Abstract
Many neotropical species have a complex history of diversification as a result of the influence of geographical, ecological, climatic, and geological factors that determine the distribution of populations within a lineage. Phylogeography identifies such populations, determines their geographic distributions, and quantifies the degree of genetic divergence. In this work we explored the genetic structure of Habia rubica populations, a polytypic taxon with 17 subspecies described, in order to obtain hypotheses about their evolutionary history and processes of diversification. We undertook multilocus analyses using sequences of five molecular markers (ND2, ACOI-I9, MUSK, FGB-I5 and ODC), and sampling from across the species’ distribution range, an area encompassing from Central Mexico throughout much of South America. With these data, we obtained a robust phylogenetic hypothesis, a species delimitation analysis, and estimates of divergence times for these lineages. The phylogenetic hypothesis of concatenated molecular markers shows that H. rubica can be divided in three main clades: the first includes Mexican Pacific coast populations, the second is formed by population from east of Mexico to Panama and the third comprises the South American populations. Within these clades we recognize seven principal phylogroups whose limits have a clear correspondence with important geographical discontinuities including the Isthmus of Tehuantepec in southern Mexico, the Talamanca Cordillera, and the Isthmus of Panama in North America. In South America, we observed a marked separation of two phylogroups that include the populations that inhabit mesic forests in western and central South America (Amazon Forest) and those inhabiting the seasonal forest from the eastern and northern regions of the South America (Atlantic Forest). These areas are separated by an intervening dry vegetation “diagonal” (Chaco, Cerrado and Caatinga). The geographic and genetic structure of these phylogroups describes a history of diversification more active and complex in the northern distribution of this species, producing at least seven well-supported lineages that could be considered species.
Keywords: Habia rubica, Birds, Multilocus analysis, Phylogenetic, Deep divergence, Phylogroups, Species diversification, Neotropics
Introduction
The origins and evolutionary drivers of neotropical diversity are one of the most studied and hotly debated topics since the first biological explorations in the 19th century (Salvin & Godman, 1879–1904). Two main hypotheses have been proposed to explain the high levels of neotropical biodiversity (Rull, 2008). On one hand, the glacial-interglacial cycles of the Pleistocene (last ∼2.6 Mya) have been suggested as the major drivers of divergence, during which allopatric speciation took place in isolated rainforest refuges, particularly during the cooler and drier conditions of the glacial maxima (Whitmore & Prance, 1987; Hooghiemstra & Van der Hammen, 1998; Hewitt, 2000; Bennett, 2004). On the other hand, the paleogeographic changes that occurred throughout the Cenozoic (i.e., the last ∼66 Mya), like the Andean orogeny and the uprising of the Isthmus of Panama (Hewitt, 2000; Willis & Niklas, 2004; Nores, 2004), have been postulated as major factors driving diversification, as new geographical barriers both increased isolation and promoted divergence. Both hypotheses have however been criticized. For instance, there is little palynological (Colinvaux et al., 2001; Bush & De Oliveira, 2006), phylogenetic (e.g., Moritz et al., 2000; Glor, Vitt & Larson, 2001), or ecological-modeling support (Cowling, Maslin & Sykes, 2001; Mayle et al., 2004) for a Pleistocene origin of tropical diversity. Indeed, most divergence times estimated so far date back to the Neogene, supporting a role for more ancient diversification events. However, differentiation patterns across taxa are highly discordant and frequently cannot be linked to specific landscape or vicariant events (Burns & Naoki, 2004; Burns & Racicot, 2009; Mauck & Burns, 2009) as this hypothesis proposes; instead, they seem more related to each species ability to persist through environmental changes and independently disperse across putative geographic barriers (e.g., Smith et al., 2014).
The role of ecological divergence in producing neotropical diversity has taken force in the last decade. For instance, alternating cycles of contracting and expanding tropical forests could have resulted in multiple bouts of differentiation that produced assemblages of “old” and “young” taxa exclusive to each habitat (e.g., Pennington et al., 2004; Smith et al., 2013). This is not only important for species diverging in situ, but also for taxa that dispersed and differentiated across newly available habitats, such as those involved in inter-continental exchange after the uplifting of the Isthmus of Panama. It has been suggested that animals tolerant to a variety of habitats and elevation zones quickly expanded through this corridor into North America, while species restricted to more humid conditions could only disperse more recently into northern latitudes (Zamudio-Beltrán & Hernández-Baños, 2015).
Another point that fuels this debate is the species concept itself. That is, evidence supporting one or another hypothesis can be dismissed or not depending on whether the taxa studied are considered (or not) independent species. Traditionally, species boundaries have been drawn based on morphology, but the onset of high-throughput molecular techniques has allowed inferring these boundaries from statistical models based on the coalescent framework (e.g., Bayesian Phylogenetics and Phylogeography; BP&P; Yang & Rannala, 2010). As both views have their own limitations (see for instance Sukumaran & Knowles, 2017), an integrative approach based on different types of data (morphological, ethological, ecological, molecular, etc.) seems necessary to operationally determine whether lineages have been evolving separately and therefore can be considered different species (De Queiroz, 2007).
The Red-crowned Ant Tanager (Habia rubica, Cardinalidae) is a highly polytypic taxon with marked geographical variation; it comprises up to 17 subspecies, (https://avibase.bsc-eoc.org/avibase.jsp?lang=EN) most of which were described based on the variation in the hue and intensity of plumage coloration (Hilty, 2011). Its current distribution ranges from central Mexico to north-eastern Argentina and southeastern Brazil, and encompasses regions with very different ecological conditions and/or separated by recognized biogeographical barriers (Hilty, 2011). A previous phylogeographic survey (Lavinia et al., 2015) suggested that this species originated in South America, where it bears at least two clearly differentiated clades, one in the Atlantic Forest of Brazil and another in the rainforests of the Amazon basin. However, a limited sampling precluded the authors to explore the colonization and diversification within Central America and Southern Mexico.
The objective of this paper is to evaluate the nature and geographic structuring of genetic variation within and among populations of H. rubica, using both nuclear and mitochondrial genetic markers in order to provide a phylogenetic hypothesis about the evolutionary history of the species. We used Bayesian and coalescent methods to generate a phylogeny and estimate divergence times between clades, to assess the validity of the genetic groupings identified, using species delimitation methods (Rannala & Yang, 2003; Yang & Rannala, 2010). Emphasis was made on the missing parts of the biogeographical history of the species.
Material & Methods
Taxon sampling and laboratory procedures
We sequenced 125 individuals of H. rubica species that covered the species distribution, including samples from Mexico (using field collection on permit from Instituto Nacional de Ecología, SEMARNAT: FAUT-0169), Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica, Panama, Peru, Bolivia, Paraguay, Brazil, Argentina and Venezuela (Fig. 1A). We also included 16 samples from the genus Chlorothraupis (C. olivacea, C. carmioli and C. stolzmanni), the sister group of H. rubica (Klicka, Burns & Spellman, 2007), and three congeneric samples that were used as outgroups (H. fuscicauda, H. atrimaxillaris, and H. gutturalis; see Table S1).
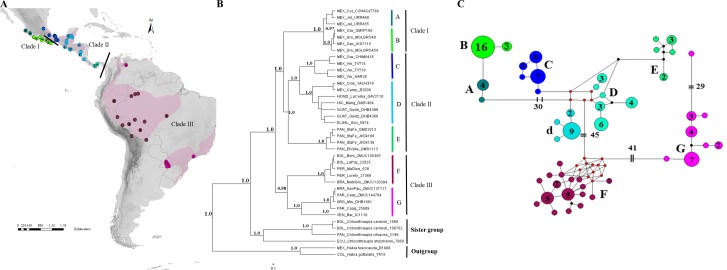
Figure 1. Geographical distribution, phylogenetic consensus tree and haplotypes network.
(A) Geographical distribution (indicated by pink shading) and sampling points of the H. rubica species; the mitochondrial DNA sampling is represented by dots color and the nuclear DNA sampling is highlighted with a black dot on the dots color. ArcGIS (ArcMAP 10.2.2; Esri, Redlands, CA, USA). (B) Phylogenetic consensus tree representing the relationship among populations from H. rubica, based on Bayesian inference from a multilocus dataset. Values above branches denote posterior probabilities (PP). (C) Haplotypes network, where the phylogroup “D” corresponds with individuals from Chiapas-Yucatan peninsula to Costa Rica and “d” corresponds with individuals from Guatemala and El Salvador (the number inside of circles indicate the number of individuals that shared each haplotype).
We isolated genomic DNA from tissues using Qiagen DNAeasy kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol. We amplified five molecular markers via the polymerase chain reaction (PCR) in 12.5 µl volumes. The mitochondrial marker ND2 (1,041 bp), was generated for all individuals (n = 144) using the primers L5219 and H6313 (Sorenson et al., 1999). We then amplified four nuclear markers for a subset of individuals with the highest DNA quality (n = 37). These nuclear loci included two Z-linked introns, the aconitase I gene (ACOI-I9, 852 bp) amplified using the primers ACOI-I9F and ACOI-I9R (Kimball et al., 2009), and the receptor of the tyrosine kinase MUSK gene (MUSK, 576 bp) amplified using the primers MUSK-I3F and MUSK-I3R (Kimball et al., 2009). We also amplified two autosomal loci, a beta-fibrinogen gene (FGB-I5, 577), using the primers Fib5 and Fib6 (Kimball et al., 2009) plus the Ornithine Decarboxylase ODC gene (ODC, 711 bp) using the primers ODCF and ODCR (Primmer et al., 2002). We used the following annealing temperatures: 54 °C (ND2), 56 °C (MUSK), 60 °C (ACOI and FGB-I5) and 65 °C (ODC). PCR success was verified on 1% agarose gels.
DNA sequencing was performed in the High Throughput Genomic Unit Service of the University of Washington. We edited and aligned chromatograms with Sequencher v4.8 (GeneCodes Corporation, Ann Arbor, MI, USA). We corroborated the origin of all of our sequences by combining at least two of the following methods: amplifying overlapping gene segments, amplifying or sequencing one region with different primer sets, sequencing both DNA strands for all amplified fragments, or using multiple individuals of a single species. No evidence of NUMT contamination was found (Zhang & Hewitt, 1996; Sorenson & Quinn, 1998; Bensasson et al., 2001), and all nuclear sequences could be easily aligned with the corresponding genes from other avian species. The overall length of the concatenated mitochondrial and nuclear sequences was ∼3,800 bp. All sequences were deposited in FigShare (https://figshare.com/s/fe9f9f6fed1686782f62).
Phylogenetic analyses
Phylogenies were constructed from two different matrices; the first matrix included 144 sequences of the mitochondrial marker ND2 (hereafter referred to as the mtDNA dataset), and the second matrix included 37 concatenated sequences of the mitochondrial marker ND2 plus the four nuclear markers describe above (hereafter referred to as multilocus dataset). Phylogenetic analyses were performed for each matrix using a Bayesian Inference method (BI), with independent partitions and evolutionary rates defined for each gene region. The model parameters of nucleotide evolution for each DNA region were obtained with jModeltest v3.8 (Posada, 2008) using the Akaike Information Criterion (Akaike, 1973). Analyses were conducted in MrBayes v3.2 (Ronquist et al., 2012), and consisted of four MCMC chains of 50 million generations each, starting from random trees and using a uniform prior distribution of parameters. Trees were sampled every 250 generations, using Tracer v1.6.0 (Rambaut & Drummond, 2013), after discarding the initial 20% as burn-in. The remaining trees were used to construct a majority rule consensus tree with a posterior probability distribution. The final tree was visualized in FigTree v1.2.3 (http//tree.bio.ed.ac.uk/software/figtre/). Finally, we used MEGA v7 (Kumar, Stecher & Tamura, 2016) to estimate the pairwise genetic distances between lineages from mitochondrial and nuclear markers, applying a bootstrap of 1,000 iterations; this allowed us to indirectly determine the relative amounts of information provided by the mitochondrial and nuclear markers.
Diversity and genetic structure
A haplotype network was obtained for the mtDNA dataset with the Median-Joining algorithm available in Network v4.6 (Bandelt, Foster & Röhl, 1999). We used DnaSP v5.0 (Librado & Rozas, 2009) for estimating the number of haplotypes (H) and segregating sites (S), the haplotype (Hd) and nucleotide (π) diversities, and the Tajima’s D (Tajima, 1989) and Fu’s F (Fu, 1997) values for the genetic clusters recovered in the BI mitochondrial phylogeny mentioned above. Significance for the last two summary statistics was inferred from 1,000 replicates of the coalescent algorithm.
Species delimitation
The phylogeny obtained from the multilocus dataset was used to assess species delimitations under the Bayesian framework implemented in BP&P (Yang & Rannala, 2010). This method allows the estimation of posterior distributions of competing models with differing number of species. It incorporates a reversible-jump Markov chain Monte Carlo (rjMCMC) algorithm and a user-specified tree, where it assigns speciation probability values (Rannala & Yang, 2003; Yang & Rannala, 2010). BP&P assumes constant population sizes, no gene flow among lineages, and no population structure within lineages after speciation occurred. It furthers includes divergence time (τ) and mutation-scaled effective population size (θ) parameters. We first performed several preliminary runs with the two available algorithms (0: fixed tree, and 1: tree as guide tree) and different seed numbers to confirm consistency and fine-tuning the priors (ε and τ). After selecting algorithm 0, we conducted a first set of analyses allowing the program to make automatic adjustments to the priors (finetune = 1). Because different values of τ and θ can affect the model posterior probabilities for a same guide tree (Leaché & Fujita, 2010; McKay et al., 2013), and to explore a wide range of speciation histories, we performed a series of simulations using three different parameter combinations: 1) large population size/deep divergence [G (1, 10) for both θ and τ], 2) small population size/ shallow divergence [G (2, 2000) for both θ and τ], and 3) large population size/shallow divergence [G (1, 10) for θ and G (2, 2000) for τ] (Leaché & Fujita, 2010; Smith et al., 2013). We then performed a final set of analyses that incorporated the finetune parameters determined in the previous analysis (finetune = 0) with the same three different combinations for θ and τ parameters described above. Each set was composed of 100,000 generations, and samples were taken every 5 steps after discarding the initial 50,000 iterations as burn-in. All analyses were performed on the BPPX graphics user interface (http://abacus.gene.ucl.ac.uk/software) using the command line.
Tests of divergence times
Divergence times were inferred from the multilocus dataset. Analyses were performed in Beast v1.7 (Drummond et al., 2013) using the previously defined gene partitions with the corresponding evolutionary rates and models as estimated above (Ellegren, 2007; Smith & Klicka, 2010) and the seven phylogroups identified with the multilocus dataset. Clade divergence was simulated as a Yule’s first tree (Yule, 1924) with a lognormal relaxed molecular clock (Drummond et al., 2006). Analyses included 100 million iterations, with samples taken every 1,000 generations after eliminating 25% of the trees as burn-in. Mixing and likelihood stability were confirmed with TRACER v1.6 (Rambaut & Drummond, 2013) to make sure that the appropriate effective sample size (ESS) was over 200 units for each parameter (Drummond & Rambaut, 2007). The best supported tree was chosen with TreeAnnotator 1.8.0 (Drummond & Rambaut, 2007) through Maximum Clade Credibility.
Given the absence of fossils for H. rubica, or any close relatives, two calibrations based on geological data were used. First, the closing of the Isthmus of Panama, estimated between 3.1 and 4.0 Ma (Daza, Castoe & Parkinson, 2010; Smith & Klicka, 2010), was used for the separation of South and Central American populations; and second, the uprising of the Talamanca Cordillera near the Costa Rica–Panama border, estimated to have occurred between 2.5 to 3.9 Ma (Marshall & Liebherr, 2000; Daza, Castoe & Parkinson, 2010), was employed as the time of divergence between populations in Panama and those occurring elsewhere throughout Mesoamerica. In addition, as estimating divergence times based on external event that could be unrelated to a species biology can result in wrong inferences and circular reasoning, we estimated divergence dates a second time but using only the mtDNA dataset (which has a higher sample size) and no calibration points. This could be seen as an independent test of confidence for the estimates obtained for the multilocus analysis with geological constraints. We assumed a mutation rate of 1.25 ×10−2 substitutions/site/lineage/Myr (Smith & Klicka, 2010), other parameters and priors were kept identical.
Results
Phylogenetic analyses
We obtained mitochondrial (1,041 bp) and concatenated (3,757 bp) datasets. The best-fit models for each molecular markers were follows: ND2, TrN+I+G; ACOI-I9, TIM2+G; MUSK, TrN+G; FGB-I5, HKY+G and ODC, TrN.
The phylogenies obtained with mitochondrial and multilocus dataset revealed well-supported topologies, independently of the method employed. Both topologies are composed of three main clades with seven and eight subclades (considered here as “phylogroups”) for the multilocus and mitochondrial datasets, respectively. The first clade in the multilocus phylogeny (I) is comprised of two phylogroups with an apparent separation between populations occupying the western (A; Fig. 1B) and southern (B; Fig. 1B) Pacific coast of Mexico. The second clade (II) was composed of three well-supported phylogroups distributed from eastern Mexico to Panama. The first includes all individuals from the Mexican Gulf Coast, west of the Isthmus of Tehuantepec (C; Fig. 1B); the second one comprised samples collected east of this Isthmus and southward into Nicaragua (D; Fig. 1B); and the third one was composed by samples from Panama (E; Fig. 1B). The last clade (III) was composed of (at least) two phylogroups, one with individuals from the Amazon basin (F; Fig. 1B) and another one with birds from eastern South America (G; Fig. 1B). A single sample from Venezuela appears distinctive and is sister to this eastern clade. However, additional samples from Venezuela will be required for a proper systematic assessment.
The mitochondrial phylogeny, although based on many more samples, was almost identical to the one obtained with the multilocus dataset (Fig S1); it did however, show that phylogroup D was composed of two subgroups: one comprised of individuals from the Yucatan Peninsula, Honduras, Nicaragua and Costa Rica, and the other one of individuals from Guatemala and El Salvador.
The pairwise genetic distances showed significant genetic differentiation in the mitochondrial but not in the nuclear markers (Table 1). That is, the genetic distances were smaller for nuclear than for mitochondrial markers (Table 1). This implies that the mitochondrial marker provides larger amounts of relative information than the nuclear gene-regions (Table 2).
Table 1. Corrected pairwise genetic p-distances between lineages of H. rubica based on mitochondrial and nuclear markers.
| Phylogroups | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| mtDNA | |||||||
| A | |||||||
| B | 0.7** | ||||||
| C | 4.1* | 4.4* | |||||
| D | 4.1* | 4.5* | 1.7** | ||||
| E | 5.1* | 5.5* | 2.7** | 2.4** | |||
| F | 6.4* | 6.4* | 5.7* | 5.8* | 6.6* | ||
| G | 6.8* | 6.6* | 5.7* | 5.8* | 6.7* | 5.4* | |
| nDNA | |||||||
| A | |||||||
| B | 0.0** | ||||||
| C | 0.1** | 0.1** | |||||
| D | 0.1** | 0.1** | 0.1** | ||||
| E | 0.1** | 0.1** | 0.1** | 0.1** | |||
| F | 0.2** | 0.2** | 0.1** | 0.2** | 0.2** | ||
| G | 0.6** | 0.6** | 0.5** | 0.6** | 0.6** | 0.4** | |
Notes.
<0.05.
<0.005.
Table 2. Relative amounts of information in the mitochondrial and nuclear DNA.
| Molecular marker | Samples | Sequences | Bases pair | Monomorphic sites | Polymorphic sites | More than two variants | Gaps | Missing data |
|---|---|---|---|---|---|---|---|---|
| Mitochondrial DNA | 125 | 125 | 1,041 | 782 | 259 | 229 | – | – |
| ACOI-I9 | 38 | 76 | 852 | 452 | 112 | 8 | 7 | 281 |
| MUSK | 39 | 78 | 576 | 181 | 54 | 2 | 4 | 337 |
| FGB-I5 | 38 | 76 | 577 | 236 | 60 | 4 | 2 | 279 |
| ODC | 39 | 78 | 711 | 584 | 46 | 1 | 8 | 73 |
| Nuclear DNA | 37 | 74 | 2,716 | 1,733 | 268 | 17 | 20 | 695 |
Diversity and genetic structure
The haplotype mtDNA network (Fig. 1C) revealed the same eight groups obtained with BI phylogeny (Fig S1). A high number of haplotypes had to be reconstructed to link some of these groups. For instance, 30 mutational steps separated the two groups from the Mexican Pacific coast from the other clusters, 45 more steps were needed to link the clades from South and Central America, while the two groups from South America were 41 steps apart; an additional 29 mutations were necessary to join the lone sample from Venezuela to the southeastern South America clade.
Overall, diversity estimates differed greatly between mtDNA phylogroups, with the South American and Panamanian ones being the more diverse, and the groups from the Mexican Pacific coast the least genetically rich. Tajimas D and Fu’s F values were negative in all phylogroups, excepting phylogroup D (eastern Mexico to northern Central America). However, these values were significant only for the two South American Clades (F & G; Fig. S1); thus suggesting recent population expansions (Table S2).
Species delimitation
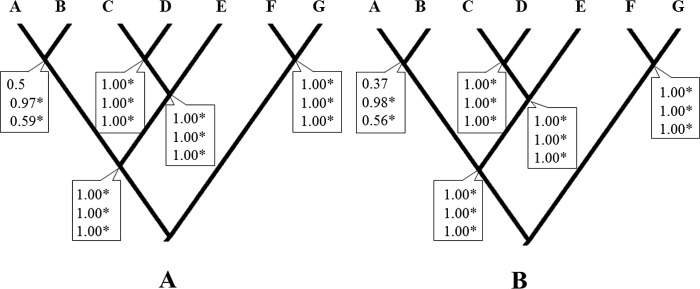
Considering a hypothesis of seven species for this taxon, the Bayesian species delimitation supported the guide tree displaying speciation probabilities of 1.0 in most nodes, independently of the finetune parameter used (finetune = 1 or 0, Figs. 2A and 2B respectively). Seven species were supported with high speciation probabilities (0.97 to1.0) on the tree guide. These species corresponded to the lineages A, B, C, D, E, F and G. The different prior distributions for θ and τ had no effect on most nodes, the only exception being that of lineages A and B which were influenced by at least one of the prior combinations (from 0.37 to 0.97). However, while these and other analyses with similar topologies and highly supported clades suggest a multi-species complex within H. rubica, other types of data are still necessary to support this hypothesis (Sukumaran & Knowles, 2017; see below).
Figure 2. Bayesian species delimitation.
(A) Bayesian species delimitation results assuming seven species (lineages) from multilocus phylogeny of H. rubica (Finetune = 1). (B) Bayesian species delimitation results assuming seven species (lineages) from multilocus phylogeny of H. rubica (Finetune = 0, parameters estimated previously). The speciation probabilities are provided for each node under each combination of priors for τ and θ : top, priors θ ∼ G(1, 10) and τ0 ∼ G(1, 10); middle, priors θ ∼ G(2, 2000) and τ0 ∼ G(2, 2000); bottom, priors θ ∼ G(1,10) and τ0 ∼ G(2, 2000). We consider speciation probability values >0.95 as strong support for a speciation event.
Tests of divergence times
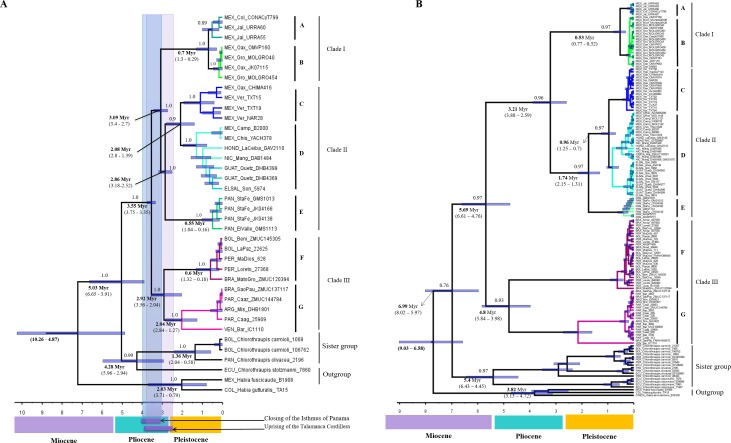
According the multilocus dataset, the divergence between H. rubica and Chlorothraupis dated back to the late Neogene, between 6.65 and 3.91 Myr ago (Fig. 3A), and the ensuing diversification of H. rubica was rather rapid. The first split was estimated some 3.75 to 3.35 Myr, between the South American (clade III) and Central/Mesoamerican lineages (clades I and II); these two groups split again around 3 Myr ago. In Central America, the first divergence event (3.4–2.7 Myr) occurred between the lineages from the Mexican Pacific coast (Clade I) and the other phylogroups (Clade II). Within clade II, there was a gradual and northward divergence of lineages from Panama to eastern Mexico; these three diversification events during the last ∼3Myr or ∼2Myr. An independent analysis performed on the mtDNA dataset and with no calibration constraints, produced an identical topology with older mean divergence times (i.e., divergence within H. rubica occurred during the last 5.6 Myr). However, as confidence intervals were much wider (Fig. 3B) and overlapped with those obtained with the multilocus dataset, they provide support for the dates estimated based on geological times.
Figure 3. Divergence times trees.
(A) For multilocus dataset (confidence interval values are inside parentheses), calibration points are shown in the time bar. (B) For ND2 dataset, we use sustitution rates of 2.5 × 10−2 substitutions/site/lineage/Myr.
Discussion
Our results show that H. rubica has considerable geographic genetic structure. We found seven lineages through its distribution; five of them are in Mesoamerica, where the evolutionary history has been more dynamic and complex, and two in South America (this last results is similar with was found by Lavinia et al., 2015). Most lineages are delimited by recognized geographic and ecological barriers, like the Rio Balsas depression in Mexico, the Central American Volcanic Arc, the Isthmus of Panama in Central America, or the dry vegetation diagonal in South America. We recovered a reciprocally monophyletic relationship between H. rubica and the genus Chlorothraupis, which coincides with previous studies (Klicka, Burns & Spellman, 2007; Klicka, Johnson & Lanyon, 2000; Barker et al., 2015; Lavinia et al., 2015); therefore the genus Habia must be considered paraphyletic.
Phylogeny, diversity and genetic structure
The mitochondrial DNA topology indicates that populations of Mexico, Central and South America are profoundly differentiated. In this topology eight phylogroups were identified, six in Mesomerica and two in South America (Fig S1). The relationship between mitochondrial haplotypes could be determined by the action of various historical processes that promoted a deep genetic structure in Mesoamerica, resulting in a clear example of cryptic speciation. This complicated phylogenetic structure in Mesoamerica is consistent with the complex geological and biogeographic history of the region (Coates & Obando, 1996), and the emergence of geographic features such as the Rio Balsas depression, the Isthmus of Tehuantepec in southern Mexico, the Motagua-Polochic-Jocotán fault system in the North of Central America, and the Talamanca Cordillera in Costa Rica, which presumably played roles in shaping the observed geographic and genetic patterns (Daza, Castoe & Parkinson, 2010; Gutiérrez-García & Vázquez-Domínguez, 2012; Gutiérrez-García & Vázquez-Domínguez, 2013; Suárez-Atilano, Burbrink & Vázquez-Domínguez, 2014). Our results together with the study of Lavinia et al. (2015) provide a robust hypothesis of the evolutionary history of H. rubica. For instance, our study confirms the main results of Lavinia et al. (2015) for South America, and improves the resolution for Mesoamerica given our larger sample size and wider area covered.
In South America, our results, in combination with those of Lavinia et al. (2015), showed two distinct phylogroups that reflected population divergence according to the habitats preferred by H. rubica (Pennington et al., 2004; Pennington, Lavin & Oliveira-Filho, 2009; Lavin, 2006; Smith et al., 2013; Smith et al., 2014; Pennington & Lavin, 2016): one western phylogroup distributed in the mesic forest of the Amazon basin, and another one in the seasonal forests of the eastern and northern parts of South America. This last lineage included the single sample from Venezuela, instead of the western clade that is geographically more proximate (Prates et al., 2016; Prates et al., 2017). This could be indicating that the deep evolutionary history of the seasonal forests in South America (Pennington et al., 2004; Pennington, Lavin & Oliveira-Filho, 2009) are driving the divergence of other taxa. Additional sampling in this region is thus necessary to test this hypothesis.
The multilocus DNA topology identified seven of the eight phylogroups found with the mitochondrial DNA (Fig. 1B), in all cases the phylogroups are the same with the exception of an additional separation of a Yucatan Peninsula and northern of Central America clade identified using the more rapidly evolving mtDNA marker. All identified phylogroups (mitochondrial and multilocus dataset) are consistent with clear geographical discontinuities and are supported with high posterior probabilities.
Although, the haplotype network recovered the same groups identified in the mitochondrial DNA phylogeny (Fig. 1C), it was necessary to invoke five hypothetical haplotypes between Mexican and Central American groups. These results support the idea about the complex geological history in Mesoamerica resulting in a high degree of genetic structure in the northernmost distribution of this species. In South America, 12 hypothetical haplotypes were required, but unlike the previous case, this could be explained by the lack of sampling in this specific area.
The genetic distances between phylogroups were relatively high and supported the groups identified in our phylogenetic and BPP analyses. The largest difference found was between the group from the Western Pacific Coast of Mexico (group A) and the group from the Southeast of South America (group G) (Table 1). This result is not surprising because the geographical distance between them is also the largest and is congruent with the divergence date (Figs. 3A and 3B). On the other hand, the more similar phylogroups were the Western (A) and the Southern Pacific Coast of Mexco (B) groups, which represent the most recent divergence event (Figs. 3A and 3B). As expected the mitochondrial DNA provides more polymorphic sites because its rate of evolution is faster (Table 2) (Brown, George Jr & Wilson, 1979).
Species delimitation and divergence times
The deep divergence times among lineages estimated herein (Figs. 3A and 3B), most of which dated back to Pleistocene and Pliocene, support the criticisms raised regarding the allopatric speciation hypothesis that invoked the Pleistocene glacial cycles as the major drivers of neotropical bird diversity and closing of the Isthmus of Panama (e.g., Moritz et al., 2000; Cowling, Maslin & Sykes, 2001; Glor, Vitt & Larson, 2001; Mayle et al., 2004). According to the ND2 divergence analyses, the first split was during the Miocene (5.69 Myr), and this is the main discordance with respect to the calibrated multilocus divergence analyses (3.55 Myr). These dates are also consistent with the compelling evidence for speciation drawn from the BP&P analyses, which suggest that enough differences have accumulated between most of the H. rubica lineages to consider them a complex of cryptic species (Fig. 2).
At present, intraspecific delimitation within H. rubica is based on geographic variation in plumage color, with 17 subspecies currently recognized (https://avibase.bsc-eoc.org/avibase.jsp?lang=EN). Many of these subspecies are not supported by the lineages described herein (Fig. 2). For instance, the subspecies rosea and rubicoides are paraphyletic (lineages A&B, and C&D, from BP&P analysis, respectively). The first taxon is further intermingled with ssp. affinis individuals in lineage B, while the second one is combined with individuals of ssp. holobrunnea and nelsoni in the lineages C and D. Some subspecies also appear indistinguishable with the markers that we used (e.g., ssp. hesterna, peruviana and rhodinolaema within lineage F). Overall, our results support a taxonomic revision of H. rubica.
Our test for species delimitation suggests that H. rubica should minimally be split into the following species: (1) H. rosea, distributed along the Pacific coast of western Mexico (Jalisco, Nayarit and Colima; lineage A); (2) H. affinis, distributed along the Pacific coast of southern Mexico (Michoacan, Guerrero and Oaxaca; lineage B); (3) H. holobrunnea, distributed over the slope of Gulf of Mexico (lineage C); (4) H. rubicoides, distributed from eastern Mexico to southern Nicaragua (lineage D); (5) H. vinacea, allopatric in Panama (lineage E); (6) H. rhodinolaema, distributed in the Amazon basin (lineage F); and (7) H. rubica, distributed in eastern Brazil, Argentina, and Paraguay (lineage G). Further tests and sampling should be necessary for a more adequate description of the Venezuela’s individuals, currently classified as spp. perijana (Hilty, 2011).
There are criticisms to the BP&P analysis implemented herein. For instance, simulation data have shown that it tends to misidentify population structure as species boundaries (Sukumaran & Knowles, 2017). However, it must be noted that our delimitation not only detected genetics lineages, but lineages whose distribution coincides with the geographical barriers and ecological differences (habitat preference) of each taxon. That is, it highlights the role of other factors, like vicariance, dispersal and ecology, under a framework of ecological allopatric speciation, which is one the drivers proposed for generating bird species diversity in the neotropics (Hooghiemstra & Van der Hammen, 1998; Hewitt, 2000; Bennett, 2004; Smith et al., 2013). It also shows that this bird diversity is underestimated, and that species cannot be described or estimated based only on morphological criteria. From this point of view, diversifying the techniques for species delimitation, like incorporating bioacoustics, high-throughput sequencing, niche modelling and advanced statistical tools, will also help addressing consequential questions in taxonomy and biogeography. For instance, such multifactorial data should help determining operationally whether the H. rubica lineages inferred herein are indeed evolving separately (see De Queiroz, 2007).
Conclusions
The phyogenies obtained independently with mitochondrial and multilocus datasets revealed well-supported topologies for the polytypic taxon H. rubica. Both topologies are composed of three main clades with eight and seven phylogroups (lineages), respectively. The mtDNA haplotype network produced the same eight groups obtained with the mitochondrial data. The Bayesian species delimitation analysis found seven species highly supported: (1) H. rosea, distributed along the Pacific coast of western Mexico; (2) H. affinis, distributed along the Pacific coast of southern Mexico; (3) H. holobrunnea, distributed distributed over the slope of Gulf of Mexico; (4) H. rubicoides, distributed from eastern Mexico to southern Nicaragua; (5) H. vinacea, allopatric in Panama; (6) H. rhodinolaema, distributed in the Amazon basin and (7) H. rubica, distributed in eastern Brazil, Argentina and Paraguay. Further tests and sampling should be necessary for a more adequate description of the samples from Venezuela, currently classified as spp. perijana. The species subdivision of H. rubica coincides with geographical barriers and ecological differences between taxa, highlighting the role of vicariance, dispersal and ecology in bird species diversification in the neotropics.
Supplemental Information
List of individuals sequenced, collection numbers, localities and georeferences. Museo de Zoología Alfonso L. Herrera (MZFC-UNAM); El Colegio de la frontera Sur, Unidad Chetumal (ECOSUR-CH); University of Washington, The Burke Museum (UWBM); (JK); Museum of Natural Science (Louisiana State University, LSUMNH) and The Natural History Museum (University of Kansas, KU).
Genetic diversity by phylogroups from mitochondrial DNA phylogeny. Numbers of individuals (n), haplotypes (H), segregation sites (s), haplotype diversity (Hd) nucleotide diversity (π), Tajima and Fu parameters.
Phylogenetic consensus tree representing the relationships among Habia rubica populations based on Bayesian inference from mitochondrial dataset. Values above branches denote posterior probabilities. Thin branches of the tree denote low values of posterior probabilities (PP < 0.6).
Acknowledgments
We thank the following institutions for providing samples: Museo de Zoología “Alfonso L. Herrera (UNAM)”, The Burke Museum (UWBM), El Colegio de la Frontera Sur (ECOSUR-Ch), the Louisiana Museum of Natural History (Louisiana State University) and the Natural History Museum (University of Kansas). We are also grateful to Isabel Vargas Fernández, Raul Ivan Martínez, Luz Estela Zamudio Beltrán, Ernesto Espinosa Jaramillo, Alejandro Gordillo Martínez and Fabiola Ramírez Corona for technical help.
Funding Statement
The research was supported by PAPIIT/DGAPA, UNAM through a grant to Blanca E. Hernández-Baños (IN215614). Sandra M. Ramírez-Barrera was supported by a scholarship from CONACyT, Mexico. This paper was written during the sabbatical leave of Blanca E. Hernández-Baños at the Museo Nacional de Ciencias Naturales, Madrid, supported by PASPA/DGAPA UNAM and CONACyT (Mexico). Juan P. Jaramillo-Correa was on a sabbatical leave during the writing of this paper at UMR INRA-Université de Bordeaux, supported by PASPA/DGAPA UNAM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Sandra M Ramírez-Barrera conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Blanca E Hernández-Baños conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Juan P. Jaramillo-Correa and John Klicka authored or reviewed drafts of the paper, approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The field collection permit was provided by Instituto Nacional de Ecologia, SEMARNAT, Mexico (FAUT-0169).
Data Availability
The following information was supplied regarding data availability:
Ramirez, Marisol (2018): Habia rubica sequences. figshare. Dataset. https://doi.org/10.6084/m9.figshare.5926591.v1.
References
- Akaike (1973).Akaike H. Information theory and an extension of the maximum likelihood principle. Second international symposium on information theory; Budapest. 1973. pp. 267–281. [Google Scholar]
- Bandelt, Foster & Röhl (1999).Bandelt HJ, Foster P, Röhl A. Median-Joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Barker et al. (2015).Barker FK, Burns KJ, Klicka J, Lanyon SM, Lovette IJ. New insights into New World biogeography: an integrated view from the phylogeny of blackbirds, cardinals, sparrows, tanagers, warblers, and allies. The Auk. 2015;132:333–348. doi: 10.1642/AUK-14-110.1. [DOI] [Google Scholar]
- Bennett (2004).Bennett KD. Continuing the debate on the role of quaternary environmental change of macroevolution. Philosophical Transactions of the Royal Society, Series B: Biological Sciences. 2004;359:295–303. doi: 10.1098/rstb.2003.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensasson et al. (2001).Bensasson D, Petrov DA, Zhang DX, Hartl DL, Hewitt GM. Genomic gigantism: DNA loss is slow in mountain grasshoppers. Molecular Biology and Evolution. 2001;18:246–253. doi: 10.1093/oxfordjournals.molbev.a003798. [DOI] [PubMed] [Google Scholar]
- Brown, George Jr & Wilson (1979).Brown WM, George Jr M, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proceedings of National Academy of Sciences of the United States of America. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns & Naoki (2004).Burns KJ, Naoki K. Molecular phylogenetics and biogeography of Neotropical tanagers in the genus Tangara. Molecular Phylogenetic and Evolution. 2004;32:838–854. doi: 10.1016/j.ympev.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Burns & Racicot (2009).Burns KJ, Racicot RA. Molecular phylogenetics of a clade of lowland tanagers: implications for avian participation in the Great Interchange. The Auk. 2009;126:635–648. doi: 10.1525/auk.2009.08195. [DOI] [Google Scholar]
- Bush & De Oliveira (2006).Bush MB, De Oliveira PE. The rise and fall of the refugial hypothesis of Amazonian speciation: a paleoecological perspective. Biota Neotropica. 2006;6:1–17. doi: 10.1590/S1676-06032006000100002. [DOI] [Google Scholar]
- Coates & Obando (1996).Coates AG, Obando JA. The geologic evolution of the Central American isthmus. In: Jackson JBC, Ludd AF, Coates AG, editors. Evolution and environment in tropical America. University of Chicago Press; Chicago: 1996. pp. 21–56. [Google Scholar]
- Colinvaux et al. (2001).Colinvaux PA, Irion G, Räsänen ME, Bush MB, Nunes de Mello JAS. A paradigm to be discarded: geological and paleoecological data falsify the HAFFER and PRANCE refuge hypothesis of Amazonian speciation. Amazoniana. 2001;16:609–646. [Google Scholar]
- Cowling, Maslin & Sykes (2001).Cowling SA, Maslin MA, Sykes M. Paleovegetation simulations of lowland Amazonia and implications for Neotropical allopatry and speciation. Quaternary Research. 2001;55:140–149. doi: 10.1006/qres.2000.2197. [DOI] [Google Scholar]
- Daza, Castoe & Parkinson (2010).Daza JM, Castoe TA, Parkinson CL. Using regional comparative phylogenetic data from snake lineages to infer historical processes in Middle America. Ecography. 2010;33:343–354. doi: 10.1111/j.1600-0587.2010.06281.x. [DOI] [Google Scholar]
- De Queiroz (2007).De Queiroz K. Species concepts and species delimitation. Systematic Biology. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- Drummond et al. (2006).Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLOS Biology. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond & Rambaut (2007).Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond et al. (2013).Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution. 2013;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren (2007).Ellegren H. Molecular evolutionary genomics of birds. Cytogenetics and Genome Research. 2007;117:120–130. doi: 10.1159/000103172. [DOI] [PubMed] [Google Scholar]
- Fu (1997).Fu YX. Statistical test of neutrality of mutation against population growth, hitchhiking and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glor, Vitt & Larson (2001).Glor RE, Vitt LJ, Larson A. A molecular phylogenetic analysis of diversification in Amazonian Anolis lizards. Molecular Ecology. 2001;10:2661–2668. doi: 10.1046/j.0962-1083.2001.01393.x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-García & Vázquez-Domínguez (2012).Gutiérrez-García TA, Vázquez-Domínguez E. Biogeographically dynamic genetic structure bridging two continents in the monotypic Central American rodent Ototylomys phyllotis. Biological Journal of the Linnean Society. 2012;107:593–610. doi: 10.1111/j.1095-8312.2012.01966.x. [DOI] [Google Scholar]
- Gutiérrez-García & Vázquez-Domínguez (2013).Gutiérrez-García TA, Vázquez-Domínguez E. Consensus between genes and stones in the biogeographic and evolutionary history of Central America. Quaternary Research. 2013;79:311–324. doi: 10.1016/j.yqres.2012.12.007. [DOI] [Google Scholar]
- Hewitt (2000).Hewitt G. The genetic legacy of the quaternary ice ages. Nature. 2000;405:907–913. doi: 10.1038/35016000. [DOI] [PubMed] [Google Scholar]
- Hilty (2011).Hilty SL. Family Thraupidae (Tanagers) In: Del Hoyo J, Elliott A, Christie DA, editors. vol. 16. Lynx Editions; Barcelona: 2011. pp. 46–329. (Handbook of the birds of the world. Tanagers to new world blackbirds). [Google Scholar]
- Hooghiemstra & Van der Hammen (1998).Hooghiemstra H, Van der Hammen T. Neogene and Quaternary development of the neotropical rain forest: the forest refugia hypothesis, and a literature overview. Earth-Science Reviews. 1998;44:147–183. doi: 10.1016/S0012-8252(98)00027-0. [DOI] [Google Scholar]
- Kimball et al. (2009).Kimball RT, Braun EL, Barker FK, Bowie RCK, Braun MJ, Chojnowski JL, Hackett SJ, Han KL, Harshman J, Heimer-Torres V, Holznagel W, Huddlestone CJ, Marks BD, Miglia KJ, Moore WS, Reddy S, Sheldon FH, Smith JV, Witt CC, Yuri T. A well-tested set of primers to amplify regions spread across the avian genome. Molecular Phylogenetics and Evolution. 2009;50:654–660. doi: 10.1016/j.ympev.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Klicka, Burns & Spellman (2007).Klicka J, Burns K, Spellman GM. Defining a monophyletic Cardinalini: a molecular perspective. Molecular Phylogenetics and Evolution. 2007;45:1014–1032. doi: 10.1016/j.ympev.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Klicka, Johnson & Lanyon (2000).Klicka J, Johnson KP, Lanyon SM. New World nine-primaried oscine relationships: constructing a mitochondrial DNA framework. The Auk. 2000;117:321–336. doi: 10.1642/0004-8038(2000)117[0321:NWNPOR]2.0.CO;2. [DOI] [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for datasets. Molecular Biology and Evolucion. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin (2006).Lavin M. Floristic and geographic stability of discontinuous seasonally dry tropical forests explains patterns of plant phylogeny and endemism, Chapter 19. In: Pennington RT, Ratter JA, Lewis GP, editors. Neotropical savannas and seasonally dry forests: plant biodiversity, biogeographic patterns and conservation. CRC Press; Boca Raton: 2006. pp. 433–447. [Google Scholar]
- Lavinia et al. (2015).Lavinia PD, Escalante P, García NC, Barreira AS, Trujillo-Arias N, Tubaro PL, Naoki K, Miyaki CY, Santos FR, Lijtmaer DA. Continental-scale analysis reveals Deep diversification within the polytypic Red-crowned Ant Tanager (Habia rubica, Cardinalidae) Molecular Phylogenetics and Evolution. 2015;89:182–193. doi: 10.1016/j.ympev.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Leaché & Fujita (2010).Leaché AD, Fujita MK. Bayesian species delimitation in West African forest geckos (Hemidactylus fasciatus) Proceedings of the Royal Society. 2010;277:3071–3077. doi: 10.1098/rspb.2010.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Marshall & Liebherr (2000).Marshall CJ, Liebherr JK. Cladistic biogeography of Mexican transition zone. Journal of Biogeography. 2000;27:203–216. doi: 10.1046/j.1365-2699.2000.00388.x. [DOI] [Google Scholar]
- Mauck & Burns (2009).Mauck WM, Burns KJ. Phylogeny, biogeography, and recurrent evolution of divergent bill types in the nectar-stealing flowerpiercers (Thraupini: Diglossa and Diglossopis) Biological Journal of the Linnean Society. 2009;98:14–28. doi: 10.1111/j.1095-8312.2009.01278.x. [DOI] [Google Scholar]
- Mayle et al. (2004).Mayle FE, Beerling DJ, Gosling WD, Bush MB. Responses of Amazonian ecosystems to climatic and atmospheric carbon dioxide changes since the last glacial maximum. Philosophical Transactions of the Royal Society of London B. 2004;359:499–514. doi: 10.1098/rstb.2003.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay et al. (2013).McKay BD, Mays Jr HL, Wu Y, Li H, Yao CT, Nishiumi I, Zou F. An empirical comparison of character-based and coalescent-based approaches to species delimitation in a young avian complex. Molecular Ecology. 2013;22:4943–4957. doi: 10.1111/mec.12446. [DOI] [PubMed] [Google Scholar]
- Moritz et al. (2000).Moritz C, Patton JL, Schneider CJ, Smith BT. Diversification of rainforest faunas: an integrated molecular approach. Annual Review of Ecology and Systematics. 2000;31:533–563. doi: 10.1146/annurev.ecolsys.31.1.533. [DOI] [Google Scholar]
- Nores (2004).Nores M. The implications of Tertiary and Quaternary sea level rise events for avian distribution patterns in the lowlands of northern South America. Global Ecology and Biogeography. 2004;13:149–161. doi: 10.1111/j.1466-882X.2004.00076.x. [DOI] [Google Scholar]
- Pennington & Lavin (2016).Pennington RT, Lavin M. The contrasting nature of woody plant species in different neotropical forest biomes reflects differences in ecological stability. The New Phytologist. 2016;210:25–37. doi: 10.1111/nph.13724. [DOI] [PubMed] [Google Scholar]
- Pennington, Lavin & Oliveira-Filho (2009).Pennington RT, Lavin M, Oliveira-Filho A. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annual Review of Ecology, Evolution and Systematics. 2009;40:437–457. doi: 10.1146/annurev.ecolsys.110308.120327. [DOI] [Google Scholar]
- Pennington et al. (2004).Pennington RT, Lavin M, Prado DE, Pendry CA, Pell SK, Butterworth CA. Historical climate change and speciation: neotropical seasonally dry forest plants show patterns of both Tertiary and Quaternary diversification. Philosophical Transactions of the Royal Society of London B. 2004;359:515–537. doi: 10.1098/rstb.2003.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada (2008).Posada D. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Prates et al. (2017).Prates I, Melo-Sampaio PR, De Oliveira L, Teixeira Jr M, Rodrigues MT, Carnaval AC. Biogeographic links between southern Atlantic Forest and western South America: rediscovery, re-description, and phylogenetic relationships of two rare montane anole lizards from Brazil. Molecular Phylogenetics and Evolution. 2017;113:49–58. doi: 10.1016/j.ympev.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Prates et al. (2016).Prates I, Rivera D, Rodrigues MT, Carnaval AC. A mid-Pleistocene rainforest corridor enabled synchronous invasions of the Atlantic Forest by Amazonia anole lizards. Molecular Ecology. 2016;25:5174–5186. doi: 10.1111/mec.13821. [DOI] [PubMed] [Google Scholar]
- Primmer et al. (2002).Primmer CR, Borge T, Lindell J, Saetre GP. Single-nucleotide polymorphism characterization in species with limited available sequence information: high nucleotide diversity revealed in the avian genome. Molecular Ecology. 2002;11:603–612. doi: 10.1046/j.0962-1083.2001.01452.x. [DOI] [PubMed] [Google Scholar]
- Rambaut & Drummond (2013).Rambaut A, Drummond A. Tracer v16.0. 2013. http://beast.bio.ed.ac.uk/Tracer http://beast.bio.ed.ac.uk/Tracer
- Rannala & Yang (2003).Rannala B, Yang Z. Bayes estimation of species divergence times and ancestral population sizes using DNA sequences from multiple loci. Genetics. 2003;164:1645–1656. doi: 10.1093/genetics/164.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull (2008).Rull V. Speciation timing and neotropical biodiversity: the tertiary—quaternary debate in the light of molecular phylogenetic evidence. Molecular Ecology. 2008;17:2722–2729. doi: 10.1111/j.1365-294X.2008.03789.x. [DOI] [PubMed] [Google Scholar]
- Salvin & Godman (1879–1904).Salvin O, Godman FD. Biologia Centrali-Americana, Aves. Vol. 4. Taylor and Francis; London: 1879–1904. [Google Scholar]
- Smith & Klicka (2010).Smith BT, Klicka J. The profound influence of the Late Pliocene Panamanian uplift on the exchange, diversification, and distribution of New World birds. Ecography. 2010;33:333–342. [Google Scholar]
- Smith et al. (2014).Smith BT, McCormack JE, Cuervo AM, Hickerson MJ, Aleixo A, Cadena CD, Pérez-Emán J, Burney CW, Xie X, Harvey MG, Faircloth BC, Glenn TC, Derryberry EP, Prejean J, Fields S, Brumfield RT. The drivers of tropical speciation. Nature. 2014;515:406–409. doi: 10.1038/nature13687. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2013).Smith BT, Ribas CC, Whitney BM, Hernández-Baños BE, Klicka J. Identifying biases at different spatial and temporal scales of diversification: a case study in the Neotropical parrotlet genus Forpus. Molecular Ecology. 2013;22:483–494. doi: 10.1111/mec.12118. [DOI] [PubMed] [Google Scholar]
- Sorenson et al. (1999).Sorenson MD, Ast CJ, Dimcheff DE, Yuri T, Mindell DP. Primers for PCR-based approach to mitochondrial genome sequencing in birds and other vertebrates. Molecular Phylogenetics and Evolution. 1999;12:105–114. doi: 10.1006/mpev.1998.0602. [DOI] [PubMed] [Google Scholar]
- Sorenson & Quinn (1998).Sorenson MD, Quinn TW. Numts: a challenge for avian systematics and population biology. The Auk. 1998;115:214–221. doi: 10.2307/4089130. [DOI] [Google Scholar]
- Suárez-Atilano, Burbrink & Vázquez-Domínguez (2014).Suárez-Atilano MA, Burbrink F, Vázquez-Domínguez E. Phylogeographical structure within Boa constrictor imperator across the lowlands and mountains of Central America and Mexico. Journal of Biogeography. 2014;41:2371–2384. doi: 10.1111/jbi.12372. [DOI] [Google Scholar]
- Sukumaran & Knowles (2017).Sukumaran J, Knowles LL. Multispecies coalescent delimits structure, not species. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:1607–1612. doi: 10.1073/pnas.1607921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima (1989).Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA poly-morphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore & Prance (1987).Whitmore TC, Prance GT. Biogeography and quaternary history in tropical America. Clarendon Press; Oxford: 1987. [Google Scholar]
- Willis & Niklas (2004).Willis KJ, Niklas KJ. The role of Quaternary environmental change in plant macroevolution, the exception or the rule? Philosophical Transactions of the Royal Society, Series B: Biological Sciences. 2004;359:159–172. doi: 10.1098/rstb.2003.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang & Rannala (2010).Yang Z, Rannala B. Bayesian species delimitation using multilocus sequence data. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9264–9269. doi: 10.1073/pnas.0913022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule (1924).Yule GU. A mathematical theory of evolution: based on the conclusions of Dr. J. C. Willis. Philosophical Transactions of the Royal Society of London B. 1924;213:21–87. [Google Scholar]
- Zamudio-Beltrán & Hernández-Baños (2015).Zamudio-Beltrán LE, Hernández-Baños BE. A multilocus analysis provides evidence for more than one species within Eugenes fulgens (Aves: Trochilidae) Molecular Phylogenetics and Evolution. 2015;90:80–84. doi: 10.1016/j.ympev.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Zhang & Hewitt (1996).Zhang DX, Hewitt GM. Nuclear integrations: challenges for mitochondrial DNA markers. Trends in Ecology and Evolution. 1996;11:247–251. doi: 10.1016/0169-5347(96)10031-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of individuals sequenced, collection numbers, localities and georeferences. Museo de Zoología Alfonso L. Herrera (MZFC-UNAM); El Colegio de la frontera Sur, Unidad Chetumal (ECOSUR-CH); University of Washington, The Burke Museum (UWBM); (JK); Museum of Natural Science (Louisiana State University, LSUMNH) and The Natural History Museum (University of Kansas, KU).
Genetic diversity by phylogroups from mitochondrial DNA phylogeny. Numbers of individuals (n), haplotypes (H), segregation sites (s), haplotype diversity (Hd) nucleotide diversity (π), Tajima and Fu parameters.
Phylogenetic consensus tree representing the relationships among Habia rubica populations based on Bayesian inference from mitochondrial dataset. Values above branches denote posterior probabilities. Thin branches of the tree denote low values of posterior probabilities (PP < 0.6).
Data Availability Statement
The following information was supplied regarding data availability:
Ramirez, Marisol (2018): Habia rubica sequences. figshare. Dataset. https://doi.org/10.6084/m9.figshare.5926591.v1.