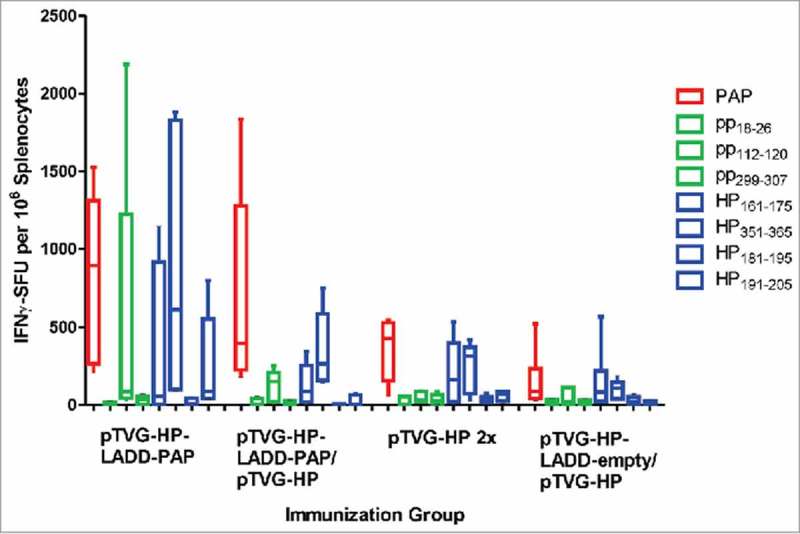
Increased immunogenicity of prime-boost immunization with LADD boost not due to increased antigen load or inflammatory signals from Listeria alone. Six- to ten-week old A2/DR1 mice were immunized with 100 µg pTVG-HP intradermally and then three weeks later with either 1× 106 cfu LADD-PAP (n = 6), 100 µg of pTVG-HP (n = 5), or pTVG-HP administered intradermally with an intraperitoneal injection with 1× 106 cfu LADD-PAP (n = 5) or LADD-empty (n = 5). One week after the last immunization splenocytes were collected for IFNγ ELISPOT analysis as above. Shown are box and whisker plots with the number of IFNγ spot-forming units (SFU) per million splenocytes for each stimulating antigen for all animals per treatment group. Statistical comparisons were made between the first two treatment groups, and third and fourth treatment groups, using a Mann-Whitney U test and no comparisons were found to have p < 0.05.
Differences in T-cell immunity following DNA priming were not due to antigen presentation by B lymphocytes
Antigens expressed by Lm vectors have been demonstrated to be directly targeted and presented by CD8α dendritic cells.10 The immunogenicity of DNA vaccines depends primarily on antigen expression in bystander cells that is cross-presented by dendritic cells,26,27 however we and others have demonstrated that B lymphocytes have the capacity to directly take up and present DNA-encoded antigens.28,29 Thus, we next investigated whether the differences in magnitude and diversity of T-cell immune response following prime/boost immunization might be due to DNA priming using B cells as additional antigen-presenting cells, potentially presenting different epitopes than DC. Using an anti-CD20 antibody that was demonstrated to deplete B cells in vivo for greater than 20 days (Supplemental Fig. 2 and30), A2/DR1 mice were depleted of B cells (or received IgG as a negative control) during the initial priming immunization with LADD-PAP or pTVG-HP. As shown in Fig. 4, the magnitude and type (MHC class I versus MHC class II) of immune response elicited with LADD-PAP, pTVG-HP, or pTVG-HP/LADD-PAP were similar to that observed in Fig. 2A, and not affected by depletion of B cells during priming. These results suggest that differences in the breadth of immunity elicited were not due to B cells acting as antigen-presenting cells during priming.
Figure 4.
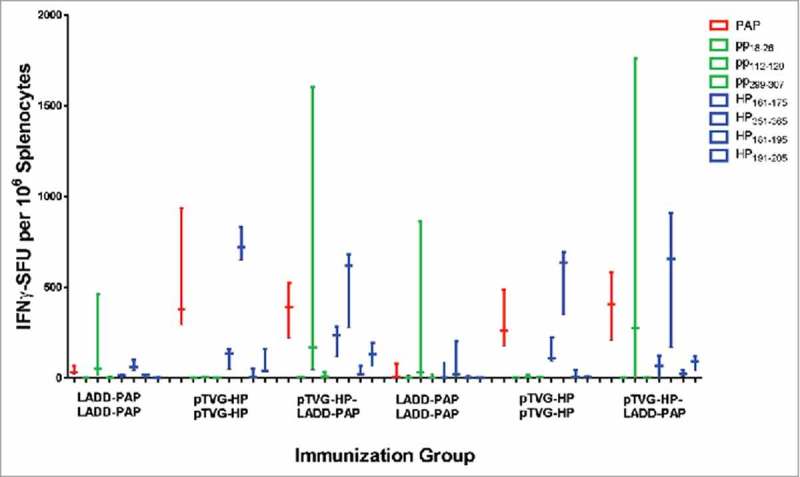
Differences in T-cell immunity were not due to antigen presentation by B lymphocytes. Six-to ten-week old A2/DR1 mice were intravenously injected with 250 µg anti-CD20 (clone SA271G2 antibody, panel A), or IgG as a negative control (panel B), nine days before vaccination. Mice were immunized twice three weeks apart with 1× 106 cfu LADD-PAP, 100 µg pTVG-HP, or pTVG-HP followed by LADD-PAP (n = 3 per group). One week after the last immunization splenocytes were collected for IFNγ ELISPOT analysis as above. Shown are box and whisker plots with the number of IFNγ spot-forming units (SFU) per million splenocytes for each stimulating antigen for all animals per group. Statistical comparisons were made using Mann-Whitney U test, and no comparisons between groups treated with IgG versus anti-CD20 were found to have p < 0.05.
The anti-tumor response elicited by pTVG-HP and LADD-PAP prime/boost was dependent on CD4+ T cells elicited
As shown in Fig. 2, the primary difference between Lm and DNA immunization was the generation of MHC class II-restricted T cells with DNA immunization. To determine whether the presence of antigen-specific CD4+ T cells, possibly elicited early with DNA priming, might account for differences in anti-tumor efficacy observed, A2/DR1 mice, implanted with tumor cell line expressing PAP, were depleted of CD4+ T cells using an anti-CD4+ antibody (or IgG as a negative control) and then immunized weekly with LADD-PAP, pTVG-HP, or pTVG-HP followed by LADD-PAP. The greater anti-tumor protection elicited with immunization of pTVG-HP and the prime/boost schedule (pTVG-HP/LADD-PAP) was eliminated after the depletion of CD4+ T cells, demonstrating that the anti-tumor activity was dependent on CD4+ T cells (Fig. 5, and Supplemental Fig. 3). It has been demonstrated by others that low antigen burden can drive the preferential induction of CD4+ T cells over CD8+ T cells.31 Consequently, we questioned whether the generation of CD4+ T cells by DNA priming was potentially due to DNA being relatively less efficient in cell uptake relative to Lm, leading to less antigen presentation. We have previously demonstrated that immunization with higher pTVG-HP plasmid dose can lead to greater magnitude of IgG response to PAP.19 Consequently, to test this, mice were immunized with different amounts of DNA prior to Lm boost. As shown in Fig. 6, there was no evidence of difference in PAP-specific MHC class II-restricted immunity using higher doses of plasmid DNA, suggesting that the generation of CD4+ T cells at the time of priming is intrinsic to DNA immunization, and not necessarily due to the dose of antigen presented by the DNA.
Figure 5.
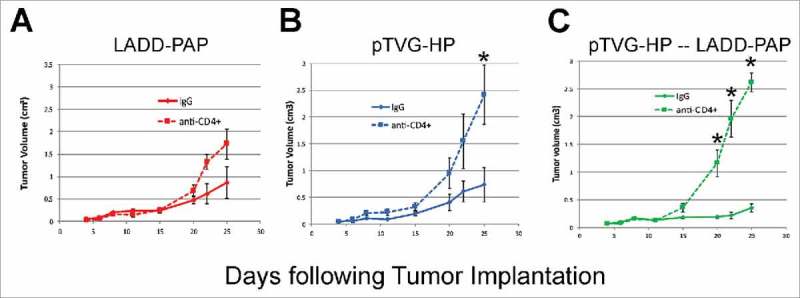
The anti-tumor response elicited by pTVG-HP and LADD-PAP prime/boost was dependent on CD4± T cells elicited. A2/DR1 mice were injected with 250 µg of anti-CD4+ antibody (clone GK1.5) (or IgG as a negative control) one day before tumor implantation (−1), on days three and six after implantation, and weekly afterwards. Tumors were implanted and animals immunized as in Fig. 1. Tumor volumes were measured three times a week. Shown are tumor growth curves (average volume (cm3) +/- SE) for mice immunized weekly with LADD-PAP (panel A), pTVG-HP (panel B), or one immunization with pTVG-HP followed by weekly immunization with 1× 106 cfu LADD-PAP (panel C), in the presence of anti-CD4+ antibody or IgG control (n = 5–6 for each treatment group). Statistical comparisons were made using Mann-Whitney U test at each time point, and comparisons with p<0.05 are represented with an asterisk.
Figure 6.
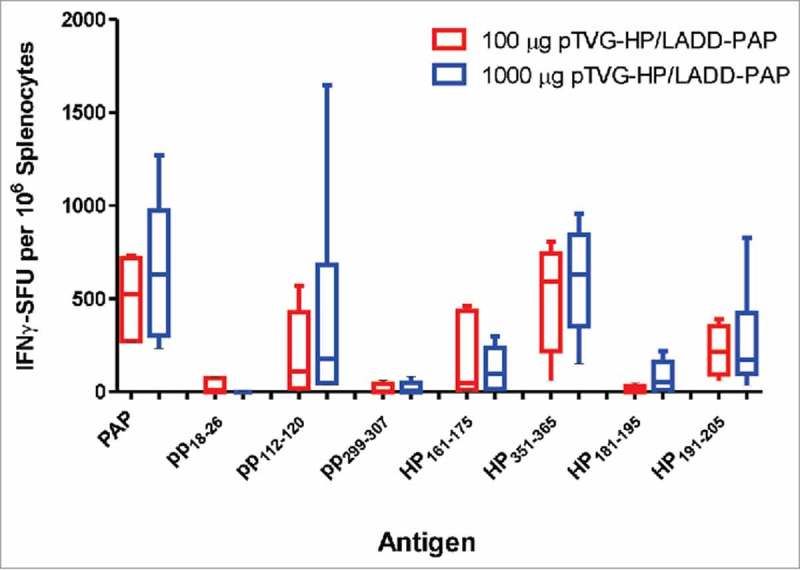
Induction of PAP-specific MHC class II-restricted immunity was not reduced using higher concentration of DNA for priming immunization. A2/DR1 mice were immunized with 100 µg (n = 5, red) or 1000 µg of pTVG-HP (n = 6, blue) followed three weeks later with 1× 106 cfu LADD-PAP. One week after the last immunization splenocytes were collected for IFNγ ELISPOT analysis as in Fig. 2. Shown are box and whisker plots with the number of IFNγ spot-forming units (SFU) per million splenocytes for each stimulating antigen for all animals per dose treatment group. Statistical comparisons were made using Mann-Whitney U test, and no comparisons between groups treated with the different DNA concentrations were found to have p < 0.05.
Discussion
PAP is a prostate tumor antigen, and currently the only target of an FDA-approved cancer treatment vaccine, sipuleucel-T. Sipuleucel-T has been demonstrated to elicit broad PAP-specific immunity, characterized by both IFNγ-secreting T cells (Th1-biased) and also production of antibodies and Th2-associated cytokines, both of which have been associated with improved clinical outcome.32,33 However, given the cost and complexity of this autologous vaccine, off-the-shelf vaccine approaches are highly desirable. In particular, DNA and bacterial vector vaccines that may favor the induction of a Th1 cytolytic type immune response should theoretically elicit greater anti-tumor immunity. In this report, we evaluated two vaccine approaches, each undergoing clinical evaluation as treatments for prostate cancer, using plasmid DNA or Lm as a bacterial vector, and each encoding the prostate tumor antigen PAP. These were evaluated alone and in combination for anti-tumor efficacy in a murine tumor model expressing HLA-A2 and HLA-DR1, permitting an evaluation of the magnitude and breadth of T-cell immune response. We found that while either approach elicited antigen-specific T cells and anti-tumor responses, a greater anti-tumor response was observed using DNA and using a directional prime-boost regimen. In this model, DNA immunization favored induction of MHC class II-restricted T cells, and the induction of antigen-specific CD4+ T cells was found to be important to the improved anti-tumor immunity observed. These findings have importance for the design of future anti-tumor vaccines, and PAP-targeted vaccines in particular.
While PAP is a relevant human tumor antigen, there is not a murine prostate-specific homologue. Hence, limitations of our study were that PAP is a foreign antigen in the mouse, and our tumor model was not of prostate origin. However, by using a transgenic model expressing only one MHC class I and one MHC class II molecule, and ones for which we have already defined PAP-specific epitopes,22,23 we were effectively able to detect all relevant PAP-specific T cells elicited with vaccination. In addition, we have previously used this same syngeneic tumor cell line expressing another tumor antigen and demonstrated that anti-tumor responses are dependent on CD8+ T cells elicited with vaccination, as deletion of HLA-A2 specific epitopes eliminated any anti-tumor efficacy.21 Thus, we conclude that while both DNA and Lm methods of immunization elicited CD8+ T cells able to lyse tumor cells, the magnitude of CD8+ T cell immunity was greater when DNA priming and Lm boosting were used, and this difference was mediated or facilitated by the presence of antigen-specific CD4+ cells elicited at the time of priming.
Several other groups have reported that heterologous immunization, using plasmid DNA as a priming immunization, induces greater antigen-specific immunity. Nearly all of these studies have been in preclinical models of infectious diseases with the goal of eliciting protective immunity. Sin and colleagues first reported that DNA priming with protein vaccine boosting targeting a herpes simplex antigen elicited a Th1 immune response, whereas protein priming followed by DNA boost elicited a Th2-biased response.34 Similar findings favoring DNA priming have been reported using DNA priming followed by protein or viral vaccine boosters in models of protection from adenovirus, tuberculosis, malaria, influenza, HIV, and other pathogens.35-39 Neeson and colleagues reported that DNA priming followed by oral administration of a Lm vaccine targeting SIV could elicit antigen-specific CD8+ T cells in rhesus macaques.40 These findings have also been translated to human trials in which it has been demonstrated the DNA priming can affect the response to subsequent booster immunizations by broadening the antibody and T cell response against HIV-1 or influenza.38,41 There have been few, if any, studies evaluating this DNA priming vaccine approach in tumor systems, and none evaluating prime-boost approaches using DNA and Lm for anti-tumor immunity. Moreover, while all of these studies have demonstrated a difference with respect to the sequence of administering DNA vaccines with other booster vaccines, there has been little exploration of the mechanism for this difference. Our study demonstrates, for the first time, the specific importance of eliciting antigen-specific CD4+ T cells with the priming immunization as a possible general mechanism.
Separate from the issue of DNA priming, other groups have reported that Lm-based vaccines can specifically serve as booster vaccines in infectious disease and tumor models. Badinovac and colleagues have reported that booster infection with Lm could augment memory CD8+ T cells following priming with DC coated with Lm-derived epitopes.16 Jia and colleagues have demonstrated that a priming immunization with a live vaccine strain of Francissella tularensis followed by booster immunization with a recombinant Lm strain encoding a single F. tularensis antigen augmented the T-cell response and protective immunity than immunization with live vaccine alone.17 Similarly, this same group recently demonstrated that immunization with Lm encoding an antigen from Mycobacterium tuberculosis could significant boost the efficacy of BCG immunization, protecting animals from subsequent M. tuberculosis exposure.42 In the B16 murine melanoma tumor model, Lim and colleagues demonstrated that local radiation therapy, followed by Lm vaccination encoding a tumor antigen, led to anti-tumor responses greater than that observed with vaccination or radiation therapy alone.43 Finally, in clinical trials for patients with pancreatic cancer, Lm encoding a tumor antigen has been used as a booster immunization following a priming immunization with an allogeneic whole cell tumor vaccine, with evidence of clinical activity.9 Taken together, these findings suggest that there may be a specific advantage of using Lm as a booster vaccine in prime/boost strategies.
The importance of antigen-specific Th1-biased CD4+ T cells in anti-tumor immunity has been known for many years, including their ability to license cytolytic CD8+ T cells and recruit other anti-tumor effector cells.44,45 In our studies, we found that MHC class II-restricted T cells, presumably CD4+ T cells, were elicited with DNA vaccination in either sequence of administration. Depleting CD4+ T cells had a greater impact on the anti-tumor response using DNA immunization than using Lm vaccination alone (Fig. 5), suggesting that eliciting this response early with vaccine priming may be important to superior anti-tumor immunity. These findings have relevance to the design of DNA vaccines, as well as other anti-tumor vaccines, suggesting that inclusion of antigen-specific MHC class II epitopes could be critically important. Future studies will explore whether the induction of CD4+ T cells with DNA priming affects the proliferation and long-term effector and memory functions of CD8+ T cells.
The current studies have other implications for the next generation of anti-tumor vaccines. As described above, many investigations are being conducted in clinical trials to explore heterologous prime-boost approaches, and the optimal timing of such approaches, using DNA vaccine priming immunizations for protection from infectious agents.41 Certainly these approaches could be applied to anti-tumor trials. A prime-boost strategy evaluating DNA and Lm targeting PAP as a treatment approach for prostate cancer is certainly feasible given that both agents are in clinical testing. Notably, we found that the breadth of T-cell response was increased following heterologous prime-boost vaccination. Anti-tumor efficacy was also greatest following prime-boost, but not significantly superior to DNA immunization alone, at least in this murine model. Generation of robust populations of both antigen-specific CD4+ and CD8+ T cells is likely beneficial for successful cancer immunotherapy. These findings suggest that other means to broaden the antigen-specific CD4+ T cell response with Lm-based vaccines could potentially improve their efficacy. Similarly, DNA vaccination might be further improved by efforts to increase the CD8+ T cell response after priming, possibly by using two different DNA vaccines, or by introducing intracellular targeting or changes to specific MHC class I-encoded epitopes, as we have previously tested.46 Additionally, other alternative approaches could use two different LADD Lm vaccines, or prime-boost approaches with other vaccine platforms that are being tested clinically (e.g., rare serotype adenovirus, RNA-based vaccines, or synthetic long peptide vaccines). Ultimately, it will be important to define optimal combinations that can treat tumors in patients. Hence, evaluation of different prime-boost approaches will be directions of future clinical research.
Materials and methods
Animals
HLA-A2.01/HLA-DRB1*0101-expressing, murine MHC class I/II knock-out (A2/DR1) transgenic mice have been previously described.21,47,48 All mice were housed in microisolator cages under aseptic conditions and fed a chow diet (2018 Envigo, Indianapolis, IN) and consumed distilled water ad libitum. All experimental protocols were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC).
Peptides
Previously identified PAP-specific, HLA-A2-restricted (pp18–26, pp112–120, and pp299–307) (9 amino acids in length) and class II HLA-DR1-restricted (HP161–175, HP351–365, HP181–195, and HP191–205) (15 amino acids in length) peptides were synthesized (Proimmune Oxford, UK and Lifetein, Somerset, NJ).22,49 The purity and identity of each peptide was confirmed by mass spectrometry and gas chromatography.
Listeria strains
Lm strains were constructed in the live attenuated double deleted (LADD) ΔactA ΔinlB strain background.13 The human coding sequence for PAP was codon optimized for expression from Listeria monocytogenes and a synthetic gene was synthesized de novo (ATUM Bio, Newark, CA). LADD-PAP, the Lm strain expressing amino acids 33–386 of the PAP protein, was constructed by cloning the synthetic PAP gene downstream of the actA promoter and in-frame with the amino terminus of the ActA protein and integrated into the LADD chromosome as described.50
Immunization schemes
Six- to ten-week old A2/DR1 mice were immunized two to four times at 1–3 week intervals (1 week intervals in tumor-bearing animals, and two immunizations 3 weeks apart for non tumor-bearing animal studies) using a DNA vaccine and/or a live, attenuated Listeria monocytogenes (Lm) vaccine. Specifically, a DNA vaccine encoding human prostatic acid phosphatase, pTVG-HP, used for immunization was described previously.19 Immunization was performed intradermally two to four times with 100 µg of pTVG-HP at intervals as indicated. A2/DR1 mice were immunized intraperitoneally up to two times 21 days apart with 5× 106 cfu of LADD-PAP. Control vaccines included a DNA vector not encoding PAP (pTVG4) or a Lm parental strain not encoding PAP (LADD-empty). One week after the last immunization, mice were euthanized and spleens were collected for immunological analysis. Where described, A2/DR1 mice were intravenously injected once with 250 µg anti-mouse CD20 (clone SA271G2, Biolegend) nine days before the first vaccination.to deplete B lymphocytes.
Tumor studies
We have previously described the generation of sarcomas in A2/DR1 mice from which we derived a transplantable tumor cell line.20,21 This cell line was transfected with a lentiviral construct encoding human PAP, and a clonal cell line was isolated. PAP expression was found in tumor cells and secreted in culture supernatants, and stable after multiple passages (Supplemental Fig. 4). For tumor therapy studies, A2/DR1 mice were injected subcutaneously in the right flank with 1× 104 of this syngeneic PAP-expressing sarcoma cell line in 50% high concentration, LDEV-free matrigel (BD Biosciences, San Jose, CA) as previously described.21 The next day, mice were immunized as described above. Tumors were measured three times a week and the tumor volume was calculated according to the following equation: (π/6)(long axis)(short axis).2 For the CD4+ T cell depletion studies, A2/DR1 mice were injected with 250 µg of anti-CD4+ antibody (clone GK1.5, BioXCell, West Lebanon, NH) or IgG as a negative control one day (day −1) before tumor implantation and then every four to seven days as previously described.51 Tumors obtained at necropsy were digested in media containing 1 mg/ml collagenase and 20 µg/ml DNase for 2 hours at 37°C and filtered through a 100 µm screen to obtain a single cell suspension. Tumor suspensions were stained with anti-CD45 (30-F11, Tonbo Biosciences, San Diego, CA), anti-CD3 (17A2, BD Biosciences), anti-CD8 (53.67, BD Biosciences), anti-CD4 (GK1.5, BD Biosciences), anti-4-1BB (17B5, eBioscience, San Diego, CA), anti-PD-1 (J43, BD Biosciences), and Ghost Dye 780 (Tonbo Biosciences) and analyzed by flow cytometry.
Immune analysis-interferon gamma (IFNγ) ELISPOT
Splenocytes were isolated by centrifugation after red blood cell osmotic lysis with ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1M EDTA) and then resuspended in RPMI medium supplemented with 2% penicillin/streptomycin, 50 mM ß-mercaptoethanol, and 10% fetal calf serum (Gemini Bio Products, Sacramento, CA and Omega Scientific, Inc. Tarzana, CA). 2× 105 splenocytes were co-cultured with media only, 2 µg/ml hPAP protein (Fitzgerald Industries International, Acton MA), 2 µg/ml PAP-specific peptides, or 5 µg/ml Concanavalin A (Con A) (Sigma-Alrich, St. Louis, MO) on an anti-mouse IFNγ antibody precoated, PDVF ELISPOT 96 well plate (EMD Millipore, Billerica, MA) for 48 hours at 37°C, 5% CO2. IFNγ production was detected using an anti-mouse IFNγ ELISPOT according to manufacturer's instructions (R & D Systems, Minneapolis, MN). Spots were enumerated with an automated plate reader (Autoimmun Diagnostika). The number of spots was corrected for media alone negative control, and reported as the mean number of peptide-specific IFNγ spot-forming units (SFU) per 106 splenocytes. All assays were conducted in triplicate, and were conducted with splenocytes obtained directly ex vivo, without prior in vitro stimulation. For all studies, Con A resulted in positive response.
Immune analysis-cytokine bead array
Splenocytes from immunized mice were pooled and stimulated with protein or peptide antigens as above for 48 hours. Additional groups were stimulated with 2 µg/mL PAP protein in the presence of 10 µg/mL antibodies blocking HLA-A2 (BD Biosciences), HLA-DR (eBiosciences), or IgG control. Supernatants were collected and analyzed for cytokine expression using a BD Cytometric Bead Array Th1/Th2/Th 17 cytokine kit according to manufacturer's instructions (BD Biosciences).
Statistical analyses
For all statistical analysis, comparison of group medians was performed using a Mann-Whitney U test (for non-normally distributed data) as indicated (GraphPad Prism software, v5.01). Log-rank tests were used to compare groups in time-to-event analyses. For all comparisons, P values equal to or less than 0.05 were considered statistically significant.
Supplementary Material
Funding Statement
This work was supported by the Department of Defense Prostate Cancer Research Program (W81XWH-12-1-0439 and W81XWH-15-1-0492), an AAI Careers in Immunology Fellowship, and by the National Institutes of Health (R01 CA188034,F30 CA210912 and P30 CA014520). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflicts of interest
DGM has ownership interest, receives research support, and serves as consultant to Madison Vaccines, Inc which has licensed material described in this manuscript. DGB, ML, and PL are employees of and hold stock in Aduro Biotech which is developing LADD Lm as an immunotherapy in multiple indications. The other authors have no relevant potential conflicts of interest.
Acknowledgments
We thank Bill Hanson for technical help in construction of the LADD-PAP vaccine strain.
References
- 1.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. PMID:20818862. [DOI] [PubMed] [Google Scholar]
- 2.Zahm CD, Colluru VT, McNeel DG. DNA vaccines for prostate cancer. Pharmacology & therapeutics. 2017;174:27–42. doi: 10.1016/j.pharmthera.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LE, Frye TP, Chinnasamy N, Chinnasamy D, McNeel DG. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol Immunother. 2007;56:885–95. doi: 10.1007/s00262-006-0241-8. PMID:17102977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, et al.. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–54. doi: 10.1200/JCO.2008.19.9968. PMID:19636017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeel DG, Becker JT, Eickhoff JC, Johnson LE, Bradley E, Pohlkamp I, Staab MJ, Liu G, Wilding G, Olson BM. Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res. 2014;20:3692–704. doi: 10.1158/1078-0432.CCR-14-0169. PMID:24850844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33:639–47. doi: 10.1097/CJI.0b013e3181dda23e. PMID:20551832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva AJ, Zangirolami TC, Novo-Mansur MT, Giordano Rde C, Martins EA. Live bacterial vaccine vectors: An overview. Brazilian journal of microbiology : [publication of the Brazilian Society for Microbiology]. 2014;45:1117–29. doi: 10.1590/S1517-83822014000400001. PMID:25763014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood LM, Paterson Y. Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Frontiers in cellular and infection microbiology. 2014;4:51. doi: 10.3389/fcimb.2014.00051. PMID:24860789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, et al.. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–33. doi: 10.1200/JCO.2014.57.4244. PMID:25584002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapadia D, Sadikovic A, Vanloubbeeck Y, Brockstedt D, Fong L. Interplay between CD8alpha+ dendritic cells and monocytes in response to Listeria monocytogenes infection attenuates T cell responses. PloS one. 2011;6:e19376. doi: 10.1371/journal.pone.0019376. PMID:21559416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leong ML, Hampl J, Liu W, Mathur S, Bahjat KS, Luckett W, Dubensky TW Jr, Brockstedt DG. Impact of preexisting vector-specific immunity on vaccine potency: characterization of listeria monocytogenes-specific humoral and cellular immunity in humans and modeling studies using recombinant vaccines in mice. Infection and immunity. 2009;77:3958–68. doi: 10.1128/IAI.01274-08. PMID:19528221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecologic oncology research and practice. 2017;4:9. doi: 10.1186/s40661-017-0046-9. PMID:28588899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW Jr. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–7. doi: 10.1073/pnas.0406035101. PMID:15365184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahan T, Hassan R, Alley E, Kindler H, Antonia S, Whiting C, Coussens L, Murphy AL, Thomas A, Brockstedt DG, et al.. 208O_PR: CRS-207 with chemotherapy (chemo) in malignant pleural mesothelioma (MPM): Results from a phase 1b trial. J Thorac Oncol. 2016;11:S156. doi: 10.1016/S1556-0864(16)30330-6. PMID:27198358. [DOI] [Google Scholar]
- 15.Shete AT, M., Mehendale S, Paranjape R. Is Prime Boost Strategy a Promising Approach in HIV Vaccine Development? J AIDS Clin Res. 2014;5:1000294. [Google Scholar]
- 16.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11:748–56. doi: 10.1038/nm1257. PMID:15951824. [DOI] [PubMed] [Google Scholar]
- 17.Jia Q, Bowen R, Sahakian J, Dillon BJ, Horwitz MA. A heterologous prime-boost vaccination strategy comprising the Francisella tularensis live vaccine strain capB mutant and recombinant attenuated Listeria monocytogenes expressing F. tularensis IglC induces potent protective immunity in mice against virulent F. tularensis aerosol challenge. Infection and immunity. 2013;81:1550– 61. doi: 10.1128/IAI.01013-12. PMID:23439306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham NL, Pewe LL, Fleenor CJ, Langlois RA, Legge KL, Badovinac VP, Harty JT. Exploiting cross-priming to generate protective CD8 T-cell immunity rapidly. Proc Natl Acad Sci U S A. 2010;107:12198–203. doi: 10.1073/pnas.1004661107. PMID:20616089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson LE, Frye TP, Arnot AR, Marquette C, Couture LA, Gendron-Fitzpatrick A, McNeel DG. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP). Vaccine. 2006;24:293–303. doi: 10.1016/j.vaccine.2005.07.074. PMID:16115700. [DOI] [PubMed] [Google Scholar]
- 20.Olson BM, Johnson LE, McNeel DG. The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunol Immunother. 2013;62:585–96. doi: 10.1007/s00262-012-1363-9. PMID:23108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer immunology research. 2015;3:946–55. doi: 10.1158/2326-6066.CIR-14-0206. PMID:26041735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson BM, Frye TP, Johnson LE, Fong L, Knutson KL, Disis ML, McNeel DG. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol Immunother. 2010;59:943–53. doi: 10.1007/s00262-010-0820-6. PMID:20140431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson LE, McNeel DG. Identification of prostatic acid phosphatase (PAP) specific HLA-DR1-restricted T-cell epitopes. Prostate. 2012;72:730–40. doi: 10.1002/pros.21477. PMID:22529020. [DOI] [PubMed] [Google Scholar]
- 24.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–42. doi: 10.1073/pnas.161293498. PMID:11470918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aubry C, Corr SC, Wienerroither S, Goulard C, Jones R, Jamieson AM, Decker T, O'Neill LA, Dussurget O, Cossart P. Both TLR2 and TRIF contribute to interferon-beta production during Listeria infection. PloS one. 2012;7:e33299. doi: 10.1371/journal.pone.0033299. PMID:22432012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauterbach H, Gruber A, Ried C, Cheminay C, Brocker T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J Immunol. 2006;176:4600–7. doi: 10.4049/jimmunol.176.8.4600. PMID:16585550. [DOI] [PubMed] [Google Scholar]
- 27.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–78. doi: 10.1084/jem.189.1.169. PMID:9874573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colluru VT, McNeel DG. B lymphocytes as direct antigen-presenting cells for anti-tumor DNA vaccines. Oncotarget. 2016;7:67901–18. doi: 10.18632/oncotarget.12178. PMID:27661128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filaci G, Gerloni M, Rizzi M, Castiglioni P, Chang HD, Wheeler MC, Fiocca R, Zanetti M. Spontaneous transgenesis of human B lymphocytes. Gene therapy. 2004;11:42–51. doi: 10.1038/sj.gt.3302132. PMID:14681696. [DOI] [PubMed] [Google Scholar]
- 30.Moyron-Quiroz JEL, L., Oida T, Garcia-Mojica S, Yang X. Kinetic study of B cell-depletion with a novel mAb anti-mouse CD20, clone SA271G2. The Journal of Immunology. 2016;196:23. [Google Scholar]
- 31.Billeskov R, Wang Y, Solaymani-Mohammadi S, Frey B, Kulkarni S, Andersen P, Agger EM, Sui Y, Berzofsky JA. Low Antigen Dose in Adjuvant-Based Vaccination Selectively Induces CD4 T Cells with Enhanced Functional Avidity and Protective Efficacy. J Immunol. 2017;198:3494–506. doi: 10.4049/jimmunol.1600965. PMID:28348274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, et al.. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–47. doi: 10.1007/s00262-012-1317-2. PMID:22865266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeel DG, Gardner TA, Higano CS, Kantoff PW, Small EJ, Wener MH. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer immunology research. 2014;2:988–99. doi: 10.1158/2326-6066.CIR-14-0073. PMID:25189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sin JI, Bagarazzi M, Pachuk C, Weiner DB. DNA priming-protein boosting enhances both antigen-specific antibody and Th1-type cellular immune responses in a murine herpes simplex virus-2 gD vaccine model. DNA and cell biology. 1999;18:771–9. doi: 10.1089/104454999314917. PMID:10541436. [DOI] [PubMed] [Google Scholar]
- 35.Xiang ZQ, Pasquini S, Ertl HC. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J Immunol. 1999;162:6716–23. PMID:10352290. [PubMed] [Google Scholar]
- 36.Wang QM, Sun SH, Hu ZL, Yin M, Xiao CJ, Zhang JC. Improved immunogenicity of a tuberculosis DNA vaccine encoding ESAT6 by DNA priming and protein boosting. Vaccine. 2004;22:3622–7. doi: 10.1016/j.vaccine.2004.03.029. PMID:15315841. [DOI] [PubMed] [Google Scholar]
- 37.Sedegah M, Brice GT, Rogers WO, Doolan DL, Charoenvit Y, Jones TR, Majam VF, Belmonte A, Lu M, Belmonte M, et al.. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8(+)-T-cell populations. Infection and immunity. 2002;70:3493–9. doi: 10.1128/IAI.70.7.3493-3499.2002. PMID:12065488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khurana S, Wu J, Dimitrova M, King LR, Manischewitz J, Graham BS. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. The Journal of infectious diseases. 2013;208:413–7. doi: 10.1093/infdis/jit178. PMID:23633404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joachim A, Nilsson C, Aboud S, Bakari M, Lyamuya EF, Robb ML, Marovich MA, Earl P, Moss B, Ochsenbauer C, et al.. Potent functional antibody responses elicited by HIV-I DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PloS one. 2015;10:e0118486. doi: 10.1371/journal.pone.0118486. PMID:25874723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neeson P, Boyer J, Kumar S, Lewis MG, Mattias L, Veazey R, Weiner D, Paterson Y. A DNA prime-oral Listeria boost vaccine in rhesus macaques induces a SIV-specific CD8 T cell mucosal response characterized by high levels of alpha4beta7 integrin and an effector memory phenotype. Virology. 2006;354:299–315. doi: 10.1016/j.virol.2006.06.036. PMID:16904153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledgerwood JE, Zephir K, Hu Z, Wei CJ, Chang L, Enama ME. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. The Journal of infectious diseases. 2013;208:418–22. doi: 10.1093/infdis/jit180. PMID:23633407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia Q, Dillon BJ, Maslesa-Galic S, Horwitz MA. Listeria-vectored vaccine expressing the Mycobacterium tuberculosis 30 kDa major secretory protein via the constitutively active prfA* regulon boosts BCG efficacy against tuberculosis. Infect Immun. 2017. pii: . doi: 10.1128/IAI.00245-17. PMID: 28630063. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim JY, Brockstedt DG, Lord EM, Gerber SA. Radiation therapy combined with Listeria monocytogenes-based cancer vaccine synergize to enhance tumor control in the B16 melanoma model. Oncoimmunology. 2014;3:e29028. doi: 10.4161/onci.29028. PMID:25083327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toes REM, Ossendorp F, Offringa R, Melief CJM. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–6. doi: 10.1084/jem.189.5.753. PMID:10049938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4(+) T cells have a major “Post-Licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–55. doi: 10.4049/jimmunol.165.11.6047. PMID:11086036. [DOI] [PubMed] [Google Scholar]
- 46.Smith HA, Rekoske BT, McNeel DG. DNA vaccines encoding altered peptide ligands for SSX2 enhance epitope-specific CD8+ T-cell immune responses. Vaccine. 2014;32:1707–15. doi: 10.1016/j.vaccine.2014.01.048. PMID:24492013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pajot A, Michel ML, Fazilleau N, Pancre V, Auriault C, Ojcius DM, Lemonnier FA, Lone YC. A mouse model of human adaptive immune functions: HLA-A2.1-/HLA-DR1-transgenic H-2 class I-/class II-knockout mice. Eur J Immunol. 2004;34:3060–9. doi: 10.1002/eji.200425463. PMID:15468058. [DOI] [PubMed] [Google Scholar]
- 48.Pajot A, Pancre V, Fazilleau N, Michel ML, Angyalosi G, Ojcius DM, Auriault C, Lemonnier FA, Lone YC. Comparison of HLA-DR1-restricted T cell response induced in HLA-DR1 transgenic mice deficient for murine MHC class II and HLA-DR1 transgenic mice expressing endogenous murine MHC class II molecules. International immunology. 2004;16:1275–82. doi: 10.1093/intimm/dxh129. PMID:15249541. [DOI] [PubMed] [Google Scholar]
- 49.Johnson LE, McNeel DG. Identification of prostatic acid phosphatase (PAP) specific HLA-DR1-restricted T-cell epitopes. The Prostate. 2012;72:730–40. doi: 10.1002/pros.21477. [DOI] [PubMed] [Google Scholar]
- 50.Sinnathamby G, Lauer P, Zerfass J, Hanson B, Karabudak A, Krakover J, Secord AA, Clay TM, Morse MA, Dubensky TW Jr, et al.. Priming and activation of human ovarian and breast cancer-specific CD8+ T cells by polyvalent Listeria monocytogenes-based vaccines. J Immunother. 2009;32:856–69. doi: 10.1097/CJI.0b013e3181b0b125. PMID:19752748. [DOI] [PubMed] [Google Scholar]
- 51.Kruisbeek AM. In vivo depletion of CD4- and CD8-specific T cells. Current protocols in immunology / edited by John E Coligan et al 2001; Chapter 4:Unit 4 1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


