Abstract
A roadblock to successful treatment for anxiety and depression is the high proportion of individuals that do not respond to existing treatments. Different underlying neurobiological mechanisms may drive similar symptoms, so a more personalized approach to treatment could be more successful. There is increasing evidence that sex is an important biological variable modulating efficacy of antidepressants and anxiolytics. We review evidence for sex-specific effects of traditional monoamine based antidepressants and newer pharmaceuticals targeting kappa opioid receptors (KOR), oxytocin receptors (OTR), and N-methyl-D-aspartate receptors (ketamine). In some cases, similar behavioral effects are observed in both sexes while in other cases strong sex-specific effects are observed. Most intriguing are cases such as ketamine which has similar behavioral effects in males and females, perhaps through sex-specific neurobiological mechanisms. These results show how essential it is to include both males and females in both clinical and preclinical evaluations of novel antidepressants and anxiolytics.
Keywords: antidepressants, depression, kappa opioid receptor, ketamine, oxytocin social behavior, sex differences
Introduction
Depression and anxiety disorders place a large burden on society [1]. Through a combination of serendipity and systematic research, a variety of pharmaceutical and counseling based therapies have been developed. For those who respond positively to these treatments, the results can lead to dramatically positive outcomes. However, over half of individuals that seek treatment do not respond positively to currently available pharmacotherapy treatment options [2]. This heterogeneity in treatment outcomes has driven research for new therapeutics as well as understanding of the mechanisms that contribute to positive behavioral outcomes. There is growing awareness that there are important sex differences in the prevalence of depression and anxiety disorders [3], and in the pharmacokinetics and pharmacodynamics of antidepressants [4; 5; 6]. Differences in pharmacokinetics may lead to sex differences in the efficacy of antidepressant treatments [7]. An important risk factor for anxiety and depression-related disorders is exposure to psychosocial stress, and there are important sex differences in physiological responses to stress [8; 9; 10]. Together, it is likely that there are important sex differences in the mechanisms contributing to the behavioral symptoms associated with anxiety and depression. Until recently most studies examining the neurobiological mechanisms of mood disorders and antidepressants have focused only on males. However, growing awareness of sex differences in neurobiology and behavior [11; 12; 13; 14; 15] and new research policies in the United States [16] have increased focus on how sex as a biological variable can modulate behavioral responses to pharmaceuticals with antidepressant or anxiolytic properties. This shift should ultimately contribute to better treatments for mood and anxiety disorders.
Here we review the growing literature assessing how sex affects the efficacy and neurobiological mechanisms of pharmaceuticals with antidepressant and anxiolytic properties. While medications targeting monoamine systems are the most established, depression-like and anxiety-like behaviors can be induced independently of monoamine systems [17]. Additionally, serotonin-based therapeutics can have important adverse effects [18]. Alternative therapeutic targets could provide a mechanism for treating individuals that do not respond well to standard monoamine-targeting treatments. Anxiety and depression are often comorbid [19; 20], which suggests at least some neurobiological mechanisms contribute to both disorders. However, the extent of comorbidity is variable, suggesting that there is some degree of independence in mechanisms. Thus, as novel antidepressants and anxiolytics are identified and studied, it is critical to understand their mechanisms of action. Here we discuss compounds targeting opioid receptors, neuropeptide receptors, and glutamate receptors, which have anxiolytic and/or antidepressant properties. A consistent theme across all of these classes of pharmaceuticals is that sex-specific behavioral or neurobiological effects have been reported. Below we review evidence for sex differences in the behavioral and neurobiological effects of these novel classes of therapeutics.
Selective Serotonin Reuptake Inhibitors
The anti-depressant properties of the most commonly prescribed medications were in some ways discovered by accident [21; 22]. The first selective-serotonin reuptake inhibitor (SSRI), fluoxetine, was discovered in the 1970s [23; 24], and the development of SSRIs continued into the 1980s [25; 26; 27]. While these drugs have been the most prescribed medication for depression for decades, their mechanisms of action are only partially understood. Sex differences in mechanisms of antidepressant therapeutics have been reviewed previously [28], so we briefly review two primary mechanisms: pharmacokinetics and pharmacodynamics, and sex hormones [7].
It has been suggested that physiological and molecular differences in males and females affect the pharmacokinetics and pharmacodynamics of antidepressant drugs [6]. One possible contributing factor may be enzymes that metabolize drugs, cytochrome P450s (CYP). Sex differences in the expression or activity of these enzymes [4; 5] can cause faster drug metabolism and affect drug clearance (but see [29]). For example, the SSRI sertraline has a longer half-life in women compared to men [30], which suggests that a single dose of sertraline has a longer duration of action in women. Consistent with this, plasma concentrations of sertraline are at least 50% higher in women compared to men [30; 31]. Higher plasma concentrations in women could be clinically significant, and may be relevant to reports that behavioral responses to SSRIs are stronger in women compared to men [32]. This finding suggests that women are more sensitive to lower doses of SSRIs than men. This sex differences has also been observed in animal models of depression and anxiety [33; 34; 35]. In additional to sex differences in drug metabolism, gonadal hormones are important modulators of SSRI action.
The relationship between serotonin and estrogens has been noted in the context of affect and mood and cognition [36; 37], as well as in the context of mood disorders [38]. In particular, estrogen receptor β (ERβ) is typically associated with having both antidepressant [39] and anxiolytic effects on behavior [40; 41; 42] in both male and female rodents. One study suggested that in female rodents, estrogens may facilitate the action of SSRIs [43]. Although the mechanism of action through which this occurs is unknown, treatment with estradiol in the dorsal raphe nucleus (DRN) has been to shown to increase the expression of tryptophan hydroxylase 2 (TPH2) [41], the brain-specific serotonin synthesizing enzyme [44]. This finding suggested that estradiol may have an indirect effect of increasing serotonin synthesis in the DRN through its modulation of TPH2 expression.
Given the established relationship between ERβ and depression- and anxiety-like behavior, ERβ modulation of TPH2 expression may contribute to the effects of estrogens on these behaviors. One study showed that treatment with 17β-estradiol or an ERβ agonist in the DRN increased TPH2 mRNA [39]. Similarly, an in vitro study using cultured medullary raphe serotonin neurons shows that 17β-estradiol activation of ERβ increased transcription of TPH2 [45]. This same study also showed that an estrogen response element (ERE) exists within the TPH2 5′-untranslated region. It is possible estrogen receptor binding to this ERE alters TPH2 expression. Further study is needed to test whether this promoter modulates to the ability of estrogens to facilitate the action of SSRIs.
In humans, ovarian hormones may also modulate antidepressant efficacy. Clinical studies show that premenopausal women respond better to SSRIs than both men [46], older women [47], and postmenopausal women [48]. In a study comparing SSRI use in combination with hormone replacement therapy, postmenopausal women responded better to SSRI treatment while undergoing this therapy than postmenopausal women receiving an SSRI alone [49]. Taken together, these data support the hypothesis that ovarian hormones play a role in the efficacy of SSRI treatments. However, not every study has reported effects of sex [50] or of menopausal stage [51; 52] on antidepressant response. An issue with many of these studies is a lack of a proper placebo control, small sample sizes, uneven age-matched groups, and a lack of randomly assigned groups. Additionally, the treatment periods observed between studies vary widely, so it is difficult to determine whether the inconsistencies in findings are because sex and menopausal stage do not actually effect drug response, or whether the lack of differences observed in these studies are an artifacts of differences in methodology. Finally, a major weakness of many studies is the use of premarine as hormone replacement therapy, which is a complex preparation of estrogen and progestin metabolites. More studies examining this topic, using larger sample sizes and consistent methods (such as purer hormone treatments) are necessary to determine the extent to which estrogens modulate SSRI treatment efficacy.
Kappa Opioid Receptors
Kappa-opioid receptors (KOR) have generated strong interest as a potentially novel therapeutic target for the treatment of mood disorders and anxiety. KOR are located widely throughout the brain, including throughout areas in the limbic system as well as the prefrontal cortex, dorsal raphe, and the periaqueductal gray [53]. The activation of these receptors by agonists induces depression-like states in humans [54] as well as in rodents [55]. In contrast, preclinical data indicate that antagonists have the potential to block many behavioral phenotypes induced by stress. These KOR-induced responses to stress are likely mediated by dynorphin, the endogenous ligand for KOR [56]. The role of the dynorphin/KOR system in stress-induced behaviors has been studied extensively (for review, see [57; 58]). Overall, there is strong evidence that KOR plays a role in the physical, psychological, and aversive experiences of stress. Despite these promising foundations, initial tests of KOR antagonists in clinical trials were unsuccessful due to the onset of short-term cardiac diseases, bradycardia and ventricular tachycardia, in some patients [59; 60]. Although several factors may have contributed to this unsuccessful outcome, the prolonged inhibition of KOR characteristic of many antagonists is likely an important factor. Despite this initial setback, there are some exciting new directions.
The KOR antagonists JDTic and norBNI were considered promising pharmaceuticals based on their performance in behavior tests predictive of antidepressant efficacy, and their ability to reduce the impact of stress on behavior. A single infusion of the KOR antagonist norBNI reduced immobility in male C57BI/6J mice and in male rats [61; 62]. Consistent with these results, the KOR agonist U-69593 dose-dependently increased immobility while norBNI pretreatment blocked this effect in male rats [63], indicating a distinct role of KOR activity in stress-induced immobility. Further support for the therapeutic potential of KOR antagonists came from animal models of stress-induced depression and addiction relapse. For example, norBNI blocked the formation of learned helplessness following inescapable shock in male rats [64], and reduced submissive behavior in male mice exposed to social defeat stress [55; 65]. In the reinstatement model of relapse, stressors such as forced swim can reinstate an extinguished place preference for cocaine, and KOR antagonists can prevent the reinstatement of this preference [61; 65; 66]. Taken together, these results suggest that activation of KOR during stress facilitates depression- and anxiety-like behaviors. One question that has been difficult to answer is how the time course of KOR activation impacts behavioral responses to stress. The most common KOR antagonists, norBNI and JDTic, inhibit KOR for weeks after a single dose. Because of this long-lasting action it’s been unclear whether the effects of KOR activity on behavior are due to the activity of KOR during stress exclusively, or if prolonged KOR activity is necessary for depression-like phenotypes. Recent work with new short-acting KOR antagonists have shed light on this important question.
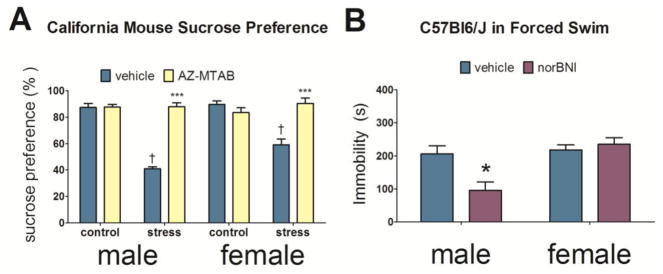
Previous work with long-lasting KOR antagonists have suggested that KOR antagonists have greater efficacy if administered before stress exposure. Interestingly, when the short-acting KOR antagonist, CERC-501, was administered after social defeat stress, social avoidance was unaffected [67]. In contrast, short-acting KOR antagonists administered immediately before episodes of social defeat stress had strong effects on behavior. Male and female California mice treated with a short-acting KOR antagonist, AZ-MTAB, 2h prior to episodes of social defeat stress blocked the development of anhedonia two weeks later (Fig. 1A) [68]. In females, AZ-MTAB also prevented the development of social avoidance. Importantly, AZ-MTAB treatment that was administered not during stress, but immediately before anhedonia or social interaction testing 2 weeks later, had no effect on behavior. Together these results suggest that KOR activation during stressful events induces long lasting neurobiological changes that promote depression-like behaviors for at least two weeks. Interestingly, treatment with the long-acting KOR antagonist JDTic prior to 10 days of chronic social defeat stress did not prevent the on-set of social withdrawal behavior or anhedonia in male mice [69]. This suggests that there are important differences between acute and prolonged inhibition of KOR. In addition, there is growing evidence that stress may alter the behavioral effects of KOR.
Figure 1. Effects of KOR antagonists in the forced swim test and on anhedonia.
Effects of AZ-MTAB on percent preference levels for sucrose water two weeks following social defeat stress in California mice (A, 7–14 per group). Effects of norBNI on time spent immobile in the forced swim test in C57Bl6/J mice (B, n=5–10 per group). *p<0.05 versus vehicle; ***p<0.0001 versus same-sex stress/vehicle; † p < 0.01 vs. same-sex control/vehicle.
While there is strong evidence that acute activation of KOR induces depression-like behavior and aversion, there is growing evidence that these effects become weaker after exposure to stress. Many of the early studies examining the ability of KOR antagonists to reverse the effects of stress on behavior conducted tests within 24h of stress exposure. For example, experiments showing KOR-dependent social avoidance in male mice assessed social interaction immediately after a single episode of social defeat [70]. However, other studies with longer periods of stress exposure or a delay between stress exposure and behavioral testing have reported different outcomes. In male c57BI/6J mice that lost aggressive encounters over a period of 3 weeks, the KOR agonist U50,488 actually increased social interaction behavior [71; 72]. Consistent with these results, infusion of U50,488 in to the dorsal raphe of male California mice exposed to social defeat reduced freezing behavior in a resident-intruder test [73]. In California mice [74] and hamsters [75], social defeat increases freezing behavior in residents tested in the resident-intruder test. Other evidence suggests that different forms of stress can reduce the aversive properties of KOR. Both chronic mild stress and chronic social defeat stress weakened KOR-dependent reinstatement of cocaine and nicotine place preference in male c57BI/6 mice [76]. Similar results were seen in female California mice, where control females but not defeated females formed a place aversion to a low (2.5mg/kg) dose of U50,488 [77]. Taken together, this suggests that over time, stress-induced neuroadaptations may change the behavioral effects of KOR.
A series of studies have identified molecular pathways that mediate the short-term effects of KOR on behavior. Activation of KOR results in coupling to inhibitory Gi/o proteins [78], which in turn can induce activation of mitogen activated protein kinase (MAPK) signaling pathways via phosphorylation [79; 80; 81]. Interestingly, several MAPK can be activated by KOR and it’s thought that selective activation of specific signaling cascades can mediate different behavioral outcomes [82]. For example, forced swim stress induces KOR-dependent phosphorylation of p38 MAPK and extracellular signal-regulated (ERK 1/2) in male C57Bl/6J mice [83]. Infusion of the p38 MAPK inhibitor intracerebroventricularly (icv) prevented U50,488-induced immobility in the FST. Similarly, KOR activated p38 MAPK in serotonin neurons in the dorsal raphe, and dopamine neurons in the ventral tegmental area, are necessary for KOR- and stress-induced conditioned place aversion [84; 85]. Together these data suggest that p38 MAPK signaling following KOR activation is necessary for acute stress-induced behavioral responses. The extent to which this mechanism generalizes is still unclear, since all of these studies were performed in male C57Bl/6J mice. Male and female California mice treated with U50,488 show dose dependent increases in phospho-p38 immunoreactive cells in the nucleus accumbens, but only in microglia [86]. It has been proposed that the binding of different ligands to KOR may result in different signaling cascades being activated, and that these different signaling pathways may have differing effects on behavior [81]. The development of biased agonists could potentially provide novel therapeutic tools. It will be necessary to test the effects of new KOR targeting pharmaceuticals in both sexes because there is growing evidence for important sex differences in KOR function.
While the few studies examining KOR function in females cited above found similar behavioral effects of KOR in females and males, several lines of evidence indicate the potential for important sex differences. For example, sex differences exist in behavioral sensitivity of KOR agonists in males and females. Most evidence suggests that KOR activation has stronger effects on behavior in males than females [87]. U50,488 induces anhedonia for intracranial self-stimulation at lower doses in male rats compared to females [88] and female rats take longer to discriminate a KOR agonist from vehicle using a fixed ratio schedule of reinforcement [89]. Consistent with these data, U50,488 infusions in to the dorsal raphe have stronger effects on behavior in male California mice than in females [73]. Finally, across a range of doses, norBNI does not reduce immobility in the FST in female California mice or c57BI/6 mice as it does in males (Fig. 1B) [90].
Across these studies KOR agonists and antagonists have stronger effects in males than in females. However, different results have been observed in place aversion studies. In California mice, females naïve to defeat form a CPA to a 2.5mg/kg dose of U50,488. However, at a higher 10mg/kg dose, this aversion is no longer observed. In stressed females, two weeks following social defeat stress, CPA is not formed towards either dose of U50,488 [77]. In contrast, male California mice naïve to defeat form a CPA to a high but not low dose of U50,488, and following stress, males continue to show CPA to the high dose [77; 86]. This suggests that before stress, females show a higher sensitivity to KOR agonists than males but that that following stress, KOR agonists no longer show aversive properties in females. In these studies, place aversion is induced after repeated exposure to U50,488 and develops over several days, whereas most studies reporting stronger effects of KOR manipulations examine behavior over a shorter timeline. Further study is needed to determine whether there are consistent sex differences in the short- and long-term effects of KOR activation on behavior.
Moving forward, more data are needed on the mechanisms of action for KOR ligands in males and females. For example, while norBNI induces phosphorylation of JNK in the NAc of male C57Bl6/J, this effect is not observed in females [90]. Even less is known about the mechanism of action for short-acting KOR antagonists. To our knowledge this has not been studied in female animals or cells. Additional studies examining sex differences in behavioral responses to KOR activation or inhibition in different contexts, as well as in mechanisms of action for KOR, is needed in order to understand whether this line of potential therapeutics can be successful in both sexes. At present, it would appear that short-acting KOR antagonists may prove more valuable if used in a prophylactic approach before stress exposure [91].
Oxytocin and oxytocin receptors
Converging lines of evidence suggest that pharmaceuticals targeting the oxytocin (OT) system could have important therapeutic uses. First, studies in nonhuman animals show that OT can reduce physiological stress response. Second, work in humans shows that intranasal OT is capable of having anxiolytic effects, at least in men. However, studies testing the efficacy of intranasal OT in clinical populations have been underwhelming (see [92] for review). One factor has been that most studies were small and focused on short term outcomes. A broader challenge is that the mechanisms through which OT modulates anxiety and social behavior are not understood. In rodents, oxytocin receptors (OTR) are expressed in hypothalamic and limbic circuits that modulate social behavior as well as stress responses [93; 94]. In the few primate species that have been examined, OTR is more widely expressed in new world primate species [95] compared to old world primates such as rhesus monkey [96]. Studies in rodents have identified neural circuits in which OT can exert anxiolytic effects. However, the majority of these studies focus exclusively on males and there is growing evidence for strong sex differences in OT modulation of anxiety, especially in aversive social contexts. Furthermore, it is usually assumed that the behavioral effects of OT are mediated by OTR. Several lines of evidence indicate that behavioral effects of OT can be mediated through activation of the vasopressin V1a receptor [97; 98; 99]. Thus, it is critical to consider how any OT-related ligand interacts with both OTR and V1aR. Overall, while the OT system remains a promising target for novel therapeutics, many unanswered questions remain.
The idea that OT might act as a damper of physiological responses to stress grew in part out of findings in rodents that various forms of stressors increased OT levels in the blood [100; 101; 102]. When an OTR antagonist was infused in to the lateral ventricle, adrenocorticotropin hormone and corticosterone levels in both male and female rats were increased [103]. This effect was observed under stressful conditions like forced swim as well as under baseline conditions. Similarly, chronic systemic [104] or central [105] administration of OT reduced stress-induced corticosterone in female rodents. While the HPA axis is frequently disrupted in disorders such as depression and PTSD, roughly half of patients do not exhibit disrupted cortisol secretion [106]. Nonetheless, the inhibitory effects of OT and OTR on the HPA axis sparked investigations of oxytocin modulation of anxiety-like behavior.
Overall, most evidence suggest that in both males and females OT and OTR activation reduce anxiety behaviors in novel environments. While an initial study reported no effect of icv infusion of an OTR antagonist in the elevated plus maze [103], protocols that increased the novelty of the testing environment observed anxiolytic effects of chronic central infusion of OT in female rats [105]. In male mice a single icv infusion of OT had anxiolytic effects in the elevated zero maze [78] while in male rats, a single infusion of OT into the paraventricular nucleus (PVN) had anxiolytic effects in the elevated plus maze (EPM) [107]. These effects appear to be mediated by OTR, as icv infusion of the OTR-specific agonist carbetocin also had anxiolytic effects in male rats tested in the EPM [108]. Site-specific infusion of OT in to the central nucleus of the amygdala (CEA) also had anxiolytic effects in non-social contexts in female rats [109; 110]. While these studies generally report similar results in males and females, sex differences emerge in social contexts, especially aversive contexts.
In males, most data point towards anxiolytic effects of OT and OTR in social contexts. Male rats that were exposed to social defeat stress showed reduced social avoidance after receiving an icv infusion of OT [111]. Consistent with this effect, intranasal OT also decreased social avoidance behavior in male California mice that were exposed to social defeat [112] while icv OTR antagonist treatment reduced social interaction in unstressed male rats [111]. A related approach pairs foot shocks with unfamiliar social interactions to induce a conditioned avoidance of novel social contexts [114]. Infusion of a selective OTR agonist in to the lateral ventricle reduced this conditioned social-fear response in male rats [89]. In addition, OT infusions in to the lateral septum facilitated extinction of conditioned social fear trials conducted in a familiar home cage [115]. In human studies, OT is most often administered intranasally. Although OT is clearly behaviorally active, it’s still uncertain whether OT crosses the blood brain barrier and if so, the extent to which OT diffuses into different brain structures [9]. These uncertainties aside, intranasal OT was found to induce “calmness” [116] and reduce negative affect [117] in men following the Trier Social Stress Test (TSST) in which the audience consisted of two women. Imaging experiments showed that in men, intranasal OT reduced amygdala responses to emotional faces of both positive and negative valences [118], including angry faces [119; 120]. Similar inhibitory effects of intranasal OT on amygdala activity were observed in men with generalized anxiety disorder, although there was no effect of OT on subjective mood [121]. Together, these results suggest OT and OTR are anxiolytic in social contexts, at least for males. However, analyses of OT function during stressful social contexts indicate that OT can have anxiogenic effects.
Social defeat increases c-fos expression in OT neurons within the PVN [112; 122] in both males and females, suggesting that these neurons are activated by social defeat. Consistent with these results, social defeat is associated with increased OT release in the lateral septum [123]. Interestingly, activation of OTR in the lateral septum enhances contextual fear conditioning six hours later [124]. The enhancement of adverse memories by OTR may alter behavior in future social encounters, as OTR knockout mice showed reduced submissive behaviors during a second episode of defeat [122]. In humans, men treated with intranasal OT reported higher levels of perceived stress while receiving negative evaluative feedback during a mental arithmetic task [125]. Intranasal OT also had an anxiogenic effect in men diagnosed with depression who were instructed to discuss a distressful personal incident with a therapist [126]. Together, these results suggest that in the short term, activation of OT or OTR during aversive social contexts amplify the effects of this experience on behavior in males. This is consistent with the social salience hypothesis of OT, which posits that OT enhances the salience of both positive and negative social experiences [127]. Less is known about how defeat affects OT and OTR systems in females. Recent evidence indicates that the long term effects of defeat on OT and OTR systems show important sex differences [112].
While OT and OTR are generally anxiolytic in social contexts for males, experiments across multiple species have failed to detect anxiolytic effects in females. Systemic OT treatment did not reduce anxiety-inducing effects of social isolation [128] while icv infusion of OT did not reduce social avoidance in stressed female rats [129]. Furthermore, the same dose of intranasal OT that reduced social avoidance in male California mice exposed to social defeat induced social avoidance in female California mice that were naïve to defeat [112]. Interestingly, analyses of OT neurons in the PVN and anteroventral bed nucleus of the stria terminalis (BSTav) showed that social defeat increases the reactivity of these neurons (as estimated by OT/c-fos immunohistochemistry) in female but not male California mice [112]. This effect was seen up to 10 weeks after the last episode of social defeat, indicating that these changes are persistent. Immediate early gene analyses suggested the nucleus accumbens (NAc) and anteromedial BST (BSTam) as possible sites mediating OT-induced social avoidance in females. Site specific infusions of an OTR antagonist in stressed females showed that OTR inactivation in the BSTam but not NAc reduced social avoidance responses. Together these results suggest that OTR antagonists might have potential for reducing social anxiety. Supporting this hypothesis, a single i.p. treatment of 5 mg/kg the brain-accessible OTR antagonist L-368,899 reduced social avoidance in stressed females [130]. This is an exciting result because 4 weeks of daily treatment are necessary to obtain the same response with sertraline [35]. Women are generally under-represented in intranasal OT studies, even though depression and anxiety disorders are more common in women. What little evidence is available suggests that the effects on intranasal OT are sex dependent in humans. While intranasal OT reduced negative affect following the TSST in men, the same dose of OT increased negative affect in women [117]. Consistent with these data, imaging studies show that intranasal OT enhances amygdala [87; 131; 132] and anterior cingulate responses [133; 134] to fearful faces and scenes. Curiously, one study reported that while intranasal OT increased amygdala reactivity to emotional faces in healthy trauma-exposed women, women diagnosed with PTSD showed reduced responses [135]. Although the mechanism for these inconsistent results is unknown, one report showed that social defeat stress reduced sex differences in DNA methyltransferase 1 mRNA within the central nucleus of the amygdala [136]. If this result generalizes to other species and stressors, a change in DNA methylation could potentially have important effects on how amygdala nuclei respond to neuropeptides such as OT. In summary, it is clear that there are important sex differences in the behavioral effects of OT and OTR in aversive social contexts but the mechanisms underlying these differences have not yet been identified.
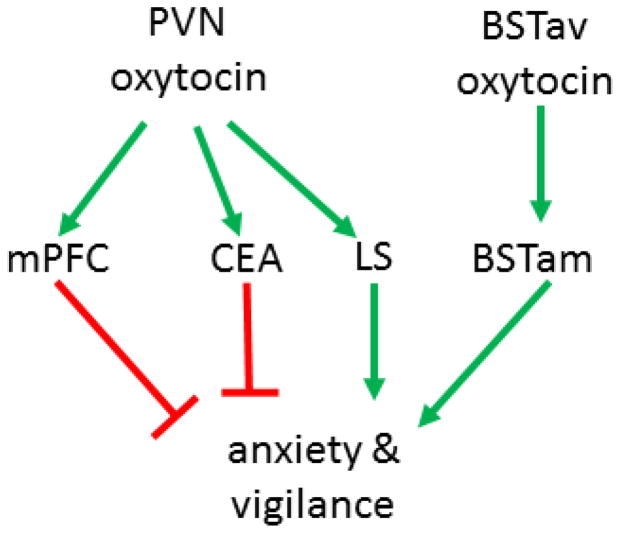
Observations that increased OT activity can have anxiogenic effects suggests alternate interpretations for some clinical findings. Women diagnosed with depression [137; 138] or PTSD [139] have been found to have elevated OT levels in plasma, and this result is usually interpreted as a homeostatic response to cope with stressful experiences. Mechanistic experiments with OTR ligands suggest that enhanced activation of OTR might actually have anxiogenic effects, at least in aversive social contexts. Familiar social contexts may be different. Female California mice exposed to social defeat showed reduced freezing behavior if treated with intranasal OT in the home cage [112]. Activation of OTR also had anxiolytic effects in female prairie voles while recovering from restraint stress in a familiar home cage [140]. One possible explanation for these diverse behavioral effects is that different neural circuits may mediate anxiogenic and anxiolytic effects of OT (Fig. 2). While inhibition of OTR in the BST increased social interaction in stressed female California mice, infusions of OT into prelimbic cortex had anxiolytic effects in female rats [141]. While the endogenous source of OT may differ between the mPFC and BSTam, the CEA and LS both receive OT fibers from the PVN. Currently, it is not clear whether different OT neurons within the PVN innervate the CEA and LS or whether the behavioral effects of OTR activation are context dependent. Further characterization and manipulation of these circuits is needed to understand how OT exert such different effects on behavior. Overall it appears that OTR agonists and antagonists have potential to reduce social anxiety, as well as other disorders with social deficits [142; 143]. The continued development of novel OTR ligands also holds great promise for more selective approaches for modulating OTR function [144]. The preclinical data suggest that successful use of OTR ligands to treat social anxiety will require a personalized approach as opposed to a one-size fits all strategy.
Figure 2. Proposed oxytocin circuit in female California mice.
Neural circuits mediating anxiolytic and anxiogenic effects of OT. Oxytocin neurons within the PVN send projections to the mPFC and CEA where activation of OTR exerts anxiolytic effects. Oxytocin neurons in the BSTav are activated by stress and OTR in the adjacent BSTam induces social anxiety responses. The LS also received OT projections from the PVN, but activation of OT in LS can promote social fear. PVN: paraventricular nucleus, mPFC: medial prefrontal cortex, CEA: central nucleus of the amygdala, LS: lateral septum, BSTav: bed nucleus of the stria terminalis anteroventral, BSTam: bed nucleus of the stria terminalis anteromedial.
Ketamine
In the mid-1990’s several studies reported that antagonists for the N-methyl-D-aspartate (NMDA) receptor had antidepressant-like effects in animal model systems such as learned helplessness [145] and forced swim [146; 147]. One of the first small clinical trials on men and women diagnosed with treatment-resistant depression showed that a single infusion of ketamine (0.5 mg/kg) improved depression ratings up to 72 hours later [148]. A subsequent randomized clinical trial reported that 0.5 mg/kg improved depression ratings in 71% of patients (12 women, 6 men) with treatment resistant depression [149]. Similar antidepressant effects of ketamine were observed in a clinical trial of patients (12 women, 6 men) diagnosed with treatment resistant bipolar depression [150]. One of the first larger clinical trials reported that ketamine reduced depression ratings in patients (35 men, 37 women) with treatment resistant depression [151]. Recent evidence also suggests that ketamine treatment (0.5 mg/kg) reduced suicidal thoughts in patients (48 women, 32 men) diagnosed with depression [152]. An important feature of these studies is that participants were diagnosed with treatment resistant depression, meaning that they did not respond to standard antidepressant medications. Thus, the fact that positive responses to ketamine were observed is of high clinical significance. None of these studies conducted analyses to determine whether effects of ketamine differed between men and women. However, emerging evidence in rodents suggest that there could be important sex differences in dose-response curves as well as mechanism of action. The doses tested in these rodent studies are generally 10 times higher than doses used in humans based in part on higher metabolic rates of smaller animals (see [153] for discussion).
One of the first studies to consider sex differences in the antidepressant effects of ketamine reported that female Sprague-Dawley rats showed reduced immobility in the forced swim test and reduced latency to feed in the novelty suppressed feeding test following 2.5 mg/kg i.p. of ketamine [154]. This dose had no effect on behavior in male rats. Female C57Bl6/J mice also showed reduced immobility in the forced swim test following treatment with lower doses of ketamine (3 mg/kg) that were not behaviorally active in males [155; 156]. In female rats, effects of low doses of ketamine on sucrose preference were dependent on gonadal hormones [157]. Ovariectomized females showed no change in response to ketamine treatment while ovariectomized females treated with implants containing estradiol and progesterone showed increased sucrose consumption following ketamine treatment. In the forced swim test, effects of ketamine are enhanced by estrogen receptors. During diestrus, when endogenous estrogen levels are at lower levels, estrogen receptor α or β agonists increased sensitivity to low doses of ketamine in the forced swim test [106]. An important aspect of these studies is that behavioral results come from assays that are predictive of antidepressant action, such as forced swim [158]. One disadvantage of some of these assays is that behavioral effects may rely on different mechanisms than is required for clinical efficacy. For example, acute SSRI treatment reduces immobility in the forced swim test while chronic SSRI treatment is required for a clinical response in humans. Complementary approaches like chronic mild stress [159] and social defeat stress [160] can induce depression- and anxiety-like behavioral responses that allow for the assessment of antidepressant action.
Only a few studies have tested the effects of ketamine in rodent models of stress-induced psychiatric illness. In male rats exposed to chronic mild stress (CMS), acute ketamine (10 mg/kg) increased sucrose preferences and decreased latency to feed in a novel environment [161]. However, acute ketamine treatment (15 mg/kg) also increased sucrose consumption in unstressed males, suggesting that these responses may not be specific to stress [162]. Similar ketamine-induced decreases in immobility were observed in male C57Bl6/J mice exposed to control or CMS, although stronger effects were observed in stressed males [155]. Acute ketamine treatment (20 mg/kg) also blocked social defeat-induced social avoidance, but this effect was not sustained one week later [163]. To achieve this same response with SSRI, four weeks of chronic treatment is required [164]. Subsequent studies suggested that individual differences in behavioral responses to defeat moderate the impact of ketamine. In male C57Bl6/J mice exposed to social defeat, some mice exhibit a susceptible phenotype defined as reduced interaction with an unfamiliar mouse while mice presenting with an unsusceptible maintain high levels of social interaction [165]. Mice showing a susceptible phenotype showed increased social interaction following a single treatment of ketamine [156]. Ketamine treatment had no effects on social interaction in stressed male mice exhibiting an unsusceptible phenotype, although this may have been due to a ceiling effect. In susceptible males acute ketamine treatment (10 mg/kg) was also found to ameliorate defeat-induced sucrose anhedonia and increased immobility in the forced swim test for up to 3 days [166; 167]. In these studies, mice exhibiting an unsusceptible phenotype based on the social interaction test were excluded. Other studies of male C57Bl/6NTac mice showed that ketamine (30 mg/kg) administered before social defeat or inescapable shock prevented stress-induced social avoidance and learned helplessness respectively [168]. Curiously, ketamine administered after defeat stress did not reduce stress-induced immobility, although social avoidance was not examined. The lack of efficacy of post-stress ketamine treatment on immobility would appear to conflict with other studies in male mice [166; 167]. Furthermore, another study of male C57Bl/6J mice reported that pre-defeat ketamine did not block the development of social avoidance [163]. One important factor contributing to these variable results is that different strains of mice were used across the different studies. There are many genetic differences between different C57Bl6 lines [169], including entire deletions of exons [170]. Currently it is not clear how genetic differences between inbred mouse lines may impact responses to ketamine. One possibility is that higher corticosterone levels in C57Bl/6NTac mice compared to C57Bl/6J are required for the prophylactic effects of ketamine. While this hypothesis has not been tested directly, further study of sex differences in behavioral and neural responses to ketamine could be extremely informative on this question. In most species of rodents corticosterone levels are higher in females than males [171; 172].
For females, ketamine also exerts antidepressant effects in model systems of depression or anxiety, but the effects are usually weaker than in males. In rats, social isolation stress increased immobility in the forced swim test for both males and females, and a moderate dose of ketamine (5 mg/kg) reduced immobility for both sexes [173]. However, while the effect of ketamine reduced immobility in stressed males to the levels of controls, the effect was weaker in females. Similar weaker effects of ketamine in females during the forced swim test were also observed in C57Bl6/J mice exposed to chronic mild stress [155]. In a witness defeat approach, female C57Bl6/J mice treated with a single dose of ketamine (20 mg/kg) showed higher levels of social interaction with an unfamiliar female than saline treated females [174]. While the dose of ketamine used here was higher than other studies, there were no differences in locomotor behavior. So far, no study has tested whether pre-stress ketamine treatment has the same prophylactic effects that have been observed in male C57Bl/6NTac mice. A potentially concerning outcome was observed when repeated ketamine administration (10 mg/kg daily for 3 weeks) was tested in C57Bl/6J mice [175]. For males, this dosing regimen reduced immobility in the forced swim test and had anxiolytic effects in the open field test. However, for females this dosing regimen increased immobility in the forced swim test. It’s not clear whether lower doses would have the same effect in females, or whether females exposed to CMS or witness defeat would show a different response. Still, these observations highlight the relative lack of knowledge on the anti-depressive effects of ketamine in females. Even less is known about the mechanisms underlying the effects of ketamine in females.
One of the first studies to examine molecular mechanisms of the antidepressant effects of ketamine focused on neuroplasticity in the hippocampus [176]. Ketamine induces rapid and sustained increases in brain derived neurotrophin factor (BDNF) protein in the hippocampus. This response was blocked with the translation inhibitor anisomycin but not the transcriptional inhibitor actinomycin D, suggesting that ketamine modulates synthesis of BDNF protein synthesis rather than increasing Bdnf gene expression. Consistent with this translational mechanism, ketamine also decreases phosphorylation of eukaryotic elongation factor 2 (eEF2). Dephosphorylation of eEF2 leads to enhanced ribosomal translocation and protein synthesis. A subsequent report presented evidence that the ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) could induce antidepressant effects in forced swim, novelty suppressed feeding as well as defeat-induced social avoidance [177]. This is a potentially very important finding since (2R,6R)-HNK does not have many of the adverse side effects associated with ketamine. As reported for ketamine, (2R,6R)-HNK was also found in decrease phosphorylation of eEF2 and increase BDNF protein in the hippocampus. However, in this case changes were only observed 24 hr after treatment and not 1 hr after treatment. There is disagreement over the precise mechanism of action for (2R,6R)-HNK. One the one hand, (2R,6R)-HNK did not displace tritiated MK-801 from NMDA receptors in vitro and was found to not functionally inhibit NMDA receptors in hippocampal slices. These findings have been interpreted as evidence that (2R,6R)-HNK may work independently of NMDA receptors. On the other hand higher doses of (2R,6R)-HNK were found to reduce NMDA receptor currents in cultured hippocampal neurons [143]. Further study of these intracellular mechanisms is needed to sort out the precise role of ketamine and its metabolites. An additional blind spot in this literature is that it’s not clear whether electrophysiological studies have been conducted on neurons from both males and females. Some initial analyses suggest that there could be sex differences in the mechanism of ketamine action.
In female C57Bl6/J mice, 3 mg/kg of ketamine increased BDNF in the hippocampus during diestrus but not proestrus even though ketamine reduced immobility during both phases of the estrus cycle [106]. This suggests the possibility that there could be complementary mechanisms of ketamine actions. In addition, systemic ketamine treatment results in higher brain concentrations of (2R,6R)-HNK in females than males [177]. Despite evidence for important sex differences in sensitivity to and metabolism of ketamine, it is not clear whether any electrophysiological studies of ketamine have considered sex as a biological variable. Sex differences in signal transduction of G-protein receptors have been observed in cortical neurons [178], so future studies should include neurons from both males and females. Thus, although ketamine has similar behavioral effects in male and female rodents, effects on brain function are quite different. At this time, most clinical trials have not detected strong differences in antidepressant responses to ketamine between men and women. However, these trials may not have been sufficiently powered to detect sex differences and preclinical data suggest that the mechanisms underlying antidepressant responses to ketamine could be quite different in men and women.
Conclusions
After decades of heavy reliance on monoamine-based therapies, emerging data suggest that pharmaceuticals targeting opioid, oxytocin, or NMDA receptors could have exciting applications for treating stress-related disorders including anxiety and depression. In some cases, compounds with anxiolytic or antidepressant properties have similar effects in males and females (Table 1). In other cases striking differences in the behavioral effects of these compounds are observed between males and females. Sufficiently powered studies or metanalyses should assess whether clinical and neurobiological responses to novel antidepressants differ between men and women. Overall, these findings highlight how essential it is to include both sexes in preclinical and clinical assessment of novel pharmaceuticals. As the field moves towards more personalized approaches for treating mental illness, consideration of sex as a biological variable should be a key piece in solving this puzzle.
Table 1.
Summary of findings of sex differences for the effects of different classes of pharmaceuticals with antidepressant and anxiolytic properties.
| Target | Administration timing for efficacy | Males more sensitive | Females more sensitive | Behavior test where targets show efficacy |
|---|---|---|---|---|
| SSRI | Chronic | X | Forced Swim Test Elevated Plus Maze Open Field |
|
| KOR | Acute (before stress) | X | Forced Swim Test (males) Social Interaction Test Sucrose Preference Test Autogrooming Assays Inescapable Shock |
|
| OTR | Acute | OT | OTA | Elevated Plus Maze Elevated Zero Maze Social Interaction Test |
| Ketamine | Acute | ? | Forced Swim Test Novelty-Suppressed Feeding Sucrose Preference Test Social Interaction Test |
Highlights.
Antidepressants targeting monoamines are not efficacious for many
Novel pharmaceuticals have different behavioral effects in males and females
Novel targets include kappa opioid and oxytocin receptors
Ketamine may have sex differences in underlying neurobiological mechanisms
Acknowledgments
The authors were supported by NIH R01 MH103322 during the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 2.Culpepper L. Why do you need to move beyond first-line therapy for major depression? J Clin Psychiatry. 2010;71:4–9. doi: 10.4088/JCP.9104su1c.01. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res. 2012;44:607–618. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolbold R, Klein K, Burk O, Nussler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003;38:978–988. doi: 10.1053/jhep.2003.50393. [DOI] [PubMed] [Google Scholar]
- 5.Cheung C, Yu AM, Chen CS, Krausz KW, Byrd LG, Feigenbaum L, Edwards RJ, Waxman DJ, Gonzalez FJ. Growth hormone determines sexual dimorphism of hepatic cytochrome P450 3A4 expression in transgenic mice. Journal of Pharmacology and Experimental Therapeutics. 2006;316:1328–1334. doi: 10.1124/jpet.105.094367. [DOI] [PubMed] [Google Scholar]
- 6.Damoiseaux VA, Proost JH, Jiawan VC, Melgert BN. Sex differences in the pharmocokinetics of antidepressants: influence of female sex hormones and oral contraceptives. Clin Pharmacokinet. 2014;53:509–19. doi: 10.1007/s40262-014-0145-2. [DOI] [PubMed] [Google Scholar]
- 7.Sramek JJ, Murphy MF, Cutler NR. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci. 2016;18:447–457. doi: 10.31887/DCNS.2016.18.4/ncutler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bale TN, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laman-Maharg A, Trainor BC. Stress, sex, and motivated behaviors. J Neurosci Res. 2017;95:83–92. doi: 10.1002/jnr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shansky RM, Woolley CS. Considering sex as a biological variable will be valuable for neuroscience research. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:11817–11822. doi: 10.1523/JNEUROSCI.1390-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:2241–7. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, Lorsch ZS, Hamilton PJ, Calipari ES, Hodes GE, Issler O, Kronham H, Pfau M, Obradovic ALJ, Dong Y, Neve RL, Russo SJ, Kazarskis A, Tamminga C, Mechawar N, Turecki G, Zhang B, Shen L, Nestler EJ. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jazin E, Cahill M. Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci. 2010;11:9–17. doi: 10.1038/nrn2754. [DOI] [PubMed] [Google Scholar]
- 15.Cahill L. Sex differences on the brain: an issue whose time has come. Neuron. 2015;88:1084–1085. doi: 10.1016/j.neuron.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angoa-Pérez M, Kane MJ, Briggs DI, Herrera-Mundo N, Sykes CE, Francescutti DM, Kuhn DM. Mice genetically depleted of brain serotonin do not display a depression-like behavioral phenotype. ACS Chem Neurosci. 2014;5:908–19. doi: 10.1021/cn500096g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, Iversen M, Banke MB, Petersen IJ, Klingenberg SL, Krogh J, Ebert SE, Timm A, Lindschou J, Gluud C. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and trial sequential analysis. BMC Psychiatry. 2017;17:1–28. doi: 10.1186/s12888-016-1173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007;3:137–158. doi: 10.1146/annurev.clinpsy.3.022806.091444. [DOI] [PubMed] [Google Scholar]
- 20.Lamers F, van Oppen P, Comijs HS, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman AT, Penninx BW. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA) J Clin Psychiatry. 2011;72:341–348. doi: 10.4088/JCP.10m06176blu. [DOI] [PubMed] [Google Scholar]
- 21.Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci. 2006;8:335–344. doi: 10.31887/DCNS.2006.8.3/tban. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23:1–21. doi: 10.1037/a0038550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong DT, Bymaster FP, Horng JS, Molloy BB. A new selective inhibitor for uptake of serotonin into synaptosomes of rat brain: 3-(p-trifluoromethylphenoxy). N- methyl-3-phenylpropylamine. Journal of Pharmacology and Experimental Therapeutics. 1975;193:804–811. [PubMed] [Google Scholar]
- 24.Wong DT, Horng JS, Bymaster FP, Hauser KL, Molloy BB. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-Trifluoromethylphenoxy)-n-methyl-3- phenylpropylamine. Life sciences. 1974;15:471–479. doi: 10.1016/0024-3205(74)90345-2. [DOI] [PubMed] [Google Scholar]
- 25.Aberg-Wistedt A. The antidepressant effects of 5-HT uptake inhibitors. Br J Psychiatry Suppl. 1989;8:32–40. [PubMed] [Google Scholar]
- 26.Doogan DP, Caillard V. Sertraline: a new antidepressant. J Clin Psychiatry. 1988;49:46–51. [PubMed] [Google Scholar]
- 27.Charney DS, Krystal JH, Delgado PL, Heninger GR. Serotonin-specific drugs for anxiety and depression disorders. Annu Rev Med. 1990;41:437–46. doi: 10.1146/annurev.me.41.020190.002253. [DOI] [PubMed] [Google Scholar]
- 28.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106:226–33. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmucker DL, Woodhouse KW, Wang RK, Wynne H, James OF, McManus M, Kremers P. Effects of age and gender on in vitro properties of human liver microsomal monooxygenases. Clin Pharmacol Ther. 1990;48:365–74. doi: 10.1038/clpt.1990.164. [DOI] [PubMed] [Google Scholar]
- 30.Ronfeld RA, Tremaine LM, Wilner KD. Pharmacokinetics of sertraline and its N-demethyl metabolite in elderly and young male and female volunteers. Clin Pharmacokinet. 1997;32:22–30. doi: 10.2165/00003088-199700321-00004. [DOI] [PubMed] [Google Scholar]
- 31.Hartter S, Wetzel H, Hammes E, Hiemke C. Inhibition of antidepressant demethylation and hydroxylation by fluvoxamine in depressed patients. Psychopharmacology. 1993;110:302–308. doi: 10.1007/BF02251285. [DOI] [PubMed] [Google Scholar]
- 32.Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005;25:318–324. doi: 10.1097/01.jcp.0000168879.03169.ce. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Guasti A, Olivares-Nazario M, Reyes R, Martínez-Mota L. Sex and age differences in the antidepressant-like effect of fluoxetine in the forced swim test. Pharmacology, biochemistry, and behavior. 2017;152:81–89. doi: 10.1016/j.pbb.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Kokras N, Antoniou K, Mikhail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C. Forced swim test: what about females? Neuropharmacology. 2015;99:408–421. doi: 10.1016/j.neuropharm.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behavioral and Cognitive Neuroscience reviews. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 37.Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biological Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 38.Young E, Korszun A. Sex, trauma, stress hormones and depression. Molecular Psychiatry. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- 39.Donner N, Handa RJ. Estrogen receptor beta regulated the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–18. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. Photoperiod reverses the effects of estrogens on male aggression via genomic and non-genomic pathways. Proc Natl Acad Sci USA. 2007;104:9840–9845. doi: 10.1073/pnas.0701819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biological Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Handa RJ, Mani SK, Uht RM. Estrogen receptors and the regulation of neural stress responses. Neuroendocrinology. 2012;96:111–118. doi: 10.1159/000338397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology (Berl) 2004;173:139–45. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- 44.Hasegawa H, Nakamura K. CHAPTER 2.3 - Tryptophan hydroxylase and serotonin synthesis regulation. In: Müller C, Jacobs BL, editors. Handbook of the behavioral neurobiology of serotonin. Elsevier; 2010. pp. 183–202. [Google Scholar]
- 45.Hiroi R, Handa RJ. Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J Neurochem. 2013;127:487–495. doi: 10.1111/jnc.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berlanga C, Flores-Ramos M. Different gender response to serotonergic and noradrenergic antidepressants. Journal of affective disorders. 2006;95:119–123. doi: 10.1016/j.jad.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Grigoriadis S, Kennedy SH, Bagby RM. A comparison of antidepressant response in younger and older women. J Clin Psychopharmacol. 2003;23:405–407. doi: 10.1097/01.jcp.0000085415.08426.c6. [DOI] [PubMed] [Google Scholar]
- 48.Pae CU, Mandelli L, Kim TS, Han C, Masand PS, Marks DM, Patkar AA, Steffens DC, De Ronchi D, Serretti A. Effectiveness of antidepressant treatments in pre-menopausal versus post-menopausal women: a pilot study on differential effects of sex hormones on antidepressant effects. Biomed Pharmacother. 2009;63:228–235. doi: 10.1016/j.biopha.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Am J Geriatr Psychiatry. 1997;5:97–106. [PubMed] [Google Scholar]
- 50.Parker G, Parker K, Austin MP, Mitchell P, Brotchie H. Gender differences in response to differing antidepressant drug classes: two negative studies. Psychological medicine. 2003;33:1473–1477. doi: 10.1017/s0033291703007918. [DOI] [PubMed] [Google Scholar]
- 51.Quitkin FM, Stewart JW, McGrath PJ, Taylor BP, Tisminetzky MS, Petkova E, Chen Y, Ma G, Klein DF. Are there differences between women’s and men’s antidepressant responses? Am J Psychiatry. 2002;159:1848–1854. doi: 10.1176/appi.ajp.159.11.1848. [DOI] [PubMed] [Google Scholar]
- 52.Kornstein SG, Pedersen RD, Holland PJ, Nemeroff CB, Rothschild AJ, Thase ME, Trivedi MH, Ninan PT, Keller MB. Influence of sex and menopausal status on response, remission, and recurrence in patients with recurrent major depressive disorder treated with venlafaxine extended release or fluoxetine: analysis of data from the PREVENT study. J Clin Psychiatry. 2014;75:62–68. doi: 10.4088/JCP.12m07841. [DOI] [PubMed] [Google Scholar]
- 53.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends in Neurosciences. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 54.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by κ opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 55.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:407–14. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–5. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 57.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa-opioid receptor: from addiction to depression, and back. Front Psychiatry. 2014;5:1–17. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buda JJ, Carroll FI, Kosten TR, Swearingen D, Walters BB. A double-blind, placebo-controlled trial to evaluate the safety, tolerability, and pharmacokinetics of single, escalating oral doses of JDTic. Neuropsychopharmacology. 2015;40:2059–65. doi: 10.1038/npp.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chavkin C, Martinez D. Kappa Antagonists JDTic in Phase 1 Clinical Trial. Neuropsychopharmacology. 2015;40:2057–8. doi: 10.1038/npp.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-Like Effects of [kappa]-Opioid Receptor Antagonists in Wistar Kyoto Rats. Neuropsychopharmacology. 2009;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Therapeutics. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 64.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve RL, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Browne CA, Falcon E, Robinson SA, Berton O, Lucki I. Reversal of stress-induced social interaction deficits by buprenorphine. International Journal of Neuropsychopharmacology. 2017 doi: 10.1093/ijnp/pyx079. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, Trainor BC. Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:166–174. doi: 10.1016/j.pnpbp.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA., Jr Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behavioural pharmacology. 2015;26:654–63. doi: 10.1097/FBP.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kudryavtseva NN, Gerrits MAFM, Avgustinovich DF, Tenditnik MV, Van Ree JM. Modulation of anxiety-related behaviors by μ- and κ-opioid receptor agonists depends on the social status of mice. Peptides. 2004;25:1355–1363. doi: 10.1016/j.peptides.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Kudryavtseva NN, Gerrits MAFM, Avgustinovich DF, Tenditnik MV, Van Ree JM. Anxiety and ethanol consumption in victorious and defeated mice; effect of κ-opioid receptor activation. Eur Neuropsychopharmacol. 2006;16:504–511. doi: 10.1016/j.euroneuro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 73.Wright EC, Parks TV, Alexander J, Supra R, Trainor BC. Activation of kappa opioid receptors in the dorsal raphe have sex dependent effects on social behavior in California mice. Behav Brain Res. 2018;351:83–92. doi: 10.1016/j.bbr.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK, Trainor BC. Hypothalamic vasopressin systems are more sensitive to social defeat in males versus females. Psychoneuroendocrinology. 2015;51:122–134. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray CL, Krebs-Kraft DL, Solomon MB, Norvelle A, Parent MB, Huhman KL. Immediate post-defeat infusions of the noradrenergic receptor antagonist propranolol impair the consolidation of conditioned defeat in male Syrian hamsters. Physiol Behav. 2015;152:56–61. doi: 10.1016/j.physbeh.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Hasani R, McCall JG, Bruchas MR. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front Pharmacology. 2013;4:96. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laman-Maharg AR, Copeland T, Ordones Sanchez E, Campi KL, Trainor BC. The long-term effects of stress and kappa opioid receptor activation on conditioned place aversion in male and female California mice. Behavioural Brain Research. 2017;332:299–307. doi: 10.1016/j.bbr.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 79.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3-and arrestin-dependent in neurons and astrocytes. Journal of Biological Chemistry. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruchas MR, Xu M, Chavkin C. Repeated swim stress induced kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruchas M, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology. 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-Induced p38 Mitogen-Activated Protein Kinase Activation Mediates k-Opioid-Dependent Dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PE, Chavkin C. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:12917–31. doi: 10.1523/JNEUROSCI.2444-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robles CF, McMackin MZ, Campi KL, Doig IE, Takahashi EY, Pride MC, Trainor BC. Effects of kappa opioid receptors on conditioned place aversion and social interaction in males and females. Behav Brain Res. 2014;262:84–93. doi: 10.1016/j.bbr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chartoff EH, Mavrikaki M. Sex differences in kappa opioid receptor function and their potential impact on addiction. Front Neurosci. 2015;9:1–16. doi: 10.3389/fnins.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russell SE, Rachlin AB, Smith KL, Muschamp JW, Berry L, Zhao Z, Chartoff EH. Sex difference in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry. 2013;76:213–222. doi: 10.1016/j.biopsych.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Craft RM, Kruzich PJ, Boyer JS, Harding JW, Hanesworth JM. Sex differences in discriminative stimulus and diuretic effects of the kappa opioid agonist U69,593 in the rat. Pharmacol Biochem Behav. 1998;61:395–403. doi: 10.1016/s0091-3057(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 90.Laman-Maharg A, Williams AV, Zufelt MD, Minie VA, Ramos-Maciel S, Hao R, Ordones Sanchez E, Copeland T, Silverman JL, Leigh A, Snyder R, Carroll FI, Fennell TR, Trainor BC. Kappa opioid receptor antagonists reduce immobility in male but not female rodents in the forced swim test. Front Pharmacol. 2018;9:1–11. doi: 10.3389/fphar.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacDonald K, Feifel D. Oxytocin’s role in anxiety: a critical appraisal. Brain Res. 2014;1580:22–56. doi: 10.1016/j.brainres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 93.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neurosci. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- 95.Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song Z, McCann KE, McNeill JKt, Larkin TEn, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anacker AM, Christensen JD, LaFlamme EM, Grunberg DM, Beery AK. Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology. 2016;68:156–162. doi: 10.1016/j.psyneuen.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song Z, Albers HE. Cross-talk among oxytocin and arginine-vasopressin receptors: relevance for basic and clinical studies of the brain and periphery. Front Neuroendocrinol. 2018 doi: 10.1016/j.yfrne.2017.10.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of petidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 101.Lang RE, Heil JWE, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- 102.Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. European Journal of Neuroscience. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- 103.Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 104.Detillion C, Craft T, Glasper E, Prendergast B, DeVries A. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central Oxytocin Administration Reduces Stress-Induced Corticosterone Release and Anxiety Behavior in Rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 106.Dossat AM, Wright KN, Strong CE, Kabbaj M. Behavioral and biochemical sensitivity to low doses of ketamine: influence of estrous cycle in C57Bl/6 mice. Neuropharmacology. 2018;130:30–41. doi: 10.1016/j.neuropharm.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK 1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 108.Loyd DR, Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Experimental Neurology. 2014;259:57–63. doi: 10.1016/j.expneurol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Knobloch HS, CA, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, SPH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 111.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EL, Walch K, Bales KL, Trainor BC. Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry. 2016;80:406–414. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL, Trainor BC. Anxiolytic effects of vasopressin V1a receptor in the medioventral bed nucleus of the stria terminalis: sex specific effects in social and nonsocial contexts. Neuropharmacology. 2016;110:59–68. doi: 10.1016/j.neuropharm.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Toth I, Neumann ID, Slattery DA. Social fear conditioning as an animal model of social anxiety disorder. Current protocols in neuroscience/editorial board, Jacqueline N. Crawley … [et al.] 2013;63:9.42.1–9.42.13. doi: 10.1002/0471142301.ns0942s63. [DOI] [PubMed] [Google Scholar]
- 115.Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology. 2014;39:3027–35. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 117.Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfealt response: oxytocin effects on response to social stress in men and women. Biol Psychol. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attentuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 119.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Radke S, Volman I, Kokal I, Roelofs K, de Bruijn ERA, Toni I. Oxytocin reduces amygdala responses during threat approach. Psychoneuroendocrinology. 2017;79:160–166. doi: 10.1016/j.psyneuen.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 121.Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nasanbuyan N, Yoshida M, Takayanagi Y, Inutsuka A, Nishimori K, Yamanaka A, Onaka T. Oxytocin-Oxytocin Receptor Systems Facilitate Social Defeat Posture in Male Mice. Endocrinology. 2018;159:763–775. doi: 10.1210/en.2017-00606. [DOI] [PubMed] [Google Scholar]
- 123.Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- 124.Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16:1185–7. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eckstein M, Scheele D, Weber KS, Stoffel-Wagner B, Maier W, Hurlemann R. Oxytocin facilitates the sensation of social stress. Hum Brain Mapp. 2014;35:4741–4750. doi: 10.1002/hbm.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.MacDonald K, MacDonald TM, Brune M, Lamb K, Wilson MP, Golshan S, Feifel D. Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology. 2013;38:2831–43. doi: 10.1016/j.psyneuen.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 127.Shamay-Tsoory S, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. 2016;79:197–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 128.Grippo AJ, Pournajafi-Nazarloo H, Sanzenbacher L, Trahanas DM, McNeal N, Clarke DA, Porges SW, Carter CS. Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress. 2012;15:149–161. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lukas M, Neumann ID. Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvment of brain oxytocin and vasopressin. J Neurosci Methods. 2014;234:101–107. doi: 10.1016/j.jneumeth.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 130.Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, Trainor BC. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol Psychiatry. 2018;83:203–213. doi: 10.1016/j.biopsych.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 132.Lischke A, Gamer M, Berger C, Grossman A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 133.Luo L, Becker B, Geng Y, Zhao Z, Gao S, Zhao W, Yao S, Zheng Z, Ma Z, Gao Z, Hu J, Kendrick KM. Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. NeuroImage. 2017;162:127–137. doi: 10.1016/j.neuroimage.2017.08.079. [DOI] [PubMed] [Google Scholar]
- 134.Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93:1245–54. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 135.Koch SBJ, Van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal Oxytocin Administration Dampens Amygdala Reactivity towards Emotional Faces in Male and Female PTSD Patients. Neuropsychopharmacology. 2016;41:1495–1504. doi: 10.1038/npp.2015.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wright EC, Johnson SA, Hao R, Kowalczyk AS, Greenberg GD, Ordoñes Sanchez E, Laman-Maharg A, Trainor BC, Rosenfeld CS. Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala. J Neuroendocrinol. 2017;29:1–9. doi: 10.1111/jne.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holt-Lunstad J, Brirmingham W, Light KC. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology. 2011;36:1249–1256. doi: 10.1016/j.psyneuen.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 138.Cryanowski JM, Hofkins TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin regulation among depressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pierrehumber B, Torrisi R, Laufer D, Halfon O, Ansermet F, Beck Popovic M. Oxytocin response to an experimental psychosocial challenge in adults exposed to traumatic experiences during childhood or adolescence. Neuroscience. 2010;166:168–177. doi: 10.1016/j.neuroscience.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 140.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sabihi S, Durosko NE, Dong SM, Leuner B. Oxytocin in the prelimbic medial prefrontal cortex redcues anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 2014;45:31–42. doi: 10.1016/j.psyneuen.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andari E, Hurlemann R, Young LJ. Current Topics in Behavioral Neurosciences. Springer; Berlin: 2017. A precision medicine approach to oxytocin trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–E3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 144.Muttenthaler M, Andersson A, Vetter I, Menon R, Busnelli M, Ragnarsson L, Bergmayr C, Arrowsmith S, Deuis JR, Chiu HS, Palpant NJ, O’Brien M, Smith TJ, Wray S, Neumann ID, Gruber CW, Lewis RJ, Alewood PF. Subtle modificiations to oxytocin produce ligands that retain potency and improved selectivity across species. Sci Signal. 2017;10:eaan3398. doi: 10.1126/scisignal.aan3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meloni D, Gambara C, Graziella de Montis M, Pra P, Taddei I, Tagliamonte A. Dizoclipine antagonizes the effect of chronic imipramine on learned helplessness in rats. Pharm Biochem Behav. 1993;46:423–426. doi: 10.1016/0091-3057(93)90374-3. [DOI] [PubMed] [Google Scholar]
- 146.Layer RT, Popik P, Olds T, Skolnick P. Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715) Pharm Biochem Behav. 1995;52:621–627. doi: 10.1016/0091-3057(95)00155-p. [DOI] [PubMed] [Google Scholar]
- 147.Moryl E, Danysz W, Quack G. Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol. 1993;72:394–397. doi: 10.1111/j.1600-0773.1993.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 148.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 149.Zarate JCA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 150.Diazgranados N, Ibrahim LB, NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate JCA. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:792–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CM, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grunebaum MF, Galfalvy HC, Choo T-H, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. American Journal of Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17060647. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boxenbaum H, DiLea C. First-time-in-human dose selection: allometric thoughts and perspectives. J Clin Pharmacol. 1995;35 doi: 10.1002/j.1552-4604.1995.tb04011.x. [DOI] [PubMed] [Google Scholar]
- 154.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 155.Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 156.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang X-P, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate JCA, Gould TD. NMDAR inhibition-independent antidepressan actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saland SK, Schoepfer KJ, Kabbaj M. Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci Rep. 2016;6:21322. doi: 10.1038/srep21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 159.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Golden SA, Convington HEI, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, Li X-Y, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits cause by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 163.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76:550–558. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 165.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 166.Zhang K, Dong C, Fujita Y, Fujta A, Hashimoto K. 5-hydroxytryptamine-independent antidepressan actions of (R)-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol. 2018 doi: 10.1093/ijnp/pyx100. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qu Y, Yang C, Ren Q, Ma M, Hashimoto K. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnromal composition of gut microbiota in a social defeat stress model. Sci Rep. 2017;7:15725. doi: 10.1038/s41598-017-16060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, Gardier AM, Mendez-David I, David DJ, Hen R, Denny CA. Ketamine as a prohylactic against stress-induced depressive-like behavior. Biol Psychiatry. 2016;79:776–786. doi: 10.1016/j.biopsych.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–94. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A. Genetic differences among C57Bl/6 substrains. Exp Anim. 2009;58:141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- 171.Trainor BC, Takahashi EY, Silva AL, Crean KK, Hostetler C. Sex differences in hormonal responses to social conflict in the monogamous California mouse. Horm Behav. 2010;58:506–512. doi: 10.1016/j.yhbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sarkar A, Kabbaj M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry. 2016;80:448–456. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, Castillo SA. Vicarious social defeat strress induces depression-related outcomes in female mice. Biol Psychiatry. 2018;83:9–17. doi: 10.1016/j.biopsych.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res. 2016;312:305–312. doi: 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 176.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-f, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang X-P, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CAJ, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bangasser DA, Curtis A, Reyes BAS, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]