Abstract
Memory functioning undergoes dynamic changes between childhood and adulthood. Spontaneous use of elaborative strategies, which can enhance the recall of information, expands with age and contributes to age-associated improvement in memory functioning. Findings from lesion and neuroimaging studies suggest that the ability to use elaborative strategies is dependent upon intact functioning of the prefrontal cortex (PFC), particularly the dorsolateral PFC region. Because the PFC undergoes protracted maturation, we examined whether age difference in the structure of the PFC is correlated with age-associated increase in strategy use. Here, we investigated the relationship between PFC volume and spontaneous strategy use in a sample of 120 participants aged 5–25 years. We assessed semantic clustering during recall with a standardized word-list recall task (California Verbal Learning Task children’s version, CVLT-C) and computed PFC regional volumes from participants’ structural brain images. We observed an age-associated increase in the use of semantic clustering and an age-associated decrease in volumes of the PFC. Further, we found that smaller PFC volume was linked to increased use of semantic clustering. Importantly, the volume of the right dorsolateral PFC partially explained the relation between age and the use of semantic clustering. These findings suggest that PFC maturation supports the development of strategy use and lends further support for the notion that brain-behavior relations change across development.
Keywords: Memory Development, Recall, Brain Development, Structural MRI, CVLT, children
1. Introduction
Memory functioning undergoes profound changes over the typical course of development. One of the contributing factors to improvement in memory functioning is the increased ability for spontaneous use of elaborative mnemonic strategies (Bjorklund & Douglas, 1997; Ofen, Yu, & Chen, 2016). This ability increases significantly from childhood into adulthood (Bjorklund & Douglas, 1997; Schneider & Pressley 1997; Gathercole, 1998; Schneider et al., 2004; Daugherty & Ofen, 2015) and benefits memory performance in adults (Camp et al. 1983; Dunlosky & Hertzog, 1998). In one developmental study, Daugherty and Ofen (2015) reported an increase in the spontaneous use of elaborative strategies in adolescents and young adults compared to children: children tend to rely on simple or no (“shallow”) strategies such as “say the word pair once,” whereas adolescents and young adults predominantly depend on elaborative (“deep”) mnemonic strategies during word pair encoding such as “associate with a personal experience”. Age-associated increase in spontaneous use of elaborative mnemonic strategies may underlie the robust age-associated enhancement in memory performance between childhood and adulthood (Ofen et al., 2016).
The spontaneous use of elaborative mnemonic strategies is thought to be supported by the prefrontal cortex (PFC; Fletcher et al., 1998; Blumenfeld & Ranganath, 2007). Beyond its putative role in supporting the spontaneous use of elaborative mnemonic strategies, the PFC is a key region supporting the typical development in memory functioning (Ofen, 2012; Shing et al., 2010; Ofen, Yu, & Chen, 2016). Age-associated differences in PFC activation have been specifically linked to the age-associated increase in the formation of complex and more detailed memory (Ofen et al., 2007; Tang, Shafer, & Ofen, 2017). Longitudinal studies (Sowell et al., 2004; Giedd et al., 1999; Gogtay et al., 2004) have demonstrated protracted maturation, evidenced as age-associated reduction in gray matter volume between childhood to adulthood, which likely contributes to age-associated increase in memory functioning. Interestingly, findings reported in prior studies also suggest that the protracted maturation trajectory of the PFC is more prominent in the right hemisphere (Sowell et al., 2004; Gogtay et al. 2004). Little evidence exists in studies of child development about the relationship between protracted structural maturation of the right PFC and facilitation of spontaneous elaborative mnemonic strategy use. Evidence supporting the notion of a link between structural integrity of the PFC and spontaneous use of mnemonic strategies is available from studies in adults. For example, a recent study by Husa and colleagues (Husa et al., 2017) compared older participants (65–80 years) to younger participants (18–25 years) in their spontaneous use of elaborative strategy, such as sentence generation, personal relevance, and visual imagery. The authors found that age-associated reduction in PFC volume, specifically in the left caudal middle frontal lobe, partially explained age-associated decrease in spontaneous use of elaborative strategy.
Semantic elaborative mnemonic strategies refer to the processes one may use to organize encoded, to-be-remembered, information based on meaning (e.g., Hertzog, McGuire, Horhota, & Jopp, 2010). Use of semantic strategies can be demonstrated when during recall information is clustered by semantic meaning. Like general spontaneously used elaborative mnemonic strategies, the spontaneous use of semantic strategies facilitates memory in adults (Hertzog et al., 2010; Kirchhoff, Gordon, & Head, 2014). Moreover, children’s memory performance also benefits from spontaneously using semantic strategy (Schleepen & Jonkman, 2012). The ability for spontaneous semantic strategy use increases with age. Older children (10–12-year old) spontaneously use semantic strategy more frequently than younger children (6–9-year old) during both encoding and retrieval (Schleepen & Jonkman, 2012). In a longitudinal study, Schneider et al. (2004) showed that the spontaneous use of semantic strategies in 6-year-olds increased over a one-year period.
The spontaneous use of semantic strategy has also been shown to be related to the PFC. In an adult lifespan sample, age-associated decrease in spontaneous use of semantic strategy is partially explained by age difference in PFC volumes (Kirchhoff et al., 2014). Within the PFC, the dorsolateral region (DLPFC) is particularly important in supporting the spontaneous use of mnemonic semantic strategies. Studies in adults with DLPFC lesions (Gershberg & Shimamura, 1995; Alexander et al., 2003) and healthy adults (Hirst & Volpe, 1988; Gershberg & Shimamura, 1995; Baldo et al., 2002; Hawco, Berlim, & Lepage, 2013; Fletcher et al., 1998; Hazlett et al., 2004; Nohara et al., 2000) have identified the DLPFC as critical for the spontaneous use of semantic strategies. In addition, a repetitive transcranial magnetic stimulation (rTMS) study showed that left DLPFC function interference affects memory performance only when participants need to spontaneously initiate semantic organization of information, but not when they are cued to do so (Hawco, Berlim, & Lepage, 2013). This finding further support the view that the DLPFC provides critical support for spontaneous use of semantic strategy.
The fact that the same neural substrate, the PFC, has been linked to both spontaneous use of semantic strategy and age-associated increase in memory performance may further support the notion that development of spontaneous use of elaborative mnemonic strategy is a contributing factor to memory development. However, currently we are lacking in knowledge about the neural correlates of age-associated increase in spontaneous semantic strategy use in developing population. Based on the above described findings linking chronological age, PFC indices, and differences in spontaneous use of semantic strategy, it is possible that age-linked increase in the spontaneous use of semantic strategy is partially explained by age-associated differences in PFC structure. Because the right lateral PFC is characterized by a more protracted maturation compared to other PFC regions (Gogtay et al., 2004), this developmental pattern may be the biological process underlying the extended developmental trajectory of cognitive abilities such as the spontaneous use of semantic strategies.
In sum, evidence of age difference in PFC structure and spontaneous use of semantic strategy, combined with findings of PFC involvement in spontaneous use of semantic strategy, provides the background and impetus for the current investigation of the association between age difference in PFC structure and age increase in spontaneous use of semantic strategy. Although in adult lifespan the relation between age-associated decrease in PFC volumes and age-associated decrease in spontaneous use of semantic strategy has been documented (18–91 years; Kirchhoff et al., 2014), the relation between age differences in PFC structures and age differences in spontaneous semantic strategy use remains unexplored in a developing population.
Here, in a sample of healthy participants aged 5–25 years, we evaluated the spontaneous use of semantic strategy during word-list retrieval and measured the volumes of anatomically defined regions within the PFC. The inclusion of a wide age range sample allowed us to examine age differences and potential nonlinear age differences in PFC structure and spontaneous semantic strategy use. In addition, results based on this developmental sample, together with findings in adult lifespan (Kirchhoff et al., 2014), provide complementary knowledge about spontaneous semantic strategy use over lifespan development. We then investigated how age difference in PFC structure is associated with age difference in spontaneous semantic strategy use. The latter construct was indexed by semantic clustering score based on California Verbal Learning Test. Based on previous findings of age difference in PFC gray matter over development, we predicted an age-associated decrease in PFC ROI volumes. We also predicted the semantic clustering score to increase with older age and with smaller volumes of the DLPFC, specifically the right DLPFC. As a comparison index, we also measured serial clustering score, a measure reflecting the extent to which the participants recall words in the same order as presented during encoding. As serial clustering, in contrast to semantic clustering, requires no additional information reorganization, we predicted younger participants to show few, if any, performance differences from their older counterparts. Additionally, we did not expect serial clustering score to correlate with PFC volumes. Finally, to associate semantic strategy development in the context of memory development, we also evaluated the effects of age and semantic strategy use on memory performance and examined the contribution of the PFC to age-associated increase in memory performance.
2. Methods
2.1. Participants
One hundred and thirty individuals (51% females) ages 5 to 25 years (M = 13.60, SD = 5.90) were recruited from Metro Detroit, as part of a larger study of neural correlates of memory development. Participants spoke English as a native language, had no reported developmental or neurological disorders, and had no reported history of head trauma. Assuring MRI compatibility and safety, participants were included only if they had no metallic implants, braces, or permanent retainers. Participants consented following procedures as approved by the Wayne State University Institutional Review Board, which included parental consent for all minors, written assent for ages 13–17 years, and oral assent for ages 5–12 years. Participants were compensated $15/hour for their time during behavioral data collection and $30/hour for their time during brain imaging data collection. The participants or the parents of the minors were paid in cash or gift card. Participants’ IQ did not vary with age (M = 109.41, SD = 13.45; r = −0.09, p = 0.34).
Two participants (ages 12.59 and 16.97 years) did not complete the behavioral session. Five participants were excluded from the imaging data analyses due to poor image quality (age 6.67 years, possibly due to excessive motion), technical problem in reading the data (age 15.61 years), or not contributing a complete data set (ages 7.04, 7.06, and 8.40 years). Overall, 123 of the 130 participants had contributed both complete behavioral and imaging data (M = 13.79, SD = 5.92, 50% females).
2.2. Mnemonic strategies: California Verbal Learning Test – Children (CVLT – C)
Participants’ use of semantic strategy was assessed by measuring their clustering of semantically related words during their free recall of a word list. A standardized administration procedure was followed (CVLT – C, Delis, Kramer, Kaplan, & Ober, 1994; this instrument is designed for ages 5–16). Participants were read a list of 15 words comprised of five words from each of three semantic categories: fruit, things to wear, and things to play with. The number of recalled words and the recall order were recorded in each of the participants’ trials. This procedure was repeated five times using the same word list. The CVLT – C was used across the entire age range for three reasons. First, it was important to use the same task for all participants to avoid confounding of potential age-associated differences in strategy use by discrepancies in measurement instrument between age subsamples. Second, we used raw scores, rather than standardized scores as performance indices to preserve age-associated variance. Third, the use of the children’s version of this task ensured that the youngest participants would be able to complete the task at a sufficient level, minimizing possible confounds from basal/floor level. Our choice to use the same instrument across all participants is further supported by the finding that the total number of words recalled over five trials was not reflecting basal/floor (0 words recalled) or ceiling (75 words recalled) levels (Mean across all participants = 48.68, range = 5–72; Mean in participants age 5–8 = 35.58, range = 5–57; Mean in participants age 18–25 = 58.61, range = 44–72).
Semantic and serial clustering scores (the latter served as a comparison measure) were calculated for each of the participants’ recall trials based on the standard CVLT-C scoring protocol (Delis et al., 1994). This protocol defined a semantic cluster as two words from the same category that were recalled sequentially, regardless of the presentation order. This protocol defined a serial cluster as two words recalled in the same order as they were presented. The number of observed clusters was corrected for chance clustering for each trial using a list-based correction (LBC) because the LBC allows for the potential impact of spontaneous strategy use on memory performance, thus allows correlation between the length of clusters and the number of words recalled (Stricker et al., 2002). Semantic clusters were corrected for chance as follows: the number of observed semantic clusters – ((the number of correctly recalled words − 1)×2/7). Serial clusters were corrected for chance as follows: the number of observed serial clusters – ((the number of correctly recalled words − 1)/15). The total number of words recalled and the corrected semantic and serial clusters for all five trials were used in the statistical analyses.
2.3. MRI Acquisition and Post-Acquisition Processing
Measures of PFC volumes were obtained from structural images (T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence) and were collected using a 32-channel head coil in a 3 Tesla Siemens Verio MR scanner (Siemens Medical AG, Erlangen, Germany) at Wayne State University. The T1 MPRAGE was acquired in the coronal plane, perpendicular to the anterior-posterior commissural axis with the following parameters: echo time = 4.26 ms; repetition time = 2200 ms; inversion time = 1200 ms; flip angle = 9.0°; pixel bandwidth = 130 Hz/pixel; GRAPPA acceleration factor PE = 2; interpolated voxel size 0.5 mm × 0.5 mm × 1.0 mm. The total scan time of T1 sequence was 235 seconds.
2.4. Volumetry of Prefrontal Cortex
Regional PFC volumes were generated by FreeSurfer v5.3 (https://surfer.nmr.mgh.harvard.edu/fswiki; Fischl et al., 2002). Six regions of interest (ROIs) were identified using the Desikan-Killiany atlas (Desikan et al., 2006) within FreeSurfer and included superior frontal lobe, rostral middle frontal lobe, caudal middle frontal lobe (rostral and caudal middle frontal lobes correspond to the dorsolateral PFC cortex in previous literature), inferior frontal lobe (a combination of pars orbitalis, pars opercularis, and pars triangularis), lateral orbitofrontal lobe, and medial orbitofrontal lobe (see Figure 1 for location of ROIs). All reconstruction data were visually checked for segmentation accuracy. No manual interventions were conducted on the data. We assessed the quality of the segmentation by Euler numbers extracted from FreeSurfer (Dale et al., 1999; Rosen et al., 2018) for each hemisphere of each participant. Based on Euler numbers we determined that three cases (age 5.13, 6.74, and 10.78 years) should be treated as outliers and excluded from further analyses (averaged Euler number across left and right hemispheres < 3.29SD below the sample mean). With all the data screening steps, we retrained 120 participants (M = 13.94, SD = 5.90, 49% female) who had complete behavioral data and brain data of good image quality. All data analyses were conducted on data from the 120 participants.
Figure 1. Prefrontal cortical regions of interest (ROIs).
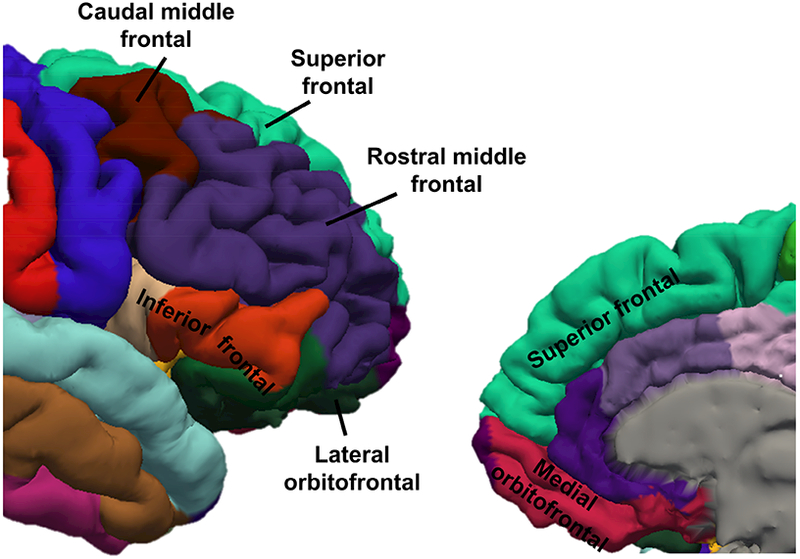
Six ROIs were identified within the prefrontal cortex in each hemisphere for each participant. ROIs were identified by FreeSurfer using Desikan-Killiany atlas and are depicted on restricted lateral (left) and medial (right) views of an example parcellation from the right hemisphere of a 17-year-old female. ROIs depicted: Superior frontal, Rostral middle frontal, Caudal middle frontal, Inferior frontal (a combination of pars orbitalis, pars opercularis, and pars triangularis), Lateral orbitofrontal, Medial orbitofrontal lobes.
2.5. Intracranial Volume Measurement and Volumetry Correction
The influence of individual differences in intracranial volume (ICV) on the outcome measures of PFC ROI volumes was accounted for in accordance with accepted procedures (Raz et al., 2003). ICV was measured using manual demarcation to avoid the potential confound presented by using the aggregate measure of ICV provided by FreeSurfer (http://surfer.nmr.mgh.harvard.edu/fswiki/eTIV). Manual demarcation of ICV was performed on the T1 MPRAGE that aligned anterior-posterior commissures, corrected for differences in head tilt and yaw, and resampled voxel size to 0.5 mm3. Independent raters manually demarcated ICV following procedures adapted from Raz et al. (2004) with high reliability (ICC(2) = 0.99; Shrout and Fleiss, 1979). ICV was measured beginning with the most dorsal slice on which brain tissue was visualized and extending ventrally. The whole demarcation range covered 200 slices, and demarcation was conducted and sampled on every 20th slice for 10 slices in total. The volume of sampled slices were added and multiplied by 20 to calculate ICV.
Bilateral ROI volumes were corrected for differences in ICV via analysis of covariance (Jack et al., 1989): volumeadj = volumei − b*(ICVi − ICVmean), where i denotes a measurement for an individual, b is the unstandardized coefficient of whole sample volume regressed on ICV, and ICVmean is the sample mean. Although age correlated with ICV in this sample (r = 0.62, p < 0.001), we verified that the slope of regional volumes regressed on ICV was not significantly different within subsamples defined by age, i.e., young children: 5.00–8.99 years, older children: 9.00–12.99 years, adolescents: 13.00–17.99 years, and adults: 18.00 years and up. The interaction of ICV by age subsample were not significant for any of the ROIs (F(1, 116) ≤ 2.89, p ≥ 0.09), except for the left rostral middle frontal lobe (ICV by age subsample interaction: F(1, 116) = 5.48, p = 0.02). False discovery rate (FDR, Benjamini & Hochberg, 1995) corrections were made for multiple comparisons for the 12 analyses based on the two hemispheres of the six ROIs, and no FDR-corrected ICV by age subsample interaction was significant (q ≥ 0.25). Therefore, the assumption of homogeneous slopes across the age subsamples was met and the same correction was applied to the whole sample.
2.6. Data Conditioning and Statistical Analysis
Outliers were defined as values outside the range of 3.29 standard deviations away from the mean of given variables within the subsamples defined by age (see above, Methods Section 2.5 for ranges of the age subsamples). No outliers were identified by this standard.
The relation between age and serial and semantic clustering scores were tested in a repeated measures GLM. This GLM included age and cluster type (semantic and serial) as independent variables and the clustering score as dependent variables. Potential significant interaction between age and cluster type was followed up with two separate univariate GLMs. The univariate GLMs results were further verified with Pearson’s correlation analyses. The relation between age and volume in each ROI was tested via separate Pearson’s correlation analyses. Given no a priori expectation for sex difference in memory performance or in use of mnemonic strategy, all analyses were controlled for sex by entering sex as a control variable. Potential non-linear (quadratic) relation between age and behavioral and brain measures were also tested. We were primarily interested in assessing whether age differences in semantic strategy use are partly accounted for by age differences in volumes of PFC ROIs. Therefore, a mediation analysis (Preacher & Hayes, 2008) was conducted to test the potential mediating effect of volumes of PFC ROIs on the relation between age and semantic clustering score. Only the ROIs that significantly correlated with both age and semantic clustering score were entered in mediation analyses models. As a comparison, we also tested whether age difference in volumes of PFC ROIs explains age difference in serial clustering score. All mediation analyses were controlled for sex by using variables residualized of sex.
Age differences in overall memory performance were assessed by testing the relation of the number of words recalled with age. The benefit of elaborative mnemonic strategy to memory performance was assessed by testing the relation between semantic clustering score and words recalled. The contribution of the PFC to age-associated difference in overall memory performance was assessed by testing the relation between PFC ROI volumes and number of words recalled. Finally, for the regions that were significantly related to both age and number of words recalled, we assessed possible mediating effects of volumes of these regions on the relation between age and number of words recalled.
Due to the correlations among volumes in selected ROIs, regional volumes of each hemisphere were assessed in separate models to avoid multicollinearity. FDR correction was conducted for multiple comparisons (q-values) following the classical one-stage method for multiple comparison correction (Benjamini & Hochberg, 1995), for each series of 12 analyses on the two hemispheres of the six ROIs. To avoid spurious effects, the mediation analysis was bootstrapped with bias-correction (5,000 draws of the original sample with replacement). Analyses were based on data with pairwise deletion of missing data.
3. Results
3.1. Assessing Age Difference in Semantic and Serial Clustering Scores During Recall
Using repeated measure GLM, we tested the statistical effect of age on the two types of clustering scores (semantic vs. serial) during recall and examined whether the association to age varied depending on cluster type. The effect of age on clustering score was significantly different for the two cluster types (F(1, 117) = 13.87, p < 0.001). Follow-up analyses of the differential effect of age on the two cluster types, using separate univariate GLMs, showed that there was an age-associated increase in semantic clustering score during recall (F(1, 117) = 30.42, p < 0.001; r(117) = 0.45, p < 0.001, Figure 2). Serial clustering score during recall was not related to age (F(1, 117) = 1.27, p = 0.26; r(117) = 0.10, p = 0.26, Figure 2). Quadratic relations between age and serial and semantic clustering scores were not significant (F(1, 116) ≤ 1.67, p ≥ 0.20) and were excluded from the GLM analyses.
Figure 2. Age-associated increase in the use of semantic clustering.
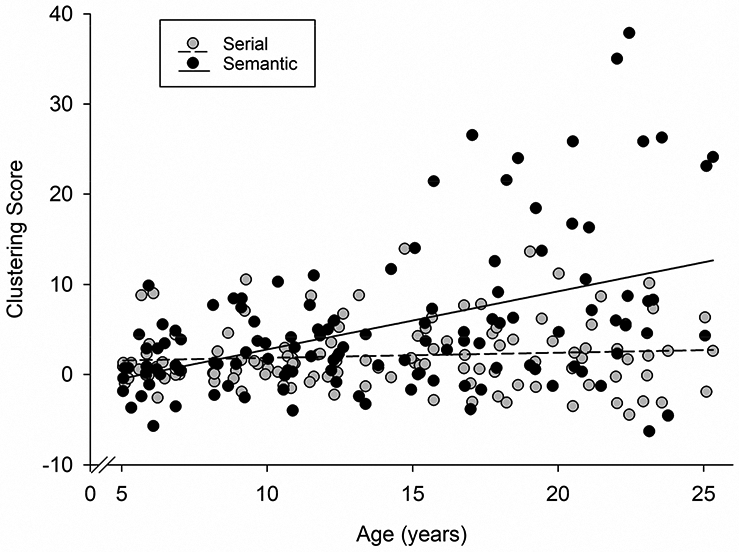
Participant’s total clustering score during recall, adjusted for chance clustering (Stricker et al., 2002), depicted as a function of participant’s age (years). Total semantic clustering score, but not serial, showed a significant increase with age (Age by cluster type interaction: F(1, 117) = 13.87, p < 0.001). Black, total semantic clustering score; Gray, total serial clustering score.
3.2. Linking Age, Prefrontal Cortex Volumes, and Clustering Scores During Recall
3.2.1. Age and PFC Volume
Volumes in all ROIs showed significant correlations with age with FDR correction (Table 1). No ROI volume showed significant quadratic relation to age (p ≥ 0.28). In the subsequent mediation analyses, only the regions that showed significant relation to age were tested for mediating effects.
Table 1.
Relations among age, recall output and volume of PFC ROIs.
| PFC Region | Age | # Semantic Clustering Score | # Serial Clustering Score | # Words Recalled | |||||
|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | ||||||
| Left | <0.001 | 0.003 | 0.88 | <0.001 | |||||
| Right | <0.001 | 0.03 | 0.54 | 0.001 | |||||
| Left | <0.001 | 0.04 | 0.63 | 0.001 | |||||
| Right | <0.001 | 0.001 | 0.27 | 0.002 | |||||
| Left | 0.004 | 0.08 | 0.47 | 0.07 | |||||
| Right | <0.001 | 0.001 | 0.11 | 0.02 | |||||
| Left | <0.001 | 0.003 | 0.93 | <0.001 | |||||
| Right | <0.001 | 0.17 | 0.60 | <0.001 | |||||
| Left | <0.001 | 0.07 | 0.52 | <0.001 | |||||
| Right | <0.001 | 0.29 | 0.56 | <0.001 | |||||
| Left | <0.001 | 0.37 | 0.26 | <0.001 | |||||
| Right | <0.001 | 0.26 | 0.99 | <0.001 | |||||
Partial correlation coefficients and significance presented for relationship between volume of each PFC ROI with age, semantic clustering score, serial clustering score, and number of words recalled. Effects were tested separately for left and right volumes for all the ROIs, including superior frontal, rostral middle frontal, caudal middle frontal, inferior frontal, lateral orbitofrontal and medial orbitofrontal lobes. All correlations are controlled for sex. Bold, significant correlations after FDR correction for multiple comparisons.
regional volumes significantly mediated the relation between age and performance indices.
3.2.2. PFC Volume and Clustering Scores During Recall
Volumes of the left superior frontal, right rostral middle frontal, right caudal middle frontal and left inferior frontal lobes negatively correlated with semantic clustering score during recall (r(117) ≤ −0.27, p ≤ 0.003, FDR q ≤ 0.009; Table 1). In contrast, among all six PFC ROIs, volumes of none of the ROIs were correlated with serial clustering score (|r(117)| ≤ 0.15, p ≥ 0.11; Table 1).
3.2.3. PFC Volume Mediated Relation between Age and Semantic Clustering Score
Next, we tested whether variability in PFC regional volume mediated the relation between age and semantic clustering score in regions in which volume was related to both age and semantic clustering use. There was a significant indirect effect between age and semantic clustering score through the volume of the right rostral middle frontal (standardized β = 0.05, SE = 0.03, 95% bias-corrected bootstrapped CI: [0.01/0.11], Figure 3), and the right caudal middle frontal (standardized β = 0.05, SE = 0.03, 95% bias-corrected bootstrapped CI [0.01, 0.13], Figure 4). There was no significant indirect effect between age and semantic clustering score through the volume of the left superior frontal region (standardized β = 0.03, SE = 0.04, 95% bias-corrected bootstrapped CI [−0.05, 0.10]; Figure 5A) or left inferior frontal region (standardized β = 0.03, SE = 0.04, 95% bias-corrected bootstrapped CI [−0.06, 0.11]; Figure 5B). Quadratic age term was not included in the mediation models because quadratic age was not related to PFC regional volumes or to semantic clustering score. Mediation analyses of the relation between age and serial clustering score were not tested because serial clustering score did not correlate with age or regional PFC volumes.
Figure 3. Age-associated increase in spontaneous use of semantic clustering is partially accounted by the volume of the right rostral middle frontal lobe.
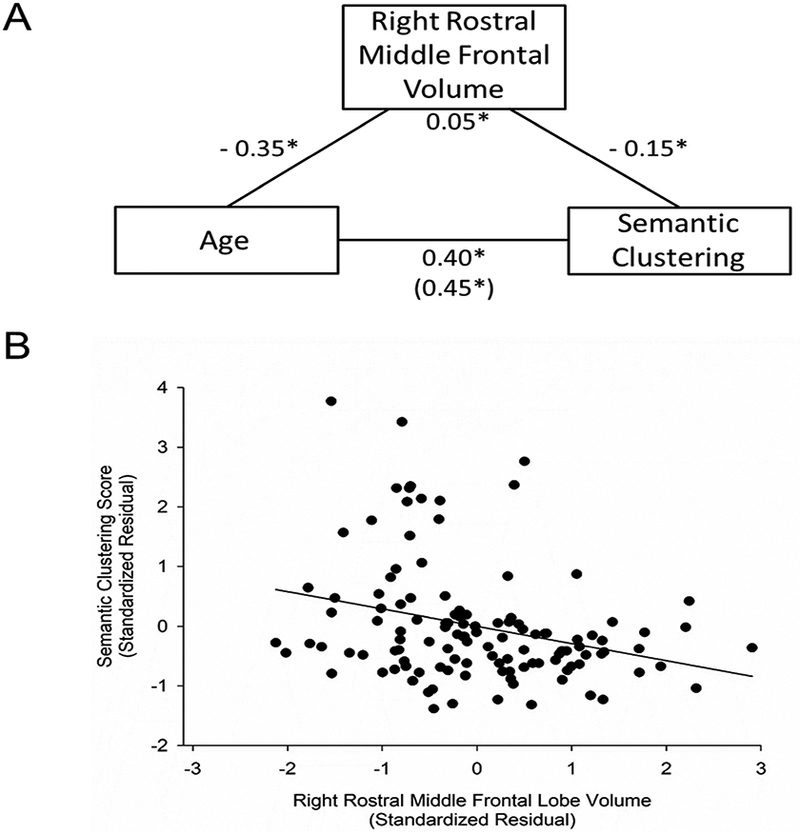
A. Volume of the right rostral middle frontal lobe partially explained the effect of age on the total semantic clustering score during recall as evident by a significant indirect effect (standardized β = 0.05, SE = 0.03, 95% bias-corrected bootstrapped CI [0.01, 0.11]). Numbers for each path shown in the figure are the standardized beta weights. SE, standard error; CI, confidence interval. *indicates significant paths with a 95% bias-corrected bootstrapped CI that does not overlap with zero. Number in parenthesis indicates direct effect of age on semantic clustering. B. Total semantic clustering score during recall trials for each participant is depicted as a function of participant’s right rostral middle frontal lobe volume. The values depicted were standardized residuals adjusted for sex. Total semantic clustering score showed a significant relation with volume in this region (r = −0.29, p = 0.001, FDR q = 0.008), smaller volumes were associated with greater use of semantic clustering during recall.
Figure 4. Age-associated increase in spontaneous use of semantic clustering is partially accounted by the volume of the right caudal middle frontal lobe.
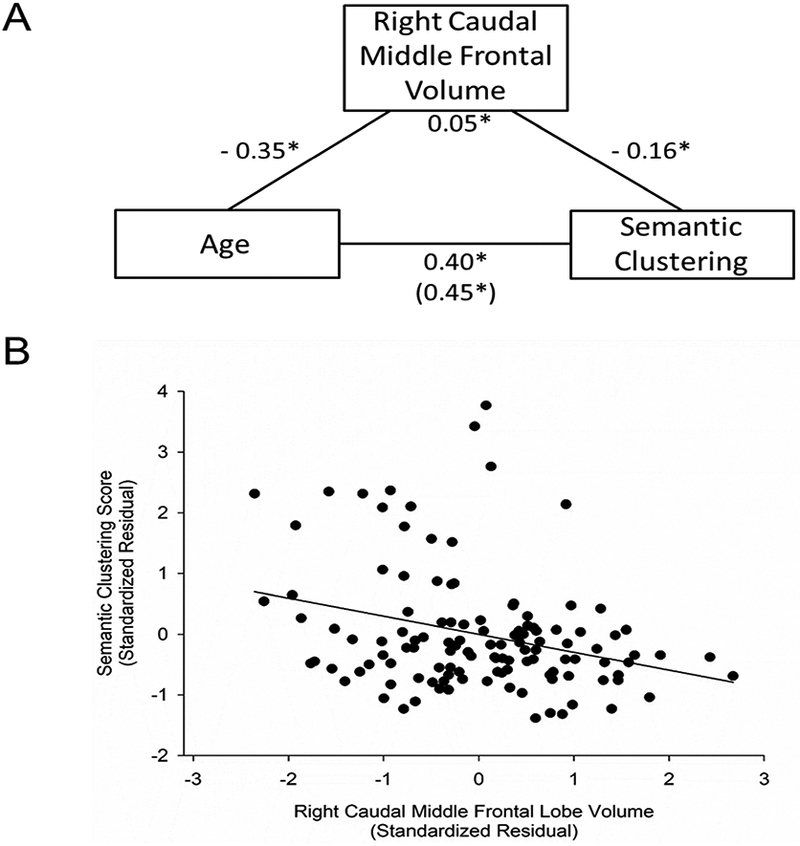
A. Volume of the right caudal middle frontal lobe partially explained the effect of age on total semantic clustering score during recall as evident by a significant indirect effect (standardized β = 0.05, SE = 0.03, 95% bias-corrected bootstrapped CI [0.01, 0.13]). Numbers for each path shown in the figure are the standardized beta weights. SE, standard error; CI, confidence interval. *indicates significant paths with a 95% bias-corrected bootstrapped CI that does not overlap with zero. Number in parenthesis indicates direct effect of age on semantic clustering. B. Total semantic clustering score during recall trials for each participant is depicted as a function of participant’s right caudal middle frontal lobe volume. The values depicted were standardized residuals adjusted for sex. Total semantic clustering score showed a significant relation with volume in this region (r = −0.30, p = 0.001, FDR q = 0.008), smaller volumes were associated with greater use of semantic clustering during recall.
Figure 5. Insignificant indirect effects of volumes of left superior frontal and left inferior frontal lobes on age-associated increase in spontaneous use of semantic clustering.
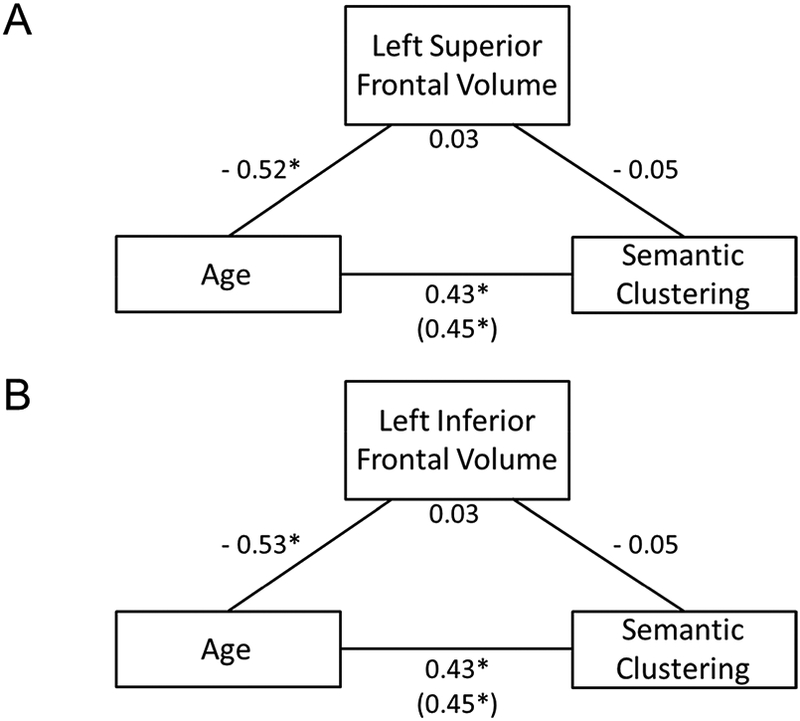
A. Volume of the left superior frontal lobe did not explain significant amount of variance of the relation between age and total semantic clustering score during recall (standardized β = 0.03, SE = 0.04, 95% bias-corrected bootstrapped CI [−0.05, 0.10]). B. Volume of the left inferior frontal lobe did not explain significant amount of variance of the relation between age and total semantic clustering score during recall (standardized β = 0.03, SE = 0.04, 95% bias-corrected bootstrapped CI [−0.06, 0.11]). Numbers for each path shown in the figure are the standardized beta weights. SE, standard error; CI, confidence interval. *indicates significant paths with a 95% bias-corrected bootstrapped CI that does not overlap with zero. Number in parenthesis indicates direct effect of age on semantic clustering.
3.3. Number of Words Recalled Related to Semantic Strategy Use, Age, and PFC Volume
Given that semantic strategy use is thought to enhance recall, we assessed the link between recall and semantic strategy use in this developmental sample. Indeed, increase in semantic clustering score during recall was associated with greater number of words recalled (r(117) = 0.50, p < 0.001). In contrast, the use of serial clusters was not related to the number of words recalled (r(117) = 0.12, p = 0.21). Effects of age on number of words recalled were assessed and identified as significant for both linear (F(1, 116) = 156.88, p < 0.001) and quadratic (F(1, 116) = 14.06, p < 0.001, age = 22.95 at inflection point) terms. Thus, among participants younger than 22.95 years, older participants recalled more words compared to their younger counterparts. Finally, the number of words recalled was also related to regional PFC volume in nearly all PFC regions (Table 1). The exception to this general pattern of association between PFC volume and number of words recalled was noted in the left caudal middle frontal region. Given that the number of words recalled was associated with age and PFC regional volume, we further tested possible mediation effects of PFC volumes on the relation between age and number of words recalled, following the same steps in assessing mediation as described above. None of the models identified a significant indirect effect of PFC volumes on the relation between age and number of word recalled.
4. Discussion
In this study, we investigated the relationship between individual differences in PFC volume and age increase in elaborative mnemonic strategy use between kindergarten age and early adulthood (5–25 years). The use of semantic clustering, an elaborative mnemonic strategy, increased with age. Consistent with the literature, PFC volumes showed a robust age-associated decrease. We also found that smaller volume of the left superior frontal lobe, left inferior frontal lobe, and right DLPFC (right rostral and caudal middle frontal lobe) was associated with increased use of semantic clustering in this developing sample. Critically, age-associated reduction in the volume of the right DLPFC partially explained age-associated increase in the use of semantic clustering. Our findings provide novel evidence highlighting the role of the DLPFC in supporting an age-associated increase in mnemonic strategy use. To understand the use of semantic strategy in the context of memory performance, we confirmed that increased use of semantic clustering was related to improved recall across all participants. Recall performance was related to individual differences in volume of PFC regions, however, age-associated reduction in PFC volumes did not partially explain the age-associated improvement in recall.
In the PFC, we focused our investigation in six anatomically defined ROIs comprising the extent of this region. Consistent with our hypotheses, age-associated increase in semantic strategy use was uniquely accounted for by the age-associated decrease in right DLPFC volume. The specificity of the findings to the DLPFC suggests a distinct role for the DLPFC in supporting the development of memory through its involvement in the use of elaborative mnemonic strategies. This finding is consistent with an existing body of research revealing the critical role of the DLPFC in supporting spontaneous use of semantic strategies (Hirst & Volpe, 1988; Gershberg & Shimamura, 1995; Baldo et al., 2002; Hawco, Berlim, & Lepage, 2013; Fletcher et al., 1998; Hazlett et al., 2004; Nohara et al., 2000; Hawco, Berlim, & Lepage, 2013). The finding that age-associated increase in semantic mnemonic strategy use was selectively explained by the right, but not left, DLPFC volume, argues for an instrumental role of the right hemisphere in supporting semantic mnemonic functions, from kindergarten age to early adulthood. The biological mechanism involved in this age-associated increase in semantic strategy use may be linked to the protracted maturation trajectory of the right PFC, as evidenced by a stronger relation between age and reduction in gray matter thickness and density of the right PFC compared to the left PFC (Sowell et al., 2004; Gogtay et al., 2004). The right lateralized finding may also reflect the involvement of the right DLPFC in general mnemonic control during retrieval (Anderson et al., 2004; Anderson & Hanslmayr, 2014; Paz-Alonso et al., 2013), which is an essential function for spontaneous use of semantic strategies. Further studies are required to explore the neural maturational correlates of age-associated increase in the use of elaborative mnemonic strategies other than semantic clustering. Additionally, future studies can investigate whether the observed age-associated reduction in the right DLPFC volume plays a domain-specific, or domain-general, developmental role. Our findings are consistent with prior work highlighting cortical gray matter thinning as related to age-associated improvements on executive function (Kharitonova et al., 2013). Future studies in multiple cognitive domains are needed to determine whether right DLPFC maturational changes underlie age-associated enhancement that is limited to semantic strategy use, or do such changes account for age-associated enhancement in all elaborative mnemonic strategies that require mnemonic control.
The direction of the mediating effect that we found in the current study of PFC volumes on the relation between age and spontaneous semantic strategy use is different from that found in adults. In an adult lifespan sample age 18–91 years, Kirchhoff et al. (2014) found that age-associated decrease in PFC regional volumes partially accounts for age-associated decrease in spontaneous semantic strategy use. In another study comparing spontaneous elaborative strategy use in younger and older adults, Husa et al. (2017) found similar pattern such that smaller PFC volume in older participants partially accounts for older participants’ lesser use of spontaneous elaborative strategies. Taken together, this may indicate that age-associated decrease in PFC volume is beneficial in development, potentially reflecting the neuronal pruning process over development, whereas less age-associated decrease in PFC volume is beneficial in aging. This possibility based on cross-sectional data across studies requires evidence based on lifespan longitudinal data.
In contrast to semantic clustering, we did not find an age-associated increase in serial clustering, nor did we find a relation between PFC volume and serial clustering, or a mediating effect of PFC volume on the relation between age and serial clustering. Consistent with our hypothesis, these null findings may reflect the fact that serial clustering relies predominantly on rote memorization or passive memory processing that lacks in semantic strategy use, and thus requires minimal strategic manipulation of the information to be memorized and recalled. The absence of significant relationship between age and serial clustering suggests that the ability to recall information as presented may be fully developed early on (Gathercole, 1998). The absence of significant statistical association between serial clustering and the PFC is consistent with previous findings that patients with PFC lesions and healthy controls are comparable in using serial clusters (Alexander et al., 2003; Baldo et al., 2002). Though the PFC seems unessential for serial clustering, other brain regions may nonetheless be critical. Future studies may investigate neural correlates of serial clustering other than PFC morphology.
The differential relation of semantic and serial, or rote, clustering to age and PFC volumes may reflect the two-component framework of episodic memory development across the lifespan (Shing et al., 2010). Shing and colleagues (2010) proposed a two-component framework of episodic memory development that consists of an associative component that allows for the details of an event to be combined into a single unified event, and a strategic component that requires cognitive control to encode discrete details and organize them based on their association to other details. According to this framework, in the context of lifespan development, memory function during childhood is limited by the strategic component, linked to the protracted development of the PFC and the stable function of the medial temporal lobe (MTL; Shing et al., 2010), yet is not limited by the associative component. In contrast, the memory function in older adults is limited by both strategic and associative components, linked to changes in both the PFC and MTL (Shing et al., 2010). Consistent with this notion, a recent study suggests that from childhood to young adulthood, successful encoding relies on the protracted activation increase in the PFC, but successful encoding in older adults relies on maintained activation level in both the PFC and the MTL (Shing, Brehmer, Heekeren, Backman, & Lindenberger, 2016). It is possible that semantic clustering development is more dependent on the strategic component of episodic memory, whereas serial clustering is more reliant on the associative component. The differential relationship of the two clustering types to age and PFC volumes observed in the current investigation may reflect the two components of episodic memory process and the different developmental trajectories of the two components. Future studies examining the relation between serial clustering and MTL structure and function may provide insight for the relation between the two types of clusters and the two-component framework.
To understand the use of semantic strategy in the context of memory performance, we tested the relation between semantic clustering score and words recalled. There was a positive correlation between semantic clustering score and words recalled, supporting the notion that using semantic strategy benefits memory performance. Though we did not find significant mediating effects of volumes of any PFC regions on age-associated increase in the number of words recalled, volumes of all the tested PFC regions, except the left caudal middle frontal lobe, showed a significant negative correlation with number of words recalled, supporting that PFC structure plays an important role in memory performance. The lack of a significant mediating effect of DLPFC volume on the association between age and recall, together with the significant effect of DLPFC volume on the association between age and semantic strategy use, may reflect a unique contribution of DLPFC structure to spontaneous semantic strategy use in developing population. However, we caution this interpretation given the difficulty in interpreting null findings.
Our findings of age-associated increase in the use of semantic strategy, reduction in PFC volume, and a mediating effect of PFC volume in age-associated difference in semantic strategy use are largely consistent with prior findings (Bjorklund & Douglas, 1997; Gogtay et al., 2004; Giedd et al., 1999; Kirchhoff et al., 2014). Nonetheless, the limitation of the current cross-sectional study design should be noted. First, the age-associated differences we found cannot by interpreted as demonstrating a neural mechanism of developmental (Lindenberger, von Oertzen, Ghisletta, & Hertzog, 2011) or a direct age-associated mediating effect (Maxwell & Cole, 2007). Future studies using longitudinal designs are necessary to test the contribution of PFC maturation to the development of semantic strategy use. Future longitudinal studies may also examine potential association between PFC maturation and non-linear and step-like developmental trajectories of strategy use (Schlagmuller & Schneider, 2002). Second, in our investigation we used the robust measure of strategy use across the five repetitions of the word-list recall trials. Future research using multilevel modeling will be instrumental in providing insight about how strategy use changes over repeated learning trials, whether these changes differ depending on age or the structure of the PFC. Third, although by using the CVLT, we were able to measure spontaneous mnemonic strategy use and specifically assess an individual’s level of semantic strategy, we were only able to measure individual differences in mnemonic strategy use that are specific to administration parameters, such as three semantic categories, five repetitions, etc. Other modalities, and task characteristics may influence individual differences in spontaneous strategy use. Further work can be directed to assessing the relationship between brain measures and age differences in instructed use of mnemonic strategies, or benefits of training in specific mnemonic strategies. In addition, the semantic clustering measure based on CVLT, though measured during recall, may reflect processes operation during encoding, the maintenance of represented information or recall. Future studies using tasks that are able to distinguish encoding and recall processes are necessary to study the neural correlates of semantic strategy use over development.
5. Conclusion
We provide evidence that age-associated reduction in PFC volume is linked to age-associated increase in the use of semantic strategy. Specifically, decrease in the volume of the right middle PFC partially accounted for an age-associated increase in semantic clustering use during recall. These findings in a developmental sample are consistent with findings in adult lifespan samples showing link between the age-associated decline in functional contributions of PFC and decline in cognition in aging (West, 1996; Buckner, 2004; Kirchhoff et al., 2014). More broadly our findings support the notion that cognitive development is linked to structural and functional changes in the PFC (Ofen, 2012; Tang et al, 2017; Kharitonova et al., 2013) and point to the use of semantic strategy in underscoring the role of the PFC in supporting memory development.
Acknowledgments
The authors declare no competing financial interests. We thank A. Daugherty for helpful discussions; PA Sam, D Brush, A Hardwick, J Piercy, A Heitzer, B Thompson, S Ramesh, P Jella and D Khatib for help with data collection. The work was partially supported by funding from the National Institutes of Health (R01MH107512 to NO) and funding from the Institute of Gerontology and the Department of Psychology at Wayne State University (to NO and QY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander MP, Stuss DT, & Fansabedian N (2003). California Verbal Learning Test: Performance by patients with focal frontal and non-frontal lesions. Brain, 126(6), 1493–1503. [DOI] [PubMed] [Google Scholar]
- Anderson MC, & Hanslmayr S (2014). Neural mechanisms of motivated forgetting. Trends in Cognitive Sciences, 18(6), 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, … Gabrieli JDE (2004). Neural systems underlying the suppression of unwanted memories. Science, 303(5655), 232–235. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Delis D, Kramer J, & Shimamura AP (2002). Memory performance on the California Verbal Learning Test-II: findings from patients with focal frontal lesions. Journal of the International Neuropsychological Society, 8(4), 539–546. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Krieger AM, & Yekutieli D (2006). that control the false Adaptive linear procedures rate discovery. Biometrika, 93(3), 491–507. [Google Scholar]
- Bjorklund DF, & Douglas RN (1997). The development of memory strategies In Cowan N (Ed.), The development of memory in childhood: Studies in developmental psychology (pp. 201–246). New York: Psychology Press. [Google Scholar]
- Blumenfeld RS, & Ranganath C (2007). Prefrontal cortex and long-term memory encoding: an integrative revkhiew of findings from neuropsychology and neuroimaging. Neuroscientist, 13(3), 280–291. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2004). Memory and executive function in aging and ad: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44(1), 195–208. [DOI] [PubMed] [Google Scholar]
- Camp CJ, Markley RP, & Kramer JJ (1983). Naive mnemonics: What the “do-nothing” control group does. American Journal of Psychology, 96(4), 503–511. [Google Scholar]
- Dale AM, Fischl B, Sereno MI, (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Daugherty AM, & Ofen N (2015). That’s a good one! Belief in efficacy of mnemonic strategies contributes to age-related increase in associative memory. Journal of Experimental Child Psychology, 136, 17–29. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (1994). California Verbal Learning Test Manual. San Antonio, TX: Pearson. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Dunlosky J, & Hertzog C (1998). Aging and deficits in associative memory: what is the role of strategy production? Psychology and Aging, 13(4), 597–607. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole Brain Segmentation: Neurotechnique Automated Labeling of Neuroanatomical Structures in the Human Brain. Neurotechnique, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, & Dolan RJ (1998). The functional roles of prefrontal cortex in episodic memory. Brain, 121(7), 1239–1248. [DOI] [PubMed] [Google Scholar]
- Gathercole SE (1998). The development of memory. Journal of Child Psychology and Psychiatry, 39, 3–27. [PubMed] [Google Scholar]
- Gershberg FB, & Shimamura AP (1995). Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia, 33(10), 1305–1333. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2(10), 861–863. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis a C., … Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 707(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawco C, Berlim MT, & Lepage M (2013). The dorsolateral prefrontal cortex plays a role in self-initiated elaborative cognitive processing during episodic memory encoding: RTMS evidence. PLoS One, 8(9), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Hsieh P, Haznedar MM, Platholi J, LiCalzi EM, … Hollander E (2004). Regional glucose metabolism within cortical Brodmann areas in healthy individuals and autistic patients. Neuropsychobiology, 49(3), 115–125. [DOI] [PubMed] [Google Scholar]
- Hertzog C, McGuire CL, Horhota M, & Jopp D (2010). Does believing in “use it or lose it” relate to self-rated memory control, strategy use and recall? The International Journal of Aging and Human Development, 70(1), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst W, Volpe BT (1988). Memory strategies with brain damage. Brain and Cognition, 8, 379–408. [DOI] [PubMed] [Google Scholar]
- Husa RA, Gordon BA, Cochran MM, Bertolin M, Bond DN, & Kirchhoff BA (2017). Left caudal middle frontal gray matter volume mediates the effect of age on self-initiated elaborative encoding strategies. Neuropsychologia, 106, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, & Cascino GD (1989). Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology, 172, 549–554. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JD, & Sheridan MA (2013). Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Developmental cognitive neuroscience, 6, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Gordon BA, & Head D (2014). Prefrontal gray matter volume mediates age effects on memory strategies. NeuroImage, 90, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, & Beaulieu C (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience, 31(30), 10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Von Oertzen T, Ghisletta P, & Hertzog C (2011). Cross-sectional age variance extraction: What’s change got to do with it? 26(1), 34–47. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, & Cole DA (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods, 12(1), 23–44. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, & Levy O (2007). The associative memory deficit of older adults: the role of strategy utilization. Psychology and Aging, 22(1), 202–208. [DOI] [PubMed] [Google Scholar]
- Nohara S, Suzuki M, Kurachi M, Yamashita I, Matsui M, Seto H, & Saitoh O (2000). Neural correlates of memory organization deficits in schizophrenia. A single photon emission computed tomography study with 99mTc-ethyl-cysteinate dimer during a verbal learning task. Schizophrenia Research, 42(3), 209–222. [DOI] [PubMed] [Google Scholar]
- Ofen N (2012). The development of neural correlates for memory formation. Neuroscience and Biobehavioral Reviews, 36(7), 1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, & Gabrieli JDE (2007). Development of the declarative memory system in the human brain. Nature Neuroscience, 10(9), 1198–1205. [DOI] [PubMed] [Google Scholar]
- Ofen N, Yu Q, & Chen D (2016). Memory and the developing brain: are insights from cognitive neuroscience applicable to education? Current Opinion in Behavioral Sciences, 10, 81–88. [Google Scholar]
- Paz-Alonso PM, Bunge SA, Anderson MC, & Ghetti S (2013). Strength of Coupling within a Mnemonic Control Network Differentiates Those Who Can and Cannot Suppress Memory Retrieval. Journal of Neuroscience, 33(11), 5017–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, & Acker JD (2004). Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging, 25(3), 377–396. [DOI] [PubMed] [Google Scholar]
- Rosen AFG, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa LP, … Satterthwaite TD (2018). Quantitative assessment of structural image quality. NeuroImage, 169(October 2017), 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagmuller M, & Schneider W (2002). The development of organizational strategies in children: evidence from a microgenetic longitudinal study. Journal of Experimental Child Psychology, 81(3), 298–319. [DOI] [PubMed] [Google Scholar]
- Schleepen TMJ, & Jonkman LM (2012). Children’s use of semantic organizational strategies is mediated by working memory capacity. Cognitive Development, 27(3), 255–269. [Google Scholar]
- Schneider W, Kron V, Hunnerkopf M, & Krajewski K (2004). The development of young children’s memory strategies: First findings from the Wurzburg Longitudinal Memory Study. Journal of Experimental Child Psychology, 88(2), 193–209. [DOI] [PubMed] [Google Scholar]
- Schneider W, & Pressley M (1997). Memory Development Between Two and Twenty. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Schneider W, & Sodian B (1997). Memory strategy development: Lessons from longitudinal research. Developmental Review, 17(4), 442–461. [Google Scholar]
- Shing YL, Brehmer Y, Heekeren HR, Backman L, & Lindenberger U (2016). Neural activation patterns of successful episodic encoding: reorganization during childhood, maintenance in old age. Developmental Cognitive Neuroscience, 20, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Brehmer Y, Muller V, Li SC, & Lindenberger U (2010). Episodic memory across the lifespan: The contributions of associative and strategic components. Neuroscience and Biobehavioral Reviews, 34(7), 1080–1091. [DOI] [PubMed] [Google Scholar]
- Shrout PE, & Fleiss J (1979). Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin, 86(2), 420–428. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, & Jernigan TL (2001). Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. Journal of the International Neuropsychological Society, 7(3), 312–322. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, & Toga AW (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24(38), 8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker JL, Brown GG, Wixted J, Baldo JV, & Delis DC (2002). New semantic and serial clustering indices for the California Verbal Learning Test-Second Edition: Background, rationale, and formulae. Journal of the International Neuropsychological Society, 8(3), 425–435. [DOI] [PubMed] [Google Scholar]
- Tang L, Shafer AT, & Ofen N (2017). Prefrontal Cortex Contributions to the Development of Memory Formation. Cerebral Cortex, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL (1996). An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin, 120(2), 272–292. [DOI] [PubMed] [Google Scholar]


