Abstract
Introduction
Evidence suggests that the energy efficiency of key ATPases involved in skeletal muscle contractile activity are improved in a hypothermic condition. However, it is unclear how a decrease in temperature affects skeletal muscle O2 consumption (mVO2) induced by muscle contraction.
Methods
Isolated mouse extensor digitorum longus (EDL) muscles were incubated in a temperature-controlled (37°C or 25°C) bath that included an O2 probe. EDL muscles from one limb were subjected to the measurement of resting mVO2, and the contralateral EDL muscles were used for the measurement of mVO2 with electrically-stimulated contraction. For the resting protocol, muscles were suspended at resting tension for 15 mins with continuous O2 recordings. For the contraction protocol, EDL muscles underwent ten electrically-stimulated isometric contractions with continuous O2 recordings for 15 mins. The rate of O2 disappearance was quantified as μmole O2 per minute and normalized to the wet weight of the muscle.
Results
Resting mVO2 was greater at 37°C than at 25°C, consistent with the idea that lower temperature reduces basal metabolic rate. Electrically-stimulated contraction robustly increased mVO2 at both 37°C and 25°C, which was sustained for ~3 min post-contraction. During that period, mVO2 was elevated ~5-fold at both 37°C and 25°C. Greater contraction-induced mVO2 at 37°C compared to 25°C occurred despite lower force generated at 37°C than at 25°C.
Conclusion
Together, O2 cost for muscle contraction (force-time integral per O2 consumed) was greater at 37°C than at 25°C. Levels of high-energy phosphates were consistent with greater energy demand at 37°C compared to 25°C. In conclusion, these results indicate that muscle contraction that occurs at subnormal temperature requires less O2 than at 37°C.
Keywords: Energy expenditure, O2 consumption, efficiency, force production
Introduction
Skeletal muscle accounts for ~25% of resting and up to ~90% of exercise-induced whole-body energy expenditure (1–4). As such, alteration in skeletal muscle energy expenditure can promote a substantial change in whole-body metabolic rate (4–11). Specifically, energy efficiency of contracting skeletal muscle is thought to be an important player in weight regulation (9, 10). Myofibrillar ATPase and sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) become highly activated during muscle contraction, accounting for the majority of the increase in skeletal muscle energy expenditure. At temperature below euthermia (37°C), ATP requirements for both myofibrillar ATPase and SERCA are decreased (12, 13). Thus, it is predicted that muscle contraction that occurs at lower temperature would require less ATP or O2 consumption. In frog muscles, the energetic cost of contraction is decreased in a hypothermic condition for Rana pipiens species (14), but the reverse is true for Xenopus laevis (15). Thus, it is unclear how temperature affects energy requirement for contractile activity in mammalian skeletal muscle. In this study, we examined the effect of temperature on contraction-stimulated O2 consumption in mouse skeletal muscle.
Methods to quantify skeletal muscle O2 consumption (mVO2) has been previously developed with hindlimb perfusion in situ (16, 17), with cultured flexor digitorum brevis muscle fibers in vitro (18), and with isolated skeletal muscle ex vivo (8, 19–24). Among these, there has only been one study that quantified contraction-stimulated mVO2 in isolated skeletal muscle (24). The isolated muscle preparation provides distinct advantages that allows direct measurements of force production and mVO2. Thus, we instrumented a similar setup to examine the effect of temperature on resting and contraction-stimulated mVO2 ex vivo. We performed these experiments at both 37°C and 25°C and derived the O2 cost for contracting skeletal muscle at both temperatures. Based on evidence that hypothermic condition requires less ATP for myofibrillar ATPase and SERCA (12, 13), we hypothesized that muscle contraction at 25°C would require less mVO2 per force produced compared to contraction at 37°C.
Methods
Animals
C57BL/6J mice (male, ~12 wks of age) were used for all experiments. In the morning of the experiment, all animals were fasted for exactly 4 hrs before the anesthetization. All procedures were approved by Institutional Animal Care and Use Committee at East Carolina University and University of Utah. All mice were fed standard chow diet ad libitum.
Calibration of O2 sensor
Krebs-Henseleit buffer (KHB) (without CaCl2) was gassed with either 100% N2 or 95% O2/5% CO2 either at 37°C or 25°C for a minimum of 20 mins (oxygenation longer than 20 mins did not result in greater % O2 saturation). The O2 sensor (MI-730 Dip-type, Microelectrode) was placed in each buffer (0% or 100% O2) and equilibrated for ~5 mins. Voltage readings from the O2 probe were recorded at 0% or 100% O2 saturation at the beginning of each experimental day to account for a small variability in voltage reading. A linear regression from these numbers was used to convert voltage readings into % O2 saturation for each temperature. The linearity between voltage readings and % O2 saturation was confirmed using a buffer solution that has been equivalated to the atmospheric O2 (20.95%).
Extensive pilot experiments were performed to test whether sensitivity of the O2 probe may be compromised with tissue bath electrical stimulation. As described below, while tissue bath electrical stimulation inactivates the O2 probe for ~45 sec, the sensitivity of the probe returns normal thereafter (verified with O2 leak measurement before and after stimulation). The manufacturer recommends replacing the O2 probe after one-month use, but we replaced them every week to ensure required sensitivity.
Tissue-bath for measurements of force production and mVO2
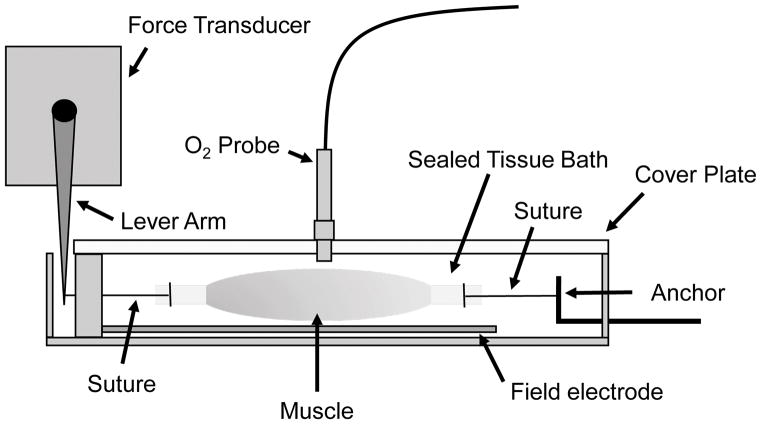
A schematic for the tissue-bath is shown in Figure 1. The horizontal tissue bath system was purchased from Aurora Scientific, Inc. (Model: 801C). We chose this system based on its smaller bath size (1.9 mL), which has allowed us to detect changes in O2 with greater sensitivity. In previous studies, resting mVO2 in isolated muscles were measured in a ~13 mL bath (8, 23), which required muscles to be incubated for a longer duration (for O2 changes to be detectable). The horizontal orientation of the tissue bath also provided better access for the O2 probe. This tissue bath system was modified by adding a plexiglass cover, a cover that can be quickly removed or placed to close the chamber. We then drilled an air-tight hole in the plexiglass cover to allow access for the O2 probe (MI-730 Dip-type, Microelectrode). The tissue-bath was used in conjunction with a force transducer, electrodes for field stimulation, and a thermocouple meter for precise temperature control.
Figure 1.
A sagittal view of the tissue bath. Mouse EDL muscles were suspended at 15–20 mN in the 1.9 mL tissue bath. After muscle placement, fresh oxygenated KHB was reintroduced, and a plexiglass cover plate was screwed onto the tissue bath. The O2 probe was inserted through an air-tight hole in the cover plate. The tissue bath was completely sealed except for small slits on both ends, one for an adjustable anchor and another for a suture that connected to a lever arm. O2 leak from these sites were accounted for when quantifying O2 consumption.
Prior to recording mVO2, we quantified the rate of O2 leak from the system (the tissue bath is exposed to atmosphere at both ends of muscle suspension). In the absence of muscle, fresh oxygenated KHB was pipetted into the tissue bath and the chamber was closed immediately with a cover plate and the O2 probe. After 5 mins of equilibration, the rate of O2 leak was quantified by continuous O2 recording for 15 mins.
Measurements of resting or contraction-stimulated mVO2
After anesthetization with a ketamine (90 mg/kg body weight) and xylazine (10 mg/kg body weight) cocktail, extensor digitorum longus (EDL) muscles were excised from mice and tendons were sutured on both ends (SP117, Surgical Specialties Corporation). One end of the muscle was suspended to an anchor, and the other end was tied to a lever arm connected to a force transducer. The anchor was positioned for muscles to be suspended at a resting tension between 15–20 mN, and muscle’s contractile viability was tested with a pulse electric stimulation (20 V, 0.2 ms). A voltage of 20 V elicited maximal force at both 37°C and 25°C. After the muscle was sets up, buffer solution was replaced with fresh oxygenated KHB so that the recording could begin as close to 100% O2 saturation as possible. The bath was immediately closed and the O2 probe was equilibrated for 5 mins. At the end of the 5-min equilibration, one of the EDL muscles used for resting mVO2 measurements remained in its place for a continuous O2 recording. The contralateral EDL muscles were used for the measurements of contraction-stimulated mVO2 measurements. These muscles underwent 10 trains of electrically-stimulated tetanic contractions (20 V, 0.1 ms pulse, 100 Hz pulse frequency, 1 s train, 4 s between trains, 46 s total, protocol modified from (25)). For both resting and electrically-stimulated muscles, O2 concentration was recorded continuously for 15 mins. For both resting and electrically-stimulated muscles, muscle lengths and wet weights were measured before being frozen in liquid N2. Previous reports found that O2 diffusion is not rate-limiting to muscle tissues that are at or below 30 mg (26, 27), and all the muscle samples in these experiments were below 15 mg. For all experiments, the O2 content in the buffer solution remained above ~600 mmHg or 79% O2, or 6-fold above the O2 concentration found in the arterial blood so that oxygen concentration is not rate-limiting to mVO2. Thus, O2 availability is presumably in excess in our experiments. All O2 recordings were performed at Greenville, NC (56 ft. sea level).
Calculation of mVO2
The rate of O2 consumption (mol•L−1•g−1•min−1) was derived from the rate of decrease in O2 saturation using the following equation (28):
R = Rate of O2 consumption (mol•L−1•min−1)
r = Rate of a decrease in O2 saturation (accounting for leak) (% O2•min−1)
a = Absorption coefficient of gas at temperature (% O2−1•mm Hg−1): 0.02831 at 25°C and 0.02419 at 37°C
V = Volume that 1 mole of gas occupies (L•mol−1): 22.414
p = Vapor pressure of H2O (mm Hg): 23.8 at 25°C and 47.1 at 37°C
The calculated R values were converted to moles•min−1•g−1 (final units for mVO2) by multiplying by 1.9 mL (volume of tissue bath) and dividing by the wet muscle weight in grams.
Measurements of force production
Forces produced by electrically-stimulated muscle contractions were recorded in real-time via a force transducer (Aurora Scientific Inc., Model: 400A), using Aurora Scientific Dynamic Muscle Control software (DMCv5.500) for data acquisition and Dynamic Muscle Analysis software (DMAv5.321) for data analyses. Specific force was calculated using cross sectional area of the muscle tissue, which was estimated from the weight and length of the muscle (mN/mm2) (29–31). The O2 cost for contracting skeletal muscle was calculated by dividing the energy output (force produced) by the energy input (O2 consumed). For the energy output, total force produced over time (force-time integral or FTI) was derived from area under the curve of absolute force produced over the period of 10 tetanic contractions (mN•s). For the energy input, O2 consumed was derived from a sum of total O2 consumption (moles O2) while mVO2 remained above the resting level (starting at the onset of muscle contraction and including post-contraction phase when mVO2 remained elevated). Values were expressed as FTI/O2 (mN•s•moles O2−1).
Quantification of high-energy phosphates
In a separate set of experiments, EDL muscles were prepared for the measurement of high-energy phosphates with or without contraction. After 5 mins of equilibration, muscles underwent either 46 seconds of resting or electrical stimulation followed by immediate removal and freeze-clamping. Samples were extracted using perchloric acid and concentrations of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), inosine monophosphate (IMP) and creatine were determined by ultra-performance liquid chromatography as previously described (32).
Statistical analyses
Values are expressed as mean ± standard error of the mean. Statistical comparisons were performed using an unpaired two-tailed Students t-test for 2-group analyses, one-way ANOVA or two-way ANOVA with Tukey’s post hoc test for multiple comparisons (GraphPad Prism 7.03).
Results
Resting mVO2 with or without MgCl2
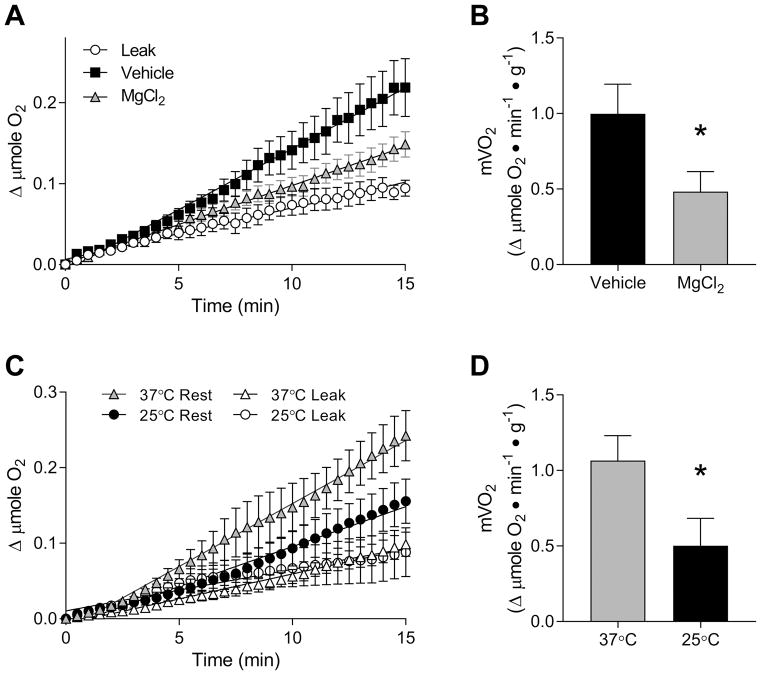
To demonstrate validity of our O2 measurements, EDL muscles were incubated in either the presence or absence of MgCl2. Mg2+ represses ryanodine receptor-dependent Ca2+ release (33), thereby indirectly halting the energy expended by sarco/endoplasmic reticulum Ca2+ ATPase (SERCA). Inhibition of SERCA by MgCl2 is known to reduce resting mVO2 by 40~50% (23). For our experiments, isolated EDL muscles were incubated in the oxygenated KHB with or without 10 mM of MgCl2. In the absence of MgCl2, mVO2 in the EDL muscles were 0.999 ± 0.196 μmole O2•min−1•g−1 at 37°C (R2 = 0.9728 ± 0.0090), which was reduced to 0.485 ± 0.133 μmole O2•min−1•g−1 with MgCl2 (~50% reduction, R2 = 0.9466 ± 0.0215, Figure 2). Thus, our system appears to be capable of accurately detecting changes in resting mVO2 values.
Figure 2.
Resting mVO2 in mouse EDL muscles. (A) Changes in O2 content were quantified over 15 mins with or without MgCl2 at 37°C. Filled squares: vehicle, gray triangles: with MgCl2, empty circles: O2 leak. (B) Calculated values for resting mVO2 in EDL muscles with or without MgCl2. (C) Resting mVO2 at 37°C and 25°C over 15 mins. Gray triangles: mVO2 at 37°C, empty triangles: O2 leak at 37°C, black circles: mVO2 at 25°C, empty circles: O2 leak at 25°C. (D) Calculated values for resting mVO2 at 37°C and 25°C. Data are means ± SEM. n = 8/group. * p < 0.05.
The effect of temperature on resting mVO2
Isolated EDL muscles were incubated for 15 min at 37°C or 25°C for the measurement of mVO2. Resting length (Lo, 37°C: 13.58 ± 0.24 mm, 25°C: 13.46 ± 0.13 mm) or resting tension at Lo (37°C: 13.29 ± 0.51 mN, 25°C: 14.31 ± 0.96 mN) was not different between the temperatures. At both temperatures, O2 consumption remained roughly linear for the entire duration of O2 recording (Figure 2C). The rate of resting mVO2 for both temperatures was calculated by taking the slope of the line of best fit. Consistent with previous reports (12, 19), resting mVO2 was greater at 37°C compared to 25°C (Figure 2D). The resting mVO2 values were comparable to values in previously performed studies in mice and other species (17, 19, 21, 22, 24, 34).
The effect of temperature on force production
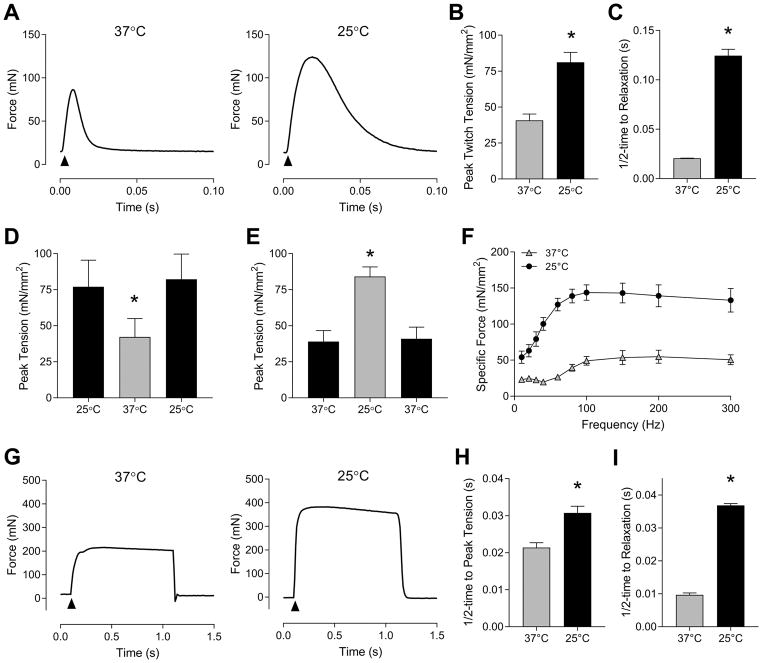
Force generating properties of EDL muscles were characterized at 37°C and 25°C. Consistent with previous reports (27, 35), pulse stimulation-induced force production was greater at 25°C (81.1 ± 6.9 mN/mm2) than at 37°C (40.7 ± 4.4 mN/mm2) (Figure 3A and B). Muscle relaxation was slower at 25°C compared to 37°C (Figure 3A and C), reflecting reduced SERCA activity in hypothermic conditions (13). We also found that the effect of temperature on force production is reversible. After electrically-stimulated contraction at 25°C, the same muscle was warmed to 37°C for another contraction, followed by cooling of the muscle to 25°C for yet another contraction. Forces generated at 25°C were the same for pre- and post- 37°C stimulation (Figure 3D), suggesting that lower force production at 37°C is likely not due to damage sustained from electrical stimulation at warmer temperature. We also similarly observed that force production is reversible when the temperature is changed from 25°C to 37°C to 25°C (Figure 3E).
Figure 3.
Force generating properties of mouse EDL muscles at 37°C and 25°C. (A) Representative force tracings of twitch contraction (0.1 ms single pulse) at 37°C and 25°C. (B) Peak specific twitch tension at 37°C and 25°C. n = 6/group. (C) Half-time to relaxation of twitch tension at 37°C and 25°C. n = 4/group. (D) Reversible effect of temperature on force production from 25°C to 37°C to 25°C. n = 3/group. (E) Reversible effect of temperature on force production from 37°C to 25°C to 37°C. n = 3/group. (F) Force-frequency curves at 37°C and 25°C. n = 4–5/group. (G) Representative force tracings of tetanic contraction (0.1 ms pulse, 100 Hz pulse frequency, 1 s train) at 37°C and 25°C. (H) Half-time to peak tetanic tension at 37°C and 25°C. n = 8/group. (I) Half-time to relaxation of tetanic contraction. n = 8/group. Gray bars or triangles: 37°C, black bars or circles: 25°C. Data are means ± SEM. * p <0.05.
The force-frequency curve was derived for EDL muscles at 37°C and 25°C (Figure 3F). Consistent with previous reports (27, 35, 36), muscles incubated at 25°C reached maximal force earlier than muscles incubated at 37°C. Forces generated at 100 Hz were not statistically different from forces generated at higher frequencies, suggesting that muscles reached maximal force production at both 37°C and 25°C. At 100 Hz frequency, peak tetanic tension (Figure 3F and G), half-time to peak tension (Figure 3H), and half-time to relaxation (Figure 3I) were all greater at 25°C than at 37°C.
Contraction-stimulated mVO2 at 37°C vs. 25°C
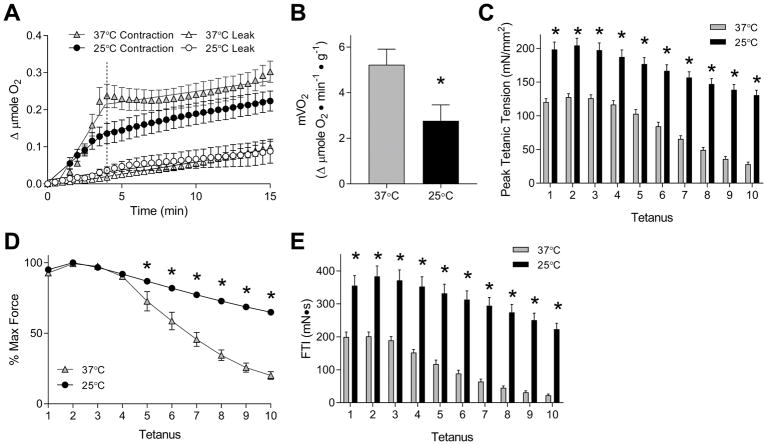
Contraction-stimulated mVO2 was assessed with tetanic contractions induced at 100 Hz stimulation. At both 37°C and 25°C, electrical stimulation induced a substantial increase in O2 consumption (Figure 4A). Likely due to tetanic contractions largely deriving immediate energy sources from anaerobic metabolism, mVO2 remained elevated until ~4-min time point (46 seconds of electrical stimulation and ~3-min post-contraction) for both temperatures. This is analogous to a large increase in O2 consumption observed after a cessation of exercise, also known as the excess post-exercise oxygen consumption (EPOC) (37). EPOC accounts for the O2 deficit accrued for the anaerobic portion of energy requirement during exercise. Similarly, sustained increase in mVO2 after cessation of ex vivo muscle contraction reflects the aerobic equivalence of its energy expenditure. Contraction-stimulated mVO2 values were surprisingly linear for the first 4 mins, then quickly returned to the resting mVO2 values thereafter. In a separate set of experiments, we left electrically-stimulated muscles in the tissue bath for 40 mins post-contraction, and confirmed that mVO2 does not increase again at a later time point. Thus, the O2 cost for muscle contraction was mostly accounted for in the first 4 mins. Contraction-stimulated mVO2 were calculated by taking the slope of the line of best fit between 0- and 4-min time points. Contraction-stimulated mVO2 was 5.226 ± 0.681 μmole O2•min−1•g−1 at 37°C (R2 = 0.8777 ± 0.0182, Figure 4B), and 2.766 ± 0.700 μmole O2•min−1•g−1 at 25°C (R2 = 0.8692 ± 0.0738, Figure 4B). Thus, compared to resting mVO2, contraction-stimulated mVO2 was 5- to 6-fold greater at both temperatures. Contraction-stimulated mVO2 values from our studies were relatively comparable to values from previously performed studies (8, 17, 34).
Figure 4.
Contraction-stimulated mVO2 and force production in mouse EDL muscles at 37°C and 25°C. (A) Contraction-stimulated mVO2 at 37°C and 25°C over 15 mins. Gray triangles: mVO2 at 37°C, empty triangles: O2 leak at 37°C, black circles: mVO2 at 25°C, empty circles: O2 leak at 25°C. n = 5/group. (B) Calculated values for contraction-stimulated mVO2 at 37°C and 25°C. (C) Peak specific tension of ten electrically-stimulated tetanic contractions (0.1 ms pulse, 100 Hz pulse frequency, 1 s train, 4 s between trains, 10 trains) at 37°C and 25°C. n = 8–9/group. (D) Fatigue over 10 tetanic contractions, derived from C. n = 8–9/group. (E) Force-time integral (FTI) derived from area under the curve of force produced over 10 tetanic contractions. n = 8–9/group. Gray bars or triangles: 37°C, black bars or circles: 25°C. Data are means ± SEM. * p <0.05.
Real-time recordings of electrically-stimulated force production were made for our study on contraction-stimulated mVO2. Just as we observed in the force-frequency curve study, the electrical stimulation elicited greater force production at 25°C (204.4 ± 10.8 mN/mm2) than at 37°C (127.8 ± 5.0 mN/mm2) (Figure 4C). Over ten tetanic contractions, muscles at 37°C fatigued more quickly than at 25°C (Figure 4D) (27, 36). Together, FTI over ten tetanic contractions was markedly greater at 25°C (2898 ± 267 mN•s) than at 37°C (1180 ± 111 mN•s) (Figure 4E and 5A).
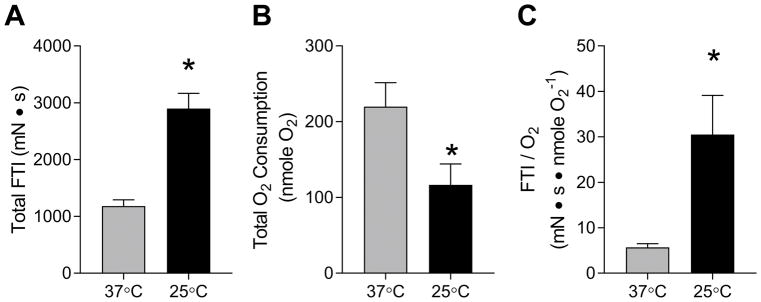
Figure 5.
The O2 cost for electrically-stimulated contractions for EDL muscles at 37°C and 25°C. (A) Total FTI over 10 tetanic contractions. (B) Total O2 consumed between 0-min and 4-min time points. (C) O2 cost of electrically-stimulated contraction at 37°C and 25°C. Values were calculated by dividing total FTI by total O2 consumption. n = 4–5/group. Gray bars: 37°C, black bars: 25°C. Data are means ± SEM. * p <0.05.
The effect of temperature on FTI/O2
Total O2 consumption was calculated over the period of time immediately before and four mins after the onset of first tetanus. The 4-min time point was used based on the observation that contraction-induced increase in mVO2 was completely reversed approximately at that time point (Figure 3B). Total O2 consumed was approximately 2-fold greater at 37°C (219.9 ± 31.6 nmole O2) compared to 25°C (116.7 ± 27.6 nmole O2) (Figure 5B). This was surprising based on the observation that FTI was approximately 2.5-fold greater at 37°C than at 25°C. Together, FTI/O2 was greater at 25°C (30.52 ± 8.59 mN•s•nmole O2−1) than at 37°C (5.69 ± 0.82 mN•s•nmole O2−1) (Figure 5C).
The effect of temperature on high-energy phosphates
Our experiments showed greater contraction-stimulated O2 cost at 37°C compared to 25°C despite greater force production at 25°C than at 37°C. This discrepancy prompted the possibility that O2 consumption may not proportionately represent energy demand at these two temperatures. As both aerobic and anaerobic metabolism converge at the level of high-energy phosphate pools, we quantified the levels of ATP, ADP, AMP, IMP and creatine in rest and contraction-stimulated EDL muscles (Table 1). At rest, creatine was greater at 37°C than at 25°C, likely reflecting the greater energy expenditure at 37°C compared to 25°C. Levels of adenosine nucleotides were not significantly different between 37°C and 25°C at rest. At 37°C, contraction induced a decrease in ATP (an observation that usually does not occur with in vivo exercise but does with ex vivo contraction), as well as increases in creatine, ADP, AMP, IMP, ADP/ATP, AMP/ATP, consistent with the idea that muscle contraction induced lower-energy state in the muscle. At 25°C, creatine, ADP, AMP, IMP, and ADP/ATP was increased with contraction, but ATP and AMP/ATP remained unchanged compared to rest. Together, these data confirm that energy supply/demand mismatch was greater at 37°C compared to 25°C, and that greater contraction-stimulated O2 consumption (Figure 5B) likely accurately reflects the greater energy requirement at 37°C than at 25°C.
Table 1.
High-energy phosphates in resting and contraction-stimulated EDL muscles at 37°C and 25°C.
| Resting | Post-Contraction | |||
|---|---|---|---|---|
|
| ||||
| 37°C | 25°C | 37°C | 25°C | |
| Creatine | 16.93±0.955 | 13.11±1.313* | 22.94±0.575† | 23.09±1.042† |
| ATP | 8.521±0.239 | 7.620±0.360 | 6.104±0.248† | 8.095±0.422* |
| ADP | 1.756±0.058 | 1.557±0.051 | 2.519±0.120† | 2.545±0.180† |
| AMP | 0.052±0.008 | 0.040±0.007 | 0.132±0.010† | 0.093±0.017† |
| IMP | 0.045±0.011 | 0.075±0.043 | 0.964±0.115† | 0.253±0.046†* |
| ADP/ATP | 0.207±0.011 | 0.207±0.011 | 0.418±0.033† | 0.315±0.019†* |
| AMP/ATP | 0.0061±0.0011 | 0.0056±0.0013 | 0.0221±0.0027 | 0.0115±0.0022†* |
Values are μmole•g tissue (wet mass)−1 and shown as mean ± SEM. n = 5–6/group.
p < 0.05 for the effect of temperature.
p < 0.05 for the effect of contraction.
Discussion
The premise of this study was to determine whether the O2 cost for electrical-stimulated contraction (during and immediately post-contraction) in isolated mouse skeletal muscle is reduced in a hypothermic condition. We found that mVO2 was lower at 25°C than at 37°C despite greater force production at 25°C than 37°C, indicating that a decrease in temperature decreases the O2 cost for contracting skeletal muscle. These findings are consistent with previous findings that myofibrillar and SERCA ATPases are more energy efficient at subnormal temperature (12, 13). To our knowledge, this is only the second study that reported the measurements of O2 consumption in contracting mouse skeletal muscle. Below we describe important methodological considerations for subsequent studies by other groups who wish to utilize this technique.
Previously, an in situ technique to measure O2 consumption by hindlimb perfusion and sciatic nerve stimulation had been developed (16, 17). While this procedure remains valuable for our studies on skeletal muscle respiration (38, 39), this procedure cannot conclusively attribute changes in O2 extraction to changes in skeletal muscle alone. O2 measured by perfusion can be affected by O2 delivery, which in turn can be influenced by density and availability of skeletal muscle capillaries. Force measured by in situ contraction may be imprecise due to simultaneous activation of synergist and antagonist muscles that are not perfectly aligned with each other. Furthermore, perfusion surgeries are highly involved and may create unwanted physiological responses that alter O2 measurements or force production. Comparatively, the method described in this manuscript provides an alternative, more direct, and less technically complicated procedure to quantify O2 cost of skeletal muscle contraction.
A number of labs have incubated isolated mouse skeletal muscles ex vivo to quantify resting O2 consumption (8, 19–23). To our knowledge, there has been only one study that reported the measurement of contraction-stimulated O2 consumption in isolated mouse skeletal muscle (24). There were several hurdles to overcome when designing the apparatus and protocol that would appropriately provide such measurements. For instance, the rate of mVO2 was quite slow, necessitating that the tissue bath be small enough to produce detectable changes in the O2 saturation. For this reason, we abandoned the idea of tissue bath perfusion as such a system would require a higher rate of O2 disappearance for detectability. We also optimized our setup to minimize O2 leakage as much as possible so that rates for mVO2 were substantially faster than the O2 leak. Another problem we overcame was sensor interference. Field electrical stimulation, used to induce muscle contraction, interfered with the O2 sensor. While tissue bath perfusion would circumvent this problem, perfusion systems would also require much higher rates of O2 disappearance as previously explained. We tested the viability of O2 sensor after field stimulation in the absence of muscle and observed that it took ~45 sec to be responsive again. Thus, the field stimulation needed to be short but robust enough for the increased mVO2 to be sustained for at least a few minutes post-stimulation. With our protocol, real-time O2 recording was made throughout muscle suspension but values during the first minute, when the sensor might not be fully recovered, were discarded. Last but not least, it was necessary to validate our methods for resting and contraction-stimulated mVO2 at both 37°C and 25°C.
A large portion of contraction-stimulated O2 consumption occurred after cessation of electric stimulation, analogous to EPOC (37). It is curious that contraction-stimulated mVO2 returned to resting values around the 4-min time point for both 37°C and 25°C. A greater O2 deficit (such that occurred at 37°C) may predict greater mVO2 or a greater length of time for mVO2 to return to resting values. We believe that lower contraction-stimulated mVO2 at 25°C is likely due to the effect that lower temperature has on slowing down mitochondrial oxidative phosphorylation (40, 41). Thus, the observation that contraction-stimulated mVO2 return to resting values around 4-min time point for both 37°C and 25°C may be coincidental. One limitation of our study is that we were only able to quantify static values of high-energy phosphates at the beginning and at the end of ex vivo muscle contraction. While our lab cannot currently perform these experiments, complementary measurements of real-time turnover of high-energy phosphates (using 31P-NMR) would provide further insights into how a prolonged increase in contraction-stimulated mVO2 relates to the kinetics of the ATP turnover. Alternatively, VCO2 measurements or substrate flux analyses may be used to understand how skeletal muscle relies on anaerobic and aerobic metabolism to derive energy.
In this study, we quantified mVO2 and force production at 37°C or 25°C as these are the two most common temperatures used to study skeletal muscle metabolism or contractile properties (25, 42). Consistent with previous studies (19, 27), resting mVO2 increased as temperature rose from 25°C to 37°C. The difference in the rate of resting mVO2 presumably comes from the effect that temperature has on mitochondrial enzymes (40, 41) as well as on O2 diffusion to mitochondria (19). The rate of mitochondrial enzymes in turn is also likely affected by lower ATP demand due to reduced activity of ATPases at 25°C compared to 37°C (12). Electrical stimulation increased mVO2 to a similar extent in both temperatures, that was reversed to resting levels after 4 mins. EPOC can last for hours (37), but our observations suggest that the O2 deficit from ten tetanic ex vivo contractions was recovered relatively quickly.
Forces produced during tetanic contractions were substantially lower at 37°C than at 25°C. Results from the force-frequency curve experiments clearly show that tetanic force productions were greater at 25°C than 37°C across all stimulation frequencies that were tested. Consistent with previous findings (27, 35), muscles incubated at 25°C developed tetanic tensions at lower stimulation frequencies than muscles at 37°C. However, this does not explain the differences in force production observed for the experiments on the mVO2 assessment, as muscles incubated at both temperatures produced maximal tension at the 100 Hz frequency. Greater force production at 25°C compared to 37°C was also observed for the peak twitch tension. Consistent with data that SERCA activity is greater at euthermia than in hypothermic condition (13), muscle relaxation occurred much more quickly at 37°C than at 25°C. Somewhat unexpectedly, muscles incubated at 37°C exhibited more rapid fatigue compared to 25°C, even though these muscles produced less tension.
We quantified the O2 cost for contracting skeletal muscle by dividing FTI by total O2 consumed, and found that muscles were approximately 5-fold more efficient at 25°C than at 37°C. This suggests that temperature has a surprisingly robust effect on how energy derived from oxidative phosphorylation is channeled to produce force. This is consistent with the idea that mitochondrial efficiency is increased at a lower temperature (40, 41). It was unexpected that total O2 consumed was greater at 37°C than at 25°C, when muscles incubated at 25°C produced greater force than at 37°C. To confirm that O2 values accurately reflected energy expenditure, we measured levels of high-energy phosphates and confirmed that the rate of total cellular ATP hydrolysis was indeed likely greater at 37°C than at 25°C. These observations agree with the idea that higher temperature reduces the energy efficiency of myofibrillar and SERCA ATPases (12, 13). Thus, we conclude that energy requirement of contracting muscles are higher at 37°C than at 25°C, even with lower force production at 37°C than at 25°C.
In conclusion, muscle contraction at 25°C was more energy-efficient compared at 37°C. Such observation may be relevant for athletic performance as well as for strategies to augment energy expenditure during exercise (6, 9). While these findings are exciting, it is worthwhile to note that the environment for electrically-stimulated contraction ex vivo differs from exercise in many physiological and biomechanical factors that affect skeletal muscle contractile activity. One potentially very important distinction is that the current study was performed with isometric contraction, while muscle performance is largely dependent on power (not just force) generated during concentric or eccentric contraction. Another difference is the excess availability of O2 in the buffer solution which may be conducive to more oxidative stress (41). A detailed description of our methods to quantify force production and O2 consumption of contracting skeletal muscle can be used by other groups to study other mechanisms that affect O2 cost for skeletal muscle contraction. Future studies are necessary to more comprehensively understand the effect of temperature on energy efficiency of contracting skeletal muscle, including a greater range of temperature, skeletal muscle with different fiber-type compositions (11, 43, 44), and alternate contraction protocols. We are especially interested in how interventions such as diet, weight alteration, and exercise training affects energy efficiency of skeletal muscle.
Acknowledgments
This work was funded by NIH grants DK095774, DK107397, and DK109888 to KF and AR070200 to JJB, and by American Heart Association grant 18PRE33960491 to ARPV.
The authors would like to thank Dr. David Brown for assistance with O2 recording and Dr. Espen Spangenburg for assistance with muscle electrical stimulation. This work was funded by NIH grants DK095774, DK107397, and DK109888 to KF and AR070200 to JJB, and by American Heart Association grant 18PRE33960491 to ARPV.
Footnotes
Conflict of Interest: We have no conflict of interest to report. The results of the present study do not constitute endorsement by ACSM and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. The Journal of clinical investigation. 1990;86(5):1423–7. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. The American journal of clinical nutrition. 2010;92(6):1369–77. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asmussen E, Christensen EH, Nielsen M. The O2 uptake of resting and working skeletal muscles [In German: Die O2-Aufnahme der ruhenden und der arbeitenden Skelettmuskeln] Acta Physiol. 1939;82(2):212–20. [Google Scholar]
- 4.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological reviews. 1997;77(3):731–58. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, et al. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nature medicine. 2000;6(10):1115–20. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- 6.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England journal of medicine. 1995;332(10):621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 7.Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nature medicine. 2012;18(10):1575–9. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurya SK, Bal NC, Sopariwala DH, Pant M, Rowland LA, Shaikh SA, et al. Sarcolipin Is a Key Determinant of the Basal Metabolic Rate, and Its Overexpression Enhances Energy Expenditure and Resistance against Diet-induced Obesity. The Journal of biological chemistry. 2015;290(17):10840–9. doi: 10.1074/jbc.M115.636878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285(1):R183–92. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith R, Joanisse DR, Gallagher D, Pavlovich K, Shamoon E, Leibel RL, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298(1):R79–88. doi: 10.1152/ajpregu.00053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barclay CJ. The basis of differences in thermodynamic efficiency among skeletal muscles. Clinical and experimental pharmacology & physiology. 2017;44(12):1279–86. doi: 10.1111/1440-1681.12850. [DOI] [PubMed] [Google Scholar]
- 12.Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. The Journal of physiology. 1996;493(Pt 2):299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barata H, de Meis L. Uncoupled ATP hydrolysis and thermogenic activity of the sarcoplasmic reticulum Ca2+-ATPase: coupling effects of dimethyl sulfoxide and low temperature. The Journal of biological chemistry. 2002;277(19):16868–72. doi: 10.1074/jbc.M200648200. [DOI] [PubMed] [Google Scholar]
- 14.Rome LC, Kushmerick MJ. Energetics of isometric contractions as a function of muscle temperature. The American journal of physiology. 1983;244(1):C100–9. doi: 10.1152/ajpcell.1983.244.1.C100. [DOI] [PubMed] [Google Scholar]
- 15.Seebacher F, Tallis JA, James RS. The cost of muscle power production: muscle oxygen consumption per unit work increases at low temperatures in Xenopus laevis. The Journal of experimental biology. 2014;217(Pt 11):1940–5. doi: 10.1242/jeb.101147. [DOI] [PubMed] [Google Scholar]
- 16.Stainsby WN. Oxygen uptake for isotonic and isometric twitch contractions of dog skeletal muscle in situ. The American journal of physiology. 1970;219(2):435–9. doi: 10.1152/ajplegacy.1970.219.2.435. [DOI] [PubMed] [Google Scholar]
- 17.Hood DA, Gorski J, Terjung RL. Oxygen cost of twitch and tetanic isometric contractions of rat skeletal muscle. The American journal of physiology. 1986;250(4 Pt 1):E449–56. doi: 10.1152/ajpendo.1986.250.4.E449. [DOI] [PubMed] [Google Scholar]
- 18.Schuh RA, Jackson KC, Khairallah RJ, Ward CW, Spangenburg EE. Measuring mitochondrial respiration in intact single muscle fibers. American journal of physiology Regulatory, integrative and comparative physiology. 2012;302(6):R712–9. doi: 10.1152/ajpregu.00229.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentley TB, Meng H, Pittman RN. Temperature dependence of oxygen diffusion and consumption in mammalian striated muscle. The American journal of physiology. 1993;264(6 Pt 2):H1825–30. doi: 10.1152/ajpheart.1993.264.6.H1825. [DOI] [PubMed] [Google Scholar]
- 20.Barker SB, Klitgaard HM. Metabolism of tissues excised from thyroxine-injected rats. The American journal of physiology. 1952;170(1):81–6. doi: 10.1152/ajplegacy.1952.170.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Ellsworth ML, Pittman RN. Heterogeneity of oxygen diffusion through hamster striated muscles. The American journal of physiology. 1984;246(2 Pt 2):H161–7. doi: 10.1152/ajpheart.1984.246.2.H161. [DOI] [PubMed] [Google Scholar]
- 22.Bombardier E, Smith IC, Vigna C, Fajardo VA, Tupling AR. Ablation of sarcolipin decreases the energy requirements for Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases in resting skeletal muscle. FEBS letters. 2013;587(11):1687–92. doi: 10.1016/j.febslet.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Smith IC, Bombardier E, Vigna C, Tupling AR. ATP consumption by sarcoplasmic reticulum Ca(2)(+) pumps accounts for 40–50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PloS one. 2013;8(7):e68924. doi: 10.1371/journal.pone.0068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren GL, 3rd, Williams JH, Ward CW, Matoba H, Ingalls CP, Hermann KM, et al. Decreased contraction economy in mouse EDL muscle injured by eccentric contractions. J Appl Physiol (1985) 1996;81(6):2555–64. doi: 10.1152/jappl.1996.81.6.2555. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47(8):1369–73. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg AL, Martel SB, Kushmerick MJ. In vitro preparations of the diaphragm and other skeletal muscles. Methods in enzymology. 1975;39:82–94. doi: 10.1016/s0076-6879(75)39012-5. [DOI] [PubMed] [Google Scholar]
- 27.Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. The American journal of physiology. 1985;248(3 Pt 1):C265–70. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- 28.Steen H. Determinations of the Solubility of Oxygen in Pure Water. Limnol Oceanogr. 1958;3(4):423–6. doi: 10.4319/lo.1958.3.4.0423. [DOI] [Google Scholar]
- 29.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. The Journal of physiology. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez JKA. Density and composition of mammalian muscle. Metabolism. 1960;9:184–8. [Google Scholar]
- 31.Moorwood C, Liu M, Tian Z, Barton ER. Isometric and eccentric force generation assessment of skeletal muscles isolated from murine models of muscular dystrophies. Journal of visualized experiments : JoVE. 2013;(71):e50036. doi: 10.3791/50036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brault JJ, Pizzimenti NM, Dentel JN, Wiseman RW. Selective inhibition of ATPase activity during contraction alters the activation of p38 MAP kinase isoforms in skeletal muscle. Journal of cellular biochemistry. 2013;114(6):1445–55. doi: 10.1002/jcb.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laver DR, Baynes TM, Dulhunty AF. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. The Journal of membrane biology. 1997;156(3):213–29. doi: 10.1007/s002329900202. [DOI] [PubMed] [Google Scholar]
- 34.Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of VO2 kinetics. J Appl Physiol (1985) 2008;105(2):575–80. doi: 10.1152/japplphysiol.01129.2007. [DOI] [PubMed] [Google Scholar]
- 35.Lannergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. The Journal of physiology. 1987;390:285–93. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Ruiter CJ, Jones DA, Sargeant AJ, de Haan A. Temperature effect on the rates of isometric force development and relaxation in the fresh and fatigued human adductor pollicis muscle. Experimental physiology. 1999;84(6):1137–50. doi: 10.1017/s0958067099018953. [DOI] [PubMed] [Google Scholar]
- 37.Laforgia J, Withers RT, Shipp NJ, Gore CJ. Comparison of energy expenditure elevations after submaximal and supramaximal running. J Appl Physiol (1985) 1997;82(2):661–6. doi: 10.1152/jappl.1997.82.2.661. [DOI] [PubMed] [Google Scholar]
- 38.Hepple RT, Hagen JL, Krause DJ. Oxidative capacity interacts with oxygen delivery to determine maximal O(2) uptake in rat skeletal muscles in situ. The Journal of physiology. 2002;541(Pt 3):1003–12. doi: 10.1113/jphysiol.2001.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ. Oxygen generating biomaterials preserve skeletal muscle homeostasis under hypoxic and ischemic conditions. PloS one. 2013;8(8):e72485. doi: 10.1371/journal.pone.0072485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Temperature, skeletal muscle mitochondrial functions, and oxygen debt. The American journal of physiology. 1971;220(4):1053–9. doi: 10.1152/ajplegacy.1971.220.4.1053. [DOI] [PubMed] [Google Scholar]
- 41.Jarmuszkiewicz W, Woyda-Ploszczyca A, Koziel A, Majerczak J, Zoladz JA. Temperature controls oxidative phosphorylation and reactive oxygen species production through uncoupling in rat skeletal muscle mitochondria. Free radical biology & medicine. 2015;83:12–20. doi: 10.1016/j.freeradbiomed.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3710–4. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophysical journal. 2000;79(2):945–61. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bottinelli R, Canepari M, Reggiani C, Stienen GJ. Myofibrillar ATPase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. The Journal of physiology. 1994;481(Pt 3):663–75. doi: 10.1113/jphysiol.1994.sp020472. [DOI] [PMC free article] [PubMed] [Google Scholar]