Abstract
Mitochondrial function has been examined in insulin-resistant (IR) states including type 2 diabetes mellitus (T2DM). Previous studies using phosphorus-31 magnetic resonance spectroscopy (31P-MRS) in T2DM reported results as relative concentrations of metabolite ratios, which could obscure differences in phosphocreatine ([PCr]) and adenosine triphosphate concentrations ([ATP]) between T2DM and normal glucose tolerance (NGT) individuals. We used an image-guided 31P-MRS method to quantitate [PCr], inorganic phosphate [Pi], phosphodiester [PDE], and [ATP] in vastus lateralis (VL) muscle in 11 T2DM and 14 NGT subjects. Subjects also received oral glucose tolerance test, euglycemic insulin clamp, 1H-MRS to measure intramyocellular lipids [IMCL], and VL muscle biopsy to evaluate mitochondrial density. T2DM subjects had lower absolute [PCr] and [ATP] than NGT subjects (PCr 28.6 ± 3.2 vs. 24.6 ± 2.4, P < 0.002, and ATP 7.18 ± 0.6 vs. 6.37 ± 1.1, P < 0.02) while [PDE] was higher, but not significantly. [PCr], obtained using the traditional ratio method, showed no significant difference between groups. [PCr] was negatively correlated with HbA1c (r = −0.63, P < 0.01) and fasting plasma glucose (r = −0.51, P = 0.01). [PDE] was negatively correlated with Matsuda index (r = −0.43, P = 0.03) and M/I (r = −0.46, P = 0.04), but was positively correlated with [IMCL] (r = 0.64, P < 0.005), HbA1c, and FPG (r = 0.60, P = 0.001). To summarize, using a modified, in vivo quantitative 31P-MRS method, skeletal muscle [PCr] and [ATP] are reduced in T2DM, while this difference was not observed with the traditional ratio method. The strong inverse correlation between [PCr] vs. HbA1c, FPG, and insulin sensitivity supports the concept that lower baseline skeletal muscle [PCr] is related to key determinants of glucose homeostasis.
Keywords: ATP, insulin resistance, mitochondrial function, phosphocreatine, phosphorus-31 magnetic resonance spectroscopy, skeletal muscle metabolism, type 2 diabetes
INTRODUCTION
Skeletal muscle mitochondrial function and impaired ATP production have been described in insulin-resistant (IR) states, including type 2 diabetes mellitus (T2DM) (3, 46). However, there is controversy as to whether the mitochondrial dysfunction is the cause of or result of insulin resistance. Phosphorus-31 magnetic resonance spectroscopy (31P-MRS) has been used to study mitochondrial function by measuring phosphorus metabolites under a range of physiological conditions and relating intramyocellular levels to the presence of skeletal muscle insulin resistance (55). Using MR spectroscopic techniques in vivo, impaired skeletal muscle mitochondrial function has been demonstrated in a variety of insulin-resistant states, including the elderly (41), insulin-resistant offspring of T2DM individuals (9, 35, 42), T2DM subjects (2, 43, 52, 53, 55), and obese subjects (38, 46, 61).
Traditionally, results from in vivo 31P-MRS in T2DM individuals have been expressed as ratios of phosphorus metabolites, under the assumption that the ATP concentration ([ATP]) in resting muscle is uniform and constant across all subjects. Data to support the constancy of muscle ATP levels are lacking and reliance on this assumption could obscure simultaneous changes in phosphorus metabolites, attenuating or enhancing metabolic differences between T2DM and NGT. Therefore, development of a 31P-MRS method to provide absolute concentrations of intramyocellular phosphorous metabolites is essential to precisely assess the contribution of mitochondrial function to metabolic processes (28).
The aims of this study were 1) to develop a reproducible method to quantitate phosphorus metabolites in human vastus lateralis (VL) muscle using MRS, 2) to compare differences between NGT and T2DM subjects in basal [ATP], inorganic phosphate [Pi], phosphocreatine [PCr], phosphodiester [PDE] and creatine [Cr], and 3) to examine the relationship between phosphorous metabolite concentrations and measures of glycemic control, insulin resistance, mitochondrial density, and intramyocellular lipid content.
MATERIALS AND METHODS
Subjects.
Fourteen NGT and 11 T2DM subjects, matched for age, sex and body mass index (BMI), participated in the study (Table 1). All subjects had normal liver, cardiopulmonary, and kidney function as determined by medical history, physical examination, screening blood tests, electrocardiogram, and urinalysis. No subject was taking any medication known to affect glucose tolerance. Body weight was stable (±1.4 kg) for at least 3 mo before the study in all subjects, and no subject participated in an excessively heavy exercise regimen or took part in any exercise program over the 48 h before study. NGT subjects had no family history of T2DM. T2DM subjects were on a stable dose (at least 3 mo) of monotherapy with metformin or metformin combined with sulfonylurea. All patients were diagnosed with T2DM for at least 1 yr.
Table 1.
Clinical characteristics of the participants
| NGT | T2DM | P Value | |
|---|---|---|---|
| N | 14 | 11 | |
| Sex, M/F | 7/7 | 7/4 | |
| Age, yr | 47.0 ± 12.8 | 55.1 ± 11.1 | 0.14 |
| Diabetes duration, yr | NA | 3.8 ± 1.5 | |
| Weight, kg | 83.1 ± 13.8 | 88.6 ± 13.1 | 0.33 |
| BMI, kg/m2 | 28.6 ± 3.9 | 30.8 ± 5.0 | 0.22 |
| FPG, mg/dl | 93 ± 6 | 140 ± 23 | <0.001 |
| HbA1c, % | 5.5 ± 0.3 | 7.5 ± 0.7 | <0.001 |
| Total cholesterol, mg/dl | 173 ± 38 | 173 ± 57 | 0.99 |
| LDL cholesterol, mg/dl | 99 ± 39 | 93 ± 41 | 0.73 |
| HDL cholesterol, mg/dl | 56 ± 12 | 37 ± 5 | <0.001 |
| Triglycerides, mg/dl | 93 ± 63 | 221 ± 135 | 0.006 |
| Matsuda index of insulin sensitivity | 4.76 ± 2.75 | 1.80 ± 1.18 | 0.003 |
| IS/IR index | 5.91 ± 2.95 | 0.66 ± 0.71 | <0.001 |
| TGD*, mg·kg−1·min−1 | 8.37 ± 2.07 | 4.39 ± 2.17 | <0.001 |
| TGD/SSPI, (mg·kg−1·min−1)/(μU/ml) | 4.03 ± 1.52 | 1.77 ± 0.68 | <0.001 |
| Mitochondrial density†, mito/µm2 | 0.19 ± 0.04 | 0.19 ± 0.02 | 0.96 |
NGT, normal glucose tolerance; T2DM, type 2 diabetes mellitus; IS/IR index, insulin secretion/insulin resistance (disposition) index; TGD, total body glucose disposal; SSPI, steady-state plasma insulin concentration; FPG, fasting plasma glucose; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HbA1c, glycated hemoglobin.
Subset of 12 NGT and 9 T2DM subjects participated in the euglycemic insulin clamp.
8 NGT and 6 T2DM subjects had muscle biopsies.
Studies were performed at 0800 following a 10-h overnight fast. Subjects received a 2-h, 75-g oral glucose tolerance test (OGTT). 1H- and 31P-MRS were performed on the VL muscle at the Research Imaging Institute within 1 wk of the OGTT. On a separate day, a subset of subjects (9 T2DM and 12 NGT) received euglycemic insulin clamp and VL muscle biopsy. The study protocol was approved by the Institutional Review Board of University of Texas Health Science Center, San Antonio, Texas, and informed written consent was obtained from all subjects before participation.
Oral glucose tolerance test.
Before the start of the OGTT, a catheter was placed into an antecubital vein and blood samples were collected at −30, −15, and 0 min. Subjects then ingested 75 g of glucose, and blood samples were obtained at 30, 60, 90, and 120 min for determination of plasma glucose, free fatty acids (FFA), and insulin concentrations. Insulin sensitivity during the OGTT was assessed by the Matsuda index (MI) (33). The insulin secretion/insulin resistance (disposition) index (IS/IR index), an indicator of beta cell function, was calculated as (∆AUC-I/∆AUC-G) × MI (1).
Magnetic resonance spectroscopy.
At 0800 after an overnight fast, 31P-MRS measurements of hydrogen- and of phosphorus-containing metabolites were obtained from the right VL muscle using a 3-T MRI system (TIM Trio, Siemens Medical, Malvern PA). Quantitation of intramyocellular lipids [IMCL] was determined using single-voxel 1H-MRS with the internal reference method. Subjects were positioned in a supine, feet-first orientation with the right upper leg as close to the center of the magnet as possible. A 4-channel receive-only array flex coil (Siemens) was wrapped around the right thigh, and the leg was stabilized. Two stimulated echo acquisition mode (STEAM) MRS sequences were acquired, one with the water peak intact [15 mm × 15 mm × 15 mm, pulse repetition time (TR) = 3 s, echo time (TE) = 30 ms, number of signals averaged (NSA) = 16] and another at the same location with the water peak suppressed (15 mm × 15 × 15 mm, TR = 3 s, TE = 270 ms, NSA = 128).
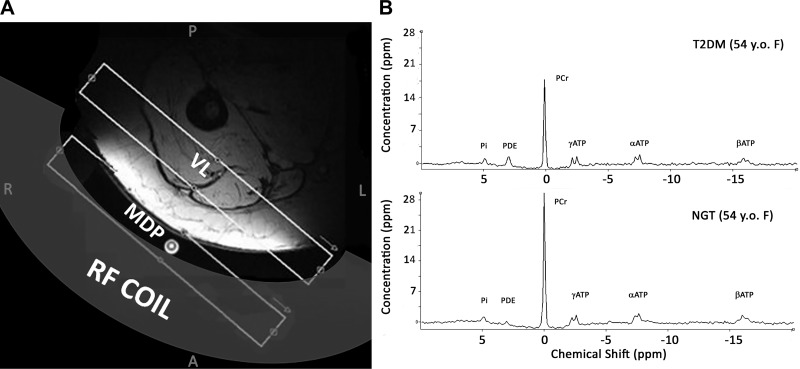
For the 31P-MRS measurements, a rigid 31P/1H dual-tuned circular, saddle-shaped (18-cm diameter) surface coil (Rapid Biomedical, Rimpar, Germany) was used. Radiofrequency (RF) coil testing indicated that the shape of the sensitive volume changed with movements that alter the RF coil orientation relative to the main, Bo field. To ensure study-to-study reproducibility, a holder was devised that fixed the RF coil to the table to maintain the shape and size of the RF coil sensitive constant. Tests of the excitation pulse flip angle with spatial depth and applied RF power demonstrated a broad maximum over the range of 3–5 cm deep from RF coil midline for 50–70 V applied to produce a putative 160° flip angle. Since the VL muscle is in an anterior compartment of the quadriceps muscle group, the RF coil was positioned as close as possible to isocenter under the right VL muscle with the subjects positioned head first and prone in the magnet. A 6-ml plastic vial with an 850 mM concentration of methylenediphosphonic acid (MDP) was fixed at the center of the coil and used as an external reference (Fig. 1A). MDP was used because of its resonance frequency of ~22 ppm downfield from PCr, to avoid overlapping relevant metabolite peaks. In addition, MDP is safe when diluted, is water soluble, and can be placed in a sealed container.
Fig. 1.
A: depiction of 31P-MRS experimental set-up based on axial 1H-MR image of right leg used to guide placement of MRS slab. Subject is prone and coil is attached to table to ensure reproducibility of results. B: representative 31P-MR spectra from two 54-yr-old women. Top: subject with type 2 diabetes mellitus (T2DM). Bottom: normal glucose tolerance (NGT) subject. PCr and ATP peaks are noticeably lower in the T2DM skeletal muscle. MDP, methylenediphosphonic acid; VL, vastus lateralis.
An axial 1H-MRI localizer [35 slices, 200 × 169 × 5 mm, TR/TE = 498/6.47 ms, receiver bandwidth (BW) = 120 Hz/px] was acquired for guidance of the spectroscopy slab placement. A 1H-MRS voxel (20 × 20 × 10 mm3, TR/TE = 3,000/150 ms, NSA = 8, BW = 1200 Hz), placed in the right VL muscle, was shimmed to a FWHM of water ~20 Hz. The acquired shim values were also used for the 31P-MRS sequences. The axial images were used to position a paracoronal, five-slice 1H-MRI scan (TR/TE = 450/6.15 ms; thickness 5 mm; field of view = 187 × 250 mm; matrix = 192 × 256, BW = 120 Hz/pixel) which was positioned in the same way as the subsequent 31P-MRS slab, both in the muscle and the phantom, to estimate the volume of muscle tissue contained within the 31P-MRS slice. Using the reference, axial 1H-MRI images of the upper right leg, a 31P-MRS slab was positioned in the VL muscle to exclude as much subcutaneous fat and bone as possible (Fig. 1A). However, if signal from adipose tissue was inadvertently included, it should not affect the results since there is no PCr in adipose tissue and [ATP] is only ~50 μmol/g wet wt (20). A slice-selective 31P-MRS sequence (TR/TE = 10,000/2.3 ms, NSA = 16, slice thickness 25 mm, BW = 3,000 Hz) was performed in the quadriceps muscle of the subject and in the leg phantom, as described below.
After the subject was scanned, a 15-cm diameter, 4-liter plastic cylindrical leg phantom containing 35 mM phosphoric acid (H3PO4) was placed on the coil and a 5-slice, paracoronal MRI scan was acquired followed by an MRS scan. Both the phantom 5-slice MRI and phantom 31P-MRS slabs were scanned with the same parameters and slab positions, relative to the RF coil, that were used previously so that the data were collected from the same area within the RF excitation field of the coil. Also, the same 31P-MRS sequence was used with the position centered over the vial of MDP, first with the subject and then with the leg phantom. The change in the MDP peak area, which is due to the changes of the coil’s RF field, was used to correct for the effect of coil loading on the metabolite peak height (28). The position of the MDP vial did not change relative to the coil throughout the experiment. The experimental set-up and representative 31P-MRS muscle spectra are shown in Fig. 1B.
To examine the reproducibility of the method, five healthy NGT subjects (2 men/3 women; age = 39 ± 22 yr; BMI = 25.1 ± 4.8 kg/m2) were studied on two separate occasions within an interval of 5–7 days. Reproducibility was expressed using the coefficient of variation (CV) calculated as the standard deviation (SD) between an individual’s two visits divided by the mean of the two visits. The intrasubject CV and the intersubject CV also were calculated for each parameter. The same sequence using the MRS slab with varying TR values (0.5, 1, 3, 6, 10 s) in these volunteers also was used to validate that TR = 10,000 ms was sufficient avoid T1 corrections in the quantitation calculation.
Euglycemic insulin clamp and muscle biopsy.
Approximately 1 wk after the MRS study, subjects returned at 0800 for a 4-h euglycemic insulin clamp (17). A prime-continuous insulin infusion (80 mU/kg⋅min) was started at time 0 via a catheter placed into the antecubital vein and continued throughout the study. A second catheter was placed retrogradely into a vein on the dorsum of hand, which was placed in a heated box (60°C). Baseline arterialized venous blood samples for determination of plasma glucose and insulin were drawn at −30, −20, −10, −5, and 0 min. After the start of insulin, plasma glucose concentration was allowed to decrease to 100 mg/dl, at which level it was maintained by variable infusion of 20% glucose solution. During the insulin clamp, blood samples were drawn every 5–15 min for determination of plasma insulin and glucose concentrations. At the high insulin infusion rate employed in the present study, endogenous glucose production is suppressed by >90% in NGT and T2DM subjects, and the mean glucose infusion rate during the last 60 min provides a measure of total body glucose disposal (TGD). TGD divided by the steady-state plasma insulin (SSPI) concentration during the 180- to 240-min duration also was calculated.
VL muscle biopsy was performed 60 min before the start of insulin infusion. The VL muscle specimen was immediately fixed in phosphate-buffered 4% formaldehyde, 1% glutaraldehyde (pH 7.4) at 4°C for several hours, and postfixed in 1% osmium tetroxide for 1 h at room temperature, dehydrated in a series of ethanol dilutions, and embedded in epoxy resin (EMBed 812). Electron microscopy was performed on a JEOL 1230 by an operator blinded to the study. The density of mitochondria was estimated using the point-counting method in a blinded fashion. For each subject, the number density measurements from a minimum of seven images were averaged.
Mitochondrial ATP synthesis.
Mitochondrial ATP synthesis rate was measured ex vivo with the chemiluminescence technique, as previously described (3). Briefly, mitochondria were isolated from fresh muscle tissue by differential centrifugation, with 4 mg of mitochondrial protein aliquoted to each reaction well. Substrates were added as follows: 2.5 mM pyruvate, 2.5 mM glutamate, 5 mM succinate plus 0.001 mM rotenone, and palmitoyl-l-carnitine. Malate (2.5 mM) was added to complex I substrates. Luciferine/luciferase was added to monitor ATP production. Substrates were added after 5 min of incubation at 37°C, and the reaction was started by addition of ADP.
Analytical determinations.
Plasma glucose was measured by the glucose oxidase method (Beckman Instruments, Fullerton, CA). Plasma insulin was measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA). Plasma FFA was determined by the enzymatic colorimetric quantification method (Wako Chemicals, Neuss, Germany).
MR spectroscopy calculations.
The processing of [IMCL] data assumed only a slight variation in the water content in muscle tissue among individuals. The H2O signal from the unsuppressed 1H-MRS acquisition was corrected for proton density and relaxation effects to calculate the concentration of IMCL. The concentration of water was calculated as:
| (1) |
where WC is the water content in muscle tissue (76%), ρM is the density of muscle tissue (1.06 g/ml wet wt), ρW is the density of water (1.0 g/ml), and MMW is the molecular weight of water (18 g/mol). Then [IMCL] was calculated (in mmol/kg wet wt) by
| (2) |
where [H2O] is from Eq. 1, AIMCL and AH2O are the fitted amplitudes from AMARES, n is the number of protons for IMCL, and H2O (62 protons/molecule and 10 protons/molecule, respectively), and kH2O and kIMCL are the T1 and T2 relaxation corrections for water and IMCL, respectively, with
| (3) |
where TE and TR are the 1H-MRS STEAM scan parameters, and T1 and T2 relaxation times for water and IMCL are the published values for VL muscle (60).
Decreased in vivo mitochondrial oxidative phosphorylation (OxPhos) has been inferred from changes in the apparent flux of ATP, reported from 31P-MRS studies in a variety of IR states, including T2DM. 31P-MRS allows the evaluation of muscle energetics in vivo 1) by methods using magnetization transfer; 2) by measuring the concentrations of phosphorus-containing metabolites and 3) by measuring the rate of recovery of the PCr signal following exercise which is a metric for the rate of oxidative ATP synthesis assuming that the CK reaction is much faster than oxidative ATP production; and 4) in conjunction with 1H-MRS measurements of total creatine concentration [tCr], allowing calculations of adenosine diphosphate concentrations, [ADP], which can yield important information regarding the free energy of ATP hydrolysis. [ADP] is calculated in the literature either assuming a constant level of total creatine [tCr] = [PCr] + [Cr] (5) or that 15% of the total creatine is phosphorylated (49). It is not known whether these assumptions are appropriate for all subjects, especially those with metabolic disorders, so the absolute concentration of creatine, [Cr], was determined directly from the 1H-MRS data in this study:
| (4) |
where [H2O] is the concentration of the muscle water, ACr is the amplitude of the creatine metabolite peak from the water suppressed scan, Aw is the amplitude of the H2O peak from the water reference spectrum, nw and nCr are the number of protons in water and creatine, and kw and kCr are the T1 and T2 relaxation corrections for water and Cr, respectively, with k = e−TE/T2 × (1 − e−TR/T1). [H2O] and WC, the water content factor, were assumed to be 55,556 mM and 77%, respectively (63).
For the 31P-MR spectra (Fig. 1B), the volume of the subject’s muscle in the MRS slab was determined from the paracoronal, five-slice MR image data from the patient. A similar calculation was performed on the five-slice MR image data from the H3PO4 phantom. Hand-drawn contours in the inferior-superior extrema on each of the five images were used to determine the muscle area in each slab. Variability in the definition in muscle margins on these images led to an estimated error of 3–5%. The signal drop-off changes due to differences in RF coil coupling to the subject vs. the H3PO4 phantom were compensated by performing the same measurement on 35 mM H3PO4 phantom and using the signal from the MDP vial to compensate for coil loading.
The AMARES fitting algorithm within jMRUI 5.0 was used to analyze all spectra (37). [PCr] was determined using our modified, quantitative method as well as with the conventional relative determination, previously described (48). [ATP] was determined from the fitted height of the γATP peak. Muscle pH was measured based on the chemical shift difference between Pi and PCr. In a subset of subjects, muscle creatine concentration ([Cr]) and intramyocellular lipid concentrations ([IMCL]) were determined using single-voxel 1H-MRS as previously described (11, 62). Thus the [PCr] (and by analogy, [ATP], [PDE], and [Pi]) was determined by modifying an equation, proposed by Kemp et al. (28) based on the method of Roth et al. (47):
| (5) |
where [H3PO4] is the concentration of phosphoric acid in the leg phantom, A is the amplitude of the metabolite peak in the subject, Ap is the amplitude of the H3PO4 peak from the phantom, AMDPref is the amplitude of the MDP peak with the phantom, AMDP is the amplitude of the MDP peak with the coil on the leg, Vp is the volume of the phantom in the slab, and V is the volume of the subject’s muscle in the slab. [PCr]conv also was calculated using the conventional assumption that [ATP]conv is 8.2 mM (24, 48). [PCr]conv = 8.2 mM × (APCr/AATP), where APCr is the amplitude of the PCr peak and AATP is the amplitude of the γATP peak in the subject. Similarly, [PDE]conv was also calculated using the [ATP]conv = 8.2 mM assumption.
Muscle pH was determined from the chemical shift difference between PCr and Pi (δ), in parts per million (ppm), in the 31P-MRS spectrum with the formula:
| (6) |
[ADP] was calculated as [ADP] = ([ATP][tCr])/([PCr][H+]·Keq), using the calculated pH (H+ = 10pH), a creatine kinase equilibrium constant (Keq) of 1.66 × 109 /mol (47), and the [ATP], total creatine concentration ([tCr]), and [PCr] values from the quantitated MRS measurements. [ADP]conv also was calculated using the conventional assumptions of [ATP]conv = 8.2 mM and total creatine concentration ([PCr] + [Cr]) is 42.5 mM (24, 28, 48).
Statistics.
Values are expressed as means ± SD. Between-group comparisons were performed using the Student’s two-tailed t-test. Correlation analysis was performed using Pearson’s product-moment correlation method for the baseline metabolite concentrations with the following parameters: glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), Matsuda index (MI), IS/IR index, insulin sensitivity index (M/I) during the clamp, and mitochondrial density. All statistical analyses were performed using the R statistical package (43a) with significance at P < 0.05.
RESULTS
Subjects did not differ significantly in age, weight, sex, and BMI (Table 1). As expected, T2DM subjects had higher FPG, HbA1C, fasting plasma triglycerides, and lower high-density lipoprotein (HDL) cholesterol (Table 1). The Matsuda index of insulin sensitivity (OGTT) and TGD and TGD/SSPI during insulin clamp were significantly reduced in T2DM vs NGT (Table 1).
The absolute quantitation of [PCr] had a mean coefficient of variation for repeated measurements in the same individual of 5.2%. Measured muscle volumes for the T2DM and NGT subjects were 430.2 ± 64.6 and 428.4 ± 68.5 cm3, respectively (P = 0.95). The average PCr linewidth was 21.6 ± 8.7 Hz.
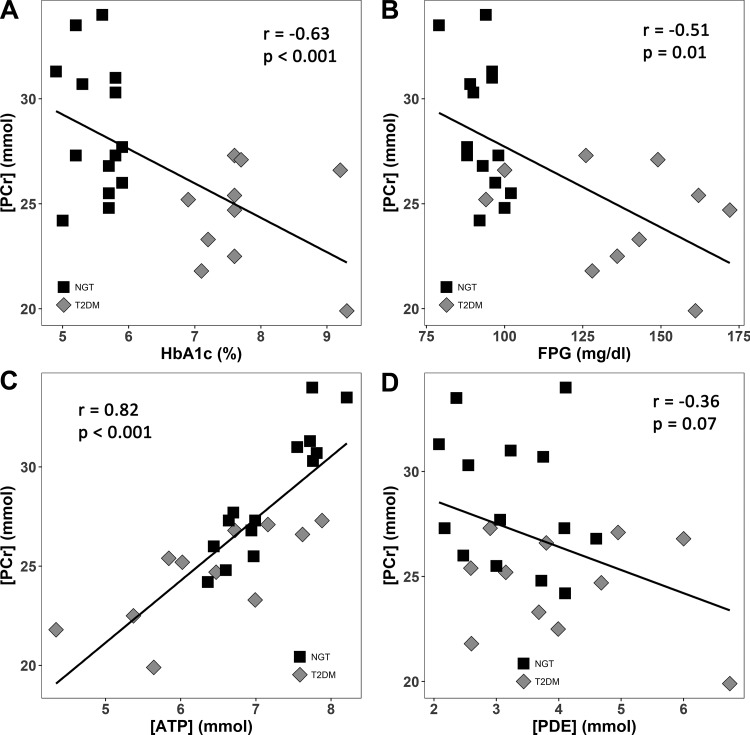
Resting VL muscle [PCr] was reduced in T2DM (24.6 ± 2.4 mM) vs. NGT (28.6 ± 3.2 mM, P = 0.002) (Table 2) and correlated inversely with FPG (r = −0.51, P = 0.01) and HbA1c (r = −0.63, P < 0.001) (Fig. 2, A and B). VL muscle [ATP] was reduced in T2DM (6.37 ± 1.05 mM) compared with NGT (7.18 ± 0.60 mM, P = 0.02) subjects. Of note, the [ATP] in VL muscle in both NGT and T2DM subjects was lower than the reference concentration of ATP (8.2 mM) used in the literature (24, 48). [PCr] was significantly and positively correlated with [ATP] (Fig. 2C) and negatively correlated with [PDE] (Fig. 2D). [ADP] did not differ significantly between NGT and T2DM subjects using our quantitative method, whereas it was significantly higher in T2DM using the conventional method which assumes a constant concentration for ATP (Table 2).
Table 2.
Phosphorus metabolites measured by 31P-MRS and intramyocellular lipid concentration ([IMCL]) by 1H-MRS
| Parameter | NGT | T2DM | P Value |
|---|---|---|---|
| [Pi], mM | 2.80 ± 0.57 | 2.79 ± 0.41 | 0.93 |
| [ATP], mM | 7.18 ± 0.6 | 6.37 ± 1.05 | 0.02 |
| [PCr], mM | 28.6 ± 3.2 | 24.6 ± 2.4 | 0.002 |
| [PCr]conv*, mM | 32.5 ± 1.7 | 32.2 ± 4.1 | 0.82 |
| [PDE], mM | 3.11 ± 0.82 | 4.04 ± 0.98 | 0.02 |
| [PDE]conv*, mM | 3.72 ± 1.05 | 5.35 ± 1.6 | 0.005 |
| pH | 7.00 ± 0.03 | 7.00 ± 0.05 | 0.99 |
| [Cr], mM | 21.8 ± 5.6 | 19.7 ± 2.5 | 0.28 |
| [ADP], µM | 33 ± 9 | 31 ± 6 | 0.45 |
| [ADP]conv**, µM | 26 ± 8 | 35 ± 9 | 0.03 |
| [IMCL]***, mmol/kg | 6.87 ± 1.15 | 8.99 ± 1.46 | 0.004 |
[PCr]conv and [PDE]conv use the ratio of the desired metabolite to the amplitude of ATP × [ATP], using the assumption that [ATP]conv = 8.2 mM.
[ADP]conv assumes [ATP]conv = 8.2 mM and [PCr] + [Cr] = 42.5 mM.
Determined in a subset of 9 NGT and 8 T2DM subjects.
Fig. 2.
Inverse relationship between [PCr] and hemoglobin A1c (A) and fasting plasma glucose (FPG) (B). Positive correlation between [PCr] and [ATP] in subjects with normal glucose tolerance (NGT) and type 2 diabetes mellitus (T2DM) (C). Weak negative correlation between [PCr] and [PDE] is shown in D.
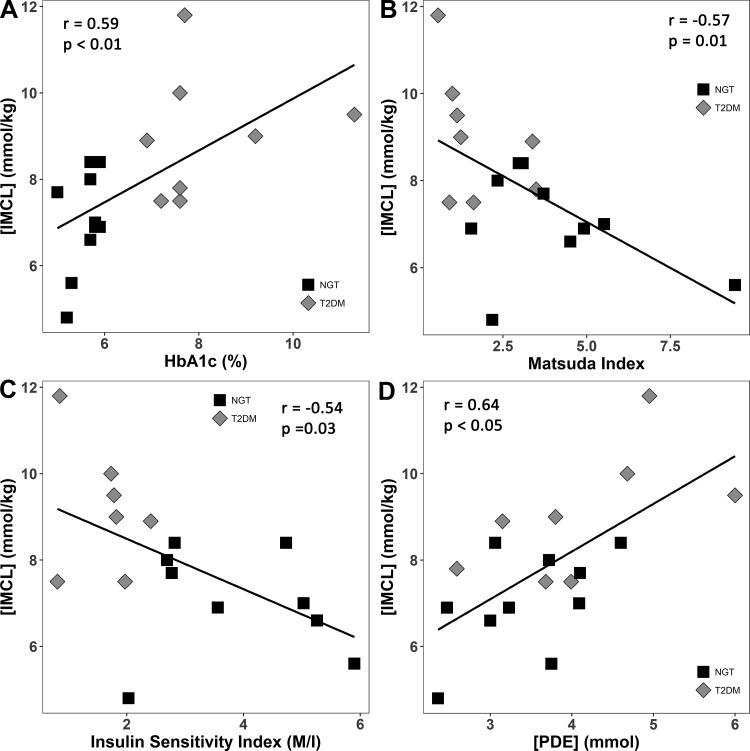
There were no differences in [Pi], pH, and [Cr] between T2DM and NGT subjects. [IMCL] was significantly increased (P = 0.004) in T2DM (8.99 ± 1.46 mmol/kg) vs NGT subjects (6.87 ± 1.15 mmol/kg) (Table 2). [IMCL] was significantly and positively correlated with fasting plasma glucose (r = 0.53, P = 0.03) and hemoglobin A1c (r = 0.59, P = 0.007) (Fig. 3A). [IMCL] was significantly and negatively correlated with the Matsuda index of insulin sensitivity (r = −0.60, P = 0.009) (Fig. 3B) and insulin sensitivity (M/I) measured with the insulin clamp (r = −0.54, P = 0.03) in subjects with NGT and T2DM (Fig. 3C). [IMCL] also was negatively correlated with [PCr] (r = −0.43, P = 0.074) and was significantly and positively correlated with [PDE] (r = 0.64, P < 0.005) (Fig. 3D).
Fig. 3.
Relationship between intramyocellular lipid concentration ([IMCL]) and hemoglobin A1c (A), Matsuda index of insulin sensitivity (B), and insulin sensitivity index (M/I) measured with the insulin clamp (C) in subjects with normal glucose tolerance (NGT) and type 2 diabetes mellitus (T2DM). [IMCL] was also strongly and positively correlated with [PDE] obtained with 31P-MRS (D).
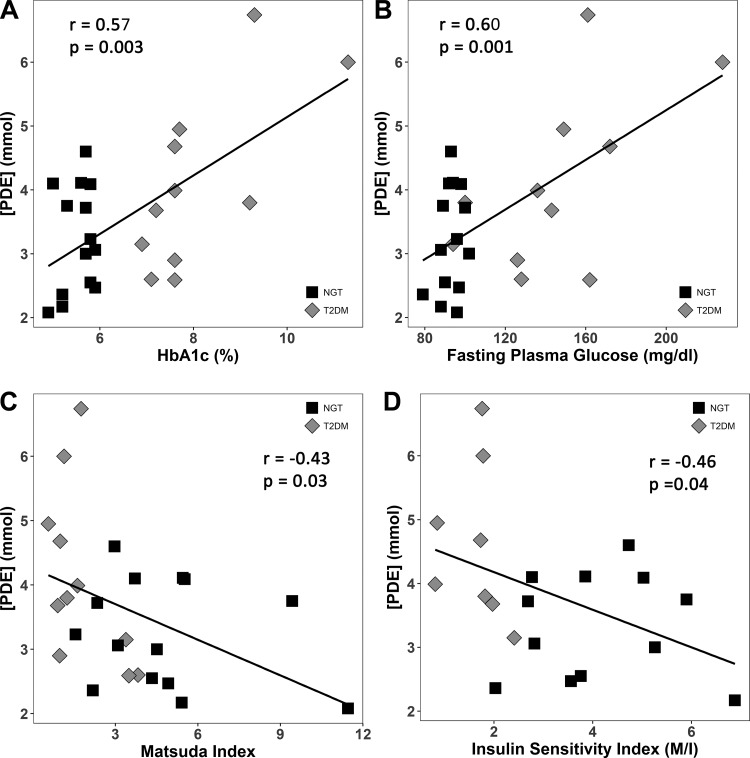
Myocellular [PDE] was significantly higher in the T2DM vs. NGT (4.04 ± 0.98 vs. 3.11 ± 0.82 mM, P = 0.02). [PDE]conv also was significantly greater (P = 0.005) in T2DM VL muscles (5.35 ± 1.6 mM) compared with NGT (3.72 ± 1.05 mM). PDE was positively associated with HbA1c (Fig. 4A) and FPG (r = 0.60, P = 0.001) across all participants (Fig. 4B). [PDE] correlated negatively with Matsuda index and insulin sensitivity index (Fig. 4, C and D). However, [PDE] was not associated with BMI or mitochondrial density.
Fig. 4.
Relationships between [PDE] and hemoglobin A1c (A), fasting plasma glucose (B), Matsuda index of insulin sensitivity (C), and the insulin sensitivity index (M/I) (D) in subjects with normal glucose tolerance (NGT) and type 2 diabetes mellitus (T2DM).
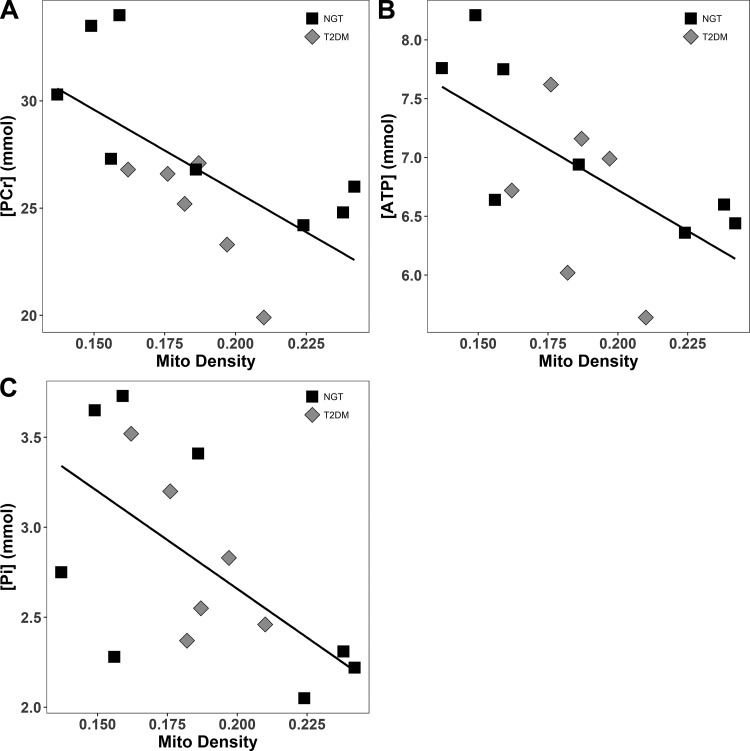
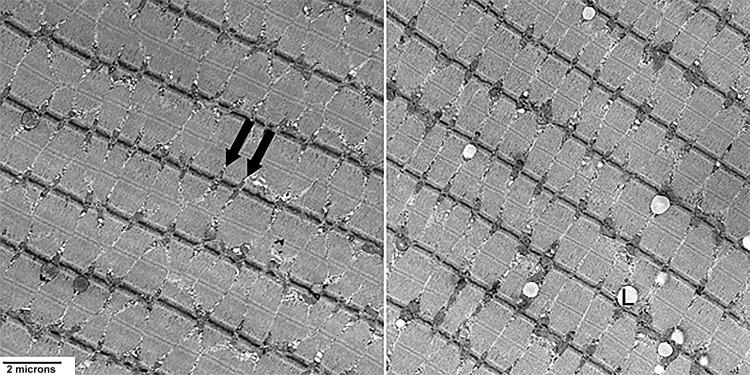
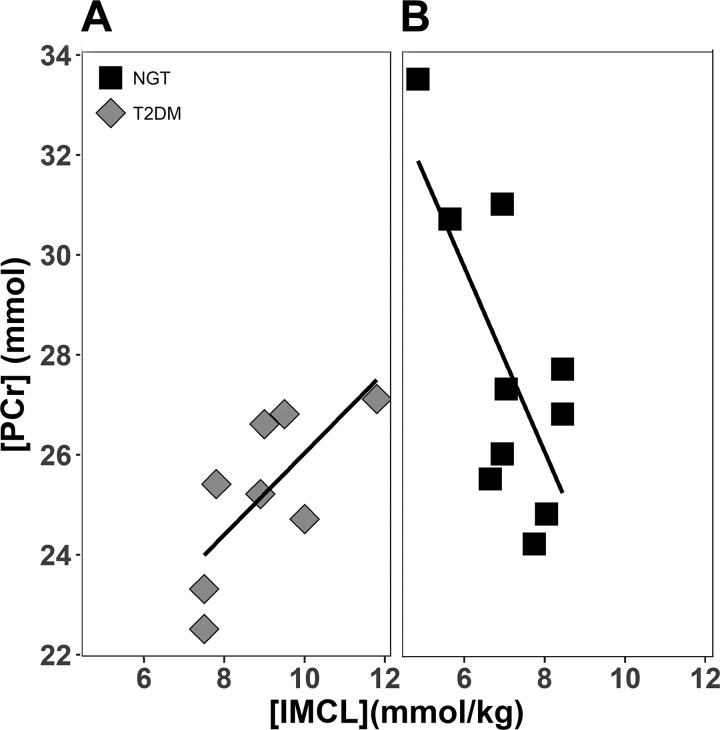
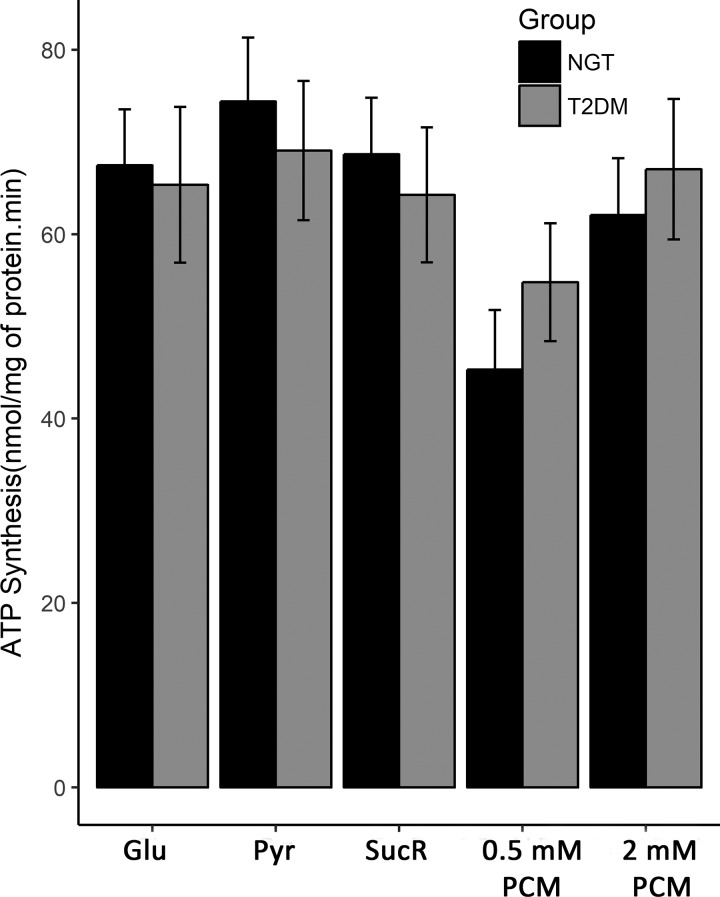
Mitochondrial density was measured with transmission electron microscopy (TEM) in 8 NGT and 6 T2DM subjects; no difference was noted between the two groups (P = 0.96). However, mitochondrial density was negatively correlated with [PCr] (r = −0.67, P = 0.009), [Pi] (r = −0.61, P = 0.02), and [ATP] (r = −0.63, P = 0.02) (Fig. 5). TEM examination revealed a loss of muscle mitochondrial structural arrangement and increased lipid droplets in T2DM subjects (Fig. 6). [IMCL] correlated negatively with [PCr] in the NGT group, whereas it correlated positively with [PCr] in the T2DM group (Fig. 7, A and B), even though the two groups were well-matched for age (P = 0.92) and BMI (P = 0.82). No difference in ATP synthesis rate was observed between T2DM and NGT subjects in VL muscle ex vivo with any substrate (Fig. 8).
Fig. 5.
Significant correlations were observed between mitochondrial density and three phosphorus metabolites: [PCr]: r = −0.67, P = 0.009 (A); [ATP]: r = −0.63, P = 0.02 (B); [Pi]: r = −0.61, P = 0.02 (C). Similar correlations were found when each group was examined individually. There was no correlation of [PDE] with mitochondrial density.
Fig. 6.
Representative transmission electron micrographs at 15,000×. A normal glucose tolerance (NGT) (62-yr-old male) subject (left) and a type 2 diabetes mellitus (T2DM) (59 yr-old male) subject (right) showing mitochondria in longitudinal vastus lateralis (VL) muscle fibers. These images are probably of slow-twitch fibers, which are characterized by thick Z lines and mitochondria paired along either side in at regular intervals (black arrows). The T2DM sample on the right exhibits large lipid droplets (L) and a less regular mitochondrial arrangement.
Fig. 7.
Correlation between [PCr] and intramyocellular lipid concentration ([IMCL]) in type 2 diabetes mellitus (T2DM) [r = 0.72, P = 0.04 (A)] and normal glucose tolerance (NGT) [r = −0.77, P = 0.02 (B)] subjects.
Fig. 8.
ATP synthesis rate in normal glucose tolerance (NGT) and type 2 diabetes mellitus (T2DM) subjects. Glu, glutamate; Pyr, pyruvate; SucR, succinate plus rotenone; PCM, palmitoyl-l-carnitine.
DISCUSSION
31P-MRS is a noninvasive method commonly used to provide information about concentrations of intramyocellular high-energy phosphate compounds and to assess mitochondrial function. Few human studies have attempted to measure absolute concentrations of these metabolites in the postabsorptive state, and conflicting results have been reported (15, 52, 55). De Feyter et al. (15) measured resting PCr, Pi, and ADP concentrations using a constant value for [ATP] = 8.2 mM and reported no difference between NGT, prediabetic, and long-standing insulin-treated type 2 diabetic subjects. Schrauwen-Hinderling et al. (53) reported a 45% delay in PCr recovery half-time in T2DM vs. NGT individuals but found no difference in resting Pi/PCr between the two groups. Scheuermann-Freestone et al. (50) reported lower baseline metabolite concentrations in cardiac but not skeletal muscle. Wu et al. (64) reported reduced resting skeletal muscle [Pi], [PCr], and [ATP] by assuming that the β-ATP for the control subjects at rest was equivalent to 5.5 mmol/kg of wet muscle weight.
One possibility that could explain the inconsistent data reported in the literature relates to the use of the conventional ratio method instead of absolute [PCr] and [ATP] quantitation. Use of an assumed constant value for [ATP] can obscure simultaneous decreases in both [PCr] and [ATP] and enhance otherwise marginal differences. Furthermore, the assumption of a uniform and constant [ATP] = 8.2 mM has not been validated in human skeletal muscle. This value originates from a study of 81 young healthy human subjects of indeterminate sex with an age range of 18–30 yr (24). The muscle samples were obtained from the VL muscle using a percutaneous needle biopsy and were frozen within 4.2 ± 0.8 s, powdered, and assayed. The investigators showed a highly significant variance between individuals, suggesting that a single value should not be assumed for all individuals. Further, metabolite concentrations were reported in millimoles per kilogram dry mass, and assumptions were used to convert the values into millimoles per liter of intracellular water, finally arriving at a mean ATP concentration of 8.2 mM.
In the current study, we developed a reproducible method to quantitate 31P-MRS measurements in human VL muscle. Using this modified in vivo quantitative imaging method, we found significantly lower [PCr] and [ATP] in VL muscle in T2DM compared with NGT subjects. This finding has important physiological as well as clinical implications. Although reduced muscle [PCr] and [ATP] should not be construed to be synonymous with impaired mitochondrial function, they are consistent with a defect in mitochondrial function and could contribute to the insulin resistance in T2DM individuals (2, 3, 9, 14, 26, 34, 46). Controversy exists about whether the defect in mitochondrial function is responsible for the insulin resistance, i.e., primary or secondary to the insulin resistance (2, 3). Incubation of mitochondria with FFA can cause impaired mitochondrial function, whereas infusion of lipid to elevate the plasma FFA concentration in vivo impairs mitochondrial function and induces insulin resistance (2, 3). Conversely, in a GWAS study, arylamine N-acetlytransferase 2 has been identified as an insulin sensitivity gene in humans (29), and deficiency of Nat2 (mouse homolog of Nat1) in mice leads to reduced mitochondrial function, increased muscle lipid deposition, and insulin resistance (13). Thus it appears that defective mitochondrial function can both be the cause of, or result from, insulin resistance. Lower [PCr] and [ATP] concentrations have been reported from a freeze-clamped human skeletal muscle biopsy study taken from T2DM subjects following 10-wk exposure to metformin (36). However, the significance of this observation is unclear since metformin is not an insulin-sensitizing drug in muscle (4) and, when [11C]metformin is given intravenously, it cannot be detected in skeletal muscle (25). In mice, metformin has been reported to inhibit respiratory chain complex I, resulting in decreased hepatic ATP levels and activation of AMPK (22). However, the dose of metformin used in these studies was in the supraphysiological range. Therefore, we do not believe that background metformin can explain the reduced levels of [PCr] and [ATP] in the present study.
In the current study, differences in mitochondrial number or density between the T2DM and NGT groups were not observed. However, there were clear alterations in muscle architecture of T2DM patients, characterized by intermittent loss of pairing along the Z line and altered regularity of the pairing. Mitochondrial density correlated negatively with the resting concentration of phosphorus metabolites [PCr], [Pi], and [ATP], indicating that individuals with a greater quantity of mitochondria had lower baseline cytosolic phosphorus levels. This could be related to the previously described reduction in the number of subsarcolemmal mitochondria in T2DM patients, which are hypothesized to be functionally and structurally distinct from intermyofibrillar mitochondria (45).
Previous light and electron microscopy studies in healthy NGT subjects have demonstrated that mitochondria in skeletal muscle are arranged in a highly ordered manner, with the highest mitochondrial density found in the subsarcolemmal region of type I fibers (54). Reduced size, but not lower density, of mitochondria has been reported in human skeletal muscle in T2DM (26), although one study reported reduced mitochondrial densities in offspring of IR individuals (35). Intergenerational manifestations of insulin-resistant phenotypes may result from genetic (13, 29, 40) or epigenetic causes (19, 31).
We also found [IMCL] to be significantly higher in T2DM subjects than in age- and BMI-matched NGT subjects, consistent with previous publications from our laboratory (6, 7) and others (16, 23, 30, 39, 57, 59). [IMCL] correlated strongly with measures of glycemic control and insulin sensitivity, including HbA1c, FPG, Matsuda index, and M/I, insulin sensitivity index (Fig. 3). The increased [IMCL] is consistent with previous findings with MRS (7, 16, 21, 23, 30) and with increased intramyocellular levels of fatty acyl CoA, diacylglycerol (DAG), and ceramides with muscle biopsy (3, 6, 26, 32). Further, increased intramyocellular levels of toxic lipid metabolites have been shown to inhibit insulin signaling and to correlate closely with defects in insulin resistance in T2DM and obese NGT individuals (8, 10).
In the present study we also observed increased skeletal muscle PDE concentrations by 31P-MRS. Although less well appreciated than increased intramyocellular levels of FACoAs, DAG, and ceramides, elevated muscle [PDE] also has been associated with advancing age, lower resting mitochondrial activity, obesity, and insulin resistance (56, 61). Using high-resolution 31P-MRS (7 T), the main component of the PDE peak in skeletal muscle has been shown to be glycerophosphocholine (GPC) (61). In the present study we also observed an increase in muscle [PDE] in T2DM vs. NGT subjects (Table 2). Further, muscle [PDE] was closely associated with insulin resistance using both the Matsuda index of insulin sensitivity and insulin resistance index (M/I) during the insulin clamp, as well as with indexes of glycemia and [IMCL] (Fig. 4). These results suggest that increased [PDE] should be added to the list of intramyocellular metabolites that contribute to insulin resistance in T2DM individuals. In T2DM individuals, [IMCL] was increased and correlated positively with [PCr], whereas in NGT individuals it correlated negatively with [PCr]. Because diabetic skeletal muscle is resistant to insulin-stimulated glucose uptake (18), it is forced to switch to lipid oxidation to generate energy in the form of ATP. This could explain the elevated [IMCL] and positive correlation between [IMCL] and [PCr]. In contrast, skeletal muscle in NGT subjects is normally sensitive to insulin-stimulated glucose uptake and relies upon glucose as its primary energy source. This would explain the negative relationship between [IMCL] and [PCr] and reduced (compared with T2DM subjects) muscle [IMCL]. This study has several limitations. The number of subjects is relatively small, obese nondiabetic subjects were not studied, and the age range did not include very young or very old individuals (range = 31–70 yr) (5, 21, 37, 44). Maximal rate of oxygen consumption (V̇o2max) was not measured, although all subjects were considered to be sedentary on the basis of a routine exercise questionnaire and no subject participated in a routine or excessively heavy exercise program. A dedicated study to examine the effect of V̇o2max and exercise training on phosphorus metabolite concentrations in VL muscle would be of great interest (5, 21, 38, 44), since the V̇o2max is reduced in T2DM individuals (43). Further studies examining the relationship between body fat composition/distribution and muscle phosphorus metabolites also would be of interest. Due to the limited size of the biopsy samples, direct measurements of lipid content/type could not be performed and subsarcolemmal and intermyofibrillar fractions were not examined (45). Future studies using the absolute quantitative method to measure PCr recovery time to assess in vivo mitochondrial capacity in combination with measurement of the activity of mitochondrial oxidative enzymes and mRNA expression of phosphodiesterase and regulators of mitochondrial biogenesis in muscle biopsy specimens would provide additional insights.
In summary, using a modified in vivo quantitative imaging method, we have demonstrated that skeletal muscle [PCr] and [ATP] are reduced in T2DM subjects, while [PDE] is increased. Of note, these differences could not be appreciated using the traditional ratio method for in vivo 31P-MRS. The strong correlation between [PCr] vs. HbA1c, FPG, and measures of insulin sensitivity and beta cell function supports the concept that lower baseline skeletal muscle [PCr] is related to key determinants of glucose homeostasis. Adoption of precise, quantitative 31P-MRS measurements in metabolic studies will allow this information to substantively contribute to modeling of complex, in vivo metabolic processes in skeletal muscle (12).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-24092-34 (R. A. DeFronzo) and K25-DK-089012 (G. D. Clarke).
DISCLOSURES
R. A. DeFronzo is on the Advisory Board for Astra Zeneca, Novo Nordisk, Janssen, Boehringer-Ingelheim, Intarcia, and Elcelyx; received research support from Boehringer-Ingelheim, Takeda, Astra Zeneca, and Janssen; and is on the Speaker’s Bureau of Novo-Nordisk and Astra Zeneca.
AUTHOR CONTRIBUTIONS
E.M.R., G.D.C., V.H., F.D.S., C.S., S.D., M.A.-G., D.T., and R.A.D. edited and revised manuscript; E.M.R., G.D.C., R.A.M., F.D.S., C.S., S.D., M.A.-G., D.T., and R.A.D. approved final version of manuscript; V.H. analyzed data; V.H. interpreted results of experiments; V.H. and R.A.D. drafted manuscript; R.A.D. conceived and designed research.
REFERENCES
- 1.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55: 1430–1435, 2006. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep 8: 173–178, 2008. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, DeFronzo RA. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab 295: E678–E685, 2008. doi: 10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Ghani M, DeFronzo RA. Is it time to change the type 2 diabetes treatment paradigm? Yes! GLP-1 RAs should replace metformin in the type 2 diabetes algorithm. Diabetes Care 40: 1121–1127, 2017. doi: 10.2337/dc16-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med 1: 307–315, 1984. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 54: 3148–3153, 2005. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW, Monroy A, Koul S, Sriwijitkamol A, Musi N, Shulman GI, DeFronzo RA. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95: 1916–1923, 2010. doi: 10.1210/jc.2009-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bays H, Mandarino L, DeFronzo RA. Role of the adipocytes, free fatty acids, and ectopic fat in the pathogenesis of type 2 diabetes mellitus: peroxisome proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 89: 463–478, 2004. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 9.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56: 1376–1381, 2007. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, DeFronzo RA, Cusi K. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 54: 1640–1648, 2005. doi: 10.2337/diabetes.54.6.1640. [DOI] [PubMed] [Google Scholar]
- 11.Boesch C, Machann J, Vermathen P, Schick F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed 19: 968–988, 2006. doi: 10.1002/nbm.1096. [DOI] [PubMed] [Google Scholar]
- 12.Bordbar A, Monk JM, King ZA, Palsson BO. Constraint-based models predict metabolic and associated cellular functions. Nat Rev Genet 15: 107–120, 2014. doi: 10.1038/nrg3643. [DOI] [PubMed] [Google Scholar]
- 13.Camporez JP, Wang Y, Faarkrog K, Chukijrungroat N, Petersen KF, Shulman GI. Mechanism by which arylamine N-acetyltransferase 1 ablation causes insulin resistance in mice. Proc Natl Acad Sci USA 114: E11285–E11292, 2017. doi: 10.1073/pnas.1716990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniele G, Eldor R, Merovci A, Clarke GD, Xiong J, Tripathy D, Taranova A, Abdul-Ghani M, DeFronzo RA. Chronic reduction of plasma free fatty acid improves mitochondrial function and whole-body insulin sensitivity in obese and type 2 diabetic individuals. Diabetes 63: 2812–2820, 2014. doi: 10.2337/db13-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol 158: 643–653, 2008. doi: 10.1530/EJE-07-0756. [DOI] [PubMed] [Google Scholar]
- 16.De Feyter HM, Lenaers E, Houten SM, Schrauwen P, Hesselink MK, Wanders RJ, Nicolay K, Prompers JJ. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. FASEB J 22: 3947–3955, 2008. doi: 10.1096/fj.08-112318. [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA. Banting lecture: From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deiuliis JA. MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes 40: 88–101, 2016. doi: 10.1038/ijo.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denton RM, Yorke RE, Randle PJ. Measurement of concentrations of metabolites in adipose tissue and effects of insulin, alloxan-diabetes and adrenaline. Biochem J 100: 407–419, 1966. doi: 10.1042/bj1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369, 2010. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 24.Harris RC, Hultman E, Nordesjö LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974. doi: 10.3109/00365517409082477. [DOI] [PubMed] [Google Scholar]
- 25.Jensen JB, Gormsen LC, Sundelin E. Organ-specific uptake and elimination of metformin can be determined in vivo in mice and humans by PET-imaging using a novel 11C-metformin tracer. Diabetes 64, Suppl 1: 128-LB, 2015.25190567 [Google Scholar]
- 26.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 27.Kemp GJ, Thompson CH, Taylor DJ, Hands LJ, Rajagopalan B, Radda G. K. Quantitative analysis by 31P MRS of abnormal mitochondrial oxidation in skeletal muscle during recovery from exercise. NMR Biomed 6: 302–310, 1993. doi: 10.1002/nbm.1940060504. [DOI] [PubMed] [Google Scholar]
- 28.Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed 20: 555–565, 2007. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- 29.Knowles JW, Xie W, Zhang Z, Chennamsetty I, Assimes TL, Paananen J, Hansson O, Pankow J, Goodarzi MO, Carcamo-Orive I, Morris AP, Chen YD, Mäkinen VP, Ganna A, Mahajan A, Guo X, Abbasi F, Greenawalt DM, Lum P, Molony C, Lind L, Lindgren C, Raffel LJ, Tsao PS, Schadt EE, Rotter JI, Sinaiko A, Reaven G, Yang X, Hsiung CA, Groop L, Cordell HJ, Laakso M, Hao K, Ingelsson E, Frayling TM, Weedon MN, Walker M, Quertermous T; The RISC (Relationship between Insulin Sensitivity and Cardiovascular Disease) Consortium; EUGENE2 (European Network on Functional Genomics of Type 2 Diabetes) Study; GUARDIAN (Genetics UndeRlying DIAbetes in HispaNics) Consortium; SAPPHIRe (Stanford Asian and Pacific Program for Hypertension and Insulin Resistance) Study . Identification and validation of N-acetyltransferase 2 as an insulin sensitivity gene. J Clin Invest 125: 1739–1751, 2015. doi: 10.1172/JCI74692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Shulman GI, Roden M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42: 113–116, 1999. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz CL, Peck BC, Fannin EE, Beysen C, Miao J, Landstreet SR, Ding S, Turaga V, Lund PK, Turner S, Biddinger SB, Vickers KC, Sethupathy P. MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes 63: 3141–3148, 2014. doi: 10.2337/db13-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin K, Daa Schroeder H, Alford FP, Beck-Nielsen H. Morphometric documentation of abnormal intramyocellular fat storage and reduced glycogen in obese patients with Type II diabetes. Diabetologia 44: 824–833, 2001. doi: 10.1007/s001250100545. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 34.Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56: 1592–1599, 2007. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 35.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51: 2074–2081, 2002. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 37.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA 12: 141–152, 2001. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 38.Newcomer BR, Larson-Meyer DE, Hunter GR, Weinsier RL. Skeletal muscle metabolism in overweight and post-overweight women: an isometric exercise study using (31)P magnetic resonance spectroscopy. Int J Obes Relat Metab Disord 25: 1309–1315, 2001. doi: 10.1038/sj.ijo.0801673. [DOI] [PubMed] [Google Scholar]
- 39.Nowotny B, Zahiragic L, Krog D, Nowotny PJ, Herder C, Carstensen M, Yoshimura T, Szendroedi J, Phielix E, Schadewaldt P, Schloot NC, Shulman GI, Roden M. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes 62: 2240–2248, 2013. doi: 10.2337/db12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Praet SF, De Feyter HM, Jonkers RA, Nicolay K, van Pul C, Kuipers H, van Loon LJ, Prompers JJ. 31P MR spectroscopy and in vitro markers of oxidative capacity in type 2 diabetes patients. MAGMA 19: 321–331, 2006. doi: 10.1007/s10334-006-0060-0. [DOI] [PubMed] [Google Scholar]
- 43a.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing, 2013. (Available online at http://www.R-project.org).
- 44.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc 27: 875–881, 1995. doi: 10.1249/00005768-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 46.Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298: E49–E58, 2010. doi: 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth K, Hubesch B, Meyerhoff D, Naruse S, Gober J, Lawry T, Boska MD, Matson G, Weiner M. Noninvasive quantitation of phosphorus metabolites in human tissue by NMR spectroscopy. J Magn Reson 81: 299–311, 1989. doi: 10.1016/0022-2364(89)90062-0. [DOI] [Google Scholar]
- 48.Rothman DL, Shulman RG, Shulman GI. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest 89: 1069–1075, 1992. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmid AI, Schrauwen-Hinderling VB, Andreas M, Wolzt M, Moser E, Roden M. Comparison of measuring energy metabolism by different 31P-magnetic resonance spectroscopy techniques in resting, ischemic, and exercising muscle. Magn Reson Med 67: 898–905, 2012. doi: 10.1002/mrm.23095. [DOI] [PubMed] [Google Scholar]
- 50.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107: 3040–3046, 2003. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 51.Schrauwen-Hinderling VB, Hesselink MK, Schrauwen P, Kooi ME. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring) 14: 357–367, 2006. doi: 10.1038/oby.2006.47. [DOI] [PubMed] [Google Scholar]
- 52.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 50: 113–120, 2007. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 53.Schrauwen-Hinderling VB, Roden M, Kooi ME, Hesselink MK, Schrauwen P. Muscular mitochondrial dysfunction and type 2 diabetes mellitus. Curr Opin Clin Nutr Metab Care 10: 698–703, 2007. doi: 10.1097/MCO.0b013e3282f0eca9. [DOI] [PubMed] [Google Scholar]
- 54.Shaw CS, Jones DA, Wagenmakers AJ. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol 129: 65–72, 2008. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 55.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176, 2000. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szendroedi J, Schmid AI, Chmelik M, Krssak M, Nowotny P, Prikoszovich T, Kautzky-Willer A, Wolzt M, Waldhäusl W, Roden M. Skeletal muscle phosphodiester content relates to body mass and glycemic control. PLoS One 6: e21846, 2011. doi: 10.1371/journal.pone.0021846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, Jelenik T, Müller J, Herder C, Nowotny P, Shulman GI, Roden M. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci USA 111: 9597–9602, 2014. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab 287: E558–E565, 2004. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 60.Valaparla SK, Gao F, Daniele G, Abdul-Ghani M, Clarke GD. Fiber orientation measurements by diffusion tensor imaging improve hydrogen-1 magnetic resonance spectroscopy of intramyocellular lipids in human leg muscles. J Med Imaging (Bellingham) 2: 026002, 2015. doi: 10.1117/1.JMI.2.2.026002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valkovič L, Chmelík M, Ukropcová B, Heckmann T, Bogner W, Frollo I, Tschan H, Krebs M, Bachl N, Ukropec J, Trattnig S, Krššák M. Skeletal muscle alkaline Pi pool is decreased in overweight-to-obese sedentary subjects and relates to mitochondrial capacity and phosphodiester content. Sci Rep 6: 20087, 2016. doi: 10.1038/srep20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Salibi N, Fayad LM, Barker PB. Proton magnetic resonance spectroscopy of skeletal muscle: a comparison of two quantitation techniques. J Magn Reson 243: 81–84, 2014. doi: 10.1016/j.jmr.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J Biomech 38: 2317–2320, 2005. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Wu FY, Tu HJ, Qin B, Chen T, Xu HF, Qi J, Wang DH. Value of dynamic 31P magnetic resonance spectroscopy technique in in vivo assessment of the skeletal muscle mitochondrial function in type 2 diabetes. Chin Med J (Engl) 125: 281–286, 2012. [PubMed] [Google Scholar]