Abstract
G protein-coupled receptor signaling mechanisms are implicated in many aspects of cardiovascular control, and dysfunction of such signaling mechanisms is commonly associated with disease states. Investigators have identified a large number of regulator of G protein signaling (RGS) proteins that variously contribute to the modulation of intracellular second-messenger signaling kinetics. These many RGS proteins each interact with a specific set of second-messenger cascades and receptor types and exhibit tissue-specific expression patterns. Increasing evidence supports the contribution of RGS proteins, or their loss, in the pathogenesis of cardiovascular dysfunctions. This review summarizes the current understanding of the functional contributions of RGS proteins, particularly within the B/R4 family, in cardiovascular disorders of pregnancy including gestational hypertension, uterine artery dysfunction, and preeclampsia.
Keywords: B/R4 family, pregnancy, regulator of G protein signaling, RGS2, RGS5
INTRODUCTION
Pregnancy induces profound physiological changes in nearly every organ system (30, 33). In particular, the cardiovascular system undergoes significant adaptations. Most of the changes, such as increases in heart mass (ventricular wall mass), occur within the first trimester. Myocardial contractility and cardiac compliance, as well as heart rate and stroke volume all increase significantly. Overall, these changes increase cardiac output by 30–50% and preferentially increase uterine blood flow by 10-fold compared with prepregnancy. Total blood volume increases similarly by 40–50% during pregnancy (33). In early to midgestation of normal pregnancies, vascular resistance decreases, accompanied by blood pressure reductions (75) in response to nitric oxide and prostaglandins (29, 167). Failure to reduce blood pressure in midgestation carries increased risk for stillbirth, proteinuria, hypertension, intrauterine growth restriction, and preeclampsia (PreE) (132). Thus, it follows that mechanisms that regulate vascular function in early and midgestation may represent causal mechanisms for, and potential therapeutic targets to prevent, such complications of pregnancy.
Women who develop complications during pregnancy, such as hypertension, often receive suboptimal treatment because of the acute changes in pharmacokinetics and pharmacodynamics of drugs during pregnancy (30, 33). Cardiovascular adaptations and organ-specific changes that influence pharmacokinetics and pharmacodynamics such as increased cardiac output during pregnancy result in a 50% increase in renal plasma flow, glomerular filtration rate, and creatinine clearance. Increases in maternal plasma volume cause a progressive decrease in plasma albumin, which significantly alters binding, volume, and distribution of drugs administered during pregnancy (42, 50). In addition, expression of drug-metabolizing enzymes such as cytochrome P450 are increased during pregnancy, and overall metabolism of drugs in the liver is increased (30, 42, 50).
Hypertension during pregnancy is associated with perinatal death, placental abruption, indicated preterm birth, small fetuses, and miscarriage. Treatment with antihypertensive drugs can improve these risks and decrease hypertensive morbidities like stroke. However, in more severe disorders of pregnancy that include hypertension, such as PreE, treatment with antihypertensive drugs does not stop progression of the disorder (1, 106). PreE affects 5–7% of all pregnancies and requires more acute treatments to avoid maternal and fetal death as well as preventing intracerebral hemorrhage, placental abruption, or intrauterine growth restriction (30). However, current treatments for disorders such as PreE do not stop the progression of the disorder. Furthermore, preventative strategies for PreE are modest.
Daily low-dose aspirin (81 mg per day) has been endorsed as a potential preventative intervention for women who are at increased risk of developing PreE. The risk reduction for development of PreE at this dose, however, is only ~10–15% (8, 112). Recently, the ASPRE trial (The Combined Multimarker Screening and Randomized Patient Treatment with Aspirin for Evidence-Based Preeclampsia Prevention) has further examined higher-dose aspirin as a preventative for PreE. The study identified women who were at high risk for developing preterm PreE by measuring uterine artery function via Doppler ultrasound. Women identified at high risk for PreE in the treatment group received 150 mg aspirin per day, and this treatment resulted in significantly reduced risk of developing PreE (149). Identifying women at highest risk of PreE by uterine artery Doppler measurements remains difficult, however, and is not feasible for many or most clinical settings. Therefore, continued identification of additional novel risk factors and therapeutic targets is still desperately needed for disorders such as PreE.
Increasing evidence implicates arginine vasopressin (AVP) (158, 160, 205), endothelin-1 (ET-1) (43, 186), and angiotensin II (ANG) (43, 78, 96) in the pathogenesis of pregnancy-associated cardiovascular disorders such as PreE. The major receptor subtypes that transduce signaling in response to these vascular hormones, including the V1A (2, 165, 171, 182), V2 (145), ETA/ETB (91, 111, 184), and AT1 receptors (74, 173), respectively, activate G proteins and their second-messenger cascades in target tissues. Downstream mediators or modulators of G protein signaling, such as the regulator of G protein signaling (RGS) family (Fig. 1), may represent a common mechanism contributing to cardiovascular disorders of pregnancy and may offer new targets for more effective treatment of pregnancy-associated cardiovascular disorders. The purpose of the current review is to summarize the current understanding of the status and contribution of various RGS family members in cardiovascular function during pregnancy. We propose the general hypothesis that dysregulation or dysfunction of RGS family members may mechanistically contribute to the pathogenesis of cardiovascular disorders of pregnancy, and thus RGS family members may represent novel screening or therapeutic targets for such disorders.
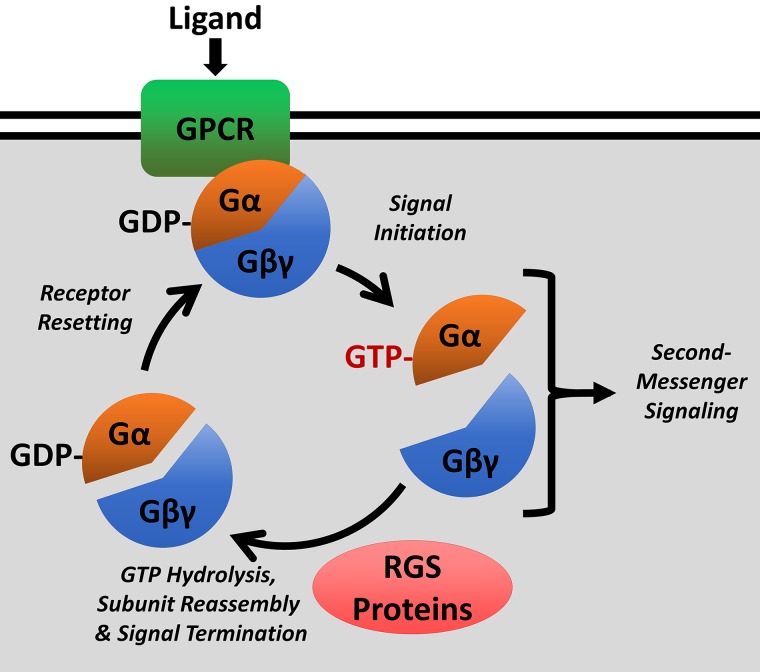
Fig. 1.
Canonical function of regulator of G protein signaling (RGS) proteins. RGS proteins function to accelerate hydrolysis of GTP bound to the Gα subunit, thereby promoting reassembly of the Gα and Gβγ heterotrimer, and termination of second-messenger signaling. GPCR, G protein-coupled receptor.
G PROTEIN SIGNALING
G protein-coupled receptors (GPCRs) remain core targets of pharmaceutical drug development with over 40% of currently marketed drugs targeting GPCRs (45). These plasma membrane receptors regulate virtually all physiological processes including vision, taste, smell, neurotransmission, metabolism, blood clotting, cardiac output, blood pressure, and cell growth and development (208). Disrupted GPCR function or expression can lead to aberrant signaling, inactivation of the receptor, desensitization, and altered ligand binding, which can result in cardiovascular disorders, hypertension, diabetes, neurodegenerative disorders, anxiety, and cancer (166). All GPCRs contain a seven-transmembrane domain (7TM) consisting of seven transmembrane helices. Ligand binding to a GPCR induces a conformational change, and the receptor acts as a GTP exchange factor for G proteins by exchanging GDP for GTP on the Gα subunit of the G protein (166, 180). This GDP-to-GTP exchange on the Gα subunit stimulates dissociation of the Gα and Gβγ subunits, which then activate or inhibit various downstream effectors and result in a number of cellular responses. There are several subfamilies of Gα subunits including Gαq, Gαs, Gαi (including Gαz and Gαo), and Gα12/13. Each Gα subunit can differ in specific effector activation/inactivation (180).
Duration of activated GPCR signaling can be regulated many ways including posttranslational modification of the receptor by G protein-coupled receptor kinases (189). Phosphorylation of GPCRs results in recruitment of β-arrestins and internalization of the activated receptor (189). G proteins can be modified, as well, to regulate second messenger activation. Protein kinase C phosphorylates Gαz preferentially in the GDP bound state and prevents association of Gαz with the Gβγ subunits, thereby potentially leading to aberrant signaling through Gαz (201). In addition, blocking myristoylation of Gα subunits alters their affinity for the cell membrane, altering their activation and function (23, 140). Hydrolysis of GTP to GDP on the Gα subunit controls the duration of second messenger signaling, as this allows for their reassociation with the Gβγ subunits and termination of signaling (180).
RGS Proteins
In some tissues, termination of GPCR signaling activation is 100-fold faster than the predicted rate of GTP hydrolysis based on rates calculated from in vitro experiments (9). Additionally, rates of deactivation for G protein activated calcium and potassium channels can be much faster in vivo than in vitro (210). This indicates there are additional mechanisms by which G protein signaling can be regulated. Other such regulators of G protein signaling were discovered in yeast. Mutants of the gene SST2 were found to be hypersensitive to G protein coupled stimulation to induce growth arrest. Up to 200 times less ligand could be used to elicit an exaggerated growth arrest response compared with wild type, and SSST2P was subsequently defined as a negative regulator of G protein signaling. Other negative regulators were also discovered in Caenorhabditis elegans (EGL-10), and monocytes (GOS8) (66, 176, 210). Additionally, the nucleotide sequences of 15 mammalian genes from a cDNA library revealed a conserved 130-amino acid core domain within SSST2P, EGL-10, and GOS8. These proteins were defined as “regulators of G protein signaling,” and the core domain was, therefore, deemed the “RGS” domain.
Approximately 37 RGS proteins have been identified, and an additional 14 contain a nonfunctional RGS homology domain. Proteins with a functional RGS domain are classified into subfamilies (180). There are two types of classification systems. The first classification system organizes subfamilies by letter (A–F) (66, 214). The second classification system derives the name of a subfamily from the representative RGS member. For example, the RZ family is named for the representative RGSZ protein (66, 150). Each family grouping is based on amino acid sequence identity, structure, and function. For instance, members of the C/R7 family have a unique disheveled, EGL-10, pleckstrin (DEP) domain, known as the R7H domain. In contrast, the A/RZ and B/R4 RGS proteins have little more than a functional RGS domain. The main function of RGS proteins is to stabilize the Gα subunit in its GTP hydrolysis transition state and lower the free energy needed to complete the reaction. Hydrolysis of GTP allows for the Gα protein, bound to GDP, to reassociate with the Gβγ subunits and terminate the second messenger signaling cascade (Fig. 1). Though RGS proteins bind to the transition state, but not active conformation of G proteins, some RGS proteins (such as RGS4) interfere with interaction of activated Gα subunits with downstream effectors, functioning as effector antagonists (64).
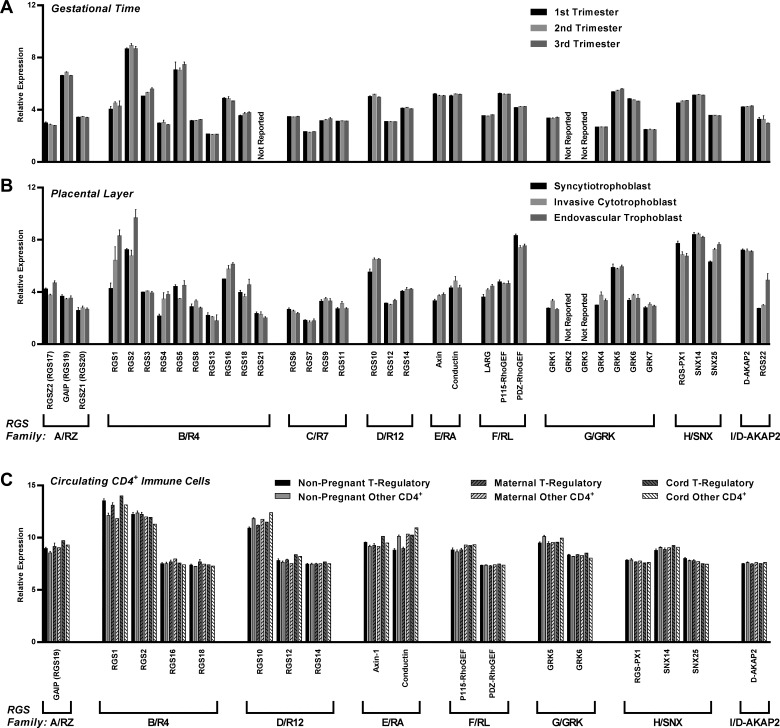
Expression of RGS protein mRNA, due to transcription factor regulation, can be ubiquitous or cell-type specific. Rgs1, Rgs2, and Rgs3 mRNA is expressed in both cardiac myocytes and fibroblasts of the heart. RGS2 is even more ubiquitously expressed and can be found in smooth muscle and endothelial cells of the vasculature (127). However, some RGS protein expression is restricted, such as in the brain, where several RGS proteins are limited to specific regions of the brain (51, 211). Within the placenta, most of the 37 RGS genes are expressed at detectable levels throughout gestation and across various layers (Fig. 2, A–B). RGS genes are also expressed in circulating human CD4+ T cells in both the nonpregnant and pregnant states and in cord blood (Fig. 2C). Although the placenta and T cell functions (and dysfunctions) are clearly implicated in cardiovascular complications of pregnancy, the functions of RGS proteins during pregnancy have been largely unstudied to date, with the recent exceptions of RGS2 and RGS5 (72, 88, 96). Future studies to elucidate the role of RGS proteins in these tissues in pathogenic complications of pregnancy are clearly required.
Fig. 2.
Expression of mRNA for various RGS family members in placenta and immune cells during human pregnancy. In silico reanalysis of published processed data sets GSE9984 (108), GSE93839 (53), and GSE31976 (161) reveals differential expression of the various RGS family members in whole-thickness human placenta across gestation (A), in different layers of term human placenta (B), and in subsets of human CD4+ T cells from nonpregnant women, and maternal and cord blood samples at term (C). While many genes were either not detected or reported in circulating CD4+ cells in the GSE31976 data set, the concordance of relative expression levels of individual genes tested, across cell types, is notable.
The B/R4 Family of RGS Proteins
There are a total of 10 RGS proteins that make up the B/R4 family, RGS-1 to -5, -8, -13, -16, -18, and -21, as summarized in Table 1 (13, 180). These are the smallest RGS proteins in terms of size (with the exception of RGS3), and they primarily regulate Gαi or Gαq signaling pathways (13, 180). RGS1 functions mainly in the immune system, with high concentrations in germinal centers of B lymphocyte maturation (13, 70). RGS1 functions to regulate B lymphocyte maturation induced by chemokines and can also be found in T lymphocytes, natural killer (NK) cells, dendritic cells, and monocytes (4, 38, 94, 113, 169). The next RGS protein, Rgs2, is ubiquitously expressed, with expression documented in the CNS, heart, vasculature, kidney, immune system, lungs, bone and ovaries (13, 89, 98, 125, 163). The main function of RGS2 is to regulate Gαq and Gαi signaling (64, 89, 92, 180). Loss of RGS2 effects T lymphocyte proliferation and reduces inflammation responses to viral challenge. In addition, Rgs2-deficient mice exhibit hypertension with increased vascular resistance, hypertrophy of renal vasculature, and extended calcium signaling in the presence of vasoconstrictors (13, 124). As for RGS3, of the various isoforms, both short isoforms contain barely more than an RGS domain, while the longer isoforms contain other protein interaction domains including a PDZ domain (13, 90). Similarly to Rgs1, Rgs3 is mainly found in immune cells and regulates immune cell function (143, 144, 162). Alternatively, RGS4 expression is highest in the brain and heart (13, 44, 212). RGS4 has been implicated in regulating downstream signaling of opioid, cholinergic, and serotoninergic receptors and has been linked to schizophrenia by microarray and genotype single nucleotide polymorphism (SNP) analysis (109, 117). RGS5 expression appears to be highest in the vasculature as well, with high levels in pericytes and vascular smooth muscle cells. This RGS protein can be found in microvasculature as well, such as capillaries and arterioles, the aorta, and carotid artery (13, 16, 101). Like most other B/R4 members, Rgs8 is highly enriched in the brain. Various isoforms of Rgs8 have been implicated in receptor type-specific inhibition of Gαq signaling in which the shorter form of RGS8 yielded a less potent inhibition response compared with the other isoforms (156). RGS13 is found in T and B lymphocytes as well as mast cells (13, 170). Like most other B/R4 members, Rgs16 has a fairly ubiquitous expression and can be found in the heart, brain, liver, NK cells, and T lymphocytes (13). Conversely, RGS18 is mainly found in bone marrow-derived cells such as hematopoietic stem cells, megakaryocytes, platelets, granulocytes, as well as osteoclasts (13, 47, 119, 134). RGS18, like most RGS B/R4 members, regulates both Gαi- and Gαq-mediated signaling (13, 180). The last B/R4 member, RGS21, is found in sensory taste cells, while others have reported expression in various tissues such as brain, heart, placenta, lung, liver, and others (13, 102).
Table 1.
RGS B/R4 family members
| Member | Interactions | Vascular | Receptor Association | Refs. |
|---|---|---|---|---|
| RGS1 | Gαi/Gαq | no | ANG, sphingosine-1 | (13, 89, 122) |
| RGS2 | Gαi/Gαq (and AC) | yes | ANG, ET-1, AVP, α1/β2AR, muscarinic, CCK2 | (15, 23, 64, 89, 107, 122, 128, 151, 152, 188, 191, 193, 198, 199) |
| RGS3 | Gαi/Gαq/Gαz/Gαo | yes | ET-1, sphingosine-1, GnRH, M1/M3 muscarinic, βAR | (21, 89, 90, 120, 121, 139, 164) |
| RGS4 | Gαi/Gαq/Gαz | yes | Kir3, ANG, ET-1, muscarinic | (81, 133, 151, 153, 185, 192, 209) |
| RGS5 | Gαi/Gαq/Gαo | yes | ANG, ET-1, muscarinic | (10, 26, 54, 68, 141, 215) |
| RGS8 | Gαi/Gαq/Gαo | yes | ANG, GIRK, M1 muscarinic | (79, 154, 179, 187) |
| RGS13 | Gαi/Gαq/Gαo | yes | sphingosine-1, muscarinic, CXCR | (12, 85, 170) |
| RGS16 | Gαi/Gαq/Gαo | yes | PAF, rhodopsin, GIRK | (14, 22, 86, 213) |
| RGS18 | Gαi/Gαq | no | ANG, M1 muscarinic, OGR1, CCR2 | (80, 119, 134, 207) |
| RGS21 | Gαi/Gαq/Gαo | no | T2R | (20, 31, 194) |
ANG, angiotensin II; AC, adenylyl cyclase; ET-1, endothelin-1; AVP, arginine vasopressin; AR, adrenergic receptor; CCK, cholecystokinin; GnRH, gonadotropin-releasing hormone; Kir, potassium inwardly-rectifying channel; GIRK, G protein-coupled inwardly-rectifying potassium channel; CXCR, C-X-C motif chemokine receptor; PAF, platelet-activating factor receptor; OGR1, Ovarian cancer G-protein coupled receptor 1; CCR2, C-C chemokine receptor type 2; T2R, (TAS2R) taste receptor type 2.
ROLE FOR B/R4 FAMILY MEMBERS IN THE CARDIOVASCULAR SYSTEM
The RGS B/R4 family members are highly expressed in the cardiovascular system in contrast to other RGS protein families (48). All of the RGS B/R4 members have been reported to be expressed at the mRNA level in cardiac tissue, with the exception of RGS13. RGS2, RGS3, RGS5, and RGS16 are the most highly expressed in the heart and vasculature. However, RGS protein expression varies in that many of the RGS B/R4 family members have not been detected (181). RGS8 has been detected in cardiac tissue, albeit at very low levels (181, 211). Rgs8 mRNA is primarily expressed in the brain, with the most densely expressed region being the cerebellum. Specifically, RGS8 seems to be localized to Purkinje cells where it presumably regulates Gαo and Gαi signaling (155, 157). RGS21 is also not expressed in cardiovascular tissue. In most recent studies it appears to be selectively expressed in circumvallate taste papillae or lung airway epithelial cells (31, 194). RGS21 was found to interact with Gαq, Gαo, Gαi, and Gαz subunits but not with Gαs subunits (194). However, since RGS21 is a newly recognized B/R4 family member and as few studies have been published on its function, a cardiovascular function of RGS21 cannot be ruled out.
Although some of the RGS B/R4 members cannot be detected in cardiovascular tissue directly, many of the B/R4 members are expressed in various immune cells (12, 137, 143). Both the innate and adaptive immune systems have been implicated in cardiovascular disorders such as hypertension and PreE (123). For example, T cells are implicated in kidneys of hypertensive rodents and humans (123, 146). In addition, deletion of T and B cells by RAG1 knockout in mice blunts hypertension induced by challenge with ANG II infusion or deoxycorticosterone (DOCA)-salt, and such animals are protected from renal damage (55). Transfer of T cells back into this model restores the hypertensive phenotype (55). In addition, B cells are implicated in hypertension. Specifically, autoantibodies to the AT1A receptor have been detected in humans with PreE, and experimental models of PreE, and studies have demonstrated CD4+ T cells are required for production of these autoantibodies (36, 37, 97, 195). Similar antibodies can also be detected in humans with secondary malignant hypertension (46). A more comprehensive review of immune cell function/dysfunction in hypertension (123) highlights numerous studies where immunosuppression blunts hypertensive responses in humans and in various animal models. As such, although RGS B/R4 members may not be detected in cardiovascular tissue per se, their roles in immune cells may contribute to cardiovascular function and dysfunction.
Regulator of G Protein Signaling-1
Regulator of G protein signaling 1 (RGS1), as mentioned previously, is primarily expressed in lymphoid-derived immune cells such as B lymphocytes, T cells, NK cells, and dendritic cells. Expression of RGS1 (mRNA and protein) in these cells has been detected in peripheral blood, germinal centers, and in bone marrow (4, 94, 99, 103, 114, 142, 148, 169, 190). Due to high expression of RGS1 in lymphoid derived cells, RGS1 is implicated in many immune-related diseases such as celiac disease, multiple sclerosis, and multiple myeloma with mutations within the RGS1 gene being associated with development of these immune related diseases (73, 77, 148). In addition, a SNP in the 5′-region of the RGS1 gene (rs2816316) was recently associated with development of celiac disease and Type 1 diabetes. Biopsies of intestinal regions indicate RGS1 is expressed in intraepithelial lymphocyte compartments (73, 178). Interestingly, another SNP located within the 5′-region of RGS1 gene (rs2760524) is significantly associated with risk for developing multiple sclerosis. The multiple sclerosis SNP (rs2760524) has also been found to exist in strong linkage disequilibrium with the celiac disease SNP (rs2816316) (77). Within B lymphocytes, RGS1 mainly mediates Gαi-induced signaling and is linked to regulating migration of immune cells (58).
RGS1 mRNA expression can be observed in the heart at low levels, and it is more highly expressed in the aorta than heart tissue (25). However, this expression has been shown not to be in cardiovascular cells (smooth muscle or endothelial cells), but in resident immune cells of aorta and heart (137). Despite little expression in cardiovascular cells, RGS1 is still linked to cardiovascular function by its role in immune cells. RGS1 expression is significantly reduced in monocytes from patients with early-onset coronary artery disease, and increased RGS1 expression has been implicated in diabetes mellitus (19, 49, 175). In a study performed to analyze genetically dysregulated signaling pathways in obesity and atherosclerosis, microarray analysis of white adipose tissue from obese patients and atherosclerotic aortae demonstrated RGS1 was one of the highest ranked genes involved in inflammatory pathways of these disorders (115). High expression of RGS1 can be found in atherosclerotic plaques and aortic aneurysms (137). RGS1 was shown to regulate leukocyte recruitment, and mice lacking RGS1 had significantly reduced trafficking to the aortic wall after ANG infusion than mice with intact RGS1 and ANG infusion. In addition, loss of trafficking reduced the occurrence of ANG induced aortic aneurysm rupture in RGS1 knockout mice (137). Furthermore, RGS1 can functionally regulate ANG-II induced ERK activation, although RGS1 has little effect on ET-1-induced signaling (25). Although RGS1 is linked to cardiovascular-related disorders, its role appears to be primarily mediated through effects in immune cells.
Regulator of G Protein Signaling-2
Regulator of G protein signaling-2 (RGS2) is highly expressed in brain, kidneys, heart, and vascular smooth muscle cells (84, 89, 126). Within the brain, Rgs2 expression can be seen in the hippocampus, striatum, and cortex. Expression of RGS2 can be significantly induced by activation of synaptic activity and is implicated in regulation of both Gαq- and Gαi-induced M2 muscarinic acetylcholine receptor (AChR) activation (76). Both heterozygous and homozygous RGS2 knockout mice exhibit a hypertensive phenotype. This leads to a hypertensive phenotype that is indistinguishable between the heterozygous and homozygous knockouts (62). In addition, loss of RGS2 results in an increase in medial thickness of renal arterial walls, which is characteristic of humans that have chronic hypertension (62). After challenge with vasodilator agonists, the rate of blood pressure decrease was significantly lower in RGS2 knockout mice than wild-type mice. Furthermore, vasoconstrictor challenge of RGS2 knockout aortic vascular smooth muscle cells results in increased peak calcium responses and prolonged decline of intracellular calcium levels postchallenge (62). Hypertension mediated by loss of RGS2 is due to increased vascular tone as demonstrated by increased contraction of mesenteric resistance arteries, renal interlobar arteries, and increased hypertension responses to ANG (62, 63, 127). Although RGS2 mainly functions to inhibit Gαq signaling in vascular smooth muscle, RGS2 was shown to regulate Gαi/o signaling in the vascular endothelium as well. Gαi/o signaling inhibits vasodilation, and loss of RGS2 results in enhanced Gαi/o signaling and impaired vasodilation in response to acetylcholine (127). Alternatively, RGS2 was shown to alter Gαs signaling as well. However, it does not interact with Gαs itself. RGS2 has been demonstrated to inhibit the production of cAMP in olfactory membranes independently of Gαs, and so is thought to regulate adenylyl cyclase itself. Of the various adenylyl cyclases, RGS2 has been shown to regulate adenylyl cyclase III, V, and VI (174). Additionally, RGS2 has been implicated in regulating AVP-induced V2 receptor (Gαs)-mediated signaling. In the kidneys, RGS2 expression is localized to parts of the nephron that express V2 receptors, and kidneys from RGS2 knockout mice exhibit increased levels of cAMP, the main signaling molecule of V2 receptors. In the same study, RGS2 knockout mice that were water restricted followed by acute water loading exhibited significantly lower urine volume output after water restriction and more urine volume output after acute water loading compared with wild-type mice. This effect was lost when acute water loading injections contained a V2 antagonist, suggesting RGS2 functionally regulates vasopressin-induced Gαs signaling through the V2 receptor in the kidneys (216).
Regulator of G Protein Signaling-3
As mentioned previously, the regulator of G protein signaling-3 (RGS3) gene generates multiple protein isoforms, and it is the largest B/R4 family member (13, 180). RGS3 mRNA is expressed in various different cells and tissues and functionally regulates Gαi/o and Gαq activation. Similar to RGS1, RGS3 can be found in immune-derived cells such as bone marrow derived and peripheral blood B and T lymphocytes as well as macrophages (143, 144, 162). Within these cells, RGS3 has been implicated in regulating B and T lymphocyte chemotaxis by lymphoid-derived chemokines (143). In addition, Rgs3 is expressed in various regions of the brain, as detected by in situ hybridization, and has been reported in several regions of the brain stem, cerebellum, dorsal thalamus, and hypothalamus (51). In addition, RGS3 expression can be found in several neuroblastoma cell lines and has been implicated in negative regulation of chemokine signaling in some of these cell lines (5).
RGS3 can be found in cardiovascular tissues as well and exhibits differential expression among the various isoforms. The PDZ-containing isoform of RGS3 can be found in the atria of the heart, whereas the long and short isoforms of RGS3 are more highly expressed in the ventricles of the heart (25). RGS3 expression is more highly localized within heart endothelial cells but has also been documented in smooth muscle cells, cardiomyocytes, and cardiac fibroblasts (25, 211). In smooth muscle cells, RGS3 regulates signaling induced through sphingosine 1-phosphate receptors and also attenuates signaling induced by ET-1 and ANG (25). In the presence of cardiovascular dysfunction RGS3 expression changes, RGS3 can be found in cardiomyocytes of adult rats and expression is significantly elevated in rat models of cardiac hypertrophy (104). In spontaneously hypertensive heart failure (SHHF) rats, RGS3 is significantly elevated in the failing myocardium (212). In contrast, the SHHF rat model that also develops congestive heart failure exhibits loss of Rgs3 mRNA and protein in failing hearts (212). In line with these findings, RGS3 mRNA and protein expression are significantly increased in myocardial samples from human end-stage failing hearts, suggesting a role in regulating cellular responses to hypertrophy in humans as well (131). Overall these studies suggest RGS3 may hold an important role in mediating cardiac hypertrophy and regulating cardiac function and failure.
Regulator of G Protein Signaling-4
Regulator of G protein Signaling-4 (RGS4) is expressed in various tissues, similar to RGS3. Expression of RGS4 mRNA and protein has been documented in immune cells such as T lymphocytes and B cell-derived cell lines where it is implicated in regulating chemotaxis signaling and differential regulation of G protein activation by varying localization within cells (40, 162). RGS4 expression by in situ hybridization reveals dense mRNA within specific areas of the brain as well. The densest regions include the neocortex, piriform cortex, caudoputamen and ventrobasal thalamus, and hippocampus (28, 51). In the cardiovascular system, Rgs4 is most densely located in the heart near the sinoatrial and atrioventricular nodal regions, the endogenous pacemakers for the heart (28, 181). RGS4 knockout mice exhibit reduced renal blood flow that can be reversed with ET-1 antagonists, implicating RGS4 function in regulating ET-1 activation as well (172). Additionally, exogenous expression of RGS4 in cardiomyocytes resulted in a dampening of ET-1 activation of PLC signaling, reducing myocyte contractility (181). In addition, similar to RGS3, RGS4 expression is increased in rat models of hypertrophy including cultured primary cardiomyocytes treated with growth factor and pulmonary artery banded mice. Increases in RGS4 can be seen in myocardium of SHHF rats as well (212). In humans, upregulation of RGS4 mRNA expression can be observed in patients with dilated and ischemic cardiomyopathy or end-stage heart failure (110, 131). RGS4 has been implicated in heart failure and hypertrophy, as well as in vitro cardiomyocyte contractility; however, more studies could be done to elucidate a role for RGS4 in control of cardiovascular function.
Regulator of G Protein Signaling-5
The highest expression of regulator of G protein signaling-5 (RGS5) appears to be within the brain and cardiovascular tissues. In situ hybridization reveals Rgs5 mRNA is densely expressed within three neuronal populations located in the paraventricular and supraoptic hypothalamic nuclei, as well as in the basolateral amygdala. Furthermore, Rgs5 has been detected within glial cell rich regions, suggesting RGS5 may also be expressed within glial cells of the brain (28). RGS5 has been implicated in the function of arteries, small renal arteries, and resistance arteries of the kidneys (48). RGS5 is mainly a Gαi/o and Gαq regulator and does not regulate or bind Gαs subunits (59, 215).
In the adult human, RGS5 is strongly expressed in peripheral arterial smooth muscle cells and regulates Gαi/Gαq activation in response to cardiovascular hormones, such as ANG (48, 197). RGS5 can also be found in mural cell populations, a mix of pericytes and smooth muscle cells. However, RGS5 expression is highest in medial smooth muscle cells of arteries (48). RGS5 has been implicated in regulating responses to ANG and ET-1, controlling calcium responses to these hormones as well as regulating activation of TGF-β and MEK/ERK pathways. Loss of RGS5 leads to enhanced activation of these pathways and results in cardiac hypertrophy in mice and increased renal hypertrophy and cardiovascular damage in the two-kidney one-clip model (48, 60, 100). In addition, SNPs in the RGS5 gene have been correlated with hypertensive populations. However, knockout models of RGS5 can display hypotension, hypertension, or normotensive phenotypes. In hypertensive models, loss of RGS5 leads to arterial hypercontractility, vessel stiffening, and increased vascular resistance. RGS5 has also been implicated in arteriogenesis. When femoral arteries were ligated to cause arterial growth, RGS5 expression in vascular smooth muscle cell significantly increased. In addition to being involved in cardiac tissue repair, RGS5 has been implicated in angiogenesis pathways as well. RGS5 is highly expressed in angiogenic vessels of solid tumors, in a model of pancreatic cancer, and is also highly expressed in angiogenic neovessels in brain tumors. It has even been suggested that RGS5 could be used as a pericyte marker for pathological neovascularization of tumors (48). Overall, RGS5 is a critical regulator of vascular function.
Regulator of G Protein Signaling-13
Regulator of G protein signaling-13 (RGS13) mRNA expression is mainly documented in lymphoid tissues with highest expression in B cells and monocytes (12). RGS13 activity has been characterized mainly within immune-derived cells. RGS13 was found to negatively regulate Gαi and Gαo signaling, as well as associate with Gαq subunits (85). In addition, RGS13 attenuates MAPK activation by Gαi and Gαq stimulation (85). Interestingly, RGS13, along with RGS2, typically displays nuclear localization. It was recently demonstrated upon activation of adenylyl cyclase and cAMP protein-dependent kinase (PKA), RGS13 translocates to the nucleus where it complexes with phosphorylated CREB to repress gene transcription (202). In addition, PKA activity phosphorylates RGS13, which results in stabilization of RGS13 and inhibits its degradation (204). In terms of vascular expression, there are only low levels of RGS13 found in the heart, and mostly found in cardiac fibroblasts (211).
Regulator of G Protein Signaling-16
Regulator of G protein signaling-16 (RGS16) mRNA and protein are expressed in the heart in both cardiac myocytes and fibroblasts, but at much lower levels than RGS2, -3, and -5 (87, 138, 211). RGS16 mRNA and protein are strongly expressed in the liver as well, and this expression can be controlled diurnally by feeding and fasting activity. In the liver, RGS16 regulates fatty acid oxidation metabolism (71, 136). Within cardiovascular tissue, RGS16 is implicated in regulation of Gaq activation. Bacterial endotoxin (LPS) is associated with impairment of myocardial contractility and acute heart failure. Treatment of cultured rat cardiomyocytes with LPS results in an increase of Rgs16 mRNA expression. Induction of Rgs16 expression by LPS resulted in blunted activation of PLC activity by ET-1. Additionally, LPS treatment of vascular smooth muscle cells and rat aortic rings can also induce Rgs16 mRNA expression (61). LPS treatment results in dampened contractile responses to vasoconstrictors such as ANG, suggesting RGS16 regulation of ANG receptors as well (61). Moreover, ETB receptor has been implicated in regulating RGS16 expression, whereby activation of the receptor leads to increases in RGS16 expression (183). Overall, these studies implicate RGS16 in cardiac function.
Regulator of G Protein Signaling-18
Expression of regulator of G protein signaling-18 (RGS18) mRNA can be detected, but is very low, in the heart. RGS18 is not found in cardiomyocytes and exhibits only low expression in cardiac fibroblasts (47, 134, 211). Like RGS1, RGS18 is mostly expressed in immune cells. RGS18 mRNA and protein expression appears to be highest in bone marrow-derived cells, especially platelets. It can also be found in megakaryocytes and leukocytes (47). RGS18 functions to regulate Gαi and Gαq signaling in these cells but did not interact with Gαs, Gαz, or Gα12 (47). Additionally, Rgs18 is also expressed in osteoclasts where it functions as a negative regulator of osteoclast differentiation (80). However, to date, no studies have revealed a function for RGS18 within cardiovascular tissues.
GESTATIONAL DISORDERS AND THE RGS B/R4 FAMILY
Recently, various RGS B/R4 members have been implicated in vascular disorders of pregnancy. During normal pregnancy, the cardiovascular system changes dramatically. Cardiac output significantly increases, and by week 24 of gestation, the renin-angiotensin system is activated and ANG is secreted. AVP secretion is also activated, and both hormones act to increase water intake and retention. Retained fluids can be increased as much as 45%, resulting in a reduction in osmolality. Blood pressure also decreases up to 5–10 mm Hg during pregnancy, and most of these changes occur early during the 6–8 wk range of gestation (159). Despite activation of vasoconstrictor hormones, pregnancy displays an overall reduction in blood pressure thought to be mediated by relaxin, progesterone, or nitric oxide (27, 159). Furthermore, during pregnancy, there are attenuated vasoconstriction responses to ANG (32).
ANG receptors (AT1) and AVP receptors (V1A and V1B, respectively) function by activating Gαq and Gαi/o pathways (39, 67), with the exception of AVP receptor V2, which can activate Gαs (17, 93). Both hormones, in addition to other G protein-activating hormones such as ET-1, have been implicated in gestational cardiovascular conditions such as PreE (78, 82, 160). A disorder classified by elevated blood pressure and proteinuria, PreE affects ~10% of pregnancies each year and in some areas remains the leading cause of maternal and fetal death (41, 56). Yet, the cause of PreE remains unknown. Currently, there is a lack of adequate diagnostic tools to predict risk of PreE and the only cure is delivery of the fetus and placenta. RGS proteins function to regulate signaling by many of the cardiovascular hormones implicated in PreE, and therefore there is growing interest in the role of RGS proteins in the pathogenesis of cardiovascular complications of pregnancy such as PreE.
RGS5 has recently been linked to hypertensive disorders of pregnancy such as PreE (68). Myometrial arteries examined from hypertensive and PreE pregnancies display reduced expression of RGS5. Loss of RGS5 during pregnancy in mice induces gestational hypertension and reduces flow through uterine arteries, a key characteristic of PreE. In addition, RGS5 knockout pregnancies displayed an increased sensitivity to ANG challenge, and when treated with an AT1 receptor antagonist, hypertensive phenotypes were ameliorated. Uterine arteries also displayed increased production of reactive oxygen species with ANG challenge (indicating oxidative stress) and an increased sensitivity to sodium nitroprusside (SNP, consistent with a nitric oxide signaling deficiency). Although there was no difference in fetal weight or placental weight with RGS5 deficiency, developmental effects were observed in the placenta. RGS5 knockout pregnancies displayed reduced labyrinth layer thickness and increased proliferation of spongiotrophoblast layers compared with normal pregnancies. Additionally, the gestational hypertension and vascular dysfunction observed in RGS5 knockout pregnancies could be attenuated by treatment with a peroxisome proliferating factor gamma (PPARγ) agonist. This supports links PPARγ, ANG, and RGS5 in the regulation of blood pressure during pregnancy (68).
RGS2 was also recently linked to PreE. A SNP (rs4606) in the 3′-untranslated region (UTR) of the RGS2 gene is associated with hypertension in selected human populations (168). This same SNP, rs4606, was correlated with increased risk of developing PreE, specifically in a population of overweight women (88, 96, 168). Women who carry rs4606 were at increased odds of developing PreE and cardiovascular disorders later in life (95). This SNP in the 3′-UTR of the RGS2 gene has been associated with decreased levels of RGS2 transcript expression (168). Interestingly, as noted previously, RGS2 heterozygous and knockout mice both display hypertensive phenotypes, suggesting that the RGS2 gene displays haplo-insufficiency. Moreover, both heterozygous and homozygous changes in the rs4606 C1114G SNP were linked to increased odds of developing human PreE.
Decreased uterine artery flow is a characteristic of PreE, and RGS2 function has recently been implicated in uterine artery flow control. Previously, it was demonstrated that reducing RGS2 increases blood pressure and decreases blood flow in kidneys (126). Most recently, Jie et al. (84) used Doppler ultrasonography to assess uterine artery blood flow in RGS2 wild-type, heterozygous, or knockout mice. In both heterozygous mice and homozygous nonpregnant female RGS2 mice, loss of RGS2 results in increased resistive index in uterine arteries. In addition, this group assessed myogenic responses (physiological response of arteries to differences in pressure) of uterine arteries. They determined that with increasing pressure, uterine artery myogenic responses in RGS2 knockout and heterozygous females was significantly increased as well as prolonged in the presence of calcium. In addition, RGS2 knockout and heterozygous mice had increased sensitivity to increases in intraluminal pressure. Lastly, they implicated loss of RGS2 in control of internal calcium release. Smooth muscle cells derived from wild-type RGS2 mice released reduced amounts of calcium compared with smooth muscle cells from RGS2 knockout mice (84). The same group also previously used RGS2 endothelial- and smooth muscle-specific knockout mice to delineate the mechanism by which RGS2 loss could cause cardiovascular dysfunction. Loss of RGS2 in smooth muscle of mesenteric arteries did not have much effect on vasodilation of the vessel. However, endothelial-specific reduction of RGS2 caused impaired vasodilatory responses to acetylcholine (ACh) in mesenteric arteries. In addition, loss of RGS2 in endothelial cells impaired vasodilation responses to endothelial-derived hyperpolarizing factor, and they could recover vasodilation responses by blocking Gαi/o activation. However, endothelial-specific and smooth muscle-specific loss of RGS2 had minor affects, overall, on blood pressure (127). Thus, although associated with gestational hypertensive disorders, the molecular and physiological mechanisms by which RGS proteins such as RGS2 and RGS5 contribute to these pathological states require further study.
CONTROL OF RGS PROTEIN FUNCTION
As highlighted throughout this review, RGS proteins are ubiquitously expressed. Yet recent studies have provided evidence for relatively specific interactions of various RGS proteins with individual GPCRs and G proteins. For example, RGS21 is known to interact with very few receptors, while RGS2 is known to interact with many GPCRs (Table 1). Furthermore, RGS1 regulates Gαi and Gαq signaling but not Gαo, whereas RGS4 and RGS16 preferentially interact with Gαq (Table 1).
How these RGS proteins mediate receptor-specific signaling is still under investigation. Selectivity of RGS proteins may be affected by subcellular localization. For example, if we focus on RGS2 as an exemplar member of the RGS family, this RGS protein can be found either in the nucleus or cytosol. Upon coexpression in HEK293T cells with β2-adrenergic receptor or angiotensin AT1A receptor, RGS2 is recruited to the plasma membrane, even in the absence of agonist treatment (151). Splice variants can also regulate RGS protein specificity. RGS3 has a splice variant containing a PDZ domain, which mediates interactions with the Ephrin-B receptor (105). NH2-terminal structure can additionally alter RGS interactions with various GPCRs. RGS2 interacts with the M1 AChR through its NH2 terminus (15). Furthermore, another study confirmed the NH2-terminal domain of RGS2 was responsible for directly binding the α1A-adrenergic receptor. However, this does not occur with the α1B-adrenergic receptor (57). Instead of directly binding, a scaffolding protein known as spinophilin recruits RGS2 to the α1B-adrenergic receptor/Gαq complex to regulate calcium signaling (199). Overall, subcellular localization, splice variants, NH2-terminal structure, and other complexing proteins can mediate the specificity of RGS protein response to various GPCRs.
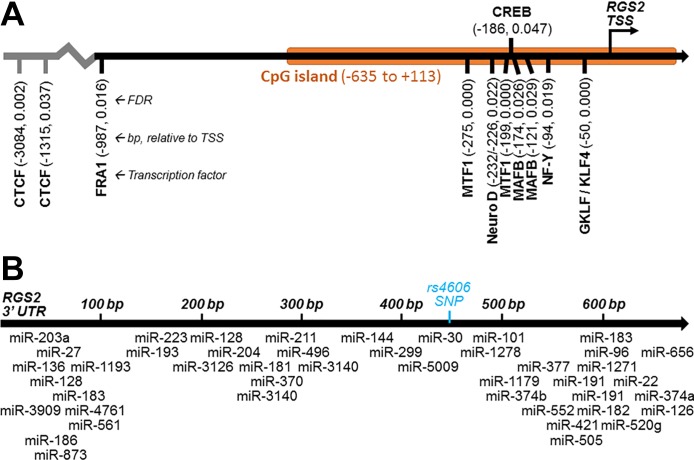
RGS proteins can also be modified or regulated directly, to alter GPCR signaling. NH2-terminal posttranslational modifications can affect RGS protein interactions with GPCRs. Once again with RGS2 as an example, its protein half-life is regulated by the NH2 end rule pathway. Some hypertensive populations carry mutations in the second amino acid of RGS2, which targets them for NH2-terminal acetylation (Met-Gln-RGS2 to Met-Arg-RGS2) or NH2-terminal Arginine degradation pathways. A mutation to leucine (Met-Leu-RGS2) targets RGS2 for both NH2-end degradation pathways and significantly affects RGS2 protein half-life (135). In addition, RGS proteins can be phosphorylated to regulate activity. RGS5 is phosphorylated at a basal state in HEK293T cells, and with ET-1 activation, increases phosphorylation at Serine166. This phosphorylation interferes with RGS5 GAP activity but can be rescued with protein kinase C (PKC) inhibitors (116). In addition, RGS2 can be phosphorylated by PKC. Similar to RGS5, phosphorylation of RGS2 inhibits its GAP activity (34). Transcriptional regulation also controls RGS protein expression and function. A CRE element in the RGS2 promoter was found to regulate RGS2 expression in vascular smooth muscle cells, and treatment with forskolin increases RGS2 mRNA (203). Heat shock factor 1 has also been implicated in the transcription of RGS2. The RGS2 promoter contains a heat shock element. HeLa cells subjected to 1 h of heat shock had induced RGS2 mRNA, and chromatin immunoprecipitation revealed increased HSF1 at the RGS2 promoter (129). Similarly, in silico analyses reveal several other transcription factors that are also predicted to regulate RGS2 transcription (Fig. 3A).
Fig. 3.
Predicted regulators of RGS2 mRNA. A: transcription factor binding sites between −5 kb and +1 kb of the human RGS2 gene transcription start site (TSS), predicted using the MotifMap software (35), with false discovery rate (FDR) < 0.05. B: microRNA binding sites within the 681 base pair 3′-untranslated region (UTR) of the human RGS2 mRNA transcript, predicted using the TargetScan 7.1 software (3).
Epigenetic regulation of RGS proteins has also been reported. More specifically, DNA methylation of the RGS2 promoter, contributing to gene repression via a CpG island (Fig. 3A), has been demonstrated in various malignancies including ovarian cancer (18), bladder cancer (206), and prostate cancer (200), highlighting the importance of transcriptional fine-turning of these molecules. Alqinyah and Hooks (6) also recently published a review of the current state of knowledge regarding epigenetic regulation of RGS proteins.
Posttranscriptional mechanisms have also been demonstrated to regulate the function RGS proteins. MicroRNA (miRNA)-mediated mechanisms inhibit translation of proteins and reduce stability of mRNA transcripts by binding to the 3′-UTR of the RNA transcript (7). Of note, many of the RGS B/R4 members have been predicted, or evidenced, to be targets of various miRNAs (11, 24, 52, 147, 196). In silico analysis of the 3′-UTR of the RGS2 gene, again as a convenient example, identifies many conserved miRNA consensus binding sequences that are predicted to be targeted by miRNAs (Fig. 3B), with the most well characterized of these being miR-4717, miR-1271, miR-183, miR-96, and miR-22. MiR-1271 is highly expressed in the brain and shown to decrease Rgs2 expression (83). Furthermore, control of RGS2 by these miRNAs within the brain is associated with anxiety, panic disorders, and neurodegenerative disorders, such as Huntington’s disease. Studies have demonstrated links between RGS2 mutations, anxiety, and panic disorders (130, 177), and between miR-4717 or miR-22 and panic and anxiety disorders (69, 118), but direct mechanistic demonstrations through the miRNAs and RGS2 to mediate the disorders are yet to be demonstrated. Mutations in the RGS2 3′-UTR itself (such as rs4606) are located near these predicted miRNA binding sites. rs4606 is associated with anxiety disorders, hypertension, and PreE (96, 168, 177). It is possible that this SNP interferes with miRNA regulatory processes, but that effect also remains to be demonstrated experimentally. In addition to the 3′-UTR of the RGS2 gene, it is conceivable that mutations within miRNA themselves, or other regions of the RGS gene, may also play important roles in regulating expression or regulation of the RGS2 transcript in the disorders associated with this gene.
Thus, substantial work is needed to comprehensively understand developmental-, tissue-, cell-, and context-specific regulation RGS proteins, and more specifically, how dysregulation of these proteins may contribute to disease states such as pregnancy-associated cardiovascular diseases.
CONCLUSIONS AND FUTURE DIRECTIONS
RGS proteins are strongly expressed in cardiovascular and immune tissues, and its members have been variably implicated in cardiovascular diseases of the nonpregnant state. A few of the RGS B/R4 family members (such as RGS2 and RGS5) have been associated with the pathogenesis of hypertensive disorders of pregnancy. Substantial work is required to characterize the normal function(s) of RGS proteins in cardiovascular and immune adaptations to normal pregnancy and the ways in which dysfunctions in the expression, activity, or localization of RGS proteins may contribute to cardiovascular pathologies of pregnancy.
GRANTS
This work and the authors were supported by National Institutes of Health (NIH) Grants HL-134850, HL-084207, R21HD-091458, AA-025919, CA-161882; the American Heart Association (15SFRN23730000, 17PRE33660633, 18EIA33890055); NIH Reproductive Scientist Development Program; the University of Iowa Graduate College (2017 Post-Comprehensive Research Fellowship); and the University of Iowa Center for Hypertension Research.
DISCLOSURES
M.K.S. and J.L.G. have submitted patent applications describing potential use of the AVP system in the diagnosis and treatment of PreE.
AUTHOR CONTRIBUTIONS
K.J.P., G.D., and J.L.G. conceived and designed research; K.J.P. and G.D. analyzed data; K.J.P. and J.L.G. prepared figures; K.J.P. drafted manuscript; K.J.P., G.D., R.A.F., K.N.G.-C., M.K.S., and J.L.G. edited and revised manuscript; K.J.P., G.D., R.A.F., K.N.G.-C., M.K.S., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical and intellectual contributions of Jeremy A. Sandgren, Danny W. Linggonegoro, Sabrina M. Scroggins, Nicole A. Pearson, Donna A. Santillan, Gary L. Pierce, and Curt D. Sigmund.
REFERENCES
- 1.Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (1): CD002252, 2007. doi: 10.1002/14651858.CD002252.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Abel A, Wittau N, Wieland T, Schultz G, Kalkbrenner F. Cell cycle-dependent coupling of the vasopressin V1a receptor to different G proteins. J Biol Chem 275: 32543–32551, 2000. doi: 10.1074/jbc.M002171200. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4: e05005, 2015. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agenès F, Bosco N, Mascarell L, Fritah S, Ceredig R. Differential expression of regulator of G-protein signalling transcripts and in vivo migration of CD4+ naïve and regulatory T cells. Immunology 115: 179–188, 2005. doi: 10.1111/j.1365-2567.2005.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Airoldi I, Raffaghello L, Piovan E, Cocco C, Carlini B, Amadori A, Corrias MV, Pistoia V. CXCL12 does not attract CXCR4+ human metastatic neuroblastoma cells: clinical implications. Clin Cancer Res 12: 77–82, 2006. doi: 10.1158/1078-0432.CCR-05-1376. [DOI] [PubMed] [Google Scholar]
- 6.Alqinyah M, Hooks SB. Regulating the regulators: Epigenetic, transcriptional, and post-translational regulation of RGS proteins. Cell Signal 42: 77–87, 2018. doi: 10.1016/j.cellsig.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists.. Task Force Report: Hypertension in Pregnancy. p. 100, 2013. [DOI] [PubMed]
- 9.Arshavsky VY, Wensel TG. Timing is everything: GTPase regulation in phototransduction. Invest Ophthalmol Vis Sci 54: 7725–7733, 2013. doi: 10.1167/iovs.13-13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahrami AJ, Gunaje JJ, Hayes BJ, Riehle KJ, Kenerson HL, Yeung RS, Stempien-Otero AS, Campbell JS, Mahoney WM Jr. Regulator of G-protein signaling-5 is a marker of hepatic stellate cells and expression mediates response to liver injury. PLoS One 9: e108505, 2014. doi: 10.1371/journal.pone.0108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banaei-Esfahani A, Moazzeni H, Nosar PN, Amin S, Arefian E, Soleimani M, Yazdani S, Elahi E. MicroRNAs that target RGS5. Iran J Basic Med Sci 18: 108–114, 2015. [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal G, DiVietro JA, Kuehn HS, Rao S, Nocka KH, Gilfillan AM, Druey KM. RGS13 controls g protein-coupled receptor-evoked responses of human mast cells. J Immunol 181: 7882–7890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther 116: 473–495, 2007. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beadling C, Druey KM, Richter G, Kehrl JH, Smith KA. Regulators of G protein signaling exhibit distinct patterns of gene expression and target G protein specificity in human lymphocytes. J Immunol 162: 2677–2682, 1999. [PubMed] [Google Scholar]
- 15.Bernstein LS, Ramineni S, Hague C, Cladman W, Chidiac P, Levey AI, Hepler JR. RGS2 binds directly and selectively to the M1 muscarinic acetylcholine receptor third intracellular loop to modulate Gq/11alpha signaling. J Biol Chem 279: 21248–21256, 2004. doi: 10.1074/jbc.M312407200. [DOI] [PubMed] [Google Scholar]
- 16.Bondjers C, Kalén M, Hellström M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 162: 721–729, 2003. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch 456: 1005–1024, 2008. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacan E. Epigenetic regulation of RGS2 (Regulator of G-protein signaling 2) in chemoresistant ovarian cancer cells. J Chemother 29: 173–178, 2017. doi: 10.1080/1120009X.2016.1277007. [DOI] [PubMed] [Google Scholar]
- 19.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One 8: e59701, 2013. [Erratum in PLoS One 9: 2014]. doi: 10.1371/journal.pone.0059701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 444: 288–294, 2006. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee TK, Eapen AK, Fisher RA. A truncated form of RGS3 negatively regulates G protein-coupled receptor stimulation of adenylyl cyclase and phosphoinositide phospholipase C. J Biol Chem 272: 15481–15487, 1997. doi: 10.1074/jbc.272.24.15481. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Zheng B, Han J, Lin SC. Characterization of a novel mammalian RGS protein that binds to Galpha proteins and inhibits pheromone signaling in yeast. J Biol Chem 272: 8679–8685, 1997. doi: 10.1074/jbc.272.13.8679. [DOI] [PubMed] [Google Scholar]
- 23.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene 20: 1643–1652, 2001. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Du G, Wang Y, Shi L, Mi J, Tang G. Integrative analysis of mRNA and miRNA expression profiles in oral lichen planus: preliminary results. Oral Surg Oral Med Oral Pathol Oral Radiol 124: 390– 402, 2017. doi: 10.1016/j.oooo.2017.05.513. [DOI] [PubMed] [Google Scholar]
- 25.Cho H, Harrison K, Schwartz O, Kehrl JH. The aorta and heart differentially express RGS (regulators of G-protein signalling) proteins that selectively regulate sphingosine 1-phosphate, angiotensin II and endothelin-1 signalling. Biochem J 371: 973–980, 2003. doi: 10.1042/bj20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17: 440–442, 2003. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- 27.Chu ZM, Beilin LJ. Nitric oxide-mediated changes in vascular reactivity in pregnancy in spontaneously hypertensive rats. Br J Pharmacol 110: 1184–1188, 1993. doi: 10.1111/j.1476-5381.1993.tb13939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, Heximer SP. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res 103: 527–535, 2008. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- 29.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, Pivarnik J, Spillman T, DeVore GR, Phelan J, Hankins GDV, Benedetti TJ, Tolley D. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol 161: 1439–1442, 1989. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 30.Clark SM, Dunn HE, Hankins GD. A review of oral labetalol and nifedipine in mild to moderate hypertension in pregnancy. Semin Perinatol 39: 548–555, 2015. doi: 10.1053/j.semperi.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Cohen SP, Buckley BK, Kosloff M, Garland AL, Bosch DE, Cheng G Jr, Radhakrishna H, Brown MD, Willard FS, Arshavsky VY, Tarran R, Siderovski DP, Kimple AJ. Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J Biol Chem 287: 41706–41719, 2012. doi: 10.1074/jbc.M112.423806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad KP, Morganelli PM, Brinck-Johnsen T, Colpoys MC. The renin-angiotensin system during pregnancy in chronically instrumented, conscious rats. Am J Obstet Gynecol 161: 1065–1072, 1989. doi: 10.1016/0002-9378(89)90785-0. [DOI] [PubMed] [Google Scholar]
- 33.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 5: 65, 2014. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham ML, Waldo GL, Hollinger S, Hepler JR, Harden TK. Protein kinase C phosphorylates RGS2 and modulates its capacity for negative regulation of Galpha 11 signaling. J Biol Chem 276: 5438–5444, 2001. doi: 10.1074/jbc.M007699200. [DOI] [PubMed] [Google Scholar]
- 35.Daily K, Patel VR, Rigor P, Xie X, Baldi P. MotifMap: integrative genome-wide maps of regulatory motif sites for model species. BMC Bioinformatics 12: 495, 2011. doi: 10.1186/1471-2105-12-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation 101: 2382–2387, 2000. doi: 10.1161/01.CIR.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 37.Dechend R, Müller DN, Wallukat G, Homuth V, Krause M, Dudenhausen J, Luft FC. AT1 receptor agonistic antibodies, hypertension, and preeclampsia. Semin Nephrol 24: 571–579, 2004. doi: 10.1016/j.semnephrol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Denecke B, Meyerdierks A, Böttger EC. RGS1 is expressed in monocytes and acts as a GTPase-activating protein for G-protein-coupled chemoattractant receptors. J Biol Chem 274: 26860–26868, 1999. doi: 10.1074/jbc.274.38.26860. [DOI] [PubMed] [Google Scholar]
- 39.Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci 100: 481–492, 2001. [PubMed] [Google Scholar]
- 40.Druey KM, Sullivan BM, Brown D, Fischer ER, Watson N, Blumer KJ, Gerfen CR, Scheschonka A, Kehrl JH. Expression of GTPase-deficient Gialpha2 results in translocation of cytoplasmic RGS4 to the plasma membrane. J Biol Chem 273: 18405–18410, 1998. doi: 10.1074/jbc.273.29.18405. [DOI] [PubMed] [Google Scholar]
- 41.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 33: 130–137, 2009. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Easterling TR. Pharmacological management of hypertension in pregnancy. Semin Perinatol 38: 487–495, 2014. doi: 10.1053/j.semperi.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egidy G, Robert B, Corvol P, Ferré F, Pinet F. The endothelin system and renin in human fetal membranes. Cell Mol Biol (Noisy-le-grand) 51, Suppl: OL839–OL847, 2005. [PubMed] [Google Scholar]
- 44.Erdely HA, Lahti RA, Lopez MB, Myers CS, Roberts RC, Tamminga CA, Vogel MW. Regional expression of RGS4 mRNA in human brain. Eur J Neurosci 19: 3125–3128, 2004. doi: 10.1111/j.0953-816X.2004.03364.x. [DOI] [PubMed] [Google Scholar]
- 45.Filmore D. It's a GPCR world: Cell-based screening assays and structural studies are fueling G-protein coupled receptors as one of the most popular classes of investigational drug targets. Mod Drug Discov 7: 2004. [Google Scholar]
- 46.Fu ML, Herlitz H, Schulze W, Wallukat G, Micke P, Eftekhari P, Sjögren KG, Hjalmarson A, Müller-Esterl W, Hoebeke J. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens 18: 945–953, 2000. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 47.Gagnon AW, Murray DL, Leadley RJ Jr. Cloning and characterization of a novel regulator of G protein signalling in human platelets. Cell Signal 14: 595–606, 2002. doi: 10.1016/S0898-6568(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 48.Ganss R. Keeping the Balance Right: Regulator of G Protein Signaling 5 in Vascular Physiology and Pathology. Prog Mol Biol Transl Sci 133: 93–121, 2015. doi: 10.1016/bs.pmbts.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Invest Ophthalmol Vis Sci 46: 349–357, 2005. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- 50.Ghanem FA, Movahed A. Use of antihypertensive drugs during pregnancy and lactation. Cardiovasc Ther 26: 38–49, 2008. doi: 10.1111/j.1527-3466.2007.00036.x. [DOI] [PubMed] [Google Scholar]
- 51.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci 17: 8024–8037, 1997. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong Y, Wu CN, Xu J, Feng G, Xing QH, Fu W, Li C, He L, Zhao XZ. Polymorphisms in microRNA target sites influence susceptibility to schizophrenia by altering the binding of miRNAs to their targets. Eur Neuropsychopharmacol 23: 1182–1189, 2013. doi: 10.1016/j.euroneuro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Gormley M, Ona K, Kapidzic M, Garrido-Gomez T, Zdravkovic T, Fisher SJ. Preeclampsia: novel insights from global RNA profiling of trophoblast subpopulations. Am J Obstet Gynecol 217: 200.e1–200.e17, 2017. doi: 10.1016/j.ajog.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Gu S, He J, Ho WT, Ramineni S, Thal DM, Natesh R, Tesmer JJ, Hepler JR, Heximer SP. Unique hydrophobic extension of the RGS2 amphipathic helix domain imparts increased plasma membrane binding and function relative to other RGS R4/B subfamily members. J Biol Chem 282: 33064–33075, 2007. doi: 10.1074/jbc.M702685200. [DOI] [PubMed] [Google Scholar]
- 55.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haché S, Takser L, LeBellego F, Weiler H, Leduc L, Forest JC, Giguère Y, Masse A, Barbeau B, Lafond J. Alteration of calcium homeostasis in primary preeclamptic syncytiotrophoblasts: effect on calcium exchange in placenta. J Cell Mol Med 15: 654–667, 2011. doi: 10.1111/j.1582-4934.2010.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hague C, Bernstein LS, Ramineni S, Chen Z, Minneman KP, Hepler JR. Selective inhibition of alpha1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem 280: 27289–27295, 2005. doi: 10.1074/jbc.M502365200. [DOI] [PubMed] [Google Scholar]
- 58.Han SB, Moratz C, Huang NN, Kelsall B, Cho H, Shi CS, Schwartz O, Kehrl JH. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity 22: 343–354, 2005. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Hao J, Michalek C, Zhang W, Zhu M, Xu X, Mende U. Regulation of cardiomyocyte signaling by RGS proteins: differential selectivity towards G proteins and susceptibility to regulation. J Mol Cell Cardiol 41: 51–61, 2006. doi: 10.1016/j.yjmcc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17: 1576–1578, 2003. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 61.Hendriks-Balk MC, Tjon-Atsoi M, Hajji N, Alewijnse AE, Peters SL. LPS differentially affects vasoconstrictor responses: a potential role for RGS16? J Physiol Biochem 65: 71–83, 2009. doi: 10.1007/BF03165971. [DOI] [PubMed] [Google Scholar]
- 62.Hercule HC, Tank J, Plehm R, Wellner M, da Costa Goncalves AC, Gollasch M, Diedrich A, Jordan J, Luft FC, Gross V. Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp Physiol 92: 1014–1022, 2007. doi: 10.1113/expphysiol.2007.038240. [DOI] [PubMed] [Google Scholar]
- 63.Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin AJ, Steinberg TH, Wyss JM, Mecham RP, Blumer KJ. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J Clin Invest 111: 445–452, 2003. doi: 10.1172/JCI15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci USA 94: 14389–14393, 1997. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev 54: 527–559, 2002. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 67.Holmes CL, Landry DW, Granton JT. Science review: Vasopressin and the cardiovascular system part 1–receptor physiology. Crit Care 7: 427–434, 2003. doi: 10.1186/cc2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holobotovskyy V, Chong YS, Burchell J, He B, Phillips M, Leader L, Murphy TV, Sandow SL, McKitrick DJ, Charles AK, Tare M, Arnolda LF, Ganss R. Regulator of G protein signaling 5 is a determinant of gestational hypertension and preeclampsia. Sci Transl Med 7: 290ra88, 2015. doi: 10.1126/scitranslmed.aaa5038. [DOI] [PubMed] [Google Scholar]
- 69.Hommers L, Raab A, Bohl A, Weber H, Scholz CJ, Erhardt A, Binder E, Arolt V, Gerlach A, Gloster A, Kalisch R, Kircher T, Lonsdorf T, Ströhle A, Zwanzger P, Mattheisen M, Cichon S, Lesch KP, Domschke K, Reif A, Lohse MJ, Deckert J. MicroRNA hsa-miR-4717-5p regulates RGS2 and may be a risk factor for anxiety-related traits. Am J Med Genet B Neuropsychiatr Genet 168B: 296–306, 2015. doi: 10.1002/ajmg.b.32312. [DOI] [PubMed] [Google Scholar]
- 70.Hong JX, Wilson GL, Fox CH, Kehrl JH. Isolation and characterization of a novel B cell activation gene. J Immunol 150: 3895–3904, 1993. [PubMed] [Google Scholar]
- 71.Huang J, Pashkov V, Kurrasch DM, Yu K, Gold SJ, Wilkie TM. Feeding and fasting controls liver expression of a regulator of G protein signaling (Rgs16) in periportal hepatocytes. Comp Hepatol 5: 8, 2006. doi: 10.1186/1476-5926-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang ZP, Ni H, Yang ZM, Wang J, Tso JK, Shen QX. Expression of regulator of G-protein signalling protein 2 (RGS2) in the mouse uterus at implantation sites. Reproduction 126: 309–316, 2003. doi: 10.1530/rep.0.1260309. [DOI] [PubMed] [Google Scholar]
- 73.Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, Bruinenberg M, Romanos J, Dinesen LC, Ryan AW, Panesar D, Gwilliam R, Takeuchi F, McLaren WM, Holmes GK, Howdle PD, Walters JR, Sanders DS, Playford RJ, Trynka G, Mulder CJ, Mearin ML, Verbeek WH, Trimble V, Stevens FM, O’Morain C, Kennedy NP, Kelleher D, Pennington DJ, Strachan DP, McArdle WL, Mein CA, Wapenaar MC, Deloukas P, McGinnis R, McManus R, Wijmenga C, van Heel DA. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet 40: 395–402, 2008. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20: 953–970, 2006. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 75.Iacobaeus C, Andolf E, Thorsell M, Bremme K, Jörneskog G, Östlund E, Kahan T. A longitudinal study of vascular structure and function during normal pregnancy. Ultrasound Obstet Gynecol 49:46–53, 2016. doi: 10.1002/uog.17326. [DOI] [PubMed] [Google Scholar]
- 76.Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, Barnes CA, Lanahan AA, Siderovski DP, Ross EM, Gilman AG, Worley PF. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J Neurosci 18: 7178–7188, 1998. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.International Multiple Sclerosis Genetics Consortium (IMSGC) IL12A, MPHOSPH9/CDK2AP1 and RGS1 are novel multiple sclerosis susceptibility loci. Genes Immun 11: 397–405, 2010. doi: 10.1038/gene.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irani RA, Xia Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta 29: 763–771, 2008. doi: 10.1016/j.placenta.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Itoh M, Nagatomo K, Kubo Y, Saitoh O. Alternative splicing of RGS8 gene changes the binding property to the M1 muscarinic receptor to confer receptor type-specific Gq regulation. J Neurochem 99: 1505–1516, 2006. doi: 10.1111/j.1471-4159.2006.04220.x. [DOI] [PubMed] [Google Scholar]
- 80.Iwai K, Koike M, Ohshima S, Miyatake K, Uchiyama Y, Saeki Y, Ishii M.. RGS18 acts as a negative regulator of osteoclastogenesis by modulating the acid-sensing OGR1/NFAT signaling pathway. J Bone Miner Res 22: 1612–1620, 2007. doi: 10.1359/jbmr.070612. [DOI] [PubMed] [Google Scholar]
- 81.Jaén C, Doupnik CA. RGS3 and RGS4 differentially associate with G protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. J Biol Chem 281: 34549–34560, 2006. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- 82.Jain A. Endothelin-1: a key pathological factor in pre-eclampsia? Reprod Biomed Online 25: 443–449, 2012. doi: 10.1016/j.rbmo.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 83.Jensen KP, Covault J. Human miR-1271 is a miR-96 paralog with distinct non-conserved brain expression pattern. Nucleic Acids Res 39: 701–711, 2011. doi: 10.1093/nar/gkq798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jie L, Owens EA, Plante LA, Fang Z, Rensing DT, Moeller KD, Osei-Owusu P. RGS2 squelches vascular Gi/o and Gq signaling to modulate myogenic tone and promote uterine blood flow. Physiol Rep 4: e12692, 2016. doi: 10.14814/phy2.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson EN, Druey KM. Functional characterization of the G protein regulator RGS13. J Biol Chem 277: 16768–16774, 2002. doi: 10.1074/jbc.M200751200. [DOI] [PubMed] [Google Scholar]
- 86.Johnson EN, Seasholtz TM, Waheed AA, Kreutz B, Suzuki N, Kozasa T, Jones TL, Brown JH, Druey KM. RGS16 inhibits signalling through the G alpha 13-Rho axis. Nat Cell Biol 5: 1095–1103, 2003. doi: 10.1038/ncb1065. [DOI] [PubMed] [Google Scholar]
- 87.Kardestuncer T, Wu H, Lim AL, Neer EJ. Cardiac myocytes express mRNA for ten RGS proteins: changes in RGS mRNA expression in ventricular myocytes and cultured atria. FEBS Lett 438: 285–288, 1998. doi: 10.1016/S0014-5793(98)01319-2. [DOI] [PubMed] [Google Scholar]
- 88.Karppanen T, Kaartokallio T, Klemetti MM, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, Staff AC, Laivuori H. An RGS2 3'UTR polymorphism is associated with preeclampsia in overweight women. BMC Genet 17: 121, 2016. doi: 10.1186/s12863-016-0428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kehrl JH, Sinnarajah S. RGS2: a multifunctional regulator of G-protein signaling. Int J Biochem Cell Biol 34: 432–438, 2002. doi: 10.1016/S1357-2725(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 90.Kehrl JH, Srikumar D, Harrison K, Wilson GL, Shi CS. Additional 5′ exons in the RGS3 locus generate multiple mRNA transcripts, one of which accounts for the origin of human PDZ-RGS3. Genomics 79: 860–868, 2002. doi: 10.1006/geno.2002.6773. [DOI] [PubMed] [Google Scholar]
- 91.Kilts JD, Grocott HP, Kwatra MM. Gαq-coupled receptors in human atrium function through protein kinase C epsilon and delta. J Mol Cell Cardiol 38: 267–276, 2005. doi: 10.1016/j.yjmcc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 92.Kimple AJ, Soundararajan M, Hutsell SQ, Roos AK, Urban DJ, Setola V, Temple BR, Roth BL, Knapp S, Willard FS, Siderovski DP. Structural determinants of G-protein alpha subunit selectivity by regulator of G-protein signaling 2 (RGS2). J Biol Chem 284: 19402–19411, 2009. doi: 10.1074/jbc.M109.024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klingler C, Preisser L, Barrault MB, Lluel P, Horgen L, Teillet L, Ancellin N, Corman B. Vasopressin V2 receptor mRNA expression and cAMP accumulation in aging rat kidney. Am J Physiol Regul Integr Comp Physiol 272: R1775–R1782, 1997. doi: 10.1152/ajpregu.1997.272.6.R1775. [DOI] [PubMed] [Google Scholar]
- 94.Kveberg L, Ryan JC, Rolstad B, Inngjerdingen M. Expression of regulator of G protein signalling proteins in natural killer cells, and their modulation by Ly49A and Ly49D. Immunology 115: 358–365, 2005. doi: 10.1111/j.1365-2567.2005.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kvehaugen AS, Melien Ø, Holmen OL, Laivuori H, Dechend R, Staff AC. Hypertension after preeclampsia and relation to the C1114G polymorphism (rs4606) in RGS2: data from the Norwegian HUNT2 study. BMC Med Genet 15: 28, 2014. doi: 10.1186/1471-2350-15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Oian P, Andersgaard AB, Dechend R, Staff AC. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension 61: 655–661, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00331. [DOI] [PubMed] [Google Scholar]
- 97.LaMarca B, Wallace K, Granger J. Role of angiotensin II type I receptor agonistic autoantibodies (AT1-AA) in preeclampsia. Curr Opin Pharmacol 11: 175–179, 2011. doi: 10.1016/j.coph.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Le TH, Coffman TM. RGS2: a “turn-off” in hypertension. J Clin Invest 111: 441–443, 2003. doi: 10.1172/JCI200317836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Le Y, Honczarenko M, Glodek AM, Ho DK, Silberstein LE. CXC chemokine ligand 12-induced focal adhesion kinase activation and segregation into membrane domains is modulated by regulator of G protein signaling 1 in pro-B cells. J Immunol 174: 2582–2590, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Li H, He C, Feng J, Zhang Y, Tang Q, Bian Z, Bai X, Zhou H, Jiang H, Heximer SP, Qin M, Huang H, Liu PP, Huang C. Regulator of G protein signaling 5 protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Proc Natl Acad Sci USA 107: 13818–13823, 2010. [Erratum in PNAS 115: E3858–E3861, 2018]. doi: 10.1073/pnas.1008397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J, Adams LD, Wang X, Pabon L, Schwartz SM, Sane DC, Geary RL. Regulator of G protein signaling 5 marks peripheral arterial smooth muscle cells and is downregulated in atherosclerotic plaque. J Vasc Surg 40: 519–528, 2004. doi: 10.1016/j.jvs.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Chen L, Ji C, Liu B, Gu J, Xu J, Zou X, Gu S, Mao Y. Isolation and expression pattern of RGS21 gene, a novel RGS member. Acta Biochim Pol 52: 943–946, 2005. [PubMed] [Google Scholar]
- 103.Liao F, Shirakawa AK, Foley JF, Rabin RL, Farber JM. Human B cells become highly responsive to macrophage-inflammatory protein-3 alpha/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J Immunol 168: 4871–4880, 2002. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Huang H, Zhang Y, Zhu XY, Zhang R, Guan LH, Tang Q, Jiang H, Huang C. Regulator of G protein signaling 3 protects against cardiac hypertrophy in mice. J Cell Biochem 115: 977–986, 2014. doi: 10.1002/jcb.24741. [DOI] [PubMed] [Google Scholar]
- 105.Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 105: 69–79, 2001. doi: 10.1016/S0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 106.Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Gafni A, Helewa M, Hutton E, Koren G, Lee SK, Logan AG, Ganzevoort W, Welch R, Thornton JG, Moutquin JM; CHIPS Study Group* . The CHIPS Randomized Controlled Trial (Control of Hypertension in Pregnancy Study): Is Severe Hypertension Just an Elevated Blood Pressure? Hypertension 68: 1153–1159, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsuzaki N, Nishiyama M, Song D, Moroi K, Kimura S. Potent and selective inhibition of angiotensin AT1 receptor signaling by RGS2: roles of its N-terminal domain. Cell Signal 23: 1041–1049, 2011. doi: 10.1016/j.cellsig.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 108.Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, Unadkat JD. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci 15: 866–877, 2008. doi: 10.1177/1933719108322425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry 6: 293–301, 2001. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- 110.Mittmann C, Chung CH, Höppner G, Michalek C, Nose M, Schüler C, Schuh A, Eschenhagen T, Weil J, Pieske B, Hirt S, Wieland T. Expression of ten RGS proteins in human myocardium: functional characterization of an upregulation of RGS4 in heart failure. Cardiovasc Res 55: 778–786, 2002. doi: 10.1016/S0008-6363(02)00459-5. [DOI] [PubMed] [Google Scholar]
- 111.Mizuno N, Kokubu H, Sato M, Nishimura A, Yamauchi J, Kurose H, Itoh H. G protein-coupled receptor signaling through Gq and JNK negatively regulates neural progenitor cell migration. Proc Natl Acad Sci USA 102: 12365–12370, 2005. doi: 10.1073/pnas.0506101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mone F, Mulcahy C, McParland P, McAuliffe FM. Should we recommend universal aspirin for all pregnant women? Am J Obstet Gynecol 216: 141.e1–141.e5, 2017. doi: 10.1016/j.ajog.2016.09.086. [DOI] [PubMed] [Google Scholar]
- 113.Moratz C, Hayman JR, Gu H, Kehrl JH. Abnormal B-cell responses to chemokines, disturbed plasma cell localization, and distorted immune tissue architecture in Rgs1−/− mice. Mol Cell Biol 24: 5767–5775, 2004. doi: 10.1128/MCB.24.13.5767-5775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moratz C, Kang VH, Druey KM, Shi CS, Scheschonka A, Murphy PM, Kozasa T, Kehrl JH. Regulator of G protein signaling 1 (RGS1) markedly impairs Gi alpha signaling responses of B lymphocytes. J Immunol 164: 1829–1838, 2000. [DOI] [PubMed] [Google Scholar]
- 115.Moreno-Viedma V, Amor M, Sarabi A, Bilban M, Staffler G, Zeyda M, Stulnig TM. Common dysregulated pathways in obese adipose tissue and atherosclerosis. Cardiovasc Diabetol 15: 120, 2016. doi: 10.1186/s12933-016-0441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moroi K, Nishiyama M, Kawabata S, Ichiba H, Yajima T, Kimura S. Phosphorylation of Ser166 in RGS5 by protein kinase C causes loss of RGS function. Life Sci 81: 40–50, 2007. doi: 10.1016/j.lfs.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 117.Morris DW, Rodgers A, McGhee KA, Schwaiger S, Scully P, Quinn J, Meagher D, Waddington JL, Gill M, Corvin AP. Confirming RGS4 as a susceptibility gene for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 125B: 50–53, 2004. doi: 10.1002/ajmg.b.20109. [DOI] [PubMed] [Google Scholar]
- 118.Muiños-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipilä T, Maron E, Pettai K, Kananen L, Navinés R, Martín-Santos R, Gratacòs M, Metspalu A, Hovatta I, Estivill X. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol Psychiatry 69: 526–533, 2011. doi: 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 119.Nagata Y, Oda M, Nakata H, Shozaki Y, Kozasa T, Todokoro K. A novel regulator of G-protein signaling bearing GAP activity for Galphai and Galphaq in megakaryocytes. Blood 97: 3051–3060, 2001. doi: 10.1182/blood.V97.10.3051. [DOI] [PubMed] [Google Scholar]
- 120.Neill JD, Duck LW, Sellers JC, Musgrove LC, Kehrl JH. A regulator of G protein signaling, RGS3, inhibits gonadotropin-releasing hormone (GnRH)-stimulated luteinizing hormone (LH) secretion. BMC Cell Biol 2: 21, 2001. doi: 10.1186/1471-2121-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neill JD, Duck LW, Sellers JC, Musgrove LC, Scheschonka A, Druey KM, Kehrl JH. Potential role for a regulator of G protein signaling (RGS3) in gonadotropin-releasing hormone (GnRH) stimulated desensitization. Endocrinology 138: 843–846, 1997. doi: 10.1210/endo.138.2.5034. [DOI] [PubMed] [Google Scholar]
- 122.Neitzel KL, Hepler JR. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin Cell Dev Biol 17: 383–389, 2006. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 123.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med 215: 21–33, 2018. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oliveira-Dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, Mariathasan S, Sasaki T, Wakeham A, Ohashi PS, Roder JC, Barnes CA, Siderovski DP, Penninger JM. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Natl Acad Sci USA 97: 12272–12277, 2000. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Osei-Owusu P, Blumer KJ. Regulator of G Protein Signaling 2: A Versatile Regulator of Vascular Function. Prog Mol Biol Transl Sci 133: 77–92, 2015. doi: 10.1016/bs.pmbts.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osei-Owusu P, Owens EA, Jie L, Reis JS, Forrester SJ, Kawai T, Eguchi S, Singh H, Blumer KJ. Regulation of Renal Hemodynamics and Function by RGS2. PLoS One 10: e0132594, 2015. doi: 10.1371/journal.pone.0132594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Osei-Owusu P, Sabharwal R, Kaltenbronn KM, Rhee MH, Chapleau MW, Dietrich HH, Blumer KJ. Regulator of G protein signaling 2 deficiency causes endothelial dysfunction and impaired endothelium-derived hyperpolarizing factor-mediated relaxation by dysregulating Gi/o signaling. J Biol Chem 287: 12541–12549, 2012. doi: 10.1074/jbc.M111.332130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Osei-Owusu P, Sun X, Drenan RM, Steinberg TH, Blumer KJ. Regulation of RGS2 and second messenger signaling in vascular smooth muscle cells by cGMP-dependent protein kinase. J Biol Chem 282: 31656–31665, 2007. doi: 10.1074/jbc.M706360200. [DOI] [PubMed] [Google Scholar]
- 129.Ota A, Sawai M, Sakurai H. Stress-induced transcription of regulator of G protein signaling 2 (RGS2) by heat shock transcription factor HSF1. Biochimie 95: 1432–1436, 2013. doi: 10.1016/j.biochi.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 130.Otowa T, Shimada T, Kawamura Y, Sugaya N, Yoshida E, Inoue K, Yasuda S, Liu X, Minato T, Tochigi M, Umekage T, Kasai K, Tanii H, Okazaki Y, Kaiya H, Sasaki T.. Association of RGS2 variants with panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet 156b: 430–434, 2011. [DOI] [PubMed] [Google Scholar]
- 131.Owen VJ, Burton PB, Mullen AJ, Birks EJ, Barton P, Yacoub MH. Expression of RGS3, RGS4 and Gi alpha 2 in acutely failing donor hearts and end-stage heart failure. Eur Heart J 22: 1015–1020, 2001. doi: 10.1053/euhj.2000.2578. [DOI] [PubMed] [Google Scholar]
- 132.Page EW, Christianson R. The impact of mean arterial pressure in the middle trimester upon the outcome of pregnancy. Am J Obstet Gynecol 125: 740–746, 1976. doi: 10.1016/0002-9378(76)90839-5. [DOI] [PubMed] [Google Scholar]
- 133.Pang P, Jin X, Proctor BM, Farley M, Roy N, Chin MS, von Andrian UH, Vollmann E, Perro M, Hoffman RJ, Chung J, Chauhan N, Mistri M, Muslin AJ, Bonventre JV, Siedlecki AM. RGS4 inhibits angiotensin II signaling and macrophage localization during renal reperfusion injury independent of vasospasm. Kidney Int 87: 771–783, 2015. doi: 10.1038/ki.2014.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park IK, Klug CA, Li K, Jerabek L, Li L, Nanamori M, Neubig RR, Hood L, Weissman IL, Clarke MF. Molecular cloning and characterization of a novel regulator of G-protein signaling from mouse hematopoietic stem cells. J Biol Chem 276: 915–923, 2001. doi: 10.1074/jbc.M005947200. [DOI] [PubMed] [Google Scholar]
- 135.Park SE, Kim JM, Seok OH, Cho H, Wadas B, Kim SY, Varshavsky A, Hwang CS. Control of mammalian G protein signaling by N-terminal acetylation and the N-end rule pathway. Science 347: 1249–1252, 2015. doi: 10.1126/science.aaa3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pashkov V, Huang J, Parameswara VK, Kedzierski W, Kurrasch DM, Tall GG, Esser V, Gerard RD, Uyeda K, Towle HC, Wilkie TM. Regulator of G protein signaling (RGS16) inhibits hepatic fatty acid oxidation in a carbohydrate response element-binding protein (ChREBP)-dependent manner. J Biol Chem 286: 15116–15125, 2011. doi: 10.1074/jbc.M110.216234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel J, McNeill E, Douglas G, Hale AB, de Bono J, Lee R, Iqbal AJ, Regan-Komito D, Stylianou E, Greaves DR, Channon KM. RGS1 regulates myeloid cell accumulation in atherosclerosis and aortic aneurysm rupture through altered chemokine signalling. Nat Commun 6: 6614, 2015. doi: 10.1038/ncomms7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patten M, Stübe S, Thoma B, Wieland T. Interleukin-1beta mediates endotoxin- and tumor necrosis factor alpha-induced RGS16 protein expression in cultured cardiac myocytes. Naunyn Schmiedebergs Arch Pharmacol 368: 360–365, 2003. doi: 10.1007/s00210-003-0798-0. [DOI] [PubMed] [Google Scholar]
- 139.Pedram A, Razandi M, Kehrl J, Levin ER. Natriuretic peptides inhibit G protein activation. Mediation through cross-talk between cyclic GMP-dependent protein kinase and regulators of G protein-signaling proteins. J Biol Chem 275: 7365–7372, 2000. doi: 10.1074/jbc.275.10.7365. [DOI] [PubMed] [Google Scholar]
- 140.Preininger AM, Kaya AI, Gilbert JA III, Busenlehner LS, Armstrong RN, Hamm HE. Myristoylation exerts direct and allosteric effects on Gα conformation and dynamics in solution. Biochemistry 51: 1911–1924, 2012. doi: 10.1021/bi201472c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qin M, Liu X, Liu T, Wang T, Huang C. Potential Role of Regulator of G-Protein Signaling 5 in the Protection of Vagal-Related Bradycardia and Atrial Tachyarrhythmia. J Am Heart Assoc 5: e002783, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Müller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol 36: 1892–1903, 2006. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 143.Reif K, Cyster JG. RGS molecule expression in murine B lymphocytes and ability to down-regulate chemotaxis to lymphoid chemokines. J Immunol 164: 4720–4729, 2000. [DOI] [PubMed] [Google Scholar]
- 144.Riekenberg S, Farhat K, Debarry J, Heine H, Jung G, Wiesmüller KH, Ulmer AJ. Regulators of G-protein signalling are modulated by bacterial lipopeptides and lipopolysaccharide. FEBS J 276: 649–659, 2009. doi: 10.1111/j.1742-4658.2008.06813.x. [DOI] [PubMed] [Google Scholar]
- 145.Rochdi MD, Vargas GA, Carpentier E, Oligny-Longpré G, Chen S, Kovoor A, Gitelman SE, Rosenthal SM, von Zastrow M, Bouvier M. Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis: implications for treatments. Mol Pharmacol 77: 836–845, 2010. doi: 10.1124/mol.109.061804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 147.Rodriguez-Lebron E, Liu G, Keiser M, Behlke MA, Davidson BL. Altered Purkinje cell miRNA expression and SCA1 pathogenesis. Neurobiol Dis 54: 456–463, 2013. doi: 10.1016/j.nbd.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roh J, Shin SJ, Lee AN, Yoon DH, Suh C, Park CJ, Huh J, Park CS. RGS1 expression is associated with poor prognosis in multiple myeloma. J Clin Pathol 70: 202–207, 2017. doi: 10.1136/jclinpath-2016-203713. [DOI] [PubMed] [Google Scholar]
- 149.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum-Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 377: 613–622, 2017. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 150.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem 69: 795–827, 2000. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 151.Roy AA, Lemberg KE, Chidiac P. Recruitment of RGS2 and RGS4 to the plasma membrane by G proteins and receptors reflects functional interactions. Mol Pharmacol 64: 587–593, 2003. doi: 10.1124/mol.64.3.587. [DOI] [PubMed] [Google Scholar]
- 152.Roy AA, Nunn C, Ming H, Zou MX, Penninger J, Kirshenbaum LA, Dixon SJ, Chidiac P. Up-regulation of endogenous RGS2 mediates cross-desensitization between Gs and Gq signaling in osteoblasts. J Biol Chem 281: 32684–32693, 2006. doi: 10.1074/jbc.M604416200. [DOI] [PubMed] [Google Scholar]
- 153.Ruiz de Azua I, Scarselli M, Rosemond E, Gautam D, Jou W, Gavrilova O, Ebert PJ, Levitt P, Wess J. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc Natl Acad Sci USA 107: 7999–8004, 2010. doi: 10.1073/pnas.1003655107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature 390: 525–529, 1997. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- 155.Saitoh O, Masuho I, Itoh M, Abe H, Komori K, Odagiri M. Distribution of regulator of G protein signaling 8 (RGS8) protein in the cerebellum. Cerebellum 2: 154–160, 2003. doi: 10.1080/14734220309409. [DOI] [PubMed] [Google Scholar]
- 156.Saitoh O, Murata Y, Odagiri M, Itoh M, Itoh H, Misaka T, Kubo Y. Alternative splicing of RGS8 gene determines inhibitory function of receptor type-specific Gq signaling. Proc Natl Acad Sci USA 99: 10138–10143, 2002. doi: 10.1073/pnas.152085999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saitoh O, Odagiri M. RGS8 expression in developing cerebellar Purkinje cells. Biochem Biophys Res Commun 309: 836–842, 2003. doi: 10.1016/j.bbrc.2003.08.083. [DOI] [PubMed] [Google Scholar]
- 158.Sandgren JA, Scroggins SM, Santillan DA, Devor EJ, Gibson-Corley KN, Pierce GL, Sigmund CD, Santillan MK, Grobe JL. Vasopressin: the missing link for preeclampsia? Am J Physiol Regul Integr Comp Physiol 309: R1062–R1064, 2015. doi: 10.1152/ajpregu.00073.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 130: 1003–1008, 2014. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 160.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension 64: 852–859, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Santner-Nanan B, Straubinger K, Hsu P, Parnell G, Tang B, Xu B, Makris A, Hennessy A, Peek MJ, Busch DH, da Costa CP, Nanan R. Fetal-maternal alignment of regulatory T cells correlates with IL-10 and Bcl-2 upregulation in pregnancy. J Immunol 191: 145–153, 2013. doi: 10.4049/jimmunol.1203165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sato K, Kawasaki H, Morimoto C, Yamashima N, Matsuyama T.. An abortive ligand-induced activation of CCR1-mediated downstream signaling event and a deficiency of CCR5 expression are associated with the hyporesponsiveness of human naive CD4+ T cells to CCL3 and CCL5. J Immunol 168: 6263–6272, 2002. [DOI] [PubMed] [Google Scholar]
- 163.Sayasith K, Sirois J, Lussier JG. Expression and regulation of regulator of G-protein signaling protein-2 (RGS2) in equine and bovine follicles prior to ovulation: molecular characterization of RGS2 transactivation in bovine granulosa cells. Biol Reprod 91: 139, 2014. doi: 10.1095/biolreprod.114.121186. [DOI] [PubMed] [Google Scholar]
- 164.Scheschonka A, Dessauer CW, Sinnarajah S, Chidiac P, Shi CS, Kehrl JH. RGS3 is a GTPase-activating protein for g(ialpha) and g(qalpha) and a potent inhibitor of signaling by GTPase-deficient forms of g(qalpha) and g(11alpha). Mol Pharmacol 58: 719–728, 2000. doi: 10.1124/mol.58.4.719. [DOI] [PubMed] [Google Scholar]
- 165.Schöneberg T, Kostenis E, Liu J, Gudermann T, Wess J. Molecular aspects of vasopressin receptor function. Adv Exp Med Biol 449: 347–358, 1998. doi: 10.1007/978-1-4615-4871-3_44. [DOI] [PubMed] [Google Scholar]
- 166.Schöneberg T, Schulz A, Biebermann H, Hermsdorf T, Römpler H, Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther 104: 173–206, 2004. doi: 10.1016/j.pharmthera.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 167.Seely EW, Ecker J. Clinical practice. Chronic hypertension in pregnancy. N Engl J Med 365: 439–446, 2011. doi: 10.1056/NEJMcp0804872. [DOI] [PubMed] [Google Scholar]
- 168.Semplicini A, Lenzini L, Sartori M, Papparella I, Calò LA, Pagnin E, Strapazzon G, Benna C, Costa R, Avogaro A, Ceolotto G, Pessina AC. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. J Hypertens 24: 1115–1124, 2006. doi: 10.1097/01.hjh.0000226202.80689.8f. [DOI] [PubMed] [Google Scholar]
- 169.Shi GX, Harrison K, Han SB, Moratz C, Kehrl JH. Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: implications for G protein-coupled receptor signaling. J Immunol 172: 5175–5184, 2004. [DOI] [PubMed] [Google Scholar]
- 170.Shi GX, Harrison K, Wilson GL, Moratz C, Kehrl JH. RGS13 regulates germinal center B lymphocytes responsiveness to CXC chemokine ligand (CXCL)12 and CXCL13. J Immunol 169: 2507–2515, 2002. [DOI] [PubMed] [Google Scholar]
- 171.Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol 293: R191–R199, 2007. doi: 10.1152/ajpregu.00047.2007. [DOI] [PubMed] [Google Scholar]
- 172.Siedlecki A, Anderson JR, Jin X, Garbow JR, Lupu TS, Muslin AJ. RGS4 controls renal blood flow and inhibits cyclosporine-mediated nephrotoxicity. Am J Transplant 10: 231–241, 2010. doi: 10.1111/j.1600-6143.2009.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Singh KD, Karnik SS. Angiotensin Receptors: Structure, Function, Signaling and Clinical Applications. J Cell Signal 1: 111, 2016. doi: 10.4172/jcs.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sinnarajah S, Dessauer CW, Srikumar D, Chen J, Yuen J, Yilma S, Dennis JC, Morrison EE, Vodyanoy V, Kehrl JH. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature 409: 1051–1055, 2001. doi: 10.1038/35059104. [DOI] [PubMed] [Google Scholar]
- 175.Sivapalaratnam S, Basart H, Watkins NA, Maiwald S, Rendon A, Krishnan U, Sondermeijer BM, Creemers EE, Pinto-Sietsma SJ, Hovingh K, Ouwehand WH, Kastelein JJ, Goodall AH, Trip MD. Monocyte gene expression signature of patients with early onset coronary artery disease. PLoS One 7: e32166, 2012. doi: 10.1371/journal.pone.0032166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sjögren B. Regulator of G protein signaling proteins as drug targets: current state and future possibilities. Adv Pharmacol 62: 315–347, 2011. doi: 10.1016/B978-0-12-385952-5.00002-6. [DOI] [PubMed] [Google Scholar]
- 177.Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker D, Biederman J, Rosenbaum JF, Gelernter J, Stein MB. Influence of RGS2 on anxiety-related temperament, personality, and brain function. Arch Gen Psychiatry 65: 298–308, 2008. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- 178.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 359: 2767–2777, 2008. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song D, Nishiyama M, Kimura S. Potent inhibition of angiotensin AT1 receptor signaling by RGS8: importance of the C-terminal third exon part of its RGS domain. J Recept Signal Transduct Res 36: 478–487, 2016. doi: 10.3109/10799893.2015.1130056. [DOI] [PubMed] [Google Scholar]
- 180.Stewart A, Fisher RA. Introduction: G Protein-coupled Receptors and RGS Proteins. Prog Mol Biol Transl Sci 133: 1–11, 2015. doi: 10.1016/bs.pmbts.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 181.Stewart A, Huang J, Fisher RA. RGS Proteins in Heart: Brakes on the Vagus. Front Physiol 3: 95, 2012. doi: 10.3389/fphys.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Strakova Z, Kumar A, Watson AJ, Soloff MS. A new linear V1A vasopressin antagonist and its use in characterizing receptor/G protein interactions. Mol Pharmacol 51: 217–224, 1997. doi: 10.1124/mol.51.2.217. [DOI] [PubMed] [Google Scholar]
- 183.Stuebe S, Wieland T, Kraemer E, Stritzky A, Schroeder D, Seekamp S, Vogt A, Chen CK, Patten M. Sphingosine-1-phosphate and endothelin-1 induce the expression of rgs16 protein in cardiac myocytes by transcriptional activation of the rgs16 gene. Naunyn Schmiedebergs Arch Pharmacol 376: 363–373, 2008. doi: 10.1007/s00210-007-0214-2. [DOI] [PubMed] [Google Scholar]
- 184.Takigawa M, Sakurai T, Kasuya Y, Abe Y, Masaki T, Goto K. Molecular identification of guanine-nucleotide-binding regulatory proteins which couple to endothelin receptors. Eur J Biochem 228: 102–108, 1995. doi: 10.1111/j.1432-1033.1995.tb20236.x. [DOI] [PubMed] [Google Scholar]
- 185.Tamirisa P, Blumer KJ, Muslin AJ. RGS4 inhibits G-protein signaling in cardiomyocytes. Circulation 99: 441–447, 1999. doi: 10.1161/01.CIR.99.3.441. [DOI] [PubMed] [Google Scholar]
- 186.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab 71: 1675–1677, 1990. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 187.Taylor VG, Bommarito PA, Tesmer JJ. Structure of the Regulator of G Protein Signaling 8 (RGS8)-Gαq Complex: MOLECULAR BASIS FOR Gα SELECTIVITY. J Biol Chem 291: 5138–5145, 2016. doi: 10.1074/jbc.M115.712075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tikhonova IG, Boulègue C, Langer I, Fourmy D. Modeled structure of the whole regulator G-protein signaling-2. Biochem Biophys Res Commun 341: 715–720, 2006. doi: 10.1016/j.bbrc.2005.12.221. [DOI] [PubMed] [Google Scholar]
- 189.Tobin AB. G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol 153, Suppl 1: S167–S176, 2008. doi: 10.1038/sj.bjp.0707662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Toh HC, Sun L, Soe Y, Wu Y, Phoon YP, Chia WK, Wu J, Wong KY, Tan P. G-CSF induces a potentially tolerant gene and immunophenotype profile in T cells in vivo. Clin Immunol 132: 83–92, 2009. doi: 10.1016/j.clim.2009.03.509. [DOI] [PubMed] [Google Scholar]
- 191.Tseng CC, Zhang XY. Role of regulator of G protein signaling in desensitization of the glucose-dependent insulinotropic peptide receptor. Endocrinology 139: 4470–4475, 1998. doi: 10.1210/endo.139.11.6282. [DOI] [PubMed] [Google Scholar]
- 192.Tu Y, Woodson J, Ross EM. Binding of regulator of G protein signaling (RGS) proteins to phospholipid bilayers. Contribution of location and/or orientation to Gtpase-activating protein activity. J Biol Chem 276: 20160–20166, 2001. doi: 10.1074/jbc.M101599200. [DOI] [PubMed] [Google Scholar]
- 193.Tuomi JM, Chidiac P, Jones DL. Evidence for enhanced M3 muscarinic receptor function and sensitivity to atrial arrhythmia in the RGS2-deficient mouse. Am J Physiol Heart Circ Physiol 298: H554–H561, 2010. doi: 10.1152/ajpheart.00779.2009. [DOI] [PubMed] [Google Scholar]
- 194.von Buchholtz L, Elischer A, Tareilus E, Gouka R, Kaiser C, Breer H, Conzelmann S. RGS21 is a novel regulator of G protein signalling selectively expressed in subpopulations of taste bud cells. Eur J Neurosci 19: 1535–1544, 2004. doi: 10.1111/j.1460-9568.2004.03257.x. [DOI] [PubMed] [Google Scholar]
- 195.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang J, Zhou Y, Fei X, Chen X, Zhu Z. Regulator of G-protein signaling 3 targeted by miR-126 correlates with poor prognosis in gastric cancer patients. Anticancer Drugs 28: 161–169, 2017. doi: 10.1097/CAD.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 197.Wang Q, Liu M, Mullah B, Siderovski DP, Neubig RR. Receptor-selective effects of endogenous RGS3 and RGS5 to regulate mitogen-activated protein kinase activation in rat vascular smooth muscle cells. J Biol Chem 277: 24949–24958, 2002. doi: 10.1074/jbc.M203802200. [DOI] [PubMed] [Google Scholar]
- 198.Wang X, Huang G, Luo X, Penninger JM, Muallem S. Role of regulator of G protein signaling 2 (RGS2) in Ca(2+) oscillations and adaptation of Ca(2+) signaling to reduce excitability of RGS2-/- cells. J Biol Chem 279: 41642–41649, 2004. doi: 10.1074/jbc.M406450200. [DOI] [PubMed] [Google Scholar]
- 199.Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, Muallem S. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol 7: 405–411, 2005. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 200.Wolff DW, Xie Y, Deng C, Gatalica Z, Yang M, Wang B, Wang J, Lin MF, Abel PW, Tu Y. Epigenetic repression of regulator of G-protein signaling 2 promotes androgen-independent prostate cancer cell growth. Int J Cancer 130: 1521–1531, 2012. doi: 10.1002/ijc.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ho MK, Wong YH. G(z) signaling: emerging divergence from G(i) signaling. Oncogene 20: 1615–1625, 2001. doi: 10.1038/sj.onc.1204190. [DOI] [PubMed] [Google Scholar]
- 202.Xie Z, Geiger TR, Johnson EN, Nyborg JK, Druey KM. RGS13 acts as a nuclear repressor of CREB. Mol Cell 31: 660–670, 2008. doi: 10.1016/j.molcel.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xie Z, Liu D, Liu S, Calderon L, Zhao G, Turk J, Guo Z. Identification of a cAMP-response element in the regulator of G-protein signaling-2 (RGS2) promoter as a key cis-regulatory element for RGS2 transcriptional regulation by angiotensin II in cultured vascular smooth muscles. J Biol Chem 286: 44646–44658, 2011. doi: 10.1074/jbc.M111.265462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xie Z, Yang Z, Druey KM. Phosphorylation of RGS13 by the cyclic AMP-dependent protein kinase inhibits RGS13 degradation. J Mol Cell Biol 2: 357–365, 2010. doi: 10.1093/jmcb/mjq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yeung EH, Liu A, Mills JL, Zhang C, Männistö T, Lu Z, Tsai MY, Mendola P. Increased levels of copeptin before clinical diagnosis of preeclampsia. Hypertension 64: 1362–1367, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ying L, Lin J, Qiu F, Cao M, Chen H, Liu Z, Huang Y. Epigenetic repression of regulator of G-protein signaling 2 by ubiquitin-like with PHD and ring-finger domain 1 promotes bladder cancer progression. FEBS J 282: 174–182, 2015. doi: 10.1111/febs.13116. [DOI] [PubMed] [Google Scholar]
- 207.Yowe D, Weich N, Prabhudas M, Poisson L, Errada P, Kapeller R, Yu K, Faron L, Shen M, Cleary J, Wilkie TM, Gutierrez-Ramos C, Hodge MR. RGS18 is a myeloerythroid lineage-specific regulator of G-protein-signalling molecule highly expressed in megakaryocytes. Biochem J 359: 109–118, 2001. doi: 10.1042/bj3590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zalewska M, Siara M, Sajewicz W. G protein-coupled receptors: abnormalities in signal transmission, disease states and pharmacotherapy. Acta Pol Pharm 71: 229–243, 2014. [PubMed] [Google Scholar]
- 209.Zeng W, Xu X, Popov S, Mukhopadhyay S, Chidiac P, Swistok J, Danho W, Yagaloff KA, Fisher SL, Ross EM, Muallem S, Wilkie TM. The N-terminal domain of RGS4 confers receptor-selective inhibition of G protein signaling. J Biol Chem 273: 34687–34690, 1998. doi: 10.1074/jbc.273.52.34687. [DOI] [PubMed] [Google Scholar]
- 210.Zerangue N, Jan LY. G-protein signaling: fine-tuning signaling kinetics. Curr Biol 8: R313–R316, 1998. doi: 10.1016/S0960-9822(98)70196-4. [DOI] [PubMed] [Google Scholar]
- 211.Zhang P, Mende U. Regulators of G-protein signaling in the heart and their potential as therapeutic targets. Circ Res 109: 320–333, 2011. doi: 10.1161/CIRCRESAHA.110.231423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang S, Watson N, Zahner J, Rottman JN, Blumer KJ, Muslin AJ. RGS3 and RGS4 are GTPase activating proteins in the heart. J Mol Cell Cardiol 30: 269–276, 1998. doi: 10.1006/jmcc.1997.0591. [DOI] [PubMed] [Google Scholar]
- 213.Zhang Y, Neo SY, Han J, Yaw LP, Lin SC. RGS16 attenuates galphaq-dependent p38 mitogen-activated protein kinase activation by platelet-activating factor. J Biol Chem 274: 2851–2857, 1999. doi: 10.1074/jbc.274.5.2851. [DOI] [PubMed] [Google Scholar]
- 214.Zheng B, De Vries L, Gist Farquhar M. Divergence of RGS proteins: evidence for the existence of six mammalian RGS subfamilies. Trends Biochem Sci 24: 411–414, 1999. doi: 10.1016/S0968-0004(99)01474-7. [DOI] [PubMed] [Google Scholar]
- 215.Zhou J, Moroi K, Nishiyama M, Usui H, Seki N, Ishida J, Fukamizu A, Kimura S. Characterization of RGS5 in regulation of G protein-coupled receptor signaling. Life Sci 68: 1457–1469, 2001. doi: 10.1016/S0024-3205(01)00939-0. [DOI] [PubMed] [Google Scholar]
- 216.Zuber AM, Singer D, Penninger JM, Rossier BC, Firsov D. Increased renal responsiveness to vasopressin and enhanced V2 receptor signaling in RGS2-/- mice. J Am Soc Nephrol 18: 1672–1678, 2007. doi: 10.1681/ASN.2007010032. [DOI] [PubMed] [Google Scholar]