Summary
Redox-active metals are thought to be implicated in neurodegenerative diseases including amyotrophic lateral sclerosis (ALS). To address this point, we measured the concentrations of 12 elements and, for the first time, the stable isotope compositions of copper (redox-active) and zinc (redox-inactive) in human cerebrospinal fluids of 31 patients with ALS, 11 age-matched controls (CTRL), and 14 patients with Alzheimer disease. We first show that metal concentrations weakly discriminate patients with ALS from the two other groups. We then report that zinc isotopic compositions are similar in the three groups, but that patients with ALS have significantly 65copper-enriched isotopic compositions relative to CTRL and patients with AD. This result unambiguously demonstrates that copper is implicated in ALS. We suggest that this copper isotopic signature may result from abnormal protein aggregation in the brain parenchyma, and propose that isotopic analysis is a potential tool that may help unraveling the molecular mechanisms at work in ALS.
Subject Areas: Nuclear Medicine, Isotope Chemistry, Neuroscience, Clinical Neuroscience
Graphical Abstract
Highlights
-
•
Redox-active metals are implicated in ALS through oxidative stress
-
•
Concentrations of these metals in CSFs of patients with ALS are non-specific
-
•
Copper stable isotope composition in CSFs of patients with ALS are specific
-
•
Isotopic balance between CSFs and brain is probably the mechanism
Nuclear Medicine; Isotope Chemistry; Neuroscience; Clinical Neuroscience
Introduction
Amyotrophic lateral sclerosis (ALS) is one of the most harmful neurodegenerative diseases characterized by the progressive deterioration of the upper and lower motor neurons, leading to muscle weakness and atrophy, severe paralysis, and finally death typically within 3–5 years after symptom onset (Narasimhan, 2015). Currently, although genetic factors have been identified to play a role in familial ALS, which represents only 10% of the reported cases (Taylor et al., 2016), the leading mechanisms accounting for motor neuron degeneration in sporadic ALS (i.e., 90% of ALS cases) remains unknown. In addition, because of the complexity of the pathology, reliable markers (Savage, 2017) and consequently efficient treatments are still missing (Scott, 2017). Recently, biomarkers in cerebrospinal fluid (CSF) including concentrations of neurofilament light chain (Lu et al., 2015) and chemokines (Lind et al., 2016) have been identified. However, concentrations of neurofilament light chain in CSF have also been reported to be elevated in other neurological disorders such as Alzheimer disease (AD) (Zetterberg et al., 2016) and Parkinson disease (Bäckström et al., 2015). Robust and specific ALS markers are still scarce.
As for other neurodegenerative diseases, the progression of ALS is associated with the production of free radicals such as reactive oxygen species (Barnham and Bush, 2014), the production of which can be favored by the presence of free, redox-active metals like copper (Cu) (Barnham and Bush, 2014). Conversely, these metals can also be catalytic cofactors of several enzymes, like Cu/Zn superoxide dismutase (SOD1), involved in free radical detoxification by catalyzing highly toxic products (i.e., superoxide) to less dangerous species such as dioxygen and hydrogen peroxide (Valentine et al., 2005). Redox-active metals thus play a pivotal role in both the pro- and anti-oxidant homeostasis, and it is expected that dysregulation affecting these pathways will be characterized by significant elemental impairment. In mice models of familial ALS caused by mutations in SOD1, Cu has been observed to accumulate in the spinal cord (Tokuda et al., 2013). Metal dysregulations have also been observed for other redox-active metals in CSFs of patients with ALS including iron and manganese (Barnham and Bush, 2014, Roos et al., 2013). Zinc (Zn), which is not redox active, but binds to SOD1, has also been reported to be dysregulated in CSFs of patients with ALS (Hozumi et al., 2011). Comparing published results generally leads to equivocal conclusions about the usefulness of metal concentrations in CSFs to diagnose and study ALS (Figure S1).
Contrary to concentrations, stable isotope compositions may offer a more comprehensive view on biological reactions and neurological disease progression. This is due to two main reasons. First, stable isotope compositions are measured with a precision of about two orders of magnitude better than concentrations. Second, the intensity of enrichment or depletion of a metal in a given compartment is hardly predictable, whereas isotopic fractionation, i.e., the variation of the natural abundances of stable isotope ratios between coexisting compartments, can usually be quantitatively predicted by ab initio calculations (Albarède et al., 2016). In blood, Cu isotope compositions (δ65Cu) exhibit, for example, significant differences between men and women (Jaouen et al., 2012) as well as during aging (Jaouen et al., 2013). In addition, the δ65Cu value varies in pathological conditions such as cancer (Balter et al., 2015), Wilson disease, or liver cirrhosis (Costas-Rodriguez et al., 2016). Regarding neurodegenerative disorders, although Cu isotope compositions seem to be insensitive to the SOD1G93A mutation in the brain of mouse model (Enge et al., 2017), they are highly responsive in models of prion protein knockout (PrP-KO) mice (Büchl et al., 2008, Miller et al., 2016). Zinc isotope compositions (δ66Zn) were also scrutinized in mouse models of neurodegenerative diseases. In PrP-KO mice, the brain δ66Zn values were heavier than in wild-type controls (Büchl et al., 2008), a pattern also observed in mutant mouse (APPswe/PSEN1dE9) developing Alzheimer-like disease (Moynier et al., 2017).
In the present work, to further explore the potential of elemental concentrations and isotopic compositions for clinical studies of ALS, we measured the concentration of 12 major and trace elements and the Cu and Zn isotopic compositions in the CSFs of patients with ALS (n = 31), age-matched controls (CTRL, n = 11), and patients with AD (n = 14).
Results
Major and trace element concentrations as well as Cu and Zn isotopic compositions measured in CSFs of subjects with ALS, CTRL, and subjects with AD are all reported in Data S1.
ALS-Related Dysregulations of Major and Trace Element Concentrations
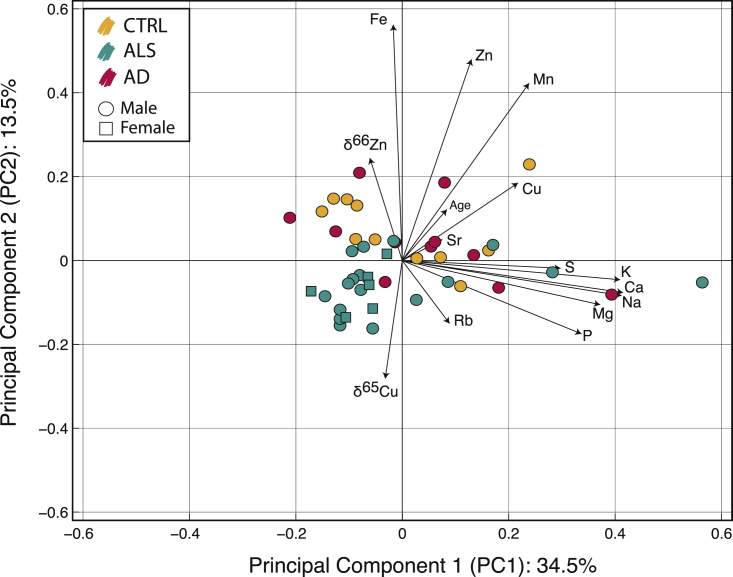
To evaluate the whole pattern of variations, we used a principal component analysis (PCA) (Figure 1). The most noticeable feature is the chemical distinction between CSFs of patients with ALS and CTRL as illustrated by the y principal component axis (Figure 1). Subjects with ALS have, for example, significant lower Fe concentrations (Wilcoxon-Mann-Whitney, p value = 2.426 × 10−3) and Mn concentrations (Wilcoxon-Mann-Whitney, p value = 2.206 × 10−3) (Figure 1) but higher Rb concentrations (Wilcoxon-Mann-Whitney, p value = 1.003 × 10−2) (Figure 1 and Data S1). Conversely, Zn concentrations (Wilcoxon-Mann-Whitney, p value = 0.0715) and Cu concentrations (Wilcoxon-Mann-Whitney, p value = 0.1610) do not exhibit any systematics (Figure S1), an observation that also holds for other trace and major elements such as Sr, S, P, or Na (Data S1). Although these results are in line with a couple of studies, e.g., Ostachowicz et al., 2006, Kanias and Kapaki, 1997, and Kapaki et al. (1997), opposite or equivocal results have also been reported (Figure S1 and references therein).
Figure 1.
Principal Component Analysis of the Results
The PCA allows the distinction of patients with ALS (green points) from CTRL subjects (yellow points) and subjects with AD (pink points). Concentrations and isotopic compositions are normalized to their SD. The two principal components (PC1 and PC2) are represented and explain ∼50% of the total variance in chemical composition. For each component, black straight lines show the loading factors (i.e., the weight of each variable on the principal components). Circles and squares points stand for male and female subjects, respectively. No significant variation is observed between the male and the female subjects within the ALS group.
ALS-Related Dysregulations of Cu-Zn Isotopic Compositions
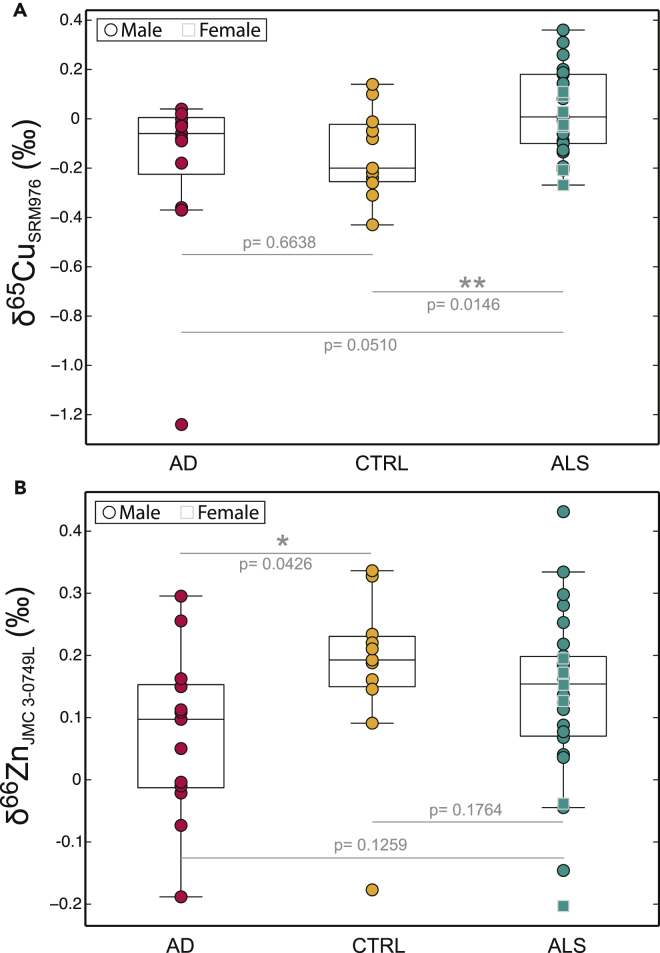
Regarding the Cu and Zn isotopic compositions, Cu isotopic ratios in ALS (median δ65CuALS = 0.01‰, 25th percentile = −0.10‰; 75th percentile = 0.18‰) are significantly heavier (Wilcoxon-Mann-Whitney, p value = 0.0146) than CTRL (median δ65CuCTRL = −0.20‰, 25th percentile = −0.26‰; 75th percentile = −0.02‰) (Figure 2A). By contrast, no significant difference is observed between Zn isotopic compositions of ALS (median δ66ZnALS = 0.15‰, 25th percentile = 0.07‰; 75th percentile = 0.20‰) and CTRL (median δ66ZnCTRL = 0.19‰, 25th percentile = 0.15‰; 75th percentile = 0.23‰) (Wilcoxon-Mann-Whitney, p value = 0.1764) (Figure 2B).
Figure 2.
Copper (δ65Cu) and Zinc (δ66Zn) Isotopic Variability in Cerebrospinal Fluids
(A and B) (A) Copper (δ65Cu) and (B) zinc (δ66Zn) isotopic compositions measured in CSFs of CTRL (yellow dots), patients with AD (red dots), and patients with ALS (green dots). Circle and square points are for male and female subjects, respectively. For each boxplot, the central mark is the median; the edges of the box are the first (i.e., 25th percentiles) and the third quartiles (i.e., 75th percentiles), respectively; and the points outside the boxes extend to the most extreme data points (i.e., not considered outliers). Significance level (i.e., p value) was determined using non-parametric “two-sided,” Wilcoxon-Mann-Whitney U tests. **p < 0.005, *p < 0.05.
Similar observations can be made by comparing the δ65Cu value of patients with ALS and AD (median δ65CuAD = −0.06‰, 25th percentile = −0.23‰; 75th percentile = 0.01‰), patients with AD being chemically indistinguishable from CTRL subjects (Wilcoxon-Mann-Whitney, p value = 0.6638) (Figure 2A). However, subjects with AD tend to have a slightly lower δ66Zn value (median δ66Zn = 0.10‰, 25th percentile = −0.01‰; 75th percentile = 0.15‰) than CTRL subjects (Wilcoxon-Mann-Whitney, p value = 0.0426).
Effect of Biological and Clinical Parameters on Elemental Concentrations and Isotopic Compositions
Biological (gender and age at sample collection) and clinical (localization of first symptoms, Awaji criteria, ALSFRS-R score, delay between sampling date and first visible symptoms) parameters are given in Table S1. Focusing on the ALS and CTRL groups, neither clinical parameters nor biological parameters show significant association with elemental concentrations and Cu-Zn isotopic compositions. An illustration is the similar δ65Cu values observed between male and female subjects in the ALS group (median δ65CuALS-female = −0.02‰, 25th percentile = −0.16‰, 75th percentile = 0.07‰; median δ65CuALS-male = +0.01‰, 25th percentile = −0.10‰, 75th percentile = 0.20‰) (Wilcoxon-Mann-Whitney, p value = 0.2721) as well as between patients with ALS characterized by different site at onset (i.e., lower limbs, ML; upper limbs, MS; bulbar, bulb) (median δ65CuALS-MI = 0.08‰, 25th percentile = 0.01‰, 75th percentile = 0.14‰; median δ65CuALS-MS = −0.06‰, 25th percentile = −0.15‰, 75th percentile = 0.07‰; and median δ65CuALS-bulb = −0.03‰, 25th percentile = −0.13‰, 75th percentile = 0.18‰) (Table S1).
Discussion
Disruption of the homeostasis of metals such as Cu and Zn is a key feature of neurodegenerative diseases, including ALS, leading to multiple abnormalities in the CSF, brain, and spinal cord, where they are inappropriately redistributed (Barnham and Bush, 2014). The changes in the distribution of metals seem to be controlled by their sequestration within misfolded protein aggregates as shown, for example, in amyloid-β (Aβ) plaques within the AD brain (Pithadia and Lim, 2012). In ALS, misfolded protein aggregates including TAR DNA-binding protein 43 (TDP-43), fused in sarcoma (FUS) (Pokrishevsky et al., 2012), and SOD1 (Valentine et al., 2005) have been reported to be present in the brain. Described as the main pathological hallmark of ALS, these proteins, like Aβ for AD, may concentrate metals. The local accumulation is likely to deplete the surrounding environment, ultimately scavenging the CSF burden, leading to decreased CSF concentrations. However, no change in elemental concentrations in patients with ALS, or AD, compared with CTRL, is observed in the present study, and more generally in the literature (Figure S1 and reference therein). The absence of any systematics may result from the natural wide range of metal concentrations in human fluids (e.g., Iyengar and Wolttiez, 1988). A more robust pattern of variations of metal concentrations is probably specific to each patient and would be more accessible through analysis of cohorts. The absence of any systematics may also result from exogenous contamination. Collection, storage, and preparation of CSFs can be sensitive to trace element contamination (Garçon et al., 2017). CSFs are made of 99% water and have very low metal contents; hence the risk of contamination is high. In this study, we ensure the absence of external contamination by quantifying the chemical content that may be released by sampling procedures and storage in tubes as well as dropper-type pipettes used to collect and conserve CSF. A complete description of the procedure is given in Transparent Methods. We also took care of low-acid blanks before and after each column chemistry, and we only used vinyl gloves as suggested by Garçon et al. (2017).
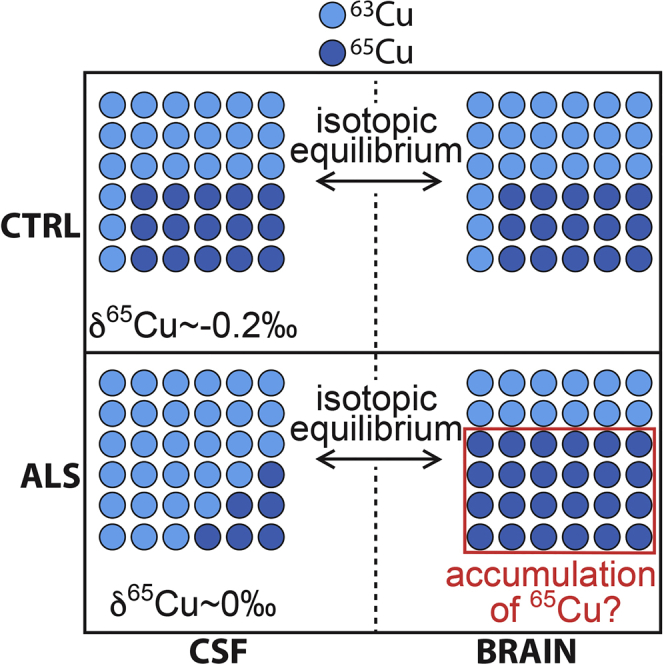
One advantage of using isotopic composition over concentration for a given element is that isotopic fractionation is not dependent on the amount of the element. Isotopic compositions are thus theoretically more reliable biomarkers than concentrations. Here, we found that CSFs of patients with ALS have significantly heavier Cu isotopic composition compared with age-matched CTRL (Wilcoxon-Mann-Whitney, p value = 0.0146) and also tend to be different from those of patients with AD by having slightly heavier δ65Cu (Wilcoxon-Mann-Whitney, p value = 0.0510) (Figure 2A), whereas again no distinction is observed for Cu concentration (Figure S1). Using receiver operating characteristic (ROC) analysis, we determined a δ65Cu cutoff value of −0.05‰ with a sensitivity and a specificity of 73% and 65% respectively, and an accuracy of 76% (Figure S2). This is a modest score compared, for instance, with the results obtained by Pasinetti et al. (2006), with a sensitivity, specificity, and accuracy of 91%, 97%, and 95%, respectively, using three protein concentrations in CSF. Noteworthy is the specificity of the CSF δ65Cu values of ALS as illustrated by the absence of significant δ65Cu difference between subjects with AD and CTRL subjects (Wilcoxon-Mann-Whitney, p value = 0.6638) (Figure 2A). Further additional studies and evidence of the mechanisms at work are undoubtedly needed to improve the present results achieved by the ROC test.
Recently, Moynier et al. (2017) showed that brains of mice with AD have higher δ66Zn value than wild-type mice and suggested that this isotopic enrichment may result from the formation of Aβ plaques in the brain parenchyma binding preferentially heavier Zn isotopes. Following this assumption, if the binding of a metal in the protein aggregates of the brain is associated with an isotopic fractionation, this must be balanced in the CSF, the brain and CSF being two complementary reservoirs in a closed system, the CNS. At first glance, our results on patients with AD support the hypothesis of an isotopic equilibrium between brain and CSF, because patients with AD exhibit CSF depleted in heavier Zn isotopes relative to CTRL subjects. However, this requires further analysis of CSF of patients with AD and CTRL subjects, which is beyond the scope of the present work. Focusing on ALS, a similar reasoning can be proposed with aggregation of SOD1 in the brain associated with a Cu isotopic fractionation. Direct evidence could be obtained by measuring the Cu isotope composition of aggregates, but here we can test if the assumption of an isotopic equilibrium between brain and CSF resists mass conservation laws. The concentration of Cu in brain varies between 3 and 5 μg/g (Scheiber et al., 2014). The brain volume ranges from 1,300 to 1,500 mL leading to a brain Cu burden (MB) of 3.9–7.5 mg. Regarding CSF, the concentration of Cu ranges from 0.02 to 0.2 μg/mL (Table S1; Iyengar and Wolttiez, 1988) and, with a volume of 150–250 mL, this gives a total Cu mass (MCSF) of 3–5 μg. As a first approximation, the CNS can be considered as a closed system at steady state and, δ65CuB and δ65CuCSF being the Cu isotopic composition in brain and CSF, respectively, one can write that:
| MB. δ65CuB ⇔ MCSF. δ65CuCSF |
If the isotopic composition of Cu varies in one compartment by a factor Δ, this must be balanced in the other one such that:
| MB. Δδ65CuB = MCSF. Δδ65CuCSF |
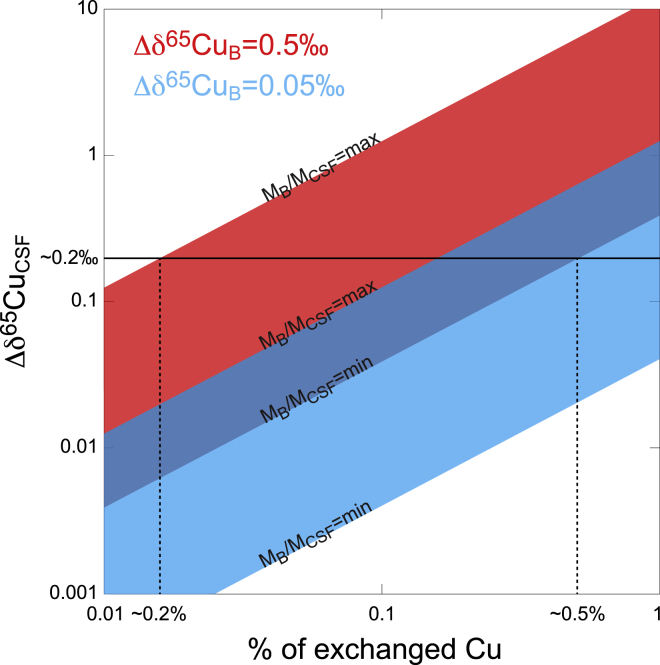
It is thus possible to calculate the Cu isotopic offset in the CSF (Δδ65CuCSF) that would result from the incorporation of Cu in the brain with an isotopic fractionation Δδ65CuB. The assumption that the CNS is a closed system is made for the sake of simplicity for purposes of mass balance calculations. Physiologically, metals and other molecules are supplied in the CNS by blood, but classically stay there and accumulate with time. The inward flux of Cu in the CNS by time unit is unknown but should be very small compared with the mass of the CNS, considering that the Cu amount in blood (∼5 μg) is three orders of magnitude less than that in CNS. Results of the calculations are illustrated by Figure 3. Intuitively, because there are between 78 and 2,500 more Cu in the brain than in the CSF, the Δδ65CuB value must be very small. Indeed, when the highest MB/MCSF ratio is considered (2,500), a contribution of only 0.01% of pathological Cu associated with a Δδ65CuB value of 0.5‰ can trigger a Δδ65CuCSF offset of almost 0.2‰, which is the observed Cu isotopic difference between ALS and CTRL. An identical Δδ65CuCSF offset is obtained with a contribution of pathological Cu of 0.5% when the lowest MB/MCSF ratio of 78 is considered. Altogether, these results show that minute proportions of aggregates formation associated with relevant Cu isotopic fractionation can likely explain the observed differences between the CSFs δ65Cu values of CTRL and patients with ALS. Whatever the proportion of formed aggregates, the increase of the ALS δ65Cu values implies for mass balance requirement that 63Cu preferentially binds in aggregates. Measuring the Cu isotopic composition of protein aggregates and normal adjacent areas in autopsies of brains of patients with ALS, and/or in brains of ALS animal model like the SOD1G93A mice, can be the aim of future experiments to challenge this hypothesis.
Figure 3.
Cu Isotope Mass Balance between CSF and Brain
Calculation of the Cu isotopic offset in the CSF (Δδ65CuCSF) that results from the incorporation of Cu in the brain (% of exchanged Cu) associated with an isotopic fractionation Δδ65CuB. The calculations are made with two values of Δδ65CuB (0.5‰ in red and 0.05‰ in blue) and two extreme values of the MB/MCSF ratio. The results thus define two areas in red and blue that overlap giving the dark blue area. The results discussed in the text corresponding to some proportions of exchanged Cu for a Δδ65CuCSF value of 0.2‰, which represents the difference between CTRL and ALS δ65Cu mean values, are illustrated.
Another way to explain the specific Cu isotopic composition of ALS would involve a differential Cu isotope fractionation between detergent-soluble SOD1 and aggregated, insoluble, SOD1. In mutant (G37R and G93A) SOD1 proteins from SOD1-ALS transgenic mice spinal cords, Lelie et al. (2011) found that aggregated, insoluble, SOD1 is metal depleted, whereas soluble SOD1 is highly Cu metallated. Exchange of Cu between soluble and aggregated SOD1 is unlikely because soluble SOD1 is thought to be highly stable. Lelie et al. (2011) also argued that aggregation occurs when SOD1 is in its immature, unmodified, apo form (Shaw et al., 2008). All these results suggest that the aggregation of SOD1 leads to the over-metallation of soluble SOD1. The abnormal Cu metallation of soluble SOD1 can be accompanied by different isotopic fractionation from normal conditions, which can explain the present results. Measurement of the Cu isotope composition of the soluble and insoluble fractions of SOD1-ALS transgenic mice spinal cords would help to confirm this hypothesis.
To conclude, our results, i.e., the first to report Cu and Zn isotopic compositions in CSFs of patients with ALS and AD and CTRL subjects, demonstrate that Cu isotopic measurements in CSF may offer a more comprehensive view of the ALS metallome than elemental concentrations and may potentially reinforce any diagnosis. Similarly, the lighter δ66Zn value observed in the CSF of patients with AD compared with that of CTRL subjects may also offer promising information regarding Zn dyshomeostasis in AD. Increasing the number of Cu and Zn isotopic measurements in CSFs of ALS and AD, respectively, as well as in other neurodegenerative pathologies, such as Parkinson or Huntington diseases, would undoubtedly challenge the proposition that δ65Cu and δ66Zn values in CSF are probably future candidate biomarkers of neurodegenerative diseases.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank three anonymous reviewers for their very helpful comments, Philippe Télouk for his assistance during isotopic measurements, and Mélanie Simon for her help regarding chemical procedures. Thanks are also due to the clinicians Dr. Anne Nove-Josserand and Dr. Dominique Minier, the chiefs of the Division of Neurology at Hospital North of Villefranche sur Saône and at William Morey Hospital in Châlon sur Saône, respectively. V.B. and L.S. are grateful to Fondation Bullukian and Fondation Mérieux as well as the Ecole Polytechnique Fédérale of Lausanne (EPFL) for their financial support. P.L. received support from the Association pour la Recherche sur la SLA (ARSLA), the NeuroDis Foundation, the AFM (AFM-MyoNeuralp), the CNRS, and INSERM.
Author Contributions
Conceptualization, E. Bernard, E. Broussolle, P.L., and V.B.; Methodology, L.S. and V.B.; Investigation, L.S., E. Bernard, A.P.-L. I.Q. A.V., P.K.-S., and E. Broussolle; Resources, E. Bernard, A.P.-L., I.Q., A.V., P.K.-S., E. Broussolle, and V.B.; Writing – Original Draft, L.S., E. Bernard, E. Broussolle, P.L., and V.B.; Writing – Reviews and Editing, L.S., E. Bernard, E. Broussolle, P.L., and V.B.; Funding Acquisition, V.B.
Declaration of Interests
The authors have no financial interests to declare.
Published: August 31, 2018
Footnotes
Supplemental Information includes Transparent Methods, three figures, one table, and two data files and can be found with this article online at https://doi.org/10.1016/j.isci.2018.07.023.
Supporting Citations
Costa et al., 2012, del Campo et al., 2012, Ihara et al., 2013, Jaouen et al., 2016, Kapaki et al., 1989, Maréchal et al., 1999, McKhann et al., 2011.
Supplemental Information
References
- Albarède F., Télouk P., Balter V., Bondanese V.P., Albalat E., Oger P., Bonaventura P., Miossec P., Fujii T. Medical applications of Cu, Zn, and S isotope effects. Metallomics. 2016;8:1056–1070. doi: 10.1039/c5mt00316d. [DOI] [PubMed] [Google Scholar]
- Bäckström D.C., Eriksson Domellöf M., Linder J., Olsson B., Öhrfelt A., Trupp M. Cerebrospinal Fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 2015;72:1175–1182. doi: 10.1001/jamaneurol.2015.1449. [DOI] [PubMed] [Google Scholar]
- Balter V., Nogueira da Costa A., Bondanese V.P., Jaouen K., Lamboux A., Sangrajrang S. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA. 2015;112:982–985. doi: 10.1073/pnas.1415151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham K.J., Bush A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014;43:6727–6749. doi: 10.1039/c4cs00138a. [DOI] [PubMed] [Google Scholar]
- Büchl A., Hawkesworth C.J., Ragnarsdottir K.V., Brown D.R. Re-partitioning of Cu and Zn isotopes by modified protein expression. Geochem. Trans. 2008;9:11. doi: 10.1186/1467-4866-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J., Swash M., de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis. Arch. Neurol. 2012;69:1410. doi: 10.1001/archneurol.2012.254. [DOI] [PubMed] [Google Scholar]
- Costas-Rodriguez M., Delanghe J., Vanhaecke F. High-precision isotopic analysis of essential mineral elements in biomedicine: natural isotope ratio variations as potential diagnostic and/or prognostic markers. Trends Anal. Chem. 2016;76:182–193. [Google Scholar]
- del Campo M., Mollenhauer B., Bertolotto A., Engelborghsn S., Hampel H., Simonsen A.H. Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: an update. Biomarkers Med. 2012;6:419–430. doi: 10.2217/bmm.12.46. [DOI] [PubMed] [Google Scholar]
- Enge T.G., Ecroyd H., Jolley D.F., Yerbury J.J., Dosseto A. Longitudinal assessment of metal concentrations and copper isotope ratios in the G93A SOD1 mouse model of amyotrophic lateral sclerosis. Metallomics. 2017;9:161–174. doi: 10.1039/c6mt00270f. [DOI] [PubMed] [Google Scholar]
- Garçon M., Sauzéat L., Carlson R.W., Shirey S.B., Simon M., Balter V. Nitrile, latex, neoprene and vinyl gloves: a primary source of contamination for trace element and Zn isotopic analyses in geological and biological samples. Geostand. Geoanal. Res. 2017;41:367–380. [Google Scholar]
- Hozumi I., Hasegawa T., Honda A., Ozawa K., Hayashi Y., Hashimoto K. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci. 2011;303:95–99. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Nobukuni K., Takata H., Hayabara T. Oxidative stress and metal content in blood and cerebrospinal fluid of amyotrophic lateral sclerosis patients with and without a Cu, Zn-superoxide dismutase mutation. Neurol. Res. 2013;27:105–108. doi: 10.1179/016164105X18430. [DOI] [PubMed] [Google Scholar]
- Iyengar V., Wolttiez J. Trace elements in Human clinical specimens: evaluation of literature data to identify reference values. Clin. Chem. 1988;34:474–481. [PubMed] [Google Scholar]
- Jaouen K., Balter V., Herrscher E., Lamboux A., Télouk P., Albarède F. Fe and Cu stable isotopes in archeological human bones and their relationship to sex. Am. J. Phys. Anthropol. 2012;148:334–340. doi: 10.1002/ajpa.22053. [DOI] [PubMed] [Google Scholar]
- Jaouen K., Gibert M., Lamboux A., Télouk P., Fourel F., Albarède F. Is aging recorded in blood Cu and Zn isotope compositions? Metallomics. 2013;5:1016–1024. doi: 10.1039/c3mt00085k. [DOI] [PubMed] [Google Scholar]
- Jaouen K., Beasley M., Schoeninger M., Hublin J.-J., Richards M.P. Zinc isotope ratios of bones and teeth as new dietary indicators: results from a modern food web (Koobi Fora, Kenya) Sci. Rep. 2016;6:1–8. doi: 10.1038/srep26281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanias G.D., Kapaki E. Trace elements, age, and sex in amyotrophic lateral sclerosis disease. Biol. Trace Elem. Res. 1997;56:187–201. doi: 10.1007/BF02785392. [DOI] [PubMed] [Google Scholar]
- Kapaki E., Segditsa C., Papageorgiou C. Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acat. Neurol. Scand. 1989;79:373–378. doi: 10.1111/j.1600-0404.1989.tb03803.x. [DOI] [PubMed] [Google Scholar]
- Kapaki E., Zournas C., Kanias G., Zambelis T., Kakami A., Papageorgiou C. Essential trace element alterations in amyotrophic lateral sclerosis. J. Neurol. Sci. 1997;147:171–175. doi: 10.1016/s0022-510x(96)05334-8. [DOI] [PubMed] [Google Scholar]
- Lelie H.L., Liba A., Bourassa M.W., Chattopadhyay M., Chan P.K., Gralla E.B., Miller L.M., Borchelt D.R., Valentine J.S., Whitelegge J.P. Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice. J. Biol. Chem. 2011;286:2795–2806. doi: 10.1074/jbc.M110.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind A.-L., Wu D., Freyhult E., Bodolea C., Ekegren T., Larssson A. A multiplex protein panel applied to cerebrospinal fluid reveals three new biomarker candidates in ALS but none in neuropathic pain patients. PLoS One. 2016;11:e0149821. doi: 10.1371/journal.pone.0149821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.H., Macdonald-Wallis C., Gray E., Pearce N., Petzold A., Norgren N. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal C.N., Télouk P., Albarède F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 1999;156:251–273. [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.A., Keenan C.M., Martin G.R., Jirik F.R., Sharkey K.A., Wieser M.E. The expression levels of cellular prion protein affect copper isotopic shifts in the organs of mice. J. Anal. Spectrom. 2016;31:2015–2022. [Google Scholar]
- Moynier F., Foriel J., Shaw A.S., Le Borgne M. Distribution of Zn isotopes during Alzheimer’s disease. Geochem. Persp. Lett. 2017;3:142–150. [Google Scholar]
- Narasimhan S.D. A brief history of ALS. Cell. 2015;161:181–183. [Google Scholar]
- Ostachowicz B., Lankosz M., Tomik B., Adamek D., Wobrauschek P., Streli C. Analysis of some chosen elements of cerebrospinal fluid and serum in amyotrophic lateral sclerosis patients by total reflection X-ray fluorescence. Spectrochim. Acta Part B At. Spectrosc. 2006;61:1210–1213. [Google Scholar]
- Pasinetti G.M., Ungar L.H., Lange D.J., Yemul S., Deng H., Yuan X., Brown R.H., Cudkowicz M.E., Newhall K., Peskind E. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66:1218–1222. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- Pithadia A., Lim M.H. Metal-associated amyloid-β in Alzheimer's disease. Curr. Opin. Chem. Biol. 2012;16:67–73. doi: 10.1016/j.cbpa.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Pokrishevsky E., Grad L.I., Yousefi M., Wang J., Mackenzie I.R., Cashman N.R. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos P.M., Vesterberg O., Syversen T., Flaten T.P., Nordberg M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 2013;151:159–170. doi: 10.1007/s12011-012-9547-x. [DOI] [PubMed] [Google Scholar]
- Savage N. Calculating disease. Nature. 2017;550:S115. doi: 10.1038/550S115a. [DOI] [PubMed] [Google Scholar]
- Scheiber I.F., Mercer J.F.B., Dringen R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014;116:33–57. doi: 10.1016/j.pneurobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Scott A. On the treatment trail for ALS. Nature. 2017;550:S120. doi: 10.1038/550S120a. [DOI] [PubMed] [Google Scholar]
- Shaw B.F., Lelie H.L., Durazo A., Nersissian A.M., Xu G., Chan P.K., Gralla E.B., Tiwari A., Hayward L.J., Borchelt D.R. Detergent-insoluble aggregates associated with amyotrophic lateral sclerosis in transgenic mice contain primarily full-length, unmodified superoxide dismutase-1. J. Biol. Chem. 2008;283:8340–8350. doi: 10.1074/jbc.M707751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.P., Brown R.H., Cleveland D.W. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda E., Okawa E., Watanabe S., Ono S.-I., Marklund S.L. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase-1s regardless of their copper-binding abilities. Neurobiol. Dis. 2013;54:308–319. doi: 10.1016/j.nbd.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Valentine J.S., Doucette P.A., Zittin Potter S. Copper-Zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- Zetterberg H., Skillbäck T., Mattsson N., Trojanowski J.Q., Portelius E., Shaw L.M. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73:60. doi: 10.1001/jamaneurol.2015.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.