Abstract
While there is a growing consensus that insulin has diverse and important regulatory actions on the brain, seemingly important aspects of brain insulin physiology are poorly understood. Examples include: what is the insulin concentration within brain interstitial fluid under normal physiologic conditions; whether insulin is made in the brain and acts locally; does insulin from the circulation cross the blood-brain barrier or the blood-CSF barrier in a fashion that facilitates its signaling in brain; is insulin degraded within the brain; do privileged areas with a “leaky” blood-brain barrier serve as signaling nodes for transmitting peripheral insulin signaling; does insulin action in the brain include regulation of amyloid peptides; whether insulin resistance is a cause or consequence of processes involved in cognitive decline. Heretofore, nearly all of the studies examining brain insulin physiology have employed techniques and methodologies that do not appreciate the complex fluid compartmentation and flow throughout the brain. This review attempts to provide a status report on historical and recent work that begins to address some of these issues. It is undertaken in an effort to suggest a framework for studies going forward. Such studies are inevitably influenced by recent physiologic and genetic studies of insulin accessing and acting in brain, discoveries relating to brain fluid dynamics and the interplay of cerebrospinal fluid, brain interstitial fluid, and brain lymphatics, and advances in clinical neuroimaging that underscore the dynamic role of neurovascular coupling.
Keywords: blood-brain barrier, blood CSF barrier, endothelium, insulin, insulin resistance
INTRODUCTION AND RATIONALE
Pancreatic insulin secretion critically regulates body glucose, fat, and protein metabolism. While the brain is not a classical insulin target tissue, an increasing body of evidence implicates the brain in insulin’s actions on metabolism and cognition. In rodents, brain insulin receptor (IR) mRNA expression is highest in the olfactory bulb, followed by the cortex, hypothalamus, hippocampus, and cerebellum, suggesting that insulin could affect multiple brain regions. Indeed, insulin signaling has been implicated for numerous actions in the brain, including feeding behavior and body weight regulation, hepatic metabolism, and cognition. Insulin can act on hypothalamic nuclei to decrease food intake, an effect that is blunted in rats eating a moderate- or high-fat diet (HFD).The more recent observations that people with type 2 diabetes or insulin resistance are at increased risk for dementia and Alzheimer’s disease suggest that insulin action on the brain is widespread, including the cerebral cortex and hippocampus. Epidemiological evidence for cognitive impairment in diabetes and experimental studies demonstrating “brain insulin resistance” led to clinical trials of intranasal insulin administration, which may slow cognitive decline. Effects on feeding behavior and metabolism appear to be largely mediated by insulin’s hypothalamic actions, whereas cognitive function and memory changes are attributed to its actions in the cerebral cortex and hippocampus. These brain regions differ in function and in blood-brain barrier (BBB) structure. While some data suggest that the brain can produce insulin, pancreatic insulin is likely the dominant source for brain insulin signaling.
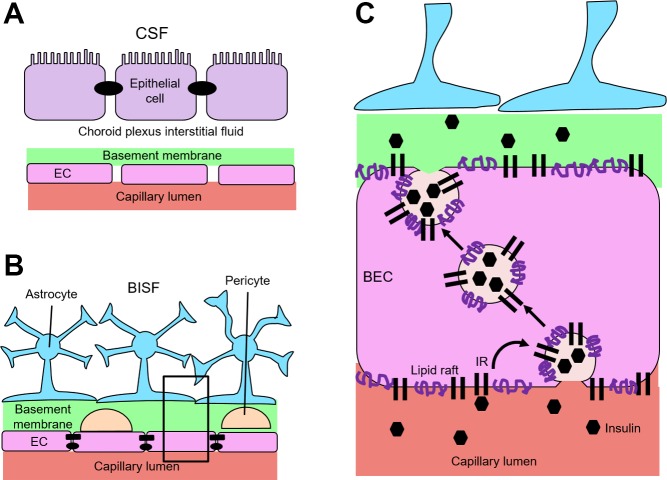
Direct insulin administration into cerebrospinal fluid (CSF) has long been employed to investigate insulin’s actions in the brain. This method, however, sidesteps the question of how insulin reaches the brain and ignores both historical and recent findings regarding CSF secretion, circulation, and clearance from the brain. Thus, it is unlikely to represent a major physiological pathway of pancreatic insulin entry into brain interstitial fluid (BISF). Moreover, extremely high insulin concentrations are often used, which, although experimentally expedient, do not replicate normal physiology. Some blood-borne chemicals can enter the BISF by crossing the fenestrated endothelium and overlying epithelium of the choroid plexus (the blood-CSF barrier, Fig. 1A) to enter CSF circulation with eventual movement through the paravascular Virchow-Robin space (VRS) to brain parenchyma to enter BISF (Fig. 2). Alternatively, substances can cross the highly restrictive endothelium of the BBB (Fig. 1B) to reach the VRS directly before entering BISF. Some substances may use both pathways to reach BISF. Although recent work has begun to elucidate how circulating insulin enters the BISF to reach neuronal tissue and exert its central nervous system (CNS) effects (Fig. 1C), much remains unknown.
Fig. 1.
Barriers of the brain and insulin movement across the blood-brain barrier. A: the blood-cerebrospinal fluid (CSF) barrier has fenestrated capillaries in the choroid plexus that lack tight junctions and allow para- and transcellular transport across the endothelium. Substances secreted into ventricular cerebrospinal fluid are actively transported across the epithelial cells (joined by tight junctions). B: architecture of the blood-brain barrier at the capillary level. Tight junctions (ovals) and adherens junctions (rectangles) between brain endothelial cells (BECs) where the tight junctions prevent paracellular movement. Pericytes and basement membrane material largely fill the Virchow-Robin space (VRS) between the BEC and the astrocyte boundary. BISF, brain interstitial fluid. C: magnified image of box in B demonstrating how insulin transits the BEC. Circulating insulin (hexagons) interacts with insulin receptor (IR) on the luminal membrane of the microvascular BEC. Subsequently, it is internalized by a lipid raft-mediated endocytotic process and shuttled to the antiluminal membrane. It is released there and can permeate the VRS, which at the capillary level is filled with basement membrane material. Once across the endothelium it may interact with pericytes, astrocytes, or enter the BISF and act directly on neurons. The proximity of astrocyte endfeet to the pericyte and endothelial cell (EC) may facilitate neurovascular coupling.
Fig. 2.
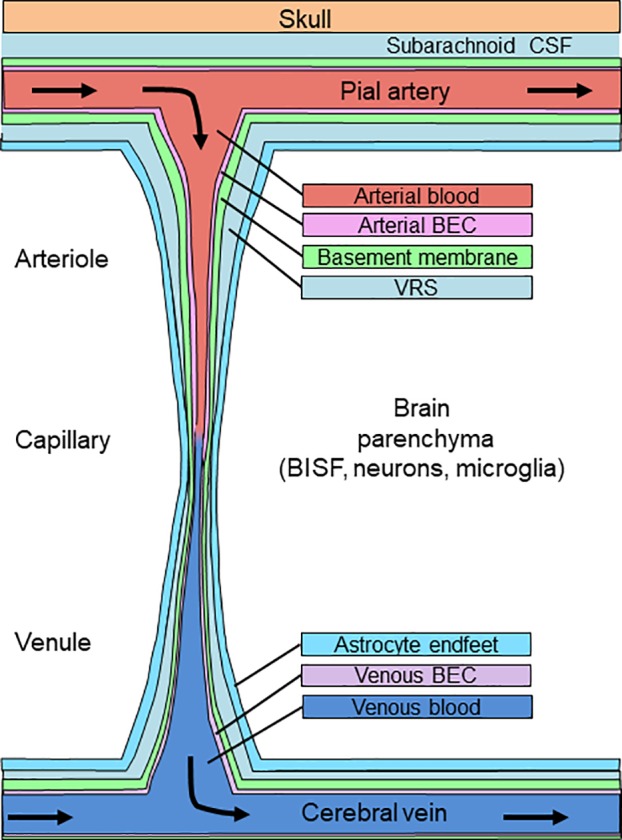
A simplified schematic of the circulation of blood through the brain originating in pial arteries which are bathed by subarachnoid cerebrospinal fluid (CSF). As pial vessels penetrate into brain parenchyma as arterioles, then capillaries, then venules, they are accompanied by a paravascular sheath (Virchow-Robin space, VRS) whose medial boundary (green) is the blood vessel basement membrane and whose lateral wall is the astrocyte foot processes (light blue). BEC, brain endothelial cell; BISF, brain interstitial fluid.
This review provides a status report on historical and recent work that begins to address selected issues related to the physiology of brain insulin. First, we review evidence for insulin’s entry into BISF either via CSF secretion and subsequent circulation or by crossing the BBB. While discussing each, we review effects of HFD and insulin resistance on brain insulin transport, access, and action. We highlight the impact of new findings regarding CSF circulation through the “glymphatic” system and newly characterized brain lymphatic system. We emphasize the difficulty of interpreting many previous studies of brain insulin action due to their methodological and technical limitations and believe that this field warrants renewed investigation. Finally, we pose several questions to consider as investigators plan future studies to clarify the role of circulating insulin within the brain.
INSULIN MOVEMENT ACROSS THE BLOOD-CSF BARRIER
Cerebrospinal fluid functions to provide buoyancy to cushion the brain within the skull, to transport nutrients throughout the brain, and to clear waste. Its composition is similar to plasma but with a lower protein concentration. Biological sampling of the CSF is often used as a surrogate for the BISF that bathes neuronal tissue. While CSF can intermix with BISF, and vice versa, they are not identical. Here, we discuss movement of blood-borne substances into CSF in the cerebral ventricles and the pathway of CSF flow throughout the brain.
Choroid Plexus Secretion of CSF
Cerebrospinal fluid is produced by the highly vascularized choroid plexus tissue in the lateral, third, and fourth ventricles. Fenestrated capillaries in the choroid plexus (Fig. 1A) allow blood-borne substances to enter the choroid plexus interstitium rapidly, and blood flow through the choroid plexus normalized to tissue mass is 5 times higher than that of bulk brain tissue. Cuboidal epithelial cells, joined by tight and adherens junctions, line the choroid plexus villi and numerous transporters and aquaporins facilitate movement of water, electrolytes and other blood-born substances into the ventricle, creating CSF. In humans, the CSF secretion rate is estimated at 0.37 ml/min or ~500–600 ml/day. With a total CSF volume between 90 and 150 ml in humans, bulk CSF turns over on average 4 times per day. Across multiple species, despite differences in CSF volume, the secretion rate (in ml/min) is estimated at 0.5% of total CSF volume. In adult rats, the species often used for examining insulin entry into brain, CSF volume is between 150 and 250 µl, with an estimated secretion rate of 0.8–1.25 µl/min, and bulk turnover 5.5–7 times per day. Estimates from more recent work are slightly lower than early reports.
Proteins and peptides can be transported across the blood-CSF barrier, including albumin and IgG, though the mechanism(s) mediating their transport remain under debate. For example, circulating insulin-like growth factor-I (IGF-I) and leptin each cross the choroid plexus by processes involving their respective receptors. In addition, low-density lipoprotein-related proteins (LRP)-1 and -2 can also contribute to IGF-I and leptin transport, respectively. Autoradiographic data indicate that insulin binds to the choroid plexus, presumably to its receptor that is widely expressed in the brain. Beyond binding, direct evidence of IR-mediated transport across the choroid plexus is lacking. To date, there is no evidence that LRP-1 or -2 facilitates insulin transport into the CSF. However, LRP-2 (also known as megalin) is implicated in insulin reabsorption by renal proximal tubules. This notwithstanding, as insulin is found at low concentrations in CSF (see below), it is likely that some transits the choroid plexus. However, the cellular and/or paracellular pathways involved and whether this transport is regulated are not known. Leptin and IGF-I are also transported across the BBB, albeit at rates different than into CSF, indicating that some peptides can cross both the blood-CSF barrier and the BBB, though through different mechanisms. Recent work suggests that insulin BBB transport occurs, yet blood-CSF transport remains to be demonstrated and its quantitative contribution to either CSF or BISF insulin concentrations is currently unclear.
Circulation of Secreted CSF
The routes and regulation of CSF flow through the brain have been investigated for nearly 100 years, and our understanding continues to evolve as improved imaging techniques and novel animal models are employed. Following its secretion from the choroid plexus, CSF flows through the ventricles and then enters the subarachnoid space. Recent in vivo imaging studies with fluorescent tracers demonstrated that subarachnoid CSF flows alongside penetrating arteries through the paravascular channels of the Virchow-Robin space (VRS, Fig. 2). The vascular wall and astrocyte endfeet form the medial and lateral borders of the VRS, respectively. At the capillary, the basement membrane mostly fills the VRS. When this occurs the VRS is sometimes referred to as the “perivascular space,” whereas “paravascular VRS” refers to the VRS channel through which CSF flows parallel to arteriolar and venous blood vessels. These terms have not always been used consistently in the literature.
Some studies suggest that subarachnoid CSF must cross a leptomeningeal pial sheath, which serves as a sieve, to gain entry to the VRS surrounding penetrating arteries in the cortex. Pial sheaths lining the penetrating arteries were reported in human brain tissue, and this cell layer becomes increasingly fenestrated along the penetrating arterioles and ultimately disappeared at the capillary. Pial sheaths have not been detected in rodent brain, which may reflect use of differing fixation and imaging techniques or species variation. The physiological importance of pial sheaths to CSF flow is uncertain.
Studies of CSF flow through the VRS have made it clear that CSF is a distinct pool, different from the BISF that bathes neuronal tissue. Substances that cross the brain endothelium mix with paravascular CSF in the VRS, and components of this admixture may cross the astrocyte endfeet to reach the BISF (Fig. 2). Components in the VRS may also flow back into subarachnoid CSF (see below).
Insulin in the CSF
The first study to demonstrate appearance of intravenously injected insulin in CSF reported low concentrations (~25% lower than in plasma) that took 3–4 hours to stabilize. Subsequent studies in multiple species confirm that fasting CSF insulin concentrations are much (typically 80–90%) lower than those measured simultaneously in plasma. Furthermore, hyperinsulinemic-euglycemic clamp studies in multiple species (including humans) indicate that CSF insulin concentrations increase very slowly, peaking only after several hours at values <10% of those simultaneously measured in plasma (57, 83, 114, 131). The very low insulin concentrations found in CSF raise the issue of whether the insulin receptor in brain might be more sensitive to insulin relative to peripheral IR. Mammalian IR is present in two isoforms (A and B, with the former missing exon 11). Isoform A is predominantly expressed during fetal life, whereas in the adult, isoform B predominates in some tissues (e.g., liver) while isoform A dominates in brain (87). The affinity of isoform A for insulin is perhaps 2-fold greater than B and it has ~10-fold greater affinity for IGF-II (40). Insulin receptor glycation may be different in brain than in the periphery (1), but there are no data to indicate that the brain IR affinity for insulin differs significantly from the periphery. This, together with the observation that postabsorptive and postprandial CSF insulin concentrations are very low and generally do not reach levels that would be expected to significantly increase insulin signaling, suggests that the BISF insulin concentration is likely higher than, and not well reflected by, CSF insulin measurements. Despite this, many studies of brain insulin action continue to use CSF as a surrogate for BISF. As noted, however, this discounts insulin transport across the BBB.
Schwartz and colleagues (114) fit kinetic models to intravenously infused insulin’s appearance in cisterna magna CSF. They postulated that insulin crossed the BBB and then transited through an intermediate compartment (i.e., BISF) between plasma and CSF, suggesting that CSF might provide an index of BISF composition. However, these authors noted that only 10–20% of labeled albumin injected into brain parenchyma is recovered in cisternal CSF, suggesting an alternative exit pathway (120). Given the small contributions of BISF to CSF, sampling cisternal, ventricular, or lumbar CSF gives a poor estimate of BISF composition or of BBB transport processes but may reasonably reflect substances that have been transported into CSF via choroid plexus secretion. At present, whether insulin crossing the BBB contributes substantially to sampled subarachnoid CSF insulin concentrations and just how much insulin is transported across the blood-CSF barrier remains unknown. Despite this uncertainty, CSF insulin concentrations are presented as an expedient surrogate for BISF insulin. Interestingly, the CSF-to-plasma insulin concentration ratio is inversely related to obesity (12, 66) and directly related to insulin sensitivity (57). This suggests that insulin resistance adversely affects insulin transport into CSF by whatever pathway is used. Brain interstitial fluid is a difficult compartment to sample directly without causing significant tissue damage (35, 52), hence, the routine use of CSF insulin as a proxy for insulin transported across the BBB or for BISF insulin concentration. However, caution is needed as this does not appreciate the complexity of CSF flow and likely distorts quantitative measures of insulin transport from blood into BISF.
Beyond CSF sampling, intracerebroventricular (ICV) insulin infusion is commonly used to study insulin action on feeding behavior and hepatic metabolism. Giving insulin ICV reduces food intake in multiple animal species. Dose-dependent reductions of food intake and body weight are seen consistently with ICV-administered insulin, though it is unclear whether changes in food intake fully account for weight loss (15, 38, 132). Reduced food intake is seen both in ad libitum fed and upon refeeding food-deprived rats (18). Fat intake may be more sensitive to ICV insulin than intake of carbohydrates or protein (19). Overfed and genetically obese rodents are less responsive to ICV insulin (3, 19, 23, 24, 60), yet may regain sensitivity after reversing diet-induced obesity (12). These effects likely occur via hypothalamic IR action (16, 94) and changes in hypothalamic pro-opiomelanocortin (POMC) gene expression (24). A possible, but essentially unexplored, shortcut for ICV-administered insulin into brain parenchyma involves crossing the ependymal cells that line the ventricular/brain interface. Transiting these cells would provide CSF insulin access to BISF at least in the immediate periventricular region. Just how much of a role this type of transport plays in delivering substances secreted at the choroid plexus is unknown (see also section Brain Areas With Incomplete Blood-Brain Barrier).
Beyond affecting feeding behavior over days or weeks, ICV insulin may acutely affect plasma glucose concentrations and hepatic glucose production. Insulin’s effects on hepatic glucose production may be species- and dose-dependent (Table 1). In rodents, hypothalamic IRs (75), downstream signaling through phosphoinositide 3-kinase (PI3K) (94, 95), and increased vagal nerve efferent activity (103) may mediate this process. Genetic reduction of arcuate nucleus IRs (>90% loss) increased hepatic glucose production (75) and selective reintroduction of IRs into Agouti-related protein (AgRP) or POMC neurons decreased and increased hepatic glucose production, respectively (75). Detailed metabolic studies in dogs using lower (yet still supraphysiologic) ICV insulin concentrations (Table 1) found minimal (107) or no effect on endogenous glucose production, whole body glucose disappearance or net hepatic glucose output (106, 108). In these studies, insulin increased hepatic mRNA concentrations of phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), and pyruvate carboxylase (PC) significantly but there were no significant changes in protein level for these gluconeogenic enzymes (107). In contrast, infusing insulin selectively into the brain vascular circulation to achieve physiologic arterial (but much lower ICV) insulin concentrations suppressed hepatic glucose output by promoting liver glycogen synthesis, suggesting that arterial insulin may act at sites not reached by ICV insulin delivery (107). For self-evident methodologic and ethical reasons, there are no data available on insulin's acute CNS effects mediating metabolic function in humans comparable to that in dogs and rodents. Collectively, ICV insulin studies suggest that insulin can play a role in feeding behavior and metabolism, yet these reports should be interpreted with caution.
Table 1.
Studies infusing insulin into CSF of mammals
| Author | Species | Insulin Dosage, mU/day | Total Dose, µU·ml−1·day−1 | CSF Insulin, nM |
|---|---|---|---|---|
| Woods et al. (132) | Baboon | 0.001–0.1 | 15–1,500* | 0.1–10.8 |
| Ramnanan et al. (107) | Dog | 69.5† | 0.5 | |
| Obici et al. (95) | Rat | 0.03 | 148 | 1.1 |
| Pocai et al. (103) | Rat | 0.03 | 148 | 1.1 |
| Foster et al. (38) | Sheep | 255† | 1.8 | |
| Ikeda et al. (60) | Rat | 2 | 9,836 | 71 |
| Clegg et al. (23, 24) | Rat | 4–8 | 39,344 | 141–282 |
| Brief and Davis (15) | Rat | 5–10 | 24,590 | 177–353 |
| Chavez et al. (18, 19) | Rat | 6 | 29,508 | 212 |
| Begg et al. (11, 12)‡ | Rat | 8 | 39,344 | 282 |
| Arase et al. (3), Chavez et al. (19) | Rat | 10 | 49,180 | 353 |
Cerebrospinal fluid (CSF) insulin concentration is based on CSF volume of 0.203 ml (30, 91, 104) and using a conversion factor of 7.174 from µU to pM.
Reported body weight of 15 kg and estimated CSF volume at 1 ml.
Measured and reported CSF insulin concentrations from cisterna magna puncture.
Begg et al. (11) used insulin detemir and NPH insulin, which may have different clearance rates than porcine insulin or humulin.
Caveats to using ICV insulin infusion include: 1) CSF insulin rises minimally during peripheral hyperinsulinemia, 2) ICV administration bypasses the restrictive brain endothelium and likely affords insulin more direct access to the BISF compared with insulin that crosses the BBB, 3) ICV insulin reaches pharmacological CSF insulin concentrations in nearly every study reported (Table 1), and 4) insulin’s half-life in CSF is unknown and with the slow CSF circulation, extremely high insulin concentrations may persist for hours. Since the clearance rate of ICV insulin, either by local degradation or passage back through systemic circulation, is unknown, the concentration estimates in Table 1 only suggest an order of magnitude effect. These pharmacologic concentrations may initiate IR-dependent and -independent effects. Despite these significant limitations, ICV insulin administration continues to be widely employed.
Clinically, giving ICV insulin is not a feasible approach to test insulin’s effects on the human brain. Instead, investigators have examined intranasal insulin’s effects on metabolism and cognition. Typically, a 40–80 IU insulin dose is given; however, it is difficult to estimate how much of this enters CSF (77). Intranasal insulin reportedly increased peripheral insulin sensitivity in fasted adults (56) and modestly reduced endogenous glucose production during a hyperinsulinemic-euglycemic clamp (32, 58). However, these effects may occur hours after giving nasal insulin (32) and have not been uniformly observed (41). Obese and type 2 diabetic subjects may be resistant to intranasal insulin’s effects on liver glucose production and lipid content (41, 58). Effects on feeding behavior and metabolism may occur by insulin-induced increases in hypothalamic and striatal activity (56, 58). In chronic studies, intranasal insulin administration may slow cognitive decline (26, 27, 93). Exactly how or whether intranasal insulin reaches higher cortical areas directly remains unknown. Intranasal insulin may act at the olfactory bulb neuron IRs (70) or enter the CSF circulation through transcellular or paracellular pathways in the olfactory epithelium to reach subarachnoid CSF beyond the cribriform plate (77). Additionally, there is debate whether nasal insulin spills into blood to exert peripheral and central effects (32, 58). Given its clinical potential, there is need to explore the physiology of intranasal insulin transport.
In summary, although it is likely that insulin can cross the choroid plexus into the CSF, this route is unlikely physiologically relevant for acute brain responses to increases in circulating insulin. Therefore, more focus is needed on the contribution of insulin transport across the BBB to physiological changes in brain insulin concentrations.
INSULIN MOVEMENT ACROSS THE BLOOD-BRAIN BARRIER
The brain endothelial cell (BEC) is the cellular component most responsible for the highly restrictive properties of the BBB. However, other cells and perivascular structures provide supplemental barrier function, including the basement membrane, pericytes, and, at the arteriole level, smooth muscle cells (Fig. 1B). Vascular elements form the medial boundary of the VRS. The outer boundary is formed by astrocyte endfeet, which also may restrict entry of large molecules found in the CSF into BISF. The aggregate of these vascular and astrocyte elements form the neurovascular unit, which can regulate BBB transport and function. As discussed above, substances crossing the vascular endothelium will mix with CSF in the paravascular VRS. Components of this admixture may cross the astrocytic endfeet and ultimately reach BISF. For insulin, BBB transport could augment any insulin entering the VRS via the CSF circulation, and may provide the major pathway for insulin transfer to BISF. In humans, given the slow rate of CSF secretion (500–600 ml/day) (112) and the high blood flow through brain tissue (~700 ml/min) (67), crossing the endothelium potentially provides a much quicker way for blood-borne substances to reach the VRS and eventually BISF.
Components of the Blood-Brain Barrier and Neurovascular Unit
Dominating the restrictive properties of the BBB is the BEC, which prevents free solute movement between blood and BISF. As shown in Fig. 1B, at the capillary level, the endothelium has a basement membrane and is partly covered by pericytes and the basement membrane mostly fills the perivascular space, which is 6–8 µm in diameter (82). Astrocytic endfeet form the outer boundary of the VRS throughout the brain microvasculature and at the capillary level they abut the basement membrane. These endfeet form a near continuous sheath around the vessels (81) and are the final obstacle before entering BISF.
The endothelium is the first barrier faced by circulating substances and it is more restrictive in brain than in the peripheral vasculature (28, 29). A standard method for quantifying this is the transendothelial electrical resistance (TEER), a measure of endothelial electrolyte barrier permeability. In muscle, TEER values approximate 30 Ω cm2 (96), while brain endothelium TEER is >1,800 Ω cm2 (28). The restrictive properties of the BEC layer result principally from the presence of tight intercellular junctional complexes and efflux pumps. Paracellular movement between BECs is prevented by tight and adherens junctions as well as angulin and tricellulin at tricellular junctions (63). Beyond their critical function to form a permeability barrier, these junctional structures likely have roles in cell-to-cell communication, trafficking membrane proteins and/or molecules across the BEC, and intracellular signaling (123). In addition, efflux pumps are highly abundant on the luminal and abluminal aspects of the BEC and serve to limit transendothelial movement of multiple solutes (78). These efflux pumps are members of the ATP-binding cassette (ABC) family that hydrolyze ATP to power movement of chemicals out of the BEC back to plasma. These transporters are highly expressed in BECs and exhibit some redundancy regarding substrate specificity, and their expression increases in response to environmental factors (84). While offering a clear brain protective role, efflux pumps can also be an obstacle to delivering therapeutics and can also impede use of pharmacologic agents to probe BEC function. Thus, investigations into BEC insulin transport must consider this property of this unique endothelium.
Early autoradiographic studies demonstrated that insulin binds to the brain endothelium (127). In addition, isolated human and bovine brain microvessels can endocytose radiolabeled insulin (39, 99). Whether this insulin was transcytosed or simply degraded following endocytosis was not determined as microvessel isolations may induce capillary damage and promote insulin degradation (39). In vivo, intravenous infusion of a specific IR blocker (S-961) inhibits radiolabeled insulin uptake by brain tissue, suggesting receptor-mediated transport (83), consistent with earlier reports indicating saturable insulin uptake by brain (9). These studies did not investigate the cellular pathways involved in BEC insulin transport or its regulation. More recently, we observed that polarized, isolated BECs transport insulin bidirectionally and this movement was mediated by IR and lipid rafts, but it did not involve downstream insulin signaling through PI3K or MEK (48). The BEC IR also mediates insulin transport in vivo, as endothelial-specific knockout of IR in mice decreased IR phosphorylation in hypothalamus, hippocampus, and prefrontal cortex in response to intravenous insulin (71). Thus, the IR appears critical for insulin transport across the BEC as pharmacologic blockade and genetic ablation can reduce insulin transport and IR activation.
Pericytes cover approximately 30% of the brain capillary endothelium (81). Given their limited coverage of the endothelium, they should not per se pose a barrier to transport into the brain. However, within the neurovascular unit, pericytes assist with BBB integrity, support angiogenesis, and regulate blood flow (50, 101, 119). Pericytes express markers similar to smooth muscle cells (e.g., PDGF-β and α-smooth muscle actin), which are absent from capillaries, and these cells may respond to neuron or astrocyte stimulation to alter capillary perfusion. How this occurs remains an area of active research. Pericyte morphology differs throughout the vasculature (54) and it has been proposed that they be separated into subtypes, depending on their location in the vasculature (i.e., artery, capillary, vein) (6). The differential morphology may reflect diverse roles in maintaining BBB function and/or transport. Improved pericyte phenotyping may allow a refinement of our understanding of their role on the BBB and within the neurovascular unit. Pericyte loss is implicated for breakdown of BBB function and defects in neurovascular coupling and blood flow (69). Retinal pericyte death is an early manifestation of microvascular damage during diabetes (119) and whether similar pericyte loss occurs at the BBB in diabetes is unknown. Retinal pericytes bind radiolabeled insulin (68), but it is unknown whether IRs on brain pericytes regulate insulin transport or other pericyte functions.
Astrocyte endfeet form the outermost boundary for blood-born substances to enter BISF. These endfeet encapsulate the endothelium, pericytes, and basement membrane. They form a near continuous covering of the microvasculature with small (~20 nm) clefts between adjacent endfeet (81) and may impede the movement of large molecules into brain parenchyma. Astrocyte density varies throughout the brain vasculature, with arteries and arterioles having more astrocytic processes per unit of length than capillaries or veins (82). Gap junctions between astrocytes may allow signal propagation throughout the astrocyte sheath of the vasculature (13, 47). Studies of “glymphatic” paravascular CSF flow have demonstrated that larger tracers (either fluorescent- or gadolinium-labeled) pass the astrocyte endfeet more slowly than smaller molecules, supporting the idea that the astrocytic endfeet can regulate solute movement between VRS and BISF (61, 62). Astrocytes express IRs and astrocyte function may be affected by HFD feeding and insulin resistance (37, 43), yet it is unknown whether this affects insulin movement from VRS to BISF.
In summary, although it is likely that some insulin is transported across the choroid plexus and into the CSF, the process is slow and may not be physiologically relevant for acute brain responses to increases in circulating insulin concentrations. Conversely, it appears that transport across the BBB contributes the major flux of insulin into the brain (48, 71, 83) and deserves more extensive study.
Neurovascular Coupling Facilitates Transport Across the Blood-Brain Barrier
Neurovascular coupling is the interplay of neurons with astrocytes, pericytes, and endothelial cells to coordinate physiological processes related to the brain vasculature. It may affect vasodilation, vasoconstriction, and movement of substances from blood, across the BBB, and into the BISF. The current model for neurovascular coupling proposes a feed-forward mechanism, whereby local neuronal activation regulates cerebral blood flow, which alters oxygen and glucose delivery to the area (5). This coupling is the basis of functional magnetic resonance (fMRI) imaging. Recent reports suggest that neurovascular coupling may also regulate movement of other nutrients and hormones into brain. Although the majority of the literature has focused on the penetrating arteries and arterioles in the brain, microvascular vessels may also have a role in neurovascular coupling (82).
Interactions between neurons and astrocytes and subsequent effects on the endothelium may regulate BBB transport. Glutamate, a key regulator of neurovascular coupling, is secreted from presynaptic neurons (59). Acting on N-methyl-d-aspartate receptors on neurons, it can increase neuronal nitric oxide synthase activity and release nitric oxide, which dilates smooth muscle cells and acts on astrocytes to influence neurovascular coupling. Direct glutamate action on astrocytes can release endoplasmic reticulum calcium stores and activate plasma membrane Na+/Ca+ exchanger or TRPA1 channels in endfeet processes (10). Cytosolic calcium increases can induce the secretion of factors termed “gliotransmitters” that act on neighboring neurons and BBB cells (10, 53). Gliotransmitters may include ATP, glutamate (14, 51, 102), arachidonic acid (89), and other factors (10, 25, 49, 100). Arachidonic acid can provoke smooth muscle contraction and result in vasoconstriction (89). Alternatively, within the astrocyte, arachidonic acid may be converted to prostaglandin E2 (PGE2) or epoxyeicosatrienoic acids, which can relax smooth muscle cells (5).
Whereas smooth muscle cells mediate neurovascular coupling at the level of arteries and arterioles, they are absent at the capillaries. Here, pericytes and astrocytes may coordinate neuronal activity with vasodilation or vasoconstriction. Brain capillaries are closer than arterioles to neurons (79). Given the pericyte’s proximity to the endothelium, astrocytic endfeet, and neurons, as well as its contractile ability and signaling capabilities, it could have a role in neurovascular coupling (51, 119). Glutamate can dilate capillaries through its pericyte action, causing retrograde dilation in the penetrating arterioles (50). Few studies have investigated neurovascular coupling at the capillary level and many questions remain regarding the specific functions of neurons, astrocytes, and pericytes during this process.
Although neurovascular coupling is generally considered as a mechanism to increase oxygen and nutrient availability to active brain regions through local vasodilation, it may also facilitate hormone and peptide movement across the BBB. This could occur either through increased delivery by vasodilation, specific effects on BEC transport processes, or a combination of both. Neuronal activity and subsequent increased neurovascular coupling increase transport of IGF-I across the BEC (92). Glutamate stimulation of astrocytes in a transwell endothelial-astrocyte coculture system increased insulin transcytosis across BEC monolayer, suggesting there may be a role for neurovascular coupling to facilitate insulin transport into the brain independent of blood flow (48). Regional increases of cerebral blood flow in humans (measured using fMRI imaging) are observed when plasma insulin rises in response to glucose ingestion (98), highlighting the potential for neurovascular coupling to increase insulin uptake by the brain by increased delivery as well.
Interactions between BBB cell types are complex and it is clear that neurons, astrocytes, pericytes, smooth muscle cells, and endothelial cells collaboratively maintain barrier function and neurovascular coupling capabilities. It is possible that the mechanisms regulating neurovascular coupling differ along the vasculature tree, throughout areas of the brain, and in response to various neuronal stimuli. Indeed, a recent, elegant single transcriptomic study identified genotypic “zonation” of BECs along the brain’s vasculature from arteries through capillaries to veins, implying significant regional functional differentiation of the BECs (130).
Brain Areas With Incomplete Blood-Brain Barrier
The highly restrictive BBB protects nearly the entire brain but several small areas are characterized by having a more permissive, fenestrated endothelium. This can allow substances to move more freely from blood into the nearby BISF (34). These circumventricular organs (CVO) line the third and fourth ventricles and include the neurohypophysis, vascular organ of the lamina terminalis, subfornical organ, subcommissural organ, pineal gland, median eminence, and area postrema (34). Blood-borne radiolabeled insulin binds to the median eminence, area postrema, and other CVOs (125, 126, 128). It also binds to vessels in the arcuate nucleus (21, 22) of the hypothalamus where insulin can act on AgRP and/or POMC neurons (70, 113) and has known anorectic actions. Exactly how insulin (and other hormones with anorectic or orexigenic actions) moves from blood to arcuate neurons is debated. Potential pathways include: 1) crossing this fenestrated capillary endothelium of the median eminence and entering CSF in the third ventricle before gaining access to the arcuate nucleus or 2) a proposed route through the subependymal plexus (a vasculature plexus between the third ventricle, median eminence, and arcuate nucleus) (2, 22), or a combination of these. The third and fourth ventricles are lined by tanycytes, which are specialized ependymal cells. In the third ventricle, they form a barrier between the median eminence and arcuate nucleus and may regulate transport of substances between fenestrated capillaries, cerebrospinal fluid, and the hypothalamic BISF (73, 88, 111). Subsets of tanycytes (β1 and β2) possess endocytotic machinery (caveolae and clathrin-coated pits) and may transport fluid and hormones by receptor-mediated transport (110). For example, after crossing the fenestrated endothelium of the median eminence, leptin is taken up by tanycytes in a leptin receptor-dependent process and is transported to ventricular CSF where it can then reach leptin sensitive neurons in the arcuate nucleus (7). Whether insulin could be transferred to the arcuate via a similar pathway has not to our knowledge been defined.
Evan’s blue dye injected into CSF can enter the arcuate nucleus but not the median eminence, and intravenously injected Evan’s blue dye enters the median eminence but not the arcuate nucleus (88). This suggests that the BBB is intact in the arcuate nucleus and that fenestrated capillaries are present in the median eminence alone. Recent work has highlighted the dynamic qualities of endothelial cell fenestrations within the median eminence and the arcuate nucleus in response to fasting (72). During fasting, the arcuate nucleus vasculature allows for greater diffusion of blood-borne substances, whereas in the fed state, this increased diffusion is limited to the median eminence alone (72). Other cellular architecture may also be affected by nutritional status. Tanycyte structure may change in response to nutrients and HFD may affect tanycyte proliferation (20). These studies highlight the plasticity of this system and complex regulation of the brain vasculature in the hypothalamus. Recently, increased BBB permeability in the median eminence was described in the endothelial IR knockout mouse (71). It is not known whether insulin can enter or act on CVO(s) to initiate neuronal signaling that could affect brain regions with an intact BBB. Such a system could potentially obviate the need for insulin transport across either the choroid plexus or the BBB and remains to be tackled experimentally. However, the recent findings of decreased insulin action in multiple brain regions of the VE-cadherin driven endothelial specific IR knockout mouse might argue against this possibility (71). Further investigation of insulin transport to and within the hypothalamus is clearly warranted.
INSULIN WITHIN BRAIN TISSUE
While circulating insulin may be secreted with CSF or cross the BBB to reach BISF, there are also limited studies proposing neuronal insulin production. In insects, insulin-like peptides are produced exclusively in the brain (133); however, evidence for this in higher-order organisms is scant. Early studies using radioimmunoassay methods reported insulin concentrations in CNS homogenates that exceeded those in circulating plasma, suggesting either local production or concentration of insulin from the circulation (55). Later studies reported far lower concentrations (74) and neuronal insulin production continues to be questioned (8). Recently, insulin transcripts were detected by single-cell PCR in GABAergic neurogliaform cells in the cortex (86). These investigators also provided evidence for insulin acting on nearby neurons. The possibility that insulin is synthesized and acts in discreet brain regions requires further study.
To our knowledge, microdialysis sampling methods have been only occasionally used to estimate BISF immunoreactive insulin, principally in the hypothalamus. There, estimated insulin BISF concentrations ranged from 2 to 18 pM (44, 45, 97) and were greater than that of cerebellum (45), supporting the thesis that hypothalamic vasculature is more permissive to circulating insulin entry. Fasting hypothalamic concentrations varied considerably (44, 97) and it is unclear whether macronutrients or meal anticipation affected hypothalamic levels (45, 97). There are major caveats, however, to microdialysis insulin sampling that limit its use. First, microdialysis catheter placement almost certainly damages neural tissue and blood vessels (35, 52) and BBB integrity was not verified in these studies (44–46, 97). In addition, the microdialysis membrane used in these studies (0.5 mm in diameter) is very large compared with the hypothalamus and insulin transfer across the microdialysis membrane is slow and incomplete. Thus, microdialysis methods have provided limited insight regarding BISF insulin concentrations and have not allowed quantitation of insulin transport from blood into brain tissue. The complex architecture of brain tissue with extensive fluid (blood and CSF) circulation, and variation in BBB integrity (e.g., CVOs) prevents sampling of uncontaminated BISF. Consequently, current microdialysis methods are unlikely to provide insight into insulin BBB transcytosis.
Insulin, applied directly to postmortem brain sections, activates canonical insulin signaling pathways. This effect is notably decreased in the cerebral cortex and hippocampus of patients with Alzheimer’s disease (121), suggesting brain insulin resistance. In mice, insulin resistance provoked by HFD feeding impaired insulin-stimulated PI3K signaling in the cortex and decreased dendritic spine abundance and spatial memory (4). Similarly, rats fed a combination of high-fat and high-fructose diet became insulin resistant and had impaired hippocampus-dependent spatial learning and reduced dendritic spine density (117). Mice with Alzheimer’s disease neuropathology (3xTg-AD) fed HFD exhibit increased diabetic-like pathology in the pancreas compared with wild type controls and increased brain amyloid content compared with 3xTg-AD mice fed a normal diet (129). In these mice, a single intraperitoneal dose of insulin restored memory function and reduced soluble amyloid-β in the cortex and hippocampus. These changes in insulin signaling, anatomical neuron dendrite morphology, behavior, learning, and cognition strongly support a role for diet and insulin action in brain function. Considering that insulin’s effects on cognition and memory occur in areas of the brain behind an intact BBB, the ability of intraperitoneal insulin to improve memory (129) suggests that peripheral insulin movement across the BBB may be a key pathway for understanding the relationship of insulin resistance and Alzheimer’s disease progression and exploring potential treatments. Given the relatively late-in-life onset of both type 2 diabetes and dementia, deconvoluting the contribution of one to the other will be difficult (118). However, these and other findings emphasize the potential importance of insulin action in the brain for these diseases.
INSULIN CLEARANCE FROM THE BRAIN
Insulin transported to, or made within, the brain may be degraded in CNS target cells or cleared from the BISF via the blood or CSF circulation. Insulin’s clearance from brain has not been well studied and to our knowledge, the roles of local degradation or return to the systemic insulin pool are unexplored. As stated earlier, the half-life of insulin injected into CSF or brain parenchyma has not been reported. Radiolabeled tracers or dye (e.g., Evan’s blue and horseradish peroxidase) injected into brain parenchyma drain into the VRS/perivascular space (120). Tracer clearance may depend on size (31) and proximity of injection site to CSF sampling location (e.g., cisterna magna) (120), yet does not differ between white and gray matter (120). Sampling from the cisterna magna may include 10–20% of BISF drainage (31, 120). Thus, use of cisterna magna CSF sampling includes freshly produced CSF and BISF clearance. As noted earlier, this further diminishes subarachnoid CSF’s usefulness as a proxy for BISF.
Morphologic studies indicate that tracers injected into brain parenchyma may localize to the arterial VRS/perivascular space (120) or basement membrane (17), suggesting that BISF may drain para-arterially. While care has been taken in these studies to inject minimal volumes, whether or not parenchymal injections affect tracer flow pathways is unresolved. Since the VRS parallels the venous vasculature, BISF solutes could enter the VRS by crossing astrocyte endfeet and be cleared from VRS by either crossing the BEC to enter plasma or flow through the VRS and terminate on arachnoid granulations. Thus, paravenous flow of BISF solutes affords a potential clearance pathway for solutes leaving BISF (65); however, little is known as to whether or how much tracer or solute clearance may occur along venous routes. Thus, the contributions of para-arterial, paravenous, or a combination of both processes to BISF clearance remain unknown and this knowledge gap extends to insulin. Clearance of BISF and CSF through dural lymphatics has been reviewed elsewhere (109) and it is unknown how or whether insulin is cleared from the brain through this pathway.
FUTURE DIRECTIONS AND OPEN QUESTIONS
Insulin action in the brain, resistance to insulin action, and the relation of each to normal and abnormal brain function constitute a new investigative frontier. The question of how peripheral insulin actually reaches the brain and whether processes responsible for brain insulin entry are altered in different physiologic and pathologic states requires study as part of this overarching effort. Studies have only begun to elucidate this transport (48, 83). In closing, we highlight some unresolved or previously unapproached questions relating to brain insulin entry that deserve further experimental study. For some, appropriate experimental methods exist while others necessitate new approaches.
Further resolving insulin’s transport across the BBB is of clear interest. After crossing the BEC, insulin may cross the astrocytic endfeet processes reaching BISF, have localized effects on astrocytes, or indirectly act upon neurons. Discerning insulin’s cellular location during its BBB transit may be approached using fluorescent-tagged insulin and high-resolution microscopy. Autoradiography lacks sufficient spatial resolution of radiolabeled insulin. Several fluorescent-tagged insulins are commercially available and others continue to be developed (64). The bioactivity of these compounds must be confirmed before use in physiologic studies. Combined use of fluorescent-tagged insulin and antibodies against the cell types of the BBB could perhaps clarify the location of transported insulin in brain sections. Moreover, such experiments could test whether insulin moves differently at various levels of the vasculature (e.g., arteriole versus capillary) or in different areas of the brain (e.g., the fenestrated median eminence as compared with the BBB in the cerebellum).
Interactions between the several types of BBB cells, i.e., neurovascular coupling, related to insulin’s BBB transport warrant further investigation. Exercise (90), environmental enrichment (124), and whisker stimulation (92) in rodents induce neurovascular coupling and IGF-I BBB transcytosis. Circulating IGF-I concentrations can affect neuronal IGF-I production and IGF-I receptor expression (124). Whether putative neuronal insulin and/or brain insulin receptors are regulated in response to increased circulating insulin is unknown. Functional imaging studies indicate that nutrients such as glucose increase perfusion in selected brain regions (76). On a cellular level, whether this affects BBB insulin transcytosis is unknown. In vivo imaging of neurovascular coupling in superficial brain regions may provide some insight. Vasodilation and vasoconstriction can be visualized with AlexaFluor 633, which binds to the elastin in arteries and arterioles, and changes in neuronal calcium may be revealed using Oregon Green 488 Bapta-1 acetoxymethyl ester (115). At this point, resolution at the capillary level has not been reported. However, using fluorescent-tagged compounds to label anatomical landmarks in combination with fluorescent insulin could yield valuable insights as to how circulating insulin enters the brain.
Insulin turnover within the brain remains an outstanding question. Insulin is generally degraded intracellularly in lysosomes, though there is some evidence that insulin-degrading enzyme (IDE) can degrade circulating insulin (33). Despite its name, IDE degrades numerous peptides and hormones (122). Its ability to degrade amyloid-β protein (36, 122) has made it an attractive target for Alzheimer’s disease research. Insulin-degrading enzyme is present in BECs (42, 80) and within brain tissue (85). Additionally, it may be secreted from many cell types (134), including astrocytes (116). Insulin-degrading enzyme has been detected in CSF of healthy controls and individuals with dementia (105). Whether IDE in brain tissue or CSF serves to regulate insulin degradation in the brain is unknown. Additionally, the role of this protease in both type 2 diabetes and Alzheimer’s disease progression appears complex (122).
Currently, much remains to be learned about how circulating insulin moves into the BISF. Insulin’s role in the brain is clearly important—from feeding behavior to hepatic metabolism to cognition. Testing the role of insulin in these functions requires attention to use of physiologic doses and routes of insulin administration which has not routinely been observed in past studies. Given the inadequacy of CSF as a surrogate for insulin reaching BISF, there is a strong impetus for examining insulin transport across the BBB. An understanding of the mechanisms that govern this transit and how they may be altered by diet, by insulin resistance and by diabetes will advance our understanding of insulin BBB transport and provide new avenues for clinical interventions.
GRANTS
This work was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-073059 and American Diabetes Association Grant 11-BS6 (to E. J. Barrett) and NIH NIDDK Grant F31 DK-104521 (to S. M. Gray).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.G. and E.J.B. prepared figures; S.M.G. and E.J.B. drafted manuscript; S.M.G. and E.J.B. edited and revised manuscript; S.M.G. and E.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Thorner for thoughtful critique of the manuscript.
REFERENCES
- 1.Adamo M, Raizada MK, LeRoith D. Insulin and insulin-like growth factor receptors in the nervous system. Mol Neurobiol 3: 71–100, 1989. doi: 10.1007/BF02935589. [DOI] [PubMed] [Google Scholar]
- 2.Ambach G, Palkovits M, Szentágothai J. Blood supply of the rat hypothalamus. IV. Retrochiasmatic area, median eminence, arcuate nucleus. Acta Morphol Acad Sci Hung 24: 93–119, 1976. [PubMed] [Google Scholar]
- 3.Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol Regul Integr Comp Physiol 255: R974–R981, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis 67: 79–87, 2014. doi: 10.1016/j.nbd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab 36: 451–455, 2016. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prévot V. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19: 293–301, 2014. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks WA. The source of cerebral insulin. Eur J Pharmacol 490: 5–12, 2004. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides 18: 1423–1429, 1997. doi: 10.1016/S0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 10.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci 19: 182–189, 2016. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 11.Begg DP, May AA, Mul JD, Liu M, D’Alessio DA, Seeley RJ, Woods SC. Insulin detemir is transported from blood to cerebrospinal fluid and has prolonged central anorectic action relative to NPH insulin. Diabetes 64: 2457–2466, 2015. doi: 10.2337/db14-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begg DP, Mul JD, Liu M, Reedy BM, D’Alessio DA, Seeley RJ, Woods SC. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology 154: 1047–1054, 2013. doi: 10.1210/en.2012-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett MV, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26: 610–617, 2003. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7: 613–620, 2004. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 15.Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull 12: 571–575, 1984. doi: 10.1016/0361-9230(84)90174-6. [DOI] [PubMed] [Google Scholar]
- 16.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 17.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34: 131–144, 2008. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 18.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci 109: 528–531, 1995. doi: 10.1037/0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- 19.Chavez M, Riedy CA, Van Dijk G, Woods SC. Central insulin and macronutrient intake in the rat. Am J Physiol Regul Integr Comp Physiol 271: R727–R731, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Chowen JA, Argente J, Horvath TL. Uncovering novel roles of nonneuronal cells in body weight homeostasis and obesity. Endocrinology 154: 3001–3007, 2013. doi: 10.1210/en.2013-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciofi P. The arcuate nucleus as a circumventricular organ in the mouse. Neurosci Lett 487: 187–190, 2011. doi: 10.1016/j.neulet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prévot V, Levine JE. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology 150: 5509–5519, 2009. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 24.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav 103: 10–16, 2011. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 26.Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. J Alzheimers Dis 57: 1325–1334, 2017. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69: 29–38, 2012. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res 241: 49–55, 1982. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- 29.Crone C, Thompson AM. Comparative studies of capillary permeability in brain and muscle. Acta Physiol Scand 87: 252–260, 1973. doi: 10.1111/j.1748-1716.1973.tb05388.x. [DOI] [PubMed] [Google Scholar]
- 30.Cserr H. Potassium exchange between cerebrospinal fluid, plasma, and brain. Am J Physiol 209: 1219–1226, 1965. [DOI] [PubMed] [Google Scholar]
- 31.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol Renal Physiol 240: F319–F328, 1981. [DOI] [PubMed] [Google Scholar]
- 32.Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 64: 766–774, 2015. doi: 10.2337/db14-0685. [DOI] [PubMed] [Google Scholar]
- 33.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 19: 608–624, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Brain Res Rev 56: 119–147, 2007. doi: 10.1016/j.brainresrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Dykstra KH, Hsiao JK, Morrison PF, Bungay PM, Mefford IN, Scully MM, Dedrick RL. Quantitative examination of tissue concentration profiles associated with microdialysis. J Neurochem 58: 931–940, 1992. doi: 10.1111/j.1471-4159.1992.tb09346.x. [DOI] [PubMed] [Google Scholar]
- 36.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA 100: 4162–4167, 2003. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez AM, Hernandez-Garzón E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T, Santi A, Trueba-Saiz A, García-Guerra L, Pose-Utrilla J, Fielitz J, Olson EN, Fernandez de la Rosa R, Garcia Garcia L, Pozo MA, Iglesias T, Araque A, Soya H, Perea G, Martin ED, Torres Aleman I. Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes 66: 64–74, 2017. doi: 10.2337/db16-0861. [DOI] [PubMed] [Google Scholar]
- 38.Foster LA, Ames NK, Emery RS. Food intake and serum insulin responses to intraventricular infusions of insulin and IGF-I. Physiol Behav 50: 745–749, 1991. doi: 10.1016/0031-9384(91)90012-D. [DOI] [PubMed] [Google Scholar]
- 39.Frank HJ, Pardridge WM, Morris WL, Rosenfeld RG, Choi TB. Binding and internalization of insulin and insulin-like growth factors by isolated brain microvessels. Diabetes 35: 654–661, 1986. doi: 10.2337/diab.35.6.654. [DOI] [PubMed] [Google Scholar]
- 40.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 19: 3278–3288, 1999. doi: 10.1128/MCB.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gancheva S, Koliaki C, Bierwagen A, Nowotny P, Heni M, Fritsche A, Häring HU, Szendroedi J, Roden M. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes 64: 1966–1975, 2015. doi: 10.2337/db14-0892. [DOI] [PubMed] [Google Scholar]
- 42.Gao W, Eisenhauer PB, Conn K, Lynch JA, Wells JM, Ullman MD, McKee A, Thatte HS, Fine RE. Insulin degrading enzyme is expressed in the human cerebrovascular endothelium and in cultured human cerebrovascular endothelial cells. Neurosci Lett 371: 6–11, 2004. doi: 10.1016/j.neulet.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 43.García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166: 867–880, 2016. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerozissis K, Orosco M, Rouch C, Nicolaidis S. Basal and hyperinsulinemia-induced immunoreactive hypothalamic insulin changes in lean and genetically obese Zucker rats revealed by microdialysis. Brain Res 611: 258–263, 1993. doi: 10.1016/0006-8993(93)90511-K. [DOI] [PubMed] [Google Scholar]
- 45.Gerozissis K, Rouch C, Nicolaïdis S, Orosco M. Brain insulin response to feeding in the rat is both macronutrient and area specific. Physiol Behav 65: 271–275, 1998. doi: 10.1016/S0031-9384(98)00158-9. [DOI] [PubMed] [Google Scholar]
- 46.Gerozissis K, Orosco M, Rouch C, Nicolaidis S. Insulin responses to a fat meal in hypothalamic microdialysates and plasma. Physiol Behav 62: 767–772, 1997. doi: 10.1016/S0031-9384(97)00195-9. [DOI] [PubMed] [Google Scholar]
- 47.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11: 87–99, 2010. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 48.Gray SM, Aylor KW, Barrett EJ. Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia 60: 1512–1521, 2017. doi: 10.1007/s00125-017-4285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grima G, Benz B, Do KQ. Glutamate-induced release of the nitric oxide precursor, arginine, from glial cells. Eur J Neurosci 9: 2248–2258, 1997. doi: 10.1111/j.1460-9568.1997.tb01643.x. [DOI] [PubMed] [Google Scholar]
- 50.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508: 55–60, 2014. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11: 227–238, 2010. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 52.Hammarlund-Udenaes M. Microdialysis as an important technique in systems pharmacology—a historical and methodological review. AAPS J 19: 1294–1303, 2017. doi: 10.1208/s12248-017-0108-2. [DOI] [PubMed] [Google Scholar]
- 53.Harada K, Kamiya T, Tsuboi T. Gliotransmitter release from astrocytes: functional, developmental and pathological implications in the brain. Front Neurosci 9: 499, 2016. doi: 10.3389/fnins.2015.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartmann DA, Underly RG, Grant RI, Watson AN, Lindner V, Shih AY. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2: 041402, 2015. doi: 10.1117/1.NPh.2.4.041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci USA 75: 5737–5741, 1978. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heni M, Kullmann S, Ketterer C, Guthoff M, Linder K, Wagner R, Stingl KT, Veit R, Staiger H, Häring HU, Preissl H, Fritsche A. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 55: 1773–1782, 2012. doi: 10.1007/s00125-012-2528-y. [DOI] [PubMed] [Google Scholar]
- 57.Heni M, Schopfer P, Peter A, Sartorius T, Fritsche A, Synofzik M, Haring HU, Maetzler W, Hennige AM. Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol 51: 679–681, 2014. doi: 10.1007/s00592-013-0546-y. [DOI] [PubMed] [Google Scholar]
- 58.Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, Stefan N, Preissl H, Häring HU, Fritsche A. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66: 1797–1806, 2017. doi: 10.2337/db16-1380. [DOI] [PubMed] [Google Scholar]
- 59.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D Jr, Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite 7: 381–386, 1986. doi: 10.1016/S0195-6663(86)80006-X. [DOI] [PubMed] [Google Scholar]
- 61.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123: 1299–1309, 2013. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwamoto N, Higashi T, Furuse M. Localization of angulin-1/LSR and tricellulin at tricellular contacts of brain and retinal endothelial cells in vivo. Cell Struct Funct 39: 1–8, 2014. doi: 10.1247/csf.13015. [DOI] [PubMed] [Google Scholar]
- 64.Jacob D, Joan Taylor M, Tomlins P, Sahota TS. Synthesis and identification of FITC-insulin conjugates produced using human insulin and insulin analogues for biomedical applications. J Fluoresc 26: 617–629, 2016. doi: 10.1007/s10895-015-1748-1. [DOI] [PubMed] [Google Scholar]
- 65.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res 40: 2583–2599, 2015. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, Fehm HL, Hallschmid M. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia 49: 2790–2792, 2006. doi: 10.1007/s00125-006-0409-y. [DOI] [PubMed] [Google Scholar]
- 67.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 27: 476–483, 1948. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King GL, Buzney SM, Kahn CR, Hetu N, Buchwald S, Macdonald SG, Rand LI. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. J Clin Invest 71: 974–979, 1983. doi: 10.1172/JCI110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadžić S, Zlokovic BV. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 20: 406–416, 2017. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63: 2232–2243, 2014. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, Rask-Madsen C, Kahn CR. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proc Natl Acad Sci USA 114: E8478–E8487, 2017. doi: 10.1073/pnas.1710625114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, Bouret SG, Prevot V, Dehouck B. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 17: 607–617, 2013. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 521: 3389–3405, 2013. doi: 10.1002/cne.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le Roith D, Hendricks SA, Lesniak MA, Rishi S, Becker KL, Havrankova J, Rosenzweig JL, Brownstein MJ, Roth J. Insulin in brain and other extrapancreatic tissues of vertebrates and nonvertebrates. Adv Metab Disord 10: 303–340, 1983. doi: 10.1016/B978-0-12-027310-2.50017-7. [DOI] [PubMed] [Google Scholar]
- 75.Lin HV, Plum L, Ono H, Gutiérrez-Juárez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59: 337–346, 2010. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Gao JH, Liu HL, Fox PT. The temporal response of the brain after eating revealed by functional MRI. Nature 405: 1058–1062, 2000. doi: 10.1038/35016590. [DOI] [PubMed] [Google Scholar]
- 77.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 64: 614–628, 2012. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Löscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 6: 591–602, 2005. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 79.Lovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience 92: 47–60, 1999. doi: 10.1016/S0306-4522(98)00737-4. [DOI] [PubMed] [Google Scholar]
- 80.Lynch JA, George AM, Eisenhauer PB, Conn K, Gao W, Carreras I, Wells JM, McKee A, Ullman MD, Fine RE. Insulin degrading enzyme is localized predominantly at the cell surface of polarized and unpolarized human cerebrovascular endothelial cell cultures. J Neurosci Res 83: 1262–1270, 2006. doi: 10.1002/jnr.20809. [DOI] [PubMed] [Google Scholar]
- 81.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58: 1094–1103, 2010. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 82.McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab 31: 795–806, 2011. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meijer RI, Gray SM, Aylor KW, Barrett EJ. Pathways for insulin access to the brain: the role of the microvascular endothelial cell. Am J Physiol Heart Circ Physiol 311: H1132–H1138, 2016. doi: 10.1152/ajpheart.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev 60: 196–209, 2008. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miners JS, Kehoe PG, Love S. Immunocapture-based fluorometric assay for the measurement of insulin-degrading enzyme activity in brain tissue homogenates. J Neurosci Methods 169: 177–181, 2008. doi: 10.1016/j.jneumeth.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Molnár G, Faragó N, Kocsis AK, Rózsa M, Lovas S, Boldog E, Báldi R, Csajbók É, Gardi J, Puskás LG, Tamás G. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci 34: 1133–1137, 2014. doi: 10.1523/JNEUROSCI.4082-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J 9: 2409–2413, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol 518: 943–962, 2010. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431: 195–199, 2004. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 90.Munive V, Santi A, Torres-Aleman I. A concerted action of estradiol and insulin like growth factor I underlies sex differences in mood regulation by exercise. Sci Rep 6: 25969, 2016. doi: 10.1038/srep25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, Spratt NJ, McLeod DD. Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 11: 12, 2014. doi: 10.1186/2045-8118-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JMG, Leroy F, Soya H, Nuñez A, Torres-Aleman I. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 67: 834–846, 2010. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 93.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 37: 751–759, 2014. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 95.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 96.Olesen SP, Crone C. Electrical resistance of muscle capillary endothelium. Biophys J 42: 31–41, 1983. doi: 10.1016/S0006-3495(83)84366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orosco M, Gerozissis K, Rouch C, Nicolaïdis S. Feeding-related immunoreactive insulin changes in the PVN-VMH revealed by microdialysis. Brain Res 671: 149–158, 1995. doi: 10.1016/0006-8993(94)01347-K. [DOI] [PubMed] [Google Scholar]
- 98.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 309: 63–70, 2013. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem 44: 1771–1778, 1985. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 100.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 101.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317: 1083–1086, 2007. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 103.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic KATP channels control hepatic glucose production. Nature 434: 1026–1031, 2005. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 104.Preston JE. Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech 52: 31–37, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 105.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem 273: 32730–32738, 1998. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 106.Ramnanan CJ, Edgerton DS, Cherrington AD. Evidence against a physiologic role for acute changes in CNS insulin action in the rapid regulation of hepatic glucose production. Cell Metab 15: 656–664, 2012. doi: 10.1016/j.cmet.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramnanan CJ, Saraswathi V, Smith MS, Donahue EP, Farmer B, Farmer TD, Neal D, Williams PE, Lautz M, Mari A, Cherrington AD, Edgerton DS. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest 121: 3713–3723, 2011. doi: 10.1172/JCI45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramnanan CJ, Kraft G, Smith MS, Farmer B, Neal D, Williams PE, Lautz M, Farmer T, Donahue EP, Cherrington AD, Edgerton DS. Interaction between the central and peripheral effects of insulin in controlling hepatic glucose metabolism in the conscious dog. Diabetes 62: 74–84, 2013. doi: 10.2337/db12-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raper D, Louveau A, Kipnis J. How Do Meningeal Lymphatic Vessels Drain the CNS? Trends Neurosci 39: 581–586, 2016. doi: 10.1016/j.tins.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodríguez EM, Blázquez JL, Pastor FE, Peláez B, Peña P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247: 89–164, 2005. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 111.Rodríguez EM, Blázquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31: 757–776, 2010. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg 25: 430–436, 1966. doi: 10.3171/jns.1966.25.4.0430. [DOI] [PubMed] [Google Scholar]
- 113.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 114.Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ Jr, Fisher LD, Sipols AJ, Woods SC, Steil GM, Porte D Jr. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest 88: 1272–1281, 1991. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen Z, Lu Z, Chhatbar PY, O'herron P, Kara P. An artery-specific fluorescent dye for studying neurovascular coupling. Nat Methods 9: 273–276, 2012. doi: 10.1038/nmeth.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Son SM, Kang S, Choi H, Mook-Jung I. Statins induce insulin-degrading enzyme secretion from astrocytes via an autophagy-based unconventional secretory pathway. Mol Neurodegener 10: 56, 2015. doi: 10.1186/s13024-015-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18: 1085–1088, 2008. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sutherland GT, Lim J, Srikanth V, Bruce DG. Epidemiological approaches to understanding the link between type 2 diabetes and dementia. J Alzheimers Dis 59: 393–403, 2017. doi: 10.3233/JAD-161194. [DOI] [PubMed] [Google Scholar]
- 119.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19: 771–783, 2016. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Szentistványi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol Renal Physiol 246: F835–F844, 1984. [DOI] [PubMed] [Google Scholar]
- 121.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122: 1316–1338, 2012. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang WJ. Targeting insulin-degrading enzyme to treat type 2 diabetes mellitus. Trends Endocrinol Metab 27: 24–34, 2016. doi: 10.1016/j.tem.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol 209: 493–506, 2015. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trueba-Saiz A, Fernandez AM, Nishijima T, Mecha M, Santi A, Munive V, Aleman IT. Circulating insulin-like growth factor I regulates its receptor in the brain of male mice. Endocrinology 158: 349–355, 2017. doi: 10.1210/en.2016-1468. [DOI] [PubMed] [Google Scholar]
- 125.van Houten M, Posner BI. Specific binding and internalization of blood-borne [125I]-iodoinsulin by neurons of the rat area postrema. Endocrinology 109: 853–859, 1981. doi: 10.1210/endo-109-3-853. [DOI] [PubMed] [Google Scholar]
- 126.van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin-binding sites in the rat brain: in vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology 105: 666–673, 1979. doi: 10.1210/endo-105-3-666. [DOI] [PubMed] [Google Scholar]
- 127.van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature 282: 623–625, 1979. doi: 10.1038/282623a0. [DOI] [PubMed] [Google Scholar]
- 128.van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin binding sites localized to nerve terminals in rat median eminence and arcuate nucleus. Science 207: 1081–1083, 1980. doi: 10.1126/science.6986652. [DOI] [PubMed] [Google Scholar]
- 129.Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrançois D, Virgili J, Planel E, Giguere Y, Marette A, Calon F. Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes 63: 4291–4301, 2014. doi: 10.2337/db14-0375. [DOI] [PubMed] [Google Scholar]
- 130.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554: 475–480, 2018. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 131.Wallum B, Taborsky G Jr, Porte D Jr, Figlewicz D, Jacobson L, Beard J, Ward W, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 64: 190–194, 1987. [DOI] [PubMed] [Google Scholar]
- 132.Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282: 503–505, 1979. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 133.Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol 51: 1–24, 2006. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 134.Zhao J, Li L, Leissring MA. Insulin-degrading enzyme is exported via an unconventional protein secretion pathway. Mol Neurodegener 4: 4, 2009. doi: 10.1186/1750-1326-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]