Abstract
Beyond avoiding risky behavior—smoking, substance abuse, obesity—and embracing healthy habits like exercise, a balanced diet, and non-obese body weight, are there things we each do today to significantly extend our lifespan? Caloric restriction is the only behavioral intervention consistently shown to extend both mean and maximal lifespan across a wide range of species. In most cases, the lifespan extension is accompanied by a marked delay in the onset of age-associated disease and infirmity.
“If you live to be one hundred, you’ve got it made. Very few people die past that age.”
-George Burns
Introduction
Public health measures like sanitary sewers and water purification, together with the discovery of antibiotics and vaccines, have greatly extended human lifespan. An American born today can expect to live an average of 78.7 years, although significant regional and racial disparities exist. Have humans living in western societies in the 21st century achieved maximum lifespan, or are there additional interventions that could extend lifespan further?
There are many things everyone can still do to increase their likelihood of a long life: don’t smoke, wear sunscreen, engage in regular exercise, maintain a healthy body weight, don’t drive while texting. Some factors are beyond our control, such as genetics. But which of us would not eat or drink a magic elixir that would make us live longer? The best medical evidence suggests that it’s not what we eat, but what we don’t eat, that could add years to our lives.
Aging is the confluence of age-associate conditions ranging from wrinkles and baldness to cardiovascular disease and cancer. Aging-associated diseases can and do occur in young patients with either genetic predisposition or due to environmental insults. However, these diseases will eventually develop in aging individuals even without any specific genetic or environmental etiology if they live long enough. The result is an age-dependent increase in personal suffering and a substantial societal burden imposed by the aging demographic. Increased lifespan free of chronic illness is a highly desirable goal.
In a previous issue of Missouri Medicine, I discussed the theory that progressive loss of DNA from the ends of our chromosomes could be mechanistically associated with aging.1 As I pointed out then, “[t]he most consistent predictor of longevity in every organism tested is caloric restriction.” Here, I summarize some of the evidence that caloric restriction can extend healthy lifespan.
Caloric Restriction and Longevity
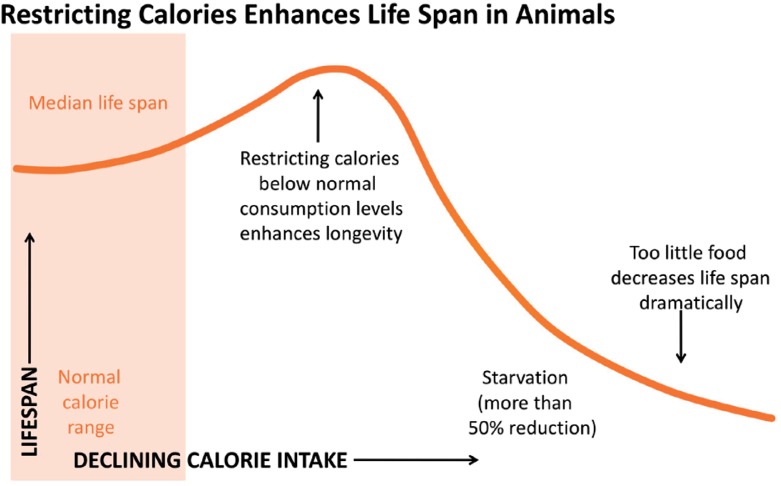
Caloric restriction refers to reduction in caloric intake short of malnutrition, typically a reduction of 20–50% compared to ad libidum. The association between caloric restriction and longevity has been known for over eighty years.2 Studies in yeast, protozoa, nematodes, insects, fish and mammals have so far shown that caloric restriction is the only known intervention consistently and reproducibly associated with prolonging lifespan.3,4 In some experiments, the lifespan extension can be 1.5–3 fold! The longevity benefit of caloric restriction follows a U-shaped curve; modest-to-significant caloric restriction can significantly extend lifespan, but when caloric restriction becomes frank starvation, the longevity benefit is reversed (Figure 1).
Figure 1.
The benefits of caloric restriction follow a U-shaped curve.
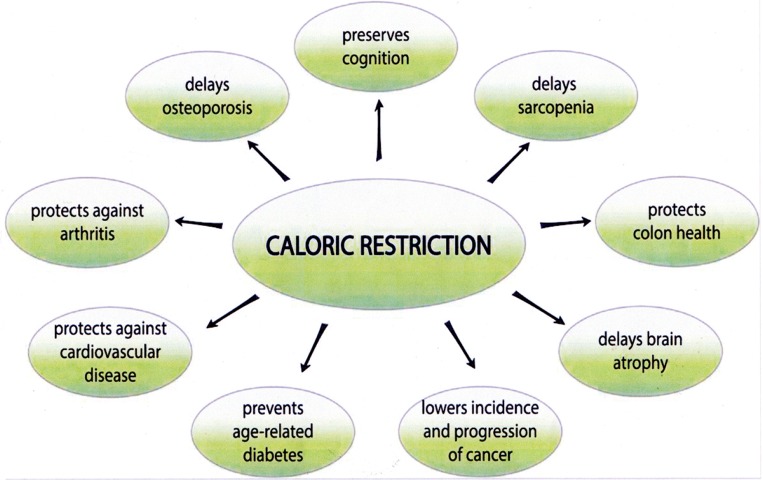
But how relevant are the data in model organisms, which tend to be genetically homogeneous and are tested under highly constrained environmental conditions, to genetically diverse, free-living humans? Various large- and small-scale studies over the past 60 years suggest that caloric restriction can slow age-related degeneration in metabolic regulation, muscloskeletal systems and cognition (Figure 2).5 The most complete study on the effect of caloric restriction in humans was the CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) study, which ran from 2007 to 2015. The goal of the CALERIE study was to test the hypothesis that two years of sustained 25% caloric restriction in men age 21–50 and women age 21–47 can slow aging and protect against age-related cardiovascular disease risk, insulin sensitivity and secretion, immune function, neuroendocrine function, cognition and quality of life.6 Two hundred and thirty-eight volunteers were enrolled, 220 were randomized, and 218 started the assigned intervention. The results demonstrated some of the caloric restriction-dependent effects reported for mammalian models, such as improving cardiovascular biomarkers and insulin sensitivity.7,8 It seems likely that at least some of the exceptions are due to the more modest caloric restriction achieved by participants in the CALERIE study, compared to the enforced restriction imposed in the animal studies.7
Figure 2.
Proposed benefits from long-term caloric restriction.
From Reference 5.
All Calories Are Not Equal
A standard human diet contains various classes of macronutrients (carbohydrates, proteins, fats) and micronutrients (vitamins, minerals). Recent studies in performed on the macronutrients involved in caloric restriction indicate that the ratio of protein to carbohydrates is important.9–11 Specifically, Simpson et al.11 conclude that enhanced longevity is associated with an optimum ratio of protein to carbohydrates of about 1:10, with the protein content of the diet as low as 10%. Within the category of dietary protein, there is evidence that reducing the intake of the essential amino acids methionine and tryptophan, as well as branched chain amino acids, can delay aging in several animal models.10,12 The typical American diet consists of 15% protein, 50% carbohydrate and 35% fat,13 so even without caloric restriction, this would entail a 33% reduction in average calories from protein.
Better Living Through Geroprotective Chemistry?
Let’s face it: caloric restriction is uncomfortable. Sure, you get to live longer, but is that really “living?” If we could take a pill that mimicked the anti-aging effects of caloric restriction without the discomfort of eating less, lots of folks would be on board. Not surprisingly, there is considerable interest in research and development of caloric restriction mimetics. Also called “geroprotectors,” these compounds have been shown to prolong lifespan in model systems, making them candidates for geroprotecting drugs in humans. Some examples that have attracted recent attention.
Resveritrol
Resveritrol is a naturally occurring polyphenol and secondary plant metabolite enriched in peanuts, pistachios, grapes, red and white wine, blueberries, cranberries, cocoa and dark chocolate. In cells, resveritrol enhances the activity of Sirt1, an enzyme that removes acetyl groups from selected proteins, thereby changing cell metabolism and increasing mitochondrial function.14 Evidence that resveritrol is geroprotective comes from a number of model organism studies, although results have been inconsistent.15 Some human studies showed promising improvements in markers associated with longevity using controlled dosages of resveritrol.15 However, field studies using dietary resveritrol are not so promising. For example, a cohort of 283 men and women over the age of 65 in the Chianti region of Italy were followed from 1998–2009, and total urinary resveritrol metabolites and all-cause mortality, as well as markers for inflammation, were recorded.16 No significant association was found between total urinary resveritrol metabolites and inflammatory markers, cardiovascular disease or cancer, nor were they predictive of all-cause mortality. This suggests that dietary levels of resveritrol may not have the lifespan-extending effects in humans that were found in studies of superdietary levels of resveritrol in laboratory studies of model organisms.15
Rapamycin
Rapamycin was initially isolated as an antibiotic secreted by the soil bacterium Streptomyces hygroscopicus found on Easter Island (known locally as Rapa Nui).17 Rapamycin has long been used clinically as an immunosuppressant to prevent organ transplant rejection. Several studies found that rapamycin can extend lifespan in mice.18–22 One report describes the use of rapamycin to improve immune parameters in aged humans.23 However, adverse metabolic side effects such as insulin resistance and dyslipidemia make chronic rapamycin use as a lifespan-extending drug in humans untenable.24 Alternatives, such as combinations of low-dose rapamycin with other drugs, as well as rapamycin analogs that lack the side effects, are the subject of active research.25,26
Metformin
The oral drug metformin is a safe frontline therapy for prevention and treatment of type 2 diabetes. Compared to alternative anti-diabetics, it is also reported to decrease cardiovascular disease risk, cancer incidence, and overall mortality.27,28 The association of metformin with longevity has been extensively documented in nematodes29 and rodent models.30–32 In mice, metformin causes similar changes in liver gene expression profiles as caloric restriction.33
Can Metformin extend longevity in humans? An interdisciplinary consortium is currently pursuing a large-scale clinical trial called the “Targeting Aging with MEtformin (TAME)” project, sponsored by the American Federation for Aging Research.34 TAME is a double-blind, placebo-control study to test whether metformin can delay the onset of diseases such as cancer, cardiovascular disease and cognitive decline, as well as delay mortality. If so, it will catalyze the development of additional drugs aimed at treating human aging and improving resiliency in older adults.35
For cancer and HIV drugs, the current approach is to use combination therapy with multiple drugs that target different pathways. Among its advantages, combination therapies can mean reduced dosages compared to using a single drug. Thus, the future of geroprotectives may lie in the compounding of several molecules, perhaps tailored to the specific physiology of each patient.36
Caloric Restriction vs. Intermittent Fasting
For free-living animals in the wild (including our ancestors at the time Homo sapiens first appeared), intermittent aperiodic feeding and fasting based on food availability is a constant reality. Given the chronic discomfort that most people experience from caloric restriction, considerable interest has focused on whether the benefits associated with chronic caloric restriction can be achieved through intermittent fasting. Intermittent fasting entails going for ca. 16–48 hours without energy intake, alternating with periods of normal feeding.37
In rodents, lifespan can increase by up to 30% as a result of a 24-hour fast every other day or twice a week and can slow or reverse cancer, cardiovascular disease, diabetes, and neurodegenerative disorders in animal models.38,39 Reliable data on the effects of intermittent fasting and longevity in humans would require large numbers of fasting and matched control individuals followed over several decades. But somewhat more limited studies using biological markers of age-related decline have shown promising results. In a study of 107 overweight or obese women aged 30–45 years, the effects on various biomarkers was compared for 25% energy restriction over a six month period using either continuous energy restriction (∼6276 kJ/d, 7d/wk) or intermittent energy restriction (∼2710 kJ/d, 2 d/wk).40 Both groups showed similar weight loss, reductions in C-reactive protein, total and LDL cholesterol, triglycerides, insulin, insulin resistance and blood pressure, suggesting that intermittent fasting is an plausible alternative to chronic caloric restriction in reducing markers of aging. Whether similar benefits accrue to people with healthy body mass is still an open question.
Caloric Restriction and Exercise
The typical consequence of caloric restriction is a loss of lean body mass. Does this imply a loss of robustness as a necessary consequence? In humans, physical activity has been shown to lower mortality rates.41,42 The benefits of moderate exercise, like the benefits of caloric restriction or intermittent fasting, are thought to be examples of “hormesis,” the chronic or intermittent exposure to low-grade negative stress that leads to long-term enhanced resilience.43,44 As such, the lifespan-extending benefit of exercise may lie primarily in opposing the negative stress of obesity and metabolic disorders such as diabetes and cardiovascular disease. Still, exercise is proven treatment to reverse or prevent age-dependent muscle wasting.45 A randomized control study of 48 non-obese individuals found that both caloric restriction and exercise-induced negative energy balance results in similar and substantial reduction in risk factors for coronary heart disease.46 Interestingly, a literature survey of 44 observational studies concluded that there is an inverse linear dose-response relation between volume of physical activity and all-cause mortality rates in humans.47 Thus, like in many things, moderation in the dose and duration of exercise is likely to provide the optimal benefit.
Inflammaging
Normally, inflammation is a protective response our bodies mount to infection and tissue injury. This response is self-limiting, abating after a period of hours or days. Dysregulation of inflammation, however, can lead to pathological conditions. Acute dysregulation of the inflammatory response can lead to septic shock. Chronic dysregulation of the normal inflammatory response during aging, called “inflammaging,” results in a low-grade inflammatory state that can drive such age-associated conditions as rheumatoid arthritis, atherosclerosis, asthma and various autoimmune syndromes, cancer and Alzheimer disease.48
A general decline in immune function accompanies aging, a phenomenon called immunosenescence. Immunosensence leads to a general decline in immune function with a higher risk of infection and of cancer. But paradoxically, immunosenescence also leads to immune hyper-responsiveness, with increased risk of autoimmune and inflammatory diseases.49
Inflammaging is mediated primarily by small molecules called cytokines that trigger the recruitment and activation of immune cells (T cells, B cells, macrophages) to respond to the infection. Secretion of cytokines is in turn triggered by the innate immune system. It appears that aging may be accompanied by age-associated reprogramming of cells of the adaptive immune system to innate immune responsiveness.50 Age-associated increase in adiposity and the recruitment of macrophages in inflamed tissues contribute to chronic insulin resistance and metabolic dysregulation by increasing levels of pro-inflammatory cytokines.51
Recent evidence suggests that caloric restriction can attenuate the cellular markers of inflammaging.52 Although the mechanisms are still being defined, they may include the elevation of anti-inflammatory glucocorticoids, reduction of blood glucose levels (reducing oxidation and oxidative stress) and activation of the nuclear hormone superfamily of peroxisome proliferator-activated receptors.52
“Lifespan” vs. “Healthspan”
In the Greek myth of Eos (goddess of the dawn) and her lover, Tithonus, Eos asks Zeus to grant Tithonus immortality, but forgets to ask also for eternal youth. Accordingly, Tithonus ages like all mortals, gradually shrivelling and shrinking with age and begins to babble continuously. Eventually he is transformed into a cicada. None of us wants to spend an extended lifespan shriveling through those extra years. The goal of geroscience is to increase “healthspan” as well as lifespan. Healthspan is the length of time during a person’s life when they are in optimal health/functional capacity (Figure 3). Put another way, geroscience seeks to add life to years and not just years to life. The promise of caloric restriction and intermittent fasting is a delay in the decline in physical and cognitive functions that accompany aging. Based on the current evidence, continued regular exercise should contribute to improved healthspan.
Figure 3.
The goal of lifespan/healthspan extension is to extend both the age before death and to maintain functional capacity while increasing lifespan. This may also be accompanied by a slowing of the age-related decline in functional capacity.
From Reference 56.
Conclusion
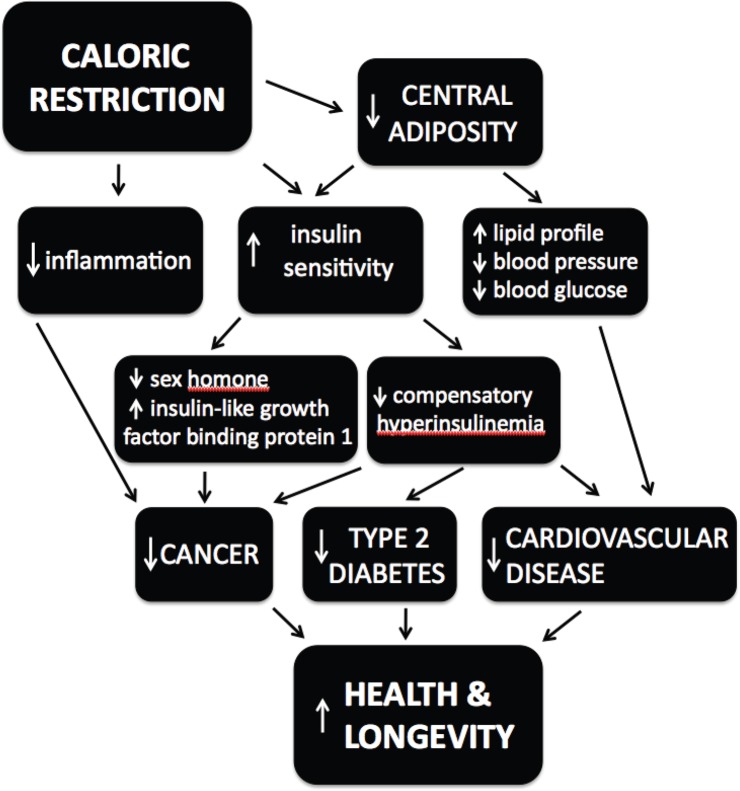
Taken together, the weight of evidence supports the conclusion that if you want to increase your lifespan and maintain good health into old age, watch what you eat and how much of it. Caloric restriction acts through multiple pathways and mechanisms to attenuate the primary causes of age-related disease (Figure 4). It remains unclear to what extent the effects on longevity and healthspan reported for caloric restriction in humans are due primarily to decreased caloric intake versus a high quality diet.53 However, the data suggest that reduced caloric intake in humans prior to the onset of old age improves healthspan54 and delays cardiovascular aging, one of the main causes of death in humans.55 Whether there is any longevity benefit for caloric restriction begun after the age of 60 is unknown; indeed, weight loss may increase mortality in this demographic.
Figure 4.
Hierarchical model for the effects of caloric restriction on health and longevity.
Modified from Reference 8.
The appeal of increasing lifespan through caloric restriction is balanced by the reality that caloric restriction is unpleasant; it may or may not extend your life, but it will certainly make your life feel longer. This difficulty is compounded by the fact that in western cultures, three meals a day with between-meal snacks are a cultural norm. Extending healthspan is a goal worth striving for, in the face of habit, discomfort, and peer pressure. With or without caloric restriction, healthy diet, regular exercise, and positive social engagement are proven strategies to attain that goal.
Biography
Joel C. Eissenberg, PhD, is a Professor and Associate Dean for Research, Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine.
Contact: eissenjc@slu.edu

References
- 1.Eissenberg JC. Telomeres, cancer & aging: Live long & prosper? Missouri Med. 2013;110:11–16. [PMC free article] [PubMed] [Google Scholar]
- 2.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition. 1935;5:155–171. [PubMed] [Google Scholar]
- 3.Le Bourg E. Predicting whether dietary restriction would increase longevity in species not tested so far. Ageing Res Rev. 2010;9:289–297. doi: 10.1016/j.arr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Testa G, Biasi F, Poli G, Chiarpotto E. Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity. Curr Pharm Des. 2014;20:2950–2977. doi: 10.2174/13816128113196660699. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian P, Howell PR, Anderson RM. Aging and caloric restriction research: A biological perspective with translational potential. EBioMedicine. 2017 doi: 10.1016/j.ebiom.2017.06.015. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, Hadley EC, Kraus WE. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66:97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein R, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Robers SB, for the CALERIE Study Group A 2-Year randomized controlled trial of human caloric restriction: Feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.08.005. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson SJ, Le Couteur DG, Raubenheimer D, Solon-Biet SM, Conney GJ, Cogger VC, Fontana L. Dietary protein, aging and nutritional geometry. Aging Res Rev. 2017 doi: 10.1016/j.arr.2017.03.001. [DOI] [Google Scholar]
- 12.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Alexander CM, Kimple ME, Lamming DW. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16:520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Last AR, Wilson SA. Low-carbohydrate diets. Am Fam Physician. 2006;73:1942–1948. [PubMed] [Google Scholar]
- 14.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallauf K, Rimbach G, Rupp PM, Chin D, Wolf IMA. Resveratrol and lifespan in model organisms. Curr Med Chem. 2016;23:4639–4680. doi: 10.2174/0929867323666161024151233. [DOI] [PubMed] [Google Scholar]
- 16.Semba RD, Ferrucci L, Bartali B, Urpi-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S, Andres-Lacueva C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Int Med. 2014;174:1077–1084. doi: 10.1001/jamainternmed.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 18.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon L, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandezr E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature. 2009;460:392–396. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wildinson JE, Nadon L, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous 53+/− mice. Aging. 2012;4:709–714. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH, 3rd, Becker KG, Perez VI, Richardson A. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2013;9:e83988. doi: 10.1371/journal.pone.0083988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, Tung H, Vizzini N, Chen B, Smith K, Meza D, Yajima M, Beyer RP, Kerr KF, Davis DJ, Gillespie CH, Snyder JM, Treuting PM, Kaeberlein M. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife. 2016;5:e16351. doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009892. 268ra179. [DOI] [PubMed] [Google Scholar]
- 24.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, Lamming DW. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016;15:28–38. doi: 10.1111/acel.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Ivest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskalev A, Chernyagina E, Tsvetkov V, Fedintsev A, Shaposhnikov M, Krut’ko V, Zhavoronkov A, Kennedy BK. Developing criteria for evaluation of geroprotectors as a key stage toward translation to the clinic. Aging Cell. 2016;15:407–415. doi: 10.1111/acel.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 30.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DL, Jr, Elam CF, Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010;65:468–474. doi: 10.1093/gerona/glq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23:343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- 34.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huffman DM, Schafer MJ, LeBrasseru NK. Energetic interventions for healthspan and resiliency with aging. Expt Geront. 2016;86:73–83. doi: 10.1016/j.exger.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blagosklonny MV. From rapalogs to anti-aging formula. Oncotarget. 2017;8:35492–35507. doi: 10.18632/oncotarget.18033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Re Rev. 2016 doi: 10.1016/j.arr.2016.10.005. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FAJL, Seyfried TN, Varady KA, Panda S. Meal frequency and timing in health and disease. Proc Natl Acad Sci USA. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longo VD, Mattson MP. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers. A randomized trial in young overweight women. Int J Obesity. 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol. 2011;40:1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 42.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 2012;9:e1001335. doi: 10.1371/journal.pmed.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging. 2011;3:821–828. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci. 2010;1211:25–36. doi: 10.1111/j.1749-6632.2010.05809.x. [DOI] [PubMed] [Google Scholar]
- 46.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO, Washington Uniersity School of Medicine CALERIE Group Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 47.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose– response relation? Med Sci Sports Exerc. 2001;33:S459–S471. doi: 10.1097/00005768-200106001-00016. [DOI] [PubMed] [Google Scholar]
- 48.Goto M. Inflammaging (inflammation+aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? BioSci Trends. 2008;2:218–230. [PubMed] [Google Scholar]
- 49.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Pereira BI, Akbar AN. Convergence of innate and adaptive immunity during human aging. Frontiers Immun. 2016;7:445. doi: 10.3389/fimmu.2016.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riera CE, Dillin A. Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol. 2015;17:196–203. doi: 10.1038/ncb3107. [DOI] [PubMed] [Google Scholar]
- 52.Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. In: Mobbs CV, Yen K, Hof PR, editors. Interdiscipl Top erontol. Vol. 35. Basel: Karger; 2007. pp. 83–97. In: “Mechanisms of Dietary Restriction in Aging and Disease. [DOI] [PubMed] [Google Scholar]
- 53.Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. 2014;13:38–45. doi: 10.1016/j.arr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Anderson RM, Weindruch R. The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol. 2012;24:101–106. doi: 10.1002/ajhb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bales CW, Kraus WE. Caloric restriction: implications for human cardiometabolic health. J Cardiopulm Rehabil Prev. 2013;33:201–208. doi: 10.1097/HCR.0b013e318295019e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brett JO, Rando TA. Alive and well? Exploring disease by studying lifespan. Curr Opin Genet Dev. 2014;26:33–40. doi: 10.1016/j.gde.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]