Abstract
Zika virus (ZIKV) has been linked to intrauterine growth restriction (IUGR), spontaneous miscarriage, and microcephaly in infants of women infected during pregnancy. To determine how ZIKV affects the fetus, we infected pregnant mice subcutaneously (mimicking a mosquito bite) with ZIKV. Multiple techniques revealed that ZIKV replicated within placental trophoblasts, fetal endothelial cells, and the fetal neocortex. We also noted severe placental defects, IUGR, and fetal death. Thus, our mouse model recapitulated ZIKV infection in human pregnancy and demonstrated that ZIKV can be transmitted from mother to fetus via the placenta.
Introduction to Zika Virus
Zika virus (ZIKV) is an insect-borne virus similar to Dengue, yellow fever, Japanese encephalitis, and West Nile viruses. Like other members of the family flaviviridae and the genus flavivirus, ZIKV is enveloped and icosahedral and has a non-segmented, single-stranded, positive-sense RNA genome. It is transmitted to humans and non-human primates by the Asian tiger mosquito Aedes aegypti.1
Origins and Symptoms of ZIKV
ZIKV is named after the place where it was first identified and isolated in 1947: the Ziika Forest in Uganda, Central Africa. The virus was identified as a causal agent in small outbreaks in a narrow equatorial belt from Africa to Asia in the 1950s and 1960s. In 2007, an epidemic was recorded on the island of Yap in the Federated States of Micronesia. This outbreak was initially thought to be Dengue virus, but serological analyses identified the virus as ZIKV. During the first 50 years after it was discovered, ZIKV was not regarded as a significant pathogen, as the majority of infections were asymptomatic. Even symptomatic cases of ZIKV present with mild symptoms that clear within three to five days, including fever, headache, muscle and joint pain, conjunctivitis, and skin rash.2 However, in 2007, a ZIKV outbreak in French Polynesia received attention from the Centers for Disease Control because a number of infected individuals presented with neurological symptoms including temporary paralysis, which were later diagnosed as Guillain-Barré Syndrome. Between 2007 and 2016, ZIKV spread eastward to the Americas, leading to the current global epidemic. In early 2015, a major ZIKV outbreak was recorded in Bahia, Brazil, and was confirmed as resulting from viral transmission from French Polynesia. The Bahia ZIKV outbreak was followed by similar outbreaks in neighboring regions, leading the Brazilian Ministry of Health to issue a ZIKV alert in April 2015.
Fetal Outcomes Associated with Maternal ZIKV Infection
In the past year, the incidences of Guillain Barré syndrome in all age groups 3 and microcephaly in newborns of infected mothers4 have both increased. As a result, the World Health Organization declared ZIKV a public health emergency in February 2016.5 Although the relationship between ZIKV infection in pregnant women and microcephaly in their infants was at first unclear, data now strongly indicate that ZIKV infection during pregnancy causes microcephaly. For example, a prospective cohort study of symptomatic, ZIKV-infected pregnant women showed that 29% of fetuses exhibited developmental abnormalities including microcephaly, IUGR, fetal demise, and stillbirth.6 The first trimester of pregnancy is a crucial period for brain development, and ZIKV infection during this time is likely to be more strongly associated with microcephaly than infections later in pregnancy, as demonstrated in French Polynesia. 7, 8
Scope of the Problem
At present, no specific vaccines or treatment for ZIKV infection are available because, in part, we have limited knowledge of the molecular pathogenesis of the virus. Because of the growing public health concern, we urgently needed to establish animal models of ZIKV infection that define mechanisms of maternal-fetal transmission, satisfy the criteria for proof of teratogenicity8, and facilitate testing of therapeutic modalities and candidate vaccines (reviewed in9).
A Mouse Model of ZIKV Infection in Pregnancy
Recently, we developed a mouse model of ZIKV infection during pregnancy.10 We used ZIKV strain H/ PF/2013 (isolated in French Polynesia in 2013) propagated in kidney epithelial cells (called Vero cells) under biosafety level 2 or 3 containment. Flaviviruses such as ZIKV have evolved to cause disease by overcoming one of the host immune defense pathways in humans and non-human primates, type I interferon–receptor (IFNAR1) signaling. However, ZIKV cannot efficiently overcome murine IFNAR1 signaling and thus normally would not infect a mouse. Therefore, we used mice in which both copies of the Ifnar1 gene were defective (Ifnar1−/−). Because we wanted the fetuses to have a largely intact interferon response, we mated Ifnar1−/− females to wild-type males, thereby producing fetuses that had one functioning copy of the Ifnar1 gene. Mice were allowed to breed for a short time so that we could accurately follow fetal development. At embryonic day 6.5 (E6.5; gestation in mice is 19–21 days, thus E6.5 is analogous to the first trimester of pregnancy in women), we injected ZIKV into the mice subcutaneously to mimic the route of infection from a mosquito bite. We sacrificed the mice between E13.5 and E16.5, which would be equivalent to the late second or early third trimester, dissected out the placentas and fetuses, and assessed them in detail.
ZIKV Infection During Pregnancy Causes Murine IUGR and Fetal Death
By E13.5, the majority of fetuses of ZIKV-infected Ifnar1−/− mice had died and been resorbed (resorption in mice is analogous to spontaneous abortion in humans). We measured the crown-rump length and occipito-frontal head diameter of the remaining fetuses and found that they were significantly smaller than fetuses of uninfected mothers, indicating that ZIKV infection causes IUGR in mice as it does in humans6, 11. To assess viral load in the pregnant mothers and fetuses, we isolated RNA (ZIKV is an RNA virus) from maternal spleen and blood, placentas, and fetal heads at E13.5. We detected high levels of ZIKV in all tissues examined. Of note, the placentas contained ~1,000-fold higher concentration of ZIKV RNA than the maternal serum, suggesting that ZIKV preferentially replicates within the placenta.
ZIKV Infection Severely Damages the Placenta
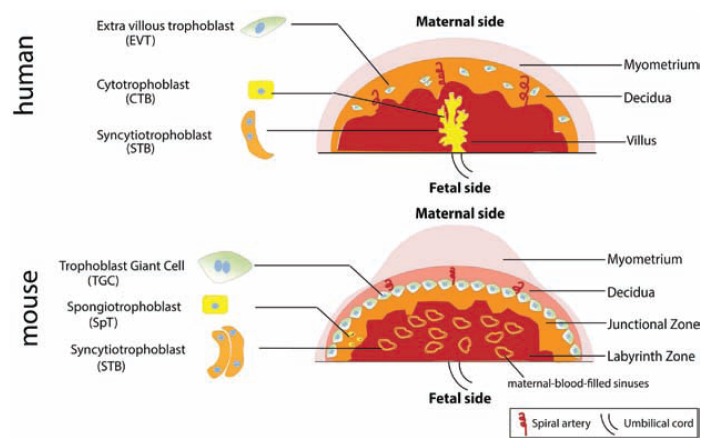
Similar to the human placenta, the mouse placenta comprises both maternal and fetal-derived components; in the mouse, these are termed the junctional and labyrinth zones, respectively (see Figure 1). In the labyrinth zone, fetal capillaries lined by fetal blood vessel endothelium are separated from maternal sinusoids by a layer of mononuclear trophoblasts and a syncytiotrophoblast (STB) bilayer.12, 13 In both human and mouse placentas, STBs separate fetal and maternal blood and mediate exchange of nutrients, waste, and gases. The mouse placenta lacks cells analogous to the human cytotrophoblasts and instead contains spongiotrophoblasts found in the junctional zone. Analogous to the human extravillous trophoblasts are the mouse trophoblast giant cells, which invade the maternal decidua to facilitate implantation of the placenta into the uterine wall (see Figure 1).
Figure 1.
Illustration depicting structure of human versus mouse placenta with analogous cell types indicated.
To determine at a cellular level how ZIKV reached the fetus, we asked where in the placentas ZIKV was located. We found ZIKV RNA in different trophoblast cells including spongiotrophoblasts (see Figure 2). Transmission electron microscopy of placentas revealed ZIKV virion particles assembling within the endoplasmic reticulum of trophoblasts and fetal endothelial cells that line fetal capillaries. In addition, histopathological analysis of the placentas showed extensive vascular damage with irregularly shaped fetal capillaries, damaged placental microvasculature, and a number of nucleated fetal erythrocytes, key indicators of fetal stress. Finally, we examined fetal brains and found extensive neuronal death (reviewed in9). From these findings, we concluded that maternal ZIKV infection compromises the placental barrier by infecting fetal trophoblasts and thereby enters the fetal circulation and impairs development.
Figure 2.
Image depicting the Zika virus particles in red encased inside a spongiotrophoblast with nuclei stained green.
Perspectives and Future Directions
Our study was the first to administer ZIKV to mice early in gestation (at E6.5, equivalent to the first trimester in human gestation); other investigators infected mice at E13.5 (closer to late second-early third trimester in human pregnancy).14–17 The high rate of fetal death we observed is consistent with the observation in humans that the most severe manifestations of ZIKV-induced fetal abnormalities are associated with infections in the first and second trimesters.6, 7, 18 Additionally, our study unequivocally demonstrated that ZIKV can take a transplacental route to infect fetuses. Consistent with our findings, recent studies have demonstrated that ZIKV infects human placental trophoblasts, endothelial cells, and placental macrophages. 19–22
Principal findings from our murine studies support observations in human pregnancy that ZIKV infection during pregnancy causes transplacental infections and fetal demise. Furthermore, we demonstrate that ZIKV spreads to the fetal brain where it leads to neuropathologies that have been observed in human neonates. Our ongoing studies are focused on determining the mechanisms of viral entry into the placenta and fetal endothelium. Further, we will use our mouse model as a foundation for defining viral pathogenic mechanisms and testing candidate vaccines, anti-ZIKV antibodies, and other therapeutic modalities needed to combat this rapidly expanding epidemic.
Biography
Indira U. Mysorekar, PhD, is in the Departments of Obstetrics and Gynecology and Pathology and Immunology, and is Associate Director, Centre for Reproductive Health Sciences, Washington University School of Medicine.
Contact: indira@wustl.edu

Footnotes
Disclosure
None reported.
References
- 1.Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94:675–686C. doi: 10.2471/BLT.16.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao-Lormeau VM, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–9. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mlakar J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–8. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 5.Calvet GA, Santos FB, Sequeira PC. Zika virus infection: epidemiology, clinical manifestations and diagnosis. Curr Opin Infect Dis. 2016;29:459–66. doi: 10.1097/QCO.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 6.Brasil P, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauchemez S, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387:2125–32. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–7. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 9.Mysorekar IU, Diamond MS. Modeling Zika Virus Infection in Pregnancy. N Engl J Med. 2016;375:481–4. doi: 10.1056/NEJMcibr1605445. [DOI] [PubMed] [Google Scholar]
- 10.Miner JJ, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–91. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarno M, et al. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis. 2016;10:e0004517. doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–93. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 14.Cugola FR, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–71. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016;19:120–6. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Wu KY, et al. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016;26:645–54. doi: 10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yockey LJ, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166:1247–1256 e4. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cauchemez S, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016 doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabata T, et al. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe. 2016;20:155–66. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz. 2016;111:287–93. doi: 10.1590/0074-02760160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurado KA, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quicke KM, et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]