Abstract
Aims
To explore the potential of the skin microbiome as biomarker in six dermatological conditions: atopic dermatitis (AD), acne vulgaris (AV), psoriasis vulgaris (PV), hidradenitis suppurativa (HS), seborrhoeic dermatitis/pityriasis capitis (SD/PC) and ulcus cruris (UC).
Methods
A systematic literature review was conducted according to the PRISMA guidelines. Two investigators independently reviewed the included studies and ranked the suitability microbiome implementation for early phase clinical studies in an adapted GRADE method.
Results
In total, 841 papers were identified and after screening of titles and abstracts for eligibility we identified 42 manuscripts that could be included in the review. Eleven studies were included for AD, five for AV, 10 for PV, two for HS, four for SD and 10 for UC. For AD and AV, multiple studies report the relationship between the skin microbiome, disease severity and clinical response to treatment. This is currently lacking for the remaining conditions.
Conclusion
For two indications – AD and AV – there is preliminary evidence to support implementation of the skin microbiome as biomarkers in early phase clinical trials. For PV, UC, SD and HS there is insufficient evidence from the literature. More microbiome‐directed prospective studies studying the effect of current treatments on the microbiome with special attention for patient meta‐data, sampling methods and analysis methods are needed to draw more substantial conclusions.
Keywords: biomarkers, clinical drug development, microbiota, surrogate parameter
Introduction
The escalating number of therapeutic candidates in drug development programs require strategies that optimize the process of clinical development. A common approach is the use of biomarkers in clinical trials. A biomarker is defined as a characteristic that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention 1, 2. Clinical biomarkers are thought to reflect disease activity and pathophysiology 3, 4. A useful biomarker in any class has to comply with the following general criteria: (i) there must be a consistent response of the biomarker across studies (preferably from different research groups) and drugs from the same mechanistic class; (ii) the biomarker must respond clearly to therapeutic (not supratherapeutic) doses; (iii) there must be a clear dose‐ or concentration‐response relationship; and (iv) there must a plausible relationship between the biomarker, pharmacology of the drug class and disease pathophysiology 4. Validated biomarkers are often being used to guide drug development programmes from human pharmacology studies, i.e. phase 1 trials, to confirmatory trials, i.e. phase 3 studies 2. For dermatological diseases the drug developers often rely on clinical efficacy scores, e.g. the Eczema Area and Severity Index (EASI) for atopic dermatitis (AD), Psoriasis Area and Severity Index for psoriasis vulgaris (PV) and inflammatory lesion count for acne vulgaris (AV) or investigator global assessments. However, more objective outcome measures including validated biomarkers would have great added value in this field. One of these potential new biomarkers is the human skin microbiome, which has the potential to monitor disease activity and drug specific (mechanistic) effects.
The human microbiome refers to the combined genomic information of all microbial communities living on or in the human body. Collectively, this encompasses fungi (mycobiota), bacteria (microbiota), viruses, bacteriophage, archaea and protozoa. This, along with the human genome, completes what is now termed the human microbial superorganism 5. The skin microbiome harbours vast microbial communities living in a range of both physiologically and topographically distinct niches and microenvironments 6, 7. Actinobacteria (52%), Firmicutes (24%), Proteobacteria (17%) and Bacteroidetes (7%) are the four most abundant species identified on the skin 8. Previous studies have shown that it is not only skin topography that influences microbial colonization, but also a vast range of host‐specific factors including age and sex, and environmental factors such as occupation, clothing choice, antibiotic use, cosmetics, soaps, environmental temperature, humidity, and longitudinal and/or latitudinal variation in UV exposure, which can all contribute to the variability seen in the microbial flora of the skin 9, 10, 11, 12, 13, 14, 15. Moreover, changes or aberrations in the skin microbiome have been implicated in the pathophysiology of numerous skin diseases such as AD and AV 16.
Several reviews have described the role and impact of skin microbiome on disease 17, 18, 19, 20, 21, 22. However, to date, no structured review has been conducted to evaluate the feasibility, suitability and potential use of the skin microbiome as biomarker for early phase clinical drug development. Therefore, we conducted a systemic literature review with predefined search terms according to the PRISMA guidelines, with focus on six relevant disorders, i.e. AD, seborrhoeic dermatitis and pityriasis capitis (dandruff; SD/PC), AV, hidradenitis suppurativa (HS), PV and ulcus cruris/chronic wounds (UC). In addition, we evaluated and ranked the conditions regarding the potential as clinical biomarker. Lastly, we provided recommendations for prospective microbiome investigations in clinical drug development programmes.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) 23. In collaboration with a trained librarian from the Leiden University Medical Centre, a structured electronic literature search was composed, using a combination of two main search criteria: microbiome and the targeted skin condition (i.e. AD, SD/PC, HS, AV, UC and PV). For each search term, all relevant keyword variations were used in conjunction with free text word variations. The search strategy was optimized for all consulted databases, taking into account the differences of the various controlled vocabularies, as well as the differences of database‐specific technical variations (e.g. the use of quotation marks). The final search was performed on 29 September 2017, using bibliographic databases including PubMed (incl. MEDLINE), Embase (OVID‐version), Web of Science, Cochrane Library, CENTRAL, Academic Search Premier and ScienceDirect. Animal‐only studies, reviews without original data, non‐English studies and case studies were excluded. Moreover, culture‐based methods were excluded since the objective of this review was to explore the full microbiome profile and relative abundances compared to other genus as biomarker. The remaining studies were fully reviewed. The overall quality of evidence was rated using pre‐defined criteria (group size, type of control, method of sampling, serial sampling available, well defined metadata, analysis method). Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines were used as guidance for rating the quality of evidence 24. This was done by two investigators independently and the final outcome was determined by discussion once discrepancies occurred.
Results
The search resulted in 841 titles. After duplicates were removed, 443 papers were screened for inclusion. Four‐hundred‐and‐one manuscripts were excluded based on the exclusion criteria with mostly culture‐based studies that were not eligible. The remaining 42 studies were identified as using nonculture‐based methods to analyse microbiome populations in one of the targeted skin conditions and fully reviewed, Figure 1. All 42 were included in the review, the study characteristics can be found in Table 1.
Figure 1.
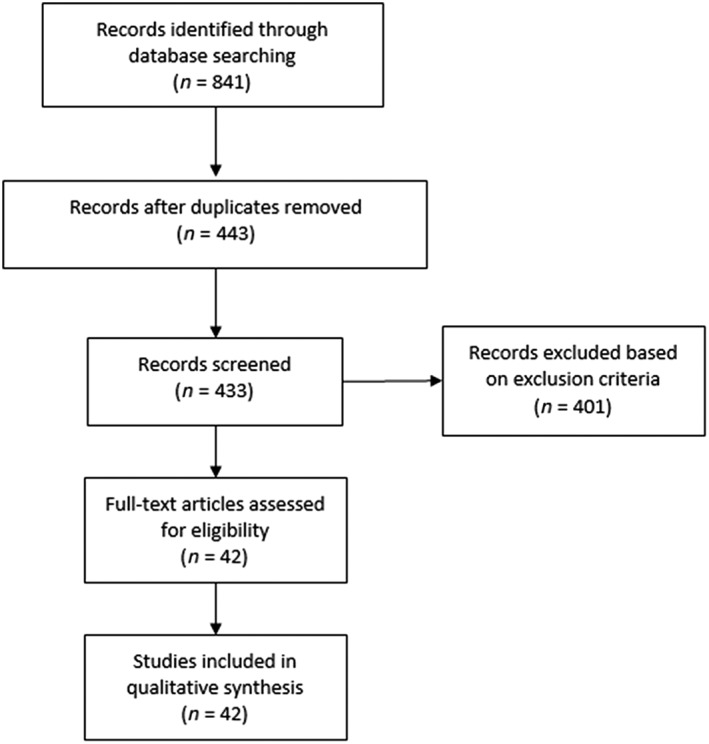
Flowchart of the study
Table 1.
Summary table of the studies included in the review
| Source First author, year [ref] | Disease | Study design No. of patients | Sample collection methods | Analysis | Key findings | Weaknesses | Level evidence |
|---|---|---|---|---|---|---|---|
| Paulino et al . 2006 18 | PV |
Case control 3 PV/5 HV |
Sterile swabs Lesional and nonlesional skin Multiple sampling in one PV and 2 HV |
18S rRNA 5.8S rDNA | ▪ Malassezia mycobiota substantially different PV vs. HV | ▪ Small cohort | Low |
| Amaya et al . 2007 19 | PV |
Case control 22 PV/36 AD/30 HV |
OpSite® transparent adhesive dressings Lesional and nonlesional skin |
5.8S rDNA | ▪ Malassezia species detected in overall sites higher in PV and AD compared to HV |
▪ Small cohort ▪ PV patients on treatment ▪ Limited analysis ▪ Different skin site collection PV vs. AD and HV |
Low |
| Paulino et al . 2008 20 | PV |
Case control 1 PV/1 HV |
Sterile swabs Lesional and nonlesional skin Multiple time points |
5.8S rDNA |
▪ Mycobiota relatively stable over time. ▪ No significant dichotomy between PV and HV. |
▪ Small cohort ▪ Limited analysis |
Low |
| Gao et al . 2008 21 | PV |
Case control 6 PV/6 HV |
Sterile swabs Lesional and nonlesional skin |
16S rRNA V1‐V9 |
▪ Firmucutes more abundant in lesional skin PV vs. nonlesional skin and HV. ▪ Actinobacteria less abundant in lesional skin PV vs. nonlesional skin and HV. |
▪ Small cohort ▪ No serial sampling |
Low |
| Fahlen et al . 2011 22 | PV |
Case control 10 PV/12 HV |
2‐mm skin punch biopsies | 16S rRNA V3‐V4 |
▪ Most common phyla in PV and HV: Firmicutis, Proteobacteria, Actinobacteria. ▪ Staphylococci and Propionibacteria were less common in psoriatic lesions |
▪ Small cohort ▪ No serial sampling ▪ Variation in skin sample sites |
Low |
| Alekseyenko et al . 2013 23 | PV |
Case control & Prospective longitudinal cohort study CC: 54 PV/37 HV PC: 17 PV/15 HV |
Sterile swabs Lesional and nonlesional skin HV matched sites Multiple sampling |
16S rRNA V1‐V3 |
▪ Most common phyla in PV and HV: Firmicutis, Proteobacteria, Actinobacteria. ▪ Combined relative abundance of Corynebacterium, Streptococcus and Staphylococcus was increased in psoriatic skin, compared to unaffected skin and healthy control skin |
▪ Some patients on active treatment ▪ Mainly severe patients |
Low to moderate |
| Statnikov et al . 2013 24 | PV |
Case control 54 PV/37 HV |
Sterile swabs Lesional and nonlesional skin HV matched sites |
16S rRNA V1‐V3 and V3‐V5 | ▪ Microbiome signatures could be used to diagnose psoriasis | ▪ No serial sampling | Low to moderate |
| Takemoto et al . 2015 25 | PV |
Case control 12 PV/12 HV |
PV: psoriatic scales by tweezer HV: OpSite® transparent adhesive dressings |
26S rRNA D1 – D2 |
▪ Psoriatic lesions exhibited significantly greater diversity compared to HV ▪ Malassezia restricta levels were significantly higher in psoriatic lesions, compared to healthy controls |
▪ Small cohort ▪ No serial sampling ▪ Only male patients ▪ Different sample method PV and HV |
Low |
| Salava et al . 2017 26 | PV |
Case control 13 PV |
Sterile swabs Lesional and nonlesional skin |
16S rRNA V1‐V3 | ▪ No significant differences microbial diversity between lesional and nonlesional skin |
▪ Small cohort ▪ No serial sampling ▪ Variation in skin sample sites |
Low |
| Tett et al . 2017 27 | PV |
Case control 28 PV |
Sterile swabs Lesional and nonlesional skin |
WMS sequencing |
▪ Plaques at the ear had a significant decrease in microbial diversity, and increase in Staphylococcus abundance ▪ At species level, no differences between lesional and nonlesional skin were observed |
▪ Small cohort ▪ No serial sampling ▪ Some patients on active treatment |
Low |
| Ring et al . 2017 28 | HS |
Case control 30 HS 24 HV |
Biopsies Lesional and nonlesional skin |
16S rRNA V3‐V4 18S rDNA V3‐V4 |
▪ Microbiome in HS significantly different from HV in lesional and nonlesional skin ▪ Five microbiome types identified ▪ Lesional skin consisted predominantly of Corynebacterium species (type I) and Peptoniphilus species (type IV) ▪ Propionibacterium showed a significant higher abundance in HV |
▪ Small cohort ▪ No serial sampling |
Low |
| Guet‐Revillet et al . 2017 29 | HS |
Prospective cohort 65 HS |
Sterile swabs Lesional and nonlesional skin |
16S rRNA V1‐V2 |
▪ Lesional skin consisted predominantly of anaerobes (Porphyromonas and Prevotella species) ▪ Clinical severity significantly associated with variations in lesional microbiota ▪ Fusobacterium associated with severe HS |
▪ Small cohort | Low |
| Dowd et al . 2008 30 | UC |
Prospective cohort 10 VLU/10 DFU/ 10 PU |
Debridement samples | 16S rRNA V4 |
▪ Major populations include of all wound include: Staphylococcus, Pseudomonas, Peptoniphilus, Enterobacter, Strenotrophomonas, Finegoldia and Serratia species ▪ Each wound type different profile, dependent on oxygen tolerance of the bacterial population |
▪ Small study ▪ No serial sampling |
Low |
| Price et al . 2009 31 | UC |
Prospective cohort 7 DFU/7 NU/3 VLU/3 PSU/4 OTH |
Wound base curette Multiple time points |
16S rRNA V3 |
▪ Fastidious anaerobic bacteria of the Clostridiales family XI were the most prevalent bacteria in wounds ▪ Wound microbiota from antibiotic treated patients were significantly different from untreated patients ▪ In diabetic patients, Streptococcus was more abundant |
▪ Small study ▪ Sampling time point variable ▪ Patients on wide variety of treatments |
Low |
| Price et al . 2011 32 | UC |
Cross‐sectional 4 DFU/3 NU/3 VLU/2 OTH |
Wound base curette Multiple samples taken |
16S rRNA V3‐V4 |
▪ The 10 most common genera included Staphylococcus, Pseudomonas, Streptococcus, Anaerococcus, Ralstonia, Morganella, Porphyromonas, Peptoniphilus, Janthinobacterium and Corynebacterium
▪ Samples from different sites within individual wounds shared similarities in bacterial community compositions ▪ Samples taken from different wounds were less similar than those taken from different sites within the same wound |
▪ Small cohort ▪ Patients on active treatment ▪ No serial sampling |
Low |
| Rhoads et al . 2012 33 | UC |
Cross‐sectional 4 DFU/3 NU/3 VLU/2 OTH |
Wound base curette | 16S rRNA V1‐V3 |
▪ The ten most common genera included Staphylococcus, Pseudomonas, Streptococcus, Anaerococcus, Ralstonia, Morganella, Porphyromonas, Peptoniphilus, Janthinobacterium and Corynebacterium
▪ Samples from different sites within individual wounds shared similarities in bacterial community compositions ▪ Samples taken from different wounds were less similar than those taken from different sites within the same wound |
▪ Small cohort ▪ Patients on active treatment ▪ No serial sampling |
Low |
| Gjodsbol et al . 2012 34 | UC |
Comparative 46 VLU |
Filter paper pad & punch biopsies | 16S rRNA V1‐V3 |
▪ Staphylococcus aureus most found species ▪ Multiple sampling over time lead to identification of additional species ▪ No difference in outcomes different sample techniques |
▪ No controls | Low |
| Gardner et al . 2013 35 | UC |
Cross‐sectional 52 DFU |
Sterile swabs | 16S rRNA V1‐V3 |
▪ The most abundant OTU was Staphylococcus, with S. aureus the most common species ▪ Ulcer closing was positively correlated with number of species level OTUs, higher microbial diversity, relative abundance of Proteobacteria, and negatively correlated with relative abundance of Staphylococcus ▪ Ulcer depth was negatively associated with Staphylococcus abundance and positively associated with anaerobic bacteria relative abundance |
▪ No serial sampling ▪ No controls |
Low |
| Wolcott et al . 2016 37 | UC |
Cohort 2963 910 DFU/916 VLU/676 DU/370 PSU |
Sharp debridement at surface wound bed | 16S rRNA V1‐V3 |
▪ Neither patient demographics (age, gender, race, diabetes status) nor wound type influenced the bacterial composition of the chronic wound microbiome ▪ Staphylococcus and Pseudomonas comprise the most prevalent genera present in the microbiota of chronic wounds, with S. aureus and S. epidermidis the most predominant species ▪ Chronic wounds are frequently colonized by communalistic and anaerobic bacteria, including coagulation‐negative Staphylococcus, Corynebacterium, and Propionibacterium species |
▪ Unclear whether patients were on treatment | Low to moderate |
| Smith et al . 2016 36 | UC |
Cohort 20 DFU |
Sterile swabs | 16S rRNA V4 |
▪ The most commonly detected bacteria in all ulcers were Peptoniphilus, Anaerococcus and Corynebacterium species ▪ In new ulcers, the most commonly detected bacteria were the above and Staphylococcus species ▪ The majority of OTUs residing in both new and recurrent ulcers (>67%) were mostly Gram‐positive cocci (Staphylococcus, Streptococcus, Anaerococcus, Peptoniphilus and Finegoldia ▪ Lower HbA1c values and shorter duration of diabetes correlated with higher diversity within the ulcer |
▪ Small cohort ▪ No serial sampling ▪ No controls |
Low |
| Kalan et al . 2016 38 | UC |
Prospective longitudinal cohort 100 DFU |
Sterile swabs Multiple time point sampling |
ITS1 rRNA |
▪ Fungal microbiome was highly heterogeneous over time and between subjects ▪ Fungal diversity increased with antibiotic administration ▪ The proportion of the phylum Ascomycota were significantly greater at the beginning of the study in wounds that took >8 weeks to heal |
▪ No controls ▪ Most patients on active treatment |
Low to moderate |
| Loesche et al . 2017 39 | UC |
Prospective longitudinal cohort 100 DFU |
Sterile swabs Multiple time point sampling |
16S rRNA V1‐V3 |
▪ The most abundant genus identified was Staphylococcus, followed by Streptococcus, Corynebacterium and Anaerococcus
▪ The major OTU attributed to Staphylococcus was S. aureus ▪ Ulcer microbiota was highly dynamic, with community type transitions occurring approximately every 3.52 weeks ▪ Microbiota community instability was associated with faster healing and improved outcomes ▪ Exposure to systemic antibiotics destabilize wound microbiota, rather than altering overall diversity or relative abundance of specific taxa |
▪ No controls ▪ Most patients on active treatment |
Low to moderate |
| Kuk Park et al . 2012 40 | SD/PC |
Case control 4 PC 3 HV |
Sterile swabs | 26S rRNA D1‐D2 |
▪ P. meleagrinum and P. chrusogenum detected on dandruff scalp ▪ Malassezia spp. 2 times more abundant on dandruff scalp |
▪ Small cohort ▪ No serial sampling |
Low |
| Clavaud et al . 2013. 41 | SD/PC |
Case–control 29 PC 20 HV |
Sterile swabs In 20 PC patients lesional and nonlesional sampling |
16S 28S‐ITS |
▪ M. restricta major fungal species on scalp PC and HV ▪ M. restricta and s. epidermidis significantly more abundant on PC scalp ▪ Propionibacterium acnes significantly less abundant on PC scalp ▪ M. restricta/P. acnes ratio significantly higher in PC scalp |
▪ Small cohort ▪ No serial sampling |
Low |
| Soares et al . 2015 42 | SD/PC |
Case control 9 SD (5 mild, 4 severe) 5 HV |
Sterile swabs Scalp, forehead chin, shoulder and interface samples |
5.8S/ITS2 rDNA |
▪ In general, no association between Malassezia mycobiota and SD was found ▪ Higher m. globosa abundance was found in nonscalp lesions of severe SD patients |
▪ Small cohort ▪ No serial sampling |
Low |
| Park et al . 2017 43 | SD/PC |
Case control 29 SD 28 PC 45 HV |
Sterile swabs Scalp samples |
16 s rRNA V4‐V5 ITS1 rDNA |
▪ Higher abundance of Staphylococcus sp. and m. restricta, and lower abundance of Propionibacterium associated with scalp disease | ▪ No serial sampling | Low |
| Bek‐Thomsen et al . 2008 44 | AV |
Case control 5 AV/3 HV |
Cyanoacrylate biopsy AV acne lesion face HV nose area |
16S rRNA V1‐V9 | ▪ Acne skin higher diversity, P. acnes and S. epidermidis most common species |
▪ Small cohort ▪ Only moderate to severe patients ▪ No serial sampling ▪ No nonlesional patient sampling |
Low |
| Fitz‐Gibbon et al . 2013 45 | AV |
Case control 49 AV/52 HV |
Bioré® Deep Cleansing Pore strips Nose area |
16S rRNA V1‐V9 |
▪ No difference relative abundance P. acnes AV in HV. ▪ Association specific P. acnes strain and acne. |
▪ Some patients on active treatment ▪ No serial sampling ▪ No nonlesional patient sampling |
Low |
| Barnard et al . 2016 46 | AV |
Case control 38 AV/34 HV |
Bioré® Deep Cleansing Pore strips Nose area |
WMS sequencing | ▪ Association specific P. acnes strain and acne. |
▪ Some patients on active treatment ▪ No serial sampling ▪ No nonlesional patient sampling |
Low |
| Dreno et al . 2017 47 | AV |
Single‐center, randomized‐controlled, double‐blind Erythromycin 4% OR Dermatocosmetic 26 AV |
Sterile swabs Lesional and nonlesional skin Multiple time points |
16S rRNA V4 |
▪ Different microbiota profiles on different sites. ▪ Erythromycin treatment reduced the number of Actinobacteria, and dermocosmetic reduced Actinobacteria and Staphylococcus spp. |
▪ Small cohort ▪ Multiple samples excluded due to insufficient bacterial material |
Moderate |
| Kelhala et al . 2017 48 | AV |
Single‐centre, controlled study isotretinoin 0.4–0.6 mg kg–1 or lymecycline 300 mg twice daily 17 isotretinoin 11 lymecycline 16 HV |
Sterile swabs Predose and after 6 weeks Cheek, back and armpit |
16S rRNA V1‐V3 |
▪ Positive correlation Propionibacterium abundance and acne severity grade ▪ Both treatments reduced clinical acne grades ▪ Propionibacterium decreased in cheek samples after both treatments ▪ Propionibacterium decreased in back samples after lymecycline, but not isotretinoin treatment ▪ Diversity increased after treatment |
▪ Small cohort ▪ No nonlesional patient sampling |
Moderate |
| Sugita et al . 2004 58 | AD |
Case control 13 AD/12 HV |
OpSite® transparent adhesive dressings Lesional skin HV matched sites |
26S and 5S rRNA intergenic spacer region 1 | ▪ M. restricta colonizes both AD and HV |
▪ Small cohort ▪ No serial sampling ▪ Limited analysis ▪ Patients on active treatment |
Low |
| Dekio et al . 2007 49 | AD |
Case control 13 AD/10 HV |
Sterile swabs Forehead skin |
16S rRNA |
▪ In both AD and HV there was a high rate of Streptococcus species ▪ In AD Strenotrophomonas maltophilia was significantly more common |
▪ Small cohort ▪ No serial sampling ▪ Patients on active treatment |
Low |
| Kaga et al. 2009 50 | AD |
Case control 56 AD/32 HV |
OpSite® transparent adhesive dressings Lesional skin AD Face HV |
26S and 5S rRNA intergenic spacer region 1 |
▪ In mild and moderate AD, M. restricta was predominant over M. globose
▪ In patients with severe AD, proportions of M. restricta and M. globose were almost identical |
▪ Limited analysis ▪ No serial sampling ▪ Variation in skin sample sites ▪ Patients possibly on active treatment |
Low to moderate |
| Yim et al . 2010 51 | AD |
Prospective cohort 60 |
Sterile swabs 5 body sites |
26S | ▪ There were no significant differences between positive Malassezia culture, Malassezia species, and severity of AD |
▪ Limited analysis ▪ Patients on emollient treatment |
Low to moderate |
| Akaza et al . 2010 52 | AD |
Case control 67 |
Sterile swabs Lesional and nonlesional skin Face and trunk |
26S | ▪ For the total number of Malassezia species, there were no significant differences between lesional and nonlesional areas |
▪ No serial sampling ▪ Patients on active treatment |
Low to moderate |
| Kong et al . 2012 60 | AD |
Prospective cohort 12 AD/11 HV |
Sterile swabs Multiple time points Baseline, flare, post‐flare |
16S rRNA V1‐V9 |
▪ Flare ups were associated with an increased proportion of Staphylococcus sequences, particularly S. aureus, and correlated with disease severity ▪ Increases in Streptococcus, Propionbacterium, and Corynebacterium species were observed following therapy |
▪ Small cohort ▪ Only moderate to severe patients ▪ Different treatments regimens during flare |
Low to moderate |
| Seite et al . 2014 54 | AD |
Prospective cohort Emolliens treatment 46 |
Sterile swabs Lesional and nonlesional skin Multiple time points |
16S rRNA V1‐V2 |
▪ Affected skin harboured a greater relative abundance of Staphylococcus, and in particular S. epidermis, compared to healthy skin ▪ Responders had increased microbial diversity and decrease in Staphylococcus species |
▪ Large time between first and second sample ▪ Only moderate patients |
Low to moderate |
| Chng et al . 2016 55 | AD |
Case control 19 medical history AD/15 HV/5 positive skin prick |
Tape stripping anti‐cubital fossa |
16S rRNA V3‐V6 WMS |
▪ Nonflare, baseline skin microbiome signatures enriched for Streptococcus and Gemella in AD prone skin versus normal skin ▪ Increased percentage of S. aureus carriers noted in AD cohort over control subjects |
▪ Small cohort ▪ No serial sampling ▪ No lesional samples |
Low |
| Gonzalez et al . 2016 56 | AD |
Randomized, placebo‐controlled, single‐blinded Topical steroid or Topical steroid + dilute bleach bath 21 AD/14 HV |
Sterile swabs Lesional and nonlesional skin Multiple time points |
16S rRNA V4 |
▪ Affected skin harboured a greater relative abundance of S. aureus
▪ Microbial diversity at all lesional sites inversely correlated with overall EASI Index score ▪ Taxonomic normalization occurred on lesional following treatments ▪ Bacterial communities on lesional skin resemble nonlesional skin but remain distinct from healthy control skin |
▪ Small study | Moderate |
| Seite et al . 2017 57 | AD |
Double‐blind, Randomized, comparative Emollient A or Emollient B 53 |
Sterile swabs Lesional and nonlesional skin Multiple time points |
16S rRNA V1‐V2 |
▪ Significant increased levels of Xanthomonas genus in patients treated with emollient A ▪ Levels of Staphylococcus genus increased between Day 1 and Day 28 in patients treated with emollient B |
▪ Only moderate patients ▪ No wash‐out other treatments |
Moderate |
| Kim et al . 2017 59 | AD |
Prospective cohort Wet dressings Topical steroids Antihistamines Antibiotics 27 AD 6 HV |
Saline soaked gauzes | 16S rRNA V1‐V3 |
▪ Proportion of Staphylococcus significantly decreased after treatment ▪ Diversity (Shannon Index) significantly increased after treatment |
▪ Small study ▪ Patients on wide variety of treatments ▪ No nonlesional skin analysis |
Low to moderate |
AD, atopic dermatitis; AV, acne vulgaris; DFU, diabetic foot ulcer; HS, hidradenitis suppurativa; NU, neuropathic ulcer; OTH, other; OTU, operational taxonomic unit; PSU, post‐surgical ulcer; PU, pressure ulcer; PV, psoriasis vulgaris; SD/PC, seborrhoeic dermatitis/pityriasis capitis; UC, ulcus cruris; VLU, venous leg ulcer
Psoriasis vulgaris
In 10 studies, the cutaneous microbiome in PV patients was investigated, Table 1 25, 26, 27, 28, 29, 30, 31, 32, 33, 34. In addition to microbiota, these studies have focused on the mycobiota. An increased diversity in the fungal flora in psoriatic skin lesions, compared to healthy skin was reported by Paulino et al. 25 and Amaya et al. 26. No differences in the abundance of specific species was observed. Controversially, a significant dichotomy between the relative abundances of specific Malassezia species between healthy skin, and psoriatic skin lesions was found by Takemoto et al. 32. Similar inconsistencies in findings were also observed in those studies assessing the microbiota 28, 29, 30, 31, 34.
Hidradenitis suppurativa
To date, only two studies have been published that investigated the skin microbiome in HS (Table 1) 35, 36. Both studies report a significant dysbiosis in HS lesional skin with more abundance of anaerobic genera. Five lesional microbiome types were identified of which type 1 (Corynebacterium species) and type IV (Porphyromonas and Peptoniphilus species) were most prevalent 35. Porphyromonas was also found as predominantly abundant on lesional skin by Guet‐Revillet et al. 36, together with Prevotella species. In addition, clinical severity significantly correlated with Fusobacterium and Parvimonas species variation in this study.
Ulcus cruris
The role of the skin microbiome in UC was explored in 10 different studies, Table 1 37, 38, 39, 40, 41, 42, 43, 44, 45, 46. Current research into UC microbiome, comprises larger, longitudinal studies, compared to those in PV and HS. The skin mycobiota of diabetic foot ulcers was longitudinally assessed and was observed to be highly heterogeneous over time and between subjects while the diversity increased upon antibiotic treatment 45. There have been similar efforts to reveal correlations between patient metadata, treatment and/or clinical outcomes and the cutaneous microbiome in studies investigating the microbiota in UC 38, 42, 43, 44, 46. Overall, the most common found genus in these studies was Staphylococcus, with Staphylococcus aureus the most common species. Ulcer closing in diabetic patients was found to be positively correlated with higher microbial diversity and relative abundance of Proteobacteria, while a relative abundance of Staphylococcus was correlated negatively in a study by Gardner et al. 42. Although Staphylococcus was consistently reported to be the most common genus, inconsistencies exist regarding other genus that are important in CU.
Seborrheic dermatitis/Pityriasis capitis
Four case–control studies investigated the microbiome in SD patients 47, 48, 49, 50, Table 1. In general, Malassezia spp. were found to be more abundant on dandruff scalp compared to healthy scalp 47, 48, 50. In addition to the mycobiota, a dysbiosis in Staphylococcus and Propionibacterium spp. was described in microbiota analysis 48, 50. One of the four studies did not find a general association between Malassezia spp. and SD but did find a higher abundance of M. globate in severe SD patients 49.
Acne vulgaris
Five studies investigated the skin microbiome in patients with AV, Table 1 51, 52, 53, 54, 55. Three (3) were case–control studies and two (2) were small single‐centre, controlled studies, of whom one was a double‐blind, randomized‐controlled trial. In general, all case–control studies demonstrated similarly an increased microbial abundance of Propionibacterium acnes in the skin microbiome of patients with AV, compared to healthy 51, 52, 53. In addition, an association between a specific P. acnes strains and acne affected skin, and healthy skin respectively was demonstrated 51, 52. Acne improved and Propionibacterium abundance decreased after various treatments, together with an increase of microbial diversity in the two controlled studies. Moreover, a positive correlation between Propionibacterium abundance and acne severity grade was found 54, 55.
Atopic dermatitis
The skin microbiome in patients with AD was assessed in 11 studies, Table 1 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66. A greater proportion of longitudinal studies and 2 completed randomized controlled trials were performed in AD patients. There is general consensus across studies that skin affected by AD exhibits decreased bacterial diversity, as a result of an increased abundance of S. aureus 60, 61, 62, 63, 64, 66. In particular, AD flare ups were associated with an increased proportion of Staphyloccocus sequences, and S. aureus abundance correlated with disease severity 60. In line with these results, microbial diversity in AD lesions was inversely correlated with overall eczema severity as observed by the EASI 63, with several further studies also reporting taxonomic normalization and increased bacterial diversity in AD lesional skin, following various treatments 60, 61, 63, 66.
Discussion
This systematic review provides an overview of the clinical studies that have investigated nonculture skin microbiome associated outcomes in AD, SD, AV, HS, PV and UC with the goal to explore its potential as biomarker in early phase clinical drug development with drug specific or disease specific application, as also referred to as type 3 or type 6 biomarker according to the classic definition of Danhof et al. 67.
Potential for microbiome as biomarker: AD and AV
From our analysis, there is some preliminary evidence that the skin microbiota may be a suitable disease specific biomarker for clinical trials of AD. This is due to the correlation between Staphylococcus abundance, microbiome diversity profile and disease severity that seems to exist in multiple trials, therewith complying with most of the criteria for a useful biomarker, Table 2 4. Objective data on the change of the microbiota may be valuable to support subjective AD efficacy scores in early phase clinical trials. However, it must be noted that the cause and effect relationship between skin microbiota dysbiosis and AD remains incompletely elucidated 68. Currently, no evidence of benefit of antimicrobial interventions directed at reduction of Staphylococcus in patients with AD exists, only in secondarily impetiginized AD 69, 70, 71. As multiple studies included in this review indicate that the skin microbiota within an individual patient varies over time 60, 61, 63, 64, there is need for longitudinal, frequent sampling and standard analysis studies. Nevertheless, it has proven its potential value and is recommended to apply in AD clinical trials, in particular when microbiota can serve also as drug‐specific biomarker, i.e. for drugs with antimicrobial activity such as antimicrobial peptides that are currently in clinical trials for AD.
Table 2.
Evaluation of the microbiome as clinical biomarker for each dermatological disease included in the review based on the criteria of a useful biomarker as defined by de Visser et al. 4
| Indication | Manuscripts (N) | Evidence level overall | Consistency | Therapeutic response | Dose–response relation | Relationship with disease | Recommendation for trial implementation |
|---|---|---|---|---|---|---|---|
| PV | 10 | Low | – | 0 | 0 | 0 | Negative, more evidence needed |
| HS | 2 | Low | + | 0 | 0 | + | Negative, more evidence needed |
| UC | 10 | Low | + | 0 | 0 | + | Negative, more evidence needed |
| SD | 4 | Low | – | 0 | 0 | + | Negative, more evidence needed |
| AV | 5 | Moderate | + | + | 0 | + | Positive |
| AD | 11 | Moderate | + | + | 0 | + | Positive |
AD, atopic dermatitis; AV, acne vulgaris; HS, hidradenitis suppurativa; PV, psoriasis vulgaris; SD/PC, seborrhoeic dermatitis/pityriasis capitis; UC, ulcus cruris
Scoring system indicated as follows: +, studies in general report a positive outcome; 0, no studies available; –, studies in general report a negative outcome
In AV, a strong, positive correlation between Propionibacterium and acne severity grade is reported 55. Moreover, acne improved and Propionibacterium decreased after treatment, while the microbial diversity increased 54, 55. Taking into account that a clear pathophysiological role of P. acnes exists and antimicrobial interventions are effective in AV 72, 73, the adoption of the skin microbiome as biomarker in acne drug development programmes is, although still in its infancy, suggested by our review (Table 2). Lesion clearance often takes a long time; therefore, the inclusion of microbiota is a valid option to monitor subclinical treatment effects and restoration of normal bacterial profile, i.e. rebiosis. Although a small uncertainty remains regarding the exact relationship between aberrations in the skin microbiome and acne 74, we conclude that there is definitely a potential for the microbiota as biomarker in clinical trials (Table 2). Another option would be to culture P. acnes instead of profiling the whole skin microbiota in clinical trials; however, with this approach a comprehensive overview and insight in the diversity will be missed.
PV, UC, hidradenitis and SD are lacking evidence
Although dysbiosis in psoriasis seems to exist in the micro‐ as well as the mycobiota, study findings are heterogeneous. Wide variability in study design, sampling methods, controllable factors and sequencing techniques between groups, in conjunction with small sample populations, could provide a possible explanation for this. Therefore, no clear recommendations can be made at this time. Future work focusing on serial sampling and longitudinal studying of skin microbiome populations it PV patients, may provide information on its potential applicability as biomarker, Table 2. From a clinical perspective, we know that antimicrobial and antifungal agents are not successful in the treatment of psoriasis, which suggests that it is less attractive to explore 75, 76. However, since immune dysregulation is the key of psoriasis and recent investigations describe the extensive cross talk between the immune system and the microbiome, there may still be potential that should be explored 77. For UC inconsistencies in study design, sampling methods and the heterogeneity of the disease group also limit the comparability of study findings. There appears to be a relationship between certain species, types of ulcers and ulcer duration 42, 46. However, longitudinal studies with frequent standard sampling and standard analysis procedures are necessary to make a recommendation. The finding of dysbiosis in HS skin microbiome mostly regarding anaerobic species that is mostly consistent in two different studies opens up opportunities for the skin microbiome as biomarker in this field, Table 2 35, 36. However, future studies will have to confirm this potential. In SD, three different sequencing methods were used in the three different studies 47, 49, 50. This, together with the small sample populations, single time point sampling and poor study designs, might explain the heterogeneity in findings. Since there is a clear evidence that antifungal agents such as ketoconazole are effective in SD 78, it is recommended to further explore the skin microbiome's potential in this disease in future clinical trials.
Limitations and considerations
It is important to note that in all included studies, there was a high variability in study design and sampling methods between groups, which makes comparisons of specific findings difficult. Case–control studies (25/42, 60%) dominate research into the skin microbiome and skin disease. Patients are compared with healthy controls, capturing microbial profiles at a particular time, but have little predictive value in determining functionality, looking more at associations, and not causation. The small patient sample sizes across all studies may fail to account for interindividual differences within the study population. The poorly defined inclusion and exclusion criteria, with certain studies including actively treated patients in their sample population, could also confound potential findings. The standardization of controllable factors to reduce confounders was not well documented or maybe not performed in most of the included studies. As simple factors including but not limiting of age, ethnicity, environmental factors, soap use, hand‐washing and the use of topical (antimicrobial) agents before sampling have been shown to alter microbial skin communities; documentation of these metadata is essential to draw valid conclusions 5, 8, 12, 60, 61, 79, 80, 81. Multiple methods were used for skin microbiome sampling across the studies (i.e. swabs, biopsies, tape strips, wound curettes). Interestingly, all have been shown to exhibit a wide variation in biomass yield, microbial profile, human DNA contribution/contamination, sampling depth and discomfort level for the test subject 19, 62, 82, 83, 84, 85, 86, 87. In addition to the sampling method, the selection of sampling sites and sampling frequency are important factors that were not always considered in the included studies. Consistent sampling of the same anatomical area of skin in all individuals in study cohorts is essential in order to limit confounders, and allow for the accurate comparison of skin microbiome populations. Moreover, regarding analysis, only consistent use of specific primers to target specific hypervariable V regions, will allow for collation of data and comparison between multiple studies. It is clear that broadly used analysis methods in this review as shown in Table 1 count as a limitation for comparison. Taken all the above together, based on the level of evidence it is clear that our recommendations should be made with some caution. A standard approach for skin microbiome study design, collection, storage, processing and analysis as proposed by Kong et al. should be followed in future studies 17. However, although the list of limitations and sometimes poor evidence might be assessed as a weak recommendation for the inclusion of cutaneous microbiome in dermatological trials, the recent finding that the gut microbiome partially explains the response/nonresponse to PD‐1 immunotherapy in different cancer patients will foster research into microbiome in general 88, 89. In addition, the relation between the gut microbiome in inflammatory bowel disease and response to infliximab was also recently highlighted 90. In particular, when considering the reports about the role of the gut‐skin axis that might influence many diseases including the here investigated skin disorders 91, 92, 93.
Conclusion
Only a small number of studies have consistently reported the cutaneous microbiome for skin diseases and chronic wounds. Our findings reveal that for two indications – AD and AV – there is preliminary evidence to support implementation of the skin microbiome as biomarker in early phase clinical trials. For PV, UC, SD and HS, there is insufficient evidence. More standardized microbiome‐directed studies studying the effect of current treatments on the microbiome are needed to draw conclusions.
Competing Interests
There are no competing interests to declare.
Niemeyer ‐ van der Kolk, T. , van der Wall, H. E. C. , Balmforth, C. , Van Doorn, M. B. A. , and Rissmann, R. (2018) A systematic literature review of the human skin microbiome as biomarker for dermatological drug development. Br J Clin Pharmacol, 84: 2178–2193. 10.1111/bcp.13662.
References
- 1. Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, et al Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 2. Cohen AF, Burggraaf J, van Gerven JM, Moerland M, Groeneveld GJ. The use of biomarkers in human pharmacology (Phase I) studies. Annu Rev Pharmacol Toxicol 2015; 55: 55–74. [DOI] [PubMed] [Google Scholar]
- 3. Lee JW, Weiner RS, Sailstad JM, Bowsher RR, Knuth DW, O'Brien PJ, et al Method validation and measurement of biomarkers in nonclinical and clinical samples in drug development: a conference report. Pharm Res 2005; 22: 499–511. [DOI] [PubMed] [Google Scholar]
- 4. de Visser SJ, van der Post JP, de Waal PP, Cornet F, Cohen AF, van Gerven JM. Biomarkers for the effects of benzodiazepines in healthy volunteers. Br J Clin Pharmacol 2003; 55: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Human Microbiome Project C . A framework for human microbiome research. Nature 2012; 486: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selwyn S. Microbiology and ecology of human skin. Practitioner 1980; 224: 1059–1062. [PubMed] [Google Scholar]
- 7. Tagami H. Location‐related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int J Cosmet Sci 2008; 30: 413–434. [DOI] [PubMed] [Google Scholar]
- 8. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al Topographical and temporal diversity of the human skin microbiome. Science (New York, NY) 2009; 324: 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leyden JJ, McGinley KJ, Mills OH, Kligman AM. Age‐related changes in the resident bacterial flora of the human face. J Invest Dermatol 1975; 65: 379–381. [DOI] [PubMed] [Google Scholar]
- 10. Somerville DA. The normal flora of the skin in different age groups. Br J Dermatol 1969; 81: 248–258. [DOI] [PubMed] [Google Scholar]
- 11. Marples RR. Sex, constancy, and skin bacteria. Arch Dermatol Res 1982; 272: 317–320. [DOI] [PubMed] [Google Scholar]
- 12. Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A 2008; 105: 17994–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giacomoni PU, Mammone T, Teri M. Gender‐linked differences in human skin. J Dermatol Sci 2009; 55: 144–149. [DOI] [PubMed] [Google Scholar]
- 14. McBride ME, Duncan WC, Knox JM. The environment and the microbial ecology of human skin. Appl Environ Microbiol 1977; 33: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faergemann J, Larko O. The effect of UV‐light on human skin microorganisms. Acta Derm Venereol 1987; 67: 69–72. [PubMed] [Google Scholar]
- 16. Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011; 9: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong HH, Andersson B, Clavel T, Common JE, Jackson SA, Olson ND, et al Performing skin microbiome research: a method to the madness. J Invest Dermatol 2017; 137: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edmonds‐Wilson SL, Nurinova NI, Zapka CA, Fierer N, Wilson M. Review of human hand microbiome research. J Dermatol Sci 2015; 80: 3–12. [DOI] [PubMed] [Google Scholar]
- 19. Zeeuwen PL, Boekhorst J, van den Bogaard EH, de Koning HD, van de Kerkhof PM, Saulnier DM, et al Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol 2012; 13: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol 2013; 21: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jo JH, Kennedy EA, Kong HH. Research techniques made simple: bacterial 16S Ribosomal RNA gene sequencing in cutaneous research. J Invest Dermatol 2016; 136: e23–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paulino LC. New perspectives on dandruff and seborrheic dermatitis: lessons we learned from bacterial and fungal skin microbiota. Euro J Dermatol EJD 2017; 27: 4–7. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paulino LC, Tseng CH, Strober BE, Blaser MJ. Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. J Clin Microbiol 2006; 44: 2933–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amaya M, Tajima M, Okubo Y, Sugita T, Nishikawa A, Tsuboi R. Molecular analysis of Malassezia microflora in the lesional skin of psoriasis patients. J Dermatol 2007; 34: 619–624. [DOI] [PubMed] [Google Scholar]
- 27. Paulino LC, Tseng CH, Blaser MJ. Analysis of Malassezia microbiota in healthy superficial human skin and in psoriatic lesions by multiplex real‐time PCR. FEMS Yeast Res 2008; 8: 460–471. [DOI] [PubMed] [Google Scholar]
- 28. Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One 2008; 3: e2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fahlen A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res 2012; 304: 15–22. [DOI] [PubMed] [Google Scholar]
- 30. Alekseyenko AV, Perez‐Perez GI, De SA, Strober B, Gao Z, Bihan M, et al Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013; 1: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Statnikov A, Alekseyenko AV, Li Z, Henaff M, Perez‐Perez GI, Blaser MJ, et al Microbiomic signatures of psoriasis: feasibility and methodology comparison. Sci Rep 2013; 3: 2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takemoto A, Cho O, Morohoshi Y, Sugita T, Muto M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J Dermatol 2015; 42: 166–170. [DOI] [PubMed] [Google Scholar]
- 33. Salava A, Pereira P, Aho V, Vakeva L, Paulin L, Auvinen P, et al Skin microbiome in small‐ and large‐plaque parapsoriasis. Acta Derm Venereol 2017; 97: 685–691. [DOI] [PubMed] [Google Scholar]
- 34. Tett A, Pasolli E, Farina S, Truong DT, Asnicar F, Zolfo M, et al Unexplored diversity and strain‐level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microbiomes 2017; 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ring HC, Thorsen J, Saunte DM, Lilje B, Bay L, Riis PT, et al The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatology 2017; 153: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guet‐Revillet H, Jais JP, Ungeheuer MN, Coignard‐Biehler H, Duchatelet S, Delage M, et al The microbiological landscape of anaerobic infections in Hidradenitis Suppurativa: a prospective metagenomic study. Clin Infect Dis Official Pub Infectious Dis Soc Am 2017; 65: 282–291. [DOI] [PubMed] [Google Scholar]
- 37. Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, James GA, et al Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol 2008; 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Price LB, Liu CM, Melendez JH, Frankel YM, Engelthaler D, Aziz M, et al Community analysis of chronic wound bacteria using 16S rRNA gene‐based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One 2009; 4: e6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price LB, Liu CM, Frankel YM, Melendez JH, Aziz M, Buchhagen J, et al Macroscale spatial variation in chronic wound microbiota: a cross‐sectional study. Wound Repair Regen 2011; 19: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rhoads DD, Cox SB, Rees EJ, Sun Y, Wolcott RD. Clinical identification of bacteria in human chronic wound infections: culturing vs. 16S ribosomal DNA sequencing. BMC Infect Dis 2012; 12: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gjodsbol K, Skindersoe ME, Christensen JJ, Karlsmark T, Jorgensen B, Jensen AM, et al No need for biopsies: comparison of three sample techniques for wound microbiota determination. Int Wound J 2012; 9: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes 2013; 62: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolcott RD, Hanson JD, Rees EJ, Koenig LD, Phillips CD, Wolcott RA, et al Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen 2016; 24: 163–174. [DOI] [PubMed] [Google Scholar]
- 44. Smith K, Collier A, Townsend EM, O'Donnell LE, Bal AM, Butcher J, et al One step closer to understanding the role of bacteria in diabetic foot ulcers: characterising the microbiome of ulcers. BMC Microbiol 2016; 16: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kalan L, Loesche M, Hodkinson BP, Heilmann K, Ruthel G, Gardner SE, et al Redefining the chronic‐wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. MBio 2016; 7: e01058‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Loesche M, Gardner SE, Kalan L, Horwinski J, Zheng Q, Hodkinson BP, et al Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol 2017; 137: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park HK, Ha MH, Park SG, Kim MN, Kim BJ, Kim W. Characterization of the fungal microbiota (mycobiome) in healthy and dandruff‐afflicted human scalps. PLoS One 2012; 7: e32847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clavaud C, Jourdain R, Bar‐Hen A, Tichit M, Bouchier C, Pouradier F, et al Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One 2013; 8: e58203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soares RC, Zani MB, Arruda AC, Arruda LH, Paulino LC. Malassezia intra‐specific diversity and potentially new species in the skin microbiota from Brazilian healthy subjects and seborrheic dermatitis patients. PLoS One 2015; 10: e0117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park T, Kim HJ, Myeong NR, Lee HG, Kwack I, Lee J, et al Collapse of human scalp microbiome network in dandruff and seborrhoeic dermatitis. Exp Dermatol 2017; 26: 835–838. [DOI] [PubMed] [Google Scholar]
- 51. Bek‐Thomsen M, Lomholt HB, Kilian M. Acne is not associated with yet‐uncultured bacteria. J Clin Microbiol 2008; 46: 3355–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fitz‐Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol 2013; 133: 2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep 2016; 6: 39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dreno B, Martin R, Moyal D, Henley JB, Khammari A, Seite S. Skin microbiome and acne vulgaris: staphylococcus, a new actor in acne. Exp Dermatol 2017; 26: 798–803. [DOI] [PubMed] [Google Scholar]
- 55. Kelhala HL, Aho VTE, Fyhrquist N, Pereira PAB, Kubin ME, Paulin L, et al Isotretinoin and lymecycline treatments modify the skin microbiota in acne. Exp Dermatol 2018; 27: 30–36. [DOI] [PubMed] [Google Scholar]
- 56. Dekio I, Sakamoto M, Hayashi H, Amagai M, Suematsu M, Benno Y. Characterization of skin microbiota in patients with atopic dermatitis and in normal subjects using 16S rRNA gene‐based comprehensive analysis. J Med Microbiol 2007; 56: 1675–1683. [DOI] [PubMed] [Google Scholar]
- 57. Kaga M, Sugita T, Nishikawa A, Wada Y, Hiruma M, Ikeda S. Molecular analysis of the cutaneous Malassezia microbiota from the skin of patients with atopic dermatitis of different severities. Mycoses 2011; 54: e24–8. [DOI] [PubMed] [Google Scholar]
- 58. Yim SM, Kim JY, Ko JH, Lee YW, Choe YB, Ahn KJ. Molecular analysis of malassezia microflora on the skin of the patients with atopic dermatitis. Ann Dermatol 2010; 22: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Akaza N, Akamatsu H, Sasaki Y, Takeoka S, Kishi M, Mizutani H, et al Cutaneous Malassezia microbiota in atopic dermatitis patients differ by gender and body part. Dermatology 2010; 221: 253–260. [DOI] [PubMed] [Google Scholar]
- 60. Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012; 22: 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seite S, Flores GE, Henley JB, Martin R, Zelenkova H, Aguilar L, et al Microbiome of affected and unaffected skin of patients with atopic dermatitis before and after emollient treatment. J Drugs Dermatol 2014; 13: 1365–1372. [PubMed] [Google Scholar]
- 62. Chng KR, Tay AS, Li C, Ng AH, Wang J, Suri BK, et al Whole metagenome profiling reveals skin microbiome‐dependent susceptibility to atopic dermatitis flare. Nat Microbiol 2016; 1: 16106. [DOI] [PubMed] [Google Scholar]
- 63. Gonzalez ME, Schaffer JV, Orlow SJ, Gao Z, Li H, Alekseyenko AV, et al Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J Am Acad Dermatol 2016; 75: 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seite S, Zelenkova H, Martin R. Clinical efficacy of emollients in atopic dermatitis patients – relationship with the skin microbiota modification. Clin Cosmet Investig Dermatol 2017; 10: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sugita T, Tajima M, Amaya M, Tsuboi R, Nishikawa A. Genotype analysis of Malassezia restricta as the major cutaneous flora in patients with atopic dermatitis and healthy subjects. Microbiol Immunol 2004; 48: 755–759. [DOI] [PubMed] [Google Scholar]
- 66. Kim MH, Rho M, Choi JP, Choi HI, Park HK, Song WJ, et al A metagenomic analysis provides a culture‐independent pathogen detection for atopic dermatitis. Allergy Asthma Immunol Res 2017; 9: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Danhof M, Alvan G, Dahl SG, Kuhlmann J, Paintaud G. Mechanism‐based pharmacokinetic‐pharmacodynamic modeling‐a new classification of biomarkers. Pharm Res 2005; 22: 1432–1437. [DOI] [PubMed] [Google Scholar]
- 68. Huang YJ, Marsland BJ, Bunyavanich S, O'Mahony L, Leung DY, Muraro A, et al The microbiome in allergic disease: current understanding and future opportunities‐2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol 2017; 139: 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Birnie AJ, Bath‐Hextall FJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema. Cochrane Database Syst Rev 2008; CD003871. [DOI] [PubMed] [Google Scholar]
- 70. Bath‐Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol 2010; 163: 12–26. [DOI] [PubMed] [Google Scholar]
- 71. Hepburn L, Hijnen DJ, Sellman BR, Mustelin T, Sleeman MA, May RD, et al The complex biology and contribution of Staphylococcus aureus in atopic dermatitis, current and future therapies. British J Dermatol 2017; 177: 63–71. [DOI] [PubMed] [Google Scholar]
- 72. Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016; 74: 945–973, e33. [DOI] [PubMed] [Google Scholar]
- 73. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012; 379: 361–372. [DOI] [PubMed] [Google Scholar]
- 74. Shaheen B, Gonzalez M. A microbial aetiology of acne: what is the evidence? Br J Dermatol 2011; 165: 474–485. [DOI] [PubMed] [Google Scholar]
- 75. Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 2009; 61: 451–485. [DOI] [PubMed] [Google Scholar]
- 76. Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60: 643–659. [DOI] [PubMed] [Google Scholar]
- 77. Chen YE, Fischbach MA, Belkaid Y. Skin microbiota‐host interactions. Nature 2018; 553: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gupta AK, Versteeg SG. Topical treatment of facial seborrheic dermatitis: a systematic review. Am J Clin Dermatol 2017; 18: 193–213. [DOI] [PubMed] [Google Scholar]
- 79. Oh J, Conlan S, Polley EC, Segre JA, Kong HH. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med 2012; 4: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Oh J, Byrd AL, Park M, NISC Comparative Sequencing Program , Kong HH, Segre JA. Temporal stability of the human skin microbiome. Cell 2016; 165: 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Perez Perez GI, Gao Z, Jourdain R, Ramirez J, Gany F, et al Body site is a more determinant factor than human population diversity in the healthy skin microbiome. PLoS One 2016; 11: e0151990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009; 326: 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al Topographic diversity of fungal and bacterial communities in human skin. Nature 2013; 498: 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oh J, Byrd AL, Deming C, Conlan S, NISC Comparative Sequencing Program , Kong HH, et al Biogeography and individuality shape function in the human skin metagenome. Nature 2014; 514: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grice EA, Kong HH, Renaud G, Young AC, NISC Comparative Sequencing Program , Bouffard GG, et al A diversity profile of the human skin microbiota. Genome Res 2008; 18: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A 2007; 104: 2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, Gallo RL. The microbiome extends to subepidermal compartments of normal skin. Nat Commun 2013; 4: 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science (New York, NY) 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]
- 89. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science (New York, NY) 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhou Y, Xu ZZ, He Y, Yang Y, Liu L, Lin Q, et al Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems 2018; 3: e00188–e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nylund L, Satokari R, Nikkila J, Rajilic‐Stojanovic M, Kalliomaki M, Isolauri E, et al Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at‐risk for atopic disease. BMC Microbiol 2013; 13: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies‐level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol 2016; 137: 852–860. [DOI] [PubMed] [Google Scholar]
- 93. Eppinga H, Sperna Weiland CJ, Thio HB, van der Woude CJ, Nijsten TE, Peppelenbosch MP, et al Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and inflammatory bowel disease, but not in hidradenitis suppurativa. J Crohns Colitis 2016; 10: 1067–1075. [DOI] [PubMed] [Google Scholar]


