Abstract
Glioblastoma multiforme (GBM) is the most common type of malignant brain tumors in adults and has a dismal prognosis. The highly aggressive invasion of malignant cells into the normal brain parenchyma renders complete surgical resection of GBM tumors impossible, increases resistance to therapeutic treatment, and leads to near-universal tumor recurrence. We have previously demonstrated that TROY (TNFRSF19) plays an important role in glioblastoma cell invasion and therapeutic resistance. However, the potential downstream effectors of TROY signaling have not been fully characterized. Here, we identified PDZ-RhoGEF as a binding partner for TROY that potentiated TROY-induced nuclear factor kappa B activation which is necessary for both cell invasion and survival. In addition, PDZ-RhoGEF also interacts with Pyk2, indicating that PDZ-RhoGEF is a component of a signalsome that includes TROY and Pyk2. PDZ-RhoGEF is overexpressed in glioblastoma tumors and stimulates glioma cell invasion via Rho activation. Increased PDZ-RhoGEF expression enhanced TROY-induced glioma cell migration. Conversely, silencing PDZ-RhoGEF expression inhibited TROY-induced glioma cell migration, increased sensitivity to temozolomide treatment, and extended survival of orthotopic xenograft mice. Furthermore, depletion of RhoC or RhoA inhibited TROY- and PDZ-RhoGEF–induced cell migration. Mechanistically, increased TROY expression stimulated Rho activation, and depletion of PDZ-RhoGEF expression reduced this activation. Taken together, these data suggest that PDZ-RhoGEF plays an important role in TROY signaling and provides insights into a potential node of vulnerability to limit GBM cell invasion and decrease therapeutic resistance.
Abbreviations: GBM, glioblastoma multiforme; PRG, PDZ-RhoGEF; NF-κB, nuclear factor kappa B; GEF, guanine nucleotide exchange factor
Introduction
Glioblastoma multiforme (GBM), a grade IV astrocytoma, is the most common primary central nervous system tumor in human adults and remains largely incurable with a median life expectancy of approximately 15 months [1], [2]. Despite advances in therapeutic treatments of GBM, including surgical resection, chemotherapy, and radiation therapy, overall patient survival has not shown significant improvement over the past decade [1]. A hallmark of malignant gliomas is the extensive invasion of tumor cells into the normal brain parenchyma [3], which implies that even extensive resection of the primary tumor mass is not curative. Moreover, the invading cells are highly resistant to current therapeutic modalities, consequently leading to tumor recurrence [4]. However, the molecular mechanisms underlying glioma cell invasion have remained elusive. Thus, a deeper understanding of the signaling pathways that drive glioma cell invasion as well as the identification and specific targeting of the crucial signaling effectors is needed to ultimately improve the treatments for this disease.
Among the important mediators of glioblastoma cell invasion is TROY, an orphan member of the tumor necrosis factor receptor superfamily, which is widely expressed during embryonic development but whose postnatal expression is tightly regulated [5], [6], [7], [8]. Increased expression of TROY has been implicated in several invasive cancers, including melanoma, nasopharyngeal carcinoma, lung cancer, colorectal cancer, and GBM [9], [10], [11], [12], [13], [14]. We have previously shown that expression of TROY protein is low in non-neoplastic brain tissue but increases with glial tumor grade and inversely correlates with patient survival [13]. We also noted that TROY mRNA expression was elevated in invasive glioma cells relative to cells in the matched tumor core [14]. Increased expression of TROY stimulated glioma cell invasion in vitro and invasion ex vivo in brain slices, and induced astrocyte migration in situ. TROY-stimulated migration correlated with increased glioma cell resistance to temozolomide (TMZ) or radiation in vitro via activation of Akt and the nuclear factor kappa B (NF-κB) [14]. Conversely, knockdown of TROY expression inhibited glioma cell migration and increased sensitivity to TMZ [14]. Furthermore, knockdown of TROY expression alone significantly increased survival in an intracranial xenograft model [14]. Recently, we found that TROY forms a novel complex with epidermal growth factor receptor and that TROY was capable of modulating epidermal growth factor receptor signaling in GBM [15]. However, the signaling pathways and specific downstream effectors involved in TROY-stimulated cell migration and invasion remain largely undefined.
The Rho GTPases, a subgroup of the Ras superfamily, play important roles in a wide spectrum of cellular functions such as actin cytoskeletal reorganization, cell cycle progression, and vesicle trafficking [16]. They act as molecular switches by cycling between an active (GTP-bound) and an inactive (GDP-bound) conformational state. The switch is primarily regulated by guanine nucleotide exchange factors (GEFs), catalyzing the exchange of GDP for GTP, and GTPase-activating proteins, promoting the hydrolysis of GTP bound to Rho GTPases to deactivate the Rho GTPases [17]. Emerging evidence has demonstrated that Rho GEFs link many receptor tyrosine kinases to Rho GTPase activation [18], [19]. Given their central role as regulators of the cytoskeleton, cell cycle, cellular polarity, cell adhesion, and cell migration, RhoGEFs have been implicated in cancer cell invasion and tumor progression [20].
In this study, we sought to identify downstream effectors involved in TROY-induced glioma cell migration and invasion. We identified PDZ-RhoGEF (ARHGEF11) as a component of a signalsome that includes TROY and the non–receptor tyrosine kinase Pyk2 [13]. PDZ-RhoGEF expression is significantly increased in GBM tumors and stimulates the migration of TROY-expressing GBM cells. PDZ-RhoGEF can exchange for both RhoA and RhoC linking TROY signaling to Rho activation. The current results substantiate a role for PDZ-RhoGEF as an effector of TROY signaling and suggest that PDZ-RhoGEF may represent a novel target to inhibit GBM cell invasion.
Materials and Methods
Cell Culture
Authenticated human astrocytoma cell lines U87MG and T98G (American Type Culture Collection), human kidney epithelial cell line 293 cells, and T98G cells transduced with a shRNA targeting TROY [14] as well as the 293/NF-κB-luc reporter cell line [15] were maintained in Dulbecco's modified Eagle medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated FBS (Invitrogen), 1% nonessential amino acids, 2 mmol/l glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2. When indicated, cells were serum starved by replacing the culture media with DMEM supplemented with 0.1% bovine serum albumin (BSA). GBM43 and GBM10 are primary GBM patient-derived xenografts (PDX) obtained from the Mayo Clinic Brain SPORE [21]. These PDX were established directly from patient surgical samples and maintained as subcutaneous flank xenografts through serial passaging in immune-deficient mice. Extensive phenotypic and genotypic characterizations of these models as well as their growth properties in flank and brain and the response of orthotopic tumors to various therapies are available at https://www.mayo.edu/research/labs/translational-neuro-oncology/mayo-clinic-brain-tumor-patient-derived-xenograft-national-resource. Fresh flank tumors were resected, processed to single cell suspension by mechanical dissociation, and maintained in neurosphere media (DMEM/F12 containing 2% B-27 supplement, 20 ng/ml bFGF, and 20 ng/ml EGF).
Antibodies, Expression Constructs, and Reagents
A polyclonal PDZ-RhoGEF antibody was purchased from Novus Biologicals (Littleton, CO). Antibodies to HA-epitope tag, α-tubulin, β-tubulin, and RhoC were purchased from Cell Signaling Technologies (Beverly, MA). A rabbit polyclonal antibody to TROY was produced by Cocalico Biologicals (Reamstown, PA) using a peptide mapping to the TROY amino terminus conjugated to KLH. The anti-RhoA antibody and the anti–PDZ-RhoGEF monoclonal antibody were obtained from Santa Cruz biotechnology (Dallas, TX). The anti-Myc monoclonal antibody (9E10), the anti-Rac1 monoclonal antibody, and the anti-Pyk2 polyclonal antibody were obtained from Millipore (Bedford, MA). The anti-FLAG antibody was obtained from Sigma (St. Louis, MO). The polyclonal anti-AU1 epitope antibody and the polyclonal anti-Myc epitope antibody were obtained from Bethyl Laboratories (Montgomery, TX). The anti-phosphotyrosine mAb pY20 was from BD Biosciences (San Jose, CA). The β-actin monoclonal antibody was obtained from ThermoFisher Scientific (San Jose, CA). Alexa Fluor 546–labeled goat anti-rabbit antibody and Alexa Fluor 488–labeled goat anti-mouse antibody were purchased from Invitrogen (Carlsbad, CA). The 3X HA epitope-tagged wild-type (WT) TROY construct was constructed as previously described [13]. The AU1 epitope-tagged TROY was generated by replacing the 3X HA epitope with the AU1 epitope (DTYRYI) by PCR. Generation of the FLAG-epitope tagged wild-type Pyk2 and kinase-deficient Pyk2 K457A variant has been previously described [22]. Plasmids encoding rat PDZ-RhoGEF and leukemia-associated Rho guanine nucleotide exchange factor (LARG) [23] were generously provided by Zhekang Ying (University of Maryland School of Medicine). To generate FLAG epitope-tagged wild-type PDZ-RhoGEF, the coding sequence of PDZ-RhoGEF was cloned in-frame downstream of a 3X FLAG epitope in pcDNA3. Recombinant E1-deleted adenovirus for this construct was prepared using the Ad-Easy system as previously described [24]. Collagen was obtained from Advanced Biomatrix (San Diego, CA). EGF was obtained from Invitrogen (Carlsbad, CA).
Generation of NF-κB Response Element–Driven Firefly Luciferase Reporter Stable Cell Lines Overexpressing HA Tagged TROY
A cDNA fragment encoding WT TROY with a C-terminal 3X HA epitope-tag was subcloned into the lentiviral transfer vector pCDH GFP (System Biosciences) as previously described [14]. The empty pCDH lentiviral construct expressing only GFP was used as control. Recombinant lentiviruses encoding TROY-HA were produced by transient transfection of 293T packing cells with pCDH construct encoding HA tagged TROY and the pPACKH1 plasmid packing mix (System Biosciences). 293/NF-κB-luc cells, a NF-κB response element–driven firefly luciferase reporter cell line [15], and T98G/NF-κB-luc cells were transduced with the recombinant TROY-HA lentiviruses and selected by mass sorting the GFP-positive cells on a FACS Aria cell sorter (BD Biosciences) to generate the reporter cell lines designated 293/NF-κB-luc/TROY-HA and T98G/NF-κB-luc/TROY-HA, respectively.
Immunoblotting and Immunoprecipitation
Immunoblotting of cell lysates and protein determination were performed as described [15], [25]. Briefly, cells were washed with ice-cold PBS once and lysed on ice in the presence of protease and phosphatase inhibitors, lysates were clarified by centrifugation, and protein concentrations were measured using bicinchoninic acid assay (Pierce) with bovine serum albumin as a standard. For immunoblotting, equal amounts of cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and incubated with the appropriate primary antibody. Protein detections were performed using IRDye-conjugated secondary antibodies with the Odyssey CLx Infrared Imaging System (LI-COR Biosciences).
For immunoprecipitation experiments, cells were lysed 24 hours after transfection with RIPA buffer (50 mM Tris, pH 8.0, 135 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, and 5% glycerol) containing protease and phosphatase inhibitors. Eight hundred micrograms of cell lysates was precleared with protein G-agarose beads (Millipore) for 1 hour at 4°C. Precleared lysates were incubated with the appropriate antibodies at a dilution of 1:100 overnight at 4°C followed by incubation with protein G-agarose beads for 1 hour. The immune complexes were washed five times with ice-cold RIPA buffer, eluted with 2× SDS sample buffer, boiled in the presence of 2-mercaptoethanol (Sigma), and resolved by SDS-PAGE. Immunoblotting of resolved immunoprecipitates was performed as described above.
Immunofluorescence
T98G, GBM10, and GBM43 cells were plated onto four-chamber glass slides. Twenty-four hours later, cells were fixed in 4% formaldehyde, permeabilized with 0.1% Triton-X100, blocked in 5% normal goat serum, and incubated with a 1:100 dilution of polyclonal rabbit anti-TROY antibody and mouse anti–PDZ-RhoGEF antibody at 4°C overnight. The following day, the samples were washed three times in PBS and incubated with a 1:1000 dilution of Alexa Fluor 546–labeled goat anti-rabbit antibody and Alexa Fluor 488–labeled goat anti-mouse antibody in dark for 1 hour at room temperature. Slides were mounted with ProLong Gold Antifade Reagent with DAPI (Cell Signaling Technology). Images were collected using a Zeiss LSM 800 microscope equipped with a 63× objective, ZEN image analysis software, and Adobe Photoshop CC 2015.
Glioblastoma Tissue Microarray and Immunohistochemistry
The preparation of the glioblastoma tissue microarray and immunohistochemistry protocols used to examine PDZ-RhoGEF expression in glioblastoma tumor samples has been described previously [26]. A standard histological scoring system of 0, negative; 1, weak; 2, moderate; and 3, strong was used to grade the staining by individuals blinded to the sample identity.
Small Interfering RNA (siRNA) Transfection
siRNA oligonucleotides specific for GL2 luciferase were described previously [27]. siRNA target sequences for PDZ-RhoGEF (PRG), RhoA, and RhoC were as follows: PRG-1 (5′-ACCGUACAAACCACCAAAUCCUCUG-3′), PRG-2 (5′-GCGAUUCAAUCCUGAUCAA-3′), RhoA-1 (5′-AUGGAAAGCAGGUAGAGUU-3′), RhoA-2 (5′-CCCAGAUACCGAUGUUAUACUGAUG-3′), RhoC-1 (5′-GAACUAUAUUGCGGACAUU-3′), and RhoC-2 (5′-GGACAUGGCGAACCGGAUC-3′). The negative control siRNA (Silencer Negative Control #1 siRNA, AM4611) was obtained from Ambion (Austin, TX). The cells were transfected with siRNA using Lipofectamine RNAiMax (Invitrogen) according to the manufacture's protocols. Two or 3 days after transfection, depletion of the respective proteins was confirmed by immunoblotting using the indicated antibodies.
shRNA Transduction
Lentivirus constructs encoding a validated short hairpin RNA (shRNA)-targeting human TROY (Clone ID: V2LHS 30848) or a nonsilencing control shRNA (Catalog #RHS4348) were purchased from Thermo Fisher Open Biosystems. Three different shRNAs targeting PDZ-RhoGEF (Clone IDs: V3LHS 373445, V3LHS 373447, or V2LHS 95367) were also purchased from Thermo Fisher Open Biosystems. Preliminary experiments indicated V3LHS 373445 exhibited the greatest knockdown efficacy and was used in subsequent studies. shRNA transduction was performed as previously described [14]. Briefly, recombinant lentiviruses encoding shRNAs were produced by transient transfection of 293T packaging cells with the shRNA expression constructs and the Trans-Lentiviral packaging kit (Open Biosystems). For lentiviral transduction, media from the packing cells were harvested 48 hours after transfection; recombinant lentiviruses were concentrated by polyethylene glycol precipitation and added to GBM10 or GBM43 cells with 8 μg/ml polybrene for 6 hours at 37°C. Positively transduced cells were enriched by mass sorting the GFP-positive cells using a FACS Aria cell sorter (BD Biosciences).
Intracranial Xenograft Tumor Model
The orthotopic intracranial xenograft model was conducted under a protocol approved by the Mayo Institutional Animal Care and Use Committee. The procedure was performed as previously described [14]. Power analysis indicated that a sample size of 8 animals for each group will have 80% power to detect a probability of 0.90 that the time until onset of a moribund state in one group is less than the time until onset of a moribund state in another group using a Wilcoxon (Mann-Whitney) rank sum test with a .05 two-sided significance level. Female athymic nude mice were randomized into groups of 10 that received either GBM10 cells transduced with a nontargeting control shRNA or GBM10 cells transduced with a shRNA-targeting PDZ-RhoGEF. Extent of knockdown of PDZ-RhoGEF was examined by immunoblotting analysis of transduced cells 48 hours after injections. Cells (5 × 105 cells) were injected into the right basal ganglia of anesthetized mice with a stereotaxic frame. Mice were weighed daily and monitored for the onset of neurologic symptoms and euthanized when they reached a moribund condition.
Organotypic Brain Slice Invasion Assay
Preparation and culture of adult brain slice were performed as described previously [28]. Briefly, GFP-labeled U87MG glioma cells transfected with siRNA targeting PDZ-RhoGEF or a control siRNA were placed onto the putamen of the brain slice. After 48 hours, glioma cell invasion into rat brain slices was quantified by confocal microscopy, and the depth of invasion (z-axis stacks) was calculated as described previously [22], [28].
Transwell Migration Assays
Glioma cells were seeded in 100-mm–diameter dishes and incubated overnight at 37°C. Subsequently, the culture media were replaced with DMEM and 0.1% BSA for additional 16 hours at 37°C. Cells were harvested, resuspended in DMEM and 0.1% BSA (1 × 105 cells/200 μl), added in triplicate to collagen-coated Transwell chambers (8-μm pore size), and allowed to migrate towards 10% serum. After incubation for 24 hours at 37°C, cells on the upper surface of the membrane were scraped off. Migrated cells on the lower surface of the membrane were fixed with 4% paraformaldehyde and stained with ProLong Gold Antifade reagent with DAPI (Invitrogen). Nuclei of migrated cells were counted in five high-power fields with a 20× objective.
Cell Viability Assays
The CellTiter-Glo assay (Promega) was used to examine the cell viability after TMZ treatment as previously described [29]. Briefly, GBM43 cells transduced with a shRNA-targeting TROY or GBM43 cells transduced with a nontargeting control shRNA were seeded in 96-well plates in DMEM with 10% FBS at a density of 3000 cells/well. Increasing concentration of TMZ was added to the different wells with six replicates under each condition and incubated for 72 hours at 37°C. Subsequently, CellTiter-Glo reagent was added to each well, and luminescence was measured using Cytation 5 Multi-Mode Reader (BioTek). The data were normalized to control cells (TMZ 0 μM, 100% cell viability) and plotted as mean +/− SD.
Rho Activation Assay
GTP loading of Rho activity was measured using the Rho Activation Assay Kit (Cytoskeleton) according to the manufacturer's instructions. Briefly, glioma cells were plated in 100-mm dishes and incubated overnight. Cells were 0.1% BSA serum starved for 16 hours, cell lysates were harvested, and equal amounts of proteins were assessed for Rho activation.
Luciferase Reporter Assays
Reporter cells were plated in complete DMEM in six-well plates. The cells were transfected in triplicate with indicated plasmids using Effectene (Qiagen). Cells were serum starved (0.1% BSA in DMEM) for 16 hours and then lysed in Reporter Lysis Buffer (Promega). Luciferase assays were performed using the luciferase reporter assay system (Promega) according to the manufacturer's instructions.
Statistical Analysis
Statistical analyses were conducted by the two-sample t test using GraphPad Prism 7.0 (GraphPad, Inc.). A P value less than .05 was considered significant. Survival of mice with intracranial xenografts was determined by Kaplan-Meier analysis using GraphPad Prism 7.0. Differences between survival curves were compared with the log-rank test. Statistical significance was set at criterion level P < .01.
Results
Identification of PDZ-RhoGEF as a Component of a Signalsome That Includes TROY and Pyk2
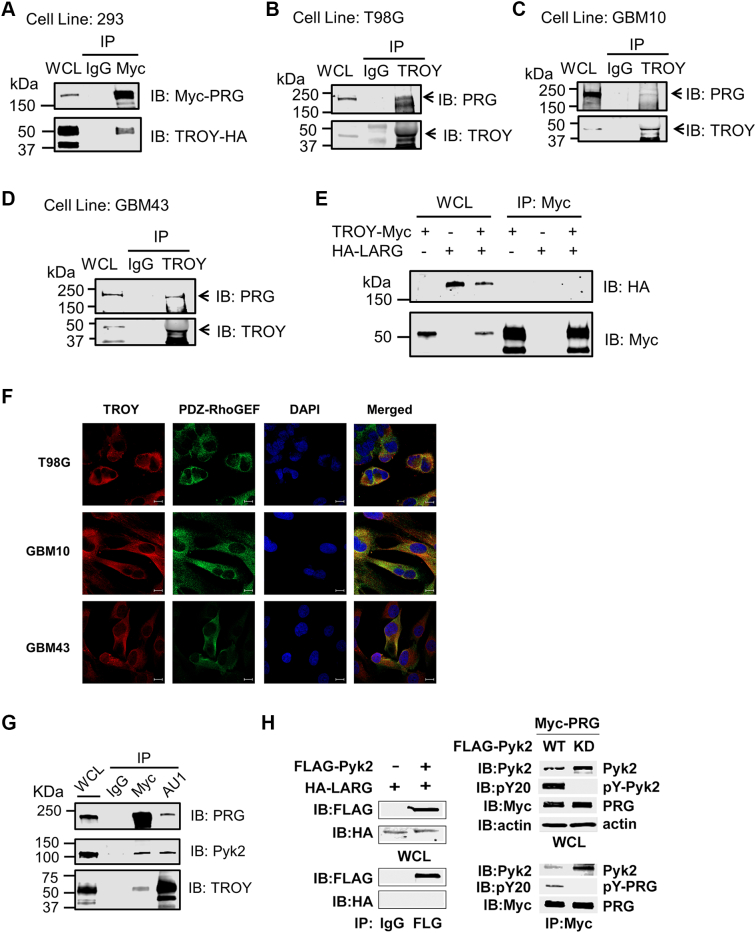
To determine possible mechanisms through which TROY induces GBM cell invasion, we performed immunoprecipitation of TROY from T98G glioma cells overexpressing TROY and analyzed the precipitates with MALDI-TOF and MS/MS to identify proteins that are capable of interacting with TROY and potentially mediating TROY signaling [13]. Several proteins were identified in the TROY immunoprecipitates, among which PDZ-RhoGEF was of particular interest because it has previously been reported to be involved in cellular motility [30], [31]. To confirm the interaction between TROY and PDZ-RhoGEF, 293 cells co-transfected with HA-tagged TROY and Myc-tagged PDZ-RhoGEF were immunoprecipitated with an anti-Myc antibody, and the precipitates were immunoblotted with anti-HA and anti-Myc antibodies. Immunoblotting demonstrated that HA-tagged TROY co-immunoprecipitated with Myc-tagged PDZ-RhoGEF, substantiating an interaction between TROY and PDZ-RhoGEF (Figure 1A). Endogenous PDZ-RhoGEF was found to co-immunoprecipitate with endogenous TROY in T98G glioma cells as well as primary GBM patient-derived xenografts GBM10 and GBM43 cells (Figure 1, B-D). The expression pattern of endogenous TROY and TROY interactants in the different cells lines is shown in Supplementary Figure 1. To explore the specificity of the interaction between TROY and PDZ-RhoGEF, we examined whether TROY could interact with LARG, a closely related GEF belonging to the same RhoGEF subfamily as PDZ-RhoGEF. 293 cells were co-transfected with HA-tagged LARG and/or Myc-tagged TROY. Immunoprecipitation of the transfected cell lysates with an anti-Myc antibody followed by immunoblotting of the precipitates with the anti-HA antibody showed that LARG was not present in the TROY-Myc immunoprecipitates, suggesting a specific interaction of TROY with PDZ-RhoGEF (Figure 1E). To further confirm the interaction, we examined the colocalization of TROY and PDZ-RhoGEF by immunofluorescence analysis. T98G, GBM10, and GBM43 cells were stained with anti-TROY and anti–PDZ-RhoGEF antibodies and examined by confocal microscopy (Figure 1F). Consistent with previous results [13], TROY staining was predominately near the cell periphery and enriched in lamellipodia, and the merged images indicated colocalization of TROY and PDZ-RhoGEF.
Figure 1.
TROY associates with PDZ-RhoGEF (PRG). (A) 293 cells were co-transfected with HA-tagged TROY plasmid and Myc-tagged PDZ-RhoGEF plasmid, and the lysates were immunoprecipitated with an anti-Myc antibody or mouse IgG. The immunoprecipitates were analyzed for the presence of PDZ-RhoGEF by immunoblot analysis. The expression level of Myc-tagged PDZ-RhoGEF and HA-tagged TROY in the cells is shown by immunoblotting of whole cell lysates (WCL). (B-D) The lysates of T98G, GBM10, and GBM43 cells were immunoprecipitated with an anti-TROY antibody or rabbit IgG. The immunoprecipitates were analyzed for the presence of PDZ-RhoGEF by immunoblot analysis. The expression level of PDZ-RhoGEF and TROY in the cells is shown by immunoblotting of WCL. (E) TROY does not co-immunoprecipitate with LARG in 293 cells. 293 cells were transfected with HA-tagged LARG or Myc-tagged TROY, or co-transfected with HA-tagged LARG and Myc-tagged TROY. Twenty-four hours after transfection, cells were lysed and immunoprecipitated with anti-Myc antibody. Immunoprecipitates or WCL were immunoblotted with the indicated antibodies. (F) Colocalization of TROY and PDZ-RhoGEF in T98G, GBM10, and GBM43 cells. Cells were stained and examined by confocal microscopy. TROY was visualized with Alexa Fluor 546–conjugated antibody, and PDZ-RhoGEF was visualized with Alexa Fluor 488–conjugated antibody. The colocalization of TROY (red) with PDZ-RhoGEF (green) appears as a yellow color in the merged image. Cells were counterstained with DAPI (blue) (bars = 10 μm). (G) 293 cells were co-transfected with AU1-tagged TROY, Myc-tagged PRG, and HA-tagged Pyk2, and 24 hours later, cells were lysed and immunoprecipitated with goat anti-Myc or goat anti-AU1 antibodies or goat IgG. Immunoprecipitates or WCL were immunoblotted (IB) with the indicated antibodies. (H) Left panel, 293 cells were transfected with HA-tagged LARG and/or FLAG-tagged Pyk2. Twenty-four hours later, the cells were lysed and immunoprecipitated with anti-FLAG antibody or mouse IgG. Immunoprecipitates or WCL were IB with anti-FLAG or anti-HA antibodies. Right panel, 293 cells were co-transfected with Myc-tagged PRG and FLAG-tagged WT Pyk2 or kinase-deficient (KD) Pyk2 for 24 hours. Cells were lysed and immunoprecipitated with anti-Myc antibody. Immunoprecipitates or WCL were IB with the indicated antibodies.
In a previous study using MALDI-TOF and MS/MS analysis of immunoprecipitates of TROY from T98G glioma cells, we identified the non–receptor tyrosine kinase Pyk2 in the TROY immunoprecipitate and verified their association by co-immunoprecipitation [13]. In addition, we demonstrated that TROY-induced cell migration and invasion were dependent upon Pyk2 expression and activity. Given that both Pyk2 and PDZ-RhoGEF were found to associate with TROY, we examined whether we could observe a signaling complex that includes TROY, Pyk2, and PDZ-RhoGEF. Cells were transfected with epitope tagged variants of TROY, Pyk2, and PDZ-RhoGEF; cell lysates were immunoprecipitated with antiepitope antibodies; and the precipitates were immunoblotted with antibodies for each of the components (Figure 1G). Both the anti-TROY and the anti–PDZ-RhoGEF immunoprecipitates contained TROY, PDZ-RhoGEF, and Pyk2, indicating the presence of a TROY signalsome that includes Pyk2 and PDZ-RhoGEF.
Previous studies have reported that tyrosine phosphorylation is among several mechanisms described for the activation of RhoGEFs [17], [18]. Notably, phosphorylation has been previously reported to regulate the activity of PDZ-RhoGEF in 293 cells [32]. Therefore, we investigated further the interaction between Pyk2 and PDZ-RhoGEF. Studies examining the specificity of the interaction demonstrated that Pyk2 co-immunoprecipitated with PDZ-RhoGEF but failed to immunoprecipitate with the closely related RhoGEF LARG (Figure 1H) consistent with the inability of TROY to associate with LARG. Moreover, PDZ-RhoGEF present in the co-immunoprecipitate with wild-type Pyk2 was phosphorylated but not in the co-immunoprecipitate with a kinase inactive Pyk2 (Figure 1H). Together, these results suggest a potential TROY signaling pathway that includes Pyk2 and PDZ-RhoGEF.
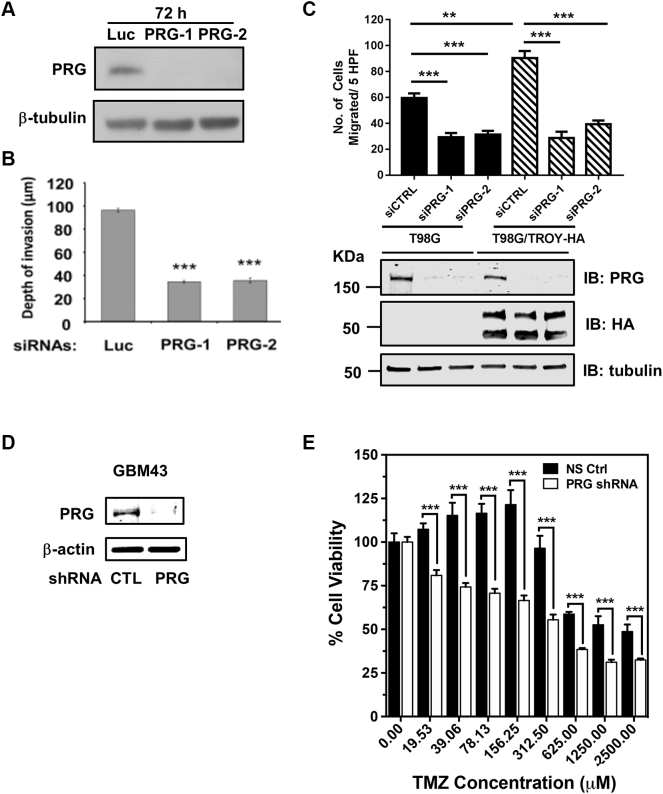
Depletion of PDZ-RhoGEF Inhibits TROY-Induced Glioblastoma Cell Invasion and Increases Sensitivity to TMZ
To examine the role of PDZ-RhoGEF in glioma cell invasion, we used RNA interference to knock down the expression of PDZ-RhoGEF and examined the effect on cell invasion using an ex vivo rat brain slice model [22], [28]. U87MG cells were transfected with two independent siRNA oligonucleotides targeting PDZ-RhoGEF for 72 hours, and the cell lysates were subjected to immunoblotting with an anti–PDZ-RhoGEF antibody. Immunoblotting analysis showed that siRNA-induced knockdown of PDZ-RhoGEF expression in U87MG with both siRNA oligonucleotides was >90% effective relative to cells transfected with a control siRNA targeting firefly luciferase (luc) (Figure 2A). U87MG cells transfected with siRNA targeting PDZ-RhoGEF or the control siRNA (luc) were seeded onto the brain slice, and cell invasion into the brain slice was quantified after 48 hours using confocal microscopy [28]. Knockdown of PDZ-RhoGEF in U87MG cells resulted in a significant inhibition in the depth of invasion relative to U87MG cells expressing the control siRNA, supporting a role for PDZ-RhoGEF in glioma cell invasion signaling (P < .001; Figure 2B). To further determine the role of PDZ-RhoGEF in TROY-induced glioma cell migration, we used RNA interference to knockdown the expression of PDZ-RhoGEF in T98G cells and T98G cells overexpressing TROY (T98G/TROY-HA) and examined the migratory behavior of these cells using Transwell migration assay. These cells were transfected with two independent siRNAs targeting PDZ-RhoGEF (siPRG-1, siPRG-2) or a nontargeting control siRNA (siCTRL) for 48 hours, serum starved overnight, and seeded on the top of transwell chambers. Ten percent serum was added into the lower chamber as chemoattractant. Cell migration into the lower chamber was quantified after 24 hours. Knockdown of PDZ-RhoGEF was confirmed by immunoblotting analysis (Figure 2C). Migration assays showed that increased expression of TROY increased cell migration consistent with previous results [13]. Knockdown of PDZ-RhoGEF expression inhibited the migration of T98G cells and also significantly inhibited the TROY-stimulated increased cell migration (Figure 2C), supporting a role of PDZ-RhoGEF in TROY-induced glioma cell migration.
Figure 2.
Depletion of PDZ-RhoGEF expression inhibits glioma cell migration and invasion and TROY-stimulated glioma cell migration. (A) U87MG cells were transfected with two independent siRNAs targeting PDZ-RhoGEF (PRG-1, PRG-2) or with a control siRNA targeting firefly luciferase (Luc). Cell lysates were analyzed for PDZ-RhoGEF by immunoblotting. Immunoblotting of β-tubulin protein was used as a loading control. (B) Invasion of U87MG cells transfected with siRNAs targeting PDZ-RhoGEF (PRG-1, PRG-2) or luciferase (Luc). Forty-eight hours after cell seeding onto the brain slice, glioma cell invasion into the brain slices was quantified using confocal microscopy. The data are depicted as the mean values (+/− SEM) from three separate experiments. ***P < .001. (C) Migration of T98G cells and T98G cells overexpressing TROY (T98G/TROY-HA) transfected with siRNAs targeting PDZ-RhoGEF (siPRG-1, siPRG-2) or a nonsilencing control (siCTRL). Data represent the mean values (+/− SEM) (n = 3, **, P < .01; ***, P < .001). Cell lysates were analyzed by immunoblotting with indicated antibodies. Immunoblotting of tubulin was used as a loading control. (D) Knockdown of PDZ-RhoGEF expression in patient xenograft GBM43 cells transduced with the shRNA-targeting PDZ-RhoGEF (PRG) or the control (CTL) nontargeting shRNA. Cell lysates were analyzed by immunoblotting with indicated antibodies. Immunoblotting of β-actin protein was used as a loading control. (E) Primary xenograft GBM43 cells transduced with a control nonsilencing shRNA (NS Ctrl) or shRNA targeting PDZ-RhoGEF (PRG) were treated with the indicated concentration of TMZ. Cell viability was assessed after 72 hours by CellTiter-Glo assay. Data are depicted as the mean values (+/− SD) of six replicates. ***P < .001.
Since elevated TROY expression stimulates cell migration/invasion and increases therapeutic resistance to TMZ [13], [14], we hypothesized that if PDZ-RhoGEF is a downstream effector of TROY signaling, knockdown of PDZ-RhoGEF would inhibit survival signaling and increase sensitivity to therapeutic agents. For this experiment, we utilized GBM43 which is MGMT promoter unmethylated by qMS-PCR and thus relatively resistant to TMZ therapy compared to PDX with MGMT promoter hypermethylation. GBM43 cells expressing a control nonsilencing shRNA or a shRNA targeting PDZ-RhoGEF were treated with increasing concentrations of TMZ. Knockdown of PDZ-RhoGEF in GBM43 cells was verified by immunoblotting analysis (Figure 2D). Results of cell viability after 72 hours showed that knockdown of PDZ-RhoGEF significantly reduced GBM43 cell viability at all doses of TMZ treatment (Figure 2E). These data suggest that PDZ-RhoGEF plays an important role in glioma cell invasion and functions as a downstream effector for the TROY signaling.
PDZ-RhoGEF Expression Is Upregulated in GBM, and Knockdown of PDZ-RhoGEF Expression Increases Survival of Orthotopic Xenograft Mice
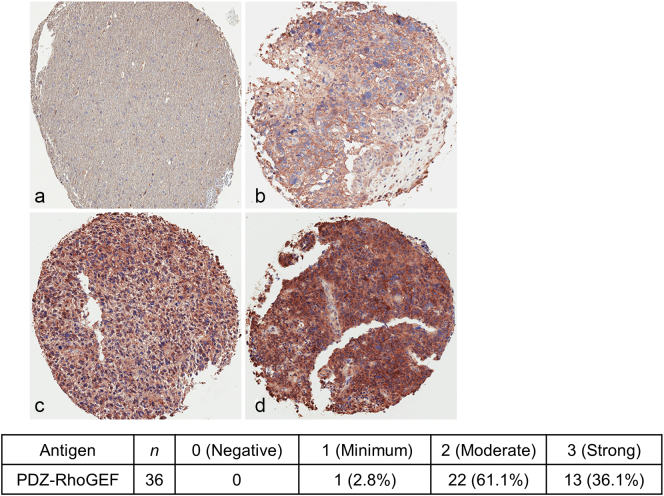
We have demonstrated that specific Rac1 GEFs (Trio, Ect2, and Vav3) are upregulated in GBM [27]. To determine the protein expression of PDZ-RhoGEF in GBM, we examined its expression in GBM tumors in situ by immunohistochemical (IHC) analysis on a GBM tissue microarray. IHC analysis of PDZ-RhoGEF expression in 36 clinical GBM specimens indicated increased expression of PDZ-RhoGEF in nearly all samples, with 61% of samples exhibiting a moderate staining score of 2 and 36% a strong score of 3 (on a 0-3 scale) (Figure 3).
Figure 3.
PDZ-RhoGEF expression is increased in GBM. (A) IHC analysis of PDZ-RhoGEF expression in non-neoplastic brain and GBM biopsy samples (upper panel). Samples are representative of 36 matched biopsy samples on a tissue microarray. a, negative, score 0; b, minimum, score 1; c, moderate, score 2; d, strong, score 3. Neuropathology scoring of the percentage distribution of staining intensity of PDZ-RhoGEF in GBM patient specimens (lower panel).
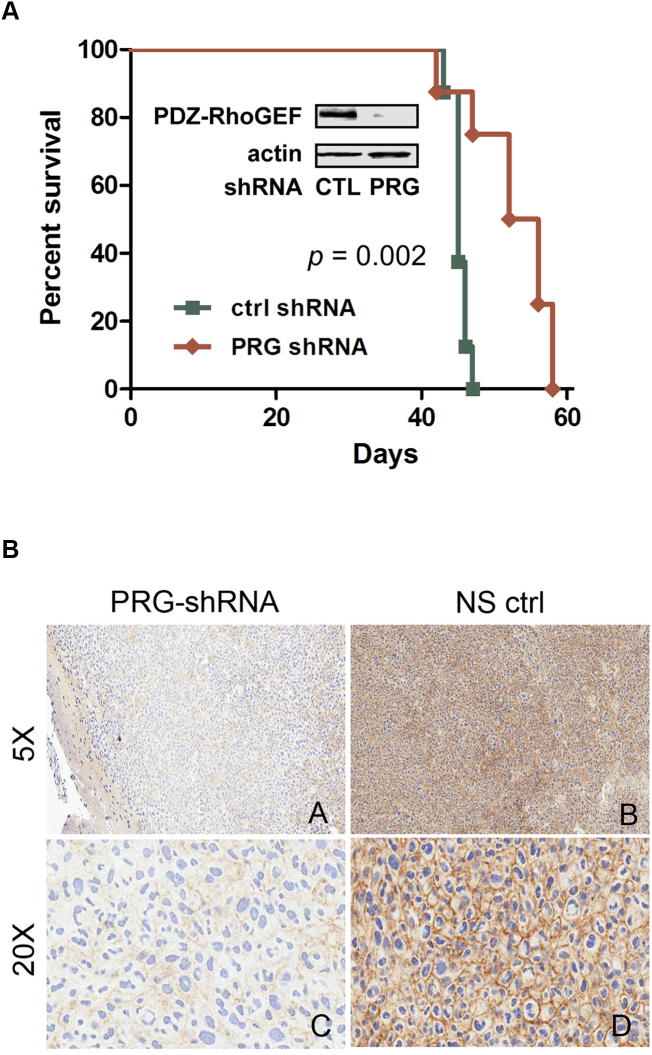
To determine the effect of PDZ-RhoGEF expression on glioma tumor progression in vivo, we examined the effect of silencing PDZ-RhoGEF expression on the survival of mice with intracranial xenografts established with GBM10 from a panel of serially in vivo passaged GBM xenografts established from patient tumors that maintain characteristic morphologic and molecular properties of the original tumor [21], [33]. GBM10 cells, which express both TROY and PDZ-RhoGEF (Supplementary Figure 1), were transduced with shRNA targeting PDZ-RhoGEF or a nontargeting shRNA. Immunoblotting analysis showed that GBM10 cells transduced with a shRNA-targeting PDZ-RhoGEF (PRG) showed more than 90% reduction in PDZ-RhoGEF expression compared to GBM10 cells transduced with a control nontargeting shRNA (CTL) (Figure 4A). Following intracranial implantation, mice with intracranial xenografts established with GBM10 cells transduced with the shRNA-targeting PDZ-RhoGEF exhibited significantly increased survival compared to mice with xenografts established with GBM10 cells transduced with the control nontargeting shRNA (P = .002). Immunohistochemical analysis of tumors resected from moribund animals at the time of sacrifice demonstrated a marked reduction in staining for PDZ-RhoGEF in tumors between the PDZ-RhoGEF targeted and control groups (Figure 4B). These data support a role for PDZ-RhoGEF expression in GBM tumor progression.
Figure 4.
Knockdown of PDZ-RhoGEF expression increases xenograft survival. (A) Kaplan-Meier survival curves of athymic nude mice with intracranial xenografts of primary GBM10 cells transduced with a control nontargeting shRNA (CTL) or a shRNA-targeting PDZ-RhoGEF (PRG). Curves show a significant survival benefit for mice with xenografts with silencing PDZ-RhoGEF expression (P = .002). Insert: Western blot of whole cell lysates of transduced GBM10 cells used for the intracranial xenografts immunoblotted with the indicated antibodies. (B) IHC of GBM10 tumors transduced with shRNA targeting PDZ-RhoGEF (PRG-shRNA) or a nonsilencing control shRNA (NS ctrl).
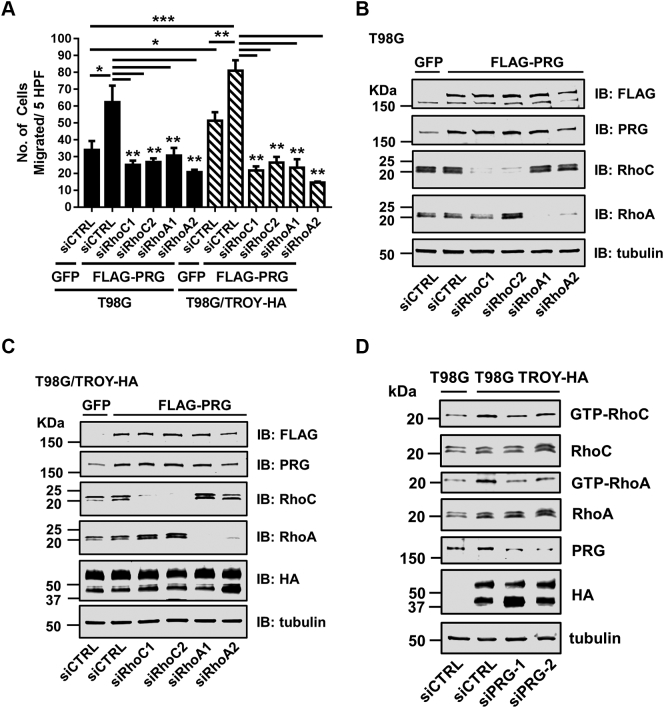
Depletion of RhoA and RhoC Inhibits PDZ-RhoGEF and TROY-Induced Cell Migration
It has been reported that PDZ-RhoGEF is a specific GEF for Rho but not for Rac1 and Cdc42 [34], [35]. Knockdown of PDZ-RhoGEF had no effect on Rac1 activation in T98G cells (Supplementary Figure 2), supporting that PDZ-RhoGEF exhanges mainly RhoA and RhoC. To more closely examine the role of PDZ-RhoGEF, RhoC, and RhoA in TROY-induced glioma cell migration, we knocked down RhoC and RhoA expression in T98G cells and T98G cells overexpressing TROY (T98G/TROY-HA) in the presence of increased PDZ-RhoGEF expression. T98G and T98G/TROY-HA cells were transfected with two independent siRNAs targeting RhoC (siRhoC-1, siRhoC-2) or two independent siRNAs targeting RhoA (siRhoA-1, siRhoA-2) or a nonsilencing control RNA (siCTRL). After 24 hours, these cells were infected with FLAG-tagged PDZ-RhoGEF adenovirus or a control adenovirus expressing GFP for an additional 24 hours. Cells were serum starved overnight and seeded on top of Transwell chamber. Cell migration to lower chamber was quantitated after 24 hours. Increased PDZ-RhoGEF expression enhanced cell migration in both T98G and T98G/TROY-HA cells (Figure 5A). The increased migration of T98G and T98G/TROY-HA cells induced by increased PDZ-RhoGEF expression was significantly inhibited by siRNA-mediated depletion of RhoC or RhoA (Figure 5A), indicating that RhoC and RhoA may function as downstream effectors of a TROY/PDZ-RhoGEF signaling axis. The knockdown of RhoC and RhoA and overexpression of FLAG-tagged PDZ-RhoGEF in T98G and T98G/TROY-HA cells were verified by immunoblotting analysis (Figure 5, B and C).
Figure 5.
Depletion of Rho suppresses TROY and PDZ-RhoGEF-induced glioma cell migration.
(A) T98G and T98G/TROY-HA cells were transfected with a nontargeting control siRNA (siCTRL) or two independent siRNAs targeting PDZ-RhoGEF (siPRG-1, siPRG-2) for 24 hours and infected with FLAG-tagged PRG lentivirus or GFP lentivirus for additional 24 hours. The cells were serum starved overnight and then seeded on top of a Transwell chamber. The number of migrated cells post 24 hours was quantified using DAPI staining. Data represent the mean values (+/− SEM) (n = 3, *P < .05; **P < .01; ***P < .001). (B) The lysates of T98G used for migration assay in A were immunoblotted with indicated antibodies. (C) The lysates of T98G/TROY-HA used for migration assay in A were immunoblotted with indicated antibodies. (D) T98G and T98G/TROY-HA cells were transfected with a nontargeting control siRNA (siCTRL) or two independent siRNAs targeting PDZ-RhoGEF (siPRG-1, siPRG-2) for 2 days and then serum starved overnight. Cells were lysed, and the Rho activities in lysates were measured using GST-Rhotekin-RBD pull-down assay. Immunoblot is a representation of two independent experiments.
As knockdown of RhoC and RhoA inhibited TROY and PRG-induced cell migration, we examined the relationship between Rho activation status and TROY in T98G and T98G/TROY-HA cells. A Rho activation assay showed that increased TROY expression enhanced both RhoA and RhoC activation in serum starved cells (Figure 5D). Moreover, depletion of PDZ-RhoGEF reduced the TROY-induced RhoA and RhoC activation (Figure 5D). Taken together, these data suggest that TROY promotes Rho activation through PDZ-RhoGEF.
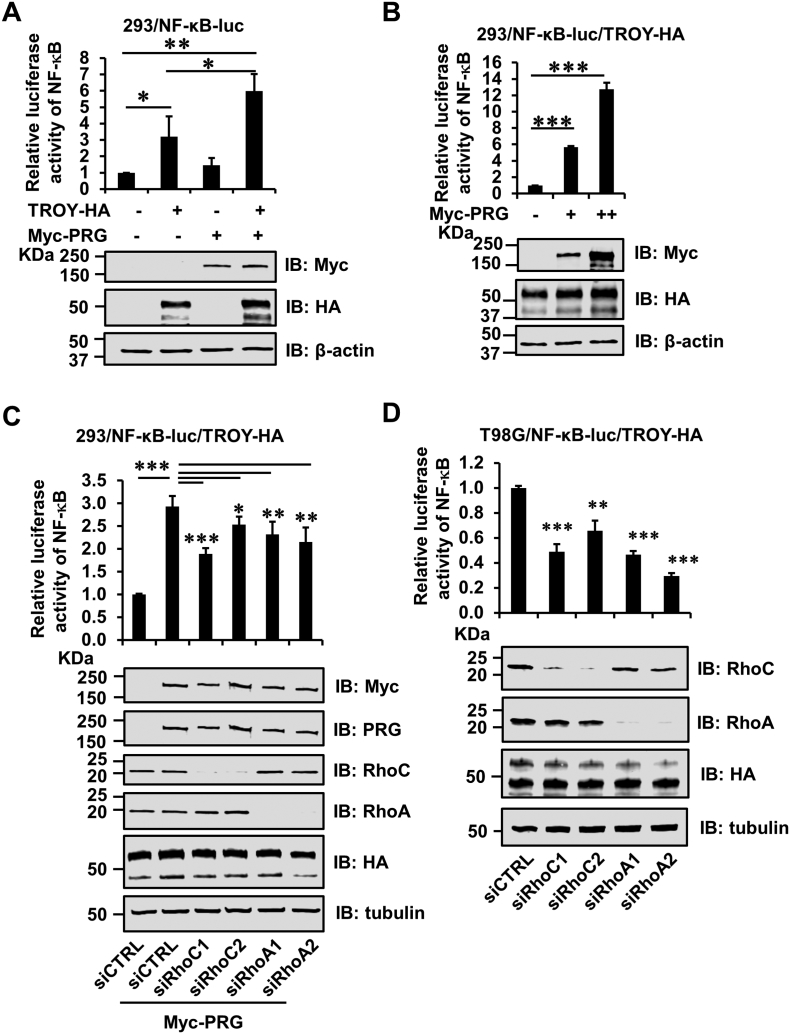
PDZ-RhoGEF Enhances TROY-Induced NF-κB Activation
We have previously demonstrated that increased TROY expression activates the NF-κB pathway in glioblastoma cell lines [14]. We next examined the effect of association of PDZ-RhoGEF with TROY on TROY-induced NF-κB activation. 293/NF-κB-luc reporter cells were transfected with HA-tagged TROY or Myc-tagged PDZ-RhoGEF or co-transfected with HA-tagged TROY and Myc-tagged PDZ-RhoGEF. Twenty-four hours after transfection, cells were serum starved for an additional 16 hours and then subjected to immunoblot analysis and NF-κB reporter assay. Immunoblotting analysis showed the expression of TROY and PDZ-RhoGEF in transfected cells (Figure 6A, bottom panel). Consistent with previous results, increased expression of TROY stimulated NF-κB activity (Figure 6A, upper panel). In contrast, increased expression of PDZ-RhoGEF alone did not induce NF-κB activation above that of control cells. However, coexpression of PDZ-RhoGEF along with TROY potentiated NF-κB activation above that of TROY expression alone (Figure 6A, upper panel). PDZ-RhoGEF expression enhanced activation of NF-κB in a dose-dependent manner in 293/NF-κB-luc cells stably coexpressing TROY (Figure 6B), corroborating that PDZ-RhoGEF facilitates TROY-induced NF-κB activation. We also examined whether RhoC or RhoA is involved in TROY- and PDZ-RhoGEF–induced NF-κB activation. Notably, knockdown of RhoC or RhoA attenuated the PDZ-RhoGEF–induced activation of NF-κB in 293/NF-κB-luc/TROY-HA cells (Figure 6C, upper panel). Immunoblotting analysis showed that siRNA-mediated knockdown of RhoC or RhoA in 293/NF-κB-luc/TROY-HA cells with two independent siRNA oligonucleotides was >90% effective (Figure 6C, bottom panel). We also examined the effects of RhoA or RhoC on TROY- and PDZ-RhoGEF–induced NF-κB activation in T98G glioma cells transduced with the NF-κB luciferase reporter and with increased expression of TROY (T98G/NF-κB-luc/TROY-HA cells). Consistent with the results observed in 293/NF-κB-luc/TROY-HA cells, knockdown of RhoC or RhoA reduced the TROY-induced NF-κB activation in the T98G/NF-κB-luc/TROY-HA cells (Figure 6D, upper panel), which have endogenous PDZ-RhoGEF expression (see Figure 2C). Immunoblotting analysis showed the RhoC or RhoA in T98G/NF-κB-luc/TROY-HA cells with two independent siRNAs was >90% effective (Figure 6D, bottom panel). These data further suggest that PDZ-RhoGEF functions as a downstream effector of TROY and the association of PDZ-RhoGEF with TROY potentiates TROY-induced NF-κB activation, in part, via Rho.
Figure 6.
PDZ-RhoGEF (PRG) enhances TROY-induced NF-κB activation. (A) 293 cells expressing a NF-κB-luciferase reporter (293/NF-κB-luc) were transfected with the indicated plasmids. Twenty-four hours after transfection, cells were serum starved (0.1% BSA) for 16 hours and lysed, and NF-κB-luc reporter expression was measured using a luciferase reporter assay kit. Luciferase activity was normalized to the vector-transfected cells. The data are depicted as the mean values (+/− SD) (n = 3, *P < .05; **P < .01) (upper panel). The expression of HA-tagged TROY and Myc-tagged PDZ-RhoGEF in lysates was detected by immunoblotting (bottom panel). (B) 293/NF-κB-luc reporter cells overexpressing TROY (293/NF-κB-luc/TROY-HA) were transfected with either vector or 0.8 or 1.6 μg Myc-tagged PDZ-RhoGEF. Twenty-four hours after transfection, cells were serum starved (0.1% BSA) for 16 hours and lysed, and luciferase activity was measured using luciferase reporter assay kit. Luciferase activity was normalized to the vector-transfected cells. The data are depicted as the mean values (+/− SD) (n = 3, ***P < .001) (upper panel). Expression levels of PDZ-RhoGEF and TROY were determined by immunoblot analysis (bottom panel). (C) 293/NF-κB-luc/TROY-HA cells were transfected with either two independent siRNAs targeting RhoC or RhoA or a control siRNA (siCTRL). Twenty-four hours after transfection, cells were transfected with Myc-tagged PRG plasmid for an additional 24 hours. Cells were serum starved (0.1% BSA) for 16 hours and lysed, and luciferase activity was measured using a luciferase reporter assay kit. Luciferase activity was normalized to the control cells. The data are depicted as the mean values (+/− SD) (n = 3, *P < .05; **P < .01; ***P < .001) (upper panel). Knockdown of RhoC and RhoA expression and overexpression of PDZ-RhoGEF was verified by immunoblotting (bottom panel). (D) T98G/NF-κB-luc/TROY-HA cells were transfected with either two independent siRNAs targeting RhoC or RhoA or a control siRNA (siCTRL). Twenty-four hours after transfection, cells were serum starved (0.1% BSA) for additional 16 hours and lysed, and luciferase activity was measured using a luciferase reporter assay kit. Luciferase activity was normalized to the control cells. The data are depicted as the mean values (+/− SD) (n = 3, **P < .01; ***P < .001) (upper panel). Knockdown of RhoC and RhoA expression was verified by immunoblotting (bottom panel).
Discussion
Our previous studies have reported that TROY plays an important role in the regulation of GBM migration and invasion, thereby promoting GBM survival signaling and therapeutic resistance [13], [14]. In this study, we sought to identify important downstream effector(s) for TROY signaling in glioblastoma cells. The major findings of this study are as follows: 1) PDZ-RhoGEF forms a complex with TROY and is component of a signaling complex that includes Pyk2; 2) PDZ-RhoGEF expression is upregulated in GBM tumors; 3) knockdown of PDZ-RhoGEF suppressed TROY-induced glioma cell migration and decreased therapeutic resistance to TMZ; 4) depletion of RhoA and RhoC inhibited TROY- and PDZ-RhoGEF–induced cell migration; 5) depletion of PDZ-RhoGEF suppressed TROY-induced Rho activation; and 6) PDZ-RhoGEF potentiated TROY-induced NF-κB activation. Together, these results substantiate the role of PDZ-RhoGEF as an important effector for TROY signaling and suggest that targeting PDZ-RhoGEF may represent a novel strategy for GBM treatment.
This study is the first to identify PDZ-RhoGEF as a component of a signalsome that includes TROY and the non–receptor tyrosine kinase Pyk2. Previously, we demonstrated that TROY-induced cell migration and invasion were dependent upon Pyk2 expression and kinase activity [13]. Here, we observed via immunoprecipitation that TROY, Pyk2, and PDZ-RhoGEF could all be found in the same immunoprecipitate. Moreover, we observed that PDZ-RhoGEF immunoprecipitated with Pyk2 was phosphorylated but was not phosphorylated when immunoprecipitated with a kinase dead Pyk2 variant. This association appears to be specific as neither TROY nor Pyk2 co-immunoprecipitated with the closely related GEF LARG. Further studies are planned to investigate the temporal assembly of this complex, the domains that mediate the different interactions, and the functional significance of the phosphorylation of PDZ-RhoGEF by Pyk2.
It is well appreciated that members of the small family of Rho GTPases regulate cell motility through their effects on actin dynamics and cytoskeletal organization [39]. Rho GTPases such as RhoA, Cdc42, and Rac1 are master regulators of cell migration and proliferation and are involved in the formation of stress fibers, induction of lamellipodia, and filopodia protrusion [40]. In addition, Rho GTPases have been implicated in glioma cell invasion and tumor progression [20]. Indeed, our earlier study showed that TROY activates Rac1 signaling in a Pyk2–dependent fashion, leading to enhanced GBM cell motility [13]. In this study, our data showed that PDZ-RhoGEF knockdown does not affect Rac1 activation, suggesting that TROY-mediated Rac1 activation is independent of PDZ-RhoGEF. In contrast, knockdown of PDZ-RhoGEF inhibited TROY-stimulated RhoA and RhoC activation in serum-starved glioma cells. Moreover, knockdown of RhoA or RhoC expression inhibited TROY- and PDZ-RhoGEF–induced glioma cell migration.
Activity of Rho GTPases is regulated by a large number of Rho guanine nucleotide exchange factors (RhoGEFs), which activate Rho GTPases by promoting the exchange of GDP for GTP [17]. To date, it has been reported that seven Rac1-activating GEFs, including Ect2, Trio, Vav3, SWAP-70, Dock7, Dock180-ELMO1, and SGEF, enhance GBM cell migration and invasion [27], [41], [42], [43]. Six of these GEFs have been shown to be overexpressed in glioblastoma compared to non-neoplastic brain (Ect2, Trio, Vav3, SWAP-70, Dock7, and SGEF) [27], [41], [43], whereas overexpression of ELMO1 and Dock180 was observed in invasive glioma cells compared to glioma cells in the tumor core [42]. Here, our observations add PDZ-RhoGEF to this growing list of GEFs that are involved in GBM cell invasion.
PDZ-RhoGEF, p115RhoGEF, and the leukemia-associated RhoGEF (LARG) constitute a RhoGEF subfamily (RGS-RhoGEFs) characterized by the presence of the regulator of G protein signaling (RGS) domain. Emerging evidence suggests that the role of these RGS-RhoGEFs is varied in cancer cell invasion and their functions may be tumor specific. Expression of p115RhoGEF is upregulated in prostate cancer cell lines (DU145 and PC-3) and invasive prostate tumors [44], whereas LARG expression is found to be decreased in breast and colon carcinomas, potentially functioning as a putative tumor suppressor [45]. Overexpression of PDZ-RhoGEF has been noted in several cancers, including breast, colon, and gallbladder cancers, as well as glioma [31], [46], [47], [48]. Existing evidence supports an important role for PDZ-RhoGEF in cancer cell migration and invasion. For example, PDZ-RhoGEF is required for CXCR4-promoted breast cancer cell migration and invasion [48]. Immunohistochemistry studies further showed that elevated expression of PDZ-RhoGEF correlates with an invasive phenotype in human breast cancer [48]. Here, we demonstrated that PDZ-RhoGEF expression is upregulated in patient GBM specimens and that depletion of PDZ-RhoGEF inhibited TROY-induced glioma cell invasion.
Consistent with the observation that migratory/invasive glioma cells exhibit increased resistance to cytotoxic agents [49], [50], the current results demonstrated that depletion of PDZ-RhoGEF inhibited cellular invasion and sensitized glioma cells to TMZ-induced apoptosis. This result is supported by the results of a high-throughput siRNA screen assay that identified PDZ-RhoGEF as 1 of the 55 survival genes in T98G glioma cell line, implicating a role of PDZ-RhoGEF in glioma cell survival signaling [47]. Activation of NF-κB has been shown to promote cell survival in GBM tumors [14], [25], [51], and the current results demonstrate that the association of PDZ-RhoGEF with TROY further enhanced TROY-induced NF-κB activation. However, we observed that the knockdown of RhoA or RhoC expression alone does not completely inhibit TROY- and PDZ-RhoGEF–induced NF-κB activation. This partial inhibition of NF-κB induced by TROY and PDZ-RhoGEF suggests that RhoA and RhoC can compensate for each other in the activation of NF-κB. In addition, silencing PDZ-RhoGEF by shRNA significantly prolonged survival in a glioma xenograft model. Together, these data further support that PDZ-RhoGEF may play an important role in GBM cell survival.
Immunohistochemical analysis of a glioma tissue microarray demonstrated that PDZ-RhoGEF expression is significantly increased in GBM versus non-neoplastic brain. The increased expression of PDZ-RhoGEF in GBM tumors and its role in GBM cell invasion and cell survival suggest that PDZ-RhoGEF may represent a novel potential target for treatment. There is emerging evidence that supports the feasibility of targeting RhoGEFs as a promising anticancer therapy [52]. For example, it has been reported that selective inhibition of Trio via targeting the TrioGEFD2 domain by the inhibitor TRIPα inhibits RhoA activation, thereby inhibiting the neurite retraction phenotype induced by TrioGEFD2 in PC12 cells [53]. Furthermore, PDZ-RhoGEF inhibitors are likely to have a wide therapeutic window since mice deficient in PDZ-RhoGEF do not display any noticeable phenotype [54].
In summary, the current data establish an important role for PDZ-RhoGEF as an effector of TROY signaling. The results of this study show that PDZ-RhoGEF is a component of a signalsome including TROY, and expression of PDZ-RhoGEF is upregulated in GBM tumors and potentiates TROY-induced migration and NF-κB activation. In contrast, depletion of PDZ-RhoGEF suppresses RhoA and RhoC activation, which results in inhibition of glioma cell invasion and increases the sensitivity of GBM cells to TMZ treatment. Taken together, these suggest that PDZ-RhoGEF may represent a potential node of vulnerability to limit GBM cell invasion and decrease therapeutic resistance.
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgements
The authors thank Dr. Zhekang Ying (University of Maryland School of Medicine) for the gift of the rat PDZ-RhoGEF and LARG plasmids. This works was supported in part by National Institutes of Health grants R01 NS086853 and U01 CA220378-01 (J.C. Loftus and N.L. Tran).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2018.08.008.
Contributor Information
Nhan L. Tran, Email: Tran.Nhan@mayo.edu.
Joseph C. Loftus, Email: Loftus.Joseph@mayo.edu.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 4.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Tamada K, Ni J, Vincenz C, Chen L. Characterization of TNFRSF19, a novel member of the tumor necrosis factor receptor superfamily. Genomics. 1999;62:103–107. doi: 10.1006/geno.1999.5979. [DOI] [PubMed] [Google Scholar]
- 6.Pispa J, Mikkola ML, Mustonen T, Thesleff I. Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr Patterns. 2003;3:675–679. doi: 10.1016/s1567-133x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 7.Hisaoka T, Morikawa Y, Kitamura T, Senba E. Expression of a member of tumor necrosis factor receptor superfamily, TROY, in the developing mouse brain. Brain Res Dev Brain Res. 2003;143:105–109. doi: 10.1016/s0165-3806(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 8.Hisaoka T, Morikawa Y, Kitamura T, Senba E. Expression of a member of tumor necrosis factor receptor superfamily, TROY, in the developing olfactory system. Glia. 2004;45:313–324. doi: 10.1002/glia.10323. [DOI] [PubMed] [Google Scholar]
- 9.Spanjaard RA, Whren KM, Graves C, Bhawan J. Tumor necrosis factor receptor superfamily member TROY is a novel melanoma biomarker and potential therapeutic target. Int J Cancer. 2007;120:1304–1310. doi: 10.1002/ijc.22367. [DOI] [PubMed] [Google Scholar]
- 10.Bei JX, Li Y, Jia WH, Feng BJ, Zhou G, Chen LZ, Feng QS, Low HQ, Zhang H, He F. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Wu C, Shi Y, Guo H, Zhao X, Yin Z, Yang L, Dai J, Hu L, Tan W. A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet. 2011;43:792–796. doi: 10.1038/ng.875. [DOI] [PubMed] [Google Scholar]
- 12.Schon S, Flierman I, Ofner A, Stahringer A, Holdt LM, Kolligs FT, Herbst A. beta-catenin regulates NF-kappaB activity via TNFRSF19 in colorectal cancer cells. Int J Cancer. 2014;135:1800–1811. doi: 10.1002/ijc.28839. [DOI] [PubMed] [Google Scholar]
- 13.Paulino VM, Yang Z, Kloss J, Ennis MJ, Armstrong BA, Loftus JC, Tran NL. TROY (TNFRSF19) is overexpressed in advanced glial tumors and promotes glioblastoma cell invasion via Pyk2-Rac1 signaling. Mol Cancer Res. 2010;8:1558–1567. doi: 10.1158/1541-7786.MCR-10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftus JC, Dhruv H, Tuncali S, Kloss J, Yang Z, Schumacher CA, Cao B, Williams BO, Eschbacher JM, Ross JT. TROY (TNFRSF19) promotes glioblastoma survival signaling and therapeutic resistance. Mol Cancer Res. 2013;11:865–874. doi: 10.1158/1541-7786.MCR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Z, Roos A, Kloss J, Dhruv H, Peng S, Pirrotte P, Eschbacher JM, Tran NL, Loftus JC. A novel signaling complex between TROY and EGFR mediates glioblastoma cell invasion. Mol Cancer Res. 2018;16:322–332. doi: 10.1158/1541-7786.MCR-17-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 17.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 18.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases–GEFs what's the link. Cell Signal. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Goicoechea SM, Awadia S, Garcia-Mata R. I'm coming to GEF you: regulation of RhoGEFs during cell migration. Cell Adh Migr. 2014;8:535–549. doi: 10.4161/cam.28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortin Ensign SP, Mathews IT, Symons MH, Berens ME, Tran NL. Implications of Rho GTPase signaling in glioma cell invasion and tumor progression. Front Oncol. 2013;3:241. doi: 10.3389/fonc.2013.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA, Galanis E, Giannini C, Wu W, Dinca EB. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, Berens ME, Loftus JC. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying Z, Giachini FR, Tostes RC, Webb RC. PYK2/PDZ-RhoGEF links Ca2+ signaling to RhoA. Arterioscler Thromb Vasc Biol. 2009;29:1657–1663. doi: 10.1161/ATVBAHA.109.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran NL, McDonough WS, Savitch BA, Sawyer TF, Winkles JA, Berens ME. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J Biol Chem. 2005;280:3483–3492. doi: 10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- 26.Fortin SP, Ennis MJ, Savitch BA, Carpentieri D, McDonough WS, Winkles JA, Loftus JC, Kingsley C, Hostetter G, Tran NL. Tumor necrosis factor-like weak inducer of apoptosis stimulation of glioma cell survival is dependent on Akt2 function. Mol Cancer Res. 2009;7:1871–1881. doi: 10.1158/1541-7786.MCR-09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salhia B, Tran NL, Chan A, Wolf A, Nakada M, Rutka F, Ennis M, McDonough WS, Berens ME, Symons M. The guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate the invasive behavior of glioblastoma. Am J Pathol. 2008;173:1828–1838. doi: 10.2353/ajpath.2008.080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakada M, Niska JA, Miyamori H, McDonough WS, Wu J, Sato H, Berens ME. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64:3179–3185. doi: 10.1158/0008-5472.can-03-3667. [DOI] [PubMed] [Google Scholar]
- 29.Roos A, Dhruv HD, Mathews IT, Inge LJ, Tuncali S, Hartman LK, Chow D, Millard N, Yin HH, Kloss J. Identification of aurintricarboxylic acid as a selective inhibitor of the TWEAK-Fn14 signaling pathway in glioblastoma cells. Oncotarget. 2017;8:12234–12246. doi: 10.18632/oncotarget.14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, Parsons JT. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci. 2008;121:895–905. doi: 10.1242/jcs.020941. [DOI] [PubMed] [Google Scholar]
- 31.Patel M, Kawano T, Suzuki N, Hamakubo T, Karginov AV, Kozasa T. Galpha13/PDZ-RhoGEF/RhoA signaling is essential for gastrin-releasing peptide receptor-mediated colon cancer cell migration. Mol Pharmacol. 2014;86:252–262. doi: 10.1124/mol.114.093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- 33.Dhruv HD, Roos A, Tomboc PJ, Tuncali S, Chavez A, Mathews I, Berens ME, Loftus JC, Tran NL. Propentofylline inhibits glioblastoma cell invasion and survival by targeting the TROY signaling pathway. J Neurooncol. 2016;126:397–404. doi: 10.1007/s11060-015-1981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togashi H, Nagata K, Takagishi M, Saitoh N, Inagaki M. Functions of a rho-specific guanine nucleotide exchange factor in neurite retraction. Possible role of a proline-rich motif of KIAA0380 in localization. J Biol Chem. 2000;275:29570–29578. doi: 10.1074/jbc.M003726200. [DOI] [PubMed] [Google Scholar]
- 35.Rumenapp U, Blomquist A, Schworer G, Schablowski H, Psoma A, Jakobs KH. Rho-specific binding and guanine nucleotide exchange catalysis by KIAA0380, a dbl family member. FEBS Lett. 1999;459:313–318. doi: 10.1016/s0014-5793(99)01270-3. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 40.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 41.Seol HJ, Smith CA, Salhia B, Rutka JT. The guanine nucleotide exchange factor SWAP-70 modulates the migration and invasiveness of human malignant glioma cells. Transl Oncol. 2009;2:300–309. doi: 10.1593/tlo.09172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarzynka MJ, Hu B, Hui KM, Bar-Joseph I, Gu W, Hirose T, Haney LB, Ravichandran KS, Nishikawa R, Cheng SY. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007;67:7203–7211. doi: 10.1158/0008-5472.CAN-07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fortin Ensign SP, Mathews IT, Eschbacher JM, Loftus JC, Symons MH, Tran NL. The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2. J Biol Chem. 2013;288:21887–21897. doi: 10.1074/jbc.M113.468686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang C, Liu S, Miller RT. Role of p115RhoGEF in the regulation of extracellular Ca(2+)-induced choline kinase activation and prostate cancer cell proliferation. Int J Cancer. 2011;128:2833–2842. doi: 10.1002/ijc.25633. [DOI] [PubMed] [Google Scholar]
- 45.Ong DC, Ho YM, Rudduck C, Chin K, Kuo WL, Lie DK, Chua CL, Tan PH, Eu KW, Seow-Choen F. LARG at chromosome 11q23 has functional characteristics of a tumor suppressor in human breast and colorectal cancer. Oncogene. 2009;28:4189–4200. doi: 10.1038/onc.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JH, Kim HN, Lee KT, Lee JK, Choi SH, Paik SW, Rhee JC, Lowe AW. Gene expression profiles in gallbladder cancer: the close genetic similarity seen for early and advanced gallbladder cancers may explain the poor prognosis. Tumour Biol. 2008;29:41–49. doi: 10.1159/000132570. [DOI] [PubMed] [Google Scholar]
- 47.Thaker NG, Zhang F, McDonald PR, Shun TY, Lewen MD, Pollack IF, Lazo JS. Identification of survival genes in human glioblastoma cells by small interfering RNA screening. Mol Pharmacol. 2009;76:1246–1255. doi: 10.1124/mol.109.058024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Struckhoff AP, Rana MK, Kher SS, Burow ME, Hagan JL, Del Valle L, Worthylake RA. PDZ-RhoGEF is essential for CXCR4-driven breast tumor cell motility through spatial regulation of RhoA. J Cell Sci. 2013;126:4514–4526. doi: 10.1242/jcs.132381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joy AM, Beaudry CE, Tran NL, Ponce FA, Holz DR, Demuth T, Berens ME. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409–4417. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- 50.Demuth T, Reavie LB, Rennert JL, Nakada M, Nakada S, Hoelzinger DB, Beaudry CE, Henrichs AN, Anderson EM, Berens ME. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther. 2007;6:1212–1222. doi: 10.1158/1535-7163.MCT-06-0711. [DOI] [PubMed] [Google Scholar]
- 51.Tran NL, McDonough WS, Savitch BA, Fortin SP, Winkles JA, Symons M, Nakada M, Cunliffe HE, Hostetter G, Hoelzinger DB. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res. 2006;66:9535–9542. doi: 10.1158/0008-5472.CAN-06-0418. [DOI] [PubMed] [Google Scholar]
- 52.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt S, Diriong S, Mery J, Fabbrizio E, Debant A. Identification of the first Rho-GEF inhibitor, TRIPalpha, which targets the RhoA-specific GEF domain of Trio. FEBS Lett. 2002;523:35–42. doi: 10.1016/s0014-5793(02)02928-9. [DOI] [PubMed] [Google Scholar]
- 54.Mikelis CM, Palmby TR, Simaan M, Li W, Szabo R, Lyons R, Martin D, Yagi H, Fukuhara S, Chikumi H. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem. 2013;288:12232–12243. doi: 10.1074/jbc.M112.428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures