Abstract
Purpose
This single-arm, open label Phase II study evaluated the safety and efficacy of taselisib (GDC-0032) plus fulvestrant in postmenopausal women with locally advanced or metastatic HER2-negative, hormone receptor (HR)-positive breast cancer.
Experimental design
Patients received 6 mg oral taselisib capsules daily plus intramuscular fulvestrant (500 mg) until disease progression or unacceptable toxicity. Tumor tissue (if available) was centrally evaluated for PIK3CA mutations. Adverse events (AEs) were recorded using NCI-CTCAE v4.0. Tumor response was investigator-determined using RECIST v1.1.
Results
Median treatment duration was 4.6 (range: 0.9–40.5) months. All patients experienced ≥1 AE, 30 (50.0%) had grade ≥3 AEs, and 19 (31.7%) experienced 35 serious AEs. Forty-seven of sixty patients had evaluable tissue for central PIK3CA mutation testing (20 had mutations, 27 had no mutation detected [MND]). In patients with baseline measurable disease, clinical activity was observed in tumors with PIK3CA mutations (best confirmed response rate: 38.5% [5/13; 95% CI 13.9–68.4]; clinical benefit rate [CBR]: 38.5% [5/13; 95% CI 13.9–68.4]), PIK3CA-MND (best confirmed response rate: 14.3% [3/21; 95% CI 3.0–36.3]; CBR: 23.8% [5/21; 95% CI 8.2–47.2]), and unknown PIK3CA mutation status (best confirmed response rate: 20.0% [2/10; 95% CI 2.5–55.6]; CBR: 30.0% [3/10; 95% CI 6.7–65.2]).
Conclusions
Taselisib plus fulvestrant had clinical activity irrespective of PIK3CA mutation status, with numerically higher objective response rate and CBR in patients with PIK3CA-mutated (versus -MND) locally advanced or metastatic HER2-negative, HR-positive breast cancer. No new safety signals were reported. A confirmatory Phase III trial is ongoing.
Keywords: Taselisib, GDC-0032, PI3K, breast cancer, Phase I–III clinical trials, breast cancer
Introduction
The phosphatidylinositol 3-kinase (PI3K) pathway is essential for normal cell growth and is implicated in many cancers (1, 2), including hormone receptor (HR)-positive breast cancer (3, 4). The gene encoding phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit-alpha (PIK3CA) is a commonly mutated human oncogene in breast cancer (5). Activating mutations in PIK3CA have been detected in ~40% of patients with HR-positive breast cancer (6, 7). Thus, the PI3K pathway is an attractive target for drug development.
Taselisib (GDC-0032) is a potent and selective PI3K inhibitor, with enhanced efficacy in cell lines that harbor a PIK3CA (p110α) somatic mutation (8–11). Clinical studies demonstrated that taselisib, when administered as an oral capsule formulation at doses of 3–16 mg once daily to patients with locally advanced or metastatic solid tumors in a Phase Ia dose-escalation trial, had a linear exposure profile and an elimination half-life of approximately 40 hours (12). Taselisib was well tolerated and had clinical activity over the dosage range evaluated (12). Hyperglycemia, diarrhea, rash, and stomatitis were common adverse events (AEs) observed in the trial, consistent with toxicities observed with other PI3K inhibitors (12).
Inhibition of PI3K signaling in HR-positive breast cancer results in upregulation of estrogen receptor (ER)-dependent function (13, 14). The mechanism by which this occurs has been recently elucidated and involves increased KMT2D activity, which in turn stimulates ER-dependent transcription (14). The combination of PI3K inhibition using BYL719 (alpelisib) and ER inhibition using fulvestrant resulted in marked tumor regression in a xenograft model that was more robust than either agent alone (13). Importantly, in a proof-of-concept study in patients with ER-positive breast cancer, the combination of anti-estrogen therapy using exemestane with inhibition of mammalian target of rapamycin (mTOR), a downstream target of PI3K, with everolimus significantly increased progression-free survival (PFS) compared with everolimus plus placebo (15). Collectively, these observations provide a rationale for dual PI3K and ER inhibition in patients with breast cancer.
Data from a phase Ib trial in patients with HR-positive breast cancer demonstrate that there is no pharmacokinetic drug–drug interaction between taselisib and fulvestrant, and suggest that the combination has clinical activity and acceptable tolerability (16). On this basis, we designed the present phase II study to evaluate the clinical efficacy and safety of taselisib plus fulvestrant in postmenopausal women with locally advanced or metastatic human epidermal growth factor receptor 2 (HER2)-negative, HR-positive breast cancer.
Materials and Methods
Patients
Eligible patients were postmenopausal women with locally advanced or metastatic HER2-negative, HR-positive breast cancer who had progressed or failed to respond to ≥1 prior endocrine therapy in the adjuvant or metastatic setting. Patients were also required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, a fasting plasma glucose level ≤120 mg/dL, granulocyte count ≥1500/µL, platelet count ≥100,000/µL, hemoglobin concentration ≥9 g/dL, serum albumin concentration ≥2.5 g/dL, and both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels ≤1.5 times the upper limit of normal. As an exception, patients with documented liver metastases were eligible if their AST and/or ALT levels were ≤5.0 times the upper limit of normal. The presence of measurable or evaluable disease, defined by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 criteria, was required. Prior treatment with everolimus was allowed.
Patients were excluded if they had received: prior therapy with fulvestrant; >1 cytotoxic chemotherapy regimen for breast cancer in the metastatic setting; prior therapy with a PI3K inhibitor, or oral endocrine therapy within 2 weeks prior to initiation of study treatment. Patients with active inflammatory diseases requiring immunosuppressant agents, including Crohn’s disease or ulcerative colitis; known and untreated, or active central nervous system metastases (progressing or requiring anticonvulsants for symptomatic control); and/or type 1 or 2 diabetes mellitus requiring anti-diabetic medication, were also excluded.
The study was performed after approval by an institutional review board and conducted in accordance with the principles of the Declaration of Helsinki, International Conference on Harmonisation Guidelines, and the laws and regulations of the countries in which it was conducted. All patients provided written informed consent before undergoing any study procedures.
Study design and treatment
This was a Phase II, open-label, multicenter, single-arm study (clinicaltrials.gov: NCT01296555). Patients received 6 mg oral taselisib capsules once daily in combination with fulvestrant until the occurrence of disease progression or unacceptable toxicity. Fulvestrant was administered as a 500 mg intramuscular injection on days 1 and 15 during cycle 1 and thereafter on day 1 of each 28-day cycle.
Dosing with taselisib or fulvestrant could be interrupted for up to 28 days in the event of toxicity or unanticipated medical events not associated with study drug toxicity or disease progression. Step-wise reductions in the dose of taselisib were permitted to manage study drug-related toxicity (first reduction: 3 mg every day; second reduction: 3 mg every other day). Dose reductions were not allowed for fulvestrant, although patients were allowed to temporarily suspend treatment with fulvestrant for ≤28 days. Study treatment was discontinued in patients who experienced disease progression or unacceptable toxicity.
Safety Assessment
Safety was assessed by monitoring and recording protocol-defined AEs and serious AEs (SAEs), and by monitoring protocol-specified laboratory parameters and vital signs. AEs were graded according to the National Cancer Institute – Common Terminology Criteria for Adverse Events Version 4.0 (NCI-CTCAE v4.0) (17).
The protocol-defined AEs of special interest (AESI) for taselisib were hyperglycemia, colitis, diarrhea, rash, and pneumonitis. The search strategy for AESIs was based on Sponsor-specific AE group terms, based on the Medical Dictionary for Regulatory Activities (MedDRA). (Supplemental Methods).
PIK3CA-mutation testing
Patients were enrolled based on PIK3CA mutation status by local or central testing, with central PIK3CA-mutation testing performed retrospectively on all available samples. Formalin-fixed paraffin-embedded tumor tissue samples (blocks or slides) either from prior tumor excisions or fresh biopsies, if available, were requested for all patients for central mutation testing, although availability of archival tissue was not required for enrollment. Tumors were classified as being PIK3CA-mutated if a positive result was obtained from central analysis of archival tumor tissue using the cobas® PIK3CA Mutation Test (Roche Molecular Diagnostics, Pleasanton, CA) (4), which uses real-time polymerase chain reaction to detect frequent hotspot mutations in exons 1, 4, 7, 9, and 20. The following substitution mutations were included: E542K, E545X (A, D, G, or K), Q546X (K, R, E, or L), N345K, C420R, R88Q, H1047X (L, R, or Y), G1049R, and M1043I. Helical domain mutations were defined as E542K, E545X (A, D, G, or K), and Q546X (K, R, E, or L). Kinase domain mutations were defined as H1047X (L, R, or Y), G1049R, and M1043I. Tumors were classified as PIK3CA-mutation-not-detected (MND) if no mutations were detected by the cobas® test, and PIK3CA mutation status unknown if there was no tissue available or assay failure.
Tumor response assessments and criteria
Measurable or evaluable disease was documented at screening and at each subsequent tumor evaluation on the basis of physical examinations, imaging studies, and laboratory results. The same radiographic procedure used at baseline was used throughout the study for each patient. Post-baseline tumor assessments were conducted at the end of cycles 2, 4, 6, and 8 and every 12 weeks thereafter. Bone scans and brain scans were performed if clinically indicated. Tumor response was determined by investigators using RECIST v1.1 criteria (18).
Outcomes
The primary endpoints were clinical benefit rate (CBR), defined as confirmed complete response, confirmed partial response, or stable disease lasting for ≥6 months in all patients, and objective response rate (ORR) in all patients and in patients with PIK3CA-mutated breast cancer per central cobas® test. The secondary endpoints, in all patients and in patients with PIK3CA-mutated breast cancer, included safety (all treated patients), duration of objective response (DoR), PFS, and overall survival (OS).
Statistical considerations
The planned enrollment was 60 patients, including a minimum of 30 patients with PIK3CA-mutated breast cancer as determined by local or central testing.
The study was designed to estimate the ORR and CBR of the combination of taselisib and fulvestrant and to allow for a comparison with historical studies of fulvestrant [ORR: 7–10%; CBR: 32–46% (19, 20)]. Assuming that 30% of patients (9 out of 30) had non-measurable, bone-only disease, an observed ORR of ≥30% in the remaining 21 patients with PIK3CA-mutated tumors was estimated to have a lower bound of the 95% confidence interval (CI) ≥14.6%, excluding an ORR of 10%. An observed CBR of 67% (n = 30) was estimated to have a 95% CI of 47.2–82.7, excluding a CBR of 46%.
All safety and efficacy analyses were based on the safety-evaluable population, defined as all patients who received at least one dose of study drug (taselisib or fulvestrant).
95% CIs were estimated for ORR and DoR, with DoR estimated by Kaplan–Meier methodology.
Results
Patient characteristics
Overall, 60 patients were enrolled between July 9, 2013 and May 8, 2014 and treated with taselisib plus fulvestrant. The data-analysis cut-off was December 1, 2016. Baseline demographic and patient characteristics are shown by PIK3CA mutation status in Table 1. Patient demographics were well balanced between PIK3CA mutation status groups. The median age of women enrolled in the trial was 61.5 years (range: 31–82), the median time from initial diagnosis was 64.2 months (range: 6.7–315.3), and 56.7% of women had an ECOG PS of 0 (Table 1).
Table 1.
Baseline demographics and patient characteristics by PIK3CA mutation status
| Characteristic |
PIK3CA-mutated (n = 20) |
PIK3CA-MND (n = 27) |
PIK3CA mutation status unknown (n = 13) |
All patients (N = 60) |
|---|---|---|---|---|
| Median age, years (range) | 61.0 (41–78) | 57.0 (31–82) | 63.0 (44–75) | 61.5 (31–82) |
| ECOG PS, n (%) | ||||
| 0 | 9 (45.0) | 15 (55.6) | 10 (76.9) | 34 (56.7) |
| 1 | 11 (55.0) | 12 (44.4) | 3 (23.1) | 26 (43.3) |
| Median time from primary diagnosis, months (range) | n = 17 | n = 23 | n = 12 | n = 52 |
| 65.3 (6.7–183.1) | 56.7 (11.6–315.3) | 81.7 (16.0–167.7) | 64.2 (6.7–315.3) | |
| Bone-only disease, n (%) | ||||
| Yes | 1 (5.0) | 4 (14.8) | 2 (15.4) | 7 (11.7) |
| No | 19 (95.0) | 23 (85.2) | 11 (84.6) | 53 (88.3) |
| Visceral disease, n (%) | ||||
| Yes | 14 (70.0) | 17 (63.0) | 8 (61.5) | 39 (65.0) |
| No | 6 (30.0) | 10 (37.0) | 5 (38.5) | 21 (35.0) |
| Endocrine sensitivity, n (%)a | ||||
| Yes | 7 (35.0) | 9 (33.3) | 1 (7.7) | 17 (28.3) |
| No | 13 (65.0) | 18 (66.7) | 12 (92.3) | 43 (71.7) |
| Median number of prior hormonal therapies, (range) | n = 20 | n = 26 | n = 13 | n = 59 |
| 2.0 (1.0–5.0) | 2.0 (1.0–4.0) | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | |
| Prior treatment, n (%)b | ||||
| Aromatase inhibitor | 18 (90.0) | 21 (77.8) | 13 (100.0) | 52 (86.7) |
| Everolimus | 4 (20.0) | 3 (11.1) | 3 (23.1) | 10 (16.7) |
| Letrozole | 13 (65.0) | 14 (51.9) | 8 (61.5) | 35 (58.3) |
| Anastrozole | 6 (30.0) | 7 (25.9) | 6 (46.2) | 19 (31.7) |
| Exemestane | 10 (50.0) | 6 (22.2) | 8 (61.5) | 24 (40.0) |
| Tamoxifen | 10 (50.0) | 19 (70.4) | 7 (53.8) | 36 (60.0) |
| Prior chemotherapy, n (%) | ||||
| Adjuvant setting | 8 (40.0) | 15 (55.6) | 6 (46.2) | 29 (48.3) |
| Metastatic setting | 4 (20.0) | 8 (29.6) | 4 (30.8) | 16 (26.7) |
| Prior hormonal therapy, n (%) | ||||
| Adjuvant setting | 12 (60.0) | 21 (77.8) | 9 (69.2) | 42 (70.0) |
| Metastatic setting | 14 (70.0) | 12 (44.4) | 11 (84.6) | 37 (61.7) |
Endocrine sensitivity was defined based on a positive response to either of the following: 1) In patients with at least one hormonal treatment in the metastatic setting: treatment duration was ≥24 weeks from the most recent hormonal therapy in the metastatic setting, if the best response was missing. Documented complete/partial response or stable disease ≥24 weeks from the most recent hormonal therapy in the metastatic setting, if the best response was available. 2) In patients without hormonal therapy in the metastatic setting, but who received hormonal treatment in the adjuvant setting: treatment duration of the most recent hormonal treatment in the adjuvant setting was ≥24 months.
Patients received prior treatment in the adjuvant or metastatic setting. For the “median time from primary diagnosis” and “median number of prior hormonal therapies” categories, the number of patients for whom data were available is presented.
Among the 60 patients included in the analysis, 47 had evaluable tumor tissue for central PIK3CA mutation testing (25 from metastatic tissue and 22 from primary tissue; Supplementary Fig. S1). Based on central testing, 20 patients (33.3%) were PIK3CA-mutated, 27 (45.0%) were PIK3CA-MND, and 13 (21.7%) had an unknown PIK3CA mutation status (12 samples without sufficient evaluable tumor tissue and one assay failure). A numerically higher number of patients with an unknown PIK3CA mutation status had an ECOG PS of 0 (10/13 [76.9%]) versus patients with a known mutation status (24/47 [51.1%]).
The disposition of patients is shown in Supplementary Table S1. At the data cut-off, 42 patients (70.0%) had been discontinued from the study: 36 (60.0%) had died, four (6.7%) were lost to follow-up, and two (3.3%) had withdrawn from the study. The remaining patients were being followed for OS.
Safety
Treatment with taselisib and fulvestrant was generally well tolerated (Table 2). Sixty patients (100.0%) experienced ≥1 AE (Table 2 and Table 3). The most common AEs, regardless of attribution, were diarrhea (42 patients [70.0%]), nausea (27 patients [45.0%]), fatigue (25 patients [41.7%]), and decreased appetite (19 patients [31.7%]) (Table 3). Thirty patients (50.0%) experienced grade ≥3 AEs (Table 2 and Table 3), and the most common were colitis (8 patients [13.3%]; median onset of grade ≥3 AEs was 4.5 months [range: 3.7–8.2]), diarrhea (7 patients [11.7%]; median onset of grade ≥3 AE was 4.7 months [range: 3.7–12.2]), and hyperglycemia (4 patients [6.7%]; median onset of grade ≥3 AE was 4.2 months [range: 1.9–5.3]) (Table 3). Overall, a total of 35 SAEs were observed in nineteen patients (31.7%) (Supplementary Table S2). SAEs that occurred in more than one patient included colitis in six patients (10.0%), and pneumonia in three patients (5.0%) (Supplementary Table S2).
Table 2.
Overview of AEs in the safety population
| AE, n (%) | All patients (N = 60) |
|---|---|
| Any-grade AEs | 60 (100.0) |
| Grade ≥3 AEs | 30 (50.0) |
| Grade 5 AEs | 4 (6.7) |
| Serious AEs | 19 (31.7) |
| AEs leading to taselisib dose modifications | |
| AE leading to taselisib dose reduction | 14 (23.3) |
| AE leading to taselisib dose interruption | 27 (45.0) |
| AE leading to taselisib discontinuation | 12 (20.0) |
Table 3.
AEs occurring in ≥10% of patients or grade ≥3 AEs occurring in ≥2% of patients regardless of attribution in the safety population
| AE, n (%) | All-grade AEs (N = 60) |
Grade ≥3 AEs (N = 60) |
|---|---|---|
| Total number of patients with at least one AE | 60 (100.0) | 30 (50.0) |
| Total number of AEs | 901 | 69 |
| Diarrhea | 42 (70.0) | 7 (11.7) |
| Nausea | 27 (45.0) | |
| Fatigue | 25 (41.7) | |
| Decreased appetite | 19 (31.7) | 1 (1.7) |
| Mucosal inflammation | 17 (28.3) | 2 (3.3) |
| Dry skin | 16 (26.7) | |
| Rash | 15 (25.0) | 1 (1.7) |
| Dyspepsia | 14 (23.3) | |
| Colitis | 13 (21.7) | 8 (13.3) |
| Hyperglycemia | 13 (21.7) | 4 (6.7) |
| Asthenia | 12 (20.0) | 2 (3.3) |
| Abdominal pain | 11 (18.3) | |
| Stomatitis | 11 (18.3) | |
| Back pain | 10 (16.7) | 1 (1.7) |
| Cough | 10 (16.7) | |
| Headache | 10 (16.7) | |
| Arthralgia | 9 (15.0) | |
| Insomnia | 9 (15.0) | |
| Dyspnea | 8 (13.3) | |
| Urinary tract infection | 8 (13.3) | |
| Alopecia | 7 (11.7) | |
| Dizziness | 7 (11.7) | |
| Dysgeusia | 7 (11.7) | |
| Muscle spasms | 7 (11.7) | |
| Constipation | 6 (10.0) | |
| Dry mouth | 6 (10.0) | |
| Gastroesophageal reflux disease | 6 (10.0) | |
| Hypokalemia | 6 (10.0) | 1 (1.7) |
| Musculoskeletal pain | 6 (10.0) | |
| Pyrexia | 6 (10.0) | |
| Vomiting | 6 (10.0) | |
| Weight decreased | 6 (10.0) | |
| Aspartate aminotransferase increased | 5 (8.3) | 2 (3.3) |
| Rash maculopapular | 5 (8.3) | 2 (3.3) |
| Pneumonia | 4 (6.7) | 3 (5.0) |
| Hyponatremia | 2 (3.3) | 2 (3.3) |
All AE categories are preferred terms, encoded using MedDRA version 20.0. For frequency counts by preferred term, multiple occurrences of the same AE in an individual are counted once. For the total number of events, multiple occurrences of the same AE in an individual were counted separately.
AESIs in the safety population were reported in 48 patients (80.0%) (Supplementary Table S3). AESIs included diarrhea (42 patients [70.0%]), colitis (14 patients [23.3%]), stomatitis (25 patients [41.7%]), rash (18 patients [30.0%]), hyperglycemia (13 patients [21.7%]), and pneumonitis (1 patient [1.7%]) (Supplementary Table S3).
Overall, fourteen patients (23.3%) had AEs leading to taselisib dose reduction. AEs that led to taselisib dose reductions included colitis in four patients (6.7%); diarrhea in four patients (6.7%); mucosal inflammation in three patients (5.0%); and asthenia, decreased appetite, rash, and stomatitis, each in one patient (1.7%). In the four patients who had a dose reduction of taselisib due to diarrhea, all experienced a subsequent episode of diarrhea following the initial dose reduction. In the three patients who had a dose reduction due to mucosal inflammation, one patient experienced an additional event of mucositis following the initial dose reduction. In the patient who had a dose reduction of taselisib due to asthenia, this patient had one more reported event of asthenia following resolution of the first event. Overall, seven of the fourteen patients who had a dose reduction of taselisib did not experience another episode of the event that initially led to the dose reduction, including the four patients with colitis.
Taselisib treatment was discontinued due to sixteen AEs in 12 patients (20.0%) for the following: colitis in five patients (8.3%); diarrhea in three patients (5.0%); and increased ALT (1.7%), increased AST (1.7%), atrial fibrillation (1.7%), enterocolitis (1.7%), fatigue (1.7%), nausea (1.7%), pneumonitis (1.7%), and maculopapular rash (1.7%), each in one patient.
The median time to onset of any-grade colitis was 4.7 months (range: 3.2–8.2). Colitis was diagnosed by imaging studies and/or by endoscopy, and the observed pathology demonstrated ulcerations, and lymphocytic and/or eosinophilic infiltration. These events resolved or improved after interruption of study treatment, reduction of the taselisib dose, and/or initiation of corticosteroid therapy. In 13 patients with colitis, five (38.5%) were able to resume treatment following resolution of the event and either dose reduction (four) or interruption (one), without reoccurrence of colitis.
The median onset of any-grade diarrhea was 1.7 months (range: 0.1–8.3). All but two events of the 127 (98.4%) reports of diarrhea resolved after interruption of study treatment, reduction of the taselisib dose, and/or initiation of anti-diarrheal and/or corticosteroid therapy.
Four patients (6.7%) experienced a total of five grade 5 AEs during study treatment (one patient experienced grade 5 sepsis, two patients had grade 5 pneumonia, and one patient experienced both grade 5 device-related infection and grade 5 pericardial effusion). None of these events were related to taselisib treatment in the opinion of the investigator.
Time on treatment and exposure to taselisib
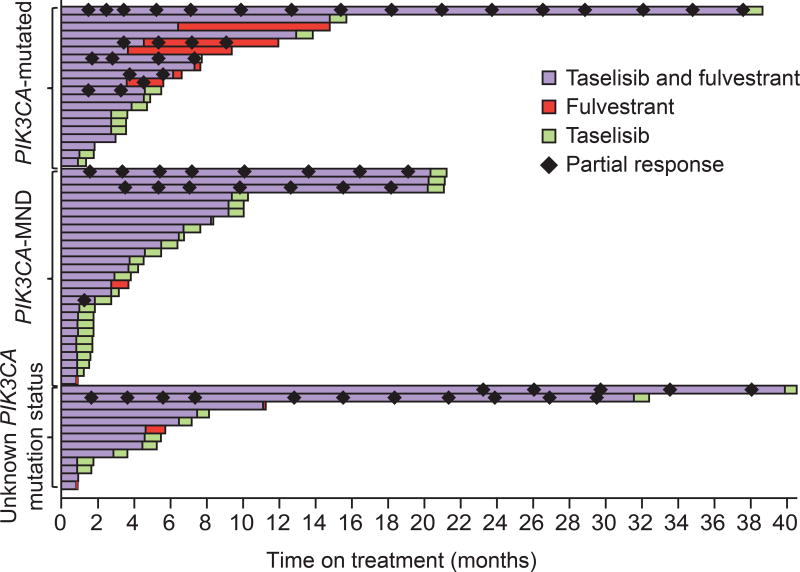
The duration of treatment with taselisib and fulvestrant is depicted by mutation status in Fig. 1. Twenty-two patients (37.0%) out of 60 received more than 6 months of treatment with taselisib. The overall median duration of treatment was 4.6 months (range 0.9 to 40.5) (Supplementary Table S4 and Supplementary Fig. S2). The median dose intensity with taselisib was 97.1% (range: 42–100) (Supplementary Table S4), and the median dose intensity of fulvestrant was 100% (range: 88–150).
Figure 1. Time on treatment by PIK3CA mutation status.
Many patients are shown as having received taselisib alone after their last dose of fulvestrant treatment. This is because they stopped taselisib treatment mid-cycle (typically for progression) and fulvestrant was received on days 1 and 15 of cycle 1, then on day 1 of each 28-day cycle.
Clinical activity
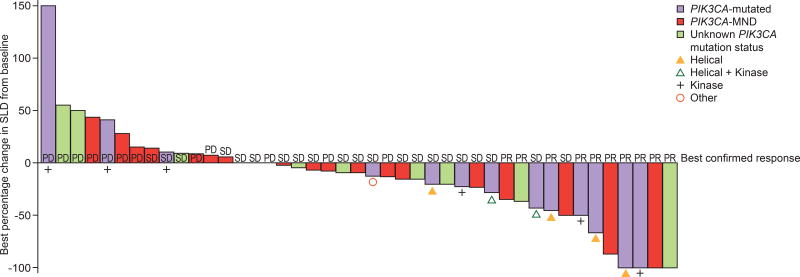
Among the subset of patients who had their PIK3CA mutation status evaluated by tumor tissue analysis, 44 patients had measurable disease at baseline; of these, 13 (29.5%) were PIK3CA-mutated, 21 (47.7%) had PIK3CA-MND, and 10 (22.7%) had unknown PIK3CA mutation status (Table 4). None had a complete response. Confirmed partial responses were observed in 5 of 13 patients with PIK3CA-mutated tumors (38.5%; 95% CI 13.9–68.4), in 3 of 21 patients with PIK3CA-MND tumors (14.3%; 95% CI 3.0–36.3), and in 2 of 10 patients with unknown PIK3CA tumor mutation status (20.0%; 95% CI 2.5–55.6; Fig. 2 and Table 4).
Table 4.
Clinical activity in patients with measurable disease at baseline
| n (%) |
PIK3CA-mutated (n = 13) |
PIK3CA-MND (n = 21) |
PIK3CA mutation status unknowna (n = 10) |
All patients (N = 44) |
|---|---|---|---|---|
| Best confirmed response | ||||
| Responders | 5 (38.5) | 3 (14.3) | 2 (20.0) | 10 (22.7) |
| 95% CI for response rate | 13.9–68.4 | 3.0–36.3 | 2.5–55.6 | 11.5–37.8 |
| Non-responders | 8 (61.5) | 18 (85.7) | 8 (80.0) | 34 (77.3) |
| Complete response | 0 | 0 | 0 | 0 |
| 95% CI | 0.00–24.7 | 0.0–16.1 | 0.0–30.8 | 0.0–8.0 |
| Partial response | 5 (38.5) | 3 (14.3) | 2 (20.0) | 10 (22.7) |
| 95% CI | 13.9–68.4 | 3.0–36.3 | 2.5–55.6 | 11.5–37.8 |
| Clinical benefit rate | 5 (38.5) | 5 (23.8) | 3 (30.0) | 13 (29.5) |
| 95% CI | 13.9–68.4 | 8.2–47.2 | 6.7–65.2 | 16.8–45.2 |
| Median duration of response, months | 8.8 | 18.5 | 30.5 | 19.6 |
| 95% CI | 3.7–36.1 | 17.4–19.6 | NE | 8.8–31.4 |
| Patients with disease progression | 2 (15.4) | 8 (38.1) | 2 (20.0) | 12 (27.3) |
One patient had missing or unavailable response data; they died prior to receiving a post-baseline tumor assessment, as a result of pericardial effusion related to study disease and device-related infection. 95% CI for median duration of response was calculated using the method of Brookmeyer and Crowley; all others used the Clopper–Pearson method. Patients were classified as missing or NE if no post–baseline response assessments were available or all post–baseline response assessments were unevaluable. Clinical benefit was defined as an objective response or stable disease lasting for ≥24 weeks since first study treatment.
Figure 2. Antitumor activity of taselisib plus fulvestrant in patients with measurable or evaluable disease at baseline (safety evaluable population).
PD, progressive disease; PR, partial response; SD, stable disease; SLD, sum of the longest diameter
In patients with measurable disease at baseline, the overall CBR was 29.5% (13/44; 95% CI 16.8–45.2) (Table 4). The CBR was 38.5% (5/13; 95% CI 13.9–68.4) in patients with PIK3CA mutations, 23.8% (5/21; 95% CI 8.2–47.2) in patients with PIK3CA-MND and 30.0% (3/10; 95% CI 6.7–65.2) in patients with unknown PIK3CA mutation status (Table 4). Overall response rates and CBR for all 60 patients, including patients with and without measurable disease at baseline, are included in Supplementary Table S5.
Kaplan–Meier estimates of the median DoR in responders (n = 10), regardless of PIK3CA mutation status, was 19.6 months (range: 1.4 [censored]–36.1). Median DoR was 8.8 months (range: 3.7–36.1) in the subgroup of responding patients with PIK3CA-mutated tumors (n = 5) and 18.5 months (range: 1.4 [censored]–19.6) in responding patients with PIK3CA-MND (n = 3).
Median PFS was 7.6 months in patients with PIK3CA-mutated tumors (95% CI 4.9–13.7), 5.4 months in patients with PIK3CA-MND tumors (95% CI 1.8–10.0), and 5.3 months (95% CI 1.8–not evaluable [NE]) in patients with unknown PIK3CA mutation status. Median PFS was 6.5 months (95% CI 4.9–7.8) in all patients, irrespective of PIK3CA status.
Median OS was 19.2 months (95% CI 17.7–26.9) in 20 patients with PIK3CA-mutated tumors and 27.0 months (95% CI 15.2–32.9) in 27 patients with PIK3CA-MND tumors. OS was NE (95% CI 17.6–NE) in 13 patients with unknown PIK3CA mutation status.
Discussion
This phase II open-label study evaluated the efficacy and safety of taselisib plus fulvestrant in postmenopausal women with locally advanced or metastatic HER2-negative, HR-positive breast cancer. Overall, the combination of taselisib plus fulvestrant was generally well tolerated and clinical activity was observed. The safety profile of taselisib in combination with fulvestrant was consistent with previous reports (12, 16), with the most common AEs being diarrhea, nausea, fatigue, and decreased appetite. PI3K inhibitor class effects, as reported in other clinical trials of pan-PI3K inhibitors, also included AEs such as hyperglycemia, rash, stomatitis, and gastrointestinal toxicities (diarrhea), and these toxicities led to frequent dose modifications (21–24).
Treatment-emergent diarrhea was the most common AE in the present trial. The median time to onset of all-grade diarrhea was 1.7 months, and the median time to onset of grade ≥3 diarrhea was 4.7 months, suggesting that diarrhea with a higher grade had a longer latency. Diarrhea was manageable and reversible with anti-diarrheal medications, corticosteroids, and/or taselisib dose interruption and reduction.
Treatment-emergent colitis was the most common grade ≥3 AE and SAE, and the most common AE leading to dose reduction and discontinuation of taselisib in the present trial. Colitis was observed in patients treated with a pan-PI3K inhibitor (22), as well as with the delta-isoform-specific inhibitor, idelalisib (25). Pathological examination of tissue obtained from patients who experienced colitis showed that this AE was associated with infiltration of inflammatory cells and ulceration in some patients; similar to that reported with idelalisib (26). Our results, and those from a previous report (12), suggest that taselisib-associated treatment-emergent colitis can be managed by dose reductions and/or corticosteroid treatment, although some patients (5/60; 8.3%) discontinued therapy permanently due to this AE. Additionally, therapy was reinitiated after resolution of colitis in some patients, without subsequent additional events of colitis. As such, it is recommended that early patient reporting, close monitoring, and early intervention may lead to reduced severity of treatment-related toxicities, particularly diarrhea and colitis, thereby maintaining greater duration of therapy.
Antitumor activity was observed in this trial and the confirmed response rate of 22.7% (95% CI 11.5–37.8) and CBR of 29.5% (95% CI 16.8–45.2) in patients with baseline measurable disease were promising, although the sample size was small. Among patients with baseline measurable disease, there was a numerically higher ORR and CBR in patients with PIK3CA-mutated tumors (ORR: 38.5% [95% CI 13.9–68.4]; CBR: 38.5% [95% CI 13.9–68.4]) compared with patients with PIK3CA-MND tumors (ORR: 14.3% [95% CI 3.0–36.3]; CBR: 23.8% [95% CI 8.2–47.2]). The response rate of 38.5% (95% CI 13.9–68.4) in patients with PIK3CA mutations is promising given that the lower limit of the 95% CI exceeds the historical response rate achieved with fulvestrant alone (approximately 7–10%) in a similar patient population, including an ORR of 8.2% in patients with PI3K pathway-activated tumors treated with fulvestrant plus placebo in the BELLE-2 trial (19, 20, 24).
In the Phase Ia study of single-agent taselisib, objective responses were observed only in patients with PIK3CA-mutated tumors (12). In contrast, responses were reported in patients with both PIK3CA-mutated and PIK3CAMND when treated with taselisib in combination with fulvestrant in the present study. It is difficult, however, to draw conclusions regarding the magnitude of benefit conferred by taselisib in patients with PIK3CA-MND tumors as, in the present study, patients also received fulvestrant. Potential activity of combined PI3K and ER inhibition in both PIK3CA-mutated and -wild-type tumors may reflect the cross-talk between these two pathways in breast cancer.
As an open-label, single-arm Phase II trial, this study has several limitations. No comparator group was included and the cohort was highly selected. While the study enrolled 60 patients, only 47 patients had suitable tissue samples for PIK3CA mutation testing. The absence of information regarding the PIK3CA mutation status in 13 patients limits the power of the mutation analysis. Tissue samples were tested using the cobas® test that detects 17 PIK3CA hotspot mutations, potentially missing some tumors with non-hotspot rare PIK3CA mutations. Archival tumor samples were tested for PIK3CA mutations, with samples provided from primary tumor tissue or a metastatic site. In our study, approximately half of the samples submitted for testing were from metastatic tissue; however, primary tissue may be used as a surrogate for absent or unavailable metastatic tissue as there is generally a high concordance between PIK3CA mutation status from primary tumor samples compared to samples from metastatic sites or relapses (4, 27). Overall, 44 of 60 patients had baseline measurable disease, limiting the number of patients evaluable for objective response. Analysis by PIK3CA mutation status was retrospective and there were differences in baseline characteristics among subjects with different PIK3CA mutation status. This trial was also conducted prior to the approval of the cyclin-dependent kinase 4/6 inhibitors (palbociclib, ribociclib, abemaciclib) and, while this is not in itself a limitation of the study, these agents have been shown to increase PFS in patients with HR-positive, HER2-negative breast cancer (28–31). It remains to be seen how any new agent such as taselisib may be used either sequentially or in combination with cyclin-dependent kinase 4/6 inhibitors to further improve outcomes in this population.
Taselisib may offer an improved therapeutic window with a more favorable toxicity profile than pan-PI3K inhibitors, where higher rates of treatment discontinuations were reported in the pictilisib (22, 23) and buparlisib (24) treatment arms compared with the placebo arms. While preliminary activity of taselisib plus fulvestrant was observed in this single-arm trial, further study in a larger, randomized cohort study is required to determine whether taselisib has additive efficacy when combined with fulvestrant, and differential antitumor activity in PIK3CA-mutated versus PIK3CA-MND tumors. The efficacy and safety of the combination of taselisib plus fulvestrant is currently being evaluated in postmenopausal women with ER-positive, HER2-negative locally advanced or metastatic breast cancer in the Phase III randomized SANDPIPER study (clinicaltrials.gov: NCT02340221) (32). The Phase III trial is recruiting a population that is enriched with patients who have PIK3CA-mutated tumors as determined prior to enrollment by the centrally assessed cobas® PIK3CA Mutation Test in tumor tissue (32). Taselisib dosing for SANDPIPER (4 mg tablet daily) had an exposure equivalent to the 6 mg daily capsule used in this Phase II trial (33).
In conclusion, the results of this Phase II trial demonstrated that the combination of taselisib plus fulvestrant was tolerable in postmenopausal women with locally advanced or metastatic HER2-negative, HR-positive breast cancer. Preliminary clinical activity was observed; however, further study in a larger, ongoing randomized Phase III study will determine whether taselisib has additive efficacy when combined with fulvestrant in PIK3CA-mutated tumors.
Supplementary Material
Statement of Translational Relevance
The phosphatidylinositol 3-kinase (PI3K) pathway is essential for normal cell function and is implicated in the pathogenesis of hormone receptor (HR)-positive breast cancer. Taselisib (GDC-0032) is a potent and selective PI3K inhibitor with greater efficacy against mutant than wild-type phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit-alpha (PIK3CA). Inhibition of PI3K signaling in HR-positive breast cancer results in upregulation of estrogen receptor (ER)-dependent function. The combination of PI3K inhibition and ER inhibition with fulvestrant produces marked tumor regression in breast cancer models, providing a rationale for dual PI3K and ER inhibition in breast cancer. In this trial, women with advanced HR-positive breast cancer received oral taselisib plus intramuscular injections of fulvestrant. The adverse event profile was typical of that previously reported with PI3K inhibitors, and objective responses were obtained in women with PIK3CA-mutated and -mutation-not-detected tumors. On this basis, taselisib plus fulvestrant is being evaluated in a Phase III study.
Statement of significance (a one- or two-sentence summary of the manuscript’s salient findings)
Taselisib plus fulvestrant had clinical activity in patients with locally advanced or metastatic HER2-negative, hormone receptor-positive breast cancer, with a numerically higher objective response rate in PIK3CA mutant vs mutation-not-detected tumors. No new safety signals were reported and a confirmatory Phase III trial is ongoing.
Acknowledgments
Financial support: This study was funded by Genentech, Inc. / F. Hoffmann-La Roche Ltd. Maura N. Dickler is supported in part by the Memorial Sloan Kettering Cancer Center (MSKCC) Core Grant (P30 CA 008748).
M.N. Dickler has received fees in a consultant or advisory role from Novartis, Pfizer, Genentech/Roche, AstraZeneca, TapImmune, PUMA Biotechnology, Hengrui, Celldex, G1 Therapeutics, and Eli Lilly, and research funding from Novartis, Eli Lilly, Sanofi Aventis, and Genentech/Roche. C. Saura has received fees in a consultant or advisory role from Genentech/Roche, Pfizer and PUMA Biotechnology, and institutional research funding from Roche/Genentech. I.E. Krop has received fees in a consultant or advisory role, and research funding, from Genentech/Roche. P.L. Bedard has received institutional fees in a consultant or advisory role from Pfizer, Genentech/Roche, and Sanofi, and institutional research funding from Bristol-Myers Squibb, Sanofi, Genentech/Roche, AstraZeneca, SERVIER, GlaxoSmithKline, Oncothyreon, Novartis, SignalChem, and PTC Therapeutics. L. Pusztai has received institutional research funding from Genentech. M. Oliveira has received fees in a consultant or advisory role from Genentech/Roche and PUMA Biotechnology, and institutional research funding from Roche/Genentech. A.K. Cardenas has received fees in a consultant or advisory role from Roche, fees from speakers’ bureau from Roche, research funding from Roche/Genentech, and travel, accommodation, or expenses from Roche. A.K. Cardenas, N. Cui, T.R. Wilson, T.J. Stout, M.C. Wei, and J.Y. Hsu are employees of Genentech, Inc., South San Francisco, CA. A.K. Cardenas, T.R. Wilson, T.J. Stout, and M.C. Wei own stock in Roche. The funding organization participated in and supported the preparation, review, and approval of the manuscript.
This study was funded by Genentech, Inc., South San Francisco, CA. Support for third-party writing assistance for this manuscript, furnished by Islay Steele, PhD, and Blair Jarvis, MSc, of Health Interactions, was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland. We thank Roche Molecular Diagnostics (Pleasanton, CA) for developing the cobas® PIK3CA Mutation Test (research use only).
Footnotes
Disclosure of Potential Conflicts of Interest
The remaining authors declare no potential conflicts of interest.
Disclosure of prior publication
These analyses have been presented in part: Maura N. Dickler, Cristina Saura, Donald A. Richards, Ian E. Krop, Andrés Cervantes, Philippe L. Bedard, Manish R. Patel, Lajos Pusztai, Mafalda Oliveira, Joseph A. Ware, Huan Jin, Timothy R. Wilson, Thomas Stout, Michael C. Wei, Jerry Y. Hsu, José Baselga. A phase II study of the PI3K inhibitor taselisib (GDC-0032) combined with fulvestrant (F) in patients (pts) with HER2-negative (HER−), hormone receptorpositive (HR+) advanced breast cancer (BC). American Society of Clinical Oncology Annual Meeting 2016, June 3–7, 2016, Chicago, IL; Abstract 520. Published in J Clin Oncol 2016;34(15 Suppl):Abstract 520: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.520.
Authors’ contributions
Conception and design:
Development of methodology:
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M.N. Dickler, C. Saura, D.A. Richards, I.E. Krop, A. Cervantes, P.L. Bedard, M.R. Patel, L. Pusztai, M. Oliveira, T.R. Wilson
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M.N. Dickler, P.L. Bedard, A.K. Cardenas, T.R. Wilson, T.J. Stout, M.C. Wei
Writing, review, and/or revision of the manuscript: M.N. Dickler, C. Saura, D.A. Richards, I.E. Krop, A. Cervantes, P.L. Bedard, M.R. Patel, L. Pusztai, M. Oliveira, A.K. Cardenas, N. Cui, T.R. Wilson, T.J. Stout, M.C. Wei, J.Y. Hsu, J. Baselga
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases):
Study supervision: A.K. Cardenas, M.C. Wei, J.Y. Hsu
References
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 3.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur LM, Turnbull AK, Renshaw L, Keys J, Thomas JS, Wilson TR, et al. Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine-treated breast cancer. Breast Cancer Res Treat. 2014;147:211–9. doi: 10.1007/s10549-014-3080-x. [DOI] [PubMed] [Google Scholar]
- 5.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–19. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): A β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem. 2013;56:4597–610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- 9.Olivero AG, Heffron TP, Baumgardner M, Belvin M, Ross LB, Blaquiere N, et al. Discovery of GDC-0032: A beta-sparing PI3K inhibitor active against PIK3CA mutant tumors. Cancer Res. 2013;73 Abstract DDT02-01 (and associated oral presentation) [Google Scholar]
- 10.Savage H, O'Brien C, Spoerke J, Huw L, Wallin J, Friedman L, et al. Development of a predictive biomarker gene expression signature for the PIK3CA inhibitor, GDC-0032, in breast cancer cells. Cancer Res. 2013;73 Abstract P6-05-09. [Google Scholar]
- 11.Wallin JJ, Edgar KA, Guan J, Sampath D, Nannini M, Belvin M, et al. The PI3K inhibitor GDC-0032 is selectively potent against PIK3CA mutant breast cancer cell lines and tumors. Cancer Res. 2013;73 Abstract P2-17-01 (and associated poster presentation) [Google Scholar]
- 12.Juric D, Krop I, Ramanathan RK, Wilson TR, Ware JA, Sanabria Bohorquez SM, et al. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 2017;7:704–15. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7:283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toska E, Osmanbeyoglu HU, Castel P, Chan C, Hendrickson RC, Elkabets M, et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science. 2017;355:1324–30. doi: 10.1126/science.aah6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juric D, Saura C, Cervantes A, Kurkjian C, Patel MR, Sachdev J, et al. Ph1b study of the PI3K inhibitor GDC-0032 in combination with fulvestrant in patients with hormone receptor-positive advanced breast cancer. Cancer Res. 2013;73 Abstract PD1-3 (and associated poster presentation) [Google Scholar]
- 17.US Department of Health and Human Services, National Institutes of Health (NIH) and National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010). Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: Results from EFECT. J Clin Oncol. 2008;26:1664–70. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 20.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 21.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 22.Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:811–21. doi: 10.1016/S1470-2045(16)00106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuylsteke P, Huizing M, Petrakova K, Roylance R, Laing R, Chan S, et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann Oncol. 2016;27:2059–66. doi: 10.1093/annonc/mdw320. [DOI] [PubMed] [Google Scholar]
- 24.Baselga J, Im SA, Iwata H, Cortés J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–16. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–18. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louie CY, DiMaio MA, Matsukuma KE, Coutre SE, Berry GJ, Longacre TA. Idelalisib-associated enterocolitis: Clinicopathologic features and distinction from other enterocolitides. Am J Surg Pathol. 2015;39:1653–60. doi: 10.1097/PAS.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 27.Fumagalli D, Wilson T, Salgado R, Lu X, Yu J, O'Brien C, et al. Somatic mutation, copy number and transcriptomic profiles of primary and matched metastatic estrogen receptor-positive breast cancers. Ann Oncol. 2016;27:1860–6. doi: 10.1093/annonc/mdw286. [DOI] [PubMed] [Google Scholar]
- 28.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 29.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 30.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 31.Sledger GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–84. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 32.Baselga J, Cortés J, De Laurentiis M, Dent S, Diéras V, Harbeck N, et al. SANDPIPER: Phase III study of the PI3-kinase (PI3K) inhibitor taselisib (GDC-0032) plus fulvestrant in patients (pts) with estrogen receptor (ER)-positive, HER2-negative locally advanced or metastatic breast cancer (BC) enriched for pts with PIK3CA-mutant tumors. J Clin Oncol. 2017;35 Abstract TPS1119 (and associated poster presentation) [Google Scholar]
- 33.Faber KP, Borin MT, Cheeti S, Fraczkiewicz G, Nelson E, Ran Y, et al. Impact of formulation and food on taselisib (GDC-0032) bioavailability: Powder-in-capsule formulation represents unique drug development challenge. Clin Pharmacol Ther. 2016;99(Suppl 1):S48. (Abstract P1-067) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.