Key Points
Question
Is vitamin D supplementation during pregnancy beneficial and safe for offspring?
Findings
In this systematic review and meta-analysis of 24 randomized clinical trials including 5405 individuals, vitamin D supplementation during pregnancy was associated with a lower risk of infants being small for gestational age and improved growth during infancy without an increased risk of fetal or neonatal mortality or congenital abnormality.
Meaning
Vitamin D supplementation during pregnancy may reduce the risk of infants being small for gestational age and improve growth during infancy without an increased risk of fetal or neonatal mortality or congenital abnormality.
Abstract
Importance
Whether vitamin D supplementation during pregnancy is beneficial and safe for offspring is unclear.
Objective
To systematically review studies of the effects of vitamin D supplementation during pregnancy on offspring growth, morbidity, and mortality.
Data Sources
Searches of Medline, Embase, and the Cochrane Database of Systematic Reviews were conducted up to October 31, 2017. Key search terms were vitamin D, pregnancy, randomized controlled trials, and offspring outcomes.
Study Selection
Randomized clinical trials of vitamin D supplementation during pregnancy and offspring outcomes.
Data Extraction and Synthesis
Two authors independently extracted data, and the quality of the studies was assessed. Summary risk ratio (RR), risk difference (RD) or mean difference (MD), and 95% CI were calculated using fixed-effects or random-effects meta-analysis.
Main Outcomes and Measures
Main outcomes were fetal or neonatal mortality, small for gestational age (SGA), congenital malformation, admission to a neonatal intensive care unit, birth weight, Apgar scores, neonatal 25-hydroxyvitamin D (25[OH]D) and calcium concentrations, gestational age, preterm birth, infant anthropometry, and respiratory morbidity during childhood.
Results
Twenty-four clinical trials involving 5405 participants met inclusion criteria. Vitamin D supplementation during pregnancy was associated with a lower risk of SGA (RR, 0.72; 95% CI, 0.52 to 0.99; RD, −5.60%; 95% CI, −0.86% to −10.34%) without risk of fetal or neonatal mortality (RR, 0.72; 95% CI, 0.47 to 1.11) or congenital abnormality (RR, 0.94; 95% CI, 0.61 to 1.43). Neonates with prenatal vitamin D supplementation had higher 25(OH)D levels (MD, 13.50 ng/mL; 95% CI, 10.12 to 16.87 ng/mL), calcium levels (MD, 0.19 mg/dL; 95% CI, 0.003 to 0.38 mg/dL), and weight at birth (MD, 75.38 g; 95% CI, 22.88 to 127.88 g), 3 months (MD, 0.21 kg; 95% CI, 0.13 to 0.28 kg), 6 months (MD, 0.46 kg; 95% CI, 0.33 to 0.58 kg), 9 months (MD, 0.50 kg; 95% CI, 0.01 to 0.99 kg), and 12 months (MD, 0.32 kg; 95% CI, 0.12 to 0.52 kg). Subgroup analysis by doses showed that low-dose vitamin D supplementation (≤2000 IU/d) was associated with a reduced risk of fetal or neonatal mortality (RR, 0.35; 95% CI, 0.15 to 0.80), but higher doses (>2000 IU/d) did not reduce this risk (RR, 0.95; 95% CI, 0.59 to 1.54).
Conclusions and Relevance
Vitamin D supplementation during pregnancy is associated with a reduced risk of SGA and improved infant growth without risk of fetal or neonatal mortality or congenital abnormality. Vitamin D supplementation with doses of 2000 IU/d or lower during pregnancy may reduce the risk of fetal or neonatal mortality.
This systematic review and meta-analysis assesses findings from clinical trials that evaluated the association of vitamin D supplementation during pregnancy with outcomes in the fetus and neonate.
Introduction
Low maternal vitamin D level status is common during pregnancy and is a public health issue worldwide.1,2,3 Vitamin D, a fat-soluble nutrient and prohormone,2 has classic functions of calcium absorption, metabolism, and bone health and nonclassic actions that may affect various other aspects of health.4 Low vitamin D level status during pregnancy may expose the offspring to a suboptimal nutritional environment during critical phases of fetal development and may have long-term effects on offspring health outcomes.3,5,6 Sufficient vitamin D concentrations are needed during pregnancy to address the increased demand of fetal growth and development because the mother provides all of the vitamin D for the fetus.7
During the past few decades, emerging randomized clinical trials (RCTs) have assessed the effect of vitamin D supplementation during pregnancy on maternal, neonatal, infant, or child outcomes. However, the results of the RCTs are inconsistent.2 There is a lack of evidence from systematic reviews and meta-analyses to evaluate the association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality.4 Given the high prevalence of low vitamin D level status during pregnancy and the public health importance of clarifying the role of vitamin D during pregnancy in offspring health, we conducted a systematic review and meta-analysis of RCTs with aims to evaluate the effectiveness and safety of vitamin D supplementation during pregnancy on offspring outcomes.
Methods
Data Sources and Searches
This systematic review is presented according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.8 Medline, Embase, and the Cochrane Database of Systematic Reviews were searched up to October 31, 2017. The key words used were vitamin D, pregnancy, randomized controlled trials, and offspring outcomes. References cited in these articles were manually searched to identify additional RCTs.
Study Selection
Two investigators (W.G.B. and S.Q.W.) independently scrutinized the electronic searches and obtained full articles of all citations that were potentially eligible studies for inclusion. Full-length articles of studies evaluating maternal vitamin D supplementation in pregnancy and offspring outcomes were examined and subsequently selected if they fulfilled the following inclusion criteria: (1) the design was an RCT; (2) population was healthy, pregnant women without prior vitamin D supplementation of more than 400 IU/d; (3) vitamin D protocol was specified in the treatment group; (4) outcomes were offspring growth, morbidity, and mortality; (5) the study contained relevant data to calculate the effect size; and (6) the study met the methodologic quality assessment criteria for RCTs.9 Articles were excluded if (1) they were reviews, observational studies, case reports, letters, or comments; (2) there was no appropriate control group; (3) vitamin D dose in the intervention group was 400 IU/d or less; or (4) data were incomplete or conflicting.
Primary outcomes were (1) small for gestational age (SGA), indicated by birth weight less than the 10th percentile for gestational age, and (2) fetal or neonatal mortality. Secondary outcomes were (1) neonatal 25-hydroxyvitamin D (25[OH]D) levels, (2) congenital malformation, (3) admission to a neonatal intensive care unit (NICU), (4) Apgar scores, (5) neonatal calcium levels, (6) birth weight, (7) low birth weight, (8) gestational age, (9) preterm birth, (10) infant growth, (11) asthma, (12) respiratory infection, (13) eczema, and (14) allergy.
Quality Assessment
We evaluated the methodologic quality of each eligible RCT using the Cochrane Risk Assessment Tool (eTable in the Supplement).9 The following items were evaluated: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. For all RCTs, each item was described as having a low risk of bias, a high risk of bias, or an unclear risk of bias.9
Data Extraction and Synthesis
The following information was extracted from the study reports: the first author’s last name, year of publication, country of origin, study design, total sample size, characteristics of participants, timing of supplementation, interventions, and outcomes. When the study had 2 or more intervention groups with different doses of vitamin D supplementation, we combined them into 1 intervention group. Two of us (W.G.B. and S.Q.W.) extracted the data independently and in duplicate. Discrepancies were resolved through discussion to achieve a consensus.
Subgroup analyses were performed according to timing (initiation at <20 or ≥20 weeks’ gestation), dose (>2000 or ≤2000 IU/d), and method (regular or bolus doses) of vitamin D supplementation for the outcomes of SGA, fetal or neonatal mortality, neonatal blood 25(OH)D concentration, and birth weight.
Statistical Analysis
Data on dichotomous outcomes were combined using the Mantel-Haenszel method, and measures of effect are presented as risk ratios (RRs) or risk differences with 95% CIs. For continuous data, we calculated the sample size–weighted mean difference (MD) when outcomes were measured in the same way between studies. We used forest plots to show the point estimate (95% CIs) for each study. The I2 statistic (percentage of variability in the results that is due to heterogeneity) was used to quantify the degree of heterogeneity across studies.10 If the I2 value was 50% or greater, the heterogeneity was considered significant and we pooled results using a random-effects model. Otherwise, a fixed-effect model was applied. Funnel plots were applied to evaluate publication bias. The data were extracted and statistical analysis was carried out using Review Manager, version 5.3 (RevMan).11 Two-tailed P < .05 values were considered statistically significant.
Results
Study Selection
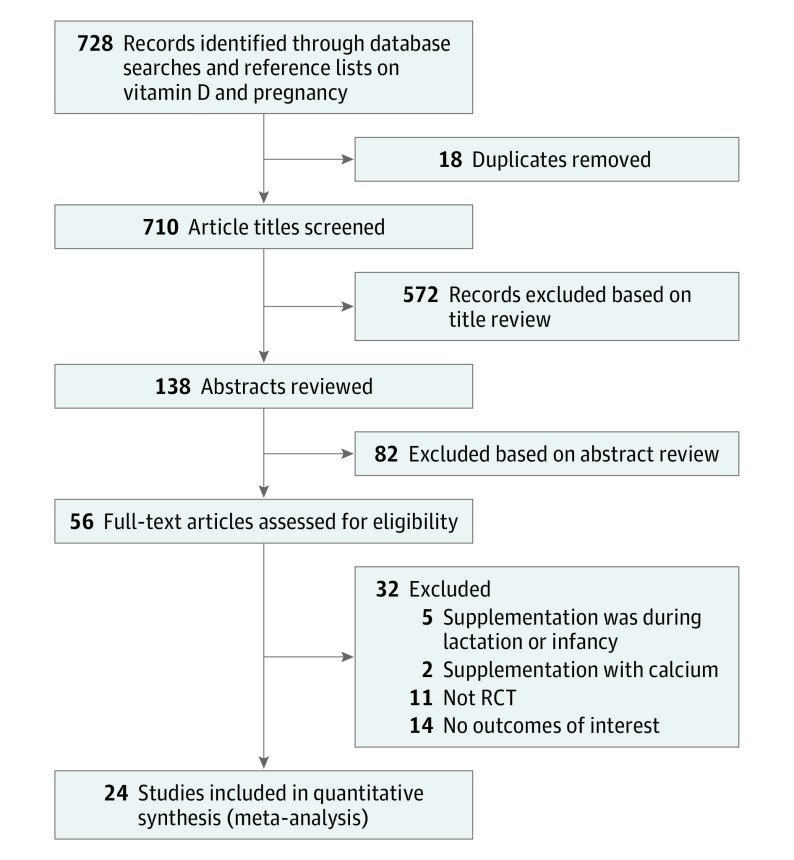
The search strategy resulted in 728 potentially relevant citations. The PRISMA flow diagram (Figure 1) summarizes the process of the literature search and selection of studies. After screening the titles and abstracts, we read 56 articles. Twenty-four RCTs12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36 comprising 5405 participants met the inclusion criteria. Two of these trials are from the same RCT with different outcomes.17,36 The assessment of methodologic quality of each eligible RCT by the Cochrane Risk Assessment Tool is summarized in the eTable in the Supplement.
Figure 1. Flowchart of Study Selection Process.
RCT indicates randomized clinical trial.
Study Characteristics
The characteristics of the included RCTs are summarized in the Table. Vitamin D supplementation was in the form of cholecalciferol in 22 RCTs13,14,15,16,17,18,19,20,21,22,23,24,26,27,28,29,30,31,32,33,34,35 and in the form of ergocalciferol in 3 RCTs.12,17,25 For the intervention group, the daily doses were 800 IU in 1 RCT,17 1000 IU in 6 RCTs,12,14,16,18,25,26 1200 IU in 1 RCT,34 2000 IU in 7 RCTs,15,18,20,26,29,34,35 2800 IU in 1 RCT,13 4000 IU in 4 RCTs,15,20,21,29 4400 in 1 RCT,24 or 5000 IU in 1 RCT33; the weekly doses were 35 000 IU30 or 50 000 IU19; the fortnightly dose was 50 000 IU in 2 RCTs23,28; the monthly dose was 60 000 IU32; the bimonthly dose was 60 000 IU32; and the bolus doses were 60 000 IU in 3 RCTs,22,27,31 120 000 IU in 2 RCTs,22,31 or 200 000 IU in 2 RCTs.17,25 In studies comprising 3 or more groups, as was the case in 9 RCTs,15,17,18,20,22,25,26,32,34 the higher-dose groups were combined into 1 cohort as the intervention group and the lowest dose groups as the control cohort. There were no toxic effects on offspring in the included RCTs and no evidence of publication bias.
Table. Characteristics of Included Randomized Clinical Trials.
| Source | Country | Total Sample Size | Participants | Initiation and Timing of Supplementation | Interventions | Outcomes |
|---|---|---|---|---|---|---|
| Brooke et al,12 1980 | United Kingdom | 126 | Pregnant Asian women | Third trimester | Ergocalciferol, 1000 IU/d, vs placebo | Cord blood 25(OH)D concentration, neonatal anthropometry, SGA, LBW, gestational age at birth, anthropometry at birth, 3, 6, 9, and 12 mo |
| Chawes et al,13 2016 | Denmark | 623 | Pregnant women not after wk 26; without endocrine, cardiovascular, or nephrologic disorders; vitamin D3 intake no more than 600 IU/d | 24 wk of gestation to 1 wk postpartum | Cholecalciferol, 2800 IU/d, vs 400 IU/d | Fetal or neonatal death, congenital malformation, admission to a NICU, preterm birth, wheeze, asthma, upper and lower respiratory tract infections, eczema, allergy skin prick test, allergy-specific IgE at age 3 y |
| Cooper et al,14 2016 | United Kingdom | 965 | Pregnant women >18 y, singleton pregnancy, gestation <17 weeks, serum 25(OHD) level 10-40 ng/mL at 10-17 wk of gestation | 14 wk of gestation or as soon as possible before 17 wk of gestation if recruited later until delivery | Cholecalciferol, 1000 IU/d, vs placebo | Fetal or neonatal death, congenital malformation, neonatal anthropometry, preterm birth |
| Dawodu et al,15 2013 | United States | 126 | Arab expectant mothers, 12-16 wk of gestation, singleton pregnancy | 12-16 wk of gestation until delivery | Cholecalciferol, 2000 or 4000 IU/d, vs 400 IU/d | Cord blood 25(OH)D concentration, SGA |
| Delvin et al,16 1986 | France | 30 | Pregnant women | Third trimester | Cholecalciferol, 1000 IU/d, vs no treatment | Cord blood 25(OH)D concentration |
| Goldring et al,17 2013; Yu et al,36 2009 | United Kingdom | 179 | Pregnant women | 27 wk of gestation until delivery | Ergocalciferol, 800 IU/d, or cholecalciferol, 200 000 IU (1 dose), vs no treatment (control) | Fetal or neonatal death, cord blood 25(OH)D concentration, neonatal anthropometry, SGA, gestational age at birth, wheeze, eczema, upper and lower respiratory tract infections at age 3 y |
| Grant et al,18 2014 | New Zealand | 258 | Pregnant women, 26-30 wk of gestation, singleton pregnancy, no vitamin D supplementation >200 IU/d, history of renal stones, hypercalcemia, or any serious pregnancy complication at enrollment | 27 wk of gestation until delivery | Cholecalciferol, 1000 or 2000 IU/d, vs placebo | Fetal or neonatal death, cord blood 25(OH)D concentration, preterm birth, asthma, upper and lower respiratory tract infections, allergy skin prick test, allergy-specific IgE at age 3 y |
| Hashemipour et al,19 2014 | Iran | 110 | Iranian pregnant women with vitamin D deficiency | Start at 26-28 wk of gestation; duration, 8 wk | Cholecalciferol, 50 000 IU/wk, vs 400 IU/d | Fetal or neonatal death, cord blood 25(OH)D concentration, neonatal anthropometry, SGA, preterm birth |
| Hollis et al,20 2011 | United States | 350 | Women with a singleton pregnancy | 12-16 wk of gestation until delivery | Cholecalciferol, 2000 or 4000 IU/d, vs 400 IU/d | Fetal or neonatal death, admission to a NICU, cord blood 25(OH)D concentration, neonatal anthropometry, gestational age at birth |
| Hossain et al,21 2014 | Pakistan | 175 | Women with singleton pregnancy | 20 wk of gestation until delivery | Cholecalciferol, 4000 IU/d, vs routine care | Fetal or neonatal death, Apgar score, cord blood 25(OH)D concentration, neonatal anthropometry, SGA, gestational age at birth, preterm birth |
| Kalra et al,22 2012 | Zimbabwe | 109 | Pregnant women | 12-24 wk of gestation until delivery | Cholecalciferol, 120 000 IU (1 dose) or 60 000 IU (2 doses), vs standard care | Neonatal anthropometry, infant anthropometry at 3, 6, and 9 mo |
| Karamali et al,23 2015 | Iran | 60 | Pregnant women prima gravida, aged 18-40 y, at risk for preeclampsia, without abnormal fetal anomaly scan | 20-30 wk of gestation | Cholecalciferol, 50 000 IU/fortnight, vs placebo | Apgar score, neonatal anthropometry, LBW, gestational age at birth, preterm birth |
| Litonjua et al,24 2016 | United States | 835 | Pregnant women aged 18-39 y; gestational age 10-18 wk; history of asthma, eczema, or allergic rhinitis; nonsmoker; English or Spanish speaking | 10-18 wk of gestation until delivery | Cholecalciferol, 4400 vs 400 IU/d | Fetal or neonatal death, congenital malformation, admission to a NICU, cord blood 25(OH)D concentration, neonatal anthropometry, preterm birth, asthma, lower respiratory tract infections, eczema, allergy skin prick test, allergy-specific IgE at age 3 y |
| Mallet et al,25 1986 | France | 77 | White pregnant women | 7 mo of gestation | Ergocalciferol, 1000 IU/d or 200 000 IU (1 dose), vs control | Cord blood 25(OH)D concentration |
| March et al,26 2015 | Canada | 105 | Pregnant women aged 18-45 y, healthy, 13-24 wk of gestation, exclusion of women receiving supplements >400 IU/d | 13-24 wk of gestation until delivery | Cholecalciferol, 1000 or 2000 IU/d, vs 400 IU/d | Cord blood 25(OH)D concentration |
| Marya et al,27 1988 | India | 200 | Pregnant women aged 22-35 y | 7 mo of gestation | Cholecalciferol, 600 000 IU (2 doses), vs no supplementation | Neonatal anthropometry, LBW, gestational age at birth |
| Mojibian et al,28 2015 | Iran | 389 | Pregnant women, 12-16 wk of gestation, serum 25(OH)D <30 ng/mL | 12 wk of gestation until delivery | Cholecalciferol, 50 000 IU/fortnight, vs 400 IU/d | Apgar score, cord blood 25(OH)D concentration, neonatal anthropometry, LBW, preterm birth |
| Rodda et al,29 2015 | Australia | 45 | Pregnant women, singleton pregnancy, serum 25(OH)D <30 ng/mL | 12-16 wk of gestation until delivery | Cholecalciferol, 2000 IU/d (adjusted to 4000 IU/d if serum vitamin D level remains <75 nmol/L), vs standard care | Cord blood 25(OH)D concentration |
| Roth et al,30 2013 | United States | 147 | Pregnant women | Third trimester | Cholecalciferol, 35 000 IU/wk, vs placebo | Fetal or neonatal death, 25(OH)D concentration, neonatal anthropometry, gestational age at birth, preterm birth, infant anthropometry at 12 mo, weight, length, and head circumference z scores in infants at age 1 y |
| Sablok et al,31 2015 | India | 165 | Prima gravida with singleton pregnancy at 14-20 wk, without preexisting osteomalacia, known hyperparathyroidism, renal or liver dysfunction, tuberculosis, or sarcoidosis | 20 wk of gestation until delivery | Cholecalciferol, 60 000 IU (1 dose), 120 000 IU (2 doses), or 120 000 IU (4 doses), vs no supplementation | Cord blood 25(OH)D concentration, neonatal anthropometry, SGA, preterm birth |
| Sahoo et al,32 2017 | India | 52 | Pregnant women aged >18 y, singleton pregnancy, <20 wk of gestation, no known bone diseases or complicated pregnancy, no vitamin D supplementation within previous 3 mo | 14-20 wk of gestation until delivery | Cholecalciferol, 60 000 IU/4 wk or 60 000 IU/8 wk, vs 400 IU/d | Cord blood 25(OH)D concentration, neonatal anthropometry, weight, length, and head circumference z scores in infants at age 1 y |
| Yap et al,33 2014 | Australia | 179 | Women with singleton pregnancies, aged ≥18 y and gestational age <20 wk, no history of diabetes, calcium or vitamin D metabolism disorders, hypercalcemia, or significant renal impairment, no vitamin D supplements ≥1000 IU/d | 20 wk of gestation until delivery | Cholecalciferol, 5000 IU/d, vs 400 IU/d | Fetal or neonatal death, cord blood 25(OH)D concentration, neonatal anthropometry, gestational age at birth, preterm birth |
| Yesiltepe Mutlu et al,34 2014 | Turkey | 51 | Pregnant women aged >16 y, singleton pregnancy, no previously known calcium metabolism or untreated thyroid disorders | 13-32 wk of gestation until delivery | Cholecalciferol, 1200 or 2000 IU/d, vs 600 IU/d | Neonatal anthropometry, neonatal 25(OH)D concentration |
| Zerofsky et al,35 2016 | United States | 49 | Participants aged >18 y with a singleton pregnancy <20 wk | No later than 20 wk of gestation until delivery | Cholecalciferol, 2000 IU/d, vs 400 IU/d | Apgar score, neonatal anthropometry, gestational age at birth |
Abbreviations: IgE, immunoglobulin E; LBW, low birth weight; NICU, neonatal intensive care unit; RCT, randomized clinical trial; SGA, small for gestational age; 25(OH)D, 25-hydroxyvitamin D.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
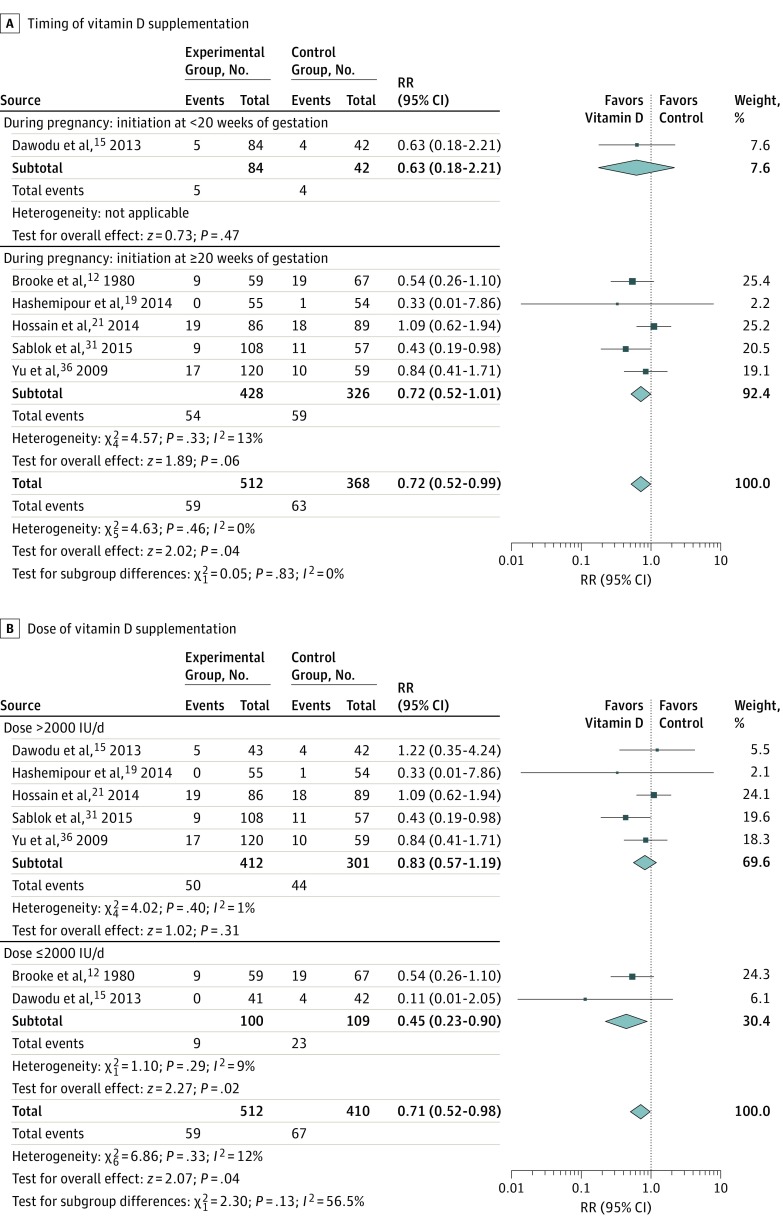
The vitamin D supplementation group had a significantly lower risk of SGA (RR, 0.72; 95% CI, 0.52 to 0.99; I2 = 0%) in 6 RCTs12,15,19,21,31,36 with 898 participants (Figure 2). Risk difference was −5.60%; 95% CI, −0.86% to −10.34%. Subgroup analysis by doses showed that vitamin D supplementation at 2000 IU/d or lower was associated with a reduced risk of SGA (RR, 0.45; 95% CI, 0.23 to 0.90), while vitamin D supplementation at doses larger than 2000 IU/d was not associated with a reduced risk of SGA (RR, 0.83; 95% CI, 0.57 to 1.19). Testing of subgroups showed no significant differences, but significant heterogeneity was present (P = .13; I2 = 56.5%) (Figure 2B). Timing (early or late) (Figure 2A) and method (regular or bolus doses) (eFigure 1 in the Supplement) of vitamin D supplementation had no association with the risk of SGA.
Figure 2. Summary Risk Ratio (RR) of the Association Between Vitamin D Supplementation and Small for Gestational Age (SGA) .
Subgroup analyses by timing (initiation at <20 or ≥20 weeks of gestation) (A) and dose (>2000 or ≤2000 IU/d) (B). Diamond at the bottom represents the pooled point estimate (95% CIs) for each outcome of interest.
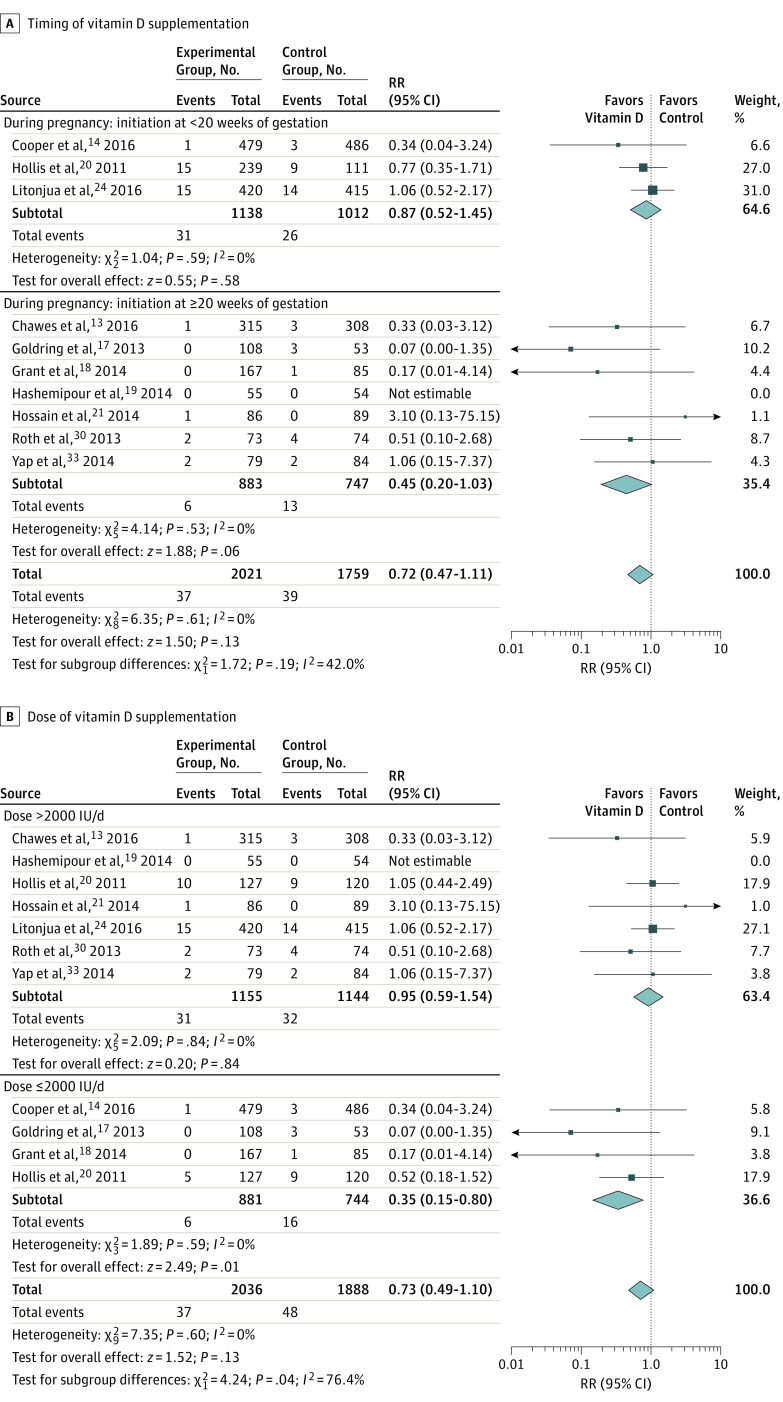
Vitamin D supplementation during pregnancy was not associated with a risk of fetal or neonatal mortality (RR, 0.72; 95% CI, 0.47-1.11; I2 = 0%) in 10 RCTs13,14,17,18,19,20,21,24,30,33 with 3780 participants (Figure 3). Subgroup analysis by doses showed that vitamin D supplementation at 2000 IU/d or less was associated with reduced risk of fetal or neonatal mortality (RR, 0.35; 95% CI, 0.15-0.80), while vitamin D supplementation at doses larger than 2000 IU/d did not reduce the risk of fetal or neonatal mortality (RR, 0.95; 95% CI, 0.59-1.54). Testing for subgroup difference was statistically significant (RR, 0.73; 95% CI, 0.49-1.10; P = .04) (Figure 3B). Timing (early or late) (Figure 3A) and method (regular or bolus doses) (eFigure 2 in the Supplement) of vitamin D supplementation had no association with the risk of fetal or neonatal mortality.
Figure 3. Summary Risk Ratio (RR) of the Association Between Vitamin D Supplementation and Fetal or Neonatal Mortality .
Subgroup analyses by timing (initiation at <20 or ≥20 weeks of gestation) (A) and dose (>2000 or ≤2000 IU/d) (B). Diamond at the bottom represents the pooled point estimate (95% CIs) for each outcome of interest.
There was no significant difference between neonates who received prenatal vitamin D supplementation and those who had not in the outcomes of congenital malformation (RR, 0.94; 95% CI, 0.61-1.43; I2 = 0%) in 3 RCTs13,14,24 with 2355 participants and admission to a NICU (RR, 1.11; 95% CI, 0.82-1.51; I2 = 0%) in 3 RCTs13,20,24 with 1740 participants; however, the supplementation group had significantly higher Apgar scores at 1 minute (MD, 0.09; 95% CI, 0.01-0.17; I2 = 40%) in 4 RCTs21,23,28,35 with 670 participants and at 5 minutes (MD, 0.08; 95% CI, 0.02-0.14; I2 = 13%) in 4 RCTs21,23,28,35 with 668 participants.
Results show that, compared with the control group, the vitamin D supplementation group had higher 25(OH)D concentrations (MD, 13.50 ng/mL; 95% CI, 10.12-16.87 ng/mL; I2 = 97%) in 14 RCTs12,16,17,19,20,24,25,26,28,29,30,32,33,34 with 2361 participants and had more neonates achieving levels of 20 ng/mL (RR, 2.81; 95% CI, 1.92-4.12; I2 = 68%) in 7 RCTs15,17,18,20,30,31,34 with 1107 participants or 30 ng/mL (RR, 5.20; 95% CI, 3.34-8.10; I2 = 47%) in 3 RCTs18,19,21 with 485 participants. Subgroup analysis for neonatal 25(OH)D concentrations by supplementation timing, dose, or method showed that vitamin D supplementation increased neonatal blood 25(OH)D levels whether the supplementation was initiated early (<20 weeks’ gestation) or late (≥20 weeks’ gestation), at higher doses (>2000 IU/d) or lower doses (≤2000 IU/d), or as bolus or regular doses (eFigure 3 in the Supplement).
Neonates who received prenatal vitamin D supplementation had higher calcium concentrations (to convert to millimoles per liter, multiply by 0.25) than those who received no intervention or placebo (MD, 0.19 mg/dL; 95% CI, 0.003-0.38 mg/dL; I2 = 74%) in 9 RCTs12,16,18,22,25,27,32,33,34 with 1007 participants) (eFigure 4 in the Supplement).
Neonates who received prenatal vitamin D supplementation had significantly greater birth weight (MD, 75.38 g; 95% CI, 22.88 to 127.88 g; I2 = 44%) in 17 RCTs12,14,17,19,20,21,22,23,24,27,28,30,31,32,33,34,35 with 4087 participants, greater neonatal femur length (MD, 0.12 cm; 95% CI, 0.01 to 0.23 cm; I2 = 0%) in 2 RCTs30,33 with 316 participants, and greater skinfold thickness (MD, 0.34 mm; 95% CI, 0.17 to 0.51 mm; I2 = 34%) in 2 RCTs12,27 with 326 participants, but no significant difference was observed for crown heel length (MD, 0.33 cm; 95% CI, −0.05 to 0.70 cm; I2 = 74%) in 12 RCTs12,14,19,21,22,23,24,27,28,30,32,33 with 3301 participants or head circumference (MD, 0.20 cm; 95% CI, −0.04 to 0.43 cm; I2 = 78%) in 11 RCTs12,14,19,21,22,23,24,27,28,30,33 with 3240 participants. Subgroup analysis by supplementation timing showed that vitamin D supplementation increased birth weight only in the group with therapy initiated late (≥20 weeks’ gestation) (MD 97.74 g; 95% CI, 29.40 to 166.08 g). Test for subgroup differences between early and late supplementation showed significant differences (χ2 = 5.911; P = .02; I2 = 83.1%) (eFigure 5A in the Supplement). Test for subgroup differences by dose showed no significant difference between higher dose and lower dose (χ2 = 0.131; P = .72; I2 = 0%) (eFigure 5B in the Supplement). Test for subgroup differences by supplementation method showed no significant difference in effect between regular and bolus dose (χ2 = 0.071; P = .79; I2 = 0%) (eFigure 5C in the Supplement).
There was no significant difference between neonates who received prenatal vitamin D supplementation and those who had not in the outcomes of low birth weight (RR, 0.52; 95% CI, 0.20 to 1.37; I2 = 65%) in 4 RCTs12,23,27,28 with 775 participants, gestational age (MD, −0.08 weeks; 95% CI, −0.68 to 0.53 weeks; I2 = 81%) in 9 RCTs12,17,20,21,23,27,30,33,35 with 1441 participants, or preterm birth (RR, 0.98; 95% CI, 0.77 to 1.26; I2 = 33%) in 11 RCTs13,14,18,19,21,23,24,28,30,31,33 with 3822 participants).
On infant anthropometry, 2 RCTs12,22 reported on outcomes at 3 months (216 participants), 6 months (199 participants), and 9 months (179 participants), and 2 RCTs12,30 reported at 12 months. Results showed that infants who received prenatal vitamin D supplementation had significantly greater weight at 3 months (MD, 0.21 kg; 95% CI, 0.13 to 0.28 kg; I2 = 0%) (eFigure 6A in the Supplement), 6 months (MD, 0.46 kg; 95% CI, 0.33 to 0.58 kg; I2 = 0%) (eFigure 6B in the Supplement), 9 months (MD, 0.50 kg; 95% CI, 0.01 to 0.99 kg; I2 = 89%) (eFigure 6C in the Supplement), and 12 months (MD, 0.32 kg; 95% CI, 0.12 to 0.52 kg; I2 = 47%; 252 participants) (eFigure 6D in the Supplement); significantly greater height at 3 months (MD, 1.09 cm; 95% CI, 0.64 to 1.54; cm; I2 = 16%), 9 months (MD, 1.47 cm; 95% CI, 0.13 to 2.82 cm; I2 = 80%), and 12 months (MD, 1.36 cm; 95% CI, 0.81 to 1.92 cm; I2 = 40%; 251 participants) but not at 6 months (MD, 1.35 cm; 95% CI, −0.30 to 3.00 cm; I2 = 87%); and significantly greater head circumference at 3 months (MD, 0.71 cm; 95% CI, 0.23 to 1.18 cm; I2 = 64%) but not at 6 months (MD, 0.54 cm; 95% CI, −0.04 to 1.13 cm; I2 = 72%), 9 months (MD, 0.36 cm; 95% CI, −0.16 to 0.88 cm; I2 = 15%), or 12 months (MD, 0.09 cm; 95% CI, −0.28 to 0.45 cm; I2 = 0%; 248 participants).
Vitamin D supplementation showed no association with the infants’ outcomes of asthma (RR, 0.63; 95% CI, 0.36-1.11; I2 = 71%) in 3 RCTs13,18,24 with 1591 participants, eczema (RR, 0.92; 95% CI, 0.77-1.11; I2 = 0%) in 3 RCTs13,17,24 with 1538 participants, upper respiratory tract infection (RR, 0.94; 95% CI, 0.79-1.12; I2 = 27%) in 2 RCTs17,18 with 389 participants, lower respiratory tract infection (RR, 0.97; 95% CI, 0.85-1.12; I2 = 0%) in 4 RCTs13,17,18,24 with 1769 participants, allergy skin prick test (RR, 0.88; 95% CI, 0.52-1.49; I2 = 60%) in 3 RCTs13,18,24 with 1304 participants, or presence of allergy-specific immunoglobulin E (RR, 0.80; 95% CI, 0.39-1.67; I2 = 78%) in 3 RCTs13,18,24 with 1298 participants. The funnel plots for the primary outcomes showed no publication bias in SGA and fetal or neonatal mortality (eFigure 7 in the Supplement).
Discussion
The main finding of this systematic review and meta-analysis of RCTs was that vitamin D supplementation during pregnancy was associated with a reduced risk of SGA (RR, 0.72) without an increased risk of fetal or neonatal mortality and congenital malformation. Vitamin D supplementation during pregnancy with lower doses (≤2000 IU/d) was associated with a reduced risk of fetal and neonatal mortality. Vitamin D supplementation was associated with higher neonatal vitamin D status (bolus- or regular-dose supplement and early or late timing were equally effective in attaining improvement in vitamin D levels), higher calcium levels, higher Apgar scores, greater neonatal skinfold thickness, greater weight (at birth, 3 months, 6 months, 9 months, and 12 months), and greater height (at 3 months, 9 months, and 12 months) in the offspring. Timing of vitamin D supplementation affected birth weight. There was no significant difference in the offspring outcomes of gestational age, preterm birth, asthma, eczema, respiratory tract infection, or allergy. Based on the results from this meta-analysis, the number needed to treat for SGA was 18: 1 offspring SGA case could be avoided for every 18 pregnant women receiving vitamin D supplementation during pregnancy.
The quality of systematic reviews depends on the quality of the studies included. We evaluated the risk of bias in the RCTs analyzed. Methodologic issues may affect the study quality. We scrutinized the selected studies of good methodologic quality using strict quality assessment criteria.9 Our systematic review is a comprehensive quantitative review of 24 RCTs that reported the effects of maternal vitamin D supplementation in offspring health outcomes, including SGA, fetal or neonatal mortality, congenital malformation, admission to a NICU, Apgar scores, neonatal 25(OH)D and calcium concentrations, preterm birth, anthropometric indicators (weight, height, head circumference, or skinfold thickness) during infancy (at birth and ages 3, 6, 9, and 12 months), asthma, eczema, respiratory tract infection, and allergy in the first 3 years of life.
Previous systematic reviews3,37,38 reported that vitamin D supplementation during pregnancy increased maternal 25(OH)D levels3,37 or neonatal 25(OH)D concentrations.38 One systematic review3 evaluated the outcome of vitamin D supplementation during pregnancy for maternal 25(OH)D levels, risk of preeclampsia, gestational diabetes, and other maternal complications but lacked review on offspring outcomes. A Cochrane review39 studied the association between supplementing vitamin D in pregnant women alone or in combination with calcium along with maternal complications and neonatal outcomes and showed no association between vitamin D supplementation and birth weight in 5 RCTs. Another systematic review40 assessed maternal and neonatal outcomes and showed that birth weight in 8 RCTs and length in 6 RCTs were greater in the vitamin D supplementation group; however, this review had no information on infant follow-up.
The present review adds to the existing literature by including a greater number of recent RCTs and, to our knowledge, is the first meta-analysis of RCTs reporting that vitamin D supplementation during pregnancy was safe (without increased risk of fetal or neonatal mortality, congenital abnormality, or admission to a NICU) and effective in reducing the risk of SGA and improving neonatal calcium levels, skinfold thickness, and postnatal growth (greater weight and height at ages 3, 6, 9, or 12 months). We found that maternal vitamin D supplementation timing, dose, and administration method did not affect cord blood vitamin D concentration. Late vitamin D supplementation (initiation at ≥20 weeks’ gestation) improved birth weight, but early supplementation (initiation at <20 weeks’ gestation) did not. Most importantly, we found that the lower dose of vitamin D supplementation (≤2000 IU/d) reduced the risk of fetal or neonatal mortality and SGA, but the higher dose (>2000 IU/d) did not.
Our findings that maternal vitamin D supplementation during pregnancy reduced the risk of SGA and improved infant growth are biologically plausible. Maternal vitamin D levels during pregnancy positively affect infant bone formation41 as well as skeletal muscle42 and adiposity development,43 which are important for infant growth and development. Vitamin D is needed in maintaining normal levels of calcium and phosphate in blood, which in turn facilitate the process of mineral ion homeostasis and bone formation during early life.44 Increased maternal vitamin D status improved fetal skeletal muscle development and myoblast activity.42 Members of the Southampton Developmental Origins of Health and Disease research group reported that low maternal vitamin D status at 34 weeks’ gestation was associated with lower fat mass at birth.43 Vitamin D also plays an important role in the modulation of the immune function45 and oxidative stress46 that may link to fetal growth. In addition, vitamin D regulates genes responsible for trophoblast invasion and angiogenesis critical for placental implantation and function,47,48,49 which is important for fetal growth.
Vitamin D during pregnancy has been linked to fetal lung maturation in animal models.50,51 Maternal vitamin D may exert its influence during pregnancy on the respiratory and immune systems during lung development in early childhood.52 However, the results of this meta-analysis show that vitamin D supplementation during pregnancy was not associated with childhood respiratory or immune outcomes, including upper or lower respiratory tract infections, asthma, eczema, or allergy, in children at age 3 years. Christensen et al53 conducted a meta-analysis of maternal vitamin D supplementation during pregnancy and infant respiratory tract infections; their results were in line with ours with respect to respiratory tract infections, but they did not have results on asthma. Long-term follow-up of children is needed to determine the effect of vitamin D supplementation during pregnancy on other health outcomes.
Limitations
This study has limitations. First, there were limited data on maternal vitamin D supplementation during pregnancy regarding long-term offspring outcomes, and the longest follow-up in the included RCTs was 3 years. Second, there were only 2 studies on the outcomes of infant growth at age 3 months,12,22 6 months,12,22 9 months,12,22 and 12 months12,30; this result has to be interpreted cautiously. In addition, there was heterogeneity in the result of weight in infants at age 9 months; it is not clear why these 2 studies show different patterns in infants at this age. From a developmental perspective, at 9 months, infants’ weight may differ because of transition to solid food and the total intake, and some infants will begin to walk. Third, the included RCTs differed in several aspects, such as the population studied, ethnicity, altitude, latitude, the outcomes chosen, the clinical setting, the timing of the intervention, and the dose of vitamin D administered during pregnancy. Fourth, the variability in the assay methods for 25(OH)D measurement in each study may contribute to the heterogeneity of the neonatal vitamin D levels. Finally, there were limited data on adherence to the respective protocols.
Conclusions
Vitamin D supplementation during pregnancy was associated with reduced risk of SGA, improved infant growth, and no risk of fetal or neonatal mortality and congenital abnormality. Vitamin D supplementation (≤2000 IU/d) during pregnancy may reduce the risk of fetal or neonatal mortality.
eTable. Assessment of Bias Risk of Randomized Clinical Trials
eFigure 1. Summary Crude Risk Ratio (RR) of the Association Between Vitamin D Supplementation and Small for Gestational Age (SGA) Stratified by Administration Method of Intervention (Regular or Bolus Dose)
eFigure 2. Summary Risk Ratio of the Association 1 Between Vitamin D Supplementation Group and Fetal or Neonatal Mortality Stratified by Administration Method of Intervention (Regular or Bolus Dose).
eFigure 3. Forest Plots of Summary Mean Difference (MD) of Neonatal 25-Hydroxyvitamin D (25[OH]D) (ng/mL) Between Vitamin D Supplementation Group and Control Group
eFigure 4. Forest Plots of Summary Mean Difference (MD) of Neonatal Calcium (mg/dL) Between Vitamin D Supplementation Group and Control Group
eFigure 5. Forest Plots of Summary Mean Difference (MD) of Birth Weight (g) Between Vitamin D Supplementation Group and Control Group
eFigure 6. Forest Plots of Summary Mean Difference (MD) of Postnatal Weight (kg) Between Vitamin D Supplementation Group and Control Group in Infants at Age 3 Months; 6 Months; 9 Months; and 12 Months
eFigure 7. The Funnel Plots of the Primary Outcomes
References
- 1.Urrutia-Pereira M, Solé D. Vitamin D deficiency in pregnancy and its impact on the fetus, the newborn and in childhood [Portuguese]. Rev Paul Pediatr. 2015;33(1):104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3(2):118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palacios C, De-Regil LM, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26(suppl 1):75-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429.e1-429.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladipo OA. Nutrition in pregnancy: mineral and vitamin supplements. Am J Clin Nutr. 2000;72(1)(suppl):280S-290S. [DOI] [PubMed] [Google Scholar]
- 7.Kaushal M, Magon N. Vitamin D in pregnancy: a metabolic outlook. Indian J Endocrinol Metab. 2013;17(1):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. [DOI] [PubMed] [Google Scholar]
- 11.Manager R. (RevMan) [computer program]. Version 5.3. Copenhagen, Denmark: The Cochrane Collaboration; 2014.
- 12.Brooke OG, Brown IR, Bone CD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. BMJ. 1980;280(6216):751-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawes BL, Bønnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353-361. [DOI] [PubMed] [Google Scholar]
- 14.Cooper C, Harvey NC, Bishop NJ, et al. ; MAVIDOS Study Group . Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4(5):393-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab. 2013;98(6):2337-2346. [DOI] [PubMed] [Google Scholar]
- 16.Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. J Pediatr. 1986;109(2):328-334. [DOI] [PubMed] [Google Scholar]
- 17.Goldring ST, Griffiths CJ, Martineau AR, et al. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PLoS One. 2013;8(6):e66627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant CC, Stewart AW, Scragg R, et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics. 2014;133(1):e143-e153. [DOI] [PubMed] [Google Scholar]
- 19.Hashemipour S, Ziaee A, Javadi A, et al. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2014;172:15-19. [DOI] [PubMed] [Google Scholar]
- 20.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hossain N, Kanani FH, Ramzan S, et al. Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J Clin Endocrinol Metab. 2014;99(7):2448-2455. [DOI] [PubMed] [Google Scholar]
- 22.Kalra P, Das V, Agarwal A, et al. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108(6):1052-1058. [DOI] [PubMed] [Google Scholar]
- 23.Karamali M, Beihaghi E, Mohammadi AA, Asemi Z. Effects of high-dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm Metab Res. 2015;47(12):867-872. [DOI] [PubMed] [Google Scholar]
- 24.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315(4):362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallet E, Gügi B, Brunelle P, Hénocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68(3):300-304. [DOI] [PubMed] [Google Scholar]
- 26.March KM, Chen NN, Karakochuk CD, et al. Maternal vitamin D3 supplementation at 50 μg/d protects against low serum 25-hydroxyvitamin D in infants at 8 wk of age: a randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. Am J Clin Nutr. 2015;102(2):402-410. [DOI] [PubMed] [Google Scholar]
- 27.Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res. 1988;88:488-492. [PubMed] [Google Scholar]
- 28.Mojibian M, Soheilykhah S, Fallah Zadeh MA, Jannati Moghadam M. The effects of vitamin D supplementation on maternal and neonatal outcome: a randomized clinical trial. Iran J Reprod Med. 2015;13(11):687-696. [PMC free article] [PubMed] [Google Scholar]
- 29.Rodda CP, Benson JE, Vincent AJ, Whitehead CL, Polykov A, Vollenhoven B. Maternal vitamin D supplementation during pregnancy prevents vitamin D deficiency in the newborn: an open-label randomized controlled trial. Clin Endocrinol (Oxf). 2015;83(3):363-368. [DOI] [PubMed] [Google Scholar]
- 30.Roth DE, Al Mahmud A, Raqib R, et al. Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: the AViDD trial. Nutr J. 2013;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sablok A, Batra A, Thariani K, et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf). 2015;83(4):536-541. [DOI] [PubMed] [Google Scholar]
- 32.Sahoo SK, Katam KK, Das V, Agarwal A, Bhatia V. Maternal vitamin D supplementation in pregnancy and offspring outcomes: a double-blind randomized placebo-controlled trial. J Bone Miner Metab. 2017;35(4):464-471. [DOI] [PubMed] [Google Scholar]
- 33.Yap C, Cheung NW, Gunton JE, et al. Vitamin D supplementation and the effects on glucose metabolism during pregnancy: a randomized controlled trial. Diabetes Care. 2014;37(7):1837-1844. [DOI] [PubMed] [Google Scholar]
- 34.Yesiltepe Mutlu G, Ozsu E, Kalaca S, et al. Evaluation of vitamin D supplementation doses during pregnancy in a population at high risk for deficiency. Horm Res Paediatr. 2014;81(6):402-408. [DOI] [PubMed] [Google Scholar]
- 35.Zerofsky MS, Jacoby BN, Pedersen TL, Stephensen CB. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. J Nutr. 2016;146(11):2388-2397. [DOI] [PubMed] [Google Scholar]
- 36.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf). 2009;70(5):685-690. [DOI] [PubMed] [Google Scholar]
- 37.Chakhtoura M, El Ghandour S, Shawwa K, et al. Vitamin D replacement in children, adolescents and pregnant women in the Middle East and North Africa: a systematic review and meta-analysis of randomized controlled trials. Metabolism. 2017;70:160-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang N, Wang L, Li Z, Chen S, Li N, Ye R. Effects of vitamin D supplementation during pregnancy on neonatal vitamin D and calcium concentrations: a systematic review and meta-analysis. Nutr Res. 2015;35(7):547-556. [DOI] [PubMed] [Google Scholar]
- 39.De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016;(1):CD008873. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-López FR, Pasupuleti V, Mezones-Holguin E, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015;103(5):1278-1288.e4. [DOI] [PubMed] [Google Scholar]
- 41.Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95(4):1749-1757. [DOI] [PubMed] [Google Scholar]
- 42.Hines EA, Coffey JD, Starkey CW, Chung TK, Starkey JD. Improvement of maternal vitamin D status with 25-hydroxycholecalciferol positively impacts porcine fetal skeletal muscle development and myoblast activity. J Anim Sci. 2013;91(9):4116-4122. [DOI] [PubMed] [Google Scholar]
- 43.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM; SWS Study Group . Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2012;96(1):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832-872. [DOI] [PubMed] [Google Scholar]
- 45.Ji JL, Muyayalo KP, Zhang YH, Hu XH, Liao AH. Immunological function of vitamin D during human pregnancy. Am J Reprod Immunol. 2017;78(2). [DOI] [PubMed] [Google Scholar]
- 46.Farhangi MA, Mesgari-Abbasi M, Hajiluian G, Nameni G, Shahabi P. Adipose tissue inflammation and oxidative stress: the ameliorative effects of vitamin D. Inflammation. 2017;40(5):1688-1697. [DOI] [PubMed] [Google Scholar]
- 47.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31(12):1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen TP, Yong HE, Chollangi T, Borg AJ, Brennecke SP, Murthi P. Placental vitamin D receptor expression is decreased in human idiopathic fetal growth restriction. J Mol Med (Berl). 2015;93(7):795-805. [DOI] [PubMed] [Google Scholar]
- 49.Chan SY, Susarla R, Canovas D, et al. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta. 2015;36(4):403-409. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen M, Trubert CL, Rizk-Rabin M, et al. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89-90(1-5):93-97. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol. 1996;271(3, pt 1):L392-L399. [DOI] [PubMed] [Google Scholar]
- 52.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9(3):202-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christensen N, Søndergaard J, Fisker N, Christesen HT. Infant respiratory tract infections or wheeze and maternal vitamin D in pregnancy: a systematic review. Pediatr Infect Dis J. 2017;36(4):384-391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Assessment of Bias Risk of Randomized Clinical Trials
eFigure 1. Summary Crude Risk Ratio (RR) of the Association Between Vitamin D Supplementation and Small for Gestational Age (SGA) Stratified by Administration Method of Intervention (Regular or Bolus Dose)
eFigure 2. Summary Risk Ratio of the Association 1 Between Vitamin D Supplementation Group and Fetal or Neonatal Mortality Stratified by Administration Method of Intervention (Regular or Bolus Dose).
eFigure 3. Forest Plots of Summary Mean Difference (MD) of Neonatal 25-Hydroxyvitamin D (25[OH]D) (ng/mL) Between Vitamin D Supplementation Group and Control Group
eFigure 4. Forest Plots of Summary Mean Difference (MD) of Neonatal Calcium (mg/dL) Between Vitamin D Supplementation Group and Control Group
eFigure 5. Forest Plots of Summary Mean Difference (MD) of Birth Weight (g) Between Vitamin D Supplementation Group and Control Group
eFigure 6. Forest Plots of Summary Mean Difference (MD) of Postnatal Weight (kg) Between Vitamin D Supplementation Group and Control Group in Infants at Age 3 Months; 6 Months; 9 Months; and 12 Months
eFigure 7. The Funnel Plots of the Primary Outcomes