Summary
Transition metal oxides/carbon (TMOs/C) composites are important for high-performance lithium-ion batteries (LIBs), but the development of interface-stable TMOs/C composite anodes for robust lithium storage is still a challenge. Herein, mesoporous TiO2/TiC@C composite membranes were synthesized by an in situ carbothermic reduction method. TiC nanodots with high conductivity and electrochemical inactivity at the TiO2-C interface can significantly enhance the electrical conductivity and structural stability of the membranes. Finite element simulations demonstrate that the TiO2/TiC@C membranes can effectively alleviate tensile and compression stress effects upon lithiation, which is beneficial for robust lithium storage. When used as additives and binder-free electrodes, the TiO2/TiC@C membranes show excellent cycling capability and rate performance. Moreover, a flexible full battery can be assembled by employing the TiO2/TiC@C membranes and shows good performance, highlighting the potential of these membranes in flexible electronics. This work opens an avenue to constructing interface-stable composite structures for the next-generation high-performance LIBs.
Subject Areas: Composite Materials, Nanomaterials, Energy Materials
Graphical Abstract
Highlights
-
•
Mesoporous TiO2/TiC@C membranes were synthesized by a simple method
-
•
This method can be extended to the synthesis of other metal oxide/metal carbide@C
-
•
The TiC nanodots can alleviate tensile and compression stress effect upon lithiation
-
•
Long working life and excellent rate performance can be achieved
Composite Materials; Nanomaterials; Energy Materials
Introduction
Lithium-ion batteries (LIBs) are widely used in portable electric devices and electric vehicles (Armand and Tarascon, 2008, Aricò et al., 2005, Larcher and Tarascon, 2015). Extensive research has been carried out to develop transition metal oxides (TMOs)-based composite materials as LIB anodes (Yu et al., 2017a, Jeong et al., 2013, Su et al., 2013, Yu et al., 2017b, Peng et al., 2012, Wang et al., 2016a, Wang et al., 2013, Wang et al., 2015, Gu et al., 2015, Cai et al., 2014, Guan et al., 2016, Armstrong et al., 2006). In most cases, carbon materials including mesoporous carbon, carbon nanotubes, and graphene are employed as ideal matrixes for TMOs anodes owing to their unique properties such as excellent conductivity and flexibility, which can facilitate stable and fast lithium storage (Zhang et al., 2014, Fang et al., 2016, Mo et al., 2017). However, current TMOs/C composite anodes still suffer from poor cycling stability due to unstable TMOs-C interfaces resulting from the volume change difference between carbon and TMOs upon Li+ insertion/extraction. The unstable TMOs-C interfaces may cause aggregation of TMOs nanoparticles as well as collapse of carbon frameworks. As a result, the cycling life over 1,000 cycles based on TMOs/carbon composites have been extremely limited. Therefore the construction of stable TMOs-carbon interfaces is the key for stable and robust lithium storage, which remains a considerable challenge.
Among the different kinds of TMOs, TiO2 is an attractive material for LIBs owing to its natural abundance, low cost, and environmental benignancy (Liu and Chen, 2014, Liu et al., 2015a, Zhang et al., 2012). TiO2/C composite anode materials with various dimensions and structures have been fabricated, which include hierarchical TiO2/C nanocomposite monoliths (Huang et al., 2016), ordered mesoporous TiO2/C nanocomposites (Zeng et al., 2013), graphitic carbon conformal coating of mesoporous TiO2 hollow spheres (Liu et al., 2015b), and mesoporous TiO2 coating on flexible graphitized carbon (Liu et al., 2016). However, all TiO2/C composites also suffer from severe structural collapse stemming from unstable TiO2-C interfaces. In this regard, TiO2/C composite represents a typical class of LIB anode materials facing the problem of serious structure disintegration. Therefore there is a pressing need to solve the aforementioned problem of TiO2/C composite.
Herein, to construct stable TiO2-C interfaces, TiC nanodots with high conductivity and electrochemical inactivity (Wang et al., 2016b, Yao et al., 2011, Peng et al., 2016, Allcorn and Manthiram, 2015) are introduced to TiO2-C interfaces by an in situ carbothermic reduction (Yu et al., 2007) that occurs in TiO2-nanocrystals-embedded mesoporous carbon framework (TiO2@C) membranes. The designed strategy leads to the formation of stable TiO2-C interfaces where TiC nanodots act as a bridge to link TiO2 nanocrystals and carbon frameworks accurately. The obtained mesoporous TiO2/TiC@C composite membranes have a conductive, robust, and mesoporous framework. Besides, TiO2 nanocrystals and TiC nanodots are interconnected and highly dispersed in the mesoporous carbon frameworks. When used as additive-free and binder-free electrodes, the TiO2/TiC@C membranes deliver a high capacity of ∼237 mA⋅h⋅g−1 at a current density of 0.4 A⋅g−1. More importantly, an ultra-long cycling life (up to 5,000 cycles with over 68.4% reversible capacity retention) and superior rate performance can be achieved. Furthermore, a flexible full battery with impressive battery performance was assembled by using the TiO2/TiC@C membranes as the anode, highlighting the great potential of the composite membranes in flexible devices.
Results
Design and Material Synthesis
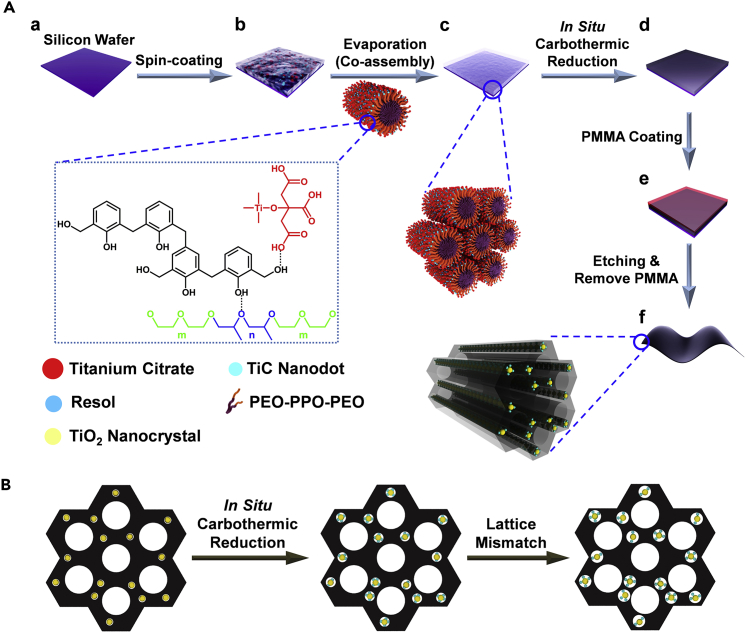
The mesoporous TiO2/TiC@C composite membranes can be synthesized through a simple co-assembly/in situ carbothermic reduction route (see Transparent Methods) by using resol, titanium citrate complex, and pluronic F127 as the carbon precursor, titanium precursor, and template, respectively. First, a pre-treated silicon wafer (Figure 1A, panel a) with thin oxidation layers on the surface was selected as a substrate, and the homogeneous solution containing resol, titanium citrate complex, and F127 was coated on the substrate by a spin-coating method (Figure 1A, panel b). A dry thin membrane was obtained after evaporation of solvents (Figure 1A, panel c), and mesoporous TiO2/TiC@C composite membranes (Figure 1A, panel d) were obtained after further carbothermic reduction in nitrogen atmosphere at 900°C (Huang et al., 2010). In this process, titanium citrate clusters was transformed into TiO2 nanocrystals and mesoporous TiO2@C composite membranes were formed first. With the temperature increasing, an in situ carbothermic reduction occurred at the TiO2-C interface and then TiC nanodots were formed between the TiO2 nanocrystals and carbon frameworks (Figure 1B). To obtain free-standing membranes, a polymethyl methacrylate (PMMA) thin film was introduced onto the surface of TiO2/TiC@C membranes (Figure 1A, panel e). After the silica layer was etched by using a KOH solution (10 wt %) and the PMMA thin film was removed by anisole solvent (Feng et al., 2011), a free-standing membrane was obtained (Figure 1A, panel f). To deeply understand the role of the unique structure of the TiO2/TiC@C membranes in lithium storage, mesoporous TiO2@C composite membranes were selected and examined for comparison.
Figure 1.
Design and Material Synthesis
(A) Schematic illustration of the synthesis of mesoporous TiO2/TiC@C composite membranes via a facile in situ carbothermic reduction strategy.
(B) Schematic illustration of structure evolution during the in situ carbothermic reduction.
Structural and Composition Characterizations
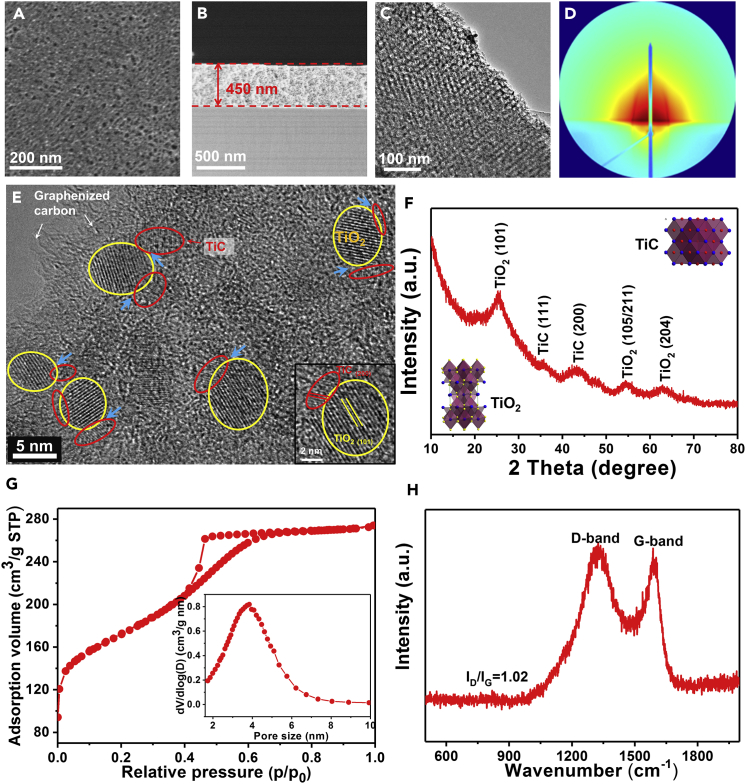
The top-view field emission scanning electron microscopic (FESEM) images (Figures 2A and S1A) show that the TiO2@C and TiO2/TiC@C membranes have a smooth and continuous surface with stripe-like, hexagonally arranged mesopores. A uniform thickness of ∼450 nm of the TiO2/TiC@C membrane is observed by a cross-sectional FESEM image (Figure 2B), showing a small shrinkage of 10% compared with ∼500 nm of the pristine TiO2@C membranes (Figure S1B). It should be noted here that the thickness of the membranes can be easily controlled by changing the rotation speed. Transmission electron microscopic (TEM) images (Figures 2C and S1C) of the TiO2@C and TiO2/TiC@C membranes show a well-defined 2D porous structure consisting of ordered hexagonal-patterned mesopores with an average diameter of ∼4 nm, which is consistent with the observation in the top-view FESEM images (Figures 2A and S1A). Further evidences for the ordered mesoporous structure can be obtained from glancing incidence small-angle X-ray diffraction (GISAXS, Figures 2D and S1D) and small-angle X-ray diffraction (SAXS, Figure S2) patterns.
Figure 2.
Structure and Composition Characterizations of the Mesoporous TiO2/TiC@C Composite Membranes
(A) Top-view scanning electron microscopic image.
(B) Cross-sectional scanning electron microscopic image.
(C) Low-magnification TEM image.
(D) GISAXS image.
(E)High-resolution TEM image. The blue arrows indicate the TiO2-TiC interfaces.
(F) XRD pattern, the insets in (F) show the molecular structures of TiO2 and TiC crystals.
(G) N2 adsorption-desorption isotherms and the corresponding pore size distribution (inset).
(H) Raman spectrum.
See also Figures S1–S10.
A high-resolution transmission electron microscopic (HRTEM) image of the TiO2/TiC@C membranes (Figure 2E) shows that TiC nanodots (∼2 nm) are formed at the TiO2-C interface as a bridge between TiO2 nanocrystals and carbon frameworks. Compared with pristine TiO2 nanocrystals (∼7 nm) in the TiO2@C membranes (Figure S1E), the size of TiO2 nanocrystals decreases because the formation of TiC nanodots is at the expense of TiO2 nanodots in the in situ carbothermic reduction. The crystal lattices are clearly observed (inset Figure 2E), with distances of 0.21 and 0.35 nm corresponding to the (200) plane of cubic structured TiC (JCPDS No. 32-1383) and the (101) plane of tetragonal structured anatase TiO2 (JCPDS No. 21-1272), respectively. The phase transformation (from TiO2 to TiC) is further confirmed by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and X-ray absorption fine structure spectroscopy (XANFS). The XRD pattern of the mesoporous TiO2/TiC@C composite membranes (Figure 2F) exhibits broad diffraction peaks indexed to TiO2 and TiC phases, in accordance with the results of HRTEM. No rutile can be detected, because amorphous carbon can act as an inhibitor for grain growth of TiO2 nanocrystals and causes the suppression of phase transition from anatase to rutile nanocrystals (Huang et al., 2010). The XPS spectra (Figure S3) of the TiO2@C and TiO2/TiC@C membranes reveal three main peaks at approximately 284, 458, and 530 eV, which are associated with the spin states of C1s, Ti2p, and O1s, respectively. From high-resolution XPS spectra (Figures S4 and S5), a shift of Ti2p and C1s can be observed between TiO2@C and TiO2/TiC@C composite, which may be attributed to the formation of the TiC nanodots. The Ti2p peak at a low bonding energy (Figure S6) is assigned to O-Ti-C bond (Shan et al., 2015, Kiran and Sampath, 2012), implying that TiO2 nanocrystals and TiC nanodots are interconnected through chemical bonds. The Ti K-edge pre-edge XANFS (Figure S7) shows that the A1 and A2 per-peaks of the TiO2/TiC@C membrane have a little shift compared with the TiO2@C membranes, indicating the difference of coordination environment (Angelomé et al., 2007). The uniform distribution of C, Ti, and O can be observed by elemental mapping (Figures S8 and S9). These results clearly demonstrate that TiC nanodots are produced at the TiO2-C interface through an in situ carbothermic reduction process.
The nitrogen sorption isotherms of the TiO2@C and TiO2/TiC@C composite membranes (Figures 2G and S1G) show representative type IV curves with H2 hysteresis loops, similar to those of the typical ordered mesoporous materials, revealing uniform pore size distribution. The pore size distribution derived from the adsorption branch using the Barrett-Joyner-Halenda (BJH) model shows uniform mesopores centered at 4.0 and 3.8 nm for the TiO2@C and TiO2/TiC@C membranes, respectively (insets in Figures 2G and S1G). The Brunauer-Emmett-Teller (BET) surface area of the TiO2/TiC@C membranes is calculated to be 674 m2⋅g−1, which is slightly larger than that for the pristine TiO2@C membranes (501 m2⋅g−1, Table S1), implying that more micropores are generated during the in situ carbothermic reduction process. In addition, the mass ratios of C, TiO2, and TiC for the mesoporous TiO2/TiC@C composite membranes are calculated to be 60:28:12 on the basis of thermogravimetric analysis (TGA) (Figure S10) before and after removal of the TiO2 nanocrystals by using concentrated H2SO4. Raman spectra of the TiO2@C and TiO2/TiC@C membranes (Figures 2H and S1H) show sharp D and G peaks with a D/G intensity ratio of ∼1:1, indicating the partial graphitization of the carbon matrixes (Kong et al., 2016).
Processing Feasibility and Scalability
It is worth mentioning that the membranes have a set of interesting properties, such as transferability, flexibility, tailorability, and large-scale production. Membranes supported by silicon wafer with an area of 1.5 cm × 1.5 cm can be peeled off to form an intact membrane with good flexibility (Figures S11A and S11B). These membranes can be transferred onto other substrates with the protection of PMMA, such as glass slides and glass rods, and can be bent without cracking (Figures S11C–S11F). Furthermore, the membranes supported by soft substrates can be conveniently tailored into desired shapes, such as square, circle, or triangle (Figures S11G–S11I). The high processing feasibility highlights the potential applications in a variety of important fields (e.g., sensor, battery, and adsorption).
More importantly, the synthesis for the mesoporous composite membranes can be scaled up. For instance, by using a large Ti foil (12 cm × 12 cm) as a substrate, a membrane with an area of ∼144 cm2 was produced (Figure S12). Moreover, the synthetic method can be extended to a large variety of substrates with different dimensions from one to three dimensions, such as W wires, Ti foils, and Cu foams (Figure S13). Therefore the synthetic strategy is highly versatile, which could be used for the synthesis of other mesoporous membranes.
Extension of the Synthetic Strategy to Other TMO/Carbon System
Significantly, this in situ carbothermic method is general and can be extended to the synthesis of other TMO/metal carbide@C systems. For example, the mesoporous MoO2.80/Mo2C@C composites can be synthesized by using molybdenyl acetylacetonate, phenolic resol, and pluronic F127 as molybdenum, carbon precursors, and template, respectively. The SAXS results show that the resultant Mo2C/MoO2.80@C composites possess ordered mesoporous structure (space group p6mm, Figure S14A). The XRD pattern (Figure S14B) of the mesoporous Mo2C/MoO2.80@C composites exhibits well-defined diffraction peaks, which can be indexed to Mo2C (JCPDS No.15-0457) and MoO2.80 (JCPDS No.12-0517), indicating the success of carbothermic reduction. The ordered mesoporous structure can be further confirmed by the TEM image (Figure S14C). Moreover, small nanoparticles with a uniform size of ∼3 nm are embedded in the mesoporous carbon frameworks. The HRTEM image shows that Mo2C nanodots are formed at the MoO2.80-C interface (Figure S14D); the interplanar distances of ∼0.24 and 0.29 nm are well matched with the d-spacing of the (111) plane of Mo2C and the (1620) plane of MoO2.80, respectively. These results demonstrate that the mesoporous Mo2C/MoO2.80@C composite has been successfully prepared by the in situ carbothermic reduction method and that the proposed synthetic strategy may be generally applicable to other TMO/carbon systems.
Electrochemical Performances
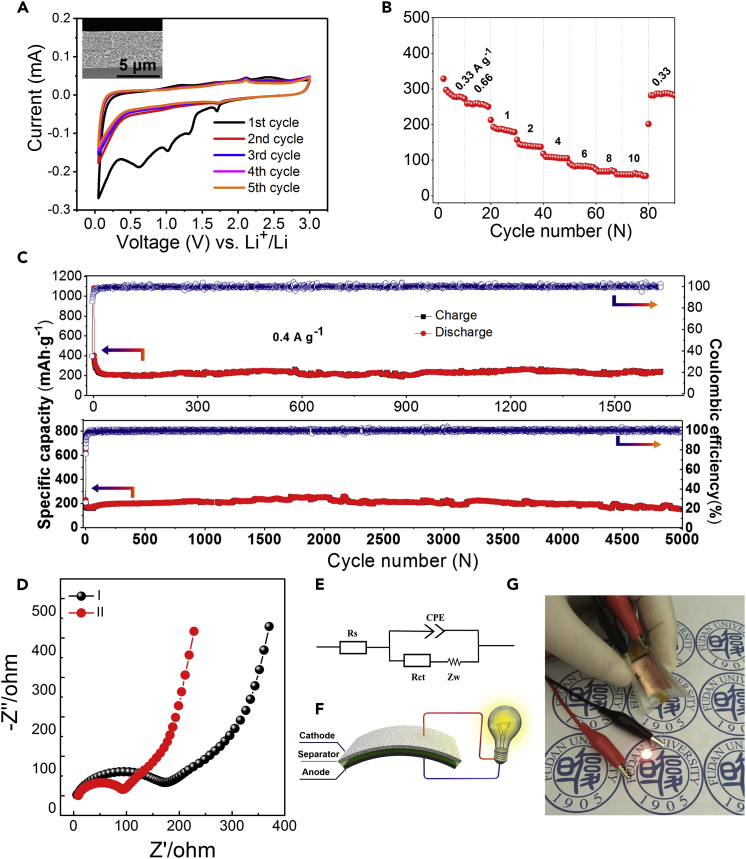
The synthesized mesoporous composite TiO2@C and TiO2/TiC@C membranes with thickness of ∼5 μm (inset in Figure 3A) standing on Cu foil were directly used as anodes of LIBs without any conducting additives and binders. The electrochemical behaviors of the representative TiO2/TiC@C and TiO2@C membrane electrodes were characterized by cyclic voltammetry (CV) at a scanning rate of 0.5 mV⋅s−1 between 0.01 and 3.0 V (Figures 3A and S15). The first cathodic scan of the mesoporous TiO2/TiC@C composites shows four peaks. The peak at 1.70 V can be assigned to Li+ insertion into the lattices of anatase. Besides, the peaks at 1.31 and 0.62 V resulted from the formation of solid-electrolyte interface (SEI) on TiO2 nanocrystals and carbon frameworks, respectively (Fang et al., 2013), while the peak at 1.0 V is attributed to the combined effect of the formation of SEI on TiO2 and carbon (Zeng et al., 2013, Wang et al., 2016b). After the first cycle, two peaks, i.e., one anodic peak at 1.70 V and one cathodic peak at 2.05 V, are observed in the CV curves (Figure 3A), which are associated with the Li+ insertion/extraction into/from the lattices of anatase TiO2. It is found that the peak current in the first scan is much higher than the following ones owing to the formation of SEI layer on the surface of the electrode (Kaskhedikar and Maier, 2009). From the second cycle onward, the CV scans overlap substantially, indicating the outstanding reversibility of lithiation/delithiation over the TiO2/TiC@C membrane electrode.
Figure 3.
Electrochemical Performances
(A) Cyclic voltammograms of the mesoporous TiO2/TiC@C composite membrane electrode in a voltage range of 0.01–3 V at a scanning rate of 1 mV⋅s−1 and the corresponding cross-section scanning electron microscopic image (inset).
(B) Rate performance of the mesoporous TiO2/TiC@C composite membrane electrodes.
(C) Cycling performance of the mesoporous TiO2/TiC@C composite membrane electrodes at current densities of 0.4 and 1.5 A⋅g−1.
(D) Nyquist plots of the mesoporous composite membrane electrodes: (I) TiO2@C and (II) TiO2/TiC@C.
(E) Equivalent circuit of the mesoporous TiO2/TiC@C composite membrane electrodes.
(F) Schematic diagram of a flexible full battery.
(G) Photograph of a red LED lightened by the flexible battery under bending. See also Figures S15–S26 and Table S2.
Rate performance of the mesoporous composite TiO2/TiC@C electrodes was conducted at drastically varying current densities from 0.33 A⋅g−1 to as high as 10 A⋅g−1. At an extremely high current density of 10 A⋅g−1, a high capacity of 57 mA⋅h⋅g−1 can be still retained, demonstrating the excellent rate performance (Figure 3B). The mesoporous TiO2/TiC@C membrane electrode also demonstrates superior long cycling stability and high reversibility. The composite membrane electrode was examined up to 1,600 cycles at a discharge/charge current of 0.4 A⋅g−1 (Figure 3C). It can be seen that the TiO2/TiC@C membrane electrode is able to maintain a specific reversible capacity of 244 mA⋅h⋅g−1 after 1,600 cycles, with only 0.009% fading per cycle, which is ∼23.7% of that (0.038% fading per cycle) for the pristine TiO2@C membrane electrode at the same current density (Figure S16, Table S2). In addition, when an extremely long cycling test of 5,000 cycles is applied at a very high current density of 1.5 A⋅g−1, the TiO2/TiC@C membrane electrode can still exhibit outstanding cycling stability. After a slow capacity fading in the initial dozens of cycles, a reversible capacity of 150 mA⋅h⋅g−1 remains unchanged during the subsequent cycles, with coulombic efficiencies of nearly 100% (Figure 3C). Here, to clarify the effect of carbon on the performance of the TiO2/TiC@C composites, mesoporous carbon annealed at 900°C with a high surface area (806 m2g−1) and uniform pore size (4.0 nm) was synthesized and its electrochemical properties were measured (Figure S17). Compared with the TiO2/TiC@C composites, the mesoporous carbon shows a much lower capacity (∼70 mA⋅h⋅g−1 at a current of 1.5 A g−1, Figure S18) and initial coulomb efficiency (∼12.8%), which are only ∼46.7% and ∼48.4% of those of the TiO2/TiC@C electrode, respectively. Besides, the mesoporous carbon shows poor rate performance and the capacity delivered at specific current densities is ∼21%–61% of that of the TiO2/TiC@C composite electrode (Figure S18, Table S3). Furthermore, to clarity the effect of mass loading on the electrochemical performance, the mesoporous TiO2/TiC@C membrane electrode as thick as ∼30 μm was fabricated (Figure S19) and evaluated with related battery performance. It is found that the thicker TiO2/TiC@C membrane electrode exhibits high capacity and excellent cycling stability (Figure S20), comparable to that of the typical thin membrane electrode.
Nyquist plots for the TiO2/TiC@C membrane electrode (Figures 3D and 3E) exhibit a lower charge transfer resistance (Rct, 92 Ω) than those of the TiO2@C membrane electrode (175 Ω), which can lead to faster lithium-ion diffusion. The sheet resistances of the TiO2@C and TiO2/TiC@C membranes (Figure S21) are measured to be 22.62 and 1.37 kΩ⋅sq−1, respectively. To verify the effect of the mesoporous carbon component in the composites, the sheet resistance of the pristine mesoporous carbon (Figure S22) annealed at 700°C and 900°C was measured, and it was found to be 8.62 and 0.47 kΩ⋅sq−1, respectively. Therefore the enhanced electric conductivity of the composite membrane can be assigned to the enhanced graphitization of carbon at a higher annealing temperature and the existence of the TiC nanodots owing to the significantly higher conductivity of TiC (∼104 S⋅cm−1) than TiO2 (∼10−10 S⋅cm−1).
Assembly of a Flexible Full Cell
To solve the large Li consumption problem caused by SEI formation, the mesoporous TiO2/TiC@C membranes were pre-lithiated before being used as an anode material for the assembly of full battery (Figure S23). The optical images (Figures 3F, 3G, S24, and S25A, Video S1) illustrate that the flexible full battery can successfully be assembled and is able to power a light-emitting diode (LED) under both flat and bent states with different bent angles (0, 30, 60, 90, 120, and 150°). The charge/discharge curves of the battery cycled at a current density of ∼20 mA g−1 in the voltage window of 2.8–4.2 V is shown in Figure S24B. The battery exhibits good performance, and acceptable specific capacities of 116, 108, 103, 78, 62, and 53 mAh g−1 can be achieved with different bent angles of 0, 30, 60, 90, 120, and 150°, respectively. The results demonstrate the potential of the membranes in practical flexible electronics applications.
Electrochemical Kinetics Analysis
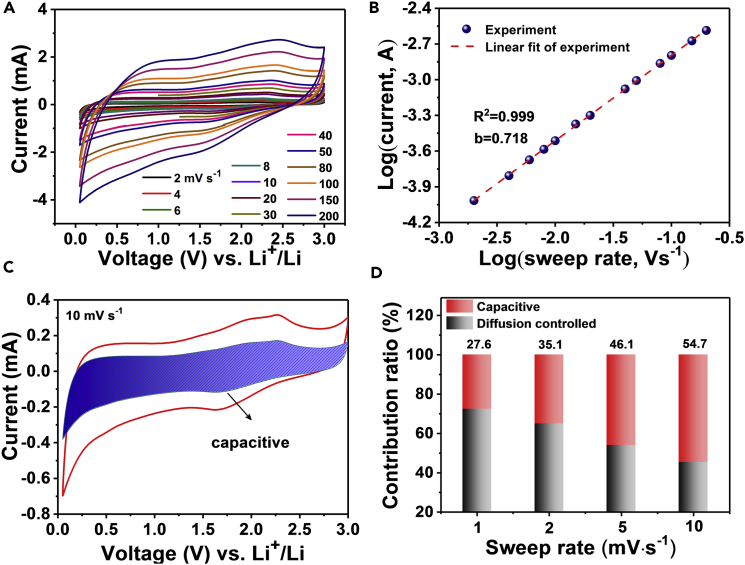
Kinetics analysis based on CV was carried out to gain further insight into the electrochemistry of the mesoporous TiO2/TiC@C membrane electrode. The CV curves of the TiO2/TiC@C membrane electrode at various scan rates from 2 to 200 mV⋅s−1 display similar shapes with broad peaks during both cathodic and anodic scans (Figure 4A). According to the relationship between the measured current (i) and the scan rate (v): i = avb, where a and b are adjustable values (Augustyn et al., 2013), b can be determined by the slope of the log(v)–log(i) plots. A b value of 0.718 can be obtained, indicating that both the capacitive and diffusion-controlled processes contribute to the total capacity of the TiO2/TiC@C membrane electrodes (Figure 4B). Quantitatively, 54.7% of the total charge (or capacity) is capacitive at a scan rate of 10 mV⋅s−1 (Figure 4C). Contribution ratios between the two different processes at other scan rates can also be quantified. The results show that the capacitance is improved gradually with increasing scan rate (Figure 4D). For the mesoporous carbon annealed at 900°C, the b value is calculated to be 0.705 (Figures S26 and S27), which is similar to that of the TiO2/TiC@C composite. However, the capacitive contribution of the mesoporous carbon (35.3%) at 10 mV⋅s−1 is lower than that of the TiO2/TiC@C composite. The higher capacitive contribution in the composite is probably caused by extra capacitive contribution of the TiO2 nanocrystals (Kim et al., 2010). The prominent pseudocapacitive behavior in the TiO2/TiC@C electrode is beneficial for fast charge storage and long-term cyclability.
Figure 4.
Electrochemical Kinetics Analysis
(A) CV curves at various scan rates from 2 to 200 mV s−1 of the mesoporous TiO2/TiC@C composite membrane electrodes.
(B) Log(i) versus log (v) plots of the cathodic current response at ∼ 2.05 V of the mesoporous TiO2/TiC@C membrane electrodes.
(C) Separation of the capacitive and diffusion currents in the mesoporous TiO2/TiC@C composite membrane electrodes at a scan rate of 10 mV s−1.
(D) Contribution ratio of the capacitive and diffusion-controlled charge versus scan rate. See also Figures S27 and S28.
Structural Evolution of Electrodes
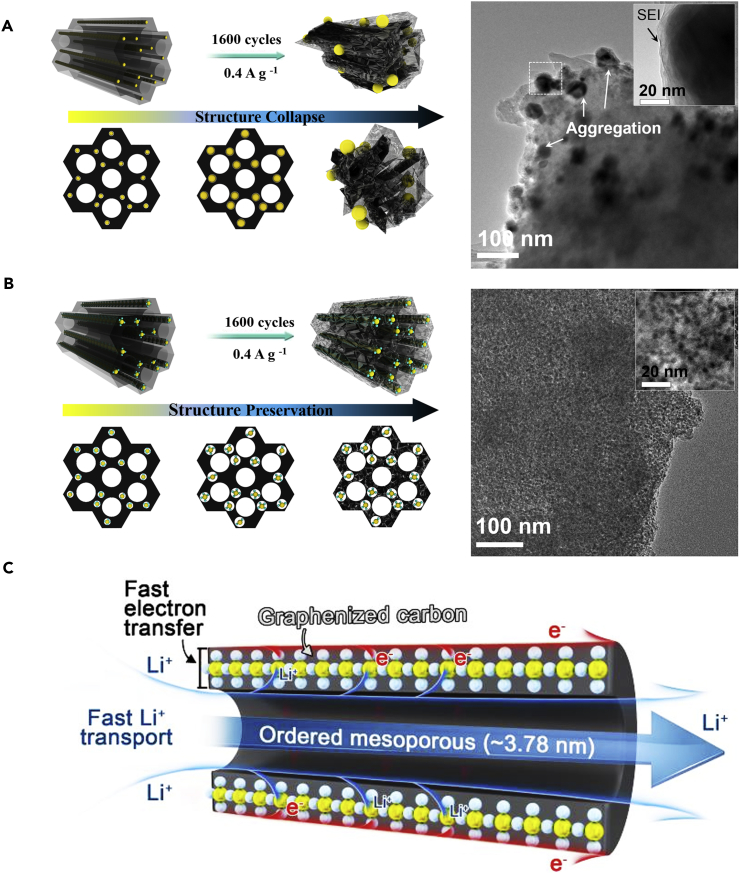
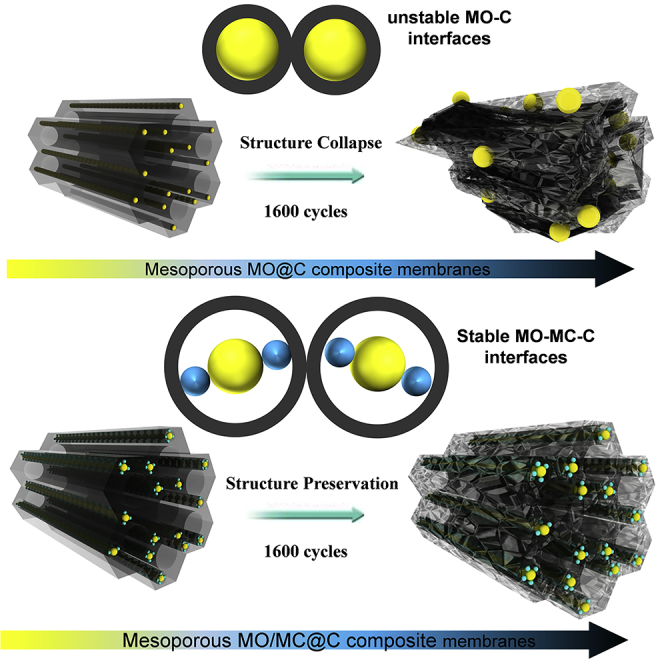
The TEM image of the mesoporous TiO2@C membrane electrode after 1,600 cycles at a current density of 0.4 A⋅g−1 (Figure 5A) shows that the size of TiO2 nanocrystals largely increases and the mesostructure is completely collapsed. This result clearly indicates that TiO2 nanocrystals got aggregated during Li+ insertion/extraction processes, which in turn results in the collapse of the mesoporous structure of the carbon matrix. Unfortunately, a thick SEI film is formed on the surface of the TiO2 nanocrystals, which would decrease the conductivity of the electrode. A similar structure damage is also observed in mesoporous carbon electrode after 1,000 cycles, where the ordered mesopores are totally distorted or even collapsed (Figure S28). However, it is found that the structure of the mesoporous TiO2/TiC@C composite membrane can still be well retained after 1,600 cycles. The TiO2 nanocrystals and TiC nanodots with a thin SEI film and size less than 5 nm are still dispersed uniformly in the mesoporous carbon matrix (Figures 5B and S29), which is almost the same as that before the cycling.
Figure 5.
Structural Evolution and Lithium Storage Mechanism
(A) Schematic illustrations of the structural evolution of mesoporous TiO2@C composite membrane electrodes upon cycling and the corresponding TEM image.
(B) Schematic illustrations of the structural evolution of the mesoporous TiO2/TiC@C composite membrane electrodes upon cycling and the corresponding TEM image.
(C) Charge-discharge mechanism for the mesoporous TiO2/TiC@C composite membrane electrodes as the high-performance lithium battery. See also Figure S29.
Mechanics Simulations
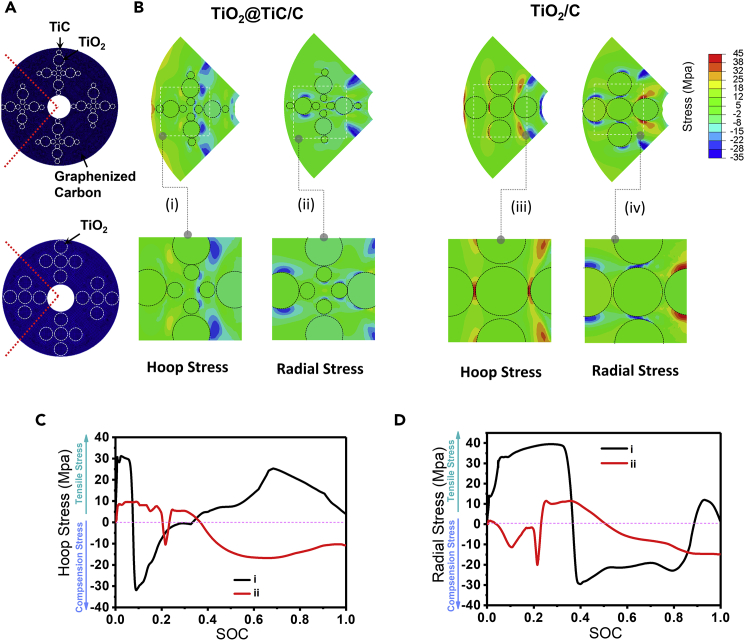
To deeply understand the origin of the anti-collapse property of the TiO2/TiC@C membrane electrode during the lithiation process, a simulation of elastic-plastic evolution coupled to Li diffusion was adopted to evaluate the lithiation-induced deformation and stress states. The simulated results for the TiO2@C and TiO2/TiC@C composites are shown (Figure 6). It should be noted that our diffusion simulations mainly serve to generate the deformation of the structures for the stress analyses, rather than provide a precise description of the dynamic lithiation process. The configurations of the TiO2@C and TiO2/TiC@C composites for simulations and stress evolution during lithiation process are shown (Figure 6A). The regions filled with different colors from blue to red signify different stress states of tensile stresses (quantitated with positive values) and compressive stresses (quantitated with negative values) (Figure 6B). The green region means stress-free state, the red region means high-magnitude tensile stress, and the colors between green and red regions are the stress-excessive boundary. Similarly, the blue region means high-magnitude compressive stress and the colors between green and blue regions are the stress-excessive boundary. During the process of lithiation, inside channels are filled with a constant Li-ion concentration and subjected to an invariant lithium flux (J0) inside the channels. The stress simulation (Figure 6C) reveals that, after the lithiation of the TiO2/TiC@C composite, when the state of charge value (SOC) (SOC = 0 represents the lithium-free state, and SOC = 1, the fully lithiated state) ranges from 0 to 0.1, a hoop maximum tensile stress (∼9.7 MPa) drastically generates at the TiO2-C interface. For the TiO2@C composite, a similar structural deformation occurs during lithiation. However, the maximum hoop tensile stress generated at the TiO2-C interface is ∼31.1 MPa, three times that of the TiO2-C interface of TiO2/TiC@C composite, which may lead to the structure collapse of the TiO2@C composite at the very beginning of the lithiation process. It is noted that when the SOC value is in the range of 0.1–0.4, the TiO2-C interface of the TiO2@C composite undergoes a higher maximum radial tensile stress (∼39.5 MPa) than that (∼11.5 MPa) of the TiO2-C interface of TiO2/TiC@C composite (Figure 6D), clearly demonstrating that the TiO2/TiC@C composite possesses higher structural stability due to the stable TiO2-C interface.
Figure 6.
Mechanics Simulations
(A) Finite element simulations model of the mesoporous TiO2@C and TiO2/TiC@C models.
(B) Stress distributions from finite element simulations of quarter of the mesoporous carbon frameworks during lithiation process. The steps (i) and (ii) and (iii) and (iv) correspond to radial and hoop stress values for the two mesoporous composites, respectively. Top panels depict the quarter of mesoporous carbon framework; bottom panels are enlarged views of the regions marked by rectangles in (i)–(iv).
(C and D) Evolution of hoop (C) and radial stress (D) in the TiO2@C (I, black curve) and TiO2/TiC@C (II, red curve) composites after lithiation.
Discussion
On the basis of substantial data and discussion, the possible mechanism for superior lithium storage performance over the mesoporous TiO2/TiC@C composite membrane electrodes has been proposed (Figure 5C). The stiff TiC nanodots attached on the surface of TiO2 nanocrystals can effectively avoid the aggregation of TiO2 nanocrystals and prevent the collapse of the composite frameworks, which provide a stable TiO2-C interface to withstand lithium-ion insertion and extraction, thus enabling a stable cycling performance. On the other hand, the highly conductive TiC nanodots serve as a bridge between the TiO2 nanocrystals and mesoporous carbon matrix to facilitate electron transfer, which is conducive to forming a thickness-reduced SEI film on the surface of the TiO2 nanocrystals, giving rise to an improved rate performance. Furthermore, the mesoporous structure of the membranes offers direct and short pathways for lithium-ion diffusion and electrolyte penetration, beneficial for fast and robust lithium storage. Accordingly, the mesoporous TiO2/TiC@C composite membranes exhibit outstanding lithium storage performance, such as high capacity, superior high-rate capability, and long cycling stability. Moreover, a flexible full battery can be assembled by using the TiO2/TiC@C membrane as an anode, highlighting the great potentials of the composite membranes in practical energy applications such as portable bendable electronics and flexible energy storage devices. We expect that this work can open an avenue to constructing interface-stable composite structures by introducing transition metal carbide nanodots with electrochemical inactivity and conductivity for the development of new-generation LIBs with both high power and long cycling life.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the State Key Basic Research Program of the PRC (2012CB224805, 2013CB934104, and 2017YFA0207300), Shanghai Science and Technology Committee (14JC1400700), NSF of China (Grant Nos. 21210004, 21603036, and 21273161), the Natural Science Foundation of Shanghai (Grant No. 17ZR1447800), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, Hundred Youth Talent Plan of Tongji University, and the Fundamental Research Funds for the Central Universities.
Author Contributions
D.Z proposed and supervised the project. W.Z., L.Z., J.Y. and D.Z. conceived the study and co-wrote the manuscript. W.Z. and L.Z carried out the synthesis and electrochemical evaluation. B.C. and L.Z. preformed the mechanics simulations. B.K., H.H., K.L., and Y.L. helped with material characterization and manuscript preparation. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no conflict of interests.
Published: May 25, 2018
Footnotes
Supplemental Information includes Transparent Methods, 29 figures, 3 tables, and 1 video and can be found with this article online at https://doi.org/10.1016/j.isci.2018.04.009.
Contributor Information
Jinhu Yang, Email: yangjinhu@tongji.edu.cn.
Dongyuan Zhao, Email: dyzhao@fudan.edu.cn.
Supplemental Information
References
- Allcorn E., Manthiram A. High-rate, high-density FeSb–TiC–C nanocomposite anodes for lithium-ion batteries. J. Mater. Chem. A. 2015;3:3891–3900. [Google Scholar]
- Angelomé P.C., Andrini L., Calvo M.E., Requejo F.G., Bilmes S.A., Soler-Illia G.J. Mesoporous anatase TiO2 films: use of Ti K XANES for the quantification of the nanocrystalline character and substrate effects in the photocatalysis behavior. J. Phys. Chem. C. 2007;111:10886–10893. [Google Scholar]
- Aricò A.S., Bruce P., Scrosati B., Tarascon J.-M., Van Schalkwijk W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005;4:366–377. doi: 10.1038/nmat1368. [DOI] [PubMed] [Google Scholar]
- Armand M., Tarascon J.-M. Building better batteries. Nature. 2008;451:652–657. doi: 10.1038/451652a. [DOI] [PubMed] [Google Scholar]
- Armstrong G., Armstrong A.R., Bruce P.G., Reale P., Scrosati B. TiO2(B) nanowires as an improved anode material for lithium-ion batteries containing LiFePO4 or LiNi0.5Mn1.5O4 cathodes and a polymer electrolyte. Adv. Mater. 2006;18:2597–2600. [Google Scholar]
- Augustyn V., Come J., Lowe M.A., Kim J.W., Taberna P.-L., Tolbert S.H., Abruña H.D., Simon P., Dunn B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013;12:518–522. doi: 10.1038/nmat3601. [DOI] [PubMed] [Google Scholar]
- Cai Z., Xu L., Yan M., Han C., He L., Hercule K.M., Niu C., Yuan Z., Xu W., Qu L. Manganese oxide/carbon yolk–shell nanorod anodes for high capacity lithium batteries. Nano Lett. 2014;15:738–744. doi: 10.1021/nl504427d. [DOI] [PubMed] [Google Scholar]
- Fang Y., Lv Y., Che R., Wu H., Zhang X., Gu D., Zheng G., Zhao D. Two-dimensional mesoporous carbon nanosheets and their derived graphene nanosheets: synthesis and efficient lithium ion storage. J. Am. Chem. Soc. 2013;135:1524–1530. doi: 10.1021/ja310849c. [DOI] [PubMed] [Google Scholar]
- Fang Y., Lv Y., Gong F., Elzatahry A.A., Zheng G., Zhao D. Synthesis of 2D-mesoporous-carbon/MoS2 heterostructures with well-defined interfaces for high-performance lithium-ion batteries. Adv. Mater. 2016;28:9385–9390. doi: 10.1002/adma.201602210. [DOI] [PubMed] [Google Scholar]
- Feng D., Lv Y., Wu Z., Dou Y., Han L., Sun Z., Xia Y., Zheng G., Zhao D. Free-standing mesoporous carbon thin films with highly ordered pore architectures for nanodevices. J. Am. Chem. Soc. 2011;133:15148–15156. doi: 10.1021/ja2056227. [DOI] [PubMed] [Google Scholar]
- Gu D., Li W., Wang F., Bongard H., Spliethoff B., Schmidt W., Weidenthaler C., Xia Y., Zhao D., Schüth F. Controllable synthesis of mesoporous peapod-like Co3O4@carbon nanotube arrays for high-performance lithium-ion batteries. Angew. Chem. Int. Ed. 2015;54:7060–7064. doi: 10.1002/anie.201501475. [DOI] [PubMed] [Google Scholar]
- Guan B.Y., Yu L., Li J., Lou X.W.D. A universal cooperative assembly-directed method for coating of mesoporous TiO2 nanoshells with enhanced lithium storage properties. Sci. Adv. 2016;2:e1501554. doi: 10.1126/sciadv.1501554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Huang C.-H., Gu D., Zhao D., Doong R.-A. Direct synthesis of controllable microstructures of thermally stable and ordered mesoporous crystalline titanium oxides and carbide/carbon composites. Chem. Mater. 2010;22:1760–1767. [Google Scholar]
- Huang H.-B., Yang Y., Chen L.-H., Wang Y., Huang S.-Z., Tao J.-W., Ma X.-T., Hasan T., Li Y., Xu Y. Hierarchical TiO2/C nanocomposite monoliths with a robust scaffolding architecture, mesopore–macropore network and TiO2–C heterostructure for high-performance lithium ion batteries. Nanoscale. 2016;8:10928–10937. doi: 10.1039/c5nr09149g. [DOI] [PubMed] [Google Scholar]
- Jeong J.M., Choi B.G., Lee S.C., Lee K.G., Chang S.J., Han Y.K., Lee Y.B., Lee H.U., Kwon S., Lee G. Hierarchical hollow spheres of Fe2O3@ polyaniline for lithium ion battery anodes. Adv. Mater. 2013;25:6250–6255. doi: 10.1002/adma.201302710. [DOI] [PubMed] [Google Scholar]
- Kaskhedikar N.A., Maier J. Lithium storage in carbon nanostructures. Adv. Mater. 2009;21:2664–2680. doi: 10.1002/adma.200901079. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Zhu K., Yan Y., Perkins C.L., Frank A.J. Microstructure and pseudocapacitive properties of electrodes constructed of oriented NiO-TiO2 nanotube arrays. Nano Lett. 2010;10:4099–4104. doi: 10.1021/nl102203s. [DOI] [PubMed] [Google Scholar]
- Kiran V., Sampath S. Enhanced Raman spectroscopy of molecules adsorbed on carbon-doped TiO2 obtained from titanium carbide: a visible-light-assisted renewable substrate. ACS Appl. Mater. Interfaces. 2012;4:3818–3828. doi: 10.1021/am300349k. [DOI] [PubMed] [Google Scholar]
- Kong B., Zu L., Peng C., Zhang Y., Zhang W., Tang J., Selomulya C., Zhang L., Chen H., Wang Y. Direct superassemblies of freestanding metal–carbon frameworks featuring reversible crystalline-phase transformation for electrochemical sodium storage. J. Am. Chem. Soc. 2016;138:16533–16541. doi: 10.1021/jacs.6b10782. [DOI] [PubMed] [Google Scholar]
- Larcher D., Tarascon J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015;7:19–29. doi: 10.1038/nchem.2085. [DOI] [PubMed] [Google Scholar]
- Liu L., Chen X. Titanium dioxide nanomaterials: self-structural modifications. Chem. Rev. 2014;114:9890–9918. doi: 10.1021/cr400624r. [DOI] [PubMed] [Google Scholar]
- Liu Y., Luo Y., Elzatahry A.A., Luo W., Che R., Fan J., Lan K., Al-Enizi A.M., Sun Z., Li B. Mesoporous TiO2 mesocrystals: remarkable defects-induced crystallite-interface reactivity and their in situ conversion to single crystals. ACS Cent. Sci. 2015;1:400–408. doi: 10.1021/acscentsci.5b00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Li W., Shen D., Zhao D., Wang G. Graphitic carbon conformal coating of mesoporous TiO2 hollow spheres for high-performance lithium ion battery anodes. J. Am. Chem. Soc. 2015;137:13161–13166. doi: 10.1021/jacs.5b08743. [DOI] [PubMed] [Google Scholar]
- Liu Y., Elzatahry A.A., Luo W., Lan K., Zhang P., Fan J., Wei Y., Wang C., Deng Y., Zheng G. Surfactant-templating strategy for ultrathin mesoporous TiO2 coating on flexible graphitized carbon supports for high-performance lithium-ion battery. Nano Energy. 2016;25:80–90. [Google Scholar]
- Mo R., Rooney D., Sun K., Yang H.Y. 3D nitrogen-doped graphene foam with encapsulated germanium/nitrogen-doped graphene yolk-shell nanoarchitecture for high-performance flexible Li-ion battery. Nat. Commun. 2017;8:13949. doi: 10.1038/ncomms13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C., Chen B., Qin Y., Yang S., Li C., Zuo Y., Liu S., Yang J. Facile ultrasonic synthesis of CoO quantum dot/graphene nanosheet composites with high lithium storage capacity. ACS Nano. 2012;6:1074–1081. doi: 10.1021/nn202888d. [DOI] [PubMed] [Google Scholar]
- Peng H.J., Zhang G., Chen X., Zhang Z.W., Xu W.T., Huang J.Q., Zhang Q. Enhanced electrochemical kinetics on conductive polar mediators for lithium–sulfur batteries. Angew. Chem. Int. Ed. 2016;55:12990–12995. doi: 10.1002/anie.201605676. [DOI] [PubMed] [Google Scholar]
- Shan Z., Archana P.S., Shen G., Gupta A., Bakker M.G., Pan S. NanoCOT: low-cost nanostructured electrode containing carbon, oxygen, and titanium for efficient oxygen evolution reaction. J. Am. Chem. Soc. 2015;137:11996–12005. doi: 10.1021/jacs.5b05367. [DOI] [PubMed] [Google Scholar]
- Su Q., Xie D., Zhang J., Du G., Xu B. In situ transmission electron microscopy observation of the conversion mechanism of Fe2O3/graphene anode during lithiation–delithiation processes. ACS Nano. 2013;7:9115–9121. doi: 10.1021/nn403720p. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang N., Tang H., Dong Z., Jin Q., Yang M., Kisailus D., Zhao H., Tang Z., Wang D. Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew. Chem. Int. Ed. 2013;52:6417–6420. doi: 10.1002/anie.201301622. [DOI] [PubMed] [Google Scholar]
- Wang D., Yu Y., He H., Wang J., Zhou W., Abruna H.D. Template-free synthesis of hollow-structured Co3O4 nanoparticles as high-performance anodes for lithium-ion batteries. ACS Nano. 2015;9:1775–1781. doi: 10.1021/nn506624g. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yu L., Lou X.W.D. Formation of triple-shelled molybdenum–polydopamine hollow spheres and their conversion into MoO2/carbon composite hollow spheres for lithium-ion batteries. Angew. Chem. Int. Ed. 2016;55:14668–14672. doi: 10.1002/anie.201608410. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang Y., Ang H., Zhang Y., Tan H.T., Zhang Y., Guo Y., Franklin J.B., Wu X.L., Srinivasan M. A high-energy lithium-ion capacitor by integration of a 3D interconnected titanium carbide nanoparticle chain anode with a pyridine-derived porous nitrogen-doped carbon cathode. Adv. Funct. Mater. 2016;26:3082–3093. [Google Scholar]
- Yao Y., Huo K., Hu L., Liu N., Cha J.J., McDowell M.T., Chu P.K., Cui Y. Highly conductive, mechanically robust, and electrochemically inactive TiC/C nanofiber scaffold for high-performance silicon anode batteries. ACS Nano. 2011;5:8346–8351. doi: 10.1021/nn2033693. [DOI] [PubMed] [Google Scholar]
- Yu T., Deng Y., Wang L., Liu R., Zhang L., Tu B., Zhao D. Ordered mesoporous nanocrystalline titanium-carbide/carbon composites from in situ carbothermal reduction. Adv. Mater. 2007;19:2301–2306. [Google Scholar]
- Yu L., Wu H.B., Lou X.W.D. Self-templated formation of hollow structures for electrochemical energy applications. Acc. Chem. Res. 2017;50:293–301. doi: 10.1021/acs.accounts.6b00480. [DOI] [PubMed] [Google Scholar]
- Yu L., Hu H., Wu H.B., Lou X.W.D. Complex hollow nanostructures: synthesis and energy-related applications. Adv. Mater. 2017 doi: 10.1002/adma.201604563. [DOI] [PubMed] [Google Scholar]
- Zeng L., Zheng C., Xia L., Wang Y., Wei M. Ordered mesoporous TiO2–C nanocomposite as an anode material for long-term performance lithium-ion batteries. J. Mater. Chem. A. 2013;1:4293–4299. [Google Scholar]
- Zhang R., Elzatahry A.A., Al-Deyab S.S., Zhao D. Mesoporous titania: from synthesis to application. Nano Today. 2012;7:344–366. [Google Scholar]
- Zhang R., Du Y., Li D., Shen D., Yang J., Guo Z., Liu H.K., Elzatahry A.A., Zhao D. Highly reversible and large lithium storage in mesoporous Si/C nanocomposite anodes with silicon nanoparticles embedded in a carbon framework. Adv. Mater. 2014;26:6749–6755. doi: 10.1002/adma.201402813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.