Abstract
Apolipoprotein A-I (apoA-I) shares with other exchangeable apolipoproteins a high level of structural plasticity. In the lipid-free state, the apolipoprotein amphipathic α-helices interact intra- and intermolecularly, providing structural stabilization by self-association. We have reported that lipid-free apoA-I becomes amyloidogenic upon physiologically relevant (myeloperoxidase-mediated) Met oxidation. In this study, we established that Met oxidation promotes amyloidogenesis by reducing the stability of apoA-I monomers and irreversibly disrupting self-association. The oxidized apoA-I monomers also exhibited increased cellular cholesterol release capacity and stronger association with macrophages, compared to nonoxidized apoA-I. Of physiologic relevance, preformed oxidized apoA-I amyloid fibrils induced amyloid formation in nonoxidized apoA-I. This process was enhanced when self-association of nonoxidized apoA-I was disrupted by thermal treatment. Solid state NMR analysis revealed that aggregates formed by seeded nonoxidized apoA-I were structurally similar to those formed by the oxidized protein, featuring a β-structure-rich amyloid fold alongside α-helices retained from the native state. In atherosclerotic lesions, the conditions that promote apoA-I amyloid formation are readily available: myeloperoxidase, active oxygen species, low pH, and high concentration of lipid-free apoA-I. Our results suggest that even partial Met oxidation of apoA-I can nucleate amyloidogenesis, thus sequestering and inactivating otherwise antiatherogenic and HDL-forming apoA-I.—Witkowski, A., Chan, G. K. L., Boatz, J. C., Li, N. J., Inoue, A. P., Wong, J. C., van der Wel, P. C. A., Cavigiolio, G. Methionine oxidized apolipoprotein A-I at the crossroads of HDL biogenesis and amyloid formation.
Keywords: self-association, protein structure, solid-state NMR, myeloperoxidase, cholesterol efflux
In recent years, our knowledge of the impact of oxidation on the multiple physiologic functions of apolipoprotein A-I (apoA-I) has expanded significantly. In particular, myeloperoxidase (MPO), a macrophage-originated heme enzyme strongly implicated in the development of atherosclerosis (1, 2), has been shown to target several residues of apoA-I with detrimental effects on the protein’s antiatherogenic function (3). Tyr chlorination and nitration (4–6), Lys chlorination (7, 8), Trp hydroxylation (8–10), and Met oxidation (11–13) are reactions catalyzed by MPO with apoA-I as a substrate. All these modifications have also been detected in apoA-I extracted from plasma and atheroma, with a marked increase in specific oxidative modifications of apoA-I residing in atherosclerotic lesions, compared to circulating apoA-I (6, 10, 14).
Together, these data indicate that physiologic levels of MPO impair apoA-I-induced release of cholesterol from cholesterol-laden macrophages and activation of lecithin-cholesterol acyl transferase (LCAT). Such loss of function impacts reverse cholesterol transport, the antiatherogenic pathway wherein apoA-I generates HDL by extracting cholesterol from arterial macrophages and delivers it to the liver for excretion.
Which specific apoA-I modifications lead to loss of function is still the subject of intense debate, with contradictory results from different laboratories. The Heinecke laboratory identified apoA-I Met-148 as the oxidation target that causes the observed reduction in LCAT activation (12), whereas the Hazen and Smith laboratories attributed LCAT activation dysfunction to modifications of Tyr-166 (15).
In the case of ATP binding cassette transporter A1 (ABCA1)–mediated release of cellular cholesterol toward apoA-I, its extracellular recipient, the disagreement is even more pronounced (3, 4, 6, 8, 9, 11, 14, 16–19). In vitro, chlorination levels of apoA-I Tyr residues tightly correlate with a reduction in cellular cholesterol release capacity (3, 4, 11) and in vivo, they correlate with the incidence of cardiovascular events and atherosclerosis (14, 16, 17). However, results from the Hazen and Smith laboratories suggest that chlorination of Tyr residues is a good marker, but not the primary cause, of dysfunction (6). In fact, the impact of MPO-mediated oxidation on an apoA-I variant in which all Tyr residues were conservatively replaced with Phe was equivalent to the impact on wild-type apoA-I (8). In contrast, an apoA-I variant in which all 4 Trp were conservatively replaced with Phe (4WF-ApoA-I) was immune to MPO-mediated impairment of the cholesterol acceptor function (9, 18, 19). This observation indicates that MPO-mediated oxidation of Trp plays a central role in apoA-I loss of function (9).
We previously reported that MPO-mediated oxidation of apoA-I in relatively mild oxidative conditions sufficed to promote amyloid formation by the oxidized lipid-free protein (20). Such an amyloidogenic outcome is exclusively dependent on oxidation of apoA-I Met residues and does not require modification of any other residues (20). Although lipid-free apoA-I has a relatively short lifetime in circulation (21–23), in atherosclerotic lesions it accumulates at concentrations up to 100-fold higher than in plasma (24). Furthermore, in this environment, apoA-I Met residues are easily exposed to oxidation by macrophage-secreted MPO and active oxygen species (6, 10, 14).
With aging, amyloid material accumulates in the media and intima layers of human arteries (25–28). Such amyloids occur with high incidence (25, 29–32): for example, amyloids were found in 97% of media aortic samples from patients older than 50 yr (26). Although a causal link between the process of amyloid deposition in the arteries and atherosclerosis progression has been often postulated (33), so far, no study has been conducted with sufficient observed power to demonstrate this causality (26, 28, 29).
Several amyloidogenic proteins colocalize in amyloids associated with atherosclerotic lesions (33). ApoA-I is the most abundant component of this material (27–29, 31). Furthermore, apoA-I is the main constituent of amyloids isolated not only from aortic valves (32), aorta atherosclerotic plaques (27, 28), and peripheral atherosclerotic plaques (29), but also from knee joints (34), lung nodules (35), and peripheral neural tissues (sciatic nerve) (36). The Met oxidation state of apoA-I in amyloid deposits is largely unknown, mainly because of the difficulty in preserving the original oxidation state of proteins during the elaborate process of dissecting amyloid material ex vivo and purifying amyloids from other tissue components. In a study of amyloids from a lumbosacral radiculoplexus amyloidoma, magnetic resonance imaging–guided fascicular nerve biopsy combined with laser-dissected mass spectrometric analysis identified complete oxidation of apoA-I Met-112, but no information was reported regarding the oxidation state of the other 2 Met residues (36). The frequency of apoA-I amyloid deposits in different tissues and disease states, such as atherosclerosis, combined with our lack of knowledge of the process of apoA-I amyloid formation and deposition in vivo warrant further investigation of the mechanism of apoA-I oxidation and amyloid formation.
In this study we investigated the structural effects of Met oxidation on apoA-I and how these structural changes affect the cellular functions and amyloidogenic potential of the protein. Met-oxidized apoA-I is a competent recipient of cellular lipids in HDL biogenesis, but it can also form amyloids that deposit in the atheroma with potentially deleterious consequences. Lipid-free Met-oxidized apoA-I is therefore an atheroma-relevant molecular species that resides at the crossroads of 2 possible reactions: HDL biogenesis by binding of cell membrane lipids and aggregation into amyloid fibrils.
MATERIALS AND METHODS
Materials
MPO from human polymorphonuclear leukocytes was purchased from Calbiochem (San Diego, CA, USA), ultrapure grade thioflavin T (ThT) from AnaSpec (Fremont, CA, USA), and Sypro Orange from MilliporeSigma (Billerica, MA, USA). Sequencing grade modified trypsin was obtained from Promega (Madison, WI, USA). Isotopically labeled peptides were synthesized by CPC Scientific (Sunnyvale, CA, USA). [U-13C]-d-glucose and [15N]-ammonium chloride were purchased from Cambridge Isotope Laboratories (Xenia, OH, USA). TopFluor Cholesterol (23-(dipyrrometheneboron difluoride)-24-norcholesterol, previously known as BODIPY cholesterol) was purchased from Avanti Polar Lipids (Alabaster, AL, USA). Except where indicated, all other materials were purchased from MilliporeSigma. TEV protease was produced as previously described (37).
Production of recombinant proteins
Multiple Met to Leu and Trp to Phe mutations were generated as described in refs. 19, 20. See Supplemental Data for additional information.
Production of isotopically labeled apoA-I
[U-13C, 15N]-labeled wild-type apoA-I (wt-13C,15N-ApoA-I) with N-terminal His tag was expressed in BL21(DE3)pLysS Escherichia coli (Agilent Technologies, Santa Clara, CA, USA), containing the pET-20b bacterial expression vector (19, 20). Cells were grown for 6 h in 5 ml of full NZYCM medium (TekNova, Hollister, CA, USA), and then 280 µl of culture medium was used to inoculate 25 ml of minimal medium (pregrowth cell culture) in which 15N-ammonium chloride and [U-13C]-d-glucose were the only source of nitrogen and carbon, respectively (38, 39). Upon overnight incubation at 37°C, 0.5 ml of the pregrowth cell culture was used to inoculate 238 ml of final minimal medium. When the optical density (OD) of the final cell culture was 0.67–0.70, protein expression was induced with 0.8 mM isopropyl β-d-1-thiogalactopyranoside (TekNova). The induced cell culture was incubated for 4 h at 18°C before cell harvesting. Purification of intracellularly expressed His-tagged protein and removal of His-tag via TEV protease reaction were performed as previously described (20, 37). After confirming protein purity by SDS-PAGE, protein preparations were frozen in 6 M guanidine chloride buffer for storage. The average protein yield was ∼5 mg of wt-13C,15N-ApoA-I per 238 ml of minimal medium cell culture. Protein refolding and analyses were performed before each experiment (Supplemental Data).
Protein oxidation
ApoA-I samples, typically at concentrations of 1.5–2.0 mg/ml, were oxidized in oxidation buffer [OB; 10 mM sodium phosphate, 100 μM diethylenetriaminepentaacetic acid, 100 mM NaCl, (pH 7.5)] (20). Exhaustive Met oxidation (H2O2-ApoA-I) was achieved in OB by overnight incubation at 37°C with a 1000:1 molar excess of H2O2. The concentration of H2O2 was determined spectrophotometrically (ε240 = 39.4 M−1 cm−1) (40). MPO-mediated oxidation was performed in OB in the presence of the MPO-H2O2-Cl− system for 90 min at 37°C. The MPO concentration was 60 nM, and H2O2:apoA-I molar ratios were 3:1 and 10:1 for MPO-3:1-H2O2-ApoA-I and MPO-10:1-H2O2-ApoA-I, respectively. Enzymatic oxidations were terminated by adding a 10-fold molar excess (relative to H2O2) of l-Met. After oxidation, the protein samples were extensively dialyzed against fibrillation buffer (10 mM sodium phosphate; pH 6.0) or the experiment-specific buffer for 2 d with 2 buffer exchanges. Control samples were subjected to the same treatment as for H2O2 oxidation, but in the absence of H2O2.
Size-exclusion chromatography
Protein analysis was performed on a Superdex 200 Increase 10/300 GL column (GE Healthcare, Waukesha, WI, USA) controlled by an AKTA Pure FPLC system (GE Healthcare). Elution by isotonic PBS (iPBS) [10 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl, and 2.7 mM KCl (pH 7.4)] was performed at a flow rate of 0.9 ml/min. The column was calibrated with globular proteins of known mass: bovine thyroid thyroglobulin, 670 kDa; equine spleen apoferritin, 481 kDa; bovine liver catalase, 240 kDa (tetramer); bovine heart l-lactic dehydrogenase type XVII, 142 kDa (tetramer); bovine albumin fraction V, 66 kDa (monomer); chicken ovalbumin, 44.3 kDa; and cytochrome c, 12.4 kDa (19, 41, 42). Distribution coefficients (Kav) were calculated as: (Ve − V0)/(Vt – V0), where V0 is the outer bead volume, Vt is the total interstitial volume, and Ve is the elution volume.
Amyloid fibril formation
Samples in fibrillation buffer (10 mM sodium phosphate; pH 6.0) were diluted to 1.0 mg/ml in sterile 1.5 ml microfuge tubes and incubated at 37°C with continuous vortexing (20). At the indicated time points, sample aliquots were withdrawn for analysis. Yields of protein aggregation were determined by comparing the residual concentration of soluble protein upon incubation of a sample under fibrillation conditions and the original protein concentration of the clear sample before fibrillation incubation. After incubation under fibrillation conditions, samples were spun at 15,000 g for 10 min and the protein concentration of the clear supernatants was measured by the bicinchoninic acid assay.
Fluorescence spectroscopy
Fluorescence emission spectra of 50 µg/ml protein samples in iPBS were recorded on a Jobin-Yvon FluoroMax-4 spectrofluorometer (Horiba, Piscataway, NJ, USA), between 300 and 450 nm, with 280 nm excitation wavelength and 2.5 nm slit width for both excitation and emission monochronomators. In amyloid formation experiments, ThT fluorescence emission spectra were collected by single-time-point dilutions (20). At selected time points a sample aliquot was withdrawn from the fibrillation mixture and rapidly diluted with stock ThT (500 μM in 50 mM sodium phosphate, pH 7.4) to obtain a final concentration of 18 µM ThT and 80 µg/ml apoA-I (2.84 μM). The intensity of ThT fluorescence emission at the wavelength of maximum fluorescence (WMF) was plotted against time to construct ThT kinetics curves. Replications of ThT experiments were performed as described in Data processing and statistical analysis.
Protein stability by Trp fluorescence thermal shift analysis
Trp fluorescence emission spectra (excitation 295 nm; protein concentration 50 µg/ml) were recorded upon sample heating from 20 to 90°C or 4 to 90°C, for nonoxidized wild-type apoA-I (wt-ApoA-I) or H2O2-oxidized wild-type apoA-I (H2O2-wt-ApoA-I), respectively, at a constant heating rate of 1°C/min in a sample heater/cooler Peltier thermocouple drive equipped with a 300B temperature controller (Newport Corp., Bozeman, MT, USA). Sigmoidal melting curves were obtained by plotting at each temperature the ratio (FWMF (F)/FWMF (U)) of the fluorescence intensities at the WMF of the folded state (F) and at the WMF of the unfolded state (U). To measure WMF of F and U, fluorescence spectra were collected at 20 and 90°C, respectively. First and second derivative curves were used to calculate the melting temperature (Tm) values reported in Table 3 as mean ± sem of ≥3 independent experiments. Representative melting curves are illustrated in Supplemental Fig. S4A, B.
TABLE 3.
Tm of monomeric nonoxidized- and H2O2-wt-ApoA-I
| Sample name | Trp fluorescence | SYPRO orange fluorescence |
|---|---|---|
| wt-ApoA-I | 58.5 ± 1.2 | 57.5 ± 2.0 |
| H2O2-wt-ApoA-I | 34.3 ± 1.7 | 37.3 ± 0.8 |
The concentration of apoA-I samples was 50 µg/ml in iPBS. Thermal shift analysis was performed by 2 independent methods: using temperature-dependent changes in Trp fluorescence (ex 295 nm) and SYPRO orange fluorescence (ex 492 nm) as reporter signals. Data are Tm values reported as mean ± sem of 3 or more independent experiments.
Protein stability by SYPRO orange fluorescence thermal shift analysis
SYPRO orange is an environmentally sensitive fluorescent dye that binds low dielectric/hydrophobic regions of proteins with a significant increase in fluorescence emission quantum yield (43, 44). In the case of apoA-I, SYPRO orange displays a much higher quantum yield when bound to the native protein (20°C), compared to the unfolded state (90°C). The sigmoidal reduction of SYPRO orange emission intensity at the WMF (excitation 492 nm) when the protein samples were heated at a constant rate of 1°C/min was used to calculate the Tm of nonoxidized- and H2O2-wt-ApoA-I. Protein samples were diluted to 50 µg/ml and SYPRO orange was added at final 500-fold dilution of the commercial DMSO stock (MilliporeSigma). SYPRO orange fluorescence emission spectra were recorded upon sample heating from 25 to 90, or 20 to 90°C, for nonoxidized apoA-I and H2O2-wt-ApoA-I, respectively. First and second derivative curves were used to calculate the Tm values reported in Table 3, as mean and sem ≥3 independent experiments. Representative melting curves are illustrated in Supplemental Fig. S4C, D.
Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) absorbance spectra were recorded between 4000 and 800 cm−1 on a Direct Detect spectrometer (MilliporeSigma) (20).
Solid-state NMR spectroscopy
Hydrated samples of protein aggregates generated by H2O2-oxidized wt-13C,15N-ApoA-I (H2O2-wt-13C,15N-ApoA-I) and those produced by seeding of nonoxidized wt-13C,15N-ApoA-I with unlabeled preformed H2O2-wt-ApoA-I amyloid seeds were separately packed into thin-wall 3.2 mm Zirconia magic-angle spinning (MAS) rotors (Bruker Biospin, Billerica, MA, USA). The hydrated aggregates were packed into the rotors at 100,500 g for 60 min using a home-built swinging-bucket ultracentrifugal packing device in an Optima L-100 XP ultracentrifuge (Beckman Coulter, Jersey City, NJ, USA) (45). The rotors were sealed with epoxy, and the samples were studied in a fully hydrated and unfrozen state. All MAS solid-state NMR spectroscopy (MAS ssNMR) experiments were performed on a wide-bore Bruker Avance I NMR spectrometer operating at a 1H Larmor frequency of 600 MHz (14.1 T) and equipped with a 3.2 mm MAS ssNMR probe with a 1H/13C/15N EFree coil (Bruker Biospin). The sample temperature was controlled by a constant flow of cooled gas. One-dimensional (1D) ramped 1H-13C cross-polarization (CP) spectra and direct 13C excitation spectra were obtained at 13 kHz MAS. Intraresidue through-space 13C–13C correlations were obtained from 2-dimensional (2D) ramped 1H-13C CP followed by 20 ms dipolar-assisted rotational resonance 13C–13C mixing (46). 1D J-coupling-based 13C spectra were obtained at 8.333 kHz MAS using a rotor-synchronized refocused insensitive nuclei enhanced by polarization transfer (INEPT) scheme. The INEPT experiments employed τ1 = 0.9 ms and τ2 = 0.5 ms for the H2O2-wt-13C,15N-ApoA-I aggregates and τ1 = 1.4 ms and τ2 = 0.8 ms for seeded nonoxidized wt-13C,15N-ApoA-I. Spectra were acquired with Bruker Topspin software, processed with NMRPipe (47), and analyzed with the CcpNmr Analysis program (48). The 13C chemical shifts were referenced to 4,4-dimethyl-4-silapentane-1-1 sulfonic acid based on external reference measurements of the 13C signals of adamantine (49). Spectral analysis was performed with the help of empirical reference data and software tools (50, 51). Synthetic simulated spectra were prepared from the α-helical structure of C terminus truncated apoA-I [PDB 3R2P (52); Supplemental Fig. S7] or fully α-helical or β-sheet models of the full-length protein generated by the Chimera program (53) (see Fig. 9D), using previously described protocols (54, 55) and standard NMR programs (47, 56).
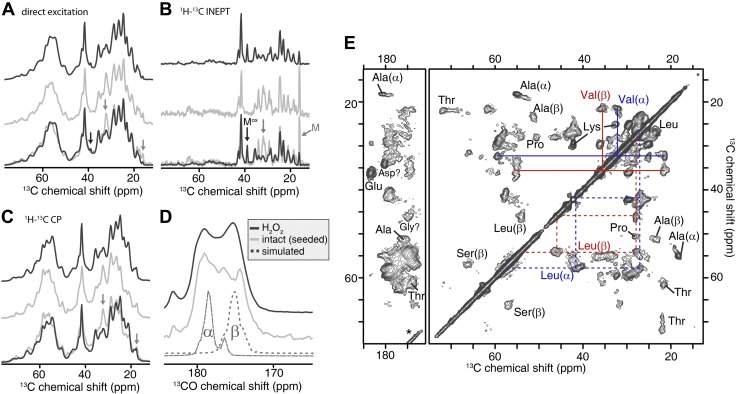
Figure 9.
MAS ssNMR spectra of pelleted [U-13C,15N] wt-ApoA-I aggregates. A) Direct excitation 13C spectra of H2O2-wt-13C,15N-ApoA-I aggregates (black trace) and aggregates formed by nonoxidized wt-13C,15N-ApoA-I incubated for 8 d with 10% (molar) of unlabeled H2O2-wt-ApoA-I seeds (light gray trace). An overlay of the 2 spectra is shown at the bottom. Black and gray arrows: the 13C signals of oxidized and unoxidized Met side chains, respectively. B) 13C spectra obtained via 1H-13C refocused INEPT, which shows only highly dynamic residues. C) 13C spectra of the same samples using 1H-13C cross polarization, which is selective for the more rigid parts of the pelleted protein samples. A–C) Only aliphatic spectral regions are shown; full spectra are reported in Supplemental Fig. S6. D) 1H-13C CP spectra in the carbonyl region of H2O2-wt-13C,15N-ApoA-I (black trace) and nonoxidized wt-13C,15N-ApoA-I after seeding (light gray trace). Dotted lines (bottom) show simulated spectra for hypothetical fully α-helical and β-sheet structural models of ApoA-I, to illustrate the secondary structure dependence. E) 2D 13C-13C spectrum of the same H2O2-wt-13C,15N-ApoA-I as in A–D, using 20 ms dipolar-assisted rotational resonance mixing. The amino acid type assignments of select cross peaks are indicated. Color-coded lines connect sets of peaks from coexisting α-helical and β-sheet Val and Leu. All spectra were acquired at 600 MHz (1H frequency).
Quantification of Met oxidation by liquid-chromatography mass-spectrometry
ApoA-I preparations were digested with a modified trypsin as described (20). Peptides were separated by reversed-phase chromatography (1290 Infinity LC system; Agilent Technologies) on an AdvanceBio Peptide Map column thermostated at 30°C (100 × 2.1 mm; Agilent Technologies) and interfaced with a 6490 triple-quadrupole mass spectrometer (Agilent Technologies) operating in Agilent jet stream-electrospray ionization (AJS ESI) mode. A binary gradient of mobile phase A (water containing 0.1% formic acid) and B (acetonitrile containing 0.1% formic acid) was delivered at a flow rate of 0.4 ml/min. After an isocratic step of 1 min at 0% B, a linear gradient from 0 to 40% B was run over the next 6 min. The column was then washed with 90% B for 2 min and re-equilibrated with 0% B for 3 min. Column eluate was monitored by mass spectrometry (MS) over the 0.5–7.0 min portion of the run. MS analysis was performed using a dynamic multiple-reaction monitoring. The general source settings were as follows: positive ionization mode; gas temperature, 180°C; gas flow, 18 l/min; nebulizer, 35 ψ; sheath gas temperature, 300°C; and sheath gas flow, 11 l/min. Capillary, fragmentor, and cell accelerator voltage were 1.5 kV, 380 V, and 5 V, respectively. Collision energies (CEs) for the selected transitions of the peptides, 84QEMSK, 108WQEEMELYR, 141LSPLGEEMR, and 124AELQEGAR, were optimized with the aid of Skyline software (57). Three isotope-labeled peptides, QEMSK(U-13C6, 15N2), WQEEMEL(U-13C6, 15N)YR, and LSPL(U-13C6, 15N)GEEMR and their analogs with oxidized Met were used as internal standards to correct the peak area of oxidized peptide. Data for 2 or 3 transitions per peptide were collected, each of them separately corrected and then averaged. Usually, 5 pM of tryptic digest was analyzed in 1 run. Total ion current of AELQEGAR peptide was used to control for trypsinization efficiency. Identity and chromatographic characteristic of the precursor peptides and transitions used for quantification of Met oxidation are reported in the Supplemental Table S1. Data processing was performed with MassHunter software version B.06.00 (Agilent Technologies).
ABCA1-mediated cellular cholesterol release
The TopFluor cholesterol method was used for measuring release of cellular cholesterol to lipid-free apoA-I as described (58, 59) (BODIPY cholesterol has been renamed TopFluor cholesterol by Avanti Polar Lipids). The detailed procedure is reported in Supplemental Data.
Mouse bone marrow–derived macrophages preparation and incubation with apoA-I samples
All mouse procedures were approved by the UCSF Benioff Children’s Hospital Oakland Institutional Animal Care and Use Committee. Bone marrow–derived macrophages (BMDMs) were isolated from wild-type C57BL/6 mice and from Cd36−/− mice on a C57BL/6 background (60). Bone marrow cells were isolated by flushing the bone marrow from the femurs of the hind legs of 3- to 6-mo-old mice with Dulbecco’s PBS (D-PBS; MilliporeSigma). To lyse red blood cells, the collected bone marrow cells were washed with D-PBS and treated with 155 mM ammonium chloride, 12 mM sodium bicarbonate, and 0.1 mM EDTA (pH 7.1–7.4). The remaining cells were washed twice with D-PBS containing 5% fetal bovine serum (FBS), suspended in the differentiation medium [high-glucose DMEM (AQmedia, with l-Ala-l-Gln, NaHCO3, and pyridoxine; MilliporeSigma), 1 mM sodium pyruvate, 10% FBS, 20% L-929 conditioned medium (61), 100 U/ml penicillin, and 0.1 mg/ml streptomycin) at 0.4–0.5 × 106 cells/ml and distributed into 90 × 15 mm Petri dishes (62), 10 ml/dish. After 7 d of incubation, the attached cells were washed twice with D-PBS, incubated in 3 ml of 10 mM EDTA-D-PBS for 10 min at 37°C, and removed by means of a scraper. The cells were suspended in the seeding medium [high-glucose DMEM (AQmedia), 1 mM sodium pyruvate, 2% FBS, 7.5% L-929 conditioned medium, 100 U/ml penicillin, and 0.1 mg/ml streptomycin] at 1 × 106 cells/ml and portioned in aliquots to a 96-well plate at a volume of 0.2 ml/well. BMDM were incubated in 5% CO2 at 37°C for 18–20 h, washed twice with warm D-PBS and treated with 0.1 ml/well of tested reagents [apoA-I protein samples, 29 µg/ml (1 µM)] diluted in HBSS without phenol red and containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 100 U/ml penicillin. Before each experiment, the HBSS–HEPES medium was preincubated in the CO2 incubator overnight and its pH adjusted to 7.3 just before treatment. After 4 h of incubation in the presence of the tested reagents, the wells were washed 3 times with PBS, and the cells solubilized in 0.1 ml RIPA buffer (Boston BioProducts, Ashland, MA, USA) with protease inhibitors (Biotool.com). Cell extracts were separated by SDS-PAGE, transferred to PVDF membrane and stained with anti-apoA-I (goat polyclonal, 1:5000 dilution; Meridian Life Science, Memphis, TN, USA) or anti-β-actin antibody (mouse monoclonal, 1:7500 dilution; MilliporeSigma), followed by incubation with horseradish peroxidase–conjugated secondary antibodies. Upon reaction with Advansta Western Bright ECL, horseradish peroxidase-generated light was detected by a FluorChem Q instrument (Alpha Innotech, San Leandro, CA, USA).
Data processing and statistical analysis
Independent experiments were performed by preparing new recombinant apoA-I and repeating oxidation, liquid chromatography (LC-MS) quantification, fibrillation incubation, and sample analysis. Origin software (OriginLab, Northampton, MA, USA) was used to fit ThT kinetic data. SAS 9.3 (SAS Institute, Cary, NC, USA) was used for statistical analyses. Means ± sem were calculated for all variables. Differences in cellular cholesterol release capacities between apoA-I groups were assessed using the appropriate ANOVA model. If a significant overall difference was found, the Tukey-Kramer’s post hoc method for multiple comparisons was used to determine which groups differed. The significance level was set at 0.01. In our single time-point dilution ThT method (see Fluorescence Spectroscopy), only 1 dilution was performed and 1 fluorescence spectrum was collected at each time point. Experimental replications were obtained by repeating the full ThT kinetics experiment with new samples. Mean t1/2 (defined as the time when ThT fluorescence reached half of the maximum fluorescence level) and sem values from at least 3 independent experiments are reported in the figures.
RESULTS
Exhaustive Met oxidation of apoA-I promotes rapid amyloid formation and enhances the cellular cholesterol release properties of the protein
MPO-mediated oxidation impairs apoA-I-induced release of cellular cholesterol (8, 17). To achieve such an effect in vitro, MPO oxidation is usually performed with the MPO-H2O2-Cl− system at high enough concentration of H2O2 (i.e., >3:1 H2O2:apoA-I mol:mol) to significantly modify residues other than Met; namely, to hydroxylate Trp and chlorinate Tyr (8, 9, 40, 63). These oxidative conditions are commonly regarded as similar to the physiologic conditions that occur in atherosclerosis (14, 63). In milder oxidative conditions, which are achieved in vitro by lowering the concentration of H2O2 (i.e., ≤3:1 H2O2:apoA-I mol:mol), the 3 apoA-I Met residues, often regarded as an endogenous antioxidant system (11, 64), efficiently scavenge the hydroxyl radicals and prevent modification of residues other than Met (40).
At 3:1 H2O2:apoA-I mol:mol (MPO-3:1-H2O2-ApoA-I), Met residues were only partially oxidized, whereas at 10:1 H2O2:apoA-I mol:mol (MPO-10:1-H2O2-ApoA-I), Met residues were oxidized exhaustively (Table 1). As expected, a significant reduction in fluorescence emission intensity (Supplemental Fig. S1A) and red shift of WMF (Table 2) was detected only for MPO-10:1-H2O2-ApoA-I, indicating that besides Met, other residues (i.e., Trp and Tyr) were also significantly modified under these conditions.
TABLE 1.
Met sulfoxide [Met(O)] formation in apoA-I samples oxidized with different methods and quantified by LC-MS
| Sample name | Oxidation system | H2O2:apoA-I (mol:mol) | Met(O)86 | Met(O)112 | Met(O)148 |
|---|---|---|---|---|---|
| wt-ApoA-I control | None | 0:1 | 3.3 ± 3.0 (4) | 2.8 ± 0.5 (7) | 4.3 ± 0.9 (7) |
| MPO-3:1-H2O2-wt-ApoA-I | MPO-H2O2-Cl− | 3:1 | 67.4 ± 2.3 (5) | 88.5 ± 2.1 (5) | 69.9 ± 2.3 (5) |
| MPO-10:1-H2O2-wt-ApoA-I | MPO-H2O2-Cl− | 10:1 | 98.2 ± 0.4 (5) | 99.1 ± 0.1 (5) | 99.4 ± 0.1 (5) |
| H2O2-wt-ApoA-I | H2O2 | 1000:1 | 95.9 ± 1.1 (3) | 99.0 ± 0.3 (8) | 98.9 ± 0.4 (3) |
| H2O2-4WF-ApoA-I | H2O2 | 1000:1 | 93.2 | 99.0 | 98.4 |
| 90C-wt-ApoA-I | None | 0:1 | 5.5 ± 0.2 (3) | 3.6 ± 0.3 (3) | 0.9 ± 0.1 (3) |
The percentage of single Met residue oxidation is reported as mean and sem (n); n is the number of independent experiments.
TABLE 2.
WMF of monomeric ApoA-I samples at 50 µg/ml in iPBS
| Sample name | WMF (nm) |
|---|---|
| wt-ApoA-I | 339.6 ± 0.2 |
| 90C-wt-ApoA-I | 353.3 ± 0.5 |
| 90C-wt-ApoA-I+20C 1h | 342.0 ± ND |
| MPO-3:1-H2O2-wt-ApoA-I | 342.8 ± 0.2 |
| MPO-10:1-H2O2-wt-ApoA-I | 372.5 ± 0.4 |
| H2O2-wt-ApoA-I | 342.9 ± 0.4 |
| 90C-H2O2-wt-ApoA-I | 353.7 ± 0.3 |
| 90C-H2O2-wt-ApoA-I+20C 1h | 345.0 ± ND |
The excitation wavelength was 280 nm. 90C-wt-ApoA-I+20C 1h, 90C-H2O2-wt-ApoA-I+20C 1h: wt-ApoA-I, and H2O2-wt-ApoA-I samples, respectively, heated at 90°C for 1 h and then incubated at 20°C for 1 h before fluorescence analysis. Full spectra are reported in Supplemental Fig. S1. ND, not determined.
In contrast, in the absence of MPO, H2O2 exclusively targets apoA-I Met residues. Even at molar excesses of H2O2 as large as 1000:1 (H2O2-wt-ApoA-I), Trp and Tyr are not significantly modified (Supplemental Fig. S1A), whereas Met are exhaustively oxidized (Table 1). We previously reported that both MPO-3:1-H2O2-wt-ApoA-I and H2O2-wt-ApoA-I readily form amyloid fibrils when incubated under fibrillation conditions (pH 6.0; 37°C and continuous vortexing) (20). This amyloidogenic outcome is solely promoted by Met oxidation, as a Met-to-Leu variant in which all 3 Met residues were substituted with Leu (3ML-ApoA-I) was unable to form amyloid upon oxidation under similar conditions. The notion that Met oxidation is sufficient for amyloid formation is also supported by the amyloidogenic character of MPO-3:1-H2O2-wt-ApoA-I, where Met residues are only partially oxidized, and other residues are not significantly modified (Supplemental Fig. S1A, Table 1, and ref. 20).
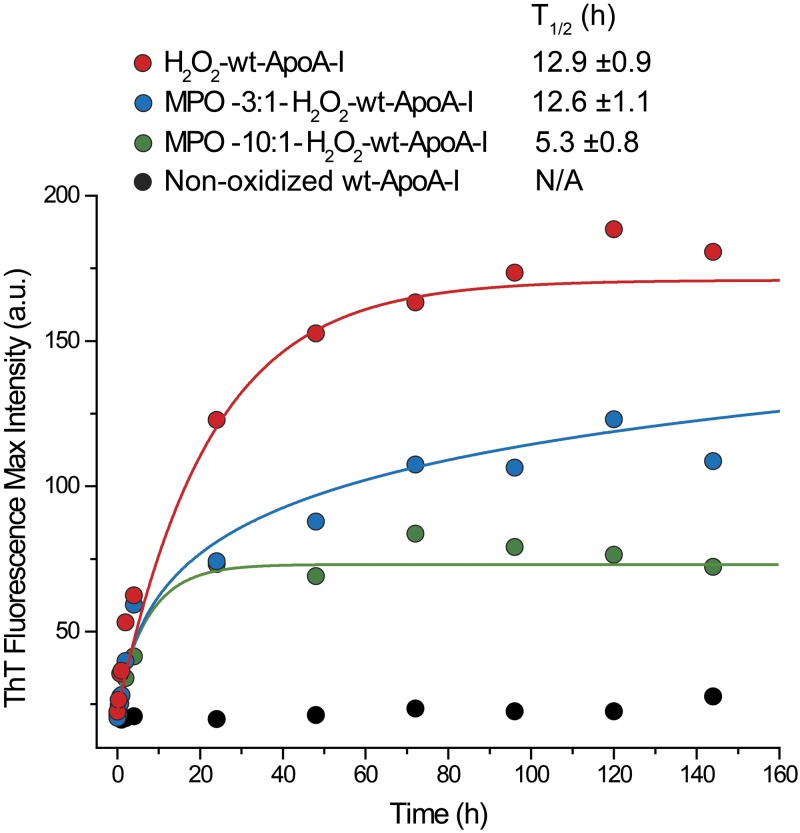
To test how different oxidative modifications impact the propensity of the protein to aggregate into amyloids, we incubated variably oxidized wt-ApoA-I under fibrillation conditions and measured the kinetics of amyloid formation by ThT fluorescence analysis (Fig. 1). ThT is an amyloid specific dye that fluoresces in the presence of amyloids. Full Met oxidation in the absence of other residue modifications (H2O2-wt-ApoA-I) afforded the highest increase in ThT fluorescence. Partial Met oxidation with nonsignificant modification of other residues (MPO-3:1-H2O2-wt-ApoA-I) also promoted amyloid formation, but to a lesser extent than exhaustive Met oxidation. MPO-10:1-H2O2-wt-ApoA-I, wherein Met is fully oxidized but Trp and Tyr are also significantly modified (Supplemental Fig. S1A, Table 1, and ref. 40), was the least prone to forming amyloids among all oxidized apoA-I variants.
Figure 1.
Kinetics of amyloid fibril formation measured by ThT fluorescence. Representative ThT kinetics curves for H2O2-wt-ApoA-I, MPO-3:1-H2O2-wt-ApoA-I, MPO-10:1-H2O2-ApoA-I, and nonoxidized wt-ApoA-I. A portion of fibrillation incubation mixture was combined with the ThT stock solution at the indicated time points and the fluorescence emission spectrum of the sample recorded. Solid lines are fitting of the experimental values by exponential (H2O2-wt-ApoA-I and MPO-10:1-H2O2-ApoA-I) or logarithmic (MPO-3:1-H2O2-wt-ApoA-I) curves. T1/2 data (means ± sem) from ≥3 independent experiments are shown.
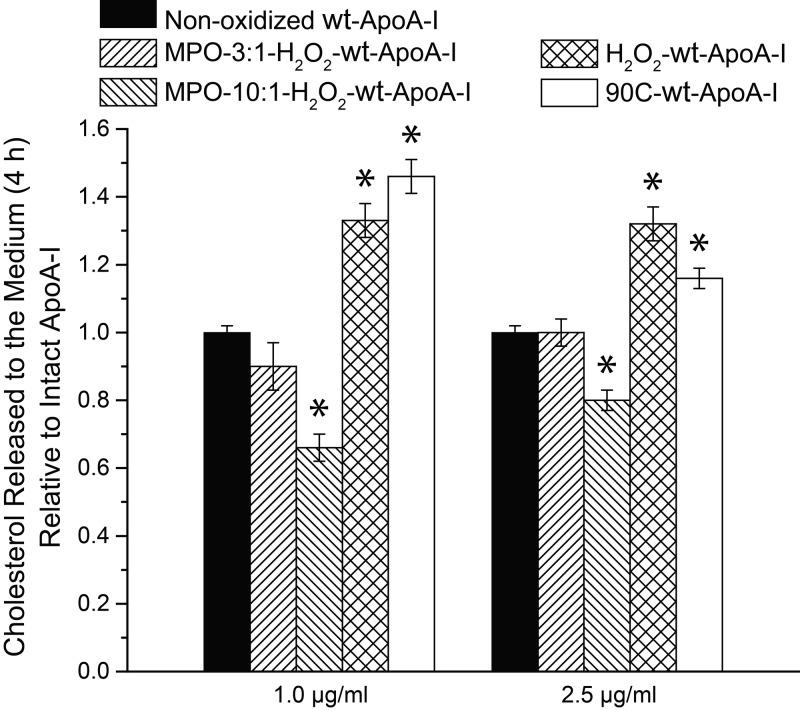
To investigate whether these modifications affect the main physiologic function of the protein, oxidized apoA-I samples were used as acceptors of cellular cholesterol from macrophages (58). It is to be noted that apoA-I at the low concentrations used for these experiments (1.0–2.5 µg/ml) is monomeric (see the following sections). The cholesterol release ability of MPO-10:1-H2O2-wt-ApoA-I was significantly reduced when compared to nonoxidized wt-ApoA-I (Fig. 2), whereas that of MPO-3:1-H2O2-wt-ApoA-I was not significantly affected. In contrast, the cholesterol release ability of H2O2-wt-ApoA-I was significantly enhanced when compared to nonoxidized wt-ApoA-I, consistent with previous findings from the Stocker laboratory (65) for apoA-I carrying 2 oxidized Met residues. These results indicate that functional impairment occurs only upon modification of residues other than Met (8, 9), and that Met oxidation promotes structural changes that facilitate, rather than impair, lipid binding (65), or interaction with the ABCA1/cell membrane system (66), or both. Notably, upon thermal treatment of wt-ApoA-I at 90°C for 1 h (90C-wt-ApoA-I) (19), its cholesterol release ability was also significantly increased, when compared to the untreated protein.
Figure 2.
Release of cholesterol from cells into cell culture medium during a 4 h incubation in the presence of 1.0 and 2.5 µg/ml ApoA-I samples. To induce expression of ABCA1, cells were preincubated overnight with cAMP, as described in the Supplemental Data. Data are normalized to the cholesterol levels released in the presence of nonoxidized wt-ApoA-I at the indicated concentration. Data are reported as means ± sem of 3 independent experiments with 3 replications per experiment. *P ≤ 0.01, probability that the reported means are not significantly different from the cholesterol release efficiency of nonoxidized wt-ApoA-I (which is normalized to 1). Remaining histograms represent values not significantly different from nonoxidized wt-ApoA-I. P > 0.01.
ApoA-I interaction with macrophages
To investigate whether Met oxidation alters the interaction mechanism of apoA-I with macrophages, cells that are strongly implicated in atherosclerosis development, we measured the association of apoA-I with mouse BMDMs. The cells were incubated in the presence of apoA-I for 4 h, which is sufficient time to promote the efflux of a significant amount of cellular cholesterol toward lipid-free apoA-I (Fig. 2). After the macrophages were extensively washed with iPBS, cell extract analysis by Western blot (Fig. 3A) revealed that H2O2-wt-ApoA-I was stably associated with the cells, whereas nonoxidized and MPO-oxidized apoA-I were not detected in the cell extracts. Remarkably, 90C-wt-ApoA-I was associated with cells to an even higher degree than H2O2-wt-ApoA-I. Thus, structural changes promoted by complete Met oxidation or by thermal treatment, rather than the oxidation state of Met residues per se, are the likely cause of the increased protein association with macrophages. In the case of MPO-oxidized apoA-I samples, incomplete Met oxidation or other protein modifications may alter the ability of the modified protein to associate with macrophages.
Figure 3.
Western blot analysis of BMDM cell extracts. Cell-association of apoA-I samples with BMDM from wild-type mice (A) and CD36-KO mice (B).
To test whether CD36, a cell membrane scavenger receptor implicated in recognition of amyloid precursor peptides and proinflammatory activation of macrophages (67–72), is responsible for the observed cell association, BMDM experiments were performed with macrophages derived from CD36-knockout (C36-KO) mice. Similar to what was observed with wild-type BMDMs, H2O2-wt-ApoA-I was clearly present in CD36-KO BMDM cell extracts, whereas wt-ApoA-I was nearly undetectable (Fig. 3B). These results suggest that CD36 is not responsible for the enhanced cell association of Met(O)-ApoA-I.
Impact of Met oxidation and thermal treatment on apoA-I self-association
As the state of apoA-I self-association affects the protein function (19), we used size-exclusion chromatography (SEC; Fig. 4, Supplemental Fig. S2, and Supplemental Table S2) and nondenaturing gradient gel electrophoresis (NDGGE) (Fig. 5 and Supplemental Fig. S3) analyses to investigate whether oxidation alters apoA-I self-association.
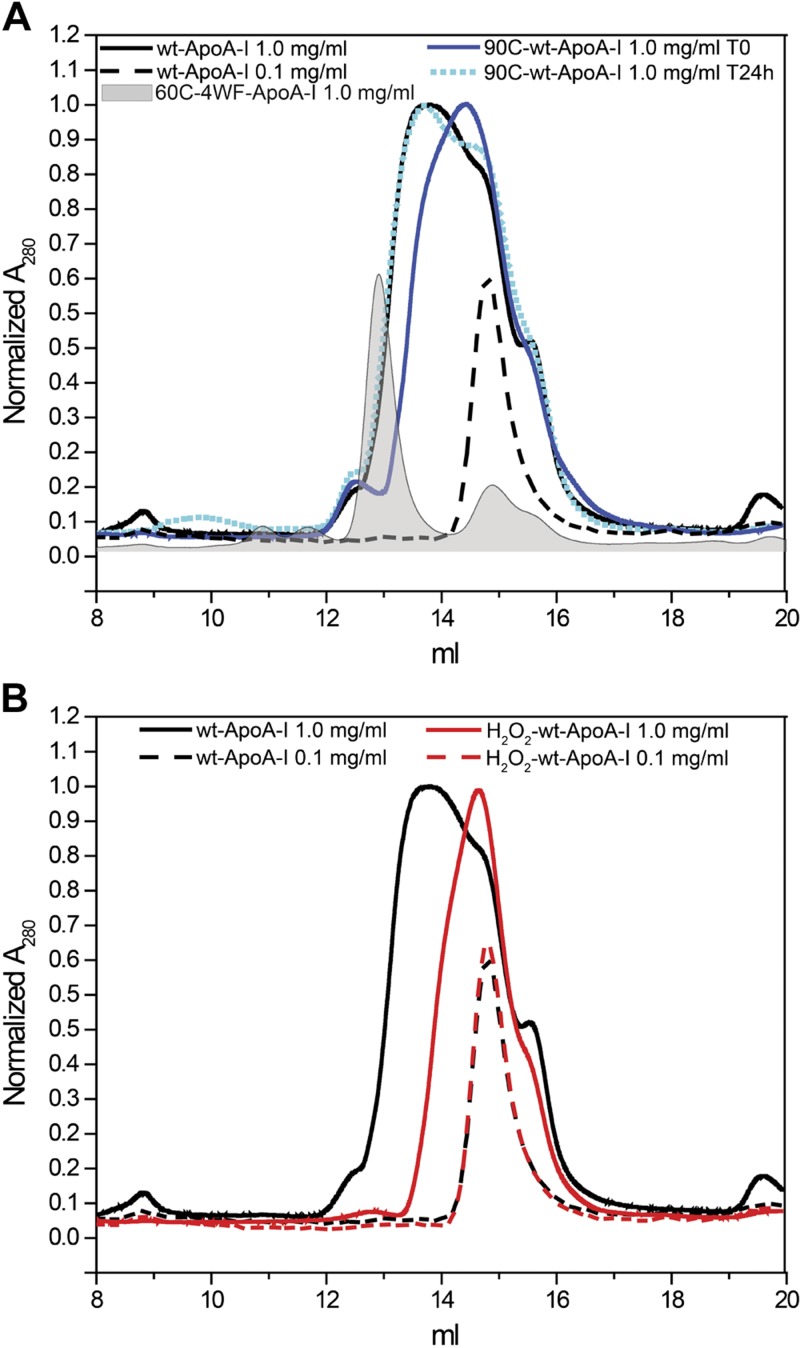
Figure 4.
SEC analysis of 100 µl apoA-I samples at 1.0 or 0.1 mg/ml in iPBS. A) 90C-wt-ApoA-I was injected into the column after incubation at 90°C for 1 h and 10 min cooling at room temperature. B) After oxidation, H2O2-wt-ApoA-I was dialyzed against iPBS for 2 d with 2 buffer exchanges at 4°C before SEC analysis. To facilitate the comparison of different chromatograms, the y-axes were manually adjusted to normalize the intensity of the most prominent peak in each chromatogram to 1. 60C-4WF-ApoA-I and the 0.1 mg/ml sample chromatograms were arbitrarily normalized to 0.6.
Figure 5.
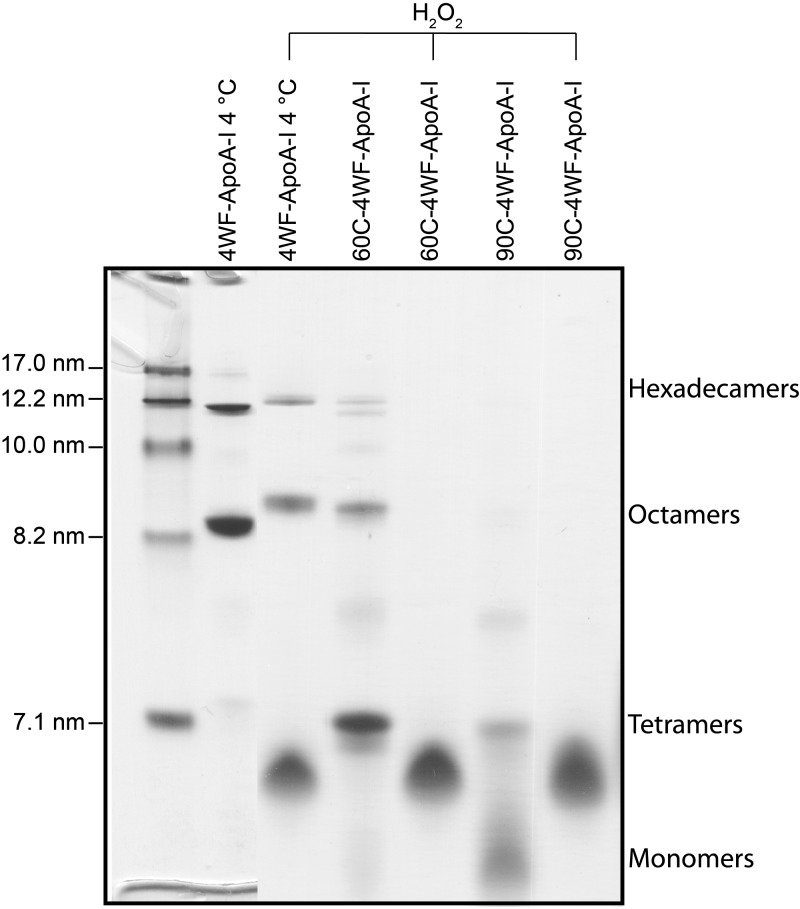
NDGGE analysis of heated and oxidized 4WF-ApoA-I samples. Protein samples at 1.0 mg/ml were incubated for 1 h at the indicated temperatures. After cooling at room temperature for 10 min, the samples were loaded on gel (3 µg/lane) for NDGGE analysis or oxidized by 1000:1 molar excess of H2O2, dialyzed against iPBS for 2 d with 2 buffer exchanges and then analyzed. Left lane: molecular weight markers. Molecular weight marker diameters (nm) are indicated on the left. Labels on the right identify the different oligomeric species of nonoxidized 4WF-ApoA-I (19). Met-oxidized oligomers and monomers have slightly reduced electrophoretic mobilities compared to nonoxidized samples.
Nonoxidized wt-ApoA-I at 1.0 mg/ml was largely self-associated and eluted on a Superdex 200 column as a very broad peak with the main component at Ve = 13.8 ml (Fig. 4A and Supplemental Table S2) (19). This broad band comprises self-associated species ranging from tetramers to monomers, as illustrated by comparison with the chromatogram of 4WF-ApoA-I treated at 60°C for 1 h (60C-4WF-ApoA-I) (Fig. 4A), which has been characterized [by dynamic light scattering (19)] to contain predominantly tetramers (Ve = 13.0 ml).
As expected (73, 74), wt-ApoA-I self-association was completely disrupted upon dilution to 0.1 mg/ml. The diluted protein eluted as a narrow peak corresponding to monomers (Ve = 14.8 ml) (Fig. 4A) (19).
Notably, heating at 90°C for 1 h also significantly reduced the self-association of 1.0 mg/ml wt-ApoA-I, but such effect was completely reversible. After 24 h at 4°C, the SEC chromatogram of 90C-wt-ApoA-I was almost indistinguishable from that of wt-ApoA-I. In contrast to thermal treatment, Met oxidation disrupted self-association of apoA-I irreversibly. Even after oxidation, 48 h dialysis at 4°C, and an extra 24 h storage at 4°C, H2O2-wt-ApoA-I was still predominantly monomeric (Fig. 4B), with the main peak component eluting at Ve = 14.6 ml. These results are consistent with the loss of ability to form tetramers and dimers by H2O2-wt-ApoA-I, as observed by Wong et al. (75). MPO oxidation also disrupted self-association, with the main SEC peak of MPO-3:1-H2O2-wt-ApoA-I eluting at the same Ve (14.7 ml) of the monomeric peak of H2O2-wt-ApoA-I (Supplemental Fig. S2 and Supplemental Table S2). The persistence of higher molecular weight species in MPO-3:1-H2O2-wt-ApoA-I (Ve = 14.0 ml) suggests that disruption of self-association is proportional to the degree of Met oxidation in the samples. MPO-10:1-H2O2-wt-ApoA-I on the other hand, completely lost any oligomeric structure, consistent with the total Met oxidation of this sample. However, the main peak was centered at a Ve (15.0 ml) larger than the Ve of monomeric H2O2-wt-ApoA-I and MPO-3:1-H2O2-wt-ApoA-I (Supplemental Fig. S2 and Supplemental Table S2), indicating that in the case of MPO-10:1-H2O2-wt-ApoA-I, modifications other than Met oxidation (e.g., crosslinking, Tyr, Trp, and Lys oxidation) further altered the structure of the protein monomers.
The state of self-association of apoA-I was also investigated by NDGGE. The self-associated species of wt-ApoA-I are not stable under the NDGGE running conditions. Most of them are disrupted during the electrophoretic run and only a heavily smeared band is detected on the gel (Supplemental Fig. S3) (19, 76, 77). To assess the impact of Met oxidation and heating on the self-association state of apoA-I by NDGGE, we used 4WF-ApoA-I in lieu of wt-ApoA-I, as the self-associated species of 4WF-ApoA-I are stable during NDGGE and detectable as well-defined bands (Supplemental Fig. S3). We previously established that 4WF-ApoA-I stored at 4°C is mostly composed of hexadecamers (16×), octamers (8×), and tetramers (4×), (∼27%, ∼54%, and ∼9%, respectively, as calculated by densitometric analysis of NDGGE in Supplemental Fig. S3), with virtually undetectable monomers (≤5%) (19). The high-molecular-weight self-associated species of 4WF-ApoA-I are disrupted by thermal treatment at 60 and 90°C (90C-4WF-ApoA-I) for 1 h, with preferential generation of tetramers in 60C-4WF-ApoA-I and monomers in 90C-4WF-ApoA-I (∼75 and ∼59%, respectively; Supplemental Fig. S3 and ref. 19).
In agreement with the SEC results, NDGGE analysis revealed that exhaustive Met oxidation also disrupts self-association, with ∼72% (by densitometric analysis) of H2O2-oxidized 4WF-ApoA-I (H2O2-4WF-ApoA-I) in the monomeric state and complete breakdown of tetramers (Fig. 5). It is to be noted that, upon Met oxidation, the mobility of the octamer, tetramer, and monomer bands was slightly reduced, compared to unoxidized samples. We propose that such a change in electrophoretic mobility is produced by Met oxidation–promoted structural changes that affect the protein’s Stokes diameter, or superficial charges, or both. As all oligomer bands show this reduction in electrophoretic mobility, all oligomers must contain at least some oxidized apoA-I molecules. Furthermore, oxidation of 4WF-ApoA-I samples preincubated at 60 and 90°C (i.e., 60C-4WF-ApoA-I and 90C-4WF-ApoA-I) completely broke apart the residual oligomers that survived heating of (nonoxidized) 4WF-ApoA-I, yielding ∼100% of the oxidized proteins in the monomeric state.
SDS-PAGE analysis of apoA-I samples oxidized with different methods revealed the presence of irreversibly cross-linked higher molecular weight bands in MPO-10:1-H2O2-wt-ApoA-I (Fig. 6), whereas no high-molecular-weight species were detectable in oxidized samples in which only Met was modified (i.e., MPO-3:1-H2O2-wt-ApoA-I and H2O2-wt-ApoA-I). Furthermore, high-molecular-weight bands were also visible in SDS-PAGE of the Met-to-Leu variant, 3ML-ApoA-I, oxidized by the MPO-10:1-H2O2 system, suggesting that formation of intermolecular covalent bonds involves residues other than Met.
Figure 6.
SDS-PAGE analysis of wt-ApoA-I, 3ML-ApoA-I, and 4WF-ApoA-I (1.0 mg/ml) upon oxidation by different methods. At the end of oxidation incubations (overnight for H2O2 oxidation and 90 min for MPO-mediated oxidation), 3 μg/lane of samples were loaded on gel. Electrophoresis was executed as described in Supplemental Data. Molecular masses of protein standards are indicated on the right. Met-oxidized apoA-I samples have slightly reduced electrophoretic mobility vs. nonoxidized samples.
Taken together, these results indicate that Met oxidation induces disruption of apoA-I self-association, with no other apparent irreversible modifications of the protein, such as covalent cross-linking.
Impact of Met oxidation and thermal treatment on apoA-I tertiary structure
We previously reported that exhaustive Met oxidation does not produce any secondary structure change detectable by infrared or circular dichroism spectroscopy (20). Formation of β-structure in Met(O)-ApoA-I was apparent only upon protein aggregation in fibrillation conditions (20). To investigate structural changes induced by oxidation and heating in the protein monomers, fluorescence spectroscopy analysis was performed on protein samples at 50 µg/ml (Supplemental Fig. S1 and Table 2), a concentration at which apoA-I is monomeric (Fig. 4). Monomer stability was also assessed by Trp and SYPRO orange fluorescence thermal shift analyses (Supplemental Fig. S4 and Table 3).
Met oxidation, in the absence of other residue modifications (H2O2-wt-ApoA-I and MPO-3:1-H2O2-wt-ApoA-I), produced a moderate but significant WMF red-shift (∼ +3 nm) when compared to nonoxidized wt-ApoA-I (Supplemental Fig. S1A and Table 2). The red shift suggests that aromatic residues become more solvent exposed upon Met oxidation (78, 79), perhaps as a consequence of a partial destabilization of the 4-helix bundle in the N-terminal domain of apoA-I (79–82). This observation was corroborated by a significant overall protein destabilization as indicated by a ∼20°C reduction in protein melting temperature upon Met oxidation (Δ-24.2 and Δ-20.2°C by Trp and SYPRO orange fluorescence thermal shift analyses, respectively; Table 3).
Upon heating at 90°C for 1 h, the WMFs of both nonoxidized and H2O2-wt-ApoA-I were red shifted to ∼353 nm, as expected for completely solvent exposed Trp and Tyr (Supplemental Fig. S1B, C and Table 2) (78). Subsequent incubation at 20°C for 1 h largely reversed the red shift, suggesting that the protein was able to refold into a native-like structure. Both 90C-wt-ApoA-I and 90C-H2O2-wt-ApoA-I incubated 1 h at 20°C displayed a 3 nm residual red shift, compared to the spectra of the same samples before melting, indicating that the protein structure was still partially altered. These conditions are similar to those experienced by the 90°C-treated samples used for cellular cholesterol release experiments (Fig. 2), as the normal sample preparation time between heating and cell treatment was about 1 h at room temperature. Notably, the WMFs of both 90C-wt-ApoA-I+20°C 1 h and H2O2-wt-ApoA-I were ∼342 nm. A more open and solvent exposed N-terminal helix bundle, promoted either by Met oxidation or 90°C heating, may explain the increased activity of those 2 samples as cellular cholesterol recipients.
Amyloid formation occurs in nonoxidized apoA-I upon seeding with preformed amyloid fibrils
The presence of suitable amyloid fibril nuclei may induce fibril growth under conditions where such growth is virtually undetectable in the absence of seeding. For instance, we reported that lowering of the pH is not essential for amyloid formation by H2O2-oxidized apoA-I. Seeding of H2O2-wt-ApoA-I with as little as 1% of preformed oxidized apoA-I amyloid fibrils leads to amyloid formation at pH 7.0 (20). Prompted by this observation, we hypothesized that seeding could induce amyloid fibril formation in nonoxidized apoA-I as well. To test this hypothesis, we incubated 1 part of preformed H2O2-wt-ApoA-I amyloid fibrils with 99 parts of nonoxidized wt-ApoA-I under fibrillation conditions at pH 6.0. Seeded nonoxidized apoA-I formed amyloids, although with slower kinetics compared to seeding of H2O2-oxidized apoA-I (20), as indicated by an increase in ThT fluorescence starting after about 14 d of incubation (Fig. 7). The seeding efficiency was dependent on the amount of seeds used: when 10% of the total initial protein was composed of preformed aggregates, seeding kinetics were faster, with gain of ThT fluorescence beginning after about 8 d of incubation. Unseeded nonoxidized wt-apoA-I was perfectly stable for the duration of the fibrillation incubation. No increase in ThT fluorescence or visible aggregation occurred, indicating that long-term incubation under these conditions (e.g., vortexing at 800 rpm) does not induce amyloidogenic modifications in apoA-I, in the absence of seeds.
Figure 7.
Seeding of nonoxidized wt-ApoA-I with molar 10% and 1% of preformed H2O2-wt-ApoA-I aggregates. As a control, nonoxidized wt-ApoA-I was incubated in the absence of seeds. Total protein concentration in each sample was 1.0 mg/ml. The samples were incubated in fibrillation buffer (pH 6.0) at 37°C with continuous vortexing at 800 rpm. ThT fluorescence was measured at the indicated time points. Solid lines show fitting of the experimental values by sigmoidal curves. T1/2 data (means ± sem) from ≥3 independent experiments are shown.
In general, amyloid-like aggregates formed by Met-oxidized apoA-I display high morphologic heterogeneity among different preparations and within the same sample, with straight fibrils often interspersed with more curly structures (20, 75). As expected, the EM micrographs of H2O2-wt-ApoA-I seeds used for the seeding experiments illustrate morphologic heterogeneity (Supplemental Fig. S5A), also present in micrographs of seeded aggregates (Supplemental Fig. S5B).
Yields of nonoxidized apoA-I aggregation upon seeding were calculated by measuring the residual amount of soluble protein after seeded fibril formation (Supplemental Table S3). Although with lower aggregation yields compared to oxidized apoA-I (H2O2-ApoA-I = 91.3%), a large fraction of the nonoxidized protein aggregated upon seeding. Yields of seeded aggregation were dependent on the amount of seeds used (55.0 and 45.6% for seeding with 10 and 1% seeds, respectively, Supplemental Table S3).
Structural transformations of nonoxidized apoA-I upon seeded amyloid formation
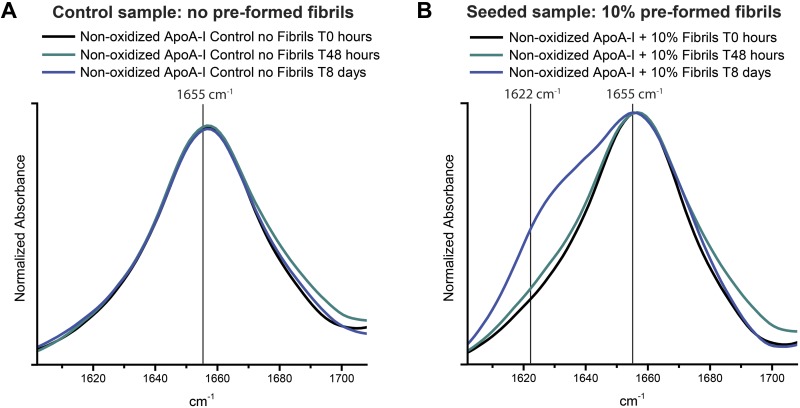
Consistent with the ThT assays, FTIR spectra revealed the time-dependent appearance of β-structures when nonoxidized wt-ApoA-I was seeded with preformed oxidized aggregates (Fig. 8). The single peak at about 1655 cm−1 in the amide I region of the FTIR spectrum of a control wt-ApoA-I sample, incubated in the absence of preformed oxidized apoA-I aggregates, suggests that the predominantly α-helical and random-coil secondary structure of the original protein was unchanged upon incubation (Fig. 8A). In contrast, in the seeded sample, the new band visible at about 1622 cm−1 after 8 d of incubation indicates formation of a significant amount of β-structures (Fig. 8B) (20, 83). Such IR β-signal and the increased ThT fluorescence are both characteristic of amyloid-like structures. The FTIR spectra of the assemblies formed by seeded nonoxidized wt-ApoA-I appear similar to our previously reported FTIR data on oxidized (by excess of H2O2 or by the MPO system) wt-ApoA-I amyloid fibrils (20). One notable and characteristic feature, which is also apparent from our ssNMR studies (see below), is the coexistence of both (amyloid-like) β- and non–β-structure, with the latter including α-helical signals that persist even after fibril maturation.
Figure 8.
FTIR spectra in the amide I region (1602–1708 cm−1) of nonoxidized wt-ApoA-I after seeding with preformed H2O2-wt-ApoA-I aggregates. Control (A) and 10% (molar) seeded (B) mixtures before incubation under fibrillation conditions (T0) and after 48 h and 8 d incubations.
To further probe the degree of similarity between aggregates formed by seeded nonoxidized wt-ApoA-I and the original oxidized H2O2-wt-ApoA-I aggregates (seeds), we compared such samples by MAS ssNMR spectroscopy. MAS ssNMR is a powerful means of examining the structure of noncrystalline insoluble protein aggregates (84). The observed NMR resonance frequencies are sensitive to changes in structure and report on the local secondary structure of individual residues or residue types (55, 85, 86). The 13C MAS ssNMR spectrum of H2O2-wt-13C,15N-ApoA-I aggregates and that of nonoxidized wt-13C,15N-ApoA-I seeded with 10% of unlabeled preaggregated H2O2-wt-ApoA-I are compared in Fig. 9A. Because the seeds were not labeled with 13C/15N, the 13C NMR spectra of the seeded preparations can be attributed for ∼99% to the recruited labeled nonoxidized wt-ApoA-I (which is confirmed by the spectral analysis below). The 2 spectra are very similar, as can be appreciated by the overlay shown in Fig. 9A (bottom trace). The most obvious difference is the intensity of the peak near 33 ppm (indicated by a gray arrow). This resonance frequency is consistent with that of Cγ/Cβ of unoxidized Met. Met Cγ/Cβ chemical shifts change dramatically upon oxidation of the side chain (87, 88). Gray (∼17 ppm) and black (∼39 ppm) arrows indicate less prominent peaks attributed to Met in the nonoxidized and oxidized forms, respectively (see also below). The spectra of the 2 samples show few other significant differences (full 1D spectra are presented in Supplemental Fig. S6). Additional MAS ssNMR experiments separately identified rigid and mobile protein domains and informed on dynamic differences between samples (55, 89, 90). First, spectra of the same 2 aggregated samples were obtained with a solution-NMR-like experiment (INEPT) that detects only the most dynamic parts of the aggregated protein (Fig. 9B). Again, there is a striking similarity between the 2 samples, with the most obvious differences affecting peaks that align with the expected chemical shifts of nonoxidized Met (34.6, 32.5, and 16.7 ppm for Cβ, Cγ, and Cε, respectively). All of the peaks in the INEPT spectra reflect chemical shifts typical of amino acid side chains, including specifically Met, Lys, and the aromatic residues Phe and Tyr (Supplemental Fig. S6). The published solution structure of the monomeric nonoxidized protein presents a highly unstructured C-terminal domain (82, 91). Nevertheless, our analysis of the INEPT spectra of the aggregated samples indicates that the number of highly dynamic and unstructured residues is relatively small. It should be noted, however, that unstructured segments can be (partially) immobilized by surrounding ordered domains, thus becoming invisible in the INEPT experiments (84, 90).
Separately, we probed the more rigid parts of the aggregates using cross-polarization (CP) experiments (Fig. 9C–E). Although these spectra are still similar, differences are observed between the oxidized and the nonoxidized (but seeded) protein aggregates. Such differences are apparent not only in the Met side chain signals, but also in the Cα and CO backbone peaks (CO shown in Fig. 9D). Given that these CP spectra selectively enhance the signal of the most rigid parts of the sample, we interpret these results as revealing subtle differences in the structure and dynamics of the core of the recruited wt-ApoA-I aggregates. The chemical shifts of the CO and Cα CP signals (Fig. 9C, D) are sensitive to the secondary structure (92) and point to a difference in the secondary structure content of the immobilized cores of the assemblies. Alongside substantial α-helical signal, there is a significant but variable amount of β-sheet structure, consistent with these data and earlier studies (20, 86). The β-sheet content is larger in the immobilized cores of the oxidized aggregates than the seeded nonoxidized sample. The residues affected by the mixed α/β structure are observed in the CP-based 2D 13C-13C MAS ssNMR spectrum of aggregated 13C,15N-labeled H2O2-wt-ApoA-I (Fig. 9E). Several amino acid types show cross-peaks of both β- and α-structured residues, such as Ala, Val, Ser, and Leu (marked in Fig. 9E). This is consistent with the above analysis and prior reports (20, 83, 86). The presence of β-sheet peaks not expected for the native fold [which is predominantly α-helical (52)] can be appreciated from a comparison to simulated 2D data for the latter (Supplemental Fig. S7). At the same time, the ssNMR experiments clearly show that extensive α-helical, and probably native-like, structure is present in the seeded nonoxidized and oxidized aggregates alike. The ssNMR peaks of α-helical residues are narrower than those of the β-sheet structured amino acids. This difference may be attributed to a relatively homogeneous α-helical structure, whereas there is more disorder in the nonnative β-sheet structure. It is important to note that the α-helical signals observed in the CP-based ssNMR experiments cannot be caused by residual soluble protein, given that the CP polarization transfer process requires the protein to be immobilized.
Mechanism of amyloid fibril formation
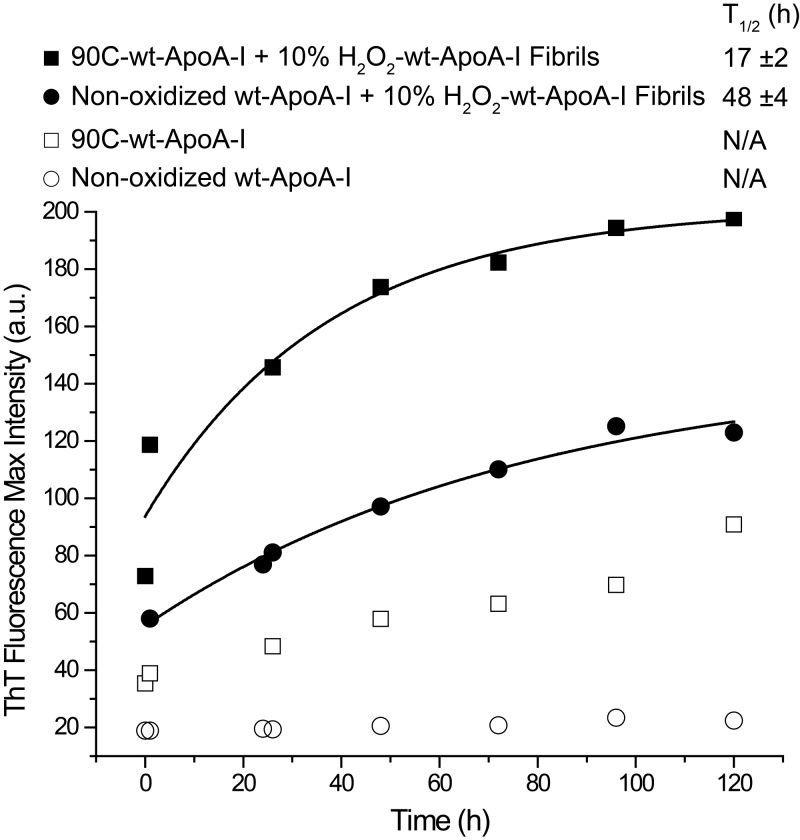
To test the hypothesis that amyloid fibril elongation proceeds through a monomeric-apoA-I-dependent mechanism we seeded nonoxidized wt-ApoA-I and 90C-wt-ApoA-I with 10% of preformed H2O2-wt-ApoA-I aggregates and compared their amyloid formation kinetics via the ThT fluorescence assay. In case of a monomer-dependent mechanism, the large population of monomers in 90C-wt-ApoA-I (Figs. 4A, 5 and Supplemental Fig. S3) should aggregate into amyloid fibrils more rapidly than highly self-associated nonoxidized apoA-I. Confirming this hypothesis, ThT fluorescence kinetics of seeded 90C-wt-ApoA-I were faster than those of seeded wt-ApoA-I (Fig. 10). An increase in ThT fluorescence was also observed upon incubation of unseeded 90C-wt-ApoA-I under these fibrillation conditions, albeit at significantly lower rates than in the seeded samples. This result suggests an intrinsic propensity of monomeric apoA-I toward aggregating into amyloid-like structures, even in the absence of fibril seeds. It should be noted that the observed kinetics in Fig. 10 are enhanced relative to those in Fig. 7, because of the stronger agitation conditions applied in this experiment (1100 rpm). Sample agitation has a notable impact on the aggregation rates, both in our hands and in aggregation kinetics studies of other amyloidogenic proteins (93–96). Independent of the conditions, the oxidized seeds accelerate and initiate further aggregation, possibly through a combination of prion-like propagation and secondary nucleation processes (97).
Figure 10.
Seeding of nonoxidized wt-ApoA-I and 90C-wt-ApoA-I with molar 10% of preformed H2O2-wt-ApoA-I aggregates. In control experiments, nonoxidized wt-ApoA-I and 90C-wt-ApoA-I were incubated in the absence of seeds. Total protein concentration was 1.0 mg/ml. The samples were incubated in fibrillation buffer (pH 6.0) at 37°C with continuous vortexing at 1100 rpm. ThT fluorescence was measured at the indicated time points. Solid lines show fitting of the experimental values by monomolecular growth exponential curves. T1/2 data (means ± sem) from ≥3 independent experiments are shown.
DISCUSSION
This study was inspired by the seemingly contradictory observation that Met oxidation, besides promoting amyloid formation in apoA-I (20, 75), also increases apoA-I ability to extract cellular cholesterol (65). Our results establish a structural mechanism that reconciles the apparent paradox of increased HDL-biogenesis function and enhanced amyloidogenic dysfunction.
Almost 2 decades ago, Panzenböck et al. (65) studied oxidized apoA-I isolated from human HDL upon reaction with 2,2′-azobis(2-amidinopropane) hydrochloride, a generator of aqueous peroxyl radicals. Only Met-86 and Met-112 were oxidized under such conditions (apoA-I+32), but these modifications were sufficient to increase the lipid-binding affinity and the cellular cholesterol release capacity of the protein, compared to nonoxidized apoA-I. Notably, apoA-I+32 was able to form HDL particles with the same size and physical–chemical characteristics of those produced by nonoxidized apoA-I. Furthermore, HDL particles reconstituted with apoA-I+32 and nonoxidized apoA-I demonstrated similar abilities to extract cellular cholesterol, suggesting that the enhanced properties of apoA-I+32 were caused by specific features of the lipid-free protein that were normalized when the protein was HDL associated.
In the current study, we observed that Met oxidation significantly reduces the overall stability of the protein by altering its tertiary structure (Supplemental Figs. S1 and S4, and Tables 2 and 3) and disrupting self-association (Fig. 4).
It is plausible that at higher concentrations, the altered tertiary structure of oxidized monomers produce the irreversible loss of self-association. We propose that destabilization of the tertiary structure of the monomers and disruption of self-association increase the probability of alternative protein–protein interactions, thereby significantly enhancing the amyloidogenic character of Met(O)-ApoA-I.
The Met-oxidation–promoted tertiary structure changes can also explain the enhancement of the cellular cholesterol release capacity of H2O2-wt-ApoA-I, as a less stable N-terminal α-helix bundle may increase accessibility of lipids to the hydrophobic regions of the amphipathic α-helices of apoA-I. Similarly, the increased cholesterol release ability of 90C-wt-ApoA-I can also be explained by a transiently destabilized tertiary structure, as indicated by the similar fluorescence spectra of H2O2-wt-ApoA-I and 90C-wt-ApoA-I incubated at 20°C for 1 h. However, 90C-wt-ApoA-I was significantly less amyloidogenic than H2O2-wt-ApoA-I (Figs. 1 and 10). This could relate in some way to the ability of the nonoxidized monomers of 90C-wt-ApoA-I to fully refold, upon longer times (>1 h), into the native quaternary structure (Fig. 4A). Disruption of self-association by Met oxidation was, in contrast, completely irreversible (Fig. 4B).
It is to be noted that Met oxidation per se did not induce amyloid formation nor any detectable secondary structure change, until oxidized samples were incubated under fibrillation conditions. Similar to other amyloidogenic proteins, Met(O)-ApoA-I aggregation kinetics were notably enhanced by sample agitation. Upon incubation under agitated fibrillation conditions, a dramatic increase in β-structure component occurred rapidly (20). Once aggregated, Met(O)-ApoA-I amyloid-like fibrils promoted amyloid formation also in nonoxidized apoA-I via prion-like propagation. This process was expedited when the native self-association of nonoxidized apoA-I was disrupted by thermal treatment. Thus, when the monomer population was increased, either by oxidation or thermal treatment, recruitment of apoA-I into the aggregates was enhanced, suggesting that amyloid formation proceeds through addition onto growing fibrils of monomers rather than oligomers.
Such latent propensity of lipid-free apoA-I to form amyloids has important physiologic implications, as all the components and conditions needed for inducing amyloid formation by nonoxidized apoA-I exist within the atherosclerotic lesions: MPO (98), active oxygen species (99), lower pH (100), and a higher than in circulation concentration of lipid-free apoA-I (24). This amyloidogenic activity would remove functional protein and deposit amyloid material within the atherosclerotic plaques, a process whose contribution to atherosclerosis progression is presently unknown but postulated to correlate with the incidence of the disease (25–29). An important finding of the current work is that, although potentially deleterious, the role that apoA-I Met oxidation plays in atherosclerosis progression may have been undervalued so far, because it does not directly impact the cholesterol binding function of the protein, the most common functional test for apoA-I.
Furthermore, the observation that 90C-wt-ApoA-I and H2O2-wt-ApoA-I, but not nonoxidized wt-ApoA-I, associate with BMDMs has potentially highly relevant physiologic implications. Other amyloid precursor peptides, such as Aβ peptide and islet amyloid polypeptide, induce a proinflammatory response in macrophages through a CD36 dependent pathway (68, 101). Met(O)-ApoA-I, as a soluble precursor to apoA-I amyloids, could also interact with specific cell membrane receptors and promote atherosclerosis-relevant cellular processes. Although our results indicate that CD36 is not implicated in the enhanced association of Met(O)-ApoA-I with macrophages, other inflammation relevant receptors [e.g., other scavenger receptors (SR-B1) or TLRs] could be responsible for the increased cell association. This hypothesis is under investigation.
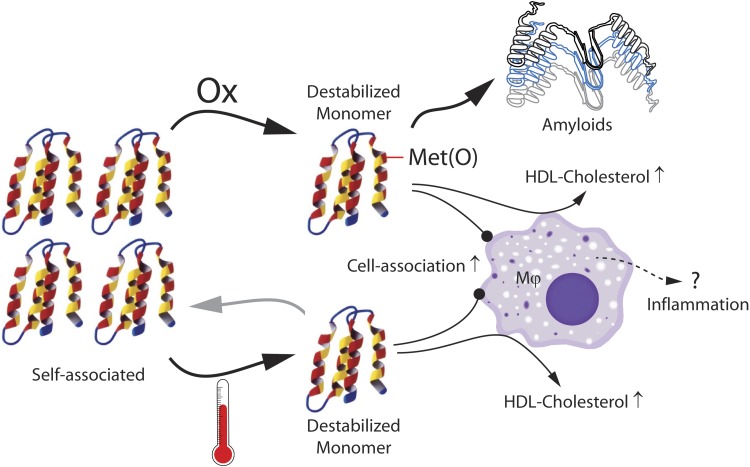
To transition between the lipid-free and lipid-bound states and to perform their physiologic function, exchangeable apolipoproteins ought to display flexibility and conformational adaptability. Such structural plasticity can be described as a carefully tuned instability that, as a side-effect, also increases the amyloidogenic potential of the protein, a common characteristic of several exchangeable apolipoproteins. Our experimental findings, which are summarized in Fig. 11, suggest that the propensity to self-associate in solution, another common feature of exchangeable apolipoproteins (e.g., apoA-I, apoE, apoC-II, apoA-IV), provides stabilization to the protein native structure. Met oxidation alters apoA-I tertiary structure and disrupts apoA-I self-association, yielding soluble oxidized monomers that retain their secondary structure, but are more competent in releasing cellular cholesterol (Fig. 2), associate with macrophages more strongly (Fig. 3), and aggregate into amyloids more readily (Fig. 1). Thermal treatment of apoA-I, used as an in vitro tool to validate these observations, produced similar structural changes (Supplemental Fig. S1 and Table 3) that were accompanied by analogous functional changes (Figs. 2, 3, and 10). Thus, the native structure of lipid-free apoA-I, comprised its quaternary features, protects the protein monomers from engaging in alternative amyloidogenic interactions. We note that this is reminiscent of analogous observations for the amyloidogenic protein transthyretin (102), which have formed the basis for the development of tetramer-stabilizing drugs that protect against cardiac amyloidosis (103).
Figure 11.
Summary of the presented results. ApoA-I native self-association is disrupted by thermal treatment or Met oxidation. Destabilized monomeric apoA-I associates with macrophages more strongly and extracts cholesterol more efficiently than native apoA-I. Destabilized monomeric Met(O)-ApoA-I is also amyloidogenic.
An interesting aspect of aggregated apoA-I samples is that they feature the canonical hallmarks of amyloid-like fibrils (ThT reactivity, β-sheet-structure, and seeding activity), while also containing large amounts of α-helical structure. Several specific amino acid types are seen to adopt β-sheet resonance frequencies in the 2D ssNMR data, suggesting that only a small, and likely specific, segment of the protein ends up in the β-sheet-based amyloid core. This is not uncommon for amyloidogenic proteins (84). In the case of apoA-I, it seems possible that α-helical segments outside the amyloid core retain a native-like structure. Although the location of the amyloid segment is not revealed by the current experimental results, various algorithms that try to predict amyloidogenic sequences suggest specific segments that feature the amino acid residues seen as β-structured by ssNMR (Supplemental Fig. S8) (104–106).
Our electron microscopy results indicate variability and heterogeneity in the morphology of the aggregates, in line with prior studies (20, 75). Our ssNMR and FTIR data also reveal differences in the amount of β-structure between the oxidized apoA-I aggregates and the (seeded) nonoxidized protein assemblies. A key feature of the ssNMR studies of the seeded preparations is that the (unlabeled) seed material is effectively invisible, permitting us to unequivocally identify the signals of the seeded (and labeled) nonoxidized protein. As noted, the seeded aggregates show a reduced amount of β-sheet structure, which cannot be caused by residual soluble native proteins that would be invisible in the CP ssNMR spectra. We speculate that this finding may be explained by an ability of the (oxidized) aggregates to recruit protein not only through a typical β-sheet–based amyloid elongation process, but also by sequestering nonoxidized protein via α-helical segments that decorate the fibrils’ β-sheet amyloid core. Thus, native-like intermonomer interactions may protect from amyloid-based aggregation in the absence of preformed nucleation sites, but could also promote recruitment of monomers to fibrils that can then facilitate (secondary) nucleation events. These complex and partly non-amyloid–based self-assembly processes may contribute to the morphologic heterogeneity seen by electron microscopy.
Environmental conditions compatible with the physiology of atherosclerosis, such as low pH and the presence of catalytic surfaces (i.e., provided by cellular membranes), other atherosclerosis-associated amyloidogenic proteins (107) [e.g., medin (108), transthyretin (109), serum amyloid A (110)], microcalcifications (111), or cholesterol crystals (112)] could further enhance the kinetics of the apoA-I sequestration, with potentially deleterious consequences for the progression of the disease. These consequences could include not only the loss of functional apoA-I (upon aggregation into amyloid-like products), but also intracellular effects of amyloid precursor species, which are currently under investigation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Gordon L. Watson [Children’s Hospital Oakland Research Institute (CHORI)] for contributing the wild-type mice for bone marrow extraction and Dorothy Tabron (CHORI) for taking care of the animals and extracting the bone marrow. The CD36-KO mice were generously provided by Prof. Andreas Stahl (University of California at Berkeley, Berkeley, CA, USA). The authors are also grateful to Prof. Shinji Yokoyama and Prof. Rui Lu (Nutritional Health Science Research Center, Chubu University, Kasugai, Japan) for assistance in troubleshooting the cellular cholesterol release protocol and to Dr. Trudy M. Forte (CHORI) and Dr. Shobini Jayaraman (Boston University School of Medicine. Boston, MA, USA) for useful discussions. This work was supported in whole or part by U.S. National Institutes of Health (NIH), National Heart, Lung, and Blood Institute Grant R01HL113059 (to G.C)., and NIH National Institute of General Medical Sciences Grants R01GM112678 (to P.C.A.V.D.W.) and T32 GM088119 (to J.C.B.). The Agilent 6490 triple quadrupole mass spectrometer was purchased through Grant 1S10OD018070, from the Office of the Director of the NIH. The article’s content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- 2D
2-dimensional
- ABCA1
ATP binding cassette transporter A1
- apoA-I
apolipoprotein A-I
- BMDM
bone marrow–derived macrophages
- CP
cross-polarization
- D-PBS
Dulbecco’s PBS
- FBS
fetal bovine serum
- FTIR
Fourier transform infrared
- H2O2-wt-ApoA-I
H2O2-oxidized wild-type apoA-I
- INEPT
insensitive nuclei enhanced by polarization transfer
- iPBS
isotonic PBS
- LC-MS
liquid chromatography-mass spectrometry
- LCAT
lecithin-cholesterol acyl transferase
- MAS
magic-angle spinning
- MAS ssNMR
MAS solid-state NMR
- Met(O)
Met sulfoxide
- MPO
myeloperoxidase
- NDGGE
nondenaturing gradient gel electrophoresis
- OB
oxidation buffer
- SEC
size-exclusion chromatography
- ThT
thioflavin T
- WMF
wavelength of maximum fluorescence
- wt-ApoA-I
wild-type apoA-I
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
G. Cavigiolio conceived and coordinated the study; G. Cavigiolio, A. Witkowski and G. K. L. Chan designed and analyzed all the experiments, except for the MAS ssNMR and the electron microscopy studies, which were designed, performed, and analyzed by J. C. Boatz and P. C. A. van der Wel; G. K. L. Chan produced the protein samples and contributed to the amyloid formation experiments; N. J. Li and G. Cavigiolio executed the cellular cholesterol release experiments; A. P. Inoue, J. C. Wong, and G. Cavigiolio performed the protein melting and fluorescent studies; A. Witkowski executed the BMDM experiments; and A. Witkowski, P. C. A. van der Wel, and G. Cavigiolio wrote the paper.
REFERENCES
- 1.Lau D., Baldus S. (2006) Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 111, 16–26 10.1016/j.pharmthera.2005.06.023 [DOI] [PubMed] [Google Scholar]
- 2.Podrez E. A., Abu-Soud H. M., Hazen S. L. (2000) Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic. Biol. Med. 28, 1717–1725 10.1016/S0891-5849(00)00229-X [DOI] [PubMed] [Google Scholar]
- 3.Shao B. (2012) Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim. Biophys. Acta 1821, 490–501 10.1016/j.bbalip.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao B., Bergt C., Fu X., Green P., Voss J. C., Oda M. N., Oram J. F., Heinecke J. W. (2005) Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J. Biol. Chem. 280, 5983–5993 10.1074/jbc.M411484200 [DOI] [PubMed] [Google Scholar]
- 5.Zheng L., Nukuna B., Brennan M. L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P. L., Ischiropoulos H., Smith J. D., Kinter M., Hazen S. L. (2004) Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114, 529–541 10.1172/JCI200421109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiDonato J. A., Aulak K., Huang Y., Wagner M., Gerstenecker G., Topbas C., Gogonea V., DiDonato A. J., Tang W. H., Mehl R. A., Fox P. L., Plow E. F., Smith J. D., Fisher E. A., Hazen S. L. (2014) Site-specific nitration of apolipoprotein A-I at tyrosine 166 is both abundant within human atherosclerotic plaque and dysfunctional. J. Biol. Chem. 289, 10276–10292 10.1074/jbc.M114.556506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker G., Peng D. Q., Somerlot B., Abdollahian D. J., Smith J. D. (2006) Apolipoprotein A-I lysine modification: effects on helical content, lipid binding and cholesterol acceptor activity. Biochim. Biophys. Acta 1761, 64–72 10.1016/j.bbalip.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Peng D. Q., Wu Z., Brubaker G., Zheng L., Settle M., Gross E., Kinter M., Hazen S. L., Smith J. D. (2005) Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J. Biol. Chem. 280, 33775–33784 10.1074/jbc.M504092200 [DOI] [PubMed] [Google Scholar]
- 9.Peng D. Q., Brubaker G., Wu Z., Zheng L., Willard B., Kinter M., Hazen S. L., Smith J. D. (2008) Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler. Thromb. Vasc. Biol. 28, 2063–2070 10.1161/ATVBAHA.108.173815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y., DiDonato J. A., Levison B. S., Schmitt D., Li L., Wu Y., Buffa J., Kim T., Gerstenecker G. S., Gu X., Kadiyala C. S., Wang Z., Culley M. K., Hazen J. E., Didonato A. J., Fu X., Berisha S. Z., Peng D., Nguyen T. T., Liang S., Chuang C. C., Cho L., Plow E. F., Fox P. L., Gogonea V., Tang W. H., Parks J. S., Fisher E. A., Smith J. D., Hazen S. L. (2014) An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 20, 193–203 10.1038/nm.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao B., Oda M. N., Bergt C., Fu X., Green P. S., Brot N., Oram J. F., Heinecke J. W. (2006) Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J. Biol. Chem. 281, 9001–9004 10.1074/jbc.C600011200 [DOI] [PubMed] [Google Scholar]
- 12.Shao B., Cavigiolio G., Brot N., Oda M. N., Heinecke J. W. (2008) Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc. Natl. Acad. Sci. USA 105, 12224–12229 10.1073/pnas.0802025105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao B., Tang C., Sinha A., Mayer P. S., Davenport G. D., Brot N., Oda M. N., Zhao X. Q., Heinecke J. W. (2014) Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ. Res. 114, 1733–1742 10.1161/CIRCRESAHA.114.303454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao B., Pennathur S., Heinecke J. W. (2012) Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J. Biol. Chem. 287, 6375–6386 10.1074/jbc.M111.337345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z., Wagner M. A., Zheng L., Parks J. S., Shy J. M., III, Smith J. D., Gogonea V., Hazen S. L. (2007) The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction (published correction in Nat. Struct. Mol. Biol. (2008) 15, 651). Nat. Struct. Mol. Biol. 14, 861–868 10.1038/nsmb1284 [DOI] [PubMed] [Google Scholar]
- 16.Pennathur S., Bergt C., Shao B., Byun J., Kassim S. Y., Singh P., Green P. S., McDonald T. O., Brunzell J., Chait A., Oram J. F., O’brien K., Geary R. L., Heinecke J. W. (2004) Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 279, 42977–42983 10.1074/jbc.M406762200 [DOI] [PubMed] [Google Scholar]
- 17.Bergt C., Pennathur S., Fu X., Byun J., O’Brien K., McDonald T. O., Singh P., Anantharamaiah G. M., Chait A., Brunzell J., Geary R. L., Oram J. F., Heinecke J. W. (2004) The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 101, 13032–13037 10.1073/pnas.0405292101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berisha S. Z., Brubaker G., Kasumov T., Hung K. T., DiBello P. M., Huang Y., Li L., Willard B., Pollard K. A., Nagy L. E., Hazen S. L., Smith J. D. (2015) HDL from apoA1 transgenic mice expressing the 4WF isoform is resistant to oxidative loss of function. J. Lipid Res. 56, 653–664 10.1194/jlr.M056754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraman S., Abe-Dohmae S., Yokoyama S., Cavigiolio G. (2011) Impact of self-association on function of apolipoprotein A-I. J. Biol. Chem. 286, 35610–35623 10.1074/jbc.M111.262485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan G. K., Witkowski A., Gantz D. L., Zhang T. O., Zanni M. T., Jayaraman S., Cavigiolio G. (2015) Myeloperoxidase-mediated methionine oxidation promotes an amyloidogenic outcome for apolipoprotein A-I. J. Biol. Chem. 290, 10958–10971 10.1074/jbc.M114.630442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanjee M. N., Crouse J. R., King J. M., Hovorka R., Rees S. E., Carson E. R., Morgenthaler J. J., Lerch P., Miller N. E. (1996) Effects of intravenous infusion of lipid-free apo A-I in humans. Arterioscler. Thromb. Vasc. Biol. 16, 1203–1214 10.1161/01.ATV.16.9.1203 [DOI] [PubMed] [Google Scholar]
- 22.Kee P., Rye K. A., Taylor J. L., Barrett P. H., Barter P. J. (2002) Metabolism of apoA-I as lipid-free protein or as component of discoidal and spherical reconstituted HDLs: studies in wild-type and hepatic lipase transgenic rabbits. Arterioscler. Thromb. Vasc. Biol. 22, 1912–1917 10.1161/01.ATV.0000038485.94020.7F [DOI] [PubMed] [Google Scholar]
- 23.Horowitz B. S., Goldberg I. J., Merab J., Vanni T. M., Ramakrishnan R., Ginsberg H. N. (1993) Increased plasma and renal clearance of an exchangeable pool of apolipoprotein A-I in subjects with low levels of high density lipoprotein cholesterol. J. Clin. Invest. 91, 1743–1752 10.1172/JCI116384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiDonato J. A., Huang Y., Aulak K. S., Even-Or O., Gerstenecker G., Gogonea V., Wu Y., Fox P. L., Tang W. H., Plow E. F., Smith J. D., Fisher E. A., Hazen S. L. (2013) Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation 128, 1644–1655 10.1161/CIRCULATIONAHA.113.002624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornwell G. G., III, Murdoch W. L., Kyle R. A., Westermark P., Pitkänen P. (1983) Frequency and distribution of senile cardiovascular amyloid: a clinicopathologic correlation. Am. J. Med. 75, 618–623 10.1016/0002-9343(83)90443-6 [DOI] [PubMed] [Google Scholar]
- 26.Mucchiano G., Cornwell G. G., III, Westermark P. (1992) Senile aortic amyloid: evidence for two distinct forms of localized deposits. Am. J. Pathol. 140, 871–877 [PMC free article] [PubMed] [Google Scholar]
- 27.Westermark P., Mucchiano G., Marthin T., Johnson K. H., Sletten K. (1995) Apolipoprotein A1-derived amyloid in human aortic atherosclerotic plaques. Am. J. Pathol. 147, 1186–1192 [PMC free article] [PubMed] [Google Scholar]
- 28.Mucchiano G. I., Häggqvist B., Sletten K., Westermark P. (2001) Apolipoprotein A-1-derived amyloid in atherosclerotic plaques of the human aorta. J. Pathol. 193, 270–275 10.1002/1096-9896(2000)9999:9999%3c::AID-PATH753%3e3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 29.Mucchiano G. I., Jonasson L., Häggqvist B., Einarsson E., Westermark P. (2001) Apolipoprotein A-I-derived amyloid in atherosclerosis: its association with plasma levels of apolipoprotein A-I and cholesterol. Am. J. Clin. Pathol. 115, 298–303 10.1309/PJE6-X9E5-LX6K-NELY [DOI] [PubMed] [Google Scholar]
- 30.Röcken C., Tautenhahn J., Bühling F., Sachwitz D., Vöckler S., Goette A., Bürger T. (2006) Prevalence and pathology of amyloid in atherosclerotic arteries. Arterioscler. Thromb. Vasc. Biol. 26, 676–677 10.1161/01.ATV.0000201930.10103.be [DOI] [PubMed] [Google Scholar]
- 31.Kristen A. V., Schnabel P. A., Winter B., Helmke B. M., Longerich T., Hardt S., Koch A., Sack F. U., Katus H. A., Linke R. P., Dengler T. J. (2010) High prevalence of amyloid in 150 surgically removed heart valves: a comparison of histological and clinical data reveals a correlation to atheroinflammatory conditions. Cardiovasc. Pathol. 19, 228–235 [DOI] [PubMed] [Google Scholar]
- 32.Audet A., Côté N., Couture C., Bossé Y., Després J. P., Pibarot P., Mathieu P. (2012) Amyloid substance within stenotic aortic valves promotes mineralization. Histopathology 61, 610–619 10.1111/j.1365-2559.2012.04265.x [DOI] [PubMed] [Google Scholar]
- 33.Howlett G. J., Moore K. J. (2006) Untangling the role of amyloid in atherosclerosis. Curr. Opin. Lipidol. 17, 541–547 10.1097/01.mol.0000245260.63505.4f [DOI] [PubMed] [Google Scholar]
- 34.Solomon A., Murphy C. L., Kestler D., Coriu D., Weiss D. T., Makovitzky J., Westermark P. (2006) Amyloid contained in the knee joint meniscus is formed from apolipoprotein A-I. Arthritis Rheum. 54, 3545–3550 10.1002/art.22201 [DOI] [PubMed] [Google Scholar]
- 35.Murphy C., Kestler D., Weiss D., Solomon A. (2011) Non-hereditary apolipoprotein AI-associated pulmonary amyloid. Amyloid 18(Suppl 1), 219–220 10.3109/13506129.2011.574354082 [DOI] [PubMed] [Google Scholar]
- 36.Loavenbruck A. J., Chaudhry V., Zeldenrust S. R., Spinner R. J., Theis J. D., Klein C. J. (2012) Mass spectrometry analysis reveals non-mutated apolipoprotein A1 lumbosacral radiculoplexus amyloidoma. Muscle Nerve 46, 817–822 10.1002/mus.23415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tubb M. R., Smith L. E., Davidson W. S. (2009) Purification of recombinant apolipoproteins A-I and A-IV and efficient affinity tag cleavage by tobacco etch virus protease. J. Lipid Res. 50, 1497–1504 10.1194/jlr.D900003-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoofnagle A. N., Becker J. O., Oda M. N., Cavigiolio G., Mayer P., Vaisar T. (2012) Multiple-reaction monitoring-mass spectrometric assays can accurately measure the relative protein abundance in complex mixtures. Clin. Chem. 58, 777–781 10.1373/clinchem.2011.173856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoop C. L., Lin H. K., Kar K., Hou Z., Poirier M. A., Wetzel R., van der Wel P. C. (2014) Polyglutamine amyloid core boundaries and flanking domain dynamics in huntingtin fragment fibrils determined by solid-state nuclear magnetic resonance. Biochemistry 53, 6653–6666 10.1021/bi501010q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao B., Heinecke J. W. (2008) Using tandem mass spectrometry to quantify site-specific chlorination and nitration of proteins: model system studies with high-density lipoprotein oxidized by myeloperoxidase. Methods Enzymol. 440, 33–63 10.1016/S0076-6879(07)00803-8 [DOI] [PubMed] [Google Scholar]
- 41.Cavigiolio G., Shao B., Geier E. G., Ren G., Heinecke J. W., Oda M. N. (2008) The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses. Biochemistry 47, 4770–4779 10.1021/bi7023354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nanjee M. N., Brinton E. A. (2000) Very small apolipoprotein A-I-containing particles from human plasma: isolation and quantification by high-performance size-exclusion chromatography. Clin. Chem. 46, 207–223 [PubMed] [Google Scholar]
- 43.Niesen F. H., Berglund H., Vedadi M. (2007) The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2, 2212–2221 10.1038/nprot.2007.321 [DOI] [PubMed] [Google Scholar]
- 44.Huynh K., Partch C. L. (2015) Analysis of protein stability and ligand interactions by thermal shift assay. Curr. Protoc Protein Sci. 79, 28.9. 1–28.9.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandal A., Boatz J. C., Wheeler T. B., van der Wel P. C. A. (2017) On the use of ultracentrifugal devices for routine sample preparation in biomolecular magic-angle-spinning NMR. J. Biomol. NMR 67, 165–178 10.1007/s10858-017-0089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takegoshi K., Nakamura S., Terao T. (2001) 13C-1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett. 344, 631–637 10.1016/S0009-2614(01)00791-6 [DOI] [Google Scholar]
- 47.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 10.1007/BF00197809 [DOI] [PubMed] [Google Scholar]
- 48.Vranken W. F., Boucher W., Stevens T. J., Fogh R. H., Pajon A., Llinás M., Ulrich E. L., Markley J. L., Ionides J., Laue E. D. (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 10.1002/prot.20449 [DOI] [PubMed] [Google Scholar]
- 49.Harris R. K., Becker E. D., De Menezes S. M., Granger P., Hoffman R. E., Zilm K. W.; International Union of Pure and Applied Chemistry Physical and Biophysical Chemistry Division . (2008) Further conventions for NMR shielding and chemical shifts (IUPAC Recommendations 2008). Magn. Reson. Chem. 46, 582–598 10.1002/mrc.2225 [DOI] [PubMed] [Google Scholar]
- 50.Fritzsching K. J., Hong M., Schmidt-Rohr K. (2016) Conformationally selective multidimensional chemical shift ranges in proteins from a PACSY database purged using intrinsic quality criteria. J. Biomol. NMR 64, 115–130 10.1007/s10858-016-0013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H., Neal S., Wishart D. S. (2003) RefDB: a database of uniformly referenced protein chemical shifts. J. Biomol. NMR 25, 173–195 10.1023/A:1022836027055 [DOI] [PubMed] [Google Scholar]
- 52.Mei X., Atkinson D. (2011) Crystal structure of C-terminal truncated apolipoprotein A-I reveals the assembly of high density lipoprotein (HDL) by dimerization. J. Biol. Chem. 286, 38570–38582 10.1074/jbc.M111.260422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 54.Mandal A., Hoop C. L., DeLucia M., Kodali R., Kagan V. E., Ahn J., van der Wel P. C. (2015) Structural changes and proapoptotic peroxidase activity of cardiolipin-bound mitochondrial cytochrome c. Biophys. J. 109, 1873–1884 10.1016/j.bpj.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boatz J. C., Whitley M. J., Li M., Gronenborn A. M., van der Wel P. C. A. (2017) Cataract-associated P23T γD-crystallin retains a native-like fold in amorphous-looking aggregates formed at physiological pH. Nat. Commun. 8, 15137 10.1038/ncomms15137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen Y., Bax A. (2010) SPARTA+: a modest improvement in empirical NMR chemical shift prediction by means of an artificial neural network. J. Biomol. NMR 48, 13–22 10.1007/s10858-010-9433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maclean B., Tomazela D. M., Abbatiello S. E., Zhang S., Whiteaker J. R., Paulovich A. G., Carr S. A., Maccoss M. J. (2010) Effect of collision energy optimization on the measurement of peptides by selected reaction monitoring (SRM) mass spectrometry. Anal. Chem. 82, 10116–10124 10.1021/ac102179j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sankaranarayanan S., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Asztalos B. F., Bittman R., Rothblat G. H. (2011) A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid Res. 52, 2332–2340 10.1194/jlr.D018051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rohatgi A., Khera A., Berry J. D., Givens E. G., Ayers C. R., Wedin K. E., Neeland I. J., Yuhanna I. S., Rader D. R., de Lemos J. A., Shaul P. W. (2014) HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371, 2383–2393 10.1056/NEJMoa1409065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Febbraio M., Abumrad N. A., Hajjar D. P., Sharma K., Cheng W., Pearce S. F., Silverstein R. L. (1999) A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274, 19055–19062 10.1074/jbc.274.27.19055 [DOI] [PubMed] [Google Scholar]
- 61.Weischenfeldt J., Porse B. (2008) Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008, pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X., Goncalves R., Mosser D. M. (2008) The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. Chapter 14, Unit 14.1 10.1002/0471142735.im1401s83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hewing B., Parathath S., Barrett T., Chung W. K., Astudillo Y. M., Hamada T., Ramkhelawon B., Tallant T. C., Yusufishaq M. S., Didonato J. A., Huang Y., Buffa J., Berisha S. Z., Smith J. D., Hazen S. L., Fisher E. A. (2014) Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 34, 779–789 10.1161/ATVBAHA.113.303044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine R. L., Mosoni L., Berlett B. S., Stadtman E. R. (1996) Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 93, 15036–15040 10.1073/pnas.93.26.15036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panzenböck U., Kritharides L., Raftery M., Rye K. A., Stocker R. (2000) Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J. Biol. Chem. 275, 19536–19544 10.1074/jbc.M000458200 [DOI] [PubMed] [Google Scholar]
- 66.Nagata K. O., Nakada C., Kasai R. S., Kusumi A., Ueda K. (2013) ABCA1 dimer-monomer interconversion during HDL generation revealed by single-molecule imaging. Proc. Natl. Acad. Sci. USA 110, 5034–5039 10.1073/pnas.1220703110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., Lacy-Hulbert A., El Khoury J., Golenbock D. T., Moore K. J. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 10.1038/ni.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheedy F. J., Grebe A., Rayner K. J., Kalantari P., Ramkhelawon B., Carpenter S. B., Becker C. E., Ediriweera H. N., Mullick A. E., Golenbock D. T., Stuart L. M., Latz E., Fitzgerald K. A., Moore K. J. (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 10.1038/ni.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rios F. J., Koga M. M., Pecenin M., Ferracini M., Gidlund M., Jancar S. (2013) Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm. 2013, 198193 10.1155/2013/198193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rios F. J., Koga M. M., Ferracini M., Jancar S. (2012) Co-stimulation of PAFR and CD36 is required for oxLDL-induced human macrophages activation. PLoS One 7, e36632 10.1371/journal.pone.0036632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medeiros L. A., Khan T., El Khoury J. B., Pham C. L., Hatters D. M., Howlett G. J., Lopez R., O’Brien K. D., Moore K. J. (2004) Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J. Biol. Chem. 279, 10643–10648 10.1074/jbc.M311735200 [DOI] [PubMed] [Google Scholar]
- 72.Liu W., Yin Y., Zhou Z., He M., Dai Y. (2014) OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm. Res. 63, 33–43 10.1007/s00011-013-0667-3 [DOI] [PubMed] [Google Scholar]
- 73.Vitello L. B., Scanu A. M. (1976) Studies on human serum high density lipoproteins: self-association of apolipoprotein A-I in aqueous solutions. J. Biol. Chem. 251, 1131–1136 [PubMed] [Google Scholar]
- 74.Silva R. A., Hilliard G. M., Fang J., Macha S., Davidson W. S. (2005) A three-dimensional molecular model of lipid-free apolipoprotein A-I determined by cross-linking/mass spectrometry and sequence threading. Biochemistry 44, 2759–2769 10.1021/bi047717+ [DOI] [PubMed] [Google Scholar]
- 75.Wong Y. Q., Binger K. J., Howlett G. J., Griffin M. D. (2010) Methionine oxidation induces amyloid fibril formation by full-length apolipoprotein A-I. Proc. Natl. Acad. Sci. USA 107, 1977–1982 10.1073/pnas.0910136107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rye K. A., Barter P. J. (2004) Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24, 421–428 10.1161/01.ATV.0000104029.74961.f5 [DOI] [PubMed] [Google Scholar]
- 77.Jahangiri A., Rader D. J., Marchadier D., Curtiss L. K., Bonnet D. J., Rye K. A. (2005) Evidence that endothelial lipase remodels high density lipoproteins without mediating the dissociation of apolipoprotein A-I. J. Lipid Res. 46, 896–903 10.1194/jlr.M400212-JLR200 [DOI] [PubMed] [Google Scholar]
- 78.Burstein E. A., Vedenkina N. S., Ivkova M. N. (1973) Fluorescence and the location of tryptophan residues in protein molecules. Photochem. Photobiol. 18, 263–279 10.1111/j.1751-1097.1973.tb06422.x [DOI] [PubMed] [Google Scholar]
- 79.Davidson W. S., Arnvig-McGuire K., Kennedy A., Kosman J., Hazlett T. L., Jonas A. (1999) Structural organization of the N-terminal domain of apolipoprotein A-I: studies of tryptophan mutants. Biochemistry 38, 14387–14395 10.1021/bi991428h [DOI] [PubMed] [Google Scholar]
- 80.Saito H., Dhanasekaran P., Nguyen D., Holvoet P., Lund-Katz S., Phillips M. C. (2003) Domain structure and lipid interaction in human apolipoproteins A-I and E, a general model. J. Biol. Chem. 278, 23227–23232 10.1074/jbc.M303365200 [DOI] [PubMed] [Google Scholar]
- 81.Brouillette C. G., Dong W. J., Yang Z. W., Ray M. J., Protasevich I. I., Cheung H. C., Engler J. A. (2005) Förster resonance energy transfer measurements are consistent with a helical bundle model for lipid-free apolipoprotein A-I. Biochemistry 44, 16413–16425 10.1021/bi051018v [DOI] [PubMed] [Google Scholar]
- 82.Chetty P. S., Mayne L., Lund-Katz S., Stranz D., Englander S. W., Phillips M. C. (2009) Helical structure and stability in human apolipoprotein A-I by hydrogen exchange and mass spectrometry. Proc. Natl. Acad. Sci. USA 106, 19005–19010 10.1073/pnas.0909708106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zandomeneghi G., Krebs M. R., McCammon M. G., Fändrich M. (2004) FTIR reveals structural differences between native beta-sheet proteins and amyloid fibrils. Protein Sci. 13, 3314–3321 10.1110/ps.041024904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Wel P. C. A. (2017) Insights into protein misfolding and aggregation enabled by solid-state NMR spectroscopy. Solid State Nucl. Magn. Reson. 88, 1–14 10.1016/j.ssnmr.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li J., Hoop C. L., Kodali R., Sivanandam V. N., van der Wel P. C. (2011) Amyloid-like fibrils from a domain-swapping protein feature a parallel, in-register conformation without native-like interactions. J. Biol. Chem. 286, 28988–28995 10.1074/jbc.M111.261750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Townsend D., Hughes E., Hussain R., Siligardi G., Baldock S., Madine J., Middleton D. A. (2017) Heparin and methionine oxidation promote the formation of apolipoprotein A-I amyloid comprising α-helical and β-sheet structures. Biochemistry 56, 1632–1644 10.1021/acs.biochem.6b01120 [DOI] [PubMed] [Google Scholar]
- 87.Cohen J. S., Yariv J., Kalb A. J., Jacobson L., Shechter Y. (1979) 13C NMR analysis of methionine sulfoxide in protein. J. Biochem. Biophys. Methods 1, 145–151 10.1016/0165-022X(79)90033-2 [DOI] [PubMed] [Google Scholar]
- 88.Skvortsov A. N., Zavodnik V. E., Stash A. I. (2003) Molecular structure and spectral properties of methionine sulfone, product of methionine oxidation. Russ. J. Org. Chem. 39, 170–175 10.1023/A:1025523832425 [DOI] [Google Scholar]
- 89.Helmus J. J., Surewicz K., Nadaud P. S., Surewicz W. K., Jaroniec C. P. (2008) Molecular conformation and dynamics of the Y145Stop variant of human prion protein in amyloid fibrils. Proc. Natl. Acad. Sci. USA 105, 6284–6289 10.1073/pnas.0711716105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin H. K., Boatz J. C., Krabbendam I. E., Kodali R., Hou Z., Wetzel R., Dolga A. M., Poirier M. A., van der Wel P. C. A. (2017) Fibril polymorphism affects immobilized non-amyloid flanking domains of huntingtin exon1 rather than its polyglutamine core. Nat. Commun. 8, 15462 10.1038/ncomms15462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melchior J. T., Walker R. G., Cooke A. L., Morris J., Castleberry M., Thompson T. B., Jones M. K., Song H. D., Rye K. A., Oda M. N., Sorci-Thomas M. G., Thomas M. J., Heinecke J. W., Mei X., Atkinson D., Segrest J. P., Lund-Katz S., Phillips M. C., Davidson W. S. (2017) A consensus model of human apolipoprotein A-I in its monomeric and lipid-free state. Nat. Struct. Mol. Biol. 24, 1093–1099 10.1038/nsmb.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wishart D. S., Sykes B. D. (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR 4, 171–180 10.1007/BF00175245 [DOI] [PubMed] [Google Scholar]
- 93.Knowles T. P., Waudby C. A., Devlin G. L., Cohen S. I., Aguzzi A., Vendruscolo M., Terentjev E. M., Welland M. E., Dobson C. M. (2009) An analytical solution to the kinetics of breakable filament assembly. Science 326, 1533–1537 10.1126/science.1178250 [DOI] [PubMed] [Google Scholar]
- 94.Lee C. C., Nayak A., Sethuraman A., Belfort G., McRae G. J. (2007) A three-stage kinetic model of amyloid fibrillation. Biophys. J. 92, 3448–3458 10.1529/biophysj.106.098608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dunstan D. E., Hamilton-Brown P., Asimakis P., Ducker W., Bertolini J. (2009) Shear flow promotes amyloid-beta fibrilization. Protein Eng. Des. Sel. 22, 741–746 10.1093/protein/gzp059 [DOI] [PubMed] [Google Scholar]
- 96.Sasahara K., Yagi H., Sakai M., Naiki H., Goto Y. (2008) Amyloid nucleation triggered by agitation of beta2-microglobulin under acidic and neutral pH conditions. Biochemistry 47, 2650–2660 10.1021/bi701968g [DOI] [PubMed] [Google Scholar]
- 97.Arosio P., Knowles T. P. J., Linse S. (2015) On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 17, 7606–7618 10.1039/C4CP05563B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daugherty A., Dunn J. L., Rateri D. L., Heinecke J. W. (1994) Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 94, 437–444 10.1172/JCI117342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ekstrand M., Gustafsson Trajkovska M., Perman-Sundelin J., Fogelstrand P., Adiels M., Johansson M., Mattsson-Hultén L., Borén J., Levin M. (2015) Imaging of intracellular and extracellular ROS levels in atherosclerotic mouse aortas ex vivo: effects of lipid lowering by diet or atorvastatin. PLoS One 10, e0130898 10.1371/journal.pone.0130898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Öörni K., Rajamäki K., Nguyen S. D., Lähdesmäki K., Plihtari R., Lee-Rueckert M., Kovanen P. T. (2015) Acidification of the intimal fluid: the perfect storm for atherogenesis. J. Lipid Res. 56, 203–214 10.1194/jlr.R050252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masters S. L., Dunne A., Subramanian S. L., Hull R. L., Tannahill G. M., Sharp F. A., Becker C., Franchi L., Yoshihara E., Chen Z., Mullooly N., Mielke L. A., Harris J., Coll R. C., Mills K. H., Mok K. H., Newsholme P., Nuñez G., Yodoi J., Kahn S. E., Lavelle E. C., O’Neill L. A. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 10.1038/ni.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao L., Buxbaum J. N., Reixach N. (2013) Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry 52, 1913–1926 10.1021/bi301313b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kerschen P., Planté-Bordeneuve V. (2016) Current and future treatment approaches in transthyretin familial amyloid polyneuropathy. Curr. Treat. Options Neurol. 18, 53 10.1007/s11940-016-0436-z [DOI] [PubMed] [Google Scholar]
- 104.Fernandez-Escamilla A.-M., Rousseau F., Schymkowitz J., Serrano L. (2004) Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 22, 1302–1306 10.1038/nbt1012 [DOI] [PubMed] [Google Scholar]
- 105.Tartaglia G. G., Vendruscolo M. (2008) The Zyggregator method for predicting protein aggregation propensities. Chem. Soc. Rev. 37, 1395–1401 10.1039/b706784b [DOI] [PubMed] [Google Scholar]
- 106.Sormanni P., Aprile F. A., Vendruscolo M. (2015) The CamSol method of rational design of protein mutants with enhanced solubility. J. Mol. Biol. 427, 478–490 10.1016/j.jmb.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 107.Westermark G. T., Fändrich M., Lundmark K., Westermark P. (2018) Noncerebral amyloidoses: aspects on seeding, cross-seeding, and transmission. Cold Spring Harb. Perspect. Med. 8, a024323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Larsson A., Malmström S., Westermark P. (2011) Signs of cross-seeding: aortic medin amyloid as a trigger for protein AA deposition. Amyloid 18, 229–234 10.3109/13506129.2011.630761 [DOI] [PubMed] [Google Scholar]
- 109.Ruberg F. L., Berk J. L. (2012) Transthyretin (TTR) cardiac amyloidosis. Circulation 126, 1286–1300 10.1161/CIRCULATIONAHA.111.078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.King V. L., Thompson J., Tannock L. R. (2011) Serum amyloid A in atherosclerosis. Curr. Opin. Lipidol. 22, 302–307 10.1097/MOL.0b013e3283488c39 [DOI] [PubMed] [Google Scholar]
- 111.Roijers R. B., Debernardi N., Cleutjens J. P., Schurgers L. J., Mutsaers P. H., van der Vusse G. J. (2011) Microcalcifications in early intimal lesions of atherosclerotic human coronary arteries. Am. J. Pathol. 178, 2879–2887 10.1016/j.ajpath.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Varsano N., Fargion I., Wolf S. G., Leiserowitz L., Addadi L. (2015) Formation of 3D cholesterol crystals from 2D nucleation sites in lipid bilayer membranes: implications for atherosclerosis. J. Am. Chem. Soc. 137, 1601–1607 10.1021/ja511642t [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.