Abstract
Adeno-associated virus (AAV) vectors have been successfully used for transgene delivery in clinical trials. A systemic administration of AAV vectors has been proposed in order to achieve global transduction, which requires that the AAV vector is capable of crossing the blood vessels. It has been demonstrated that serum proteins are able to directly interact with AAV virions to enhance liver transduction. In this study, we investigate whether the serum proteins have the potential to increase the capacity of AAV to diffuse through the endothelial cells and deliver the transgene into the whole body. First, we found that the direct interaction of serum with AAV9 virions increased the epithelial cell permeability of AAV9 in vitro. Several serum proteins with a potential effect on AAV vascular permeability have been identified from mass spectrometry analysis, including fibrinogen, fibronectin, von Willebrand factor (vWF), platelet factor 4, alpha-1-acid glycoprotein, and plasminogen. The incubation of these serum proteins with AAV9 enhanced the global transduction in mice after a systemic administration. To apply these findings in clinical practice, we demonstrated that the clinical product cryoprecipitate (mainly containing fibrinogen and vWF) augmented AAV9 global transduction. The mechanism study revealed that cryoprecipitate slowed down the clearance of AAV9 vectors in the blood so that the AAV9 vectors had sufficient time to travel to the peripheral organs. In summary, the results from this study suggests that serum proteins interact with AAV virions and enhance the AAV9 vascular permeability for global transduction, and, more importantly, cryoprecipitate can be immediately applied for clinical patients who need the systemic administration of AAV vectors for global transduction.
Keywords: cryoprecipitate, serum proteins, AAV, global transduction
1. Introduction
Adeno-associated virus (AAV), a non-pathogenic parvovirus, is widely used as a viral vector for gene therapy because of its safety and simplicity. The recombinant AAV vector is one of the most popular vehicles for therapeutic gene delivery and has been developed for many years. To date, 13 AAV serotypes and more than 100 variants have been identified [1, 2]. AAV genomes can persist in the nucleus of transduced cells and induce a stable transgene expression in different tissues and species. Therefore, AAV mediated gene delivery has been extensively studied in pre-clinics [1, 3, 4]. Several AAV serotypes or variants have being used in clinical trials, including AAV1, 2, 4, 5, 8, 9 and rh10 [2, 5–8]. Impressively, the US Food and Drug Administration has approved the first AAV gene therapy treatment using AAV2 as a gene delivery tool for a rare inherited retinal disease [9].
The previous studies have shown that the different tissue bio-distribution of AAV serotypes depends on the routes of delivery [10]. Several serotypes of AAV vectors are capable of inducing global transduction efficiently from one single intravascular injection [10–12]. In comparison with other serotypes, AAV9 is superior for viral genome distribution and protein expression after systemic administration [10]. AAV9 is able to efficiently transduce brain, spinal cord, muscle, and other tissues after peripheral vein injection [13, 14]. Therefore, a systemic administration of AAV9 vectors has been proposed and applied in clinical trials for patients with CNS diseases and muscular disorders, including amyotrophic lateral sclerosis, frontotemporal dementia, Rett syndrome, Huntington’s disease, and muscular dystrophy [15–17].
Although the application of AAV vectors has proven its therapeutic effect and safety in pre-clinical and clinical trials, one of the major challenges is that a large number of AAV vectors is needed because of its low transduction efficiency. Of practical concern, a high dose of AAV vectors also results in higher risks of AAV related side effects, such as AAV capsid antigen presentation and immediate innate immune response that potentially causes liver toxicities after systemic administration. Therefore, the strategies that increase transduction efficiency with a lower vector dose would be ideal for the application of AAV gene therapy. Several strategies have been developed to increase AAV transduction efficiency, such as genetically modifying the AAV capsids and the utilization of pharmacological agents. It is attractive that serum protein, which naturally exists in blood circulation, may impact the AAV transduction after its systemic administration. When AAV vectors are injected into the blood, they are first met with serum proteins before reaching the target tissues or organs for an effective transduction. Some serum proteins can bind to AAV and inhibit transduction, especially the AAV neutralizing antibodies, which have been extensively studied. Other serum proteins may interact with AAV virions and enhance transduction. We have previously demonstrated that several human serum proteins are able to directly interact with the AAV8 capsid and increase its transduction in the liver [18], [19]. AAV9 is able to cross the blood barrier and then transduce the nervous system and muscles after systemic administration [13, 14]. We hypothesize that serum proteins could interact with AAV9 and affect its transduction efficiency after systemic administration. The identification of serum proteins that enhance AAV9 global transduction has clinical significance. In this study, mass spectrometry (MS) was used to identify the serum proteins which potentially interact with AAV9 virions. Of these serum proteins, several serum proteins (fibrinogen, fibronectin, plasminogen and platelets factor 4) with a potential effect on vascular permeability have been chosen to test their impact on AAV9 global transduction [20, 21], [22, 23 ]. After the systemic administration of an AAV9 complex with these proteins, an enhanced global transduction was observed in mice. Cryoprecipitate is typically used to supplement fibrinogen in clinical practice, and we further showed that it augmented AAV9 global transduction after a systemic administration. This result indicates that cryoprecipitate would be immediately transited to clinical practice for AAV9 systemic application.
2. Materials and Methods
2.1. Cells
HEK293 cells and Caco-2 cells were maintained at 37 °C with 5 % C02 in Dulbecco’s Modified Eagle’s Medium with 10 % fetal bovine serum and 1 % penicillin–streptomycin. To establish cell culture monolayers on permeable membranes, Caco-2 cells were seeded on 0.4 μm pore size polycarbonate filters in 6-mm transwell chambers (Costar, MA) and then cultured for 14 days prior to infection. The medium was replaced at intervals of 2 to 3 days.
2.2. Recombinant AAV virus production
Recombinant AAV was produced by a triple-plasmid transfection system. A 15-cm plate of HEK293 cells was transfected with 9 μg of AAV transgene plasmid pTR/CBA-Luc, 12 μg of AAV helper plasmid containing AAV Rep and Cap genes, and 15 μg of Ad helper plasmid pXX6-80. Sixty hours post-transfection, HEK293 cells were collected and lysed. Supernatant was subjected to CsCl gradient ultra-centrifugation. Virus titer was determined by quantitative PCR.
2.3. Transwell permeability assay
Caco-2 cells have been used as a model for studying transportation and permeability in many published studies [24–26]. In this study, Caco-2 cells were used to establish cell culture monolayers on permeable membranes. AAV9 vectors were incubated with either serum, serum proteins, or phosphate-buffered saline (PBS) at 4°C for 1 hour and then placed in the upper (apical) chamber of the transwell (Corning, NY). As a control, serum was added to the upper chamber of transwell for 1 hour, and then AAV9 vectors were applied. At the indicated time-points (2- and 6-hour post-infection), the media in the lower (basolateral) chamber was collected and tested for the presence of AAV9 genome by qPCR. All of the experiments were independently performed at least twice. There were three replicates used in each experiment.
2.4. Mass spectrometric analysis
The procedure was performed as previously described [18]. Briefly, the mixtures of AAV9 with either human serum or PBS were used for the co-immunoprecipitation analysis using a Pierce co-immunoprecipitation kit (Thermo scientific, IL) following the manufacture’s instruction. This kit provided a covalent antibody immobilization onto an insoluble agarose support, and then incubated the pre-incubated mixtures containing AAV9 viruses and either human serum or PBS. We used the anti-AAV9 antibody ADK9 to immunoprecipate the proteins, which bound to the particles of AAV9. The final product was reduced, alkylated, and digested in-gel by trypsin and then analyzed by liquid chromatography tandem mass spectrometry (LC/MS). The twofold difference between AAV9 and PBS was considered as a positive result.
2.5. Animal study
Animal experiments performed in this study were conducted in C57BL/6 mice. The mice were maintained in accordance to NIH guidelines, as approved by the UNC Institutional Animal Care and Use Committee (IACUC). AAV9 vectors were incubated with serum or serum proteins or PBS at 4 °C for 2 hours. Six-week-old female C57BL/6 mice received the mixtures via retro-orbital injection. Luciferase expression was measured from the imaging taken at 3 days post-injection using a Xenogen IVIS Lumina (PerkinElmer, MA) following the i.p. injection of D-luciferin substrate (PerkinElmer, MA). Bioluminescent images were analyzed using Living Image (PerkinElmer, MA).
2.6. Quantitation of tissue luciferase expression ex vivo
Animals were sacrificed at 2 days after the last imaging and the following organs were collected: heart, liver, lung, kidney, skeletal muscle, and brain. Tissues were minced and homogenized in passive lysis buffer (Promega, WI). Tissue lysates were centrifuged at 12,000 rpm for 10 minutes to remove cellular debris. The supernatant was transferred to 96-well plates for luciferase activity analysis. Total protein concentration in tissue lysates was measured by the Bradford assay (Bio-Rad, CA).
2.7. AAV genome copy number analysis
The minced tissue samples were treated by Proteinase K. Total genomic DNA was isolated by the DNeasy Blood & Tissue kit (QIAGEN, CA). The luciferase gene was detected by qPCR assay. The mouse lamin gene served as an internal control.
2.8. Cryoprecipitate production
Human cryoprecipitate was purchased from the New York blood center. The procedure of dog and rabbit cryoprecipitate production was performed as described [27]. Briefly, the whole blood with anti-coagulant from dog and rabbit were centrifuged. The plasma were collected and stocked at −80 °C for future study. The frozen plasma was thawed in the cold room (4 °C) and centrifuged for 15 minutes at 3000 rpm at 4 °C. Most of the supernatant liquid (9/10 volume) was removed and the cryoprecipitate was labeled and immediately used for incubation with AAV9 viruses.
2.9. Statistical analysis
All of the data in this study was presented as mean ± SD. The Student t-test was used to compare the differences between two groups, while one-way ANOVA multiple comparison test was used among three or more groups. P values of < 0.05 were considered a statistically significant difference.
3. Results
3.1. Serum proteins increase the epithelial cell permeability of AAV9 in vitro
Our previous study demonstrated that human serum could enhance the liver transduction of AAV8 in vitro and in vivo [18]. Human serum also increased the AAV9 transduction in Huh7 cells and in the mouse liver [18]. It has been reported that AAV9 efficiently transduces various tissues in mice after its systemic administration [13, 14]. This finding indicates that AAV9 is able to penetrate the vascular barrier for diffuse and global transduction. In this study, we explored whether serum proteins played a role in AAV9 vector vascular permeability. First, we performed vascular permeability analysis in vitro using a transwell system. Three groups were designed: AAV9 pre-incubated with serum (Serum+AAV9 incubation), AAV9 pre-incubated with PBS (PBS+AAV9) before adding the complex to cells, and application of serum with AAV9 simultaneously to cells (Serum+AAV9 no incubation). The Caco-2 cells were seeded onto the upper chamber of the transwell system. Pre-incubation with human serum induced about a 3-fold higher AAV9 viral genome in the basolateral media at 2- and 6-hour when compared to that with PBS (Fig. 1). However, there was no significant change in the group with non-preincubation of serum and AAV9 vectors. This result suggests that the serum proteins are able to increase vascular permeability of AAV9 via the direct interaction between serum proteins and AAV9 virions.
Fig. 1. Human serum increased the permeability of AAV9 in vitro.
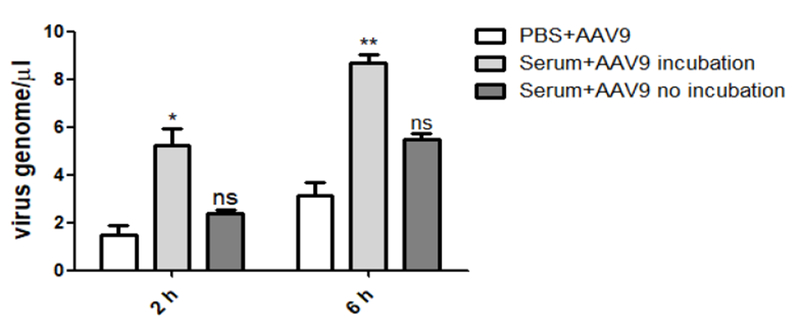
Human serum or PBS was incubated with AAV9 at 4 °C for 2 hours, and was then transferred into the upper chamber of transwell with Caco-2 cells. Serum and AAV9 were added into the upper chamber without a pre-incubation as a control. At the time points of 2- and 6-hour, the media was collected from the lower chamber and was tested by qPCR for the AAV9 virus genome. The experiment was independently performed twice. Each experiment contained a 3-replicated transwell. A representative experiment is shown.
To identify serum proteins which could bind to AAV9 virions, we performed pulldown and MS analysis (Supplemental Table 1). Among the identified serum proteins, several were selected for further investigation due to their effect on vascular permeability, including fibrinogen, fibronectin, and vWF (Table 1). AAV9 vectors were incubated with these proteins to evaluate whether they had effect on the permeability of the vector in a transwell assay. The results showed that pre-incubated fibrinogen induced an enhanced AAV9 permeability at the time point of 6 and 24 hours, while the other proteins did not have any effect (Fig. 2). The data suggested that fibrinogen might have the ability to enhance the vascular permeability of AAV9 so that it might help its global transduction after a systemic administration.
Table 1.
Serum proteins bind to AAV9
| Accession | Description | Fold increase |
|---|---|---|
| P02671 | Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 | 4.945652 |
| P02675 | Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 Fibrinogen gamma chain OS=Homo sapiens GN=FGG PE=1 |
4.974026 |
| P02679 | SV=3 Fibrinogen gamma chain (Fragment) OS=Homo sapiens |
4.853614 |
| C9JU00 | GN=FGG PE=2 SV=1 | 8.362319 |
| P02751 | Fibronectin OS=Homo sapiens GN=FN1 PE=1 SV=4 | 5.405263 |
| P00747 | Plasminogen OS=Homo sapiens GN=PLG PE=1 SV=2 Platelet factor 4 variant OS=Homo sapiens GN=PF4V1 PE=1 |
3.232278 |
| P10720 | SV=1 | 1.466667 |
| P04275 | von Willebrand factor OS=Homo sapiens GN=VWF PE=1 SV=4 Alpha-1-acid glycoprotein 2 OS=Homo sapiens GN=ORM2 |
ND |
| P19652 | PE=1 SV=2 | ND |
ND: indicates no detection of protein in control group
➢ Several serum proteins have a potential effect on AAV vascular permeability.
➢ Serum proteins enhance AAV9 global transduction in mice after systemic administration.
➢ Cryoprecipitate augment AAV9 global transduction in mice.
➢ Fibrinogen and cryoprecipitate slow down the clearance of AAV9 vector in the blood.
Fig. 2. Fibrinogen increased the permeability of AAV9 in vitro.
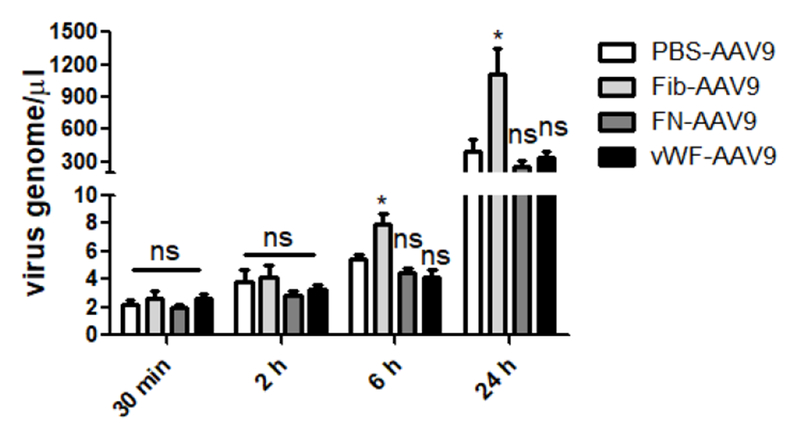
Either fibrinogen, FN, vWF, or PBS was incubated with AAV9 at 4 °C for 2 hours, and were then transferred into the upper chamber of transwell with Caco-2 cells. At the time points of 30 minutes, 2-, 6-, and 24- hours, the media was collected from the lower chamber and was tested by qPCR for the AAV9 virus genome. The experiment was independently performed twice. Each experiment contained a 3-replicated transwell. A representative experiment is shown.
3.2. Fibrinogen enhances the global transduction of AAV9 in mice
As described above, fibrinogen increased the AAV9 epithelial cell permeability in vitro (Fig. 2). Previous studies reported that fibrinogen increased pial venular permeability in mice [20]. Next, we explored whether fibrinogen could enhance the global transduction of AAV9 in mice. Fifteen C56BL/6 mice were randomly divided into three groups. AAV9 vectors were incubated with fibrinogen (Group: Fib-PBS) at a concentration of physical condition (4 mg in 1 mL blood) or PBS (Group: PBS) at 4 °C for 2 hours, then was administered via retro-orbital infection. Fibrinogen was mixed with AAV9 and was instantly injected (Group: PBS-Fib). After seven days, the images were taken and analyzed (Fig. 3A). The global transduction of AAV9 in the Fib-PBS group was 4-fold higher than that in the other two groups (PBS and PBS-Fib). An enhanced liver transduction in the Fib-PBS group was also observed compared with those in groups PBS or PBS-Fib. The data implicates that a direct interaction between fibrinogen and AAV9 is needed to enhance the global transduction of AAV9.
Fig. 3. Fibrinogen directly increased the global transduction efficiency of AAV9 vectors.
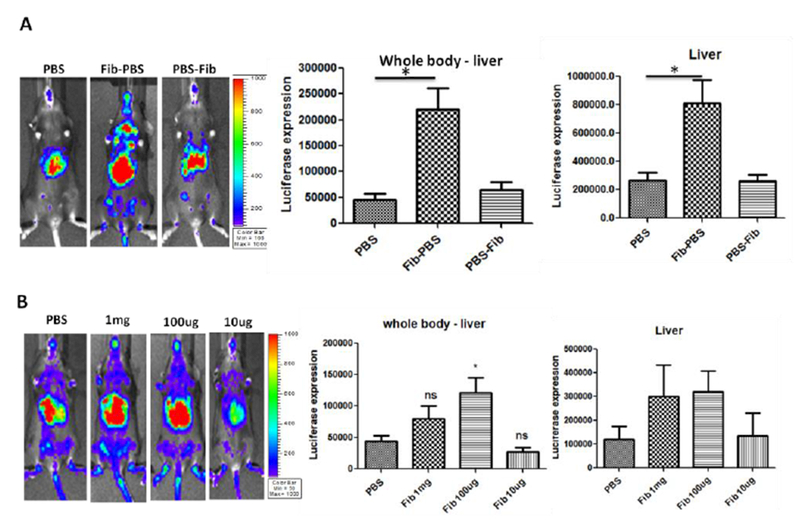
1×1010 vg of AAV9/luc were incubated with fibrinogen at the normal physiological concentration (Fib-PBS group) or PBS (PBS group) at 4 °C for 2 hours and then injected into C57BL/6 mice via the retro-orbital vein. The other group (PBS-Fib) was injected with non-incubated AAV9 and fibrinogen. Imaging was taken at day 7, and the photon signal was measured and calculated. (A) Fibrinogen enhanced AAV9 transduction. (B) The effect of AA9 transduction with fibrinogen at different concentrations. The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p < 0.05).
To study the effect of the fibrinogen concentration on AAV9 global transduction in mice, the vectors were pre-incubated with fibrinogen at the concentrations of 1 mg, 100 μg 10 μg or with PBS. The imaging for the injected mice was performed 7 days post injection (Fig. 3B). When 100 μg or 1 mg of fibrinogen was used, the transduction of AAV9 in the liver and whole body was appropriately 3-fold higher when compared to that of PBS or fibrinogen at a concentration of 10 μg (Fig. 3B). There was no difference in transduction enhancement between the doses of 100 μg and 1 mg. No enhanced transduction was achieved when a lower dose of fibrinogen was employed. This result suggests that the enhancement of AAV9 transduction requires a sufficient amount of fibrinogen for the interaction with AAV virions.
3.3. Other serum proteins enhance the global transduction of AAV9
Besides fibrinogen, other serum proteins were identified, including fibronectin (FN), plasminogen (PMG), platelet factor 4 (PF4), von Willebrand factor (vWF) and Alpha-1-acid glycoprotein 2 (AGP) (Table 1). These proteins also impact vascular permeability. To investigate whether these serum proteins increase the global transduction of AAV9 as well as fibrinogen, we pre-incubated AAV9 vectors with these serum proteins at a concentration of physical condition and then injected the complex into C57BL/6 mice via the retro-orbital vein. After 7 days post-injection, all of the serum proteins enhanced the AAV9 transduction in the whole body of the mice (Fig. 4). Similar to the results from fibrinogen, a 3-fold higher transduction of AAV9 was observed in the whole body (except liver) of the mice. Again, an enhanced AAV9 transduction in the liver was also observed in the mice treated with AAV9 pre-incubated with these proteins (Fig. 4).
Fig. 4. Other serum protein enhanced the global transduction of AAV9 vectors.
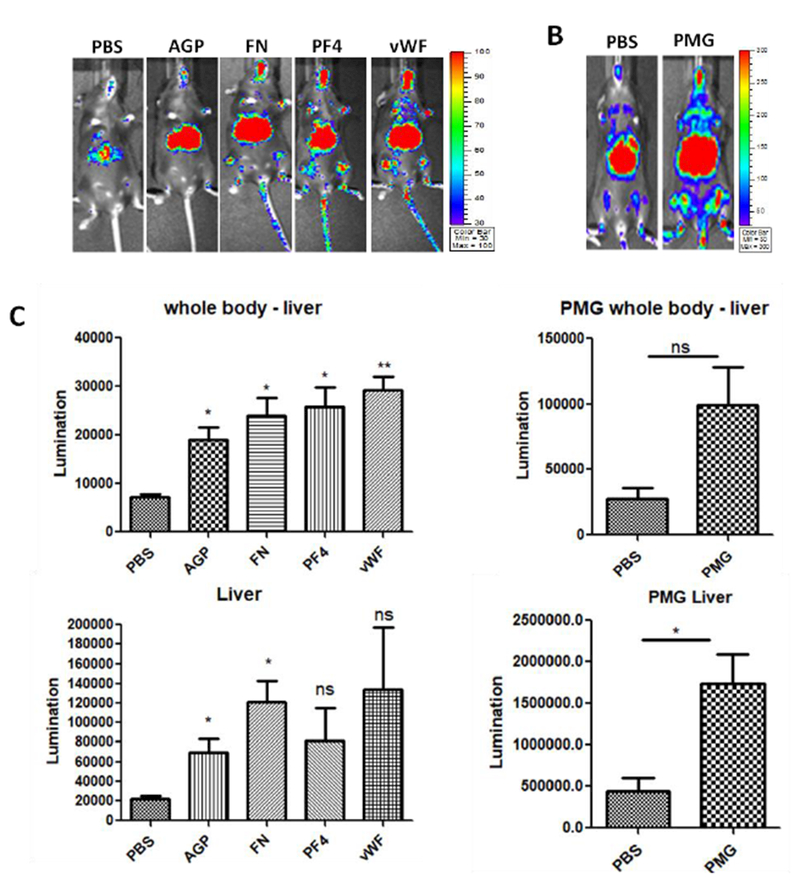
1×1010 vg of AAV9/luc were incubated with either AGP, FN, PF4, vWF, or PMG at the normal physiological concentration for 2 hours at 4 °C and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 7, and the photon signal was measured and calculated. The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
We further investigated whether the concentration of these serum proteins has an effect on the AAV9 transduction in mice. The AAV9 vectors were pre-incubated with AGP, FN, PF4, vWF, and PMG at a 10-, 100-, and 1000-fold dilution. The imaging was performed after 7 days post AAV9 injection (Fig. S1). The enhanced transduction was achieved in both the liver and other tissues in mice that only received the AAV9 vector incubated with a 10-fold diluted FN and PMG, while no difference was observed in the transduction efficiency of the mice with the diluted PF4 and vWF (Fig. S1). In the mice treated with AGP, transduction efficiency was increased in the liver, but not in any other tissues (Fig. S1).
3.4. A combination of serum proteins does not further enhance the global transduction of AAV9
The individual proteins increased the global transduction of AAV9 as shown above (Fig. 3 and 4). Next we studied whether the combination of these proteins further increased the AAV9 transduction in the whole body of mice. AAV9 vectors were incubated with a combination of three proteins at a 10-fold diluted physical concentration and the individual proteins (fibrinogen, FN, vWF) were used as control groups. After the systemic administration of the mixtures, imaging was taken 7 days post AAV injection. The imaging results showed that the combination of three serum proteins induced an enhanced global transduction of the AAV9 vector (Fig. 5B and C). However, there was no further enhancement in the combination group when compared with the individual protein groups (Fig. 5B and D).
Fig. 5. The combination of serum proteins did not further enhance the global transduction of AAV9 vectors.
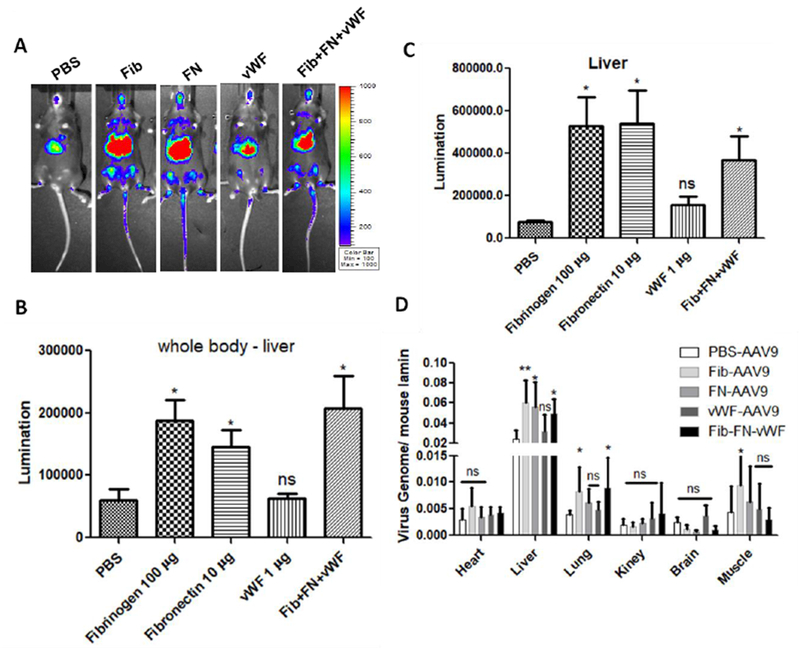
1×1010 vg of AAV9/luc were incubated with either fibrinogen, FN, vWF, or the combination of these three proteins at a 10-fold dilution of the normal physiological concentration for 2 hours at 4 °C and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 7 (A). The photon signal was measured and calculated for luciferase expression (B and C). The AAV9 genomic copy number was measured by qPCR assay (D). The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
3.5. Cryoprecipitate enhances the global transduction of AAV9
Several serum proteins interact with AAV9 virions and enhance AAV9 global transduction, as stated above. The next objective is whether we could apply this finding for clinical trials. Indeed, the blood product cryoprecipitate is mainly composed of fibrinogen, FN, and vWF. Cryoprecipitate has been used in many clinical contexts. We further investigated whether cryoprecipitate could enhance the global transduction of AAV9. 1×1010 particles of AAV9 vector were pre-incubated with 0.2-, 2-, and 20 μl (~2, 20, 200 μg fibrinogen) cryoprecipitate for 2 hours, and then were injected into mice via the retro-orbital vein. The imaging was performed at 7 days post-injection (Fig. 6A). The results showed that both 0.2- and 2- μl cryoprecipitate significantly augmented the whole body transduction of AAV9 vectors (Fig. 6B and C).
Fig. 6. Human cryoprecipitate enhances the global transduction of AAV9 vectors.
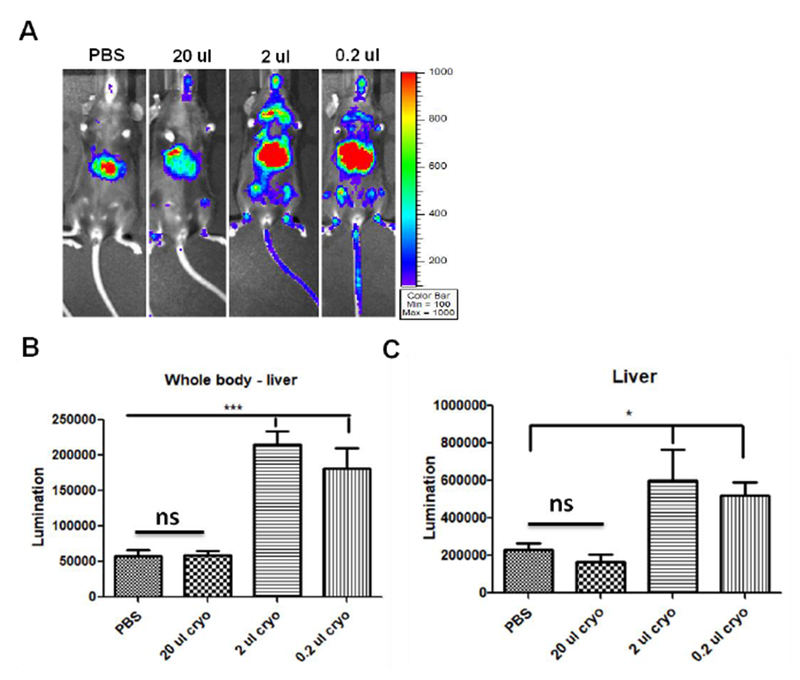
1×1010 vg of AAV9/luc were incubated with either 0.2-, 2-, or 20- μl cryoprecipitate for 2 hours at 4 °C and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 7 (A). The photon signal was measured and calculated for luciferase expression (B and C). The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
To study whether cryoprecipitate from other species also enhanced AAV9 global transduction, we made cryoprecipitate from dog and rabbit plasma following the protocol described by Sparrow, et. al. [27]. 1×1010 particles of AAV9 vector was pre-incubated with 2 μl cryoprecipitate for 2 hours, and then was used to inject into mice via the retro-orbital vein. The imaging was performed at 7 days post-injection. Consistent to human cryoprecipitate, both dog and rabbit cryoprecipitate significantly augmented the whole body transduction of AAV9 vectors (Fig. S2).
In clinical trials, very high-dose of AAV9 vector (over 5×1012 vg/kg) has been proposed in patients with CNS or muscular disorders [13, 28]. We investigated the effect of human cryoprecipitate on AAV9 global transduction when a high dose of vector was used. 1×1011 particles of AAV9 vectors were pre-incubated with 2 μl (~ 200 μg fibrinogen) of cryoprecipitate for 2 hours, and then were injected into mice via the retro-orbital vein. The imaging was carried out at 1, 3, and 7 days post-injection (Fig. 7A). The mice injected with the pre-incubated mixture of AAV9 and cryoprecipitate showed an enhancement of global transduction (Fig. 7A). At day 9 after AAV9 injection, the mice were euthanized for analysis of luciferase expression and AAV genome copy number in the heart, liver, lung, kidney, muscle, and brain (Fig. 7B and C). Both the luciferase expression and the AAV genome copy number were increased in all of these tissues from mice treated with a high-dose of AAV9 pre-incubated with cryoprecipitate. Although a high dose of the AAV vector was systemically administered and a higher liver transduction was achieved in the mice with the pre-incubated mixture of AAV9 and cryoprecipitate, there was no difference in the expression of the inflammatory cytokines IL-1β and IL-6 (Fig. S3).
Fig. 7. Cryoprecipitate increased the global transduction efficiency of the high-dose AAV9 vectors.
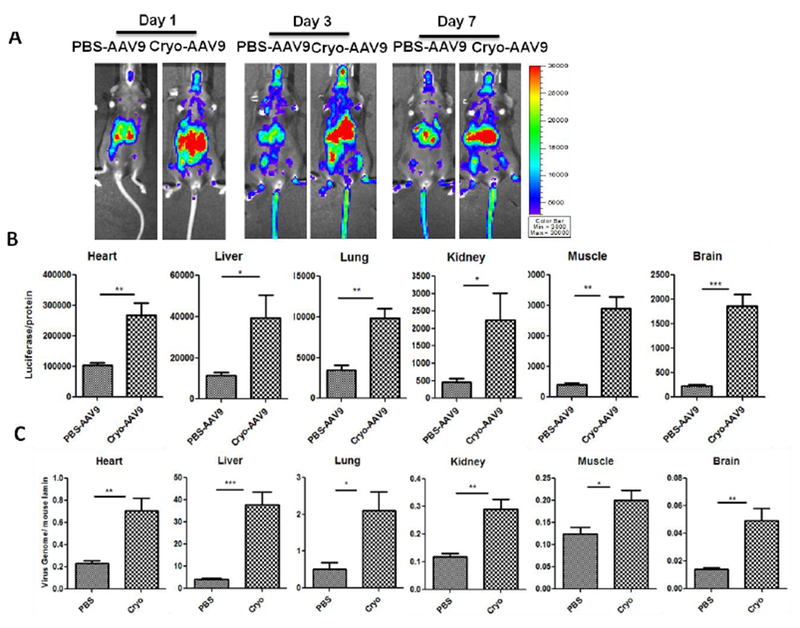
1×1011 vg of AAV9/luc were incubated with 2 μl of cryoprecipitate at 4 °C for 2 hours and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 1, 3, and 7 (A). The mice were sacrificed at 9 days post AAV9 administration. The tissues were harvested for luciferase expression assay ex vivo (B) and AAV9 genomic copy number analysis (C). The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
3.6. The interaction of AAV9 with serum proteins impact the kinetics of vector clearance in the blood
It has been suggested that a slow clearance of AAV9 in the blood may contribute to its global transduction after a systemic administration. Herein, we also investigated whether the interaction of AAV9 with serum proteins had an effect on the vector clearance in the blood. After the injection of AAV9 vector complex with fibrinogen or cryoprecipitate into the mice via the retro-orbital vein, the blood was collected at different time points. The amount of AAV9 vectors in the plasma were evaluated by quantitative PCR. There was a significantly higher AAV numbers shown in the mice receiving the fibrinogen- or cryo-AAV9 complex than that of the control mice at the early time points, but no significant difference at 24- and 48-hour post-AAV injection (Fig. 8). These results suggest that the interaction of serum proteins with AAV9 virions might slow the clearance of the vectors in the blood, which leads to an enhanced global transduction after the systemic administration.
Fig. 8. Both fibrinogen and cryoprecipitate slowed down the clearance of AAV9 vectors in bloodstream.
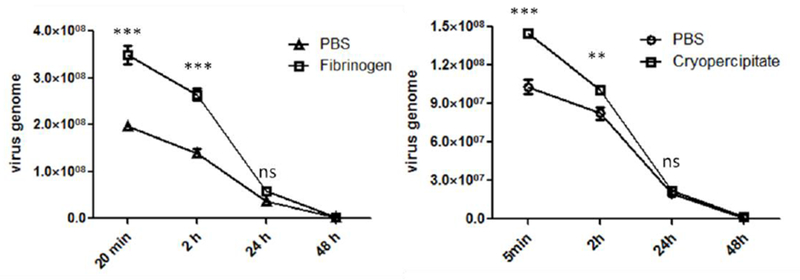
1×1011 vg of AAV9/luc were incubated with either fibrinogen or cryoprecipitate at 4 °C for 2 hours and then injected into C57BL/6 mice via the retro-orbital vein. The blood was collected from the mice with cryoprecipitate at 5 minutes, 2-, 24-, 48-, and 72- hours after AAV9 injection, or at 20 minutes, 2-, 24-, 48-, and 72- hours in the mice treated with fibrinogen and AAV9. The AAV9 gene copy number in plasma was detected by qPCR. The data represents the average and standard deviation from 5 mice. The asterisk indicates significant difference (p < 0.05), and the “ns” indicates no significant difference (p > 0.05).
4. Discussion
In this study, we demonstrated that the human serum was able to enhance the vascular permeability of AAV9. Several serum proteins (fibrinogen, fibronectin, PF4, and PMG) bound to AAV9 virions and increased the global transduction of AAV9 vectors in mice after systemic injection. The combination of the three serum proteins (fibrinogen, FN, and vWF) did not further increase the global transduction efficiency when compared with the individual serum proteins. Importantly, we found that the clinical product, cryoprecipitate, significantly augmented the global transduction of AAV9 vectors after a systemic administration.
AAV has been widely used in pre-clinical and clinical studies for the therapeutics of numerous diseases. However, a limited therapeutic transgene expression and circumventing the immune response to the vector are a major challenge. In addition, there is a 10- to 100-fold difference of transgene expression among different species (mouse, primate, human) [7, 29, 30]. Therefore, it is necessary to develop highly efficient strategies that could enhance the efficiency of gene delivery. Many efforts have been done for this purpose, including the genetic modification of AAV capsids and the utilization of pharmacological agents [18, 31–33]. However, these strategies may not be optimal in clinical trials because of the adverse effects or by changing the tropism and transduction efficiency of the vectors. The exploration of natural serum proteins to enhance AAV transduction may represent a promising strategy. The interaction of serum proteins with AAV virions does not impact the structure of the AAV virions and are also safe for patients. In this study, we used mass spectrometry analysis to identify several proteins (fibrinogen, AGP, FN, PF4, vWF, and PMG) that could potentially impact the vascular permeability. Fibrinogen binds to the intracellular adhesion molecule-1 or to the integrin on the cell surface and increases the endothelial cell permeability through extracellular signal regulated kinase signaling and by inducing F-actin formation [34]. FN is a soluble protein in plasma and an insoluble fibrillar component in basement membranes. FN has effect on cell adhesion and can bind to the Arg-Gly-Asp (RGD) sequence, which is believed to influence the integrity of the vascular barrier [35, 36]. Platelet-acting factor induced endothelial barrier leakiness through disrupting the interendothelial junctions and increasing the endothelia permeability [23]. AGP is needed for maintaining the capillary permeability by increasing the net negative charge on the microvessel walls as well as by interacting with the components of the endothelial glycocalyx [37–39]. VWF as an emerging mediator of vascular inflammation supports leukocyte and platelet recruitment in inflamed tissues, which modulates the vascular permeability [40]. Plasminogen activator increases the permeability of the blood-brain barrier through vascular endothelial growth factor-mediated endothelial endocytosis [41].These proteins were able to directly interact with the AAV9 virions and increase the vascular permeability, which resulted in an enhanced global transduction after a systemic administration (Fig. 3 and 4). We analyzed the whole body transduction after a systemic administration of AAV9 vector pre-incubated with the serum albumin in our previous experiments and found that human serum albumin did not significantly impact the global transduction efficiency of AAV9 vectors after a systemic administration (Fig. S4), even though an enhanced liver transduction was achieved [18]. This result suggests that these serum proteins studied here are able to interact with the AAV9 virions and further increase the vector vascular permeability for an enhanced global transduction. It is worthy to note that the combination of fibrinogen, FN, and vWF did not further increase the AAV9 global transduction in mice after a systemic administration (Fig. 5). Perhaps these proteins could bind to the similar location and compete with each other for the same binding sites on the surface of AAV9 virion (Fig. S5). A similar result was observed in our previous study in which serum proteins (albumin, LDL, or transferrin) competitively bind to the same locations of the AAV8 virions for an enhanced liver transduction [19].
To move forward the serum proteins, which enhanced AAV9’s ability to cross the vascular barrier, to clinical trials, we studied the effect of cryoprecipitate on the global transduction of AAV9 after a systemic administration. Cryoprecipitate is a blood product containing a high concentration of factor VIII, vWF, fibrinogen, FN, and factor XIII [42]. Cryoprecipitate was originally developed as a therapeutic treatment for patients with antihemophilic factor deficiency or hemophilia A [43]. At present, cryoprecipitate is most commonly used to replenish the fibrinogen levels in patients with acquired coagulopathy, such as in clinical settings with hemorrhage including cardiac surgery, trauma, liver transplantation, or obstetric hemorrhage [43]. Cryoprecipitate is licensed for use in the UK, USA, Canada, Australia, and New Zealand [42]. After a systemic administration of AAV9 vectors pre-incubated with cryoprecipitate, consistent to the results with individual proteins, an enhanced global transduction was achieved. It was found that the high concentration of cryoprecipitate did not show an enhanced function (Fig. 6). Cryoprecipitate is a complex product, containing multiple serum proteins, so the AAV virions incubated with high concentration of cryoprecipitate are able to conglutinate and form aggregates, which inhibit the transduction efficiency from single AAV virion. Our findings suggest that cryoprecipitate would be directly used for enhancing AAV9 transduction in future pre-clinical and clinical studies.
It has been suggested that AAV vector clearance in the blood may relate to whole body transduction after a systemic administration [10]. For example, AAV1 and AAV4 were cleared from the blood within the first hour, AAV 6-8 were slowly removed between 1-6 hours, and AAV9 was the slowest one, which was cleared between 6 - 48 hours after injection [10]. The slow clearance of AAV9 in bloodstream potentially provides sufficient time for AAV9 to travel to the target organs for efficient global transduction [44]. Additionally, it has been reported that AAV9 is able to effectively cross the capillary endothelial or blood-brain barrier via transcytosis [45, 46]. In this study, we found that fibrinogen increased the transcytosis of AAV9 in a transwell system at 6- and 24- hours (Fig. 2), and interaction of fibrinogen and AAV9 slowed down the clearance of AAV9 in the blood after a systemic administration (Fig. 8). These results suggest that these serum proteins increase AAV9 vascular permeability by a mechanism of delaying the clearance of AAV9 in the blood. Also, data from this study indicates that serum proteins can serve as another layer to interact with AAV9 for an enhanced global transduction.
There are several lines of evidence to support the complex formation of AAV9-serum protein. (1) The serum proteins in this study were identified by an AAV9 antibody after incubation with AAV9. (2) The enhancement of AAV transduction was only observed with AAV9 pre-incubated with serum proteins, but not with the mixture of AAV9 and serum proteins (Fig. 3). (3) The incubation of AAV9 with one of these serum protein blocks the other proteins binding to AAV9 (Fig. S5). In the future, we will figure out how the AAV virion interacts with serum proteins and where the binding domains are on the AAV virions surface and serum proteins.
There are several issues we did not address in this study. One is the binding affinity of AAV9 vectors with the serum proteins. Different serum proteins may have a different binding affinity with AAV. The other one is stability of the complex of the serum protein and AAV9 in the blood after systemic administration. These issues warrant future investigation.
In summary, our study demonstrated that the serum proteins increased the vascular permeability of AAV9. These serum proteins directly interacted with AAV9 vectors and increased its global transduction efficiency in mice after a systemic administration. For clinical purposes, we found that the clinical product cryoprecipitate induced a significant enhancement of AAV9 global transduction. The mechanism of this finding may be that serum proteins delay the clearance of AAV9 in blood so that AAV9 vectors get sufficient time to cross the blood barrier and transduce other organs after systemic injection. Our study strongly suggests that cryoprecipitate could be immediately used for enhancing global transgene delivery of AAV9 vectors in future clinical trials.
Supplementary Material
Fig. SI. The effect of other serum proteins at different concentrations on the global transduction of AAV9. 1×1010 vg of AAV9/luc were incubated with either AGP, FN, PF4, vWF, or PMG at the dilutions of either 1:10, 1:100, or 1:1000 of the normal physiological concentration for 2 hours at 4 °C and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 7. The photon signal was measured and calculated for luciferase expression. The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
Fig. S2. Rabbit and dog cryoprecipitate increased the global transduction of AAV9 vectors. 1×1010 vg of AAV9/luc were incubated with 2 μl of cryoprecipitate at 4 °C for 2 hours and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 7. The photon signal was measured and calculated for luciferase expression. The AAV9 gene copy number was measured by qPCR assay. The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
Fig. S3. High transduction efficacy did not induce an inflammatory response in the liver of mice. 1 × 1011 vg of AAV9/luc were incubated with 2 μl of cryoprecipitate at 4°C for 2 hours and then injected into C57BL/6 mice via the retro-orbital vein. The mice were sacrificed at 9 days post AAV9 administration, and the livers were harvested. The mRNA levels of IL-1β and IL-6 were measured by qRT-PCR. The data represents the average and standard deviation from 5 mice.
Fig. S4. Human serum albumin (HSA) did not increase the global transduction of AAV9 in mice. 1×1010 vg of AAV9/luc incubated with HSA were administered via the retro-orbital injection. The imaging was taken at day 3 post AAV injection. The data represents the average and standard deviation from 4 mice.
Fig. S5. Serum proteins competitively bound to the AAV9 virions. For the competitive binding assay of serum proteins, 1×1010 vg of AAV9/luc vector was incubated with either FN or vWF at different dilutions. Fibrinogen was added at a dilution of 100-fold at for 1 hour 4°C. The antibody against fibrinogen was used for immunoprecipitation. The vims titer was determined by qPCR.
Acknowledgements
We thank Violeta Zaric for her technical assistance. The authors acknowledge the UNC Biomedical Research Imaging Center (BRIC) Small Animal Imaging (SAI) facility for assistance of mouse imaging. This work was supported by National Institutes of Health Grants R01AI117408 and R01HL125749 (to C.L.), P01HL112761, R01AI072176 (to R.J.S.), P30-CA016086-35-37, U54-CA151652-01-04 (to the BRIC SAI facility).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflict of Interest
R. Jude Samulski is the founder, and a shareholder, at Asklepios BioPharmaceutical. He receives research support through the University of North Carolina from Asklepios BioPharmaceutical. He holds patents that have been licensed by UNC to Asklepios Biopharmaceutical, for which he receives royalties. He has consulted for Baxter Healthcare and has received payment for speaking.
References
- [1].Srivastava A, In vivo tissue-tropism of adeno-associated viral vectors, Current Opinion in Virology, 21 (2016) 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lisowski L, Tay SS, Alexander IE, Adeno-associated virus serotypes for gene therapeutics, Current Opinion in Pharmacology, 24 (2015) 59–67. [DOI] [PubMed] [Google Scholar]
- [3].DiPrimio N, McPhee SW, Samulski RJ, Adeno-associated virus for the treatment of muscle diseases: toward clinical trials, Current opinion in molecular therapeutics, 12 (2010) 553–560. [PubMed] [Google Scholar]
- [4].Duan D, Systemic delivery of adeno-associated viral vectors, Current Opinion in Virology, 21 (2016) 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miesbach WA, Meyer C, Nijmeijer B, Salmon F, Grosios N, Petry H, Leebeek FWG, Phase 1-2 Clinical Trial of a Recombinant AAV5 Vector Containing the Human FIX Gene in Patients with Severe or Moderately Severe Haemophilia B, Blood, 124 (2014) 5948–5948. [Google Scholar]
- [6].Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE, Grieger JC, Govindasamy L, Agbandje-McKenna M, Xiao X, Samulski RJ, Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector, Mol Ther, 20 (2012) 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D, Nawathe S, Waddington SN, Bronson R, Jackson S, Donahue RE, High KA, Mingozzi F, Ng CYC, Zhou J, Spence Y, McCarville MB, Valentine M, Allay J, Coleman J, Sleep S, Gray JT, Nienhuis AW, Davidoff AM, Long-term Safety and Efficacy Following Systemic Administration of a Self-complementary AAV Vector Encoding Human FIX Pseudotyped With Serotype 5 and 8 Capsid Proteins, Molecular Therapy, 19 (2011) 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Russell S, Bennett J, Wellman JA, Chung DC, Yu Z-F, Tillman A, Wittes J, Pappas J, Elci O, McCague S, Cross D, Marshall KA, Walshire J, Kehoe TL, Reichert H, Davis M, Raffini L, George LA, Hudson FP, Dingfield L, Zhu X, Haller JA, Sohn EH, Mahajan VB, Pfeifer W, Weckmann M, Johnson C, Gewaily D, Drack A, Stone E, Wachtel K, Simonelli F, Leroy BP, Wright JF, High KA, Maguire AM, Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial, The Lancet, 390 (2017) 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smalley E, First AAV gene therapy poised for landmark approval, Nature biotechnology, 35 (2017) 998. [DOI] [PubMed] [Google Scholar]
- [10].Zincarelli C, Soltys S, Rengo G, Rabinowitz JE, Analysis of AAV Serotypes 1–9 Mediated Gene Expression and Tropism in Mice After Systemic Injection, Molecular Therapy, 16 (2008) 1073–1080. [DOI] [PubMed] [Google Scholar]
- [11].Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X, Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart, Nature biotechnology, 23 (2005) 321. [DOI] [PubMed] [Google Scholar]
- [12].Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, Kazazian HH, Long-Term Efficacy of Adeno-Associated Virus Serotypes 8 and 9 in Hemophilia A Dogs and Mice, Human gene therapy, 17 (2006) 427–439. [DOI] [PubMed] [Google Scholar]
- [13].Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK, Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes, Nature biotechnology, 27 (2009) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar A-M, Fyfe J, Moullier P, Colle M-A, Barkats M, Intravenous Administration of Self-complementary AAV9 Enables Transgene Delivery to Adult Motor Neurons, Molecular Therapy, 17 (2009) 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Choudhury SR, Harris AF, Cabral DJ, Keeler AM, Sapp E, Ferreira JS, Gray-Edwards HL, Johnson JA, Johnson AK, Su Q, Stoica L, DiFiglia M, Aronin N, Martin DR, Gao G, Sena-Esteves M, Widespread Central Nervous System Gene Transfer and Silencing After Systemic Delivery of Novel AAV-AS Vector, Molecular Therapy, 24(2016) 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vagner T, Dvorzhak A, Wojtowicz AM, Harms C, Grantyn R, Systemic application of AAV vectors targeting GFAP-expressing astrocytes in Z-Q175-KI Huntington’s disease mice, Molecular and Cellular Neuroscience, 77 (2016) 76–86. [DOI] [PubMed] [Google Scholar]
- [17].Yue Y, Pan X, Hakim CH, Kodippili K, Zhang K, Shin J-H, Yang HT, McDonald T, Duan D, Safe and bodywide muscle transduction in young adult Duchenne muscular dystrophy dogs with adeno-associated virus, Human molecular genetics, 24 (2015) 5880–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang M, Sun J, Crosby A, Woodard K, Hirsch ML, Samulski RJ, Li C, Direct interaction of human serum proteins with AAV virions to enhance AAV transduction: immediate impact on clinical applications, Gene therapy, 24 (2016) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pei X, He T, Hall NE, Gerber D, Samulski RJ, Li C, AAV8 virions hijack serum proteins to increase hepatocyte binding for transduction enhancement, Virology, 518(2018)95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Muradashvili N, Qipshidze N, Munjal C, Giwimani S, Benton RL, Roberts AM, Tyagi SC, Lominadze D, Fibrinogen-Induced Increased Pial Venular Permeability in Mice, Journal of Cerebral Blood Flow & Metabolism, 32 (2012) 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Muradashvili N, Lominadze D, Role of fibrinogen in cerebrovascular dysfunction after traumatic brain injury, Brain Injury, 27 (2013) 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M, Tissue-type plasminogen activator–mediated shedding of astrocytic low-density lipoprotein receptor–related protein increases the permeability of the neurovascular unit, Blood, 109 (2007) 3270–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN, Tiam1 and Rac1 Are Required for Platelet-activating Factor-induced Endothelial Junctional Disassembly and Increase in Vascular Permeability, J. Biol. Chem, 284 (2009) 5381–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Di Pasquale G, Chiorini JA, AAV transcytosis through barrier epithelia and endothelium, Molecular Therapy, 13 (2006) 506–516. [DOI] [PubMed] [Google Scholar]
- [25].He B, Lin P, Jia Z, Du W, Qu W, Yuan L, Dai W, Zhang H, Wang X, Wang J, Zhang X, Zhang Q, The transport mechanisms of polymer nanoparticles in Caco-2 epithelial cells, Biomaterials, 34 (2013) 6082–6098. [DOI] [PubMed] [Google Scholar]
- [26].Emmerson CD, van der Vlist EJ, Braam MR, Vanlandschoot P, Merchiers P, de Haard HJW, Verrips CT, van Bergen en Henegouwen PMP, Dolk E, Enhancement of Polymeric Immunoglobulin Receptor Transcytosis by Biparatopic VHH, PloS one, 6 (2011) e26299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sparrow RL, Greening DW, Simpson RJ, A Protocol for the Preparation of Cryoprecipitate and Cryodepleted Plasma, in: Simpson RJ, Greening DW (Eds.) Serum/Plasma Proteomics: Methods and Protocols, Humana Press, Totowa, NJ, 2011, pp. 259–265. [DOI] [PubMed] [Google Scholar]
- [28].Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ, Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates, Mol Ther, 19 (2011) 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nathwani AC, Gray JT, Ng CYC, Zhou J, Spence Y, Waddington SN, Tuddenham EGD, Kemball-Cook G, McIntosh J, Boon-Spijker M, Mertens K, Davidoff AM, Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver, Blood, 107 (2006) 2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nathwani AC, Reiss UM, Tuddenham EGD, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, Riddell A, Pie J, Rangarajan S, Bevan D, Recht M, Shen YM, Halka KG, Basner-Tschakarjan E, Mingozzi F, High KA, Allay J, Kay MA, Ng CYC, Zhou J, Cancio M, Morton CL, Gray JT, Srivastava D, Nienhuis AW, Davidoff AM, Long-Term Safety and Efficacy of Factor IX Gene Therapy in Hemophilia B, The New England journal of medicine, 371 (2014) 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Monahan PE, Lothrop CD, Sun J, Hirsch ML, Kafri T, Kantor B, Sarkar R, Tillson DM, Elia JR, Samulski RJ, Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application, Mol Ther, 18 (2010) 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alexander IE, Russell DW, Miller AD, DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors, Journal of virology, 68 (1994) 8282–8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kotterman MA, Schaffer DV, Engineering adeno-associated viruses for clinical gene therapy, Nat Rev Genet, 15 (2014) 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tyagi N, Roberts AM, Dean WL, Tyagi SC, Lominadze D, Fibrinogen induces endothelial cell permeability, Molecular and cellular biochemistry, 307 (2008) 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wheatley EM, Vincent PA, McKeown-Longo PJ, Saba TM, Effect of fibronectin on permeability of normal and TNF-treated lung endothelial cell monolayers, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 264 (1993) R90–R96. [DOI] [PubMed] [Google Scholar]
- [36].Curtis TM, McKeown-Longo PJ, Vincent PA, Homan SM, Wheatley EM , Saba TM, Fibronectin attenuates increased endothelial monolayer permeability after RGD peptide, anti-alpha 5 beta 1, or TNF-alpha exposure, American Journal of Physiology-Lung Cellular and Molecular Physiology, 269 (1995) L248–L260. [DOI] [PubMed] [Google Scholar]
- [37].HARALDSSON B, RIPPE B, Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle, Acta Physiologica Scandinavica, 129 (1987) 127–135. [DOI] [PubMed] [Google Scholar]
- [38].Curry FE, Rutledge JC, Lenz JF, Modulation of microvessel wall charge by plasma glycoprotein orosomucoid, American Journal of Physiology-Heart and Circulatory Physiology, 257 (1989) H1354–H1359. [DOI] [PubMed] [Google Scholar]
- [39].Zhang S, Mark KS, al-Acid glycoprotein induced effects in rat brain microvessel endothelial cells, Microvascular Research, 84 (2012) 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gragnano F, Sperlongano S, Golia E, Natale F, Bianchi R, Crisci M, Fimiani F, Pariggiano I, Diana V, Carbone A, Cesaro A, Concilio C, Limongelli G, Russo M, Calabr ò P., The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy, Mediators of Inflammation, 2017 (2017) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Suzuki Y, Nagai N, Yamakawa K, Muranaka Y, Hokamura K, Umemura K, Recombinant Tissue-Type Plasminogen Activator Transiently Enhances Blood–Brain Barrier Permeability During Cerebral Ischemia through Vascular Endothelial Growth Factor-Mediated Endothelial Endocytosis in Mice, Journal of Cerebral Blood Flow & Metabolism, 35 (2015) 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Callum JL, Karkouti K, Lin Y, Cryoprecipitate: The Current State of Knowledge, Transfusion Medicine Reviews, 23 (2009) 177–188. [DOI] [PubMed] [Google Scholar]
- [43].Nascimento B, Goodnough LT, Levy JH, Cryo precipitate therapy, British Journal of Anaesthesia, 113 (2014) 922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kotchey NM, Adachi K, Zahid M, Inagaki K, Charan R, Parker RS, Nakai H, A Potential Role of Distinctively Delayed Blood Clearance of Recombinant Adeno-associated Virus Serotype 9 in Robust Cardiac Transduction, Molecular Therapy, 19 (2011) 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R, Xu Z, Gao G, Several rAAV Vectors Efficiently Cross the Blood–brain Barrier and Transduce Neurons and Astrocytes in the Neonatal Mouse Central Nervous System, Molecular Therapy, 19 (2011) 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Merkel SF, Andrews AM, Lutton EM, Mu D, Hudry E, Hyman BT, Maguire CA, Ramirez SH, Trafficking of adeno-associated virus vectors across a model of the blood–brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells, Journal of Neurochemistry, 140 (2017) 216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. SI. The effect of other serum proteins at different concentrations on the global transduction of AAV9. 1×1010 vg of AAV9/luc were incubated with either AGP, FN, PF4, vWF, or PMG at the dilutions of either 1:10, 1:100, or 1:1000 of the normal physiological concentration for 2 hours at 4 °C and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 7. The photon signal was measured and calculated for luciferase expression. The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
Fig. S2. Rabbit and dog cryoprecipitate increased the global transduction of AAV9 vectors. 1×1010 vg of AAV9/luc were incubated with 2 μl of cryoprecipitate at 4 °C for 2 hours and then injected into C57BL/6 mice via the retro-orbital vein. Imaging was taken at day 7. The photon signal was measured and calculated for luciferase expression. The AAV9 gene copy number was measured by qPCR assay. The data represents the average and standard deviation from 5 mice. The asterisk indicates the significant difference (p<0.05).
Fig. S3. High transduction efficacy did not induce an inflammatory response in the liver of mice. 1 × 1011 vg of AAV9/luc were incubated with 2 μl of cryoprecipitate at 4°C for 2 hours and then injected into C57BL/6 mice via the retro-orbital vein. The mice were sacrificed at 9 days post AAV9 administration, and the livers were harvested. The mRNA levels of IL-1β and IL-6 were measured by qRT-PCR. The data represents the average and standard deviation from 5 mice.
Fig. S4. Human serum albumin (HSA) did not increase the global transduction of AAV9 in mice. 1×1010 vg of AAV9/luc incubated with HSA were administered via the retro-orbital injection. The imaging was taken at day 3 post AAV injection. The data represents the average and standard deviation from 4 mice.
Fig. S5. Serum proteins competitively bound to the AAV9 virions. For the competitive binding assay of serum proteins, 1×1010 vg of AAV9/luc vector was incubated with either FN or vWF at different dilutions. Fibrinogen was added at a dilution of 100-fold at for 1 hour 4°C. The antibody against fibrinogen was used for immunoprecipitation. The vims titer was determined by qPCR.


