ABSTRACT
Natural Killer (NK) cell-based cancer immunotherapies were often disappointing in the clinic mostly due to insufficient NK cell infiltration into tumors. We found that targeting autophagy induced a massive infiltration of NK cells into melanoma tumors. These findings highlight autophagy inhibition as a cutting-edge approach to fully exploit the anti-tumor properties of NK cells in a wide variety of cancer patients.
KEYWORDS: autophagy, Beclin1, cancer immunotherapy, CCL5 cytokine, Immune infiltration, immunotherapy, melanoma, natural killer cells
The ability of Natural Killer (NK) cells to kill tumor cells is well documented and experimentally supported in vitro and in various mouse models.1 However, the clinical response to NK-based cancer immunotherapy is so far relatively low.2 Several lines of evidence suggest that these limited clinical responses could be attributed to the fact that tumors are infrequently infiltrated by NK cells and that NK cells failed to reach the tumor bed.3 Therefore, approaches that would increase the trafficking of NK cells to the tumor microenvironment combined with strategies activating their cytotoxic function would be plausible therapeutic interventions to enhance the antitumor efficacy of NK cells.
It is now well recognized that targeting autophagy inhibits the tumor growth in several preclinical mouse models.4 However, the impact of blocking autophagy on the infiltration of NK cells into tumors remains largely unknown. We reported that silencing of the autophagy gene Beclin1 decreased the tumor growth of syngeneic transplanted melanoma B16-F10 tumors by inducing a massive infiltration of functional NK cells into the tumor bed. The molecular mechanism underlying the recruitment of NK cells was related to the ability of Beclin1-defective tumor cells to overexpress and release the chemotactic cytokine CCL5/RANTES in the tumor microenvironment. Genetic silencing of CCL5 in Beclin1-defective tumors completely abrogated the infiltration of NK cells. Based on these data, an ultimate question that arises is whether the increased expression of CCL5 in Beclin1-defective cells results from its autophagic or non-autophagic role. We strongly argue for the autophagic role of Beclin1 in mediating CCL5 overexpression and subsequent enhancement of NK cell infiltration into the tumor. This argument is supported by data showing that, similar to Beclin1, targeting other autophagy-related genes such as Atg5 or Sqtsm1/p62 or pharmacological inhibition of autophagy by chloroquine significantly increase the release of CCL5 by tumor cells. The increased expression of CCL5 was also observed in human melanoma cell lines displaying low expression level of CCL5. We identified the JNK/c-jun signaling pathway as activated in Beclin1-defective cells, which transcriptionally induces the expression of CCL5 in these cells. This activation is characterized by an increased JNK phosphorylation on Thr185 and Tyr183 residues due to a decreased activity of the Protein phosphatase 2 A (PP2 A). We also revealed that activated JNK phosphorylated the CCL5 transcription factor c-JUN which subsequently bound to the CCL5 promoter and transcriptionally induced its expression (Fig. 1). The clinical significance of this study is underscored by data showing a strong positive correlation between the expression of CCL5 and the infiltration of NK cells into melanoma biopsies.5 Taken together, our study establishes the first evidence that targeting autophagy is able to modulate the tumor microenvironment to increase NK cell infiltration.
Figure 1.
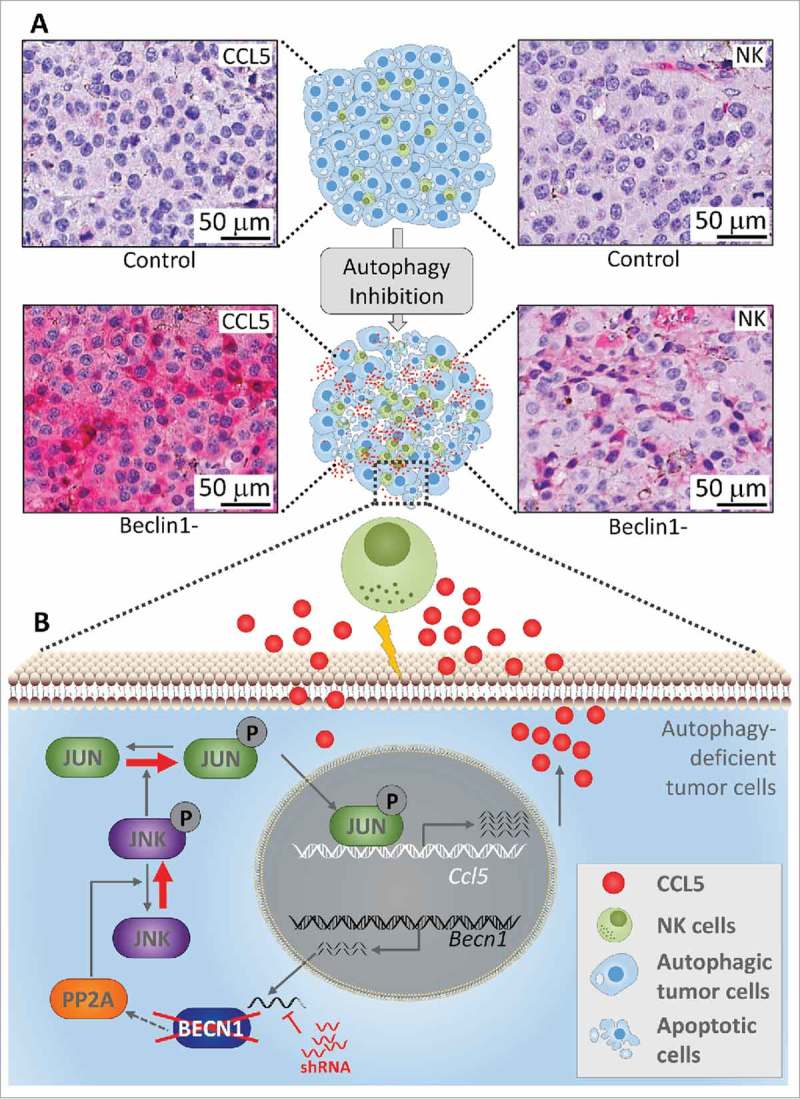
Targeting autophagy induces a massive infiltration of NK cells into melanoma tumors. The upper part (A) shows immunohistochemical staining of NK cells and CCL5 on syngeneic transplanted control or Beclin1-defective B16-F10 melanoma tumor sections. The lower part (B) describes the molecular mechanism underlying the expression of the chemotactic cytokine CCL5. Briefly, shRNA silencing Beclin1 (Becn1) leads to a decrease in the activity of the Protein phosphatase 2A (PP2A) by a mechanism not yet understood. Such a decrease enhances the phosphorylation of JNK which subsequently phosphorylates c-JUN. Phosphorylated c-JUN binds to the promoter of Ccl5 and induces its transcription. CCL5 released by Beclin1-defective tumor cells binds to CCL5 receptor on the surface of NK cells, and induces their infiltration. Functional NK cells recruited to the tumor site kill cancer cells and thereby reduce the tumor volume.
Diverse approaches are now being undertaken to enhance the cytotoxic function of NK cells by developing monoclonal antibodies able to enhance the cytotoxicity of NK cells. These antibodies block the interaction between the inhibitory Killer-cell immunoglobulin-like receptors (KIRs) expressed on the surface of NK cells and the major histocompatibility class I (MHC-I) molecules expressed on the surface of tumor cells.6 Currently considered as NK immune checkpoints, inhibitory KIRs have become targets of choice for NK-based cancer immunotherapy. Anti-KIR blocking antibodies are currently used for the treatment of both hematologic malignancies and solid tumors7 and notably in tumors in which T lymphocyte and NK cell immune checkpoints (PD-1/PD-L1 and KIR/MHC-I, respectively) contribute together to the tumor immune evasion. Humanized anti-KIR antibodies, IPH2101 and IPH2102, have been tested in several clinical trials as a single agent or in combination for the treatment of hematologic malignancies. The first phase II clinical trial in multiple myeloma patients showed no clinical response to IPH2101 as a single agent. Other clinical trials have been set-up using IPH2102 in combination with T cell immune checkpoint blockade antibodies such as anti-CTLA4 or anti-PD-L1.8 An ongoing phase I/II clinical trial include the anti-PD-1 in combination with anti-KIR (lirilumab, IPH2102/BMS-986015) is being tested in advanced refractory solid tumors with the aim to synergize NK- and T-cell mediated anti-tumor immune responses.9 NK cells are currently moving at the forefront of cancer immunotherapies. Our current data described here provide a strong rationale to design innovative NK cell-based cancer immunotherapy approaches by combining autophagy inhibitors with anti-KIR blocking antibodies. Such approaches would definitively unlock the tremendous promise of NK cell-based anti-cancer therapeutics and would significantly improve their use in the clinic.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, Cocco L, Vitale M. NK cells and cancer. J Immunol. 2007;178:4011–6. doi: 10.4049/jimmunol.178.7.4011. PMID:17371953. [DOI] [PubMed] [Google Scholar]
- 2.Carotta S. Targeting NK cells for anticancer immunotherapy: Clinical and preclinical approaches. Front Immunol. 2016;7:152. doi: 10.3389/fimmu.2016.00152. PMID:27148271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–6. doi: 10.1158/2159-8290.CD-13-0985. PMID:24795012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. PMID:28529316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mgrditchian T, Arakelian T, Paggetti J, Noman MZ, Viry E, Moussay E, Van Moer K, Kreis S, Guerin C, Buart S, et al.. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc Natl Acad Sci U S A. 2017;114:E9271–E9. doi: 10.1073/pnas.1703921114. PMID:29078276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakker AB, Phillips JH, Figdor CG, Lanier LL. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta T cells, and antigen-specific CTL. J Immunol. 1998;160:5239–45. PMID:9605119. [PubMed] [Google Scholar]
- 7.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM Jr, Blaser BW, et al.. Preclinical characterization of 1–7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–77. doi: 10.1182/blood-2009-02-206532. PMID:19553639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov. 2015;14:487–98. doi: 10.1038/nrd4506. PMID:26000725. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DB, Peng C, Sosman JA. Nivolumab in melanoma: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7:97–106. doi: 10.1177/1758834014567469. PMID:25755682. [DOI] [PMC free article] [PubMed] [Google Scholar]


