Abstract
Despite major advances in HIV testing, early detection of infection at the point of care (PoC) remains a key challenge. While rapid antibody PoC and laboratory-based nucleic acid amplification tests dominate the diagnostics market, the viral capsid protein p24 is recognized as an alternative early virological biomarker of infection. However, the detection of ultra-low levels of p24 at the PoC has proven challenging. Here we review the landscape of p24-diagnostics to identify knowledge gaps and barriers and help shape future research agendas. 574 research papers to May 2018 that propose or evaluate diagnostic assays for p24 were identified and reviewed. We give a brief history of diagnostic development, and the utility of p24 as a biomarker in different populations such as infants, the newly infected, those on pre-exposure prophylaxis and self-testers. We review the performance of commercial p24 assays and consider elements such as immune complex disruption, resource-poor settings, prevalence, and assay antibodies. Emerging and ultrasensitive assays are reviewed and show a number of promising approaches but further translation has been limited. We summarize studies on the health economic benefits of using antigen testing. Finally, we speculate on the future uses of high performance p24 assays, particularly if available in self-test format.
Keywords: HIV, capsid, point-of-care, early detection, biomarker, diagnostic tests
Introduction
With adoption by the General Assembly of the United Nations of the political declaration “On the Fast Track to Accelerating the Fight against HIV and ending the AIDS epidemic by 2020” there has been a major shift in the field of HIV medicine from optimization of palliative control, to eradication [1,2]. The UNAIDS has set ambitious targets towards this end, which include goals that 90% of people living with HIV know their status by 2020, and 95% by 2030 [2,3]. Improved testing methods including detection earlier in infection, better test accuracy, increased self-testing, and robust linkage to care have all been highlighted as key areas for improvement [4–6].
Currently only an estimated 46% of people living with HIV know their status [7]. Early detection has been proven to allow for better patient outcomes and lower rates of transmission [8,9]. Extending the window of detection facilitates prompt linkage to pathways of clinical care, and would also allow for accurate diagnosis of infant HIV; currently around a half of HIV-exposed infants are appropriately tested with a virological assay, and one third of those who require anti-retroviral therapy receive it [10–13]. Current alternative solutions include the use of dry blood spots to transfer samples to distant high-throughput laboratories and the need for a more accessible and affordable pathogen-based point of care (PoC) assay for early infant diagnosis (EID), especially in resource-limited settings, remains [11,14,15].
In this review, we present outcomes of research pertaining to use of p24 as a biomarker including a short history of p24 diagnostics, use of antigen assays for early detection, characteristics of commercial antigen assays, and we discuss reasons why detection of p24 is challenging and complex. We briefly review research laboratory-stage techniques and health-economic evaluations of antigen-detection assays and present the outlook for these in the light of emerging trends in HIV research and care. This review is focussed on assays for p24 antigen since other reviews of current and emerging molecular diagnostics for HIV are existent, including articles which specifically address their suitability for resource limited settings [16–19].
Diagnostics for HIV – a brief history
The first FDA-licensed HIV test was an ELISA in 1985 [20], followed by the rapid development of immunofluorescence assays [21], agglutination and dot blot tests [22,23]. Some early tests (e.g. the HIVCHEK (Du Pont de Nemours, USA; [24]) and the Single Use Diagnostic System (Murex, [25])) could be performed in 5-20 minutes, though results required trained interpretation. In November 2002, the OraQuick Rapid HIV-1/2 Antibody Test was FDA-approved and was CLIA-waived shortly after in January 2003, permitting diagnosis of HIV in a non-clinical setting [26]. Detection of anti-HIV antibodies by rapid PoC test remains the mainstay of testing algorithms across many settings, with high levels of the target molecules (up to mg/ml) and generally good specificity [27,28]. Algorithms that use multiple PoC tests have been shown to be accurate, reliable and cost-effective when compared to laboratory-based tests [29,30]. These tests are unable, however, to accurately diagnose infection in infants prior to clearance of maternal antibody (transmitted prenatally and in breast milk), in those who have not yet seroconverted [31,32] and sporadic cases where no or an atypical antibody response is mounted [33–37].
In an ideal scenario, a confirmatory test and/or viral load test follow a reactive HIV screening test. CD4 count and sequencing can ensue to determine the individual’s immune status and the drug-resistance phenotype of the virus, followed by appropriate therapy [38–41]. Nucleic acid amplification tests (NAAT) (e.g. viral load tests) can be either RNA- or DNA-based (DNA comes from integrated provirus) with purification and amplification usually required. These requirements render current NAAT unsuitable for PoC use, though emerging novel nucleic acid amplification technologies and miniaturization may bring this goal closer (reviewed in [42,43]).
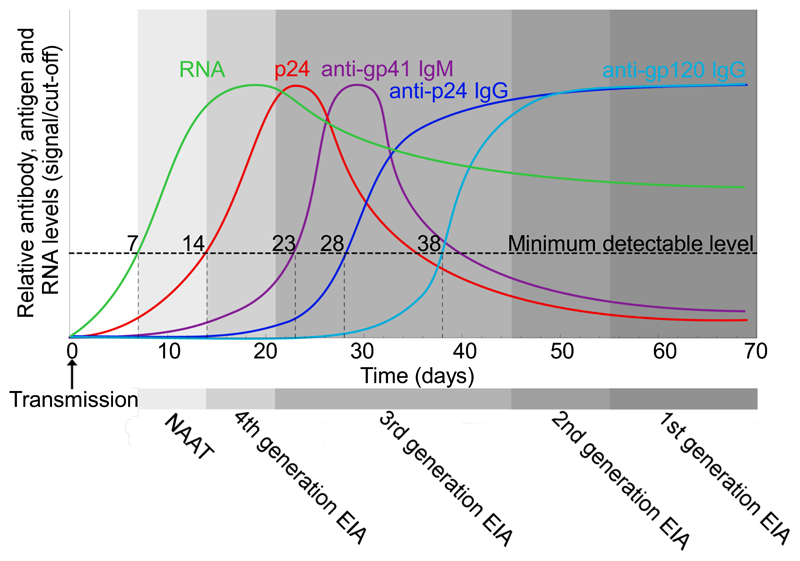
Current guidelines recommend fourth generation antibody-antigen assays (which detect p24 and the antibody response to the virus) as the preferred method of screening for HIV [44] since they have the “advantage of reducing the time between infection and testing HIV positive to [less than] one month which is one to two weeks earlier than with sensitive third generation (antibody-only detection) assays” [40,45], as illustrated schematically in Figure 1. Fourth-generation HIV antibody/antigen tests are among the list of in vitro diagnostics that the WHO considers essential for both primary healthcare and higher-level reference laboratories [46]. Several studies have investigated differences in time to first positive result for different p24-antigen assay methods including reference tests (usually serology or NAAT). These studies are summarized in Supplementary Table 1. Although Meier et al. found evidence for a second diagnostic window, a period when there is insufficient uncomplexed p24 yet too little antibody for detection, such a phenomenon has not been observed in more recent systems that can detect IgM [35,47–50].
Figure 1.
Kinetics of HIV markers during acute infection to seroconversion and time-frames of detection by generations of tests. Figure and information adapted from [31,51–57]. Refer to [58] for further information about disease staging. NAAT: nucleic acid amplification test; EIA: enzyme immunoassay.
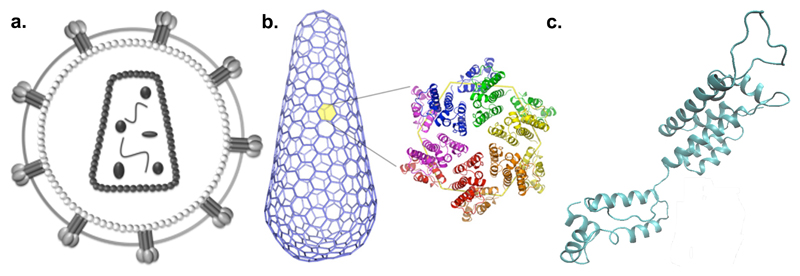
Although antibody tests have dominated the rapid PoC diagnostics market, p24 has long been recognized as an alternative virological biomarker, especially in early attempts to close the window period of detection of HIV, and for unequivocal EID [59,60]. Polymerised capsid protein forms a protective shell around the viral RNA and its structure has been elucidated (Figure 2) [61]. It is a ~24-25 kDa protein encoded by the gag gene [62], present at high copy number in HIV-1 virions; like RNA, it can be detected before seroconversion.
Figure 2.
Schematic of HIV and p24 structures. (a) Entire virion, (b) capsid fullerene cone superstructure made of hexamers and 12 pentamers, and (c) monomer unit. Sources: [63–65].
Beyond the standard, currently available options, there is an array of biomarkers that could potentially be used as surrogate markers for HIV diagnosis in the future, for example, micro-RNA, mRNA, and novel protein targets such as cytokines and other immune markers [66–70]. To date we are not aware of any approaches that have progressed beyond early stage proof of concept to commercial products, or proven themselves suitable for PoC settings.
The promise of early detection at point of care and self-testing
Acute HIV infection is defined as the period when HIV is present, but an antibody response has not yet been mounted; recent infection covers the time when the immune response is immature, and only highly sensitive diagnostic assays can detect the response [71,72]. Early detection therefore covers both acute and early infection, and up until a stable viral set point and immunological response is reached. In neonates or those up to 18 months old born to HIV-infected mothers, this period will last up until maternal antibodies have cleared [73,74]. In adults, early infection is generally between 2-6 months long, and fourth-generation assays that can detect either antigen or antibody are recommended [27,31,40,58]. During Fiebig stage I, the earliest stage of acute infection, HIV RNA is the sole viral biomarker detectable, and therefore only NAAT can be used for virological detection [58]. Though NAAT have long been used in resource-rich settings to measure viral load during patient monitoring, they are generally not approved for use as qualitative diagnostic tools in most countries [40,75]. NAAT suitable for detection of HIV in acute infection are existent and reviewed in [43,76–79]; as such, NAAT will not be considered further here.
Key potential advantages of p24 antigen-based testing in specific populations are listed in Table 1, including early detection for specific target groups and EID.
Table 1. Summary of potential benefits of p24 testing across different population groups.
| Target population or application | Current needs | Limitations of current antibody tests | Potential benefits of p24 antigen tests |
|---|---|---|---|
| Infants under 18 months old | ~1.5 million births to women with HIV [80]. Half of infected neonates die before 2 if untreated [81] | Confounding maternal antibodies up to ~18 months of age [82,83]; Early initiation of ART leads to undetectable antibody response [84] | Accurate EID, targeted care and treatment from birth [85,86] |
| Adults with acute infection (pre-seroconversion) | Increased infectiousness during acute infection [87–89] | Diagnostic window where HIV undetectable; Early initiation of ART can lead to seroreversion or undetectable antibody response [31,90–92] | Earlier detection prior to seroconversion [40,89] |
| Adults in high-risk groups (including those on pre-exposure prophylaxis) | Recommendation of yearly testing may be insufficient for high-risk practices, and is not followed [93–95] | Diagnostic window where HIV undetectable [31]; A significant minority of attendees for testing in clinics with a high proportion of high-risk groups are acutely infected [96] | Earlier detection prior to seroconversion, no confusion over concept of diagnostic window [93] |
| Adult self-testers | Recent legalization of self-testing, full effects on epidemiology unknown [94,97,98] | Approved commercial self-tests rely on antibody detection (post-seroconversion) [99] | Earlier detection prior to seroconversion, no confusion over concept of diagnostic window [93] |
| Adults and children treated during acute/early infection | Accurate identification of HIV-positivity using antibody tests, uncertainty over true status if confirmatory assays are antibody-based tests | Early initiation of antiretroviral therapy can lead to seroreversion, or failure to develop positive serological response [90,100] | Earlier detection without need for seroconversion [40,89] |
| Adults and children in HIV vaccine trials | Discrimination between host response to true infection and vaccine-induced sero-reactivity; high social impact of false-positive HIV status [101] | Antibody-only tests are unable to differentiate between vaccine-induced and virally-induced antibodies | Detection of virological components will unequivocally confirm infection. |
EID and those in acute stages of infection have long been identified as key demographics who would benefit enormously from virological tests suitable for PoC use in resource-limited settings. The confounding effect of maternal antibodies means that a positive result from an antibody-based test administered to neonates cannot be accurately interpreted, and these antibodies can persist until 12-18 months [74]. If no virological-based alternative is available at an earlier stage, the child must be recalled for testing after weaning and many do not return. Without treatment, around half of infected children will die by 2 years old [102]. Current options include testing via dry blood spots for RNA, DNA or p24 antigen; here, sensitivity is limited due to small sample volume, cross-reactions due to release of intracellular contents and high cross-contamination rates [103–106]. Sending samples away for testing in centralized laboratories additionally entails a longer turnaround time with the potential for samples or results to get lost, and extra effort and cost required to transmit results and recall patients for repeat testing or to begin therapy [12].
In the last few years, two molecular technologies described as PoC have progressed through WHO pre-qualification stages (the Alere Q HIV-1/2 Detect and the Cepheid Xpert HIV-1 Qual) and have been tested in a limited number of field trials which reflect routine clinical workflows to varying degrees [107–110]. However, while game-changers in terms of turn-around time and with fewer infants lost to follow-up and a higher proportion begun on anti-retroviral medication [14,109], these technologies are intended for ‘trained health or laboratory professionals’ and come at a cost that remains prohibitive for scale-up [11].
For adults, low-cost and PoC p24 testing would be transformative in high-risk populations to detect those acutely infected, before antibody-based tests can be used, and to initiate treatment as early as possible. Those acutely infected are key drivers of the epidemic, with the highest rates of transmission during this time [88]. Adult HIV incidence has remained stubbornly high, in a large part due to failure to routinely test for and detect acute infection at scale [89]. As the number of people taking pre-exposure prophylaxis steadily rises, the need to test for acute infections also increases to reduce the risk of drug-resistant strains emerging during monotherapy [111,112], and self-testing with rapid PoC tests alongside pre-exposure prophylaxis for high-risk groups are being discussed [113,114].
The maximum benefit of p24-based testing will only be realized when the diagnostic is available in rapid, PoC format compatible with the ASSURED criteria and suitable for use in resource-limited settings and self-testing [115]. The key challenge of detection by rapid PoC tests is the clinical range of p24 in the blood, which spans at least four orders of magnitude from under 0.1 to 103 pg/mL; though levels above 10pg/mL are only briefly reached during acute infection [116–134]. In particular, differences in population groups that present for testing mean that rapid antibody tests are less sensitive in high-income settings, as a higher proportion of infections in those presenting for testing are at the acute stage [135]. The ratio of detected infections to true infections is therefore lower.
Sensitivity and specificity of commercial p24 assays
As p24 detection is challenging, while there are a number of laboratory-based automated systems on the market, currently only one PoC assay in rapid-test format exists (Supplementary Table 2). The laboratory tests are complex, automated equipment-intensive ELISA-type assays. The Alere Determine HIV-1/2 Ag/Ab until recently was the sole “4th generation” lateral flow PoC assay and has now been replaced by the Alere HIV Combo. All the current laboratory-based assays listed show sensitivity and specificity levels approaching 100%. The performance of the Alere Determine HIV-1/2 Ag/Ab has been much more variable depending on the trial and population groups tested (Supplementary Tables 2 and 3). The range of sensitivities of 0-99.8% obscures the mostly low results from the antigen-detection portion of the test in acute infection, with sensitivity at 0% in 6/11 primary studies, and under 52% in 10/11 of these. Specificity is insufficiently high for this test to be useful as a screening test with too many false positives generated, particularly in low-prevalence settings (Supplementary Tables 2 and 3, [136–147], summarized in [148]). Follow-up studies of the Alere HIV Combo in the literature are currently insufficient in number to assess whether performance has significantly improved [149,150].
Lessons learnt
In addition to low viral loads, research has suggested that failure to detect p24 may be associated with:
Insufficient immune complex disruption (ICD). Sequestering of p24 by host anti-p24 antibody may lower the sensitivity of assays by several orders of magnitude. ICD dissociates host antibodies, allowing the assay antibodies to bind p24, and sensitivity levels may be considerably enhanced. Dissociation is conventionally via heat or acid-based techniques. Publications on these methods peaked in the early 1990s, later petering out as efforts to mainstream and consolidate advances in nucleic acid testing gained traction. A summary of methods from studies using ICD is presented in Supplementary Table 4.
Early infant diagnosis. Studies have reported widely variable sensitivities of p24 antigen tests for EID (summarized in [151]). For example, Quinn et al. found good sensitivity for infants older than 1 month, but all nine specimens under 1 month were negative [152]; Lewis et al. [153] found very poor performance in all infants under 3 months; Parpia et al. found very high sensitivity and specificity in infants despite using a test with a limit of detection of 20 pg/mL [154]. In part this variability may relate to lack of stratification between infants infected in utero, during birth or breast-feeding, which significantly alter the timing of the window period. False negatives may also result if neonates received anti-retrovirals as part of ‘prevention of mother to child transmission’ programs [155]. As a neonate born to an infected mother will have high levels of anti-HIV antibodies, use of ICD techniques have been highlighted [156,157]. Supplementary Table 3 contains details of studies for which ICD was used or compared, including for EID.
-
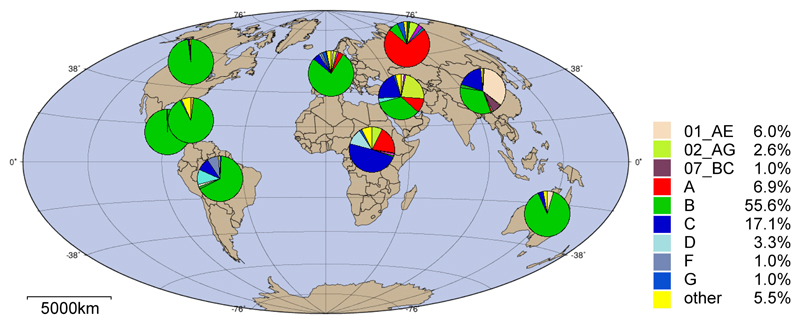
Viral subtypes (Figure 3). In assessments of non-B subtypes, Spacek et al. found poor and variable performance especially for RNA loads below 400 and above 500,000 copies/mL in Uganda [131]. Variable sensitivity depending on genotype has been found using combined assays in a French study that concluded: “many HIV Ag/Ab assays could fail to detect HIV primary infection due to HIV-1 non-B, non-M and HIV-2 strains” [158]. Beerlaert et al. found p24 assays could not detect HIV-2, and some failed to detect outlier subtypes (one group O, one subtype F and 2 subtype H out of 50 tested) [145]. However, others found good performance with multiple subtypes. For example, Pascual et al. found performance of a modified ELISA to be good compared to NAAT (the Roche Monitor RNA) including for subtypes A to F [125]. Ribas et al. demonstrated the ability of a modified ELISA to detect various subtypes and recombinant forms of p24 [130]. Subtype diversity panels (e.g. https://eqapol.dhvi.duke.edu/viral-diversity) enable researchers to ensure that their reagents are validated against a wide range of subtypes found worldwide (for example, [65]).
HIV-2 is relatively uncommon outside West Africa (~1 to 2 million infected [159]). Suppliers of antibodies often claim cross-detection, though not all demonstrate sufficient cross-reactivity to be useful, as shown by a report which found no activity for many AIDS Reagent Program antibodies against HIV-2 in a laboratory ELISA (http://www.aidsreagent.org/) [160].
Low prevalence settings. In low prevalence settings, false positives necessitate uncertainty and further testing. Currently, third generation tests have lower false-positive rates compared to fourth generation tests, suggesting that laboratory-based screening should instead be used for those at high risk of infection [137]. Tamhane et al. suggested adjusting positive readout thresholds in order to optimize a modified ELISA for a given prevalence (using receiver-operator curves) but this approach would be difficult to implement in simple PoC tests [161].
Use in resource-poor settings. A potential use of antigen detection tests is their easy adaptation for resource-poor settings compared to NAAT. A number of studies in low-income countries or non-clinical, community settings found very poor performance of the Alere Determine rapid test, inconsistent with manufacturer evaluations, but largely consistent with each other. For example, studies led by Conway, Duong, Jones, Rosenberg, Chetty, and Taegtmeyer all reported that the antigen-detection portion of the test failed to detect any cases of acute infection and Kilembe, Faraoni and Brauer found limited detection levels at 1/34, 3/17 and 3/30 respectively [137–144,146] (Supplementary Table 2). In one study the specificity was sufficiently poor at 86.1% for the test to be inappropriate as a screening tool, but in another study the antigen portion successfully highlighted 32/39 antigen-positive cases of acute infection [136,145]. Bulterys et al. further found limited sensitivity of a modified ELISA for p24 in African children compared to reports in developed countries, though this study could also be affected by patient age and HIV subtype (see point 2) [162].
Choice of antibodies. Lack of adequate antibodies may in part explain poor performance observed in earlier studies, particularly efficacy of monoclonal antibodies for binding certain subtypes [163], and variable binding affinity [65,164,165]. These issues can be potentially be overcome by careful selection from wide screening of antibodies, or potentially using multiple antibodies concurrently [160,166].
Stability of test components and target. For both research studies and clinics, samples may be stored and processed in batches. For studies in particular, analysis can occur years after collection. Storage at 4°C even for brief periods permits immune complexes to form, though freezing does not [167]. Cold-chain transportation is excluded from the criteria for ASSURED rapid tests though required for reagents of many laboratory-based assays [4,115]. Even for those that do not require refrigeration, conditions during transport or storage in resource-limited settings regularly exceed guidance on maxima for temperature (usually 30°C), sometimes by 15 degrees or more, and humidity (65%), leading to invalid results in some studies [168,169] but not others [170].
Figure 3.
Prevalence of HIV subtypes worldwide. Source: Los Alamos HIV Sequence Database (http://www.hiv.lanl.gov/). Proportions are calculated from total available sequence data available from each region and may reflect localized biases in sampling.
Emerging and ultrasensitive approaches
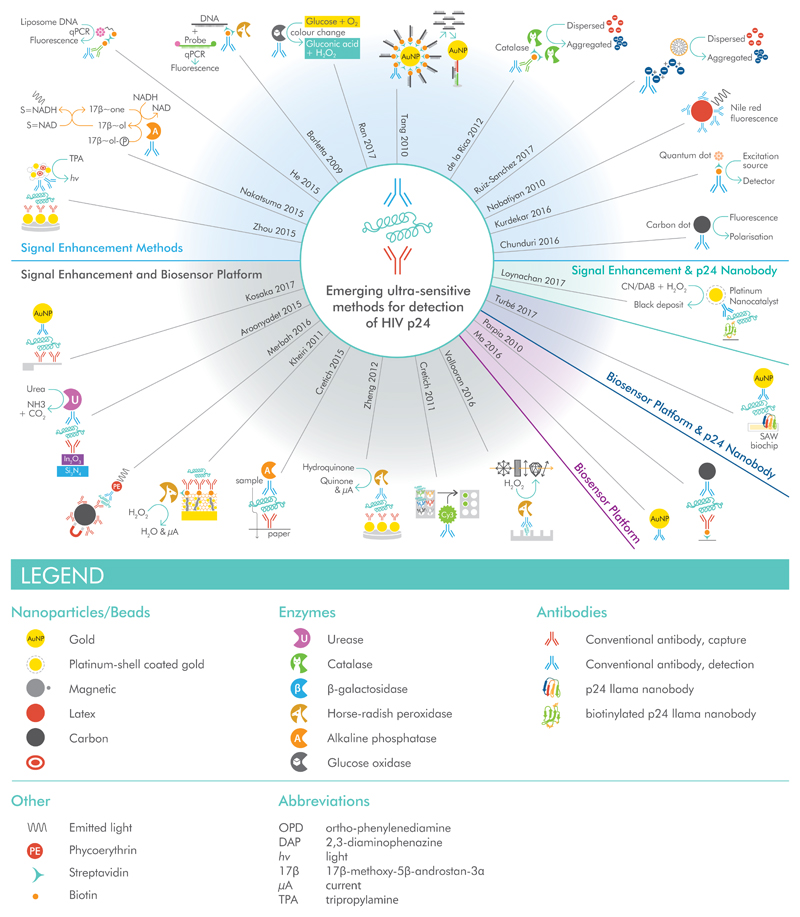
The limited sensitivity of most p24-detection assays has led to the widely-held belief that p24 tests “are relatively insensitive and therefore have a limited utility in clinical practice” [171] or that the practical limit of detection for p24 is of the order of 3 to 4 pg/mL [172]. Emerging, proof of concept assays from research laboratories have exceeded this by several orders of magnitude, but have not progressed through the product development pathway (see [79] for current or imminently launching products). Figure 4 illustrates methods for achieving sensitive detection of p24 for which quantitative limits have been reported, with details in Supplementary Table 5. A number of these tests approach single molecule limits of detection; sensitivity is therefore limited primarily by the volume of sample analyzed. Further development of these technologies into PoC format would greatly facilitate translation into the clinic.
Figure 4.
Schematics of the biosensors in Supplementary Table 5, grouped according to whether novelty is in the structural component of the biosensor, the signal enhancement mechanism, or another/multiple components. For further details and references, refer to Supplementary Table 5.
Cost-effectiveness of using p24 assays for HIV detection
Health-economic analyses of using p24 assays as screening tests for HIV include [173–179] (Table 2). Models are dependent on a wide range of variables, such as prevalence, source population (e.g. use in blood banks, use in sexual health clinics, use in accident and emergency), gross domestic product per capita, and the current cost, sensitivity and specificity of the p24 antigen test under consideration (including false-positive rate) meaning that a model may only be applicable to one country or type of clinic. Antigen tests considered were laboratory-based fourth generation antigen/antibody immunoassays, not PoC tests. Prevalence strongly affects measures of cost-effectiveness; in blood banks this depends on donor population, for example, unpaid donor vs paid donor vs familial donors [178]. Other factors that affect cost-effectiveness include rates of linkage and retention in care, as well as improvements in partner testing [6,180]. As cheaper p24 antigen tests become available, particularly those in self-test format, parameters for these studies will shift considerably requiring cost-benefit recalculation for each target population. Currently no target product profile exists that could guide development towards key goals such as sensitivity, cost or simplicity of use.
Table 2.
Cost-effectiveness studies comparing p24 antigen testing to a baseline. The assessment is made in comparison to third-generation antibody tests, to the status quo, or to no testing. US, United States; EIA, enzyme immunoassay; IDU, injecting drug user; MSM, men who have sex with men. *Cost-effectiveness was validated solely for MSM, not IDU.
| Conclusion | Reference | Study location | Study population | Reference scenario |
|---|---|---|---|---|
| p24 antigen tests are cost-effective | [174] | US | 3,030,303 outpatients | No testing |
| [173] | US | 1,500,000 people eligible for screening: 13-64yrs, no risk criteria | 3rd generation EIA | |
| [175] | US | 10,000 MSM and 10,000 IDU; testing biannually/quarterly* | Testing annually | |
| [176] | US | Entire population aged 15-64 (>186,000,000) or sub-groups | Status quo for testing frequency and method [181] | |
| p24 antigen tests are not cost-effective | [177] | US | 16,000,000 donor units | 3rd generation antibody testing |
| [178] | Ghana | Blood bank donors to 193 transfused patients | 3rd generation antibody testing and no testing considered | |
| [179] | US | 2,744 from San Diego early test program | 3rd generation antibody testing |
Monitoring of HIV infection using p24?
To date, most applications of p24 assays have focused on diagnosis during acute infection and EID. However, other utilities have been investigated such as prognosis and treatment monitoring have been previously proposed. Established (correlated, but independent) predictors of disease progression and treatment failure are CD4 count and HIV viral load [182]. Regular monitoring of patients on antiretroviral therapy is critical to prevent drug-resistant strains arising through treatment failure or poor adherence, and viral load assays are universally recommended for this purpose [183,184]. Though p24 detection for diagnosis of HIV is standard, it is not clear that p24 quantification can provide clinically meaningful data on treatment monitoring. According to a review of literature between 1997 and 2010 [66], relatively few studies investigate the relationship between p24 levels and progression of disease; just two were identified [121,124] and stated: “In both studies higher [p24] levels were associated with an increased risk of progression.” While these might suggest that using p24 levels may add value, this could only be used in situations where optimal monitoring methods (i.e. quantitative NAAT) were not routinely available, and in this era of the recommendation to ‘treat all’ it is unclear that predominantly untreated patients, as found in these studies, would be routinely encountered.
Several additional studies of correlation of p24 levels to markers of disease progression (CD4+ lymphocyte counts, RNA and quantitative viral load measurements) or health outcomes were identified for this review; the main findings from these studies are detailed in Supplementary Table 6. In general, the correlation of p24 levels with RNA viral load is not strong and varies between settings for several reasons; Erythrocytes clear immune complexes [128] and one study found limited correlation between the amount of erythrocyte-associated p24 antigen and p24 antigen in plasma [185].
On balance, p24 could in the future be used to monitor for early infection in patients belonging to high-risk groups and using pre-exposure prophylaxis, but the evidence for monitoring therapy using p24 is not as unequivocal as suggested by early enthusiasts [186,187]. As those involved in HIV care increasingly begin to look towards HIV cure, assays for p24 could yet come into focus again, especially if adequately sensitive tests can be made sufficiently simple to be used for self-testing.
Conclusion and Outlook
The future of HIV diagnostics and monitoring will include PoC testing of an increasing number and variety of target population subsets (outlined in Table 1), each with specific and diverse requirements. Though testing of large numbers of vaccine trial recipients is currently in the pipeline, the need is immediate and ongoing for EID when mothers are known to be HIV positive, and home testing on a regular basis for adults at an elevated risk of becoming infected to catch those in the window period. With the rollout of pre-exposure prophylaxis in many countries, diagnostic tests that would facilitate cheap and routine testing for those in the window period, potentially by self-testing, will become highly desirable. In multiple scenarios, PoC testing for p24 antigen offers an attractive alternative to nucleic acid detection for diagnostics that are targeted at the pathogen, rather than the host response, and a simple p24 test for the presence of the virus could be transformative. In places where the facilities and technology available do not permit adherence to recommended methods for monitoring treatment, a simplified quantitative or semi-quantitative p24 test to detect resurgence of virus could permit investigations into treatment compliance or the need to switch from a failing therapy regimen.
These advantages will only be realized when PoC tests are sufficiently sensitive so as to enable detection significantly before antibody tests, and as easy to use as current PoC rapid tests. Ideally, the test would detect p24 at fg/mL (10-15 g/mL) to ng/mL (10-9 g/mL) levels, with a minimum detection limit of the order of 10-10 g/mL. Many approaches currently at the research stage of development from the last decade can detect p24 at these levels. However, many of these are early stage experimental studies and have not yet made a successful transition out of the research laboratory, and through clinical trials. The challenge to develop these into simple low-cost diagnostics in the field is substantial.
Many of the studies which assessed the properties of p24 antigen during infection, particularly the correlation of p24 antigen with other biomarkers, were conducted over a decade ago. More investment into fundamental clinical research of this kind, as well as for translating cutting-edge technology for p24 detection out of the laboratory and into the field. Further clinical studies with the most up-to-date technology are merited, in addition to updated health-economic analyses for fourth generation rapid tests in different population groups.
The requirements of HIV detection technologies for diagnosis could shortly be radically altered by the introduction of cure therapies and vaccination programmes. As the next generation of technologies comes through, with many intended for use in resource-limited settings, HIV p24 could yet see a fresh role as a virological target.
Methods
This is a scoping review, though a systematic search was initially used to identify articles of interest. PubMed was searched for papers from the outset of the HIV epidemic to January 2018 using the following keywords: (HIV OR AIDS OR autoimmune diseases syndrome OR human immunodeficiency virus) AND (p24) AND (test OR diagnostic). Identified studies were further screened. Studies were excluded if they met any of the following criteria:
Not in English
Not about HIV
Not about use of p24 as a clinical and/or diagnostic marker
Reports on chemical modification or inhibition of p24 (drug-type studies)
Purely immunological studies
Unable to obtain full-text
Conference abstracts alone
574 were scrutinized further for potential inclusion in the review, and associated citations pursued if not identified in the original search. In addition, WHO and UNAIDS reports were sourced as appropriate.
Supplementary Material
Supplemental Digital Content 1. .docx file. Contains:
Table 1 summarising the timing of detection of different assays to detect HIV in comparison to reference and p24 assays
Table 2 summarising studies of commercial assays for p24 antigen detection.
Table 3 with details of trials of p24 antigen detection with reported sensitivity and specificity from selected studies.
Table 4 of immune complex disruption methods.
Table 5 of emerging and ultrasensitive approaches to p24 antigen detection.
Table 6 summarising studies investigating correlation between p24 levels and other biomarkers.
Acknowledgements
RB wrote the original draft, with input from EG. EG then extensively rewrote the manuscript, with input from RB, RM, MS, RP and OV. All authors had the opportunity to review the manuscript before submission. We thank Mark Yeager and the Scripps Institute for permission to use the image of capsid in Figure 2. Funding: EPSRC i-sense Early Warning Sensing Systems in Infectious Disease (EP/K031953/1) (all authors); MRC Global Challenges Research Fund (m-Africa) MR/P024378/1 (RM, MS); Royal Society Wolfson Merit Award (RM).
Funding:
EPSRC i-sense Early Warning Sensing Systems in Infectious Disease (EP/K031953/1)
MRC Global Challenges Research Fund (m-Africa) MR/P024378/1
Royal Society Wolfson Merit Award
Footnotes
ORCID:
References
- 1.UN General Assembly. Political Declaration on HIV and AIDS: On the Fast-Track to Accelerate the Fight against HIV and to End the AIDS Epidemic by 2030. 2016 [Google Scholar]
- 2.UNAIDS. Fast Track: Ending the AIDS epidemic by 2030. 2014 [Google Scholar]
- 3.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2014 [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on HIV testing services. 2015 [Google Scholar]
- 5.Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health. 2016;1:e000010–11. doi: 10.1136/bmjgh-2015-000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah M, Risher K, Berry SA, Dowdy DW. The Epidemiologic and Economic Impact of Improving HIV Testing, Linkage, and Retention in Care in the United States. Clinical Infectious Diseases. 2015;62:220–229. doi: 10.1093/cid/civ801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. GLOBAL AIDS UP DATE 2016. 2016:1–16. [Google Scholar]
- 8.Mehta SR, Murrell B, Anderson CM, Kosakovsky Pond SL, Wertheim JO, Young JA, et al. Using HIV Sequence and Epidemiologic Data to Assess the Effect of Self-referral Testing for Acute HIV Infection on Incident Diagnoses in San Diego, California. Clinical Infectious Diseases. 2016;63:101–107. doi: 10.1093/cid/ciw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien M, Markowitz M. Should We Treat Acute HIV Infection? Curr HIV/AIDS Rep. 2012;9:101–110. doi: 10.1007/s11904-012-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 11.Jean-Philippe P, Spiegel H, Gnanashanmugam D, Fitzgibbon J, D'Souza P, Crawford KW, et al. HIV birth testing and linkage to care for HIV-infected infants. AIDS. 2017;31:1797–1807. doi: 10.1097/QAD.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 12.Sibanda EL, Weller IVD, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care. AIDS. 2013;27:2787–2797. doi: 10.1097/QAD.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dube Q, Dow A, Chirambo C, Lebov J, Tenthani L, Moore M, et al. Implementing early infant diagnosis of HIV infection at the primary care level: experiences and challenges in Malawi. Bull World Health Org. 2012;90:699–704. doi: 10.2471/BLT.11.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Technau K-G, Kuhn L, Coovadia A, Murnane PM, Sherman G. Xpert HIV-1 point-of-care test for neonatal diagnosis of HIV in the birth testing programme of a maternity hospital: a field evaluation study. The Lancet HIV. 2017;4:e442–e448. doi: 10.1016/S2352-3018(17)30097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majors CE, Smith CA, Natoli ME, Kundrod KA, Richards-Kortum R. Point-of-care diagnostics to improve maternal and neonatal health in low-resource settings. Lab on a Chip. 2017;17:3351–3387. doi: 10.1039/c7lc00374a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pai NP, Pai M. Point-of-care diagnostics for HIV and tuberculosis: landscape, pipeline, and unmet needs. Discov Med. 2012;13:35–45. [PubMed] [Google Scholar]
- 17.Haleyur Giri Setty MK, Hewlett IK. Point of Care Technologies for HIV. AIDS Research and Treatment. 2014;2014:1–20. doi: 10.1155/2014/497046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornett JK, Kirn TJ. Laboratory Diagnosis of HIV in Adults: A Review of Current Methods. Clinical Infectious Diseases. 2013;57:712–718. doi: 10.1093/cid/cit281. [DOI] [PubMed] [Google Scholar]
- 19.Drain PK, Rousseau C. Point-of-care diagnostics: extending the laboratory network to reach the last mile. Current Opinion in HIV and AIDS. 2017;12:175–181. doi: 10.1097/COH.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Council on Scientific Affairs. Status report on the acquired immunodeficiency syndrome. Human T-cell lymphotropic virus type III testing. JAMA. 1985;254:1342–1345. [PubMed] [Google Scholar]
- 21.Hedenskog M, Dewhurst S, Ludvigsen C, Sinangil F, Rodriguez L, Wu YT, et al. Testing for antibodies to AIDS-associated retrovirus (HTLV-III/LAV) by indirect fixed cell immunofluorescence: specificity, sensitivity, and applications. Journal of medical virology. 1986;19:325–334. doi: 10.1002/jmv.1890190405. [DOI] [PubMed] [Google Scholar]
- 22.Spielberg F, Kabeya CM, Ryder RW, Kifuani NK, Harris J, Bender TR, et al. Field testing and comparative evaluation of rapid, visually read screening assays for antibody to human immunodeficiency virus. The Lancet. 1989;1:580–584. doi: 10.1016/s0140-6736(89)91610-3. [DOI] [PubMed] [Google Scholar]
- 23.Starkey CA, Yen-Lieberman B, Proffitt MR. Evaluation of the Recombigen HIV-1 Latex Agglutination Test. Journal of Clinical Microbiology. 1990;28:819–822. doi: 10.1128/jcm.28.4.819-822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josefsen D, Myrmel H. Evaluation of a rapid test for detection of antibodies against human immunodeficiency virus (HIV) APMIS. 1989;97:95–96. doi: 10.1111/j.1699-0463.1989.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Constantine NT, Zhang X, Smialek JE. Determination of human immunodeficiency virus antibody status in forensic autopsy cases using a rapid and simple FDA-licensed assay. J Forensic Sci. 1993;38:798–805. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (U.S.) Morbidity and Mortality Weekly Report. 2002:1051–1052. [Google Scholar]
- 27.Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory testing for the diagnosis of HIV infection: Updated recommendations. Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 28.Nath N, Wunderlich C, Darr FW, Douglas DK, Dodd RY. Immunoglobulin level in donor blood reactive for antibodies to human immunodeficiency virus. Journal of Clinical Microbiology. 1987;25:364–369. doi: 10.1128/jcm.25.2.364-369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato PA, Maskill WJ, Tamashiro H, Heymann DL. Strategies for laboratory HIV testing: An examination of alternative approaches not requiring Western blot. Bull World Health Org. 1994;72:129–134. [PMC free article] [PubMed] [Google Scholar]
- 30.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, et al. Diagnostic point-of-care tests in resource-limited settings. The Lancet Infectious Diseases. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. The American Journal of Medicine. 1997;102:117–24. doi: 10.1016/s0002-9343(97)00077-6. discussion 125–6. [DOI] [PubMed] [Google Scholar]
- 32.Read JS, the Committee on Pediatric AIDS Diagnosis of HIV-1 Infection in Children Younger Than 18 Months in the United States. PEDIATRICS. 2007;120:e1547–e1562. doi: 10.1542/peds.2007-2951. [DOI] [PubMed] [Google Scholar]
- 33.Jindai K, Kunzer B, Van TT, Striker R. Human immunodeficiency virus testing pitfalls and clinical suspicion. Am J Emerg Med. 2014;32:1442.e1–2. doi: 10.1016/j.ajem.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Brown P, Merline JR, Levine D, Minces LR. Repeatedly false-negative rapid HIV test results in a patient with undiagnosed advanced AIDS. Ann Intern Med. 2008;149:71–72. doi: 10.7326/0003-4819-149-1-200807010-00028. [DOI] [PubMed] [Google Scholar]
- 35.Chin BS, Lee SH, Kim GJ, Kee MK, Suh SD, Kim SS. Early Identification of Seronegative Human Immunodeficiency Virus Type 1 Infection with Severe Presentation. Journal of Clinical Microbiology. 2007;45:1659–1662. doi: 10.1128/JCM.00166-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathews AA, Mambatta AK, Appalaraju B, Menon S. Implications of p24 antigen in HIV testing. Indian J Med Microbiol. 2016;34:119. doi: 10.4103/0255-0857.167681. [DOI] [PubMed] [Google Scholar]
- 37.Lang R, Charlton C, Beckthold B, Kadivar K, Lavoie S, Caswell D, et al. HIV misdiagnosis: A root cause analysis leading to improvements in HIV diagnosis and patient care. J Clin Virol. 2017;96:84–88. doi: 10.1016/j.jcv.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Waters L, Ahmed N, Angus B, Boffito M, Bower M, British HIV Association BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update) 2016 doi: 10.1111/hiv.12426. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Department of HIV/AIDS. Progress Report 2016: PREVENT HIV, TEST AND TREAT ALL. Geneva: 2016. [Google Scholar]
- 40.Public Health England. HIV screening and confirmation. 11 ed. 2017.
- 41.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2018.
- 42.Shafiee H, Wang S, Inci F, Toy M, Henrich TJ, Kuritzkes DR, et al. Emerging Technologies for Point-of-Care Management of HIV Infection. Annu Rev Med. 2015;66:387–405. doi: 10.1146/annurev-med-092112-143017. [DOI] [PubMed] [Google Scholar]
- 43.Mauk M, Song J, Bau HH, Gross R, Bushman FD, Collman RG, et al. Miniaturized devices for point of care molecular detection of HIV. Lab on a Chip. 2017;17:382–394. doi: 10.1039/c6lc01239f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander TS. Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution. Clinical and Vaccine Immunology. 2016;23:249–253. doi: 10.1128/CVI.00053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.British HIV Association, British Association of Sexual Health and HIV, British Infection Society. UK National Guidelines for HIV Testing 2008. 2008.
- 46.WHO. World Health Organization Model List of Essential In Vitro Diagnostics. 1st ed. Geneva: 2018. [Google Scholar]
- 47.Meier T, Knoll E, Henkes M, Enders G, Braun R. Evidence for a diagnostic window in fourth generation assays for HIV. Journal of Clinical Virology. 2001;23:113–116. doi: 10.1016/s1386-6532(01)00183-4. [DOI] [PubMed] [Google Scholar]
- 48.Speers D, Phillips P, Dyer J. Combination Assay Detecting both Human Immunodeficiency Virus (HIV) p24 Antigen and Anti-HIV Antibodies Opens a Second Diagnostic Window. Journal of Clinical Microbiology. 2005;43:5397–5399. doi: 10.1128/JCM.43.10.5397-5399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George CRR, Robertson PW, Lusk MJ, Whybin R, Rawlinson W. Prolonged Second Diagnostic Window for Human Immunodeficiency Virus Type 1 in a Fourth-Generation Immunoassay: Are Alternative Testing Strategies Required? Journal of Clinical Microbiology. 2014;52:4105–4108. doi: 10.1128/JCM.01573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallefuoco L, Mazzarella C, Portella G. Fourth generation assays for HIV testing. Expert Review of Molecular Diagnostics. 2016;16:723–732. doi: 10.1080/14737159.2016.1179115. [DOI] [PubMed] [Google Scholar]
- 51.Liao H-X, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Current Opinion in HIV and AIDS. 2009;4:373–379. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, et al. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. Journal of Virology. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yates NL, Stacey AR, Nolen TL, Vandergrift NA, Moody MA, Montefiori DC, et al. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunology. 2013;6:692–703. doi: 10.1038/mi.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allain JP, Laurian Y, Paul DA, Senn D. Serological markers in early stages of human immunodeficiency virus infection in haemophiliacs. The Lancet. 1986;2:1233–1236. doi: 10.1016/s0140-6736(86)92673-5. [DOI] [PubMed] [Google Scholar]
- 57.Delaney KP, Hanson DL, Masciotra S, Ethridge SF, Wesolowski L, Owen SM. Time Until Emergence of HIV Test Reactivity Following Infection With HIV-1: Implications for Interpreting Test Results and Retesting After Exposure. Clinical Infectious Diseases. 2017;64:53–59. doi: 10.1093/cid/ciw666. [DOI] [PubMed] [Google Scholar]
- 58.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 59.George E, Beauharnais CA, Brignoli E, Noel F, Bois G, De Matteis Rouzier P, et al. Potential of a simplified p24 assay for early diagnosis of infant human immunodeficiency virus type 1 infection in Haiti. Journal of Clinical Microbiology. 2007;45:3416–3418. doi: 10.1128/JCM.01314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sydow von M, Gaines H, Sönnerborg A, Forsgren M, Pehrson PO, Strannegård O. Antigen detection in primary HIV infection. Br Med J (Clin Res Ed) 1988;296:238–240. doi: 10.1136/bmj.296.6617.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao G, Perilla JR, Yufenyuy EL, Meng X, Chen B, Ning J, et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature. 2013;497:643–646. doi: 10.1038/nature12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 63.Pornillos O, Ganser-Pornillos BK, Kelly BN, Hua Y, Whitby FG, Stout CD, et al. X-Ray Structures of the Hexameric Building Block of the HIV Capsid. CELL. 2009;137:1282–1292. doi: 10.1016/j.cell.2009.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turbé V, Gray ER, Lawson VE, Nastouli E, Brookes JC, Weiss RA, et al. Towards an ultra-rapid smartphone-connected test for infectious diseases. Sci Rep. 2017;7:11971. doi: 10.1038/s41598-017-11887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray ER, Brookes JC, Caillat C, Turbé V, Webb BLJ, Granger LA, et al. Unravelling the Molecular Basis of High Affinity Nanobodies against HIV p24: In Vitro Functional, Structural, and in Silico Insights. ACS Infect Dis. 2017;3:479–491. doi: 10.1021/acsinfecdis.6b00189. [DOI] [PubMed] [Google Scholar]
- 66.Neaton JD, Neuhaus J, Emery S. Soluble biomarkers and morbidity and mortality among people infected with HIV: summary of published reports from 1997 to 2010. Current Opinion in HIV and AIDS. 2010;5:480–490. doi: 10.1097/COH.0b013e32833ed75d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fowler L, Saksena NK. Micro-RNA: new players in HIV-pathogenesis, diagnosis, prognosis and antiviral therapy. AIDS Rev. 2013;15:3–14. [PubMed] [Google Scholar]
- 68.Zhang ZN, Xu JJ, Fu YJ, Liu J, Jiang YJ, Cui HL, et al. Transcriptomic Analysis of Peripheral Blood Mononuclear Cells in Rapid Progressors in Early HIV Infection Identifies a Signature Closely Correlated with Disease Progression. Clinical Chemistry. 2013;59:1175–1186. doi: 10.1373/clinchem.2012.197335. [DOI] [PubMed] [Google Scholar]
- 69.Leeansyah E, Malone DFG, Anthony DD, Sandberg JK. Soluble biomarkers of HIV transmission, disease progression and comorbidities. Current Opinion in HIV and AIDS. 2013;8:117–124. doi: 10.1097/COH.0b013e32835c7134. [DOI] [PubMed] [Google Scholar]
- 70.Kincaid RP, Sullivan CS. Virus-Encoded microRNAs: An Overview and a Look to the Future. PLoS Pathog. 2012;8:e1003018. doi: 10.1371/journal.ppat.1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg NE, Pilcher CD, Busch MP, Cohen MS. How can we better identify early HIV infections? Current Opinion in HIV and AIDS. 2015;10:61–68. doi: 10.1097/COH.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen MS, Gay CL, Busch MP, Hecht FM. The Detection of Acute HIV Infection. J INFECT DIS. 2010;202:S270–S277. doi: 10.1086/655651. [DOI] [PubMed] [Google Scholar]
- 73.Rakusan TA, Parrott RH, Sever JL. Limitations in the laboratory diagnosis of vertically acquired HIV infection. J Acquir Immune Defic Syndr. 1991;4:116–121. [PubMed] [Google Scholar]
- 74.Chantry CJ, Cooper ER, Pelton SI, Zorilla C, Hillyer GV, Diaz C. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J. 1995;14:382–387. doi: 10.1097/00006454-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Pierce VM, Neide B, Hodinka RL. Evaluation of the Gen-Probe Aptima HIV-1 RNA qualitative assay as an alternative to Western blot analysis for confirmation of HIV infection. Journal of Clinical Microbiology. 2011;49:1642–1645. doi: 10.1128/JCM.02183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmad F, Hashsham SA. Miniaturized nucleic acid amplification systems for rapid and point-of-care diagnostics: A review. Analytica Chimica Acta. 2012;733:1–15. doi: 10.1016/j.aca.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 77.Lifson MA, Ozen MO, Inci F, Wang S, Inan H, Baday M, et al. Advances in biosensing strategies for HIV-1 detection, diagnosis, and therapeutic monitoring. Advanced Drug Delivery Reviews. 2016;103:90–104. doi: 10.1016/j.addr.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Craw P, Balachandran W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip. 2012;12:2469–18. doi: 10.1039/c2lc40100b. [DOI] [PubMed] [Google Scholar]
- 79.Mazzola LT, Péres-Casas C. HIV/AIDS Diagnostics Technology Landscape. 2015 [Google Scholar]
- 80.UNAIDS. The Gap Report 2014-children and pregnant women living with HIV. 2014 [Google Scholar]
- 81.Prendergast AJ, Essajee S, Penazzato M. HIV and the Millennium Development Goals. Archives of Disease in Childhood. 2015;100(Suppl 1):S48–52. doi: 10.1136/archdischild-2013-305548. [DOI] [PubMed] [Google Scholar]
- 82.Kenny J, Mulenga V, Hoskins S, Scholten F, Gibb DM. The needs for HIV treatment and care of children, adolescents, pregnant women and older people in low-income and middle-income countries. AIDS. 2012;26:S105–S116. doi: 10.1097/QAD.0b013e32835bddfc. [DOI] [PubMed] [Google Scholar]
- 83.Palasanthiran P, Robertson P, Ziegler JB, Graham GG. Decay of transplacental human immunodeficiency virus type 1 antibodies in neonates and infants. J INFECT DIS. 1994;170:1593–1596. doi: 10.1093/infdis/170.6.1593. [DOI] [PubMed] [Google Scholar]
- 84.Kuhn L, Schramm DB, Shiau S, Strehlau R, Pinillos F, Technau K, et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV-infected children when suppressed. AIDS. 2015;29:1053–1060. doi: 10.1097/QAD.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Payne H, Mkhize N, Otwombe K, Lewis J, Panchia R, Callard R, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: A retrospective analysis. The Lancet Infectious Diseases. 2015;15:803–809. doi: 10.1016/S1473-3099(15)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pilcher CD, Tien HC, Eron JJ, Vernazza PL, Leu S-Y, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J INFECT DIS. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 88.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J INFECT DIS. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 89.Rutstein SE, Ananworanich J, Fidler S, Johnson C, Sanders EJ, Sued O, et al. Clinical and public health implications of acute and early HIV detection and treatment: a scoping review. Journal of the International AIDS Society. 2017;20:e323–13. doi: 10.7448/IAS.20.1.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hare CB, Pappalardo BL, Busch MP, Karlsson AC, Phelps BH, Alexander SS, et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis. 2006;42:700–708. doi: 10.1086/500215. [DOI] [PubMed] [Google Scholar]
- 91.Killian MS, Norris PJ, Rawal BD, Lebedeva M, Hecht FM, Levy JA, et al. The effects of early antiretroviral therapy and its discontinuation on the HIV-specific antibody response. AIDS Research and Human Retroviruses. 2006;22:640–647. doi: 10.1089/aid.2006.22.640. [DOI] [PubMed] [Google Scholar]
- 92.Stefic K, Novelli S, Mahjoub N, Seng R, Molina J-M, Cheneau C, et al. Nonreactive Human Immunodeficiency Virus Type 1 Rapid Tests After Sustained Viral Suppression Following Antiretroviral Therapy Initiation During Primary Infection. J INFECT DIS. 2018;217:1793–1797. doi: 10.1093/infdis/jiy120. [DOI] [PubMed] [Google Scholar]
- 93.Witzel TC, Rodger AJ, Burns FM, Rhodes T, Weatherburn P. HIV Self-Testing among Men Who Have Sex with Men (MSM) in the UK: A Qualitative Study of Barriers and Facilitators, Intervention Preferences and Perceived Impacts. PloS one. 2016;11:e0162713–13. doi: 10.1371/journal.pone.0162713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katz DA, Cassels SL, Stekler JD. Replacing Clinic-Based Tests With Home-Use Tests May Increase HIV Prevalence Among Seattle Men Who Have Sex With Men. Sexually Transmitted Diseases. 2014;41:2–9. doi: 10.1097/OLQ.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clifton S, Nardone A, Field N, Mercer CH, Tanton C, Macdowall W, et al. HIV testing, risk perception, and behaviour in the British population. AIDS. 2016;30:943–952. doi: 10.1097/QAD.0000000000001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters PJ, Westheimer E, Cohen S, Hightow-Weidman LB, Moss N, Tsoi B, et al. Screening Yield of HIV Antigen/Antibody Combination and Pooled HIV RNA Testing for Acute HIV Infection in a High-Prevalence Population. JAMA. 2016;315:682–690. doi: 10.1001/jama.2016.0286. [DOI] [PubMed] [Google Scholar]
- 97.Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015;12:e1001873–21. doi: 10.1371/journal.pmed.1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sorensen SW, Sansom SL, Brooks JT, Marks G, Begier EM, Buchacz K, et al. A Mathematical Model of Comprehensive Test-and-Treat Services and HIV Incidence among Men Who Have Sex with Men in the United States. PloS one. 2012;7:e29098–9. doi: 10.1371/journal.pone.0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stevens DR, Vrana CJ, Dlin RE, Korte JE. A Global Review of HIV Self-testing: Themes and Implications. AIDS Behav. 2017;18:1–16. doi: 10.1007/s10461-017-1707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Souza MS, Pinyakorn S, Akapirat S, Pattanachaiwit S, Fletcher JLK, Chomchey N, et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clinical Infectious Diseases. 2016;63:555–561. doi: 10.1093/cid/ciw365. [DOI] [PubMed] [Google Scholar]
- 101.Voronin Y, Zinszner H, Karg C, Brooks K, Coombs R, Hural J, et al. HIV vaccine-induced sero-reactivity: A challenge for trial participants, researchers, and physicians. Vaccine. 2015;33:1243–1249. doi: 10.1016/j.vaccine.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newell M-L, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. The Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 103.Fajardo E, Metcalf CA, Chaillet P, Aleixo L, Pannus P, Panunzi I, et al. Prospective Evaluation of Diagnostic Accuracy of Dried Blood Spots from Finger Prick Samples for Determination of HIV-1 Load with the NucliSENS Easy-Q HIV-1 Version 2.0 Assay in Malawi. Journal of Clinical Microbiology. 2014;52:1343–1351. doi: 10.1128/JCM.03519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamers RL, Smit PW, Stevens W, Schuurman R, Rinke de Wit TF. Dried fluid spots for HIV type-1 viral load and resistance genotyping: a systematic review. Antivir Ther (Lond) 2009;14:619–629. [PubMed] [Google Scholar]
- 105.Bertagnolio S, Parkin NT, Jordan M, Brooks J, García-Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing: A review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12:195–208. [PubMed] [Google Scholar]
- 106.Kivuyo SL, Johannessen A, Trøseid M, Kasubi MJ, Gundersen SG, Naman E, et al. p24 antigen detection on dried blood spots is a feasible and reliable test for infant HIV infection in rural Tanzania. Int J STD AIDS. 2011;22:719–721. doi: 10.1258/ijsa.2009.009382. [DOI] [PubMed] [Google Scholar]
- 107.Hsiao N-Y, Dunning L, Kroon M, Myer L. Laboratory Evaluation of the Alere q Point-of-Care System for Early Infant HIV Diagnosis. PloS one. 2016;11:e0152672–11. doi: 10.1371/journal.pone.0152672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murray TY, Sherman GG, Nakwa F, MacLeod WB, Sipambo N, Velaphi S, et al. Field Evaluation of Performance of Alere and Cepheid Qualitative HIV Assays for Pediatric Point-of-Care Testing in an Academic Hospital in Soweto, South Africa. Journal of Clinical Microbiology. 2017;55:3227–3235. doi: 10.1128/JCM.01021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jani IV, Meggi B, Mabunda N, Vubil A, Sitoe NE, Tobaiwa O, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. 2014:e1–4. doi: 10.1097/QAI.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 110.Dunning L, Kroon M, Hsiao N-Y, Myer L. Field evaluation of HIV point-of-care testing for early infant diagnosis in Cape Town, South Africa. PloS one. 2017;12:e0189226–11. doi: 10.1371/journal.pone.0189226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Streeck H, Verheyen J, Storim J, Dittmer U, Jochum C, Timm J, et al. Pre-exposure prophylaxis failure with tenofovir disoproxil. AIDS. 2017;31:176–177. doi: 10.1097/QAD.0000000000001280. [DOI] [PubMed] [Google Scholar]
- 112.Lehman DA, Baeten JM, McCoy CO, Weis JF, Peterson D, Mbara G, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J INFECT DIS. 2015;211:1211–1218. doi: 10.1093/infdis/jiu677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eshleman SH, Piwowar-Manning E, Sivay MV, Debevec B, Veater S, McKinstry L, et al. Performance of the BioPlex 2200 HIV Ag-Ab assay for identifying acute HIV infection. Journal of Clinical Virology. 2018;99–100:67–70. doi: 10.1016/j.jcv.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fransen K, de Baetselier I, Rammutla E, Ahmed K, Owino F, Agingu W, et al. Performance of serological and molecular tests within acute HIV infection. Journal of Clinical Virology. 2017;93:81–84. doi: 10.1016/j.jcv.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Diagnostics for the developing world. Nat Rev Micro. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 116.Böni J, Opravil M, Tomasik Z, Rothen M, Bisset L, Grob PJ, et al. Simple monitoring of antiretroviral therapy with a signal-amplification-boosted HIV-1 p24 antigen assay with heat-denatured plasma. AIDS. 1997;11:F47–52. doi: 10.1097/00002030-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Brown AE, Vahey MT, Zhou SY, Chung RC, Ruiz NM, Hofheinz D, et al. Quantitative relationship of circulating p24 antigen with human immunodeficiency virus (HIV) RNA and specific antibody in HIV-infected subjects receiving antiretroviral therapy. The RV43 Study Group. J INFECT DIS. 1995;172:1091–1095. doi: 10.1093/infdis/172.4.1091. [DOI] [PubMed] [Google Scholar]
- 118.Daar ES, Moudgil T, Meyer RD, Ho DD. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. The New England journal of medicine. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 119.Dennin RH, Dalhoff K. HIV p24 antigen concentration in serum of 11 anti-HIV 1-positive patients before and after immune-complex dissociation: a study of a 5-year period. Clin Diagn Virol. 1995;3:131–137. doi: 10.1016/0928-0197(94)00035-s. [DOI] [PubMed] [Google Scholar]
- 120.Duiculescu DC, Geffin RB, Scott GB, Scott WA. Clinical and immunological correlates of immune-complex-dissociated HIV-1 p24 antigen in HIV-1-infected children. J Acquir Immune Defic Syndr. 1994;7:807–815. [PubMed] [Google Scholar]
- 121.Erikstrup C, Kallestrup P, Zinyama-Gutsire RBL, Gomo E, Lüneborg-Nielsen M, Gerstoft J, et al. p24 as a predictor of mortality in a cohort of HIV-1-infected adults in rural Africa. J Acquir Immune Defic Syndr. 2008;48:345–349. doi: 10.1097/QAI.0b013e31817dc3d1. [DOI] [PubMed] [Google Scholar]
- 122.Fackler OT, Schäfer M, Schmidt W, Zippel T, Heise W, Schneider T, et al. HIV-1 p24 but not proviral load is increased in the intestinal mucosa compared with the peripheral blood in HIV-infected patients. AIDS. 1998;12:139–146. doi: 10.1097/00002030-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 123.Hashida S, Hashinaka K, Nishikata I, Oka S, Shimada K, Saitoh A, et al. Measurement of human immunodeficiency virus type 1 p24 in serum by an ultrasensitive enzyme immunoassay, the two-site immune complex transfer enzyme immunoassay. Journal of Clinical Microbiology. 1995;33:298–303. doi: 10.1128/jcm.33.2.298-303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ledergerber B, Flepp M, Böni J, Tomasik Z, Cone RW, Lüthy R, et al. Human immunodeficiency virus type 1 p24 concentration measured by boosted ELISA of heat-denatured plasma correlates with decline in CD4 cells, progression to AIDS, and survival: comparison with viral RNA measurement. J INFECT DIS. 2000;181:1280–1288. doi: 10.1086/315366. [DOI] [PubMed] [Google Scholar]
- 125.Pascual A, Cachafeiro A, Funk ML, Fiscus SA. Comparison of an Assay Using Signal Amplification of the Heat-Dissociated p24 Antigen with the Roche Monitor Human Immunodeficiency Virus RNA Assay. Journal of Clinical Microbiology. 2002;40:2472–2475. doi: 10.1128/JCM.40.7.2472-2475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paul MO, Toedter G, Hofheinz D, Tetali S, Pelton S, Marecki M, et al. Diagnosis of human immunodeficiency virus type 1 infection in infants by immune complex dissociation p24 assay. Clin Diagn Lab Immunol. 1997;4:75–78. doi: 10.1128/cdli.4.1.75-78.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pokriefka RA, Manzor O, Markowitz NP, Saravolatz LD, Kvale P, Donovan RM. Increased detection of human immunodeficiency virus antigenemia after dissociation of immune complexes at low pH. Journal of Clinical Microbiology. 1993;31:1656–1658. doi: 10.1128/jcm.31.6.1656-1658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prado JG, Shintani A, Bofill M, Clotet B, Ruiz L, Martinez-Picado J. Lack of Longitudinal Intrapatient Correlation between p24 Antigenemia and Levels of Human Immunodeficiency Virus (HIV) Type 1 RNA in Patients with Chronic HIV Infection during Structured Treatment Interruptions. Journal of Clinical Microbiology. 2004;42:1620–1625. doi: 10.1128/JCM.42.4.1620-1625.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Respess RA, Cachafeiro A, Withum D, Fiscus SA, Newman D, Branson B, et al. Evaluation of an Ultrasensitive p24 Antigen Assay as a Potential Alternative to Human Immunodeficiency Virus Type 1 RNA Viral Load Assay in Resource-Limited Settings. Journal of Clinical Microbiology. 2005;43:506–508. doi: 10.1128/JCM.43.1.506-508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ribas SG, Ondoa P, Schüpbach J, van der Groen G, Fransen K. Performance of a quantitative human immunodeficiency virus type 1 p24 antigen assay on various HIV-1 subtypes for the follow-up of human immunodeficiency type 1 seropositive individuals. Journal of virological methods. 2003;113:29–34. doi: 10.1016/s0166-0934(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 131.Spacek LA, Lutwama F, Shihab HM, Summerton J, Kamya MR, Ronald A, et al. Diagnostic accuracy of ultrasensitive heat-denatured HIV-1 p24 antigen in non-B subtypes in Kampala, Uganda. Int J STD AIDS. 2011;22:310–314. doi: 10.1258/ijsa.2009.009363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stevens G, Rekhviashvili N, Scott LE, Gonin R, Stevens W. Evaluation of Two Commercially Available, Inexpensive Alternative Assays Used for Assessing Viral Load in a Cohort of Human Immunodeficiency Virus Type 1 Subtype C-Infected Patients from South Africa. Journal of Clinical Microbiology. 2005;43:857–861. doi: 10.1128/JCM.43.2.857-861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tehe A, Maurice C, Hanson DL, Borget MY, Abiola N, Maran M, et al. Quantification of HIV-1 p24 by a highly improved ELISA: An alternative to HIV-1 RNA based treatment monitoring in patients from Abidjan, Côte d’Ivoire. Journal of Clinical Virology. 2006;37:199–205. doi: 10.1016/j.jcv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 134.Kwon J-A, Yoon S-Y, Lee C-K, Lim CS, Lee KN, Sung HJ, et al. Performance evaluation of three automated human immunodeficiency virus antigen–antibody combination immunoassays. Journal of virological methods. 2006;133:20–26. doi: 10.1016/j.jviromet.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 135.Tan WS, Chow EPF, Fairley CK, Chen MY, Bradshaw CS, Read TRH. Sensitivity of HIV rapid tests compared with fourth-generation enzyme immunoassays or HIV RNA tests. AIDS. 2016;30:1951–1960. doi: 10.1097/QAD.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 136.Fox J, Dunn H, O'Shea S. Low rates of p24 antigen detection using a fourth-generation point of care HIV test. Sexually Transmitted Infections. 2011;87:178–179. doi: 10.1136/sti.2010.042564. [DOI] [PubMed] [Google Scholar]
- 137.Taegtmeyer M, MacPherson P, Jones K, Hopkins M, Moorcroft J, Lalloo DG, et al. Programmatic Evaluation of a Combined Antigen and Antibody Test for Rapid HIV Diagnosis in a Community and Sexual Health Clinic Screening Programme. PloS one. 2011;6:e28019–5. doi: 10.1371/journal.pone.0028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Conway DP, Holt M, McNulty A, Couldwell DL, Smith DE, Davies SC, et al. Multi-Centre Evaluation of the Determine HIV Combo Assay when Used for Point of Care Testing in a High Risk Clinic-Based Population. PloS one. 2014;9:e94062–8. doi: 10.1371/journal.pone.0094062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Duong YT, Mavengere Y, Patel H, Moore C, Manjengwa J, Sibandze D, et al. Poor Performance of the Determine HIV-1/2 Ag/Ab Combo Fourth-Generation Rapid Test for Detection of Acute Infections in a National Household Survey in Swaziland. Journal of Clinical Microbiology. 2014;52:3743–3748. doi: 10.1128/JCM.01989-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jones CB, Kuldanek K, Muir D, Phekoo K, Black A, Sacks R, et al. Clinical evaluation of the Determine HIV-1/2 Ag/Ab Combo test. J INFECT DIS. 2012;206:1947–9. doi: 10.1093/infdis/jis617. author reply 1949–50. [DOI] [PubMed] [Google Scholar]
- 141.Kilembe W, Keeling M, Karita E, Lakhi S, Chetty P, Price MA, et al. Failure of A Novel, Rapid Antigen and Antibody Combination Test to Detect Antigen-Positive HIV Infection in African Adults with Early HIV Infection. PloS one. 2012;7:e37154–6. doi: 10.1371/journal.pone.0037154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rosenberg NE, Kamanga G, Phiri S, Nsona D, Pettifor A, Rutstein SE, et al. Detection of Acute HIV Infection: A Field Evaluation of the Determine® HIV-1/2 Ag/Ab Combo Test. J INFECT DIS. 2011;205:528–534. doi: 10.1093/infdis/jir789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Faraoni S, Rocchetti A, Gotta F, Ruggiero T, Orofino G, Bonora S, et al. Evaluation of a rapid antigen and antibody combination test in acute HIV infection. Journal of Clinical Virology. 2013;57:84–87. doi: 10.1016/j.jcv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 144.Chetty V, Moodley D, Chuturgoon A. Evaluation of a 4th generation rapid HIV test for earlier and reliable detection of HIV infection in pregnancy. Journal of Clinical Virology. 2012;54:180–184. doi: 10.1016/j.jcv.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 145.Beelaert G, Fransen K. Evaluation of a rapid and simple fourth-generation HIV screening assay for qualitative detection of HIV p24 antigen and/or antibodies to HIV-1 and HIV-2. Journal of virological methods. 2010;168:218–222. doi: 10.1016/j.jviromet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Brauer M, De Villiers JC, Mayaphi SH. Evaluation of the Determine™ fourth generation HIV rapid assay. Journal of virological methods. 2013;189:180–183. doi: 10.1016/j.jviromet.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 147.Masciotra S, Luo W, Westheimer E, Cohen SE, Gay CL, Hall L, et al. Performance evaluation of the FDA-approved Determine™ HIV-1/2 Ag/Ab Combo assay using plasma and whole blood specimens. J Clin Virol. 2017;91:95–100. doi: 10.1016/j.jcv.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smallwood M, Vijh R, Nauche B, Lebouché B, Joseph L, Pai NP. Evaluation of a rapid point of care test for detecting acute and established HIV infection, and examining the role of study quality on diagnostic accuracy: A Bayesian meta-analysis. PloS one. 2016;11:e0149592. doi: 10.1371/journal.pone.0149592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van Tienen C, Rugebregt S, Scherbeijn S, van Kessel CG, Götz H. The Alere HIV Combo point-of-care test; Useful in clinical practice? Journal of Clinical Virology. 2016;82:S94–100. [Google Scholar]
- 150.Fitzgerald N, Cross M, O'Shea S, Fox J. Diagnosing acute HIV infection at point of care: a retrospective analysis of the sensitivity and specificity of a fourth-generation point-of-care test for detection of HIV core protein p24. Sexually Transmitted Infections. 2017;93:100–101. doi: 10.1136/sextrans-2015-052491. [DOI] [PubMed] [Google Scholar]
- 151.Wessman MJ, Theilgaard Z, Katzenstein TL. Determination of HIV status of infants born to HIV-infected mothers: A review of the diagnostic methods with special focus on the applicability of p24 antigen testing in developing countries. Scandinavian Journal of Infectious Diseases. 2011;44:209–215. doi: 10.3109/00365548.2011.627569. [DOI] [PubMed] [Google Scholar]
- 152.Quinn TC, Kline R, Moss MW, Livingston RA, Hutton N. Acid dissociation of immune complexes improves diagnostic utility of p24 antigen detection in perinatally acquired human immunodeficiency virus infection. J INFECT DIS. 1993;167:1193–1196. doi: 10.1093/infdis/167.5.1193. [DOI] [PubMed] [Google Scholar]
- 153.Lewis DE, Adu-Oppong A, Hollinger FB, Rosenblatt HM, Hanson IC, Reuben JM, et al. Sensitivity of immune complex-dissociated p24 antigen testing for early detection of human immunodeficiency virus in infants. Clin Diagn Lab Immunol. 1995;2:87–90. doi: 10.1128/cdli.2.1.87-90.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parpia ZA, Elghanian R, Nabatiyan A, Hardie DR, Kelso DM. p24 antigen rapid test for diagnosis of acute pediatric HIV infection. J Acquir Immune Defic Syndr. 2010;55:413–419. doi: 10.1097/QAI.0b013e3181f1afbc. [DOI] [PubMed] [Google Scholar]
- 155.Cachafeiro A, Sherman GG, Sohn AH, Beck-Sague C, Fiscus SA. Diagnosis of human immunodeficiency virus type 1 infection in infants by use of dried blood spots and an ultrasensitive p24 antigen assay. Journal of Clinical Microbiology. 2009;47:459–462. doi: 10.1128/JCM.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fiscus SA, Wiener J, Abrams EJ, Bulterys M, Cachafeiro A, Respess RA. Ultrasensitive p24 Antigen Assay for Diagnosis of Perinatal Human Immunodeficiency Virus Type 1 Infection. Journal of Clinical Microbiology. 2007;45:2274–2277. doi: 10.1128/JCM.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guay LA, Hom DL, Mmiro F, Piwowar EM, Kabengera S, Parsons J, et al. Detection of human immunodeficiency virus type 1 (HIV-1) DNA and p24 antigen in breast milk of HIV-1-infected Ugandan women and vertical transmission. PEDIATRICS. 1996;98:438–444. [PubMed] [Google Scholar]
- 158.Ly TD, Plantier J-C, Leballais L, Gonzalo S, Lemée V, Laperche S. The variable sensitivity of HIV Ag/Ab combination assays in the detection of p24Ag according to genotype could compromise the diagnosis of early HIV infection. Journal of Clinical Virology. 2012;55:121–127. doi: 10.1016/j.jcv.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 159.Gottlieb GS, Eholié S-P, Nkengasong JN, Jallow S, Rowland-Jones S, Whittle HC, et al. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS. 2008;22:2069–72. doi: 10.1097/QAD.0b013e32830edd44. discussion 2073–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tang S, Zhao J, Wang A, Viswanath R, Harma H, Little RF, et al. Characterization of Immune Responses to Capsid Protein p24 of Human Immunodeficiency Virus Type 1 and Implications for Detection. Clinical and Vaccine Immunology. 2010;17:1244–1251. doi: 10.1128/CVI.00066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tamhane M, Gautney B, Shiu C, Segaren N, Jeannis L, Eustache C, et al. Analysis of the optimal cut-point for HIV-p24 antigen testing to diagnose HIV infection in HIV-exposed children from resource-constrained settings. Journal of Clinical Virology. 2011;50:338–341. doi: 10.1016/j.jcv.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bulterys M, Farzadegan H, Chao A, Dushimimana A, Voltz A, Nawrocki P, et al. Diagnostic utility of immune-complex-dissociated p24 antigen detection in perinatally acquired HIV-1 infection in Rwanda. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:186–191. doi: 10.1097/00042560-199510020-00012. [DOI] [PubMed] [Google Scholar]
- 163.Tersmette M, Winkel IN, Groenink M, Gruters RA, Spence RP, Saman E, et al. Detection and subtyping of HIV-1 isolates with a panel of characterized monoclonal antibodies to HIV p24gag. Virology. 1989;171:149–155. doi: 10.1016/0042-6822(89)90521-7. [DOI] [PubMed] [Google Scholar]
- 164.Kashala O, Kayembe K, Kanki P, Mukeba P, Diese M, Kalengayi M, et al. Humoral aspects of anti-HIV immune responses in Zairians with AIDS: lower antigenemia does not correlate with immune complex levels. AIDS Research and Human Retroviruses. 1993;9:251–258. doi: 10.1089/aid.1993.9.251. [DOI] [PubMed] [Google Scholar]
- 165.Archary D, Rong R, Gordon ML, Boliar S, Madiga M, Gray ES, et al. Characterization of anti-HIV-1 neutralizing and binding antibodies in chronic HIV-1 subtype C infection. Virology. 2012;433:410–420. doi: 10.1016/j.virol.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gorny MK, Gianakakos V, Sharpe S, Zolla-Pazner S. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arens M, Meyer W, Brambilla D, Bremer J, Fiscus S, Griffith B, et al. Stabilities of free and complexed human immunodeficiency virus p24 antigens during short- and long-term storage. Journal of Clinical Microbiology. 1997;35:2413–2416. doi: 10.1128/jcm.35.9.2413-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Albertini A, Lee E, Coulibaly SO, Sleshi M, Faye B, Mationg ML, et al. Malaria rapid diagnostic test transport and storage conditions in Burkina Faso, Senegal, Ethiopia and the Philippines. Malaria Journal. 2012;11:1–1. doi: 10.1186/1475-2875-11-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bienek DR, Charlton DG. The effect of simulated field storage conditions on the accuracy of rapid user-friendly blood pathogen detection kits. Mil Med. 2012;177:583–588. doi: 10.7205/milmed-d-11-00420. [DOI] [PubMed] [Google Scholar]
- 170.Choko AT, Taegtmeyer M, MacPherson P, Cocker D, Khundi M, Thindwa D, et al. Initial Accuracy of HIV Rapid Test Kits Stored in Suboptimal Conditions and Validity of Delayed Reading of Oral Fluid Tests. PloS one. 2016;11:e0158107. doi: 10.1371/journal.pone.0158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hammer SM. Advances in antiretroviral therapy and viral load monitoring. AIDS. 1996;10(Suppl 3):S1–11. [PubMed] [Google Scholar]
- 172.Weber B. Screening of HIV infection: role of molecular and immunological assays. Expert Review of Molecular Diagnostics. 2006;6:399–411. doi: 10.1586/14737159.6.3.399. [DOI] [PubMed] [Google Scholar]
- 173.Cragin L, Pan F, Peng S, Zenilman JM, Green J, Doucet C, et al. Cost-effectiveness of a Fourth-Generation Combination Immunoassay for Human Immunodeficiency Virus (HIV) Antibody and p24 Antigen for the Detection of HIV Infections in the United States. HIV Clinical Trials. 2015;13:11–22. doi: 10.1310/hct1301-011. [DOI] [PubMed] [Google Scholar]
- 174.Coco A. The cost-effectiveness of expanded testing for primary HIV infection. Ann Fam Med. 2005;3:391–399. doi: 10.1370/afm.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hutchinson AB, Farnham PG, Sansom SL, Yaylali E, Mermin JH. Cost-Effectiveness of Frequent HIV Testing of High-Risk Populations in the United States. J Acquir Immune Defic Syndr. 2016;71:323–330. doi: 10.1097/QAI.0000000000000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Long EF. HIV Screening via Fourth-Generation Immunoassay or Nucleic Acid Amplification Test in the United States: A Cost-Effectiveness Analysis. PloS one. 2011;6:e27625–10. doi: 10.1371/journal.pone.0027625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.AuBuchon JP, Birkmeyer JD, Busch MP. Cost-effectiveness of expanded human immunodeficiency virus-testing protocols for donated blood. Transfusion. 1997;37:45–51. doi: 10.1046/j.1537-2995.1997.37197176950.x. [DOI] [PubMed] [Google Scholar]
- 178.van Hulst M, Sagoe KWC, Vermande JE, van der Schaaf IP, van der Tuuk Adriani WPA, Torpey K, et al. Cost-effectiveness of HIV screening of blood donations in Accra (Ghana) Value Health. 2008;11:809–819. doi: 10.1111/j.1524-4733.2008.00337.x. [DOI] [PubMed] [Google Scholar]
- 179.Karris MY, Anderson CM, Morris SR, Smith DM, Little SJ. Cost savings associated with testing of antibodies, antigens, and nucleic acids for diagnosis of acute HIV infection. Journal of Clinical Microbiology. 2012;50:1874–1878. doi: 10.1128/JCM.00106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang Y-LA, Lasry A, Hutchinson AB, Sansom SL. A systematic review on cost effectiveness of HIV prevention interventions in the United States. Appl Health Econ Health Policy. 2015;13:149–156. doi: 10.1007/s40258-014-0142-5. [DOI] [PubMed] [Google Scholar]
- 181.Centers for Disease Control and Prevention (CDC) Persons tested for HIV - United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:845–849. [PubMed] [Google Scholar]
- 182.Mellors JW, Rinaldo CR, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 183.WHO. What's new in treatment monitoring. 2017 http://apps.who.int/iris/bitstream/handle/10665/255891/WHO-HIV-2017.22-eng.pdf?sequence=1.
- 184.UNAIDS. Geneva: 2016. The need for routine viral load testing. [Google Scholar]
- 185.Garcia MN, Ramos Farias dos MS, Avila MM, Rabinovich RD. Presence of p24-antigen associated to erythrocyte in HIV-positive individuals even in patients with undetectable plasma viral load. PloS one. 2011;6:e14544. doi: 10.1371/journal.pone.0014544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schüpbach J. Measurement of HIV-1 p24 antigen by signal-amplification-boosted ELISA of heat-denatured plasma is a simple and inexpensive alternative to tests for viral RNA. AIDS Rev. 2002;4:83–92. [PubMed] [Google Scholar]
- 187.Schüpbach J. Viral RNA and p24 antigen as markers of HIV disease and antiretroviral treatment success. Int Arch Allergy Immunol. 2003;132:196–209. doi: 10.1159/000074552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.