Abstract
Accurately measuring respiration in laboratory rodents is essential for many fields of research, including olfactory neuroscience, social behavior, learning and memory, and respiratory physiology. However, choosing the right technique to monitor respiration can be tricky, given the many criteria to take into account: reliability, precision, and invasiveness, to name a few. This review aims to assist experimenters in choosing the technique that will best fit their needs, by surveying the available tools, discussing their strengths and weaknesses, and offering suggestions for future improvements.
Keywords: breathing, respiration, sniffing
INTRODUCTION
Whereas respiration, or breathing, is the involuntary act of inhaling through the nose, sniffing corresponds to its voluntary modulation (Kurnikova et al. 2017; Uchida and Mainen 2003; Wesson 2013; Wesson et al. 2008; Youngentob et al. 1987). When it comes to exploring their environment or interacting with each other, rodents alter their sniffing (Welker 1964; Wesson et al. 2008). Not only does sniffing govern the way odor information is sampled and processed, but also it modulates many aspects of their behavior and physiology, from whisker and head movement (Macrides et al. 1982; Moore et al. 2013; Welker 1964) to limbic operations (Macrides 1975; Macrides et al. 1982; Semba and Komisaruk 1978).
Furthermore, due to the position of the epiglottis relative to the soft palate, the oropharynx of most rodents, such as rats, mice, and guinea pigs, does not connect to the lower airway. As a consequence, these animals are obligate nose breathers: under normal conditions, they breathe through their nose only (Gautam and Verhagen 2012; Harkema et al. 2006). In other words, breathing and sniffing are related behaviors and can usually be measured with the same tools.
For numerous fields of study where rodents are extensively used, such as odor processing, social behavior, learning and memory, or respiratory physiology, finding the right method to monitor respiration is crucial. Furthermore, as of now, breathing monitoring remains an open problem and has motivated many recent innovations (for example: Bolding and Franks 2017; Esquivelzeta Rabell et al. 2017; McAfee et al. 2016; Vandivort et al. 2016). It is, therefore, surprising that the last published extensive review discussing the different methods available to measure the breathing of laboratory rodents dates back to 1979 (Likens and Mauderly 1979). In this review, we aim to address this gap in the literature. In addition to describing the principle of each method for monitoring respiration (Fig. 1), we also discuss their strengths and weaknesses in terms of reliability, precision, and invasiveness, to help readers choose the method that best fits their needs (Fig. 2; Table 1). When possible, we also provide historical context as well as suggestions for improving existing methods and exploring new techniques. The different methods discussed are organized according to the physiological variable they measure: neuronal or muscular activity, air movements through the airways, temperature changes, or body movements. First things first, let’s start with methods relying on neuronal activity.
Fig. 1.
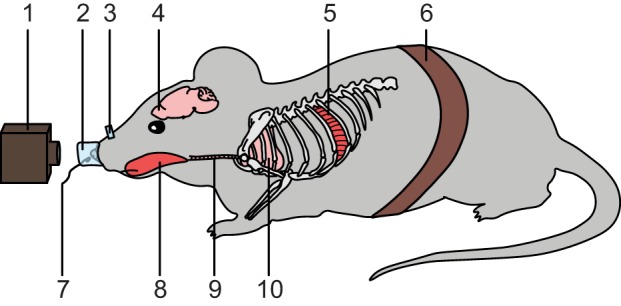
Visual summary of the methods discussed in this review. The diagram illustrates the following methods: 1) infrared camera and video monitoring, 2) face mask, 3) intranasal cannula and implanted temperature probe, 4) olfactory bulb local field potentials, 5) electromyogram (EMG) of respiratory muscles (in this case, intercostal muscles), 6) movement sensor, 7) external temperature probe, 8) EMG of nonrespiratory muscles (in this case, genioglossus), 9) intubation, and 10) lung plethysmograph.
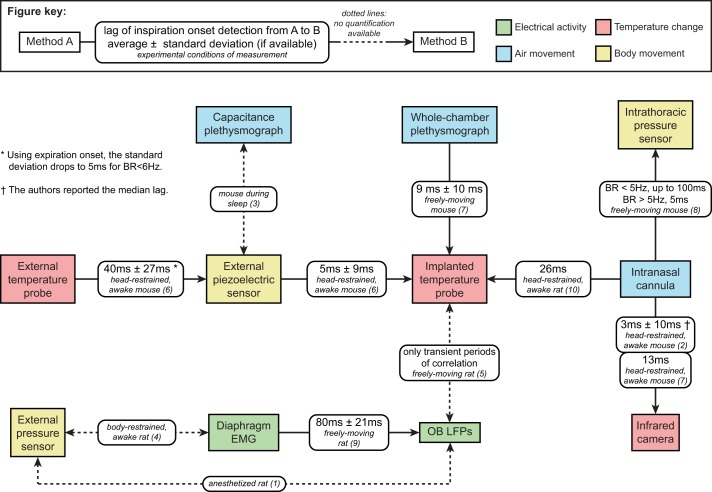
Fig. 2.
Phase shifts between breathing-monitoring methods. This diagram gives, when available in the literature, the lag time of inhalation onset detection between the methods presented here. This figure is based on the following articles: 1) Buonviso et al. 2003, 2) Esquivelzeta Rabell et al. 2017, 3) Flores et al. 2007, 4) Jourdan et al. 1997, 5) Khan et al. 2012, 6) McAfee et al. 2016, 7) Mutlu et al. 2018, 8) Reisert et al. 2014, 9) Rojas-Líbano et al. 2014, and 10) Verhagen et al. 2007. BR, breathing rate; EMG, electromyogram; OB LFPs, olfactory bulb local field potentials.
Table 1.
Summary of the methods discussed in this review
| Variable Measured | Method | Is the Technique Invasive? | Does the Instrument Touch the Animal? | Does the Method Work on Awake Animals? | Can the Animal Move Freely? | Recent Examples of the Use of This Method |
|---|---|---|---|---|---|---|
| Electrical activity | OB LFPs* | Yes | Yes | No | No | Rojas-Líbano et al. 2014 |
| EMG of respiratory muscles | Yes | Yes | Yes | Yes | Li et al. 2016 | |
| EMG of nonrespiratory muscles (genioglossus, etc.) | Yes | Yes | Yes | Yes | Cui et al. 2016 | |
| Air movement | Intubation | Yes | Yes | No | No | Cui et al. 2016 |
| Li et al. 2016 | ||||||
| Vandivort et al. 2016 | ||||||
| Intranasal cannula | Yes | Yes | Yes | Yes | Li et al. 2014 | |
| Reisert et al. 2014 | ||||||
| Sirotin et al. 2014 | ||||||
| Face mask | No | Yes | Yes | No | Bolding and Franks 2017 | |
| Lung plethysmograph | No | It depends on the design (mask, whole chamber, etc.) | Yes | It depends on the design (mask, whole chamber, etc.) | Lim et al. 2014 | |
| Li et al. 2016 | ||||||
| McAfee et al. 2016 | ||||||
| Temperature changes | Implanted temperature probe | Yes | Yes | Yes | Yes | Khan et al. 2012 |
| McAfee et al. 2016 | ||||||
| External temperature probe | No | No | Possibly not | No | Lepousez and Lledo 2013 | |
| Ito et al. 2014 | ||||||
| McAfee et al. 2016 | ||||||
| Infrared camera | No | No | Yes | Yes theoretically for short periods of time (seconds to minutes, never tested), no for longer | Esquivelzeta Rabell et al. 2017 | |
| Body movement | Movement sensor | No, except when the sensor is implanted | Yes | No, except when the sensor is implanted | No, except when the sensor is implanted | Zehendner et al. 2013 |
| Reisert et al. 2014 | ||||||
| Kapoor et al. 2016 | ||||||
| McAfee et al. 2016 | ||||||
| Video monitoring | No | No | Yes | Yes for short periods of time (seconds to minutes), no for longer | Welker 1964 (no recent example) |
The table presents the strengths and weaknesses of each method. A method was considered invasive when it required a surgery on the animal. EMG, electromyogram; OB LFPs, olfactory bulb local field potentials.
The fidelity of this method is controversial; see corresponding section of this review for more details.
BACK TO BASICS: MONITORING RESPIRATION THROUGH NEURONAL OR MUSCULAR ACTIVITY
From the Brain. . .
Let’s go back to basics: respiration is the result of volume changes in the lungs, which result from respiratory muscle activity, which is the consequence of neuronal activity conveyed through nerves, from the brain. Therefore, it would seem logical to monitor breathing using neuronal activity.
The neuronal circuits underlying respiration have been and continue to be an extensively studied field of research. Many brain areas, grouped under the name of respiratory centers, are known to be necessary for breathing. They are all located in the medulla oblongata and the pons, in the brain stem (for a review, see: Feldman et al. 2013). However, these regions are rarely, if ever, monitored for the sole purpose of respiration tracking, most probably because easier, less invasive techniques are readily available.
Another category of neuronal activity that reflects breathing consists of local field potentials (LFPs) in the olfactory bulb (OB). LFPs are electrical signals originating from the simultaneous activity of numerous nearby neurons. The observation of the similarity between OB LFPs and respiratory cycles goes back as far as the 1940s. In an article focused on odor responses in the OB of hedgehogs, Edgar Adrian (1942) noted the apparent synchrony of breathing cycles with the LFP θ-band (4–8 Hz). A few years later, as he was working on the rabbit OB, Adrian (1950) observed the same phenomenon. These observations make sense, since OB θ-cycles result from air movements on the olfactory epithelium, at the back of the nasal cavity (Grosmaitre et al. 2007).
Nonetheless, OB LFPs are not ideal for monitoring respiration: whereas some authors found great reliability between OB LFPs and diaphragm electromyograms (EMGs; Rojas-Líbano et al. 2014; Fig. 2), others highlighted that OB LFPs lose synchrony at breathing frequencies >10 Hz (Kay and Laurent 1999). More recently, a study showed that OB LFPs and breathing only show transient periods of correlation (Khan et al. 2012).
. . . To the Muscles
As discussed above, inserting an electrode in the brain of a rodent is a rather poor idea for monitoring respiration, either because it is too invasive (respiratory centers) or simply unreliable (OB LFPs). What’s left, then?
The respiratory centers are connected to the respiratory muscles through the pulmonary plexuses. In mammals, inhalation is exclusively active: at each inhalation, the diaphragm and the external intercostal muscles contract, allowing the lungs to expand. Unlike inhalation, exhalation is a mostly passive process, driven by the elastic return of the inspiratory muscles, ribcage, and lungs to their resting position. During exercise, however, active exhalation can occur through the contraction of the abdominal and internal intercostal muscles (Feldman et al. 2013).
Therefore, due to their ease of access, inspiratory muscles provide an accurate, reliable way of measuring breathing. The biggest inspiratory muscle, hence the most accessible, is the diaphragm (Fig. 2). Once electrodes have been implanted, diaphragm EMG recordings can be used to monitor precisely the different phases of the respiratory cycle in awake animals over an extensive period of time, weeks to months, depending on the quality of the electrode and the implantation. To our knowledge, the first publication reporting diaphragm EMGs in rodents dates to 1977 (Sherrey and Megirian 1977). Before then, EMGs were performed on larger animals, such as dogs and cats (for an example with cats, see: Schoolman and Fink 1963). Since the end of the 1970s, diaphragm EMGs have been extensively used on rodents, when extreme precision in the measurement of the respiratory cycle was required (a recent example: Li et al. 2016). Note that rodents usually have one “dominant” nostril (i.e., more widely open than the other). This is due to unequal production of mucus and unbalanced swelling of the nasal cavity. The dominant nostril switches every few hours (Bojsen-Moller and Fahrenkrug 1971; Eccles 2000). As a consequence, respiratory muscle output can potentially be misleading with regard to olfactory sampling through each individual nostril.
Some nonrespiratory muscles can be used to monitor respiration as well, such as the genioglossus, an easily accessible muscle of the tongue (Cui et al. 2016). Muscles involved in whisking have also been found to contract in accordance with the rhythm of breathing. Unfortunately, due to the lack of correlation between their contraction and low-rate breathing, these muscles do not appear suitable for respiration monitoring (Moore et al. 2013).
MEASURING RESPIRATION USING AIR MOVEMENT
EMG recordings of respiratory muscles are some of the most direct and precise measurements of respiration. Not only do they provide the immediate breathing frequency, but also they allow the experimenter to quantify the different phases of a given respiratory cycle as well as the respiratory volumes for those given cycles. However, these methods are usually extremely invasive, requiring the opening of the peritoneum to implant electrodes into respiratory muscles, with all the risks that such surgery implies. Many other methods allow for the accurate measurement of the instantaneous breathing rate through less invasive procedures. Some of these methods aim at quantifying the air movements in and out of the lungs.
Intubation
Intubation is relatively simple to perform for trained experimenters: a cannula is inserted in the trachea of an anesthetized animal, and the airflow going through the cannula is monitored (Vandivort et al. 2016). The trachea and the vocal cords allow for a good seal around the cannula, which leads to little air leakage. As a consequence, the volumes of air in and out of the lungs can be accurately measured. Similar to EMGs of respiratory muscles, this method provides not only breathing frequencies, but also respiratory volumes. This technique can be paired with EMG measurements to provide a broader picture of how the respiratory centers in the brain stem control breathing (Cui et al. 2016; Li et al. 2016). However, intubation is invasive and can only be performed on anesthetized rodents.
Intranasal Cannula
The use of intranasal cannulas for the monitoring of breathing is quite a recent development compared with the methods described above (Verhagen et al. 2007). Verhagen and coauthors were inspired by a paper from 1970 that described the implantation of intraoral cannulas in rats (Phillips and Norgren 1970). Instead of implanting a small piece of tubing in the mouth to feed rodents, they perforated the roof of the nasal cavity and glued a cannula on top of it. Therefore, it became possible to measure the intranasal pressure through the cannula. The authors tested their new technique against a thermocouple also implanted in the nasal cavity (see below for a detailed description of the method), in anesthetized as well as head-restrained, awake animals. In both cases, the intranasal cannula showed high reliability for monitoring instantaneous breathing rate (Fig. 2). It only took a year before the method was adapted to freely moving rodents (Wesson et al. 2008). It is now used in numerous applications (Li et al. 2014; Reisert et al. 2014; Sirotin et al. 2014).
Unfortunately, intranasal cannulas have a few disadvantages. Once implanted, the cannula needs to be cleaned daily, otherwise connective tissue can regrow and clog it. Furthermore, the implantation requires puncturing the roof of the nasal cavity, which has two potential side effects: 1) it may change the way rodents breathe and 2) it may disturb odor perception because of the partial destruction of the olfactory epithelium (these aspects have yet to be investigated).
Face Mask
The principle of the face mask is self-explanatory: the animal breathes through a mask pressed against its face. The resulting air movement can be quantified with the help of an airflow sensor. This method has the advantage over an intranasal cannula of being noninvasive.
This technique has been used for decades on many species, including humans, both for research and medical applications (Connell 1966; Ferdenzi et al. 2014; Saibene et al. 1978; Waters 1936). To our knowledge, the first description of such a mask for rodents goes back to 1987 (Youngentob et al. 1987). The authors designed an experimental chamber with a hole in which rats could poke their nose to sample odors. As the animals pressed their face against it, the odor-delivery hole acted as a face mask, allowing the experimenter to know precisely when the rats started sampling the presented odors.
Instead of having the rodent poke its nose in the mask, it is also possible to head-restrain the animal and then position the mask against its muzzle. This way, respiration can be monitored during a significantly longer period of time (Bolding and Franks 2017; Sherman et al. 2015).
Face masks for rodents have various advantages. They are cheap to make, easy to use, noninvasive, and can be used on anesthetized and awake rodents. The one requirement is that there needs to be a seal between the edges of the mask and the skin of the face to ensure maximum airflow through the sensor. This requirement can be fulfilled either by head-restraining the animal, in which case the respiration is continuously measured, or by training the rodent to poke its nose into the mask, which makes respiration measurable only during the brief episodes when the rodent’s muzzle is attached to the mask.
Lung Plethysmography
Plethysmographs refer to a large family of instruments that measure variations of volume in an organ or within the whole body. Depending on their design, plethysmographs for lungs require connecting the trachea to the instrument, pressing a mask against the face, or placing the animal in a chamber. One advantage of lung plethysmography over other methods of respiration monitoring is that it measures not only the breathing rate, but also the volume of air breathed at each inhalation and expelled at each exhalation. Three categories of lung plethysmographs exist, depending on what they measure: changes in airflow, pressure, or capacitance (Likens and Mauderly 1979).
Of the three, airflow plethysmography is the first method to have been adapted to rodents. One of the oldest publications describing a lung plethysmograph for rodents, then called a “respirograph,” dates to 1947 (Guyton 1947). In short, the animal, awake or anesthetized, was immobilized and its nose pressed against a mask, through which a stream of air was applied at a constant rate. The air stream passed through the mask unidirectionally via two one-way valves. As the animal breathed, the airflow going out of the mask was disturbed. These variations were measured with an airflow sensor. Pressure plethysmography works on the same principle, except it measures pressure fluctuation. Note that pressure plethysmographs were first invented for rabbits, not rodents (Gaddum 1941).
Guyton’s respirograph design inspired a plethysmograph model still used today: the whole body plethysmograph. First created for adult humans (Drorbaugh and Fenn 1955; DuBois et al. 1956), whole body plethysmographs have since been adapted for smaller animals such as rodents (Jacky 1978). They rely on the same principle as Guyton’s design: the measurement of fluctuations in the constant airflow the subject is given to breathe.
Barrow et al. (1971) proposed a new method for measuring the respiration of small animals: capacitance plethysmography. As the animal breathes, the volume of its lungs varies, which induces changes in the capacitance of its whole body. Capacitance plethysmographs measure such changes by incorporating the animal into an electrical circuit.
Since then, the measurement tools have become cheaper, more precise, and readily available (Flores et al. 2007; Li et al. 2016; Lim et al. 2014; Fig. 2). However, most modern plethysmographs are still based on the pressure, airflow, or capacitance designs invented decades ago. The three methods can be easily and reliably used on freely moving animals, each with its own limitations. Pressure and airflow whole body plethysmographs require the animal to remain in an enclosed chamber during the measurement, which may prove hard to adapt to some behavioral assays. On the other hand, capacitance plethysmography involves passing a small current through the rodent’s body and measuring its capacitance changes, which may interfere with electrophysiological recordings.
MEASURING THE TEMPERATURE CHANGES INDUCED BY RESPIRATION
Rodents are warm-blooded animals, and the air they breathe in a laboratory environment is usually colder than their body. Once in their airways, the inhaled air gets warmer. As a consequence, at each exhalation, the air around the nostrils gets warmer as well.
Temperature Probes
Two major categories of temperature probe can be used for breathing monitoring: thermistors and thermocouples. The resistance of the material from which a thermistor is made changes with its temperature, such that one can measure the temperature at the tip of a thermistor by assessing its resistance. Thermocouples work differently: they are made of two wires made of different metals and connected together at one end. As a consequence of the different thermoelectric characteristics of the two carefully selected metals, a temperature-dependent voltage forms at the junction of the two wires. In either case, thermistor or thermocouple, a voltage is read and converted into temperature (McAfee et al. 2016).
One of the first attempts at using temperature probes to monitor the breathing of laboratory animals (cats in this case) goes back to the end of the 1960s (Angyán and Szirmai 1967). During the following decade, the method was adapted to rats (Clarke et al. 1970) and later to mice (Crossland et al. 1977). When using temperature probes, experimenters have two options: they can either implant the probe in the nasal cavity (Clarke et al. 1970; Crossland et al. 1977; McAfee et al. 2016; Khan et al. 2012; Kurnikova et al. 2017; Uchida and Mainen 2003; Wesson 2013) or place it in front of the nostril (Ito et al. 2014; Lepousez and Lledo 2013; McAfee et al. 2016).
Classically, implantation requires making a hole through the roof of the nasal cavity. Once the animal is implanted, its respiration can be monitored over a long period of time while head-fixed or freely moving. However, in the same fashion as intranasal cannulas, the surgery can interfere with the way the rodents breathe and perceive odors, even though this has never been investigated. McAfee et al. (2016) described a new surgical procedure for the implantation of temperature probes that did not require making a hole through the roof of the nasal cavity. Instead, probes were lowered and chronically implanted in a formerly unreported hollow space right above the anterior part of the nasal cavity. Note that the surgery still required a craniotomy to reveal the skull. In addition, the authors validated their method by comparing it with three commonly used breathing-tracking methods: piezoelectric sensors (see below), thermic probes placed externally (see below as well), and whole body plethysmography (Fig. 2).
One alternative to implanting the sensor is placing it a few millimeters away from the nostrils. Unlike implanted probes, this requires the rodents to be head-restrained. The most obvious advantage of this technique is that it is not invasive at all, as the sensor does not even touch the animal.
However, this method has not yet been validated experimentally. In fact, it has been suggested that, because of the increased movements of the nares during high-frequency respiration, external thermic probes are not reliable when rodents are awake: their use should, therefore, be restricted to anesthetized animals (McAfee et al. 2016).
Infrared Cameras
Esquivelzeta Rabell et al. (2017) described a new noninvasive method for breathing monitoring. Similar to what had been done on humans a few years before (Lewis et al. 2011), they placed an infrared camera in front of the nose of head-restrained mice and tracked the breathing through the variations of temperature around the nostrils over time. Importantly, Esquivelzeta Rabell and colleagues validated their new method by comparing it with breathing signals obtained simultaneously through implanted cannulas (Esquivelzeta Rabell et al. 2017; Mutlu et al. 2018; Fig. 2).
One of the main limitations of this method is the price of the infrared camera: not only does it need to capture movies with a high enough frame rate, but also it should have good enough thermic sensitivity. As of the time this review was written, the camera Esquivelzeta Rabell et al. used in their publication (FLIR A325sc infrared camera) costs a few thousand US dollars, not including the dedicated lens. Furthermore, the camera is relatively large and needs to be placed extremely close to the rodent to capture video at a sufficient resolution to monitor the breathing. This method is currently restricted to head-restrained animals to keep the nostril images registered to the camera. Finally, extraction of the breathing signal is performed after recording through numerous video-processing steps. Despite recent improvements of the breathing-extraction algorithm (Mutlu et al. 2018), the method has yet to be implemented for real-time breathing detection, which may require developing new computational approaches.
BODY MOVEMENTS AS A MEASURE OF RESPIRATION
As animals breathe, their rib cage moves to inflate and deflate their lungs. The movements of the rib cage, often visible to the eye, can be monitored as a measure of respiration.
Movement Sensors
Movement sensors have been used for decades to monitor rodent respiration. For example, in 1997, Jourdan et al. used a small balloon, inflated between the trunk of awake, immobilized rats and the wall of their restriction box. Each time the rats inhaled, the air in the balloon was compressed. The changes of air pressure in the balloon were used as a breathing signal. Note that the authors recorded diaphragm EMGs at the same time to validate their new method (Jourdan et al. 1997; Fig. 2).
More recently, Reisert and colleagues (Reisert et al. 2014) implanted mice with an intrathoracic pressure sensor, which allowed them to monitor their respiration while moving freely and performing an odor-driven task. Importantly, they validated their method by comparing it with simultaneous recordings of intranasal pressure through an implanted cannula (Fig. 2).
Nowadays, due to their sensitivity and affordability, piezoelectric sensors are frequently used for respiration monitoring. Usually, they are incorporated into an elastic waistband (Kapoor et al. 2016) or directly placed below the belly of the animal (Flores et al. 2007; McAfee et al. 2016; Zehendner et al. 2013). However, their high sensitivity is also their biggest limitation: the rodent needs to remain completely still during recording. In practice, the use of piezoelectric sensors is mostly restricted to anesthetized or sleeping animals and inactive pups.
Video Monitoring
To our knowledge, the only attempt at using video to monitor the respiration of rodents was published in 1964. In this article, Welker (1964) manually analyzed, frame by frame, movies of rats exploring objects placed closed to the camera. Not only did he use the movements on the head, nose, vibrissae, and body to monitor the breathing, but also he gave the first accurate description of all of the stereotypical facial movements accompanying each breath and explored how different conditions can alter such movements. Even if more recent studies have also used movies to investigate respiration-related facial movements (Esquivelzeta Rabell et al. 2017; Kurnikova et al. 2017; Moore et al. 2013), they have not monitored the actual respiratory cycles through the movements captured on video.
CONCLUSION
Being able to monitor rodent respiration precisely and reliably is crucial for at least two reasons: 1) it is a key indicator of a rodent’s health and 2) breathing greatly influences the way a rodent perceives its environment and behaves. With mice and rats being some of the most common animal models, it is not surprising that, even now, older methods continue to be improved and new techniques invented. Therefore, finding the right method to monitor rodent respiration continues to be a critical and challenging question. In efforts to get closer to the ideal technique, at least two paths remain unexplored.
Future Directions
An obvious idea to anyone who has seen the Star Wars movie franchise, and is, therefore, familiar with the distinctive breathing pattern of the epic villain Darth Vader, is to track respiration through sound. Listening to the sounds made by the air circulating in the airways is not a new idea: the method has been developed and used on larger animals such as humans and dogs (Concha et al. 2014; Margolin and Kubie 1943; Thesen et al. 1993). However, to our knowledge, the idea has not been applied to rodents.
A second avenue to follow: the study by Welker (1964) is the only example of the use of video to monitor respiration through body movements. Unlike movement sensors, which usually require close contact against the abdomen, video is entirely noninvasive and contactless. Over the last few years, tremendous progress has been achieved in video motion processing. For example, it is now possible to amplify movements within a specific range of frequencies on a video (Wadhwa et al. 2013). Such advances, combined with the increasing affordability of high-speed cameras and the sophisticated machine vision algorithms, will surely make video-based breath tracking easier in the future.
When imaging the neuronal activity at the OB surface, glomeruli often show basal activity that visually follows the breathing signal, even during odor presentations (Verhagen et al. 2007; Wachowiak and Cohen 2001). To our knowledge, basal glomerular activity has yet to be formally compared with established techniques for breathing monitoring and may require the development of new computational approaches to extract the signal of interest. However, if validated, this method would allow experimenters to extrapolate a breathing signal directly from their imaging data. With any luck, this approach could even be applicable to freely moving rodents while being imaged through a head-mounted miniature microscope (Helmchen et al. 2001; Liberti et al. 2017; Sawinski et al. 2009).
Finally, respiratory monitoring can be used as part of closed-loop systems, in which a piece of equipment, usually a computer, processes the breathing signal as it is acquired and, for each respiratory cycle, automatically delivers a stimulus to the animal at a predefined moment. Such approaches have been successfully applied to odor delivery (Resulaj and Rinberg 2015; Shusterman et al. 2011; Sirotin et al. 2015) as well as electrical (Mercier et al. 2017) and optophysiological (Smear et al. 2011, 2013) neural stimulations. Closed-loop systems can be implemented with virtually any breathing-monitoring method, given the respiratory signal is simple enough to be processed on the fly.
The Ideal Method
The ideal method for respiration monitoring should be cheap, easy to set up, and noninvasive. It should work on anesthetized as well as awake rodents, restrained as well as freely moving, pups as well as adults, performing a task or not. Such a method does not exist. However, numerous techniques have been developed and perfected over the past few decades, each with its own advantages and drawbacks.
One of the largest limitations of many of these techniques is their lack of accuracy in awake animals. General anesthesia significantly reduces the animals’ movement and has notable physiological effects. In particular, when anesthetized, rodents have a slower (<2 Hz), more regular breathing pattern than when they are awake (usually 2–4 Hz, ≤12 Hz during active odor sampling; Esquivelzeta Rabell et al. 2017; Low et al. 2016; Shusterman et al. 2011; Uchida and Mainen 2003; Zehendner et al. 2013). As a consequence, whereas some techniques are suitable for anesthetized animals, they become unreliable on awake rodents, either because they lose synchrony as the breathing rate increases (OB LFPs, external temperature probes) or because they are sensitive to all body movements (external movement sensors; Table 1).
Breathing signals obtained with the various techniques reported here have different lag times compared with each other. A summary of all of these phase shifts can be found in Fig. 2. Lags between two methods range from a few milliseconds (for example: 3 ms from intranasal cannulas to infrared cameras; Esquivelzeta Rabell et al. 2017) to a few tens of milliseconds (for example: 26 ms from intranasal cannulas to implanted temperature probes; Verhagen et al. 2007). Such phase shifts can be considerable compared with the duration of a breathing cycle (250–500 ms for awake mice and rats at rest and ≤80 ms during active odor sampling; Esquivelzeta Rabell et al. 2017; Shusterman et al. 2011; Uchida and Mainen 2003).
Therefore, when choosing a method, experimenters need to take into account these shifts. For example, the mouse OB shows odor responses that are locked to the breathing cycle with a precision of 12 ms on average (Shusterman et al. 2011), in accordance with the ability for mice to perceive breath cycles with ≥10-ms precision (Smear et al. 2011, 2013). Therefore, when analyzing odor-driven activity of the OB, it may be more relevant to report the breathing as the movement of odorized air in and out of the nasal cavity rather than chest movement or external changes of temperature unless the lag between the variable of interest and the measurement is taken into account.
Picking a method that satisfies the requirements of an experiment can be challenging. The primary purpose of this review is to help experimenters choose the method that best fits their needs, by providing a comprehensive, up-to-date list of current techniques at their disposal. A summary of all of the techniques discussed throughout the review is supplied in Fig. 1 and Table 1.
GRANTS
V. N. Murthy was supported by National Institute on Deafness and Other Communication Disorders Grants R01-DC-014453 and R01-DC-01332.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G. drafted manuscript; J.G. and V.N.M. edited and revised manuscript; J.G. and V.N.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Andrew Maher for copy editing. We also thank Dorothy Barr from the Ernst Mayr Library of the Museum of Comparative Zoology at Harvard University for help finding a copy of some of the oldest articles cited in this review. Finally, we thank Jenelle Wallace from the Murthy laboratory at Harvard University for helpful comments on this manuscript.
REFERENCES
- Adrian ED. Olfactory reactions in the brain of the hedgehog. J Physiol 100: 459–473, 1942. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol 2: 377–388, 1950. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- Angyán L, Szirmai I. Recording of respiration with thermocouple in freely moving cats. Acta Physiol Acad Sci Hung 31: 73–76, 1967. [PubMed] [Google Scholar]
- Barrow RE, Vorwald AJ, Domier E. The measurement of small animal respiratory volumes by capacitance respirometry. Am Ind Hyg Assoc J 32: 593–598, 1971. doi: 10.1080/0002889718506511. [DOI] [PubMed] [Google Scholar]
- Bojsen-Moller F, Fahrenkrug J. Nasal swell-bodies and cyclic changes in the air passage of the rat and rabbit nose. J Anat 110: 25–37, 1971. [PMC free article] [PubMed] [Google Scholar]
- Bolding KA, Franks KM. Complementary codes for odor identity and intensity in olfactory cortex. eLife 6: e22630, 2017. doi: 10.7554/eLife.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonviso N, Amat C, Litaudon P, Roux S, Royet JP, Farget V, Sicard G. Rhythm sequence through the olfactory bulb layers during the time window of a respiratory cycle. Eur J Neurosci 17: 1811–1819, 2003. doi: 10.1046/j.1460-9568.2003.02619.x. [DOI] [PubMed] [Google Scholar]
- Clarke S, Panksepp J, Trowill J. A method of recording sniffing in the free-moving rat. Physiol Behav 5: 125–126, 1970. doi: 10.1016/0031-9384(70)90024-7. [DOI] [PubMed] [Google Scholar]
- Concha A, Mills DS, Feugier A, Zulch H, Guest C, Harris R, Pike TW. Using sniffing behavior to differentiate true negative from false negative responses in trained scent-detection dogs. Chem Senses 39: 749–754, 2014. doi: 10.1093/chemse/bju045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell JT. An instrument for measuring the effective cross-sectional nasal airway. J Allergy 37: 127–134, 1966. doi: 10.1016/0021-8707(66)90087-6. [DOI] [PubMed] [Google Scholar]
- Crossland NJ, Horsfall GB, Oxenham ST, Shaw JS, Turnbull MJ. A simple device for measurement of respiratory rate in the mouse. Br J Pharmacol 61: 490P–491P, 1977. [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL. Defining preBötzinger complex rhythm- and pattern-generating neural microcircuits in vivo. Neuron 91: 602–614, 2016. doi: 10.1016/j.neuron.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- DuBois AB, Botelho SY, Bedell GN, Marshall R, Comroe JH Jr. A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. J Clin Invest 35: 322–326, 1956. doi: 10.1172/JCI103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. Nasal airflow in health and disease. Acta Otolaryngol 120: 580–595, 2000. doi: 10.1080/000164800750000388. [DOI] [PubMed] [Google Scholar]
- Esquivelzeta Rabell J, Mutlu K, Noutel J, Martin Del Olmo P, Haesler S. Spontaneous rapid odor source localization behavior requires interhemispheric communication. Curr Biol 27: 1542–1548.e4, 2017. doi: 10.1016/j.cub.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdenzi C, Poncelet J, Rouby C, Bensafi M. Repeated exposure to odors induces affective habituation of perception and sniffing. Front Behav Neurosci 8: 119, 2014. doi: 10.3389/fnbeh.2014.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AE, Flores JE, Deshpande H, Picazo JA, Xie X, Franken P, Heller HC, Grahn DA, O’Hara BF. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng 54: 225–233, 2007. doi: 10.1109/TBME.2006.886938. [DOI] [PubMed] [Google Scholar]
- Gaddum JH. A method of recording the respiration. J Physiol 99: 257–264, 1941. doi: 10.1113/jphysiol.1941.sp003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam SH, Verhagen JV. Direct behavioral evidence for retronasal olfaction in rats. PLoS One 7: e44781, 2012. doi: 10.1371/journal.pone.0044781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci 10: 348–354, 2007. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC. Measurement of the respiratory volumes of laboratory animals. Am J Physiol 150: 70–77, 1947. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol 34: 252–269, 2006. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope. High-resolution brain imaging in freely moving animals. Neuron 31: 903–912, 2001. doi: 10.1016/S0896-6273(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Ito J, Roy S, Liu Y, Cao Y, Fletcher M, Lu L, Boughter JD, Grün S, Heck DH. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat Commun 5: 3572, 2014. doi: 10.1038/ncomms4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J Appl Physiol Respir Environ Exerc Physiol 45: 644–647, 1978. doi: 10.1152/jappl.1978.45.4.644. [DOI] [PubMed] [Google Scholar]
- Jourdan D, Ardid D, Chapuy E, Le Bars D, Eschalier A. Audible and ultrasonic vocalization elicited by a nociceptive stimulus in rat: relationship with respiration. J Pharmacol Toxicol Methods 38: 109–116, 1997. doi: 10.1016/S1056-8719(97)00067-1. [DOI] [PubMed] [Google Scholar]
- Kapoor V, Provost AC, Agarwal P, Murthy VN. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels. Nat Neurosci 19: 271–282, 2016. doi: 10.1038/nn.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci 2: 1003–1009, 1999. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Khan AG, Sarangi M, Bhalla US. Rats track odour trails accurately using a multi-layered strategy with near-optimal sampling. Nat Commun 3: 703, 2012. doi: 10.1038/ncomms1712. [DOI] [PubMed] [Google Scholar]
- Kurnikova A, Moore JD, Liao SM, Deschênes M, Kleinfeld D. Coordination of orofacial motor actions into exploratory behavior by rat. Curr Biol 27: 688–696, 2017. doi: 10.1016/j.cub.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepousez G, Lledo PM. Odor discrimination requires proper olfactory fast oscillations in awake mice. Neuron 80: 1010–1024, 2013. doi: 10.1016/j.neuron.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Gatto RG, Porges SW. A novel method for extracting respiration rate and relative tidal volume from infrared thermography. Psychophysiology 48: 877–887, 2011. doi: 10.1111/j.1469-8986.2010.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Gire DH, Bozza T, Restrepo D. Precise detection of direct glomerular input duration by the olfactory bulb. J Neurosci 34: 16058–16064, 2014. doi: 10.1523/JNEUROSCI.3382-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL. The peptidergic control circuit for sighing. Nature 530: 293–297, 2016. doi: 10.1038/nature16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti WA 3rd, Perkins LN, Leman DP, Gardner TJ. An open source, wireless capable miniature microscope system. J Neural Eng 14: 045001, 2017. doi: 10.1088/1741-2552/aa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likens SA, Mauderly JL. Respiratory Measurements in Small Laboratory Mammals: A Literature Review. National Technical Information Service, 1979. [Google Scholar]
- Lim R, Zavou MJ, Milton PL, Chan ST, Tan JL, Dickinson H, Murphy SV, Jenkin G, Wallace EM. Measuring respiratory function in mice using unrestrained whole-body plethysmography. J Vis Exp 90: e51755, 2014. doi: 10.3791/51755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LA, Bauer LC, Klaunberg BA. Comparing the effects of isoflurane and alpha chloralose upon mouse physiology. PLoS One 11: e0154936, 2016. doi: 10.1371/journal.pone.0154936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F. Temporal relationships between hippocampal slow waves and exploratory sniffing in hamsters. Behav Biol 14: 295–308, 1975. doi: 10.1016/S0091-6773(75)90419-8. [DOI] [PubMed] [Google Scholar]
- Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic θ rhythm during odor discrimination reversal learning. J Neurosci 2: 1705–1717, 1982. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin S, Kubie LS. An acoustic respirograph. A method for the study of respiration through the graphic recording of the breath sounds. J Clin Invest 22: 221–224, 1943. doi: 10.1172/JCI101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee SS, Ogg MC, Ross JM, Liu Y, Fletcher ML, Heck DH. Minimally invasive highly precise monitoring of respiratory rhythm in the mouse using an epithelial temperature probe. J Neurosci Methods 263: 89–94, 2016. doi: 10.1016/j.jneumeth.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier LM, Gonzalez-Rothi EJ, Streeter KA, Posgai SS, Poirier AS, Fuller DD, Reier PJ, Baekey DM. Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury. J Neurophysiol 117: 767–776, 2017. doi: 10.1152/jn.00721.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Deschênes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature 497: 205–210, 2013. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu K, Rabell JE, Martin Del Olmo P, Haesler S. IR thermography-based monitoring of respiration phase without image segmentation. J Neurosci Methods 301: 1–8, 2018. doi: 10.1016/j.jneumeth.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Phillips M, Norgren R. A rapid method for permanent implantation of an intraoral fistula in rats. Behav Res Methods Instrum 2: 124, 1970. doi: 10.3758/BF03211020. [DOI] [Google Scholar]
- Reisert J, Golden GJ, Matsumura K, Smear M, Rinberg D, Gelperin A. Comparing thoracic and intra-nasal pressure transients to monitor active odor sampling during odor-guided decision making in the mouse. J Neurosci Methods 221: 8–14, 2014. doi: 10.1016/j.jneumeth.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resulaj A, Rinberg D. Novel behavioral paradigm reveals lower temporal limits on mouse olfactory decisions. J Neurosci 35: 11667–11673, 2015. doi: 10.1523/JNEUROSCI.4693-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Líbano D, Frederick DE, Egaña JI, Kay LM. The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci 8: 214, 2014. doi: 10.3389/fnbeh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibene F, Mognoni P, Lafortuna CL, Mostardi R. Oronasal breathing during exercise. Pflügers Arch 378: 65–69, 1978. doi: 10.1007/BF00581959. [DOI] [PubMed] [Google Scholar]
- Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JN. Visually evoked activity in cortical cells imaged in freely moving animals. Proc Natl Acad Sci USA 106: 19557–19562, 2009. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolman A, Fink BR. Permanently implanted electrode for electromyography of the diaphragm in the waking cat. Electroencephalogr Clin Neurophysiol 15: 127–128, 1963. doi: 10.1016/0013-4694(63)90048-8. [DOI] [PubMed] [Google Scholar]
- Semba K, Komisaruk BR. Phase of the theta wave in relation to different limb movements in awake rats. Electroencephalogr Clin Neurophysiol 44: 61–71, 1978. doi: 10.1016/0013-4694(78)90105-0. [DOI] [PubMed] [Google Scholar]
- Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci 18: 408–414, 2015. doi: 10.1038/nn.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrey JH, Megirian D. State dependence of upper airway respiratory motoneurons: functions of the cricothyroid and nasolabial muscles of the unanesthetized rat. Electroencephalogr Clin Neurophysiol 43: 218–228, 1977. doi: 10.1016/0013-4694(77)90129-8. [DOI] [PubMed] [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci 14: 1039–1044, 2011. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Sirotin YB, Costa ME, Laplagne DA. Rodent ultrasonic vocalizations are bound to active sniffing behavior. Front Behav Neurosci 8: 399, 2014. doi: 10.3389/fnbeh.2014.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotin YB, Shusterman R, Rinberg D. Neural coding of perceived odor intensity. eNeuro 2: 1–16, 2015. doi: 10.1523/ENEURO.0083-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smear M, Resulaj A, Zhang J, Bozza T, Rinberg D. Multiple perceptible signals from a single olfactory glomerulus. Nat Neurosci 16: 1687–1691, 2013. doi: 10.1038/nn.3519. [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O’Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature 479: 397–400, 2011. doi: 10.1038/nature10521. [DOI] [PubMed] [Google Scholar]
- Thesen A, Steen JB, Døving KB. Behaviour of dogs during olfactory tracking. J Exp Biol 180: 247–251, 1993. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229, 2003. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Vandivort TC, An D, Parks WC. An improved method for rapid intubation of the trachea in mice. J Vis Exp 108: 53771, 2016. doi: 10.3791/53771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci 10: 631–639, 2007. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32: 723–735, 2001. doi: 10.1016/S0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wadhwa N, Rubinstein M, Durand F, Freeman WT. Phase-based video motion processing. ACM Trans Graph 32: 80, 2013. doi: 10.1145/2461912.2461966. [DOI] [Google Scholar]
- Waters RM. Carbon dioxide absorption from anaesthetic atmospheres. Proc R Soc Med 30: 11–22, 1936. [PMC free article] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour 22: 223–244, 1964. doi: 10.1163/156853964X00030. [DOI] [Google Scholar]
- Wesson DW. Sniffing behavior communicates social hierarchy. Curr Biol 23: 575–580, 2013. doi: 10.1016/j.cub.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chem Senses 33: 581–596, 2008. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiol Behav 41: 59–69, 1987. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Zehendner CM, Luhmann HJ, Yang JW. A simple and novel method to monitor breathing and heart rate in awake and urethane-anesthetized newborn rodents. PLoS One 8: e62628, 2013. doi: 10.1371/journal.pone.0062628. [DOI] [PMC free article] [PubMed] [Google Scholar]