Significance
Obesity is a widespread heritable health condition. Evidence from psychology, cognitive neuroscience, and genetics has proposed links between obesity and the brain. The current study tested whether the heritable variance in body mass index (BMI) is explained by brain and behavioral factors in a large brain imaging cohort that included multiple related individuals. We found that the heritable variance in BMI had genetic correlations 0.25–0.45 with cognitive tests, cortical thickness, and regional brain volume. In particular, BMI was associated with frontal lobe asymmetry and differences in temporal-parietal perceptual systems. Further, we found genetic overlap between certain brain and behavioral factors. In summary, the genetic vulnerability to BMI is expressed in the brain. This may inform intervention strategies.
Keywords: brain morphology, cortical thickness, cognition, twins, body mass index
Abstract
Recent molecular genetic studies have shown that the majority of genes associated with obesity are expressed in the central nervous system. Obesity has also been associated with neurobehavioral factors such as brain morphology, cognitive performance, and personality. Here, we tested whether these neurobehavioral factors were associated with the heritable variance in obesity measured by body mass index (BMI) in the Human Connectome Project (n = 895 siblings). Phenotypically, cortical thickness findings supported the “right brain hypothesis” for obesity. Namely, increased BMI is associated with decreased cortical thickness in right frontal lobe and increased thickness in the left frontal lobe, notably in lateral prefrontal cortex. In addition, lower thickness and volume in entorhinal-parahippocampal structures and increased thickness in parietal-occipital structures in participants with higher BMI supported the role of visuospatial function in obesity. Brain morphometry results were supported by cognitive tests, which outlined a negative association between BMI and visuospatial function, verbal episodic memory, impulsivity, and cognitive flexibility. Personality–BMI correlations were inconsistent. We then aggregated the effects for each neurobehavioral factor for a behavioral genetics analysis and estimated each factor’s genetic overlap with BMI. Cognitive test scores and brain morphometry had 0.25–0.45 genetic correlations with BMI, and the phenotypic correlations with BMI were 77–89% explained by genetic factors. Neurobehavioral factors also had some genetic overlap with each other. In summary, obesity as measured by BMI has considerable genetic overlap with brain and cognitive measures. This supports the theory that obesity is inherited via brain function and may inform intervention strategies.
Obesity is a widespread condition leading to increased mortality (1) and economic costs (2). Twin and family studies have shown that individual differences in obesity are largely explained by genetic variance (3). Gene enrichment patterns suggest that obesity-related genes are preferentially expressed in the brain (4). While it is unclear how these brain-expressed genes lead to obesity, several lines of research show that neural, cognitive, and personality differences have a role in vulnerability to obesity (5, 6). Here, we seek to test whether these neurobehavioral factors could explain the genetic variance in obesity.
In the personality literature, obesity is most often negatively associated with conscientiousness (self-discipline and orderliness) and positively with neuroticism (a tendency toward negative affect) (7). In the cognitive domain, tests capturing executive function, inhibition, and attentional control have a negative association with obesity (5–8). Neuroanatomically, obesity seems to have a negative association with the gray matter volume of prefrontal cortex and, to a lesser extent, the volume of parietal and temporal lobes, as measured by voxel-based morphometry (9). It has also been suggested that structural and functional asymmetry of the prefrontal cortex might underlie overeating and obesity (10). For genetic analysis, cortical thickness estimates of brain structure from magnetic resonance imaging (MRI) have been preferred over volumetric measures (11). However, to date, reports of cortical thickness patterns associated with obesity have been inconsistent (12, 13). As a prerequisite to our goal of ascertaining the heritability of brain-based vulnerability to obesity, we sought to extend previous neurobehavioral findings in a large multifactor dataset from the Human Connectome Project (HCP). We also measured volumetric estimates of medial temporal lobe and subcortical structures, which have been implicated in appetitive control (e.g., ref. 14).
The main goal was to assess whether the aforementioned obesity–neurobehavioral associations are of genetic or environmental origin. Recent evidence from behavioral and molecular genetics suggests that there is considerable genetic overlap among obesity, cognitive test scores, and brain imaging findings (15–20). However, the evidence so far is not comprehensive across all neurobehavioral factors discussed. A recent paper assessed the heritability of obesity-associated regional brain volumes (21). However, the study did not analyze the heritability of the association between brain and obesity. The latter analysis is crucial for understanding whether brain anatomy and obesity could have a genetic overlap, which would suggest that the heritability of vulnerability to obesity is expressed in the brain.
In addition, we sought to estimate the genetic overlap between the different BMI-related neurobehavioral factors. Performance on cognitive tests and personality must originate from the brain (e.g., ref. 22) and, therefore, personality and cognition could be expected to explain brain–morphometry associations with BMI (6). However, brain–behavior associations are far from certain (23), and even different measurement traditions in both behavior (personality and cognitive tests) and brain morphometry (cortical thickness or brain volume) are often conceptualized as providing independent sources of information (7, 11). Documenting the degree of genetic overlap between behavioral and brain measures would shed light on whether similar underlying processes lead to obesity’s associations with different neurobehavioral factors.
Taken together, the goal of the current analysis was to use a large multifactor dataset to analyze the heritability of the associations between obesity and brain/behavior. We further tested genetic overlap between the different neurobehavioral factors themselves.
Results
Background.
We analyzed data from 895 participants from the Human Connectome Project S900 release (24), including 111 pairs of monozygotic twins and 188 pairs of dizygotic twins and siblings. Similar to many previous reports (3) we modeled BMI heritability with the AE model (A, additive genetics; E, unique environment), as opposed to the ACE model (C, common environment), as AE had the lowest Akaike Information Criterion (Dataset S1, section 9). BMI heritability was A = 71% [95% CI: 61%; 78%], which is close to the published meta-analytic estimate (A = 75%, ref. 3).
In all analyses below, we controlled for age, gender, race, ethnicity, handedness, and evidence of drug consumption on day of testing, which mostly associated with BMI (SI Appendix, SI Results and Fig. S2). When presenting and interpreting phenotypic associations, we controlled for family structure to avoid inflated effect sizes and SEs (e.g., ref. 25). The behavioral genetics analysis did not control for family structure, since this information is needed for modeling heritability. As socio-economic status (SES) is intertwined with cognitive test scores (26), personality (27), and brain morphometry (28), we also present phenotypic associations controlling for SES (education and income) in SI Appendix, Supplementary Material. All in-text P values are provided without correcting for multiple comparisons. False discovery rate (FDR) correction was applied when screening for features within cognitive, personality, and brain factors (Figs. 1 and 2).
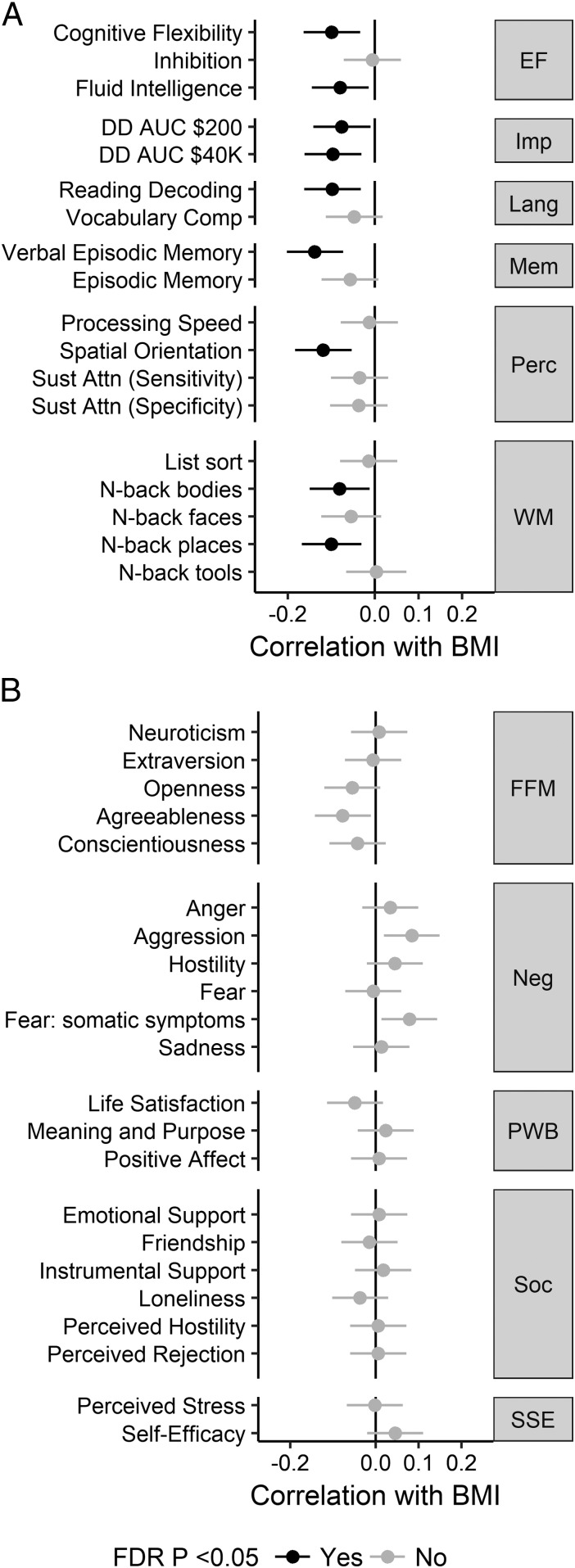
Fig. 1.
Associations between BMI and cognitive test scores (A) and personality traits (B). Error bars represent 95% confidence intervals. See Dataset S1, section 1 for explanation of cognitive tests. Numerical values are reported in Dataset S1, section 2. EF, executive function; FDR, false discovery rate; FFM, Five-Factor Model; Imp, (lack of) impulsivity; Lang, language; Mem, memory; Neg, negative affect; Perc, perception; PWB, psychological well-being; Soc, social relationships; SSE, stress and self efficacy; WM, working memory.
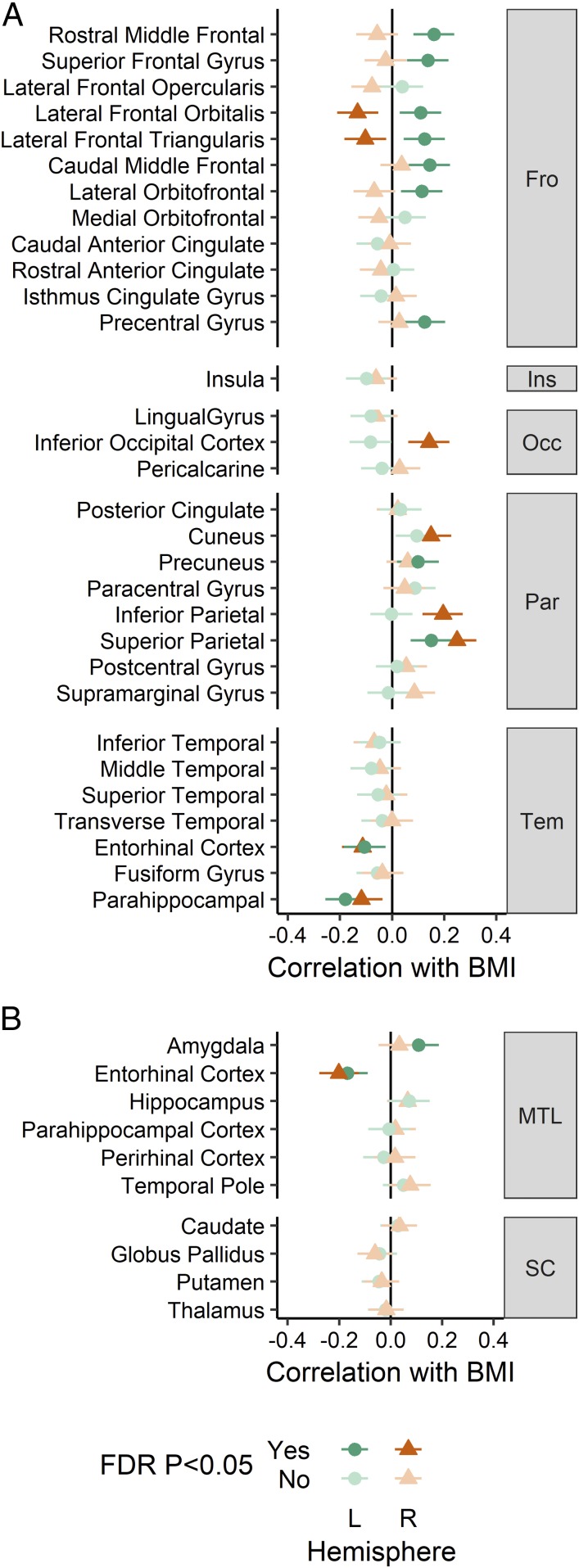
Fig. 2.
Associations between BMI and brain morphometry. (A) Cortical thickness. (B) Medial temporal and subcortical regional brain volume. Error bars represent 95% confidence intervals. Numerical values are reported in Dataset S1, section 2. FDR, false discovery rate; Fro, frontal, Ins, insula; L, left; MTL, medial temporal lobe; Occ, occipital; Par, parietal; R, right; SC, subcortical; Tem, temporal.
Cognitive and Personality Factors.
BMI was negatively correlated with the following tests of executive function: cognitive flexibility, fluid intelligence, inability to delay gratification, reading abilities, and working memory. Intriguingly, the strongest effects were present for nonexecutive tasks measuring visuospatial ability and verbal memory (Fig. 1A). These tasks remained associated with BMI after controlling for SES; controlling for SES reduced the number of executive function tests involved with BMI to cognitive flexibility and inability to delay gratification (SI Appendix, Fig. S3A, Left). No personality test score correlated with BMI when FDR correction was applied (Fig. 1B).
Brain Morphology.
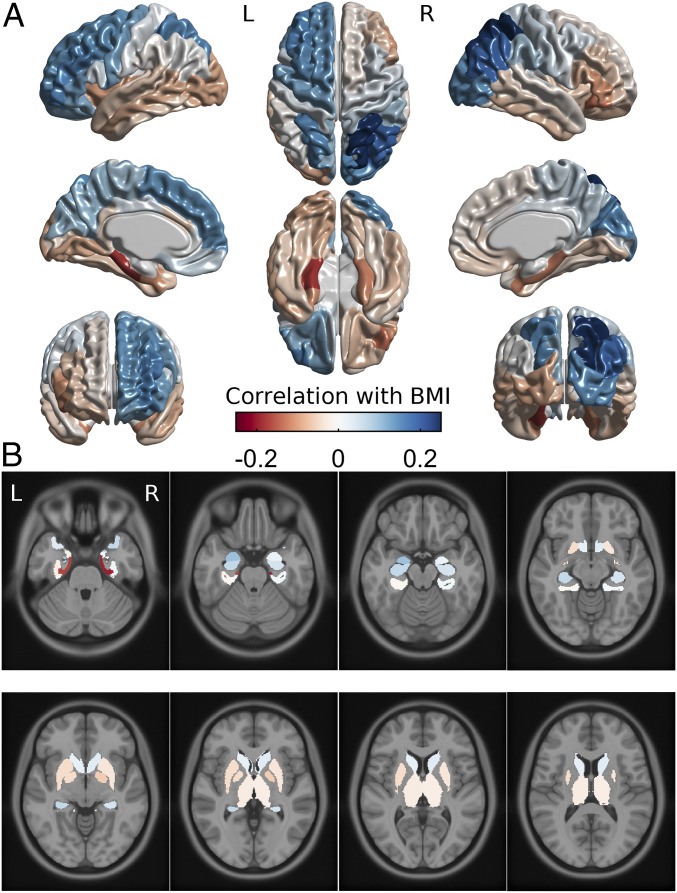
Cortical thickness was estimated from each T1-weighted MRI using CIVET 2.0 software (29). Parcel-based analysis identified negative associations with BMI in right inferior lateral frontal cortex and bilateral entorhinal-parahippocampal cortex (Figs. 2A and 3A). Positive associations with BMI were found with the left superior frontal cortex, left inferior lateral frontal cortex, and bilateral parietal cortex parcels. Controlling for SES did not change these results (SI Appendix, Fig. S4A, Left). The frontal lobe asymmetry in the BMI association (thinner on the right, thicker on the left) mostly involved the inferior lateral prefrontal areas, such as inferior frontal gyrus.
Fig. 3.
Brain maps of the associations between BMI and cortical thickness (A) and medial temporal and subcortical regional brain volume (B) on a standard brain template in Montreal Neurological Institute space. Values are the same as in Fig. 2. Color bar applies to both subplots. L, left; R, right.
Regional brain volumes were measured for estimation of brain morphology–obesity associations in brain structures not covered by the CIVET cortical thickness algorithm. Medial temporal lobe and subcortical volumes were individually segmented and measured by registering each brain to a labeled atlas using ANIMAL software (30). Volumetric results demonstrated an association between BMI and lower volume of the entorhinal cortex bilaterally and a positive association of left amygdala volume with BMI (Figs. 2B and 3B). No subcortical region had a significant association with BMI, and results did not change when controlling for SES (SI Appendix, Fig. S4B, Left).
Creating Poly-Phenotype Scores.
We performed dimension reduction for heritability analyses to reduce measurement noise and avoid multiple testing with redundant measures. Similar to other recent papers (20, 27), we used the weights of each individual feature within a neurobehavioral factor (personality test, cognitive test, brain parcel) to create an aggregate BMI risk score or poly-phenotype score (PPS). This is similar to the polygenic score approach in genetics, where the small effects of several polymorphisms are aggregated to yield a total effect score (15, 19, 20, 27). We used the correlation values as weights to multiply each participant’s scaled measurements and aggregated the results into a single composite variable, the PPS. The PPS reflects the total association of each neurobehavioral factor with BMI. To avoid overfitting, we assigned each 10% of participants the PPS weights obtained from the other 90% (see SI Appendix, Data Analysis for details).
The associations between BMI and the PPS-s for cognition (correlation with BMI: r = 0.16, P < 0.001, n = 798) and personality (r = 0.08, P = 0.017, n = 888) are slightly higher than the meta-analytic estimates of the pooled association between BMI and cognitive test scores (r = 0.10, ref. 8) and personality factors (r = 0.05, ref. 8). BMI had stronger associations with the PPS-s for cortical thickness (r = 0.26, P < 0.001, n = 591) and medial temporal brain volume (r = 0.23, P < 0.001, n = 594). There was no association between BMI and subcortical brain volume (r = −0.05, P = 0.169, n = 828). To test the generalizability of the PPS approach, we used weights obtained from the full S900 release (SI Appendix, Fig. S3, Right and SI Appendix, Fig. S4, Right) to test PPS–BMI correlation among the unseen additional participants in the S1200 release (referred to as S1200n, n = 236). Cortical thickness PPS had essentially unchanged effect size when correlated with BMI in S1200n (SI Appendix, SI Results and Fig. S7). At the same time, cognitive and personality PPS-s were less stable (SI Appendix, SI Results and Fig. S7), likely because the smaller effect sizes of individual features need larger training datasets to reduce inaccuracies, or that the true PPS-BMI effect size was too small to be found just within the S1200n sample.
Heritability.
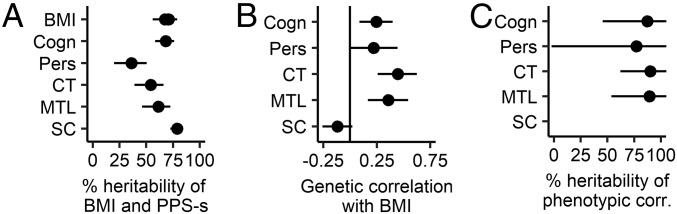
Bivariate heritability was similarly conducted with the AE model, since the main goal was to explain variance in BMI, for which AE was the best model. All PPS-s were found to be highly heritable, with the A component explaining 36–79% of the variance (Fig. 4A and Dataset S1, section 10). Significant genetic correlations (rg) were found between BMI and cognitive test scores [rg = 0.25 (P = 0.002), cortical thickness (rg = 0.45, P < 0.001), and medial temporal brain volume (rg = 0.36, P < 0.001) (Fig. 4B and Dataset S1, section 11). The personality PPS genetic correlation with BMI was not significant (rg = 0.22, P = 0.052). Molecular evidence relying on linkage disequilibrium score regression has reported effects of similar magnitude between higher cognitive test scores and BMI (rg = −0.22, ref. 15; rg = −0.18, ref. 18). Environmental correlations (i.e., correlations between environmental variances) were small and not significant (Dataset S1, section 11). As expected from high heritability of the traits and high genetic correlations, the phenotypic BMI–PPS correlations described in the previous sections were 77–89% explained by genetic factors (Fig. 4C and Dataset S1, section 10).
Fig. 4.
Heritability analysis of the association between PPS and BMI. (A) Heritability of each trait. BMI has multiple estimates, since it was entered into a bivariate analysis with each PPS separately. (B) Genetic correlations between BMI and each PPS. The genetic correlations are positive, because the PPS-s are designed to positively predict BMI. (C) Heritability of the significant phenotypic correlation between BMI and PPS. Horizontal lines depict 95% confidence intervals. Cogn, PPS of cognitive tests; corr, correlation; CT, PPS of cortical thickness; MTL, PPS of medial temporal lobe volume; Pers, PPS of personality tests; SC, PPS of subcortical structure volumes.
The results broadly replicated when repeating the analysis with just the top features within a PPS, suggesting that PPS-based findings summarize the effects of the underlying individual features (SI Appendix, Fig. S8). We further replicated the heritability patterns in a separate analysis focused only on the additional participants from the S1200 HCP release (SI Appendix, Fig. S9). Additionally, controlling for SES (education and income) did not change the results for brain-based PPS-s. However, the estimates for cognitive test scores and personality became lower and not significant in the S900 release (SI Appendix, Fig. S10). However, the same estimates were significant in the combined sample S900+S1200, suggesting that the effects of cognition and personality were reduced but not eliminated when controlling for SES.
Genetic Overlap Between Neurobehavioral Factors.
Phenotypically, certain PPS-s had small but significant intercorrelations (SI Appendix, Fig. S11, upper triangle). After FDR correction, we were able to find two genetic correlations between PPS-s of cognition and cortical thickness (rg = 0.35), as well as cognition and personality (rg = 0.33, SI Appendix, Fig. S11, lower triangle). Taken together, while the neurobehavioral factors have mostly independent effects on BMI, cognitive test scores may have a small genetic overlap with brain structure and personality.
Discussion
Cortical thickness, medial temporal lobe volume, and cognitive measures all had covariation with BMI, and their effect on BMI was almost entirely heritable. Similarly, we found genetic correlations between obesity risk scores of cognition, cortical thickness, and personality. Together, our results from a large sample support the role of brain and psychological constructs in explaining genetic variance in BMI.
BMI correlated with increased cortical thickness in the left prefrontal cortex and decreased thickness in the right prefrontal cortex, supporting the “right brain” hypothesis for obesity (10). The effect was most prominent in the inferior frontal gyrus (Figs. 2A and 3A). Only preliminary support for the right brain hypothesis has been previously available (13). Right prefrontal cortex has been implicated in inhibitory control (22) and possibly bodily awareness (10). Many neuromodulation interventions (e.g., transcranial magnetic stimulation) aimed at increasing self-regulation capacity often target right prefrontal cortex. However, effects have also been demonstrated in studies targeting left prefrontal cortex (31).
Cortical thickness results also highlighted the role of temporo-parietal perceptual structures in obesity. Namely, BMI was associated with bilaterally decreased thickness of the parahippocampal and entorhinal cortices, and with mostly right-lateralized increased thickness of parietal and occipital lobes. Volumetric results within the medial temporal lobe supported the role of entorhinal cortex and also suggested that obesity is positively associated with the volume of left amygdala. Emergence of the effects of the right parietal structures together with right prefrontal structures hint at the role of the ventral frontoparietal network, thought to be especially important for detection of behaviorally relevant visual stimuli (32). The parahippocampal and entorhinal cortex are associated with episodic memory and context mediation (33). Similarly, the hippocampus has been associated with the modulation of food cue reactivity by homeostatic and contextual information, and hippocampal dysfunction is postulated to promote weight gain in the western diet environment (34). The amygdala is implicated in emotional and appetitive responses to sensory stimuli, including food cues (35).
Integrating these findings, one could envision a model where obesity is associated with a certain cognitive profile (36). The model starts with a hyperactive visual attention system attributing heightened salience to food stimuli, implicating the ventral visual stream and amygdala. These signals are then less optimally tied into relevant context by the parahippocampal and entorhinal structures, and less well moderated (or filtered) by the prefrontal executive system. This could result in consummatory behavior driven by the presence of appetitive food signals, which are ubiquitous in our obesogenic environment. An impaired response inhibition and salience attribution model of obesity has been suggested based on the functional neuroimaging literature. Namely, functional MRI studies have consistently identified obesity to associate with heightened salience response to food cues, coupled with reduced activation in prefrontal and executive systems involved in self-regulation and top-down attentional control (e.g., ref. 35). A similar conclusion emerged from a recent resting state network analysis of the HCP data (37), in which obesity was associated with alterations in perceptual networks and decreased activity of default mode and central executive networks.
This brain morphology-derived model has some support from cognitive tests. The role of prefrontal executive control is outlined by our finding of a negative association between BMI and scores on several executive control tasks. Surprisingly, there was no effect of motor inhibition as measured by the Flanker inhibitory task. A relation between obesity and reduced motor inhibition, while often mentioned, has been inconsistent even across meta-analyses (7, 8). However, we found a relationship between decisional impulsivity, measured by delay discounting, and BMI, replicating previous literature (6, 7, 18). While controlling for education reduced the number of executive tasks associated with BMI, the overall pattern remained the same, suggesting that education level is a proxy for certain executive function abilities.
Intriguingly, BMI was found to be negatively associated with spatial orientation and verbal episodic memory. These tasks tap into the key functions associated with entorhinal and parahippocampal regions implicated in our study (33). Therefore, both cognitive and brain morphology features propose that the increased salience of food stimuli could be facilitated by dysregulated context representation in obesity.
Regarding personality, we were unable to find any questionnaire-specific effects, notably with respect to neuroticism and conscientiousness, both often thought to be associated with obesity (5–7). There are potential explanations for this negative finding. First, the meta-analytical association between various personality tests and BMI is small (r = 0.05, ref. 7), for which we might have been underpowered after P value correction. Second, controlling for family structure likely further reduced the effect sizes (25). Third, the personality–obesity associations tend to pertain to more specific facets and nuances than broad personality traits (38), therefore, further analysis with more detailed and eating-specific personality measures is needed in larger samples.
All of the associations discussed here were largely due to shared genetic variance between neurobehavioral factors and BMI. This is in accordance with recent molecular genetics evidence that 75% of obesity-related genes express preferentially in the brain (4). Similarly, the genetic correlation between cognition and BMI uncovered in our sample is at the same magnitude as molecular estimates of associations between more specific cognitive measures and BMI (15, 18). The current evidence further supports the brain–gene association with obesity vulnerability.
A possible explanation of the genetic correlations is pleiotropy—the existence of a common set of genes that influence variance in both obesity and brain function. It is possible that people with a higher genetic risk for obesity also have genetic propensity for the brain and cognitive patterns outlined here. It is also likely that interventions could influence both obesity and brain function. For instance, regular exercise can support weight management (39), reduce the heritability of obesity (40), and improve cognitive health (41).
However, our results could also support a causal relationship—that the genetic correlation is due to a persistent effect of heritable brain factors on overeating and, hence, BMI. For instance, we could hypothesize that the heritable obesity-related cognitive profile promotes overeating when high-calorie food is available. As high-calorie food is abundant and inexpensive, the cognitive risk profile could lead to repeated overeating, providing an opportunity for genetic obesity proneness to express. Such longitudinal environmental effects of a trait need not to be large, they just have to be consistent (ref. 42, see discussion in ref. 43). Of course, a reverse scenario is also possible—obesity leads to alterations in cortical morphology due to the consequences of cardiometabolic complications, including low-grade chronic inflammation, hypertension, and vascular disease (reviewed in refs. 9 and 44). However, we find this hypothesis less plausible as global brain atrophy due to metabolic syndrome is mostly seen in older participants, whereas the current sample had a mean age of 29. Young adults often experience “healthy or transitional obesity,” where clinical inflammation levels (45) and other cardiometabolic comorbidities have not yet developed (46).
We found neurobehavioral PPS-s to have occasional phenotypic and genetic correlations with each other. Here, it is hard to argue against pleiotropy playing a role. While one could reasonably expect that at least part of the variation in cognitive performance would be shaped by brain morphometry (22), it is also the case that engaging in education leads to improvement in cognitive test scores (26) and might also lead to changes in cortical thickness (47). The small genetic overlap between cognition, cortical thickness, and personality can probably be explained by common pleiotropic roots. At the same time, integrating morphometry and cognitive findings is difficult with this dataset.
From a practical point of view, our work suggests that evidence from psychology and neuroscience can be used to design intervention strategies for people with higher genetic risk for obesity. One way would be modifying neurobehavioral factors, e.g., with cognitive training, to improve people’s ability to resist the obesogenic environment (31, 36). Another path could be changing the immediate environment to be less obesogenic (e.g., ref. 48) so that individual differences in neurobehavioral factors would be less likely to manifest. In any case, obesity interventions should not focus solely on energy content, but also acknowledge the certain neurobehavioral profile that obesity is genetically intertwined with.
The current analysis has limitations. Due to the cross-sectional nature of the dataset, causality between neurobehavioral factors and BMI is only suggestive—longitudinal designs would enable better insight into the causal associations between brain morphology, psychological measures, and BMI or weight gain. BMI is a crude proxy for actual eating behaviors or health status. In addition, there were more normal-weight than obese participants. However, the 25% obesity rate in this sample is close to the published obesity rate of the state of Missouri (31.7%) and the United States (36.5%, ref. 49). Also, we expect that BMI itself and the neurobehavioral mechanisms behind it are continuum processes, therefore all variation in the range from normal weight to obesity is likely helping to uncover underlying associations. While the measurement of cognition and personality was exhaustive, it lacked some common behavioral tasks like the stop-signal task, or common questionnaires measuring self-control, impulsivity, and eating-specific behaviors, that have been previously associated with body weight (5, 6). Particularly, the common eating-specific behaviors such as uncontrolled eating (50) are likely better candidates for explaining brain morphology–BMI associations as they are more directly related to the hypothesized underlying behavior.
One has to be careful in translating individual differences in cortical thickness in normal populations to underlying neural mechanisms. Diverse biological processes have been suggested to influence MRI-based cortical thickness measures, ranging from synaptic density to apparent thinning due to synaptic pruning and myelination (summarized in refs. 51 and 52). A definitive model of the underlying mechanism that links normal variations in cortical thickness to differences in brain function cannot be given, as cortical thickness has not been mapped with both MRI and histology in humans (52). Still, the associations between cortical thickness and BMI in one sample were able to predict BMI in a new separate sample, suggesting that the pattern is robust. Our conceptual interpretation of the significance of cortical thickness patterns has support from measures of both brain structure and cognitive function.
Relying on PPS-s prevented us from analyzing detailed interactions between cortical thickness and cognitive function and their genetic overlap with each other. However, given the relatively small associations between PPS-s and the number of candidate measures that could be expected to interact with one another, we believe it would have been hard to find an association that would have survived multiple testing corrections. Future, focused, hypothesis-driven studies have to further elucidate the neurobehavioral mechanisms behind obesity proneness.
In summary, the current analysis provides comprehensive evidence that the obesity-related differences in brain structure and cognitive tests are largely due to shared genetic factors. Genetic factors also explain occasional overlap between neurobehavioral factors. We hope that increasingly larger longitudinal datasets and dedicated studies will help to outline more specific neurobehavioral mechanisms that confer vulnerability to obesity and provide a basis for designing informed interventions.
Methods
Data were provided by the Human Connectome Project (24). Certain people were excluded due to missing data or not fulfilling typical criteria. Exclusion details, demographics, and family structure are summarized in SI Appendix, SI Methods and Table S1. Software pipelines for obtaining features of cortical thickness and brain volume are described in SI Appendix, SI Methods. Analysis scripts to reproduce results presented are available at: https://osf.io/htx7u.
SI Appendix, Fig. S1 provides a schematic pipeline for data analysis. Details of each data analysis step are outlined in SI Appendix, SI Methods. We describe how PPS weights are obtained through cross-validation and how the weights generalize to a separate dataset (S1200n). We further describe the main principles of twin and sibling-based heritability analysis and replication of these findings using individual features instead of PPS-s and replication in a separate dataset (S1200n). Finally, the software and packages used are listed.
Supplementary Material
Acknowledgments
Supported by funding from Canadian Institutes of Health Research (A.D.) and Fonds de Recherche du Québec – Santé (U.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.H.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718206115/-/DCSupplemental.
References
- 1.Di Angelantonio E, et al. Global BMI Mortality Collaboration Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. 2010;3:285–295. doi: 10.2147/DMSOTT.S7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elks CE, et al. Variability in the heritability of body mass index: A systematic review and meta-regression. Front Endocrinol (Lausanne) 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke AE, et al. LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaud A, Vainik U, Garcia-Garcia I, Dagher A. Overlapping neural endophenotypes in addiction and obesity. Front Endocrinol (Lausanne) 2017;8:127. doi: 10.3389/fendo.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vainik U, Dagher A, Dubé L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: A systematic review. Neurosci Biobehav Rev. 2013;37:279–299. doi: 10.1016/j.neubiorev.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery RL, Levine MD. Questionnaire and behavioral task measures of impulsivity are differentially associated with body mass index: A comprehensive meta-analysis. Psychol Bull. 2017;143:868–902. doi: 10.1037/bul0000105. [DOI] [PubMed] [Google Scholar]
- 8.Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev. 2016;64:35–62. doi: 10.1016/j.neubiorev.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Willette AA, Kapogiannis D. Does the brain shrink as the waist expands? Ageing Res Rev. 2015;20:86–97. doi: 10.1016/j.arr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- 11.Winkler AM, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veit R, et al. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin. 2014;6:307–311. doi: 10.1016/j.nicl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medic N, et al. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes. 2016;40:1177–1182. doi: 10.1038/ijo.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mole TB, Mak E, Chien Y, Voon V. Dissociated accumbens and hippocampal structural abnormalities across obesity and alcohol dependence. Int J Neuropsychopharmacol. 2016;19:pyw039. doi: 10.1093/ijnp/pyw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marioni RE, et al. CHARGE Cognitive Working Group Assessing the genetic overlap between BMI and cognitive function. Mol Psychiatry. 2016;21:1477–1482. doi: 10.1038/mp.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spieker EA, et al. Shared genetic variance between obesity and white matter integrity in Mexican Americans. Front Genet. 2015;6:26. doi: 10.3389/fgene.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapuano KM, et al. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc Natl Acad Sci USA. 2017;114:160–165. doi: 10.1073/pnas.1605548113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Roige S, et al. 23andMe Research Team Genome-wide association study of delay discounting in 23,217 adult research participants of European ancestry. Nat Neurosci. 2018;21:16–18. doi: 10.1038/s41593-017-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancaster TM, Ihssen I, Brindley LM, Linden DE. Preliminary evidence for genetic overlap between body mass index and striatal reward response. Transl Psychiatry. 2018;8:19. doi: 10.1038/s41398-017-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opel N, et al. Prefrontal gray matter volume mediates genetic risks for obesity. Mol Psychiatry. 2017;22:703–710. doi: 10.1038/mp.2017.51. [DOI] [PubMed] [Google Scholar]
- 21.Weise CM, et al. The obese brain as a heritable phenotype: A combined morphometry and twin study. Int J Obes. 2017;41:458–466. doi: 10.1038/ijo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Allen TA, DeYoung CG. The Oxford Handbook of the Five Factor Model. Oxford Univ Press; Oxford: 2017. Personality neuroscience and the five factor model. [Google Scholar]
- 24.Van Essen DC, et al. WU-Minn HCP Consortium The WU-Minn human connectome project: An overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J. Personality traits and body weight: Evidence using sibling comparisons. Soc Sci Med. 2016;163:54–62. doi: 10.1016/j.socscimed.2016.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie SJ, Tucker-Drob EM. How much does education improve intelligence? A meta-analysis. Psychol Sci. 2018;29:1358–1369. doi: 10.1177/0956797618774253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mõttus R, Realo A, Vainik U, Allik J, Esko T. Educational attainment and personality are genetically intertwined. Psychol Sci. 2017;28:1631–1639. doi: 10.1177/0956797617719083. [DOI] [PubMed] [Google Scholar]
- 28.Noble KG, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ad-Dab’bagh Y, et al. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping. Elsevier; New York: 2006. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research; p. 2266. [Google Scholar]
- 30.Collins DL, Evans AC. Animal: Validation and applications of nonlinear registration-based segmentation. Int J Pattern Recognit Artif Intell. 1997;11:1271–1294. [Google Scholar]
- 31.Val-Laillet D, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 33.Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hargrave SL, Jones S, Davidson TL. The outward spiral: A vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci. 2016;9:40–46. doi: 10.1016/j.cobeha.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neseliler S, Han J-E, Dagher A. The use of functional magnetic resonance imaging in the study of appetite and obesity. In: Harris RBS, editor. Appetite and Food Intake: Central Control. 2nd Ed CRC/Taylor & Francis; Boca Raton, FL: 2017. [Google Scholar]
- 36.Jansen A, Houben K, Roefs A. A cognitive profile of obesity and its translation into new interventions. Front Psychol. 2015;6:1807. doi: 10.3389/fpsyg.2015.01807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cereb Cortex. 2017;28:988–997. doi: 10.1093/cercor/bhx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vainik U, Mõttus R, Allik J, Esko T, Realo A. Are trait–outcome associations caused by scales or particular items? Example analysis of personality facets and BMI. Eur J Pers. 2015;29:688–634. [Google Scholar]
- 39.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation. 2012;125:1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horn EE, Turkheimer E, Strachan E, Duncan GE. Behavioral and environmental modification of the genetic influence on body mass index: A twin study. Behav Genet. 2015;45:409–426. doi: 10.1007/s10519-015-9718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 42.Dickens WT, Flynn JR. Heritability estimates versus large environmental effects: The IQ paradox resolved. Psychol Rev. 2001;108:346–369. doi: 10.1037/0033-295x.108.2.346. [DOI] [PubMed] [Google Scholar]
- 43.Tucker-Drob EM, Harden KP. Intellectual interest mediates gene-by-SES interaction on adolescent academic achievement. Child Dev. 2012;83:743–757. doi: 10.1111/j.1467-8624.2011.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillemot-Legris O, Muccioli GG. Obesity-induced neuroinflammation: Beyond the hypothalamus. Trends Neurosci. 2017;40:237–253. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Cheng HL, et al. Iron, hepcidin and inflammatory status of young healthy overweight and obese women in Australia. PLoS One. 2013;8:e68675. doi: 10.1371/journal.pone.0068675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng HL, Medlow S, Steinbeck K. The health consequences of obesity in young adulthood. Curr Obes Rep. 2016;5:30–37. doi: 10.1007/s13679-016-0190-2. [DOI] [PubMed] [Google Scholar]
- 47.Engvig A, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 48.Appelhans BM, French SA, Pagoto SL, Sherwood NE. Managing temptation in obesity treatment: A neurobehavioral model of intervention strategies. Appetite. 2016;96:268–279. doi: 10.1016/j.appet.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.CDC 2017 New adult obesity maps. Cent Dis Control Prev. Available at https://www.cdc.gov/obesity/data/prevalence-maps.html. Accessed March 26, 2018.
- 50.Vainik U, Neseliler S, Konstabel K, Fellows LK, Dagher A. Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite. 2015;90:229–239. doi: 10.1016/j.appet.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Fjell AM, et al. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci USA. 2015;112:15462–15467. doi: 10.1073/pnas.1508831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT. Through thick and thin: A need to reconcile contradictory results on trajectories in human cortical development. Cereb Cortex. 2017;27:1472–1481. doi: 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.