Abstract
Purpose of the Study
We examined the effect of daily stress, affect, and adult day service (ADS) use on the daily pain experience among caregivers of individuals with dementia (IWD). Participants were interviewed for 8 consecutive days. Caregivers utilized an ADS program on some days and provided care at home on other days. We hypothesized ADS use, care-related and noncare-related subjective stress, and affect would significantly influence and interact in ways to exacerbate or buffer the experience of daily pain.
Design
Participants were 173 family caregivers of IWDs using ADS more than 2 days per week. Participants with IWDs diagnosed with “mild cognitive impairment” were excluded. Daily telephone interviews assessed stress, affect, and pain.
Methods
Multilevel models were used to examine the relation between daily stress and daily pain and interaction effects of other daily experiences within the context of ADS use.
Results
Multilevel models revealed a significant relation between care-related subjective stress and daily bodily pain as well as an interaction between noncare-related subjective stress and daily bodily pain. ADS use and affect did not predict daily pain. Lagged effects revealed a significant interaction between yesterday’s ADS use and today’s positive affect on today’s bodily pain.
Implications
Findings suggest that further studies are warranted for understanding and controlling pain among caregivers. Addressing the physical health needs through pain management interventions, positive affect maximization, and ADS use may improve the overall wellbeing of caregiving dyads.
Keywords: Caregiving–informal, Dementia, Adult day care, Caregiver stress
The population of individuals caring for someone with age-related disorders such as Alzheimer’s disease and dementia is increasing at a rapid rate (Alzheimer’s Association, 2015; Hebert, Weuve, Scherr, & Evans, 2013). Past research has identified many psychological factors associated with the caregiver role, including elevated stress levels, higher incidence of depression, and decreased quality of life (Clyburn, Stones, Hadjistavropoulos, & Tuokko, 2000; Pinquart & Sörensen, 2003; Schulz & Martire, 2004). These psychological factors are also associated with pain (Affleck, Tennen, Urrows, & Higgins, 1991). Recently, caregiver pain has been identified as a significant predictor of the emotional and physical aspects of caregiver burden in informal caregivers caring for someone with Alzheimer’s disease or dementia or characterized as physically frail or disabled (Jones, Hadjistavropoulos, Janzen, & Hadjistavropoulos, 2011); however, daily determinants for pain have yet to be investigated. These determinants have also not been examined among dementia caregivers specifically.
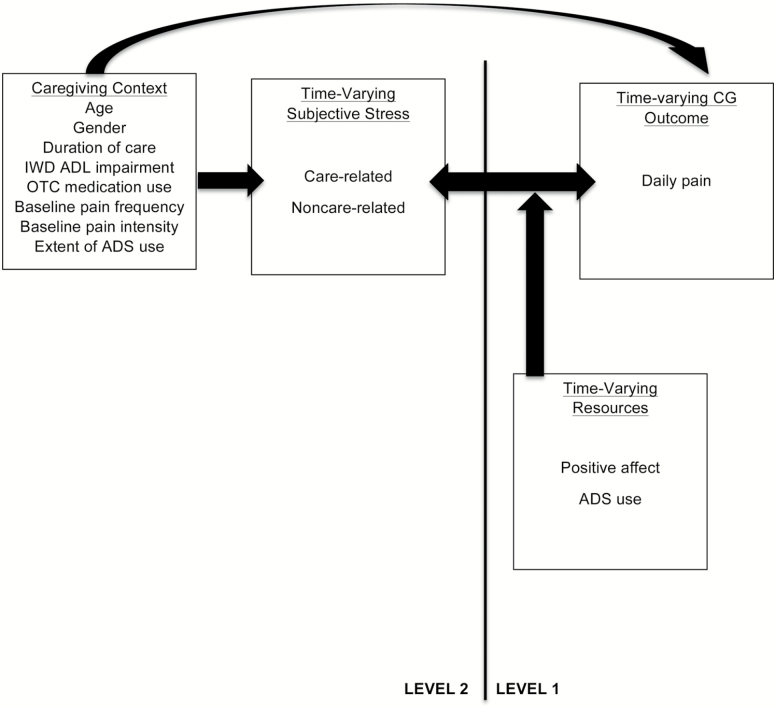
Figure 1 illustrates a condensed version of Pearlin’s stress process model (SPM; Pearlin, Mullan, Semple, & Skaff, 1990) adapted to include time-varying moderators and time-varying outcomes to account for daily associations and fluctuations of the caregiver experience. Pain has been incorporated here as an outcome of the stress process that has not been investigated among dementia caregiver populations within a daily context. To effectively target aspects of the caregiver experiences that are difficult and challenging, daily pain must be understood as it relates to all other aspects associated with the stress process (Figure 1).
Figure 1.
The conceptual model on daily stress and pain in the caregiving context (Adapted from Pearlin et al.’s [1990] SPM).
Daily Pain and Stress
Daily events have immediate and cumulative effects that influence the caregivers’ health and wellbeing. In some instances, this can result in overload and subsequent adverse physical and emotional outcomes (Yates, Tennstedt, & Chang, 1999) thereby threatening the caregiver’s ability to be resilient. This may decrease quality of life for both the caregiver and the care recipient (Everhart, Fiese, & Smyth, 2008). Care-related stressors have been identified as a significant predictor of caregiver depression and negative affective responses (Ornstein et al., 2013). This may result in other negative outcomes such as daily pain. Nearly 20%–25% of caregivers are over the age of 65 and experience pain often (Alecxih, Zeruld, & Olearczyk, 2001; Shahly et al., 2013). Jones and colleagues (2011) reported that overall pain significantly predicted levels of emotional and physical dimensions of caregiver burden in informal caregivers. They did not consider daily pain fluctuations or time-varying predictors of pain within dementia caregiver populations. Moreover, this study did not include a measure of stress, specifically, and it also did not delineate between care- and noncare-related subjective stress, highlighting a significant gap in the current literature. Although reported stress and pain have yet to be investigated among dementia caregivers, there is extensive evidence to provide a robust rationale for the importance of understanding how they are related. For example, there is evidence that catastrophizing, and its components, magnification, rumination, and helplessness, operate much like other coping mechanisms (Geisser, Robinson, & Riley, 2000; Parker et al., 1989; Sullivan et al., 2001) in their effects on the affective and sensory components of the pain experience. An individual’s stress appraisal and subsequent coping response are well-established determinants of the pain experience; thus, it is conceivable that reported care- and noncare-related subjective stress may contribute to the daily caregiving pain experience.
ADS Use and Biomarkers
Previous studies suggest the utilization of adult day services (ADS) and other programs providing respite care alleviates exposure to care-related stressors by as much as 40% (Zarit, Stephens, Townsend, & Greene, 1998; Zarit et al., 2011). Emerging studies have suggested that stress is lower for those who utilize ADS as they do not provide all the care, and stress is lowest in the evening following ADS attendance with effects lasting through the night compared to caregivers who provided all the care (Zarit et al., 2011). Past studies have further suggested ADS use induces increases in levels of dehydoepiandrosterone-sulfate (D-HEAS), a biomarker of the hypothalamic–pituitary–adrenal axis (HPA) of stress reactivity responsive to the presence of acute and chronic stressors on days following ADS use (Zarit, Whetzel, et al., 2014). Higher levels of D-HEAS are protective against effects of stressor exposure (Lennartsson, Theorell, Kushnir, Bergquist, & Jonsdottir, 2013). Those who utilize ADS experience lagged effects such that D-HEAS levels and positive mood are higher on days following ADS use (Zarit, Whetzel, et al., 2014). This variability in the effects of ADS on stressor exposure and subjective stress underscores the need to understand day-to-day associations within a varying context to fully understand the caregiver experience.
Need for Study
The present study extends this prior work by examining daily influences on pain among dementia caregivers. It further assesses potential daily interaction effects of affect and type of day (ADS/non-ADS; Figure 1). Examining daily associations and interactions allows us to more accurately assess caregivers’ response on both high and low subjective stress days and how this fluctuates based on ADS use. More specifically, we can examine whether daily pain outcomes are predicted by individual clustering of person-specific characteristics such as baseline pain and daily level of noncare- and care-related stress or to characteristics associated with ADS use. This study will examine the following hypotheses. First, we hypothesized daily reports of bodily pain would be lower on ADS days than non-ADS days. Second, we hypothesized that daily care-related and noncare-related subjective stress would predict daily pain reports. Third, we hypothesized that positive affect and negative affect would individually influence the association between care- and noncare-related subjective stress and daily pain reports. Fourth, we decided to investigate in exploratory fashion whether positive affect today would interact with ADS use yesterday to influence the experience of pain today.
Methods
Participants
The participants were 173 (86.5% of eligible participants) family caregivers of individuals with dementia (IWDs) using ADS programs who were enrolled in Daily Stress and Health (DaSH) Study. To take part in the study, participants had to be related to the IWD, live in the same household, and indicate their primary responsibility, operationally defined as spending the most time helping the IWD with daily tasks. The IWD had to have been diagnosed by a physician as having a type of dementia (e.g., Alzheimer’s disease, frontotemporal dementia) and must have been scheduled to attend ADS more than 2 days per week. Participants with relatives diagnosed with “mild cognitive impairment” or other predementia syndromes (e.g., age related cognitive decline) were excluded.
Procedure
In the primary study, ADS programs were identified through their regional state associations in five areas: Northern and Central New Jersey, the greater Philadelphia area, the greater Pittsburgh area, Northern Virginia, and Denver, Colorado. Meetings were conducted with ADS representatives to explain the study and provide informational fliers to display for potential participants with the research coordinator’s contact information. Announcements were also placed in ADS program newsletters, and reminder and study updates were given to ADS staff. A total of 57 programs provided referrals over a 3-year recruitment period.
Family caregivers who contacted the research coordinator were told about the study and screened for eligibility. Eligible caregivers were scheduled for an initial in-person interview. The interviewer obtained signed informed consent and gathered sociodemographic information and baseline data on a number of measures. The Penn State Survey Research Center conducted daily interviews. Participants received $25 for completing the initial interview and $50 for completing the eight daily interviews.
Measures
Daily Bodily Pain
Daily pain was measured by a single item from Larsen and Kasimatis’ Symptom checklist (Larsen & Kasimatis, 1991). Caregivers indicated how often they experienced headache, backache, or muscle soreness in the past day, using a 5-point scale that ranged between 1 (none of the day) and 5 (all day). This is an atypical measure of pain as single item measures of pain are generally used within the context of chronic pain conditions (e.g., rheumatoid arthritis, sickle cell disease; Affleck et al., 1999; Smith et al., 2008). This is, however, a novel outcome to consider among dementia caregivers; thus, the findings will be useful and potentially inform future daily pain assessments outside of chronic pain conditions.
Type of Day
Type of day, that is, whether the IWD used ADS (=1) or did not use ADS (=0), was confirmed at the end of each day during the telephone interview.
Extent of ADS Use
The total number of ADS days over the course of 8 consecutive days was summed.
Daily Affect
Daily positive and negative affect was assessed using an adapted inventory from the Non-Specific Psychological Distress Scale (Kessler et al., 2002; Mroczek & Kolarz, 1998). The 22-item scale assesses four affective domains relevant to caregivers: anxiety symptoms, anger, depressive symptoms, and positive affect. Positive affect was supplemented with two items (interested, attentive) from the Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988) to create a broader assessment of positive emotions. Caregivers reported the frequency of each emotion over the past day along a 5-point scale from 1 (none of the day) to 5 (all day).
A factor analysis was performed that replicated the four affective domains. Four items were dropped because they did not load on any scale or loaded approximately equally on two or more domains. The final scales in the primary study included the following: anxiety symptoms (three items, α = .84), anger (four items, α = .83), depressive symptoms, (four items, α = .84), and positive affect (nine items, α = .92). The four scales represent dimensions usually included in models of affect: negative affect scores were composed of high-activation negative emotion (anger symptoms), low-activation negative emotion (depressive symptoms), trait-related negative emotion (anxiety symptoms), and positive affect (Watson & Tellegen, 1985).
Care-Related Subjective Stress
Care-related subjective stress was measured using a 19-item version of the Daily Record of Behavior (DRB; Fauth, Zarit, Femia, Hofer, & Stephens, 2006; Femia, Zarit, Stephens, & Greene, 2007) drawn from six behavioral categories: resistance to help with activities of daily living (ADL), restless behaviors, reality problems, depressive behaviors, disruptive behaviors, and memory related behaviors. Up to three other behavioral events related to care could be added by caregivers. In daily diaries, days were divided into four periods: waking to 9:00 a.m., 9:00 a.m. to 4:00 p.m., 4:00 p.m. to bedtime, and overnight. For each period, each day, caregivers were asked if a behavior had occurred, and if yes, to rate the subjective stress severity of the behavior along a 5-point scale ranging from 1 (not at all stressful) to 5 (very stressful). A care-related stress severity score was computed by summing the stress ratings for all behaviors for each day that would include four time periods; (a) the night before, (b) waking to 9:00 a.m., (c) 9:00 a.m. to 4:00 p.m., and (d) 4:00 p.m. to bedtime that same day. A zero was assigned if no behavior occurred.
Noncare-Related Subjective Stress
Noncare-related subjective stress was assessed through the Daily Inventory of Stressful Events (DISE, Almeida, 1998; Almeida, Wethington, & Kessler, 2002). Each day, caregivers reported whether each of the eight items had occurred. They were instructed to report stressful events not related to or encountered while assisting their IWD. Items included arguments with other people, avoiding an argument, stressors affecting friends or family, health-related issues, financial issues, work-related events, or any other incidents. Caregivers then rated the subjective stress severity of each event on a 5-point scale ranging from 1 (not at all stressful) to 5 (very stressful). A noncare-related stress severity score was computed by summing the stress ratings for each event for that day. A zero was assigned if no stressful event was reported.
Covariates
We included age, gender, duration of care, IWD ADL impairment (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963; Lawton & Brody, 1969), caregiver over-the-counter (OTC) medication use, and baseline reports of bodily pain (frequency and interference; Ware & Sherbourne, 1992) as covariates. OTC medication use was measured by asking participants if they were taking OTC medication for each of the following; headaches, stomach/gastrointestinal problems, sleep problems, anxiety, tension, or depression, to improve memory, or for any other reason. A sum was then calculated for each individual. Race was not included as it was not significantly associated with daily bodily pain reports and the sample was 73% Caucasian.
Analysis
A random intercepts two-level multilevel model (SAS PROC MIXED) was conducted using daily diary data nested within persons (Littell, Milliken, Stroup, & Wolfinger, 1996) to examine daily pain experiences among caregivers. Some of the days were ADS days whereas the others were non-ADS days when caregivers took active care of the IWDs. This is the suggested approach by Hoffman and Stawski (2009). Restricted maximum likelihood (REML) was used for estimation, which is more preferable for models when comparing random intercepts models (Raudenbush & Bryk, 2002). All predictors in the Level 1 equation except ADS day were person-mean centered. ADS day was not centered because it is a dummy variable and centering would complicate interpretation. All predictors in the Level 2 equation were grand-mean centered.
To assess Hypothesis 1, Model 1 was fit for daily pain on the dth day for the ith person as a function of an intercept ( , the average score on non-ADS days), ADS use ( ), and the person-specific deviations from the intercept (εid) in Level 1 (within-person) equation. At Level 2, we included the following eight between-person covariates: caregiver’s age, gender, duration of care, IWD’s ADL impairment, OTC medication use, baseline pain frequency and interference, and extent of ADS use to control for their effects on the average daily bodily pain. Model 1 was specified as the following:
Level 1:
Level 2:
To examine the effects of care-related and noncare-related subjective stress on the daily experience of pain (Hypothesis 2), Model 2 was fit by adding the main effects of care-related subjective stress ( ), noncare-related subjective stress ( ), and ADS use ( as well as the interactions between ADS use and care-related stress ( ), and ADS and noncare-related stress ( ) as the predictors in the Level 1 model. At Level 2, we included person averages of care- and noncare-related stress along with the same set of between-person covariates as in Model 1. Model 2 was specified as the following:
Level 1:
Level 2:
To test the moderating effects of ADS use and daily affect (Hypothesis 3) on daily pain reports, Model 3 was fit by including the main effects of care- ( ) and noncare-related stress ( ), ADS use ( ), and positive ( ) and negative affect ( ), and four interaction terms ( ) between two types of daily affect and two types of subjective stress in the Level 1 (within-person) model. At Level 2, we included the same set of between-person covariates as in Model 2. Model 3 was specified as the following:
Level 1:
Level 2:
We further modeled the lagged effects of ADS use and positive affect on daily pain (exploratory Hypothesis 4, Model 4). Yesterday’s ADS use ( ), today’s positive affect ( ), and their interaction were entered in the Level 1 equation in Model 4. In the level-2 equation, we included the same set of between-person covariates as in Model 1. Model 4 was specified as the following:
Level 1:
Level 2:
Results
Characteristics of caregivers are shown in Table 1. Preliminary analysis with the empty model revealed an intraclass correlation (ICC) of .35, indicating 35% of the variance in the daily bodily pain was at the between-person level. Model 1 revealed that daily pain reports did not differ across type of day (i.e., ADS or non-ADS day, β 01 = 0.05, p = .247). Therefore, Hypothesis 1 was not supported. Table 2 shows within-person means. Table 3 shows model parameter estimates for full models addressing all tested hypotheses as well as parameter estimates from the trimmed models.
Table 2.
Daily Stress, Affect, and Pain of Family Caregivers (N = 173)
| Variable | Min | Max | M | SD |
|---|---|---|---|---|
| Care-related subjective stressa | 76 | 266 | 88.31 | 21.37 |
| Noncare-related subjective stressa | 8 | 31 | 10.75 | 3.49 |
| Positive affect | 1 | 5 | 3.02 | 0.95 |
| Daily Pain | 1 | 5 | 2.18 | 1.24 |
Note: Negative affect was excluded as it was omitted from the analyses.
aSummed scores for care and noncare-related subjective stress; rated on a 5-point scale ranging from 1 (not stressful at all) to 5 (very stressful). Care-related subjective stress ratings were given for each day for four time periods; (a) the night before, (b) waking to 9:00 a.m., (c) 9:00 a.m to 4:00 p.m., and (d) 4:00 p.m. to bedtime that same day.
Table 3.
Full and Trimmed Model Parameters
| Model 1 | Model 2 | Model 2 | Model 3 | Model 3 | Model 4 | Model 4 | |
|---|---|---|---|---|---|---|---|
| (full) | (trimmed) | (full) | (trimmed) | (full) | (trimmed) | ||
| Within-person fixed effects | |||||||
| Intercept | 2.17*** | 1.90*** | 2.21*** | 1.83*** | 2.25*** | 1.89*** | 2.25*** |
| ADS (today), β01 | 0.05 | 0.06 | 0.07 | 0.06 | |||
| Care subjective stress, β01 | 0.01*** | 0.01*** | 0.01** | 0.01*** | |||
| Noncare subjective stress, β02 | 0.02 | 0.02 | 0.04*** | 0.04*** | |||
| Positive effecta | −0.05 | −0.35*** | −0.35*** | ||||
| Negative effect, β05 | 0.07 | ||||||
| ADS × Care subjective stress, β04 | 0.00 | ||||||
| ADS × Noncare subjective stress, β05 | 0.04* | 0.04* | |||||
| Positive affect × Care subjective stress, β06 | 0.01 | ||||||
| Positive affect × Noncare subjective stress, β07 | 0.00 | ||||||
| Negative affect × Care subjective stress, β08 | 0.01 | ||||||
| Negative affect × Noncare subjective stress, β09 | −0.04 | ||||||
| ADS (yesterday), β01 | −0.06 | −0.05 | |||||
| ADS (yesterday)*Positive affect, β 03 | 0.33** | 0.33** | |||||
| Between-person fixed effects | |||||||
| Caregiver age, β10 | −0.01 | 0.00 | 0.00 | −0.01 | |||
| Caregiver gender, β20 | −0.26 | −0.29 | −0.35 | −0.10 | |||
| Duration of care, β30 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| IWD ADL impairment, β40 | 0.12 | 0.07 | 0.08 | 0.11 | |||
| OTC medication use, β50 | 0.09 | 0.09 | 0.07 | 0.11 | |||
| Caregiver pain frequency, β60 | 0.35*** | 0.29*** | 0.31*** | 0.30*** | 0.38*** | 0.34*** | 0.35*** |
| Caregiver pain interference, β70 | 0.22** | 0.14** | 0.17** | 0.10 | 0.18** | 0.17 | |
| Extent of ADS use, β80 | 0.03 | 0.09 | 0.09 | ||||
| Care subjective stress, β90 | 0.01 | 0.01 | |||||
| Noncare subjective stress, β100 | 0.08** | 0.10** | 0.05 | ||||
| Positive affect, β110 | −0.11 | ||||||
| Negative affect, β120 | 0.38 | ||||||
| Random effects | |||||||
| σ2 | 0.56*** | 0.53*** | 0.53*** | 0.53*** | 0.54*** | 0.50*** | 0.50*** |
| τ00 | 0.72*** | 0.66*** | 0.68*** | 0.64*** | 0.77*** | 0.77*** | 0.78*** |
| BIC | 3289.88 | 3114.39 | 3078.48 | 3130.69 | 3082.86 | 2683.88 | 2665.16 |
aPositive affect was estimated by β04 in Model 3 and β02 in Model 4.
**p < .01. ***p < .001.
Table 1.
Caregiver Characteristics (N = 173)
| Variable | M or freq | SD or % | Range |
|---|---|---|---|
| Age | 61.97 | 10.66 | 39–89 |
| Educationa | 4.46 | 1.20 | 1–6 |
| Incomeb | 6.68 | 3.10 | 1–11 |
| Duration of carec | 61.12 | 45.55 | 3–264 |
| Female (=1) | 151 | 87% | 0–1 |
| Relation to IWD | |||
| Spouse (=1) | 66 | 38% | 0–1 |
| Child (=1) | 100 | 58% | 0–1 |
| Other (=1) | 7 | 4% | 0–1 |
| White (=1) | 126 | 73% | 0–1 |
| Married (=1) | 119 | 69% | 0–1 |
| Employed (yes = 1) | 73 | 42% | 0–1 |
| Number of daily interview days | 7.86 | 0.65 | 3–8 |
| Number of ADS days | 4.09 | 1.46 | 1–6 |
| Number of non-ADS days | 3.77 | 1.43 | 2–7 |
Note: ADS = adult day services; IWD = individual with dementia.
aRated on a 6-point scale ranging from 1 (less than high school) to 6 (post-college degree). bRated on a 11-point scale ranging from 1 (less than $10,000) to 11 (100,000 or over). cMeasured in months.
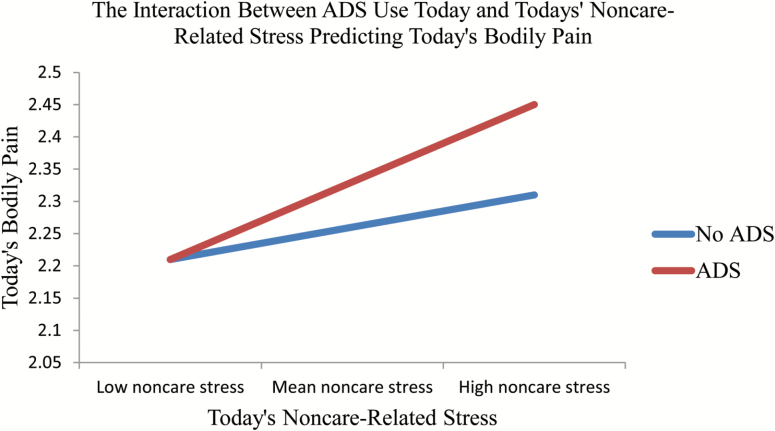
To test Hypothesis 2, we constructed Model 2 to examine the potential main effects and interactions of type of day and care- and noncare-related subjective stress on the daily experience of pain. Care-related subjective stress , p = .000) was associated with higher self-reported bodily pain for that day. In other words, for each one-unit increase in care-related subjective stress, there was a 0.01 increase in daily pain. Findings revealed a significant interaction between ADS use and noncare-related subjective stress ( p = .040). Bodily pain was higher on ADS days when noncare-related stress was high (Figure 2). To test Hypothesis 3, we constructed Model 3 to examine the main effects and interactions of positive and negative affect and type of stressors on daily pain. There were no significant findings with regard to these effects.
Figure 2.
The interaction between today’s ADS use and today’s noncare-related stress predicting bodily pain.
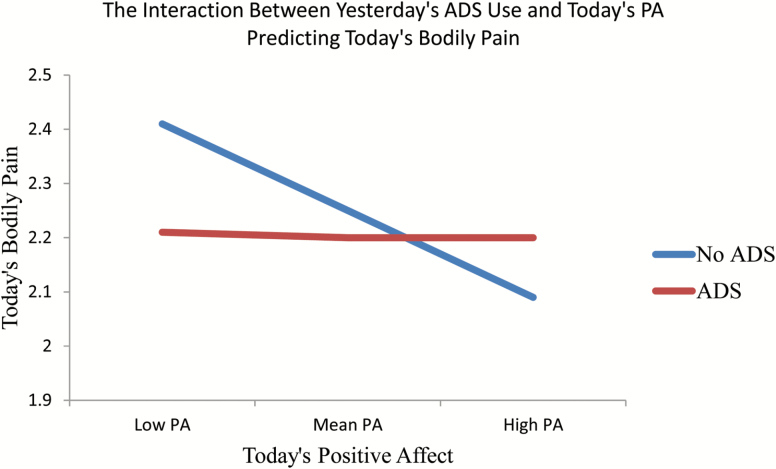
To examine Hypothesis 4, we constructed Model 4 to explore the interaction effects between today’s positive affect and yesterday’s ADS use on today’s daily pain. Model 4 revealed significant decreases in pain following ADS use if today’s positive affect was approximately one standard deviation higher than average,( 0.33, p = .002, Figure 3).
Figure 3.
The interaction between yesterday’s ADS use and today’s PA predicting bodily pain.
Discussion
To our knowledge, this is the first study to examine contributing factors to the daily reporting of bodily pain within the context of ADS use among dementia caregivers. The findings extend prior research by revealing care- and noncare-related subjective stress influence the daily experience of bodily pain. Despite prior findings that ADS use reduces the occurrence of care-related subjective stress and increases next day positive affect (Zarit, Kim, et al., 2014), ADS use does not yield significant decreases in same day bodily pain. However, results revealed that when positive affect is higher, and ADS were utilized the previous day, there were significant decreases in bodily pain. These findings underscore the importance of providing respite, which lowers exposure to care-related stressors, and stress management skills that can assist caregivers in managing daily pain.
We expected that utilizing ADS might provide respite and time for caregivers to relax and engage in other needed or meaningful activities. Instead, noncare-related subjective stress was elevated on ADS days. As indicated by the interaction of noncare-related stress and type of day on daily pain, when this stress was higher on an ADS day than an individual’s mean score, daily pain was also higher. The increase in the occurrence of noncare-related stressors was primarily due to events at work and family interactions (Zarit, Kim, et al., 2014). Other events that become possible for caregivers when care recipients are at ADS, such as gardening or house cleaning, which are not experienced as stressful, may contribute to increased pain. In sum, while ADS may alleviate care-related stress, caregivers then have more time to engage in noncare-related activities that are stressful and in enjoyable physical activities that might exacerbate the daily experience of pain.
Results also suggest that positive affect may be an important protective factor for pain. Whereas ADS use has a same day effect on negative emotions (Zarit, Kim, et al., 2014), it has a lagged effect on next day’s positive affect. The finding is consistent with Finan and Garland’s (2015) upward spiral model of positive affect, which posits that positive affect buffers maladaptive responses to pain. This is also consistent with prior findings that levels of DHEA-S were higher on days after ADS use and were significantly correlated with positive affect (Zarit, Whetzel, et al., 2014). Studies have found that DHEA-S is depleted in prolonged stress situations (Izawa, Saito, Shirotsuki, Sugaya, & Nomura, 2012; Lennartsson et al., 2013). It is possible that that relief from stressors provided by an ADS use day has a restorative effect on DHEA-S, which contributes to the lagged effects on positive affect. Additionally, a main source of noncare-related stress, like work, can also lead to positive experiences and contribute to improved positive affect the next day. Likewise, while engaging in strenuous hobbies or other leisure activities on ADS days could lead to increased pain, they also may contribute to positive affect. In future studies, it would be ideal to examine the combined effects of ADS use, noncare-related stress, and positive affect. Untangling these processes would shed light on whether interventions targeting caregivers’ appraisals of noncare-related stressors or targeting maximization of positive affect in the face of those stressors would be a more effective avenue for intervention.
Taken together, these findings are encouraging, as they identify future targets for intervention (e.g., stress management, positive affect maximization) beyond those addressed by respite alone. This has notable implications as policy makers are concerned with long-term benefits of ADS use and other respite programs. The National Family Caregiver Support Program (NFCSP) provides grants to states and territories to assist family and informal caregivers. Five types of service are covered, including providing information about available services, facilitating access to those services, counseling and support groups, caregiver training, and respite (National Family Caregiver, 2017). Caregivers might have to postpone adequately addressing other sources of stress when they are actively providing care. If caregivers are not using respite services for respite, but rather to confront other equally distressing responsibilities or relationships, chances for burnout and other negative physical and mental health outcomes may increase.
Although pain is not directly addressed by ADS use, it may be indirectly influenced. While respite services or caregiver-specific training might be effective in relieving aspects of care-related stress, it increases the likelihood of experiencing more noncare-related stressor exposure exacerbating the experience of pain. As a result, it might be beneficial for ADS and other respite programs to consider offering supplemental programs or workshops for general stress management, pain management, or financial strain management, and other stressful aspects not related to care. These findings also suggest the addition of specific intervention components that target pain and emphasize positive affect maximization, such as acceptance and commitment therapy (ACT; Hayes, Luoma, Bond, Masuda, & Lillis, 2006), cognitive behavioral therapy (CBT) for pain management (e.g., see Keefe, 1996), relaxation training, or mindfulness-based stress reduction (e.g., see Kabat-Zinn, & Hanh, 2009) to maximize the effectiveness of such programs.
As with any research, the current study has several limitations. There may be a selection bias, as caregiver enrollment was voluntary and it is unclear how many caregivers decided not to contact the research team. Our results are also limited, albeit useful, in that our sample of dementia caregivers was consistently using ADS at least two times per week, Monday through Friday. This study also did not examine positive aspects of caregiving (PACs), and past findings have found these positive appraisals have lasting effects on burden and depression among dementia caregivers (Hilgeman, Allen, DeCoster, & Burgio, 2007). Our findings, while useful in describing daily associations with pain, are not able to shed light on what a clinically meaningful decrease in pain would be. It will also be important to operationalize what would be considered a clinically meaningful decrease in daily pain, both in a single day, as well as over time to further understand cumulative effects of protective factors such as positive affect or ADS use. Our study also only used a single item as an index for pain. In the future, it is also important to use a more comprehensive measure of pain, beyond the context of chronic conditions, to garner a comprehensive understanding of differences in the daily and cumulative effects of subjective stress and ADS use on caregivers’ daily experience of pain. For example, the particular cause of the reported physical pain will also be an important consideration in future research. Specifically, pain catastrophizing is an important individual variable that should be considered as it predicts reported pain intensity and associated psychological distress independent of the objective physical ailment (Severeijns, Vlaeyen, van den Hout, & Weber, 2001)
Implications
In conclusion, this study adds to the literature by suggesting pain is an important experience among caregivers that is partly related to stressors they encounter. It is possible avenues for intervention to reduce subjective stress may indirectly address pain. Moreover, the article highlights the need to further investigate pain among caregivers to inform current available interventions such that pain might be addressed more directly. For example, treatments for caregivers might incorporate a module regarding physical activity and active coping strategies such as mindfulness for pain management (Kabat-Zinn, 1982; Morone, Greco, & Weiner, 2008; Zeidan, Gordon, Merchant, & Goolkasian, 2010). Research to date has generally underestimated the importance of pain in caregivers. Work by Jones and colleagues (2011), however, reported that pain is a robust correlate of caregiver burden and predicts coping behavior. The current study suggests that daily pain, generally, is an important facet of the dementia caregiving experience to consider in the design and delivery of interventions. Integration of treatments that address management of noncare-related stressors as well as the physical health needs of caregivers has implications for the overall health, well-being, and quality of life of caregiving dyads such that healthy caregivers have a greater capacity and resources to provide optimal care to IWDs.
Funding
This project was supported by National Institute of Aging [R01 AG031758].
Acknowledgments
Steven H. Zarit is the principal investigator. There are no known conflicts of interest and the National Institute of Aging was not involved in these secondary data analyses and development of this manuscript. A special thanks to all of the caregivers who gave generously of their time and energy to this project.
Conflict of Interest
None declared.
References
- Affleck G. Tennen H. Keefe F. J. Lefebvre J. C. Kashikar-Zuck S. Wright K.,…Caldwell D. S (1999). Everyday life with osteoarthritis or rheumatoid arthritis: Independent effects of disease and gender on daily pain, mood, and coping. Pain, 83, 601–609. [DOI] [PubMed] [Google Scholar]
- Affleck G. Tennen H. Urrows S., & Higgins P (1991). Individual differences in the day-to-day experience of chronic pain: A prospective daily study of rheumatoid arthritis patients. Health Psychology, 10, 419–426. [DOI] [PubMed] [Google Scholar]
- Alecxih L. M. B. Zeruld S., & Olearczyk B (2001). Characteristics of caregivers based on the survey of income and program participation.Washington, DC: United States Department of Health and Human Services, Administration on Aging. [Google Scholar]
- Almeida D. M. (1998). Daily Inventory of Stressful Events (DISE) expert coding manual. Tucson: Division of Family Studies and Human Development, University of Arizona. [Google Scholar]
- Almeida D. M. Wethington E., & Kessler R. C (2002). The daily inventory of stressful events: An interview-based approach for measuring daily stressors. Assessment, 9, 41–55. doi:10.1177/1073191102091006 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association (2015). 2015 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 11, 332. [DOI] [PubMed] [Google Scholar]
- Clyburn L. D. Stones M. J. Hadjistavropoulos T., & Tuokko H (2000). Predicting caregiver burden and depression in Alzheimer’s disease. Journal of Gerontology: Psychological Sciences and Social Sciences, 55, 2. doi:10.1093/geronb/55.1.S2 [DOI] [PubMed] [Google Scholar]
- Everhart R. S. Fiese B. H., & Smyth J. M (2008). A cumulative risk model predicting caregiver quality of life in pediatric asthma. Journal of Pediatric Psychology, 33, 809–818. doi:10.1093/jpepsy/jsn028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth E. B., Zarit S. H., Femia E. E., Hofer S. M., Stephens M. A. (2006). Behavioral and psychological symptoms of dementia and caregivers’ stress appraisals: Intra-individual stability and change over short-term observations. Aging & Mental Health, 10, 563–573. doi:10.1080/13607860600638107 [DOI] [PubMed] [Google Scholar]
- Femia E. E. Zarit S. H. Stephens M. A., & Greene R (2007). Impact of adult day services on behavioral and psychological symptoms of dementia. The Gerontologist, 47, 775–788. [DOI] [PubMed] [Google Scholar]
- Finan P. H., Garland E. L. (2015). The role of positive affect in pain and its treatment. The Clinical Journal of Pain, 31, 177–187. doi:10.1097/AJP.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisser M. E. Robinson M. E., & Riley J. L (2000). Pain beliefs, coping, and adjustment to chronic pain: Let’s focus more on the negative. In Pain Forum, 8, 161–168. [Google Scholar]
- Hayes S. C. Luoma J. B. Bond F. W. Masuda A., & Lillis J (2006). Acceptance and commitment therapy: Model, processes and outcomes. Behaviour Research and Therapy, 44, 1–25. doi:10.1016/j.brat.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Hebert L. E. Weuve J. Scherr P. A., & Evans D. A (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80, 1778–1783. doi:10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L., & Stawski R. S (2009). Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development, 6, 97–120. doi:10.1080/15427600902911189 [Google Scholar]
- Hilgeman M. M. Allen R. S. DeCoster J., & Burgio L. D (2007). Positive aspects of caregiving as a moderator of treatment outcome over 12 months. Psychology and Aging, 22, 361–371. doi:10.1037/0882-7974.22.2.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S. Saito K. Shirotsuki K. Sugaya N., & Nomura S (2012). Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: A study of a two-week teaching practice. Psychoneuroendocrinology, 37, 852–858. doi:10.1016/j.psyneuen.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Jones S. L., Hadjistavropoulos H. D., Janzen J. A., Hadjistavropoulos T. (2011). The relation of pain and caregiver burden in informal older adult caregivers. Pain Medicine (Malden, Mass.), 12, 51–58. doi:10.1111/j.1526-4637.2010.01018.x [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. (1982). An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry, 4, 33–47. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J., & Hanh T. N (2009). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Pub. by Dell Pub., a division of Bantam Doubleday Dell Pub. Group, Delta. [Google Scholar]
- Katz S. Ford A. B. Moskowitz R. W. Jackson B. A., & Jaffe M. W (1963). Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA, 185, 914–919. [DOI] [PubMed] [Google Scholar]
- Keefe F. J. (1996). Cognitive behavioral therapy for managing pain. Clinical Psychology, 49, 4–5. [Google Scholar]
- Kessler R. C. Andrews G. Colpe L. J. Hiripi E. Mroczek D. K. Normand S. L.,…Zaslavsky A. M (2002). Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological Medicine, 32, 959–976. doi:10.1017/S0033291702006074 [DOI] [PubMed] [Google Scholar]
- Larsen R. J., & Kasimatis M (1991). Day-to-day physical symptoms: Individual differences in the occurrence, duration, and emotional concomitants of minor daily illnesses. Journal of Personality, 59, 387–423. [DOI] [PubMed] [Google Scholar]
- Lawton M. P., & Brody E. M (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9, 179–186. doi:10.1093/geront/9.3_Part_1.179 [PubMed] [Google Scholar]
- Lennartsson A. K. Theorell T. Kushnir M. M. Bergquist J., & Jonsdottir I. H (2013). Perceived stress at work is associated with attenuated DHEA-S response during acute psychosocial stress. Psychoneuroendocrinology, 38, 1650–1657. doi:10.1016/j.psyneuen.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Littell R. C. Milliken G. A. Stroup W. W., & Wolfinger R. D (1996). SAS system for mixed models (p. 633). Cary, NC: SAS Institute. [Google Scholar]
- Morone N. E. Greco C. M., & Weiner D. K (2008). Mindfulness meditation for the treatment of chronic low back pain in older adults: A randomized controlled pilot study. Pain, 134, 310–319. doi:10.1016/j.pain.2007.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek D. K., & Kolarz C. M (1998). The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology, 75, 1333. doi:10.1037/0022-3514.75.5.1333 [DOI] [PubMed] [Google Scholar]
- National Family Caregiver Support Program (2017). Administration on Community Living. Retrieved from https://www.acl.gov/ programs/support-caregivers/national-family-caregiver- support-program. [Google Scholar]
- Ornstein K. A. Gaugler J. E. Devanand D. P. Scarmeas N. Zhu C. W., & Stern Y (2013). Are there sensitive time periods for dementia caregivers? The occurrence of behavioral and psychological symptoms in the early stages of dementia. International Psychogeriatrics, 25, 1453–1462. doi:10.1017/S1041610213000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C. Smarr K. L. Buescher K. L. Phillips L. R. Frank R. G. Beck N. C.,…Walker S. E (1989). Pain control and rational thinking. Implications for rheumatoid arthritis. Arthritis and Rheumatism, 32, 984–990. [DOI] [PubMed] [Google Scholar]
- Pearlin L. I., Mullan J. T., Semple S. J., Skaff M. M. (1990). Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist, 30, 583–594. [DOI] [PubMed] [Google Scholar]
- Pinquart M., Sörensen S. (2003). Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging, 18, 250–267. [DOI] [PubMed] [Google Scholar]
- Raudenbush S. W., & Bryk A. S (2002). Hierarchical linear models: Applications and data analysis methods (Vol. 1). Thousand Oaks, CA: Sage. [Google Scholar]
- Severeijns R. Vlaeyen J. W. van den Hout M. A., & Weber W. E (2001). Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. The Clinical Journal of Pain, 17, 165–172. [DOI] [PubMed] [Google Scholar]
- Shahly V. Chatterji S. Gruber M. J. Al-Hamzawi A. Alonso J. Andrade L. H.,…Kessler R. C (2013). Cross-national differences in the prevalence and correlates of burden among older family caregivers in the World Health Organization World Mental Health (WMH) Surveys. Psychological Medicine, 43, 865–879. doi:10.1017/S0033291712001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Martire L. M. (2004). Family caregiving of persons with dementia: Prevalence, health effects, and support strategies. The American Journal of Geriatric Psychiatry, 12, 240–249. [PubMed] [Google Scholar]
- Smith W. R. Penberthy L. T. Bovbjerg V. E. McClish D. K. Roberts J. D. Dahman B.,…Roseff S. D (2008). Daily assessment of pain in adults with sickle cell disease. Annals of Internal Medicine, 148, 94–101. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J. Thorn B. Haythornthwaite J. A. Keefe F. Martin M. Bradley L. A., & Lefebvre J. C (2001). Theoretical perspectives on the relation between catastrophizing and pain. The Clinical Journal of Pain, 17, 52–64. [DOI] [PubMed] [Google Scholar]
- Ware J. E. Jr, & Sherbourne C. D (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30, 473–483. [PubMed] [Google Scholar]
- Watson D. Clark L. A., & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Watson D., & Tellegen A (1985). Toward a consensual structure of mood. Psychological Bulletin, 98, 219–235. [DOI] [PubMed] [Google Scholar]
- Yates M. E. Tennstedt S., & Chang B. H (1999). Contributors to and mediators of psychological well-being for informal caregivers. The Journal of Gerontology: Psychological Sciences, 54, 12–22. [DOI] [PubMed] [Google Scholar]
- Zarit S. H. Kim K. Femia E. E. Almeida D. M., & Klein L. C (2014). The effects of adult day services on family caregivers’ daily stress, affect, and health: Outcomes from the Daily Stress and Health (DaSH) study. The Gerontologist, 54, 570–579. doi:10.1093/geront/gnt045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit S. H., Kim K., Femia E. E., Almeida D. M., Savla J., Molenaar P. C. (2011). Effects of adult day care on daily stress of caregivers: A within-person approach. The Journal of Gerontology: Psychological Sciences and Social Sciences, 66, 538–546. doi:10.1093/geronb/gbr030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit S. H. Stephens M. A. Townsend A., & Greene R (1998). Stress reduction for family caregivers: Effects of adult day care use. The Journal of Gerontology: Social Sciences, 53, 267–277. [DOI] [PubMed] [Google Scholar]
- Zarit S. H. Whetzel C. A. Kim K. Femia E. E. Almeida D. M. Rovine M. J., & Klein L. C (2014). Daily stressors and adult day service use by family caregivers: Effects on depressive symptoms, positive mood, and dehydroepiandrosterone-sulfate. The American Journal of Geriatric Psychiatry, 22, 1592–1602. doi:10.1016/j.jagp.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F. Gordon N. S. Merchant J., & Goolkasian P (2010). The effects of brief mindfulness meditation training on experimentally induced pain. The Journal of Pain, 11, 199–209. doi:10.1016/j.jpain.2009.07.015 [DOI] [PubMed] [Google Scholar]