Abstract
It is now generally accepted that animal studies are playing an important role in the understanding of anxiety disorders, since they contribute to the current knowledge regarding the mechanisms and possible therapeutic approaches in anxiety. In the present review we will detail some essential aspects of behavioral animal models of anxiety related to social defeat paradigm, elevated plus maze, elevated zero or T maze, light/dark box, social interaction test or tests based on predator models, considering the latest theories and methodological approaches in this area of research, as well as our previous studies focusing on anxiety manifestations in a variety of species including rats, zebrafish, dogs and pigs. Moreover, in this context, we will focus on the recent theories concerning oxidative stress, as well as importance of oxytocin administration (especially the intranasal route). This could be important considering that these two factors are currently being investigated as possible mechanisms (oxidative stress status) and related therapeutic target (both intranasal oxytocin and antioxidants) in the pathology of the anxiety disorders.
Keywords: Anxiety, Elevated plus maze, Neuropsychiatric diseases, Rat, Social defeat stress, Zebrafish
1. Introduction
Anxiety disorders are clusters of psychiatric disorders that are mainly described by excessive and unjustified fear, somatic symptoms, and related behavioral responses (escape, avoidance or assurance behaviors) [1]. It is important to mention the fact that animal models will never be perfectly replicating the complex human neuro-psycho-pathology. Still, they have a fundamental relevance in studying neuropsychiatric disorders, considering important physiological and anatomical similarities between humans and animals [2].
Thus, animal models studies are mainly based on the physiological, anatomical, and genetic structural similarities between human and animals [3]. Moreover, animal models have many positive characteristics including, the fact that they are easy to obtain, maintain, handle, relatively inexpensive, and exhibit increased reproducibility, as compared to the clinical studies [4], [5].
Considering these advantages, many rodent models of anxiety disorders have been developed and extensively used to study changes in animal behavior after exposing them to various types of stressors. Models of rodents (rat and mouse) [6] are the most commonly used models in neuropsychiatric studies, although other species of animal models have been described in neuropsychiatry research including primates [7], fish [8] or birds [9].
Studying the mechanisms of mental disorders such as anxiety on animal models is often questioned in terms of equivalence. However, as mentioned above, many evidences of similarities between rodents and human exist starting with the organization of nervous and endocrine systems [10], [11]. Additionally, aspects regarding equivalences in terms of basic cognitive process and behaviors related to anxiety such as conditional learning, anticipation of danger, avoidance, freezing, and escape are important as survival mechanisms [12], [13].
Concerning the validity type of the animal models, there are mainly three classes of models: 1- a correlation model with a predictive validity, implying that the model is sensitive to pharmacological agents [12], 2- an isomorphic model sustained by a face validity that implies an equivalence between behavioral responses in human and animals [13], and 3- a homologous model based on a construct validity that speculate similar etiology for a certain disorder between humans and animals [14]. By detailing, it is generally accepted now that especially for the rodent models of psychiatric and neurological diseases, there should be firstly a clear resemblance to the classical human symptoms (the so-called face validity), an additional resemblance to the main mechanisms of the disease (e.g. construct validity) and an identifiable response to the drugs that are generally working in humans (predictive validity), as described by Crawley in 2004 [15].
Considering all the aforementioned aspects, we will summarize both the current knowledge and our previous experience in animal models of anxiety, by focusing on various species such as rats, zebrafish, dogs, and pigs, as well as describing the relevance of oxidative stress in this context. Additionally, we will briefly describe the possible relevance of the oxytocin administration in this context, considering its increased relevance in this area of research in the last couple of years.
2. Some common tests of anxiety and their associated mechanisms
2.1. Social defeat stress
An important model in studying anxiety disorders relies on social defeat stress. Social defeat paradigm was used in large number of models that studied depressive [16], [17] and anxiety disorders [18], [19]. In this way, social defeat model is based on an interaction of the experimental animal with specific stimuli that the animal considers dangerous, without the possibility of escaping from the situation [20]. A model of social defeat is thus created by the exposure of the animal to an aggressive-type interaction that the animal considers dangerous. The classical experimental settings involve a short interaction with another larger individual, which is more aggressive, inducing a relation of coordination-submission [21].
Also, the model of social defeat could be a one-time confrontation with the threat or may be designed as chronic repeated exposures. Generally, exposure to repeated obligatory social stress (chronic stress) is fairly used in depression model, while acute exposure (acute stress) is rather investigated for anxiety disturbances [17], [22].
In addition, several methods [23], [24], [25] have been described and used for study anxiety and stress related disorders that are generally divided into conditioned [26] and unconditioned methods [23], [24]. The most common unconditioned tests are exploration-based models including elevated plus-maze, elevated zero maze, elevated T-maze, and the light/dark box or social interaction test [24], [25], and also tests based on predator models (e.g. including cat exposure or rat exposure) [23].
2.2. Elevated plus maze
Elevated plus-maze is considered an important test that evaluates anxiety behavior by counting the number of entries in the open-arms [27]. The preference for the closed arms is a sign of need for security and reveals anxiety traits. The measure for anxiety is calculated by counting the number of open-arm entries and the number of closed-arm entries expressed as a percentage of the total number of arm entries and the period of time spent on the open arms [14]. In fact, this behavioral test has more sophisticated concerns, as additional anxiety-related behaviors are evaluated including head dipping in open-arms (sticking the head below the level of the maze and towards the floor), protected stretch-attend postures (stretching) in the closed arms (the animal stretches with the forepaws while maintaining the hind paws in the same place and then retracts to the original position), and grooming (cleaning of any part of the body with the paws and/or the mouth) [27]. Our team was the first; to the best of our knowledge; to show that the intra-cerebro-ventricular administration of a drug such as angiotensin-(1-7) could result in anxiolytic-like behavior, 3 weeks after neurosurgery, when administrated 8 consecutive days, 0.1 mg/kg body weight, as demonstrated by the significant increase in the time spent in the open arms of the elevated plus maze and increased head-dipping behavior in the open arms, as well as decreased stretching in closed arms [27]. Even more, one important aspect to be studied in the elevated plus-maze test is the so-called “locomotor control” of the data, since it is difficult to differentiate between motor deficiencies and anxiety-induced suppression of exploration [25], [27]. That is the reason why the counting of the closed arms entries; which are generally considered as an index of the locomotor activity; is essential in analyzing the behavioral significance of elevated plus maze.
Furthermore, grooming behavior can sometimes be associated with both anxiogenic and anxiolytic behavior, mainly depending on the environment where it is performed (e.g. inside the elevated plus maze - an anxiogenic behavior vs. inside the home cage - anxiolytic) [28], [29].
Results from our former laboratory investigations of the anxiety-related functions of the central renin-angiotensin system, showed that blocking angiotensin II, by both its specific receptors antagonists or by blocking the angiotensin converting enzyme, resulted in anxiolytic effect [30], [31], in studies involving the administration of intracerebroventricular captopril, as a central angiotensin converting enzyme blocker [30] or losartan and PD-123177, as AT1 and AT2 specific receptors antagonists [31].
Other authors [32], [33] as well have used elevated plus-maze to study anxiety in rat models. For instance, Berton group had found that one time social defeat exposure followed by 14–18 h of sensory contact with the aggressor increased anxiety behavior in Lewis rats evaluated by elevated plus-maze [32].
2.3. Other specific behavioral testing for anxiety
Anxiogenic responses were also recorded by Bulos et al., who showed an anxiogenic effect on the rats after 5 min exposure to a predator (cat), but not 30 min after contact, as demonstrated both in elevated plus-maze and in elevated T-maze [23].
Another important test used for investigating the social anxiety is the elevated T-maze test. It is particularly used for panic and generalized anxiety disorders that investigate two types of behaviors related to anxiety (inhibitory avoidance and escape) [32].
Elevated plus-maze was successfully used by our research group in many experiments as follow:
-
1
Demonstrating an increased anxiety as a result of tartrazine administration, in which we work on to further develop a possible model of Attention Deficit Hyperactivity Disorder (ADHD) in rats (e.g. as a result of various specimens of tartrazine administration) [34], considering also the strong correlations between these manifestation, as sometimes more than half of the patients with ADHD have an co-occurring anxiety pathological manifestation [35].
-
2
Study the effect of black tea which is protective in both anxiety and fluoride-induced neurobehavioral toxicity in rats [36].
-
3
Study the anxiolytic-like effect of the deuterium depleted water, which has a concentration of deuterium 6–7 times lower than the normal water, as demonstrated by the increase in the time open arms of the elevated plus maze. In addition, this water generated an increased head-dipping suggesting a possible usage for anxiolytic actions [37].
-
4
Investigating the lesioning of the hypothalamic paraventricular nucleus through the usage of 5, 7-dihydroxytryptamine resulted in clear anxiolytic effects in rats, as mainly expressed in increased time in the open arms of the elevated maze [6].
3. Animal models commonly used in behavioral tests
3.1. The social defeat rat model
The social defeat rat model is involving a resident-intruder model, where a male rodent that plays the role of the intruder is placed in the cage of another male, which is the resident male. After the fight of the animals, a dominant and a subordinate male are established. In this way, social stress is believed to be experiences by the defeated rat model [38].
3.2. Studies of zebrafish
Another model to study behavioral changes is the zebrafish (Danio rerio) [8]. In fact, lately there is an increased concern in studying anxiety behavior in species such as the zebrafish. This could be extremely relevant, especially considering that zebrafish shares a fundamental pattern of neurodevelopment and functional brain organization with other vertebrates, including humans.
These shared features provide a basis for experimental research, demonstrating the mechanisms of disease-associated brain abnormalities [8], as current rapports are describing sleep related studies in zebrafish, associated or not with specific behavioral tests such as light and dark conditioning [39], [40] or how to generate autism models in this specie [41].
Thus, Maximino et al. [42] clearly showed that zebrafish is increasingly being used in behavioral neuroscience, neuro-psycho-pharmacology and neuro-toxicology. They showed how the behavioral tests used to model anxiety in rodents were adapted to zebrafish. Other research described how these processes are studied in this specie based on some related and conditional-associated color developed anxiety [43]. Thus, in this study, Blaser and Rosemberg [43] showed that in specific behavioral tasks for zebrafish, such as novel tank diving test (which is developed on the basic response of zebrafish to initially dive to the bottom of a novel experimental tank), anxiety could be modulated by the colors used for the tank construction (e.g. black, white or transparent, with the zebrafish always and naturally preferring the black one). In addition, specific behaviors related to anxiety, such as shuttling and immobility were affected by the aforementioned colors manipulation [43].
3.3. Models related to post-traumatic stress disorder
Another model as shown by Solanki et al. [44] shows how to generate animal models of post-traumatic stress disorder (PTSD). They demonstrated; in an original approach in rats; that witnessing a traumatic event is leading to the development of anxiety-like and depression-like behavior deficits. In this way, the aforementioned authors used their trauma witness model to subsequently interact with a socially defeated rat, generating a PTSD model [44]. Moreover they showed that anxiety and aggression can be associated and linked together by factors such as corticosterone and increased oxidative stress status [45].
3.4. Models related to ADHD and interactions
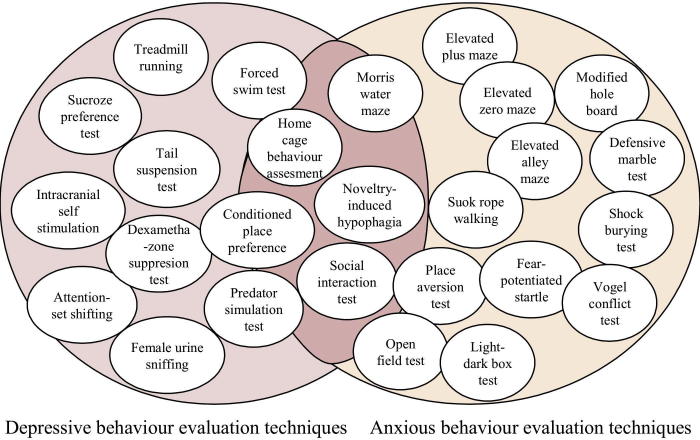
As previously shown in the case of complex interactions between ADHD and anxiety or aggression and anxiety, many of these behavioral manifestations are in tight correlations with each other, as our group previously described the interactions between anxiety and depression-related behaviors when studied in specific rat tests (Fig. 1). This also occurs when anxiety and depression-related behaviors are studied in rat models [46]. We investigated the hypothesis that oxidative stress is affecting anxiety, depression or PTSD, by mainly referring to their main biomarkers, animal models relevance, genetic perspectives and antioxidant approaches [46].
Fig. 1.
The interactions between anxiety and depression-related behaviors as studied in various tasks in rat animal models, as described by Balmus et al. [46].
3.5. Oxidative stress implications
Lately, there is an increased concern in understating the exact relevance of the oxidative stress status in most of the neuropsychiatric disorders [47], [48], [49], [50], [51], [52], [53] such as anxiety. Previous reports described for example that anxiety behavior in light/dark box task is clearly related to the oxidative status (as expressed through the ROS concentration) in both neuronal and glial cells of the cerebellum or hippocampus, as well as in the neurons of the cerebral cortex and peripheral leucocytes (monocytes, granulocytes and lymphocytes) [54], [55].
Furthermore, Hughes et al. [56] showed that the administration of vitamins C and/ or E, decreases anxiety-related behavior of rats in open-field. Likewise, in the previous studies on anxiety in rats, most of the anxiolytic effects of angiotensin-1-7 or angiotensin II blockers were very well correlated with some antioxidant actions of these drugs [30], [31], [50].
3.6. Models related to irritable bowel syndrome
There is also an increased interest in the recent literatures on the irritable bowel syndrome (IBS) [57], which besides being a modern disorder clinically characterized by cramping, abdominal pain, bloat, diarrhea, and constipation. It is also a functional gastrointestinal disorder, which means that it is not characterized by any tissue or organ damage [58], exhibiting complex and controversial pathological features. The etio-pathogenic process in IBS is not well elucidated, but stress may be an important factor, as most of the times it is associated with increased levels of anxiety [59].
What are interesting in IBS animal models is their related anxiety manifestations [60]. In this way, the generation of the IBS animal models is mainly based on stress and anxiety, as shown by Mozaffari et al. [61], by using the method of chronic contention stress to induce gastrointestinal disorders where the animals were placed in plastic containers that permit complete immobilization, without any risk of suffocation. Thus, stress exposure was 6 h/day, for one week, which resulted in high levels of anxiety [61].
Also, our research group is now working in generating a model of IBS with greater reliability, which would enable a more relevant therapeutic approach, based on alternating multiple stressors, such as applying a combination of seven persistent, daily, chronic stressor: cage tilting, water/food deprivation, pinch tail, predator sound, wet bedding, and injection mimicking. An increased advantage of this method resides in avoiding the progressive habituation that may occur when a homotypic stimulus is repeatedly consistently.
4. Oxytocin relevance
Besides its well-known role in reproduction [62], oxytocin plays a significant role in most of the neuropsychiatric dysfunctions, with increased number of reports discussing especially the intranasal oxytocin management approach in disorders such as autism [63], schizophrenia [64], fronto-temporal dementia [65], Prader Willi syndrome [66], anxiety [67], depression [68], aggression [69] or suicide-related behavior [70].
Some of these results were also similar to results observed in other animal models, in species such as dogs [71], [72], rat [73] or pigs [74], [75], [76]. In this way, some authors found changes in complex and specific behavioral tasks performed on dogs (such as Threatening Approach Test) when oxytocin was administered [71], [72]. The authors observed that after given intranasal oxytocin some modification in the way the animal reacts to several different stimuli, such as the presence or the posture of their owner or an unfamiliar experimenter may occur [71]. Furthermore, in pig models (which are actually considered much more closer resembling to human brain in anatomy, growth and development, as compared to the brains of rodent [74]) that repeated administration of oxytocin postnatally can actually affect the hypothalamo-pituitary-adrenal axis functioning [75], with increased relevance to further anxiety-related manifestations.
Also, it was previously demonstrated in a different report on dogs that the administration of intranasal oxytocin is increasing the appropriate use of human social cues and can result in decreased aversion to gazing cues in these animals [72]. Also, recently Hernadi et al., showed that positive human contact could actually generate a significant increase in cerebrospinal fluid oxytocin levels, which could last for over 120 min, after 15 min interaction [71].
All these effects of oxytocin, related to anxiety, were also demonstrated in rats, as showed by Laszlo et al. [73], who reported that 10 ng or 100 ng of oxytocin, injected in volume of 0.4 μL in the central nucleus of the amygdala (an important central area related of course to anxiety behavior [50]) resulted in anxiolytic effects, modulated by the specific oxytocin receptor located at this level. Some effects of oxytocin could be related to its antioxidant ability, considering the current arguments regarding the possible antioxidant actions of this drug in animal models and human studies [77].
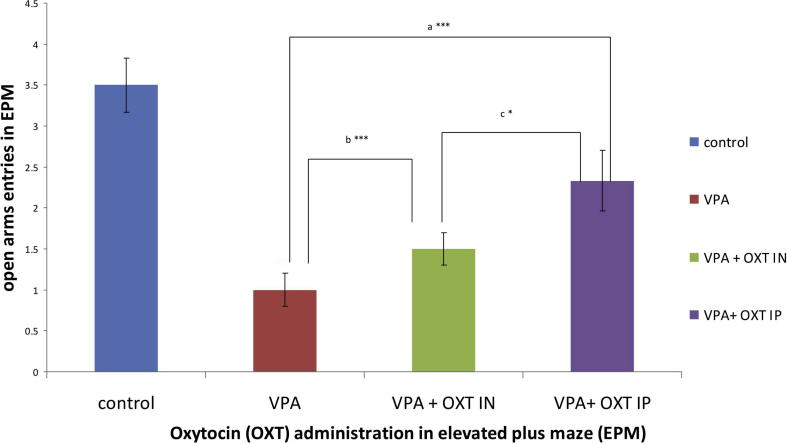
Moreover, our research group has some preliminary results (data not shown) on the effects that oxytocin administration could actually exert on the anxiety levels, as studied again in the elevated plus- maze, in an epigenetic schizophrenia rat model generated by the administration of methionine [78]. Even more, we are designing an experimental model to see if intranasal administration of oxytocin could reduce some anxiety manifestations induced in a rat model of autism (e.g. generated by the perinatal administration of valproic acid), with some preliminary positive data (Fig. 2), as seen in elevated plus - maze.
Fig. 2.
Preliminary data of the consequences of intranasal (IN) and intraperitoneal (IP) oxytocin (OXT) administration (10 UI, for one week) on the numbers of open arms entries (a parameter of anxiety) in elevated plus maze (EPM) in a rat model of autism, based on Valproic acid (VPA) administration. Post-hoc analysis are equivalent for a, b, and c on the chart (***P < .01, *P < .05).
In addition, it seems that the administration of intraperitoneal oxytocin in these rats could also decrease anxiety, as judged by the increased number of open arms entries in the elevated plus maze [79], [80] (Fig. 2).
5. Conclusions
We presented here some of our previous experience in working with behavioral animal models of anxiety, as well as some modern theories and methodological approaches regarding anxiety in lab species such as rats, zebrafish, dogs and pigs. Also, in this context, we described some current mechanism regarding oxidative stress and oxytocin administration, keeping in mind that these may represent future therapeutic targets in the pathophysiology of anxiety disorders.
Competing interests
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to disclose the PN-II-RU-TE-2014-4-1886 grant “A complex study regarding the relevance of oxytocin administration in some animal models of neuropsychiatric disorders”, number 120 from 01/10/2015.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Diagnostic and statistical manual of mental disorders american psychiatric association. 5th ed. Arlington: American Psychiatric Publishing; 2013. p. 189–195.
- 2.Lefter R., Cojocaru D., Ciobica A., Paulet I.M., Serban I.L., Anton E. Aspects of animal models for the major neuropsychiatric disorders. Arch Biol Sci Belgr. 2014;66:1105–1115. [Google Scholar]
- 3.Twigger S.N., Pruitt K.D., Fernández-Suárez X.M., Karolchik D., Worley K.C., Maglott D.R. What everybody should know about the rat genome and its online resources. Nat Genet. 2008;40(5):523–527. doi: 10.1038/ng0508-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernando A.B., Robbins T.W. Animal models of neuropsychiatric disorders. Annu Rev Clin Psychol. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- 6.Ciobica A., Hritcu L., Padurariu M., Dobrin R., Bild V. Effects of serotonin depletion on behavior and neuronal oxidative stress status in rat: relevance for anxiety and affective disorders. Adv Med Sci. 2010;55:289–296. doi: 10.2478/v10039-010-0035-2. [DOI] [PubMed] [Google Scholar]
- 7.Gonzales H.K., O'Reilly M., Lang R., Sigafoos J., Lancioni G., Kajian M. Research involving anxiety in non-human primates has potential implications for the assessment and treatment of anxiety in autism spectrum disorder: a translational literature review. Dev Neurorehabil. 2016;19:175–192. doi: 10.3109/17518423.2014.941117. [DOI] [PubMed] [Google Scholar]
- 8.Plavan G., Ciobica A., Nicoara M., Timofte D. The biomaterial relevance of oxytocin in some zebrafish studies. Key Eng Mater. 2015;660:289–293. [Google Scholar]
- 9.Calandreau L., Bertin A., Favreau-Peigné A., Richard S., Constantin P., Lansade L. Impact of high and low anxiety trait on object habituation and discrimination: evidence from selected lines of Japanese quail. Behav Brain Res. 2013;250:299–303. doi: 10.1016/j.bbr.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Jesuthasan S. Fear, anxiety, and control in the zebrafish. Dev Neurobiol. 2012;72:395–403. doi: 10.1002/dneu.20873. [DOI] [PubMed] [Google Scholar]
- 11.Ottaviani E., Franceschi C. The neuroimmunology of stress from invertebrates to man. Prog Neurobiol. 1996;48:421–440. doi: 10.1016/0301-0082(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 12.Vicens-Costa E., Martínez-Membrives E., López-Aumatell R., Guitart-Masip M., Cañete T., Blázquez G. Two-way avoidance acquisition is negatively related to conditioned freezing and positively associated with startle reactions: a dissection of anxiety and fear in genetically heterogeneous rats. Physiol Behav. 2011;103:148–156. doi: 10.1016/j.physbeh.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Díaz-Morán S., Palència M., Mont-Cardona C., Cañete T., Blázquez G., Martínez-Membrives E. Coping style and stress hormone responses in genetically heterogeneous rats: comparison with the Roman rat strains. Behav Brain Res. 2012;228:203–210. doi: 10.1016/j.bbr.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Bourin M. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues Clin Neurosci. 2015;17:295–303. doi: 10.31887/DCNS.2015.17.3/mbourin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawley J.N. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10(4):248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 16.Iñiguez S.D., Riggs L.M., Nieto S.J., Dayrit G., Zamora N.N., Shawhan K.L. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17:247–255. doi: 10.3109/10253890.2014.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker C., Zeau B., Rivat C., Blugeot A., Hamon M., Benoliel J.J. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- 18.Bader L.R., Carboni J.D., Burleson C.A., Cooper M.A. 5-HT1A receptor activation reduces fear-related behavior following social defeat in Syrian hamsters. Pharmacol Biochem Behav. 2014;122:182–190. doi: 10.1016/j.pbb.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKim D.B., Patterson J.M., Wohleb E.S., Jarrett B.L., Reader B.F., Godbout J.P. Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol Psychiatry. 2016;79:803–813. doi: 10.1016/j.biopsych.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriques-Alves A.M., Queiroz C.M. Ethological evaluation of the effects of social defeat stress in mice: beyond the social interaction ratio. Front Behav Neurosci. 2015;9:364. doi: 10.3389/fnbeh.2015.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudryavtseva N.N., Bakshtanovskaya I.V., Koryakina L.A. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 22.Hollis F., Kabbaj M. Social defeat as an animal model for depression. ILAR J. 2014;55:221–232. doi: 10.1093/ilar/ilu002. [DOI] [PubMed] [Google Scholar]
- 23.Bulos E.M., Pobbe R.L., Zangrossi H., Jr. Behavioral consequences of predator stress in the rat elevated T-maze. Physiol Behav. 2015;146:28–35. doi: 10.1016/j.physbeh.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Cruz A.P.M., Frei F., Graeff F.G. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 25.Espejo E.F. Structure of the mouse behavior on the elevated plus maze test of anxiety. Behav Brain Res. 1997;86:105–112. doi: 10.1016/s0166-4328(96)02245-0. [DOI] [PubMed] [Google Scholar]
- 26.van Meurs B., Wiggert N., Wicker I., Lissek S. Maladaptive behavioral consequences of conditioned fear-generalization: a pronounced, yet sparsely studied, feature of anxiety pathology. Behav Res Ther. 2014;57:29–37. doi: 10.1016/j.brat.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bild W., Ciobica A. Angiotensin-(1–7) central administration induces anxiolytic-like effects in elevated plus maze and decreased oxidative stress in the amygdala. J Affect Disord. 2013;145:165–171. doi: 10.1016/j.jad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Lister R.G. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 29.David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciobica A., Hritcu L., Nastasa V., Padurariu M., Bild W. Inhibition of central angiotensin converting enzyme exerts anxiolytic effects by decreasing brain oxidative stress. J Med Biochem. 2011;30:109–114. [Google Scholar]
- 31.Ciobica A., Bild V., Hritcu L., Padurariu M., Bild W. Effects of angiotensin II receptor antagonists on anxiety and some oxidative stress markers in rat. Cent Eur J Med. 2011;6:331–340. [Google Scholar]
- 32.Berton O., Durand M., Aguerre S., Mormède P., Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- 33.Walf A.A., Frye C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamel M.M., El-Lethey H.S. The potential health hazard of tartrazine and levels of hyperactivity, anxiety-like symptoms, depression and anti-social behaviour in rats. J Am Sci. 2011;7:1211–1218. [Google Scholar]
- 35.Adler B. Managing ADHD in children, adolescents, and adults with comorbid anxiety in primary care. Prim Care Companion J Clin Psychiatry. 2007;9:129–138. [PMC free article] [PubMed] [Google Scholar]
- 36.Kamel M.M., El-Lethey H.S., Shaheed I.B., Kamel G.M. Black tea forestalls sodium fluoride-induced neurobehavioral toxicity in laboratory rats. J Am Sci. 2010;6:1655–1673. [Google Scholar]
- 37.Mladin C., Ciobica A., Lefter R., Popescu A., Bild W. Deuterium-depletion induces anxiolytic-like effects in elevated plus maze in rats. Arch Biol Sci Belgrade. 2014;66:947–953. [Google Scholar]
- 38.Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:43542. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 39.Árnason B.B., Þorsteinsson H., Karlsson K.Æ. Absence of rapid eye movements during sleep in adult zebrafish. Behav Brain Res. 2015;291:189–194. doi: 10.1016/j.bbr.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Sigurgeirsson B., Thornorsteinsson H., Sigmundsdóttir S., Lieder R., Sveinsdóttir H.S., Sigurjónsson Ó.E. Sleep-wake dynamics under extended light and extended dark conditions in adult zebrafish. Behav Brain Res. 2013;256:377–390. doi: 10.1016/j.bbr.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 41.Stewart A.M., Nguyen M., Wong K., Poudel M.K., Kalueff A.V. Developing zebrafish models of autism spectrum disorder (ASD) Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:27–36. doi: 10.1016/j.pnpbp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Maximino C., de Brito T.M., da Silva Batista A.W., Herculano A.M., Morato S. Measuring anxiety in zebrafish: a critical review. Behav Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 43.Blaser R.E., Rosemberg D.B. Measures of anxiety in zebrafish (Danio rerio): dissociation of black/white preference and novel tank test. PLoS ONE. 2012;7:36931. doi: 10.1371/journal.pone.0036931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solanki N., Alkadhi I., Atrooz F., Patki G., Salim S. Grape powder prevents cognitive, behavioral and biochemical impairment in a rat model of posttraumatic stress disorder. Nutr Res. 2015;35:65–75. doi: 10.1016/j.nutres.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patki G., Atrooz F., Alkadhi I., Solanki N., Salim S. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci Lett. 2015;584:308–313. doi: 10.1016/j.neulet.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balmus I.M., Ciobica A., Antioch I., Dobrin R., Timofte D. Oxidative stress implications in the affective disorders: main biomarkers, animal models relevance, genetic perspectives, and antioxidant approaches. Oxid Med Cell Longev. 2016;2016:3975101. doi: 10.1155/2016/3975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciobica A., Padurariu M., Dobrin I., Stefanescu C., Dobrin R. Oxidative stress in schizophrenia - focusing on the main markers. Psychiatr Danub. 2011;23:237–245. [PubMed] [Google Scholar]
- 48.Ciobica A., Padurariu M., Hritcu L. The effects of short-term nicotine administration on behavioral and oxidative stress deficiencies induced by a rat model of Parkinson’s disease. Psychiatr Danub. 2012;24:194–205. [PubMed] [Google Scholar]
- 49.Stefanescu C., Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143:34–38. doi: 10.1016/j.jad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Bild W., Ciobica A. Angiotensin-(1–7) central administration induces anxiolytic-like effects in elevated plus maze and decreased oxidative stress in the amygdala. J Affect Disord. 2013;145:165–171. doi: 10.1016/j.jad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Bild W., Hritcu L., Stefanescu C., Ciobica A. Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Progr Neuropsychopharmacol Biol Psychiatry. 2013;43:79–88. doi: 10.1016/j.pnpbp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Padurariu M., Ciobica A., Lefter R., Serban I.L., Stefanescu C., Chirita R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr Danub. 2013;25:401–409. [PubMed] [Google Scholar]
- 53.Ciobica A., Olteanu Z., Padurariu M., Hritcu L. The effects of low-dose pergolide on memory and oxidative stress in a 6-OHDA induced rat model of Parkinson’s disease. J Physiol Biochem. 2012;68:59–69. doi: 10.1007/s13105-011-0119-x. [DOI] [PubMed] [Google Scholar]
- 54.Rammal H., Bouayed J., Younos C., Soulimani R. Evidence that oxidative stress is linked to anxiety related behavior in mice. Brain Behav Immun. 2008;22:1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Bouayed J., Rammal H., Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxidative Med Cell Longev. 2009;2:63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes R.N., Lowther C.L., van Nobelen M. Prolonged treatment with vitamins C and E separately and together decreases anxiety-related open-field behavior and acoustic startle in hooded rats. Pharmacol Biochem Behav. 2011;97:494–499. doi: 10.1016/j.pbb.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Ballou S., Kaptchuk T.J., Hirsch W., Nee J., Iturrino J., Hall K.T. Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial. Trials. 2017;18:234. doi: 10.1186/s13063-017-1964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weaver K.R., Melkus G.D., Henderson W.A. Irritable Bowel Syndrome. Am J Nurs. 2017;117:48–55. doi: 10.1097/01.NAJ.0000520253.57459.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee A.D., Spiegel B.M., Hays R.D., Melmed G.Y., Bolus R., Khanna D. Gastrointestinal symptom severity in irritable bowel syndrome, inflammatory bowel disease and the general population. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guenne S, Lefter R, Ciobica A, Trifan A, Compaore M, Hilou A et al. Chrysanthellum americanum extract administration is reducing depressive manifestations in an animal model of Irritable Bowel Syndrome, 1st International Congress on Evidence Based Mental Health, Corfu, Greece, May 26th - 29th, 2016;41:78–79.
- 61.Mozaffari S., Esmaily H., Rahimi R., Baeeri M., Sanei Y., Asadi-Shahmirzadi A. Effects of Hypericum perforatum extract on rat irritable bowel syndrome. Pharmacognosy Mag. 2011;7:213–223. doi: 10.4103/0973-1296.84235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mostafa T., Rashed L.A., Osman I., Marawan M. Seminal plasma oxytocin and oxidative stress levels in infertile men with varicocele. Andrologia. 2015;47:209–213. doi: 10.1111/and.12248. [DOI] [PubMed] [Google Scholar]
- 63.Guastella A.J., Hickie I.B. Oxytocin treatment, circuitry, and autism: a critical review of the literature placing oxytocin into the autism context. Biol Psychiatry. 2016;79:234–242. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 64.Guastella A.J., Ward P.B., Hickie I.B., Shahrestani S., Hodge M.A., Scott E.M. Single dose of oxytocin nasal spray improves higher-order social cognition in schizophrenia. Schizophr Res. 2015;168:628–633. doi: 10.1016/j.schres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Finger E.C., MacKinley J., Blair M., Oliver L.D., Jesso S., Tartaglia M.C. Oxytocin for frontotemporal dementia: a randomized dose-finding study of safety and tolerability. Neurology. 2015;84:174–181. doi: 10.1212/WNL.0000000000001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Einfeld S.L., Smith E., McGregor I.S., Steinbeck K., Taffe J., Rice L.J. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet A. 2014;164A:2232–2239. doi: 10.1002/ajmg.a.36653. [DOI] [PubMed] [Google Scholar]
- 67.Ciobica A., Balmus I.M., Padurariu M. Is oxytocin relevant for the affective disorders? Acta Endocrinol. 2016;12:65–71. doi: 10.4183/aeb.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Padurariu M., Ciobica A. Understanding the importance of oxytocin in depression. Nobel Medicus. 2017 [Google Scholar]
- 69.Padurariu M., Prepelita R., Ciobica A., Dobrin R., Timofte D., Stefanescu C. Short Review on the aggressive behaviour: genetical, biological aspects and oxytocin relevance. Int Lett Nat Sci. 2016;52:43–53. [Google Scholar]
- 70.Padurariu M., Prepelita R., Ciobica A., Dobrin R., Timofte D., Stefanescu C. The concept of suicide: neurophysiological/genetic theories and possible oxytocin relevance. Neurophysiology. 2016;48:312–321. [Google Scholar]
- 71.Hernádi A., Kis A., Kanizsár O., Tóth K., Miklósi B., Topál J. Intranasally administered oxytocin affects how dogs (Canis familiaris) react to the threatening approach of their owner and an unfamiliar experimenter. Behav Processes. 2015;119:1–5. doi: 10.1016/j.beproc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Oliva J.L., Rault J.L., Appleton B., Lill A. Oxytocin enhances the appropriate use of human social cues by the domestic dog (Canis familiaris) in an object choice task. Anim Cogn. 2015;18:767–775. doi: 10.1007/s10071-015-0843-7. [DOI] [PubMed] [Google Scholar]
- 73.László K., Kovács A., Zagoracz O., Ollmann T., Péczely L., Kertes E. Positive reinforcing effect of oxytocin microinjection in the ratc entral nucleus of amygdala. Behav Brain Res. 2016;296:279–285. doi: 10.1016/j.bbr.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 74.Lind N.M., Moustgaard A., Jelsing J., Vajta G., Cumming P., Hansen A.K. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Rault J.L., Carter C.S., Garner J.P., Marchant-Forde J.N., Richert B.T., Lay D.C., Jr. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol Behav. 2013;112–113:40–48. doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Rault J.L. Effects of positive and negative human contacts and intranasal oxytocin on cerebrospinal fluid oxytocin. Psychoneuroendocrinology. 2016;69:60–66. doi: 10.1016/j.psyneuen.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 77.Honceriu C., Ciobica A., Stoica B., Chirazi M., Padurariu M. Oxytocin antioxidant effects on Wistar rats. Rev Chim (Bucharest) 2016;67:2246–2249. [Google Scholar]
- 78.Padurariu M., Lefter R., Ciobica A., Paulet I.M., Dobrin R. Intraperitoneal oxytocin administration for 9 consecutive days is reducing memory deficits and anxiety in a methionine rat model of schizophrenia. Eur Neuropsychopharmacol. 2016;26:289–290. [Google Scholar]
- 79.Lefter R, Ciobica A, Padurariu M, Antioch I, Balmus I, Dobrin R. Intranasal oxytocin administration reduces memory, anxiety and depression-related deficits in a valproic acid-induced perinatal model of autism. In: 3rd Congress of the European Academy of Neurology (EAN). Amsterdam; 2017; 1:2689.
- 80.Lefter R, Ciobica A, Antioch I, Balmus I, Padurariu M, Dobrin R. Intranasal oxytocin administration reduces memory, anxiety and depression-related deficits in a valproic acid-induced rat model of autism. In: 5th International Congress on Neurobiology, Psychopharmacology and Treatment Guidance 2017; 1:108.